Abstract
Background:
Of the 38 Medicaid programs that risk adjust payments to Medicaid managed care organizations (MCOs), 33 of them use the Chronic Illness and Disability Payment System (CDPS). There has been recent interest in adding social determinants of health (SDH) into risk-adjustment models.
Objective:
To update the CDPS models using recent MCO data based on the International Classification of Diseases version 10 coding system and to explore whether indicators of SDH are predictive of expenditures.
Research Design:
Data from 3 national Medicaid MCOs and 8 states are used to update the CDPS model. We test whether spending on Medicaid beneficiaries living in economically and socially deprived communities is greater than spending on similar beneficiaries in less deprived communities.
Subjects:
Medicaid beneficiaries with full benefits and without dual eligibility under Medicare enrolled in Medicaid MCOs in 8 states during 2017–2019, including 1.4M disabled beneficiaries, 9.2M children, and 6.4M adults.
Measures:
Health care eligibility and claims records. Indicators based on the Social Deprivation Index were used to measure SDH.
Results:
The revised CDPS model has 52 CDPS categories within 19 major categories. Six major categories of CDPS were revised: Psychiatric, Pulmonary, Renal, Cancer, Infectious Disease, and Hematological. We found no relationship between health care spending and the Social Deprivation Index.
Conclusions:
The revised CDPS models and regression weights reflect the updated International Classification of Diseases-10 coding system and recent managed care delivery. States should choose alternative payment strategies to address disparities in health and health outcomes.
Key Words: risk adjustment, Medicaid, social determinants of health
Of the 38 Medicaid programs that risk adjust payments to Medicaid managed care organizations (MCOs), 33 of them use the Chronic Illness and Disability Payment System (CDPS) or a related model (Table 1). Risk-adjustment models typically use diagnosis or pharmaceutical codes from claims or encounter data to measure illness burden among groups of beneficiaries enrolled in managed care plans. The resulting risk scores are then used to adjust capitation payments received by the plans to account for differences in the severity of illness in their enrolled populations. The CDPS model maps International Classification of Disease (ICD) codes to CDPS categories.1 Medicaid Rx is a pharmacy-based system that uses National Drug Classification (NDC) data from claims and encounters to develop profiles based on types of pharmacotherapy.2 CDPS+Rx is a combination of the CDPS and Medicaid Rx (MRX) models.
TABLE 1.
Risk-Adjustment Efforts by State
| Population | Year | Classification | |
|---|---|---|---|
| State | Covered | Implemented | System |
| Arizona | SSI + TANF + EXP | 2020 | CDPS+Rx |
| California | SSI + TANF + EXP | 2009 | Medicaid Rx |
| Colorado | SSI + TANF | 1997 | CDPS |
| Delaware | SSI + TANF + EXP | 2000 | CDPS+Rx |
| District of Columbia | SSI + TANF | 2014 | CDPS+Rx |
| Florida | SSI + TANF | 2006 | CDPS+Rx |
| Georgia | TANF | 2017 | CDPS+Rx |
| Hawaii | SSI + TANF + EXP | 2014 | CDPS+Rx |
| Illinois | SSI + TANF + EXP | 2011 | CDPS+Rx |
| Indiana | SSI + TANF + EXP | 2015 | CDPS+Rx |
| Iowa | SSI + TANF + EXP | 2016 | CDPS+Rx |
| Kansas | SSI + TANF + CHIP | 2018 | CDPS+Rx |
| Kentucky | SSI + TANF + EXP | 2019 | CDPS+Rx |
| Louisiana | SSI + TANF + EXP | 2012 | ACG |
| Maryland | SSI + TANF | 1997 | ACG |
| Massachusetts | SSI + TANF | 2009 | DxCG |
| Michigan | SSI + TANF + EXP | 2000 | CDPS+Rx |
| Minnesota | TANF + EXP + BHP + SSI | 2000 | CDPS+Rx |
| Mississippi | SSI + TANF | 2017 | CDPS+Rx |
| Missouri | TANF | 2012 | CDPS+Rx |
| Nebraska | SSI + TANF | 2018 | CDPS+Rx |
| Nevada | TANF + EXP | * | CDPS+Rx |
| New Hampshire | SSI+ TANF + EXP | 2014 | CDPS+Rx |
| New Jersey | SSI + TANF + EXP | 2000 | CDPS+Rx |
| New Mexico | SSI + TANF + EXP | 2021 | CDPS+Rx |
| New York | SSI + TANF | 2008 | CRG |
| North Carolina | SSI + TANF | 2021 | CDPS+Rx |
| Ohio | SSI + TANF | 2006 | CDPS+Rx |
| Oregon | SSI + TANF + EXP | 1998 | CDPS+Rx |
| Pennsylvania | SSI + TANF + EXP | 2003 | CDPS+Rx |
| South Carolina | SSI + TANF | 2009 | CDPS+Rx |
| Tennessee | SSI + TANF | 2000 | ACG |
| Texas | SSI + TANF + CHIP | 2007 | CDPS+Rx |
| Utah | SSI | 1998 | CDPS |
| Virginia | SSI + TANF + EXP | 2003 | CDPS |
| Washington | SSI + TANF + CHIP + EXP | 2003 | CDPS+Rx |
| Wisconsin | SSI + TANF | 2011 | CDPS+Rx |
| Puerto Rico | SSI + TANF + EXP | 2018 | CDPS+Rx |
Unknown.
