Abstract
To identify Brucella antigens that are potentially involved in stimulating a protective cell-mediated immune response, a gene library of Brucella abortus 2308 was screened for the expression of antigens reacting with immunoglobulin G2a antibodies from BALB/c mice vaccinated with B. abortus RB51. One selected positive clone (clone MCB68) contained an insert of 2.6 kb; nucleotide sequence analysis of this insert revealed two open reading frames (ORFs). The deduced amino acid sequences of the first and second ORFs had significant similarities with the YajC and SecD proteins, respectively, of several bacterial species. Both the YajC and SecD proteins were expressed in Escherichia coli as fusion proteins with maltose binding protein (MBP). In Western blots, sera from mice vaccinated with B. abortus RB51 recognized YajC but not SecD. Further Western blot analysis with purified recombinant YajC protein indicated that mice inoculated with B. abortus 19 or 2308 or B. melitensis RM1 also produced antibodies to YajC. In response to in vitro stimulation with recombinant MBP-YajC fusion protein, splenocytes from mice vaccinated with B. abortus RB51 were able to proliferate and produce gamma interferon but not interleukin-4. This study demonstrates, for the first time, the involvement of YajC protein in an immune response to an infectious agent.
Brucellosis, a chronic infection resulting in abortion and infertility in animals and undulant fever in humans, is caused by Brucella species (1). Brucellae are gram-negative, facultative intracellular bacteria which can survive in macrophages of infected animals. Six well-recognized species of the genus Brucella display a certain host preference, although most can infect humans (6). Humans acquire the infection by ingesting contaminated dairy products or by contact with infected abortion-related animal tissues and secretions. Although some degree of protection can be induced in animals, mainly by vaccination with live attenuated strains, no satisfactory vaccine for humans has been described (6).
Smooth strains of Brucella have an O-polysaccharide chain attached to the core component of lipopolysaccharide, while truly rough Brucella strains completely lack such a structural moiety. Infection with smooth strains usually results in the production of antibodies against the O polysaccharide (30). These antibodies can have a protective role, at least in some animal species like the mouse (3, 4). Nevertheless, there is general consensus that a cell-mediated immune (CMI) response is necessary to induce strong protective immunity in most animal species since Brucella is able to survive within macrophages (4, 17, 36). Adoptive immunity can be induced in naive mice by the passive transfer of either CD4+ or CD8+ cells from immunized mice (4). Recent experimental evidence indicates that the induction of a Th1 type of CMI response with production of gamma interferon (IFN-γ) and generation of cytotoxic CD8+ T cells appears to play an important role in protection against brucellosis, with one major role for IFN-γ being the activation of macrophages (11, 17, 19, 37, 38). Therefore, Brucella protein antigens which can stimulate Th1 responses may have good potential to induce protective immunity if presented to the immune system in an appropriate way.
Many antigens of Brucella have been described and characterized (7, 14, 16, 19, 21, 23, 33, 34, 39), but our understanding regarding the specific antigens involved in the stimulation of a protective Th1 type of CMI response is minimal. Only two specific Brucella antigens which are able to induce a partial, protective CMI response have been described: the L7/L12 ribosomal protein (12a, 18) and certain epitopes of the Cu/Zn superoxide dismutase (31). It is therefore important to continue to search for Brucella antigens able to induce a specific Th1 type of response with IFN-γ production, since such proteins could be used to develop safe and effective vaccines against brucellosis in animals and humans.
Using recombinant DNA methods, we are identifying and characterizing the genes of Brucella abortus proteins which have the potential to stimulate a Th1 response. Our approach to isolating such proteins is screening the genomic library of B. abortus for expressed antigens that react with mouse immunoglobulin G2a (IgG2a) subisotype antibodies, since this subisotype is indicative of a Th1 response (29). By following this screening strategy, we isolated several positive recombinant clones. In this paper, we describe nucleotide sequence analysis of one such clone which contained the yajC and secD genes of B. abortus. Using purified recombinant YajC protein, we further demonstrated that Brucella-infected mice develop humoral and CMI responses to this protein.
MATERIALS AND METHODS
Bacterial strains.
B. abortus vaccine strain RB51 and virulent strain 2308 were from our culture collection and were grown either in Trypticase soy broth or on Trypticase soy agar (TSA) plates as described elsewhere (26). Escherichia coli DH5α (GIBCO BRL, Bethesda, Md.) was used for the recombinant DNA manipulation of Brucella genomic fragments. All experiments with live Brucella were performed in a biosafety level 3 facility.
Antisera.
Mouse antisera to B. abortus 2308, 19, and RB51 and to Brucella melitensis RM1 (a rough strain) were already available in our laboratory. These sera were collected at 8 weeks after intraperitoneal inoculation of BALB/c mice with viable bacteria equivalent to 1 × 106 CFU of strain 2308, 2 × 107 CFU of strain 19, 2 × 108 CFU of strain RB51, and 2 × 107 CFU of strain RM1 (36). For screening the plasmid library, mouse antisera to B. abortus RB51 were pooled and absorbed with E. coli DH5α/pBBR1MCS lysates to remove cross-reactive antibodies as described previously (23). Rabbit antiserum to maltose binding protein (MBP) of E. coli was purchased from New England Biolabs Inc., Beverly, Mass.
