Abstract
Introduction:
Human epidermal growth factor receptor type 2 (HER2) overexpression is a prognostic factor and a therapeutic target for breast cancer; however, anti-HER2 therapies are ineffective in patients with bladder cancer. The authors investigated the effect of HER2 overexpression (HER2+) on the prognosis of muscle-invasive bladder cancer (MIBC).
Materials and methods:
This retrospective cohort study included patients who underwent initial transurethral resection of bladder tumors between 2005 and 2013 and were registered in the Korea National Health Insurance Database, which provides data on overall survival (OS). Sixty-one patients with clinically nonmetastatic de novo MIBC were included in this study. As a subgroup, 33 patients who underwent immediate radical cystectomy (RC) were analyzed. Univariate and multivariate Cox proportional hazards models were used to identify prognostic factors for survival. A multivariable binary logistic regression model was used to identify the favorable T stage.
Results:
Among the 61 patients with d-MIBC, 14 were HER2+ and 47 HER2-. Age less than 70 years [hazard ratio (HR): 0.312, CI: 0.16–0.59, P<0.001] and HER2+ status (HR: 0.40, CI: 0.19–0.85, P=0.02) were favorable prognostic factors for OS after adjusting for clinical variables. In the RC subgroup, HER2+ status was a significant predictive factor for the pT2 stage (HR): 36.8, CI: 4.83–797.41, P<0.01). Age less than 70 years (HR: 0.15, CI: 0.05–0.42, P<0.001) and HER2+ status (HR: 0.11, CI: 0.02–0.54, P=0.01) were favorable prognostic factors for OS after adjusting for RC pathological variables.
Conclusions:
HER2+ status could be a marker for an indolent subset of MIBC and could predict favorable survival regardless of RC status. Moreover, HER2+ status not only consistently predicted a favorable T stage after RC, but also predicted better survival than pathological outcomes.
Keywords: HER2, neoadjuvant chemotherapy, radical cystectomy, urinary bladder neoplasms
Introduction
Highlights
Exploring human epidermal growth factor receptor type 2 (HER2+) status in de novo muscle-invasive bladder cancer.
HER2+ status is a reliable predictor for organ-confined disease.
HER2+ status could potentially guide the chemotherapy administration.
We demonstrate a different role for HER2 abundance in bladder cancer.
Paving the way for the application of effective anti-HER2 therapies.
Urothelial carcinoma is the 10th most common cancer worldwide, with 525 000 new cases annually, and is the 13th most common cause of cancer-related deaths1. Approximately 25% of bladder cancer diagnoses are of the muscle-invasive subtype (MIBC)2,3. The standard recommended treatment for MIBC is radical cystectomy (RC) following cisplatin-based neoadjuvant chemotherapy (NAC)2,3, which has an absolute 5-year overall survival (OS) benefit of 8%4. However, its utilization is limited to only ~19% of patients owing to interindividual variability in treatment response and the potential for adverse effects, which can occasionally be fatal and impede subsequent therapy5. The lack of a method for predicting which patients would respond favorably to NAC, even with the limited predictive value of radiological exams6,7, and the absence of reliable biomarkers2,3 contribute to the disparity between recommended treatments and clinical practices.
Developing a reliable biomarker for NAC therapy is crucial for optimizing MIBC treatment and improving patient outcomes. MIBC is a heterogeneous disease. It is classified into subtypes based on the expression of signature genes and oncological pathways8, which helps stratify subtypes based on their prognosis and can be used to predict the effectiveness of various treatments8–10. While the identified subtypes provide valuable insights into the efficacy of NAC within its designated therapeutic scope, the lack of clinical guidelines for determining the appropriateness of NAC administration impedes the development of alternative therapeutic strategies2,3. In an era where NAC is considered standard care, conducting studies on patients do not receive NAC becomes challenging. Assessments of disease status before NAC are often imprecise, making it difficult to determine from post-NAC RC pathology alone if a patient was initially at a low stage after TURBT and thus is a candidate for RC, or whether NAC was particularly effective. In addition to these challenges, it is not feasible to conduct RNA sequencing on past samples. Consequently, it becomes difficult to conduct studies aimed at identifying patient groups for whom NAC could be safely omitted, using subjects who have not received NAC.
Due to the reliability of immunohistochemistry (IHC) tests for assessing human epidermal growth factor receptor type 2 (HER2) expression, accurately evaluating patients from the era before NAC, who underwent RC alone. These studies provide prognostic outcomes of RC alone, in conjunction with baseline pathological result, thereby offering potential insights that could inform the decision regarding the administration of NAC. Furthermore, HER2 expression is characteristic of specific genetic subtypes8,11,12 and has been elucidated as having a unique pathway that is potentially significant to the pathogenesis of bladder cancer observed in luminal subtypes9,13–15. This makes HER2 a bridge between past patients in the era of RC alone and those with access to contemporary genetic analyses. Therefore, the potential of HER2 as a prognostic marker in the administration of NAC and as a therapeutic target for treatment should be explored.
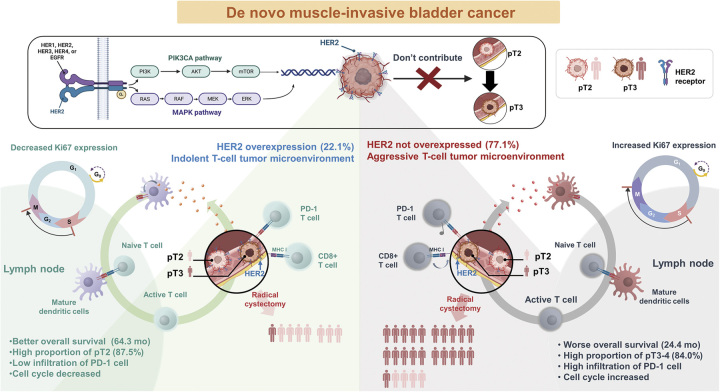
We investigated the predictive role of HER2+ in MIBC and analyzed the tumor microenvironment (TME) to assess the pathways expressed in specific molecular subtypes. We analyzed the effect of HER2 expression in patients with clinically nonmetastatic de novo MIBC (d-MIBC) corresponding to pathological stage T2N0 (PT2N0) versus PT3-4 or N1-3. We also assessed the prognosis with respect to HER2 status, density of tumor-infiltrating immune cells (PD-1, CD8), and proliferation (Ki-67).
Materials and methods
Ethics approval
Ethical approval for this study (GNAH 2018-05-017) was provided by the Institutional Review Board of Gangneung Asan Hospital, Gangneung, South Korea, on 12 May 2018. The requirement for informed consent was waived owing to the retrospective nature of the study. This study adhered to the (strengthening the reporting of cohort studies in surgery) STROCCS (Supplemental Digital Content 1, http://links.lww.com/JS9/B241) reporting guidelines16.