ACG indicates Ambulatory Care Groups; CDPS, Chronic Illness and Disability Payment System; CHIP, Children's Health Insurance Program; CRG, Clinical Risk Groups; DxCG, Diagnostic Cost Groups; EXP, Medicaid expansion population; SSI, disabled Medicaid beneficiaries; TANF, Medicaid eligibility under temporary aid to needy families or other family categories.
The CDPS model focuses on conditions more common among Medicaid beneficiaries including persons with disabilities. In this way, CDPS differs from other risk-adjustment models such as the Centers for Medicare & Medicaid Services-Hierarchical Coexisting Conditions (CMS-HCC) model that was developed for Medicare using Medicare data3 or the HHS-HCC model developed for the state health exchanges using commercial claims and encounter data.4 CDPS was originally designed to be predictive of spending while being resistant to gaming and over coding. Thus, the original CDPS model included 58 categories within 19 hierarchies compared with 76 categories used in the V22 version of the CMS-HCC model and 127 HHS-HCCs.
The CDPS was initially developed in 2000 using data from 7 fee-for-service (FFS) state Medicaid programs. The model received major updates in 2009, using national FFS Medicaid data from 2002 to 2005, and in 2014, using national FFS Medicaid data from 2011. In 2016, ICD-10 codes were incorporated into CDPS using the CMS General Equivalence Mappings. CDPS has received regular annual updates to include the most recent ICD and NDC codes.
This paper describes an update to the CDPS models. We used data from 3 national Medicaid MCOs to update the mapping of diagnoses to CDPS categories, taking advantage of the revised ICD coding system and reflecting changes in health care practice over time as well as any differences in practice in MCOs. To the extent that new treatments and technology have changed how patients are treated, the relative weights estimated with 2011 data may not accurately reflect the relative cost in 2019. In 2020, 72% of Medicaid beneficiaries were enrolled in managed care plans.5 To the extent that treatment patterns differ across managed care and FFS, the relative weights estimated in earlier versions of CDPS may not accurately reflect the treatment patterns in MCOs.
Further, we test whether spending on Medicaid beneficiaries living in economically and socially deprived communities is greater than spending on similar beneficiaries in less deprived communities. This work contributes to the growing literature exploring whether and how risk adjustment can be used to address the disparities in health and health outcomes experienced by patients with greater social needs.6–9
METHODS
Data and Setting
Health care eligibility and claims records were provided by 3 national Medicaid MCOs. The data covered 3 years, 2017–2019, and 8 states: Florida, Illinois, Kansas, Kentucky, Louisiana, Michigan, New Jersey, and Washington. The data were limited to Medicaid beneficiaries with full benefits and without dual eligibility under Medicare. The data included disabled beneficiaries and nondisabled child (age below 19) and adult (age 19 years or above) beneficiaries, including those covered under Medicaid expansions. Eligibility data included demographics (age and sex), aid category, and months enrolled during the year. Claims data included the year of service, procedure, ICD and NDC codes, and the amounts paid by the organizations to providers for their services. The resulting data include just over 17M person-year observations: 1.4M disabled beneficiaries, 9.2M children, and 6.4M adults.
