SUMMARY
The clumped distribution of resources in the world has influenced the pattern of foraging behavior since the origins of locomotion, selecting for a common search motif in which straight movements through resource-poor regions alternate with zig-zag exploration in resource-rich domains. For example, during local search, flying flies spontaneously execute rapid flight turns called body saccades, but suppress these maneuvers during long-distance dispersal or when surging upstream towards an attractive odor. Here, we describe the key cellular components of a neural network in flies that generate spontaneous turns as well as a specialized pair of neurons that inhibits the network and suppresses turning. Using 2-photon imaging, optogenetic activation, and genetic ablation, we show that only four descending neurons appear sufficient to generate the descending commands to execute flight saccades. The network is organized into two functional units—one for right turns and one for left—with each unit consisting of an excitatory (DNae014) and inhibitory (DNb01) neuron that project to the flight motor neuropil within the ventral nerve cord. Using resources from recently published connectomes of the fly, we identified a pair of large, distinct interneurons (VES041) that forms inhibitory connections to all four saccade command neurons and created specific genetic driver lines for this cell. As predicted by its connectivity, activation of VES041 strongly suppresses saccades, suggesting it promotes straight flight to regulate the transition between local search and long-distance dispersal. These results thus identify the key elements of a network that may play a crucial role in foraging ecology.
Keywords: Command neurons, locomotion, flight saccades, spontaneous activity, local search, long-distance dispersal
Graphical Abstract

eTOC blurb
The generation and regulation of flight turns play a critical role in foraging ecology. Ros et al. functionally characterize the neural network that generates spontaneous turns in fruit flies. A specialized neuron inhibits this network and suppresses flight turns, suggesting it may regulate the transition between local search and dispersal.
INTRODUCTION
When searching their environment, many species of flies execute a series of straight flight segments interspersed with extremely rapid turns lasting less than ~50 ms. When first identified, these brief turns were termed saccades, in analogy with the rapid eye movements of primates2. In flies, however, the entire body rotates during each saccade, not just the eyes. Saccades are thought to benefit flying animals in several ways. From a sensory perspective, saccades may restrict the deleterious effects of motion blur to brief moments interjected within longer sequences of gaze stabilization3–5. Brief bursts of saccades in the same direction may aid local search strategy by allowing the animal to quickly scan the local environment for salient visual and olfactory features6. More recently, it has been suggested that comparing sensory measurements immediately before and after each saccade might enable flies to estimate key parameters that are otherwise not directly measurable, such as the direction and magnitude of the ambient wind7,8. For all these hypotheses, the timing between saccades is critical, and several factors are thought to influence the frequency of the spontaneous turns. For example, tethered flies flying in a closed-loop configuration will greatly reduce the saccade rate and instead actively steer toward salient visual objects such as vertical edges—a robust fixation behavior that they maintain for several hours9. Similarly, flies also reduce saccade rates in the presence of a small bright spot, which they presumably interpret as the sun. In this case, however, they do not necessarily steer directly toward the distant bright object, but rather maintain it at an arbitrary azimuthal angle10. Patterns of polarized light that mimic those in the natural sky induce a similar reflex11,12. This orientation behavior using celestial cues, termed menotaxis, allows flies and other insects13 to maintain a constant heading over long distances (<10km) while dispersing14,15. Flies also suppress saccades in the presence of a constant odor stream to promote their progress upwind toward a food source16–18. Despite its importance with respect to multiscale search, the neural mechanisms that underlie both the generation of saccades and their suppression during dispersal and plume tracking remain largely unknown.
