Abstract
A major limitation to advances in prevention and therapy of neonatal meningitis is our incomplete understanding of the pathogenesis of this disease. In an effort to understand the pathogenesis of meningitis due to Escherichia coli K1, we examined whether environmental growth conditions similar to those that the bacteria might be exposed to in the blood could influence the ability of E. coli K1 to invade brain microvascular endothelial cells (BMEC) in vitro and to cross the blood-brain barrier in vivo. We found that the following bacterial growth conditions enhanced E. coli K1 invasion of BMEC 3- to 10-fold: microaerophilic growth, media buffered at pH 6.5, and media supplemented with 50% newborn bovine serum (NBS), magnesium, or iron. Growth conditions that significantly repressed invasion (i.e., 2- to 250-fold) included iron chelation, a pH of 8.5, and high osmolarity. More importantly, E. coli K1 traversal of the blood-brain barrier was significantly greater for the growth condition enhancing BMEC invasion (50% NBS) than for the condition repressing invasion (osmolarity) in newborn rats with experimental hematogenous meningitis. Of interest, bacterial growth conditions that enhanced or repressed invasion also elicited similar serum resistance phenotype patterns. This is the first demonstration that bacterial ability to enter the central nervous system can be affected by environmental growth conditions.
Escherichia coli is the primary gram-negative bacterium to cause neonatal meningitis (11, 29). E. coli K1 is most commonly acquired by newborns via vertical transmission from the maternal genital tract in utero or during passage through the birth canal (28). Studies have shown that following colonization, systemic invasion and development of bacteremia occurs (32); however, it is not clear how circulating E. coli K1 crosses the blood-brain barrier. In an effort to better understand the pathogenesis and pathophysiology of E. coli K1, we have employed an in vitro blood-brain barrier invasion model with brain microvascular endothelial cells (BMEC) and an in vivo newborn rat model of hematogenous meningitis. Previous studies have identified few E. coli K1 structures that contribute to bacterial invasion from the circulating blood to the central nervous system. The ibe10 locus was recently identified via TnphoA mutagenesis and screening for loss of invasiveness by use of the in vitro and in vivo model systems (14). In addition, E. coli K1 OmpA has been demonstrated to contribute to in vivo pathogenicity, in vitro BMEC invasion, and serum resistance (26, 33).
Current evidence indicates that in order for E. coli K1 to invade the blood-brain barrier, a bacteremic state must occur (17, 32). Given this evidence, it was of interest to investigate whether environmental conditions encountered within the blood, such as the presence of serum, iron, or magnesium, the pH, or the oxygen tension, could influence the ability of E. coli K1 to invade BMEC in vitro and to cross the blood-brain barrier in vivo. In this study, we show that the invasion capability of E. coli K1 can be both positively and negatively influenced by various bacterial growth conditions. In addition, we demonstrate that the ability of E. coli K1 to resist serum-mediated killing can be similarly altered by environmental growth conditions.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and chemicals.
E. coli K1 strain E44 used in this study is a spontaneous rifampin-resistant mutant derived from cerebrospinal fluid (CSF) isolate RS218 (serotype O18:K1:H7) (33). Bacteria were grown for 14 h at 37°C in brain heart infusion broth (BHI; Difco Laboratories, Detroit, Mich.) in the presence of rifampin (100 μg/ml) unless otherwise specified.
The environmental growth conditions affecting E. coli invasion were examined by using E44 grown in BHI with agitation, unless otherwise specified. Microaerophilic conditions were obtained by growing bacteria in nonagitated, tightly capped tubes. The effect of serum was tested by supplementing BHI with heat-inactivated newborn bovine serum (NBS) (Irvine Scientific). The effect of magnesium was assayed by supplementing BHI with MgSO4. The effect of iron was tested by supplementing BHI with FeSO4 or FeC6H5O7 and depleting iron with 2,2′-dipyridyl or EDDA [ethylenediamine-di(o-hydroxyphenylacetic acid]. The effect of osmolarity was examined by the use of complete M63 medium (containing 2 mg of Casamino Acids per ml, 5 μg of thiamine per ml, 1 mM MgSO4, 0.2% glucose, and 5 μg of nicotinamide per ml) supplemented with either NaCl, KCl, or sucrose at equiosmolar concentrations. The effect of pH was studied by supplementing BHI with the following sulfonate buffers with the proper pKa values: MES (morpholineethanesulfonic acid; pKa = 6.1), MOPS (morpholinepropanesulfonic acid; pKa = 7.2), and TAPS [tris(hydroxymethyl)methyl-aminopropanesulfonic acid; pKa = 8.4].
Tissue culture invasion assays.
