Abstract
Objective:
This meta-analysis aimed to compare short-term outcomes between robotic liver resection (RLR) and laparoscopic liver resection (LLR) using data collected from propensity score-matched studies.
Methods:
The PubMed, Cochrane Library, and Embase databases were searched to collect propensity score-matched studies comparing RLR and LLR. Relevant data were extracted and analyzed. Odds ratios (ORs) and standardized mean differences (SMDs) with 95% confidence intervals (CIs) were calculated using fixed-effect or random-effect models. Meta-regression analysis was performed for primary outcome measures. Subgroup analyses and sensitivity analyses were performed for outcomes exhibiting high heterogeneity. Quality of evidence was evaluated using the Grading of Recommendations, Assessment, Development and Evaluation framework.
Results:
Twenty-two propensity score-matched studies were included to comprise 5272 patients (RLR group, 2422 cases; LLR group, 2850 cases). Intraoperative blood loss (SMD=−0.31 ml, 95% CI −0.48 to −0.14; P=0.0005), open conversion (OR=0.46, 95% CI 0.37–0.58; P <0.0001), and severe complications (OR=0.76, 95% CI 0.61–0.95; P=0.02) were significantly lower in the RLR group. Operation time, odds of use, and duration of Pringle maneuver, length of hospital stay, and odds of intraoperative blood transfusion, overall complications, R0 resection, reoperation, 30-day readmission, 30-day mortality, and 90-day mortality did not significantly differ between the groups. Further subgroup and sensitivity analyses suggested that the results were stable. Meta-regression analysis did not suggest a correlation between primary outcomes and study characteristics. The quality of evidence for the primary outcomes was medium or low, while that for the secondary outcomes was medium, low, or very low.
Conclusion:
Although some short-term outcomes are similar between RLR and LLR, RLR is superior in terms of less blood loss and lower odds of open conversion and severe complications. In the future, RLR may become a safe and effective replacement for LLR.
Keywords: laparoscopy, liver resection, meta-analysis, propensity score matching, robot
Introduction
Highlights
The first meta-analysis of propensity score-matched studies to compare short-term outcomes between robotic liver resection (RLR) and laparoscopic liver resection (LLR).
The largest number of included studies, the largest sample size comparison RLR and LLR meta-analysis.
RLR is superior in terms of less blood loss and lower odds of open conversion and severe complications.
Reich et al.1 first reported laparoscopic hepatectomy in 1991. Laparoscopic hepatectomy is associated with less intraoperative bleeding, lower incidence of postoperative complications, shorter hospital stay, and faster recovery than open hepatectomy and is widely used around the world2–4. Laparoscopic hepatectomy has several limiting factors including limited angles of movement for instruments, two-dimensional visual field, poor stability, poor ergonomics, and dependence on assistants. Furthermore, liver surgery is quite complex and variable and requires intraoperative adaptability5–7. The emergence of robotic surgery has overcome these shortcomings to some extent, as it can provide increased instrument range of motion, three-dimensional visualization, and better stability and ergonomics8,9.
Several previous studies have confirmed the safety and feasibility of robotic hepatectomy as well as its advantages over open hepatectomy10–12. However, whether robotic hepatectomy is superior to laparoscopic hepatectomy is controversial. In a matched comparison of robotic liver resection (RLR) and laparoscopic liver resection (LLR) cases, Tsung et al.13 reported that both were similar in terms of safety and feasibility. Chong et al.14 compared robotic and laparoscopic right hepatectomy in a propensity score-matched analysis and found that the open conversion rate was lower and the hospital stay was shorter in the robotic cases. Two other studies have reported only that RLR was associated with less blood loss than LLR15,16. In contrast, a recent propensity score-matched analysis of patients with large hepatocellular carcinomas found no significant difference in perioperative results17. These results suggest that higher quality studies are needed to determine whether RLR or LLR is superior.
To the best of our knowledge, no randomized controlled trial (RCT) has directly compared RLR and LLR; however, meta-analyses have been conducted18–23. One reported that LLR was associated with less bleeding and shorter operation time than RLR, while two others found no such differences18,20,22. The meta-analyses reported to date have several problems: the numbers of studies and patients included were small and studies using propensity score matching were not included. Therefore, their results are not very reliable. More recently, studies have been comparing RLR and LLR using propensity score matching. We present a meta-analysis of these studies to compare short-term outcomes between RLR and LLR.
Methods
Search strategy
Based on systematic review and meta-analysis (PRISMA) and assessment of the methodological quality of systematic review (AMSTAR), it is carried out in the south24,25. The study was registered in PROSPERO. We searched the PubMed, Embase, and Cochrane Library databases to collect propensity score-matched studies that compared short-term outcomes between RLR and LLR published before 30 April 2023. Key search words included the following: robot, robotic, laparoscopy, laparoscopic, liver resection, hepatectomy, sectionectomy, propensity score-matched, and propensity score matching. Search strategies are provided in detail in Supplemental Tables S1–S3 (Supplemental Digital Content 1, http://links.lww.com/JS9/B268). References in studies identified were also searched to identify other potential studies that met the criteria.
Inclusion and exclusion criteria
The Population, Intervention, Comparator, and Outcomes model was used to determine the inclusion criteria: population − all patients who underwent hepatectomy; intervention − RLR; comparator − LLR; outcomes − operation time, intraoperative blood loss volume, conversion to laparotomy, and length of hospital stay. In addition, we only included studies published in English and those with Newcastle–Ottawa scale (NOS) score >5. Abstracts, case reports, reviews, and studies that had a matched cohort but did not use the tendency score method to match were excluded.
When there was a queue of multiple score matching ratios, data were prioritized in the following order of RLR:LLR ratios (1:1>1:2>1:3). The most recent published data were used when there were multiple related studies by the same author. Larger sample sizes were used when there were studies by the same author at the same time.
Data extraction
Three researchers extracted data according to the extraction form and reviewed it. Inconsistencies and disputes were resolved by discussion with a fourth researcher who made the decision. General data extracted included the following: article title, author, publication date and country, number of patients, male-to-female ratio, age, and other clinical data. Primary outcomes data included overall complications, severe complications (Clavien–Dindo grade II and higher), and R0 resection rate. Secondary outcomes included operation time, intraoperative blood loss volume, application and duration of Pringle maneuver, rate of conversion to laparotomy, intraoperative blood transfusion, length of hospital stay, reoperation rate, 30-day readmission rate, and 30-day and 90-day mortality rates.
Quality assessment
Study quality was evaluated by two researchers using the NOS. Any differences in evaluation were evaluated by a third researcher and resolved through negotiation.
Statistical analysis
Statistical analyses were performed using RevMan software version 5.4 (Cochrane, London, UK). Binary short-term outcomes were evaluated using odds ratios (ORs) with 95% confidence intervals (CIs); continuous outcomes were evaluated using standardized mean differences (SMDs) with 95% CIs. We used the method proposed by Hozo et al.26 to estimate only the mean and standard deviation of the median, extreme value, and quartile spacing. The Q test and heterogeneity statistic (I2) were used to evaluate study heterogeneity. When I2 was less than 50%, the fixed-effects model was used for analysis; when it was greater than or equal to 50%, the random-effects model was used27.
Meta-regression analysis was performed using Stata software version 14 (StataCorp, College Station, Texas, USA) to explore the relationship between primary outcomes and patient characteristics. The variables considered included publication year, percentage of men, average age, sample size, American Society of Anesthesiologists (ASA) physical status grade, and study NOS score. For results with high heterogeneity (I2 >50%), a leave-one-out meta-analysis of sensitivity score and subgroup analyses according to publication time, number of participating centers, and NOS score were performed using RevMan software version 5.4. Publication bias was evaluated using a funnel chart. All statistical tests were two-sided. P <0.05 was considered significant.
