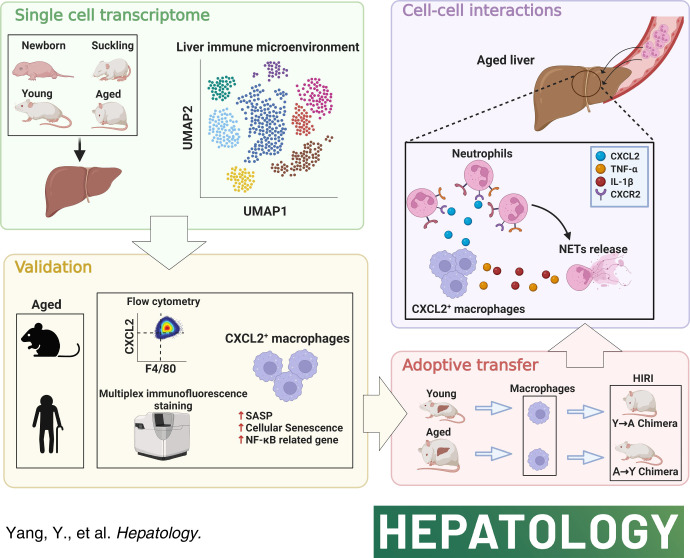
Abstract
Background and Aims:
Immune cells play a crucial role in liver aging. However, the impact of dynamic changes in the local immune microenvironment on age-related liver injury remains poorly understood. We aimed to characterize intrahepatic immune cells at different ages to investigate key mechanisms associated with liver aging.
Approach and Results:
We carried out single-cell RNA sequencing on mouse liver tissues at 4 different ages, namely, the newborn, suckling, young, and aged stages. The transcriptomic landscape, cellular classification, and intercellular communication were analyzed. We confirmed the findings by multiplex immunofluorescence staining, flow cytometry, in vitro functional experiments, and chimeric animal models. Nine subsets of 89,542 immune cells with unique properties were identified, of which Cxcl2 + macrophages within the monocyte/macrophage subset were preferentially enriched in the aged liver. Cxcl2 + macrophages presented a senescence-associated secretory phenotype and recruited neutrophils to the aged liver through the CXCL2-CXCR2 axis. Through the secretion of IL-1β and TNF-α, Cxcl2 + macrophages stimulated neutrophil extracellular traps formation. Targeting the CXCL2-CXCR2 axis limited the neutrophils migration toward the liver and attenuated age-related liver injury. Moreover, the relationship between Cxcl2 + macrophages and neutrophils in age-related liver injury was further validated by human liver transplantation samples.
Conclusions:
This in-depth study illustrates that the mechanism of Cxcl2 + macrophage-driven neutrophil activation involves the CXCL2-CXCR2 axis and provides a potential therapeutic strategy for age-related liver injury.
INTRODUCTION
Liver disease is a major cause of death worldwide,1 and its prevalence is consistently increasing.2,3 Aging is a significant risk factor for liver disease, which presents as a dramatic decline in hepatic regeneration ability, a physiological increase in lipid accumulation, and accelerated fibrosis progression.4,5 The liver serves as a vital immune organ that includes a complex network of intrahepatic immune cells and their interactions. Aging can significantly alter the composition of resident immune cells, thereby exacerbating liver aging and increasing susceptibility to aging-related diseases, such as liver cancer, cirrhosis, and fatty liver disease, leading to a vicious cycle.6 Therefore, there is a crucial need to establish an in-depth understanding of the dynamic change of the local immune microenvironment during liver aging, which would have significant scientific and clinical implications.
Scientists have commonly used bulk RNA-seq to investigate biological mechanisms. However, bulk RNA-seq of the liver can mask changes within certain cell populations that drive functional heterogeneity. Recent advances in single-cell RNA sequencing (scRNA-seq) allow the collection of cellular transcriptomic data from the whole liver and the analysis of cell type-specific changes during the development of diseases. scRNA-seq studies of the liver have been reported, supporting the conclusion that multiple cell populations contribute during aging in mice,7,8 rats,9 and humans.10 However, intrahepatic immune cells have rarely been reported in published studies, hindering a comprehensive description of their cellular functions at different age stages. In addition, the aging process is dynamic during the lifespan,11 and rather than simply dividing the aging process into 2 stages (young and old), a comprehensive description is necessary.
To address these issues, we focused on the immune microenvironment and assessed transcriptomes of 89,542 immune cells of the mouse liver from 4 stages (newborn, suckling, young, and aged), creating the largest comprehensive single-cell transcriptomic atlas in the study of liver aging. Cxcl2 + macrophages presented a senescence-associated secretory phenotype (SASP) and recruited neutrophils to the aged liver through the CXCL2-CXCR2 axis. IL-1 beta (IL-1β) and TNF alpha (TNF-α) secreted by Cxcl2 + macrophages subsequently stimulate neutrophil extracellular trap (NET) formation, and excessive NET accumulation contributes to liver injury.
In conclusion, our work provides a comprehensive understanding of the dynamic changes in the liver immune microenvironment during aging at the single-cell level and identifies the central role of the CXCL2-CXCR2 axis in ameliorating age-dependent liver injury.
METHODS
Isolation of intrahepatic mononuclear cells
Intrahepatic mononuclear cells were obtained according to previously described methods.12 The liver was first perfused in situ with HBSS (Gibco, Australia, without magnesium and calcium) by means of the portal vein in both the young and aged groups or the heart of the mice in the newborn and suckling groups (as the liver volumes in these 2 groups are too small to perfuse in situ) followed by dissection,13 mincing into 1 mm3 pieces, and digestion in buffer containing collagenase II, collagenase IV, DNase I, and CaCl2 (Sigma, USA) at 37°C with gentle agitation for 30 min. RPMI-1640 medium supplemented with heat-inactivated 10% FBS (Gibco) was added to terminate digestion, and the digested liver was successively passed through 70 μm and 40 μm cell strainers, followed by centrifugation at 400 × g and 4°C for 10 min. The samples were subsequently treated with red blood cell lysis buffer (Sigma) for 7 min on ice to lyse the remaining red blood cells. After resuspension in PBS, intrahepatic mononuclear cells from young and aged mice were sorted using mouse CD45 MicroBeads (Miltenyi Biotec) in accordance with the manufacturer’s protocol. However, we did not perform CD45+ sorting for the newborn and suckling groups due to the low count of immune cells in the liver in these 2 groups, which was necessary to mix the cells derived from multiple mice to obtain enough immune cells for sequencing if CD45+ sorting was performed. Although this approach may affect the constitution of the local immune microenvironment, it could reduce the bias derived from the batch effect as much as possible. Cell viability was determined by trypan blue staining and ranged from 85% to 90%. Notably, the livers from newborn mice and suckling mice were directly dissected, minced, and digested without perfusion in situ. More key resources can be found in Supplemental Table S4 http://links.lww.com/HEP/H973.
Statistical analyses
All experiments were performed at least 3 times, and experimental data are expressed as the means ± SD unless otherwise specified. Differences between groups were compared using Student’s t-test assuming a Gaussian distribution. Correlation analyses were performed using Pearson’s correlation coefficients (Figure 7D-F). Statistical analysis was performed using GraphPad Prism 7 software.
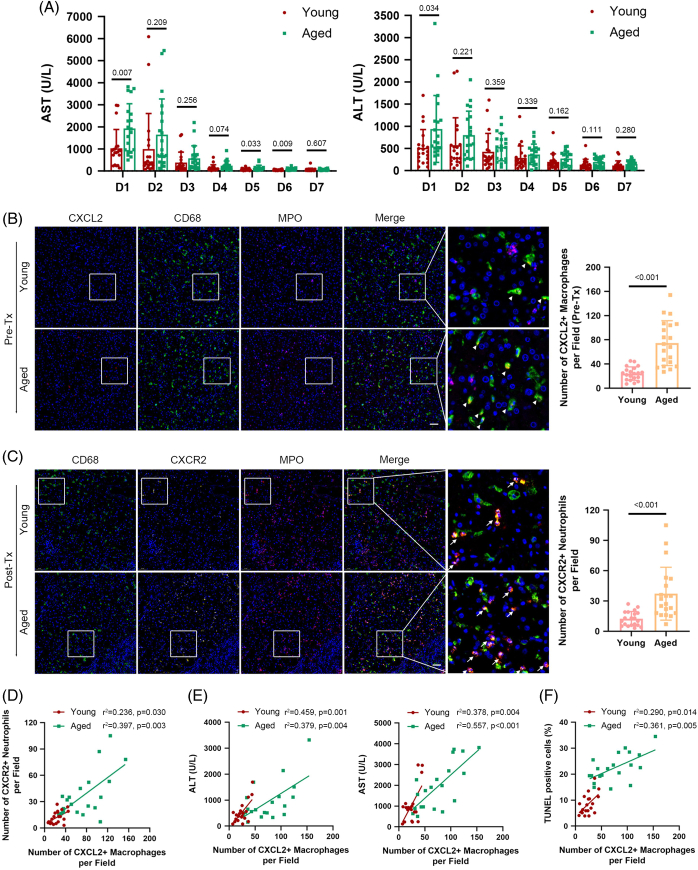
FIGURE 7.