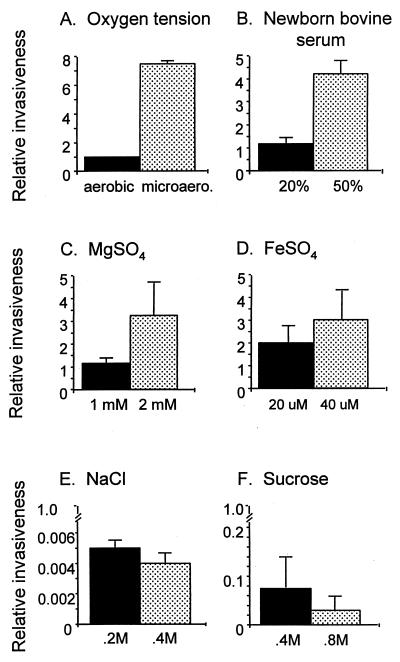
BMEC were prepared and invasion assays were performed as previously described (26, 31). Briefly, the homogenates of bovine brain cortices were centrifuged in 25% bovine serum albumin, and the pellet containing crude microvessels was digested with collagenase-dispase. Microvascular capillaries were isolated by absorption to a glass bead column, plated on collagen-fibronectin-coated dishes, and cultured in growth medium containing d-valine (to inhibit the growth of nonendothelial cells). The resulting BMEC were positive for factor VIII, uptake of fluorescently labeled acetylated low-density lipoprotein, carbonic anhydrase IV, and γ-glutamyltranspeptidase, demonstrating their brain endothelial-cell characteristics. The invasion assays utilized approximately 107 bacteria, which were added to confluent monolayers of BMEC at a multiplicity of infection of 100. The number of intracellular bacteria was determined after the extracellular bacteria were eliminated by incubation of the monolayer with experimental medium containing gentamicin (100 μg/ml). Results are presented either as percent invasion = 100 × [(number of bacteria recovered)/(number of bacteria inoculated)] or as relative invasiveness = fold effect on percent invasion in comparison to that of bacteria grown in medium alone, which has a numerical value of 1. In Fig. 1 (see below), bacteria grown in the medium-alone control condition, whose invasiveness was equated to a numerical value of 1, consistently showed 0.1 to 0.3% invasion of BMEC. It is important to note that the invasion frequency of BMEC by E. coli (approximately 0.1%) is related to enhanced entry into the central nervous system in vivo (14, 26) and thus is biologically relevant. No growth condition was found to cause bacterial clumping. Results are presented as the average ± standard deviation (SD) of at least three individual experiments performed in duplicate.
FIG. 1.
Effect of various growth conditions on the ability of E. coli K1 to invade BMEC. Bacterial invasiveness was determined for cultures grown in medium supplemented with the indicated factors. Assay values, representing the average ± SD for numerous assays, were normalized such that the invasiveness of bacteria grown aerobically in medium alone equals 1.
Newborn rat model of hematogenous E. coli meningitis.
E. coli bacteremia and meningitis were induced in 5-day-old rats as previously described (26). Briefly, at 5 days of age, all members of each litter were randomly divided into two groups to receive an intracardiac injection of E44 (107 CFU in 50 μl) grown in BHI with the indicated supplements. At 0.5 h later, blood and CSF specimens were obtained for quantitative cultures. Blood and CSF specimens were obtained by external jugular venipuncture and puncture of the cisterna magna, respectively, by using aseptic techniques as previously described (17).
Serum killing assays.
Bacteria were grown in BHI with the indicated medium supplements to stationary phase, centrifuged, and resuspended in Hanks’ balanced salt solution containing 0.4 mM MgSO4 and 1 mM CaCl2. Aliquots (10 μl) containing 107 cells per ml were mixed with either 40 or 80% pooled human serum. A 10-μl aliquot was sampled at time zero to determine the actual number of bacteria added to each tube. As a control, parallel samples were incubated with heat-inactivated serum (56°C, 30 min). The mixture was incubated at 37°C for 3 h, aliquots were serially diluted, and the number of CFU was determined. Results are expressed as percent survival = 100 × [(CFU of bacteria that survived serum treatment per milliliter)/(CFU of bacteria added per milliliter)]. Experiments were performed in triplicate and repeated several times.
Western blot analysis.
Whole-cell lysates were prepared from bacteria grown to stationary phase. Equal amounts of whole-cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose. Coomassie blue-stained gels run in parallel confirmed that equivalent amounts of protein were loaded in each lane. Blots were prepared for anti-OmpA immunoblot analysis essentially as previously described (26), except that proteins were visualized with the Amersham (Arlington Heights, Ill.) ECL Western detection system.
mRNA slot blot analysis.
