Abstract
Background:
There is no standard management for small cell esophageal carcinoma (SCEC). The purpose of this multicenter, retrospective study (ChiSCER) was to investigate the treatment, outcomes, and risk factors impacting survival endpoints in patients with limited-stage SCEC (LS-SCEC).
Materials and Methods:
Consecutive patients with LS-SCEC from 14 institutions between 2000 and 2020 in China were enrolled. Survival curves were constructed using the Kaplan–Meier method and compared using a log-rank test. Univariate and multivariate Cox regression models and propensity score matching (PSM) analysis were adopted in the prognostic analysis. Results were reported as hazard ratio (HR), 95% confidence interval (CI), and P value. Statistical significance was set as P value <0.05 in a two-tailed test.
Results:
Among 458 LS-SCEC patients, the median age was 63 [interquartile range (IQR), 57–68] years, and 318 (69%) were males. Eighty-four (18%), 167 (36%), and 207 (45%) patients received chemotherapy (CT) alone, CT plus definitive radiotherapy (CT+RT), and CT plus radical surgery (CT+S), respectively. With a median follow-up time of 58.7 (95% CI 48.9–68.6) months, the median overall survival (OS) and 3-year OS rate for all patients 24.3 (95% CI 21.6–27) months and 37.3% (95% CI 32.8–42.5%), respectively. Multivariate analysis indicated that treatment modes, Karnofsky performance status (KPS), TNM stage, and CT cycle were independent prognostic factors for OS (P<0.05). Compared with CT alone, patients treated with CT+RT (HR 0.57, 95% CI 0.41–0.8, P=0.001) or CT+S (HR 0.59, 95% CI 0.42–0.82, P=0.002) had an improved OS, with no significant survival differences between CT+S and CT+RT groups after multivariate and PSM analyses (P>0.05). Subgroup analysis indicated that compared with CT+RT, patients with tumor location at lower 1/3 (HR 0.59, 95% CI 0.37–0.93, P=0.03) or tumor length >5 cm (HR 0.52, 95% CI 0.3–0.9, P=0.02) could obtain significant OS benefit from CT+S. Patients with tumor location at middle 1/3 (HR 1.55, 95% CI 1.03–2.36, P=0.04) or tumor length ≤5 cm (HR 1.49, 95% CI 1.02–2.17, P=0.04) favored CT+RT. Distant metastasis accounted for 73.7% of all treatment failures after multidisciplinary treatments.
Conclusion:
Surgery and RT were equally effective local therapies for patients with LS-SCEC. The personalized decision of local therapy should be made after comprehensive considerations on tumor location, length, comorbidities, and organ preservation.
Keywords: chemotherapy, limited-stage, radiotherapy, small cell esophageal carcinoma, surgery
Introduction
Highlights
Surgery and radiotherapy (RT) were equally effective local therapies for LS-SCEC (limited-stage small cell esophageal carcinoma).
Patients with tumor location at lower 1/3 or length >5 cm could benefit more from CT+S (chemotherapy plus surgery).
Patients with tumor location at middle 1/3 or length ≤5 cm favored CT+RT (chemotherapy plus radiotherapy).
Small cell esophageal carcinoma (SCEC) is one of the deadliest neuroendocrine malignancies, which accounts for 0.4–2.8% of all esophageal cancers and often occurs in the middle and lower esophagus1–4. Due to the feature of rapid progression and high metastasis propensity, the prognosis is dismal, with a 5-year survival of ~10% for limited stage and practically zero for extensive stage5–8. Due to its rarity, it is not feasible to perform prospective randomized controlled trials (RCTs) on SCEC. All reports in literature are based on retrospective reviews of case reports, single-institution studies, or multi-institutional studies with a small number of patients. No consensus on standardized treatment for SCEC has been reached at present.
Previously, small retrospective series have indicated that multidisciplinary modalities by combining local therapy and systematic chemotherapy (CT) could significantly improve the prognosis of limited-stage SCEC (LS-SCEC)4–10. Compared with CT alone, the addition of radiotherapy (RT) or surgery is generally recommended for curative intent. As for local therapy, one approach is to treat as small cell lung cancer (SCLC) by utilizing definitive RT, while another is to treat as esophageal squamous cell carcinoma (ESCC) or adenocarcinoma (EAC) with radical surgery. Currently, the vast majority of patients with LS-SCECs are treated with definitive RT in Western countries3,4,6,9–11. The significant predisposition of RT in Western countries makes the direct comparison of RT and surgery difficult to perform by using local cancer registry systems, such as The Surveillance, Epidemiology, and End Results (SEER) database. One small-scale retrospective study with most patients collected from previously published literature indicated that CT plus RT (CT+RT) was associated with improved survival over CT plus surgery (CT+S) in the multidisciplinary management of LS-SCEC12. However, the high rate of missing patient data and poor quality control of treatment in this small-scale cohort study restricted its value for clinical application. The most optimal local therapy in the multidisciplinary management of LS-SCEC still remains controversial, and clinical evidence with a large sample size and strict quality control is urgently needed.
Regarding the choice of local therapies, it is more balanced in China13–15. The China Small Cell Esophageal Cancer Retrospective Study (ChiSCEC) was a large-scale, multicenter, retrospective cohort study to explore optimal treatment strategies for patients with SCEC in the era of multidisciplinary care. We hypothesized that multidisciplinary approach incorporating systemic and local treatment results in the best outcome for LS-SCEC. Specific aims of the study included: (1) describe the characteristics of patients who received different treatments; (2) compare outcomes after CT, CT+S, and CT+RT in treatment; and (3) determine patterns of failure and risk factors for treatment failure.