The correlation between the number of CXCL2+ macrophages and age-related liver damage in human OLT during the perioperative period. (A) The curves of sAST and sALT in young (n=20) and aged (n=20) groups of OLT recipients from POD 1 to 7. (B–C) Liver tissues in the young and aged groups were subjected to immunofluorescence staining for CD68 (green fluorescence, macrophages), MPO (violet fluorescence, neutrophils), CXCL2 (red fluorescence), or CXCR2 (yellow fluorescence). Representative immunofluorescence images and statistical analyses show CXCL2 staining in macrophages (triangle marker) in the preperfusion period (upper) and CXCR2 staining in neutrophils (arrowhead) in the reperfusion period (lower). Scale bar: 50 μm. (D–F) The count of CXCL2+ macrophages was positively correlated with the count of CXCR2+ neutrophils (D), the levels of sAST and sALT (E), and the proportion of TUNEL-positive cells (F) in liver tissues on POD 1 and was more notable in the aged group. Data are presented as the means ± SD. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; MPO, myeloperoxidase; POD, postoperative day; sALT, serum alanine aminotransferase; sAST, serum aspartate aminotransferase.
For further details regarding the materials and methods, please refer to the Supporting Information (Extended Materials and Methods, http://links.lww.com/HEP/H971).
RESULTS
Single-cell transcriptomic profiling identified different immune cell types and their distribution changes with age
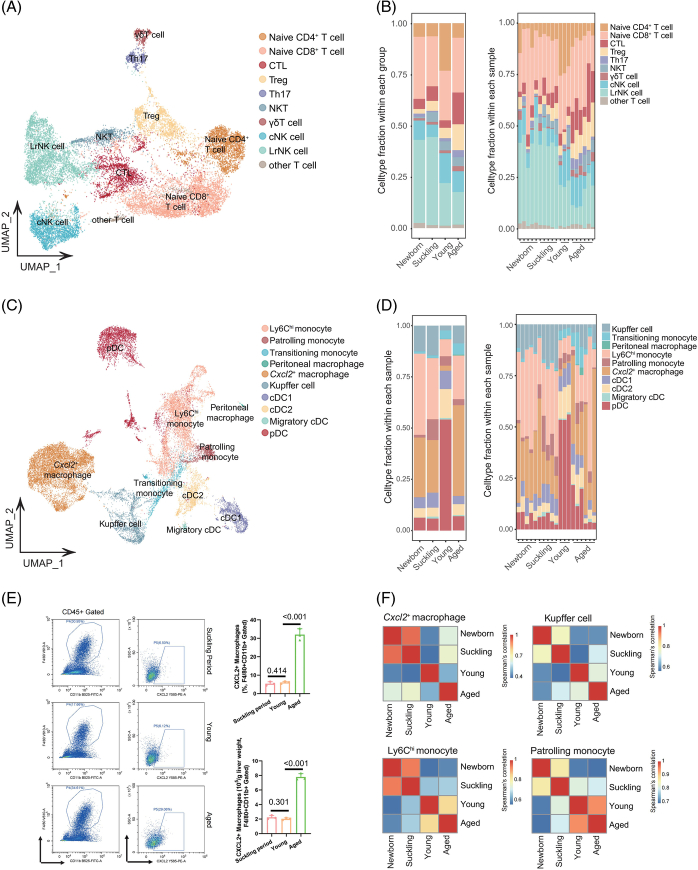
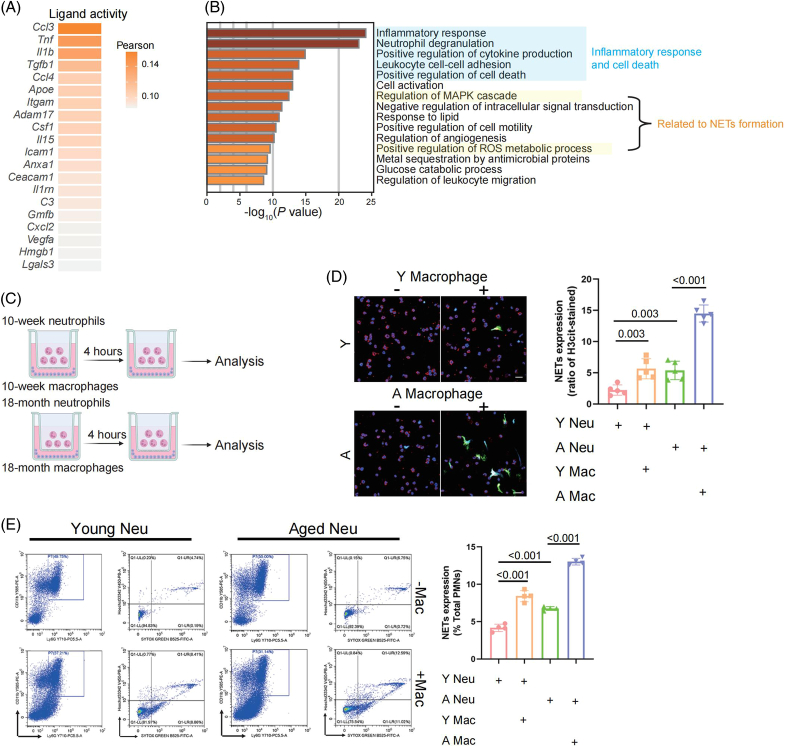
scRNA-seq was carried out on 19 livers harvested from mice at 4 different periods, namely, 5 newborns and 5 suckling (the 7th day after birth), 3 young (10 weeks), and 6 aged (18 mo) mice (Figure 1A).14,15 After qualitative filtering, 112,414 cells were obtained for further analysis, and to focus on the immune microenvironment, we further selected 89,542 immune cells with Ptprc (Cd45) expression. Based on marker gene expression, data from t-distributed Stochastic Neighbor Embedding visualization identified 9 cell types: cycling cells, T/natural killer T (NKT) cells, natural killer (NK) cells, monocytes/macrophages (mono/mac), plasmacytoid dendritic cells, conventional dendritic cells (cDCs), neutrophils, B cells, and plasma cells (Figure 1B-C and Supplemental Figure S1A, http://links.lww.com/HEP/H971, Supplemental Table S1, http://links.lww.com/HEP/H972). Notably, the cells clustered by cell identity, not the experimental batch, indicating the impact of age on the transcriptome (Figure 1B). The distribution of intrahepatic immune clusters in these 4 groups showed that the percentages of cycling cells and neutrophils were higher in both newborn mice and suckling mice, while T/NKT cells were enriched in young and aged mice (Figure 1D). Furthermore, a reduction in mono/mac accompanied by plasmacytoid dendritic cells enrichment was observed only in young livers, while B cells were uniquely decreased in the suckling period (Figure 1D). Consistent with studies published by the Tabula Muris Consortium,7 our results revealed that among the changes in immune cell types, the proportional change in mono/mac between the young and aged stages was the most notable (Figure 1D and Supplemental Figures S1B-C, http://links.lww.com/HEP/H971). This finding further supports the accuracy of our data and enhances the credibility of our subsequent analysis.
FIGURE 1.
Mouse hepatic immune cell transcriptional atlas. (A) Experimental design for scRNA-seq analysis of intrahepatic cells from mice at four age stages. Newborn, n = 5; suckling, n = 5; young, n = 3; aged, n = 6 mice. (B) t-SNE plot of all hepatic immune cells from mice at 4 different ages showing 9 clusters (left). t-SNE plots showing the distribution of hepatic immune cells from mice in different age stages (right). (C) Violin plots showing the expression levels of representative cell type-specific marker genes for the corresponding cell types in the mouse liver. (D) Relative changes in the cell ratios of hepatic immune cells across the 4 age stages (newborn, suckling, young, and aged) (left) and individuals (right). (E) Representative GO BP terms and Reactome pathways enriched with upregulated genes in each age stage in comparison to the other 3 age stages. The top 5 terms for each age group based on the −log10 (p-value) are listed. The color scale from red to gray indicates p values from low to high. DEGs were identified based on the threshold |log2(fold_change)| ≥ 0.5 and adjusted p-value < 0.05. (F) Heatmap showing the expression signatures of the top 10 specifically expressed genes in hepatic immune cells in each age stage; the value for each gene is the row-scaled Z score. Abbreviations: cDC, conventional dendritic cells; GO BP, Gene Ontology Biological Process; Mono/Mac, monocyte and macrophage; pDC, plasmacytoid dendritic cells; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed Stochastic Neighbor Embedding.
Subsequently, age-specific signatures were detected for immune cells from different age stages using pathway enrichment analysis. Genes associated with the immune response, as well as the defense response and cell death, were enriched in aged livers (Figure 1E); these included Cxcl2, encoding secreted proteins involved in immunoregulatory and inflammatory processes; Il1b, encoding the cytokine IL-1β; and Ifitm1, representing genes induced by interferon (Figure 1F and Supplemental Table S2, http://links.lww.com/HEP/H973). In addition, the upregulated genes in young livers were enriched for protein-associated activity (Figure 1E) and included Cd74, which is associated with MHC protein complex assembly,16 and Iglc2, which is involved in immune response activation (Figure 1F). The differently expressed genes (DEGs) in newborn and suckling mice overlapped greatly (Supplemental Figure S1D, http://links.lww.com/HEP/H971), indicating high similarity in intrahepatic immune cells between these two ages. Overall, the single-cell transcriptomic data analysis of intrahepatic immune cells from newborn, suckling, young, and aged mice indicated age-specific immune characteristics in the liver.
