Abstract
OBJECTIVES
It has been commonly accepted that untreated acute type A aortic dissection (ATAAD) results in an hourly mortality rate of 1–2% during the 1st 24 h after symptom onset. The data to support this statement rely solely on patients who have been denied surgical treatment after reaching surgical centres. The objective was to perform a total review of non-surgically treated (NST) ATAAD and provide contemporary mortality data.
METHODS
This was a regional, retrospective, observational study. All patients receiving one of the following diagnoses: International Classification of Diseases (ICD)-9 4410, 4411, 4415, 4416 or ICD-10 I710, I711, I715, I718 in an area of 1.9 million inhabitants in Southern Sweden during a period of 23 years (January 1998 to November 2021) were retrospectively screened. The search was conducted using all available medical registries so that every patient diagnosed with ATAAD in our region was identified. The charts and imaging of each screened patient were subsequently reviewed to confirm or discard the diagnosis of ATAAD.
RESULTS
Screening identified 2325 patients, of whom 184 NST ATAAD patients were included. The mortality of NST ATAAD was 47.3 ± 4.4%, 55.0 ± 4.4%, 76.7 ± 3.7% and 83.9 ± 4.3% at 24 h, 48 h, 14 days and 1 year, respectively. The hourly mortality rate during the 1st 24 h after symptom onset was 2.6%.
CONCLUSIONS
This study observed higher mortality than has previously been reported. It emphasizes the need for timely diagnosis, swift management and emergent surgical treatment for patients suffering an acute type A aortic dissection.
Keywords: Type A aortic dissection, Aortic dissection, Mortality
Acute type A aortic dissection (ATAAD) is an emergent condition with an incidence of 2–16 cases per 100 000 patient years [1–5].
INTRODUCTION
Acute type A aortic dissection (ATAAD) is an emergent condition with an incidence of 2–16 cases per 100 000 patient years [1–5]. Immediate surgical repair is the gold standard of care [3, 5, 6] and up to 50% of patients die before even reaching a hospital [3, 5, 7, 8]. Even with adequate surgical treatment, morbidity and mortality following ATAAD remain high with an in-hospital mortality of ∼17% in current multicentre studies [9–11]. For those arriving to the hospital but who are not eligible for surgical treatment, in-hospital mortality has been reported at 50–69% [12–15].
It has been commonly accepted that untreated ATAAD results in an hourly mortality rate of 1–2% during the first 24 h after symptom onset [13]. Although widely reported, the data to support this statement are scarce and rely solely on patients at surgical centres ineligible for surgical treatment. This inevitably leads to an underestimation of the true mortality rates as a significant proportion of patients die from the disease prehospitally at primary hospitals or during transfer to surgical centres [16].
During the last decades, significant advances have been made in clinical management, research and technology, which may affect the outcome of non-surgically treated (NST) ATAAD (e.g. emergency triage, resuscitative shock treatment, diagnostic imaging and medical treatment).
The aim of this study was to assess the fate of an all-comers cohort of NST patients with ATAAD, describe surgical decision rationale and provide contemporary data on mortality following NST ATAAD.
MATERIALS AND METHODS
Ethics statement
Ethical approval for this study was granted by the Swedish Ethical Review Agency (ref: 2021-01185, date: 23 April 2021). Informed patient consent was waived.
Study design
This was a regional, retrospective, observational study performed at the Department of Cardiothoracic Surgery, Skåne University Hospital, which is a tertiary referral centre with a strict geographical catchment area of 1.9 million inhabitants (2023).
We screened all patients in our catchment area who received one of the following diagnoses: International Classification of Diseases (ICD)-9 4410, 4411, 4415, 4416 or ICD-10 I710, I711, I715, I718 (Supplementary Material, Table S1) from January 1998 to November 2021 at any health care facility in our region. To ensure full regional coverage of eligible patients, the search was conducted using all available medical registries and carried out by the central data management department at the Skåne University Hospital. Thus, the only patients potentially left out of our search were those who succumbed to an ATAAD and did not undergo an autopsy. The charts (including autopsy reports) and available imaging of each screened patient were subsequently reviewed to confirm or discard the diagnosis of ATAAD or chronic type A aortic dissection (CTAAD). This review was performed by 1 cardiothoracic surgeon, and in complicated cases, additional cardiothoracic surgeons or a thoracic radiologist was consulted. Baseline and survival data were obtained by means of retrospective chart review. Data on patients who underwent surgery for ATAAD during the study period were collected from our departmental surgical database.