GRADE Pro software version 3.6 was used to evaluate the quality of evidence for outcomes. Quality was rated as high, medium, low, or very low based on research design, research quality, accuracy, consistency, directness, and risk of reporting bias.
Results
Study selection
After identifying 641 potential articles, 22 propensity score-matched studies were included for analysis14–17,28–45. Among these, 12 were multicenter international studies14–17,30,31,34,37,38,40,42,43, 10 were from the International Robotic and Laparoscopic Liver Resection Study Group collaborators14–17,34,37,38,40,42,43, and one was a living donor hepatectomy study36. A total of 5272 patients (2422 who underwent RLR and 2850 who underwent LLR) were analyzed. The study flow chart is shown in Fig. 1. Details and NOSs of the included studies are shown in Table 1 and Supplemental Table S4 (Supplemental Digital Content 2, http://links.lww.com/JS9/B270).
Figure 1.
PRISMA diagram for the selection of the studies.
Table 1.
General characteristics of the included studies.
| First author | Year | Country | Surgery | Cases (n) | Age (year) | Sex (male, n) | ASA score (I–II/III–IV, n) | BMI (kg/m2) | CRLM/HCC/other (n) | Median tumor size (cm) | Extent of resection |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Montalti et al.28 | 2016 | MC | RLR | 36 | 62±13 | 21 | 23/13 | N | 21/3/12 | 4.44±3.06 | S7, S8, S4a, S1 |
| LLR | 72 | 56.8±15 | 39 | 52/20 | N | 44/6/22 | 4.95±3.5 | ||||
| Salloum et al.29 | 2016 | France | RLR | 14 | 57±12 | N | 10/4 | N | N | N | N |
| LLR | 14 | 57±15 | N | 10/4 | N | N | N | ||||
| Lim et al.30 | 2019 | MC | RLR | 55 | 65±10 | 37 | N | 25±4 | 13/38/4 | 4.0±2.4 | N |
| LLR | 55 | 66±10 | 41 | N | 27±6 | 11/36/8 | 4.0±2.4 | ||||
| Beard et al.31 | 2020 | MC | RLR | 115 | 61±11 | 76 | 21/94 | 28±6 | 115/0/0 | N | N |
| LLR | 115 | 61±12 | 75 | 16/99 | 29±6 | 115/0/0 | N | ||||
| Chiow et al.15 | 2021 | MC | RLR | 88 | 60 (51–69) | 59 | 52/36 | N | 21/52/15 | 3.5 (3–5) | Right posterior sectionectomy |
| LLR | 88 | 61 (54–69) | 64 | 56/32 | N | 21/54/13 | 4 (3–5.2) | ||||
| Fagenson et al.32 | 2021 | USA | RLR | 240 | 60 (50–69) | 142 | N | 27.9(24.3–32.7) | 88/64/88 | N | N |
| LLR | 240 | 63 (51–73) | 140 | N | 27.6(24.0–32.0) | 88/64/88 | N | ||||
| Chong et al.14 | 2022 | MC | RLR | 220 | 61 (52–69) | 139 | 133/87 | N | 57/106/57 | 5 (3–7) | Right and extended right hepatectomy |
| LLR | 220 | 61 (55–71) | 144 | 128/92 | N | 59/104/57 | 5 (3–7.5) | ||||
| Cipriani et al.33 | 2022 | Italy | RLR | 288 | N | 168 | 164/124 | N | 77/115/96 | N | N |
| LLR | 864 | N | 493 | 486/378 | N | 216/307/341 | N | ||||
| D’Silva et al.16 | 2022 | MC | RLR | 104 | 62 (53–68) | 70 | 64/40 | N | 30/54/20 | 2.5 (1.6–3.5) | Posterosuperior segments |
| LLR | 104 | 63 (50–70) | 68 | 64/40 | N | 30/54/20 | 2.5 (1.8–3.5) | ||||
| Kadam et al.34 | 2022 | MC | RLR | 296 | 61 (52–67) | 191 | 173/123 | N | 58/155/83 | 2.6 (2.0–4.0) | N |
| LLR | 296 | 61 (51–70) | 196 | 173/123 | N | 58/155/83 | 2.7 (1.8–4.0) | ||||
| Kamel et al.35 | 2022 | USA | RLR | 182 | N | N | N | N | N | N | N |
| LLR | 182 | N | N | N | N | N | N | ||||
| Rho et al.36 | 2022 | USA | RLR | 19 | 29.3±10.5 | 13 | N | 22.4±2.1 | N | N | Right hepatectomy |
| LLR | 19 | 30.3±11.1 | 11 | N | 21.9±2.1 | N | N | ||||
| Sucandy et al.37 | 2022 | MC | RLR | 164 | 62±17.3 | 100 | 104/60 | N | 32/69/63 | 4.65±3.0 | Left and extended left hepatectomy |
| LLR | 164 | 63±15 | 105 | 101/63 | N | 30/66/68 | 4.1±4.28 | ||||
| Yang et al.38 | 2022 | MC | RLR | 40 | 62 (55–68) | 32 | 29/11 | N | 7/25/8 | 3.8 (3.0–4.9) | Right anterior sectionectomy and central hepatectomy |
| LLR | 40 | 62 (54–72) | 33 | 27/13 | N | 6/27/7 | 3.5 (3.0–5.0) | ||||
| Chen et al.39 | 2023 | China | RLR | 41 | 53±13 | 24 | 39/2 | 22.5±2.6 | 1/21/19 | 5.3±2.2 | S7, S8, S4a, S1 |
| LLR | 41 | 54±12 | 27 | 39/2 | 23.1±2.6 | 3/21/17 | 4.8±2.7 | ||||
| Kato et al.41 | 2023 | Japan | RLR | 91 | 71 (23–88) | 62 | 81/10 | 22.9 (15.2–30.7) | 0/60/0 | 2.2 (0.6–1.6) | N |
| LLR | 91 | 70 (26–86) | 63 | 79/12 | 23.0 (16.7–32.4) | 0/60/0 | 2.4 (0.7–1.6) | ||||
| Liu et al.43 | 2023 | MC | RLR | 221 | 61 (52–68) | 167 | 145/76 | N | 7/209/5 | 4.5 (3.0, 6.0) | N |
| LLR | 221 | 63 (52–70) | 172 | 414/80 | N | 6/210/5 | 4.0 (2.7, 7.0) | ||||
| Zhang et al.44 | 2023 | China | RLR | 43 | 48 (26–62) | 13 | N | 22.4 (18.8–32.9) | 0/0/43 | 9 (5.6–2.0) | N |
| LLR | 86 | 49 (27–66) | 26 | N | 22.5 (18.3–33) | 0/0/86 | 9 (5–2.5) | ||||
| Zhu et al.45 | 2023 | China | RLR | 56 | 52 (28–72) | 45 | 51/5 | 23.4 (15.9–30.9) | 0/56/0 | 3.3 (1.0–1.25) | N |
| LLR | 56 | 53 (24–72) | 47 | 53/3 | 23.3 (16.6–31.2) | 0/56/0 | 3.3 (1.1–1.43) | ||||
| Kwak et al.42 | 2023 | MC | RLR | 48 | 62 (53–68) | 20 | 39/9 | N | 0/0/48 | N | N |
| LLR | 48 | 63 (47–68) | 20 | 38/10 | N | 0/0/48 | N | ||||
| Chong et al.40 | 2023 | MC | RLR | 179 | 60±14 | 111 | 125/54 | N | 29/102/17 | 3.0±2.9 | Left lateral sectionectomy |
| LLR | 179 | 61±17 | 115 | 133/46 | N | 32/101/7 | 3.0±3.0 | ||||
| Cheung et al.17 | 2023 | MC | RLR | 73 | 54 (40–66) | 34 | 51/22 | 25.0 (22.6–28.5) | 8/24/41 | 11.5 (10.0–13.5) | N |
| LLR | 219 | 55 (42–68) | 105 | 159/60 | 24.0 (21.6–27.3) | 28/82/108 | 11.0 (10.0–13.0) |
ASA, American Society of Anesthesiologists; BMI, body mass index; CRLM, colorectal cancer liver metastases; HCC, hepatocellular carcinoma; MC, multicenter; N, not available; NOS, Newcastle−Ottawa scale; S, sectionectomy.