CDPS Analysis Methods
The 2000 CDPS model was developed using an iterative process that combined clinical and economic expertise. ICD codes were initially ordered within major categories corresponding to body systems or types of disease. These codes were then combined, typically at the 3-digit level, into stage 1 groups. Linear regression of stage 1 group indicators on health expenditures was used to generate estimated coefficients for these stage 1 groups. Linear regression was chosen in favor of alternatives, including nonlinear regression, multipart, or machine-learning models. Linear regression has traditionally been used in risk adjustment, and introducing a new modeling approach would add complexity to the interpretation of the coefficients and application of the models to adjust payment. In addition, the CDPS algorithm was developed using linear regression, and it is not clear if the linear function form of CDPS could be accurately applied using nonlinear models.
Two specifications of expenditures are used in the CDPS model. Concurrent expenditures are those that occur within the year that the diagnoses are assigned to CDPS categories. Prospective expenditures are those that occur within the following year. In the concurrent model, at least 6 months of enrollment is required to establish stable CDPS profiles. The prospective model additionally requires at least 1 month of enrollment in the following year. The dependent variable is the ratio of monthly health care expenditures to average expenditures by category of enrollment. Beneficiaries without any expenditures in the year are included in the analysis with a ratio of zero. Regressions are weighted by months of enrollment. Separate models are estimated for the disabled, children, and adults.
Multiple iterations of these regressions were reviewed by a team of clinical experts and health service researchers. The results were used to combine stage 1 groups into CDPS categories and to create hierarchies within major categories. Combining the stage 1 groups into CDPS categories and categories into hierarchies helps to reduce the potential overfitting of the model to the data and reduces the incentive and ability for upcoding and gaming the algorithm to maximize reimbursement. Diagnoses were excluded if they are not associated with costs or if they were clinically not well defined. Diagnoses from laboratory or radiology claims were excluded since they are sometimes listed as “rule-out” diagnoses. The 2000 model had 58 CDPS categories within 19 major categories. Categories higher in the hierarchy had greater coefficients and generally lower numbers of beneficiaries than categories lower in the hierarchy.
A similar approach was used in the 2022 revision. An initial run of the 2000 model using newer data from 3 national Medicaid MCOs showed that some of the hierarchies were not maintained. Stage 1 groups were recreated using ICD-10 codes for each of the affected major categories. Linear regression analyses were conducted by each major category after replacing the respective CDPS categories with stage 1 groups. These regression results were reviewed to determine which stage 1 groups should be reordered to improve the hierarchy while maintaining clinical accuracy. The updated CDPS model was re-estimated after each reordering. If the hierarchy still appeared insufficient, then the stage 1 groups were revisited and reordered. Iterations of this process were continued until each major category had an acceptable hierarchy.
In developing the 2000 model, to avoid overfitting the data, we used a split-sample design, whereby we developed the model on a 75% sample of the data and compared the result when applying the resulting algorithm to the remaining 25% of the data. In this revision, we decided against using a split-sample approach for the reason that this revision did not involve as much adding diagnoses as it did reordering diagnoses. Thus, we were less concerned with overfitting than we were with having an adequate sample size to revise and re-estimate the model.
MRX Analysis Methods
An initial run of the MRX model showed that the model was relatively stable over time. However, some MRX categories had very low or negative coefficients. Some of these categories were dropped from the model. Others were restricted to classes of medications appropriate for the most serious manifestations of disease in that category. Several MRX categories related to infectious diseases were incorporated into a hierarchy. Finally, a new category was created for Rare Diseases. This category includes medications that are used in patients with a disease state prevalence of fewer than 20,000 in the United States and in which the drug cost is $150,000 or more for the average weight and dosing.
CDPS+Rx Analysis Methods
The CDPS+Rx model includes all CDPS categories and a subset of 15 restricted MRX categories that we consider to be the least affected by variations in physician prescribing patterns. An initial run of the model included each of these categories along with CDPS. The resulting coefficients were examined to determine where the MRX categories would be most appropriately included in the CDPS hierarchies. An algorithm was developed to integrate the 15 MRX categories into CDPS.