Patients with NST ATAAD and patients with CTAAD were included in this study and compared to surgically treated (ST) patients. We defined ATAAD as an aortic dissection or intramural haematoma involving the aorta proximal to the aortic arch with symptom duration <14 days. Although it may be argued that intramural haematomas are not dissections per se, they share many clinical characteristics and clinical management with ATAAD, and in this study, they were defined and regarded as aortic dissections. We defined CTAAD as an aortic dissection with symptom duration exceeding 14 days. These patients were included in the NST cohort as they did not undergo surgery during the first 14 days after symptom onset and, therefore, by definition, were 14-day survivors of an ATAAD. All diagnoses were confirmed by computed tomography, transthoracic echocardiography, transoesophageal echocardiography, aortic angiography or autopsy. Survival follow-up was conducted on 30 April 2023. No patients were lost to follow-up.
Statistical analysis
Categorical variables were presented as numbers and percentages. Continuous variables with kurtosis and skewness ranging from –2 to 2 were considered normally distributed and presented with means ± standard deviations, whereas skewed data were presented with medians and interquartile ranges. The two-sample T-test was used to compare means, the Mann–Whitney U-test for medians and categorical variables were analysed using the Chi-square test, unless the expected cell count was <5 when the Fisher’s exact test, or the Fisher–Freeman–Halton exact test for contingency tables exceeding 2×2, was used. We compared the baseline variables of NST patients to those who underwent an ATAAD repair. For all tests, a P-value of <0.05 was considered statistically significant. When comparing groups, missing values were excluded. The Kaplan–Meier survival curves included only the patients with known exact times for symptom debut and death. The hourly mortality rates were generated by calculating the average hourly mortality rate resulting from the known Kaplan–Meier survival estimates at 24 h, 48 h and 7 days, respectively. The equation used for calculating hourly mortality was mortality ratehourly = 1 – (1 – mortalitydaily)1/24. Patients with CTAAD were, by definition, considered to have survived 14 days and were censored at the time of surgical repair. All statistical analyses were conducted using IBM® SPSS® Statistics version 27.0.1.0 for MacOS® (IBM Corp, Armonk, NY, USA) except for calculating the 95% confidence intervals of the Kaplan–Meier survival curves, which was done with GraphPad Prism version 9.5.1 for MacOS® (GraphPad Software, Boston, MA, USA) using the asymmetrical method (log–log transformation).
The 1st author had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
RESULTS
Between January 1998 and November 2021, 2325 patients in our catchment area received one of the following diagnoses: ICD-9 4410, 4411, 4415, 4416 or ICD-10 I710, I711, I715, I718 (Supplementary Material, Table S1) and were reviewed for inclusion. We excluded 480 patients with isolated aortic aneurysm, 230 patients with aortic rupture, 657 patients with Stanford type B aortic dissection and 223 patients with incorrect diagnosis. A total of 551 patients were ST for an ATAAD (547 of whom were treated at our department, while the 4 remaining patients were excluded as they underwent surgery at other sites). The remaining 184 patients were NST ATAAD patients (25% of all ATAAD patients). Of whom, 153 patients (83%) presented with an ATAAD and were NST, and 31 (17%) presented with CTAAD and subsequently underwent surgery (Fig. 1).
Figure 1:
Flowchart of inclusion and exclusion of patients.
The baseline characteristics of this study population are presented in Table 1. The NST patients were older than the ST patients (74.7 ± 13.3 years vs 64.4 ± 11.8 years, P < 0.01) and were more often female [50.5% vs 36.2% (P < 0.01)]. Furthermore, NST patients more often presented with cardiac arrest with return of spontaneous circulation (ROSC) [11.8% vs 3.8% (P < 0.01)], were more likely to suffer from syncope [26.2% vs 17.2% (P = 0.01)] and more often presented with cerebral malperfusion [23.6% vs 14.5% (P < 0.01)] than ST patients. There were fewer patients with DeBakey type 1 dissections in the NST group than in the ST group, [65.8% vs 78.3% (P < 0.01)], and NST patients showed a trend towards being more frequently misdiagnosed [55.8% vs 48.0% (P = 0.08)].
