Abstract
For approximately 80 years following Alzheimer’s description of the disease that bears his name, a gulf divided researchers who believed that extracellular deposits of the amyloid β (Aβ) peptide were pathogenic from those who believed that the deposits were secondary detritus. Since 1990, the discoveries of missense mutations in the Aβ peptide precursor (APP) and the APP-cleaving enzyme presenilin 1 (PS1) have enabled much progress in understanding the molecular, cellular, and tissue pathology of the aggregates that accumulate in the interstices of the brains of patients with autosomal dominant familial Alzheimer disease (AD). Clarification of the molecular basis of common forms of AD has been more elusive. The central questions in common AD focus on whether cerebral and cerebrovascular Aβ accumulation is (a) a final neurotoxic pathway, common to all forms of AD; (b) a toxic by-product of an independent primary metabolic lesion that, by itself, is also neurotoxic; or (c) an inert by-product of an independent primary neurotoxic reaction. Antiamyloid medications are entering clinical trials so that researchers can evaluate whether abolition of cerebral amyloidosis can mitigate, treat, or prevent the dementia associated with common forms of AD. Successful development of antiamyloid medications is critical for elucidating the role of Aβ in common AD.
Alzheimer disease (AD) is the most common cause of dementia, a clinicopathological state whose name literally means “loss of the ability to think.” There is much disagreement even among AD specialists about the basic nature of the disease, and that controversy is no better illustrated than in contemplation of how the disease is initiated at the molecular level. Histologically, the neuronal cytoskeleton twists, literally, into structures called neurofibrillary tangles (NFTs). Outside the cell, the amyloid β (Aβ) peptide aggregates into clumps called oligomers, which accumulate and form deposits called amyloid plaques. Based on studies of a syndrome known as mild cognitive impairment (MCI) (a possible prodrome to dementia), the development of detectable entorhinal NFTs is considered to be the histological correlate of MCI and, many believe, the harbinger of incipient AD (1). Still, levels of cortical synaptic markers correlate with cognitive status at time of death better than do either plaque load or tangle load (2), which is consistent with the concept that neurotransmission failure is the proximate cause of cognitive decline.
Amyloid is a highly ordered precipitate of extracellular protein, misnamed “starch-like” by Rudolf Virchow because of its reactivity to the PAS stain (3). Systemic amyloid deposits can occur in any organ and are often large and amorphous; cerebral amyloid deposits take the form of delimited, miliary spheres called plaques. Plaques contain a trace amount of glycosaminoglycans, which explains the PAS positivity. To the neuropathologist, the diagnosis of “amyloid” is applied to any proteinaceous tissue precipitate that binds the dye Congo red. Congophilia is a property of all amyloids and is related to the defining ability of these precipitates to form β-pleated sheets that subsequently assemble into fibrils (4).
In AD, brain amyloid is composed almost entirely of a 4 kDa amyloid β (Aβ) peptide (5) that exhibits microheterogeneity in amino acid sequence and in a variety of biophysical states (Figure 1). Most Aβ is comprised of a peptide designated Aβ40, Aβ40, Aβ1–40, or, in some cases, Aβx–40. Peptides with various amino termini, all bearing an identical carboxyl terminus, form a major proportion (greater than 95%) of the total Aβ produced by cells (6). A minor fraction (less than 5%) of the newly generated Aβ ends at residue 42 (6). This “long Aβ” (also abbreviated as Aβ1–42, Aβ42, Aβ42, or Aβx–42, the latter 3 representing species with heterogeneous amino termini) is much more aggregatable than Aβ40; hence, “long Aβ’’ is believed to initiate the formation of oligomers, fibrils, and plaques (7).
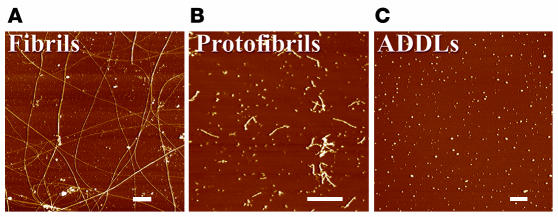
Figure 1.
Different assembly (biophysical) states of Aβ. The assembled forms obtained from incubation of synthetic Aβ are highly sensitive to preparation and incubation. Widely differing proportions of insoluble fibrils (A), soluble PFs (B), and oligomers (also known as ADDLs) are revealed by atomic force microscopy. Typical PF and fibril preparations contain varying levels of small globular molecules, putatively Aβ oligomers (ADDLs). ADDL preparations (C) initiated from monomeric dimethyl sulfoxide stock solutions are fibril- and PF-free and uniquely comprise oligomers. Scale bars: 200 nm. Figure reproduced with permission from Trends in Neurosciences (8).
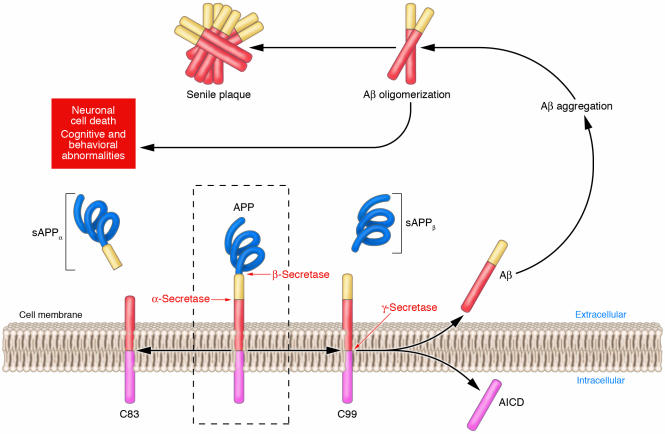
The generation of Aβ from its precursor, the Aβ peptide precursor (APP), is illustrated in Figure 2. APP is first cleaved at the amino terminus of Aβ by a membrane-bound aspartyl protease (β-secretase). This cleavage generates a large secreted derivative (soluble APPβ [sAPPβ]) and a membrane-bound β-cleaved carboxyterminal fragment of APP (CTFβ; also known as C99). Cleavage of CTFβ by γ-secretase results in the production of the Aβ40 and Aβ42 species described above. The term “soluble Aβ” generally is applied either to newly generated, cell-secreted Aβ or to that fraction of tissue or synthetic Aβ that is taken into the aqueous phase of a non–detergent-containing extraction buffer. “Misfolded” and “aggregated” Aβ are terms used to describe very early, nonspecific changes in Aβ folding states or solubility states, respectively (e.g., aggregated Aβ solutions usually scatter light to a greater extent than do solutions of soluble Aβ). “Oligomeric” Aβ refers to peptide assemblies with limited stoichiometry (e.g., dimers, trimers, etc.), while protofibrils (PFs) are structures of intermediate order between aggregates and fibrils. The term “Aβ-derived diffusible ligands” (ADDLs) is also applied to pre-protofibrillar intermediates (Figure 1), based less on a structural definition than on the neurotoxic activity of these oligomers. Indeed, oligomers, PFs, and ADDLs are believed to be the assembly states of Aβ with the most potent toxicity and are believed by many in the field to be the proximate mediators of Aβ-induced neurotoxicity, especially in primary neuronal culture models (8, 9). The final assemblies, called fibrils, are the basic building blocks of the amyloid plaque (Figure 3) and are so named because of their characteristic ultrastructural appearance.
