Abstract
Our world is undergoing rapid planetary changes driven by human activities, often mediated by economic incentives and resource management, affecting all life on Earth. Concurrently, many infectious diseases have recently emerged or spread into new populations. Mounting evidence suggests that global change—including climate change, land-use change, urbanization, and global movement of individuals, species, and goods—may be accelerating disease emergence by reshaping ecological systems in concert with socioeconomic factors. Here, we review insights, approaches, and mechanisms by which global change drives disease emergence from a disease ecology perspective. We aim to spur more interdisciplinary collaboration with economists and identification of more effective and sustainable interventions to prevent disease emergence. While almost all infectious diseases change in response to global change, the mechanisms and directions of these effects are system specific, requiring new, integrated approaches to disease control that recognize linkages between environmental and economic sustainability and human and planetary health.
Keywords: climate change, emerging infectious diseases, global change, land-use change, spillover, urbanization
1. INTRODUCTION
Emerging infectious diseases (EIDs) pose a threat to humans, domestic animals, and wildlife (Woolhouse & Gowtage-Sequeria 2005). In recent decades, the interconnectedness between global change—including climate change, land-use change, and increased global mobility— and infectious disease emergence and spread has become a more widely accepted phenomenon (Barnosky et al. 2012, Daily & Ehrlich 1996, Myers 2017, Plowright et al. 2021). Concurrently, we have witnessed an increased rate of emergence of novel infectious diseases [e.g., Hendra, Nipah, severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), swine flu (influenza A virus H1N1), and COVID-19], establishment of diseases in new geographic regions (e.g., West Nile, Zika, dengue, yellow fever, and Lyme), and a resurgence of previously controlled diseases (e.g., dengue, Ebola, malaria, and hantavirus diseases). The ongoing COVID-19 pandemic that continues to disrupt daily life, in addition to the escalating climate crisis, raises two critical questions: Why are diseases emerging and expanding so rapidly? How can we prevent future planetary health crises?
Emerging pathogens, like any invasive species, go through four distinct stages of invasion: introduction, establishment, spread, and impact (Levine 2008). For pathogens specifically, the process by which a pathogen spills over into a novel host population using the invasive species framework is as follows (Plowright et al. 2021). Introduction is when a novel species is exposed to a pathogen from a different species (usually a pathogen reservoir species). Establishment occurs when the novel species becomes infected. Next, spread occurs when the novel species is able to transmit the pathogen among individuals of the same species. Finally, impact is when pathogen transmission in the novel species causes outbreaks, either locally (epidemics) or globally (pandemics). Although there are numerous EIDs currently circulating in domestic animals and wildlife that do not infect humans, we focus on diseases that can. However, we acknowledge that EIDs in animal and plant populations are a concern, with potential implications for human health as well (Myers 2017).
Despite being unsustainable in its current form, postindustrial economic development has undoubtedly facilitated technological and scientific breakthroughs. At the same time, these advances have allowed mainstream Western research to finally illuminate the unsustainable nature of today’s resource management and the intricate connections between global change and EIDs, which other cultures have long recognized (Glidden et al. 2021, Griffin 2009). Although disease prevention practices are underway to prevent or mitigate EID outbreaks on local scales (Glidden et al. 2021, Jones et al. 2020), the future of our planet and human health would benefit from sustainable resource management and equitable economic development at a global scale (Myers 2017). Major knowledge gaps remain, especially concerning the outcomes of proposed solutions to prevent or mitigate EIDs (Myers 2017). We need to acquire a better understanding of the underlying disease ecology, understand how it intersects with socioeconomics and resource economics to guide science-based policy, and develop solutions that are simultaneously feasible, sustainable, nature-based, and society-based (Myers 2017).
As EIDs may be attributed to global change (Daily & Ehrlich 1996, Myers 2017), and global change is often driven by economic incentives, it is imperative that economists, epidemiologists, and disease ecologists work together to identify key mechanisms linking global change to disease emergence and develop economic frameworks that value planetary health outcomes. Here, we provide a review of the evidence of proposed mechanisms by which key aspects of global change—anthropogenic disturbances of ecosystems and other human activities—drive disease emergence, using well-researched disease case studies from the disease ecology literature, with an emphasis on diseases that have (re-)emerged in the last 2 5 years. To foster more collaboration between the fields of resource economics and disease ecology, we present foundational knowledge and recent case studies from disease ecology for the resource economics audience by highlighting the need to account for nonlinearity and complexity when modeling disease systems and to search for mechanisms by which anthropogenic disturbances affect disease outcomes. In addition, we briefly summarize technological and modeling advances used or developed by disease ecologists to bridge methodological approaches between the fields. Finally, we discuss the economic impacts of EIDs beyond medical and public health costs and the possible feedback of EIDs on global change.
2. INSIGHTS FROM DISEASE ECOLOGY
Infectious diseases are embedded within ecological systems in which interactions between individuals, environmental factors, and other species determine the emergence and transmission of disease. Most EIDs in humans originate in wildlife and/or are transmitted by vectors such as biting arthropods like ticks, mosquitoes, flies, and fleas (Woolhouse & Gowtage-Sequeria 2005). As a result, these diseases are connected to environmental conditions (e.g., temperature, rainfall, topography, biodiversity, and species interactions) and human social systems (e.g., the built environment, mobility, behavior, and immunology). Disease ecology—the study of how infectious disease dynamics respond to these environmental and human drivers—aims to understand and predict disease emergence and its consequences for humans and other species.
It is important to account for complexity and nonlinearity in economic systems. The same applies to infectious disease systems (Anderson & May 1979, Nova et al. 2021, Sugihara et al. 2012). Many ecological and social drivers interact to affect infectious disease dynamics, often via nonlinear responses, and all of these are stochastic, adding to uncertainty in actual outcomes. For example, secondary case distributions are strongly skewed for COVID-19 and other diseases, yielding epidemics that are highly stochastic but explosive (Lloyd-Smith et al. 2005). To facilitate collaboration among economists, disease ecologists, and epidemiologists it is important to share methods and concepts used in the respective fields. In this section, we highlight the complex nature of infectious disease systems with examples, provide an overview of the mechanistic relationship between disease and environmental change, and share recent developments in technology and methods used in disease ecology.
2.1. Nonlinearity and Complexity in Infectious Disease Systems
Key features of ecological systems, including infectious diseases, are their nonlinearity and complexity (Anderson & May 1979, Keeling & Rohani 2008). Infectious disease transmission responds nonlinearly to susceptible host availability: Transmission first grows exponentially as a pathogen emerges in a population, then reaches an inflection point as susceptible hosts are depleted by infection and immunity; it then burns out, reaches an endemic equilibrium, cycles, or exhibits other complex behaviors (Anderson & May 1979, Keeling & Rohani 2008). Transmission dynamics can be predicted based on the transmission rate, recovery or mortality rate, and the rate at which susceptible hosts are replenished through births, waning immunity, or migration (Anderson & May 1979, Keeling & Rohani 2008).
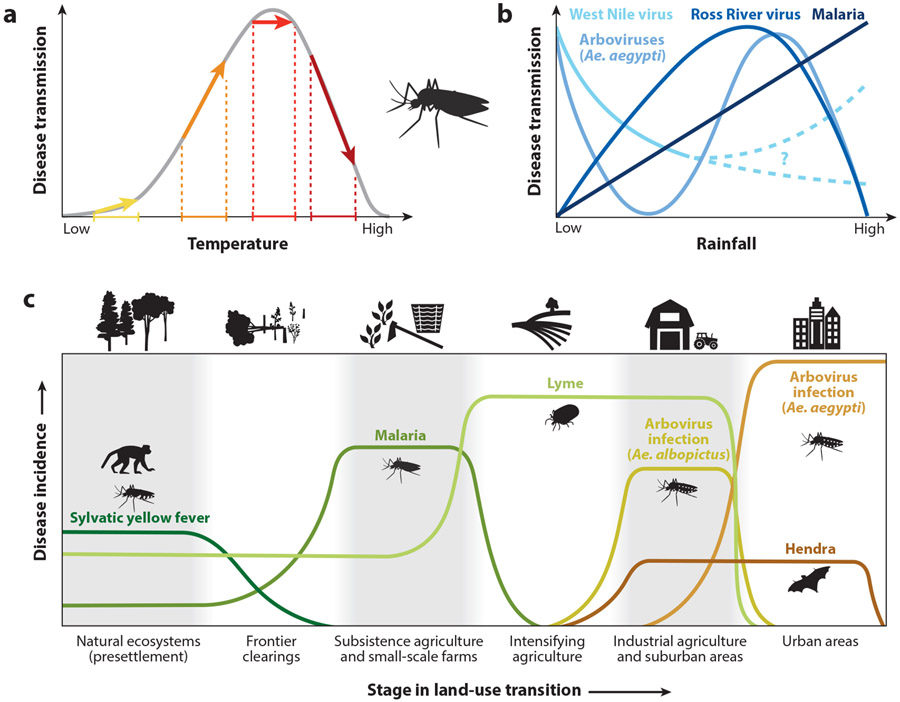
Not only does infectious disease transmission vary nonlinearly with susceptible host availability, but the parameters that govern transmission, infection, and other vital rates can also respond nonlinearly to environmental conditions (Figure 1), such as temperature and species interactions, and even in response to awareness of disease in others (Heesterbeek et al. 2015, Koelle & Pascual 2004, Weitz et al. 2020). For example, a 1°C increase in temperature can increase, decrease, or have null effects on transmission of a vector-borne disease depending on whether the current temperature is below, above, or at the thermal optimum for transmission (Caldwell et al. 2021) (Figure 1a). Moreover, many EIDs circulate through multiple hosts, including wildlife, livestock, and vectors, coupling nonlinear infectious disease dynamics in multiple populations that are linked by migration, contact, and pathogen spillover (Lloyd-Smith et al. 2009, Plowright et al. 2017).
Figure 1.