Table 1:
Baseline characteristics
| All patients, n = 731 | Non-surgically treated, n = 184 | Surgically treated, n = 547 | P-value | Missing | |
|---|---|---|---|---|---|
| Age (years)a | 67.0 ± 13.0 | 74.7 ± 13.3 | 64.4 ± 11.8 | <0.01 | 0 (0) |
| Femaleb | 291 (39.8) | 93 (50.5) | 198 (36.2) | <0.01 | 0 (0) |
| Hypertensionb | 381 (52.6) | 105 (59.0) | 276 (50.5) | 0.05 | 6 (0.8) |
| Diabetes mellitusb | 108 (14.9) | 11 (6.2) | 97 (17.7) | <0.01 | 7 (1.0) |
| COPDb | 48 (6.6) | 18 (10.1) | 30 (5.5) | 0.03 | 6 (0.8) |
| Coronary artery diseaseb | 90 (12.4) | 37 (20.6) | 53 (9.7) | <0.01 | 4 (0.5) |
| Known thoracic aneurysmb | 82 (11.2) | 22 (12.0) | 60 (11.0) | 0.72 | 1 (0.1) |
| Marfan syndromeb | 29 (4.0) | 0 (0) | 29 (5.3) | <0.01 | 6 (0.8) |
| Family history of dissectionb | 25 (3.4) | 1 (0.5) | 24 (4.4) | 0.01 | 0 (0) |
| Previous cardiac surgeryb | 26 (3.6) | 13 (7.2) | 13 (2.4) | <0.01 | 6 (0.8) |
| Previous aortic surgeryb | 21 (2.9) | 4 (2.2) | 17 (3.1) | 0.54 | 6 (0.8) |
| Syncopeb | 139 (19.4) | 45 (26.2) | 94 (17.2) | 0.01 | 13 (1.8) |
| Hypotensive shockb | 181 (25.6) | 47 (28.0) | 134 (24.8) | 0.41 | 23 (3.1) |
| Cardiac arrest with ROSCb | 41 (5.7) | 20 (11.8) | 21 (3.8) | <0.01 | 15 (2.1) |
| Any malperfusionb | 257 (36.2) | 62 (37.8) | 195 (35.7) | 0.63 | 21 (2.9) |
| Cerebral malperfusionb | 117 (16.6) | 38 (23.6) | 79 (14.5) | <0.01 | 25 (3.4) |
| Cardiac malperfusionb | 84 (12.0) | 25 (15.8) | 59 (10.8) | 0.09 | 29 (4.0) |
| Intramural haematomab | 85 (11.6) | 18 (9.8) | 67 (12.3) | 0.36 | 1 (0.1) |
| Debakey type 1b | 530 (75.5) | 104 (65.8) | 426 (78.3) | <0.01 | 29 (4.0) |
| Preoperative creatinine (μmol/l)c | 89 (73–111) | 89.5 (74–114) | 89 (73–111) | 0.78 | 57 (7.8) |
| Preoperative lactate (mmol/l)c | 1.8 (1.2–3.4) | 1.9 (1.15–3.9) | 1.8 (1.2–3.3) | 0.53 | 208 (28.5) |
| Assessed by cardiothoracic surgeonb | 665 (91.0) | 118 (64.1) | 547 (100) | <0.01 | 0 (0) |
| Accepted for acute surgeryb | 569 (77.8) | 22 (12.0) | 547 (100) | <0.01 | 0 (0) |
| Misdiagnosedb | 321 (50.2) | 101 (55.8) | 220 (48.0) | 0.08 | 92 (12.6) |
Comparison of non-surgically treated and surgically treated patients with ATAAD. Values are presented as n (%), mean ± SD or median (interquartile range). P-values represent comparison between surgically treated patients and non-surgically treated patients.
Two-sample T-test.
Chi-square test.
Mann–Whitney U-test.
ATAAD: acute type A aortic dissection; COPD: chronic obstructive pulmonary disease; ROSC: return of spontaneous circulation; SD: standard deviation.
A comparison between NST patients who survived beyond 14 days (n = 58) and patients who did not (n = 108) is presented in Table 2. The non-survivors were significantly older than the 14-day survivors (76.2 ± 11.1 vs 70.9 ± 16.2 years, P = 0.01), but there was no significant difference in gender distribution. The non-survivors were more likely to suffer from hypotensive shock, syncope, cardiac arrest with ROSC, any malperfusion, cerebral malperfusion and cardiac malperfusion. DeBakey type 1 dissection occurred more frequently (72.7% vs 44.2%, P < 0.01) and intramural haematomas were less common (2.8% vs 24.1%, P < 0.01) in non-survivors.