Social Deprivation Index
Indicators based on the Social Deprivation Index (SDI) were used to examine whether spending on Medicaid beneficiaries living in economically and socially deprived communities is greater than spending on similar beneficiaries in less deprived communities.10 The SDI is a composite measure of disparity that uses place-based data on education, employment, income, density, car and home ownership, and family structure to assign an index value to a geographic area. We used the zip code of residence to assign each beneficiary an SDI value and included these values as a set of categorical indicators (rounded up to the tens place) into the CDPS regression analysis. We considered using information on beneficiary race and ethnicity and using Z-codes to identify unhoused beneficiaries, but concerns from our MCO partners, which we shared, about incomplete and inconsistent coding caused us to use area characteristics as a measure of disadvantage. This approach follows the recommendations of a committee report from the National Academy of Medicine.3
RESULTS
The revised CDPS+Rx model is shown in Table 2. The revised model has 52 CDPS categories within 19 major categories. Six CDPS major categories were revised: psychiatric, pulmonary, renal, cancer, infectious disease, and hematological. Table 3 provides counts for the 15 restricted MRX categories and shows where they were integrated into the CDPS model. The 15 restricted MRX indicators were included in 8 major CDPS categories and at every level of severity from extra high to extra low. The MRX category for Rare Diseases did not fit naturally within any of the CDPS major categories and thus remained as an independent category with relatively few individuals but very high-estimated coefficients. More detail on changes made in updating the models is available on the CDPS website: https://hwsph.ucsd.edu/research/programs-groups/cdps.html.
TABLE 2.
Concurrent CDPS Model
| CDPS | CDPS description | SSI | TANF child | TANF adult | SSI N | TANF child N | TANF adult N |
|---|---|---|---|---|---|---|---|
| Baseline | Intercept | 0.018 | 0.159 | 0.066 | 1,438,330 | 9,246,704 | 6,417,719 |
| CARVH | Cardiovascular, very high | 2.535 | 94.17 | 7.210 | 12,841 | 1476 | 7599 |
| CARM | Cardiovascular, medium | 0.580 | 8.486 | 1.469 | 138,967 | 38,222 | 192,901 |
| CARL | Cardiovascular, low | 0.159 | 2.963 | 0.512 | 152,303 | 134,182 | 323,763 |
| CAREL | Cardiovascular, extra low | 0.068 | 0.583 | 0.120 | 338,525 | 48,853 | 1,127,054 |
| PSYH | Psychiatric, high | 0.776 | 5.839 | 2.033 | 73,896 | 2763 | 35,375 |
| PSYM | Psychiatric, medium | 0.545 | 2.721 | 1.087 | 26,082 | 30,946 | 52,120 |
| PSYL | Psychiatric, low | 0.269 | 1.206 | 0.363 | 547,021 | 903,702 | 1,310,917 |
| SKCM | Skeletal, medium | 0.706 | 1.776 | 2.336 | 78,613 | 178,683 | 115,682 |
| SKCL | Skeletal, low | 0.182 | 0.714 | 0.509 | 178,464 | 354,555 | 442,149 |
| SKCVL | Skeletal, very low | 0.041 | 0.167 | 0.226 | 103,037 | 48,727 | 280,369 |
| CNSH | CNS, high | 1.924 | 15.12 | 8.246 | 16,393 | 2875 | 9055 |
| CNSM | CNS, medium | 0.273 | 2.270 | 1.312 | 47,191 | 13,570 | 38,667 |
| CNSL | CNS, low | 0.273 | 2.209 | 0.637 | 250,565 | 134,202 | 345,727 |