In conducting research using animals, we adhered to the National Research Council’s Guide for the Care and Use of Laboratory Animals (5a).
Construction and screening of genomic library.
The molecular biologic techniques performed in this study were based on the standard procedures outlined by Sambrook et al. (24). The genomic DNA of B. abortus 2308 was partially digested with ClaI, and the resulting fragments were cloned into a broad-host-range plasmid, pBBR1MCS (12). E. coli DH5α cells transformed with these recombinant plasmids were screened for antigen expression by a previously described colony immunoblot assay (22) with mouse antisera to strain RB51 as primary antibodies and horseradish peroxidase-conjugated goat anti-mouse IgG2a as secondary antibodies (Caltag Laboratories, San Francisco, Calif.). One such clone, MCB68, was isolated for further characterization.
Sequence analysis.
The Brucella insert from clone MCB68 was subcloned into pBluescript SK(−) (Stratagene, La Jolla, Calif.), and unidirectional nested deletions from either ends of the insert were generated with the Erase-a-Base system (Promega Corp., Madison, Wis.). Nucleotide sequencing of the contigs was performed as previously described (25) with an automated DNA sequencer at the Walter Reed Army Institute for Research (D. Hoover). LaserGene sequence analysis software (DNASTAR, Inc., Madison, Wis.) was used for analyzing the nucleotide sequences for open reading frames (ORFs), inverted repeats, and hydropathy analyses and multiple alignment of protein sequences. Blast programs (2) were used for the homology searches in databases available at the National Biotechnology Information Center. Computer programs available on the Internet were used to predict the potential promoter sequence (the neural-network method of promoter prediction available at Lawrence Berkeley National Laboratory [http://www-hgc.lbl.gov/projects/promoter.html]) and potential transmembrane domains of YajC and SecD proteins (TMpred, prediction of transmembrane regions and orientation, available at the Swiss Institute for Bioinformatics [http://www.isrec.isb-sib.ch/software/TMPRED_form.html]).
Expression in E. coli.
The YajC and partial SecD proteins of B. abortus were expressed in E. coli by using expression vectors pMalP2 and pMalC2, respectively (New England Biolabs Inc.). In these vectors, the cloned foreign gene is expressed as a fusion protein with MBP at the amino terminus so that the recombinant protein can be purified by affinity chromatography with amylose resin. The yajC and secD genes were PCR amplified from the genomic DNA of strain 2308. For each gene, a primer pair consisting of one primer upstream and one primer downstream of the gene was designed based on the nucleotide sequence of the gene (see Fig. 1). A restriction site was engineered into each primer by point mutations. PCR was performed with a 50-μl volume containing assay buffer (10 mM Tris-HCl [pH 9.0], 50 mM KCl, 0.1% Triton X-100), 1.5 mM MgCl2, a mixture of the four deoxynucleoside triphosphates at 250 μM each, a 0.5 μM concentration of each primer, 50 ng of genomic DNA as a template, and 2.5 U of Taq DNA polymerase (Promega). Amplification was performed in an Omni Gene thermocycler (Hybaid Limited) at 95°C for 5 min followed by 30 cycles that each included 1 min of denaturation at 95°C, 2 min of annealing at 54°C, and 2 min of extension at 72°C. The amplified gene fragments were digested with appropriate restriction enzymes and cloned into pMalC2. The recombinant plasmids were electroporated into E. coli DH5α, and single recombinant colonies were selected. Expression and purification of the recombinant proteins were performed according to the manufacturer’s suggested procedures.
FIG. 1.
(A) Schematic diagram of clone MBP68, which contains the yajC and secD genes of B. abortus. The locations of various primers used for PCR amplification of the genes are shown as arrows. The restriction enzyme sites engineered into the primers are indicated. (B) Nucleotide sequences of the primers used for the amplification of the yajC and secD genes.
Overexpression in B. abortus.
The YajC protein was overexpressed in B. abortus RB51 by cloning the gene along with its putative promoter in a broad-host-range vector, pBBR1MCS. The yajC gene from clone MCB68 was amplified with its downstream primer and the T7 primer of the plasmid (see Fig. 1). The amplified fragment was digested with EcoRV and HindIII restriction enzymes and cloned into the same sites of pBBR1MCS. E. coli DH5α cells were transformed with the recombinant plasmid, and colonies containing the plasmid were selected on a TSA plate containing 30 μg of chloramphenicol per ml. From these colonies, plasmid DNA was extracted by using Mini Spinprep (Qiagen Inc., Valencia, Calif.). The plasmid was electroporated into B. abortus RB51 according to previously described procedures (15). B. abortus colonies containing the plasmid were obtained on a TSA plate containing 30 μg of chloramphenicol per ml. A single colony of transformed B. abortus was grown in Trypticase soy broth with chloramphenicol, and bacteria were harvested, heat killed (at 60°C for 20 min), and used for Western blot analysis. Strain RB51 harboring the pBBR1MCS plasmid containing the yajC gene was designated strain RB51/pBByajC.