Table 2:
Baseline characteristics of patients with non-surgically treated acute type A aortic dissection
| All patients, n = 166 | Non-survivors, n = 108 | 14-Day survivors, n = 58 | P-value | Missing | |
|---|---|---|---|---|---|
| Age (years)a | 74.4 ± 13.3 | 76.2 ± 11.1 | 70.9 ± 16.2 | 0.01 | 0 (0) |
| Femaleb | 85 (51.2) | 59 (54.6) | 26 (44.8) | 0.23 | 0 (0) |
| Hypertensionb | 100 (61.0) | 62 (57.9) | 38 (66.7) | 0.28 | 2 (1.2) |
| Diabetes mellitusc | 10 (6.1) | 5 (8.9) | 5 (4.7) | 0.31 | 3 (1.8) |
| COPDb | 17 (10.4) | 8 (14.0) | 9 (8.4) | 0.26 | 2 (1.2) |
| Coronary artery diseaseb | 32 (19.6) | 15 (14.0) | 17 (30.4) | 0.01 | 3 (1.8) |
| Known thoracic aneurysmb | 21 (12.7) | 14 (13.0) | 7 (12.1) | 0.87 | 0 (0) |
| Marfan syndrome | 0 (0) | 0 (0) | 0 (0) | N/A | 3 (1.8) |
| Family history of dissectionc | 1 (0.6) | 0 (0) | 1 (1.7) | 0.35 | 0 (0) |
| Previous cardiac surgeryc | 12 (7.4) | 3 (2.8) | 9 (16.1) | <0.01 | 3 (1.8) |
| Previous aortic surgeryc | 4 (2.5) | 1 (0.9) | 3 (5.4) | 0.12 | 3 (1.8) |
| Syncopeb | 43 (26.5) | 39 (36.4) | 4 (7.3) | <0.01 | 4 (2.4) |
| Hypotensive shockb | 46 (28.6) | 44 (41.5) | 2 (3.6) | <0.01 | 5 (3.0) |
| Cardiac arrest with ROSCb | 20 (12.3) | 18 (17.0) | 2 (3.6) | 0.01 | 4 (2.4) |
| Any malperfusionb | 59 (37.8) | 56 (54.9) | 3 (5.6) | <0.01 | 10 (6.0) |
| Cerebral malperfusionb | 35 (22.9) | 35 (34.7) | 0 (0) | <0.01 | 13 (7.8) |
| Cardiac malperfusionb | 25 (16.6) | 23 (23.0) | 2 (3.9) | <0.01 | 15 (9.0) |
| Intramural haematomab | 17 (10.2) | 3 (2.8) | 14 (24.1) | <0.01 | 0 (0) |
| Debakey type 1b | 91 (64.1) | 72 (72.7) | 19 (44.2) | <0.01 | 14 (8.4) |
| Chronic dissectionb | 31 (18.7) | 0 (0) | 31 (53.4) | <0.01 | 0 (0) |
| Preoperative creatinine (μmol/l)d | 89.5 (74–114) | 91 (75–111) | 86 (67–114) | 0.30 | 18 (10.8) |
| Preoperative lactate (mmol/l)d | 1.9 (1.2–3.9) | 2.9 (1.7–4.8) | 1.05 (0.7–1.7) | <0.01 | 61 (36.7) |
| Assessed by cardiothoracic surgeonb | 115 (69.3) | 66 (61.1) | 49 (84.5) | <0.01 | 0 (0) |
| Accepted for acute surgeryb | 22 (13.3) | 22 (20.4) | 0 (0) | <0.01 | 0 (0) |
| Misdiagnosedb | 94 (56.6) | 51 (47.2) | 43 (74.1) | <0.01 | 0 (0) |
Comparison of 14-day survivors and non-survivors. Values are presented as n (%), mean ± SD or median (interquartile range). P-values represent comparison between 14-day survivors and non-survivors.
Two-sample T-test.
Chi-square test.
Fisher’s exact test.
Mann–Whitney U-test.
COPD: chronic obstructive pulmonary disease; ROSC: return of spontaneous circulation; SD: standard deviation.
ATAAD was most frequently confirmed by computed tomography (71.7%) followed by autopsy (20.1%). Transoesophageal echocardiography and transthoracic echocardiography were equally common (3.3%, respectively), and 1.6% were diagnosed by aortic angiography (Table 3).
Table 3:
Diagnostic modality for non-surgically treated patients with acute type A aortic dissection
| Non-surgically treated patients, n = 184 | Missing | |
|---|---|---|
| CT | 132 (71.7) | 0 (0) |
| TEE | 6 (3.3) | 0 (0) |
| TTE | 6 (3.3) | 0 (0) |
| Aortic angiography | 3 (1.6) | 0 (0) |
| Autopsy | 37 (20.1) | 0 (0) |
Values are presented as n (%).
CT: computed tomography; TEE: transoesophageal echocardiography; TTE: transthoracic echocardiography.