Figure 2.
APP processing and Aβ accumulation. Mature APP (center, inside dashed box) is metabolized by 2 competing pathways, the α-secretase pathway that generates sAPPα and C83 (also known as CTFα; left) and the β-secretase pathway that generates sAPPβ and C99 (right). Some β-secretase cleavage is displaced by 10 amino acid residues and generates sAPPβ′ and C89 (see Figure 4). All carboxyterminal fragments (C83, C99, and C89) are substrates for γ-secretase, generating the APP intracellular domain (AICD) and, respectively, the secreted peptides p3 (not shown), Aβ (right), and Glu11 Aβ (see Figure 4). Aβ aggregates into small multimers (dimers, trimers, etc.) known as oligomers. Oligomers appear to be the most potent neurotoxins, while the end stage senile plaque is relatively inert.
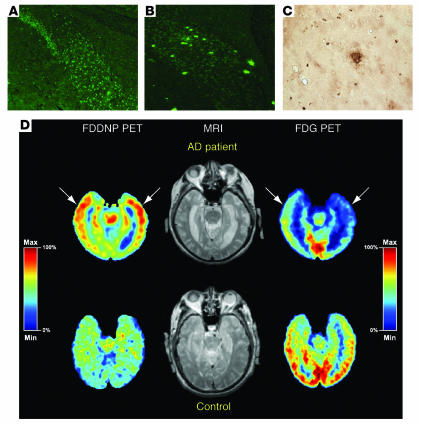
Figure 3.
Amyloid plaque–forming transgenic mice and positron emission tomography (PET) scans of amyloid plaque load in normal human subjects and subjects with AD. (A) Thioflavin staining of subiculum of control mouse, aged 14 months. Fluorescence is nonspecific and cellular. Magnification, ×20. (B) Thioflavin staining of littermate, mutant APP X mutant PS mouse, demonstrating thioflavin-positive amyloid plaques. Magnification, ×20. (C) Immunostaining of amyloid plaque from cortex from same mouse as in B. Magnification, ×40. Figures courtesy of Michelle Ehrlich (Thomas Jefferson University). (D) [18F]FDDNP PET scan (to examine amyloid plaque and NFT load), MRI, and fluoro-deoxy-glucose (FDG) PET (to examine glucose metabolism) images of a subject with AD and a control normal subject. The [18F]FDDNP and FDG (summed) images are coregistered to their respective MRI images. Areas of FDG hypometabolism (blue) are matched with the localization of amyloid plaques and NFTs as visualized by [18F]FDDNP binding. The color bar represents the scaling of the [18F]FDDNP and FDG images. FDDNP, 2-(1-[6-[(2-[18F]fluoroethyl)(methyl)amino]-2-naphthyl]ethylidene)malononitrile; max, maximum; min, minimum. Figure reproduced with permission from the American Journal of Geriatric Psychiatry (62).
Amyloids exhibit a typical exponential growth property known as seeding, which means that once a few fibrils are formed, they instruct the misfolding of other amyloidogenic peptides. The transmissibility of another amyloidosis, prion disease, can be viewed as an extreme example of seeding. In that case, the activation energy and favorability of alternative, neurotoxic forms of the prion protein are believed to permit aggressive propagation of fibrillogenesis. Of note, prion diseases, such as Creutzfeldt-Jakob disease, provide the clearest heuristic evidence that amyloids can be neurotoxic and that amyloid plaques per se are not required for neurotoxicity and clinical disease (10).
What is the relationship between amyloidosis and cognitive decline?
Neuropathologists have long recognized that — among the various structural markers of AD — plaque burden is the poorest correlate of cognitive status at the time of death. As more refined techniques for measuring Aβ levels have become available (e.g., ELISA), this correlation has been revisited again and again. Early ELISA correlations between brain Aβ levels and cognitive status were not much better than the histological correlation of cognitive status and plaque load, due, at least in part, to the seeding phenomenon: once fibrils and plaques begin to form, their concentrations rise rapidly, over several orders of magnitude. The massive levels of plaque Aβ achieved by exponential fibrillogenesis are not readily quantitatively solubilizable, and the interindividual differences in solubilizable Aβ are believed to be a source of much variability and noise in studies aimed at determining clinicopathological correlation between amyloid load and cognitive status, since small amounts of differences in amyloid solubilizabililty can cause dramatic variability in Aβ levels measured by ELISA. More recently, Naslund et al. revisited the correlation between Aβ and cognitive status using brains from well-characterized subjects at the threshold between MCI and dementia, where contamination by plaque Aβ is least problematic (11). In this study, for the first time, a strong correlation between Aβ concentration and cognitive status was documented. These results dovetail well with data obtained using transgenic mice that possess a mutant form of APP (Tg2576 mice), which show that memory deficits correlate with Aβ elevation and precede plaque formation (12). More recently, brain and cerebrospinal fluid levels of Aβ oligomers (ADDLs) have been reported to correlate closely with cognitive status (13, 14). Taken together, these 3 studies (11–14) go a long way toward resolving the heretofore apparently poor correlation between cognition and either Aβ levels or amyloid load.
