Abstract
Nonalcoholic fatty liver disease (NAFLD) is a component of the metabolic syndrome, with a clinical spectrum ranging from simple fatty liver to steatohepatitis, cirrhosis, and hepatocellular carcinoma. The primary event of NAFLD is the accumulation of triacylglycerols (TAGs) in hepatocytes. In this issue of the JCI, Donnelly et al. report on their use of stable isotope methodology to show that fatty acids stored in adipose tissue and fatty acids newly made within the liver through de novo lipogenesis are the major sources of TAGs in the liver and are secreted as lipoproteins in NAFLD.
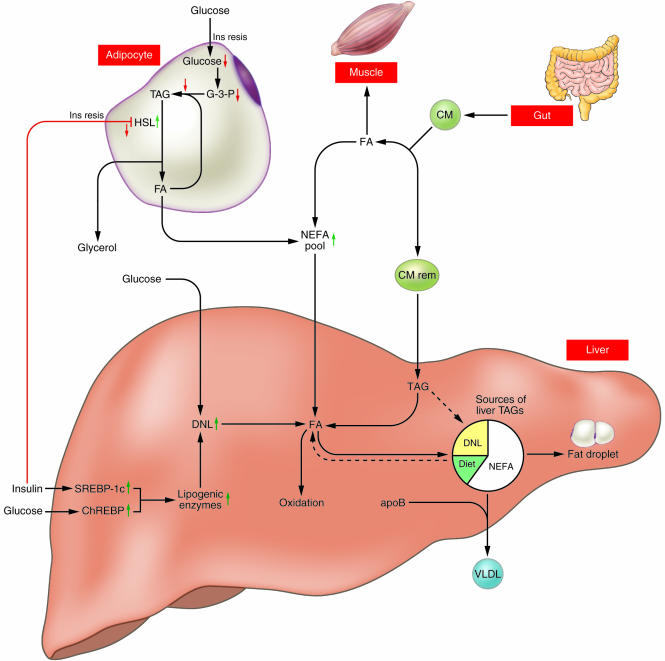
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of abnormal results in liver-function tests. This clinical situation is really a continuum of diseases that includes simple (benign) fatty liver, nonalcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (1). NAFLD is defined as liver steatosis in patients who do not consume enough alcohol to cause hepatic injury. Although some drugs or genetic abnormalities can cause NAFLD, the majority of cases are associated with obesity, insulin resistance, and type 2 diabetes. In order to clarify the pathogenesis of this disease and identify potential therapeutic targets, an increased understanding of the dynamics of triacyl-glycerol (TAG) metabolism in the liver in relation to whole-body metabolic status is needed. Sources of hepatic TAGs include dietary TAGs that are transported via chylomicrons from the intestine to adipose tissue or to the liver, where they are secreted via lipoproteins, in addition to TAGs that are synthesized from fatty acids and glycerol in the liver. Fatty acids required for TAG synthesis are available from both the plasma nonesterified fatty acid (NEFA) pool and the pool of fatty acids newly synthesized within the liver through de novo lipogenesis (DNL). TAGs present in the liver may be stored as lipid droplets or secreted into the blood as VLDLs; they may also enter the oxidation pathway (Figure 1). The study by Donnelly et al. in this issue of the JCI examines hepatic lipoprotein metabolism in humans and describes the major sources of hepatic and plasma lipoprotein TAG in NAFLD (2).
Figure 1.
A 6-month-old girl with typical clinical findings of Caffey disease, which appeared at age 7 weeks. The painful swelling of soft tissue around the legs (A) is matched by severe involvement of the tibia and fibula bilaterally (x-radiography, B). Femurs are strikingly unaffected.