Patient eligibility
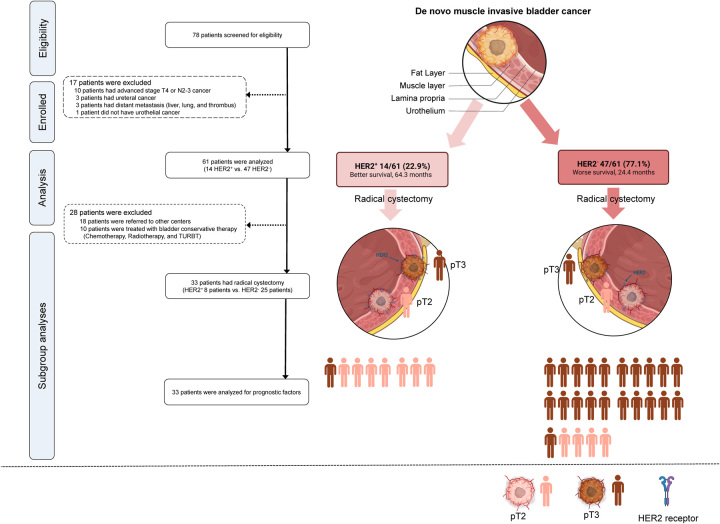
The patients were diagnosed with urothelial cancer and had no history of urothelial cancer. We included 78 patients with muscle invasion corresponding to stage pT2, as determined by transurethral resection of bladder tumor (TURBT) at Gangneung Asan hospital between January 2005 and December 2013. Patients with a local clinical stage of T4 bladder cancer, those with lymph node metastasis beyond the pelvic region (N2-3 stage), those previously diagnosed with urothelial carcinoma, and those with distant metastasis to other organs were excluded. Three patients who were diagnosed with ureteral cancer and had a prior diagnosis of urothelial carcinoma were excluded from the study. Furthermore, 10 patients were excluded based on clinical suspicion of either T4 bladder cancer or lymph node metastasis beyond the pelvic region, as evidenced by preoperative computed tomography CT scans. (Of the 10 excluded patients, six were suspected to have T4 stage bladder cancer and six were suspected to have N2-3 stage bladder cancer. Two patients were suspected to have both T4 and N2-3 stages). Although we intended to include patients with N1-stage bladder cancer based on CT findings, none were ultimately included in the study. Three patients suspected of having distant metastasis were excluded; they had liver and lung thrombus, respectively. Additionally, one patient whose pathological diagnosis was sarcoma was also excluded (Fig. 1). After the exclusion of 17 patients of these 78 patients, the clinical cohort consisted of 61 patients diagnosed with clinically nonmetastatic d-MIBC and no evidence of metastasis or adjacent organ invasion on initial examination, including physical examination, chest radiography, intravenous pyelogram, bone scan, and CT scan (Fig. 1).
Figure 1.
Flowchart showing patients and study design. HER2, human epidermal growth factor receptor 2; pT2, pathological stage T2; pT3-4, pathological stage T3-4; TURBT, transurethral resection of bladder tumor.
Study design and subgroup analyses
Survival was evaluated for all patients using clinical variables, including HER2+. In the subgroup analysis, 33 patients who underwent immediate RC were included. Of the 28 excluded patients, 18 were transferred to another center for a second opinion, and 10 received treatment other than RC, such as chemotherapy, radiotherapy, or repeated TURBT at our center (Fig. 1). Whether the subgroup reflected the characteristics of the patient cohort, including the pathological parameters of RC specimens, was evaluated, and variables were analyzed to assess predictive factors for survival. Furthermore, clinical variables and biomarkers of HER2 expression were evaluated to predict the adverse pathological outcomes of pT3 or N1-3 MIBC after RC. PD-1, CD8, and Ki-67 expressions were evaluated in the TME according to HER2+ status.
Outcomes and variables
All patients were registered in the National Health Insurance Database, which provides data on OS without loss to follow-up. Radiological and pathological analyses were performed for all patients. CT scans were not re-evaluated because of the low quality of some nonenhanced scans and because a bladder filling protocol for evaluating the radiological T stage was not performed. Pathological specimens were analyzed according to the WHO 2004 rating system17.
Tissue microarray and immunohistochemistry
Formalin-fixed, paraffin-embedded tumor samples from 61 TURBT specimens diagnosed with MIBC were collected and arrayed using a tissue microarrayer (Quick-Ray, Unitma Co., Ltd.). Briefly, representative areas of each tumor were selected and marked on hematoxylin and eosin-stained slides, and the corresponding tissue blocks were collected. Matching areas of each tumor block were punched using two tissue cylinders (2 mm diameter), and each core was transferred to the recipient microarray block. Four micrometer-thick sections were cut from TMA paraffin blocks for IHC analysis.
IHC staining for HER2 (4B5; Roche Diagnostics, Tucson, AZ, USA; predilution) and Ki-67 (SP6; Cell Marque; 1:300) was performed using TMA blocks using a Benchmark automated staining system (Ventana Medical Systems). IHC staining for PD-1 (EPR4877;; 1:100) and CD8 (SP16; Thermo Fisher Scientific; 1:100) was performed on TMA blocks using a Bond-Max automatic immunostaining device (Leica Biosystems). Tonsil tissue sections were used as positive controls for PD-1, CD8, and Ki-67. Negative controls were obtained by omitting the primary antibody.
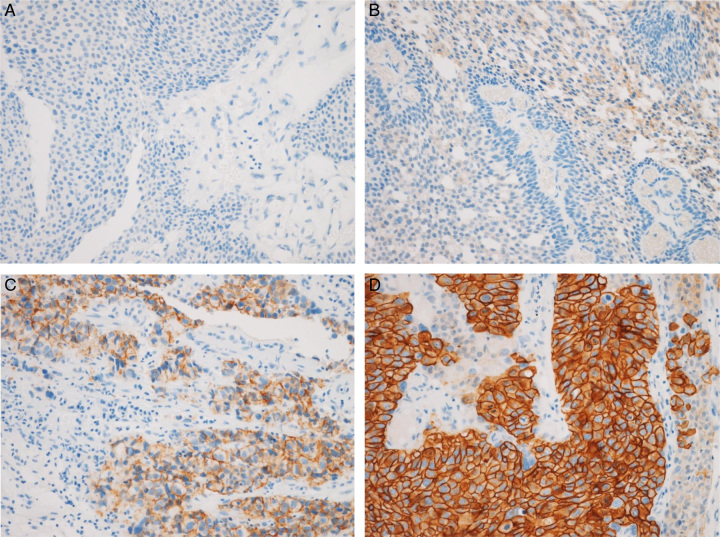
HER2 expression was evaluated according to the type of breast cancer18. These guidelines and IHC staining images according to HER2 expression are shown in Figure 2. HER2− (i.e. underexpression) included HER2 0 and HER2 1+, where HER2 0 indicated no staining (Fig. 2A; IHC score, 0) and HER2 1+ indicated weak or partial membrane staining in less than or equal to 10% tumor cells (Fig. 2B; IHC score, 1); HER2+ (i.e. overexpression) included HER2 2+ and HER2 3+, whereby HER2 2+ indicated weak or moderate complete membrane staining in greater than 10% tumor cells (Fig. 2C; IHC score, 2) and HER2 3+ indicated strong complete membrane staining in greater than 10% tumor cells (Fig. 2D; IHC score, 3).
Figure 2.
Immunohistochemical staining showing HER2 expression at 200× magnification. (A) HER2− included ʻHER2 0ʼ and ʻHER2 1+ʼ. HER2 0: no staining (IHC score 0); (B) HER2 1+: weak or partial membrane staining in less than or equal to 10% tumor cells [immunohistochemical (IHC) score, 1]. (C) HER2+ (HER2 overexpression) included ʻHER2 2+ʼ and ʻHER2 3+ʼ. HER2 2+ indicates weak or moderate complete membrane staining in less than 10% tumor cells (IHC score, 2); (D) HER2 3+ indicates strong complete membrane staining in less than 10% tumor cells (IHC, score 3). HER2, human epidermal growth factor receptor 2.