Material and methods
Study population
A total of 458 patients with LS-SCEC at 14 institutions in China were retrospectively identified from December 2000 to December 2020. The ChiSCEC study was registered in Chictr.org.cn, approved by the Institutional Review Board, and performed in accordance with the principles of the Declaration of Helsinki. The patient data were de-identified, precluding the requirement for informed patient consent. This study has been reported in line with the Strengthening the Reporting of Cohort Studies in Surgery (STROCSS) criteria16 (Supplemental Digital Content 1, http://links.lww.com/JS9/B351).
The inclusion criteria were: (1) pathologically confirmed thoracic or abdominal SCEC; (2) limited stage with no distant metastasis (cTanyNanyM0) in 8th AJCC TNM stage17; (3) curative McKeown or Ivor Lewis esophagectomy and local lymphadenectomy with negative microscopic resection margins (R0), or definitive RT, or no local therapy; (4) CT, regardless of the sequence with local therapy; and (5) adequate renal [estimated glomerular filtration rate (eGFR) ≥90 (ml/min)/1.73 m2], hepatic [alanine transaminase (ALT) 7–55 U/l, aspartate transaminase (AST) 8–48 U/l, albumin ≥35 glL, gamma-glutamyltransferase (GGT) 8–61vU/l, bilirubin 1–12 mg/l, prothrombin time (PT) 9.4–12.5 s], and bone marrow [white blood cell (WBC) 4–10×109/l, hemoglobin (HGB) 90 g/l, platelets (PLT) ≥100×109/l] functions. Patients were included only when all eligibility criteria were met.
Patients were excluded if they met any of the following: (1) cervical SCEC; (2) extensive stage with distant metastasis (M1); (3) pathologically confirmed squamous cell carcinoma, adenocarcinoma, or other pathological types; (4) esophagectomy with positive resection margins (R1/R2); (5) palliative treatment purpose; (6) no systematic CT; (7) adjuvant or neoadjuvant RT in patients receiving curative esophagectomy; and (8) incomplete medical or follow-up records.
Stage
For patients treated with CT+RT or CT alone, the clinical T and N stage was generally evaluated by medical records, chest contrast-enhanced computed tomography, positron emission tomography/computed tomography (PET/CT), and endoscopic ultrasonography (EUS). For those receiving curative surgery, TNM stage was mainly evaluated by postoperative pathological reports, as well as medical record and images. For patients diagnosed before 2017, a new 8th AJCC TNM stage17 was given to replace the original TNM stage. All TNM stages were carefully evaluated by two researchers independently.
Treatment
According to local therapy, patients were classified into three treatment groups: CT alone, CT+RT, and CT+S. In CT alone group, no local therapy RT or surgery was given. In the CT+RT group, intensity-modulated radiotherapy (IMRT), three-dimension conformal radiotherapy (3D-CRT), and conventional two-dimension radiotherapy were allowed. Gross tumor volume (GTV) included primary tumor and involved regional lymph nodes. In the CT+S group, open esophagectomy and minimally invasive esophagectomy were both allowed. To control confounding factors in treatment, neoadjuvant or adjuvant RT was not allowed in the CT+S group. For all patients, CT dose and cycle were decided at the discretion of medical oncologists.
Data extraction, evaluation, and follow-up
The data set in this study included patient demographics (age and sex), Karnofsky performance status (KPS), extent of disease (location, length, and TNM stage), pathology (pure or mixed SCEC), CT regimen and cycle, RT dose and fraction, and follow-up data (recurrence/progression site, follow-up duration, and survival status). Continuous variables were categorized according to clinical reasoning or statistical methods. Age was grouped as ≤60, 61–70, or ≥71 years old. Tumor length was grouped as ≤5 or ≥5 cm. The disease location was categorized as upper 1/3 (from the thoracic inlet to level of tracheal bifurcation; 18–23 cm from incisors), middle 1/3 (from tracheal bifurcation midway to gastroesophageal junction; 24–32 cm from incisors), or lower 1/3 (from midway between tracheal bifurcation and gastroesophageal junction to gastroesophageal junction, including abdominal esophagus; 32–40 cm from incisors). Clinicopathological information was obtained from medical records and pathology reports.
Posttreatment surveillance included routine physical, blood, and image examinations. The predominant imaging method was computed tomography. For patients suspicious for relapse or progression, PET/CT and biopsy were then recommended to make a definite diagnosis. An endoscopic examination was performed as indicated. Follow-up evaluations were generally performed every 3 months during the first 2 years, every 6 months in the next 3 years, and annually thereafter. Death information was obtained from medical records, telephone calls, or the central registry of the Chinese Bureau of Population Statistics. Treatment response was evaluated based on the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1)18.
Endpoint definition
The primary endpoint OS was calculated from the date of diagnosis to death from any cause or censored at last contact. The secondary endpoint progression-free survival (PFS) was measured from diagnosis to progression, recurrence, death from any cause, or censored at last contact. Disease recurrence/progression was categorized as locoregional (esophagus, anastomotic, or regional lymph nodes) and distant (distant lymph nodes or organs, including supraclavicular lymph nodes).