The 14-day survival in a selected cohort of NST patients, where the exact time of symptom onset and time of death were known (n = 129), is presented in Fig. 2. The Kaplan–Meier estimates of mortality were 47.3 ± 4.4% at 24 h, 55.0 ± 4.4% at 48 h, 67.4 ± 4.1% at 7 days, 76.7 ± 3.7% at 14 days, 78.9 ± 3.9% at 30 days, 81.2 ± 4.1% at 90 days and 83.9 ± 4.3% at 1 year. The hourly mortality rate in NST patients during the 1st 24 h after symptom onset was 2.6%. The hourly mortality rate from 24 to 48 h was 0.7%, and the hourly mortality rate from 48 h to 7 days was 0.3%. Figure 3 shows the 14-day survival in ST patients (n = 422). The Kaplan–Meier estimates of mortality for ST patients were 3.8 ± 0.9% at 24 h, 5.0 ± 1.0% at 48 h, 6.6 ± 1.2% at 7 days, 9.2 ± 1.4% at 14 days, 10.9 ± 1.5% at 30 days, 13.5 ± 1.7% at 90 days and 14.9 ± 1.7% at 1 year.
Figure 2:

(A and B) Kaplan–Meier estimates of 14-day and 48-h survival in patients with non-surgically treated ATAAD. Upper and lower bands display 95% confidence interval.
Figure 3:

Kaplan–Meier estimates of 14-day survival in patients with surgically treated ATAAD. Upper and lower bands display 95% confidence interval.
The most common reason not to perform acute surgery (total n = 86), as assessed by a cardiothoracic surgeon, was severe comorbidity (32.6%) followed by death during transfer to surgery (25.6%), altered state of consciousness at the time of assessment (17.4%), poor prognosis (11.6%), other (9.3%) and limited extent of the dissection (3.5%) (Fig. 4).
Figure 4:
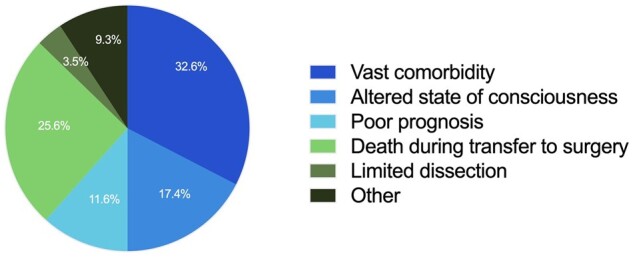
Reasons not to perform acute surgery after cardiothoracic surgeons’ assessment of patients with ATAAD (n = 86).
For some patients, the exact times of symptom debut and/or death were not known, but it was possible to determine whether the patient was alive at certain fixed time points. These data are presented in Supplementary Material, Table S2 along with the mortality rates in NST patients by subtype of ATAAD, spontaneous ATAAD, iatrogenic dissection and intramural haematoma.
DISCUSSION
In the present study, we assessed mortality of NST ATAAD in a contemporary cohort of patients not limited to individuals who were turned down for surgical repair. Our data showed a higher mortality than has previously been reported [3–5, 10, 12, 13, 17] with a 24-h mortality of 47.3%, 48-h mortality of 55.0%, 14-day mortality of 76.7% and 1-year mortality of 83.9%. The hourly mortality rate during the first 24 h after symptom onset was 2.6%.
Previous studies have reported an hourly mortality rate of 1–2% during the 1st 24 h after symptom onset in untreated ATAAD [3–5]. However, there is some level of uncertainty as to where these numbers were originally described. Most authors refer to the results from a study by Hagan et al. from 2000, with data from the International Registry of Acute Aortic Dissection (IRAD) [13], including 81 patients with medically treated ATAAD. All included patients were denied surgery as they were deemed unfit, mostly due to advanced age and comorbidity. In 2013, Booher et al. [12] published an updated study with data from IRAD, including 186 patients with medically treated ATAAD, with a 24-h mortality of 18%, a 7-day mortality of 49%, a 30-day mortality of 60% and a 60-day mortality of 62%. As IRAD collect their data from the large tertiary referral centres that participate in the registry, patients who are not referred to these centres are left out from the registries. This includes patients who were deemed unfit for surgery at the primary hospital and patients who died in their homes. This was recognized in a recent IRAD publication from 2018 by Evangelista et al. who believed the previous rates from the IRAD registry to be underestimated as >60% of patients of the patients included in IRAD were transferred from outside hospitals to IRAD facilities. Consequently, patients were not included in the registry if they died at the primary hospital or during the transfer [10]. An even more recent publication, published in 2022 by Harris et al. with data from the IRAD, compares the 48-h mortality in surgically versus medically treated ATAAD measured from hospital arrival. They found the 48-h mortality, in patients with medically treated ATAAD, to be 23.7% corresponding to an hourly mortality rate of 0.5%/h [18]. Compared to the results from IRAD, our results show substantially higher mortality at all time points. We also observed a higher hourly mortality rate during the first 24 h after symptom onset than the classically accepted 1–2%. This may be due to several reasons, but we believe that the main explanation is that the Swedish social security system with personal identification numbers and population-wide registries enables superior/excellent possibilities for patient inclusion and follow-up. With our method, we identified and included all patients in our catchment area of 1.9 million inhabitants who were diagnosed at any healthcare facility ranging from emergency departments at smaller hospitals, departments of pathology and our cardiothoracic surgical department. However, it is likely that the mortality presented in the current study is underestimated as patients who succumbed to an ATAAD were not always autopsied. This applies both to some patients dying from unclear reasons at hospitals but even more so for patients dying outside of hospitals.