Meta-analysis results
A summary of the meta-analysis results is shown in Table 2.
Table 2.
Results of meta-analysis comparison of LLR and RLR.
| Outcomes of interest | Number of studies | Number of patients | I2 (%) | Model | Overall effect size | 95% CI of overall effect size | P |
|---|---|---|---|---|---|---|---|
| Primary outcomes | |||||||
| Overall complications | 21 | 4908 | 41 | Fixed | OR=0.99 | [0.86, 1.14] | 0.91 |
| Severe complications | 20 | 4880 | 0 | Fixed | OR=0.76 | [0.61, 0.95] | 0.02 |
| R0 | 14 | 3399 | 0 | Fixed | OR=1.28 | [1.00, 1.63] | 0.05 |
| Secondary outcomes | |||||||
| Operating time (min) | 19 | 4502 | 68 | Random | SMD=0.07 | [−0.05, 0.18] | 0.25 |
| Blood loss (ml) | 17 | 3912 | 84 | Random | SMD=−0.31 | [−0.48, −0.14] | 0.0005 |
| Transfusion | 17 | 4350 | 29 | Fixed | OR=0.96 | [0.78, 1.19] | 0.72 |
| Pringle applied | 16 | 4399 | 91 | Random | OR=0.73 | [0.44, 1.22] | 0.23 |
| Pringle duration (min) | 13 | 2964 | 85 | Random | SMD=−0.01 | [−0.22, 0.19] | 0.89 |
| Open conversion | 19 | 4894 | 45 | Fixed | OR=0.46 | [0.37, 0.58] | <0.0001 |
| Hospital stay (day) | 18 | 4361 | 62 | Random | SMD=−0.02 | [−0.13, 0.08] | 0.66 |
| Reoperation | 14 | 3914 | 0 | Fixed | OR=0.67 | [0.38, 1.18] | 0.20 |
| 30-day readmission | 14 | 3950 | 0 | Fixed | OR=1.12 | [0.83, 1.51] | 0.47 |
| 30-day mortality | 13 | 3769 | 0 | Fixed | OR=1.11 | [0.55, 2.24] | 0.77 |
| 90-day mortality | 15 | 4545 | 0 | Fixed | OR=0.79 | [0.47, 1.34] | 0.38 |
Statistically significant results are shown in bold.
LLR, laparoscopic liver resection; RLR, robotic liver resection; SMD/OR, standard mean difference/odds ratio.
Primary outcomes
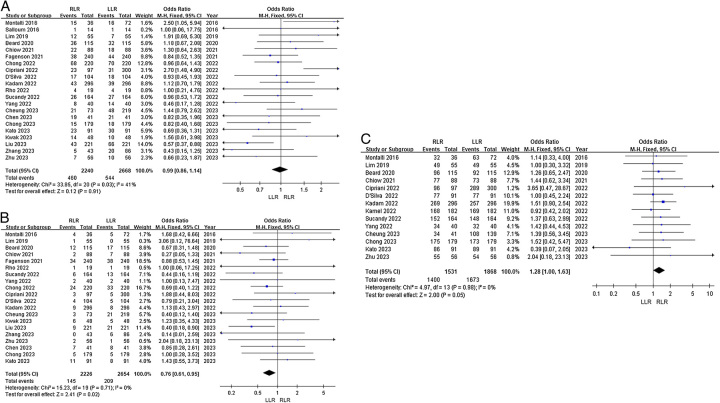
Overall complications: Twenty-one studies reported overall postoperative complications. Heterogeneity among the studies was not high (I2=41%). Fixed-effects model analysis showed no significant difference in the odds of overall complications between the groups (OR=0.99, 95% CI 0.86–1.14, P=0.91, Fig. 2A).
Figure 2.
Forest plots of primary outcomes for RLR versus LLR. (A) Overall complications; (B) severe complications; and (C) R0 resection.
Severe complications: Twenty studies reported severe postoperative complications. Heterogeneity among the studies was low (I2=0%). Fixed-effects model analysis showed that the odds of severe complications were significantly lower in the RLR group (OR=0.76, 95% CI 0.61−0.95, P=0.02, Fig. 2B).
R0 resection: Fourteen articles reported R0 resection rates. Heterogeneity among the studies was low (I2=0%). Fixed-effects model analysis showed no significant difference in odds of R0 resection between the groups (OR=1.28, 95% CI 1.00–1.63, P=0.05, Fig. 2C).
Secondary outcomes
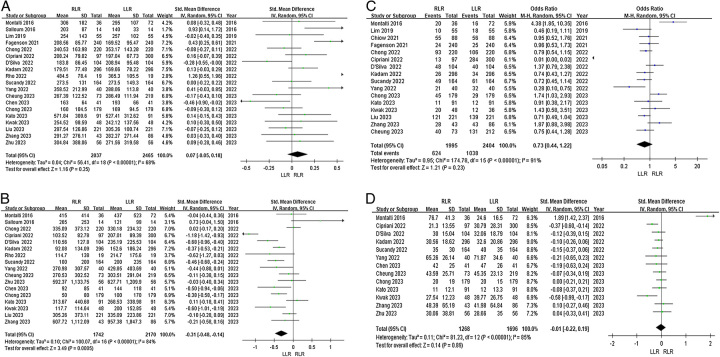
Operation time: Nineteen articles reported operation time. Heterogeneity among the studies was high (I2=68%). Random-effects model analysis showed that operation time did not significantly differ between the RLR and LLR groups (SMD=0.07, 95% CI −0.05 to 0.18, P=0.25, Fig. 3A).
Figure 3.
Forest plots of secondary outcomes for RLR versus LLR. (A) Operation time; (B) blood loss; (C) Pringle applied; and (D) duration of Pringle maneuver.
Blood loss: Seventeen articles reported blood loss. Heterogeneity among the studies was high (I2=84%). Random-effects model analysis showed that blood loss was significantly lower in the RLR group (SMD=−0.31, 95% CI −0.48 to −0.14, P=0.0005, Fig. 3B).
Pringle maneuver: Sixteen articles reported the utilization rate of the Pringle maneuver. Heterogeneity among the studies was high (I2=91%). The random-effects model showed that the odds of Pringle maneuver utilization did not significantly differ between the groups (OR=0.73, 95% CI 0.44–1.22, P=0.23, Fig. 3C).
Duration of Pringle maneuver: Thirteen articles reported duration of the Pringle maneuver. Heterogeneity among the studies was high (I2=85%). The random-effects model showed no significant difference in maneuver duration between the groups (SMD=−0.01, 95% CI −0.22 to 0.19, P=0.89, Fig. 3D).