Statistical analysis
Chi-square (χ2) test was applied for the comparison of categorical variables. Median follow-up was determined using the reverse Kaplan–Meier estimator. Survival curves were constructed using the Kaplan–Meier method and compared by log-rank test19. Univariate and multivariate Cox proportional hazards regression models were adopted in the prognostic analysis of OS and PFS. All factors on univariate analysis were then included in multivariate Cox regression analysis to test their association with potential predictors. Results were reported as hazard ratio (HR), 95% confidence interval (CI), and P value. Statistical significance was set as P value <0.05 in a two-tailed test.
Propensity score matching (PSM) analysis involving clinicopathological variables age, gender, KPS, tumor location, tumor length, TNM stage, pathology, treatment mode, CT regimen, and CT cycle were further performed to compare survival outcomes of different local therapies CT+S versus CT+RT. One-to-one matching without replacement was completed on the logit of the propensity score by using the nearest-neighbor match. Caliper width was 0.05 times the standard deviation of the logit of the propensity score. To evaluate the imbalance of baseline characteristics of the two groups, standardized mean difference (SMD) was estimated for all baseline characteristics. After matching, a SMD of less than 0.1 for a given covariate suggested a good performance of the propensity score20.
R statistical software (R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, version 3.3.2, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/, accessed on 08 November 2022) was used to perform the statistical analyses.
Results
Patient characteristics
After the screening of 916 consecutive patients, 458 patients with LS-SCEC were eligible for inclusion (Supplemental Figure 1, Supplemental Digital Content 2, http://links.lww.com/JS9/B352; Supplemental Table 1, Supplemental Digital Content 2, http://links.lww.com/JS9/B352). The median age was 63 [interquartile range (IQR), 57–68] years old. Most patients were males (318, 69%). More patients received surgery (207, 45%) than RT (167, 36%). The median follow-up time for all patients was 58.7 (95% CI 48.9–68.6) months. According to local therapy, 458 patients were classified into three treatment groups: CT alone (84, 18%), CT+RT (167, 36%), and CT+S (207, 45%). Significant imbalance in age, tumor location, tumor length, TNM stage, pathology, CT regimen, and CT cycle was observed among CT alone, CT+RT, and CT+S groups (P<0.05) (Table 1).
Table 1.
Clinicopathological characteristics of patients with LS-SCEC.
| Characteristic | Total (n=458), N (%) | CT alone (n=84), N (%) | CT+RT (n=167), N (%) | CT+S (n=207), N (%) | P |
|---|---|---|---|---|---|
| Age, years | <0.001 | ||||
| ≤60 | 164 (35.8) | 16 (19) | 61 (36.5) | 87 (42) | |
| 61–70 | 224 (48.9) | 42 (50) | 71 (42.5) | 111 (53.6) | |
| ≥71 | 70 (15.3) | 26 (31) | 35 (21) | 9 (4.3) | |
| Gender | 0.51 | ||||
| Male | 318 (69.4) | 58 (69) | 111 (66.5) | 149 (72) | |
| Female | 140 (30.6) | 26 (31) | 56 (33.5) | 58 (28) | |
| KPS | 0.84 | ||||
| 70-80 | 259 (56.6) | 49 (58.3) | 96 (57.5) | 114 (55.1) | |
| 90–100 | 199 (43.4) | 35 (41.7) | 71 (42.5) | 93 (44.9) | |
| Tumor location | <0.01 | ||||
| Upper 1/3 | 66 (14.4) | 9 (10.7) | 33 (19.8) | 24 (11.6) | |
| Middle 1/3 | 214 (46.7) | 42 (50) | 85 (50.9) | 87 (42) | |
| Lower 1/3 | 178 (38.9) | 33 (39.3) | 49 (29.3) | 96 (46.4) | |
| Tumor length | <0.001 | ||||
| ≤5 cm | 300 (65.5) | 47 (56) | 90 (53.9) | 163 (78.7) | |
| >5 cm | 158 (34.5) | 37 (44) | 77 (46.1) | 44 (21.3) | |
| TNM stage | <0.001 | ||||
| 1 | 29 (6.3) | 2 (2.4) | 3 (1.8) | 24 (11.6) | |
| 2 | 113 (24.7) | 15 (17.9) | 33 (19.8) | 65 (31.4) | |
| 3 | 257 (56.1) | 47 (56) | 110 (65.9) | 100 (48.3) | |
| 4a | 59 (12.9) | 20 (23.8) | 21 (12.6) | 18 (8.7) | |
| Pathology | 0.02 | ||||
| Pure SCEC | 414 (90.4) | 81 (96.4) | 154 (92.2) | 179 (86.5) | |
| Mixed SCEC | 44 (9.6) | 3 (3.6) | 13 (7.8) | 28 (13.5) | |
| CT regimen | 0.01 | ||||
| EP | 342 (74.8) | 68 (81) | 133 (80.1) | 141 (68.1) | |
| Others | 115 (25.2) | 16 (19) | 33 (19.9) | 66 (31.9) | |
| CT cycle | <0.01 | ||||
| 1–3 | 215 (46.9) | 47 (56) | 63 (37.7) | 105 (50.7) | |
| 4–6 | 243 (53.1) | 37 (44) | 104 (62.3) | 102 (49.3) |
CT+RT, chemotherapy plus radiotherapy; CT+S, chemotherapy plus surgery; CT, chemotherapy; EP, etoposide plus cisplatin or carboplatin; KPS, Karnofsky performance status; LS-SCEC, limited-stage small cell esophageal carcinoma; SCEC, small cell esophageal carcinoma.