Sections immunostained for PD-1 and CD8 were assessed for tumor-infiltrating lymphocytes in the tumor bed area. Each section was evaluated for immune cells under a microscope (400×; BX51). Five noncontiguous microscopic areas containing tumor-infiltrating lymphocytes were randomly selected from each sample to confirm representativeness and homogeneity. We counted the immune cells in each area and determined the mean value for one microscopic field (0.1590 mm2/field) at 200× (Supplementary Figure 1A and 1B, Supplemental Digital Content 2, http://links.lww.com/JS9/B242). The percentage of nuclear Ki-67+ cells was manually counted for 1000 cells at 400× magnification, and the Ki-67 score was evaluated using the labeling index (Supplementary Figure 1C, Supplemental Digital Content 2, http://links.lww.com/JS9/B242).
Statistical analysis
Pearson’s χ2 test or Fisher’s exact test was used to analyze variables according to HER2+ status, and an independent t-test was performed for continuous variables. The effect of clinicopathological factors and HER2+ status on OS was estimated using univariate and multivariate Cox proportional hazards models. Kaplan–Meier survival analysis was performed using the log-rank test. A logistic regression model was used to evaluate the predictive factors for the pathological stage. The Mann–Whitney U and Kruskal–Wallis tests were performed to compare nonparametric variables. Statistical analyses were performed using Prism 9.3 (GraphPad Software). P<0.05.
Results
Clinicopathological characteristics of the patients
Table 1 shows the clinical characteristics of the groups. Sixty-one patients were evaluated, of which 14 patients (22.9%) were HER2+ and 47 patients (77.1%) were in the HER2− group. The median age was significantly lower in the HER2+ group [65.5 years, interquartile range (IQR): 54.0–71.0] compared to the HER2− group (71.0 years, IQR: 66.5–78.5, P=0.031). A higher proportion of patients under 60 years were observed in the HER2+ group (35.7%) compared to the HER2− group (8.5%, P=0.037). Regarding sex distribution, the majority of patients were males in both groups (HER2+: 92.9%, HER2−: 85.1%, P=0.762). In terms of BMI, smoking, drinking, DM, and HTN, no significant differences were observed between the two groups. The radiological findings for clinical T3 (cT3) MIBC and hydronephrosis ratio before the first TURBT were comparable between the HER2+ (6/14, 42.9% and 1/14, 7.1%, respectively) and HER2− (21/47, 44.7% and 6/47, 12.8%, respectively) groups. After TURBT, the number of tumors, tumor size, and proportion of patients with concurrent carcinoma in situ (CIS) in the two groups were comparable, and all patients had high-grade MIBC. The median survival period was significantly longer in the HER2+ (64.3 months, IQR: 28.6–123.0) compared to the HER2− group (24.4 months, IQR: 7.2–43.0, P = 0.022). The rate of expiry events in the observed period was lower in the HER2+ group (64.3%) compared to the HER2− group (91.5%, P=0.037).
Table 1.
Baseline clinicopathological results according to HER2 overexpression.
| Number of patients, n (%) | Total 61 (100) | HER2+ 14 (22.9) | HER2− 47 (77.1) | P |
|---|---|---|---|---|
| Age, years | ||||
| Median (IQR) | 70.0 [64.0–77.0] | 65.5 [54.0–71.0] | 71.0 [66.5–78.5] | 0.031 |
| Age <60 (%) | 9 (14.8) | 5 (35.7) | 4 (8.5) | 0.037 |
| Sex, n (%), | 0.762 | |||
| Female | 8 (13.1) | 1 (7.1) | 7 (14.9) | |
| Male | 53 (86.9) | 13 (92.9) | 40 (85.1) | |
| BMI (kg/m2) | 22.9 [20.9–25.4] | 23.0 [21.5–25.5] | 22.9 [20.6–25.3] | 0.861 |
| Smoking, n (%) | 23 (37.7) | 4 (28.6) | 19 (40.4) | 0.625 |
| Drinking, n (%) | 13 (21.3) | 3 (21.4) | 10 (21.3) | 1.000 |
| DM, n (%) | 9 (14.8) | 1 (7.1) | 8 (17.0) | 0.627 |
| HTN, n (%) | 26 (42.6) | 6 (42.9) | 20 (42.6) | 1.000 |
| Preoperative radiologic findings | ||||
| Clinical stage of T3 | 27 (44.3) | 6 (42.9) | 21 (44.7) | 1.000 |
| Hydronephrosis | 7 (11.5) | 1 (7.1) | 6 (12.8) | 0.919 |
| TURBT findings | ||||
| T2 stage, n (%) | 61(100) | 14 (22.9) | 47 (77.1) | 1.000 |
| High-grade, n (%) | 61(100) | 14 (22.9) | 47 (77.1) | 1.000 |
| Concurrent CIS, n (%) | 4 (6.6) | 1 (7.1) | 3 (6.4) | 1.00 |
| Survival period, months, median [IQR] | 28.3 [8.9–58.4] | 64.3 [28.6–123.0] | 24.4 [7.2–43.0] | 0.022 |
| Expire event, months, n (%) | 52 (85.2) | 9 (64.3) | 43 (91.5) | 0.037 |
CIS, carcinoma in situ; DM, diabetes mellitus; HER2, human epidermal growth factor receptor 2; HTN, hypertension; IQR, interquartile range; TURBT, transurethral resection of bladder tumor.
Predictive factors for OS in all patients
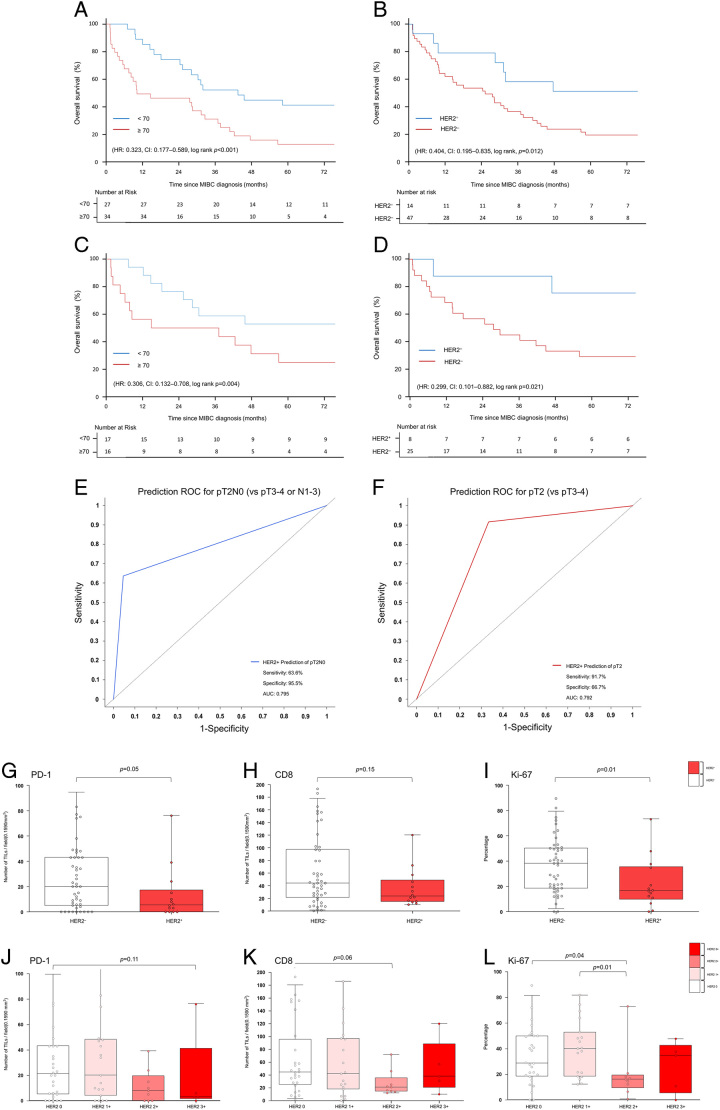
Table 2 shows the results of survival-related analyses using univariate and multivariate Cox proportional hazards models for the 61 patients. In univariate analysis, age less than 70 years [hazard ratio (HR): 0.331, CI: 0.181–0.606, P<0.001] and HER2+ status (HR: 0.411, CI: 0.198–0.851; P=0.015) were significant prognostic factors. In multivariate analysis, age less than 70 years (HR: 0.323, CI: 0.169–0.616, P<0.001) and HER2+ status (HR: 0.411, CI: 0.194–0.87; P=0.017) were favorable prognostic factors after adjusting for clinicopathological factors. Kaplan–Meier survival curves (Fig. 2) showed that age less than 70 years (log-rank, P<0.001) and HER2+ status (log-rank, P=0.012) were predictors of survival.