Western blotting.
Antigens of whole Brucella bacteria, extracts of E. coli expressing the MBP fusion proteins, and purified MBP-YajC protein were separated on 12.5% denaturing polyacrylamide gels by electrophoresis as previously described (13). From the gels the antigens were transferred to nitrocellulose membranes according to a published procedure and developed (35). Briefly, the nitrocellulose membranes were blocked with 3% bovine serum albumin and reacted with various antisera and appropriate horseradish peroxidase-conjugated secondary antibodies. The membranes were developed with substrate solution containing 1-chloro-4-naphthol and hydrogen peroxide.
Lymphocyte proliferation assay.
Assays were carried out as described elsewhere (28). Briefly, three 6-week-old female BALB/c mice (Charles River Laboratories, Wilmington, Mass.) were vaccinated intraperitoneally with 2 × 108 CFU of strain RB51 in 0.5 ml of saline. As a negative control, another three mice were inoculated with 0.5 ml of saline alone. Seven weeks postinoculation, the animals were killed by CO2 asphyxiation and the spleens were obtained. Single cell suspensions were prepared from the spleens of normal and vaccinated mice. After the erythrocytes were lysed with ACK solution (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM Na2EDTA [pH 7.3]), the splenocytes were cultured in 96-well plates at a concentration of 5 × 105 cells/well in the presence of 5 μg of recombinant YajC fusion protein, 5 μg of MBP (purified in a manner similar to that used for the YajC fusion protein), 10 μg of B. abortus RB51 crude extract, 0.5 μg of concanavalin A (ConA), or no additives (unstimulated control). RPMI 1640 medium (GIBCO BRL) supplemented with 2 mM l-glutamine, 10% heat-inactivated fetal bovine serum, and 50 μM 2-mercaptoethanol was used for culturing the cells. The cells were cultured for 3 or 5 days and then pulsed with 1 μCi of [3H]thymidine/well for 18 h. The cells were harvested onto glass fiber filters, and the radioactivity was measured in a liquid scintillation counter and expressed as counts per minute. Assays were performed in triplicate.
The B. abortus RB51 crude extract used for stimulating the lymphocytes was prepared as follows. B. abortus RB51 grown on TSA plates was harvested in sterile distilled water, and the bacteria were killed by adding an equal volume of acetone at room temperature for 3 h with continuous stirring. The killed bacteria were washed twice in distilled water by centrifugation at 8,000 × g for 10 min each time. Finally, the pellet was resuspended in a solution containing 10% NaCl, 4 M urea, and 0.1% 2-mercaptoethanol. After incubation for 24 h in a 40°C water bath with shaking, the unlysed bacterial cells were removed by centrifugation at 8,000 × g for 10 min. The clear supernatants were collected, dialyzed against several changes of distilled water, and lyophilized. The required amount of the lyophilized extract was weighed and dissolved in RPMI 1640 medium and used for lymphocyte stimulation.
Quantitation of cytokines.
After stimulation of the splenocytes with antigen or mitogen as described for the lymphocyte proliferation assay, supernatants of 3- or 5-day-old cultures were tested for the presence of IFN-γ and interleukin-4 (IL-4) by antigen-capture enzyme-linked immunosorbent assays (5). The assays were performed with plates coated with specific capture antibodies purified by protein G affinity chromatography (HiTrap Protein G column; Pharmacia Biotech, Uppsala, Sweden) from rat hybridoma culture supernatants produced in our laboratory (HB-170 [rat anti-murine IFN-γ] and HB-188 [rat anti-murine IL-4]; American Type Culture Collection, Rockville, Md.), biotinylated detection antibodies specific to IFN-γ and IL-4 (PharMingen, San Diego, Calif.), streptavidin-horseradish peroxidase conjugate (Zymed Corp.), and TMB (3,3′,5,5′-tetramethyl benzidine) one-component substrate (Dako Corp., Carpinteria, Calif.). Purified recombinant cytokines (PharMingen) were used for initial optimization of the assays and also as standards each time an assay was performed. The lower detection limits were calculated to be 100 and 10 pg for the IFN-γ and IL-4 assays, respectively. All assays were performed in triplicate, and the amounts of cytokines in the culture supernatants were calculated by using a linear-regression equation obtained from the optical density values of the standards.
Statistical analysis.
The results of lymphocyte proliferation assays were analyzed by one-way analysis of variance, followed by the Student-Newman-Keuls method of all-pairwise multiple comparison. The data for IFN-γ production were analyzed by the Wilcoxon rank sum test. A P value of ≤0.05 was considered significant.
Nucleotide sequence accession number.
The nucleotide sequence of the insert of clone MBP68 that contains the complete yajC gene and the partial secD gene has been submitted to GenBank. The accession no. is AF085217.