The NEFA pool
Donnelly et al. found that the plasma NEFA pool accounts for approximately 60% of TAG content in the livers of NAFLD patients, which reflects the importance of the NEFA pool in the pathogenesis of NAFLD (2). The hepatic uptake of fatty acids is not regulated and, as a result, plasma fatty acid concentration is directly related to the influx of fatty acids to the liver. The authors report that, in the fasted state, adipose tissue contributes approximately 80% of fatty acid content to the plasma NEFA pool, and even in the fed state, this contribution remains at approximately 60%. Thus, the overproduction of fatty acids in adipose tissues that flow to the liver via the NEFA pool is the most likely explanation for excess TAG accumulation in NAFLD. In insulin-resistant states, insulin does not fully suppress the activity of hormone-sensitive lipase, which catalyzes the hydrolytic release of fatty acids from the TAG moiety and results in enhanced lipolysis and flux of fatty acids to the plasma NEFA pool (Figure 1). In addition, reduced glucose uptake due to insulin resistance reduces glycerol-3-phosphate levels, thereby reducing the reutilization of fatty acids for TAG synthesis (Figure 1). Thus, the insulin resistance in adipose tissue is important as a pathogenic factor of NAFLD. Recently, dysregulated adipocytokines such as adiponectin and TNF-α have been examined as causative candidates of insulin resistance (3), and it was recently reported that oxidative stress in accumulated fat causes dysregulated adipocytokine production (4). In light of these findings, it seems possible that the reduction of oxidative stress as well as the use of insulin-sensitizing agents such as thiazolidinediones and metformin may prove to be successful treatments for NAFLD.
Contrary to the results reported here, Diraison et al. reported that the uptake of plasma NEFAs by the liver was not increased in NAFLD (5). However, Diraison et al. acknowledged that the rate of NEFA flux to the liver may have been underestimated due to the entry of plasma NEFAs into the liver via portal flow from visceral adipose tissue. Thus, in order to establish the contribution of NEFAs to TAG accumulation in the liver, the actual rate of NEFA uptake in the liver in NAFLD must be clarified.
De novo lipogenesis
In healthy human subjects, the contribution of DNL in the liver to TAG content in the fasted state is very small (less than 5% for VLDL-TAG) (6). It has been reported that DNL in the liver is elevated in insulin-resistant states and in NAFLD (7). Donnelly et al. found that DNL accounted for 26% of liver TAG content in hyperinsulinemic subjects with NAFLD (2). In healthy subjects, DNL is elevated following meals (23% for VLDL-TAG) (6), which can be accounted for by elevation in the circulating levels of lipogenesis precursors. However, in NAFLD, DNL was already elevated in the fasted state, and further postprandial elevation was not observed. Indeed, constant elevation of DNL was also observed in subjects fed a diet high in simple carbohydrates for 25 days (8). These observations reflect the sustained elevation of enzymes involved in hepatic DNL.
The regulation of enzymes involved in human lipogenesis can be precisely studied by experiments in animals. Insulin activates the membrane-bound transcription factor SREBP-1c, which transcriptionally activates most genes required for lipogenesis. In mice, even in the insulin-resistant state, insulin stimulates hepatic SREBP-1c transcription and increases lipogenesis (9). Lipogenesis is also regulated by glucose: glucose activates the carbohydrate response element–binding protein (ChREBP), which induces gene expression of liver-type pyruvate kinase, a key regulatory enzyme in glycolysis; this enzyme in turn provides the precursors for lipogenesis (10). ChREBP also stimulates gene expression of most enzymes involved in lipogenesis (11). Hyperinsulinemia and hyperglycemia may also induce these transcriptional factors in humans (Figure 1), although this remains to be directly demonstrated.
Other factors
Mice with peripheral insulin resistance due to adipose- and muscle-specific GLUT4 double knockout showed increased DNL accompanied by hepatic elevation of SREBP-1c and acetyl-CoA carboxylase (12). However, in these mice, increased DNL did not result in increased TAG level in the liver, nor did it increase steady-state serum levels of TAGs or NEFAs; this may be the result of a compensatory enhancement of peripheral fatty acid usage. Unlike in this animal model, such compensation is assumed to be insufficient in human subjects with type 2 diabetes (13). The reduced use of fatty acids in peripheral tissues in combination with elevated DNL may contribute to TAG accumulation in the livers of subjects with NAFLD. Muscle exercise is effective in reducing TAG accumulation in the liver not only by improving insulin resistance but also by enhancing peripheral fatty acid disposal.