What is the strongest evidence that AD can ever begin with amyloidosis?
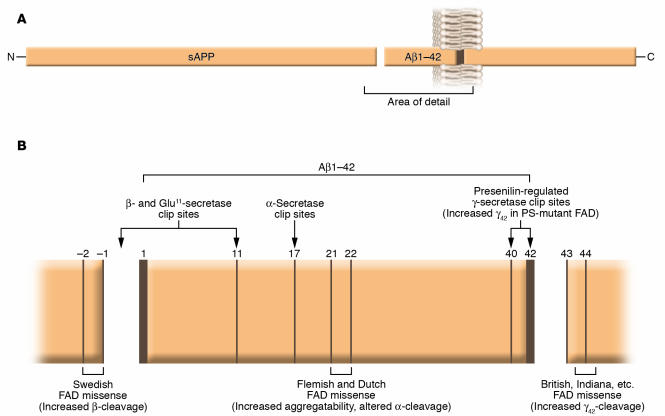
Genetic evidence links altered Aβ metabolism to the rare subset of AD that is autosomal dominant and completely penetrant. These forms of AD share a single common feature, and that is the facilitation of oligomerization of Aβ. Usually the phenotype involves an obvious change in the proteolytic processing of APP. Missense mutations within APP are clustered around the N and C termini of the Aβ domain, and the usual phenotype involves increased generation of the hyperaggregatable Aβ species that end(s) at residue 42 (Figure 4; reviewed in ref. 15). Familial AD pedigrees have been identified worldwide, and the geography of the initial discovery is often used to name the mutant molecule (e.g., Sweden, Holland, Britain, Indiana, Flanders, etc). A few mutations occur within the Aβ domain that result in increased intrinsic aggregatability of all Aβ species. A recently discovered pathogenic mutation, found in AD patients from northern Sweden, near the Arctic Circle, actually decreases the levels of total Aβ generation, yet the molecules that are generated have an increased propensity to oligomerize (16, 17). Pathogenic APP mutations are the rarest known genetic cause of familial AD, being responsible for disease in only about 50 families worldwide. Still, the existence of familial AD due to proamyloidogenic APP mutations around the Aβ domain and the final common phenotype (promotion of Aβ aggregation) provides the most compelling evidence that the entire clinicopathological picture of AD can — at least sometimes — begin with Aβ aggregation. None of these pathogenic mutations has ever been identified in any individual who failed to develop dementia, with the notable exception of a handful of members of a mutant APP family who possessed not only a mutant form of APP but also the ε2 allele of the APOE gene (18). APOE polymorphisms are discussed in more detail below, but the rare cases in which both mutant APP and the APOE ε2 polymorphism coexist in the same individuals provide the best evidence that APOE ε2 can protect against the development of AD.
Figure 4.
(A) Structure and topology of APP. (B) The fine structure around the Aβ domain, secretase cleavage sites, and locations of some selected familial AD missense mutations. Figure modified with permission from Trends in Endocrinology and Metabolism: TEM (15).
Additional support for the role of amyloid in the process of neurodegeneration comes from 2 newly described hereditary conditions, familial British and Danish dementias (FBD and FDD, respectively), in which patients suffering from either condition present with extensive amyloid deposition in the CNS that is colocalized with NFTs and associated with neurodegeneration. Although the biochemical properties of NFTs in both FBD and FDD are indistinguishable from those found in AD, the amyloid deposits in the former 2 disorders are composed of 2 new molecules, British amyloid (ABri) and Danish amyloid (ADan), which are structurally unrelated to the Aβ associated with AD (19). ABri and ADan are generated by missense mutations in the stop codons in the BRI and DAN genes and therefore only exist in nature in humans with these genetic disorders. These illnesses caused by generation of otherwise nonexistent proteins also argue against disease models that describe the origin of cerebral amyloidosis as being due to perturbation of the normal function of the respective amyloid precursors, since the gene for the amyloidogenic peptide is only present in affected individuals.
What is the usual cause of autosomal dominant, familial AD?
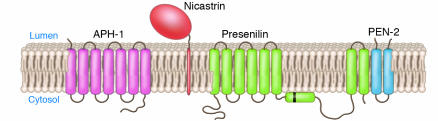
The preponderance of the familial AD for which the defect is known is attributable to completely penetrant missense mutations in the catalytic subunit of γ-secretase. The mutation is in a complex, 8-transmembrane–domain protein called presenilin (PS) that associates with other components known as nicastrin, APH-1, and PEN-2 to form the high molecular weight (>106 kDa; see Figure 5) active γ-secretase that cleaves APP fragments CTFα (the α-cleaved carboxyterminal fragment of APP, also known as C83) and CTFβ and CTFβ′ (reviewed in ref. 20).
Figure 5.
Topology of the 4 components that comprise the high molecular weight γ-secretase complex. Black bar represents the cleavage site for processing of the zymogen form of PS1 into the amino and carboxyterminal fragments that self associate and form the active enzyme. Figure modified from an image courtesy of Jan Naslund (Karolinska Institute, Stockholm, Sweden).
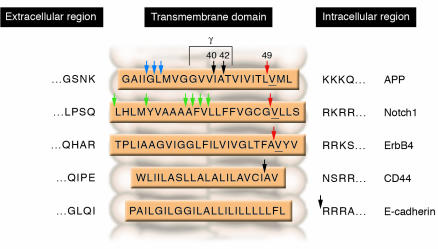
As with most APP mutations, the mutations in PS appear to act by increasing the generation of the Aβ42 species. However, unlike the relatively simple case of APP mutations (most of which are clustered around the carboxyl terminus of the Aβ domain and favor generation of Aβ42), over 150 pathogenic PS mutations, scattered throughout the PS molecule, have been described. A dozen more substrates, in addition to APP, exist for γ-secretase (Figure 6), and even within APP, γ-secretase can cleave at several sites, including a site distinct from the canonical γ-secretase site. Cleavage at this second, so-called “ε-site” liberates the cytoplasmic APP intracellular domain (AICD), which, in analogy with other PS substrates, may traffic to the nucleus and act as a transcription factor (reviewed in ref. 20).