Impacts of global change on emerging infectious diseases can be nonlinear, complex, and/or exhibit threshold effects. (a) Global changes that affect temperature, such as climate change, land-use change, and urbanization, may have nonlinear effects on disease transmission. For many mosquito-borne diseases, accumulating evidence suggests a hump-shaped relationship between temperature and disease transmission. Thus, a given temperature increase can have different effects on disease transmission in different contexts depending on the baseline, causing a small increase (yellow arrow), a large increase (orange arrow), no change (red arrow), or a decrease (dark red arrow) in disease transmission. (b) The effects of rainfall on mosquito-borne disease transmission are more complex, since the rainfall-transmission relationship (linear or nonlinear) depends on vector ecology and factors related to human behavior and the built environment, or largely remain unresolved (dashed lines). (c) Global changes that alter the landscape and habitat, such as land-use change and urbanization, may promote or suppress transmission of emerging diseases depending on the disease and its ecology. Here are some examples of how different vector-borne or zoonotic emerging diseases are expected to vary across a land-use gradient. Ae. stands for Aedes, and arboviruses are Aedes-borne viruses (e.g., dengue, Zika, and yellow fever). Figure adapted with permission from Shocket et al. (2021).
2.2. Mechanisms of Disease Emergence via Environmental Change
Human population growth, industrialization, and mobility have led to pervasive changes in the Earth’s systems, including climate, land use, species invasions and extinctions, and global movement of goods, plants, humans, and other animals (Barnosky et al. 2012). These changes affect both wild and domestic species, and human contact with them, through multiple pathways that can lead to disease emergence (Lloyd-Smith et al. 2009, Plowright et al. 2017). For example, climate change can alter the distribution, abundance, and seasonality of parasites, reservoir hosts, and vectors (Altizer et al. 2013, Lafferty & Mordecai 2016). Invasions of mosquito vectors like Aedes (Ae.) aegypti and Ae. albopictus have led to arbovirus (e.g., dengue, Zika, yellow fever, and chikungunya) outbreaks in novel regions, including dengue and chikungunya in Italy, France, the United States, and islands off the coast of Europe and Africa (Adalja et al. 2012, Amraoui & Failloux 2016, Gerardin et al. 2008, Tomasello & Schlagenhauf 2013). Land-use change creates edge habitats between human-made and wild landscapes, facilitating human contact with wildlife and vectors that can lead to disease transmission (Faust et al. 2018, MacDonald & Mordecai 2019). Loss of native species and habitats can affect the health and behavior of wild animals that are reservoirs for EIDs (Plowright et al. 2015). Mobility rapidly transports pathogens and vectors between populations and facilitates emergence (Wesolowski et al. 2015). Anthropogenic global environmental change is intricately connected to the emergence of infectious diseases in humans, highlighting the urgent imperative of connecting planetary health to human health (Myers 2017).
2.3. Recent Technological and Modeling Advances in Disease Ecology
Recent technological advances and modeling approaches in disease ecology have furthered our understanding of EIDs. Breakthroughs in computer science, statistics, and data science have enabled modelers to store, analyze, and utilize larger data sets and run more informative simulations of disease emergence. New technology, such as high-throughput sequencing and cloud-based platforms for remote sensing data (e.g., Google Earth Engine), has improved data collection and sharing, allowing disease ecologists to access and synthesize several data types to better identify spillover pathways and disease transmission at various scales. For example, using viral genomic data in addition to epidemiological data from a recent yellow fever outbreak in Brazil, Faria et al. (2018) were able to identify the source of human cases as independent spillover events from monkeys, rather than from transmission among humans as initially believed.
Modeling approaches in disease ecology often need to account for nonlinearity and complexity, which are useful for understanding mechanisms of disease emergence. In recent years, there has been an expanding array of causal inference methodologies for nonlinear systems in disease ecology often borrowed from other fields, including instrumental variable analysis from econometrics (MacDonald & Mordecai 2019), structural equation modeling from genetics (Pearl 2010), and empirical dynamic modeling (EDM) from dynamic systems theory (Nova et al. 2021, Sugihara et al. 2012). Examples of other methods developed within the field of disease ecology or epidemiology include stochastic simulation and inference algorithms for epidemic modeling using partially observed Markov processes (King et al. 2016), inference of incidence from viral genetic data using phylodynamics (Volz et al. 2009), and time-series methods for inference of disease drivers and forecasting (Becker & Grenfell 2017), including forecasting uncertainty using EDM (Nova et al. 2021). In the last two decades, sophisticated mechanistic and data-driven models—specifically tailored for infectious disease dynamics, including EIDs—have unraveled important relationships between aspects of global change and disease emergence.
3. ANTHROPOGENIC DISTURBANCES LINKING GLOBAL CHANGE TO EMERGING INFECTIOUS DISEASES
Many human activities are economically driven and occur at a global scale. Several of these activities have significant environmental impacts and cascading effects on EIDs. Here, we give an overview of how these global anthropogenic changes affect infectious disease systems, showcasing the evidence of mechanisms linking anthropogenic disturbance of the environment to disease spillover, transmission, and emergence.
3.1. Climate Change
Anthropogenic climate change is driven by human activities elevating carbon emissions into the atmosphere, and it may have profound effects on EIDs. Diseases with environmental components in their transmission cycles, including vector-borne, water-borne, food-borne, soil-borne, and environmental contaminant pathogens, are climate sensitive because of the thermal physiology of ectotherms (i.e., animals that regulate body temperature via external heat sources) and the impact of water and humidity on survival and in some cases reproduction (Altizer et al. 2013, Mordecai et al. 2019, Shocket et al. 2021). Because temperature, humidity, and rainfall affect multiple aspects of vector and host life cycles and transmission cycles, and these responses are often hump-shaped or exhibit threshold effects (Figure 1), anticipating climate-driven changes in infectious disease systems is a challenge (Caldwell et al. 2021, Lafferty & Mordecai 2016, Mordecai et al. 2019, Shocket et al. 2021). Climate change is expected to drive increases in some diseases and regions and decreases or shifts in others (Altizer et al. 2013, Lafferty & Mordecai 2016). Moreover, at-tributing changes in infectious disease dynamics to climate change is challenging because of the complexity of these systems in which multiple environmental changes are concurrent (Parmesan et al. 2013).
Here, we provide mechanistic evidence linking EIDs to climate change with an emphasis on climate warming, as it is a well-supported effect of climate change and mean temperature has the most clearly understood effect on EIDs. However, we acknowledge that other facets of climate change can also contribute to disease emergence. For example, Rift Valley fever is linked to El Nino-Southern Oscillation (ENSO), and extreme ENSO events are predicted to increase in frequency and strength with climate change (Shocket et al. 2021). Further, for many mosquito-borne diseases, the effects of rainfall on disease transmission are complex in addition to being nonlinear, since the rainfall-transmission relationship largely depends on the vector species and pathogen (Shocket et al. 2021) (Figure 1b).
The clearest evidence of climate change-mediated infectious disease emergence is for vector-borne and environmentally transmitted diseases emerging at higher latitudes and elevations with climate warming (IPCC 2022). This is because the change from disease absence to presence is the easiest to observe and connect to climate warming. For example, Ixodes spp. ticks, tick-borne encephalitis, and Lyme disease have increased in incidence and expanded in range in northern parts of Europe and North America (Caminade et al. 2019, Couper et al. 2021, Gilbert 2021). In Nepal, dengue, chikungunya, malaria, and Japanese encephalitis and their vectors have emerged and expanded upslope (Dhimal et al. 2021). Parasitic, vector-borne, and rodent-borne diseases like cryptosporidiosis, filariasis, tularemia, and hantavirus-induced hemorrhagic fever have emerged in the Arctic and sub-Arctic (Huber et al. 2020, Pauchard et al. 2016, Sachal et al. 2019). In Europe, helminth (worm) parasites and host snails are increasing and expanding with climate change (Caminade et al. 2019). These widespread changes in range limits of diseases and vectors show that climate change is already affecting human and wildlife health.
Conversely, the impacts of climate change on diseases within their currently endemic ranges are more difficult to detect in the face of multiple changing environmental conditions. Moreover, climate change is expected to expand the distributions and incidence of some diseases in some regions, while contracting them in others (Lafferty & Mordecai 2016, Mordecai et al. 2019) (Figure 1a). For 11 different pathogens transmitted by 15 mosquito vector species, the optimal temperature for transmission varied from 23°C to 29°C and the temperatures that prevented transmission ranged from 9°C to 23°C at the low end and 32–38°C at the high end (Mordecai et al. 2019). This implies that diseases with cooler thermal optima and limits—including West Nile and other temperately distributed mosquito-borne viral diseases, as well as malaria and Ross River fever, with an optimum of 25–26°C—are likely to increase in temperature suitability at their cooler-temperature range limits, while declining in warmer areas (Mordecai et al. 2019, 2020; Shocket et al. 2020). However, diseases with warmer thermal optima like dengue and Zika, which peak at 29°C, are expected to broadly expand in climate suitability and could emerge at higher elevations and latitudes, in places where housing quality, socioeconomic conditions, and lack of adequate vector control permit transmission (Mordecai et al. 2019, 2020; Ryan et al. 2019, 2021). The widespread expansions of the invasive mosquitoes Ae. aegypti and Ae. albopictus, which are competent at transmitting many viruses, pose a substantial threat of arbovirus emergence following in the tracks of the four serotypes of dengue, chikungunya, and Zika, each of which have (re)invaded across the Americas in the last three decades (Gubler 2010, Gubler et al. 2017, Lounibos 2002). Likewise, the invasion and rapid spread of West Nile virus across North America exemplify how an introduced pathogen can rapidly establish in novel vectors and wildlife hosts and cause spillover to humans when climate suitability is high (Kilpatrick et al. 2010, Paull et al. 2017).
In contrast to the anticipated changes in temperature suitability for disease transmission, realized changes in disease incidence can be much more complex. Dengue incidence is rapidly increasing globally, particularly in the Americas, Asia, and Africa (Stanaway et al. 2016), but the degree to which climate change has driven these expansions versus unplanned urbanization, population growth, and mobility remains difficult to quantify because they act synergistically (Gubler 2010, Stewart-Ibarra & Lowe 2013, Wesolowski et al. 2015). Conversely, malaria declined dramatically worldwide during the twentieth century (Gething et al. 2010) and particularly steeply in most of sub-Saharan Africa in the last 20 years due to successful intervention campaigns (Bhatt et al. 2015, Smith et al. 2013) and potentially also due to declining climate suitability (Mordecai et al. 2020). The few exceptions in which malaria has increased have been documented in highland Kenya, Ethiopia, Colombia, and Nepal, which are attributed to climate warming (Dhimal et al. 2021, Siraj et al. 2014) in concert with land-use change (Afrane et al. 2012), and in Brazil, Colombia, Peru, Guyana, French Guiana, Venezuela, and Nicaragua, which are attributed to deforestation, gold mining, and other land-use changes (Douine et al. 2020, MacDonald & Mordecai 2019). In summary, even when climate warming increases the temperature suitability for disease transmission, this does not always translate into higher incidence of disease because of the concurrent effects of socioeconomic development, land-use change, behavior, and disease control programs.