Total cellular RNA was purified with TRIzol reagent (Gibco BRL). RNA samples were treated with RNase-free DNase, and the integrity of each sample was assessed by examination of an electrophoresed aliquot. Purified RNA in amounts of 3.5 (ompA blots) and 1.5 (23S rRNA blots) μg was applied to nylon membranes (Pro-Nytran; Schleicher & Schuell) by using a slot blot apparatus (Bio-Rad). Membranes were prepared and subjected to hybridization as previously described (2). Specific DNA probes used for hybridization were a 600-bp HpaI-BamHI internal fragment of ompA (33) and a 526-bp internal fragment of E. coli 23S rRNA gene (PCR product generated by using oligonucleotide primers 23s1 [5′ GACTAAGCGTACACGGTG 3′] and 23s2 [5′ GCCTTGTACGTACACGG 3′]). mRNA slot blots were visualized and analyzed with a phosphorimager (Phosphor Analyst; Bio-Rad). The density of each slot was determined by using volume analysis. The amount of mRNA present in each sample was normalized to the signal detected by the 23S rRNA probe. Transcript levels from cultures grown in medium alone were set at 100%, and the relative amounts of transcripts from other growth conditions were determined. The data presented represent the average for serial diluted samples of an individual assay and reflect the results from several experiments.
RESULTS
Environmental growth conditions influence the ability of E. coli K1 to invade BMEC.
To determine whether incubation of E. coli K1 under conditions which may resemble the blood environment would affect the bacterium’s ability to invade BMEC, bacteria were grown under exposure to various oxygen conditions, serum, various osmolarities, and various inorganic ions. Since oxygen saturation levels are higher in arterial blood than in venous blood (i.e., 94 to 100% versus 60 to 85%, respectively) (4), we examined oxygen as a potential environmental cue. Tissue culture invasion assays were performed on cultures grown aerobically (loosely capped tubes and vigorous agitation) and microaerophilically (tightly capped tubes and nonagitation). Bacteria grown under these environmental conditions had similar growth rates, and comparable culture densities were reached after overnight growth. Compared to bacteria grown aerobically, bacteria grown microaerophilically exhibited more than a sevenfold increase in BMEC invasiveness (Fig. 1A).
Before invasion of the blood-brain barrier occurs, E. coli K1 must reach a bacteremic state (17, 27, 32), suggesting that the bacteria are thriving and replicating within the blood. To investigate the possible influences of serum on E. coli invasiveness, bacteria were grown in the presence of heat-inactivated NBS. When bacteria were grown in medium supplemented with 20% NBS, BMEC invasion was not significantly affected; however, when the serum level was raised to 50%, a fourfold increase in invasion was observed (Fig. 1B).
Because environmental factors such as magnesium, iron, chloride, and osmolarity have previously been shown to be signals for controlling virulence properties of other pathogenic bacteria (20), these and other elements were examined for potential effects on the ability of E. coli K1 to invade tissue cultures. Some plasma or serum reference values for neonates include the following: magnesium, 0.51 to 1.15 mmol/liter; iron, 4 to 52 μmol/liter; osmolarity, 274 to 305 mosmol/liter; chloride, 90 to 117 mmol/liter; zinc, 7.9 to 15.6 μmol/liter; and copper, 1.9 to 15.8 μmol/liter (4). When bacteria were grown in medium supplemented with 1 mM MgSO4, the invasion phenotype observed was not significantly different from that of bacteria grown in medium alone; however, bacteria grown in medium supplemented with 2 mM MgSO4 elicited a threefold increase in invasion relative to that of bacteria grown in medium alone (Fig. 1C). The effect of iron was investigated by utilizing FeSO4 supplementation. When bacteria were grown in medium supplemented with 20 μM FeSO4, a twofold increase in invasion was seen; furthermore, when FeSO4 supplementation was raised to 40 μM, a threefold increase in invasion was observed (Fig. 1D). When iron was chelated from the growth medium with 100 μM 2,2′-dipyridyl, the invasion phenotype was repressed by 50%. Similar results were seen when the medium was supplemented with FeC6O5H7 or the iron chelator EDDA (data not shown). The effect of osmolarity on the bacterium’s ability to invade BMEC was next examined. For this purpose, E. coli K1 was grown in M63 medium supplemented with NaCl or sucrose. Increasing concentrations of NaCl or sucrose (200 or 400 mosmol/liter) significantly repressed (13- to 250-fold) the ability of the bacteria to invade BMEC (Fig. 1E and F). Similar results were observed when bacteria were grown in medium supplemented with KCl or when cultures were grown in different media (i.e., BHI or Luria broth) and adjusted for osmolarity (data not shown). We also examined whether medium supplemented with zinc or copper could affect the tissue culture invasion phenotype of E. coli K1; invasiveness was not affected by altering the concentration of ZnSO4 or CuSO4 in the medium (data not shown). E. coli K1 cultures grown in medium supplemented with the indicated compounds had similar growth rates, and comparable culture densities were reached after overnight growth. The results described above suggest that several in vitro bacterial growth conditions that potentially mimic the in vivo environment of the blood can influence both positively and negatively the ability of E. coli K1 to invade BMEC.