Figure 6.
Intramembranous PS cleavage sites on 5 representative γ-secretase substrates. Figure modified with permission from Nature Reviews Molecular Cell Biology (61).
In addition to γ-secretase, which contains mutations that can cause AD, 2 other secretases control the cleavages that initiate APP processing. An integral aspartyl proteinase, known as β-secretase or β-APP–site cleaving enzyme (BACE; ref. 21), generates APP carboxyl fragments known as C99 (or CTFβ) and C89 (β′-cleaved carboxyterminal fragment of APP; also known as CTFβ′), which bear either Aβ [Asp1] or Aβ [Glu11] at their amino termini (see Figure 4B). These cleavage sites are sometimes known as β and β′ sites, respectively, and may be differentially generated in endosomes and the trans-Golgi network, respectively. The large ectodomain fragment generated by BACE-mediated cleavage is sAPPβ. APP is one of only 2 known BACE substrates, which makes BACE inhibition a popular anti-Aβ therapeutic strategy, and the development of safe, orally active BACE inhibitors is being aggressively pursued. To date, success has been limited; the BACE catalytic pocket is especially large, thus requiring relatively bulky molecules for efficient inhibition. To date, these BACE inhibitors have been limited by their toxicity and/or their exclusion from the CNS.
Another APP secretase proteinase, α-secretase, is comprised of cell-surface metalloproteinases, a disintegrin and metalloproteinase–10 (ADAM-10) and –17 (22). These proteases cleave several known important substrates in addition to APP, including pro–TNF-α and pro–TGF-α. The APP derivatives generated by α-secretase are the released (or shed) ectodomain fragment sAPPα and the cell-associated fragment C83, which is subsequently cleaved by γ-secretase to yield an Aβ derivative known as p3 with an amino terminus beginning at Leu17 of Aβ.
The location of α-secretase at the cell surface and β-secretase in the intracellular and endocytic pathway provides for competition between and mutual exclusivity of the 2 pathways. The α-secretase pathway is sensitive to PKC (23), MAPK/ERK (24), rho-associated protein kinase (ROCK) (ref. 25), and protein phosphatases 1 and 2A (PP1, PP2A) (ref. 23). As a result, when signals that involve activation of PKC or MAPK/ERK or inactivation of PP1, PP2A, or ROCK are transduced, APP metabolism is dramatically shifted toward the α-secretase–mediated pathway and away from the β-secretase–mediated pathway and Aβ generation (26). This phenomenon, known as regulated cleavage or regulated ectodomain shedding, appears to be due, at least in part, to redistribution of APP away from intracellular compartments and outward toward the plasma membrane (PM) where APP can encounter α-secretase. α-secretase per se may also be redistributed to the PM. Statins may lower the concentration of Aβ by stimulating α-secretase to yield a processing pattern that is indistinguishable from regulated cleavage modulated by PKC and MAPK/ERK (27). Recent evidence from Postina and colleagues using transgenic mice validates α-secretase activation as a viable therapeutic opportunity that can antagonize brain Aβ accumulation (28). These investigators showed that upregulation and downregulation of α-secretase activity can modulate amyloid burden in plaque-forming mice in the predicted, bidirectional manner.
What is the role of Aβ in common forms of AD?
Until 2004, one could justify the formulation that all autosomal dominant forms of AD could be linked to the accumulation of Aβ oligomers. A serious challenge to that model has arisen with the discovery of a family of individuals with a PS mutation manifesting clinically as frontotemporal dementia (FTD) and pathologically as “pure” NFT disease, i.e., there is no parenchymal or cerebrovascular amyloidosis in FTD (29). FTD is usually due to mutations involving the cytoskeletal protein tau, and these diseases are therefore called tauopathies (30). Since FTDs are NFT diseases, their existence has provided the strongest evidence that tangles cannot cause amyloidosis. No pathogenic PS1 mutation has heretofore been described as associating with pure NFT pathology. Furthermore, no generation of excess levels of Aβ42 was observed when the FTD mutant PS was transfected into cultured cells (29). The possibility remains that the PS1 mutation is acting by elevating the levels of toxic Aβ oligomers that induce tangle formation but not plaques, but no precedent exists for such a pathomechanism. Interest is now focused on the overexpression of this FTD mutant PS1 in transgenic mice, in order to assess further the nature of its pathogenicity.
The vast majority of all AD lacks a predictable, autosomal dominant mode of inheritance. While APP and the PSs constitute the only known AD genes, at least 1 important genetic risk factor is known for about 25% of the population with AD, and that is the APOE ε4 genotype (31). apoE (encoded by APOE) is the body’s major cholesterol transport protein and binds primarily to the LDL receptor (LDLR) and the LDLR-related protein (LRP). The most common form of APOE is the ε3 type, bearing a Cys at residue 112 and an Arg at residue 158. However, about 15% of the APOE alleles in the general population are of the ε4 type, in which the Cys at 112 is changed to an Arg. In the population of AD patients, the allele frequency is roughly tripled, with 45% of APOE alleles being of the ε4 type (31). This translates into a tripling of the risk of developing AD for every ε4 allele present, such that ε4 homozygotes have a 9- to 10-fold increased risk of developing dementia (31). However, the “ε4 effect” is modified by gender and is age-specific, with its peak effect observed at around 70 years of age (32). There are octogenarian ε4 homozygotes who appear to have escaped the “ε4 effect”, and it is this fact that causes ε4 to be described as a risk factor variant rather than a disease gene. Somewhere between 25 and 35% of the population with AD carry at least one ε4 allele (ref. 33 and references therein). Conversely, the APOE ε2 allele appears to protect against the development of AD (18). This protection is consistent with the oxidation model of AD (see “Are there other plausible hypotheses for the pathomechanisms of APOE polymorphisms?”, below).
How does APOE ε4 increase the risk for AD?