Treatment
In CT alone group, EP (etoposide plus cisplatin or carboplatin) was the most common regimen (68, 81%), and the median number of CT cycles was 3 (range, 1–6).
In CT+RT group, 52 (31%), 93 (56%), and 22 (13%) patients received 3D-CRT, IMRT, and two-dimension radiotherapy, respectively. Most (156, 93%) patients received at least 50 Gy with 1.8–2.0 Gy per fraction 5 days per week, and the average dose was 56.7 Gy [standard deviation (SD) 9.3 Gy]. Only 1 patient received prophylactic cranial irradiation (PCI). Seventy (42%) patients received sequential CT (before or after RT); 20 (12%) received concurrent CT; 77 (46%) received both sequential and concurrent CT; 133 (80%) received EP regimen. The median number of CT cycles was 4 (range, 1–6).
In CT+S group, 126 (61%) received open esophagectomy; 81 (39%) received minimally invasive esophagectomy; 143 (69%) received postoperative CT; 39 (19%) received preoperative CT; 25 (12%) received both preoperative and postoperative CT; 141 (68%) received EP regimen. The median number of CT cycles was 3 (range, 1–6). No patient received PCI.
The treatment efficacy of local therapy on survival
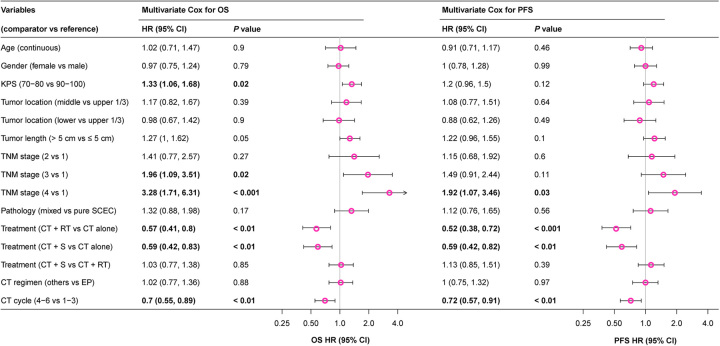
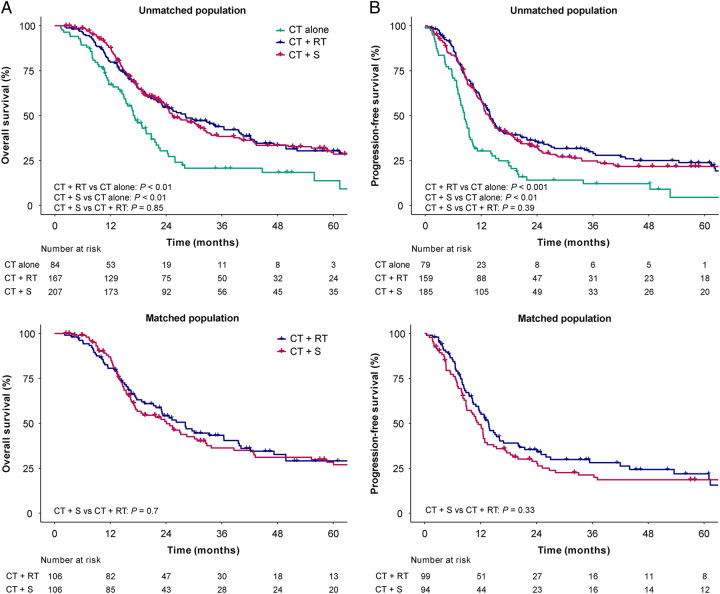
For the whole population, the median OS and 3-year OS rates were 24.3 (95% CI 21.6–27) months and 37.3% (95% CI 32.8–42.5%), respectively. Univariate and multivariate analyses demonstrated that treatment mode, KPS, TNM stage, and CT cycle were independent prognostic factors for OS (P<0.05) (Supplemental Table 2, Supplemental Digital Content 2, http://links.lww.com/JS9/B352). The addition of local therapy to CT could significantly improve OS (CT+RT vs. CT alone, HR 0.57, 95% CI 0.41–0.8, P<0.01; CT+S vs. CT alone, HR 0.59, 95% CI 0.42–0.83, P<0.01) (Fig. 1). The 3-year OS rates in CT alone, CT+RT, and CT+S groups were 20.7% (95% CI 12.9–33.1%), 43.9% (95% CI 36.5–52.8%), and 38.4% (95% CI 31.7–46.4%), respectively (Fig. 2A).
Figure 1.
Multivariate Cox HR for OS and PFS in LS-SCEC. CT, chemotherapy; CT+RT, chemotherapy plus radiotherapy; CT+S, chemotherapy plus surgery; EP, etoposide plus cisplatin or carboplatin; HR, hazard ratio; KPS, Karnofsky performance status; LS-SCEC, limited-stage small cell esophageal carcinoma; OS, overall survival; PFS, progression-free survival.
Figure 2.
Kaplan–Meier survival curves of different treatment modes in LS-SCEC. (A) OS of CT alone, CT+RT, and CT+S groups in unmatched population; (B) PFS of CT alone, CT+RT, and CT+S groups in unmatched population; (C) OS of CT+RT and CT+S groups in matched population; (D) PFS of CT+RT and CT+S groups in matched population. CT, chemotherapy; CT+RT, chemotherapy plus radiotherapy; CT+S, chemotherapy plus surgery; LS-SCEC, limited-stage small cell esophageal carcinoma; OS, overall survival; PFS, progression-free survival.