The NST patients in our study were generally older than the patients who underwent surgery. They were also more likely to have developed complications to their ATAAD such as syncope, cardiac arrest with ROSC or cerebral malperfusion. These results are expected and in line with results from other studies as the leading cause of patients not being operated is vast comorbidity [12, 13, 17]. However, in 26% of cases, the patient was accepted for surgery but died during transfer to our operating centre. In previous studies, it has been reported that 16–78% of ATAAD patients are initially misdiagnosed [19–21], potentially causing a delay in management and thereby increased risk of mortality. In this study, 56% of NST patients were initially misdiagnosed, as compared to 48% among the ST patients. This, along with the high mortality during the 1st 24 h after symptom onset emphasizes the need for swift recognition and management of ATAAD patients.
Although our study indicated that 30-day mortality after NST intramural haematomas (36%) and iatrogenic dissections (60%) are lower than that of spontaneous ATAAD (83%), our data do not encourage conservative treatment of these subsets of ATAAD, as surgery is associated with an in-hospital mortality of approximately 17% in large multicentre registries [9–11].
Our result showed that female patients constituted 51% of NST patients and significantly less often underwent surgical repair. This is consistent with previously published results [12] and may partly be explained by the known fact that female patients have their ATAADs ∼7 years later than male patients [3, 13, 22–24]. Although there is no strict age limit for performing ATAAD repair, it is a procedure carrying significant perioperative risks, and a patient presenting with advanced age could very well shift the choice of management from surgery to medical therapy. In addition, older patients have generally accumulated more co-morbidities than younger individuals. Another plausible cause is the fact that women with ATAAD tend to present with atypical symptoms [4, 23], making misdiagnosis more likely.
Limitations
This study has several limitations. First, the retrospective design carries inherent shortcomings related to the acquisition of data, e.g. missing information on time of symptom debut and information regarding co-morbidities and the single-region nature of the study may raise question about generalizability of the results. Furthermore, there is a clear selection bias as patients selected for NST of ATAAD often are the patients most unfit to receive the gold standard treatment of surgery. A prospective, randomized study is not feasible in this setting for obvious reasons. Therefore, although the outcomes of medical treatment may not be representative of all ATAAD patients, the data presented represent a real-life scenario. Another limitation is the difficulty of identifying all ATAAD patients. Considering the emergent nature of the disease, some patients will die before they can ever be diagnosed, and all patients are not autopsied. This is likely to lead to an underestimation of the true mortality rates. On the other hand, the foremost strength of this study is the large, all-comers population, collected from a period of 23 years and the benefit of a solid method for identifying diagnosed patients.
CONCLUSION
In this sizeable, contemporary, 23-year study of an all-comers cohort, we observed higher mortality after NST ATAAD than has previously been reported. This emphasizes the need of timely diagnosis, swift management and emergent surgical treatment for patients suffering an ATAAD.
Supplementary Material
ACKNOWLEDGEMENTS
The authors want to thank epidemiological statistician Daniel Oudin Åström, PhD, at Lund University, for support with the statistical analyses.
Glossary
ABBREVIATIONS
- ATAAD
Acute type A aortic dissection
- CTAAD
Chronic type A aortic dissection
- IRAD
International Registry of Acute Aortic Dissection
- NST
Non-surgically treated
- ROSC
Return of spontaneous circulation
- ST
Surgically treated
Contributor Information
Karl Teurneau-Hermansson, Department of Clinical Sciences Lund, Skåne University Hospital, Lund University, Lund, Sweden; Department of Cardiothoracic Surgery, Skåne University Hospital, Lund University, Lund, Sweden.
Jacob Ede, Department of Clinical Sciences Lund, Skåne University Hospital, Lund University, Lund, Sweden; Department of Cardiothoracic Surgery, Skåne University Hospital, Lund University, Lund, Sweden.
Mårten Larsson, Department of Clinical Sciences Lund, Skåne University Hospital, Lund University, Lund, Sweden; Department of Cardiothoracic Surgery, Skåne University Hospital, Lund University, Lund, Sweden.
Gustaf Linton, Department of Clinical Sciences Lund, Skåne University Hospital, Lund University, Lund, Sweden; Department of Cardiothoracic Surgery, Skåne University Hospital, Lund University, Lund, Sweden.