Within the central nervous system of insects, descending neurons (DNs) constitute a critical stage in the transformation of sensory input in the brain into motor commands in the ventral nerve cord (VNC)19. Drosophila possess ~650 pairs of descending neurons20–22, some of which appear to function as specialized command neurons for specific behaviors including courtship23,24, walking backward25, turning26, take off27,28, and landing29. Thus, DNs provide a logical starting point for investigating the circuits that generate and regulate flight saccades. A prior study using electrophysiology and 2-photon imaging implicated a pair of DNs, called AX, in the execution of spontaneous and visually-elicited flight saccades30. However, this work employed a broad GAL4-driver line (R65G08) and a separate study found no significant effect on saccade rate when the cells within this line were silenced via a temperature-sensitive dynamin mutation that blocks synaptic transmission31. To confirm and further investigate the role of AX in saccade generation, we constructed a sparse split-GAL4 driver for this neuron better suited for functional imaging, optogenetic activation, and genetic ablation. From here on, we will refer to the AX neuron as DNae014, as this is its identifier in the FlyWire database32,33. Because we suspected that multiple pairs of DNs might be involved in driving the bilateral wing movements exhibited during saccades, we screened a large collection of split-GAL4 lines22 for additional cells that function to trigger rapid turns. Our screen led to the discovery of one additional cell (DNb01) that together with DNae014 appears to form two functional units that drive saccades to the left and right. We systematically analyzed the upstream inputs to these DNs using emerging connectomes of the Drosophila brain32–35 and found a bilateral pair of large inhibitory interneurons (VES041) whose activation suppresses saccades thereby promoting straight flight. Thus, we have identified the core cellular components of a network that generates and regulates spontaneous flight maneuvers in flying Drosophila.
RESULTS
To confirm the role of DNae014 in generating flight saccades, we created a split-GAL4 line36 that targets this neuron for functional imaging, optogenetic activation and silencing, and genetic ablation. The split-GAL4 line we created was quite sparse (Video S1); although we occasionally saw faint expression of other cells, including one unidentified DN. To characterize the activity of individual DNae014 neurons, we used 2-photon functional imaging to record from the arbor of DNae014 cells on the left and right sides of the brain, while simultaneously tracking wing motion during flight (Figure 1A–E). The difference in GCaMP7f fluorescence between the bilateral pair of cells (R-L ΔF/F) correlated remarkable closely with the difference in wingstroke amplitude (L-R WSA) (Figure 1F, H). We used a simple classifier to determine the timing of saccades based on transient differences in WSA (dots in Figure 1H, I). We interpreted these saccades as spontaneous events because our experiments were conducted in darkness. We also recorded from the arbor of the weakly expressing, off-target DN mentioned above, but did not detect any functional activity during flight.
Figure 1. Activities of DNae014 and DNb01 correlate strongly with spontaneous saccades.

(A) Flight trajectory and photomontage illustrating a single saccade, adapted from46,53. (B) Schematic of set-up for monitoring neural activity with GCaMP7f and simultaneously tracking wingstroke amplitudes during flight. (C) Fly with central nervous system. (D) Anatomy of right DNae014 (green) and DNb01 (magenta) cells in the brain and VNC. (E) Bilateral expression patterns of DNae014 and DNb01 cells in the brain from split-GAL4 lines, showing the fields of view scanned in 2-photon experiments (dashed boxes). Black arrows indicate somata. (F) Top: left (dark gray) and right (light gray) wingstroke amplitudes. Bottom: normalized GCaMP7f fluorescence (ΔF/F) in left and right DNae014 cells during a 90 s flight epoch. (G) Similar to (F) but for DNb01. (H) Right-left difference in DNae014 signals (green) superimposed with left-right difference in wingstroke amplitudes (black). Both traces are normalized by z-score from the traces above in F. Automatically detected right and left saccades depicted as green dots above and below, respectively. (I) Similar to H, but for DNb01. (J) Pooled regressions for DNae014 (green) and DNb01 (magenta) of left vs. right cell activity (ΔF/F), normalized by z-score (n = 19 flies) and plotted as a kernel density estimate. (K) Regressions of L-R wingstroke amplitude vs. R-L ΔF/F, across 19 flies for DNae014 (green; r2 = 0.89±0.04, mean±sd) and DNb01 (magenta, r2 = 0.90±0.03, mean±sd). (L) Changes in DNae014 (left column) and DNb01 (right column) cell activity aligned to the onset of spontaneous flight saccades (vertical line). Presented as right saccades. Top traces: baseline-subtracted wingstroke amplitudes of left (dark gray), right (light gray) wings, along with left-right difference (black). Bottom traces: ΔF/F signals for right cell (light color), left cell (dark color), and right-left difference (black). Solid lines indicate mean of means for all individuals (n = 19 flies for each dataset); shaded areas indicate boot-strapped 95% CIs. See also Figure S1, Videos S1 and S2.
