Abstract
Background:
This meta-analysis aimed to evaluate the efficacy and safety of electroacupuncture (EA) in improving postoperative ileus after colorectal surgery.
Methods:
Electronic databases (e.g. Medline) were screened to identify randomized controlled trials that focused on the association between EA and postoperative ileus. Time to first flatus served as the primary outcome, while the secondary outcomes included time required for the recovery of other gastrointestinal functions (e.g. bowel sound recovery), time to tolerability of liquid/solid food, postoperative pain scores, risk of overall complications, and hospital length of stay.
Results:
Our meta-analysis focusing on 16 studies with a total of 1562 patients demonstrated positive associations of EA with shorter times to the first flatus [mean difference (MD): −10.1 h, P<0.00001, n=1562], first defecation (MD: −11.77 h, P<0.00001, n=1231), bowel sound recovery (MD: −10.76 h, P<0.00001, n=670), tolerability of liquid (MD: −16.44 h, P=0.0002, n=243), and solid food (MD: −17.21 h, P=0.005, n=582) than those who received standard care. The use of EA was also correlated with a lower risk of overall complications (risk ratio:0.71, P=0.04, n=1011), shorter hospital length of stay (MD: −1.22 days, P=0.0001, n=988), and a lower pain score on postoperative days two (standardized MD: −0.87, P=0.009, n=665) and three (standardized MD: −0.45, P<0.00001, n=795), without a difference in time to first ambulation.
Conclusion:
Our findings showed an association between EA and enhanced gastrointestinal functional recovery and reduced pain severity following colorectal surgery, highlighting the potential benefits of incorporating EA into perioperative care to enhance recovery outcomes in this setting.
Keywords: acupuncture, colorectal surgery, electroacupuncture, gastrointestinal function, postoperative ileus, recovery
Introduction
Highlights
The efficacy of electroacupuncture (EA) for postoperative ileus was investigated.
Sixteen trials with 1578 patients receiving colorectal surgery were meta-analyzed.
EA was linked to shorter times to first flatus/defecation and bowel sound recovery.
EA correlated with reduced time to food tolerance and pain score on days 2 and 3.
EA was associated with a lower risk of complications and a shorter hospital stay.
Colorectal cancer (CRC) is a highly prevalent malignancy worldwide, accounting for 9.4% of all cancer incidences in men and 10.1% in women1. More importantly, the total number of fatalities from rectal and colon cancers is speculated to increase by 60 and 71.5%, respectively2. Although surgical treatment remains the primary curative approach, various operation-related factors, including anesthesia, surgical trauma, inflammatory reactions, and the use of opioids, can impair postoperative gastrointestinal motility3. In particular, 10–30% of patients undergoing colorectal surgery reported the occurrence of postoperative ileus (POI)3,4 characterized by a range of symptoms, including abdominal distension, pain, delayed passage of gas and stool, nausea, vomiting, and an inability to tolerate oral intake5. Despite the typical resolution of POI within 5 days following open abdominal surgery and within three days following laparoscopic surgery6, its occasional presentation as a prolonged and recurrent disorder may lead to severe morbidities including electrolyte imbalances, dehydration, and sepsis7. Given the global burden of CRC and the high incidence of POI following surgical interventions, appropriate implementation of preventive strategies, close monitoring, and timely interventions for POI are crucial for minimizing associated morbidities and improving patient recovery.
Acupuncture, a traditional Chinese medicine technique originating in East Asia that has been practiced for centuries, involves the stimulation of specific acupoints to alleviate various gastrointestinal symptoms8. In recent years, acupuncture has gained popularity as a nonpharmacological and minimally invasive therapeutic option for various gastrointestinal disorders, including abdominal distension, pain, constipation, nausea, and vomiting9–11. Several meta-analyses have shown the effectiveness of acupuncture and related techniques, such as electroacupuncture (EA) or transcutaneous acupoint electrical stimulation (TEAS), in improving POI among patients undergoing abdominal surgery12–14. However, the significant heterogeneity within these meta-analyses raises concerns regarding the robustness and generalizability of the evidence generated.
EA is a contemporary clinical approach that integrates traditional acupuncture techniques with the precise application of stabilized electrical stimulation, enabling objective, and quantifiable adjustments of stimulation frequency and intensity15. This unique characteristic has garnered considerable attention, focusing on EA as a potential therapeutic intervention for patients with CRC who develop POI16. Therefore, the primary objective of this systematic review and meta-analysis was to examine the reliability and validity of currently available evidence supporting the use of EA to improve POI among patients with CRC.
Methods
The study protocol can be accessed from the PROSPERO International prospective register of the systematic review database. This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA, Supplemental Digital Contents 3 and 4, http://links.lww.com/JS9/B234, http://links.lww.com/JS9/B235)17 and AMSTAR (Supplemental Digital Content 2, http://links.lww.com/JS9/B233) (Assessing the methodological quality of systematic reviews)18 guidelines when reporting its findings.
Search strategies and data sources
A comprehensive literature search was conducted to identify randomized controlled trials (RCTs) that investigated the effectiveness of EA in improving postoperative gastrointestinal motility disorders in patients undergoing colorectal surgery. The search encompassed major databases, including PubMed, MEDLINE, Embase, and the Cochrane CENTRAL register of controlled trials, spanning from their inception dates until 8 June 2023. A search of the ClinicalTrials. gov database was also performed to ensure the inclusion of ongoing or unpublished studies. The search strategy employed a combination of keywords and medical subject headings (e.g. MeSH terms in Medline), including the following keywords: (ʻAcupunctureʼ or ʻElectroacupuncture therapyʼ or ʻElectroacupunctureʼ or ʻElectro acupuncturingʼ or ʻLaser acupunctureʼ or ʻElectro needle acupunctureʼ or ʻElectro auricular acupunctureʼ) and (ʻcolorectal cancerʼ or ʻcolorectal carcinomaʼ or ʻcolon adenocarcinomaʼ or ʻcolorectal tumorʼ or ʻcolonic neoplasmʼ or ʻrectal cancerʼ or ʻrectosigmoid tumorʼ or ʻsigmoid colon tumorʼ) and (ʻpostoperative ileusʼ or ʻpostoperative bowel dysfunctionʼ or ʻpostoperative gastrointestinal dysfunctionʼ or ʻparalytic ileusʼ). Furthermore, to prevent the omission of relevant studies, Google Scholar, the China National Knowledge Infrastructure (CNKI) database, Chongqing VIP Database (CQVIP), Chinese Biomedical Literature Database (CBM), and the Wanfang Database were also searched. To identify additional studies, the reference lists of the published systematic reviews and included RCTs were screened. No restrictions were placed on the language, sample size, publication date, or country of origin.
Inclusion criteria and study selection
The following PICOS criteria were used to screen eligible trials: (P) participants: adults receiving colorectal surgery regardless of the surgical techniques (e.g. laparoscopy or laparotomy); (I) interventions: the use of EA with or without other adjunct therapies to improve POI; (C) comparisons: sham acupuncture or standard care; (O) Outcomes: POI-related outcomes were available [for example, time to first flatus (TFF)]; and (S) study design: only RCTs were included.