Table 2.
Univariate and multivariate Cox proportional hazards model for overall survival in all patients.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | ||||
| Age ≥70 (n=34) | Reference | Reference | ||
| Age <70 (n=27) | 0.323 [0.177–0.589] | < 0.001 | 0.312 [0.164–0.592] | <0.001 |
| Sex | ||||
| Female (n=8) | Reference | Reference | ||
| Male (n=53) | 0.869 [0.39–1.934] | 0.730 | 1.195 [0.519–2.748] | 0.675 |
| HER2 expression | ||||
| HER2− (n=47) | Reference | Reference | ||
| HER2+ (n=14) | 0.404 [0.195–0.835] | 0.015 | 0.401 [0.19–0.848] | 0.017 |
| Radiologic findings | ||||
| Clinical local stage | ||||
| ≥T3 (n=27) | Reference | Reference | ||
| ≤T2 (n=34) | 0.951 [0.549–1.648] | 0.859 | 1.045 [0.589–1.855] | 0.880 |
| Hydronephrosis | ||||
| No (n=54) | Reference | Reference | ||
| Yes (n=7) | 1.513 [0.68–3.365] | 0.310 | 0.743 [0.305–1.811] | 0.513 |
| Pathologic finding of TURBT | ||||
| Carcinoma in situ | ||||
| No (n=57) | Reference | Reference | ||
| Yes (n=4) | 0.786 [0.278–2.223] | 0.650 | 0.666 [0.212–2.089] | 0.486 |
HER2, human epidermal growth factor receptor 2; HR, hazard ratio; TURBT, transurethral resection of bladder tumor.
Clinicopathological characteristics of the RC subgroup
Table 3 shows the clinical characteristics of the 33 patients who underwent RC in the subgroup analyses. The subgroup included 8/14 (57.1%) HER2+ and 25/47 (53.2%) HER2− patients. The ratio of patients with local T stage of cT3 to those with preoperative hydronephrosis (HER2+:0, 0.00%; HER2−:4, 16.0%) did not differ significantly between the HER2+ (5, 62.3%) and HER2− (9, 36.0%) groups. A significantly higher proportion of patients in the HER2+ group (7/8, 87.5%) had pathological less than or equal to pT2 stage compared to those in the HER2− group (4/25, 16.0%, P<0.001). A significantly higher proportion of patients in the HER2+ group (7/8, 87.5%) had pathological less than or equal to pT2 stage compared to those in the HER2− group (4/25, 16.0%, P<0.001). Additionally, a significantly higher proportion of one patient in the HER2+ group was adjusted to pT0 after TURBT, compared to those in the HER2- group (P=0.003). Twelve lymph nodes (median) were removed and evaluated after RC in both HER2+ (12.0, IQR: 10.5–15.0) and HER2− (12.0, IQR: 7.0–18.0) groups. There was no difference in the proportion of patients with metastatic lymph node(s) between the groups [HER2+:2/8 (25.0%) and HER2−:6/25 (24%)]. The median durations from TURBT to RC were 12 (IQR: 6.0–14.0) and 8 (IQR: 7.0–12.0) days in the HER2+ and HER2− groups, respectively. Regarding the radiological accuracy of the pathological results in predicting clinical T stage less than or equal to pT2, 6/19 patients (≤ cT2) were diagnosed with pT2, whereas 9/14 patients (≥ cT3) were diagnosed with greater than or equal to pT3. The sensitivity and specificity of the radiological findings for predicting pT2 were 40.0 and 40.9%, respectively. Patients in the HER2+ group had longer median survival (99.3 months, IQR: 64.3–128.3) than those in the HER2− group (28.2 months, IQR:7.5–89.8, P=0.013).
Table 3.
Clinicopathological results according to HER2 overexpression after radical cystectomy.
| Number of patients, n (%) | Total 33 (100) | HER2+ 8 (24.2) | HER2− 25 (75.8) | P |
|---|---|---|---|---|
| Age, years | ||||
| Median [IQR] | 69.0 [62.0–74.0] | 67.0 [56.0–74.0] | 69.0 [64.0–74.0] | 0.541 |
| Age <60 (%) | 5 (15.2) | 3 (37.5) | 2 (8) | 0.145 |
| Sex, n (%), Male | 30 (90.9) | 7 (87.5) | 23 (92.0) | 1.000 |
| BMI (kg/m2) | 22.7 [20.6–25.3] | 22.2 [21.3–25.9] | 22.7 [20.3–25.3] | 0.821 |
| Smoking, n (%) | 15 (45.5) | 5 (62.5) | 10 (40.0) | 0.481 |
| Drinking, n (%) | 10 (30.3) | 3 (37.5) | 7 (28.0) | 0.947 |
| DM, n (%) | 3 (9.1) | 1 (12.5) | 2 (8.0) | 1.000 |
| HTN, n (%) | 15 (45.5) | 3 (37.5) | 12 (48.0) | 0.911 |
| Preoperative findings | ||||
| Clinical stage of ≤T2 | 19 (57.6) | 3 (37.5) | 16 (64.0) | 0.363 |
| Clinical stage of T3 | 14 (42.4) | 5 (62.5) | 9 (36.0) | 0.363 |
| Hydronephrosis | 4 (12.1) | 0 (0) | 4 (16.0) | 0.559 |
| RC pathological results | ||||
| pT2 (VS. T3-4) | 11 (33.3) | 7 (87.5) | 4 (16.0) | <0.001 |
| T2N0 (VS. T3-4 or N1-3) | 9 (27.3) | 6 (75.0) | 3 (12.0) | 0.002 |
| No residual, n (%) | 1 (3.0) | 1 (12.5) | 0 (0.0) | 0.003 |
| T2, n (%) | 10 (30.3) | 6 (75.0) | 4 (16.0) | 0.002 |
| T3a, n (%) | 16 (48.5) | 0 (0.0) | 16 (64.0) | 0.003 |
| T3b, n (%) | 4 (12.1) | 1 (12.5) | 3 (12.0) | 1.000 |
| T4, n (%) | 2 (6.1) | 0 (0.0) | 2 (8.0) | 1.000 |
| High-grade | 33 (100) | 8 (100) | 25 (100) | 1.000 |
| Carcinoma in situ, n (%) | 4 (12.1) | 1 (12.5) | 3 (12) | 1.000 |
| Lymphadenectomy data | ||||
| Evaluated nodes per patient, median (IQR) | 12.0 [8.0–17.0] | 12.0 [10.5–15.0] | 12.0 [7.0–18.0] | 0.817 |
| Number of patients with positive lymph node | 8 (24.2) | 2 (25.0) | 6 (24.0) | 1.000 |
| Time to cystectomy, days median [IQR] | 8.0 [7.0–13.0] | 12.0 [6.0–14.0] | 8.0 [7.0–12.0] | 0.422 |
| Survival period, months, median [IQR] | 42.4 [12.0–93.1] | 99.3 [64.3–128.3] | 28.2 [7.5–89.8] | 0.013 |
| Expire event | 26 (78.8) | 4 (50.0) | 22 (88.0) | 0.073 |
DM, diabetes mellitus; HER2, human epidermal growth factor receptor 2; HTN, hypertension; IQR, interquartile range; NA, not applicable; RC, radical cystectomy.