In addition to DNae014, we identified another cell, DNb01, whose activity is also tightly correlated with the production of spontaneous saccades (Figure 1D, E, G, I). The split-GAL4 driver line for this cell (SS02383)22 is also very sparse, although the line does target some neurons that arborize in the ocellar ganglion (Video S2). Across individuals, the bilateral difference in cell fluorescence (R-L ΔF/F) explained 89±4% (for DNae014) and 90±3% (for DNb01) of the variance in differential wingstroke amplitude (L-R) (Figure 1K). The activity levels between the left and right DNae014 cells followed an inverse, highly non-linear relationship, analogous to ‘flip-flop’ components in digital electronic circuits (Figure 1J), and reminiscent of neurons identified in the steering behavior of male silkmoths37. The relationship for the two DNb01 cells was also inverse, but much more linear. Given the complex relationship between the electrical activity of a neuron and its GCaMP signal, these subtle differences in the bilateral patterns of the DNae014 and DNb01 cells may not be functionally relevant; but nevertheless, the activities of both neurons correlate remarkably well with ipsilaterally directed saccades (Figure 1l).
Despite similar activity patterns, DNae014 and DNb01 are morphologically distinct. DNae014 possesses smooth, dendritic processes in the lateral accessory lobe (LAL) and superior posterior slope (SPS) with varicose terminals in the gnathal ganglion (GNG) and wing neuropil of the VNC (Figure 1C, D). As described before based on dye fills following whole cell recordings30, all processes of the DNae014 cell are ipsilateral to the soma in both the brain and VNC. In contrast, DNb01 crosses the midline via the LAL commissure with varicose terminals in the contralateral SPS, posterior ventrolateral protocerebrum (PVLP) and LAL, and sends a descending axon to the wing neuropil of the VNC contralateral to the cell body (Figure 1D, E). Both cells arborize extensively within the LAL, a region implicated in turning behaviors in a variety of insect species38,39. Although we did not record from DNae014 and DNb01 simultaneously, transient changes in the activity of either corresponds with a rapid alteration in wing motion, and conversely, every change in wing motion corresponds with transients in the activity of the neurons. Based on this close correlation (Figure 1K), we tentatively conclude that these two pairs of DNs are co-active during flight saccades (Figure 1).
To confirm the prior observation that DNae014 responds to expanding visual patterns and test whether DNb01 exhibits a similar phenomenology, we imaged bilateral activity of the DNs while presenting a looming stimulus at different azimuthal positions (Figure 2). For both DNae014 and DNb01, a looming spot presented in one visual hemisphere increased activity in cells on the opposite side (Figure 2B). Although the absolute magnitude of the measured ΔF/F response for DNae014 was larger, this result based on Ca2+ imaging need not reflect an actual difference in the membrane-level responses of the two DNs. Recently, other descending neurons have been identified that appear to mediate looming responses during flight40, which suggests that DNs other than DNae014 and DNb01 may be involved in visually elicited avoidance reactions.
Figure 2. DNae014 and DNb01 are active during saccades elicited by visual loom.

(A) Visual azimuth directions of looming stimuli from −72°, −36°, 0°, 36°, and 72° (left to right). Traces below indicate the time course of stimulus size. (B) Normalized GCaMP7f fluorescence for DNae014 (left panels, green, n = 9), and DNb01 (right panels, magenta, n = 12) in response to each stimulus direction for left cell (L), right cell (R), and bilateral differential (black, R-L). Solid lines represent the grand mean of the mean traces for individual flies, the shaded areas indicated the boot-strapped 95% confidence intervals (CIs). (C) Wingstroke amplitude responses to every stimulus direction of left (L), right (R), and differential (L-R) wingstroke amplitudes. (D) Bilateral difference (R-L) ΔF/F for peak stimulus responses to each loom direction, plotted as mean of means with boot-strapped 95% CIs. DNae014 (green) responded more strongly but with a similar sign relative to stimulus direction, compared to DNb01 (magenta). Peak responses for DNae014 ΔF/F were larger than those for DNb01 (p < 0.001; two-way ANOVA with cell type and loom direction as the main effects and individual as a random effect). See also Video S1 and S2.