Distinct transcriptomic characteristics of immune cell subclusters among different age groups
Next, we attempted to map the characterized immune subclusters during liver aging. T, NK, and NKT cells are all lymphocytes and exhibit similar marker gene expression. Thus, we conducted unsupervised clustering of T/NKT cells and NK cells. According to canonical marker expression, a total of 10 subclusters, namely naïve CD4+ T cells, naïve CD8+ T cells, cytotoxic T lymphocytes, regulatory T cells, T helper 17 cells, NKT cells, gamma delta T cells, conventional NK cells, liver-resident NK cells, and other T cells, appeared within the T/NKT/NK-cell lineages (Figure 2A and Supplemental Figure S2A, http://links.lww.com/HEP/H971). In newborn and suckling mouse livers, there was a higher proportion of liver-resident NK cells. Naïve CD4+ T cells were predominant in the young group. Among the changes in all T/NKT/NK subsets, the proportional change in cytotoxic T lymphocytes between young and aged livers was the most significant, indicating that intracellular infections, such as viral infections and malignant cells, would be much more frequent in elderly individuals (Figure 2B). For T/NKT/NK cells, there were significantly more DEGs in the newborn and suckling mouse livers, and they overlapped greatly (Supplemental Figure S2B, http://links.lww.com/HEP/H971 and Supplemental Table S2, http://links.lww.com/HEP/H973). In addition, functional enrichment analysis revealed that the upregulated genes in the newborn and suckling mouse livers were enriched for those involved in chromosome organization and the cell cycle (Supplemental Figure S2C, http://links.lww.com/HEP/H971), including Stfa1 and Mki67 (Supplemental Figure S2D, http://links.lww.com/HEP/H971). In contrast, the cell population in aged livers highly expressed Nr4a2, Nr4a3, Tnfaip3, and Gzmk (Supplemental Figure S2D, http://links.lww.com/HEP/H971), which are associated with the leukocyte-related signaling pathway (Supplemental Figure S2C, http://links.lww.com/HEP/H971).
FIGURE 2.
Subclustering of lymphoid and myeloid lineages in the mouse liver in different age stages. (A) UMAP plot displaying T/NKT/NK cells from 19 mouse livers separated into 10 subtypes. (B) Relative changes in the cell ratios of T/NKT/NK cells across the 4 age stages (left) and samples (right). (C) UMAP plot displaying monocytes/macrophages and DCs from 19 mouse livers separated into 10 subtypes. (D) Relative changes in the cell ratios of macrophages and DCs across the 4 age stages (left) and samples (right). (E) Representative flow cytometry plots of CXCL2+ macrophages in the liver tissues of mice in the suckling, young, and aged periods (left, n = 3). Percentage and number of CXCL2+F4/80+ cells in the mouse liver in the 3 stages (right). (F) Heatmap showing the Spearman’s correlation coefficient for Cxcl2 + macrophages, KC, Ly6Chi monocytes, and patrolling monocytes among the different age groups based on gene expression levels. Abbreviations: DC, dendritic cells; NK, natural killer; NKT, natural killer T; UMAP, uniform manifold approximation and projection.
Furthermore, we analyzed myeloid-derived cells, including DCs and macrophages, as they were key components of the immune cells we identified in livers collected at different ages, and 10 subclusters were identified, namely, 4 DC (cDC1, cDC2, migratory cDC, and plasmacytoid dendritic cells) and 6 mono/mac subclusters (Figure 2C and Supplemental S2A, http://links.lww.com/HEP/H971). The mono/mac subclusters included Ly6Chi monocytes, patrolling monocytes, transitioning monocytes, peritoneal macrophages, and KCs. We additionally identified a subcluster of macrophages that displayed high expression of Cxcl2, which was not identified in a previous study.8,17–19 The DEGs of mono/mac in aged livers enriched in the immune response and cell death accounted for nearly half of the total DEGs (Supplemental Figure S3A, http://links.lww.com/HEP/H971 and Supplemental Table S2, http://links.lww.com/HEP/H973) and included Il1b, Nlrp3, and Ifitm1 (Supplemental Figure S3B, http://links.lww.com/HEP/H971). In addition, the secretion of the inflammatory cytokines IL-1β and IL-6 by macrophages was significantly increased in aged livers (Supplemental Figure S3C, http://links.lww.com/HEP/H971). We found that the proportion of plasmacytoid dendritic cells was decreased in the livers of aged mice. In contrast, the proportions of Ly6Chi monocytes were low in young livers but remarkably elevated in aged livers, indicating increased inflammation in the elderly (Figure 2D). Additionally, the most notable finding was that compared with the young group, the other 3 groups, but especially the aged group, exhibited a higher frequency of Cxcl2 + macrophages (Figure 2D).
To further validate the proportion of this cell type, we performed flow cytometry analysis to detect the frequency of CXCL2+ macrophages in CD45+ cells at different ages. As expected, the numbers of this subcluster were relatively low in the young group and dramatically elevated in the aged group (Figure 2E and Supplemental S4A, http://links.lww.com/HEP/H971). Spearman correlation coefficient analysis showed that KCs, Ly6Chi monocytes, and patrolling monocytes were similar in gene expression between young and aged mice, while for Cxcl2 + macrophages, the difference was significant, suggesting that the degree of gene expression variation in different monocyte/macrophage subtypes was different (Figure 2F). Multiplex immunofluorescence staining presenting the spatial position of CXCL2+ macrophages also confirmed that compared with other 2 groups, CXCL2+ macrophages accumulated in the aged liver, which presented senescent features (highly expressed p16, Supplemental Figure S5A, http://links.lww.com/HEP/H971) and mainly in the hepatic sinusoid (close to CD31+ cells, Supplemental Figure S5B, http://links.lww.com/HEP/H971). In addition, RNA velocity data and developmental trajectory analysis revealed a clear sequential differentiation path from KCs to Cxcl2 + macrophages (Supplemental Figure S6A, http://links.lww.com/HEP/H971). We further performed a joint analysis with other published datasets to explore the characteristics of Cxcl2 + macrophages and found that Cxcl2 + macrophages exhibited distinct differences from KC in the healthy liver but were consistent with KC in the 3,5-diethoxycarbonyl-1,4-dihydroncollidin-induced liver injury model (Supplemental Figure S6B, http://links.lww.com/HEP/H971). These cells highly expressed genes related to inflammation and immune regulation, indicating that Cxcl2 + macrophages may represent a high-inflammatory Kupffer-like cell lineage (Supplemental Figure S6C, http://links.lww.com/HEP/H971).20 Finally, the results from the flow cytometry analysis also validated that CXCL2+ macrophages highly expressed CLEC2 and TIM4 while lacking TREM2 expression and exhibiting only minimal CD9 expression, which supported that CXCL2+ macrophages were derived from KC during liver aging rather than monocytes from the blood (Supplemental Figure S6D, http://links.lww.com/HEP/H971).18,21 Overall, single-cell transcriptomic analysis of hepatic T/NKT/NK-cell and myeloid populations revealed distinct subpopulation enrichment and immunological statuses in each age group. In particular, the significantly enriched Cxcl2 + macrophage population in aged mice suggested its crucial role during liver aging.
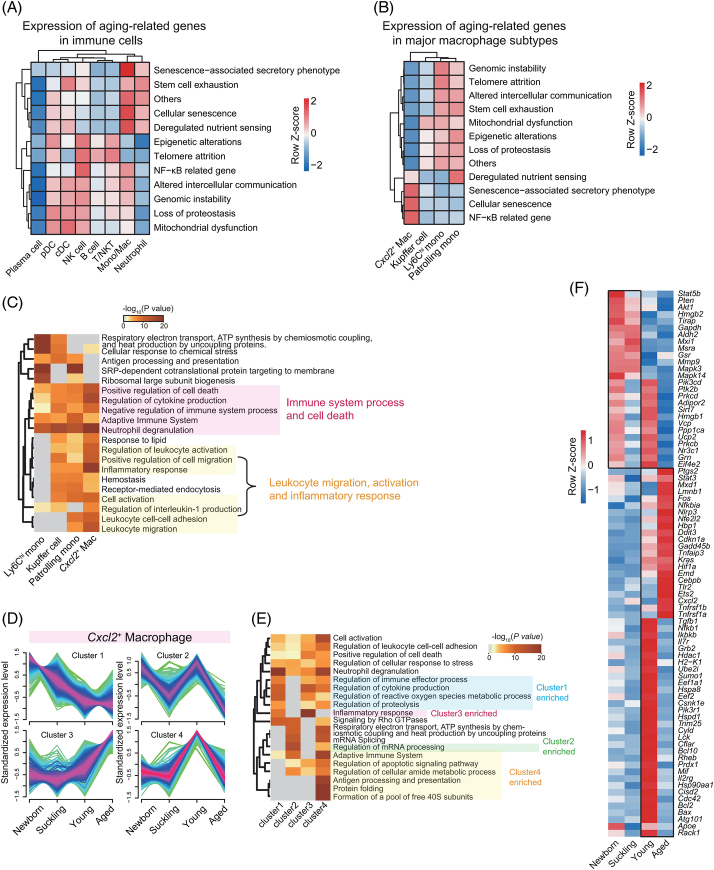
Identification of aging-related DEGs in immune cell types
We aimed to resolve the aging process from the perspective of intrahepatic immune cells. After confirming that aging did not alter the known characteristic markers of the main cell types (Supplemental Figure S7A, http://links.lww.com/HEP/H971), we identified genes that were differentially expressed across the stages of aging. According to the Aging Atlas database,22 aging-related genes were mainly expressed in mono/mac (Figure 3A, Supplemental Table S3, http://links.lww.com/HEP/H974). Notably, the highest expression of SASP-related and cellular senescence-related genes in mono/mac was observed in Cxcl2 + macrophages (Figure 3B), suggesting that this cell type is essential in liver homeostasis and aging. The SASP presents as increased secretion of numerous proinflammatory cytokines and contributes to senescence-related inflammation.23 Thus, we speculated that Cxcl2 + macrophages play roles in the induction of chronic inflammation in liver aging. Comparative functional enrichment analysis of identity-associated DEGs revealed that genes related to the immune system process and cell death were enriched in all 4 mono/mac subtypes; however, genes related to leukocyte migration, activation, and the inflammatory response were mainly enriched in Cxcl2 + macrophages (Figure 3C).