Tick-borne diseases like Lyme disease and tick-borne encephalitis have also expanded with climate warming, driven by warmer winters, northward expansions of the vector and reservoir host species, and climate-driven changes in human and other animal behavior (Caminade et al. 2019, Couper et al. 2021, Gilbert 2021). The complex tick life cycle, which requires a series of three blood meals, often on different wild animal hosts that vary in competence for transmitting pathogens, makes establishing direct links between climate change and tick-borne disease difficult (Couper et al. 2021, Ostfeld & Brunner 2015). Like other arthropods, ticks are sensitive to climate, particularly temperature and humidity, which affect survival, behavior, and activity periods (Ostfeld & Brunner 2015). Temperature and precipitation affect tick species range limits (Hahn et al. 2016, Ostfeld & Brunner 2015), and warming temperatures are implicated in the expansion of Ixodes scapularis northward (Clow et al. 2017). Climate affects seed availability, reservoir host abundance, nymphal tick abundance and, in turn, Lyme disease risk in both Europe and North America (Bregnard et al. 2020, Ostfeld et al. 2006). Like other diseases with environmental transmission cycles, tick-borne diseases are expanding in step with climate change, but the mechanisms can be complex and difficult to quantify (but see Couper et al. 2021, Gilbert 2021).
3.2. Land-Use Change and Agricultural Intensification
Land-use change is a process by which wild pristine habitat (or already human-modified land) is altered by human activities, whereby the human-modified land serves a functional role, often for economic purposes (Lambin & Meyfroidt 2011, Winkler et al. 2021). This process often involves deforestation, road construction, dam building, irrigation, mining, urbanization, and clearing of land for agriculture and livestock husbandry. Agricultural intensification may involve the expansion of land-use change geographically and/or increase the density of domestic animals locally. These activities create edge habitats at the human-wildlife interface, increasing the potential for exchange of pathogens between wildlife, domestic animals, and humans (Glidden et al. 2021, Nova 2021, Plowright et al. 2021). This is exemplified by two viruses that emerged in the 1990s at the human-wildlife interface facilitated by land-use change: Nipah and Hendra, which circulate within pteropid fruit bats in Southeastern Asia and Australia, respectively (Daszak et al. 2006). Although their ecology is different, the mechanisms by which these viruses emerged are similar, involving bats visiting trees located near human settlements (Plowright et al. 2017, Sokolow et al. 2019).
In Bangladesh, indirect bat-to-human transmission has characterized the seasonal emergence and spillover of Nipah virus since 2001. Local residents consume raw date palm sap from the silk cotton and Indian mast trees, which can be contaminated by the opportunistic feeding of pteropid bats (Hahn et al. 2014). These two trees flower during an otherwise barren season, potentially providing an oasis of resources for bats (Hahn et al. 2014). Consequently, when the pteropid bats lick shaved regions of the trees, leave urine or fecal matter in the sap collection pot, or die from falling into the pots, they can promote bat-to-human, food-borne spillover (McKee et al. 2021). Bangladeshi villages where spillover has occurred are characterized by forest fragmentation that promotes pteropid bats to shift their roosting near consistent anthropogenic food sources (i.e., household gardens, agroforests) (Faust et al. 2018, Hahn et al. 2014). The fragmentation dates back to the seventeenth century when deforestation and rice cultivation were encouraged by Mughal rulers, and the British East India Company furthered the fragmentation process through the Permanent Settlement system in the mid-to-late eighteenth century (McKee et al. 2021). Thus, gradual modifications of the natural landscape rooted in colonialism have confined pteropid bats’ native habitat to smaller roost populations embedded within a matrix of anthropogenic food resources (McKee et al. 2021).
In contrast, the spillover pathway may involve an intermediate host species, usually a domestic animal, which bridges the spillover process between the reservoir bats and humans (Plowright et al. 2017, Sokolow et al. 2019). Between the early 1970s and the late 1990s in peninsular Malaysia, pig and mango production together experienced a rapid boom, nearly tripling in output (Pulliam et al. 2012). This increased production was a result of agricultural intensification, in which land was often simultaneously cultivated with both swine and fruit. In a pig farm in northern Malaysia, hundreds of mango trees were planted adjacent to the swine enclosures, which attracted pteropid fruit bats (Pulliam et al. 2012). A drip zone tends to form around these trees, where bats’ excretions of contaminated urine, fecal matter, and saliva (via partially masticated fruit) tend to fall (Plowright et al. 2015). This provided the means for the transmission of Nipah virus into the pigsties (Daszak et al. 2006, Pulliam et al. 2012). Infected pigs served as amplifier hosts for the pathogen and the subsequent transport of infected swine from the index farm fueled a large pig-to-human outbreak in southern Malaysia and Singapore in 1998–1999 (Daszak et al. 2006).
Similarly, Hendra virus primarily spills over from bats to horses, and subsequent horse-to-human transmission often leads to fatal cases. Land-use change, human population growth, and shifting bat distributions are collectively expanding the area of overlap between bats and grazing horses (Plowright et al. 2015). Agricultural and urban development and their by-products—habitat loss, fragmentation, and edge effects—serve to redistribute and restructure the availability and quality of food resources in a given landscape (Faust et al. 2018). Urban and peri-urban areas provide alternative—albeit lower-quality—food sources for bats when ephemeral nectars in their native flowering forests diminish owing to seasonal conditions (Plowright et al. 2015). Particularly sensitive to winter and spring food shortages, bats forgo migration in favor of residing near anthropogenic sources of sustenance, such as fruiting trees planted in horse paddocks (Plowright et al. 2015). Similarly to the Nipah example above, drip zones of bat excretions or discarded saliva-laden fruit emerge around these trees (Plowright et al. 2015). Subsequently, the horses may contract Hendra by coming into contact with these excretions via either grazing underneath or adjacent to bat-visited or bat-roosting trees or consuming contaminated bat-discarded fruit (Plowright et al. 2015). Thus, land-use change, especially the emergence of urban and suburban areas, may drive the recent emergence of Hendra (Figure 1c).
Another example of an emerging disease that spills over via a domestic intermediary host is influenza A. Its natural reservoir hosts include aquatic birds and waterfowl, such as ducks and geese (Olsen et al. 2006). Intensive and extensive agricultural practices have been found to drive the likelihood of spillover (Jones et al. 2013). Anthropogenic land use in wetland areas, where rice paddies are coupled with free-grazing duck farming, for example, promotes the interspecific contact between reservoir wild waterfowl and domestic water birds (Gilbert et al. 2007). As a result, the newly infected domestic water birds can pass on the pathogen to other domestic farm poultry through the pathways of environmental contamination and direct contact (Sims et al. 2005). In other cases, livestock intensification and anthropogenic compacting of farming systems have promoted high densities of pigs that live in close proximity. As a result, influenza virus can spill over from birds to pigs through interspecific contact, and the resulting swine influenza can be subsequently passed onto humans (Ma et al. 2009). Pigs are capable of becoming infected with both avian and human influenza strains. When two viral strains infect the same cell, genomic reassortment can occur, mixing genetic elements ofthe two ancestral strains into a novel strain that can combine features of the human influenza (i.e., the ability to readily infect and transmit among humans) with elements of the avian strain (i.e., antigenic novelty that can lead to severe disease in immunologically naive people) (Ma et al. 2009). Therefore, pigs can act as mixing vessels and amplifying hosts for onward human transmission, especially when they are transported between farms—a similar mechanism to that of Nipah virus in Malaysia, 1998–1999.
Another mechanism by which land-use change has facilitated disease emergence is by providing suitable habitat for rodent disease reservoirs. For example, hantaviruses are a group of viruses that primarily infect small rodents (e.g., mice, rats, voles) but can also occur in shrews and moles. Although hantavirus infection was previously thought to be confined to Europe and Asia, since its documented emergence in 1993–1994 in the southwestern United States (Hjelle & Torres-Pérez 2010), hantaviruses are now recognized worldwide in distinct geographic clusters: North and South America (e.g., Sin Nombre virus, Choclo virus), Europe (e.g., Dobrava virus, Puumala virus), and Asia (e.g., Seoul virus, Hantaan virus). Research on hantaviruses has revealed multiple pathways by which land-use change affects hantavirus spillover, often amplifying transmission. In China, cultivation of wheat, corn, and other crops provides food for Hantaan virus reservoir rodent hosts and increases rodent density (Yan et al. 2007). Likewise, orchard land and timber forest provide adequate Hantaan virus reservoir habitats, while also facilitating higher contact between rodents and farmers or forest workers via contaminated feces and urine (Yan et al. 2007). In certain North and South American cases, cropland with food and cover can provide preferred habitats for hantavirus reservoirs, and peri-domestic buildings (e.g., storage facilities, barns, homes) may concentrate rodent urine and feces (Dearing & Dizney 2010). Similarly, peri-domestic microhabitats in the vicinity of human habitation—patios, ornamental gardens, subsistence crops, and livestock barns—can foster a higher seroprevalence of a species of Choclo virus-infected rodent (Armién et al. 2009). In Panama, forest fragmentation and crop production have shifted small animal communities to become dominated by rodents that are reservoirs of hantaviruses, leading to outbreaks in humans (Armien et al. 2004, Suzán et al. 2008). Taken together, land-use change does not uniformly affect hantavirus spillover. Indeed, a variety of geographic and land-use contexts modulate hantavirus emergence pathways in different ways.