Efforts to link APOE ε4 with Aβ accumulation have met with mixed results. apoE ε3, but not ε4, forms complexes with Aβ that are resistant to denaturation, as discovered by LaDu et al. (34), and this has been confirmed when the source of apoE is cell-culture medium, human cerebrospinal fluid, or human plasma (34–37). More recent studies, however, have raised questions about the relevance of these SDS-resistant apoE:Aβ complexes under physiological conditions since immunoprecipitation assays indicate that apoE ε3 and apoE ε4 bind (36) Aβ and stimulate fibrillogenesis (38) equivalently, at least under the conditions studied. This is bolstered by data obtained from purification and characterization of apoE:Aβ complexes from the brains of subjects with various APOE genotypes, in which we demonstrated no effect of APOE genotype on the quality or composition of the apoE:Aβ complex (39).
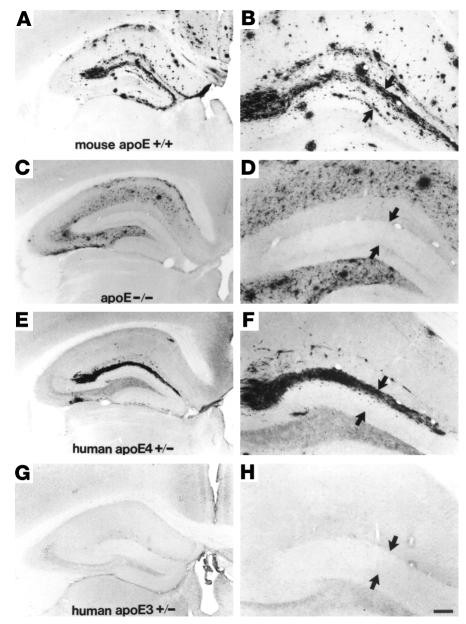
The best evidence linking APOE genotype to Aβ accumulation comes from studies of human APOE isoform–specific transgenic mice created on an APOE null background and crossed with mice bearing a pathogenic mutant form of APP (40). Plaque load was clearly enhanced in ε4 mice as compared with ε3 mice (ref. 40; Figure 7). We recently demonstrated that, unlike human ε3, human ε4 is associated with elevated levels of endogenous murine brain Aβ as a function of aging and gender, even in mice that will therefore never develop cerebral amyloidosis. These data support the conclusion that there exists an effect of ε4 on Aβ upstream of fibrillogenesis (41).
Figure 7.
APOE isoform–specific regulation of Aβ plaque burden. Aβ plaque load is highest in the hippocampi of mice expressing murine apoE (A, magnified in B) and lowest in APOE knockout animals (C, magnified in D). Plaque load is moderate in the hippocampi of human APOE ε4–expressing mice (E, magnified in F) as compared to murine apoE–expressing mice. Plaque load in the hippocampi of human APOE ε4–expressing mice is greater than plaque load in the hippocampi from human APOE ε3–expressing mice (G, magnified in H). Amyloid deposits in the dentate gyrus, indicated by arrows, never develop in the absence of apoE. Scale bar: 150 μm (A, C, E, and G) and 60 μm (B, D, F, and H). Figure modified with permission from Proceedings of the National Academy of Sciences of the United States of America (40).
Are there other plausible hypotheses for the pathomechanisms of APOE polymorphisms?
Another model that could explain, at least in part, the effects of ε4 involves the relative deficiency of ε4 as an antioxidant, since ε4 lacks the cysteine residues that are present in ε2 and ε3 and help buffer oxidant stress, when ε4 is compared with ε3 or ε2 (42). This dovetails with evidence that antioxidants can slow (43) the progression of AD and perhaps even prevent familial AD due to APP mutations (see above discussion about APOE ε2 and APP mutations; ref. 18).
We included an assessment of this possibility in our evaluation of the effect of aging and APOE genotype on levels of nonamyloidogenic endogenous mouse Aβ (41), extending the growing body of evidence indicating that oxidized prostaglandins known as isoprostanes might be useful markers for AD (reviewed in ref. 44). F2α-isoprostanes have been reported to rise in the tissues and/or body fluids of patients with AD and of transgenic plaque-forming mice at around the age of incipient amyloidosis (44–46). F2α-isoprostanes have also been shown to stimulate both generation (47) and aggregation (48) of Aβ, possibly placing isoprostane accumulation upstream of Aβ aggregation. This is an important point because human (but not murine) Aβ is reported to be a pro-oxidant (49). We were able to dissociate the isoprostane elevation from the formation of histological amyloid by using mice bearing only endogenous murine APP and Aβ (41). Thus, plaque formation is not a prerequisite for elevation of brain isoprostane levels. Indeed, one model that we propose (41) suggests that oxidized lipids might sometimes initiate Aβ aggregation.
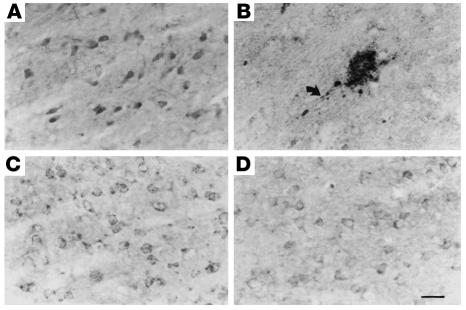
Abnormal oxidation is one of the most consistent themes in all aging-related diseases of all organ systems, and it is interesting to note that the brain lesions resulting from oxidative toxicity induced by thiamine deficiency include abnormal, amyloid precursor-containing neuritic lesions (Figure 8; ref. 50). In ongoing studies, these lesions are now being induced in plaque-competent transgenic mice in order to determine whether the neuritic brain lesions induced by the oxidative reactions of thiamine deficiency accelerate amyloid deposition.
Figure 8.