RESULTS
Clone MBP68 contains yajC and secD homologs of B. abortus.
Clone MBP68 reacted strongly with the IgG2a subisotype antibodies of mouse antisera to strain RB51. The plasmid extracted from this clone contained an insert 2.6 kb in size. Both strands of the entire insert were sequenced. Sequence analysis revealed two ORFs (Fig. 1 and 2) separated by an intergenic sequence of 100 nucleotides. The first ORF was capable of coding for a 12.5-kDa protein consisting of 113 amino acids. The second ORF was incomplete at the 3′ end and capable of coding for a 54-kDa polypeptide with 518 amino acids. Both ORFs were preceded by a purine-rich ribosomal binding site. A putative promoter sequence was identified upstream of the first ORF, but no such obvious sequences were found in the intergenic sequence. A region containing inverted repeats that can potentially form a hairpin loop was found in the intergenic sequence downstream of the first ORF (Fig. 2). Homology searches performed with the deduced amino acid sequences of first and second ORFs revealed a significant similarity with the YajC and SecD protein homologs, respectively, of several bacterial species (Fig. 3).
FIG. 2.
Nucleotide and deduced amino acid sequences of the yajC and secD genes of B. abortus, present in clone MBP68. The putative promoter region (−10 and −35), transcription start site (+1), and ribosomal binding sites (RBS) are indicated. Inverted repeat sequences (IR) with the potential to form a hairpin loop are also indicated.
FIG. 3.
Multiple alignment of deduced amino acid sequences of the YajC (A) and SecD (B) proteins. Alignment was performed with the Clustal method of the MegAlign program of LaserGene software. Aa, Aquifex aeolicus; Ba, B. abortus; Bs, Bacillus subtilis; Ec, E. coli; Ef, Enterococcus faecalis; Hi, Haemophilus influenzae; Hp, Helicobacter pylori; Rc, Rhodobacter capsulatus. Gaps are indicated by dashes. Amino acids identical for the two sequences are boxed. Based on this multiple alignment, the similarities between the YajC protein of B. abortus and those of the other bacteria are as follows: Aa, 26.5%; Bs, 30.3%; Ec, 27.9%; Ef, 31.7%; Hi, 22.7%; and Hp, 28.1%. The similarities between the SecD protein of B. abortus and those of the other bacteria are as follows: Aa, 23.4%; Ec, 24.7%; Hi, 24.9%; Hp, 26.2%; and Rc, 29.2%.
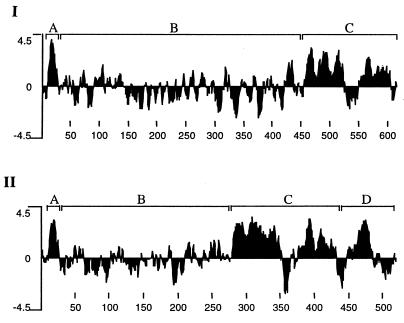
Computer analysis predicted one (amino acids 19 to 38) and six (amino acids 9 to 25, 279 to 297, 301 to 317, 382 to 401, 401 to 422, and 460 to 482) potential transmembrane regions in the YajC and SecD proteins of B. abortus, respectively. Since the multiple alignment of the sequences of SecD proteins revealed several gaps, we compared their hydropathy profiles. As shown in Fig. 4, B. abortus SecD protein contained a region (segment D; segmentation was done for the purpose of easy explanation only) not contained in E. coli SecD protein. We included only E. coli SecD protein in the figure since other SecD proteins had hydropathy profiles similar to that of E. coli SecD. For all the SecD proteins, including that of B. abortus, the profiles of segments A and C were similar. However, there was variation among all SecD proteins in the length of segment B that did not contain major hydrophobic regions. Based on the topology of E. coli SecD protein (20), this segment is probably exposed to periplasmic space. The presence of the extra region (segment D) makes it difficult to estimate the full length of B. abortus SecD protein.
FIG. 4.
Hydrophobic profiles of SecD proteins of E. coli (I) and B. abortus (II). Analysis was performed with the Kyte-Doolittle option of the Protean program of LaserGene software. The numbers at the bottom are the amino acid positions. The profiles are divided into segments for easy explanation in the text.
Expression of recombinant YajC and SecD fusion proteins.
The expression of B. abortus YajC and that of partial SecD proteins were achieved in E. coli as MBP fusions. The extracts of E. coli cells containing the recombinant plasmids were analyzed by Western blotting with rabbit antisera to MBP (Fig. 5A). The observed molecular masses of the recombinant proteins were in accordance with the estimated molecular weights of the MBP fusions: 55 kDa for the YajC function protein and 97 kDa for the SecD fusion protein. A higher degree of proteolytic degradation was observed with the SecD fusion protein than with the YajC protein. The expressed recombinant YajC protein was purified by affinity chromatography on an amylose resin column (data not shown).