Predictive factors for survival in the RC subgroup
Table 4 shows the survival analysis results using univariate and multivariate Cox proportional hazards models for patients who underwent RC. In univariate analysis, age less than 70 years (HR: 0.306, CI: 0.132–0.708; P=0.006) and HER2+ status (HR: 0.299, CI: 0.101–0.882; P=0.029) were significant prognostic factors. In the multivariate analysis, age less than 70 years (HR: 0.145, CI: 0.049–0.424; P<0.001) and HER2+ status (HR, 0.113; CI, 0.024–0.536; P=0.006) were favorable prognostic factors after adjusting for the pathological results of RC specimens. Positive lymph nodes were not a significant factor for survival in multivariate analysis (HR, 2.585; CI, 0.938–7.122; P=0.066). Figures 2C and D show the Kaplan–Meier curves resulting from the survival analysis using variables identified as significant in the multivariate analysis. Age less than 70 years (log-rank, P=0.004) and HER2+ status (log-rank, P=0.021) were found to be significant favorable prognostic factors for survival.
Table 4.
Univariate and multivariate Cox proportional hazards model for overall survival in subgroup after radical cystectomy.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR [95% CI] | P | HR [95% CI] | P | |
| Age | ||||
| Age ≥70 (n=16) | Reference | Reference | ||
| Age <70 (n=17) | 0.306 [0.132–0.708] | 0.006 | 0.145 [0.049–0.424] | <0.001 |
| Sex | ||||
| Female (n=3) | Reference | Reference | ||
| Male (n=30) | 1.542 [0.363–6.552] | 0.558 | 4.109 [0.751–22.464] | 0.103 |
| HER2 overexpression | ||||
| HER2− (n=25) | Reference | Reference | ||
| HER2+ (n=8) | 0.299 [0.101–0.882] | 0.029 | 0.113 [0.024–0.536] | 0.006 |
| Radiologic findings | ||||
| Hydronephrosis | ||||
| No (n=29) | Reference | Reference | ||
| Yes (n=4) | 1.644 [0.563–4.804] | 0.363 | 0.376 [0.101–1.393] | 0.143 |
| Pathological findings of RC | ||||
| T stage | ||||
| ≥T3 (n=22) | Reference | Reference | ||
| ≤T2 (n=11) | 0.526 [0.221–1.257] | 0.148 | 1.778[0.499–6.336] | 0.375 |
| Lymph node-positive | ||||
| No (n = 25) | reference | reference | ||
| Yes (n = 8) | 1.552 [0.643–3.743] | 0.328 | 2.585 [0.938–7.122] | 0.066 |
| Carcinoma in situ | ||||
| No (n = 29) | reference | reference | ||
| Yes (n = 4) | 1.419 [0.488–4.132] | 0.521 | 0.935 [0.294–2.976] | 0.910 |
HER2, human epidermal growth factor receptor 2; HR, hazard ratio; RC, radical cystectomy.
Predictive factors for the pathological stage after RC
We evaluated the predictive factors of pathological stage pT2N0 (vs. T3–4 or N1-3) and pT2 (vs. T3–4 or Nx) in the RC subgroup using a logistic regression model (Table 5). HER2+ status was a significant predictive factor for pT2N0 (OR: 22.0, CI: 3.45–213.66, P=0.003) and pT2 (OR: 36.75, CI: 4.83–797.41, P<0.003) after RC. Because all four hydronephrosis patients had pT3-4, the odds ratio for this could not be calculated. One patient diagnosed with CIS after TURBT was classified as pT3 after RC. HER2+ serves as an independent prognostic factor for predicting both pT2N0 and pT2. The prognostic utility of HER2+ was further corroborated by ROC analysis with the area under the curve (AUC). The model demonstrated a strong discriminatory ability in predicting pT2N0, as evidenced by an AUC of 0.795 (Fig.3E). Likewise, its performance in predicting pT2 was similarly robust, yielding an AUC of 0.792 (Fig. 3F).
Table 5.
Univariate logistic regression for pathologic results after radical cystectomy.
| Univariate (pT2N0 VS. pT3-4 or N1-3) | Univariate (pT2 VS. pT3-4) | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age | ||||
| Age ≥70 (n=16) | Reference | Reference | ||
| Age <70 (n=17) | 2.36 [0.5–13.36] | 0.29 | 1 [0.09–23.05] | 1.00 |
| Sex | ||||
| Female (n=3) | Reference | Reference | ||
| Male (n=30) | 0.73 [0.06–16.91] | 0.81 | 2.1 [0.49–10.06] | 0.33 |
| HER2 overexpression | ||||
| HER2− (n=25) | Reference | Reference | ||
| HER2+ (n=8) | 22.00 [3.45–213.66] | 0.003 | 36.75 [4.83–797.41] | 0.003 |
| Radiological findings | ||||
| Hydronephrosis | ||||
| No (n=29) | Reference | Reference | ||
| Yes (n=4) | 0.00 [0.00–∞] | 0.99 | 0.00 [0.00–∞] | 0.99 |
| Clinical local stage | ||||
| ≥T3 (n=14) | Reference | Reference | ||
| ≤T2 (n=19) | 1.69 [0.36–9.54] | 0.52 | 0.83 [0.19–3.69] | 0.80 |
| Pathological finding of TURBT | ||||
| Carcinoma in situ | ||||
| No (n=32) | Reference | Reference | ||
| Yes (n=1) | 0.00 [0.00–∞] | 0.99 | 0.00 [0.00–∞] | 0.99 |
HER2, human epidermal growth factor receptor 2; OR, odds ratio; TURBT, transurethral resection of bladder tumor.
Figure 3.
