Abstract
Objective:
Postoperative staple line leakage (SLL) after sleeve gastrectomy (SG) is a rare but serious complication. Many surgeons routinely test anastomosis with an intraoperative leak test (IOLT) as part of the SG procedure. This meta-analysis aims to determine whether an IOLT plays a role in reducing the rate of postoperative staple line related complications in patients who underwent SG.
Methods:
The authors searched the PubMed, Web of science, the Cochrane Library, and Clinical Trials.gov databases for clinical studies assessing the application of IOLT in SG. The primary endpoint was the development of postoperative SLL. Secondary endpoints included the postoperative bleeding, 30 days mortality rates, and 30 days readmission rates.
Results:
Six studies totaling 469 588 patients met the inclusion criteria. Our review found that the SLL rate was 0.38% (1221/ 324 264) in the IOLT group and 0.31% (453/ 145 324) in the no intraoperative leak test (NIOLT) group. Postoperative SLL decreased in the NIOLT group compared with the IOLT group (OR=1.27; 95% CI: 1.14–1.42, P=0.000). Postoperative bleeding was fewer in the IOLT group than that in the NIOLT group (OR 0.79; 95% CI: 0.72–0.87, P=0.000). There was no significant difference between the IOLT group and the NIOLT group regarding 30 days mortality rates and 30 days readmission rates (P>0.05).
Conclusion:
IOLT was correlated with an increase in SLL when included as a part of the SG procedure. However, IOLT was associated with a lower rate of postoperative bleeding. Thus, IOLT should be considered in SG in the situation of suspected postoperative bleeding.
Keywords: intraoperative leak test, postoperative staple line leakage, prevention, sleeve gastrectomy
Introduction
As the prevalence of obesity has continued to increase worldwide, the number of performed bariatric procedures grows in parallel1. Among all bariatric procedures, laparoscopic sleeve gastrectomy (SG) is widely used worldwide in the surgical treatment of morbid obesity. It is considered to be a minimally invasive and safe surgery with low complications and mortality rates2. In SG, the stomach has its capacity reduced by approximately two-thirds3, which results in the patient eating less and losing weight4. Many advantages were shown in SG, such as reducing serum liver enzyme concentrations5, alleviating type 2 diabetes mellitus5, decreasing blood lipids6, and improving quality of life7,8 etc.
Intraoperative leak testing (IOLT) is a common intraoperative intervention to identify staple line leaks, defects, bleeding, and stricture. IOLT is often performed using air insufflation or methylene blue dye injection via upper gastrointestinal endoscopy or nasogastric tube9. Some studies recommend routine usage of the intraoperative leak test in SG10–12. However, the utility of these tests is controversial. The international SG expert panel failed to reach a consensus (48% consensus) about whether routine intraoperative leak tests should be performed13. A study showed that an IOLT using air insufflation or methylene blue dye was performed in 81.9% of cases and the leak rate was higher in patients with air insufflation or methylene blue versus without (0.8 vs 0.4%, P<0.01)14. In addition, IOLT has the possibility to cause iatrogenic injury due to excessive dilation of the remaining gastric pouch15,16.
To the best of our knowledge, this is the first meta-analysis regarding whether the IOLT procedure carries higher risk for postoperative staple line leakage. The aim of this study was to compare postoperative staple line leakage, postoperative bleeding, 30 days mortality rates, and 30 days readmission rates of IOLT with no intraoperative leak test (NIOLT) for SG.
Methods
Literature search strategy
The literature search for this systematic review was performed in January 2023 according to the Preferred Items for Reporting of Systematic Reviews and Meta-Analyses (PRISMA) guidelines17, and Assessing the methodological quality of systematic reviews(AMSTAR) Guidelines18. The study protocol was written and registered at The International Prospective Register of Systematic Reviews (Prospero) before data extraction. A systematic review of literature was performed by two authors independently using the databases PubMed, Web-of-Science, Cochrane Library, and Clinical Trials.gov databases along with a cross-reference search of eligible papers or trials. The following search strategy was used in PubMed and modified in other databases accordingly: ((sleeve gastrectomy) and (endoscopy) and (intraoperative) and (staple line leak)) or ((sleeve gastrectomy) and (stomach tube) and (intraoperative) and (staple line leak)) or ((sleeve gastrectomy) and (endoscopy) and (intraoperative leak testing)) or ((sleeve gastrectomy) and (stomach tube) and (intraoperative leak testing)) or ((bariatric surgery) and (stomach tube) and (intraoperative leak testing)) or ((bariatric surgery) and (endoscopy) and (intraoperative) and (staple line leak)) or ((bariatric surgery) and (stomach tube) and (intraoperative) and (staple line leak)) or ((bariatric surgery) and (stomach tube) and (intraoperative) and (staple line leak)) or ((endoscopy) and (intraoperative) and (staple line leak)) or ((stomach tube) and (intraoperative) and (staple line leak)) or ((endoscopy) and (intraoperative leak testing)) or ((stomach tube and (intraoperative leak testing)).