The following criteria were used to exclude ineligible studies: (a) studies that included patients receiving both colorectal and other gastrointestinal surgeries (e.g. gastrectomy); (b) acupuncture-related therapy was also used in the control group; (c) studies that focused on manual acupuncture or TEAS; and (d) full-text or POI-related outcomes were unavailable.
Following the removal of duplicate records, potentially eligible studies underwent a screening process conducted by two independent reviewers who evaluated the titles and abstracts. Full-text articles were obtained and reviewed by the same reviewers to determine the inclusion criteria. In cases of disagreement, a third reviewer was consulted to facilitate discussion and reach consensus.
Data extraction
The data extraction process was conducted independently by two reviewers who extracted relevant information using a standardized data extraction form. The extracted data included the first author’s name, publication year, baseline characteristics of participants (e.g. age), size of the study population, surgical techniques (e.g. laparoscopy or laparotomy), details of the EA procedure, interventions in controls (e.g. sham acupuncture or standard care), and country. Outcomes, including time to first flatus (TFF), time to bowel sound recovery (TBSR), time to first defecation (TFD), time to liquid/semiliquid/solid food intake, postoperative pain score, postoperative overall complications, and hospital length of stay (LOS) were also retrieved. In cases of divergent opinions, a third reviewer made a final decision to resolve any discrepancies.
Outcomes and definitions
TFF, defined in accordance with the individual studies, was the primary endpoint of the current meta-analysis. Additionally, several secondary outcomes were evaluated, including TBSR, TFD, time to initiate intake of liquid or solid food, postoperative pain scores, postoperative complications, and hospital LOS. In cases where pain scores were measured at multiple time points, data were gathered from the first three postoperative days.
Quality of included studies
The methodological quality of the included trials was assessed by two reviewers using the Cochrane Risk of bias (ROB) tool. The RoB2 framework encompasses diverse domains crucial for the examination of potential biases, namely, the randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, selection of reported results, and overall bias. Any disagreements between the two reviewers were discussed, and if unresolved, a third reviewer was involved in the discussion until consensus was reached.
Certainty of evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was employed to evaluate the overall quality of the evidence in this study. The GRADE guidelines examine five domains: risk of bias, inconsistency, indirectness, imprecision, and potential publication bias. These domains collectively contribute to the assessment of evidence quality and are classified into four levels: high, moderate, low, and very low. To ensure objectivity and reliability, two researchers independently conducted the evaluation, whereas a third researcher reviewed the assessment process. Any discrepancies or disagreements were resolved through thorough discussion and consultation with professional specialists.
Data analysis
The meta-analysis was conducted using the Review Manager (RevMan) version 5.4.1 software. We presented categorized variables as risk ratios (RRs) with 95% CIs. Continuous variables were analyzed using the mean difference (MD) with a 95% CI, when the units were consistent. In cases where the units varied, standardized mean difference (SMD) was employed. The effect size of SMD was interpreted as follows: a value of less than 0.2 represented a small effect, 0.5 indicated a moderate effect, and a value of 0.8 or greater signified a large effect. To prevent overlap in sample size assessment in studies with more than two intervention/control arms, a strategy was employed in which participants in the intervention and control groups were divided into separate subgroups for comparison with their counterparts in the corresponding specific intervention/control arms, as described in a previous study19. This approach ensured that means and SD were preserved for continuous variables, whereas for categorical outcomes, both the event number and total number of participants were divided. If the I2 value exceeded 50%, the heterogeneity was considered significant. Given the variation in clinical settings, a random effects model was used. To investigate the sources of heterogeneity, subgroup analyses based on the type of surgery (laparoscopy vs. laparotomy), type of control group (sham-EA vs. standard care), position of acupoint (ST36-based EA vs. others), and time of EA (preoperative vs. postoperative) were conducted to analyze the primary outcome. A sensitivity analysis was performed by sequentially excluding each RCT to assess the robustness of the results. When at least 10 trials or data sets were included, a funnel plot was used to detect potential publication bias when at least ten trials or datasets were included. Statistical significance was set at a P-value of less than 0.05.
Results
Search results
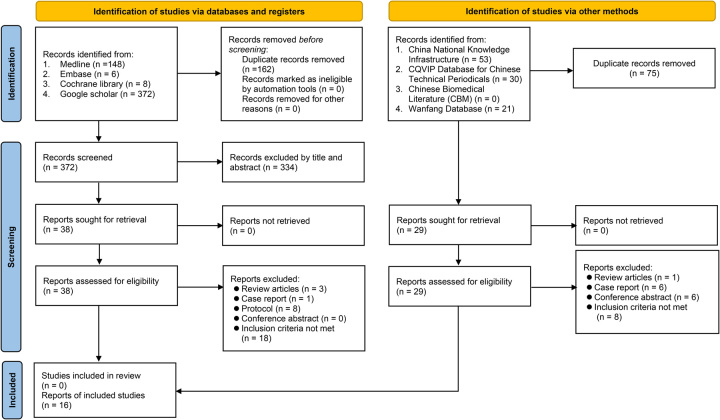
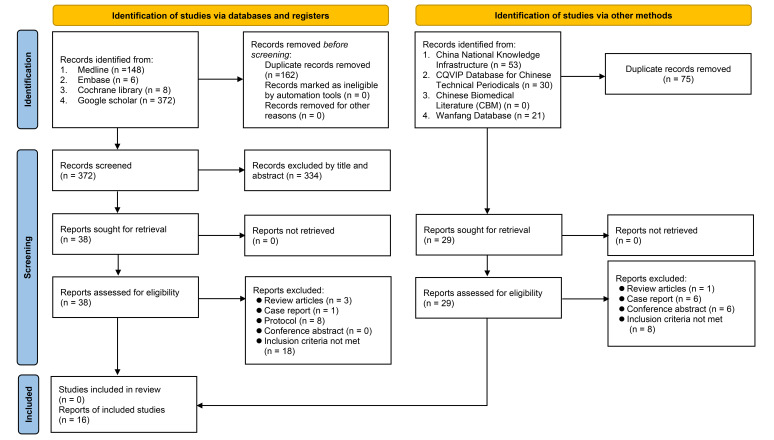
Of the initial 534 records obtained from the four electronic databases, 162 were eliminated because of duplications. Among the remaining 372 records, 334 were considered ineligible based on the assessment of their titles and abstracts. Subsequently, following a thorough full-text screening of the remaining 38 trials, an additional 30 reports were excluded, resulting in eight RCTs that met the eligibility criteria for inclusion in the present study (Fig. 1). Furthermore, during the examination of other databases, such as CNKI, an additional eight RCTs were identified. Overall, a total of 16 RCTs (n=1562 patients) conducted in China published between 2013 and 2023 were included in the analysis16,20–34.
Figure 1.
Flowchart for inclusion and exclusion of studies.
Characteristics of studies
The characteristics of the studies included in this meta-analysis are summarized in Table 1. Baseline patient characteristics, including age and sex, were available for 15 studies. Participants in the EA group were aged 49–67, while those in the control group were aged 51–69. The proportion ranged from 47.5 to 64.6%. However, one study did not provide any relevant information32. Of the 16 eligible RCTs, seven focused exclusively on laparoscopic colorectal surgery16,20,21,25,31,33,35, while four only recruited adults undergoing open colorectal surgery23,28,30,34. Two trials included patients who underwent either laparoscopic or open colorectal surgery27,29. Additionally, three trials did not provide specific details on the type of surgery conducted22,24,32. The sample size in the EA group ranged from 19 to 125 participants, whereas it ranged from 20 to 124 participants in the control group. Of the 16 trials, six employed a triple-arm study design21,23,25,27,32,33, while two utilized a four-arm design28,30.