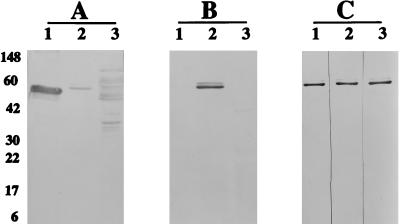
FIG. 5.
Western blot reactivities of recombinant YajC and SecD proteins. (A and B) E. coli extracts expressing MBP fusions of LacZα peptide (lane 1), YajC (lane 2), and SecD (lane 3) were reacted with rabbit antisera to MBP (A) and antisera to B. abortus RB51 (B). (C) Reaction of affinity column-purified MBP-YajC fusion protein with mouse antisera to B. abortus 2308 (lane 1) and 19 (lane 2) and B. melitensis RM1 (lane 3). Horseradish-peroxidase-conjugated secondary antibodies specific to mouse IgG whole molecules and the IgG2a subisotype were used to develop the blots in panels B and C, respectively. Numbers at the left in panel A are approximate protein molecular masses, in kilodaltons. The appearance of a double band in the MBP-YajC lanes is due to some fusion protein with uncleaved signal sequence of the MBP.
Involvement of YajC in immune responses against Brucella infections.
To verify the involvement of YajC and SecD proteins in the immune response against B. abortus, Western blotting was performed with the expressed recombinant proteins and mouse sera against strain RB51. As shown in Fig. 5B, YajC, but not SecD, reacted with the immune sera. The purified recombinant YajC fusion protein also reacted with sera from mice inoculated with live B. abortus 19 and 2308 and B. melitensis RM1 (Fig. 5C). Sera from mice immunized with killed B. abortus RB51 and 19 did not react with the recombinant YajC protein (data not shown).
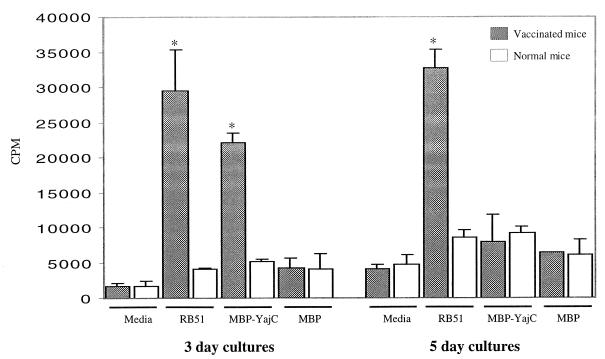
In lymphocyte proliferation assays, the purified recombinant YajC fusion protein stimulated the proliferation of splenocytes from mice vaccinated with B. abortus RB51 but not that of splenocytes from mice inoculated with saline only (Fig. 6). However, this proliferation was noticed in 3-day cultures but not in 5-day cultures. As a negative control, MBP alone was used to stimulate the splenocytes, and no significant proliferation was observed with either of the mouse groups. ConA significantly stimulated the proliferation of splenocytes from both vaccinated and normal mice (data not shown).
FIG. 6.
In vitro proliferation of splenocytes from vaccinated and naive mice. The assays were set up in triplicate, and the cells were either left unstimulated (media alone) or stimulated with antigens. The radioactivity of incorporated [3H]thymidine was measured after 3 or 5 days of culturing. Results were expressed as mean counts per minute ± standard deviation (n = 3). Groups with an asterisk are significantly different from other groups of cultures measured for the same number of days but not from each other.
Culture supernatants of immune splenocytes stimulated with the recombinant YajC protein contained IFN-γ, but the levels were approximately 10 times lower than those induced by the RB51 crude extract or ConA in 5-day cultures and negligible in 3-day cultures (Table 1). IL-4 was not detected except in the cultures stimulated with ConA (data not shown). The observed lack of correlation between splenocyte proliferation and IFN-γ secretion after stimulation with YajC fusion protein was unexpected and probably reflects the variation in the kinetics of activation of different subpopulations of lymphocytes. Experiments with purified T-cell subpopulations need to be performed to address this possibility.
TABLE 1.
Production of IFN-γ by splenocytes of vaccinated and naive mice after in vitro stimulation with specific antigens and mitogen
| Stimulant | Concn of IFN-γ (ng/ml)a
|
|||
|---|---|---|---|---|
| 3-day cultures
|
5-day cultures
|
|||
| Vaccinated mice | Naive mice | Vaccinated mice | Naive mice | |
| Media | —b | — | — | — |
| ConA | 30.55 ± 0.65* | 28.31 ± 2.00* | 27.96 ± 0.43* | 26.05 ± 0.89* |
| RB51 | 29.63 ± 1.00* | — | 29.12 ± 0.30* | — |
| MBP-YajC | 0.42 ± 0.10** | — | 2.90 ± 0.89** | — |
| MBP | — | — | — | — |
Values are means ± standard deviations for three experiments. Groups with two asterisks are significantly different from groups with one asterisk and are significantly different from each other.
—, under lower detection limit.
Immunodetection of YajC in B. abortus RB51.