All studies comparing the postoperative outcomes of IOLT with NIOLT were included. Papers published before January 2023 were included. Moreover, we attempted to find all relevant literature by thoroughly looking through the references of included clinical articles. After analyzing the full texts, we identified a total of six studies that were suitable to be included in our meta-analysis.
Study selection
Studies were included in the meta-analysis if they met the following criteria: 1) they conducted clinical trials comparing the postoperative outcomes of IOLT and NIOLT; 2) the study was published as a full-text in the English language; and 3) valid data and a full-text of the study could be obtained successfully.
Study exclusion
Studies were excluded if they included patients that underwent any of the following procedures: mini-loop gastric bypass, endoscopic therapy, intragastric balloon, clinical trial, or experimental therapy. In addition, animal studies, conference abstract, comments, reviews, guidelines, and studies with fewer patients than 20 were excluded.
Statistical extraction
Articles were first screened independently by two authors according to title and abstract, with disputes being resolved by a third author. This process was then repeated with a full-text review in which we extracted data including author, year of publication, country, study design, number of patients, sex, age, BMI, postoperative staple line leakage, postoperative bleeding, 30 days mortality rates, and 30 days readmission rates.
Outcome
The primary endpoint was the development of postoperative staple line leakage. In this study, postoperative staple line leakage was defined as a leak after SG, which included intraoperative and postoperative finding leaks. Secondary endpoints included the postoperative bleeding, 30 days mortality rates, and 30 days readmission rates. The risk of bias was assessed using the Risk of Bias in Non-Randomized Studies of Intervention Tool19, which was shown in Table 1.
Table 1.
Analysis for risk of bias of the studies included in the meta-analysis using the Risk of Bias in Non-Randomized Studies of Intervention Tool.
| Studies | Baseline confounding | Selection of participant | Classification of diagnostic tools | Deviation from intended diagnostic | Missing data | Measurement of outcomes | Selection of reported results | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| Binghan et al.20 | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Yolsuriyanwong et al.21 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Sethi et al.9 | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Mayir et al.22 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Jung et al.23 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Liu et al.24 | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
Quality assessment
Quality assessment of the included studies was completed with the Newcastle–Ottawa Scale (NOS)25. Using this scale, each study was judged on eight items, categorized into three groups: the selection of the study groups; the comparability of the groups; and the exposure evaluation groups. Stars were awarded for each quality item and the highest quality studies were awarded up to nine stars. Scores of 7–9 points indicated high-quality studies, those of 4–6 points indicated moderate-quality studies, and those of 1–3 points indicated low-quality studies.
Statistical analysis
For retrospective compared study, odds ratio (OR) was calculated. The Mantel–Haenszel method was used for dichotomous data, and the OR with 95% CIs was presented. To assess the significance in study heterogeneity, Cochran’s P statistic and I² were reported. If the data was found to be lacking in the published articles, authors were contacted for further inquiry. When heterogeneity was high, the random-effects model was used; otherwise, the fixed-effects model was used. Heterogeneity was explored using I² statistics and the analyses were illustrated with forest plots. Heterogeneity was calculated using the I² statistic and defined as low, moderate, and high when I² was more than 25, 50, and 75%, respectively26. We performed further subgroup analysis of the studies type of IOLT. Stata software (version 17.0; Stata Corporatio; College Station) was used to perform all analysis.
Results
Literature search results
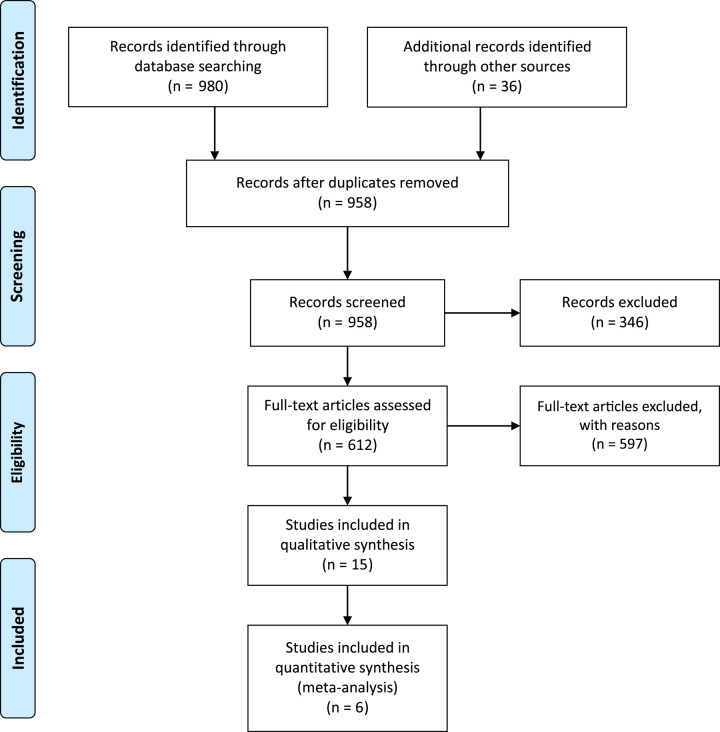
Our systematic search revealed a total of 1016 publications for possible inclusion. Based on a review of the title and abstract of each article, irrelevant publications, duplicate publications, and those not fitting our inclusion criteria were excluded. A further nine publications were excluded based on review of the full-text, leaving six retrospective studies that were included9,20–24 (Fig. 1).