FIGURE 3.
Expression of aging-related genes and dynamic characteristics of intrahepatic Cxcl2 + macrophages during aging. (A) Heatmaps showing the scaled expression patterns of aging-related genes in the Aging Atlas database for different immune cell types. (B) Heatmaps showing the scaled expression patterns of aging-related genes in the Aging Atlas database for major monocyte/macrophage subtypes. (C) Representative GO BP terms and Reactome pathways of upregulated DEGs for each major monocyte/macrophage subtype in comparison to the other subtypes. The top 5 terms for each major monocyte/macrophage subtype based on the -log10(p-value) are listed. The color scale from red to gray indicates p values from low to high. (D) Four clusters of mRNAs with different expression dynamics for Cxcl2 + macrophages were computed with Mfuzz analysis. The color of the lines from red to green represents the membership value decreasing from 1 to 0.3. Genes with high membership values were identified based on the membership value > 0.5 in each expression pattern. (E) Representative GO BP terms and Reactome pathways of genes with high membership values in each Cxcl2 + macrophage expression pattern. The top 5 terms for each expression pattern based on the -log10(p-value) are listed. The color scale from red to gray indicates p values from low to high. (F) Heatmap showing the expression signatures of aging-related genes involved in 4 expression clusters of Cxcl2 + macrophages in each age stage. Abbreviations: DEG, differently expressed genes; GO BP, Gene Ontology Biological Process.
To explore molecular changes, we performed an integrative comparison analysis of dynamic changes in gene expression, and 4 age-dependent expression patterns were identified in Cxcl2 + macrophages (Figure 3D). Cluster 1, containing 514 genes with decreasing expression from newborn to young and aged mice, was enriched in the regulation of the immune process, and cluster 2, with 471 genes highly expressed in newborn and young livers, was enriched in mRNA processing (Figure 3E). Cluster 3, containing 302 genes with increased expression in the young-to-aged stage, was significantly enriched in the inflammatory response (Figure 3E), a known hallmark of aging.24 Cluster 4, containing 936 genes highly expressed in young livers, was enriched in Ag processing and presentation (Figure 3E). Additionally, we found multiple aging-related genes highly expressed in Cxcl2 + macrophages in the aged group, with relatively low expression in the other 3 stages; these genes included many proinflammatory factors in the SASP, such as Fos, Cxcl2, Tnfrsf1a, and Tnfrsf1b (Figure 3F). Moreover, we investigated the dynamic transcriptomic changes in KC, Ly6Chi monocytes, and patrolling monocytes, which consistently identified 4 patterns in each cell type (Supplemental Figure S7B-D, http://links.lww.com/HEP/H971). However, in contrast to Cxcl2 + macrophages, these cell types did not highly express genes related to the inflammatory response and aging in aged livers (Supplemental Figure S7B-D, http://links.lww.com/HEP/H971). Apart from this, few aging-related genes were uniquely and highly expressed in these cell populations in aged livers (Supplemental Figure S8A-C, http://links.lww.com/HEP/H971). Overall, aging had a significant impact on Cxcl2 + macrophages in aged livers compared with other mono/mac subsets.
Cxcl2 + macrophages recruited neutrophils to the aged liver
Altered intercellular communication is an integrative hallmark of aging.25 To investigate intrahepatic cellular communication at different ages, we calculated the numbers and strength of secreted ligand-receptor pairs between immune cells in young and aged livers, and the results revealed that immune subclusters in the young liver displayed fewer and weaker interactions than those in the aged liver (Supplemental Figure S9A, http://links.lww.com/HEP/H971). In addition, secretory signaling pathways contributed unequally to the cells between these two groups, and these pairs included IL-10 and PROS in young mice and CXCL, ANNEXIN, BMP, GDF, BAFF, OSM, CSF, and SPP1 in aged mice (Supplemental Figure S9B, http://links.lww.com/HEP/H971), indicating dramatic differences in intercellular communication between young and aged mice.
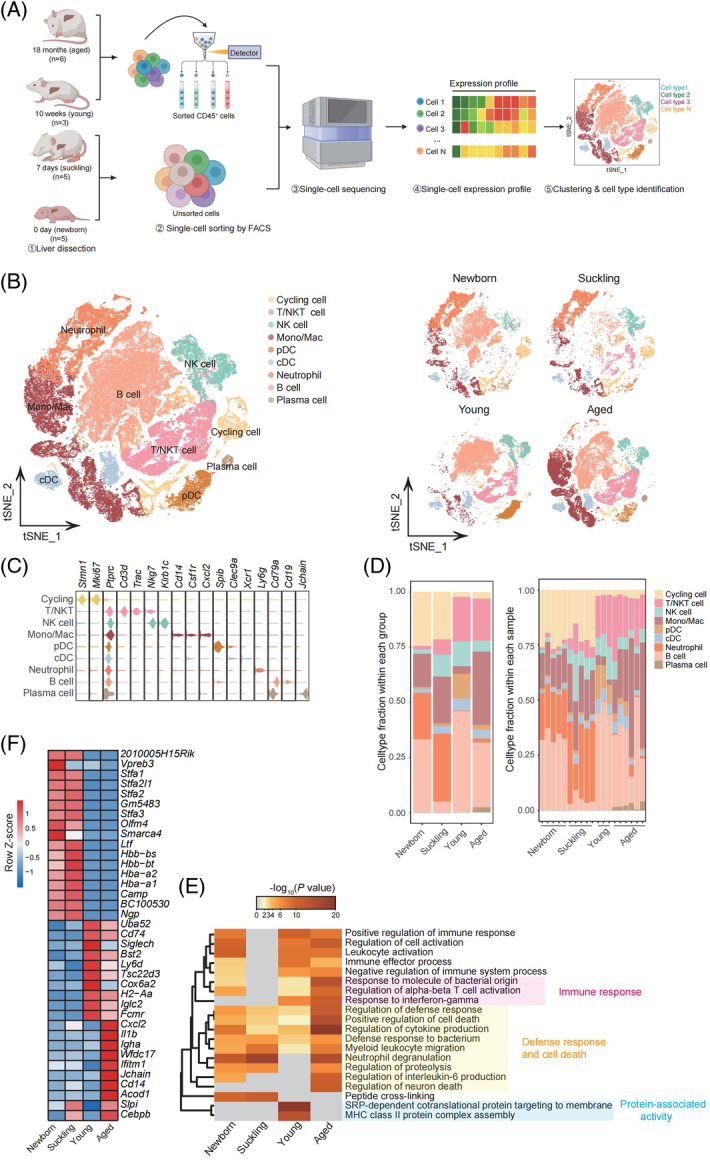
Considering the numbers and strength of ligand-receptor pairs, macrophages dominated other immune cell subsets as signal senders (Figure 4A and Supplemental Figure S9C, http://links.lww.com/HEP/H971). Notably, among the 6 monocyte/macrophage subtypes, the number of signals differed little (Supplemental Figure S9D, http://links.lww.com/HEP/H971), but the signals from Cxcl2 + macrophages and Ly6Chi monocytes to neutrophils presented enhanced interactions (Figure 4B). Combined with the finding that macrophages highly expressed SASP-related genes (Figure 3A), we speculated that macrophages have critical potential in regulating the migration and immune function of neutrophils through their ligand expression. As shown in the bubble plot representing differentially regulated signals between young and aged livers (Figure 4C), chemokine-related pairs, including Lgals9-Ighm and C3-Ccr, were present between macrophage subsets and neutrophils in young mice. In contrast, Cxcl2-Cxcr2, Ccl6-Ccr1, Ccl3-Ccr1, Anxa1-Fpr2, and Anx1-Fpr1 were more activated in the aged group. Of these ligand-receptor pairs, the Cxcl2-Cxcr2 interaction pair between Cxcl2 + macrophages and neutrophils in aged livers had the strongest interaction. Macrophages have been demonstrated to synthesize and secrete the chemoattractant CXCL1/CXCL2 to attract neutrophils during tissue infection or injury.26 Intrahepatic macrophages and Cxcl2 + macrophages showed high expression of Cxcl2 in aged mice; however, Cxcl1 expression was low at these 4 ages (Supplemental Figure S9E, http://links.lww.com/HEP/H971). Moreover, neutrophils also showed high expression of Cxcr2 in aged livers and low expression of Cxcr1 (Supplemental Figure S9E, http://links.lww.com/HEP/H971). The high expression of Cxcl2 and Cxcr2 in the aged liver suggested that the CXCL2-CXCR2 axis was crucial in the recruitment of neutrophils by Cxcl2 + macrophages.