| PULVH | Pulmonary, very high | 2.398 | 64.31 | 7.490 | 28,426 | 7078 | 15,563 |
| PULH | Pulmonary, high | 1.596 | 6.583 | 2.647 | 5960 | 20,423 | 4971 |
| PULM | Pulmonary, medium | 0.754 | 4.487 | 1.645 | 87,675 | 68,431 | 133,525 |
| PULL | Pulmonary, low | 0.089 | 0.488 | 0.256 | 283,555 | 818,127 | 572,755 |
| GIH | Gastro, high | 1.508 | 27.979 | 4.131 | 25,576 | 9663 | 18,043 |
| GIM | Gastro, medium | 0.341 | 4.612 | 0.980 | 71,167 | 19,189 | 162,910 |
| GIL | Gastro, low | 0.121 | 0.602 | 0.346 | 252,498 | 369,009 | 651,483 |
| DIA1 | Diabetes, type 1 | 0.796 | 5.951 | 1.928 | 27,790 | 14,176 | 50,078 |
| DIA2 | Diabetes, type 2 | 0.247 | 0.428 | 0.463 | 226,426 | 9320 | 493,269 |
| SKNH | Skin, high | 1.281 | 21.289 | 5.443 | 11,773 | 1007 | 6580 |
| SKNL | Skin, low | 0.416 | 4.418 | 1.342 | 18,496 | 1952 | 22,267 |
| SKNVL | Skin, very low | 0.132 | 0.410 | 0.257 | 110,561 | 333,866 | 364,461 |
| RENEH | Renal, extra high | 2.585 | 154.3 | 7.521 | 9241 | 278 | 4207 |
| RENM | Renal, medium | 0.170 | 3.227 | 0.918 | 66,143 | 6921 | 72,275 |
| RENL | Renal, low | 0.170 | 0.583 | 0.453 | 87,216 | 86,449 | 124,946 |
| SUBL | Substance abuse, low | 0.283 | 5.584 | 0.964 | 85,008 | 13,602 | 269,156 |
| SUBVL | Substance abuse, very low | 0.000 | 1.393 | 0.291 | 31,655 | 4102 | 84,225 |
| CANVH | Cancer, very high | 3.641 | 32.19 | 11.00 | 12,893 | 441 | 10,908 |
| CANH | Cancer, high | 1.352 | 9.926 | 4.557 | 20,321 | 4792 | 22,871 |
| CANM | Cancer, medium | 0.400 | 4.995 | 1.151 | 16,238 | 4608 | 27,357 |
| CANL | Cancer, low | 0.000 | 0.933 | 0.377 | 19,285 | 1793 | 38,289 |
| DDM | DD, medium | 0.287 | 3.711 | 4.807 | 6053 | 367 | 191 |
| DDL | DD, low | 0.075 | 3.661 | 0.957 | 53,914 | 21,540 | 6558 |
| GENEL | Genital, extra low | 0.059 | 0.932 | 0.273 | 66,038 | 74,100 | 331,776 |
| METH | Metabolic, high | 0.936 | 5.278 | 1.189 | 36,738 | 44,356 | 61,586 |
| METM | Metabolic, medium | 0.446 | 4.950 | 0.832 | 96,893 | 25,325 | 190,138 |
| PRGCMP | Pregnancy, complete | 0.338 | 2.327 | 1.180 | 14,030 | 17,714 | 488,479 |
| PRGINC | Pregnancy, incomplete | 0.023 | 0.709 | 0.178 | 4538 | 8285 | 119,729 |
| EYEL | Eye, low | 0.333 | 2.624 | 0.559 | 10,859 | 5809 | 23,365 |
| EYEVL | Eye, very low | 0.055 | 0.421 | 0.282 | 77,661 | 41,208 | 121,812 |
| CERM | Cerebrovascular, medium | 0.413 | 7.697 | 2.576 | 21,265 | 1334 | 13,690 |
| INFVH | Infectious, very high | 4.717 | 29.05 | 7.281 | 1,915 | 938 | 2195 |
| INFH | Infectious, high | 2.305 | 13.72 | 4.871 | 18,223 | 2966 | 37,666 |
| INFM | Infectious, medium | 1.100 | 1.473 | 2.437 | 52,775 | 101,033 | 95,030 |
| INFL | Infectious, low | 0.000 | 0.426 | 0.213 | 42,274 | 6291 | 100,388 |
| HEMEH | Hematological, extra high | 13.16 | 40.34 | 19.87 | 582 | 1115 | 762 |
| HEMVH | Hematological, very high | 2.292 | 40.34 | 5.894 | 8,190 | 1716 | 7315 |
| HEMM | Hematological, medium | 0.836 | 2.416 | 1.224 | 17,557 | 28,484 | 25,937 |
| HEML | Hematological, low | 0.638 | 2.416 | 1.224 | 28,573 | 28,219 | 58,004 |
| MRX13 | Rare diseases | 26.57 | 145.5 | 70.6 | 429 | 302 | 224 |
| R-squared | — | 0.26 | 0.11 | 0.39 | — | — | — |
Demographic indicators and interaction terms for disabled children are not shown.
All coefficients are significant at P<0.0001.