The median PFS and 3-year PFS rates for all patients were 12.7 (95% CI 11.4–13.9) months and 23.7% (95% CI 19.7–28.4%), respectively. Univariate and multivariate analyses indicated treatment mode, TNM stage, and CT cycle as independent prognostic factors for PFS (P<0.05) (Supplemental Table 3, Supplemental Digital Content 2, http://links.lww.com/JS9/B352). Compared with CT alone, the addition of local therapy significantly improved PFS (CT+RT vs. CT alone, HR 0.52, 95% CI 0.38–0.72, P<0.001; CT+S vs. CT alone, HR 0.59, 95% CI 0.42–0.82, P<0.01) (Fig. 1). The 3-year PFS rates in CT alone, CT+RT, and CT+S groups were 12% (95% CI 6.2–23.2%), 27.9% (95% CI 21.3–36.6%), and 24.8% (95% CI 19–32.4%), respectively (Fig. 2B).
Local therapy surgery versus RT on survival benefit
There were no significant survival differences in OS (HR 1.03, 95% CI 0.77–1.38, P=0.85) and PFS (HR 1.13, 95% CI 0.85–1.51, P=0.39) between CT+RT and CT+S groups in multivariate analysis (Fig. 2A). After PSM, 212 patients with local therapy were matched, with 106 in each group. PSM showed acceptable match efficacy with five variables (tumor length, TNM stage, pathology, gender, and KPS) with an SMD <0.1. After PSM, all patient characteristics except CT regimen were balanced without significant differences between CT+RT and CT+S groups (P>0.05) (Supplemental Fig. 1, Supplemental Digital Content 2, http://links.lww.com/JS9/B352 and Supplemental Table 4, Supplemental Digital Content 2, http://links.lww.com/JS9/B352). In matched population, patients treated with CT+S showed insignificant differences in OS (HR 1.07, 95% CI 0.76–1.5, P=0.7) (Fig. 2C) and PFS (HR 1.18, 95% CI 0.85–1.63, P=0.33) (Fig. 2D) when compared with those receiving CT+RT, which was consistent with multivariate analysis.
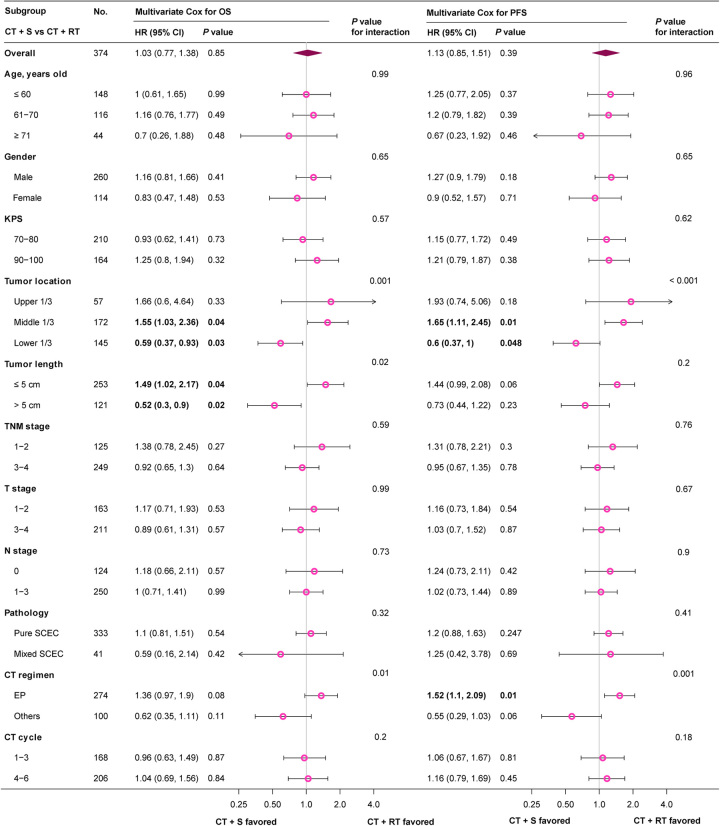
Subgroup stratification analysis was further performed to determine the treatment efficacy of surgery and RT in patients with different characteristics. Patients with tumor location at middle 1/3 favored CT+RT for significant survival benefit in both OS (HR 1.55, 95% CI 1.03–2.36, P=0.04) and PFS (HR 1.65, 95% CI 1.11–2.45, P=0.01), while those with tumor location at lower 1/3 tended to obtain significant survival benefit from CT+S (OS HR 0.59, 95% CI 0.37–0.93, P=0.03; PFS HR 0.6, 95% CI 0.37–1, P=0.048). Considering significant survival benefit in OS, patients with tumor length ≤5 cm and >5 cm were suggested to receive treatment CT+RT (HR 1.49, 95% CI 1.02–2.17, P=0.04) and CT+S (HR 0.52, 95% CI 0.3–0.9, P=0.02), respectively (Fig. 3; Supplemental Table 5, Supplemental Digital Content 2, http://links.lww.com/JS9/B352). CT+RT group showed superior PFS than CT+S in patients treated with EP regimen (HR 1.52, 95% CI 1.1–2.09, P=0.01) (Fig. 3; Supplemental Table 6, Supplemental Digital Content 2, http://links.lww.com/JS9/B352).
Figure 3.