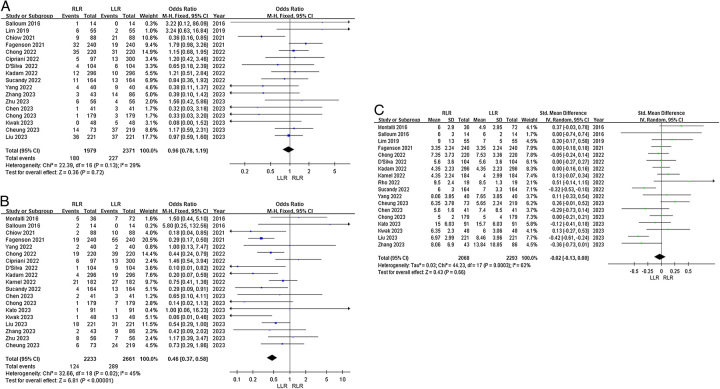
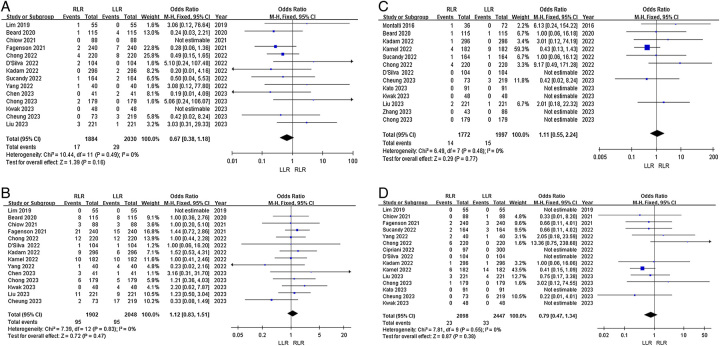
Transfusion: Seventeen articles reported incidence of intraoperative blood transfusion. Heterogeneity among the studies was low (I2=29%). Fixed-effects model analysis showed that the odds of intraoperative blood transfusion did not significantly differ between the groups (OR=0.96, 95% CI 0.78–1.19, P=0.72, Fig. 4A).
Figure 4.
Forest plots of secondary outcomes for RLR versus LLR. (A) Transfusion; (B) open conversion; and (C) postoperative hospital stay.
Open conversion: Nineteen articles reported the rate of open conversion. Heterogeneity among the studies was high (I2=45%). The fixed-effects model showed that the odds of open conversion were significantly lower in the RLR group (OR, 0.46; 95% CI 0.37–0.58, P <0.0001, Fig. 4B).
Postoperative hospital stay: Eighteen articles reported length of hospital stay. Heterogeneity among the studies was high (I2=62%). Random-effects model analysis showed no significant difference in length of hospital stay between the groups (SMD=−0.02, 95% CI −0.13 to 0.08, P=0.66, Fig. 4C).
Reoperation: Fourteen articles reported reoperation rates. Heterogeneity among the studies was low (I2=0%). Fixed-effects model analysis showed no significant difference in odds of reoperation between the groups (OR=0.67, 95% CI 0.38–1.18, P=0.20, Fig. 5A).
Figure 5.
Forest plots of secondary outcomes for RLR versus LLR. (A) Reoperation; (B) 30-day readmission; (C) 30-day mortality; and (D) 90-day mortality.
30-day readmission: Fourteen articles reported 30-day readmission rates. Heterogeneity among the studies was low (I2=0%). Fixed-effects model analysis showed no significant difference in odds of 30-day readmission between the groups (OR=1.12, 95% CI 0.83–1.51, P=0.47, Fig. 5B).
30-day mortality: Thirteen articles reported 30-day mortality. Heterogeneity among the studies was low (I2=0%). Fixed-effects model analysis showed no significant difference in odds of 30-day mortality between the groups (OR=1.11, 95% CI 0.55–2.24, P=0.77, Fig. 5C).
90-day mortality: Fifteen articles reported 90-day mortality. Heterogeneity among the studies was low (I2=0%). Fixed-effects model analysis showed no significant difference in 90-day mortality between the groups (OR=0.79, 95% CI 0.47–1.34, P=0.38, Fig. 5D).
Subgroup analysis
There was a high degree of heterogeneity in operation time, intraoperative blood loss, use of Pringle maneuver, duration of Pringle maneuver, and length of hospital stay. Studies were divided into subgroups according to date of publication (2023 and before 2023), number of research centers (single and multicenter), and study NOS score (9 and <9). The random-effects model was used for analysis.
In the 2023 publication subgroup, heterogeneity for operation time decreased significantly (I2=3%) and operation time did not significantly differ between the LLR and RLR groups (SMD= −0.05, 95% CI −0.15 to 0.05, P=0.30); moreover, heterogeneity for Pringle maneuver duration decreased significantly (I2=24%) and maneuver duration did not significantly differ between LLR and RLR (SMD=−0.08, 95% CI −0.22 to 0.05, P=0.24). There was no significant change in the heterogeneity of the remaining results (Table 3, Supplemental Figs S1–S2, Supplemental Digital Content 4, http://links.lww.com/JS9/B272; Supplemental Digital Content 5, http://links.lww.com/JS9/B273).
Table 3.
Results of published year subgroup.
| Published in 2023 | Published before 2023 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | Number of studies | Number of patients | I2 (%) | Overall effect size | 95% CI of overall effect size | P | Number of studies | Number of patients | I2 (%) | Overall effect size | 95% CI of overall effect size | P |
| Operating time (min) | 8 | 1693 | 3 | SMD=−0.05 | [−0.15, 0.05] | 0.30 | 11 | 2809 | 76 | SMD=0.17 | [−0.01, 0.34] | 0.06 |
| Blood loss (ml) | 8 | 1693 | 54 | SMD=−0.2 | [−0.36, −0.05] | 0.01 | 9 | 2219 | 89 | SMD=−0.39 | [−0.67, −0.10] | 0.009 |
| Pringle applied | 6 | 1480 | 57 | OR=1.09 | [0.75, 1.61] | 0.65 | 10 | 2919 | 94 | OR=0.56 | [0.25, 1.23] | 0.15 |
| Pringle duration (min) | 7 | 1251 | 24 | SMD=−0.08 | [−0.22, 0.05] | 0.24 | 6 | 1713 | 93 | SMD=0.12 | [−0.29, 0.52] | 0.57 |
| Hospital stay (days) | 7 | 1581 | 74 | SMD=−0.11 | [−0.32, 0.10] | 0.29 | 11 | 2780 | 41 | SMD=0.02 | [−0.09, 0.13] | 0.69 |
SMD/OR, standard mean difference/odds ratio; statistically significant results are shown in bold.
In the single-center and multicenter subgroups, heterogeneity for length of hospital stay decreased significantly (I2=39% and 47%, respectively). Length of hospital stay did not significantly differ between the single-center (SMD=−0.04, 95% CI −0.19 to 0.12, P=0.65) and multicenter subgroups (SMD=0.03, 95% CI −0.09 to 0.14, P=0.63). There was no significant change in the heterogeneity of the other results (Table 4, Supplemental Figs S3–S4, Supplemental Digital Content 6, http://links.lww.com/JS9/B274; Supplemental Digital Content 7, http://links.lww.com/JS9/B275).
Table 4.