Table 1.
Characteristics of studies (n=16).
| Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| References | Age (years)a | Male (%) | Type of surgery | Sample sizea (n=1562) | Intervention time (min) | Intervention | Control | Outcomes | Country |
| Deng et al.16 | 59 vs. 62 | 58.3 | LCS | 30 vs. 30 | 30 | EA | Standard care | 1,2,4,5,7,10,11 | China |
| Gong et al.20 | 65 vs. 66 | 52.5 | LCS | 62 vs. 58 | 30 | EA | Standard care | 1,2,4,5,11 | China |
| Li et al.21 | 61/61 vs. 61b | 47.5 | LCS | 80 vs. 40 | 30 | EA | Sham-EA | 1,2,3 | China |
| Long et al.22 | 64 vs. 62 | 53.3 | NA | 30 vs. 30 | 30 | EA | Standard care | 1,2,3,4,5,11 | China |
| Mai et al.23 | 50/52 vs. 51b | 60 | OCS | 40 vs. 20 | 30 | EA | Standard care | 1,2,3 | China |
| Meng et al.24 | 53.7 | 55 | NA | 35 vs. 40 | 20 | EA | Standard care | 1,3,7,9,10 | China |
| Ng et al.25 | 67 vs. 67/69b | 60 | LCS | 55 vs. 110 | 20 | EA | Sham-EA | 1,3,4,5,6,7,11 | China |
| Ou et al.35 | 63 vs. 64 | 60.7 | LCS | 28 vs. 28 | 20 | EA | Standard care | 1,2,5,7,11 | China |
| Shen et al.27 | 60/59 vs. 60b | 64.6 | LCS; OCS | 62 vs. 29 | 30 | EA | Standard care | 1,4,7,8 | China |
| Wang 29 | 56 vs. 55 | 51.4 | LCS; OCS | 35 vs. 35 | 30 | EA | Standard care | 1,2,3,7,8,11 | China |
| Wang et al.28 | 52/51/49 vs. 51c | 50 | OCS | 58 vs. 20 | 30 | EA | Standard care | 1,2,4,11 | China |
| Wang 30 | 65/62/62 vs. 64c | 51.3 | OCS | 60 vs. 20 | 20 | EA | Standard care | 1,2,3 | China |
| Wang et al.31 | 60 vs. 60 | 61.4 | LCS | 125 vs. 123 | 30 | EA | Sham-EA | 1,2,4,5,6,7,10,11 | China |
| Wei et al.32 | NAb | NA | NA | 85 vs. 50 | 30 | EA | Standard care | 1,2,5 | China |
| Yang et al.33 | 62/61 vs. 61b | 62.9 | LCS | 70 vs. 35 | 30 | EA | Standard care | 1,2,4,5,6,7,11 | China |
| Zhang et al.34 | 63 vs. 60 | 57.9 | OCS | 19 vs. 20 | 30 | EA | Sham-EA | 1,2,3,5,11 | China |
Present as intervention versus control groups.
Triple-arm study design.
Four-arm study design.
Outcome 1. Time to first flatus. 2. Time to first defecation. 3. Bowel sound recovery time. 4. Time tolerability of liquid, semiliquid, solid food. 5. Length of postoperative hospital stay, day. 6. Time to first ambulation, day. 7. Pain score. 8. Vomiting score. 9. Nausea score. 10. Abdominal distension score after operation. 11. Number of patients with complications.
EA, Electroacupuncture; ERS, Enhanced recovery after surgery; LCS, laparoscopic colorectal surgery; NA, not available; OCS, open colorectal surgery.
Table 2 provides further details of the acupoints used in the included studies. Among the 16 studies, the majority (i.e. 13 studies) utilized acupoint ST36 either alone or in combination with other acupoints (such as ST39) for alleviating POI16,20,21,23,25,27–29,31–35. Three studies used a variety of acupoints22,24,30. These acupoints include LU7, LU9, SJ6, GB34, LI9, ST39, CV12, and ST2522,24,30. In terms of the waveforms of EA, seven trials utilized continuous waveforms20–24,27,32, whereas two trials utilized dilatational waveforms characterized by pulsating or intermittent patterns of electrical current29,33. However, seven trials did not provide information on the waveform16,25,28,30,31,34,35. Regarding the timing of EA administration, four studies performed EA before the surgical operation21,23,27,28, while EA was administered after the operation in 12 trials16,20,22,24,25,29–35.
Table 2.
Details of acupoint (n=16).
| Author (year) | Acupoint | Waveforms | Electrical frequency | Needle Retention time (min) |
|---|---|---|---|---|
| Deng et al.16 | ST36, ST37, JM8 | NA | NA | 30a |
| Gong et al.20 | ST36 | Continuous | NA | 30a |
| Li et al.21 | ST36, SP6, PC6, GV20 | Continuous | 2/100 Hz | 30b |
| Long et al.22 | LU7, LU9 | Continuous | 2 Hz, 1–2 mA | 30a |
| Mai et al.23 | ST36, ST37, ST39 | Continuous | 2 Hz, 2–3 mA | 30b |
| Meng et al.24 | SJ6, GB34 | Continuous | 2 Hz | 20a |
| Ng et al.25 | ST36, SP6, LI4, TE6 | NA | 100Hz | 20a |
| Ou et al.35 | ST 36 | NA | NA | 20a |
| Shen et al.27 | ST36, RN16, RN17, PC6 | Continuous | 2/100 Hz, 2–3 mA | 30b |
| Wang 29 | ST36 | Dilatational | NA | 30a |
| Wang et al.28 | ST36, ST25, ST37, ST39, CV12, PC6 | NA | 2 Hz, 2–3 mA | 30b |
| Wang 30 | LI9, ST39, CV12, ST25 | NA | NA | 20b |
| Wang et al.31 | ST36, ST37, ST25, RN12 | NA | 100Hz | 30a |
| Wei et al.32 | ST36, ST37, ST39, CV6, CV8, LI4, PC6 | Continuous | 50–100Hz | 30a |
| Yang et al.33 | ST36, ST25 | Dilatational | 2/100Hz | 30a |
| Zhang et al.34 | ST36 | NA | 2 Hz | 30a |
Postoperation.
Before operation.
NA, not available.