Figure 1.
Flow diagram of study selection.
Study characteristics
The meta-analysis included 469 588 patients, of which 324 264 were assigned to the IOLT group and 145 324 to the NIOLT group. The studies were published between 2016 and 2022. One study originated from Turkey, while the other five originated from the United States. All studies performed intraoperative endoscopic or nonendoscopic methods (naso/orogastric tube insertion), which used air injection or used methylene blue to test for leakage. Details information on study characteristics are present in Table 2. Among the six studies in total, three of them reported positive results for IOLT9,20,22. Only two patients were reported as having a positive IOLT, which allowed for the reinforcement of the sutures. In the remaining three studies21,23,24, the IOLT group provided the leak rate after surgery instead of reporting positive results of IOLT. Details of distal clamp occlusion in IOLT were reported in three studies9,21,22, which showed in Table 3.
Table 2.
The basic characteristics of included studies
| Author | Country | Study period | Number of patients (IOLT/NIOLT) | Age (mean) | Sex (female/male) | BMI (kg/m2) | Type of study | Risk of Bias (NOS)☆ | Type of intraoperative leak testing | Positive postoperative leak (IOLT/NIOLT) | Postoperative bleeding (IOLT/NIOLT) | 30 days mortality rates (IOLT/NIOLT) | 30 days readmission rates (IOLT/NIOLT) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bingham et al.[20] | USA | 2010–2014 | 2376/1908 | 45 | 3599/685 | 43 | RS | 7 | AI or MBD | 23/14 | NR | NR | NR |
| Yolsuriyanwong et al.[21] | USA | 2015–2016 | 142447/42091 | 45.3 | NR | 45.9 | RS | 8 | MBD | 354/96 | NR | 103/32 | 279/83 |
| Sethi et al.[9] | USA | 2012–2014 | 1329/221 | 40 | 1235/315 | 44.2 | RS | 8 | AI or MBD | 13/2 | NR | 0/1 | NR |
| Mayir et al.[22] | Turkey | 2017–2018 | 226/226 | 40 | 358/94 | 45.9 | RS | 8 | MBD | 2/2 | 3/3 | NR | NR |
| Jung et al.[23] | USA | 2015–2017 | 55405/55405 | 44 | 87484/23326 | 45 | RS | 8 | AI or MBD | 223/183 | 335/420 | NR | 1646/1711 |
| Liu et al.[24] | USA | 2015–2016 | 122481/45473 | NR | NR | NR | ROS | 7 | EONAI or MBD | 606/157 | 721/343 | NR | 3906/1463 |
Table 3.
The details of distal clamp occlusion in intraoperative leak test.
| Studies | Types of IOLT | Distal clamp occlusion |
|---|---|---|
| Sethi et al. 9 | Air insufflation | Distal occlusion of the pylorus |
| Methylene blue dye | Distal obstruction of the duodenum | |
| Mayir et al. 22 | Methylene blue dye | Pylorus was laparoscopically closed with a bowel clamp |
| Yolsuriyanwong et al.21 | Methylene blue dye | The bowel was clamped distal to the anastomosis or staple line |
IOLT, intraoperative leak test.
Study quality
When using the NOS for case–control studies, the quality assessment of the included studies ranged from 6 to 8. All six studies had NOS quality scores greater than or equal to 6, indicating that all these studies had a high level of methodological quality. Table 4 shows the NOS quality scores of the included studies.
Table 4.
