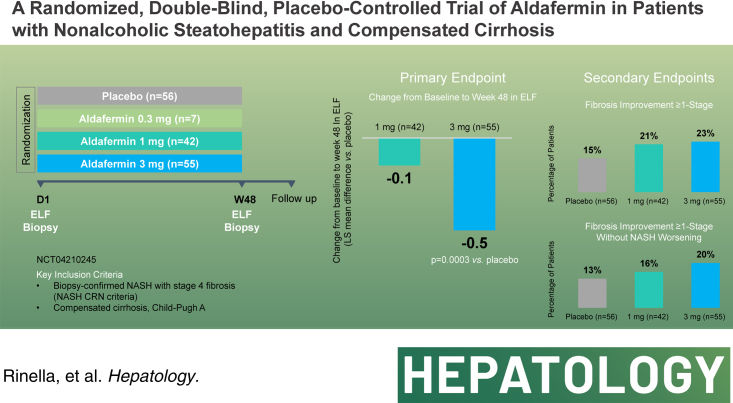
Abstract
Background and Aims:
Aldafermin, an engineered analog of the human hormone FGF19, improves liver histology in patients with noncirrhotic NASH; however, its efficacy and safety in compensated cirrhosis is unknown. No drug has yet to demonstrate benefit in the compensated NASH population.
Approach and Results:
In this multicenter, double-blind, placebo-controlled, phase 2b trial, 160 patients with compensated NASH cirrhosis were randomized to aldafermin 0.3 mg (n = 7), 1 mg (n = 42), 3 mg (n = 55), or placebo (n = 56) for 48 weeks. The 0.3 mg group was discontinued to limit exposure to suboptimal doses. The primary end point was a change in Enhanced Liver Fibrosis from baseline to week 48. The analyses were performed in the intention-to-treat population. At week 48, the least-squares mean difference in the change in Enhanced Liver Fibrosis was −0.5 (95% CI, −0.7 to −0.2; p = 0.0003) between the 3 mg group and the placebo group. 15%, 21%, and 23% of patients in the placebo, 1 mg, and 3 mg group, respectively, achieved fibrosis improvement ≥ 1 stage; and 13%, 16%, and 20% achieved fibrosis improvement ≥ 1 stage without NASH worsening. Improvement in alanine aminotransferase, aspartate aminotransferase, neoepitope-specific N-terminal pro-peptide of type III collagen, and liver stiffness favored aldefermin groups over placebo. Diarrhea was the most frequent adverse event, occurring at 26% and 40% in the 1 mg and 3 mg groups, respectively, compared to 18% in the placebo group. Overall, 0%, 2%, and 9% of patients in the placebo, 1 mg, and 3 mg group, respectively, discontinued due to treatment-related adverse events.
Conclusions:
Aldafermin 3 mg resulted in a significant reduction in Enhanced Liver Fibrosis in patients with compensated NASH cirrhosis.
INTRODUCTION
NASH is a serious and slowly progressive liver disease with an immense public health and economic burden.1 Patients can progress through varying stages of fibrosis before developing cirrhosis, and subsequently, its complications of hepatic decompensation, liver cancer, liver transplantation, and death.2 It is estimated that more than 200 million people in the world are living with NASH, and NASH is now the second most common indication for adults on the liver transplant waitlist and is the leading indication for liver transplantation for those over the age of 65 and in women in the United States.3,4 The estimated number of deaths associated with NASH cirrhosis worldwide in 2019 is 134,000.5
The journey for compounds in development for the treatment of NASH across the disease spectrum has been fraught with failures, particularly in the context of cirrhosis.6–10 Despite the tremendous disease burden, herculean effort, and investment to study promising therapeutics, there is still no approved pharmacotherapy for the disease. Even compounds that met the primary end point in noncirrhotic NASH trials still failed in the context of NASH cirrhosis,11 which may reflect the appropriateness of required regulatory end points as well as the challenges of reversing disease at advanced stages. The current regulatory guideline on drug development in NASH with compensated cirrhosis recommends the end point of outcome events for full approval.12 Cirrhosis is itself a spectrum of disease where less advanced diseases may be more likely to respond to a therapeutic intervention but have a low event rate in contrast to more advanced diseases where higher event rates need to be balanced with a lower likelihood of disease reversibility. Thus, the optimal end points for NASH cirrhosis trials are yet to be defined and evaluated, as outcome trials may take multiple years, if not decades, and thousands of patients to complete. Although data from the STELLAR and simtuzumab trials showed that fibrosis regression can predict outcome,13 histologic fibrosis assessment using current categorical scoring systems cannot capture the full breadth of anti-fibrotic response. Further, adopting a biopsy surrogate to assess treatment response is ill-advised as it is not used in routine clinical practice, particularly in cirrhosis, where liver biopsy may carry additional risks. Liver biopsy in the context of NASH is fraught with interobserver variability, particularly when samples are suboptimal in length and gauge.14 Although machine-learning technologies can improve sensitivity to detect fibrosis change and precision of biopsy read, assessment of treatment response using noninvasive tests is preferable from a cost and risk perspective. Noninvasive tests are recommended in society guidelines for the assessment and risk stratification of patients with NASH.15,16
Aldafermin is an engineered analog of the human gut hormone FGF19.17–19 Aldafermin exhibits biased FGFR4-KLB receptor signaling through modification within the amino terminus to eliminate tumorigenicity while retaining potent activity to suppress CYP7A1, which encodes the first and rate-limiting enzyme of bile acid synthesis. Additionally, aldafermin acts on the FGFR1c-KLB receptor to potentiate insulin sensitivity and fat metabolism. Since bile acid levels are elevated in NASH cirrhosis,19 and accumulation of bile acids is thought to be hepatotoxic, leading to progressive injury and dysfunction, aldafermin may improve outcomes in these patients through modulation of bile acid metabolism.20
Previous phase 2 trials in noncirrhotic NASH showed that patients who received aldafermin had significant, rapid, and dose-dependent reductions in liver fat and improved liver histology at 12 or 24 weeks.21–23 We conducted the ALPINE-4 trial (Evaluation of efficacy, safety, and tolerability of NGM282 [Aldafermin] in a phase 2b, randomized, double-blind, placebo-controlled, multicenter study in subjects with compensated cirrhosis due to NASH) to determine the efficacy and safety of aldafermin at doses of 0.3 mg, 1 mg, and 3 mg versus placebo in patients with NASH and compensated cirrhosis.
METHODS
Study design and participants
In this placebo-controlled, phase 2b trial, we randomized patients at 48 clinical centers in 8 countries (Australia, Belgium, China [Hong Kong], France, Germany, Poland, United Kingdom, and the United States) (appendix p3, http://links.lww.com/HEP/I13). The trial consisted of a 48-week treatment period and a 6-week follow-up period. Eligible patients were 18–75 years of age with compensated NASH cirrhosis. Key inclusion criteria were liver biopsy consistent with cirrhosis according to the NASH clinical research network (CRN) classification (NASH CRN fibrosis stage 4), as assessed by the central reader, definitive NASH cirrhosis,24 total bilirubin of 1.3 mg/dL or less, glycated hemoglobin no higher than 9.5%, alanine aminotransferase (ALT)/aspartate aminotransferase (AST) no more than 5 times the upper limit of normal, and creatinine clearance of at least 60 mL/min. Statin-naïve (not on statin) and statin-experienced (on statin) patients were allowed to enroll, and those on statins were switched to over-encapsulated rosuvastatin in the trial. A small number of patients (capped at 10% of planned enrollment) with a clinical diagnosis of NASH cirrhosis were allowed to enroll despite a fibrosis stage of F3 by the central reader. Clinical diagnosis of NASH cirrhosis must meet at least one of the following: platelet count ≤140,000/mm3 and liver stiffness measure (LSM) by FibroScan ≥13.6 kPa, fibrosis-4 index ≥3.25, or Agile-4 score ≥0.57. Key exclusion criteria were causes of chronic liver disease other than NASH, excessive alcohol consumption (≥ 14 U per week for women; ≥21 U per week for men), Child-Pugh class B/C, or history of hepatic decompensation. A full list of eligibility criteria is provided in the Supplemental Appendix (appendix p5-6), http://links.lww.com/HEP/I13. The study protocol was approved by the institutional review board and ethics committee at each trial site, and the trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. All the patients provided written informed consent.
Randomization and masking
Eligible patients were randomly assigned to receive a once-daily subcutaneous injection of either aldafermin (at a dose of 0.3 mg, 1 mg, or 3 mg) or placebo for a 48-week treatment period, followed by a 6-week safety follow-up period. Randomization was performed with the use of an Interactive Web-Response System and stratified according to type 2 diabetes (T2D) status (yes/no). Under a protocol amendment during the global COVID-19 pandemic, randomization into the 0.3 mg aldafermin treatment group was discontinued to limit patients’ exposure to a suboptimal dose based on a pooled analysis of prior trials of aldafermin20; this decision was approved by the Data and Safety Monitoring Board, regulatory authorities, institutional review boards, and ethics committees. Subsequently, patients were randomized in a 4:3:4 ratio to receive placebo, 1 mg, or 3 mg aldafermin, respectively. Aldafermin or placebo (of identical appearance) was administered by once-daily subcutaneous injection. Patients, investigators, site staff, sponsors, pathologists, radiologists, medical monitors, laboratories, and clinical research organizations remained masked to treatment assignment throughout the study.