Risk of bias assessment
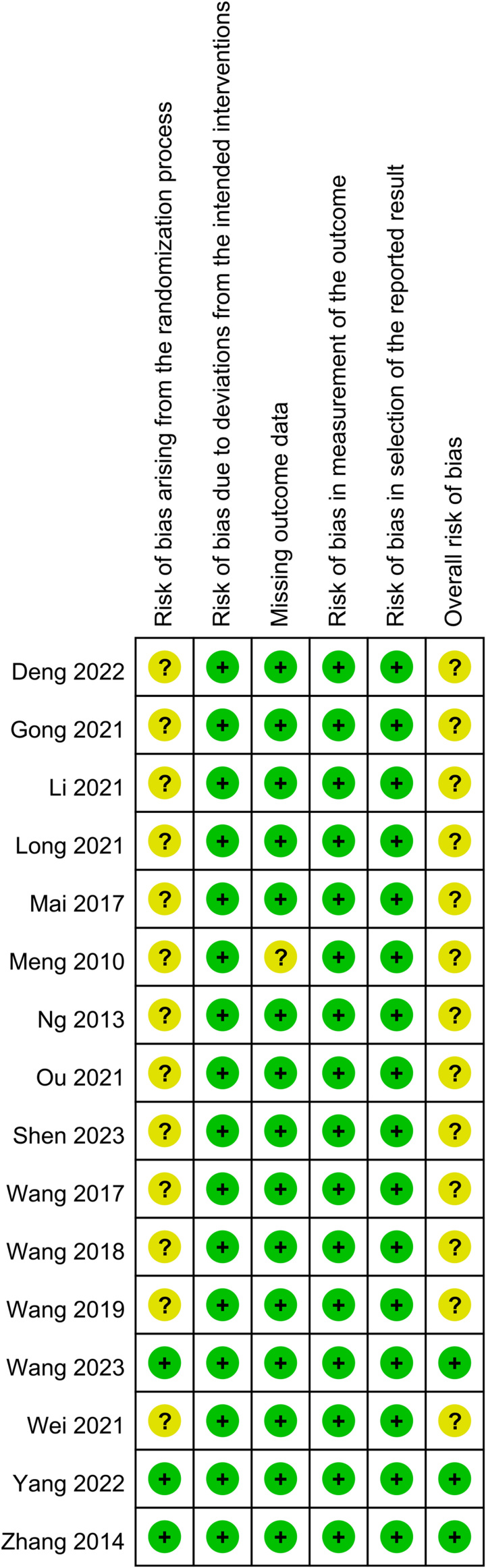
Figure 2 summarizes the results of the risk of bias assessment. Of the 16 studies, 13 were categorized as ‘having concernsʼ over the risk of bias associated with the randomization process16,20–25,27–30,32,35. For the risk of bias related to missing outcome data, one study was considered to raise ʻsome concernsʼ24. The assessment of the overall risk of bias across all studies revealed that ʻsome concernsʼ existed in 13 studies16,20–25,27–30,32,35, indicating the presence of certain methodological limitations or concerns that could impact the reliability and validity of the findings. In contrast, the overall risk of bias was considered low in three studies31,33,34.
Figure 2.
The risks of bias of individual studies.
Primary and secondary outcomes
Primary outcome: times to first flatus, first defecation, and bowel sound recovery
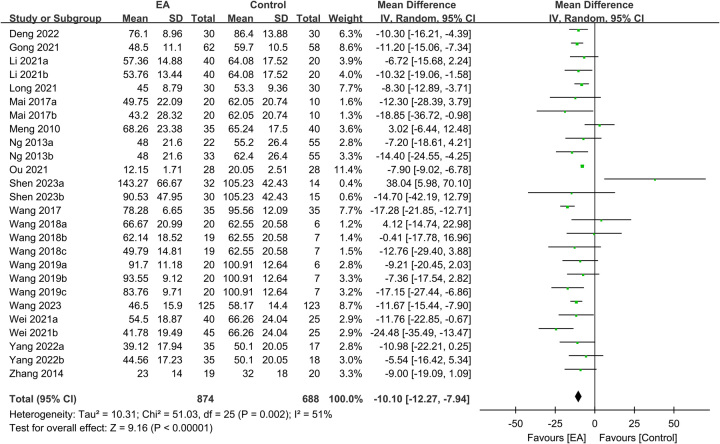
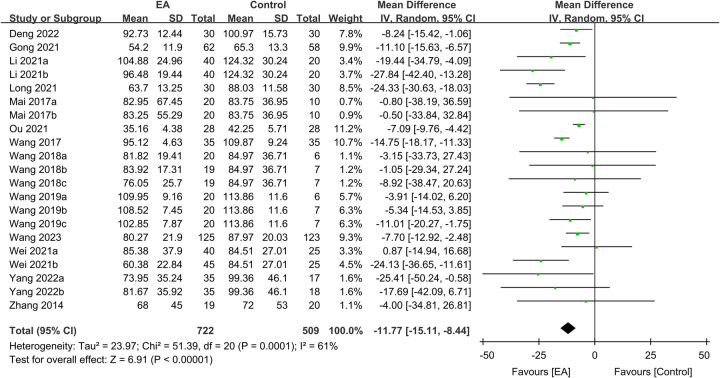
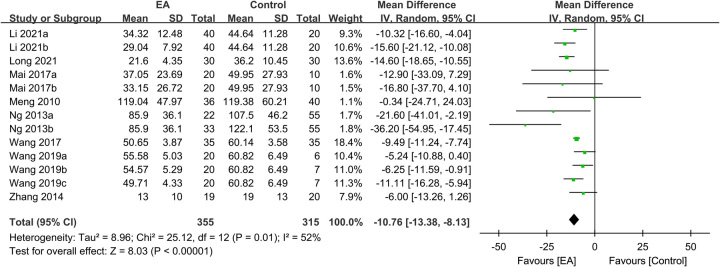
Regarding TFF, the analysis included data from 1562 participants, with 874 in the EA group and 688 in the control group. The meta-analysis revealed a significantly shorter TFF in patients receiving EA than in those receiving standard care (MD: −10.1 h, 95% CI: −12.27–−7.94, P<0.00001, I2=51%) (Fig. 3)16,20–25,27–35. The sensitivity analysis showed consistent findings. Furthermore, an analysis of 1231 participants demonstrated a shorter TFD in the EA group than in the control group (MD: −11.77 h, 95% CI: −15.11–−8.44, P<0.00001, I2=61%, sensitivity analysis: consistent) (Fig. 4)16,20–23,28–35. Additionally, pooled results from 670 patients showed a shorter TBSR in the EA group than in the control group (MD: −10.76 h, 95% CI: −13.38–−8.13, P<0.00001, I2=52%, sensitivity analysis: consistent) (Fig. 5)21–25,29,30,34. An inspection of the funnel plot suggested a low risk of publication bias for these three outcomes (Supplemental Figs. 1–3, Supplemental Digital Content 1, http://links.lww.com/JS9/B232), indicating the satisfactory reliability of the results derived from the included studies.
Figure 3.
Forest plot showing the association of electroacupuncture (EA) use with the time to first flatus (n=1562). IV, inverse variance; CI, confidence interval; SD, standard deviation.
Figure 4.
Forest plot demonstrating the correlation between electroacupuncture (EA) use and the time to first defecation (h). IV, inverse variance; CI, confidence interval; SD, standard deviation.
Figure 5.
Forest plot showing the association of electroacupuncture (EA) use with bowel sound recovery time (h). IV, inverse variance; CI, confidence interval; SD, standard deviation.