FIGURE 4.
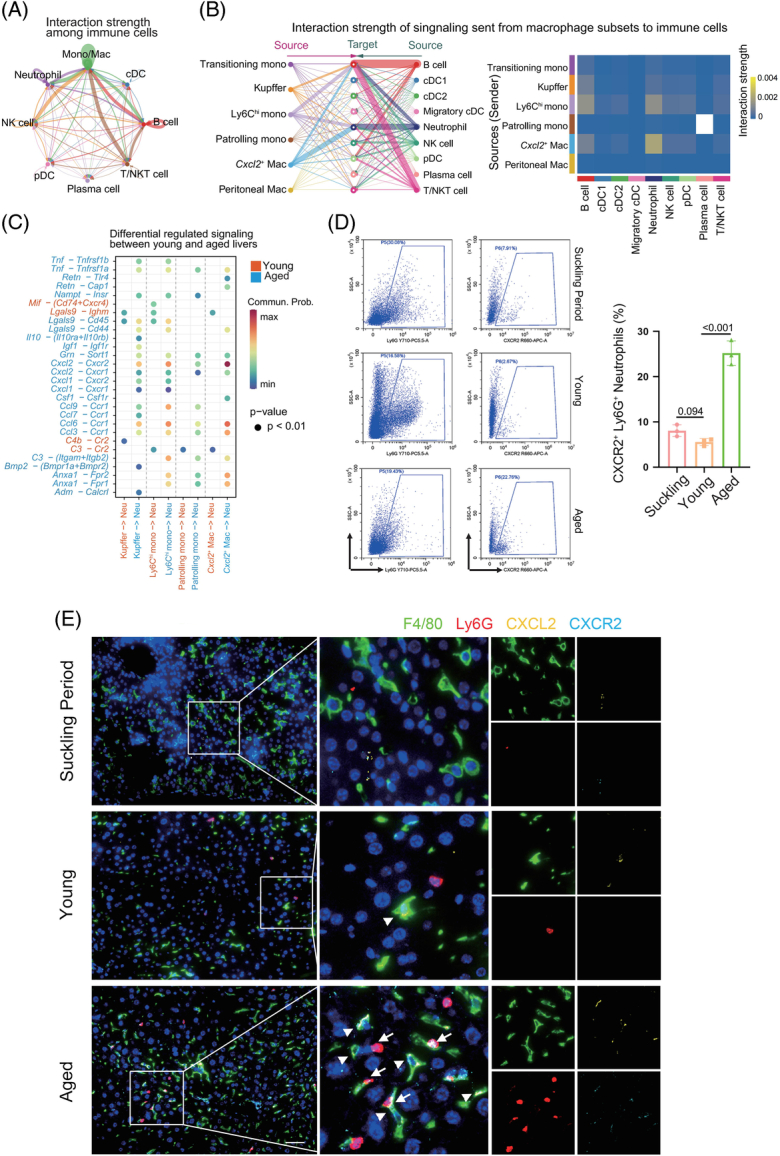
Changes in ligand‒receptor interactions between different hepatic immune cell types in mice during aging. (A) Circle plot showing ligand‒receptor interaction strength between different hepatic immune cell types. The strength of the ligand‒receptor interaction is represented by the line thickness. (B) Hierarchical plot showing the inferred intercellular communication network for secreted signaling molecules with communication from monocyte/macrophage subtypes to the remaining immune cells (left). Heatmap showing the interaction strength between the monocyte/macrophage subtypes and remaining immune cells (right). (C) Bubble plot showing major differential ligand‒receptor pairs involved in monocytes/macrophages signaling to neutrophils, showing intensity in either young (red) or aged (blue) mice. (D) Representative flow cytometry plots of CXCR2+ Ly6G+ cells (CXCR2+ neutrophils) in the liver tissues of mice in the suckling, young, and aged periods (left, n=3). Percentage of CXCR2+ Ly6G+ cells in the mouse liver at the 3 ages (right). (E) Liver tissues from mice in the suckling, young, and aged periods underwent immunofluorescence staining for F4/80 (green fluorescence, macrophages), Ly6G (red fluorescence, neutrophils), CXCL2 (yellow fluorescence), or CXCR2 (blue fluorescence). Magnified images are shown in the middle column. Representative immunofluorescence images showing CXCL2 staining within macrophages (triangle marker) and CXCR2 staining on neutrophils (arrowhead). Scale bar: 50 μm. Data are presented as the means ± SD. Abbreviation: Neu, neutrophil.
Next, we separately isolated neutrophils derived from livers at 3 different ages and performed flow cytometry analysis to compare the expression of CXCR2 in neutrophils. Although the proportion of intrahepatic neutrophils was high in suckling mice, with a decrease in young mice and an increase in aged mice, high expression of CXCR2 in neutrophils was observed only in the aged liver (Figure 4D). In addition, neutrophils in the liver were similar in the early stages but differed greatly between the young and aged stages (Supplemental Figure S9F, http://links.lww.com/HEP/H971), indicating that intrahepatic neutrophils are mainly derived from embryonic development in early-period livers and may originate from the peripheral circulation in the aged liver. Immunofluorescence staining further validated these findings and showed that CXCL2+ macrophages and CXCR2+ neutrophils were in physical proximity in the aged liver (Figure 4E). Finally, macrophages cocultured with neutrophils derived from aged livers in vitro induced significantly more neutrophil migration than those cocultured with neutrophils from young livers, while the addition of anti-CXCL2 antibody obviously blocked this phenomenon (Supplemental Figure S9G, http://links.lww.com/HEP/H971). These data suggested that macrophages promoted neutrophils migrating toward the aged liver by means of the CXCL2-CXCR2 axis.
Cxcl2 + macrophages stimulate NET formation in the aged liver
The fact that Cxcl2 + macrophages produced the strongest neutrophil-received signal revealed that in addition to affecting neutrophil recruitment, Cxcl2 + macrophages could alter the neutrophil phenotype. To prove our hypothesis, we used NicheNet to predict ligand-receptor interactions that might drive the changes in gene expression seen during the aging process.27 We obtained the top 20 predicted ligands in Cxcl2 + macrophages and their receptors in neutrophils (Figure 5A and Supplemental Figure S10A, http://links.lww.com/HEP/H971). Among them, Cxcl2 and the top 3 ranked ligands in macrophages from aged livers were validated to have the highest expression; the 3 ligands included Ccl3, encoding CCL3, a cytokine involved in the neutrophil recruitment process;28 Tnf, encoding TNF-α, an inducer of neutrophil cytotoxicity;29 and Il1b, encoding IL-1β, an important neutrophil activator30 (Supplemental Figure S10B, http://links.lww.com/HEP/H971). CCL3 could induce local neutrophil chemotaxis by interacting with CCR1; meanwhile, TNF-α and IL-1β have been demonstrated to function in eliciting NET formation,31–33 suggesting that intrahepatic Cxcl2 + macrophages might attract neutrophils through the CCL3-CCR1 ligand-receptor pair in addition to the CXCL2-CXCR2 axis and subsequently stimulate neutrophils with the proinflammatory cytokines TNF-α and IL-1β. Most ligands were significantly expressed in mono/mac compared with other immune cell types in the aged liver (Supplemental Figure S10C, http://links.lww.com/HEP/H971).
FIGURE 5.
Effect of macrophages on neutrophils in the aged mouse liver. (A) Top-ranked ligands inferred to regulate changes in gene expression in neutrophils during aging induced by Cxcl2 + macrophages, according to NicheNet. (B) Metascape bar graph showing functional enrichment of downstream target genes in neutrophils regulated by predicted ligands in Cxcl2 + macrophages. Enrichment terms sorted by −log10(p-value) are displayed by bars with a discrete color scale representing statistical significance. The top 15 enriched terms are shown. (C) Schematic depicting the experimental design. Both intrahepatic macrophages and neutrophils were isolated from young mice or aged mice and cocultured in Transwell culture dishes. (D) The degree of NET formation in each group was visualized using confocal microscopy. Red: Ly6G; green: cit-H3; blue: DAPI (left). Statistical analyses of NET formation in each group (right, n = 5). Scale bar: 20 μm. (E) Flow cytometry analysis was used to quantify NETs in each group by staining with Hoechst and Sytox Green after gating Ly6G+CD11b+ cells (left). Statistical analyses of the quantified NETs in each group (right, n = 4). Data are presented as the means ± SD. Abbreviation: NETs, neutrophil extracellular traps.
Significant activation of the inflammatory response, neutrophil degranulation, and regulation of the MAPK cascade was observed in neutrophils within aged livers in comparison to those in young livers (Figure 5B and Supplemental S11A, http://links.lww.com/HEP/H971). MAPK/ERK kinase can induce ROS to activate myeloperoxidase (MPO), neutrophil elastase, and protein-arginine deiminase type 4, which is a critical factor promoting chromatin decondensation and NETs formation.34–36 Interestingly, qPCR analysis demonstrated the upregulated expression of multiple TNF-α and IL-1β target genes, as well as MAPK signaling pathway-associated genes, in neutrophils (Supplemental Figure S11B, http://links.lww.com/HEP/H971), confirming the role of TNF-α- and IL-1β-induced activation and the activation of the MAPK signaling pathway in neutrophils in the aged liver. To further validate our findings, we isolated macrophages and neutrophils from young or aged livers, followed by 4 hours of coculture (Figure 5C). Immunofluorescence staining and flow cytometry analysis revealed that NETs formation was significantly upregulated by coculturing neutrophils with intrahepatic macrophages from aged mice compared with those from young mice (Figure 5D-E). In summary, Cxcl2 + macrophages in the aged liver could stimulate NET formation after recruiting neutrophils to the liver.