Quality assessment of included studies.
| Selection | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author | Adequate case definition | Representative of cases | Selection of controls | Definition of controls | Comparability of cases and controls on basis of design of analysis | Ascertainment of exposure | Same Method of ascertainment for cases and controls | Nonresponse rate | Score |
| Binghan et al.20 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Yolsuriyanwong et al.21 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 | |
| Sethi et al.9 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 | |
| Mayir et al.22 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 | |
| Jung et al.23 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 | |
| Liu et al.24 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | |
Primary outcome: postoperative staple line leakage
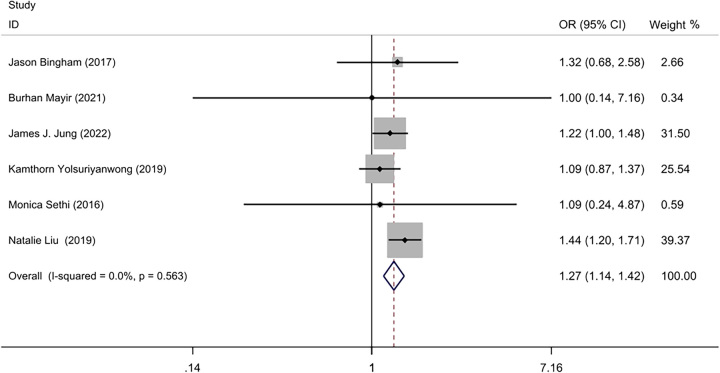
Our review9,20–24 found that the SLL rate was 0.38% (1221/ 324 264) in the IOLT group, and 0.31% (453/ 145 324) in the NIOLT group. A low statistical heterogeneity was detected between the six studies (I²=0%, P=0.56), so a fixed effect model was used for meta-analysis. The meta-analysis showed that postoperative staple line leakage was lower in the NIOLT group than the IOLT group (OR=1.27; 95% CI: 1.14–1.42, P=0.000) (Fig. 2).
Figure 2.
Forest plot of postoperative staple line leakage.
Subgroup analysis
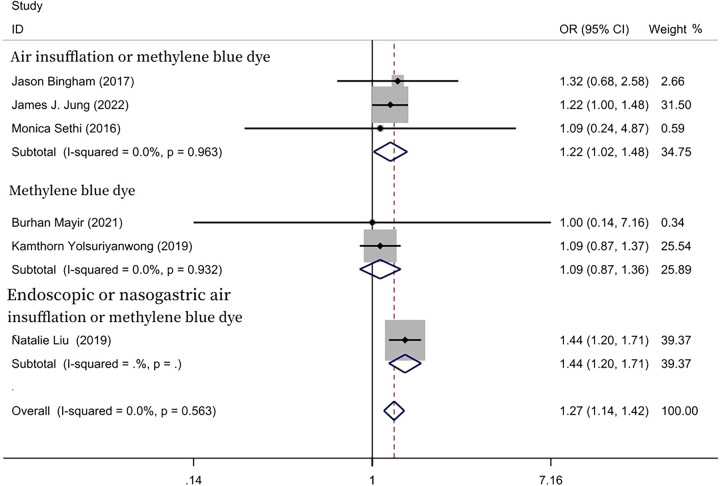
We performed further subgroup analysis of the included studies, which was done accordance with the method of every study of IOLT. In two included studies, the methylene blue test was adopted in the IOLT group21,22. The postoperative staple line leakage rate was 0.25% (356/142 673) in the IOLT group, and 0.23% (98/42 317) in the NIOLT group. The meta-analysis showed no statistically significant differences in the IOLT group and in the NIOLT group (OR=1.09; 95% CI: 0.87–1.36, P=0.458). However, in three included studies, air insufflation or methylene blue dye were adopted in the IOLT group9,20,23. The postoperative staple line leakage rate was 0.44% (259/59 110) in the IOLT group, and 0.34% (198/57 534) in the NIOLT group. The postoperative staple line leakage was lower in the NIOLT group than that in the IOLT group (OR=1.22; 95% CI: 1.02–1.48, P=0.033). Only one study was suitable for meta-analysis, which used endoscopic or nasogastric air insufflation or methylene blue in the IOLT group24 (Fig. 3).
Figure 3.
Forest plot of subgroup analysis of leaking test type.
Secondary outcomes
Postoperative bleeding
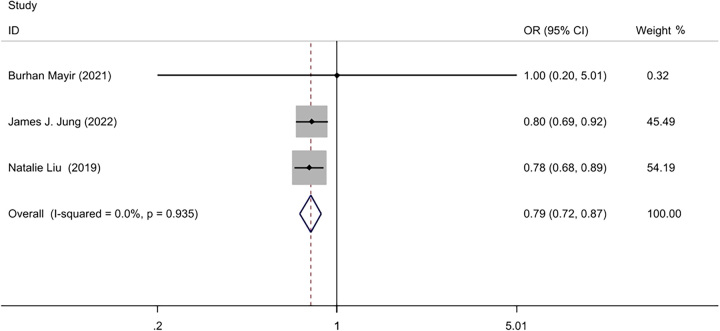
Three studies22–24 for a total of 279 216 patients reported postoperative bleeding. The postoperative bleeding rate was 0.59% (1059/178 112) in the IOLT group, and 0.76% (766/101 104) in the NIOLT group. A Fixed-effect model was used with low statistical heterogeneity (I2=0, P=0.94). The meta-analysis showed that postoperative bleeding was lower in the IOLT group than that in the NIOLT group (OR=0.79; 95% CI: 0.72–0.87, P=0.000) (Fig. 4).