To test whether DNae014 and DNb01 are sufficient to elicit saccades, we used several methods to activate individual neurons on either the left or right side of the brain while measuring the resulting changes in wing motion. First, we drove CsChrimson bilaterally in DNae014 cells, but used focal 2-photon excitation to unilaterally activate neurons on one side of the brain or the other (Figure 3A–C). Activation of the left DNae014 cell reliably elicited a saccade to the left and activation of the right cell elicited a saccade to the right, consistent with the correlation recorded during spontaneous activity (Figure 1). These evoked syndirectional saccades also confirm prior experiments that used focal injection of ATP to activate ectopically expressed P2X2 channels41 in DNae01430. Focal 2-photon CsChrimson activation was not feasible for single DNb01 cells, however; because each neuron branches extensively in both the left and right hemispheres (Figure 1D, E). Instead, we unilaterally activated the DNs using the SPARC system42 to express CsChrimson (Figure 3D). Because the SPARC reagent produced flies with CsChrimson in either both targeted cells, neither cells, the left cell only, or the right cell only, we scored the expression pattern of each individual after testing their behavioral responses to pulses of 617 nm light. A distinct advantage of the SPARC method is that it enforces a blinded experimental design, with the subset of flies in which neither of the targeted cells expressed CsChrimson serving as a convenient control group. Unilateral activation of DNae014 evoked the expected changes in wing motion based on our 2-photon experiments (Figure 3G), and unilateral activation of DNb01 also evoked syndirectional saccades. However, the saccades evoked by DNb01 activation were smaller in amplitude and longer in time course than those evoked by DNae014 activation, suggesting that the two cells drive different components of the saccade motor program within the VNC.
Figure 3. Unilateral activation of either DNae014 and DNb01 elicits directional saccades.
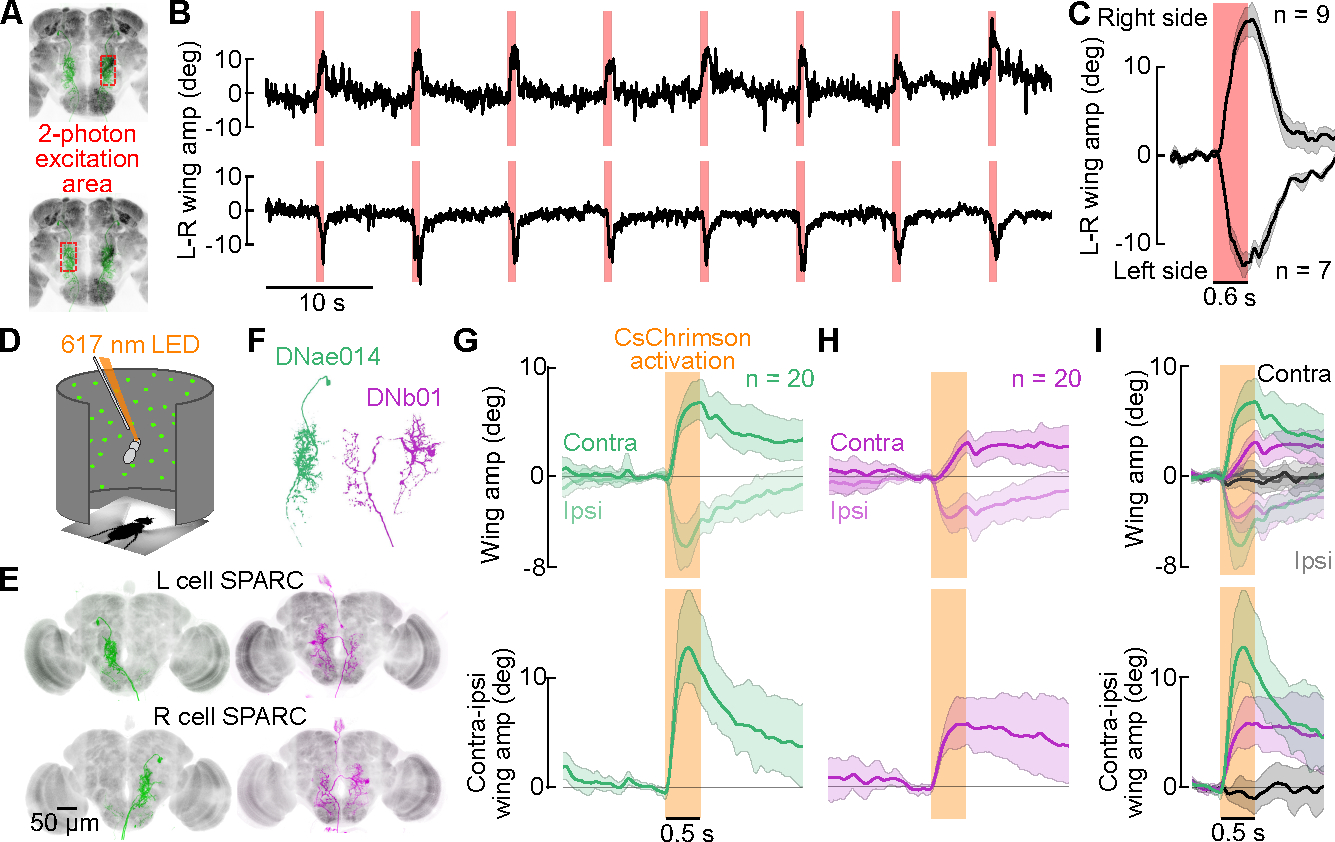
(A-C) Unilateral 2-photon excitation of either left or right DNae014 neurons. (A) Approximate 2-photon excitation areas of the right and left dendritic arbors of DNae014. (B) Example traces of L-R wingstroke amplitude during unilateral CsChrimson excitation on the left (top) or right (bottom) side of brain. (C) L-R wing amplitudes aligned to the excitation light pulse. As in all panels, solid lines indicate the mean of all individual means, the shaded patch indicates the boot-strapped 95% CI; n = 9 flies for right stimulations, n = 7 flies for left stimulations. (D-I) Unilateral activation of DNae014 or DNb01 neurons in rigidly tethered