Procedures
Screening liver biopsy was used as the baseline for histologic variables, and an additional biopsy was performed at week 48. Each biopsy was assessed centrally by an expert liver pathologist (Zachary D. Goodman) to determine the activity score for NAFLD activity score and the fibrosis stage (NASH CRN criteria) (appendix p3, http://links.lww.com/HEP/I13). The pathologist was unaware of the treatment assignments or patient characteristics. Safety and efficacy were assessed at weeks 2, 6, 12, 18, 24, 30, 36, 42, 48 (end-of-treatment), and 54 (6 weeks off-treatment).
Enhanced Liver Fibrosis (ELF) was measured in serum samples using the Siemens ADVIA Centaur XP system at Medpace central laboratory. The ELF score is derived from an algorithm that combines 3 direct markers of liver fibrosis: hyaluronic acid, type III procollagen peptide (PIIINP), and tissue inhibitor of matrix metalloproteinase 1. Serum levels of 7alphahydroxy-4-cholesten-3-one (C4) and bile acids were measured at the Mayo Clinic (Rochester, MN, USA). Concentrations of neoepitope-specific N-terminal pro-peptide of type III collagen (Pro-C3) were measured at Nordic Bioscience (Herlev, Denmark) on the Roche Elecsys platform and converted using the formula 0.17* (Roche Elecsys Pro-C3)1.27. Liver stiffness was assessed at local sites using transient elastography on a FibroScan device (Echosens, Paris, France). Esophagogastroduodenoscopy was performed locally within 1 year of screening or during screening to rule out varices; the expanded Baveno VI criteria25 was allowed as a replacement for the esophagogastroduodenoscopy during the global COVID-19 pandemic. Patients underwent liver imaging at screening for HCC detection and liver ultrasound for surveillance at weeks 24 and 48. Adverse events (AE) were recorded from the time of screening until week 54 and were graded according to the National Cancer Institute Common Terminology Criteria for AE, version 5.0.
LDL cholesterol (LDL-C) concentrations were managed with over-encapsulated rosuvastatin or placebo by an independent medical monitor (masked to treatment assignment) according to protocol-specified algorithms (appendix p3, http://links.lww.com/HEP/I13, p15, http://links.lww.com/HEP/I13). Patients, investigators, site staff, and the sponsor were masked to rosuvastatin treatment and LDL-C values. Briefly, rosuvastatin was added starting from week 2 based on the 10-year ASCVD risk score and statin status of each patient. Incremental dose titration to a maximum of 40 mg rosuvastatin daily was implemented to reach the LDL-C goal of ≤ 70 mg/dL or ≥ 50% reduction.
An independent Data and Safety Monitoring Board comprised of experienced hepatologists, independent statisticians, and other relevant specialists reviewed unblinded safety data during the trial and recommended continuation of the study according to the protocol. Three independent adjudication committees comprised of experienced clinicians adjudicated liver events, cardiovascular events, and HCC during the trial (event definitions are in appendix p3-4, http://links.lww.com/HEP/I13).
Outcomes
The primary end point was the change in the ELF score from baseline to week 48 with aldafermin versus placebo. The ELF score is the first and only FDA-approved noninvasive outcome measure for disease prognosis in patients with NASH. Secondary histologic end points included an improvement of at least 1 stage in liver fibrosis (NASH CRN classification). Histological fibrosis response was initially the primary end point, but later made a secondary end point when the protocol was amended to have a change in the noninvasive measure ELF as the primary end point. In August 2021, the FDA approved the ELF as a prognostic test for the assessment of liver-related disease progression in patients with NASH and advanced fibrosis (F3 or F4). A protocol amendment dated November 11, 2021, documented the change of the primary end point to ELF while the study was blinded and was approved by institutional review boards, ethics committees, and regulatory authorities. This change occurred more than 1 year before the last patient completed the study (the last patient’s last visit was on February 23, 2023), and the database was locked on March 28, 2023. Other secondary end points included changes from baseline to week 48 in serum C4 and bile acid levels, fibrogenesis marker Pro-C3, ALT, AST, and LSM as assessed by FibroScan. Exploratory end points included outcome events adjudicated by 3 independent, external committees (cardiovascular events, liver events, and HCC). End points were assessed at baseline and at select trial visits. A complete list of the trial end points is provided in the Supplemental Appendix (appendix p7), http://links.lww.com/HEP/I13.
Statistical analysis
The sample size for this trial was estimated based on a comparison of aldafermin and placebo with respect to the primary efficacy end point, the change from baseline at week 48 in the ELF score. On the basis of data from previous aldafermin NASH trials, and assumptions of a change in ELF of 0.1 for patients treated with placebo, a change in ELF of −0.4 at week 48 for patients treated with aldafermin 3 mg and a 15% subject drop-out rate, a sample size of 150 patients would provide at least 92% power to detect a treatment difference at the 5% significance level (2-sided). The actual enrollment was 160 patients to allow for all patients screened and eligible to enter the trial. The study was not powered for histological fibrosis end point.
Efficacy analyses were done in the intention-to-treat population, which included all patients who underwent random assignment. The primary end point of the change from baseline at week 48 in ELF score was analyzed with the use of a mixed model for repeated measures that included the baseline ELF value, baseline T2D status (yes/no), and randomization ratio as covariates, with treatment, visit, treatment-by-visit interaction as fixed effects to compare aldafermin and placebo. The primary end point was tested for statistical significance with 2-sided alpha level of 5%. A hierarchical testing procedure was used to control the family-wise type I error rate in the following order: if the between-group difference for aldafermin 3 mg and placebo was significant at the 2-sided 5% level, then 1 mg was to be tested. Missing data in ELF were imputed using a multiple imputation method under the assumption of missing-at-random. Sensitivity analysis under the assumption of missing-not-at-random was also performed. Since randomization into the aldafermin 0.3 mg group was discontinued early, there was no statistical comparison being done between the aldafermin 0.3 mg group and the placebo group, and aldafermin 0.3 mg data were included in safety analysis only. The secondary histologic outcomes of fibrosis improvement of ≥ 1 stage (NASH CRN criteria), as well as fibrosis improvement without NASH worsening, were analyzed using the Cochran-Mantel-Haenszel test stratified by baseline T2D status (Yes/No) and randomization ratio. Missing fibrosis data were imputed using a multiple imputation method under the assumption of missing-at-random. Sensitivity analyses were performed with missing-not-at-random assumption and in patients who had both baseline and end-of-treatment liver biopsies. Continuous secondary end points were analyzed using the mixed model for repeated measures model with baseline outcome value, baseline T2D status (yes/no), and randomization ratio as covariates, and treatment, visit, treatment-by-visit interaction as fixed effects. All statistical tests for the secondary analyses were performed at 2-sided alpha level of 10%. The safety analyses included all patients who received at least 1 dose of aldafermin or placebo.
Data were collected by investigators and managed, validated, and analyzed by Medpace (Cincinnati, OH, USA). The data were analyzed by means of SAS software, version 9.4. No interim analysis was performed. This trial is registered with ClinicalTrials.gov, NCT04210245, and is complete.
RESULTS
Study population
Between February 4, 2020 and January 21, 2022, 580 patients were screened, of whom 160 were randomly assigned to receive once-daily aldafermin at a dose of 0.3 mg (n = 7), 1 mg (n = 42), 3 mg (n = 55) or placebo (n = 56) for 48 weeks. In total, 137 (86%) patients completed the study. The intention-to-treat population included all 160 randomized patients (Figure 1). The safety analysis population included 160 patients.
FIGURE 1.
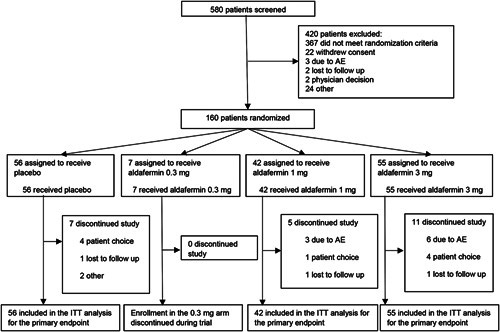
Trial profile. Abbreviations: AE, adverse event; ITT, intention-to-treat.
The demographic and clinical characteristics were similar across the groups (Table 1). The mean age was 59.6 years (SD 8.2), the mean bodyweight 96.0 kg (SD 21.3), and the mean body mass index was 34.8 (SD 6.6). Overall, 103 (64%) of the patients were female, 40 (25%) were of Hispanic or Latino ethnicity, and 121 (76%) had T2D. The baseline ELF score was 10.6 (SD 1.0), consistent with compensated cirrhosis in NASH. A total of 8 (5%) patients with a clinical diagnosis of NASH cirrhosis despite stage 3 fibrosis by central read were allowed to enroll.
TABLE 1.