Multivariate Cox HR of CT+S versus CT+RT in different subgroups. CT, chemotherapy; CT+RT, chemotherapy plus radiotherapy; CT+S, chemotherapy plus surgery; EP, etoposide plus cisplatin or carboplatin; HR, hazard ratio; KPS, Karnofsky performance status; LS-SCEC, limited-stage small cell esophageal carcinoma; OS, overall survival; PFS, progression-free survival.
Progression/recurrence pattern
Of 84 patients treated with CT alone, 54 (54%) recurrence/progression events occurred. Among 374 patients receiving local therapy, a total of 243 (65%) recurrence/progression events occurred during follow-up, with 108 and 135 recurrence/progression events in CT+RT and CT+S groups, respectively. The recurrence/progression events in locoregional, distant, and both sites of CT alone, CT+RT, and CT+S groups were 21 (38.9%), 19 (35.2%), 14 (25.9%); 30 (27.8%), 54 (50%), 24 (22.2%); and 34 (25.2%), 66 (48.9%), 35 (25.9%), respectively (Table 2). The addition of local therapy exhibited the tendency of a lower risk of locoregional progression/recurrence, but no significant differences in site distributions were found (P=0.3). Distant metastasis is the most common treatment failure in CT+RT and CT+S groups, accounting for 72.2% and 74.8%, respectively. Brain metastasis accounted for 24.2% (n=8), 17.9% (n=14), 10.9% (n=11) of all distant metastases in CT alone, CT+RT, and CT+S groups, respectively.
Table 2.
Recurrence/progression pattern in LS-SCEC.
| Recurrence/progression events | CT alone, N (%) | CT+RT, N (%) | CT+S, N (%) | P |
|---|---|---|---|---|
| Locoregional only | 21 (38.9) | 30 (27.8) | 34 (25.2) | 0.3 |
| Distant only | 19 (35.2) | 54 (50) | 66 (48.9) | |
| Both | 14 (25.9) | 24 (22.2) | 35 (25.9) |
CT+RT, chemotherapy plus radiotherapy; CT+S, chemotherapy plus surgery; LS-SCEC, limited-stage small cell esophageal carcinoma.
In CT+RT group, 34 (31.5%) and 4 (3.7%) patients received salvage RT and surgery as post-progression local treatment, respectively; and 54 (50%) patients received salvage chemotherapy. In CT+S group, 21 (15.6%) patients received salvage RT after disease progressions, and no patients received salvage surgery; and 40 (29.6%) patients received salvage chemotherapy.
Discussion
Multidisciplinary treatments by combining local therapy and systematic CT have significantly improved the survival of LS-SCEC; however, previously published case reports and small case series have not yielded a consensus opinion on the most optimal local therapy because of insufficient evidence. To the best of our knowledge, this is the first large-scale, multicenter, retrospective cohort study using individual patient data to compare RT with surgery in LS-SCECs in the era of multidisciplinary treatments. Both multivariate Cox regression and PSM analyses demonstrated insignificant survival differences between CT+S and CT+RT groups. Patients with tumor location at middle 1/3 or length ≤5 cm tended to gain survival benefit from RT, while those with tumor location at lower 1/3 or length >5 cm were priorly recommended to receive surgical resection as local therapy. Progression/recurrence site distributions were also similar between CT+S and CT+RT groups, and distant metastasis is the major reason for treatment failure. These findings provide new evidence supporting the clinical use of RT or surgery as effective local therapy to achieve equivalent survival outcomes in LS-SCEC. The personalized decision of local therapy should be made after comprehensive considerations on tumor location, length, comorbidities, and patient’s willingness for organ preservation. More effective systemic therapy is urgently needed to lower the risk of distant metastasis and improve long-term survival.
One small-scale study found that surgery could achieve clinical benefits only for localized SCEC with the stage T1-4aN0M015. The subgroup analysis of this large-scale ChiSCEC study indicated that surgery was suitable for all LS-SCEC patients (TanyNanyM0). Besides, surgery even exhibited survival benefits than RT in patients with tumor location at lower 1/3 or length >5 cm. For LS-SCEC, surgery was the principal local therapy in China, while U.S. patients mainly received RT15. This study could help to improve the status of surgery in treatment paradigms and mitigate the preconception against surgery in U.S. It needs to be noted that surgical resection is more suitable for medically fit patients with good physiologic ability. Patients with T4b (invasion of the trachea, great vessels, vertebral body, heart, or adjacent organs), multi-station bulky lymphadenopathy, comorbidities, or other risk factors are medically unsuitable for surgical resection. In this study, more than 60% of LS-SCEC patients were aged >60 years old, and the management of elderly patients posed challenges because they were likely to have multiple comorbidities and physiological changes associated with aging21,22. The prolonged negative impact of esophagectomy on quality of life should also be considered. For elderly patients with ESCC or EAC who were unable or refused to undergo surgery, CT+RT has been proven to be an effective treatment alternative with mild toxicity23–25. For LS-SCEC, RT has a wide range of indications, especially in patients with T4b, multi-station bulky lymphadenopathy, and comorbidities. Besides, RT also shows an advantage in esophagus preservation, which could help to preserve swallowing function, increase nutritional intake, and improve quality of life.
Previously, Meng et al.12 conducted a small-scale retrospective study with nearly 90% of LS-SCEC patients from the literature treated between 1989 and 2012, which demonstrated that patients treated with CT+RT had an improved OS compared to those treated with CT+S. In the previous research, missing data on patient characteristics and treatments, especially RT dose and CT cycle, were quite common, and the 3-year OS rate of patients treated with CT+S was merely 24.3%12. Significant progressions in the surgical skills and RT techniques have been achieved in recent decades, and the 3-year OS rate of patients treated with CT+S in our study was largely improved to 38.4%. The significant survival difference in patients who underwent surgery might be a major explanation for the inconsistency of conclusions. The quality control of individual patient data and modern standardized multidisciplinary care in our study further promoted its value to guide real-world clinical practice in the future.