Results of center subgroup.
| Single center | Multicenter | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | Number of studies | Number of patients | I2 (%) | Overall effect size | 95% CI of overall effect size | P | Number of studies | Number of patients | I2 (%) | Overall effect size | 95% CI of overall effect size | P |
| Operating time (min) | 8 | 1448 | 75 | SMD=0.23 | [−0.01, 0.48] | 0.06 | 11 | 3054 | 23 | SMD=−0.03 | [−0.11, 0.06] | 0.56 |
| Blood loss (ml) | 7 | 968 | 91 | SMD=−0.27 | [−0.75, 0.21] | 0.27 | 10 | 2944 | 71 | SMD=−0.3 | [−0.45, −0.16] | <0.01 |
| Pringle applied | 4 | 1188 | 98 | OR=0.34 | [0.03, 3.68] | 0.38 | 12 | 3211 | 67 | OR=0.93 | [0.69, 1.24] | 0.61 |
| Pringle duration (min) | 5 | 902 | 39 | SMD=−0.13 | [−0.32, 0.06] | 0.18 | 8 | 2062 | 90 | SMD=0.05 | [−0.25, 0.36] | 0.74 |
| Hospital stay (days) | 7 | 1307 | 39 | SMD=−0.04 | [−0.19, 0.12] | 0.65 | 11 | 2612 | 47 | SMD=0.03 | [−0.09, 0.14] | 0.63 |
SMD/OR, standard mean difference/odds ratio; statistically significant results are shown in bold.
In the NOS score 9 subgroup, heterogeneity for use of Pringle maneuver (I2=0%) and maneuver duration (I2=0%) decreased significantly. In addition, the utilization rate was significantly higher in the LLR group than the RLR group (OR= 0.74; 95% CI, 0.60–0.92, P=0.006). Duration of Pringle maneuver did not significantly differ between the groups (SMD=−0.11, 95% CI −0.21 to 0.00, P=0.90). There was no significant change in the heterogeneity of the other results (Table 5, Supplemental Figs S5–S6, Supplemental Digital Content 8, http://links.lww.com/JS9/B276; Supplemental Digital Content 9, http://links.lww.com/JS9/B277).
Table 5.
Results of NOS score subgroup.
| NOS=9 | NOS<9 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | Number of studies | Number of patients | I2 (%) | Overall effect size | 95% CI of overall effect size | P | Number of studies | Number of patients | I2 (%) | Overall effect size | 95% CI of overall effect size | P |
| Operating time (min) | 7 | 2324 | 74 | SMD=0.1 | [−0.07, 0.28] | 0.24 | 12 | 2178 | 68 | SMD=0.04 | [−0.11, 0.20] | 0.62 |
| Blood loss (ml) | 7 | 2241 | 91 | SMD=−0.37 | [−0.67, −0.07] | 0.02 | 10 | 1671 | 72 | SMD=−0.26 | [−0.46, −0.06] | 0.01 |
| Pringle applied | 6 | 2205 | 0 | OR=0.74 | [0.60, 0.92] | 0.006 | 10 | 2194 | 95 | OR=0.77 | [0.31, −1.88] | 0.56 |
| Pringle duration (min) | 5 | 1404 | 0 | SMD=−0.11 | [−0.21, 0.00] | 0.90 | 8 | 1560 | 91 | SMD=0.06 | [−0.31, 0.43] | 0.89 |
| Hospital stay (days) | 6 | 2212 | 58 | SMD=−0.02 | [−0.16, 0.12] | 0.78 | 12 | 2149 | 66 | SMD=−0.02 | [−0.18, 0.14] | 0.80 |
NOS, Newcastle−Ottawa scale; SMD/OR, standard mean difference/odds ratio; statistically significant results are shown in bold.
Sensitivity analysis and publication bias
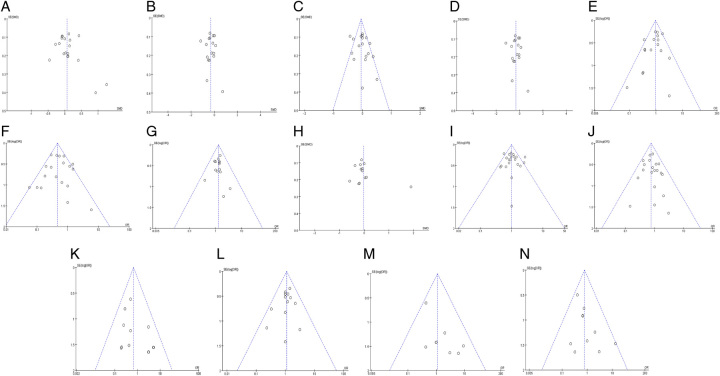
The results regarding operation time, intraoperative blood loss, use and duration of Pringle maneuver, and length of postoperative hospital stay were highly heterogeneous. Excluding the included literature one by one, the intraoperative blood loss heterogeneity had no significant change, suggesting that the analysis results were stable. After excluding the studies by Fagenson et al.32 and Rho et al.36 from the operation time data, the heterogeneity was significantly lower (I2=38%); repeat analysis using the fixed-effects model showed no significant difference in operation time between the two groups (SMD=0.00, 95% CI −0.06 to 0.06, P=0.98). After excluding Montalti et al.28 and Cipriani et al.33 from the Pringle maneuver use data, the heterogeneity was significantly lower (I2=47%); repeat analysis using the fixed-effects model still showed no significant difference in odds of Pringle maneuver use between the groups (OR=0.88, 95% CI 0.76–1.02, P=0.09). After excluding Montalti et al.28 from the Pringle maneuver duration data, the heterogeneity was significantly lower (I2=18%); repeat analysis using the fixed-effects model showed that the duration was significantly longer in the LLR group (SMD=−0.13, 95% CI −0.21 to −0.05, P=0.0008). After excluding Liu et al.43 from length of postoperative hospital stay, the heterogeneity was significantly lower (I2=41%); repeat analysis using the fixed-effects model showed no significant difference in length of postoperative hospital stay between the groups (SMD=0.00, 95% CI −0.07 to 0.06, P=0.94). A funnel chart was constructed based on the analysis results, and the publication bias test showed that the distribution of scatter spots on both sides of the funnel was basically symmetrical, suggesting that there was no obvious publication bias (Fig. 6).
Figure 6.
Funnel plot of RLR versus LLR. (A) Operation time; (B) blood loss; (C) Pringle applied; (D) duration of Pringle maneuver; (E) transfusion; (F) open conversion; (G) R0; (H) postoperative hospital stay; (I) overall complications; (J) severe complications; (K) reoperation; (L) 30-day readmission; (M) 30-day mortality; and (N) 90-day mortality.
Meta-regression analysis and quality of evidence
Meta-regression analysis showed that date of publication (P=0.059), sample size (P=0.051), male-to-female ratio (P=0.467), age (P=0.477), ASA grade (P=0.928), and study NOS score (P=0.113) had no significant effect on incidence of all complications, incidence of severe complications, nor R0 resection rate. The regression analysis results are summarized in Supplemental Tables S5–S7 (Supplemental Digital Content 3, http://links.lww.com/JS9/B271). The quality of evidence for overall and severe complications was moderate, while that for R0 resection rate was low. The quality of evidence grade of secondary outcome index was moderate in intraoperative blood transfusion, conversion to laparotomy, and reoperation, operation time, intraoperative bleeding, Pringle blocking rate, 30-day readmission, 30-day mortality, and 90-day mortality were low. The quality of evidence for duration of Pringle maneuver was very low. The quality of evidence data is summarized in Supplemental Figure S7 (Supplemental Digital Content 10, http://links.lww.com/JS9/B278).
Discussion
This meta-analysis of 22 propensity score-matched studies showed that RLR and LLR have similar operation times, durations of Pringle maneuver, intraoperative blood transfusion rates, lengths of hospital stay, and incidence of overall complications. However, RLR appears to be superior in terms of less blood loss, a lower open conversion rate, and a lower incidence of severe complications. RLR appears to be a safe and effective minimally invasive alternative to LLR.