Effect of pH on tissue culture invasiveness.
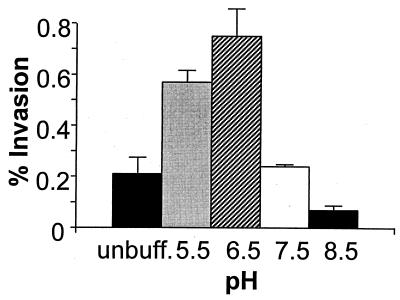
In the bacteremic state (preceding invasion of the blood-brain barrier), E. coli K1 is presumably in a blood environment with a pH of 7.33 to 7.45 (4). We therefore investigated the ability of the bacteria to invade BMEC after growth in medium buffered at various pHs. As shown in Fig. 2, there was a profound effect on the bacterium’s ability to invade when it was grown in medium buffered at pH 6.5, as compared to that in medium buffered at pH 8.5, i.e., 0.7 and 0.07% invasion, respectively. It is possible that the effects of medium supplements previously examined which showed enhancement or repression of invasion may be due to an artifact of the medium pH. To address this possibility, the pHs of the media after growth were determined. The pH of medium alone was 9.0, and likewise, the pH of medium supplemented with 50% NBS was 9.0 (data not shown). In contrast, the pH of cultures grown microaerophilically was 7.0. Furthermore, experiments using buffered medium at pH 7.5 or 5.5 and grown microaerophilically showed no significant increase in BMEC invasion in comparison to that in cultures grown aerobically (data not shown). These data suggest that the enhancement of invasion when bacteria are grown in medium supplemented with 50% NBS is probably not due to a pH effect. Notwithstanding, the effect of pH cannot be ruled out for bacterial cultures grown aerobically or microaerobically.
FIG. 2.
Effect of different pHs on the ability of E. coli K1 to invade BMEC. Bacterial invasiveness was determined for cultures grown in medium buffered at the indicated pH. Data shown for percent invasion represent the mean ± the range and are representative of numerous experiments with similar results.
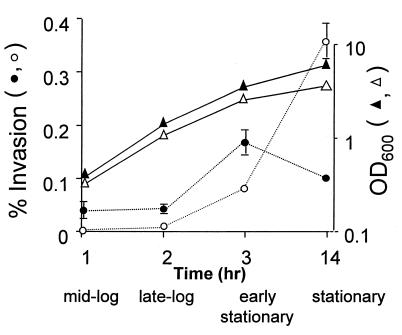
Effect of bacterial growth phase on tissue culture invasiveness.
Virulence and/or tissue culture invasiveness is growth phase regulated in many bacterial pathogens (10, 13, 15, 21, 24). We sought to determine whether the bacterial growth state influenced the ability of E. coli K1 to invade BMEC. For this purpose, assays of optical density at 600 nm, viable counts, and tissue culture invasion were performed simultaneously to determine the growth phase in which invasion was maximal. As shown in Fig. 3, the bacteria grew similarly under the different experimental conditions; however, depending on the growth medium condition, the invasiveness of E. coli K1 showed dependence on different growth phases. When the bacteria were grown in medium alone, invasion was observed for all growth phase points assayed; however, maximal invasiveness was observed in early stationary phase. In contrast, when the bacteria were grown in medium supplemented with 50% NBS, negligible invasion was demonstrated until cells entered early stationary phase. This increase in invasion continued until maximal invasion was observed for bacteria in stationary phase (overnight cultures) (Fig. 3). These results suggest that there are different growth phase-dependent BMEC tissue culture invasion phenotypes that depend on specific environmental growth conditions.
FIG. 3.
Effect of growth phase on invasion. Cultures grown for 14 h were diluted to 0.1 optical density unit at 600 nm, and aliquots were collected at the indicated growth phase points to determine culture density (▴ and ▵) and bacterial invasiveness (• and ○). Results shown for percent invasion represent the mean ± the range and are representative of several experiments performed with similar results. Bacteria grown in medium alone (• and ▴) and bacteria grown in medium supplemented with 50% NBS (○ and ▵) were assayed.
Effect of growth conditions on E. coli K1 entry into the central nervous system.
We next examined whether the aforementioned effects of environmental growth conditions on tissue culture invasiveness would be biologically relevant in our well-established newborn rat model of experimental hematogenous meningitis. We compared the bacterial growth conditions that elicited the most prominent enhancement or repression of the in vitro invasion phenotype, i.e., media supplemented with 50% NBS or 0.2 M NaCl, respectively. As shown in Table 1, the magnitudes of bacteremia were similar for the two treatment groups; however, the occurrence of meningitis as shown by positive CSF cultures was significantly higher in animals receiving bacteria grown with 50% NBS than in those receiving bacteria grown in 0.2 M NaCl. These findings indicate that medium supplemented with 50% NBS enhanced the ability of E. coli K1 to invade BMEC in vitro and to cross the blood-brain barrier in vivo.