Characterization of HER2+. (A) Kaplan–Meier curve of overall survival probability according to age in the all patients group. (B) Kaplan–Meier curve of overall survival probability according to HER2+ in the all patients group. (C) Kaplan–Meier curve of overall survival probability according to age in the radical cystectomy subgroup. (D) Kaplan–Meier curve of overall survival probability according to HER2+ in the radical cystectomy subgroup. (E) and (F): ROC curves for assessing the predictive utility of HER2+ in pathologic T Stages. (E) illustrates the ROC curve for the prediction of pT2N0. (F) illustrates the ROC curve for the prediction of pT2. (G)–(L) show tumor-infiltrating lymphocytes per field (0.1590 mm2) (PD-1, CD8) and growth fraction of cells (Ki-67) according to HER2 expression. (G) Box plots showing the number of PD-1 tumor-infiltrating lymphocytes per field (0.1590 mm2) according to HER2+ (vs. HER2−). (H) Box plots showing the number of CD8 tumor-infiltrating lymphocytes per field (0.1590 mm2) according to HER2+ (vs. HER2−). (I) Box plots demonstrating the growth fraction of cells (Ki-67) according to HER2+ (vs. HER2−). (J) Box plots showing the number of PD-1 tumor-infiltrating lymphocytes per field (0.1590 mm2) according to HER2 expression. (K) Box plots showing the number of tumor-infiltrating lymphocytes per field (0.1590 mm2) in CD8 according to HER2 expression. (L) Box plots demonstrating the growth fraction of cells (Ki-67) according to HER2 expression. Box plots indicate the bottom, upper, and lower quartiles. The middle bands indicate the median and whiskers extend to the 95th percentile. Each value is displayed on the box and whiskers plot, and for cases with a P-value, P<0.05 is displayed; for non-significant values, the smallest P-value is displayed. HER2+ included HER2 2+ and HER2 3+, and HER2− included HER2 0 and HER2 1+. MIBC, muscle-invasive bladder cancer; TIL, tumor-infiltrating lymphocyte; HER2, human epidermal growth factor receptor 2; HER2+, HER2 overexpression; ROC, receiver operating characteristic.
Characteristics of TME according to HER2 expression
Figure 3 and Supplementary File Table 1 (Supplemental Digital Content 2, http://links.lww.com/JS9/B242) show the density of tumor-infiltrating lymphocytes (PD-1 and CD8) and cells in the active phase of the cell cycle (Ki-67) according to HER2 expression. PD-1 density was lower in HER2+ patients than in HER2− patients (P=0.05; Fig. 3G), while CD8 density was not different between the HER2+ and HER2− groups (P=0.15; Fig. 2H). Ki-67 values were significantly lower in the HER2+ group than in the HER2− group (P=0.01; Fig. 3I). PD-1 density and CD8 density were not different according to each of HER2 expression level (Fig. 3J, Figure 3K). The Ki-67 value was significantly lower in the HER2 2+ group compared to the HER2 0 group (P=0.04) and HER2 3+ group (P=0.01), respectively (Fig. 3L).
Discussion
We identified an indolent subset of MIBC based on HER2+ status in patients with clinically nonmetastatic d-MIBC. HER2+ status was evaluated as a prognostic factor that could predict favorable survival regardless of subsequent RC. We found that HER2+ status was a favorable predictor of T stage less than or equal to T2 in patients with or without lymph node metastases. The incidence of HER2+ status was 7/11 (63.6%) in pT2 and 1/22 (4.5%) in pT3, demonstrating that HER2+ status does not contribute to progression from pT2 to pT3.
In clinical practice, a biomarker that can predict the pathological T stage would help determine the treatment regimen for MIBC-related therapies. HER2+ status in d-MIBC was evaluated as a critical threshold for reducing the risk of perivesical fat invasion. This is a critical stage for patients with pT2 disease for whom RC is considered the first course of treatment. It can facilitate treatment decisions by allowing patients with high-risk MIBC to select NAC and maximize therapeutic benefit while protecting low-risk patients from unnecessary exposure to cytotoxic chemotherapy2,3. The role of radiological examination is limited at this point; ~50% of patients diagnosed with T2 bladder cancer after TURBT are typically understaged2,3 and, even with the additional use of multi-detector CT, overstaging and understaging occur in 8.3 and 29.4% of patients, respectively6,7, and the accuracy of pT3 stage prediction is 49–100%7. Of the patients observed in the present study, overstaging and understaging occurred in 28.6% (4/14) and 68.3% (13/19), respectively, on CT scans. In contrast, overstaging and understaging occurred in only 12.5% (1/8) and 16.0% (4/25) of patients, respectively, when HER2+ and HER2− were used to predict T2 and T3, respectively. HER2+ status as a biomarker has the potential to predict organ-confined diseases and, therefore, could help in deciding the appropriate treatment strategy for patients with resectable bladder cancer.
NAC has achieved significant improvements in the treatment of MIBC4. However, NAC is not actively performed clinically because of potential chemotherapy-induced toxicity, delays in RC treatment in NAC nonresponders, and the lack of biomarkers to identify patients who would benefit from this treatment5,19. Cisplatin exerts its anticancer effects by inducing cellular toxicity through interference with DNA transcription and replication mechanisms, it is well known biomarker that the mutation of ERCC220 ERBB221 and DNA damage repair genes such as ATM, RB1, and FANCC20,22 are associated with increased sensitivity to NAC23. Nonetheless, the clinical interpretation of these identified biomarkers remains challenging and does not provide a definitive rationale for the administration of NAC. The absence of residual tumor (pT0) and improved pT stage observed in RC specimens after NAC are favorable predictors of survival24,25. Clinically, favorable outcomes of RC following NAC are often identified by pathologic stages such as pT0N0M020 or pT0/pTis/pTa22. While these outcomes may be interpreted as evidence of the benefits of NAC, it is crucial to note that these patient groups are heterogenous. They include individuals who initially presented with high pT stage (pT3-4) disease but responded well to NAC, as well as those whose baseline low T stage (pT2) contributed more to these outcomes. While this results support the recommendation for the enhancement of NAC, it also raises the consideration that there may be patients for whom NAC offers limited advantage, particularly when complete TURBT is achievable. As in the era of NAC as a standard treatment, the evaluation of its adequacy is restricted to the clinical results following NAC, which is challenging. Alternative research designs are essential for studies aimed at determining the NAC administration, and such studies necessitate the inclusion of patients who have not received NAC. Evaluations could be performed using historical patient data related to HER2 expression, which can be reliably assessed through archived specimens stained with IHC. Therefore, there is potential for research that connects historical patients with contemporary genetic analyses to determine NAC administration.
There is a substantial association between HER2+ and the molecular subtypes of cancers, as HER2+ is associated with specific subtypes8,11,12. MIBCs have been classified into six subtypes based on the relevant mRNA signatures for oncogenic pathways, TME, and clinical characteristics8. In this consensus molecular subtypes, HER2+ is a characteristic of luminal subtypes [luminal papillary (LumP), luminal nonspecific (LumN), and luminal unstable (LumU)]8,11,12. HER2+ in the LumP subtype differs from the other HER2+ subtypes (LumN and LumU), which is induced by an increase in ERBB2 amplification in an aggressive form8,11,26. The LumP subtype, characterized by FGFR3 mutations, is a genetically stable bladder cancer with fewer molecular alterations than FGFR3 wild-type tumors26,27. This makes LumP distinct from other subtypes, as other signature gene mutations are related to progression. Moreover, the FGFR signaling pathway regulates HER2 and EGFR, and FGFR3 amplification reduces the therapeutic effect of anti-HER2 therapy28. Given that HER2+ plays a specific role in the LumP subtype11,26, we suggest that FGFR3 is a potential modulator of HER2 expression and that the molecular mechanism is related to the indolent characteristics of LumP and HER2+. We demonstrated that the HER2+ group shared several clinical characteristics with the LumP subtype, such as a dominant ratio of T2, a high proportion of patients aged less than 60 years, similar TME with low levels of cell proliferation (Ki-67) and infiltrated activated T-cells (PD-1), and a favorable survival of 64.3 months (versus 48 months in LumP)8. Based on these commonly observed clinical characteristics and the TME, we suggest that a considerable proportion of patients overlap between the LumP and HER2+ groups.