Demographics and patient characteristics at baseline in the ITT population
| Placebo (n = 56) | Aldafermin 0.3 mg (n = 7) | Aldafermin 1 mg (n = 42) | Aldafermin 3 mg (n = 55) | |
|---|---|---|---|---|
| Age, y | 58.3 (8.1) | 59.7 (6.8) | 61.3 (7.6) | 59.6 (8.7) |
| Sex | ||||
| Female | 39 (69.6) | 5 (71.4) | 23 (54.8) | 36 (65.5) |
| Male | 17 (30.4) | 2 (28.6) | 19 (45.2) | 19 (34.5) |
| Race | ||||
| American Indian | 0 (0.0) | 0 (0.0) | 1 (2.4) | 1 (1.8) |
| Asian | 5 (8.9) | 0 (0.0) | 1 (2.4) | 4 (7.3) |
| Black | 1 (1.8) | 0 (0.0) | 0 (0.0) | 2 (3.6) |
| White | 46 (82.1) | 7 (100.0) | 38 (90.5) | 47 (85.5) |
| Other | 4 (7.1) | 0 (0.0) | 2 (4.8) | 1 (1.8) |
| Ethnicity | ||||
| Hispanic/Latino | 18 (32.1) | 2 (28.6) | 8 (19.0) | 12 (21.8) |
| Non-Hispanic/Latino | 38 (67.9) | 4 (57.1) | 33 (78.6) | 43 (78.2) |
| Type 2 diabetes | ||||
| Yes | 42 (75.0) | 5 (71.4) | 32 (76.2) | 42 (76.4) |
| No | 14 (25.0) | 2 (28.6) | 10 (23.8) | 13 (23.6) |
| Statin status | ||||
| Not on statin | 29 (51.8) | 4 (57.1) | 21 (50.0) | 17 (30.9) |
| On statin | 27 (48.2) | 3 (42.9) | 21 (50.0) | 38 (69.1) |
| ELF | ||||
| ELF score | 10.6 (1.0) | 10.8 (0.4) | 10.6 (0.8) | 10.6 (1.1) |
| Hyaluronic acid (ng/mL) | 177.5 (157.2) | 149.8 (57.8) | 155.6 (104.5) | 188.5 (213.0) |
| PIIINP (ng/mL) | 14.0 (6.0) | 15.3 (3.1) | 14.1 (5.9) | 15.9 (8.4) |
| TIMP-1 (ng/mL) | 318.4 (98.7) | 330.9 (75.6) | 337.9 (114.8) | 326.2 (96.3) |
| Histology | ||||
| Fibrosis stage | ||||
| F3 | 2 (3.6) | 0 (0.0) | 3 (7.1) | 3 (5.5) |
| F4 | 53 (94.6) | 7 (100.0) | 39 (92.9) | 52 (94.5) |
| NAS | 5.3 (1.4) | 6.0 (0.8) | 5.1 (1.5) | 5.5 (1.2) |
| Markers of target engagement | ||||
| C4, ng/mL | 45.4 (33.5) | 43.1 (19.5) | 38.7 (25.7) | 43.7 (37.9) |
| TBA, µmol/L | 10.3 (10.0) | 13.8 (6.9) | 9.2 (8.2) | 12.7 (14.1) |
| Liver enzymes | ||||
| ALT, U/L | 45.6 (31.2) | 50.0 (37.8) | 46.4 (23.2) | 51.2 (29.6) |
| AST, U/L | 36.9 (21.1) | 40.3 (20.9) | 39.5 (19.4) | 45.0 (25.7) |
| ALP, U/L | 80.9 (25.1) | 74.0 (14.7) | 74.6 (27.6) | 83.9 (32.3) |
| Total bilirubin, µmol/L | 12.8 (4.9) | 11.7 (3.9) | 12.1 (3.8) | 12.2 (5.2) |
| Fibrogenesis marker | ||||
| Pro-C3, ng/mL | 47.1 (28.3) | 53.2 (18.1) | 48.7 (27.5) | 59.2 (54.9) |
| Imaging by FibroScan | ||||
| LSM, kPa | 22.9 (12.1) | 25.8 (14.6) | 23.3 (10.8) | 22.7 (13.8) |
| Lipids | ||||
| Total cholesterol, mmol/L | 4.3 (1.0) | 4.5 (1.3) | 4.1 (1.0) | 4.2 (1.1) |
| HDL-C, mmol/L | 1.2 (0.4) | 1.1 (0.3) | 1.2 (0.3) | 1.2 (0.3) |
| LDL-C, mmol/L | 2.4 (0.9) | 2.4 (1.2) | 2.2 (0.9) | 2.3 (0.9) |
| Triglycerides, mmol/L | 1.7 (0.9) | 2.0 (1.0) | 1.7 (0.7) | 1.6 (0.5) |
| Metabolic parameters | ||||
| Weight, kg | 93.4 (19.9) | 88.2 (9.7) | 101.5 (22.2) | 95.3 (22.4) |
| BMI, kg/m2 | 34.8 (7.1) | 32.8 (3.2) | 36.0 (6.3) | 34.3 (6.7) |
| Glucose, mmol/L | 7.2 (1.9) | 8.8 (2.6) | 7.2 (2.2) | 7.8 (2.8) |
| Insulin, µIU/mL | 35.0 (29.6) | 31.0 (11.7) | 33.4 (19.9) | 50.3 (66.2) |
| HOMA-IR | 11.5 (10.9) | 12.7 (8.2) | 11.8 (10.0) | 20.0 (32.1) |
| HbA1c, % | 6.7 (1.2) | 7.8 (2.1) | 6.6 (1.1) | 6.9 (1.3) |
Notes: Values are mean (SD) or n (%).
Percentages may not total 100 because of rounding. To convert the values for cholesterol to milligram per deciliter, multiply by 38.67; to convert the values for triglycerides to milligram per deciliter, multiply by 88.57.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; C4, 7alpha-hydroxy-4-cholesten-3-one; ELF, Enhanced Liver Fibrosis; HbA1c, glycated hemoglobin; HDL-C, HDL cholesterol; HOMA-IR, homeostasis model assessment–estimated insulin resistance; ITT, intention-to-treat; LDL-C, LDL cholesterol; LSM, liver stiffness measure; NAS, NAFLD activity score; PIIINP, N-terminal pro-peptide of type III collagen; Pro-C3, neoepitope-specific N-terminal pro-peptide of type III collagen; TBA, total bile acids.
Primary end point
In the intention-to-treat population at week 48, the least-squares (LS) mean change from baseline in ELF was an increase of 0.2 (SE 0.1) in the aldafermin 1 mg group and a decrease of 0.1 (SE 0.1) in the aldafermin 3 mg group, as compared with an increase of 0.3 (SE 0.1) in the placebo group. The least-squares mean difference between the 3 mg group and the placebo group was a decrease of 0.5 (95% CI, −0.7 to −0.2; p=0.0003), and the difference in this change between the 1 mg group and the placebo group was a decrease of 0.1 (95% CI, −0.4 to 0.1; p = 0.31) (Table 2 and Figure 2A). Similar results were obtained with sensitivity analysis (appendix p8, http://links.lww.com/HEP/I13) and subgroup analysis by baseline ELF value and diabetes status (appendix p12-13, http://links.lww.com/HEP/I13). Since randomization into the aldafermin 0.3 mg group was discontinued during the global COVID-19 pandemic to limit patients’ exposure to lower, suboptimal dose,20 there was no statistical comparison being done between the aldafermin 0.3 mg group and the placebo group, and aldafermin 0.3 mg data were included in safety analysis only. Dose-dependent decreases were also seen in individual components of ELF: hyaluronic acid, PIIINP, and tissue inhibitor of matrix metalloproteinase 1 (Figure 2B-D), and at an earlier time point at week 24 (appendix p14, http://links.lww.com/HEP/I13).
TABLE 2.