TABLE 1.
Bacterial counts in blood and positive CSF cultures for animal groups receiving E. coli K1 grown in medium with 50% NBS or 0.2 M NaCl
| Group receiving bacteria grown in | n | Bacteremia (log CFU/ml of blood)a | No. (%) of animals with positive CSF culture |
|---|---|---|---|
| BHI–50% NBS | 40 | 6.86 ± 0.47 | 26 (65)b |
| BHI–0.2 M NaCl | 33 | 7.17 ± 0.60 | 12 (33) |
Values are means ± SD.
ρ = 0.038 by chi-square test with Yates’ correction.
Serum resistance is influenced by growth media.
In several pathogenic bacteria, serum resistance and invasion are coordinately regulated, as a result of either the same molecule conferring the two phenotypes or two separate factors responding similarly to environmental cues (6, 8, 19, 23, 26, 33). Thus, we sought to determine whether the bacterial growth conditions that were shown to be biologically relevant would also affect the serum resistance phenotype of E. coli K1 by growing bacteria to stationary phase in medium supplemented with 50% NBS or 0.2 M NaCl. Bactericidal assays performed with pooled human serum revealed a pattern of serum resistance analogous to the BMEC invasion phenotype. In comparison to bacteria grown in medium alone, bacteria grown in medium supplemented with 50% NBS showed more than a 1,000-fold increase in survival when exposed to 80% pooled human serum (Table 2). Conversely, bacteria grown in medium supplemented with 0.2 M NaCl elicited a 3.3-fold decrease in survival in serum in comparison to that of bacteria grown in medium alone (Table 2). Similar results were observed for bacteria grown to mid-logarithmic phase (data not shown). There was no decrease in bacterial survival when bacteria grown under either condition were exposed to heat-inactivated pooled human serum.
TABLE 2.
Serum resistance is influenced by growth conditions
| % Survivala of bacteria exposed to:
| ||
|---|---|---|
| Growth condition | 40% pooled human serum | 80% pooled human serum |
| BHI | 60 ± 13.2 | 0.30 ± 0 |
| BHI–50% NBS | 1,048 ± 114.5 | 439.5 ± 5.7 |
| BHI–0.2 M NaCl | 15.4 ± 1.3 | 0.09 ± 0 |
Bacteria were grown in medium alone or with the indicated supplements and assayed for serum resistance as described in Materials and Methods. Values are the average ± SD of a single representative assay (% survival) performed in triplicate and reflect similar results from numerous experiments.
OmpA expression is not affected by environmental growth conditions.
Due to previous observations that OmpA plays a critical role in both invasion and serum resistance (26, 33), we sought to determine if the environmental conditions mentioned above affected OmpA expression. E. coli K1 was grown to stationary phase in medium alone, in medium supplemented with 50% NBS, 0.2 M NaCl, or 40 μM FeSO4, or in medium buffered at pH 5.5. E. coli K1 cultures grown in BHI supplemented with the indicated compounds had similar growth rates, and comparable culture densities were reached after overnight growth. Whole-cell lysates were collected and subjected to SDS-PAGE. Western blot analysis with polyclonal anti-OmpA antibody (26) showed that these environmental growth conditions had little or no significant effect on OmpA protein levels (Fig. 4A). Similar results were observed for bacteria grown to mid-logarithmic phase (data not shown).
FIG. 4.
Effect of various environmental growth conditions on OmpA expression. (A) ompA transcript levels were measured by RNA slot blot analysis. RNA was prepared from mid-logarithmic-phase cells grown in the indicated media. The level of transcripts from cultures grown in medium alone was set at 100%, and relative levels of specific mRNAs for other cultures were determined (densitometry values). 23S rRNA transcript levels were determined in parallel to confirm equivalent loading. Data presented are representative of several experiments using independently prepared RNA samples. (B) Whole-cell extracts of bacteria grown in medium alone or with the indicated supplements were separated by SDS-PAGE, and OmpA levels were determined by immunoblot analysis as described in Materials and Methods.