An in vivo model of hepatic ischemia-reperfusion injury revealed that Cxcl2 + macrophages aggravated pathological injury
Next, we used an hepatic ischemia-reperfusion injury (HIRI) model to further characterize the function of macrophages in the aged liver. Previous studies demonstrated that the aged liver presented an impaired potential to respond to external stress, which resulted in age-related injuries during HIRI.37,38 We, therefore, established a HIRI model by means of 90 min of ischemia and 24 h of subsequent reperfusion. Clodronate liposomes were first applied to deplete intrahepatic macrophages, followed by the adoptive transfer of intrahepatic macrophages isolated from young or aged mice into macrophage-depleted aged or young mice, respectively (Figure 6A and Supplemental Figure S12A, http://links.lww.com/HEP/H971). To trace and assess the viability of the transferred macrophages, we first labeled the macrophages with 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide to track them by in vivo imaging and found that the transferred macrophages mainly accumulated in the spleen and liver (Supplemental Figure S12B, http://links.lww.com/HEP/H971). We further used flow cytometry analysis and found that in the livers of both aged and young recipient mice, the majority of intrahepatic macrophages were composed of transferred macrophages (Supplemental Figure S12C, http://links.lww.com/HEP/H971). Additionally, the results from TUNEL staining showed no significant difference in viability between macrophages derived from aged and young mice, as neither group exhibited obvious signs of apoptosis in the transferred macrophages (Supplemental Figure S12D, http://links.lww.com/HEP/H971). Overall, this approach yielded 2 chimeric animal groups, namely, young mice with aged macrophages (A→Y) and aged mice containing young macrophages (Y→A), for further experiments.
FIGURE 6.
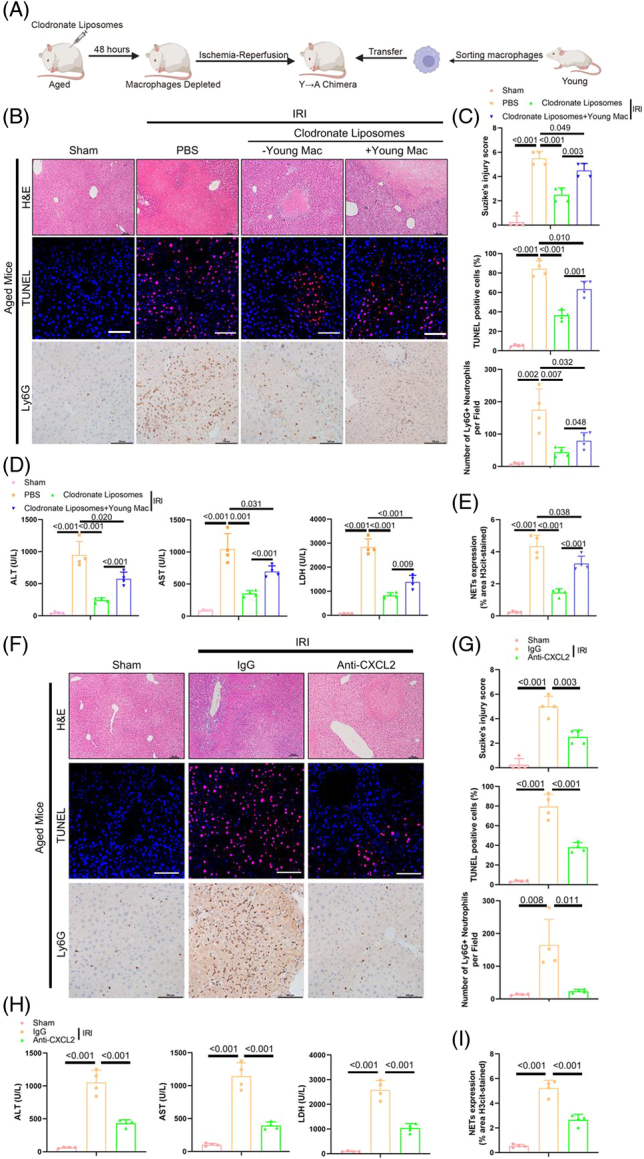
Aberrant neutrophils infiltration and NET formation promoted by aged intrahepatic macrophages to exacerbate liver damage in the HIRI model. (A) Schematic displaying the experimental design. Aged mice were pretreated with CLLs to deplete macrophages for 48 h, and the HIRI model was subsequently established, followed by a single intrasplenic injection of young intrahepatic macrophages (Y→A chimera, n = 4). (B) Histology (H&E staining, upper), TUNEL staining (middle), and IHC staining for Ly6G (lower) in liver tissues in each group. Scale bar: 100 μm. (C) The severity of liver injury in each group was scored according to Suzike’s injury criteria (upper panel). Statistical analyses of the percent of TUNEL-positive cells (middle panel) and neutrophils (lower panel) in liver tissues in each group. (D) The levels of sALT, sAST, and sLDH in each group. (E) Statistical analyses of NET formation in liver tissues in each group. (F) Histology (H&E staining, upper), TUNEL staining (middle), and IHC staining for Ly6G (lower) in liver tissues in each group. Scale bar: 100 μm. (G) Statistical analyses of Suzike’s injury criteria (upper), the percentage of TUNEL-positive cells (middle), and the number of neutrophils (lower) in each field (n = 4). (H) The levels of sALT, sAST, and sLDH in mice in the sham, anti-IgG, and anti-CXCL2 groups. (I) Statistical analyses of NET formation in liver tissues in each group. Data are presented as the means ± SD. Abbreviations: CLL, clodronate liposomes; HIRI, hepatic ischemia-reperfusion injury; NETs, neutrophil extracellular traps; sALT, serum alanine aminotransferase; sAST, serum aspartate aminotransferase; sLDH, serum lactate dehydrogenase.
As shown in Figure 6B-E, compared with the PBS group, the Y→A chimera group exhibited attenuated sinusoidal congestion and hepatic necrosis; reduced liver injury, as quantified by Suzike’s score; fewer TUNEL-positive cells; reduced neutrophil infiltration; decreased serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase; and reduced NETs formation during HIRI. Conversely, the opposite results were observed for A→Y chimeric animals, as reflected by aggravated histological injury, increased counts of apoptotic cells, higher numbers of infiltrating neutrophils, higher levels of hepatic enzymes, and increased NETs formation (Supplemental Figure S13A-D, http://links.lww.com/HEP/H971). To eliminate the influence of liver injury during the process of adoptive transfer, we also designed Y→Y chimeric animals, and the results showed a similar degree of liver injury between this group and the PBS group, indicating that adoptive transfer had little effect on the liver (Supplemental Figure S13A-D, http://links.lww.com/HEP/H971).
Finally, we also treated the aged HIRI model with an anti-CXCL2 antibody (or control IgG) to check whether intrahepatic macrophages recruit neutrophils and induce liver injury in the aged model by means of CXCL2 secretion. The liver displayed improved pathological injury, reduced neutrophil infiltration, and decreased NET formation after treatment with the anti-CXCL2 antibody compared with the IgG control (Figure 6F-I). Collectively, our results suggested that with increased age, intrahepatic macrophages aggravated pathological liver injury during ischemia-reperfusion injury by secreting CXCL2 to modulate neutrophil infiltration and stimulate NETs formation.
CXCL2+ macrophages that drove CXCR2+ neutrophils infiltration were positively correlated with liver damage in humans after OLT
The analyses above collectively showed that intrahepatic Cxcl2 + macrophages in aged mice could aggravate liver injury by recruiting neutrophils. However, whether this is applicable to human patients remains unknown. To investigate this, we enrolled 40 patients who died due to cardiac death at our center and collected paired specimens from both the pre-OLT period (after 2–10 h of cold storage) and the post-OLT stage (2 h after reperfusion) and grouped them according to donor age, namely, the young group (<30 y) and the aged group (>55 y).
As a result, we found that, unlike the young group, the aged group displayed delayed recovery of serum ALT and AST from postoperative day 1 to 7 (Figure 7A). TUNEL staining results showed that increased age positively correlated with the number of apoptotic cells in the graft after revascularization (Supplemental Figure S14A, http://links.lww.com/HEP/H971).
In addition, the counts of neutrophils (MPO+) and macrophages (CD68+) in the reperfusion graft in the aged group were markedly higher than those in the young group (Supplemental Figure S14B, http://links.lww.com/HEP/H971). Correlation analysis showed that the number of macrophages was positively correlated with the serum levels of AST and ALT as well as the numbers of neutrophils and TUNEL-positive cells (Supplemental Figure S14C-E, http://links.lww.com/HEP/H971). These correlations were more obvious in the aged group (Supplemental Figure S14C-E, http://links.lww.com/HEP/H971). Finally, we carried out multiplex immunofluorescence staining to analyze the alterations in CXCL2+ macrophages and CXCR2+ neutrophils during the peri-OLT period and found that the potential of intrahepatic macrophages to synthesize and secrete CXCL2 was dramatically upregulated in the aged group compared with the young group (preoperative period), which induced more CXCR2+ neutrophils to infiltrate the liver in the reperfusion stage (Figure 7B-C). The results also revealed that macrophages and CXCR2+ neutrophils were physically closer in the aged liver (Figure 7C). Correlation analysis also showed that the number of CXCL2+ macrophages was prevalent in the aged livers and positively correlated with the number of CXCR2+ neutrophils, the serum levels of AST and ALT, and the number of TUNEL-positive cells (Figure 7D-F). Overall, human liver transplantation validated the increased number of CXCL2+ macrophages in the aged liver and the positive correlation between their proportion and liver injury.