The accumulation of TAGs in the liver results from an imbalance among the uptake, synthesis, export, and oxidation of fatty acids. In type 2 diabetes, secretion of VLDL is increased due to insulin resistance (14). It has been reported, however, that in NAFLD, VLDL secretion is not increased compared with secretion in controls (5). Because of methodological differences, these results cannot be directly compared. Secretion rates of hepatic VLDL should be quantified in hyperinsulinemic subjects with and without hepatic steatosis. β-oxidation of fatty acids in the liver was found to be increased in patients with NASH (15). However, the increase is not sufficient for overcoming elevated rates of hepatic TAG synthesis. The increase in NEFA oxidation may account for the apparent oxidative stress that causes hepatic injury in NAFLD.
In the study by Donnelly et al. (2), considerable variation was present in liver TAG level as well as in the degrees of inflammation and fibrosis in the liver. In some cases of NASH, the amount of accumulated fat in the liver decreased as the disease progressed to cirrhosis (16). In addition, the degree of liver damage may influence lipid metabolism and apparent insulin sensitivity (15, 17). Thus, to clarify the earliest events in TAG accumulation precisely, metabolic analysis of patients with simple fatty liver (i.e., without hepatitis) is needed.
Donnelly et al. (2) show that the contributions of fatty acid sources of TAG in VLDL are almost the same as those for the TAG pool in the liver. Although these findings result from studies of NAFLD patients, the approach used will allow us to further investigate this in vivo biochemistry at various stages in the development of NAFLD, as well as the effects of diet, exercise, and pharmacological therapies on the disease.
Footnotes
See the related article beginning on page 1343.
Nonstandard abbreviations used: ChREBP, carbohydrate responsive element–binding protein; DNL, de novo lipogenesis; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NEFA, nonesterified fatty acid; TAG, triacylglycerol.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Tolman KG, Fonseca V, Tan MH, Dalpiaz A. Narrative review: hepatobiliary disease in type 2 diabetes mellitus. Ann. Intern. Med. 2004;141:946–956. doi: 10.7326/0003-4819-141-12-200412210-00011. [DOI] [PubMed] [Google Scholar]
- 2.Donnelly KL, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115:1343–1351. doi:10.1172/JCI200523621. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeda N, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa S, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004;114:1752–1761. doi:10.1172/JCI200421625. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–489. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 6.Timlin MT, Parks EJ. Temporal pattern of de novo lipogenesis in the postprandial state in healthy men. Am. J. Clin. Nutr. 2005;81:35–42. doi: 10.1093/ajcn/81.1.35. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz J-M, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am. J. Clin. Nutr. 2003;77:43–50. doi: 10.1093/ajcn/77.1.43. [DOI] [PubMed] [Google Scholar]
- 8.Hudgins LC, et al. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J. Clin. Invest. 1996;97:2081–2091. doi: 10.1172/JCI118645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimomura I, Bashmakow Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J. Biol. Chem. 1999;274:30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita H, et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Nat. Acad. Sci. U. S. A. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotani K, Peroni OD, Minokoshi Y, Boss O, Kahn BB. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J. Clin. Invest. 2004;114:1666–1675. doi:10.1172/JCI200421341. doi: 10.1172/JCI21341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 14.Cummings MH, et al. Increased hepatic secretion of very-low-density lipoprotein apolipoprotein B-100 in NIDDM. Diabetologia. 1995;38:959–967. doi: 10.1007/BF00400586. [DOI] [PubMed] [Google Scholar]
- 15.Sanyal AJ, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 16.Powell EE, et al. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 17.Schattenberg JM, Wang Y, Singh R, Rigoli RM, Czaja MJ. Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J. Biol. Chem. 2005;280:9887–9894. doi: 10.1074/jbc.M410310200. [DOI] [PubMed] [Google Scholar]