Although the Western blot analysis suggested that there was no significant difference in detectable OmpA protein levels when the bacteria were grown under various environmental conditions, it did not rule out the possibility that there are potential effects on ompA expression at the transcriptional level. For this purpose, RNA slot blot analysis was performed. RNA was isolated from mid-logarithmic-phase bacteria grown in medium alone, in medium supplemented with 50% NBS, 0.2 M NaCl, or 40 μM FeSO4, or in medium buffered at pH 5.5. Consistent with the anti-OmpA immunoblot analysis, there was no significant difference in ompA transcript levels when the cultures were grown with the various medium supplements and when the cultures were grown in medium alone (Fig. 4B). Similar results were observed for RNA collected from early stationary- and stationary-phase cultures (data not shown). These results suggest that although the BMEC invasion and the in vitro serum resistance phenotypes can be similarly altered depending on the growth environment (i.e., 50% NBS or 0.2 M NaCl), they are not due changes in OmpA expression.
DISCUSSION
The pathogenesis of E. coli K1 causing meningitis is not well characterized. Current evidence supports a requirement for hematogenous spread and development of high-grade bacteremia for invasion of the blood-brain barrier to occur (17, 27, 32). One approach to a better understanding of the pathogenic mechanisms of an organism is to determine the environmental conditions that alter the pathogen’s virulence potential. In this study, we utilized an in vitro blood-brain barrier model system to characterize and define potential environmental cues that influence the ability of E. coli K1 to invade BMEC in vitro. In addition, the biological relevance of the ability to invade BMEC in vivo was examined in the newborn rat model of hematogenous meningitis. In this experimental meningitis model, bacteria were administered via intracardiac injection, which resulted in bacteremia and subsequent entry of the bacteria into the central nervous system, which most likely occurs at the sites of the blood-brain barrier. We have previously shown that invasion of BMEC is a requirement for E. coli K1 penetration of the blood-brain barrier in vivo (14, 26). Furthermore, it is important to recognize that the frequency of in vitro invasion (0.1 to 0.3%) corresponds to the enhanced bacterial penetration of the blood-brain barrier in vivo (14, 26).
In this study, several bacterial growth conditions which potentially mimic the in vivo blood environment, including microaerophilic growth and media supplemented with NBS, magnesium, or iron, were found to enhance the invasiveness of E. coli K1. The expression of many bacterial virulence genes or properties has been shown to be regulated by similar environmental conditions. For example, oxygen tension regulates the ability of Salmonella spp. to invade tissue culture cells (3, 10, 18) and both adherence of and invasion by Listeria monocytogenes increase when bacteria are grown in iron-rich media (7).
The environmental growth conditions in which E. coli K1 invasion of BMEC was found to be repressed included iron limitation, high pH, and high osmolarity. Osmolarity has been identified as an important environmental cue for the expression of many bacterial virulence properties. For example, Salmonella invasion has been shown to be stimulated by high osmolarity (3, 12). In this study, we found that bacteria grown in medium supplemented with NaCl, KCl, or sucrose (200 mosmol/liter) demonstrated a significant decrease in their ability to invade BMEC. Of interest, the increase in invasion of BMEC due to supplementation of media with MgSO4, FeSO4, or FeC6H5O7 (data not shown) was not observed for cultures supplemented with MgCl2 or FeCl3 (although no repression was observed either) (data not shown). The decrease in E. coli K1 invasion when the medium was supplemented with NaCl and KCl suggests that in addition to osmolarity, chloride ion may negatively influence E. coli K1 invasion of BMEC.
More importantly, the enhanced tissue culture invasion of BMEC associated with NBS and the decreased invasion associated with NaCl were reproduced in vivo. In the animal model of hematogenous meningitis, significantly greater penetration of the central nervous system was observed with bacteria grown in medium supplemented with NBS than with bacteria grown in medium with NaCl. These findings support the concept that the BMEC invasion phenotype is biologically relevant and also indicate that 50% NBS supplementation may upregulate the expression of bacterial genes or proteins required for entry into the central nervous system.
Bacterial growth phase has also been shown to play a role in expression of virulence genes and properties (10, 13, 15, 21, 24). In the present study, we demonstrated that depending on the growth environment, the penetration of E. coli K1 into BMEC was markedly dependent on growth phase. The highest rate of invasion for bacteria grown in medium alone was observed for cells in early stationary phase. In contrast, when bacteria were grown in medium supplemented with 50% NBS, invasion of BMEC was negligible for mid- and late-logarithmic-phase cells. However, as bacteria progressed in the course of growth, an increase in invasion occurred, until maximal invasion was seen for stationary-phase cells. These results suggest that there are potentially different E. coli K1 growth phase-dependent invasion phenotypes whose expression is affected by the specific growth environment.