Figure 4.
Forest plot of postoperative bleeding.
30 days mortality rates
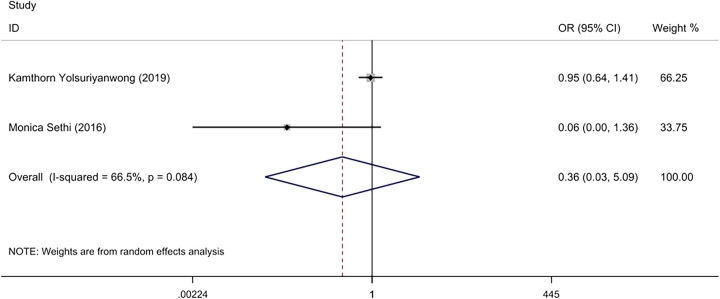
Two of the included studies9,21 reported on the 30-day mortality rates of patients. The mortality rate was 0.1% (103/143 776) in the IOLT group, and 0.1% (33/42 312) in the NIOLT group. Due to the moderate heterogeneity (I2=66%, P=0.08), a random-effect model was used for meta-analysis, which found no statistically significant differences in the 30-day mortality rates between the two groups (OR=0.36; 95% CI: 0.03–5.09, P=0.45) (Fig. 5).
Figure 5.
Forest plot of 30 days mortality rates.
30 days readmission rates
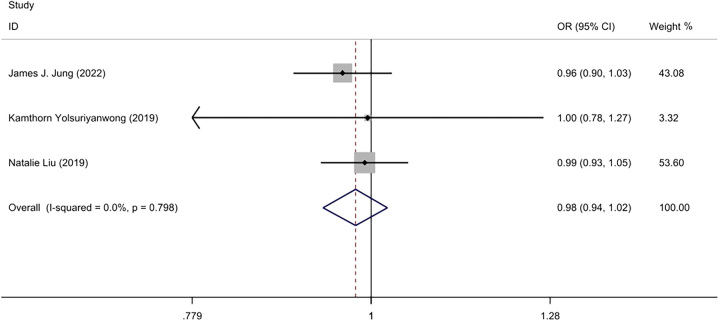
1.78% (5700/320,333) patients had 30 days readmission rates in the IOLT group, and 2.28% (1,794/142 996) in the NIOLT group21,23,24. Due to the low statistical heterogeneity (I²=0%, P=0.79), a Fixed-effect model was used. Analysis determined that there was no significant difference in the 30 days readmission rates between the IOLT group and the NIOLT group (OR=0.98; 95% CI: 0.94–1.02, P=0.33) (Fig. 6).
Figure 6.
Forest plot of 30 days readmission rates.
Discussion
SG, also known as vertical SG or gastric sleeve, was initially described in 198827 and is a commonly performed surgery for weight loss3. Postoperative SLL is one of the most severe complications following SG13. The incidence of SLL after SG is relatively low, with a reported incidence ranging from 0 to 8%28–30. To the best of our knowledge, this is the first meta-analysis regarding whether the IOLT procedure carries higher risk for postoperative staple line leakage.
In this study, we observed that NIOLT has a lower rate of postoperative staple line leakage compared to IOLT. One possible explanation for this is that postoperative leakage may occur due to a fault in the testing mechanism. For example, the calibration tube is already present in the stomach before stapling. When the test is about to be conducted, the calibration tube is gradually drawn up to the upper stomach, and then the test is performed. Therefore, there may be no need to insert it, thus reducing the risk of staple line injury.
A few different techniques for IOLT have been reported9,31. In a study by Burgos AM, IOLT involved administering methylene blue through a nasogastric tube placed after the removal of the bougie, with the goal of protecting against suture-line leaks and aiding in the evaluation of gastric capacity9. In another study by Sethi M, IOLT included methylene blue testing upon completing the sleeve, followed by the removal of the bougie and the placement of an orogastric or nasogastric tube under direct vision9. Another potential explanation for the higher leak rate is that many surgeons employ the orogastric tube method for IOLT31. This method, due to its blind insertion nature, can potentially cause trauma to the freshly constructed staple line, possibly leading to postoperative leaks. Additionally, the pressures exerted during the air insufflation leak test can weaken the staple line, increasing the risk of postoperative leak development32.
IOLT is safe and effective in gastric bypass surgery33–35. The following reasons can explain the increased leakage rate of IOLT in SG in specific. First, Roux-en-Y gastric bypass is a more complicated procedure with multiple anastomoses, making it harder to completely visualize the anastomosis, particularly on the posterior side36. Compared to gastric bypass, SG involves a simpler linear stapling. Therefore, the data supporting IOLT during Roux-en-Y gastric bypass cannot necessarily be extrapolated to SG20. Second, SG, in comparison to gastric bypass, retains the intact pylorus and has higher intraluminal pressure, which may lead to an increased leak rate28.