Comparison of the immunocytochemical distributions of APP (A and B) and PS1 (C and D) in control (A and C) and thiamine-deficient (B and D) mouse brain. Note the accumulation of APP immunoreactivity in a dystrophic neuritic cluster during thiamine deficiency (B). Arrow shows APP accumulation in an abnormal neurite arising from the neuritic cluster. PS1-immunostained sections adjacent to A and B reveal no accumulation of PS1 immunoreactivity in abnormal structures during thiamine deficiency except in areas of severe cell loss, in which the immunostaining is pale (D). Scale bar: 25 μm. Figure reproduced with permission from American Journal of Pathology (50).
What is the role of cholesterol in AD?
The involvement of APOE polymorphisms in AD immediately raised the question of whether cholesterol might play a role in the risk for the disease. In evaluating this possibility, Sparks and colleagues determined that Watanabe rabbits on a 2% cholesterol diet developed increased levels of Aβ immunoreactivity in vesicles inside the neurons of their brains (51). Elucidation of the mechanism underlying this observation began to be vigorously pursued in plaque-forming transgenic mouse models, and soon several groups demonstrated that fat feeding increases brain plaque load and that hypocholesterolemic agents such as statins lower plaque burden (52, 53). In cultured neurons, both simvastatin and atorvastatin as well as cyclodextrin, a compound used in the laboratory to deplete cellular cholesterol, were shown to lower Aβ generation while activating the alternate, nonamyloidogenic α-secretase pathway for APP metabolism (27, 54). At least some of the actions of statins on α-secretase appear to occur via the rho/ROCK pathway, which can modulate sAPPα generation (25).
The epidemiological evidence linking cholesterol and AD is confusing. Some studies have concluded that statin use lowers the risk for dementia (55), while others conclude that this association is artifactual (56). Several epidemiological studies also show that lipid levels are lower in persons who develop AD, not higher (57). Perhaps the most encouraging evidence, albeit preliminary, is the apparent ability of atorvastatin to slow progression of dementia in AD (58). The publication of this report and the results of a large National Institute on Aging–sponsored prevention trial of statins are eagerly anticipated. At the moment, caution is recommended, especially when one reflects upon the ongoing efforts to reconcile epidemiological evidence that hormone replacement therapy (HRT) could delay or prevent AD with the disappointment of clinical trials of HRT. A primary prevention trial of HRT is now underway, focusing on institution of replacement during the perimenopausal period rather than the typical, late postmenopausal protocol.
What antiamyloid medicines are in current clinical trials?
Antiamyloid strategies fall into 3 basic categories: immunotherapy agents, antiaggregants, and secretase modulators. Active immunization (vaccination) has been limited by the side effect of acute allergic encephalitis in 5% of 300 subjects (59), but phase I trials of passive immunization are now underway. The antiaggregant approach is represented by commercial efforts at blocking the promotion of amyloidogenesis by glycosaminoglycans of the extracellular matrix. Glycosaminoglycan mimetics are currently in phases II and III of clinical trials aiming to evaluate their effectiveness at either slowing the progression of AD or preventing rebleeding due to cerebral amyloid angiopathy. Statins, as mentioned above, represent the best examples of α-secretase activators that are currently in treatment and prevention trials. Flurbiprofen, a derivative of ibuprofen, is a modulator of Aβ42 generation that is believed to allosterically modify γ-secretase so that generation of Aβ42 is inhibited while an otherwise trace species, Aβ38, is generated (60). A large AD prevention trial of conventional NSAIDs was aborted in 2005 when excess cardiac mortality became associated with their use. Other γ-secretase modulators are in the pipeline at several pharmaceutical companies, but progress is especially slow with such compounds because of the known risk factor of mechanism-based toxicity due to inhibition of Notch processing (61). The website www.clinicaltrials.gov remains the best source of up-to-date information on which trials are enrolling and whom to contact locally for further information.
What is the role of Aβ accumulation in common forms of AD?
Genetic evidence indicates that accumulation of Aβ in some biophysical form is injurious to the brain. However, this formulation is most defensible in the approximately 50 families worldwide who have early onset AD due to APP mutations. Also, data from studies of other cerebral amyloidoses (e.g., FBD and FDD) support a key role for amyloidosis in causing neurodegeneration. Metabolism and trafficking of APP are tightly controlled events, and it is entirely possible that excess Aβ42 generation and/or accumulation is an initial symptom of some other primary problem (such as excess oxidation) that is itself independently neurotoxic. In such a scenario, however, it remains possible (and likely) that Aβ accumulation is a second, parallel pathway that is also toxic (Figure 9). This suggests that antiamyloid therapeutic strategies are likely to be beneficial in the treatment of AD even if they are not curative. Dissection of the genesis of the Aβ accumulation phenotype is important in order to point the way to any upstream, primary lesions. The successful development of effective antiamyloid therapies — immunomodulators, antiaggregants, secretase modulators, or some combination of these — remains a key goal, the attainment of which is required in order to elucidate the role played by cerebral accumulation of toxic Aβ oligomers in the clinical picture of AD.
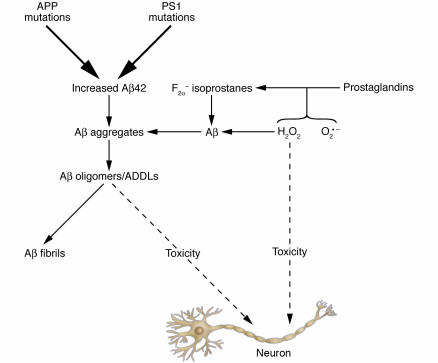
Figure 9.
Possible Aβ-dependent and Aβ-independent mechanisms of neurotoxicity. In this model, 1 route toward neurotoxicity begins with either APP mutations or PS1 mutations (top center of figure). The pathway toward toxicity flows downward via elevated Aβ42 levels and elevated oligomer levels. An alternative pathway is shown on the right side of the figure, whereby superoxide (O2•–) or hydrogen peroxide (H2O2) oxidize lipids such as prostaglandins, forming F2α-isoprostanes. Both H2O2 and F2α-isoprostanes are known to accelerate Aβ aggregation and presumably its oligomerization. This author proposes that toxicity in this pathway occurs both directly from reactive oxygen species (H2O2 and O2•–) and via acceleration of Aβ oligomerization by these reactive oxygen species.