This study provides insights into why HER-related therapeutics are ineffective for bladder cancer29, even though patients with breast or gastric cancer benefit from anti-HER2 therapies30,31. We hypothesized that HER2+ is an effective therapeutic target depending on the pathway by which it is expressed. Based on molecular subtypes in the ʻUROMOLʼ study, HER2+ in ʻClass 2 matched to Luminal unstableʼ and ʻClass 3 matched to Luminal Non-specificʼ was analyzed for ERBB2 amplification-induced forms of aggressive characteristics using reverse-phase protein lysate data from the Cancer Genome Atlas Cohort11,32. Contrary to these luminal subtypes, HER2+ of ʻClass 1 matched to Luminal papillaryʼ was not induced by ERBB2 amplification11,32. We discovered a novel form of indolent HER2+, while other HER2+ subtypes have previously been determined to be aggressive forms8,11,32. Thus, we hypothesized that there is a substantial association between HER2+ status in this study and HER2+ status in the luminal papillary subtype. We assume that clinical research on anti-HER2 therapies has been conducted in a mixed group of HER2+ patients with conflicting prognoses related to different HER2+ signatures. For patients with HER2+ in this study, the effect of HER2 therapeutics may be different because they show a good prognosis, which distinguishes them from other patients. In MIBC, it is well-documented that HER2 protein overexpression occurs more frequently than gene amplification33. This observation parallels findings in breast cancer, where it is established that HER2 therapeutics is effective against tumors with gene amplification. Thus, after excluding patients with this type of indolent HER2+, HER2 therapeutics appeared to work effectively.
Our findings are consistent with those of previous reports on de novo bladder cancer. Previous studies have reported a significant correlation between HER2 expression and the prognosis of de novo bladder cancer34,35. However, research on the relationship between HER2+ and prognosis in MIBC has yielded conflicting results. HER2 overexpression was determined to be an independent poor prognostic factor along with lymph node-positive status in MIBC36. Although other studies have reported that HER2 expression is high in recurrent and metastatic lesions, it has not yet been analyzed as a prognostic factor37,38. The complex and dynamic interactions between the HER pathway and other signaling pathways present a significant challenge in understanding their structure and function39. HER2+ status should be considered a dynamic marker, the expression pathway of which may be affected by the clinical situation of recurrence and metastatic sites. Therefore, the use of de novo specimens that are not influenced by previous clinical situations may be effective. Studies on de novo bladder cancer have demonstrated a consistent correlation between HER2+ with prognosis, and two studies on de novo non-MIBC analyzed initial HER2 positivity as a risk factor for recurrence after adjuvant intravesical therapy34,35 and progression to MIBC35. HER2 expression was higher in recurrent lesions than in primary lesions; however, only HER2+ status in primary lesions correlated with recurrence34. Although several studies have found higher HER2 expression in metastatic sites and circulating tumor cells in the peripheral blood than in primary sites, no correlation with prognosis has been reported37,38.
In the present study, we included cases of nonmetastatic de novo MIBC and found that HER2+ played a role in an indolent form of MIBC that has not been described previously. The results of this study could help with developing a treatment guideline for MIBC that utilizes IHC for HER2+ to identify patients appropriate for treatment. HER2 protein abundance is closely associated with the molecular classification of bladder cancer; it is a distinguishing feature observed exclusively in particular subtypes associated with specific pathways8,11,12. Although the available data supporting the molecular classification of bladder cancer are informative of oncological pathways, clinical characteristics, and TME8–10, they are not used for assessing prognosis or guiding therapeutic approaches owing to practical issues, such as cost and lack of standardized methods. Further studies on HER2 expression and molecular subtypes of bladder cancer are needed; however, IHC results with regard to genetic information are useful for selecting treatment strategies.
This study, characterized by its retrospective observational nature and small sample size, presents several additional limitations. Molecular signatures were inferred through a literature review; however, the absence of direct validation by RNA sequencing calls for further scrutiny. For FGFR3 mutations related to HER2 overexpression in bladder cancer, the study relied on breast cancer research due to the limited available evidence in bladder cancer. The consensus on interpreting HER2-IHC staining results is currently lacking. Therefore, guidelines for breast cancer were employed, highlighting the need for additional studies to confirm the reproducibility and utility of this approach. Nonetheless, comprehensible and reproducible results were obtained as stored specimens were immunostained for vimentin, which reflects the retained immunoreactivity of the old paraffin blocks. The sensitivity and specificity of preoperative radiological findings were less accurate (40.0 and 40.9%, respectively) than those of previous studies in predicting pT26,7. Therefore, when MIBC was initially identified and when the clinician decided the first course of treatment, no clinical factors were affected by a potential bias that influenced the selection of therapeutic approaches or surgical yields. Consequently, preoperative clinical variables were assigned to be comparable for HER2+, leading to a less biased assessment of patients.
Conclusions
In an era where NAC is considered standard care, conducting research to determine the necessity of NAC administration is challenging. We demonstrated that HER2+ status not only links past patient outcomes with current genetic characteristics but also serves as a prognostic marker for predicting the pT2 stage after RC and improved survival irrespective of RC performance. This could provide the basis for prospective studies aimed at avoiding NAC, thereby reducing unnecessary exposure to toxic chemotherapy and enhancing its efficacy in patients who would truly benefit from it. Such an approach could fundamentally improve physicians’ compliance with standard treatments in clinical practice and consequently enhance patient outcomes.
Ethical approval
Ethical approval for this study (GNAH 2018-05-017) was provided by the Institutional Review Board of Gangneung Asan Hospital, Gangneung, South Korea, on 12 June 2018. The requirement for informed consent was waived owing to the retrospective nature of the study.
Consent
The requirement for informed consent was waived owing to the retrospective nature of the study.
Sources of funding
This research was funded by Gangneung Asan Hospital Medical Institute and the Asan Foundation (2022II0014), and the National Research Foundation of Korea grants funded by the Korean government (NRF-2022R1F1A1071736) and ʻRegional Innovation Strategy (RIS)ʼ through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) in 2023 (2022RIS-005). The funding bodies were not involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author contribution
S.W.K., H.Y., H.K.C., W.N., J.Y.P., and S.J.K.: conceptualization; S.W.K., H.Y., Y.K., K.H.N., H.K.C., W.N., and S.J.K.: data curation; S.W.K., H.Y., H.K.C., W.N., J.Y.P., and S.J.K.: formal analysis; S.J.K.: funding acquisition; S.W.K., H.Y., H.K.C., W.N., J.YP., and S.J.K.: investigation; S.W.K., H.Y., and S.J.K.: methodology; S.W.K., H.Y., D.E., J.Y.P., and S.J.K.: project administration; J.Y.P., D.E., and S.J.K.: resources; S.W.K., H.Y., and S.J.K.: visualization; S.W.K., H.Y., H.K.C., W.N., and S.J.K.: software and supervision; S.W.K., H.Y., and S.J.K.: validation; S.W.K., H.Y., Y.K., K.H.N., H.K.C., W.N., and S.J.K.: writing – original draft, and writing. All authors contributed in writing – review and editing.