Change from baseline in the primary and secondary outcome measures at week 48 in the ITT population
| Placebo (n = 56) | Aldafermin 1 mg (n = 42) | Aldafermin 3 mg (n = 55) | |
|---|---|---|---|
| Primary end point | |||
| ELF score | 0.3 (0.1) | 0.2 (0.1) | −0.1 (0.1) |
| Difference vs. placebo | — | −0.1 (0.1) | −0.5 (0.1) |
| p value vs. placebo | — | 0.31 | 0.0003 |
| Individual components of ELF | |||
| Hyaluronic acid, ng/mL | 44.1 (23.8) | 46.5 (27.1) | 29.0 (24.9) |
| Difference vs. placebo | — | 2.4 (32.7) | −15.1 (31.0) |
| p value vs. placebo | — | 0.94 | 0.63 |
| PIIINP, ng/mL | 3.2 (1.1) | 0.7 (1.3) | −2.7 (1.2) |
| Difference vs. placebo | — | −2.5 (1.7) | −5.9 (1.6) |
| p value vs. placebo | — | 0.14 | 0.0003 |
| TIMP-1, ng/mL | 17.8 (13.2) | 6.8 (15.3) | −14.9 (13.8) |
| Difference vs. placebo | — | −11.0 (18.7) | −32.7 (17.8) |
| p value vs. placebo | — | 0.56 | 0.068 |
| Secondary end points | |||
| Liver histology | |||
| Fibrosis improvement of ≥ 1 stage (NASH CRN criteria) | 15% | 21% | 23% |
| Difference vs. placebo (95% CI) | — | 7% (−10 to 24) | 8% (−8 to 23) |
| p value vs. placebo | — | 0.39 | 0.36 |
| Fibrosis improvement of ≥ 1 stage without NASH worsening | 13% | 16% | 20% |
| Difference vs. placebo (95% CI) | — | 4% (−11 to 20) | 7% (−8 to 22) |
| p value vs. placebo | — | 0.54 | 0.37 |
| Markers of target engagement | |||
| C4, ng/mL | −10.2 (2.8) | −33.8 (3.3) | −37.7 (3.0) |
| Difference vs. placebo | −23.6 (4.1) | −27.5 (3.9) | |
| p value vs. placebo | <0.0001 | <0.0001 | |
| C4, %, relative | −1.8% (7.7) | −66.8% (9.4) | −73.5% (8.5) |
| Difference vs. placebo | −65.1% (11.6) | −71.8% (11.0) | |
| p value vs. placebo | <0.0001 | <0.0001 | |
| TBA, µmol/L | 0.9 (1.8) | −4.7 (2.1) | −5.4 (1.9) |
| Difference vs. placebo | −5.6 (2.4) | −6.3 (2.2) | |
| p value vs. placebo | 0.022 | 0.0053 | |
| TBA, %, relative | 31.7% (14.7) | −35.5% (17.4) | −50.5% (15.4) |
| Difference vs. placebo | −67.3% (19.5) | −82.3% (18.1) | |
| p value vs. placebo | 0.0008 | <0.0001 | |
| Liver enzymes | |||
| ALT, U/L | −5.7 (2.3) | −19.2 (2.6) | −22.7 (2.4) |
| Difference vs. placebo | −13.5 (3.3) | −17.0 (3.1) | |
| p value vs. placebo | 0.0001 | <0.0001 | |
| ALT, %, relative | −6.3% (4.0) | −35.9% (4.7) | −41.5% (4.3) |
| Difference vs. placebo | −29.6% (5.8) | −35.2% (5.6) | |
| p value vs. placebo | <0.0001 | <0.0001 | |
| AST, U/L | −2.4 (2.1) | −9.7 (2.5) | −13.9 (2.3) |
| Difference vs. placebo | −7.3 (3.0) | −11.6 (2.9) | |
| p value vs. placebo | 0.018 | 0.0001 | |
| AST, %, relative | −0.4% (4.5) | −19.2% (5.2) | −28.3% (4.7) |
| Difference vs. placebo | −18.8% (6.4) | −27.9% (6.1) | |
| p value vs. placebo | 0.0043 | <0.0001 | |
| Fibrogenesis marker | |||
| Pro-C3, ng/mL | 12.7 (6.2) | −9.3 (7.2) | −13.1 (6.6) |
| Difference vs. placebo | −22.1 (9.1) | −25.9 (8.7) | |
| p value vs. placebo | 0.017 | 0.0034 | |
| Pro-C3, %, relative | 47.6% (19.2) | −6.3% (22.3) | −12.1% (20.3) |
| Difference vs. placebo | −54.0% (29.2) | −59.7% (27.6) | |
| p value vs. placebo | 0.067 | 0.032 | |
| Liver stiffness by FibroScan | |||
| LSM, kPa | 0.9 (1.7) | −3.2 (1.9) | −1.3 (1.8) |
| Difference vs. placebo | — | −4.1 (2.3) | −2.3 (2.2) |
| p value vs. placebo | — | 0.078 | 0.32 |
| LSM, %, relative | 15.0% (10.1) | −15.1% (11.7) | −6.3% (11.0) |
| Difference vs. placebo | — | −30.1% (14.2) | −21.3% (13.7) |
| p value vs. placebo | — | 0.036 | 0.12 |
Values are LS mean (SE) or proportions of patients (%).
Fibrosis improvement was defined as ≥1 stage decrease in NASH CRN fibrosis score; no worsening of NASH was defined as no increase in NAS for ballooning, no increase in inflammation, and no increase in steatosis.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; C4, 7alpha-hydroxy-4-cholesten-3-one; ELF, Enhanced Liver Fibrosis; ITT, intention-to-treat; LS, least-squares; LSM, liver stiffness measure; NAS, NAFLD activity score; NASH CRN, NASH clinical research network; PIIINP, N-terminal pro-peptide of type III collagen; Pro-C3, neoepitope-specific N-terminal pro-peptide of type III collagen; TBA, total bile acids.
FIGURE 2.
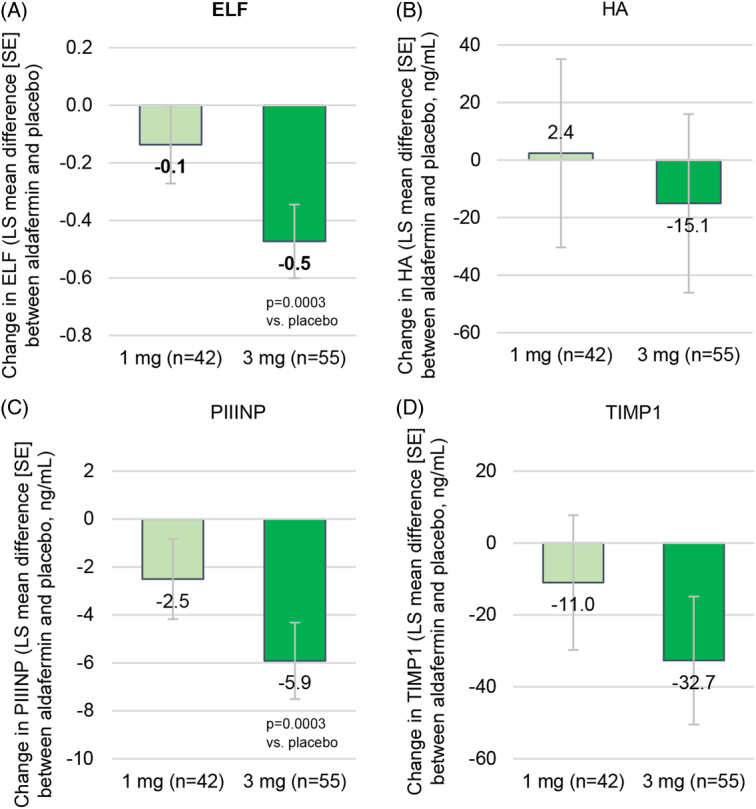
Primary end point. (A) Change from baseline in ELF score at week 48. (B–D) Change from baseline to week 48 in the individual components of ELF: HA (B), PIIINP (C), and TIMP-1 (D). Shown are LS mean differences between the aldafermin group (1 or 3 mg) and the placebo group. Enrollment in the aldafermin 0.3 mg group was discontinued during the trial. Abbreviations: ELF, Enhanced Liver Fibrosis; HA, hyaluronic acid; LS, least-squares; PIIINP, N-terminal pro-peptide of type III collagen.
Secondary end points
Overall 132 (83%) patients had biopsies at both baseline and end-of-treatment (week 48). At week 48, the percentage of patients who achieved fibrosis improvement of at least 1 stage was numerically higher in the aldafermin groups than in the placebo group [15%, 21%, and 23% in the placebo, 1 mg and 3 mg groups, respectively; with an OR of 1.7 (95% Cl, 0.5 to 5.8) for aldafermin 1 mg and 1.7 (95% CI, 0.6 to 4.8) for aldafermin 3 mg] (Table 2 and Figure 3A). Thirteen percent, 16%, and 20% in the placebo, 1 mg, and 3 mg groups, respectively, achieved fibrosis improvement without NASH worsening (Table 2 and Figure 3B). Sensitivity analyses produced similar results (appendix p9, http://links.lww.com/HEP/I13).
FIGURE 3.