Our findings are in accordance with recent studies that indicated increased postoperative leak rates when IOLT was performed in SG14,24,37. A consensus report published in the Netherlands showed that IOLT was not considered to be a key step in SG38. A 494 patients study showed that the routine use of an IOLT did not reduce the incidence of postoperative leak, and in fact was associated with a higher leak rate after SG37. Furthermore, the usage of air insufflation or methylene blue dye tests were not completely advantageous and could add operative times and unnecessary costs to the surgery9. In addition, a negative methylene blue test does not eliminate the possibility of a leak39. Some studies showed that routine tests to rule out leaks seem to be superfluous40,41. However, some studies showed that an intraoperative leak test was an effective method for detecting leakage after SG10–12. Other studies found that performing IOLT was not associated with postoperative leak in patients who underwent SG20,21,37,42.
There are patients experiencing leak postoperative even though IOLT was negative. Some possible explanations are as follows. First, IOLT can only detect the rare leaks due to technical failure in the staple line, such as stapler misfire40. Second, it has been reported that IOLT has a low sensitivity and specificity, which does not result in decreased postoperative leak rates after SG20. A study showed that upper gastrointestinal radiography found leaks, but the IOLT result was negative20.
We found that the postoperative bleeding was significantly lower in the IOLT group. Our findings are in align with recent studies indicating that IOLT during for SG was associated with lower rates of postoperative bleeding (0.6 with leak testing versus 0.8%; P<0.001)24. An observational cohort study has also demonstrated that patients who had underwent IOLT during SG had lower postoperative bleeding rates21. Furthermore, a separate study has shown that IOLT was associated with a decrease in postoperative bleeding rates in SG patients (0.6 with IOLT versus 0.8% without IOLT, P=0.002)23. One possible explanation for this is that the potential postoperative bleeding, when detected through IOLT, allows surgeons to promptly implement hemostatic measures23. It has been reported that leak testing may be justified in cases of revisional surgery, intraoperative complications, or in the case of a surgeon who is in the learning curve stage9. Thus, we suggest that IOLT should be considered in SG in the situation of suspected postoperative bleeding.
Additionally, our study showed that regardless of whether patients had received IOLT or not, the rates of postoperative 30 days mortality rates and 30 days readmission rates were not significantly different(P>0.05). Our findings are in accordance with recent studies indicating that there were no significant differences between the IOLT and NIOLT groups in terms of 30 days mortality and 30 days readmission rates21. In addition, a study found that performing IOLT was not associated with changes in rates of 30 days readmission rates23.
Our study has some limitations. First, all included studies were conducted retrospectively, which may introduce selection bias and potentially reduce the reproducibility of our results. Second, the technique of IOLT was not standardized; some studies employed endoscopy with methylene blue and air tests for IOLT while others used only an orogastric tube with methylene blue or air tests. Additionally, the pressure of IOLT in the remnant of gastric sleeve was not monitored. Third, only three studies reported the positive cases of IOLT, making it difficult to calculate the salvage rates, which would have allowed reinforcing the staple line when intraoperative leaks were detected.
In conclusion, IOLT was correlated with an increase in staple line leakage. However, IOLT was associated with a lower rate of postoperative bleeding. Prospective studies proposing a systematic way of performing IOLT are still needed. Further studies, perhaps incorporating manometric factors into IOLT, should be considered.
Ethics approval and consent to participate
All procedures followed were in accordance with the ethical standards of the ethics committee of Nanchong Central Hospital and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients.
Consent for publication
Written informed consents were obtained from the patients for publication of the two case reports and any accompanying images.
Sources of funding
This work was supposed by the Foundation of Sichuan Medical Association [S21025], the Cooperative project of Nanchong City with North Sichuan Medical College [20SXQT0321], and the Bureau of Science and Technology Nanchong City [22JCYJPT0007].
Author contribution
Y.T., L.M., and Z.G.: have made substantial contributions to the design of the work; H.L. and J.H.: interpreted the patients’ data; L.M., L.Y., and V.J.: was a major contributor in writing the manuscript; Y.T., S.K., K.L., and S.S.: have drafted the work or substantively revised it. All authors read and approved the final manuscript.
Conflicts of interest disclosure
The authors declare that they have no competing interest associated with this manuscript.
Research registration unique identifying number (UIN)
The International Prospective Register of Systematic Reviews (Prospero) (CRD:42023393776).
Guarantor
Yunhong Tian.
Data availability statement
All the data in this study are truly reliable and were done in collaboration with all the authors.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
The authors want to thank Dr Michael Hillel Kleinman from Surgical Associates of Houston, Texas Dr Michael Hillel Kleinman helped Dr Yunhong Tian dedicate work to the surgical practice and research in Gastrointestinal Surgery.