Secondary outcomes: impact of EA on postoperative pain
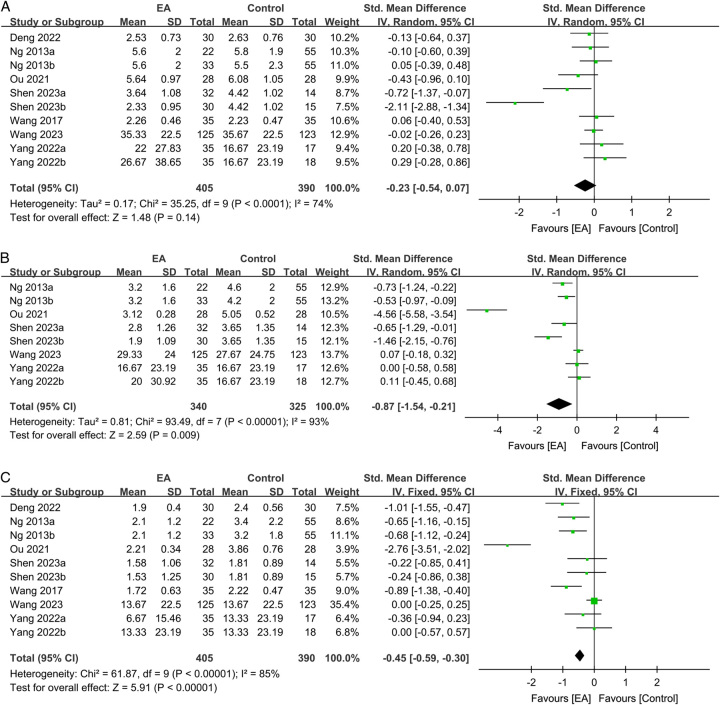
Figure 6 presents the effect of EA on the severity of pain from postoperative day one to day three. On postoperative day one, albeit not statistically significant, there was a trend indicating an association between EA use and less severe postoperative pain (SMD: −0.23, 95% CI: −0.54–0.07, P=0.14, I2=74%, n=795 patients, sensitivity analysis: consistent) (Fig. 6A)16,25,27,29,31,33,35. On postoperative day two, patients in the EA group experienced significantly less severe pain than those in the control group (SMD: −0.87, 95% CI: −1.54–−0.21, P=0.009, I2=93%, n=655 patients, sensitivity analysis: consistent) (Fig. 6B)25,27,31,33,35. Similarly, on postoperative day three, the severity of pain in patients undergoing EA was lower than that in those receiving standard care (SMD: −0.45, 95% CI: −0.59–−0.3, P<0.00001, I2=85%, n=795 patients, sensitivity analysis: consistent) (Fig. 6C)16,25,27,29,31,33,35. A low risk of publication bias for these outcomes (Supplemental Figs. 4–5, Supplemental Digital Content 1, http://links.lww.com/JS9/B232) suggested that the included studies in the current meta-analysis were representative.
Figure 6.
Forest plot showing the correlations of electroacupuncture (EA) use with pain score on (A) day 1; (B) day 2; and (C) day 3. IV, inverse variance; CI, confidence interval; Std, standardized.
Secondary outcomes: impact of EA on ambulation and food intake
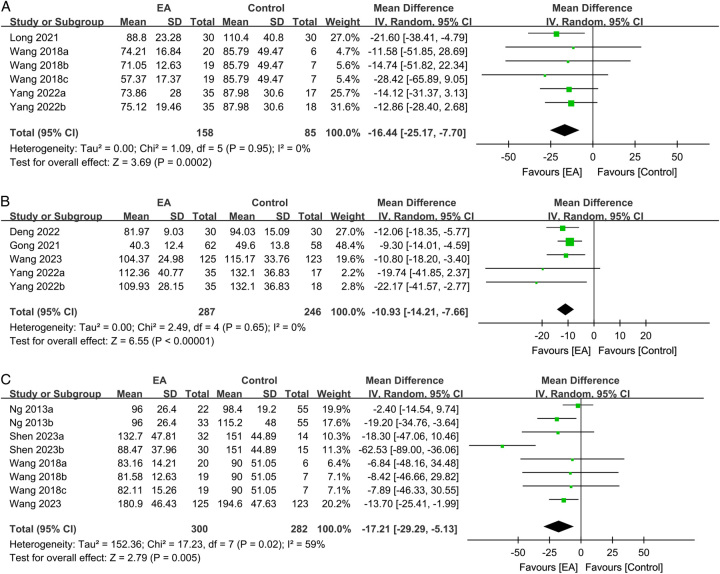
The time to first ambulation did not differ significantly between the groups receiving EA and standard care (MD: −5.9 h, 95% CI: −12.34–0.55, P=0.07, I2=52%, n=518 patients, sensitivity analysis: consistent)25,31,33. Regarding the impact of EA on postoperative liquid/food intake, the use of EA was associated with a significant reduction in the time to tolerability of liquid (MD: −16.44 h, 95% CI: −25.17–−7.7, P=0.0002, I2=0%, n=243 patients) (Fig. 7A)22,28,33. However, the sensitivity analysis showed inconsistent findings when the study by Long et al.22 was excluded. With respect to the time to tolerability of semiliquid food, EA revealed a significant benefit (MD: −10.93 h, 95% CI: −14.21–−7.66, P<0.00001, I2=0%, n=533 patients, sensitivity analysis: consistent) (Fig. 7B)16,20,31,33. Furthermore, the use of EA resulted in a shorter time to tolerability of solid food (MD: −17.21 h, 95% CI: −29.29–−5.13, P=0.005, I2=59%, n=582 patients, sensitivity analysis: consistent) (Fig. 7C)25,27,28,31.
Figure 7.
Forest plot showing the associations of electroacupuncture (EA) use with the time to tolerability of (A) liquid; (B) semiliquid; and (C) solid food. CI, confidence interval; IV, inverse variance; SD, standard deviation.
Secondary outcomes: impact of EA on postoperative complications and hospital LOS
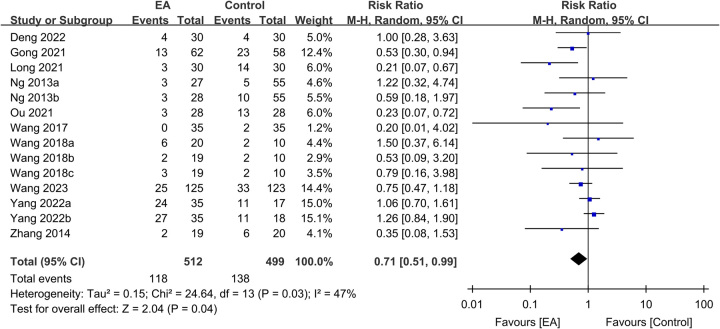
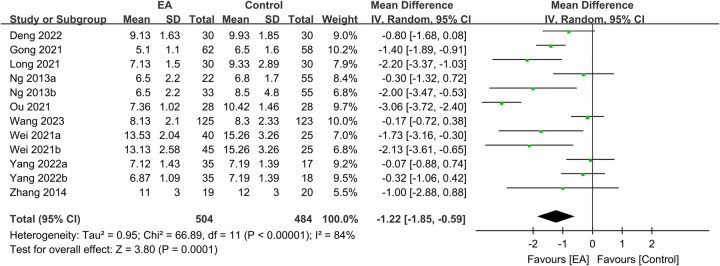
The use of EA was linked to a lower risk of overall complications than that in the controls (RR: 0.71, 95% CI: 0.51–0.99, P=0.04, I2=47%, n=1011 patients, sensitivity analysis: inconsistent) (Fig. 8)16,20,22,25,28,29,31,33–35. Pooled results based on 988 participants revealed a shorter hospital LOS in the EA group than in the control group (MD: −1.22 days, 95% CI: −1.85–−0.59, P=0.0001, I2=84%, n=988 patients, sensitivity analysis: consistent) (Fig. 9)16,20,22,25,31–35. A low risk of publication bias for these outcomes (Supplemental Figs. 6–7, Supplemental Digital Content 1, http://links.lww.com/JS9/B232) suggested that the included studies in the current meta-analysis were representative.