SSI indicates disabled Medicaid beneficiaries; TANF Child, children (age<19) eligible under temporary aid to need families or other family categories; TANF adult, adults (age 19+) eligible under temporary aid to need families or other family categories or Medicaid expansion.
TABLE 3.
Mapping of Restricted MRX Categories to CDPS
| MRX | MRX description | CDPS mapping | SSI N | TANF adult N | TANF child N |
|---|---|---|---|---|---|
| MRX1 | Anti-coagulants | CARM | 45,668 | 1506 | 62,293 |
| MRX2 | Cardiac | CAREL | 507,791 | 39,823 | 1,230,917 |
| MRX3 | Psychosis/bipolar/depression | PSYL | 486,185 | 253,063 | 1,151,716 |
| MRX4 | Diabetes | DIA2 | 202,757 | 30,113 | 443,812 |
| MRX5 | Hemophilia/von Willebrands | HEMEH | 211 | 444 | 195 |
| MRX6 | Hepatitis | INFM | 5563 | 53 | 10,005 |
| MRX7 | HIV | INFM | 10,646 | 1061 | 31,818 |
| MRX8 | Infections, high | INFH | 5318 | 935 | 6856 |
| MRX9 | Inflammatory/autoimmune | SKCM | 13,284 | 6150 | 34,048 |
| MRX10 | Malignancies | CANM | 18,260 | 4671 | 26,921 |
| MRX11 | Multiple sclerosis/paralysis | CNSH | 3849 | 206 | 5159 |
| MRX12 | Parkinsons/tremor | CNSL | 57,954 | 2192 | 53,765 |
| MRX13 | Rare diseases | MRX13 | 429 | 302 | 224 |
| MRX14 | Seizure disorders | CNSL | 60,805 | 25,400 | 50,393 |
| MRX15 | Tuberculosis | INFL | 2024 | 1845 | 5988 |
Source: Authors’ analyses of data from 3 national Medicaid Managed Care plans.
MRX indicates Medicaid Rx model; SSI, disabled Medicaid beneficiaries; TANF Child, children (age<19) eligible under temporary aid to need families or other family categories; TANF adult, adults (age 19+) eligible under temporary aid to need families or other family categories or Medicaid expansion.
The revised CDPS models result in similar predictions to the earlier versions. For the CDPS model, the correlation coefficient between the prediction with the most recent 2000 model and the prediction with the 2020 model ranges from 0.98 to 0.99 by aid category for concurrent and prospective models. The R-square values increase slightly in the new CDPS models compared with the 2000 model: from 0.21 to 0.24 among the disabled and from 0.10 to 0.11 among children, while the R-square in the adult model is the same at 0.35 (data not shown). Correlation coefficients between the most recent and revised MRX models are lower than for the CDPS model: 0.78–0.87, likely as a result of the continuous introduction of new drugs. It is challenging to interpret changes in R-square values among versions of the MRX and CDPS+Rx models since there have been significant changes in pharmacy codes over time.
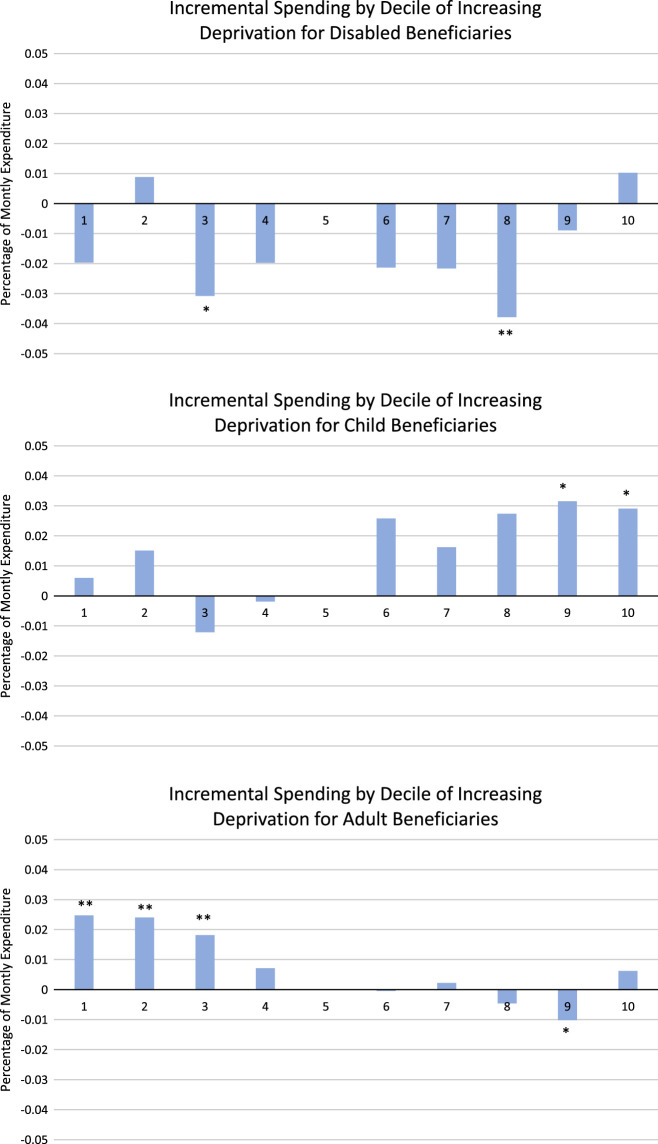
Medicaid beneficiaries living in zip codes with high levels of deprivation as measured by the SDI did not have consistently higher spending than beneficiaries living in zip codes with lower levels of deprivation. Figure 1 shows regression coefficients for SDI variables from concurrent CDPS regressions. Higher deciles of SDI indicate higher levels of deprivation. The coefficients were small (<0.05 of mean expenditure), and only 8 of 27 reached statistical significance, despite the large sample size. Only among children was there any positive relationship between deprivation and spending: among the 2 highest deciles, spending was about 3% higher than in the fifth decile (P<0.05). Among adults, spending was negatively associated with deprivation: among the 3 lowest deciles, spending was 1.8%–2.5% higher than the fifth decile (reference group, P<0.01 each). Among the disabled, deciles 3 and 8 were associated with lower spending (P<0.05 each).