The intraoperative leak test procedure carries higher risk for postoperative staple line leakage.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 20 November 2023
Contributor Information
Longyin Ma, Email: malongyin9056@163.com.
Zhenguo Gao, Email: gaozhenguo0822@163.com.
Heng Luo, Email: luoheng96@163.com.
Shien Kou, Email: koushien@126.com.
Yu Lei, Email: 1910944832@qq.com.
Victor Jia, Email: vicjia@umich.edu.
Ke Lan, Email: 1093189461@qq.com.
Subbiah Sankar, Email: sankarsubbu42@gmail.com.
Jiani Hu, Email: jianihu@yahoo.com.
Yunhong Tian, Email: drtianyunhong@126.com.
References
- 1. Głuszyńska P, Diemieszczyk I, Szczerbiński Ł, et al. Risk factors for early and late complications after laparoscopic sleeve gastrectomy in one-year observation. J Clin Med 2022;11:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guerrier JB, Dietch ZC, Schirmer BD, et al. Laparoscopic sleeve gastrectomy is associated with lower 30-day morbidity versus laparoscopic gastric bypass: an analysis of the American College of Surgeons NSQIP. Obes Surg 2018;28:3567–3572. [DOI] [PubMed] [Google Scholar]
- 3. Brajcich BC, Hungness ES. Sleeve gastrectomy. JAMA 2020;324:908. [DOI] [PubMed] [Google Scholar]
- 4. Puzziferri N, Almandoz JP. Sleeve gastrectomy for weight loss. JAMA 2018;319:316. [DOI] [PubMed] [Google Scholar]
- 5. Billmann F, El Shishtawi S, Bruckner T, et al. Combined non-alcoholic fatty liver disease and type 2 diabetes in severely obese patients-medium term effects of sleeve gastrectomy versus Roux-en-Y-gastric bypass on disease markers. Hepatobiliary Surg Nutr 2022;11:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cash JG, Konaniah E, Hegde N, et al. Therapeutic reduction of lysophospholipids in the digestive tract recapitulates the metabolic benefits of bariatric surgery and promotes diabetes remission. Mol Metab 2018;16:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nielsen HJ, Nedrebø BG, Fosså A, et al. Seven-year trajectories of body weight, quality of life and comorbidities following Roux-en-Y gastric bypass and sleeve gastrectomy. Int J Obes (Lond) 2022;46:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aminian A. Sleeve gastrectomy: metabolic surgical procedure of choice? Trends Endocrinol Metab 2018;29:531–534. [DOI] [PubMed] [Google Scholar]
- 9. Sethi M, Zagzag J, Patel K, et al. Intraoperative leak testing has no correlation with leak after laparoscopic sleeve gastrectomy. Surg Endosc 2016;30:883–891. [DOI] [PubMed] [Google Scholar]
- 10. Aggarwal S, Bhattacharjee H, Chander Misra M. Practice of routine intraoperative leak test during laparoscopic sleeve gastrectomy should not be discarded. Surg Obes Relat Dis 2011;7:e24–e25. [DOI] [PubMed] [Google Scholar]
- 11. Wahby M, Salama AF, Elezaby AF, et al. Is routine postoperative gastrografin study needed after laparoscopic sleeve gastrectomy? Experience of 712 cases. Obes Surg 2013;23:1711–1717. [DOI] [PubMed] [Google Scholar]
- 12. Nimeri A, Maasher A, Salim E, et al. The use of intraoperative endoscopy may decrease postoperative stenosis in laparoscopic sleeve gastrectomy. Obes Surg 2016;26:1398–1401. [DOI] [PubMed] [Google Scholar]
- 13. Rosenthal RJ, Diaz AA, Arvidsson D, et al. International sleeve gastrectomy expert panel consensus statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis 2012;8:8–19. [DOI] [PubMed] [Google Scholar]
- 14. Alizadeh RF, Li S, Inaba C, et al. Risk factors for gastrointestinal leak after bariatric surgery: MBASQIP analysis. J Am Coll Surg 2018;227:135–141. [DOI] [PubMed] [Google Scholar]
- 15. Deitel M, Crosby RD, Gagner M. The First International Consensus Summit for Sleeve Gastrectomy (SG), New York City, October 25-27, 2007. Obes Surg 2008;18:487–496. [DOI] [PubMed] [Google Scholar]
- 16. Deitel M, Gagner M, Erickson AL, et al. Third International Summit: current status of sleeve gastrectomy. Surg Obes Relat Dis 2011;7:749–759. [DOI] [PubMed] [Google Scholar]
- 17. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 18. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bingham J, Kaufman J, Hata K, et al. A multicenter study of routine versus selective intraoperative leak testing for sleeve gastrectomy. Surg Obes Relat Dis 2017;13:1469–1475. [DOI] [PubMed] [Google Scholar]