3.3. Urbanization
The rise of cities and peri-urban areas has led to the expansion of some infectious diseases through multiple mechanisms (Gubler 2010, Weaver 2013). Urbanization increases the density of paved and built areas, decreases species diversity and natural habitat availability, alters species composition, warms the microclimate, increases human density and mobility, and changes social structures, inequality, and behavior (Bharti et al. 2015, Cator et al. 2013, Cosner et al. 2009, Grimm et al. 2008). The resulting changes in urban environments transform the landscape for infectious disease transmission, favoring urban-adapted vectors and pathogens while decreasing the transmission potential of sylvatic and rural species (LaDeau et al. 2015, Lambin et al. 2010, Weaver 2013). However, the mechanisms by which urbanization affects vector-borne disease are complex and multivariate, and the effects may differ between urban and peri-urban areas.
The mosquito-borne viruses transmitted by Ae. aegypti—dengue, chikungunya, Zika, and others—are the canonical diseases of urbanization (Gubler 2010, Weaver 2013). Ae. aegypti is an urban-dwelling mosquito that breeds in container habitats, rests indoors, preferentially bites humans during the daytime, efficiently transmits many viral pathogens, thrives at warm temperatures, and has become established in tropical, subtropical, and even temperate environments worldwide, expanding from its native range in Africa (Gubler 2010, Weaver 2013). Multiple features of urban environments promote Ae. aegypti arbovirus transmission. First, the built environment and socioecological conditions promote vector habitat: Informal or poor-quality housing without sealed or screened windows and doors, and reliable access to piped water and sanitation, lead to the accumulation of standing water in storage containers, trash, or household items (Krystosik et al. 2020). Second, these vectors have short flight ranges and rest indoors, resulting in increased biting rates on humans (Stoddard et al. 2013). Third, population mobility is high within cities, among cities, and between cities and rural areas, resulting in high rates of virus importation and movement of epidemics between cities and the countryside (Chew et al. 2016, Cuong et al. 2013, Stoddard et al. 2013, Wesolowski et al. 2015). Fourth, urban areas have warmer microclimates, including heat island effects and warmer nights and winters, which can accelerate vector and pathogen development (Cator et al. 2013, Murdock et al. 2017, Paaijmans et al. 2010), as well as underground structures that can serve as climate refugia for vectors (Giordano et al. 2020, Lima et al. 2016). Fifth, vector control programs, which were aggressive, coordinated, and often environmentally destructive and socially oppressive but largely successful in the 1940s and 1960s, lapsed in the 1970s, resulting in the widespread resurgence and expansion of Ae. aegypti populations (Gubler 2010). As a result of these and other mechanisms acting in concert, dengue has increased exponentially since the 1970s (Gubler 2010, Stanaway et al. 2016), followed by waves of invasion of chikungunya and Zika (also transmitted by Ae. aegypti) through Latin America and the Caribbean, Oceania, and parts of Asia, Africa, North America, and Europe (Dhimal et al. 2015; LaBeaud et al. 2015; Tomasello & Schlagenhauf 2013; Weaver 2013, 2014; Weaver et al. 2016).
While Aedes-transmitted viruses are the canonical diseases of urbanization, other diseases and vectors have more complex responses to urbanization. For example, malaria often declines in cities compared to rural areas in sub-Saharan Africa, Southeast Asia, and South America as the vector’s outdoor breeding habitat and cooler optimal temperatures inhibit urban transmission, while greater accessibility of medical care may help to break transmission chains—although peri-urban areas can have substantial malaria transmission (Cator et al. 2013, Hay et al. 2005, Keiser et al. 2004). Tick-borne diseases like Lyme disease, which require tick vectors to acquire pathogens from wild animal hosts such as rodents and small mammals, often respond positively to forest fragmentation and suburbanization but decline in cities (Allan et al. 2003, Linske et al. 2018, Ostfeld et al. 2006). By contrast, some Culex mosquitoes and zoonotic mosquito-borne pathogens like West Nile virus can be present in cities, especially in urban parks, greenspaces, or abandoned buildings (Kilpatrick et al. 2010, LaDeau et al. 2015, Leisnham et al. 2014). Urbanization has had lagged effects on the establishment of the triatomine vector and Trypanosoma cruzi parasite that cause Chagas disease in Peru, where more established urban areas have higher abundances of infected vectors associated with construction materials and domestic animals, while the vector was not yet established in newly urbanizing areas (Levy et al. 2014). Natural disasters that disrupt urban infrastructure or lead to rapid, unplanned urbanization can further increase vector-borne disease risks, such as the surge in Zika transmission that occurred in Ecuador following the 2016 earthquake (Ali et al. 2017, Sorensen et al. 2017) and the increase in urban mosquito abundance following the 2010 earthquake in Haiti (Samson et al. 2015). In summary, urbanization affects vector-borne diseases by modifying the physical environment, biological diversity, microclimate, and human-vector interactions in ways that favor some vectors and diseases while suppressing others (Figure 1c).
3.4. Global Movement of Goods, Humans, and Other Animals
Global movement of goods, humans, and other animals via airline travel, international shipping, and human migration is increasing in volume, speed, and extent. This movement can facilitate disease emergence by creating conditions for spillover in the global animal trade, by rapidly turning recently emerged pathogens into global pandemics, by reintroducing pathogens into areas where previously eliminated, and by creating the conditions for disease transmission via the introduction of non-native species, especially disease vectors (Bell et al. 2004, Findlater & Bogoch 2018, Lounibos 2002, Tatem et al. 2006). While movement patterns not directly attributable to humans have also played a role in disease emergence—for example, migratory patterns of birds helped to disperse West Nile virus throughout the Americas after its emergence in New York (Swetnam et al. 2018)—here we focus on anthropogenic-driven change in global movement.
Dengue and other Aedes-transmitted arboviruses are classic examples of both the transport of goods introducing non-native species and importation of pathogens by travel of infected individuals (Tatem et al. 2006). Ae. aegypti mosquitoes were originally limited to West Africa but were imported to the Americas, Europe, and Oceania on sailing vessels associated with slave trade in the sixteenth or seventeenth century, where they subsequently established and spread throughout the Americas (Lounibos 2002). For the next 200 years, outbreaks of yellow fever would repeatedly occur, especially in port cities from Havana to New Orleans to Philadelphia where the virus was regularly reintroduced. Additionally, since the 1930s, Ae. albopictus mosquitoes have spread from their original range in Asia to the Americas, Europe, and Africa, imported in used tires and other goods that can harbor larvae and dormant eggs (Lounibos 2002, Tatem et al. 2006). The establishment of both Ae. aegypti and Ae. albopictus set the stage for subsequent arbovirus epidemics, including dengue, chikungunya, and Zika. The geographic distribution of dengue expanded in the 1980s and 1990s, most likely due to introduction by travelers, from Southeast Asia to the Pacific Islands, Caribbean, and tropical parts of the Americas and Africa, with importations in cooler regions of North America and Europe occasionally leading to limited local transmission (Findlater & Bogoch 2018, Gubler 2010, Wilder-Smith & Gubler 2008). Air travel has been implicated in the circulation and dissemination of dengue serotypes in Asia and Brazil (Nunes et al. 2014, Tian et al. 2017). Starting in 2013 and 2015, respectively, chikungunya and Zika followed similar patterns, with rapid spread likely driven by air travel causing outbreaks throughout the Pacific Islands, South and Central America, the Caribbean, and parts of North America, Asia, Africa, and Europe (Gubler et al. 2017).
The global wildlife trade also drives the national and international transport of animals and animal products. The legal and illegal wildlife trade combined is estimated to be a US$320 billion industry (Glidden et al. 2021). Wildlife hunting for trade and consumption has been linked to spillover and spread of several emerging diseases, including SARS, Ebola, and monkeypox (Glidden et al. 2021). There are two main mechanisms by which wildlife trade promotes zoonotic disease emergence. First, trade brings together high densities of species (both disease reservoirs and nonreservoirs), often kept under poor conditions during transport or in markets, facilitating both lower immunity to infection and cross-species transmission (Glidden et al. 2021). These conditions favor pathogen adaptation to new host species, including humans coming into contact with infected animals at high densities. Second, the global wildlife trade is a major contributor to the loss of biodiversity and primarily the depletion of large predators and herbivores due to the high demand for body parts (e.g., tiger bones and elephant ivory) (Baker et al. 2013). The removal of large-bodied wildlife via global trade can have implications for disease emergence locally, as a decrease in large animals has been correlated with an increase in small mammals brought to market, such as rodents and bats, which are reservoirs for many zoonotic diseases (Glidden et al. 2021). Handling and consumption of bat meat have been linked to human spillover of Ebola (Leroy et al. 2009) and may have contributed to the 2013–2016 Ebola epidemic centered in West Africa.
For directly transmitted diseases, global airline travel has also served to turn recently emerged pathogens into international epidemics (Findlater & Bogoch 2018). Over the last 20 years, coro- naviruses have become an unfortunately common example of the speed with which global human movement can spread novel pathogens around the world. In November 2002, SARS caused by the coronavirus SARS-CoV-1 was first detected in China, an emergence that has subsequently been linked to spillover from the likely reservoir in bats to the intermediate host of civets and ultimately to humans—a chain of events that was likely facilitated by the global wildlife trade (Bell et al. 2004, Glidden et al. 2021, Nova 2021). In the span of a year, SARS was reported in nearly 30 countries, with over 8,000 reported cases and over 700 reported deaths (WHO 2004). A second novel coronavirus, MERS-CoV, emerged in 2012 in Saudi Arabia, causing MERS. MERS also emerged from an animal reservoir (dromedary camels, with a potential distant origin in bats) before spreading around the world via infected travelers, with 27 countries reporting cases of MERS since 2012 (Nova 2021, WHO 2019). Unlike SARS, whose last human case was detected in May 2004, MERS has caused repeated outbreaks since its detection in 2012, with the largest outbreak outside of Saudi Arabia occurring in South Korea in 2015 when a single infected traveler returning to the country ultimately resulted in 186 additional cases (WHO 2019). Most recently, COVID-19 was detected in 2019 in Wuhan, China caused by the virus SARS-CoV-2. At its onset, the COVID-19 pandemic was fueled by international human movement, with early importations and spread associated with the high volume of air travel between countries with ongoing transmission (Lau et al. 2020, Yang et al. 2020).
In addition to short-term air travel, human migration driven by economic collapse, political instability, and climate change can become a source of disease importation and reintroduction. For example, in Venezuela, economic and healthcare collapse in combination with land-use change driven by deforestation and gold mining has led to a resurgence of malaria, with reported annual malaria cases more than tripling from 2000 to 2015 (Grillet et al. 2019). The crisis in Venezuela has also precipitated a mass migration, with more than 3.4 million Venezuelans fleeing to other countries, which has led to increases in imported cases in parts of Colombia, Brazil, Ecuador, and Peru (Doocy et al. 2019, Jaramillo-Ochoa et al. 2019, Rodríguez-Morales et al. 2019).