Many virulence properties of other bacterial pathogens are regulated by environmental pH. For example, low pH increases the expression of several Vibrio cholerae virulence factors, including cholera toxin, toxin-coregulated pili, and others (16, 22). In this study, we showed a distinct pattern of E. coli K1 invasion of BMEC that was dependent on the pH of the growth medium. Maximal invasion was observed in medium buffered at pH 6.5, whereas minimal invasion was observed in medium with a pH of 8.5. Nonetheless, prominent invasion was observed for cultures grown in medium buffered at pH 5.5. The pH of blood is 7.33 to 7.45; however, at the bacteremic state of a meningitic E. coli infection, the pH of the immediate environment surrounding the bacterium is not known. It has been noted and characterized that E. coli K1 hemagglutinates erythrocytes and produce a hemolysin(s) (1); thus, it is possible that upon lysis of erythrocytes, the pH within the immediate vicinity of the bacteria is lower than the absolute pH of the blood. Alternatively, a microbial factor(s) which may be used primarily for penetration into eukaryotic cells of a different origin (i.e., intestinal epithelial cells) could be optimally expressed at lower pHs. Recent studies suggest that the portal of entry for meningitic E. coli can be either the gastrointestinal mucosa or the pulmonary loci of the newborn (5, 32). Thus, low pH may be an environmental stimulus for E. coli K1 that mimics the environment of the host intestinal tract. Studies in our laboratory are currently in progress to address this possibility.
In several pathogenic bacteria, serum resistance and invasion phenotypes are coordinately regulated. The linked expression of these two phenotypes is due either to separate factors responding similarly to environmental cues or to one molecule conferring both phenotypes (6, 8, 19, 23, 26, 33). In the present study, we show that environmental growth conditions that elicit the most pronounced effect on the invasion phenotype (i.e., media supplemented with 50% NBS or 0.2 M NaCl) also have a similar effect on the serum resistance profile of E. coli K1. Analogous to results for many other pathogens, these results suggest that the invasion and serum resistance phenotypes of E. coli K1 are regulated in a like manner. OmpA has been shown to contribute to E. coli K1 pathogenicity, in vitro invasion, and serum resistance (26, 33), but we found that OmpA expression was not effected by these environmental growth conditions, suggesting that another factor(s) conferring serum resistance to E. coli K1 is under the influence of environmental cues. Although OmpA expression does not appear to be affected by the various growth conditions examined, it is possible that the functionality of OmpA is altered by these conditions. In addition to OmpA, the K1 capsule of E. coli has also been shown to be important in exhibiting bacteremia and serum resistance (9, 17, 25, 30, 33). Studies are in progress to determine the potential effects of environmental growth conditions on E. coli K1 capsule expression.
We speculate that the growth conditions examined in this study may be environmental cues that trigger the expression or repression of the ability of meningitic E. coli K1 to invade BMEC and serum resistance and should be useful for the identification of genes associated with these phenotypes. Moreover, a complete understanding of the basis for this environmental regulation will eventually lead to enhanced knowledge of the pathogenesis, prevention, and treatment of E. coli K1 meningitis.
ACKNOWLEDGMENTS
We thank Carol A. Wass for excellent technical assistance and Peggy A. Cotter for critically reviewing the manuscript.
This work was supported by U.S. Public Health Service grant NS 26310 to K.S.K. and a CHLA Research Institute AIDS/Host Defense Program grant to J.L.B.
REFERENCES
- 1.Achtman M, Mercer A, Kusecek B, Pohl A, Heuzenroeder M, Aaronson W, Sutton A, Silver R P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983;39:315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badger J L, Miller V L. Expression of invasin and motility are coordinately regulated in Yersinia enterocolitica. J Bacteriol. 1998;180:793–800. doi: 10.1128/jb.180.4.793-800.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajaj V, Lucas R L, Hwang C, Lee C A. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol Microbiol. 1996;22:703–714. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- 4.Barakat A, Ichikawa I. Laboratory data. In: Ichikawa I, editor. Pediatric textbook of fluids and electrolytes. Baltimore, Md: The Williams & Wilkins Co.; 1990. pp. 478–500. [Google Scholar]