Figure 8.
Forest plot showing the association between electroacupuncture (EA) use and the risks of postoperative complications. CI, confidence interval; IV, inverse variance; SD, standard deviation.
Figure 9.
Forest plot showing the association of electroacupuncture (EA) use with length of postoperative hospital stay (days). CI, confidence interval; IV, inverse variance; SD, standard deviation.
Subgroup analysis of the primary outcome
Table 3 summarizes the findings of the subgroup analysis. Subgroup analysis based on the type of surgery (laparoscopy vs. open surgery), type of control group (standard care vs. sham EA), position of acupoint (ST36 vs. other acupoints), and time of EA (postoperative vs. preoperative) demonstrated no influence of these variables on the primary outcome (TFF).
Table 3.
Subgroup analysis on primary outcome.
| Outcome or subgroup | Data sets | Participants (N) | Effect estimate; MD [95% CI] | I2 |
|---|---|---|---|---|
| Type of surgery | ||||
| Laparoscopy | 10 | 874 | −8.52 [−9.52–−7.53] | 0% |
| Open surgery | 9 | 257 | −10.02 [−14.34–−5.70] | 0% |
| Type of control group | ||||
| Standard care | 20 | 990 | −10.00 [−12.79–−7.20] | 60% |
| Sham-EA | 6 | 572 | −10.75 [−13.60–−7.90] | 0% |
| Position of acupoint | ||||
| ST36-based | 21 | 1347 | −10.73 [−13.19–−8.27] | 53% |
| Other acupoint | 5 | 215 | −7.67 [−13.28–−2.06] | 52% |
| Time of EA | ||||
| Postoperative | 17 | 1213 | −10.60 [−12.91–−8.29] | 58% |
| Preoperative | 9 | 349 | −7.01 [−13.61–−0.41] | 38% |
EA, electroacupuncture; MD, mean difference.
Certainty of evidence
Supplemental Table 1 (Supplemental Digital Content 1, http://links.lww.com/JS9/B232) summarizes the certainty of evidence across different outcomes. A high level of certainty was assigned to four outcomes (TFF, TBSR, time to tolerability of liquid, and time to tolerability of semiliquid food), indicating their robustness and reliability. Four other outcomes had a moderate level of certainty (i.e. TFD, time to first ambulation, time to tolerability of solid food, and postoperative complications), whereas the other four outcomes (i.e. postoperative pain on days 1, 2, and 3, as well as hospital LOS) had a low level of certainty.
Discussion
The current meta-analysis, focusing on 16 studies with a total of 1562 patients demonstrated several positive outcomes associated with the use of EA in patients undergoing colorectal surgeries. First, patients who received EA had shorter gastrointestinal functional recovery times, including TFF, TFD, and TBSR, than those who received standard care. Consistently, patients undergoing EA showed shorter times to tolerability of liquid, semiliquid, and solid food intake. Despite the lack of difference in time to first ambulation between the EA and control groups, the former exhibited a trend of reduced postoperative pain on day one and experienced significantly less severe pain on days two and three compared to the controls. Furthermore, our results demonstrated the association of EA with a lower risk of overall complications and a shorter hospital LOS. These findings highlight the potential benefits of incorporating EA into perioperative care to enhance recovery.
POI, which is characterized by the temporary cessation of bowel movement, is a prevalent postoperative complication, particularly following open abdominal surgery. Its etiology is believed to be an interplay between sympathetic input, mediator release, the inflammatory cascade, and analgesic impact36. Although there is no ideal treatment modality for POI, previous studies have provided compelling evidence supporting the effectiveness of EA in alleviating POI and its associated symptoms13,14. EA is a therapeutic approach that involves the insertion of fine needles into acupoints followed by the application of low-level electrical currents to achieve therapeutic outcomes by synergistically combining the stimulatory effects of acupuncture and electrostimulation. Compared with conventional acupuncture, one distinct advantage of EA is its ability to stimulate acupoints with precise modulation of frequency and intensity, thereby allowing for accurate and quantifiable adjustments25. Furthermore, EA triggers analgesic effects by activating a range of bioactive substances through peripheral, spinal, and supraspinal mechanisms37. Previous investigations have demonstrated an association of acupoints ST36 and SP6 with the cAMP-CREB pathway and the mRNA expression profile in the brainstem of morphine-tolerant mice38. Moreover, EA exhibits anti-inflammatory effects by suppressing the expression of adenosine and substance P, which contribute to inflammatory pain39. Furthermore, EA activates the cholinergic anti-inflammatory pathway, leading to a reduction in the release of proinflammatory cytokines, including TNF-α, IL-1β, and IL-10, thereby mitigating postoperative inflammatory responses and diminishing the incidence of unexplained fever in patients receiving craniotomy40.
One previous meta-analysis, including an article published before 2017, reported a correlation between acupuncture and related therapies with enhanced gastrointestinal recovery after colorectal surgery, as reflected by a shorter TFF (i.e. −15.79 h) compared to the control group41. However, the strength of the evidence of this analysis was limited by the inclusion of only four RCTs. Two other meta-analyses, which included patients undergoing various abdominal surgeries, also found a shorter TFF with the use of mixed acupuncture techniques (i.e. EA, TEA, or manual acupuncture) than with the control group. Nevertheless, the significance of their findings was impaired by high heterogeneity (I2=94% and I2=90%, respectively)13,14 probably due to the inclusion of different acupuncture techniques and various surgical procedures. Additionally, as only a small subset of studies (i.e. three and five RCTs, respectively) in those meta-analyses focused on laparoscopic procedures, their findings13,14 may not be applicable to real-world scenarios, considering the increasing popularity of laparoscopic procedures in current clinical practice42–44. Our meta-analysis of 1552 patients found a significant association between EA and a shorter TFF (i.e. −10.1 h) compared to standard care. The relatively low heterogeneity (51%) in our analysis may be attributed to the precise application of stabilized electrical stimulation during the EA procedure and the inclusion of patients specifically undergoing colorectal surgery. In addition, a substantial number of studies in our meta-analysis that included patients undergoing laparoscopic colorectal surgery (i.e. nine RCTs) may provide updated information for clinical practice.
Our current meta-analysis revealed a lower pain score on postoperative days two and three in patients who received EA than in the control group, despite only borderline significance on day one. Nevertheless, our finding of a borderline reduction in pain score on postoperative day one aligned with that of a previous study that demonstrated the effectiveness of TEAS for pain alleviation at short-term time points (i.e. 4 h, 12 h, and 24 h) following laparoscopy12. Our inclusion of studies that involved both open laparotomy and laparoscopic procedures may have hampered the significance of our findings. Considering the association between the development of POI and aggravation of postoperative pain45, our findings of a shorter TFF (i.e. lower risk of POI) in patients receiving EA may partly explain their lower pain scores observed on days two and three than those in the controls.