- 21. Yolsuriyanwong K, Ingviya T, Kongkamol C, et al. Effects of intraoperative leak testing on postoperative leak-related outcomes after primary bariatric surgery: an analysis of the MBSAQIP database. Surg Obes Relat Dis 2019;15:1530–1540. [DOI] [PubMed] [Google Scholar]
- 22. Mayir B. Is intraoperative leak test required in laparoscopic sleeve gastrectomy? J Coll Physicians Surg Pak 2021;31:318–321. [DOI] [PubMed] [Google Scholar]
- 23. Jung JJ, Jackson T, Gordon L, et al. Intraoperative leak test is associated with lower postoperative bleed rate in primary sleeve gastrectomy: a propensity matched analysis of primary and revision bariatric surgery using the MBSAQIP database. Surg Endosc 2022;36:753–763. [DOI] [PubMed] [Google Scholar]
- 24. Liu N, Cusack MC, Venkatesh M, et al. 30-day outcomes after intraoperative leak testing for bariatric surgery patients. J Surg Res 2019;242:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chung AY, Thompson R, Overby DW, et al. Sleeve gastrectomy: surgical tips. J Laparoendosc Adv Surg Tech A 2018;28:930–937. [DOI] [PubMed] [Google Scholar]
- 28. Gagner M, Brown M. Update on sleeve gastrectomy leak rate with the use of reinforcement. Obes Surg 2016;26:146–150. [DOI] [PubMed] [Google Scholar]
- 29. Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc 2012;26:1509–1515. [DOI] [PubMed] [Google Scholar]
- 30. Juza RM, Haluck RS, Pauli EM, et al. Gastric sleeve leak: a single institution’s experience with early combined laparoendoscopic management. Surg Obes Relat Dis 2015;11:60–64. [DOI] [PubMed] [Google Scholar]
- 31. Burgos AM, Braghetto I, Csendes A, et al. Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg 2009;19:1672–1677. [DOI] [PubMed] [Google Scholar]
- 32. Causey MW, Fitzpatrick E, Carter P. Pressure tolerance of newly constructed staple lines in sleeve gastrectomy and duodenal switch. Am J Surg 2013;205:571–575. [DOI] [PubMed] [Google Scholar]
- 33. Valenzuela-Salazar C, Rojano-Rodríguez ME, Romero-Loera S, et al. Intraoperative endoscopy prevents technical defect related leaks in laparoscopic Roux-en-Y gastric bypass: a randomized control trial. Int J Surg 2018;50:17–21. [DOI] [PubMed] [Google Scholar]
- 34. Cingi A, Yavuz Y. Intraoperative endoscopic assessment of the pouch and anastomosis during laparoscopic Roux-en-Y gastric bypass. Obes Surg 2011;21:1530–1534. [DOI] [PubMed] [Google Scholar]
- 35. Shin RB. Intraoperative endoscopic test resulting in no postoperative leaks from the gastric pouch and gastrojejunal anastomosis in 366 laparoscopic Roux-en-Y gastric bypasses. Obes Surg 2004;14:1067–1069. [DOI] [PubMed] [Google Scholar]
- 36. Kim J, Azagury D, Eisenberg D, et al. ASMBS position statement on prevention, detection, and treatment of gastrointestinal leak after gastric bypass and sleeve gastrectomy, including the roles of imaging, surgical exploration, and nonoperative management. Surg Obes Relat Dis 2015;11:739–748. [DOI] [PubMed] [Google Scholar]
- 37. Bingham J, Lallemand M, Barron M, et al. Routine intraoperative leak testing for sleeve gastrectomy: is the leak test full of hot air? Am J Surg 2016;211:943–947. [DOI] [PubMed] [Google Scholar]
- 38. Kaijser MA, van Ramshorst GH, Emous M, et al. A delphi consensus of the crucial steps in gastric bypass and sleeve gastrectomy procedures in the Netherlands. Obes Surg 2018;28:2634–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Casella G, Soricelli E, Rizzello M, et al. Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg 2009;19:821–826. [DOI] [PubMed] [Google Scholar]
- 40. Sakran N, Goitein D, Raziel A, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc 2013;27:240–245. [DOI] [PubMed] [Google Scholar]
- 41. Bellanger DE, Greenway FL. Laparoscopic sleeve gastrectomy, 529 cases without a leak: short-term results and technical considerations. Obes Surg 2011;21:146–150. [DOI] [PubMed] [Google Scholar]
- 42. Kirby GC, Macano CAW, Nyasavajjala SM, et al. The Birmingham experience of high-pressure methylene blue dye test during primary and revisional bariatric surgery: a retrospective cohort study. Ann Med Surg (Lond) 2017;23:32–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data in this study are truly reliable and were done in collaboration with all the authors.