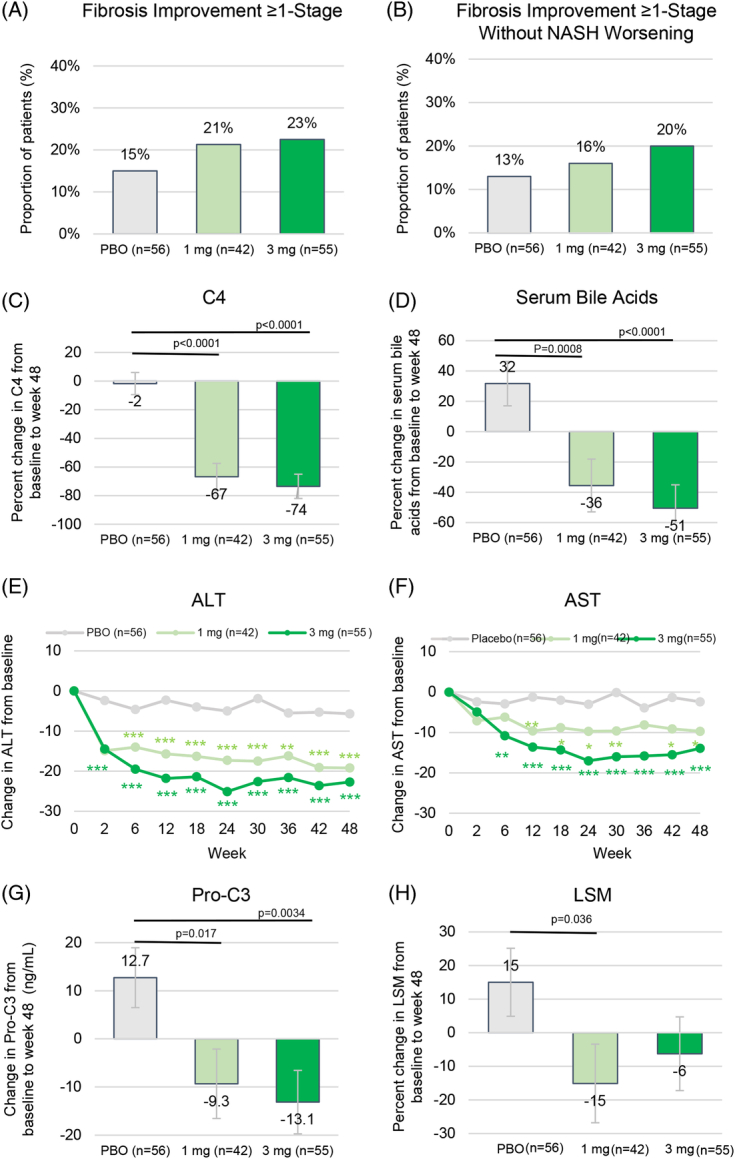
Secondary end points. (A) Proportion of patients achieving fibrosis improvement of ≥ 1 stage at week 48. (B) Proportion of patients achieving fibrosis improvement of ≥ 1 stage with no worsening of NASH at week 48. (C) Change in serum C4 from baseline to week 48. (D) Change in serum TBA from baseline to week 48. (E) Change from baseline in ALT over time. (F) Change from baseline in AST over time. (G) Change in Pro-C3 from baseline to week 48. (H) Change in LSM from baseline to week 48. Shown are LS mean (SE). Fibrosis improvement of ≥ 1 stage was defined by NASH CRN criteria. No worsening of NASH was defined as no increase in NAS score for steatosis, no increase in ballooning, and no increase in inflammation. *p<0.05, **p<0.01, ***p<0.001 vs. placebo. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; C4, 7alpha-hydroxy-4-cholesten-3-one; LS, least-squares; LSM, liver stiffness measure; NAS, NAFLD activity score; NASH CRN, NASH clinical research network; PBO, placebo; Pro-C3, neoepitope-specific N-terminal pro-peptide of type III collagen; TBA, total bile acids.
Treatment with aldafermin led to a marked, dose-depended decrease in serum levels of C4, an intermediate in the bile acid synthetic pathway, and a marker of target engagement (Table 2 and Figure 3C). The LS mean differences in percent change in C4 levels at week 48 between the aldafermin groups and the placebo group were −65.1% (SE 11.6; p < 0.0001) at the 1 mg dose and −71.8% (SE 11.0; p < 0.0001) at the 3 mg dose. Similar results were observed in serum total bile acids, with −67.3% (SE 19.5; p = 0.0008) at the 1 mg dose and −82.3% (SE 18.1; p < 0.0001) at the 3 mg dose (Table 2 and Figure 3D).
Dose-dependent reductions in levels of ALT and AST were observed with aldafermin (Table 2 and Figure 3E-F). Reductions in both ALT and AST were greater with aldafermin than with placebo (LS mean difference, −13.5 U/L and −17.0 U/L in ALT in the 1 mg and 3 mg groups, respectively; −7.3 U/L and −11.6 U/L in AST; p <0.001 for all comparisons). Levels of alkaline phosphatase and total bilirubin remained unchanged (appendix p10, http://links.lww.com/HEP/I13).
Treatment with aldafermin resulted in dose-dependent reductions in the fibrogenesis marker Pro-C3 (Table 2 and Figure 3G). At week 48, the LS mean change from baseline in Pro-C3 was a decrease of 9.3 ng/mL in the aldafermin 1 mg group, a decrease of 13.1 ng/mL in the aldafermin 3 mg group, as compared with an increase of 12.7 ng/mL in the placebo group (p = 0.017 and p = 0.0034 vs. placebo in the 1 mg and 3 mg groups, respectively).
Liver stiffness, measured by transient elastography, revealed a reduction with aldafermin treatment. The LS mean difference between the aldafermin 1 mg group and the placebo group in the percent change from baseline to week 48 in LSM was −30.1% (p = 0.036), and the difference between the aldafermin 3 mg group and the placebo group was −21.3% (p = 0.12) (Figure 3H). At week 48, 10%, 23%, and 22% of patients in the placebo, 1 mg and 3 mg groups, respectively, achieved a decrease from baseline of >10 kPa in LSM; 29%, 37%, and 32% achieved a decrease from baseline of >5 kPa in LSM.
Lipids
Aldafermin was associated with increases from baseline to week 2 in LDL-C and total cholesterol levels that were greater than those in the placebo group, consistent with the on-target inhibition of CYP7A1, the rate-limiting step in the conversion of cholesterol to bile acids. Over-encapsulated rosuvastatin/placebo was dispensed by an independent medical monitor according to the protocol-specified lipid management algorithm. At week 48, LDL-C levels were well managed and below baseline in all groups (appendix p10, http://links.lww.com/HEP/I13). Forty-nine (88%) of 56 patients in the placebo group, 36 (86%) of 42 patients in the 1 mg group, and 43 (78%) of 55 patients in the 3 mg group were receiving rosuvastatin at week 48. Numeric decreases in triglycerides were observed in the aldafermin groups. No decreases in HDL cholesterol levels were seen with aldafermin.
Cardiovascular and liver-related events
Three independent, external adjudication committees adjudicated cardiovascular events, liver events, and HCC in this 48-week study (appendix p3-4, http://links.lww.com/HEP/I13). Two (1%) patients had cardiovascular events confirmed by the cardiovascular event-adjudication committee: 1 patient had a sudden cardiac death after receiving aldafermin 1 mg for 1 week (unrelated to the drug); 1 patient in the 1 mg group had a 4-vessel coronary artery bypass grafting procedure due to pre-existing disease (unrelated to drug). No patient had ascites, jaundice, variceal bleeding, varices requiring treatment, HE of grade 2 or above according to the West Haven criteria, or liver transplantation during the study period. No HCC was observed in the study.
Safety
Overall, 5 (71%) of 7 patients who received aldafermin 0.3 mg, 41 (98%) of 42 who received aldafermin 1 mg, 52 (95%) of 55 who received 3 mg, and 49 (88%) of 56 patients who received placebo reported AEs, most of which were mild or moderate in severity (Table 3). Treatment-related AEs led to discontinuation in none (0%), 1 (2%), and 5 (9%) patients in the aldafermin 0.3 mg, 1 mg, and 3 mg groups, respectively, and none (0%) in the placebo group.
TABLE 3.
Summary of adverse events
| Placebo (n = 56) | Aldafermin 0.3 mg (n = 7) | Aldafermin 1 mg (n = 42) | Aldafermin 3 mg (n = 55) | |
|---|---|---|---|---|
| Treatment-emergent AEs | ||||
| Overall | 49 (88) | 5 (71) | 41 (98) | 52 (95) |
| Grade 1 | 17 (30) | 1 (14) | 11 (26) | 17 (31) |
| Grade 2 | 31 (55) | 3 (43) | 20 (48) | 29 (53) |
| Grade 3 | 1 (2) | 1 (14) | 9 (21) | 5 (9) |
| Grade 4 | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Grade 5 | 0 (0) | 0 (0) | 1 (2) | 0 (0) |
| Treatment-related AEs, n (%) | 22 (39) | 3 (43) | 30 (71) | 40 (73) |
| Treatment-related AEs leading to study drug discontinuation, n (%) | 0 (0) | 0 (0) | 1 (2) | 5 (9) |
| Serious AEs, n (%) | 3 (5) | 0 (0) | 11 (26) | 5 (9) |
| Most common (≥10%a) treatment-emergent AEs | ||||
| COVID-19 | 17 (30) | 1 (14) | 14 (33) | 13 (24) |
| Diarrhea | 10 (18) | 1 (14) | 11 (26) | 22 (40) |
| Nausea | 5 (9) | 2 (29) | 12 (29) | 18 (33) |
| Increased appetite | 0 (0) | 0 (0) | 2 (5) | 8 (15) |
| Headache | 5 (9) | 0 (0) | 4 (10) | 8 (15) |
| Fatigue | 5 (9) | 0 (0) | 6 (14) | 7 (13) |
| Abdominal pain upper | 10 (18) | 0 (0) | 3 (7) | 7 (13) |
| Abdominal distension | 5 (9) | 1 (14) | 3 (7) | 6 (11) |
| Injection site bruising | 6 (11) | 1 (14) | 1 (2) | 4 (7) |
| Constipation | 7 (13) | 1 (14) | 5 (12) | 8 (15) |
| Urinary tract infection | 9 (16) | 2 (29) | 4 (10) | 4 (7) |
| Cough | 4 (7) | 1 (14) | 5 (12) | 2 (4) |
| Arthralgia | 6 (11) | 0 (0) | 5 (12) | 7 (13) |
| Procedure pain | 6 (11) | 0 (0) | 2 (5) | 4 (7) |
| Pruritus | 3 (5) | 0 (0) | 1 (2) | 6 (11) |
Notes: Values are n (%).