According to the traditional meridian theory in traditional Chinese medicine, specific anatomical locations along the meridians, known as acupoints, are believed to have a direct influence on internal organs. Zusanli (ST36) is an acupoint located along the stomach meridian of the foot-yangming meridian channel. A previous RCT has demonstrated that not only could EA at ST36 alleviate inflammatory reactions, but it could also attenuate intra-abdominal pressure in septic patients46. Similarly, several animal studies have shown the effectiveness of EA in improving gastrointestinal motility46–48. These findings, therefore, suggest that EA at ST36 has therapeutic potential for preventing POI and optimizing gastrointestinal function.
This study had several limitations that should be acknowledged. First, the fact that all 16 included RCTs were conducted in China may limit the extrapolation of the findings to other populations of different ethnic and geographic backgrounds, as well as healthcare settings. Second, the wide variation in sample size (i.e. from 19 to 125 in the EA group and from 20 to 123 in the control group) may have impaired the statistical power and precision of the results. Third, although we found a significant positive effect of EA on the alleviation of POI regardless of the choice of acupoints, the majority of studies focused on acupoint ST36 alone or in combination with other acupoints, and only a few studies explored other acupoints. Therefore, the impact of EA on different acupoints cannot be differentiated. Fourth, the difference in the timing of EA administration among the studies (i.e. preoperative or postoperative) may be a potential confounder that could influence the outcomes. Finally, the finding of some concerns in our assessment of the risk of bias in the majority of studies suggested the presence of methodological limitations that may impact the reliability and validity of our results. Furthermore, the extended publication period of the included studies (i.e. from 2013 to 2023) could introduce potential biases associated with changes in clinical practice and advancements in surgical techniques over time.
In conclusion, the current meta-analysis of 16 studies involving 1562 patients demonstrated a positive association between the use of EA and enhanced gastrointestinal recovery (e.g. time to first flatus), shortened time to tolerability of liquid/food, reduced postoperative pain on day two and three, as well as a lower risk of complications and shorter hospital stays compared to patients receiving standard care. Although our findings support the multifaceted advantages of EA in optimizing the recovery trajectory in patients undergoing colorectal surgery, the inclusion of only Asian studies warrants further large-scale investigations to elucidate the benefits of EA in patients with different ethnic and geographical backgrounds.
Ethical approval
Not applicable.
Consent
Not applicable.
Sources of funding
Not applicable.
Author contribution
H.-T.C. and K.-C.H.: conceptualization, methodology, and software; Y.-T.H. and. J.-Y.W.: data curation; K.-C.H. and I.-W.C.: writing – original draft preparation; C.-H.H. and C.-M.L.: visualization, investigation; C.-K.S.: supervision; K.-C.H. and I.-W.C.: software and validation; K.-C.H. and H.-T.C.: writing – reviewing and editing; I.-W.C. and C.-K.S.: contributed equally as corresponding authors to this study; H.-T.C. and K.-C.H.: contributed equally as first authors to this study.
Conflicts of interest disclosure
The authors declare that they have no financial conflicts of interest with regard to the content of this report.
Research registration unique identifying number (UIN)
Name of the registry: PROSPERO.
Unique identifying number or registration ID: CRD42023433310.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023433310.
Guarantor
Hsiao-Tien Chen, Kuo-Chuan Hung, I-Wen Chen, and Cheuk-Kwan Sun.
Data availability statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Footnotes
H.-T.C. and K.-C.H. contributed equally as first authors to this work.
I.-W.C. and C.-K.S. contributed equally as corresponding authors to this work.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Published online 2 November 2023
Contributor Information
Hsiao-Tien Chen, Email: sandra770211@gmail.com.
Kuo-Chuan Hung, Email: ed102605@gmail.com.
Yen-Ta Huang, Email: uncleda.huang@gmail.com.
Jheng-Yan Wu, Email: Andy10271@gmail.com.
Chung-Hsi Hsing, Email: hsing@seed.net.tw.
Chien-Ming Lin, Email: chienming95@gmail.com.
I-Wen Chen, Email: CheniwenA60912@gmail.com.
Cheuk-Kwan Sun, Email: researchgate000@gmail.com.
References
- 1. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol 2019;14:89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Douaiher J, Ravipati A, Grams B, et al. Colorectal cancer-global burden, trends, and geographical variations. J Surg Oncol 2017;115:619–630. [DOI] [PubMed] [Google Scholar]
- 3. Asgeirsson T, El-Badawi KI, Mahmood A, et al. Postoperative ileus: it costs more than you expect. J Am Coll Surg 2010;210:228–231. [DOI] [PubMed] [Google Scholar]
- 4. Harnsberger CR, Maykel JA, Alavi K. Postoperative Ileus. Clin Colon Rectal Surg 2019;32:166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg 2000;87:1480–1493. [DOI] [PubMed] [Google Scholar]
- 6. Bragg D, El-Sharkawy AM, Psaltis E, et al. Postoperative ileus: recent developments in pathophysiology and management. Clin Nutr 2015;34:367–376. [DOI] [PubMed] [Google Scholar]
- 7. Venara A, Neunlist M, Slim K, et al. Postoperative ileus: pathophysiology, incidence, and prevention. J Visc Surg 2016;153:439–446. [DOI] [PubMed] [Google Scholar]
- 8. Hao JJ, Mittelman M. Acupuncture: past, present, and future. Glob Adv Health Med 2014;3:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu F, Yin S, Zhu X, et al. Acupuncture for relieving abdominal pain and distension in acute pancreatitis: a systematic review and meta-analysis. Front Psychiatry 2021;12:786401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang T, Chon TY, Liu B, et al. Efficacy of acupuncture for chronic constipation: a systematic review. Am J Chin Med 2013;41:717–742. [DOI] [PubMed] [Google Scholar]
- 11. Streitberger K, Ezzo J, Schneider A. Acupuncture for nausea and vomiting: an update of clinical and experimental studies. Auton Neurosci 2006;129:107–117. [DOI] [PubMed] [Google Scholar]
- 12. Meng D, Mao Y, Song QM, et al. Efficacy and safety of Transcutaneous Electrical Acupoint Stimulation (TEAS) for postoperative pain in laparoscopy: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med 2022;2022:9922879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ye Z, Wei X, Feng S, et al. Effectiveness and safety of acupuncture for postoperative ileus following gastrointestinal surgery: a systematic review and meta-analysis. PLoS One 2022;17:e0271580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen KB, Huang Y, Jin XL, et al. Electroacupuncture or transcutaneous electroacupuncture for postoperative ileus after abdominal surgery: a systematic review and meta-analysis. Int J Surg 2019;70:93–101. [DOI] [PubMed] [Google Scholar]
- 15. Napadow V, Makris N, Liu J, et al. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp 2005;24:193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deng Y, Wang Y, Bao S, et al. Effect of electroacupuncture and medicine combination on gastrointestinal function in patients with colorectal cancer after laparoscopic surgery based on the concept of enhanced recovery after surgery. Chin J of Oncol Prev and Treat 2022;14:194–198. [Google Scholar]