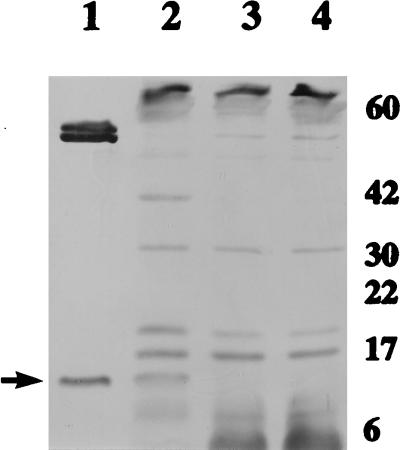
The presence of YajC protein in B. abortus RB51 was examined by Western blot analysis with mouse antisera. YajC was separated from the purified MBP-YajC fusion protein by factor Xa cleavage, and the resulting polypeptides were used as a positive antigen control for the Western blot analysis. No band of the molecular size corresponding to the YajC protein was detected in strain RB51 (Fig. 7). However, significant levels of YajC protein were detected in strain RB51/pBByajC (Fig. 7).
FIG. 7.
Western blot detection of YajC protein in B. abortus RB51. The antigens present in the lanes are as follows: lane 1, MBP-YajC fusion protein partially cleaved by factor Xa; lane 2, extracts of B. abortus RB51/pBByajC; lane 3, extracts of strain RB51 with plasmid alone; lane 4, extracts of strain RB51. The blot was reacted with mice antisera to B. abortus RB51 and horseradish-peroxidase-conjugated goat antibodies specific to mouse IgG whole molecules. The arrow at the left shows the YajC band. The numbers at the right are approximate protein molecular masses, in kilodaltons.
DISCUSSION
In order to identify B. abortus protein antigens that can invoke a Th1 type of CMI response, we screened the genomic library of B. abortus 2308 for expressed antigens that reacted with IgG2a subisotype antibodies of mice vaccinated with protective strain RB51. Clone MBP68 was one of the several positive clones isolated from the library. The deduced amino acid sequences of the two ORFs from clone MBP68 showed homology with YajC and SecD protein homologs of several bacterial species. Even though the gene sequences of these two proteins for several bacterial species are available, studies on their structural and functional characterization have been limited to E. coli. The organization of these two B. abortus genes in an operon appears to be similar to that of E. coli (20). Like in E. coli, a potential hairpin loop structure was also present downstream of the yajC gene of B. abortus. The significance of the presence of this hairpin structure within an operon is not known. Generally, a hairpin loop structure present downstream of a gene functions as a rho-independent transcription terminator. In E. coli, YajC was initially identified as a hypothetical membrane protein whose gene was present in an operon along with the genes encoding SecD and SecF, members of the translocation machinery of secretory proteins (10). Genetic studies with E. coli suggested that YajC could also be involved in the translocation of secretory proteins (8, 9, 32). However, until now, to the best of our knowledge, the presence of YajC protein in any bacteria other than E. coli has not been demonstrated. The functional role of B. abortus YajC protein may be in the translocation of periplasmic or putative secretory proteins. Deletion mutation studies are presently ongoing in our laboratory to determine the role of YajC and SecD proteins in the extra- and intracellular survival of B. abortus.
Western blots developed with sera from Brucella-infected or RB51-vaccinated mice showed strong reaction with the recombinant MBP-YajC fusion protein (Fig. 5B and C), and the reactive antibodies included the IgG2a subisotype. Further, with the recombinant MBP-YajC fusion protein it was possible to stimulate splenocytes from B. abortus RB51-vaccinated mice to proliferate in vitro and produce IFN-γ. These findings clearly indicate that the YajC protein of Brucella is involved in stimulating a Th1 type of immune response in mice, although the levels of IFN-γ induced are low. Vaccination of mice with B. abortus RB51 induces protective immunity (26), and splenocytes from such mice produce substantial levels of IFN-γ when stimulated in vitro with the antigen extract of the immunizing strain, as demonstrated in this study and by others (27). This finding is consistent with the hypothesis that a protective immune response against Brucella needs the production of the Th1 type of cytokine IFN-γ. The levels of IFN-γ produced by the MBP-YajC-stimulated splenocytes of vaccinated mice were significantly lower than those stimulated with the strain RB51 antigen extract (Table 1). Similar observations were also reported for other recombinant Brucella antigens, including the L7/L12 protein, which was shown to be involved in stimulating a protective immune response (16, 18). Hence, the observed low levels of IFN-γ produced by the splenocytes stimulated in vitro do not exclude a role for YajC in protective immunity. As demonstrated before by others with a variety of other Brucella antigens (16, 18, 38), neither the RB51 extract nor the recombinant MBP-YajC protein stimulated the production of detectable levels of IL-4, suggesting that a Th2 type of response to these antigens is not a predominant component of the immune response. In Western blots, the lack of reactivity of mouse anti-RB51 sera with the MBP-SecD protein suggests that SecD was unable to induce antibodies in these mice. Since no further immunological studies were carried out with the MBP-SecD protein, a CMI response to this protein in the absence of a humoral response cannot be ruled out at this time; further studies are needed to confirm this.