flies using split-GAL4 and SPARC expression of CsChrimson. (D) Flight arena with 617 nm excitation light. (E) Maximum intensity projection of TdTomato signal in examples of unilateral cell expression using SPARC with split-GAL4 drivers for DNae014 (green) and DNb01 (magenta). Nc82 staining shown in grey. (F) Representative morphology of right DNae014 and DNb01 cells. (G) Contralateral and ipsilateral wingstroke amplitudes (top) and contralateral-ipsilateral difference (bottom), aligned to activation pulse; n= 20 flies. (H) Similar to G, but for DNb01; n = 20 flies. (I) Combined data from G and H, including control flies (black traces, gray envelopes) in which neither cell expressed CsChrimson; n = 20, 20, and 19 for DNae014, DNb01, and controls, respectively. See also Video S1 and S2.
To assess the necessity of the two pairs of DNs to generate spontaneous saccades, we silenced the neurons via expression of the light-gated GtACR1 channel. We periodically exposed rigidly tethered flies expressing GtACR143 in DNae014 to 530 nm light pulses (Figure S2A, B). Although the experimental group exhibited a strong decrease in saccade rate when exposed to 530 nm light, empty-vector control flies, which did not express GtACR1, exhibited a substantial increase in saccade rate in response to the pulses of green light (Figure S2C, D). Whereas these silencing results support the hypothesis that suppressing DNae014 activity reduces the frequency of spontaneous saccades, the interpretation is complicated by the large increase in saccade rate exhibited by control flies in response to the activation light. For this reason, we adopted an alternative strategy to test the necessity of the two DNs to saccade by genetically ablating the cells through ectopic expression of the pre-apoptotic genes reaper and hid44.
We quantified flight saccades in rigidly tethered flies in which either DNae014 or DNb01 was ablated. Empty vector control flies exhibited a saccade frequency of 0.48 ± 0.18 Hz (mean ± S.D.), consistent with prior studies under both free and tethered flight conditions30,45,46. Genetic ablation of DNae014 reduced saccade frequency to 0.09 ± 0.09 Hz (p<0.001, Kruskal-Wallis test followed by post-hoc comparisons with Tukey’s HSD method), whereas ablation of DNb01 reduced the rate to 0.12 ± 0.14 Hz (p<0.001; Figure 4B, C). Thus, ablation of either DNae014 or DNb01 significantly reduced the rate of spontaneous saccades but did not eliminate them. This result suggests that bilateral pairs of either DNae014 or DNb01 acting alone can still generate partial saccades, albeit at a reduced spontaneous frequency, or alternatively, that spontaneous saccades that persist following ablation of either cell type might be generated by an alternate pathway.