David von Rosen, Department of Clinical Sciences Lund, Skåne University Hospital, Lund University, Lund, Sweden; Department of Cardiothoracic Surgery, Skåne University Hospital, Lund University, Lund, Sweden.
Johan Sjögren, Department of Clinical Sciences Lund, Skåne University Hospital, Lund University, Lund, Sweden; Department of Cardiothoracic Surgery, Skåne University Hospital, Lund University, Lund, Sweden.
Per Wierup, Department of Clinical Sciences Lund, Skåne University Hospital, Lund University, Lund, Sweden; Department of Cardiothoracic Surgery, Skåne University Hospital, Lund University, Lund, Sweden.
Shahab Nozohoor, Department of Clinical Sciences Lund, Skåne University Hospital, Lund University, Lund, Sweden; Department of Cardiothoracic Surgery, Skåne University Hospital, Lund University, Lund, Sweden.
Igor Zindovic, Department of Clinical Sciences Lund, Skåne University Hospital, Lund University, Lund, Sweden; Department of Cardiothoracic Surgery, Skåne University Hospital, Lund University, Lund, Sweden.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
FUNDING
This work was supported by the Tegger Foundation (to Karl Teurneau-Hermansson); the Agreement for Medical Education and Research Sweden; the Swedish Heart Lung Foundation; and Torsten Westerströms stiftelse.
Conflict of interest: none declared.
DATA AVAILABILITY
The data underlying this article cannot be shared publicly due to legislative reasons and limitations in ethical permit. The data will be shared on reasonable request to the corresponding author.
Author contributions
Karl Teurneau-Hermansson: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—original draft; Writing—review and editing. Jacob Ede: Conceptualization: Supporting; Methodology: Supporting; Validation: Equal; Writing—review and editing: Equal. Mårten Larsson: Conceptualization: Supporting; Validation: Supporting; Writing—review and editing: Equal. Gustaf Linton: Data curation: Equal; Investigation: Equal; Project administration: Supporting; Validation: Supporting; Writing—review and editing: Equal. David von Rosen: Data curation: Supporting; Investigation: Supporting; Project administration: Supporting; Writing—review and editing: Equal. Johan Sjögren: Conceptualization: Supporting; Methodology: Supporting; Supervision: Supporting; Writing—review and editing: Equal. Per Wierup: Conceptualization: Supporting; Writing—review and editing: Equal. Shahab Nozohoor: Conceptualization: Supporting; Methodology: Supporting; Supervision: Supporting; Writing—review and editing: Equal. Igor Zindovic: Formal analysis: Equal; Funding acquisition: Equal; Investigation: Supporting; Methodology: Equal; Project administration: Supporting; Resources: Equal; Supervision: Lead; Validation: Equal; Writing—original draft: Supporting; Writing—review and editing: Lead.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Giovanni Mariscalco, Ilaria Giambuzzi, Fernando Fleischman and the other, anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
- 1. Kurz SD, Falk V, Kempfert J, Gieb M, Ruschinski TM, Kukucka M. et al. Insight into the incidence of acute aortic dissection in the German region of Berlin and Brandenburg. Int J Cardiol 2017;241:326–9. [DOI] [PubMed] [Google Scholar]
- 2. Landenhed M, Engström G, Gottsäter A, Caulfield MP, Hedblad B, Newton-Cheh C. et al. Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: a prospective cohort study. J Am Heart Assoc 2015;4:e001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gudbjartsson T, Ahlsson A, Geirsson A, Gunn J, Hjortdal V, Jeppsson A et al Acute type A aortic dissection—a review. Scand Cardiovasc J 2020;54:1–13. [DOI] [PubMed] [Google Scholar]
- 4. Elsayed RS, Cohen RG, Fleischman F, Bowdish ME.. Acute type A aortic dissection. Cardiol Clin 2017;35:331–45. [DOI] [PubMed] [Google Scholar]
- 5. Nienaber CA, Clough RE.. Management of acute aortic dissection. Lancet 2015;385:800–11. [DOI] [PubMed] [Google Scholar]
- 6. Isselbacher EM, Preventza O, Hamilton Black Iii J, Augoustides JG, Beck AW, Bolen MA. et al. ; Writing Committee Members. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: a Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;80:e223–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conzelmann LO, Weigang E, Mehlhorn U, Abugameh A, Hoffmann I, Blettner M. et al. ; GERAADA Investigators. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2016;49:e44–52. [DOI] [PubMed] [Google Scholar]