FIGURE 1.
Social Deprivation Index Category Coefficients. Plotted data are regression coefficients from 3 concurrent Chronic Illness and Disability Payment System regressions, including state indicators and categories based on the Social Deprivation Index. An SDI between 50 and 59 serves as the reference category. An * indicates that the coefficient is statistically significantly different from 0 at P<0.05, and ** indicates statistical significance at P<0.01.
The lack of systematic relationship between spending and area deprivation held across multiple model specifications: concurrent versus prospective, with and without state indicators, and in CDPS, MRX, and CDPS+RX (data not shown). Results were similar in regressions restricted to beneficiaries from a single state; in none of the states in our analytic sample were expenditures consistently higher for beneficiaries in more deprived zip codes (data not shown). Further, even in regression models without CDPS indicators as explanatory variables (ie, with demographic and state indicators only), there is little indication that spending is higher on beneficiaries living in more deprived zip codes (data not shown).
DISCUSSION
We report on revisions to the CDPS family of models that reflect patterns of care delivered by 3 national Medicaid MCOs in 2017–2019 based on updated ICD-10 coding. Predicted risk scores using the revised models are similar to risk scores from the original models but can be used with greater confidence by state Medicaid programs and Medicaid MCOs. One limitation of this work is that the regression weights are based on the patterns of care in 3 large Medicaid MCOs and 8 states. It is at least possible that weights would be different if data from other states or other MCOs were available. However, the states included in the analysis encompass all geographic regions and a wide range of Medicaid programs.
We found no relationship between health care spending and social deprivation as measured by the SDI. Our results differ slightly from those of Ash et al7, who find that spending on Massachusetts Medicaid beneficiaries living in the quintile of neighborhoods that are most stressed are 2.1% greater than predicted by a diagnosis-based model. The difference between the 2% finding in Ash and colleagues and our null result may be due to differences in spending patterns between Massachusetts and the states in our analysis or to the fact that Ash and colleagues used census-tract data on neighborhood characteristics, while our analysis was limited to zip code level data.
The lack of relationship between area deprivation and spending may reflect access barriers; beneficiaries living in more deprived areas might need more care than beneficiaries in less deprived areas but might be less able to obtain needed care because of supply constraints. However, if supply constraints were the main reason that there is no gradient between area deprivation and spending, we might have expected to see some evidence of a positive gradient in states with less restrictive Medicaid programs. However, we did not see a positive gradient between area deprivation and spending in our state-specific results.