The presence of YajC in B. abortus RB51 could be demonstrated only upon overexpression of the gene (Fig. 7). This indicates that, like in E. coli, where overexpression was also needed to demonstrate the presence of the YajC protein (8), a low level of YajC expression is seen in B. abortus. This low level of expression is probably responsible for the observed lack of antibody response to YajC protein in mice vaccinated with killed B. abortus vaccines. However, during a Brucella infection, YajC is produced in quantities sufficient to stimulate the immune responses demonstrated in this study. These results demonstrate, for the first time, the involvement of YajC protein in an immune response to an infectious agent. We are presently conducting investigations in our laboratory, using the purified recombinant protein, to determine the role of YajC in the induction of immune responses in vaccinated and infected animals such as cattle, pigs, and goats.
ACKNOWLEDGMENTS
This project was supported by the U.S. Army Medical Research, Development, Acquisition and Logistics Command (Prov.) under contract DAMD 17-94-C-4042.
We thank D. Ward of the Support Laboratory for Study Design and Statistical Analysis for his assistance in the data analysis.
REFERENCES
- 1.Acha P, Szyfres B. Zoonoses and communicable diseases common to man and animals. Washington, D.C: Pan American Health Organization; 1980. pp. 28–45. [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Araya L N, Elzer P H, Rowe G E, Enright F M, Winter A J. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J Immunol. 1989;143:3330–3337. [PubMed] [Google Scholar]
- 4.Araya L N, Winter A J. Comparative protection of mice against virulent and attenuated strains of Brucella abortus by passive transfer of immune T cells or serum. Infect Immun. 1990;58:254–256. doi: 10.1128/iai.58.1.254-256.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coligan J E, Kruisbeek A M, Marulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. Vol. 1. New York, N.Y: John Wiley & Sons, Inc.; 1991. pp. 6.8.1–6.8.8. [Google Scholar]
- 5a.Committee on the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council) Guide for the care and use of laboratory animals. NIH publication no. 86-23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 6.Corbel M J. Brucellosis: an overview. Emerg Infect Dis. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denoel P A, Vo T K-O, Tibor A, Weynants V E, Trunde J-M, Dubray G, Limet J N, Letesson J-J. Characterization, occurrence, and molecular cloning of a 39-kilodalton Brucella abortus cytoplasmic protein immunodominant in cattle. Infect Immun. 1997;65:495–502. doi: 10.1128/iai.65.2.495-502.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duong F, Wickner W. Distinct catalytic roles of the SecYE, SecG and SecDFyajC subunits of preprotein translocase holoenzyme. EMBO J. 1997;16:2756–2768. doi: 10.1093/emboj/16.10.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duong F, Wickner W. The SecDFyajC domain of preprotein translocase controls preprotein movement by regulating SecA membrane cycling. EMBO J. 1997;16:4871–4879. doi: 10.1093/emboj/16.16.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardel C, Johnson K, Jacq A, Beckwith J. The secD locus of E. coli codes for two membrane proteins required for protein export. EMBO J. 1990;9:3209–3216. doi: 10.1002/j.1460-2075.1990.tb07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang X, Baldwin C L. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun. 1993;61:124–134. doi: 10.1128/iai.61.1.124-134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovach M E, Phillips R W, Elzer P H, Roop II R M, Peterson K M. pBBR1MCS: a broad-host-range cloning vector. BioTechniques. 1994;16:800–802. [PubMed] [Google Scholar]
- 12a.Kurar E, Splitter G. Nucleic acid vaccination of Brucella abortus ribosomal L7/L12 gene elicits immune response. Vaccine. 1997;15:1851–1857. doi: 10.1016/s0264-410x(97)00140-0. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Mayfield J E, Bricker B J, Godfrey H, Crosby R M, Knight D J, Halling S M, Balinsly D, Tabatabai L B. The cloning, expression, and nucleotide sequence of a gene coding for an immunogenic Brucella abortus protein. Gene. 1988;63:1–9. doi: 10.1016/0378-1119(88)90540-9. [DOI] [PubMed] [Google Scholar]
- 15.McQuiston J R, Schurig G G, Sriranganathan N, Boyle S M. Transformation of Brucella species with suicide and broad host-range plasmids. Methods Mol Biol. 1995;47:143–148. doi: 10.1385/0-89603-310-4:143. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira S C, Harms J S, Banai M, Splitter G A. Recombinant Brucella abortus proteins that induce proliferation and gamma-interferon secretion by CD4+ T cells from Brucella-vaccinated mice and delayed-type hypersensitivity in sensitized guinea pigs. Cell Immunol. 1996;172:262–268. doi: 10.1006/cimm.1996.0241. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira S C, Splitter G A. CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur J Immunol. 1995;25:2551–2557. doi: 10.1002/eji.1830250922. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira S C, Splitter G A. Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine. 1996;14:959–962. doi: 10.1016/0264-410x(96)00018-7. [DOI] [PubMed] [Google Scholar]