Previously, a small-scale retrospective study from the Netherlands reported an infield recurrence rate of 16% after the vast majority of LS-SCEC patients received an RT dose of 45–50 Gy4. However, in this study, locoregional recurrences accounted for 28% of all failure events after an average RT dose of 56.7 Gy. These inconsistent results indicate that the optimal RT dose still remains unclear and needs further exploration in the future. Distant metastasis accounted for more than 70% of all progression/recurrence events after multidisciplinary treatment in this study, and patients with extended CT cycles showed significant survival benefits, indicating the urgent need for more intensified and effective systematic therapy. Oral chemotherapy and immune checkpoint inhibitors might be promising maintenance therapies to lower the risk of distant metastasis. Brain metastasis accounted for 14% of all metastasis events in LS-SCEC, significantly higher than ESCC or EAC26, but currently, there is no sufficient evidence supporting the necessity of routine PCI. The risk stratification of LS-SCEC and the use of PCI in high-risk subgroups for brain metastasis need to be further clarified in the future.
This study should be considered in the context of certain weaknesses. First, this was a retrospective study with certain biases or confounders. Statistical optimization by multivariable and PSM analyses was applied to eliminate potential biases as much as possible. The varied patient sample size among different treatment centers might introduce some potential biases. The retrospective nature of this study should be considered when interpreting these results. Second, this study did not report quality of life or adverse effects because these data were not comprehensively collected. Third, we mainly focused on local therapies in this study, and there were no restrictions on the treatment sequence of CT, RT, and surgery, which might introduce potential bias. The clinical significance of concurrent CT in the process of RT, or neoadjuvant CT before surgery was not addressed in this study. The ChiSCER collaboration group will perform more studies in the future to comprehensively investigate the most optimal treatment sequence for patients with SCEC. Fourth, clinicopathological factors on the molecular or genetic level were absent in this study, and explorations on gene mutation profiles, tumor microenvironment, and signaling pathways are suggested for further research.
Conclusions
In conclusion, multidisciplinary treatment CT+S showed equivalent survival outcomes with CT+RT in patients with LS-SCEC. The personalized decision of local therapy should be made after comprehensive considerations of tumor location, length, comorbidities, and patient’s willingness for organ preservation. More effective systematic therapy is urgently needed to lower the risk of distant metastasis and improve survival.
Ethical approval
The ChiSCEC study was registered in Chictr.org.cn (Registration Number: ChiCTR2000040175), approved by the Institutional Review Board of Shandong Cancer Hospital (SDZLEC2020-153-01), and performed in accordance with the principles of the Declaration of Helsinki.
Consent
The retrospective study received ethical approval from the institutional review boards (SDZLEC2020-153-01). The patient data were de-identified, precluding the requirement for informed patient consent.
Sources of funding
This work was supported by the Science and Technology Department of Sichuan Province (grant number 2023YFQ0055 and 2023YFS0488) and the Academic Promotion Program of Shandong First Medical University (grant number 2019LJ004).
Author contribution
J.Z.: composed the manuscript; Y.W., H.S., Y.Z., W.Z., W.S., N.Y., B.T., X.S., L.L., W.D., J.M., J.Z., L.Z., D.S, and P.Y.: collected the data; L.P., W.H., Q.W., Z.L., and B.L.: conceived the idea of the study.
Conflicts of interest disclosure
The authors declare that they have no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: Chinese Clinical Trial Registry.
Unique identifying number or registration ID: ChiCTR2000040175.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.chictr.org.cn/showprojEN.html?proj=64519
Guarantor
Qifeng Wang.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgements
The authors would like to thank Professor Yexiong Li, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Collaborative Innovation Center for Cancer Medicine, Beijing, China; and Professor Joe Y Chang, Department of Radiation Oncology, Division of Radiation Oncology, the University of Texas MD Anderson Cancer Center, Houston, TX, USA for their suggestions in study conception.
Footnotes
Jie Zhu, Yi Wang, and Hongfu Sun have contributed equally to this work and share first authorship.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 21 November 2023
Contributor Information
Jie Zhu, Email: zhujiescch@163.com.
Yi Wang, Email: wangleo@126.com.
Hongfu Sun, Email: ice9892@163.com.
Yaowen Zhang, Email: zhangyaowen621@126.com.
Wencheng Zhang, Email: wczhang@tmu.edu.cn.
Wenbin Shen, Email: wbshen1979@sina.com.
Ning Yang, Email: yangning_sdu@163.com.
Bingxu Tan, Email: TAN-BX@163.com.
Xiujun Su, Email: 306161518@qq.com.
Lei Li, Email: leili1530@163.com.
Wei Dong, Email: weidongytly@163.com.
Jie Ma, Email: majie2316@163.com.
Jian Zhang, Email: zhangjiantsl@163.com.
Lina Zhao, Email: zhaolina@fmmu.edu.cn.
Daqing Sun, Email: dqsun1224@163.com.
Pei Yang, Email: yangpei@hnca.org.cn.
Lin Peng, Email: penglinms@126.com.
Baosheng Li, Email: bshli@sdfmu.edu.cn.