4. DISENTANGLING AND RESPONDING TO THE COMPLEX RELATIONSHIPS AMONG ECONOMIC FORCES, GLOBAL CHANGE, AND THE BURDEN OF EMERGING INFECTIOUS DISEASES
Economic development has nuanced and context-dependent impacts on global change: Increases in consumption of food (especially animal products) and material goods in general can be expected to lead to increases in land needed for food production and in resource extraction (e.g., gold mining) (Jayachandran 2021). However, as living standards rise, people are able to prioritize the environment without sacrificing basic needs or may be able to access opportunities with lower environmental impacts (Jayachandran 2021). Thus, economic development may drive disease emergence via global change, while simultaneously suppressing it via a change in perception of environmental health. In addition, feedback may occur between EIDs and global change as a result of the EIDs’ impacts on the economy. Here, we review the impacts of EIDs on the economy and management and how EIDs potentially feed back into altering global change.
4.1. Economic Impacts of Emerging Infectious Diseases and Management
The economic consequences of EIDs reach far beyond direct medical and public health costs, with cascading effects across sectors (Smith et al. 2019). The tourism sector and travel industry are often heavily impacted in places experiencing a disease outbreak or that are perceived as high risk, as governments issue travel restrictions or advisories, or people curtail travel to avoid exposure. The COVID-19 pandemic has affected the travel and tourism industry globally, the effects of which are ongoing (Vanzetti & Peters 2021). International tourism fell 74% between January and December 2020, resulting in an estimated cost of US$2.4 trillion to tourism and associated sectors in that year (Vanzetti & Peters 2021). SARS, MERS, swine flu (H1N1), andZika all have had measurable effects on tourism nationally or regionally (Joo et al. 2019, Rassy & Smith 2013, UNDP 2017, Wilder-Smith 2006).
In the case of zoonotic EIDs associated with livestock production, the agricultural sector can be affected, particularly via culling of livestock and the resulting direct and indirect costs. In the case of the 1998 Nipah virus outbreak in Malaysia—in which pigs were identified as an intermediate host—1.1 million pigs were culled, which resulted in costs to the government that included compensation to farmers and lost tax revenue (FAO & APHCA 2002), as well as costs to industries associated with pig production such as feed suppliers (Hosono et al. 2006). An alternative intervention was soon put into place: Mango trees were mandated by policy to be planted at a minimum distance from the pig enclosures (Pulliam et al. 2012). This simple, yet effective approach incurred minimal cost, while preventing further Nipah virus outbreaks (Sokolow et al. 2019). It also illustrates the benefits that can come from ecological interventions, i.e., actions that target the ecological context in which spillover processes occur, as opposed to more conventional medical or veterinary approaches such as vaccination and treatment (Sokolow et al. 2019). This provides an important alternative approach to culling of badgers to prevent spillover of tuberculosis in cattle, of vampire bats to prevent transmission of rabies to humans, and of mink infected with SARS-CoV-2 to prevent transmission back to humans, all of which were controversial and had debatable impacts on disease control (Enserink 2020, Sokolow et al. 2019).
Reductions in trade of animals or animal products, due to either government restrictions or changes in consumer behavior, can also have substantial impacts. Following an introduction of Rift Valley fever virus in Saudi Arabia and Yemen in 2000, Arabian countries banned imports of live animals from a number of African countries (Peyre et al. 2015). The ban resulted in a 75% reduction in animals exported from Somalia, which had previously made up 90% of the country’s total income (Peyre et al. 2015). Similarly, the association between H1N1 flu and pigs led to declines in Mexican pork exports to the United States and Japan, despite no official restrictions in trade (Rassy & Smith 2013).
The costs of EIDs may also include reduced health and diminished productivity over the long term, even after the disease is brought under control (Bonds et al. 2010). For example, the longterm economic costs of the 2015–2017 Zika epidemic in Latin America and the Caribbean associated with microcephaly cases and Guillain-Barré syndrome cases are estimated at US$11 billion, on par with the short-term costs (US$7–18 billion), which are dominated by lost tourism revenue and the cost of diagnosing and treating Zika (UNDP 2017). Accounting for the full range of direct and indirect economic costs associated with EIDs can help illuminate the benefits of investing in simultaneously preventative and sustainable measures.
4.2. The Feedback of Emerging Infectious Diseases on Global Change
In addition to the direct role of global change affecting EID risk, important feedback loops exist between EIDs and aspects of global change, often mediated by the economic impacts of EIDs. For example, annual global carbon emissions declined by roughly 7% in 2020 compared to 2019 as a result of restrictions that were implemented to address the COVID-19 pandemic and reduced economic activities (Friedlingstein et al. 2020). These reductions were not uniform around the world; instead, countries like the United States with more severe outbreaks experienced greater emissions reductions compared to countries that more successfully controlled COVID-19 (Tollefson 2021). In the Brazilian Amazon, deforestation increases malaria through ecological mechanisms, but increasing malaria in turn appears to reduce deforestation rates, likely through socioeconomic mechanisms (MacDonald & Mordecai 2019). EIDs also have the potential to exacerbate global change. For example, quarantine and travel restrictions associated with the 2013–2015 outbreak of Ebola in West Africa prevented Environmental Protection Agency personnel in Sierra Leone from monitoring protected areas to prevent illegal deforestation and mining, while economic instability as a result of the epidemic was expected to increase pressure on natural resources (Gov. Sierra Leone 2014).
In the face of such complexity, disentangling the relationships among global change, economic drivers, and EIDs in a particular context is important for designing effective policies and interventions to improve health and address global change. For example, the organization Health in Harmony identified the lack of access to healthcare as a driver of logging in and around a national park in Borneo (Webb et al. 2018). A program that increased local communities’ access to healthcare and lowered healthcare costs resulted in lower deforestation rates and increased visitation to health clinics (Jones et al. 2020). In the United States, where more than one-third of forest lands are privately held by individuals or families (USDA For. Serv. 2008), a survey of landowners by the Pinchot Institute identified healthcare costs as a major reason for logging or selling their lands, often for development (Mater 2008). In response, the Forest Health-Human Health Initiative is piloting a program that allows landowners to generate carbon credits for sustainable forest management in return for payments for healthcare and investments in rural health services (https://americancarbonregistry.org/resources/forest-health-human-health). The relationship between deforestation and malaria in the Brazilian Amazon suggests that reducing deforestation could both secure environmental benefits and reduce the burden of malaria, if alternative sustainable livelihood opportunities can be supported (MacDonald & Mordecai 2019).
5. CONCLUSIONS
Global change is generated by human activities at a local and global scale. The activities that lead to anthropogenic disturbances of the environment—primarily climate change, land-use change, urbanization, and global movement of humans, other organisms, and goods—affect societies and ecosystems in ways that favor the emergence of novel infectious diseases in human populations, expansions or shifts of diseases to new geographic regions, or the re-emergence of diseases in various places.
Here, we present some general mechanisms by which human-mediated disturbances of the environment drive disease (re-)emergence based on the evidence in the disease ecology literature. First, climate change will most likely shift, and may already have shifted, the geographical distribution of zoonotic and vector-borne diseases, although predicting those changes and the final disease impacts on human populations remains a major challenge. Second, land-use change frequently drives the emergence of novel diseases in humans, as it increases opportunities for diseases to spill over from wildlife, especially from disease reservoirs like rodents and bats, to human populations. Third, urbanization is expected to favor urban-adapted disease vectors and pathogens, while suppressing diseases of sylvatic or rural origin, but the effects of urbanization differ drastically between urban and peri-urban areas. Fourth, accumulating evidence suggests that the global movement of people, wild and domestic animals and plants, and goods has directly increased the emergence and spread of novel and already established human diseases via travel, migration, and trade.
By exploring the ecological and socioeconomic mechanisms driving the relationship between global change and EIDs, we are able to develop local and global ecological interventions that reduce disease burden and prevent disease (re-)emergence, complementing biomedical and public health interventions. Because diseases respond to both ecological and socioeconomic conditions, solutions require interdisciplinary systems approaches that engage with local communities. Further, the economic impacts of EIDs go far beyond medical and public health costs, and thus there are multiple ways in which disease emergence may feed back into the economy and global change via changes in policies and resource management.
In light of all this complexity, there is an urgent need for understanding interactions and feedback between ecological and economic drivers of disease emergence. Such an understanding can help to implement economic solutions that are not in isolation from disease ecology, and vice versa, in order to develop more effective strategies for disease emergence prevention. Looking ahead, interdisciplinary research that combines disease ecology and resource economics can help to identify critical gaps in our understanding and prevention of EIDs. This agenda will promote a more sustainable future where we can proactively mitigate planetary health crises.