Our study sample consists entirely of Medicaid beneficiaries, all of whom, by definition, are low income and many of whom, regardless of where they live, face substantial economic and social challenges to good health and obtaining good health care. It should not be surprising that within a Medicaid population, there is no relationship between measures of area deprivation and spending. Notwithstanding this empirical result, states could still choose to employ their Medicaid programs to address disparities in health and health outcomes. If so, we do not recommend attempting to operationalize these efforts using risk adjustment. Instead, we offer 2 alternative approaches currently being pursued in California.
One approach is to pay directly for services that address health disparities. In California, the Department of Health Care Services is addressing health disparities through their CalAIM section 1115 waiver by paying directly for services such as enhanced care management for Medi-Cal beneficiaries with high needs, community supports to help homeless beneficiaries secure and maintain housing, and targeted services for those who are justice involved before release.11 Paying directly for services requires that these services be prespecified. However, it also ensures that additional payments are used to address the identified disparities.
Another approach is to invest in community-based organizations to address health disparities. The Los Angeles County Department of Mental Health is using this approach to build community capacity to address trauma.12 Trauma is a significant public health issue, negatively impacting a range of health outcomes that disproportionately impact vulnerable populations. Recognizing the widespread experience of trauma in the community, as well as their limited ability to address community-wide trauma within traditional community mental health centers, Los Angeles County Department of Mental Health has funded 9 regionally based, community-embedded partnerships with the goal of building capacity to address trauma within their communities. Paying community organizations to address disparities relinquishes some control of funding but leverages community knowledge and trust and can more effectively engage community members in developing sustainable solutions.13
Footnotes
Funding for this work was provided by the Institute for Medicaid Innovation.
The authors declare no conflict of interest.
Contributor Information
Todd Gilmer, Email: tgilmer@ucsd.edu.
Richard Kronick, Email: rkronick@health.ucsd.edu.
REFERENCES
- 1. Kronick R, Gilmer T, Dreyfus T, et al. Improving health-based payment for Medicaid beneficiaries: CDPS. Health Care Financ Rev. 2000;21:29–64. [PMC free article] [PubMed] [Google Scholar]
- 2. Gilmer T, Kronick R, Fishman P, et al. The Medicaid Rx model: pharmacy-based risk adjustment for public programs. Med Care. 2001;39:1188–1202. [DOI] [PubMed] [Google Scholar]
- 3. Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25:119–141. [PMC free article] [PubMed] [Google Scholar]
- 4. Kautter J, Pope GC, Ingber M, et al. The HHS-HCC risk adjustment model for individual and small group markets under the Affordable Care Act. Medicare Medicaid Res Rev. 2014;4:E1–E46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaiser Family Foundation . Medicaid Managed Care Tracker. https://www.kff.org/other/state-indicator/total-medicaid-mco-enrollment/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
- 6. “National Academies of Sciences E, and Medicine” . Accounting for Social Risk Factors in Medicare Payment. The National Academies Press; 2017. [PubMed] [Google Scholar]
- 7. Ash AS, Mick EO, Ellis RP, et al. Social determinants of health in managed care payment formulas. JAMA Intern Med. 2017;177:1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Irvin JA, Kondrich AA, Ko M, et al. Incorporating machine learning and social determinants of health indicators into prospective risk adjustment for health plan payments. BMC Public Health. 2020;20:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Philips AO, Ostrovsky A, Bazemore A. Adjusting Medicaid Payments for Social Risk to Better Support Social Needs. Health Affairs Blog. 2021. DOI: 10.1377/hblog20210526.933567. [DOI]
- 10. Butler DC, Petterson S, Phillips RL, et al. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv Res. 2013;48(2 Pt 1):539–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Department of Health Care Services . Medi-Cal Transformation Initatives https://www.dhcs.ca.gov/CalAIM/Pages/Initiatives.aspx
- 12. Gilmer TP, Center K, Casteel D, et al. Developing trauma resilient communities through community capacity-building. BMC Public Health. 2021;21:1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lansing AE, Romero NJ, Siantz E, et al. Building trust: leadership reflections on community empowerment and engagement in a large urban initiative. BMC Public Health. 2023;23:1252. [DOI] [PMC free article] [PubMed] [Google Scholar]