- 17. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 18. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009;62:1013–1020. [DOI] [PubMed] [Google Scholar]
- 19. Chang CC, Yen WT, Lin YT, et al. Perioperative pregabalin for preventive analgesia in breast cancer surgery: a meta-analysis of randomized controlled trials. Clin J Pain 2020;36:968–977. [DOI] [PubMed] [Google Scholar]
- 20. Gong H, Zhang Y, Han G, et al. Clinical research on acupuncture and medicine combined with enhanced recovery after surgery on promoting recovery of patients undergoing laparoscopic colorectal cancer surgery. Shanghai J TCM 2021;55:54–57. [Google Scholar]
- 21. Li W-X, Zhao J-X, Ran D-W. Effects of electroacupuncture of different frequency on gastrointestinal function, stress response, cytokines and immune mechanism after laparoscopic radical resection of colorectal cancer with general anesthesia. J Trad Chinese Med Pharm 2021;27:93–96. [Google Scholar]
- 22. Long Y, Zhang Z-J, Huang Z-M, et al. Clinical observation on electroacupuncture at source Luo-(connecting)points in lung meridians for treatment for postoperative ileus after colorectal cancer surgery. J Guangzhou Univ Trad Chinese Med 2021;38:518–523. [Google Scholar]
- 23. Mai S, Meng J, Wang W, et al. Influence of electroacupuncture pretreatment on intestinal function in the patients of colorectal cancer surgery. Chinese Acupuncture Moxibustion 2017;37:483–487. [DOI] [PubMed] [Google Scholar]
- 24. Meng ZQ, Garcia MK, Chiang JS, et al. Electro-acupuncture to prevent prolonged postoperative ileus: a randomized clinical trial. World J Gastroenterol 2010;16:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ng S, Leung W, Hon S, et al. Electroacupuncture for ileus after laparoscopic colorectal surgery: a randomised sham-controlled study. Hong Kong Med J 2013;19:S33–S35. [PubMed] [Google Scholar]
- 26. Li X-Y, Hou H-T, Chen H-X, et al. Preoperative plasma biomarkers associated with atrial fibrillation after coronary artery bypass surgery. J Thorac Cardiovasc Surg 2021;162:851–63. e3. [DOI] [PubMed] [Google Scholar]
- 27. Shen N, Yan H, Meng J. Effect of preoperative acupoint electroacupuncture stimulation on postoperative recovery of patients undergoing radical colorectal cancer. J Ningxia Med Univ 2023;45:49–53. [Google Scholar]
- 28. Wang T-Y, Meng J-H, Mai S-C. Electroacupuncture treatment conduced before and after surgery is better in promoting recovery of gastrointestinal function in colorectal cancer patients undergoing radical resection. Acupuncture Res 2018;43:797–800. [DOI] [PubMed] [Google Scholar]
- 29. Wang Y. Research on the gastrointestinal function recovery impact of evodia hot iron combined with acupuncture therapy in treatment of colorectal cancer patients. J Liaoning Univ TCM 2017;19:195–196. [Google Scholar]
- 30. Wang Y, Li T. Study of electro–needling local and distal points on recovery of gastrointestinal function after the operation of rectum cancer. J Clin Acupuncture Moxibustion 2019;35:26–28. [Google Scholar]
- 31. Wang Y, Yang JW, Yan SY, et al. Electroacupuncture vs sham electroacupuncture in the treatment of postoperative ileus after laparoscopic surgery for colorectal cancer: a multicenter, randomized clinical trial. JAMA Surg 2023;158:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei R, Li L, Cao H, et al. Effect of hot compress of Evodia and salt combined with acupoint physiotherapy and acupuncture and massage on recovery of bowel function in postoperative patients with colorectal cancer. Chinese Nurs Res 2021;35:2597–2599. [Google Scholar]
- 33. Yang JW, Shao JK, Wang Y, et al. Effect of acupuncture on postoperative ileus after laparoscopic elective colorectal surgery: a prospective, randomised, controlled trial. Eclinical Med 2022;49:101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Z, Wang C, Li Q, et al. Electroacupuncture at ST36 accelerates the recovery of gastrointestinal motility after colorectal surgery: a randomised controlled trial. Acupunct Med 2014;32:223–226. [DOI] [PubMed] [Google Scholar]
- 35. Ou H, Ou X, Li M. Research on the efficacy of abdominal hot compress of Evodia Rutaecarpa combined with electroacupuncture at Zusanli (ST 36) on rapid rehabilitation after laparoscopic colorectal cancer surgery. China Med Pharm 2021;11:175–181. [Google Scholar]
- 36. Luckey A, Livingston E, Tache Y. Mechanisms and treatment of postoperative ileus. Arch Surg 2003;138:206–214. [DOI] [PubMed] [Google Scholar]
- 37. Zhang R, Lao L, Ren K, et al. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology 2014;120:482–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Q, Qin F, Wang H, et al. Effect of electro-acupuncture at ST36 and SP6 on the cAMP -CREB pathway and mRNA expression profile in the brainstem of morphine tolerant mice. Front Neurosci 2021;15:698967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang RY, Zhu BF, Wang LK, et al. Electroacupuncture alleviates inflammatory pain via adenosine suppression and its mediated substance P expression. Arq Neuropsiquiatr 2020;78:617–623. [DOI] [PubMed] [Google Scholar]
- 40. Liu Z, Khuong JN, Caruana CB, et al. The prognostic value of elevated perioperative neutrophil-lymphocyte ratio in predicting postoperative atrial fibrillation after cardiac surgery: a systematic review and meta-analysis. Heart Lung Circul 2020;29:1015–1024. [DOI] [PubMed] [Google Scholar]
- 41. Liu Y, May BH, Zhang AL, et al. Acupuncture and related therapies for treatment of postoperative ileus in colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med 2018;2018 3178472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buia A, Stockhausen F, Hanisch E. Laparoscopic surgery: a qualified systematic review. World J Methodol 2015;5:238–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Riviere D, Gurusamy KS, Kooby DA, et al. Laparoscopic versus open distal pancreatectomy for pancreatic cancer. Cochrane Database Syst Rev 2016;4 Cd011391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gurusamy KS, Pallari E, Midya S, et al. Laparoscopic versus open transhiatal oesophagectomy for oesophageal cancer. Cochrane Database Syst Rev 2016;3 Cd011390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Story SK, Chamberlain RS. A comprehensive review of evidence-based strategies to prevent and treat postoperative ileus. Digest Surg 2009;26:265–275. [DOI] [PubMed] [Google Scholar]
- 46. Meng JB, Jiao YN, Xu XJ, et al. Electro-acupuncture attenuates inflammatory responses and intraabdominal pressure in septic patients: a randomized controlled trial. Medicine (Baltimore) 2018;97:e0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Han X, Chen X, Wang X, et al. Electroacupuncture at ST36 improve the gastric motility by affecting neurotransmitters in the enteric nervous system in type 2 diabetic rats. Evid Based Complement Alternat Med 2021;2021 6666323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang X, Lu L, Zi L, et al. Electroacupuncture at acupoint ST36 (Zusanli) improves intestinal motility dysfunction via increasing the proportion of cholinergic neurons in rat ileal myenteric ganglia after severe acute pancreatitis. Evid Based Complement Alternat Med 2022;2022 7837711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.