ACKNOWLEDGMENTS
We thank Scott Kominers for thoughtful discussions on this topic and early feedback on this work. N.N. was supported by the Philanthropic Educational Organization (PEO) Scholar Award from the International Chapter of the PEO Sisterhood, the Stanford Data Science Scholars program, and the Predoctoral Fellowship from the Stanford Center for Computational, Evolutionary and Human Genomics. M.L.C. was supported by the Illich-Sadowsky Fellowship through the Stanford Interdisciplinary Graduate Fellowship program at Stanford University. L.M. and E.A.M. were supported by the National Science Foundation (NSF; DEB-2011147, with the Fogarty International Center). E.A.M. was also supported by the National Institute of General Medical Sciences (R35GM133439), the Terman Award, the Stanford King Center on Global Development, the Stanford Woods Institute for the Environment, and the Stanford Center for Innovation in Global Health.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Adalja AA, Sell TK, Bouri N, Franco C. 2012. Lessons learned during dengue outbreaks in the United States, 2001–2011. Emerg. Infect. Dis 18(4):608–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrane YA, Githeko AK, Yan G. 2012. The ecologyof Anopheles mosquitoes under climate change: case studies from the effects of environmental changes in East Africa highlands. Ann. N.Y. Acad. Sci 1249:204–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Gugliemini O, Harber S, Harrison A, Houle L, et al. 2017. Environmental and social change drive the explosive emergence of Zika virus in the Americas. PLOS Negl. Trop. Dis 11(2):e0005135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan BF, Keesing F, Ostfeld RS. 2003. Effect of forest fragmentation on Lyme disease risk. Conserv. Biol 17(1):267–72 [Google Scholar]
- Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. 2013. Climate change and infectious diseases: from evidence to a predictive framework. Science 341(6145):514–19 [DOI] [PubMed] [Google Scholar]
- Amraoui F, Failloux A-B. 2016. Chikungunya: an unexpected emergence in Europe. Curr. Opin. Virol 21:146–50 [DOI] [PubMed] [Google Scholar]
- Anderson RM, May RM. 1979. Population biology of infectious diseases: part I. Nature 280(5721):361–67 [DOI] [PubMed] [Google Scholar]
- Armién AG, Armién B, Koster F, Pascale JM, Avila M, et al. 2009. Hantavirus infection and habitat associations among rodent populations in agroecosystems of Panama: implications for human disease risk. Am. J. Trop. Med. Hyg 81(1):59–66 [PubMed] [Google Scholar]
- Armien B, Pascale JM, Bayard V, Munoz C, Mosca I, et al. 2004. High seroprevalence of hantavirus infection on the Azuero peninsula of Panama. Am. J. Trop. Med. Hyg 70(6):682–87 [PubMed] [Google Scholar]
- Baker SE, Cain R, van Kesteren F, Zommers ZA, D’Cruze N, Macdonald DW. 2013. Rough trade: animal welfare in the global wildlife trade. Bioscience 63(12):928–38 [Google Scholar]
- Barnosky AD, Hadly EA, Bascompte J, Berlow EL, Brown JH, et al. 2012. Approaching a state shift in Earth’s biosphere. Nature 486(7401):52–58 [DOI] [PubMed] [Google Scholar]
- Becker AD, Grenfell BT. 2017. TSIR: An R package for time-series susceptible-infected-recovered models of epidemics. PLOS ONE 12(9):e0185528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D, Roberton S, Hunter PR. 2004. Animal origins of SARS coronavirus: possible links with the international trade in small carnivores. Philos. Trans. R. Soc. B 359(1447):1107–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti N, Lu X, Bengtsson L, Wetter E, Tatem AJ. 2015. Remotely measuring populations during a crisis by overlaying two data sources. Int. Health 7(2):90–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, et al. 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526:207–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonds MH, Keenan DC, Rohani P, Sachs JD. 2010. Poverty trap formed by the ecology of infectious diseases. Proc. R. Soc. B 277(1685):1185–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregnard C, Rais O, Voordouw MJ. 2020. Climate and tree seed production predict the abundance of the European Lyme disease vector over a 15-year period. Parasites Vectors 13(1):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JM, LaBeaud AD, Lambin EF, Stewart-Ibarra AM, Ndenga BA, et al. 2021. Climate predicts geographic and temporal variation in mosquito-borne disease dynamics on two continents. Nat. Commun 12:1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminade C, McIntyre KM, Jones AE. 2019. Impact of recent and future climate change on vector-borne diseases. Ann. N.Y. Acad. Sci 1436(1):157–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator LJ, Thomas S, Paaijmans KP, Ravishankaran S, Justin JA, et al. 2013. Characterizing microclimate in urban malaria transmission settings: a case study from Chennai, India. Malar. J 12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew CH, Woon YL, Amin F, Adnan TH, Abdul Wahab AH, et al. 2016. Rural-urban comparisons of dengue seroprevalence in Malaysia. BMC Public Health 16:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow KM, Leighton PA, Ogden NH, Lindsay LR, Michel P, et al. 2017. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLOS ONE 12(12):e0189393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosner C, Beier JC, Cantrell RS, Impoinvil D, Kapitanski L, et al. 2009. The effects of human movement on the persistence of vector-borne diseases. J. Theor. Biol 258(4):550–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper LI, MacDonald AJ, Mordecai EA. 2021. Impact of prior and projected climate change on US Lyme disease incidence. Glob. Change Biol 27(4):738–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuong HQ, Vu NT, Cazelles B, Boni MF, Thai KTD, et al. 2013. Spatiotemporal dynamics of dengue epidemics, Southern Vietnam. Emerg. Infect. Dis 19(6):945–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily GC, Ehrlich PR. 1996. Global change and human susceptibility to disease. Annu. Rev. Energy Environ 21:125–44 [Google Scholar]
- Daszak P, Plowright R, Epstein JH, Pulliam J, Abdul Rahman S, et al. 2006. The emergence of Nipah and Hendra virus: pathogen dynamics across a wildlife-livestock-human continuum. In Disease Ecology: Community Structure and Pathogen Dynamics, ed. Collinge SK, Ray C, pp. 186–201. Oxford, UK: Oxford Univ. Press [Google Scholar]
- Dearing MD, Dizney L. 2010. Ecology of hantavirus in a changing world. Ann. N.Y. Acad. Sci 1195:99–112 [DOI] [PubMed] [Google Scholar]
- Dhimal M, Gautam I, Joshi HD, O’Hara RB, Ahrens B, Kuch U. 2015. Risk factors for the presence of chikungunya and dengue vectors (Aedes aegypti and Aedes albopictus), their altitudinal distribution and climatic determinants of their abundance in central Nepal. PLOS Negl. Trop. Dis 9(3):e0003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhimal M, Kramer IM, Phuyal P, Budhathoki SS, Hartke J, et al. 2021. Climate change and its association with the expansion of vectors and vector-borne diseases in the Hindu Kush Himalayan region: a systematic synthesis of the literature. Adv. Clim. Change Res 12(3):421–29 [Google Scholar]
- Doocy S, Page KR, de la Hoz F, Spiegel P, Beyrer C. 2019. Venezuelan migration and the border health crisis in Colombia and Brazil. J. Migr. Hum. Secur 7(3):79–91 [Google Scholar]
- Douine M, Lambert Y, Musset L, Hiwat H, Blume LR, et al. 2020. Malaria in gold miners in the Guianas and the Amazon: current knowledge and challenges. Curr. Trop. Med. Rep 7(2):37–47 [Google Scholar]
- Enserink M. 2020. Coronavirus rips through Dutch mink farms, triggering culls. Science 368(6496):1169. [DOI] [PubMed] [Google Scholar]
- FAO (Food Agric. Organ.), APHCA (Anim. Prod. Health Comm. Asia Pac.). 2002. Manual on the Diagnosis of Nipah Virus Infection in Animals. Rome: FAO [Google Scholar]
- Faria NR, Kraemer MUG, Hill SC, Goes de Jesus J, Aguiar RS, et al. 2018. Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science 361(6405):894–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust CL, McCallum HI, Bloomfield LSP, Gottdenker NL, Gillespie TR, et al. 2018. Pathogen spillover during land conversion. Ecol. Lett 21(4):471–83 [DOI] [PubMed] [Google Scholar]
- Findlater A, Bogoch II. 2018. Human mobility and the global spread of infectious diseases: a focus on air travel. Trends Parasitol. 34(9):772–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlingstein P, O’Sullivan M, Jones MW, Andrew RM, Hauck J, et al. 2020. Global carbon budget 2020. Earth Syst. Sci. Data 12(4):3269–40 [Google Scholar]
- Gérardin P, Guernier V, Perrau J, Fianu A, Le Roux K, et al. 2008. Estimating chikungunya prevalence in La Réunion Island outbreak by serosurveys: two methods for two critical times of the epidemic. BMC Infect. Dis 8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething PW, Smith DL, Patil AP, Tatem AJ, Snow RW, Hay SI. 2010. Climate change and the global malaria recession. Nature 465(7296):342–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L. 2021. The impacts of climate change on ticks and tick-borne disease risk. Annu. Rev. Entomol 66:373–88 [DOI] [PubMed] [Google Scholar]
- Gilbert M, Xiao X, Chaitaweesub P, Kalpravidh W, Premashthira S, et al. 2007. Avian influenza, domestic ducks and rice agriculture in Thailand. Agric. Ecosyst. Environ 119:409–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano BV, Gasparotto A, Liang P, Nelder MP, Russell C, Hunter FF. 2020. Discovery of an Aedes (Stegomyia) albopictus population and first records of Aedes (Stegomyia) aegypti in Canada. Med. Vet. Entomol 34(1):10–16 [DOI] [PubMed] [Google Scholar]
- Glidden CK, Nova N, Kain MP, Lagerstrom KM, Skinner EB, et al. 2021. Human-mediated impacts on biodiversity and the consequences for zoonotic disease spillover. Curr. Biol 31(19):R1342–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gov. Sierra Leone. 2014. The economic and social impact of Ebola virus disease in Sierra Leone.Joint Prelim. Assess. Rep., Gov. Sierra Leone, Freetown. https://reliefweb.int/sites/reliefweb.int/files/resources/Joint%20preliminary%20assessment%20socio%20economic%20impact%20of%20EVD%20in%20Sierra%20Leone.pdf [Google Scholar]
- Griffin RD. 2009. Indigenous knowledge for sustainable development: case studies of three indigenous tribes of Wisconsin. MS Thesis, Coll. Nat. Resour., Univ. Wise, Stevens Point. https://epapers.uwsp.edu/thesis/2009/griffin.pdf [Google Scholar]
- Grillet ME, Hernández-Villena JV, Llewellyn MS, Paniz-Mondolfi AE, Tami A, et al. 2019. Venezuela’s humanitarian crisis, resurgence of vector-borne diseases, and implications for spillover in the region. Lancet Infect. Dis 19(5):e149–61 [DOI] [PubMed] [Google Scholar]
- Grimm NB, Foster D, Groffman P, Grove JM, Hopkinson CS, et al. 2008. The changing landscape: ecosystem responses to urbanization and pollution across climatic and societal gradients. Front. Ecol. Environ 6(5):264–72 [Google Scholar]