The concept of minimally invasive surgery (MIS) was first proposed in 198346. At its core is less trauma, less intraoperative bleeding, shorter hospital stay, and faster postoperative recovery47. Laparoscopic surgery is minimally invasive and has been demonstrated in RCTs to be safe and feasible for abdominal operations48–50. However, it is associated with several problems, including lack of three-dimensional visualization, tremor effect, limitations related to instrumentation angles, and others. Robotic surgical platforms seem able to overcome some of these shortcomings. However, whether robotic surgery is superior to laparoscopic surgery remains debatable. Several RCTs have attempted to answer the question. Feng et al.51 suggested that robotic surgery is superior to laparoscopic for middle and low rectal cancers. In a distal gastrectomy study, Lu et al.52 suggested that robotic surgery is superior. For abdominal hernia repair, robotic and laparoscopic surgery appear to be equivalent53–55. These studies show that robotic and laparoscopic surgery have differences that vary according to the type of operation. A RCT comparing robotic and laparoscopic liver surgery has not yet been conducted, so there is currently a lack of high-level evidence to support the merits of RLR.
An early small study reported fewer complications and shorter hospital stay after robotic hepatectomy. Another suggested that outcomes were similar but the cost of robotic surgery was higher56,57. A meta-analysis of nine studies by Qiu et al.58 reported that RLR is more expensive and associated with longer operation time. Another 14 studies by Rahimli et al.22 reported that RLR and LLR outcomes were similar. It is well known that male:female ratio, age, type of disease, tumor size, extent of hepatectomy, and other factors have an effect on liver surgery outcome. At present, the published meta-analysis of RLR and LLR does not seem to match the preoperative scores of the above factors, resulting in higher heterogeneity and lower level of evidence.
To the best of our knowledge, this is the first meta-analysis of propensity score-matched studies to compare short-term outcomes between RLR and LLR. Operation time did not differ. Surgical proficiency is a crucial determinant of operation time, and operation times are generally longer for surgeons who are less experienced with a procedure. Kim et al.59 argued that the learning curve for RLR requires 16 cases, which is consistent with the 15 cases suggested by Chen et al.60. In our meta-analysis, only Salloum et al.29 had a sample size of less than 15 cases. Our results suggest that operation times are equivalent after surgeons have gained sufficient experience in the procedure. Intraoperative blood loss is another important outcome that affects hepatectomy prognosis. Use of the Pringle maneuver and its duration of application affect the amount of bleeding during hepatectomy, which is reflected by intraoperative blood transfusion volume61–63. We found no difference in use of the Pringle maneuver between RLR and LLR. However, in the LLR, maneuver duration was longer and intraoperative blood loss was lower. Intraoperative blood transfusion volume did not differ between the groups. RLR enables three-dimensional visualization, tremor filtering, and higher degrees of freedom for the instruments, which makes the operation more precise and stable. As a result, intraoperative blood loss was lower.
The most significant finding of our meta-analysis was lower odds of open conversion for RLR (OR=0.46, P <0.0001). Conversion to laparotomy increases the risk of postoperative complications64 and affects long-term outcome in patients undergoing hepatectomy65. Abdominal adhesions, tumor size and location, intraoperative bleeding, and technical operation were the reasons for the transition between RLR and LLR66,67. In our meta-analysis, confounding was reduced using propensity score matching. Previous studies also found that intraoperative bleeding was less with RLR. This indicates that the technical advantages of RLR reduces the risk of conversion to laparotomy during operation. These advantages also seem to reduce the odds of severe postoperative complications (OR=0.76, P=0.02). Although we found no difference in the odds of overall complications between RLR and LLR, the odds of severe complications were lower for RLR, which may also be the potential benefit point for the lower conversion rate of RLR to laparotomy. However, whether the lower odds of open conversion associated with RLR affect long-term outcome has not been discussed in depth. In addition, although the odds of severe complications for LLR were higher, this did not affect length of hospital stay or odds of reoperation, 30-day readmission, 30-day mortality, or 90-day mortality. We found no difference between LLR and RLR in any of these indicators, which shows that LLR is certainly a safe operation. Furthermore, short-term outcomes were only partially better with RLR, and associated costs are higher. The high cost of RLR infrastructure will probably limit the application of RLR68,69.
Our meta-analysis has several limitations. First, it does not include any RCTs. Second, there are differences between studies in the extent and location of resection (segmental vs. hepatectomy), texture of the liver, type of liver tumor, robotic and laparoscopy systems used, and patient characteristics such as sex and age that increased the heterogeneity of the results. Finally, data overlap between multicenter studies may have introduced bias. Future multicenter RCTs are needed to validate the differences between RLR and LLR.
In summary, the results of this meta-analysis of propensity score-matched studies show that although some short-term outcomes are similar between RLR and LLR, RLR is superior in terms of less blood loss and lower odds of open conversion and severe complications. In the future, RLR may become a safe and effective replacement for LLR; however, further study is needed.
Ethical approval
The study was approved by the International prospective register of systematic reviews (PROSPERO).
Sources of funding
This work was supported by grants from the National Natural Science Foundation of China (82173124, 82103533, 81972747, and 82203823), the Natural Science Foundation of Sichuan Province (2023NSFSC1877), and the Science and Technology Program of Sichuan Province (2023JDR0077).
Author contribution
F.G.: conceptualization, methodology, resources, writing – original draft, review, and editing; X.Z.: conceptualization, methodology, resources, writing – original draft, review, and editing; K.J.: investigation, formal analysis, writing – original draft and editing, and supervision; Q.X.: investigation, formal analysis, and writing – original draft and editing; M.Y.: investigation, supervision, and writing; T.M.: investigation, and supervision; H.W.: project administration, investigation, supervision, and writing.
Conflicts of interest disclosure
No conflicting relationship exists for any of the authors.
Research registration unique identifying number (UIN)
Name of the registry used: PROSPERO.
Unique identifying number or registration ID: CRD42023417941.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023417941.
Guarantor
Pro. Hong Wu, E-mail: wuhong@scu.edu.cn; Liver Transplantation Center, No. 37, Guoxue Lane, Wuhou District, Chengdu, Sichuan Province, China.
Date availability statement
All data generated or analyzed during this study are included in this article. The data are available from the corresponding author upon reasonable request.
Supplementary Material
Footnotes
Fengwei Gao and Xin Zhao contributed equally to this paper.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 3 November 2023
Contributor Information
Fengwei Gao, Email: Gaofengweigfw8@126.com.
Xin Zhao, Email: zhaoxin0502@126.com.
Qingyun Xie, Email: Dr.Xieqingyun@gmail.com.
Kangyi Jiang, Email: Jiangkangyi2014@163.com.
Tianyang Mao, Email: tianyangmao@126.com.
Manyu Yang, Email: yangmanyu2613@126.com.
Hong Wu, Email: wuhong@scu.edu.cn.