Conflicts of interest disclosure
All authors have no conflicts of interest.
Research registration unique identifying number (UIN)
We registered the study at https://cris.nih.go.kr/cris/ and the study number is KCT0008858.
Guarantor
Sung Jin Kim.
Data availability statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgement
None.
Footnotes
S.W.K. and H.Y. contributed equally to this work.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 2 November 2023
Contributor Information
So Won Kim, Email: kerorosw@gmail.com.
Hoon Yu, Email: Yuhoon721@gmail.com.
Younjuong Kim, Email: kyhj1230@gmail.com.
Kyeng Hyun Nam, Email: duhnee@naver.com.
Han Kyu Chae, Email: hanqsinopoli@gmail.com.
Wook Nam, Email: wooki6258@gmail.com.
Dae-Woon Eom, Email: edwjyh@gnah.co.kr.
Jong Yeon Park, Email: jypark11234@gmail.com.
Sung Jin Kim, Email: bop1004@hanmail.net.
References
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Int J Cancer 2021;149:778–789. [DOI] [PubMed] [Google Scholar]
- 2. Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol 2021;79:82–104. [DOI] [PubMed] [Google Scholar]
- 3. Powles T, Bellmunt J, Comperat E, et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:244–258. [DOI] [PubMed] [Google Scholar]
- 4. Yin M, Joshi M, Meijer RP, et al. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and two-step meta-analysis. Oncologist 2016;21:708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanna N, Trinh QD, Seisen T, et al. Effectiveness of neoadjuvant chemotherapy for muscle-invasive bladder cancer in the current real world setting in the USA. Eur Urol Oncol 2018;1:83–90. [DOI] [PubMed] [Google Scholar]
- 6. Tritschler S, Mosler C, Straub J, et al. Staging of muscle-invasive bladder cancer: can computerized tomography help us to decide on local treatment. World J Urol 2012;30:827–831. [DOI] [PubMed] [Google Scholar]
- 7. Mirmomen SM, Shinagare AB, Williams KE, et al. Preoperative imaging for locoregional staging of bladder cancer. Abdom Radiol (NY) 2019;44:3843–3857. [DOI] [PubMed] [Google Scholar]
- 8. Kamoun A, de Reyniès A, Allory Y, et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol 2020;77:420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woonyoung C, Sima PP, Seungchan K, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014;25:152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roland S, Al-Deen H, Nicholas A, et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. European Urology 2017;72:544–554. [DOI] [PubMed] [Google Scholar]
- 11. Tan TZ, Rouanne M, Tan KT, et al. Molecular subtypes of urothelial bladder cancer: results from a meta-cohort analysis of 2411 tumors. Eur Urol 2019;75:423–432. [DOI] [PubMed] [Google Scholar]
- 12. Dadhania V, Zhang M, Zhang L, et al. Meta-analysis of the luminal and basal subtypes of bladder cancer and the identification of signature immunohistochemical markers for clinical use. EBioMedicine 2016;12:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Cancer Genome Atlas Research Network . Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yan M, Schwaederle M, Arguello D, et al. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev 2015;34:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeffrey SD, Katherine AH, David DC, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci USA 2014;111:3110–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg Open [Internet] 2021;37:100430. [DOI] [PubMed] [Google Scholar]
- 17. Humphrey PA, Moch H, Cubilla AL, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs—part B: Prostate and bladder tumours. Eur Urol 2016;70:106–119. [DOI] [PubMed] [Google Scholar]
- 18. Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med 2018;142:1364–1382. [DOI] [PubMed] [Google Scholar]
- 19. Zaid HB, Patel SG, Stimson CJ, et al. Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology 2014;83:75–80. [DOI] [PubMed] [Google Scholar]
- 20. Liu D, Plimack ER, Hoffman-Censits J, et al. Clinical validation of chemotherapy response biomarker ERCC2 in muscle-invasive urothelial bladder carcinoma. JAMA Oncol 2016;2:1094–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Groenendijk FH, de Jong J, Fransen van de Putte EE, et al. ERBB2 mutations characterize a subgroup of muscle-invasive bladder cancers with excellent response to neoadjuvant chemotherapy. Eur Urol 2016;69:384–388. [DOI] [PubMed] [Google Scholar]
- 22. Miron B, Hoffman-Censits JH, Anari F, et al. Defects in DNA repair genes confer improved long-term survival after cisplatin-based neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol Oncol 2020;3:544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zargar H, Zargar-Shoshtari K, Lotan Y, et al. Final pathological stage after neoadjuvant chemotherapy and radical cystectomy for bladder cancer-does pT0 predict better survival than pTa/Tis/T1? J Urol 2016;195(4 Pt 1):886–893. [DOI] [PubMed] [Google Scholar]
- 25. Tilki D, Svatek RS, Novara G, et al. Stage pT0 at radical cystectomy confers improved survival: an international study of 4,430 patients. J Urol 2010;184:888–894. [DOI] [PubMed] [Google Scholar]
- 26. Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2017;171:540–556.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Rhijn BWG, Vis AN, van der Kwast TH, et al. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J Clin Oncol 2003;21:1912–1921. [DOI] [PubMed] [Google Scholar]
- 28. Hanker AB, Garrett JT, Estrada MV, et al. HER2-overexpressing breast cancers amplify FGFR signaling upon acquisition of resistance to dual therapeutic blockade of HER2. Clin Cancer Res 2017;23:4323–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hussain MHA, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol 2007;25:2218–2224. [DOI] [PubMed] [Google Scholar]
- 30. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659–1672. [DOI] [PubMed] [Google Scholar]
- 31. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–697. [DOI] [PubMed] [Google Scholar]
- 32. Hedegaard J, Lamy P, Nordentoft I, et al. Comprehensive transcriptional analysis of early-stage urothelial carcinoma. Cancer Cell 2016;30:27–42. [DOI] [PubMed] [Google Scholar]
- 33. Latif Z, Watters AD, Dunn I, et al. HER2/neu gene amplification and protein overexpression in G3 pT2 transitional cell carcinoma of the bladder: a role for anti-HER2 therapy? Eur J Cancer 2004;40:56–63. [DOI] [PubMed] [Google Scholar]
- 34. Moustakas G, Kampantais S, Nikolaidou A, et al. HER-2 overexpression is a negative predictive factor for recurrence in patients with non-muscle-invasive bladder cancer on intravesical therapy. J Int Med Res 2020;48:300060519895847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chae HK, Nam W, Kim HG, et al. Identification of new prognostic markers and therapeutic targets for non-muscle invasive bladder cancer: HER2 as a potential target antigen. Front Immunol 2022;13:903297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krüger S, Weitsch G, Büttner H, et al. HER2 overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic implications: HER2 overexpression in bladder carcinoma. Int J Cancer 2002;102:514–518. [DOI] [PubMed] [Google Scholar]
- 37. Fleischmann A, Rotzer D, Seiler R, et al. Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur Urol 2011;60:350–357. [DOI] [PubMed] [Google Scholar]
- 38. Rink M, Chun FK, Dahlem R, et al. Prognostic role and HER2 expression of circulating tumor cells in peripheral blood of patients prior to radical cystectomy: a prospective study. Eur Urol 2012;61:810–817. [DOI] [PubMed] [Google Scholar]
- 39. Grivas PD, Day M, Hussain M. Urothelial carcinomas: a focus on human epidermal receptors signaling. Am J Transl Res 2011;3:362–373. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].