- Gubler DJ. 2010. The global threat of emergent/re-emergent vector-borne diseases. In Vector Biology, Ecology and Control, ed. Atkinson PW, pp. 39–62. Dordrecht, Neth.: Springer [Google Scholar]
- Gubler DJ, Vasilakis N, Musso D. 2017. History and emergence of Zika virus. J. Infect. Dis 216(Suppl. 10):S860–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MB, Gurley ES, Epstein JH, Islam MS, Patz JA, et al. 2014. The role of landscape composition and configuration on Pteropus giganteus roosting ecology and Nipah virus spillover risk in Bangladesh. Am. J. Trop. Med. Hyg 90(2):247–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MB, Jarnevich CS, Monaghan AJ, Eisen RJ. 2016. Modeling the geographic distribution of Ixodes scapularis and Ixodespacificus (Acari: Ixodidae) in the contiguous United States. J. Med. Entomol 53(5):1176–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. 2005. Urbanization, malaria transmission and disease burden in Africa. Nat. Rev. Microbiol 3:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesterbeek H, Anderson RM, Andreasen V, Bansal S, De Angelis D, et al. 2015. Modeling infectious disease dynamics in the complex landscape of global health. Science 347(6227): aaa4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelle B, Torres-Pérez F. 2010. Hantaviruses in the Americas and their role as emerging pathogens. Viruses 2(12):2559–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosono H, Kono H, Ito S, Shirai J. 2006. Economic impact of Nipah virus infection outbreak in Malaysia. Proceedings of the 11th International Symposium on Veterinary Epidemiology and Economics, Cairns, Aust. [Google Scholar]
- Huber I, Potapova K, Ammosova E, Beyer W, Blagodatskiy S, et al. 2020. Symposium report: emerging threats for human health—impact of socioeconomic and climate change on zoonotic diseases in the Republic of Sakha (Yakutia), Russia. Int.J. Circumpolar Health 79(1):1715698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC (Intergov. Panel Clim. Change). 2022. Climate Change 2022: Impacts, Adaptation, and Vulnerability. A Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, ed. Pörtner H-O, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, et al. Cambridge, UK: Cambridge Univ. Press. https://www.ipcc.ch/report/ar6/wg2/ [Google Scholar]
- Jaramillo-Ochoa R, Sippy R, Farrell DF, Cueva-Aponte C, Beltrán-Ayala E, et al. 2019. Effects of political instability in Venezuela on malaria resurgence at Ecuador-Peru border, 2018. Emerg. Infect. Dis 25(4):834–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayachandran S. 2021. How economic development influences the environment. NBER Work. Pap. w29191 [Google Scholar]
- Jones BA, Grace D, Kock R, Alonso S, Rushton J, et al. 2013. Zoonosis emergence linked to agricultural intensification and environmental change. PNAS 110(21):8399–8404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones IJ, MacDonald AJ, Hopkins SR, Lund AJ, Liu ZYC, et al. 2020. Improving rural health care reduces illegal logging and conserves carbon in a tropical forest. PNAS 117(45):28515–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo H, Maskery BA, Berro AD, Rotz LD, Lee YK, Brown CM. 2019. Economic impact of the 2015 MERS outbreak on the Republic of Korea’s tourism-related industries. Health Secur. 17(2):100–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling MJ, Rohani P 2008. Modeling Infectious Diseases in Humans and Animals. Princeton, NJ: Princeton Univ. Press [Google Scholar]
- Keiser J, Utzinger J, de Castro MC, Smith TA, Tanner M, Singer BH. 2004. Urbanization in Sub-Saharan Africa and implication for malaria control. Am. J. Trop. Med. Hyg 71:118–27 [PubMed] [Google Scholar]
- Kilpatrick AM, Fonseca DM, Ebel GD, Reddy MR, Kramer LD. 2010. Spatial and temporal variation in vector competence of Culex pipiens and Cx. restuans mosquitoes for West Nile virus. Am. J. Trop. Med. Hyg 83(3):607–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AA, Nguyen D, Ionides EL. 2016. Statistical inference for partially observed Markov processes via the R package pomp. arXiv 1509.00503 [stat.ME] [Google Scholar]
- Koelle K, Pascual M. 2004. Disentangling extrinsic from intrinsic factors in disease dynamics: a nonlinear time series approach with an application to cholera. Am. Nat 163(6):901–13 [DOI] [PubMed] [Google Scholar]
- Krystosik A, Njoroge G, Odhiambo L, Forsyth JE, Mutuku F, LaBeaud AD. 2020. Solidwastes provide breeding sites, burrows, and food for biological disease vectors, and urban zoonotic reservoirs: a call to action for solutions-based research. Front. Public Health 7:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBeaud AD, Banda T, Brichard J, Muchiri EM, Mungai PL, et al. 2015. High rates of O’Nyong Nyong and Chikungunya virus transmission in coastal Kenya. PLOS Negl. Trop. Dis 9(2):e0003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDeau SL, Allan BF, Leisnham PT, Levy MZ. 2015. The ecological foundations of transmission potential and vector-borne disease in urban landscapes. Funct. Ecol 29(7):889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty KD, Mordecai EA. 2016. The rise and fall of infectious disease in a warmer world. F1000Research 5:2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambin EF, Meyfroidt P. 2011. Global land use change, economic globalization, and the looming land scarcity. PNAS 108(9):3465–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambin EF, Tran A, Vanwambeke SO, Linard C, Soti V. 2010. Pathogenic landscapes: interactions between land, people, disease vectors, and their animal hosts. Int. J. Health Geogr 9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau H, Khosrawipour V, Kocbach P, Mikolajczyk A, Ichii H, et al. 2020. The association between international and domestic air traffic and the coronavirus (COVID-19) outbreak. J. Microbiol. Immunol. Infect 53(3):467–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham PT, LaDeau SL, Juliano SA. 2014. Spatial and temporal habitat segregation of mosquitoes in urban Florida. PLOS ONE 9(3):e91655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy EM, Epelboin A, Mondonge V, Pourrut X, Gonzalez J-P, et al. 2009. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector-Borne Zoonotic Dis. 9(6):723–28 [DOI] [PubMed] [Google Scholar]
- Levine JM. 2008. Biological invasions. Curr. Biol 18(2):R57–60 [DOI] [PubMed] [Google Scholar]
- Levy MZ, Barbu CM, Castillo-Neyra R, Quispe-Machaca VR, Ancca-Juarez J, et al. 2014. Urbanization, land tenure security and vector-borne Chagas disease. Proc. R. Soc. B 281(1789):20141003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima A, Lovin DD, Hickner PV, Severson DW 2016. Evidence for an overwintering population of Aedes aegypti in Capitol Hill neighborhood, Washington, DC. Am. J. Trojp Med. Hyg 94(1):231–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linske MA, Williams SC, Stafford KC 3rd, Ortega IM. 2018. Ixodes scapularis (Acari: Ixodidae) reservoir host diversity and abundance impacts on dilution of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) in residential and woodland habitats in Connecticut, United States. J. Med. Entomol 55(3):681–90 [DOI] [PubMed] [Google Scholar]
- Lloyd-Smith JO, George D, Pepin KM, Pitzer VE, Pulliam JRC, et al. 2009. Epidemic dynamics at the human-animal interface. Science 326(5958):1362–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. 2005. Superspreading and the effect of individual variation on disease emergence. Nature 438(7066):355–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP. 2002. Invasions by insect vectors of human disease. Annu. Rev. Entomol 47:233–66 [DOI] [PubMed] [Google Scholar]
- Ma W, Kahn RE, Richt JA. 2009. The pig as a mixing vessel for influenza viruses: human and veterinary implications. J. Mol. Genet. Med 3(1):158–66 [PMC free article] [PubMed] [Google Scholar]
- MacDonald AJ, Mordecai EA. 2019. Amazon deforestation drives malaria transmission, and malaria burden reduces forest clearing. PNAS 116(44):22212–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mater CM. 2008. Wisconsin and Pennsylvania forestland owner offspring study results for 2007–2008. Work. Pap., Pinchot Inst., Corvallis, OR. https://www.dropbox.com/s/pwfi5qxtih67wkc/Mater 2021_NASF Offspring DC 2008.WI%26PA.pdf?dl=0 [Google Scholar]
- McKee CD, Islam A, Luby SP, Salje H, Hudson PJ, et al. 2021. The ecology of Nipah virus in Bangladesh: a nexus of land-use change and opportunistic feeding behavior in bats. Viruses 13(2):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai EA, Caldwell JM, Grossman MK, Lippi CA, Johnson LR, et al. 2019. Thermal biology of mosquito-borne disease. Ecol. Lett 22(10):1690–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai EA, Ryan SJ, Caldwell JM, Shah MM, LaBeaud AD. 2020. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet. Health 4(9):e416–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock CC, Evans MV, McClanahan TD, Miazgowicz KL, Tesla B. 2017. Fine-scale variation in microclimate across an urban landscape shapes variation in mosquito population dynamics and the potential of Aedes albopictus to transmit arboviral disease. PLOS Negl. Trop. Dis 11(5):e0005640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SS. 2017. Planetary health: protecting human health on a rapidly changing planet. Lancet 390(10114):2860–68 [DOI] [PubMed] [Google Scholar]
- Nova N. 2021. Cross-species transmission of coronaviruses in humans and domestic mammals, what are the ecological mechanisms driving transmission, spillover, and disease emergence? Front. Public Health 9:717941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nova N, Deyle ER, Shocket MS, MacDonald AJ, Childs ML, et al. 2021. Susceptible host availability modulates climate effects on dengue dynamics. Ecol. Lett 24(3):415–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes MRT, Palacios G, Faria NR, Sousa EC, Pantoja JA, et al. 2014. Air travel is associated with intracontinental spread of dengue virus serotypes 1–3 in Brazil. PLOS Negl. Trop. Dis 8(4):e2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus ADME, Fouchier RAM. 2006. Global patterns of influenza a virus in wild birds. Science 312(5772):384–88 [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, Brunner JL. 2015. Climate change and Ixodes tick-borne diseases of humans. Philos. Trans. R. Soc. B 370(1665):20140051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ, Keesing F. 2006. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLOS Biol. 4(6):e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans KP, Imbahale SS, Thomas MB, Takken W. 2010. Relevant microclimate for determining the development rate of malaria mosquitoes and possible implications of climate change. Malar J. 9:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan C, Burrows MT, Duarte CM, Poloczanska ES, Richardson AJ, et al. 2013. Beyond climate change attribution in conservation and ecological research. Ecol. Lett 16(s1):58–71 [DOI] [PubMed] [Google Scholar]