Acknowledgments
The author acknowledges support from United States Public Health Service grants P01 AG10491 and R01 NS41017. In the past, the author has also received consultant fees and/or grant support from Parke-Davis, Wyeth, F. Hoffman-LaRoche Ltd., Pfizer Inc., Eisai Inc., and Andrx Corp.
Footnotes
Nonstandard abbreviations used: Aβ, amyloid β; ABri, British amyloid; AD, Alzheimer disease; ADAM, a disintegrin and metalloproteinase; ADan, Danish amyloid; ADDL, Aβ-derived diffusible ligand; AICD, APP intracellular domain; APP, Aβ peptide precursor; BACE, β-APP site–cleaving enzyme; C89, β′-cleaved carboxyterminal fragment of APP; CTFβ, β-cleaved carboxyterminal fragment of APP (also known as C99); FBD, familial British dementia; FDD, familial Danish dementia; FTD, frontotemporal dementia; HRT, hormone replacement therapy; MCI, mild cognitive impairment; NFT, neurofibrillary tangle; PF, protofibril; PM, plasma membrane; PP, protein phosphatase; PS, presenilin; ROCK, rho-associated protein kinase; sAPPα, soluble APPα.
Conflict of interest: The author currently receives grant support from the Robert C. Atkins Foundation and Neurochem Inc. and consultant fees from representatives of the Women’s Health Research Institute of Wyeth Inc. and Elan Pharmaceuticals Inc.
References
- 1.Mitchell TW, et al. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer’s disease. Ann. Neurol. 2002;51:182–189. doi: 10.1002/ana.10086. [DOI] [PubMed] [Google Scholar]
- 2.Alford MF, Masliah E, Hansen LA, Terry RD. A simple dot-immunobinding assay for quantification of synaptophysin-like immunoreactivity in human brain. J. Histochem. Cytochem. 1994;42:283–287. doi: 10.1177/42.2.8288869. [DOI] [PubMed] [Google Scholar]
- 3.Virchow R. Zur cellulosefrage. Virchows Arch. Pathol. Anat. Physiol. 1854;6:416–426. [Google Scholar]
- 4.Glenner GG, Terry WD. Characterization of amyloid. Annu. Rev. Med. 1974;25:131–135. doi: 10.1146/annurev.me.25.020174.001023. [DOI] [PubMed] [Google Scholar]
- 5.Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem. Biophys. Res. Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 6.Naslund J, et al. Relative abundance of Alzheimer A beta amyloid peptide variants in Alzheimer disease and normal aging. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8378–8382. doi: 10.1073/pnas.91.18.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younkin SG. Evidence that A beta 42 is the real culprit in Alzheimer’s disease. Ann. Neurol. 1995;37:287–288. doi: 10.1002/ana.410370303. [DOI] [PubMed] [Google Scholar]
- 8.Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 9.Kayed R, et al. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J. Biol. Chem. 2004;279:46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- 10.Harper JD, Lansbury PT., Jr Models of amyloid seeding in Alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu. Rev. Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- 11.Naslund J, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;83:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao K, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 13.Gong Y, et al. Alzheimer’s disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georganopoulou DG, et al. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2005;102:2273–2276. doi: 10.1073/pnas.0409336102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandy S. Neurohormonal signaling pathways and the regulation of Alzheimer beta-amyloid precursor metabolism. Trends Endocrinol Metab. 1999;10:273–279. doi: 10.1016/s1043-2760(99)00166-6. [DOI] [PubMed] [Google Scholar]
- 16.Nilsberth C, et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat. Neurosci. 2001;4:887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- 17.Paivio A, Jarvet J, Graslund A, Lannfelt L, Westlind-Danielsson A. Unique physicochemical profile of beta-amyloid peptide variant Abeta1-40E22G protofibrils: conceivable neuropathogen in Arctic mutant carriers. J. Mol. Biol. 2004;339:145–159. doi: 10.1016/j.jmb.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 18.St. George-Hyslop P, et al. Alzheimer’s disease and possible gene interaction [letter] Science. 1994;263:537. doi: 10.1126/science.8290965. [DOI] [PubMed] [Google Scholar]
- 19.Ghiso J, Frangione B. Amyloidosis and Alzheimer’s disease. Adv. Drug Deliv. Rev. 2002;54:1539–1551. doi: 10.1016/s0169-409x(02)00149-7. [DOI] [PubMed] [Google Scholar]
- 20.Carter TL, et al. Alzheimer amyloid precursor aspartyl proteinase activity in CHAPSO homogenates of Spodoptera frugiperda cells. Alzheimer Dis. Assoc. Disord. 2004;18:261–263. [PubMed] [Google Scholar]
- 21.Vassar R, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 22.Buxbaum JD, et al. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J. Biol. Chem. 1998;273:27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- 23.Caporaso GL, Gandy SE, Buxbaum JD, Ramabhadran TV, Greengard P. Protein phosphorylation regulates secretion of Alzheimer beta/A4 amyloid precursor protein. Proc. Natl. Acad. Sci. U. S. A. 1992;89:3055–3059. doi: 10.1073/pnas.89.7.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills J, et al. Regulation of amyloid precursor protein catabolism involves the mitogen-activated protein kinase signal transduction pathway. J. Neurosci. 1997;17:9415–9422. doi: 10.1523/JNEUROSCI.17-24-09415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedrini S, et al. Modulation of statin-activated shedding of Alzheimer APP ectodomain by ROCK. PLoS Med. 2005;2:e18. doi:10.1371/journal.pmed.0020018. doi: 10.1371/journal.pmed.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buxbaum JD, Koo EH, Greengard P. Protein phosphorylation inhibits production of Alzheimer amyloid beta/A4 peptide. Proc. Natl. Acad. Sci. U. S. A. 1993;90:9195–9198. doi: 10.1073/pnas.90.19.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha-secretase ADAM 10. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5815–5820. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Postina R, et al. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J. Clin. Invest. 2004;113:1456–1464. doi:10.1172/JCI200420864. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dermaut B, et al. A novel presenilin 1 mutation associated with Pick’s disease but not β-amyloid plaques. Ann. Neurol. 2004;55:617–626. doi: 10.1002/ana.20083. [DOI] [PubMed] [Google Scholar]