10% AEs in any of the placebo, 0.3 mg, 1 mg, or 3 mg groups are shown.
Treatment-emergent AEs were recorded from the time of screening until 6 weeks after the end of the placebo-controlled treatment period. AEs were graded according to the National Cancer Institute CTCAE, version 5.0.
Abbreviations: AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events.
Serious AE occurred in none (0%) of patients in the aldafermin 0.3 mg group, 11 (26%) patients in the aldafermin 1 mg group, 5 (9%) patients in the aldafermin 3 mg group, and 3 (5%) patients in the placebo group. Detailed information on the serious AEs is provided in the appendix (p11), http://links.lww.com/HEP/I13. None of the serious AE were deemed to be related to the study drug by the investigators.
Aside from COVID-19 (the trial was conducted during the global Covid-19 pandemic), the most frequent AEs were gastrointestinal events. Diarrhea occurred in 10 (18%) of 56 patients in the placebo group, 1 (14%) of 7 patients in the 0.3 mg aldafermin group, 11 (26%) of 42 patients in the 1 mg group, and 22 (40%) of 55 patients in the 3 mg group. The proportion of patients experiencing headache, fatigue, abdominal pain, injection site bruising, and constipation was similar across all groups (Table 3). No increases in bodyweight, glucose, insulin, or glycated hemoglobin were seen in the aldafermin groups (appendix p10, http://links.lww.com/HEP/I13). There were no clinically relevant changes in hematological measures, vital signs, physical examinations, or electrocardiograms.
DISCUSSION
In this phase 2b trial in patients with NASH and compensated cirrhosis, treatment with aldafermin at a dose of 3 mg resulted in greater reductions in ELF score than placebo over 48 weeks, meeting the primary outcome. Furthermore, markers of liver injury (ALT and AST) and fibrogenesis (Pro-C3) were reduced in a dose-dependent manner to a greater extent with aldafermin than with placebo. All secondary outcomes, including the percentage of patients who had an improvement in fibrosis of at least 1 stage, liver stiffness, and pharmacodynamic markers C4 and serum bile acids, generally favored aldafermin. Importantly, aldafermin was generally well tolerated in patients with compensated NASH cirrhosis.
ELF is the first and only FDA-approved noninvasive test reflecting NASH prognosis. ELF measures 3 direct markers of liver fibrosis: hyaluronic acid (a glycosaminoglycan that is produced by hepatic stellate cells), PIIINP (a marker of early fibrogenesis and inflammation), and tissue inhibitor of matrix metalloproteinase 1 (the circulating inhibitor of MMP enzymes that can enhance fibrogenesis). Multiple studies have shown that ELF is predictive of hepatic decompensation, liver transplant, liver cancer, and death in chronic liver diseases,26–28 and is superior to fibrosis-4 index, MELD score and Child-Pugh score for predicting the risk of liver-related events.26 The incidence of hepatic events increased significantly with increased ELF, and lower ELF scores were associated with a reduced risk of liver-related outcomes. In a recent analysis involving 1135 patients with compensated NASH cirrhosis, reduction in ELF relative to baseline, but not fibrosis-4 index or NAFLD fibrosis score, are associated with cirrhosis regression.13 A reduction in ELF of 0.5 is considered to be clinically meaningful and correlated with 1 stage improvement in fibrosis.13,27 In the current ALPINE-4 trial, aldafermin 3 mg achieved a treatment difference of −0.5 as compared to placebo in patients with NASH and compensated cirrhosis, which compares favorably to improvements in ELF noted in NASH cirrhosis trials (eg, −0.3 for semaglutide,11 −0.2 for pegbelfermin,10 +0.1 for belapectin,8 and no change for selonsertib7 or simtuzumab6). The primary end point result was supported by all secondary end points, and the fibrogenesis marker Pro-C3 in particular. In contrast to the ELF component PIIINP, which measures an internal epitope of type III collagen during fibrosis formation and degradation, Pro-C3 only detects fibrosis formation and thus reflects true fibrogenesis activity.29 Pro-C3 has been shown to predict clinical outcome in patients with advanced chronic liver diseases including NASH and compensated cirrhosis; a 2-fold increase in Pro-C3 was associated with 2.7-fold increased hazard of liver-related events.30 In ALPINE-4, LS mean differences versus placebo in the percent change in Pro-C3 were −54% and −60% in the aldafermin 1 mg and 3 mg groups, respectively, providing further support of ELF and a potentially more precise assessment of fibrogenic activity than ELF.
The incidence of gastrointestinal AEs was higher in the aldafermin groups than in the placebo group. Diarrhea occurred in 14%, 26%, and 40% patients in the aldafermin 0.3 mg, 1 mg, and 3 mg groups, respectively, compared with 18% in the placebo group; all were mild or moderate. No serious AE were attributed by the investigators to aldafermin. The increase in LDL-C in the aldafermin groups was due to on-target inhibition of bile acid synthesis, which was mitigated by co-administration of rosuvastatin.31 LDL increases related to aldafermin are largely driven by increases in less atherogenic, large buoyant LDL particles and respond very rapidly to statin co-administration. There was no signal for adverse cardiovascular events in this trial related to aldafermin.
The ALPINE-4 study has several strengths. This is the first positive, randomized controlled trial in late-stage development in compensated NASH cirrhosis. The primary end point ELF was measured at a rigorously qualified central laboratory; thus, variability and interpretation bias were minimized. All cardiovascular and liver-related outcomes were adjudicated by independent, external committees comprised of experienced experts who were unaware of the treatment group assignments. This trial enrolled a diverse, global population, with 25% of patients of Hispanic/Latino ethnicity, and patients with a clinical diagnosis of NASH cirrhosis were allowed to participate. Finally, this trial was conducted during the height of the global COVID-19 pandemic (the first patient was randomized on March 23, 2020, and shortly after, many companies announced a pause of clinical trials due to COVID-19) and included several pragmatic adaptations through protocol amendments to enable timely completion: magnetic resonance imaging-proton density fat fraction procedures were eliminated given the de-prioritization of elective procedures in the pandemic; esophagogastroduodenoscopy was waived in patients meeting the expanded Baveno VI criteria; randomization into the 0.3 mg dose group was discontinued to limit patients’ exposure to a lower, suboptimal dose; the primary end point was changed from histology on liver biopsy to the noninvasive measure ELF; together, these strategies accelerated recruitment and reduced the burden on patients despite the hardship imposed by the COVID-19 pandemic.
This study also has some limitations. First, although an ELF score of 11.3 or above is considered a poor prognosis and high risk for liver decompensation, liver transplant, and death, and an ELF score of 9.8 or lower indicates low risk, the regulatory recognition of ELF as a surrogate end point, as well as the threshold of a minimal clinically important difference, will require further clarification. Second, a biopsy panel read methodology has been recently proposed by the FDA to reduce inter-reader and intra-reader variability32; however, given the logistic complexity and practical issues surrounding the biopsy reading process, liver biopsy was read by a central reader blinded to treatment assignment and patient information in ALPINE-4 to avoid delays in trial enrollment. Third, this trial enrolled a well-compensated population with cirrhosis, thus had a low event rate as expected and thus a reduced statistical power from which to draw firm conclusions on the impact on outcomes.2 This is a vexing issue in pivotal trial design where the selection of patients with disease that may still be responsive to therapeutic intervention needs to be balanced with the regulatory requirement to show a positive impact on clinical outcomes, which would require a very large long-term trial, or the inclusion of patients with more advanced disease and less likely to respond to an intervention. A minimum score on ELF was not required for enrollment, which potentially limited our ability to enrich the higher-risk population. Fourth, the study was not powered for pairwise comparison of histologic end point of fibrosis improvement, though numerically greater placebo-subtracted response rate (delta) was seen with aldafermin 3 mg compared with NASH cirrhosis trials (delta of 8% for aldafermin, 3% for obeticholic acid, 1% for selonsertib,7 −2% for belapectin,8 −3% for pegbelfermin,10 and −18% for semaglutide11; negative delta indicating worse fibrosis response in the drug arm; only highest doses are shown). Further analysis of histological data to assess change in fibrosis burden on a linear, rather than categorical scale, is likely to provide additional confidence that ELF change and numerical improvement in fibrosis stage reflect a true improvement in fibrosis. Finally, liver stiffness was measured at local sites on various models of FibroScan devices with no central reading, which may cause variability in the LSM data.
Among patients with compensated NASH cirrhosis, aldafermin 3 mg reduced serum ELF levels and was associated with numerically greater improvement in the fibrosis stage than placebo at week 48. Larger and longer trials are required to determine the efficacy and safety of aldafermin in this disease. The trial underscores the need for additional approaches to demonstrate efficacy and ultimately improve outcomes in our most at-risk NASH population.