Wei Huang, Email: alvinbird@163.com.
Qifeng Wang, Email: littlecancer@163.com.
Zhongxing Liao, Email: zliao@mdanderson.org.
References
- 1. Ji A, Jin R, Zhang R, et al. Primary small cell carcinoma of the esophagus: progression in the last decade. Ann Transl Med 2020;8:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brenner B, Tang LH, Klimstra DS, et al. Small-cell carcinomas of the gastrointestinal tract: a review. J Clin Oncol 2004;22:2730–2739. [DOI] [PubMed] [Google Scholar]
- 3. Kukar M, Groman A, Malhotra U, et al. Small cell carcinoma of the esophagus: a SEER database analysis. Ann Surg Oncol 2013;20:4239–4244. [DOI] [PubMed] [Google Scholar]
- 4. Jeene PM, Geijsen ED, Muijs CT, et al. Small cell carcinoma of the esophagus: a nationwide analysis of treatment and outcome at patient level in locoregional disease. Am J Clin Oncol 2019;42:534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lv J, Liang J, Wang J, et al. Primary small cell carcinoma of the esophagus. J Thorac Oncol 2008;3:1460–1465. [DOI] [PubMed] [Google Scholar]
- 6. Song Y, Wang W, Tao G, et al. Survival benefit of radiotherapy to patients with small cell esophagus carcinoma: an analysis of Surveillance Epidemiology and End Results (SEER) data. Oncotarget 2016;7:15474–15480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu L, Li Y, Liu X, et al. Treatment strategies and prognostic factors of limited-stage primary small cell carcinoma of the esophagus. J Thorac Oncol 2017;12:1834–1844. [DOI] [PubMed] [Google Scholar]
- 8. Patel S, TenNapel M, Clamon G, et al. Small cell carcinoma of the esophagus: the SEER experience from 2000–2009. Int J Radiat Oncol Biol Phys 2013;87:S292. [Google Scholar]
- 9. Verma V, Sleightholm RL, Fang P, et al. National Cancer Database report of nonmetastatic esophageal small cell carcinoma. Cancer Med 2018;7:6365–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong AT, Shao M, Rineer J, et al. Treatment and survival outcomes of small cell carcinoma of the esophagus: an analysis of the National Cancer Data Base. Dis Esophagus 2017;30:1–5. [DOI] [PubMed] [Google Scholar]
- 11. Li T, Chen S, Zhang Z, et al. Chemotherapy plus radiotherapy versus radiotherapy in patients with small cell carcinoma of the esophagus: a SEER database analysis. Cancer Control 2021;28:1073274821989321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meng MB, Zaorsky NG, Jiang C, et al. Radiotherapy and chemotherapy are associated with improved outcomes over surgery and chemotherapy in the management of limited-stage small cell esophageal carcinoma. Radiother Oncol 2013;106:317–322. [DOI] [PubMed] [Google Scholar]
- 13. Cai G, Wang J, Zou B, et al. Preoperative chemotherapy for limited-stage small cell carcinoma of the esophagus. Ann Thorac Surg 2022;114:1220–1228. [DOI] [PubMed] [Google Scholar]
- 14. Hou X, Wei JC, Wu JX, et al. Multidisciplinary modalities achieve encouraging long-term survival in resectable limited-disease esophageal small cell carcinoma. PLoS One 2013;8:e69259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao Q, Xiao H, Ouyang S, et al. Primary small cell carcinoma of the esophagus: comparison between a Chinese cohort and Surveillance, Epidemiology, and End Results (SEER) data. Cancer Med 2019;8:1074–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mathew G, Agha R, for the STROCSS Group . STROCSS 2021: Strengthening The Reporting Of Cohort, cross-sectional and case–control Studies in Surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 17. Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol 2017;12:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 19. Jager KJ, van Dijk PC, Zoccali C, et al. The analysis of survival data: the Kaplan–Meier method. Kidney Int 2008;74:560–565. [DOI] [PubMed] [Google Scholar]
- 20. Webster-Clark M, Stürmer T, Wang T, et al. Using propensity scores to estimate effects of treatment initiation decisions: state of the science. Stat Med 2021;40:1718–1735. [DOI] [PubMed] [Google Scholar]
- 21. Linde P, Mallmann M, Adams A, et al. Chemoradiation for elderly patients (≥ 65 years) with esophageal cancer: a retrospective single-center analysis. Radiat Oncol 2022;17:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Won E, Ilson DH. Management of localized esophageal cancer in the older patient. Oncologist 2014;19:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tougeron D, Di Fiore F, Thureau S, et al. Safety and outcome of definitive chemoradiotherapy in elderly patients with oesophageal cancer. Br J Cancer 2008;99:1586–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruol A, Portale G, Castoro C, et al. Management of esophageal cancer in patients aged over 80 years. Eur J Cardiothorac Surg 2007;32:445–448. [DOI] [PubMed] [Google Scholar]
- 25. Steyerberg EW, Neville B, Weeks JC, et al. Referral patterns, treatment choices, and outcomes in locoregional esophageal cancer: a population-based analysis of elderly patients. J Clin Oncol 2007;25:2389–2396. [DOI] [PubMed] [Google Scholar]
- 26. Bartelt S, Momm F, Weissenberger C, et al. Patients with brain metastases from gastrointestinal tract cancer treated with whole brain radiation therapy: prognostic factors and survival. World J Gastroenterol 2004;10:3345–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.