- 30.Hutton M. Presenilin mutations associated with fronto-temporal dementia [review] Ann. Neurol. 2004;55:604–606. doi: 10.1002/ana.20103. [DOI] [PubMed] [Google Scholar]
- 31.Mayeux R, et al. The apolipoprotein epsilon 4 allele in patients with Alzheimer’s disease. Ann. Neurol. 1993;34:752–754. doi: 10.1002/ana.410340527. [DOI] [PubMed] [Google Scholar]
- 32.Farrer LA, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 33.Martins RN, et al. ApoE genotypes in Australia: roles in early and late onset Alzheimer’s disease and Down’s syndrome. Neuroreport. 1995;6:1513–1516. [PubMed] [Google Scholar]
- 34.LaDu MJ, et al. Isoform-specific binding of apolipoprotein E to beta-amyloid. J. Biol. Chem. 1994;269:23403–23406. [PubMed] [Google Scholar]
- 35.Zhou Z, Smith JD, Greengard P, Gandy S. Alzheimer amyloid-beta peptide forms denaturant-resistant complex with type epsilon 3 but not type epsilon 4 isoform of native apolipoprotein E. Mol. Med. 1996;2:175–180. [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Z, Relkin N, Ghiso J, Smith JD, Gandy S. Human cerebrospinal fluid apolipoprotein E isoforms are apparently inefficient at complexing with synthetic Alzheimer’s amyloid-[beta] peptide (A[beta] 1–40) in vitro. Mol. Med. 2002;8:376–381. [PMC free article] [PubMed] [Google Scholar]
- 37.Yang DS, Smith JD, Zhou Z, Gandy SE, Martins RN. Characterization of the binding of amyloid-beta peptide to cell culture-derived native apolipoprotein E2, E3, and E4 isoforms and to isoforms from human plasma. J. Neurochem. 1997;68:721–725. doi: 10.1046/j.1471-4159.1997.68020721.x. [DOI] [PubMed] [Google Scholar]
- 38.Sweeney D, Martins R, LeVine H, 3rd, Smith JD, Gandy S. Similar promotion of Abeta1-42 fibrillogenesis by native apolipoprotein E epsilon 3 and epsilon 4 isoforms. J. Neuroinflammation. 2004;1:15–20. doi: 10.1186/1742-2094-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naslund J, et al. Characterization of stable complexes involving apolipoprotein E and the amyloid beta peptide in Alzheimer’s disease brain. Neuron. 1995;15:219–228. doi: 10.1016/0896-6273(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 40.Holtzman DM, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao J, et al. Aging, gender and APOE isotype modulate Alzheimer’s Aβ peptides and F2-isoprostanes in the absence of detectable amyloid deposits. J. Neurochem. 2004;90:1011–1018. doi: 10.1111/j.1471-4159.2004.02532.x. [DOI] [PubMed] [Google Scholar]
- 42.Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat. Genet. 1996;14:55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- 43.Mayeux R, Sano M. Treatment of Alzheimer’s disease. N. Engl. J. Med. 1999;341:1670–1679. doi: 10.1056/NEJM199911253412207. [DOI] [PubMed] [Google Scholar]
- 44.Pratico D, Sung S. Lipid peroxidation and oxidative imbalance: early functional events in Alzheimer’s disease. J. Alzheimers. Dis. 2004;6:171–175. doi: 10.3233/jad-2004-6209. [DOI] [PubMed] [Google Scholar]
- 45.Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J. Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao Y, et al. Brain inflammation and oxidative stress in a transgenic mouse model of Alzheimer-like brain amyloidosis [serial online] J. Neuroinflammation. 2004;1:21. http://www.jneuroinflammation.com/content/1/1/21. doi: 10.1186/1742-2094-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin W, et al. Cyclooxygenase (COX)-1 and COX-2 potentiate beta-amyloid peptide generation through mechanisms that involve gamma-secretase activity. J. Biol. Chem. 2003;278:50970–50977. doi: 10.1074/jbc.M307699200. [DOI] [PubMed] [Google Scholar]
- 48.Boutoud O, et al. Prostaglandin H2 (PGH2) accelerates formation of amyloid beta 1-42 oligomers. J. Neurochem. 2002;82:1003–1006. doi: 10.1046/j.1471-4159.2002.01064.x. [DOI] [PubMed] [Google Scholar]
- 49.Huang X, et al. The A beta peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 50.Calingasan NY, et al. Novel neuritic clusters with accumulations of amyloid precursor protein and amyloid precursor-like protein 2 immunoreactivity in brain regions damaged by thiamine deficiency. Am. J. Pathol. 1996;149:1063–1071. [PMC free article] [PubMed] [Google Scholar]
- 51.Sparks DL, et al. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp. Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 52.Refolo LM, et al. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 53.Refolo LM, et al. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2001;8:890–899. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]
- 54.Simons M, et al. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch. Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 56.Li G, et al. Statin therapy and risk of dementia in the elderly: a community-based prospective cohort study. Neurology. 2004;63:1624–1628. doi: 10.1212/01.wnl.0000142963.90204.58. [DOI] [PubMed] [Google Scholar]
- 57.Romas SN, Tang MX, Berglund L, Mayeux R. APOE genotype, plasma lipids, lipoproteins, and AD in community elderly. Neurology. 1999;53:517–521. doi: 10.1212/wnl.53.3.517. [DOI] [PubMed] [Google Scholar]
- 58.Sparks DL, et al. Benefit of atorvastatin in the treatment of Alzheimer disease. Neurobiol. Aging. 2004;25(Suppl. 1):S24. [Google Scholar]
- 59.Nicoll JA, et al. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat. Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 60.Weggen S, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 61.Fortini ME. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat. Rev. Mol. Cell Biol. 2002;3:673–684. doi: 10.1038/nrm910. [DOI] [PubMed] [Google Scholar]
- 62.Shoghi-Jadid K, et al. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am. J. Geriatr. Psychiatry. 2002;10:24–35. [PubMed] [Google Scholar]