- 8. Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM; Oxford Vascular Study. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013;127:2031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Czerny M, Schoenhoff F, Etz C, Englberger L, Khaladj N, Zierer A. et al. The impact of pre-operative malperfusion on outcome in acute type A aortic dissection: results from the GERAADA registry. J Am Coll Cardiol 2015;65:2628–35. [DOI] [PubMed] [Google Scholar]
- 10. Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U. et al. ; IRAD Investigators. Insights from the International Registry of Acute Aortic Dissection: a 20-year experience of collaborative clinical research. Circulation 2018;137:1846–60. [DOI] [PubMed] [Google Scholar]
- 11. Geirsson A, Shioda K, Olsson C, Ahlsson A, Gunn J, Hansson EC. et al. Differential outcomes of open and clamp-on distal anastomosis techniques in acute type A aortic dissection. J Thorac Cardiovasc Surg 2019;157:1750–8. [DOI] [PubMed] [Google Scholar]
- 12. Booher AM, Isselbacher EM, Nienaber CA, Trimarchi S, Evangelista A, Montgomery DG. et al. ; IRAD Investigators. The IRAD classification system for characterizing survival after aortic dissection. Am J Med 2013;126:730.e19–24. [DOI] [PubMed] [Google Scholar]
- 13. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL. et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897–903. [DOI] [PubMed] [Google Scholar]
- 14. Evangelista A, Rabasa JM, Mosquera VX, Barros A, Fernández-Tarrio R, Calvo-Iglesias F. et al. Diagnosis, management and mortality in acute aortic syndrome: results of the Spanish Registry of Acute Aortic Syndrome (RESA-II). Eur Heart J Acute Cardiovasc Care 2018;7:602–8. [DOI] [PubMed] [Google Scholar]
- 15. Yamaguchi T, Nakai M, Sumita Y, Miyamoto Y, Matsuda H, Inoue Y et al Current status of the management and outcomes of acute aortic dissection in Japan: analyses of nationwide Japanese Registry of All Cardiac and Vascular Diseases-Diagnostic Procedure Combination data. Eur Heart J Acute Cardiovasc Care 2020;9:S21–31. [DOI] [PubMed] [Google Scholar]
- 16. Zindovic I, Sjögren J, Bjursten H, Ingemansson R, Ingimarsson J, Larsson M. et al. The coagulopathy of acute type A aortic dissection: a prospective, observational study. J Cardiothorac Vasc Anesth 2019;33:2746–54. [DOI] [PubMed] [Google Scholar]
- 17. Kozai Y, Watanabe S, Yonezawa M, Itani Y, Inoue T, Takasu J. et al. Long-term prognosis of acute aortic dissection with medical treatment: a survey of 263 unoperated patients. Jpn Circ J 2001;65:359–63. [DOI] [PubMed] [Google Scholar]
- 18. Harris KM, Nienaber CA, Peterson MD, Woznicki EM, Braverman AC, Trimarchi S. et al. Early mortality in type A acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. JAMA Cardiol 2022;7:1009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pourafkari L, Tajlil A, Ghaffari S, Parvizi R, Chavoshi M, Kolahdouzan K et al The frequency of initial misdiagnosis of acute aortic dissection in the emergency department and its impact on outcome. Intern Emerg Med 2017;12:1185–95. [DOI] [PubMed] [Google Scholar]
- 20. Zaschke L, Habazettl H, Thurau J, Matschilles C, Göhlich A, Montagner M. et al. Acute type A aortic dissection: Aortic Dissection Detection Risk Score in emergency care—surgical delay because of initial misdiagnosis. Eur Heart J Acute Cardiovasc Care 2020;9:S40–7. [DOI] [PubMed] [Google Scholar]
- 21. Hirata K, Wake M, Takahashi T, Nakazato J, Yagi N, Miyagi T. et al. Clinical predictors for delayed or inappropriate initial diagnosis of type A acute aortic dissection in the emergency room. PLoS One 2015;10:e0141929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boening A, Karck M, Conzelmann LO, Easo J, Krüger T, Rylski B. et al. German Registry for Acute Aortic Dissection Type A: structure, results, and future perspectives. Thorac Cardiovasc Surg 2017;65:77–84. [DOI] [PubMed] [Google Scholar]
- 23. Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A, Petzsch M. et al. ; International Registry of Acute Aortic Dissection. Gender-related differences in acute aortic dissection. Circulation 2004;109:3014–21. [DOI] [PubMed] [Google Scholar]
- 24. Huckaby LV, Sultan I, Trimarchi S, Leshnower B, Chen EP, Brinster DR et al Sex-based aortic dissection outcomes from the International Registry of Acute Aortic Dissection. Ann Thorac Surg 2022;113:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to legislative reasons and limitations in ethical permit. The data will be shared on reasonable request to the corresponding author.