References
- 1. Reich H, McGlynn F, DeCaprio J, et al. Laparoscopic excision of benign liver lesions. Obstet Gynecol 1991;78:956–958. [PubMed] [Google Scholar]
- 2. Rao AM, Ahmed I. Laparoscopic versus open liver resection for benign and malignant hepatic lesions in adults. Cochrane Database Syst Rev 2013:CD010162. [DOI] [PubMed] [Google Scholar]
- 3. Martínez-Cecilia D, Cipriani F, Shelat V, et al. Laparoscopic versus open liver resection for colorectal metastases in elderly and octogenarian patients: a multicenter propensity score based analysis of short- and long-term outcomes. Ann Surg 2017;265:1192–1200. [DOI] [PubMed] [Google Scholar]
- 4. Yang SY, Feng JK, Yan ML, et al. Laparoscopic and open liver resection for hepatocellular carcinoma with type 2 diabetes mellitus: multicenter propensity score-matched study. Hepatol Int 2023;17:1251–1264. [DOI] [PubMed] [Google Scholar]
- 5. van der Poel MJ, Besselink MG, Cipriani F, et al. Outcome and learning curve in 159 consecutive patients undergoing total laparoscopic hemihepatectomy. JAMA Surg 2016;151:923–928. [DOI] [PubMed] [Google Scholar]
- 6. Berardi G, Aghayan D, Fretland ÅA, et al. Multicentre analysis of the learning curve for laparoscopic liver resection of the posterosuperior segments. Br J Surg 2019;106:1512–1522. [DOI] [PubMed] [Google Scholar]
- 7. Olavarria OA, Bernardi K, Shah SK, et al. Robotic versus laparoscopic ventral hernia repair: multicenter, blinded randomized controlled trial. BMJ 2020;370:m2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muaddi H, Hafid ME, Choi WJ, et al. Clinical outcomes of robotic surgery compared to conventional surgical approaches (laparoscopic or open): a systematic overview of reviews. Ann Surg 2021;273:467–473. [DOI] [PubMed] [Google Scholar]
- 9. Ojima T, Nakamura M, Hayata K, et al. Short-term outcomes of robotic gastrectomy vs laparoscopic gastrectomy for patients with gastric cancer: a randomized clinical trial. JAMA Surg 2021;156:954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Benedetto F, Petrowsky H, Magistri P, et al. Robotic liver resection: hurdles and beyond. Int J Surg 2020;82s:155–162. [DOI] [PubMed] [Google Scholar]
- 11. Gavriilidis P, Roberts KJ, Aldrighetti L, et al. A comparison between robotic, laparoscopic and open hepatectomy: a systematic review and network meta-analysis. Eur J Surg Oncol 2020;46:1214–1224. [DOI] [PubMed] [Google Scholar]
- 12. Sucandy I, Shapera E, Syblis CC, et al. Propensity score-matched comparison of robotic and open major hepatectomy for malignant liver tumors. Surg Endosc 2022;36:6724–6732. [DOI] [PubMed] [Google Scholar]
- 13. Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 2014;259:549–555. [DOI] [PubMed] [Google Scholar]
- 14. Chong CC, Fuks D, Lee KF, et al. Propensity score-matched analysis comparing robotic and laparoscopic right and extended right hepatectomy. JAMA Surg 2022;157:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chiow AKH, Fuks D, Choi GH, et al. International multicentre propensity score-matched analysis comparing robotic versus laparoscopic right posterior sectionectomy. Br J Surg 2021;108:1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D’Silva M, Han HS, Liu R, et al. Limited liver resections in the posterosuperior segments: international multicentre propensity score-matched and coarsened exact-matched analysis comparing the laparoscopic and robotic approaches. Br J Surg 2022;109:1140–1149. [DOI] [PubMed] [Google Scholar]
- 17. Cheung TT, Liu R, Cipriani F, et al. Robotic versus laparoscopic liver resection for huge (≥10 cm) liver tumors: an international multicenter propensity score-matched cohort study of 799 cases. Hepatobiliary Surg Nutr 2023;12:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montalti R, Berardi G, Patriti A, et al. Outcomes of robotic vs laparoscopic hepatectomy: a systematic review and meta-analysis. World J Gastroenterol 2015;21:8441–8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu Y, Guo K, Xu J, et al. Robotic versus laparoscopic hepatectomy for malignancy: a systematic review and meta-analysis. Asian J Surg 2021;44:615–628. [DOI] [PubMed] [Google Scholar]
- 20. Ziogas IA, Giannis D, Esagian SM, et al. Laparoscopic versus robotic major hepatectomy: a systematic review and meta-analysis. Surg Endosc 2021;35:524–535. [DOI] [PubMed] [Google Scholar]
- 21. Murtha-Lemekhova A, Fuchs J, Hoffmann K. Innovation for the sake of innovation? How does robotic hepatectomy compare to laparoscopic or open resection for HCC – a systematic review and meta-analysis. Cancers (Basel) 2022;14:3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rahimli M, Perrakis A, Andric M, et al. Does robotic liver surgery enhance R0 results in liver malignancies during minimally invasive liver surgery?-A systematic review and meta-analysis. Cancers (Basel) 2022;14:3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ziogas IA, Kakos CD, Moris DP, et al. Systematic review and meta-analysis of open versus laparoscopy-assisted versus pure laparoscopic versus robotic living donor hepatectomy. Liver Transpl 2023;29:1063–1078. [DOI] [PubMed] [Google Scholar]
- 24. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 25. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Montalti R, Scuderi V, Patriti A, et al. Robotic versus laparoscopic resections of posterosuperior segments of the liver: a propensity score-matched comparison. Surg Endosc 2016;30:1004–1013. [DOI] [PubMed] [Google Scholar]
- 29. Salloum C, Lim C, Lahat E, et al. Robotic-assisted versus laparoscopic left lateral sectionectomy: analysis of surgical outcomes and costs by a propensity score matched cohort study. World J Surg 2017;41:516–524. [DOI] [PubMed] [Google Scholar]
- 30. Lim C, Salloum C, Tudisco A, et al. Short- and long-term outcomes after robotic and laparoscopic liver resection for malignancies: a propensity score-matched study. World J Surg 2019;43:1594–1603. [DOI] [PubMed] [Google Scholar]
- 31. Beard RE, Khan S, Troisi RI, et al. Long-term and oncologic outcomes of robotic versus laparoscopic liver resection for metastatic colorectal cancer: a multicenter, propensity score matching analysis. World J Surg 2020;44:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fagenson AM, Gleeson EM, Pitt HA, et al. Minimally invasive hepatectomy in North America: laparoscopic versus robotic. J Gastrointest Surg 2021;25:85–93. [DOI] [PubMed] [Google Scholar]
- 33. Cipriani F, Fiorentini G, Magistri P, et al. Pure laparoscopic versus robotic liver resections: multicentric propensity score-based analysis with stratification according to difficulty scores. J Hepatobiliary Pancreat Sci 2022;29:1108–1123. [DOI] [PubMed] [Google Scholar]
- 34. Kadam P, Sutcliffe RP, Scatton O, et al. An international multicenter propensity score-matched and coarsened-exact matched analysis comparing robotic versus laparoscopic partial liver resections of the anterolateral segments. J Hepatobiliary Pancreat Sci 2022;29:843–854. [DOI] [PubMed] [Google Scholar]
- 35. Kamel MK, Tuma F, Keane CA, et al. National trends and perioperative outcomes of robotic-assisted hepatectomy in the USA: a propensity score-matched analysis from the National Cancer Database. World J Surg 2022;46:189–196. [DOI] [PubMed] [Google Scholar]