- 5.Bingen E, Bedu A, Brahimi N, Aujard Y. Use of molecular analysis in pathophysiological investigation of late-onset neonatal Escherichia coli meningitis. J Clin Microbiol. 1995;33:3074–3076. doi: 10.1128/jcm.33.11.3074-3076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bliska J B, Copass M C, Falkow S. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect Immun. 1993;61:3914–3921. doi: 10.1128/iai.61.9.3914-3921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conte M P, Longhi C, Polidoro M, Petrone G, Buonfiglio V, Di Santo S, Papi E, Seganti L, Visca P, Valenti P. Iron availability affects entry of Listeria monocytogenes into the enterocytelike cell line Caco-2. Infect Immun. 1996;64:3925–3929. doi: 10.1128/iai.64.9.3925-3929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelis G R, Biot T, Lambert de Rouvroit C, Michiels T, Mulder B, Sluiters C, Sory M P, Van Bouchaute M, Vanooteghem J C. The Yersinia yop regulon. Mol Microbiol. 1989;3:1455–1459. doi: 10.1111/j.1365-2958.1989.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 9.Cross A S, Kim K S, Wright D C, Sadoff J C, Gemski P. Role of lipopolysaccharide and capsule in the serum resistance of bacteremic strains of Escherichia coli. J Infect Dis. 1986;154:497–503. doi: 10.1093/infdis/154.3.497. [DOI] [PubMed] [Google Scholar]
- 10.Ernst R K, Dombroski D M, Merrick J M. Anaerobiosis, type 1 fimbriae, and growth phase are factors that affect invasion of HEp-2 cells by Salmonella typhimurium. Infect Immun. 1990;58:2014–2016. doi: 10.1128/iai.58.6.2014-2016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feigin R D. Bacterial meningitis in the newborn infant. Clin Perinatol. 1977;4:103–116. [PubMed] [Google Scholar]
- 12.Galan J E. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 13.Guiney D G, Libby S, Fang F C, Krause M, Fierer J. Growth-phase regulation of plasmid virulence genes in Salmonella. Trends Microbiol. 1995;3:275–279. doi: 10.1016/s0966-842x(00)88944-1. [DOI] [PubMed] [Google Scholar]
- 14.Huang S H, Wass C, Fu Q, Prasadarao N V, Stins M, Kim K S. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect Immun. 1995;63:4470–4475. doi: 10.1128/iai.63.11.4470-4475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iriarte M, Stainier I, Cornelis G R. The rpoS gene from Yersinia enterocolitica and its influence on expression of virulence factors. Infect Immun. 1995;63:1840–1847. doi: 10.1128/iai.63.5.1840-1847.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaper J B, Morris J G, Jr, Levine M M. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim K S, Itabashi H, Gemski P, Sadoff J, Warren R L, Cross A S. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J Clin Invest. 1992;90:897–905. doi: 10.1172/JCI115965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee C A, Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci USA. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez R J. Thermoregulation-dependent expression of Yersinia enterocolitica protein 1 imparts serum resistance to Escherichia coli K-12. J Bacteriol. 1989;171:3732–3739. doi: 10.1128/jb.171.7.3732-3739.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikulskis A V, Delor I, Thi V H, Cornelis G R. Regulation of the Yersinia enterocolitica enterotoxin Yst gene. Influence of growth phase, temperature, osmolarity, pH and bacterial host factors. Mol Microbiol. 1994;14:905–915. doi: 10.1111/j.1365-2958.1994.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 22.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pederson K J, Pierson D E. Ail expression in Yersinia enterocolitica is affected by oxygen tension. Infect Immun. 1995;63:4199–4201. doi: 10.1128/iai.63.10.4199-4201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pepe J C, Badger J L, Miller V L. Growth phase and low pH affect the thermal regulation of the Yersinia enterocolitica inv gene. Mol Microbiol. 1994;11:123–135. doi: 10.1111/j.1365-2958.1994.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 25.Pluschke G, Mayden J, Achtman M, Levine R P. Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing. Infect Immun. 1983;42:907–913. doi: 10.1128/iai.42.3.907-913.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasadarao N V, Wass C A, Weiser J N, Stins M F, Huang S H, Kim K S. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quagliarello V, Scheld W M. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327:864–872. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- 28.Sarff L D, McCracken G H, Schiffer M S, Glode M P, Robbins J B, Orskov I, Orskov F. Epidemiology of Escherichia coli K1 in healthy and diseased newborns. Lancet. 1975;i:1099–1104. doi: 10.1016/s0140-6736(75)92496-4. [DOI] [PubMed] [Google Scholar]
- 29.Siegel J D, McCracken G H., Jr Sepsis neonatorum. N Engl J Med. 1981;304:642–647. doi: 10.1056/NEJM198103123041105. [DOI] [PubMed] [Google Scholar]
- 30.Stevens P, Chu C L, Young L S. K-1 antigen content and the presence of an additional sialic acid-containing antigen among bacteremic K-1 Escherichia coli: correlation with susceptibility to opsonophagocytosis. Infect Immun. 1980;29:1055–1066. doi: 10.1128/iai.29.3.1055-1061.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stins M F, Prasadarao N V, Ibric L, Wass C A, Luckett P, Kim K S. Binding characteristics of S fimbriated Escherichia coli to isolated brain microvascular endothelial cells. Am J Pathol. 1994;145:1228–1236. [PMC free article] [PubMed] [Google Scholar]
- 32.Tunkel A R, Scheld W M. Pathogenesis and pathophysiology of bacterial meningitis. Clin Microbiol Rev. 1993;6:118–136. doi: 10.1128/cmr.6.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiser J N, Gotschlich E C. Outer membrane protein A (OmpA) contributes to serum resistance and pathogenicity of Escherichia coli K-1. Infect Immun. 1991;59:2252–2258. doi: 10.1128/iai.59.7.2252-2258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]