Supplementary Material
AUTHOR CONTRIBUTIONS
Mary E. Rinella, Hsiao D. Lieu, Arun J. Sanyal, Lei Ling, and Stephen A. Harrison participated in the study design. Mary E. Rinella, Kris V. Kowdley, Naim Alkhouri, Eric Lawitz, Vlad Ratziu, Manal F. Abdelmalek, Vincent Wai-Sun Wong, Ziad H. Younes, Aasim M. Sheikh, Donald Brannan, Bradley Freilich, Fernando Membreno, Marie Sinclair, Liza Melchor-Khan, and Stephen A. Harrison were responsible for data collection. Zachary D. Goodman performed the central reading of liver biopsy. Mary E. Rinella, Lei Ling, and Stephen A. Harrison participated in the data analysis. All authors participated in data interpretation, manuscript review, and writing and made the decision to submit the manuscript. Mary E. Rinella and Lei Ling had full access to the study data, were responsible for the preparation of the tables and figures, and vouch for the integrity of the data analyses.
ACKNOWLEDGMENTS
The authors thank all the patients who participated in this study, along with the study coordinators and staff of the participating clinical centers, for their support and assistance.
FUNDING INFORMATION
This study was funded by NGM Biopharmaceuticals.
CONFLICTS OF INTEREST
Mary E. Rinella consults for Boehringer Ingelheim, CytoDyn, GlaxoSmithKline, HistoIndex, Intercept, Madrigal, NGM Bio, Novo Nordisk, and Sonic Incytes. Hsiao D. Lieu is employed by and owns stock in NGM Bio. Kris V. Kowdley consults, is on the speakers’ bureau, and received grants from Gilead and Intercept. He consults and received grants from 89Bio, CymaBay, GENFIT, HighTide, Madrigal, Mirum, NGM Bio, Novo Nordisk, Pfizer, Terns, and Zydus. He consults and owns stock in Inipharm. He consults for Boehringer Ingelheim, Ipsen, and Kowa. He is on the speakers’ bureau for AbbVie, Gilead, and Intercept. He received grants from Boston Pharma, Corcept, GlaxoSmithKline, Hanmi, and Viking. Zachary D. Goodman advises, is on the speakers’ bureau, and received grants from Gilead, Intercept, and Perspectum. He advises and is on the speaker’s bureau for Echosens. He advises and received grants from 89Bio, Madrigal, Novo Nordisk, Pfizer, and Zydus. He is on the speakers’ bureau and received grants from AbbVie. He advises Fibronostics. He is on the speaker’s bureau for Alexion, Eisai, Excelixis, and Theratechnologies. He received grants from Akero, Arbutus, Better Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Corcept, CymaBay, DSM, Galectin, Genentech, Hepagene, Healio, Inventiva, Ionis, Merck, NGM Bio, Noom, NorthSea, Novartis, Poxel, and Viking. Naim Alkhouri consults, advises, is on the speakers’ bureau, and received grants from Gilead, Intercept, Madrigal, Novo Nordisk, Perspectum, Pfizer, and Zydus. He consults, advises, and is on the speakers’ bureau for Echosens. He consults, is on the speakers’ bureau, and received grants from AbbVie. He consults and is on the speakers’ bureau for Allergan. He advises and received grants from 89Bio. He consults for Fibronostics. He advises Fibronostics. He is on the speakers’ bureau for Alexion, Eisai, Excelixis, Salix, and Thera. He received grants from Akero, Arbutus, Better Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Corcept, DSM, Galectin, Genentech, GENFIT, Hepagene, Healio, Inventiva, Ionis, Merck, NGM Bio, Noom, NorthSea, Poxel, and Viking. Eric Lawitz consults, is on the speakers’ bureau, and received grants from Intercept. He consults for and received grants from Akero, Boehringer Ingelheim, Bristol Myers Squibb, Metacrine, Novo Nordisk, Sagimet, and Terns. He is on the speakers’ bureau and received grants from Abbvie and Gilead. He received grants from 89Bio, Allergan, Alnylam, Amgen, Ascelia, Assembly, AstraZeneca, Axcella, BioCryst, Bird Rock Bio, Conatus, CymaBay, CytoDyn, DSM, Durect, Eli Lilly, Enanta, Enyo, Exhalenz, Galectin, Galmed, GENFIT, Genentech, GlaxoSmithKline, Hanmi, HighTide, Inventiva, Janssen, Laboratory for Advanced Medicine, Loxo Oncology, Madrigal, Merck, NGM Bio, NorthSea, Novartis, Pfizer, Poxel, Roche, Synlogic, Takeda, Viking, and Zydus. Vlad Ratziu consults for Boehringer Ingelheim, Enyo, Madrigal, NGM Bio, NorthSea, Novo Nordisk, Pfizer, Poxel, Sagimet, and Terns. He consults and received grants from Gilead and Intercept. Manal F. Abdelmalek consults, advises, and received grants from Hanmi. He advises and received grants from 89Bio, Intercept, Inventiva, Madrigal, NGM Bio, and Novo Nordisk. He advises Medscape, Merck, Sonic Incytes, and Theratechnologies. He serves on the speakers’ bureaus for Chronic Liver Disease Foundation, Clinical Care Options, Fishwack, and Terra Firma. He received grants from Allergan, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Durect, Enanta, Enyo, Galmed, Gilead, Poxel, TARGET-NASH, and Viking. Vincent Wai-Sun Wong consults, serves on the speakers’ bureaus, received grants from Gilead. He consults and serves on the speakers’ bureaus AbbVie, Novo Nordisk. He consults for Boehringer Ingelheim, Echosens, Intercept, Inventiva, Pfizer, Sagimet, TARGET, and Visirna. He is on the speakers’ bureau for Abbott and Unilab. He owns stock in Illuminatio Medical Technology. Ziad H. Younes consults, advises, is on the speakers’ bureau, and received grants from Intercept. He consults and received grants from Madrigal. He consults for Gilead. He received grants from AbbVie, Allergan, Bristol Myers Squibb, CymaBay, Gilead, HighTide, Inventiva, NGM Bio, Novartis, Novo Nordisk, NST, and Poxel. Aasim M. Sheikh received grants from Akero, Altimmune, AstraZeneca, Boehringer Ingelheim, Galectin, Gilead, Hepion, Intercept, Ionis, KOWA, Madrigal, NGM, Novo Nordisk, and Viking. Fernando Membreno received grants from Akero, Ionis, and Madrigal. He is on the speakers’ bureau for Gilead. Liza Melchor-Khan is employed by and owns stock in NGM Bio. Arun J. Sanyal consults and received grants from AstraZeneca, Boehringer Ingelheim, Covance, Eli Lilly, Intercept, Novartis, Novo Nordisk, Pfizer, Salix, and Sequana. He consults and owns stock in GENFIT and Hemoshear. He consults for 89Bio, Akero, Albireo, Alnylam, Amgen, BioCellvia, Fibronest, Fractyl, Genentech, Gilead, GlaxoSmithKline, HistoIndex, Intercept, Inventiva, Jansen, Madrigal, Mallinckrodt, Merck, NGM Bio, NorthSea, PathAI, Poxel, Prosciento, Regeneron, Roche, Siemens, Takeda, TARGET, and Terns. He is employed by and owns stock in Sanyal Bio. He consults and owns stock in NorthSea. He advises Immuron. He received grants from Bristol Myers Squibb, Conatus, Galectin, and Merck. He owns stock in Durect, Exhalenz, GENFIT, Hemoshear, Indalo, Inversago, Rivus, and Tiziana. He has other interests with Echosens. Lei Ling is employed by and owns stock in NGM Bio. Stephen A. Harrison consults, advises, received grants, and owns stock in Akero, Galectin, Hepion, and NorthSea. He consults, advises, and received grants from Altimmune, Enyo, Gilead, GlaxoSmithKline, Intercept, Madrigal, Pfizer, Poxel, Sagimet, and Viking. He consults, advises, and owns stock in ChronWell, Hepta Bio, HistoIndex, and Sonic Incytes. He consults and advises Aligos, Arrowhead, Bluejay, Boxer Capital, Echosens, Foresite, Galecto, Hepagene, Humana, Ionis, Medpace, MGGM, Neurobo, Perspectum, and Terns. He consults and received grants from Novo Nordisk. He received grants and owns stock in GENFIT and NGM Bio. He received grants from Axcella, Bristol Myers Squibb, Corcept, CymaBay, Genentech, Hightide, Immuron, Inventiva, Ionis, Novartis, Novo Nordisk, Poxel, and Terns. He owns stock in Cirius, and Metacrine. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; C4, 7alphahydroxy-4-cholesten-3-one; ELF, Enhanced Liver Fibrosis; LDL-C, LDL cholesterol; LS, least-squares; LSM, liver stiffness measure; NASH CRN, NASH clinical research network; PIIINP, N-terminal pro-peptide of type III collagen; Pro-C3, neoepitope-specific N-terminal pro-peptide of type III collagen.