- 36. Rho SY, Lee JG, Joo DJ, et al. Outcomes of robotic living donor right hepatectomy from 52 consecutive cases: comparison with open and laparoscopy-assisted donor hepatectomy. Ann Surg 2022;275:e433–e442. [DOI] [PubMed] [Google Scholar]
- 37. Sucandy I, Rayman S, Lai EC, et al. Robotic versus laparoscopic left and extended left hepatectomy: an international multicenter study propensity score-matched analysis. Ann Surg Oncol 2022;29:8398–8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang HY, Choi GH, Chin KM, et al. Robotic and laparoscopic right anterior sectionectomy and central hepatectomy: multicentre propensity score-matched analysis. Br J Surg 2022;109:311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen W, Zhang X, Jiang J, et al. Robotic versus laparoscopic liver resection in posterosuperior region: a retrospective study of consecutive cases. Surg Endosc 2023;37:4728–4736. [DOI] [PubMed] [Google Scholar]
- 40. Chong Y, Prieto M, Gastaca M, et al. An international multicentre propensity score-matched analysis comparing between robotic versus laparoscopic left lateral sectionectomy. Surg Endosc 2023;37:3439–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kato Y, Sugioka A, Kojima M, et al. Initial experience with robotic liver resection: audit of 120 consecutive cases at a single center and comparison with open and laparoscopic approaches. J Hepatobiliary Pancreat Sci 2023;30:72–90. [DOI] [PubMed] [Google Scholar]
- 42. Kwak BJ, Lee JH, Chin KM, et al. Robotic versus laparoscopic liver resections for hepatolithiasis: an international multicenter propensity score-matched analysis. Surg Endosc 2023;37:5855–5864. [DOI] [PubMed] [Google Scholar]
- 43. Liu Q, Zhang W, Zhao JJ, et al. Propensity-score matched and coarsened-exact matched analysis comparing robotic and laparoscopic major hepatectomies: an international multicenter study of 4822 cases. Ann Surg 2023;25:S196. [DOI] [PubMed] [Google Scholar]
- 44. Zhang W, Liu J, Zhang Z, et al. Perioperative outcomes of robot-assisted versus laparoscopic liver resection for cavernous hemangioma: a propensity score matching study. Surg Endosc 2023;37:4505–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhu P, Liao W, Zhang WG, et al. A prospective study using propensity score matching to compare long-term survival outcomes after robotic-assisted, laparoscopic, or open liver resection for patients with BCLC stage 0-A hepatocellular carcinoma. Ann Surg 2023;277:e103–e111. [DOI] [PubMed] [Google Scholar]
- 46. Rosen M, Ponsky J. Minimally invasive surgery. Endoscopy 2001;33:358–366. [DOI] [PubMed] [Google Scholar]
- 47. Atallah S, Larach SW. Transanal minimally invasive surgery. JAMA Surg 2021;156:92–93. [DOI] [PubMed] [Google Scholar]
- 48. Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015;372:1324–1332. [DOI] [PubMed] [Google Scholar]
- 49. Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, et al. Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg 2018;267:199–207. [DOI] [PubMed] [Google Scholar]
- 50. van der Veen A, Brenkman HJF, Seesing MFJ, et al. Laparoscopic versus open gastrectomy for gastric cancer (LOGICA): a multicenter randomized clinical trial. J Clin Oncol 2021;39:978–989. [DOI] [PubMed] [Google Scholar]
- 51. Feng Q, Yuan W, Li T, et al. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol 2022;7:991–1004. [DOI] [PubMed] [Google Scholar]
- 52. Lu J, Zheng CH, Xu BB, et al. Assessment of robotic versus laparoscopic distal gastrectomy for gastric cancer: a randomized controlled trial. Ann Surg 2021;273:858–867. [DOI] [PubMed] [Google Scholar]
- 53. Prabhu AS, Carbonell A, Hope W, et al. Robotic inguinal vs transabdominal laparoscopic inguinal hernia repair: the RIVAL randomized clinical trial. JAMA Surg 2020;155:380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dhanani NH, Olavarria OA, Holihan JL, et al. Robotic versus laparoscopic ventral hernia repair: one-year results from a prospective, multicenter, blinded randomized controlled trial. Ann Surg 2021;273:1076–1080. [DOI] [PubMed] [Google Scholar]
- 55. Petro CC, Zolin S, Krpata D, et al. Patient-reported outcomes of robotic vs laparoscopic ventral hernia repair with intraperitoneal mesh: the PROVE-IT randomized clinical trial. JAMA Surg 2021;156:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Packiam V, Bartlett DL, Tohme S, et al. Minimally invasive liver resection: robotic versus laparoscopic left lateral sectionectomy. J Gastrointest Surg 2012;16:2233–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yu YD, Kim KH, Jung DH, et al. Robotic versus laparoscopic liver resection: a comparative study from a single center. Langenbecks Arch Surg 2014;399:1039–1045. [DOI] [PubMed] [Google Scholar]
- 58. Qiu J, Chen S, Chengyou D. A systematic review of robotic-assisted liver resection and meta-analysis of robotic versus laparoscopic hepatectomy for hepatic neoplasms. Surg Endosc 2016;30:862–875. [DOI] [PubMed] [Google Scholar]
- 59. Kim NR, Han DH, Choi GH, et al. Comparison of surgical outcomes and learning curve for robotic versus laparoscopic living donor hepatectomy: a retrospective cohort study. Int J Surg 2022;108:107000. [DOI] [PubMed] [Google Scholar]
- 60. Chen PD, Wu CY, Hu RH, et al. Robotic major hepatectomy: is there a learning curve? Surgery 2017;161:642–649. [DOI] [PubMed] [Google Scholar]
- 61. van den Broek MA, Bloemen JG, Dello SA, et al. Randomized controlled trial analyzing the effect of 15 or 30 min intermittent Pringle maneuver on hepatocellular damage during liver surgery. J Hepatol 2011;55:337–345. [DOI] [PubMed] [Google Scholar]
- 62. Gupta R, Fuks D, Bourdeaux C, et al. Impact of intraoperative blood loss on the short-term outcomes of laparoscopic liver resection. Surg Endosc 2017;31:4451–4457. [DOI] [PubMed] [Google Scholar]
- 63. Cao B, Hao P, Guo W, et al. A predictive model for blood transfusion during liver resection. Eur J Surg Oncol 2022;48:1550–1558. [DOI] [PubMed] [Google Scholar]
- 64. Vining CC, Al Abbas AI, Kuchta K, et al. Risk factors and outcomes in patients undergoing minimally invasive hepatectomy with unplanned conversion: a contemporary NSQIP analysis. HPB (Oxford) 2023;25:577–588. [DOI] [PubMed] [Google Scholar]
- 65. Stiles ZE, Glazer ES, Deneve JL, et al. Long-term implications of unplanned conversion during laparoscopic liver resection for hepatocellular carcinoma. Ann Surg Oncol 2019;26:282–289. [DOI] [PubMed] [Google Scholar]
- 66. Cipriani F, Ratti F, Fiorentini G, et al. Pure laparoscopic right hepatectomy: a risk score for conversion for the paradigm of difficult laparoscopic liver resections. A single-centre case series. Int J Surg 2020;82:108–115. [DOI] [PubMed] [Google Scholar]
- 67. Jo SJ, Rhu J, Kim JM, et al. Near-zero open conversion rate of laparoscopic liver resection: a high-volume single-center experience of the past 5 years. Surg Endosc 2023;37:1813–1821. [DOI] [PubMed] [Google Scholar]
- 68. Stewart C, Wong P, Warner S, et al. Robotic minor hepatectomy: optimizing outcomes and cost of care. HPB (Oxford) 2021;23:700–706. [DOI] [PubMed] [Google Scholar]
- 69. Miller HP, Hakim A, Kellish A, et al. Cost-benefit analysis of robotic vs. laparoscopic hepatectomy: a propensity-matched retrospective cohort study of American College of Surgeons National Surgical Quality Improvement Program Database. Am Surg 2022;88:2886–2892. [DOI] [PubMed] [Google Scholar]