DISCUSSION
The immune microenvironment plays a significant role in aging.8,39,40 However, due to the limited number of age groups (only young and old) and the paucity of liver-resident immune cells obtained in previous single-cell studies on liver aging,7–9 the dynamic changes in the local immune microenvironment during liver aging remain largely unexplored, and its role in liver function remains unclear. In this study, we presented the largest single-cell survey of hepatic immune cells from newborn, suckling, young, and aged mice to date and comprehensively characterized, for the first time, the dynamic changes in intrahepatic immune cells. Pathway enrichment analysis confirmed that the intrahepatic immune cells of aged mice were prone to defense response and more susceptible to death. Among the hepatic immune cell populations with different proportions in different ages, we identified a new subgroup of macrophages, Cxcl2 + macrophages, whose proportion increased from 6% in the young liver to 29% in the aged liver. These macrophages were then shown to mediate the recruitment of neutrophils and to stimulate NET formation. Finally, our conclusion was further corroborated by an in vivo HIRI experiment and human data. Overall, Cxcl2 + macrophages aggravated age-related liver injury by recruiting neutrophils to release NETs.
Previous studies have shown that compared with that in young mice, liver function in aged mice is not significantly impaired. However, the capability of the aged liver to cope with external stress (especially injury) is reduced, and the corresponding molecular mechanism has not been fully elucidated.37,38 Neutrophils play a crucial role in age-related liver injury; however, the mechanism underlying the recruitment of neutrophils to the aged liver and the function of neutrophils during liver aging remain largely unexplored.41 Our results suggested that the proportion of Cxcl2 + macrophages in the aged liver was significantly increased. It was found that this group of cells is a Kupffer-like cell cluster with inflammatory features that can recruit neutrophils by secreting CXCL2 and subsequently stimulate NETs formation by secreting TNF-α and IL-1β, thereby aggravating ischemia-reperfusion injury in the aged liver. In addition, we identified CXCL2-CXCR2 as a key ligand-receptor pair through which macrophages in the aged liver attracted circulating neutrophils, which was also confirmed in humans. The recruitment of neutrophils and the formation of NETs led to an impairment in liver repair in aged mice. Previous studies have reported that CXCR2 antagonists (eg, SB225002 and AZD5069) reduce neutrophil infiltration in the liver.42–44 Based on our findings, targeting CXCL2 led to reduced recruitment of neutrophils by intrahepatic macrophages in aged mice. Using an HIRI model, we confirmed that an anti-CXCL2 antibody effectively reduced neutrophil recruitment and NET formation, thereby significantly alleviating HIRI in the aged liver. Our work not only explains the reduced capability of the aged liver to respond to external stress but also provides novel targets for alleviating inflammatory injury in the livers of aged mice.
Multiple attempts to delay aging, including heterochronic blood exchange,45 which ameliorates age-dependent alterations in liver function and triggers a rapid decline in fibrotic region size and adiposity,45–47 have recently been made. However, due to the heterogeneity of the human body and the complexity of blood components, the clinical application of heterochronic blood exchange still faces many problems. In this study, we isolated intrahepatic macrophages from young mice and transferred them into aged, macrophage-depleted mice. Subsequently, using the ischemia-reperfusion injury model constructed by transferring macrophages from the young liver into aged mice, we observed a decreased liver injury score and decreased neutrophil infiltration in the liver. This further confirmed the important role of aging-associated intrahepatic macrophages in the response to external stress and the clinical significance of targeting aging-associated macrophages.
In conclusion, this study used scRNA-seq to comprehensively investigate the age-related dynamic changes in intrahepatic immune cells and analyzed cell populations (Cxcl2 + macrophages, neutrophils, etc.) closely associated with liver aging, as well as age-specific cell interactions. This information provides an important reference for further clarifying the role of the dynamic changes in the immune microenvironment during liver aging in humans and for alleviating inflammatory injury in the aged liver. In addition to identifying Cxcl2 + macrophages, the single-cell transcriptomes revealed the unique composition of immune cell subsets at each age stage, providing a unique resource and platform for subsequent studies on the age-dependent functions of intrahepatic immune cells.
Supplementary Material
DATA AVAILABILITY
The datasets analyzed during the current study are available from the corresponding author upon reasonable request. This study did not use any unique codes, and all analyses were performed in R and Python using standard protocols from previously published packages. All primers and other nucleic acid sequences are provided in the Methods.
AUTHOR CONTRIBUTIONS
Yang Yang and Jun Zheng initiated the project; Jianye Cai, Jiaqi Xiao, Jun Zheng, and Yasong Liu designed the experiments; Jiaqi Xiao, Jiebin Zhang, Tongyu Lu, Haitian Chen, Chenhao Jiang, Xuegang Zhao, Cuicui Xiao, and Yunguo Lei carried out the experiments and analyzed the experimental data; Yasong Liu analyzed the single-cell RNA-seq data; Jia Yao, Haibo Li, Guo Lv, Yingcai Zhang, and Yang Yang provided human hepatic samples and clinical information; Jianye Cai verified the statistics; Yasong Liu, Jun Zheng, and Jiaqi Xiao wrote the paper. Rong Li, Guihua Chen, Jinliang Liang, Xin Sui, and Jian-Rong Yang reviewed the manuscript and participated in the interpretation of data.
ACKNOWLEDGMENTS
The authors thank Zizhang Li for the helpful discussions regarding the data analyses. They also thank AccuraMed Technology Limited for providing sequencing services. Figures were created with biorender.com.
FUNDING INFORMATION
This work was supported by the grants from the National Key Research and Development Program of China (2017YFA0104304, 2021YFF1200904, 2021YFA1302500), the National Natural Science Foundation of China (82073171, 81900597, 81802897, 81901943, 81970567, 82100693, 82170631, and 81972286), the Science and Technology Program of Guangdong Province (2019B020236003, 2020B1212060019), the Science and Technology Program of Guangzhou City (201803040005), the Natural Science Foundation of Guangdong Province (2019A1515011698, 2021A1515012136, 2021A1515011156, 2021A1515010571 and 2022A1515012331), the Medical Scientific Research Foundation of Guangdong Province (A2020120), the Guangdong Basic and Applied Basic Research Foundation (2020A1515110687, 2021A1515111058, 2022A1515110316), the Guangzhou Basic and Applied Basic Research Foundation (202102020237), and the China Postdoctoral Science Foundation (2022M713617, 2022M713621).
CONFLICTS OF INTEREST
The authors have no conflicts to report.
Footnotes
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BAFF, B-cell activating factor; CCL, CC chemokine ligand; BMP, Bone morphogenetic protein; cDC, conventional dendritic cells; CLLs, clodronate liposomes; CSF, Colony-stimulating factor; CXCL, CXC chemokine ligand; CXCR, CXC chemokine receptor; DC, dendritic cells; DEGs, differently expressed genes; FBS, Fetal bovine serum; GDF, Growth differentiation factor; GO BP, Gene Ontology Biological Process; H&E, Hematoxylin and Eosin; HIRI, hepatic ischemia-reperfusion injury; IHC, Immunohistochemistry; MAPK, Mitogen-Activated Protein Kinase; MHC, Major histocompatibility complex; Mono/Mac, monocyte and macrophage; MPO, myeloperoxidase; NETs, neutrophil extracellular traps; NK, natural killer; NKT, natural killer T; OSM: Oncostatin M; pDC, plasmacytoid dendritic cells; POD, postoperative day; PROS, Protein S; ROS, Reactive Oxygen Species; RPMI, Roswell Park Memorial Institute; sALT, serum alanine aminotransferase; SASP, senescence-associated secretory phenotype; sAST, serum aspartate aminotransferase; scRNA-seq, single-cell RNA sequencing; sLDH, serum lactate dehydrogenase; t-SNE, t-Distributed Stochastic Neighbor Embedding; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling; UMAP, uniform manifold approximation and projection; SPP1, Secreted phosphoprotein 1.
Yasong Liu, Jiaqi Xiao, Jianye Cai, and Rong Li contributed equally.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.hepjournal.com.
Contributor Information
Yasong Liu, Email: liuys3@mail2.sysu.edu.cn.
Jiaqi Xiao, Email: xiaojq5@mail2.sysu.edu.cn.
Jianye Cai, Email: caijianyeah@163.com.
Rong Li, Email: tsfnlr@163.com.
Xin Sui, Email: drsuixin@126.com.
Jiebin Zhang, Email: zhjieb@mail2.sysu.edu.cn.
Tongyu Lu, Email: lutongyu@mail2.sysu.edu.cn.
Haitian Chen, Email: chenht3@mail2.sysu.edu.cn.
Guihua Chen, Email: chghua@mail.sysu.edu.cn.
Haibo Li, Email: lihb9@mail.sysu.edu.cn.