ClinicalTrials.gov number: NCT04210245
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepjournal.com.
Contributor Information
Mary E. Rinella, Email: mrinella@bsd.uchicago.edu.
Hsiao D. Lieu, Email: hlieu@ngmbio.com.
Kris V. Kowdley, Email: kkowdley@liverinstitutenw.org.
Zachary D. Goodman, Email: zackgoodman@msn.com.
Naim Alkhouri, Email: nalkhouri@azliver.com.
Eric Lawitz, Email: lawitz@txliver.com.
Vlad Ratziu, Email: vlad.ratziu@inserm.fr.
Manal F. Abdelmalek, Email: abdelmalek.manal@mayo.edu.
Vincent Wai-Sun Wong, Email: wongv@cuhk.edu.hk.
Ziad H. Younes, Email: zyounes@gastro1.com.
Aasim M. Sheikh, Email: asheikh@gigeorgia.com.
Donald Brannan, Email: dbrannan.research@gi.md.
Bradley Freilich, Email: brad@kcgastro.com.
Fernando Membreno, Email: f.membreno@dhr-rgv.com.
Marie Sinclair, Email: marie.sinclair@austin.org.au.
Liza Melchor-Khan, Email: lmelchorkhan@ngmbio.com.
Arun J. Sanyal, Email: arun.sanyal@vcuhealth.org.
Lei Ling, Email: lling@ngmbio.com.
Stephen A. Harrison, Email: stephenharrison87@gmail.com.
REFERENCES
- 1. Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–2224. [DOI] [PubMed] [Google Scholar]
- 2. Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med. 2021;385:1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am J Gastroenterol. 2018;113:1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896–904. [DOI] [PubMed] [Google Scholar]
- 5. Huang DQ, Terrault NA, Tacke F, Gluud LL, Arrese M, Bugianesi E, et al. Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20:388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harrison SA, Abdelmalek MF, Caldwell S, Shiffman ML, Diehl AM, Ghalib R, et al. Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155:1140–1153. [DOI] [PubMed] [Google Scholar]
- 7. Harrison SA, Wong VW, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J Hepatol. 2020;73:26–39. [DOI] [PubMed] [Google Scholar]
- 8. Chalasani N, Abdelmalek MF, Garcia-Tsao G, Vuppalanchi R, Alkhouri N, Rinella M, et al. Effects of Belapectin, an Inhibitor of Galectin-3, in Patients With Nonalcoholic Steatohepatitis With Cirrhosis and Portal Hypertension. Gastroenterology. 2020;158:1334–1345 e1335. [DOI] [PubMed] [Google Scholar]
- 9. Garcia-Tsao G, Bosch J, Kayali Z, Harrison SA, Abdelmalek MF, Lawitz E, et al. Randomized placebo-controlled trial of emricasan for non-alcoholic steatohepatitis-related cirrhosis with severe portal hypertension. J Hepatol. 2020;72:885–895. [DOI] [PubMed] [Google Scholar]
- 10. Abdelmalek MF, Sanyal AJ, Nakajima A, Neuschwander-Tetri BA, Goodman ZD, Lawitz EJ, et al. Pegbelfermin in Patients With Nonalcoholic Steatohepatitis and Compensated Cirrhosis (FALCON 2): A Randomized Phase 2b Study. Clin Gastroenterol Hepatol. 2023:S1542-3565(23)00311-7. doi: 10.1016/j.cgh.2023.04.012. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 11. Loomba R, Abdelmalek MF, Armstrong MJ, Jara M, Kjaer MS, Krarup N, et al. Semaglutide 2.4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: A randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FDA . Nonalcoholic Steatohepatitis with Compensated Cirrhosis: Developing Drugs for Treatment Guidance for Industry. 2019.
- 13. Sanyal AJ, Anstee QM, Trauner M, Lawitz EJ, Abdelmalek MF, Ding D, et al. Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis. Hepatology. 2022;75:1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davison BA, Harrison SA, Cotter G, Alkhouri N, Sanyal A, Edwards C, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020;73:1322–1332. [DOI] [PubMed] [Google Scholar]
- 15. Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. European Association for the Study of the Liver . Electronic address eee, Clinical Practice Guideline P, Chair, representative EGB, Panel m. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659–689. [DOI] [PubMed] [Google Scholar]
- 17. Zhou M, Wang X, Phung V, Lindhout DA, Mondal K, Hsu JY, et al. Separating Tumorigenicity from Bile Acid Regulatory Activity for Endocrine Hormone FGF19. Cancer Res. 2014;74:3306–3316. [DOI] [PubMed] [Google Scholar]
- 18. DePaoli AM, Zhou M, Kaplan DD, Hunt SC, Adams TD, Learned RM, et al. FGF19 Analog as a Surgical Factor Mimetic That Contributes to Metabolic Effects Beyond Glucose Homeostasis. Diabetes. 2019;68:1315–1328. [DOI] [PubMed] [Google Scholar]
- 19. Smirnova E, Muthiah MD, Narayan N, Siddiqui MS, Puri P, Luketic VA, et al. Metabolic reprogramming of the intestinal microbiome with functional bile acid changes underlie the development of NAFLD. Hepatology. 2022;76:1811–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanyal AJ, Ling L, Beuers U, DePaoli AM, Lieu HD, Harrison SA, et al. Potent suppression of hydrophobic bile acids by aldafermin, an FGF19 analogue, across metabolic and cholestatic liver diseases. JHEP Rep. 2021;3:100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harrison SA, Rinella ME, Abdelmalek MF, Trotter JF, Paredes AH, Arnold HL, et al. NGM282 for treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2018;391:1174–1185. [DOI] [PubMed] [Google Scholar]
- 22. Harrison SA, Neff G, Guy CD, Bashir MR, Paredes AH, Frias JP, et al. Efficacy and Safety of Aldafermin, an Engineered FGF19 Analog, in a Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Nonalcoholic Steatohepatitis. Gastroenterology. 2021;160:219–231 e211. [DOI] [PubMed] [Google Scholar]
- 23. Harrison SA, Abdelmalek MF, Neff G, Gunn N, Guy CD, Alkhouri N, et al. Aldafermin in patients with non-alcoholic steatohepatitis (ALPINE 2/3): A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol. 2022;7:603–616. [DOI] [PubMed] [Google Scholar]
- 24. Noureddin M, Chan JL, Barradas K, Dimick-Santos L, Schabel E, Omokaro SO, et al. Attribution of Nonalcoholic Steatohepatitis as an Etiology of Cirrhosis for Clinical Trials Eligibility: Recommendations From the Multi-stakeholder Liver Forum. Gastroenterology. 2020;159:422–427 e421. [DOI] [PubMed] [Google Scholar]
- 25. Petta S, Sebastiani G, Bugianesi E, Vigano M, Wong VW, Berzigotti A, et al. Non-invasive prediction of esophageal varices by stiffness and platelet in non-alcoholic fatty liver disease cirrhosis. J Hepatol. 2018;69:878–885. [DOI] [PubMed] [Google Scholar]
- 26. Are VS, Vuppalanchi R, Vilar-Gomez E, Chalasani N. Enhanced Liver Fibrosis Score Can Be Used to Predict Liver-Related Events in Patients With Nonalcoholic Steatohepatitis and Compensated Cirrhosis. Clin Gastroenterol Hepatol. 2021;19:1292–1293 e1293. [DOI] [PubMed] [Google Scholar]
- 27. Sanyal AJ, Harrison SA, Ratziu V, Abdelmalek MF, Diehl AM, Caldwell S, et al. The Natural History of Advanced Fibrosis Due to Nonalcoholic Steatohepatitis: Data From the Simtuzumab Trials. Hepatology. 2019;70:1913–1927. [DOI] [PubMed] [Google Scholar]
- 28. Trembling PM, Apostolidou S, Gentry-Maharaj A, Parkes J, Ryan A, Tanwar S, et al. The Enhanced Liver Fibrosis test is associated with liver-related outcomes in postmenopausal women with risk factors for liver disease. BMC Gastroenterol. 2020;20:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nielsen MJ, Nedergaard AF, Sun S, Veidal SS, Larsen L, Zheng Q, et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res. 2013;5:303–315. [PMC free article] [PubMed] [Google Scholar]
- 30. Nielsen MJ, Dolman GE, Harris R, Frederiksen P, Chalmers J, Grove JI, et al. PRO-C3 is a predictor of clinical outcomes in distinct cohorts of patients with advanced liver disease. JHEP Rep. 2023;5:100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rinella ME, Trotter JF, Abdelmalek MF, Paredes AH, Connelly MA, Jaros MJ, et al. Rosuvastatin improves the FGF19 analogue NGM282-associated lipid changes in patients with non-alcoholic steatohepatitis. J Hepatol. 2019;70:735–744. [DOI] [PubMed] [Google Scholar]
- 32. Anania FA, Dimick-Santos L, Mehta R, Toerner J, Beitz J. Nonalcoholic Steatohepatitis: Current Thinking From the Division of Hepatology and Nutrition at the Food and Drug Administration. Hepatology. 2021;73:2023–2027. [DOI] [PubMed] [Google Scholar]