- Pauchard A, Milbau A, Albihn A, Alexander J, Burgess T, et al. 2016. Non-native and native organisms moving into high elevation and high latitude ecosystems in an era of climate change: new challenges for ecology and conservation. Biol. Invasions 2(18):345–53 [Google Scholar]
- Paull SH, Horton DE, Ashfaq M, Rastogi D, Kramer LD, et al. 2017. Drought and immunity determine the intensity of West Nile virus epidemics and climate change impacts. Proc. R. Soc. B 284(1848):20162078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl J. 2010. An introduction to causal inference. Int.J. Biostat 6(2):1–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyre M, Chevalier V, Abdo-Salem S, Velthuis A, Antoine-Moussiaux N, et al. 2015. A systematic scoping study of the socio-economic impact of Rift Valley fever: research gaps and needs. Zoonoses Public Health 62(5):309–25 [DOI] [PubMed] [Google Scholar]
- Plowright RK, Eby P, Hudson PJ, Smith IL, Westcott D, et al. 2015. Ecological dynamics of emerging bat virus spillover. Proc. R. Soc. B 282(1798):20142124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, et al. 2017. Pathways to zoonotic spillover. Nat. Rev. Microbiol 15(8):502–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Reaser JK, Locke H, Woodley SJ, Patz JA, et al. 2021. Land use-induced spillover: a call to action to safeguard environmental, animal, and human health. Lancet Planet. Health 5(4):e237–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam JRCC, Epstein JH, Dushoff J, Rahman SA, Bunning M, et al. 2012. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J. R. Soc. Interface 9(66):89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassy D, Smith RD. 2013. The economic impact of H1N1 on Mexico’s tourist and pork sectors. Health Econ. 22(7):824–34 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Morales AJ, Suárez JA, Risquez A, Villamil-Gómez WE, Paniz-Mondolfi A. 2019. Consequences of Venezuela’s massive migration crisis on imported malaria in Colombia, 2016–2018. Travel Med. Infect. Dis 28:98–99 [DOI] [PubMed] [Google Scholar]
- Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. 2019. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLOS Negl. Trop. Dis 13(3):e0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ, Carlson CJ, Tesla B, Bonds MH, Ngonghala CN, et al. 2021. Warming temperatures could expose more than 1.3 billion new people to Zika virus risk by 2050. Glob. Change Biol 27(1):84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachal S, Deilgat M, Speechley M. 2019. Case 11: crypto climate creep: the movement of tropical infectious disease to the Arctic. In Western Public Health Casebook 2019, ed. Sibbald SL, McKinley G, pp. 135–49. London, ON: Public Health Casebook Publ. [Google Scholar]
- Samson DM, Archer RS, Alimi TO, Arheart KL, Impoinvil DE, et al. 2015. New baseline environmental assessment of mosquito ecology in northern Haiti during increased urbanization. J. Vector Ecol 40(1):46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shocket MS, Anderson CB, Caldwell JM, Childs ML, Couper LI, et al. 2021. Environmental drivers of vector-borne diseases. In Population Biology of Vector-Borne Diseases, ed. Drake JM, Bonsall M, Strand M, pp. 85–118. Oxford, UK: Oxford Univ. Press [Google Scholar]
- Shocket MS, Verwillow AB, Numazu MG, Slamani H, Cohen JM, et al. 2020. Transmission ofWestNile virus and other temperate mosquito-borne viruses occurs at lower environmental temperatures than tropical mosquito-borne diseases. eLife 9:e58511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims LD, Domenech J, Benigno C, Kahn S, Kamata A, et al. 2005. Origin and evolution of highly pathogenic H5N1 avian influenza in Asia. Vet. Rec 157(6):159–64 [DOI] [PubMed] [Google Scholar]
- Siraj AS, Santos-Vega M, Bouma MJ, Yadeta D, Ruiz Carrascal D, Pascual M. 2014. Altitudinal changes in malaria incidence in highlands of Ethiopia and Colombia. Science 343(6175):1154–58 [DOI] [PubMed] [Google Scholar]
- Smith DL, Cohen JM, Chiyaka C, Johnston G, Gething PW, et al. 2013. A sticky situation: the unexpected stability of malaria elimination. Philos. Trans. R. Soc. B 368(1623):20120145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Machalaba CC, Seifman R, Feferholtz Y, Karesh WB. 2019. Infectious disease and economics: the case for considering multi-sectoral impacts. One Health 7:100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolow SH, Nova N, Pepin KM, Peel AJ, Manlove K, et al. 2019. Ecological interventions to prevent and manage zoonotic pathogen spillover. Philos. Trans. R. Soc. B 374(1782):20180342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen CJ, Borbor-Cordova MJ, Calvello-Hynes E, Diaz A, Lemery J, Stewart-Ibarra AM. 2017. Climate variability, vulnerability, and natural disasters: a case study of Zika virus in Manabi, Ecuador following the 2016 earthquake. Geo Health 1(8):298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, et al. 2016. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis 16(6):712–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Ibarra AM, Lowe R. 2013. Climate and non-climate drivers of dengue epidemics in southern coastal Ecuador. Am.J. Trop. Med. Hyg 88(5):971–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, et al. 2013. House-to-house human movement drives dengue virus transmission. PNAS 110(3):994–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara G, May R, Ye H, Hsieh C, Deyle E, et al. 2012. Detecting causality in complex ecosystems. Science 338(6106):496–500 [DOI] [PubMed] [Google Scholar]
- Suzán G, Marcé E, Giermakowski JT, Armién B, Pascale J, et al. 2008. The effect of habitat fragmentation and species diversity loss on hantavirus prevalence in Panama. Ann. N.Y. Acad. Sci 1149:80–83 [DOI] [PubMed] [Google Scholar]
- Swetnam D, Widen SG, Wood TG, Reyna M, Wilkerson L, et al. 2018. Terrestrial bird migration and West Nile virus circulation, United States. Emerg. Infect. Dis 24(12):2184–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatem AJ, Rogers DJ, Hay SI. 2006. Global transport networks and infectious disease spread. Adv. Parasitol 62:293–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Sun Z, Faria NR, Yang J, Cazelles B, et al. 2017. Increasing airline travel may facilitate co-circulation of multiple dengue virus serotypes in Asia. PLOS Negl. Trop. Dis 11(8):e0005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson J. 2021. COVID curbed carbon emissions in 2020—butnot by much. Nature 589(7842):343. [DOI] [PubMed] [Google Scholar]
- Tomasello D, Schlagenhauf P. 2013. Chikungunya and dengue autochthonous cases in Europe, 2007–2012. Travel Med. Infect. Dis 11(5):274–84 [DOI] [PubMed] [Google Scholar]
- UNDP (United Nations Dev. Progr.). 2017. A socio-economic impact assessment of the Zika virus in Latin America and the Caribbean: with a focus on Brazil, Colombia and Suriname. Rep., UNDP, New York. http://www.undp.org/content/undp/en/home/librarypage/hiv-aids/a-socio-economic-impact-assessment-of-the-zika-virus-in-latin-am.html [Google Scholar]
- USDA For. Serv (US Dep. Agric. For. Serv.). 2008. Who owns America’s forests? Forest ownership patterns and family forest highlights from the National Woodland Owner Survey. Rep. NRS-INF-06–8, North. Res. Stn., USDA For. Serv. https://www.fs.usda.gov/treesearch/pubs/15794 [Google Scholar]
- Vanzetti D, Peters R. 2021. COVID-19 and tourism: an update. Assessing the economic consequences. Rep., United Nations Conf. Trade Dev., Geneva. https://unctad.org/system/files/official-document/ditcinf2021d3_en_0.pdf [Google Scholar]
- Volz EM, Pond SLK, Ward MJ, Brown AJL, Frost SDW. 2009. Phylodynamics of infectious disease epidemics. Genetics 183(4):1421–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC. 2013. Urbanization and geographic expansion of zoonotic arboviral diseases: mechanisms and potential strategies for prevention. Trends Microbiol. 21(8):360–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC. 2014. Arrival of Chikungunya virus in the New World: prospects for spread and impact on public health. PLOS Negl. Trop. Dis 8(6):e2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, et al. 2016. Zika virus: history, emergence, biology, and prospects for control. Antiviral Res. 130:69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb K, Jennings J, Minovi D. 2018. A community-based approach integrating conservation, liveli-hoods, and health care in Indonesian Borneo. Lancet Planet. Health 2 10.1016/S2542-5196(18)30111-6 [DOI] [Google Scholar]
- Weitz JS, Park SW, Eksin C, Dushoff J. 2020. Awareness-driven behavior changes can shift the shape of epidemics away from peaks and toward plateaus, shoulders, and oscillations. PNAS 117(51):32764–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski A, Qureshi T, Boni MF, Sundsoy PR, Johansson MA, et al. 2015. Impact of human mobility on the emergence of dengue epidemics in Pakistan. PNAS 112(38):11887–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organ.). 2004. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Meet. Rep, July 24, WHO, Geneva. https://www.who.mt/publications/rn/item/summary-of-probable-sars-cases-with-onset-of-inness-from-1-november-2002-to-31-july-2003 [Google Scholar]
- WHO (World Health Organ.). 2019. WHO MERS global summary and assessment of risk—July 2019. Situat. Rep., WHO, Geneva. https://www.who.int/publications-detail-redirect/10665-326126 [Google Scholar]
- Wilder-Smith A. 2006. The severe acute respiratory syndrome: impact on travel and tourism. Travel Med. Infect. Dis 4(2):53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A, Gubler DJ. 2008. Geographic expansion of dengue: the impact of international travel. Med. Clin. N. Am 92(6):1377–90 [DOI] [PubMed] [Google Scholar]
- Winkler K, Fuchs R, Rounsevell M, Herold M. 2021. Global land use changes are four times greater than previously estimated. Nat. Commun 12:2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse MEJ, Gowtage-Sequeria S. 2005. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis 11(12):1842–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Fang L-Q, Huang H-G, Zhang L-Q, Feng D, et al. 2007. Landscape elements and Hantaan virus-related hemorrhagic fever with renal syndrome, People’s Republic of China. Emerg. Infect. Dis 13(9):1301–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li J, Lai S, Ruktanonchai CW, Xing W, et al. 2020. Uncovering two phases of early intercontinental COVID-19 transmission dynamics. J. Travel Med 27(8):taaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]