Chenhao Jiang, Email: jiangchh7@mail2.sysu.edu.cn.
Xuegang Zhao, Email: zhaoxg7@mail2.sysu.edu.cn.
Cuicui Xiao, Email: xiaocc@mail2.sysu.edu.cn.
Yunguo Lei, Email: leiyg@mail2.sysu.edu.cn.
Jia Yao, Email: yaojia6@mail.sysu.edu.cn.
Guo Lv, Email: 564787306@qq.com.
Jinliang Liang, Email: ljlj73094@126.com.
Yingcai Zhang, Email: zhangyc3@mail.sysu.edu.cn.
Jian-Rong Yang, Email: yangjianrong@mail.sysu.edu.cn.
Jun Zheng, Email: zhengj67@mail2.sysu.edu.cn.
Yang Yang, Email: yysysu@163.com.
REFERENCES
- 1. Wang F-S, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology (Baltimore, Md). 2014;60:2099–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 3. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. Journal of Hepatology. 2019;70:151–171. [DOI] [PubMed] [Google Scholar]
- 4. Sheedfar F, Biase SD, Koonen D, Vinciguerra M. Liver diseases and aging: friends or foes? Aging Cell. 2013;12:950–954. [DOI] [PubMed] [Google Scholar]
- 5. Hoare M, Das T, Alexander G. Ageing, telomeres, senescence, and liver injury. Journal of Hepatology. 2010;53:950–961. [DOI] [PubMed] [Google Scholar]
- 6. Yao J, Li Y, Wang H. The roles of myeloid cells in aging-related liver diseases. Int J Biol Sci. 2023;19:1564–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tabula Muris C. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature. 2020;583:590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mogilenko DA, Shpynov O, Andhey PS, Arthur L, Swain A, Esaulova E, et al. Comprehensive profiling of an aging immune system reveals clonal GZMK(+) CD8(+) T Cells as conserved hallmark of inflammaging. Immunity. 2021;54:99–115 e12. [DOI] [PubMed] [Google Scholar]
- 9. Ma S, Sun S, Geng L, Song M, Wang W, Ye Y, et al. Caloric restriction reprograms the single-cell transcriptional landscape of rattus norvegicus aging. Cell. 2020;180:984–1001 e22. [DOI] [PubMed] [Google Scholar]
- 10. Brazhnik K, Sun S, Alani O, Kinkhabwala M, Wolkoff AW, Maslov AY, et al. Single-cell analysis reveals different age-related somatic mutation profiles between stem and differentiated cells in human liver. Sci Adv. 2020;6:eaax2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. da Costa JP, Vitorino R, Silva GM, Vogel C, Duarte AC, Rocha-Santos T. A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res Rev. 2016;29:90–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zheng J, Lu T, Zhou C, Cai J, Zhang X, Liang J, et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells protect liver ischemia/reperfusion injury by reducing CD154 Expression on CD4+ T Cells via CCT2. Adv Sci (Weinh). 2020;7:1903746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang Y, Kaneko K, Xin B, Lee J, Sun X, Zhang K, et al. Temporal analyses of postnatal liver development and maturation by single-cell transcriptomics. Dev Cell. 2022;57:398–414.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bilkei-Gorzo A, Albayram O, Draffehn A, Michel K, Piyanova A, Oppenheimer H, et al. A chronic low dose of Δ-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat Med. 2017;23:782–787. [DOI] [PubMed] [Google Scholar]
- 15. Wang S, Lai X, Deng Y, Song Y. Correlation between mouse age and human age in anti-tumor research: Significance and method establishment. Life Sciences. 2020;242:117242. [DOI] [PubMed] [Google Scholar]
- 16. Basha G, Omilusik K, Chavez-Steenbock A, Reinicke AT, Lack N, Choi KB, et al. A CD74-dependent MHC class I endolysosomal cross-presentation pathway. Nature Immunology. 2012;13:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guilliams M, Bonnardel J, Haest B, Vanderborght B, Wagner C, Remmerie A, et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell. 2022;185:379–396 e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Remmerie A, Martens L, Thoné T, Castoldi A, Seurinck R, Pavie B, et al. Osteopontin expression identifies a subset of recruited macrophages distinct from kupffer cells in the fatty liver. Immunity. 2020;53:641–657 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakashima H, Nakashima M, Kinoshita M, Ikarashi M, Miyazaki H, Hanaka H, et al. Activation and increase of radio-sensitive CD11b+ recruited Kupffer cells/macrophages in diet-induced steatohepatitis in FGF5 deficient mice. Sci Rep. 2016;6:34466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li L, Cui L, Lin P, Liu Z, Bao S, Ma X, et al. Kupffer-cell-derived IL-6 is repurposed for hepatocyte dedifferentiation via activating progenitor genes from injury-specific enhancers. Cell Stem Cell. 2023;30:283–299.e9. [DOI] [PubMed] [Google Scholar]
- 21. Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, et al. Lipid-associated macrophages control metabolic homeostasis in a trem2-dependent manner. Cell. 2019;178:686–698.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aging Atlas C. Aging Atlas: a multi-omics database for aging biology. Nucleic Acids Res. 2021;49(D1):D825–D830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirkland JL, Tchkonia T. Cellular senescence: A translational perspective. EBioMedicine. 2017;21:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–S9. [DOI] [PubMed] [Google Scholar]
- 25. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. [DOI] [PubMed] [Google Scholar]
- 27. Browaeys R, Saelens W, Saeys Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nature Methods. 2020;17:159–162. [DOI] [PubMed] [Google Scholar]
- 28. Ramos CDL, Canetti C, Souto JT, Silva JS, Hogaboam CM, Ferreira SH, et al. MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4. J Leukoc Biol. 2005;78:167–177. [DOI] [PubMed] [Google Scholar]
- 29. Comen E, Wojnarowicz P, Seshan VE, Shah R, Coker C, Norton L, et al. TNF is a key cytokine mediating neutrophil cytotoxic activity in breast cancer patients. NPJ Breast Cancer. 2016;2:16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prince LR, Allen L, Jones EC, Hellewell PG, Dower SK, Whyte MKB, et al. The role of interleukin-1beta in direct and toll-like receptor 4-mediated neutrophil activation and survival. Am J Pathol. 2004;165:1819–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keshari RS, Jyoti A, Dubey M, Kothari N, Kohli M, Bogra J, et al. Cytokines induced neutrophil extracellular traps formation: Implication for the inflammatory disease condition. PloS One. 2012;7:e48111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dinallo V, Marafini I, Di Fusco D, Laudisi F, Franzè E, Di Grazia A, et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohns Colitis. 2019;13:772–784. [DOI] [PubMed] [Google Scholar]
- 33. Wang L, Liu Y, Dai Y, Tang X, Yin T, Wang C, et al. Single-cell RNA-seq analysis reveals BHLHE40-driven pro-tumour neutrophils with hyperactivated glycolysis in pancreatic tumour microenvironment. Gut. 2022;72:958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7:75–77. [DOI] [PubMed] [Google Scholar]
- 35. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nature Reviews Immunology. 2018;18:134–147. [DOI] [PubMed] [Google Scholar]
- 36. Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aravinthan AD, Alexander GJM. Senescence in chronic liver disease: Is the future in aging? J Hepatol. 2016;65:825–834. [DOI] [PubMed] [Google Scholar]
- 38. Chun SK, Lee S, Flores-Toro J, U RY, Yang MJ, Go KL, et al. Loss of sirtuin 1 and mitofusin 2 contributes to enhanced ischemia/reperfusion injury in aged livers. Aging Cell. 2018;17:e12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen M, Luo C, Zhao J, Devarajan G, Xu H. Immune regulation in the aging retina. Prog Retin Eye Res. 2019;69:159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 2018;48:380–395.e6. [DOI] [PubMed] [Google Scholar]
- 41. Lagnado A, Leslie J, Ruchaud‐Sparagano MH, Victorelli S, Hirsova P, Ogrodnik M, et al. Neutrophils induce paracrine telomere dysfunction and senescence in ROS-dependent manner. EMBO J. 2021;40:e106048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Phillips BE, Lantier L, Engman C, Garciafigueroa Y, Singhi A, Trucco M, et al. Improvement in insulin sensitivity and prevention of high fat diet-induced liver pathology using a CXCR2 antagonist. Cardiovasc Diabetol. 2022;21:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Konishi T, Schuster RM, Goetzman HS, Caldwell CC, Lentsch AB. Cell-specific regulatory effects of CXCR2 on cholestatic liver injury. Am J Physiol Gastrointest Liver Physiol. 2019;317:G773–g783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kuboki S, Shin T, Huber N, Eismann T, Galloway E, Schuster R, et al. Hepatocyte signaling through CXC chemokine receptor-2 is detrimental to liver recovery after ischemia/reperfusion in mice. Hepatology. 2008;48:1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rebo J, Mehdipour M, Gathwala R, Causey K, Liu Y, Conboy MJ, et al. A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nat Commun. 2016;7:13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu A, Guo E, Yang J, Yang Y, Liu S, Jiang X, et al. Young plasma reverses age-dependent alterations in hepatic function through the restoration of autophagy. Aging Cell. 2018;17:e12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jeon OH, Mehdipour M, Gil TH, Kang M, Aguirre NW, Robinson ZR, et al. Systemic induction of senescence in young mice after single heterochronic blood exchange. Nat Metab. 2022;4:995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]