- 19.Oliveira S C, Zhu Y, Splitter G A. Recombinant L7/L12 ribosomal protein and γ-irradiated Brucella abortus induce a T-helper 1 subset response from murine CD4+ T cells. Immunology. 1994;83:659–664. [PMC free article] [PubMed] [Google Scholar]
- 20.Pogliano K J, Beckwith J. Genetic and molecular characterization of the Escherichia coli secD operon and its products. J Bacteriol. 1994;176:804–814. doi: 10.1128/jb.176.3.804-814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roop R M, II, Fletcher T W, Sriranganathan N M, Boyle S M, Schurig G G. Identification of an immunoreactive Brucella abortus HtrA stress response protein homolog. Infect Immun. 1994;62:1000–1007. doi: 10.1128/iai.62.3.1000-1007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roop R M, II, Preston-Moore D, Bagchi T, Schurig G G. Rapid identification of smooth Brucella species with a monoclonal antibody. J Clin Microbiol. 1987;25:2090–2093. doi: 10.1128/jcm.25.11.2090-2093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roop R M, II, Price M L, Dunn B E, Boyle S M, Sriranganathan N, Schurig G G. Molecular cloning and nucleotide sequence analysis of the gene encoding the immunoreactive Brucella abortus Hsp60 protein, BA60K. Microb Pathog. 1992;12:47–62. doi: 10.1016/0882-4010(92)90065-v. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schurig G G, Roop R M, Bagchi T, Boyle S, Buhrman D, Sriranganathan N. Biological properties of RB51: a stable rough strain of Brucella abortus. Vet Microbiol. 1991;28:171–188. doi: 10.1016/0378-1135(91)90091-s. [DOI] [PubMed] [Google Scholar]
- 27.Stevens M G, Olsen S C, Palmer M V, Pugh G W., Jr Immune responses and resistance to brucellosis in mice vaccinated orally with Brucella abortus RB51. Infect Immun. 1996;64:4534–4541. doi: 10.1128/iai.64.11.4534-4541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens M G, Olsen S C, Pugh G W, Jr, Palmer M V. Immune and pathologic responses in mice infected with Brucella abortus 19, RB51, or 2308. Infect Immun. 1994;62:3206–3212. doi: 10.1128/iai.62.8.3206-3212.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens T L, Bossie A, Sanders V M, Fernandez-Botran R, Coffman R L, Mosmann T R, Vitetta E S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland S S, Searson J. The immune response to Brucella abortus: the humoral immune response. In: Nielsen K, Duncan J R, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 65–81. [Google Scholar]
- 31.Tabatabai L B, Pugh G W., Jr Modulation of immune responses in Balb/c mice vaccinated with Brucella abortus Cu-Zn superoxide dismutase synthetic peptide vaccine. Vaccine. 1994;12:919–924. doi: 10.1016/0264-410x(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 32.Taura T, Akiyama Y, Ito K. Genetic analysis of SecY: additional export-defective mutations and factors affecting their phenotypes. Mol Gen Genet. 1994;243:261–269. doi: 10.1007/BF00301061. [DOI] [PubMed] [Google Scholar]
- 33.Tibor A, Saman E, de Wergifosse P, Cloeckaert A, Limet J N, Letesson J-J. Molecular characterization, occurrence, and immunogenicity in infected sheep and cattle of two minor outer membrane proteins of Brucella abortus. Infect Immun. 1996;64:100–107. doi: 10.1128/iai.64.1.100-107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tibor A, Weynants V, Denoel P, Lichtfouse B, De Bolle X, Saman E, Limet J N, Letesson J-J. Molecular cloning, nucleotide sequence, and occurrence of a 16.5-kilodalton outer membrane protein of Brucella abortus with similarity to PAL lipoproteins. Infect Immun. 1994;62:3633–3639. doi: 10.1128/iai.62.9.3633-3639.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winter A J, Schurig G G, Boyle S M, Sriranganathan N, Bevins J S, Enright F M, Elzer P H, Kope J D. Protection of BALB/c mice against homologous and heterologous species of Brucella by rough strain vaccines derived from Brucella melitensis and Brucella suis biovar 4. Am J Vet Res. 1996;57:677–683. [PubMed] [Google Scholar]
- 37.Zhan Y, Cheers C. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect Immun. 1993;61:4899–4901. doi: 10.1128/iai.61.11.4899-4901.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhan Y, Yang J, Cheers C. Cytokine response of T-cell subsets from Brucella abortus-infected mice to soluble brucella proteins. Infect Immun. 1993;61:2841–2847. doi: 10.1128/iai.61.7.2841-2847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y, Oliveira S C, Splitter G A. Isolation of Brucella abortus ssb and uvrA genes from a genomic library by use of lymphocytes as probes. Infect Immun. 1993;61:5339–5344. doi: 10.1128/iai.61.12.5339-5344.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]