Figure 4. Ablation of either DNae014 or DNb01 alters saccade dynamics.

(A) Riged-tether flight arena with IR backlighting to track wingstroke amplitudes. (B) Transient changes in L-R wingstroke amplitude indicate fictive saccades. Example control fly (top, grey), DNae014-ablated fly (middle, green), and DNb01-ablated fly (bottom, magenta) (C) Individual saccade rates in the rigid tethered arena (n = 26, 25, 36, respectively). (D) Magnotether flight arena with IR backlighting to track body angle and a static visual pattern. (E) Example traces with saccades apparent as rapid changes in body angle and angular velocity transients (black traces). (F-J) Color codes as in B. n = 22, 23, 22 flies, respectively. (F) Saccade rates in the magnotether arena, plotted as mean of individual fly means and boot-strapped 95% CI. (G) Cumulative histogram of inter-saccade intervals. (H) Maximum inter-saccade interval (ISImax) for each 2-minute trial. (I) Fraction of saccades syndirectional with the previous saccade. (J) Relationship between peak saccade speed and saccade magnitude. Dashed line: Orthogonal-distance regression fit of a 2nd order polynomial to pooled data of control flies. (K) Peak saccade speed normalized to fit in I, plotted as mean of individual fly means and boot-strapped 95% CI. Statistical differences were assessed using Kruskal-Wallis tests with Tukey’s HSD method for post-hoc comparisons (C, F, H, I, K; *** = p<0.001; n.s. = not significant). See also Figure S2, Video S1 and S2.
To gain further insight into the effect of cell ablation on saccade generation, we repeated the experiments using a magnotether arena, in which both rapid turns and epochs of straight flight are easier to observe and classify45 (Figure 4D, E). Ablating the DNs reduced the saccade rate in the magnotether from 0.37 ± 0.10 Hz in empty vector controls to 0.20 ± 0.06 Hz for DNae014-ablated flies (p<0.001) and 0.19 ± 0.10 Hz for DNb01-ablated flies (p<0.001; Figure 4E, F). In addition, genetic ablation of either cell type resulted in a qualitatively different temporal pattern of flight behavior distinguished by long sequences of straight flight interspersed with rapid bursts of saccades, typically in the same direction for DNae014-ablated flies (Figure 4E, G, I). We quantified this influence of cell ablation on saccade timing by determining the maximum inter-saccade interval (ISImax) for each two-minute trial of each fly. The ISImax for control flies was 13.2 ± 4.9 s, compared to 33.1 ± 15.2 s for DNae014-ablated flies (p<0.0001) and 33.1 ± 22.1 s for DNb01-ablated flies (p<0.0001, Figure 4H). DNae014-ablated flies saccaded more frequently in the same direction as their preceding saccades, with a syndirectional saccade ratio (i.e. the proportion of saccades in one direction immediately following a saccade in that same direction) of 0.73 ± 0.13 compared to 0.55 ± 0.10 for control flies (p=0.0005). The syndirectional saccade ratio for DNb01-ablated flies was 0.64 ± 0.20, which was not significantly different from either controls or DNae014-ablated flies (Figure 4I). As observed previously45, the speed and magnitude of saccades in the magnotether were highly correlated, with faster saccades being larger (Figure 4I). The saccades after DNae014 ablation were slower than controls, as were those following DNb01 ablation (p<0.001 and p=0.009, respectively; Figure 4J). The altered saccade dynamics and temporal distribution after ablation support the hypothesis that each DN can produce saccades independently of the other, but with different dynamics than those of control flies. The results support a working hypothesis that the two DNs play complementary roles by activating different components of the motor circuit in the VNC responsible for generating saccades. Together, functional imaging, unilateral activation, and ablation experiments suggest that two pairs of descending interneurons, DNae014 and DNb01, function together as saccade-generating units (SGUs) to execute commands for spontaneous turns during flight.
To further examine the underlying circuitry in the brain, we identified the two pairs of DNs in two available connectomes. Comparing confocal image stacks of GFP expression in DNae014 to the FlyWire and hemibrain connectomes32–35 revealed at least four DN pairs with similar morphology, all within the DNa class as defined by Namiki and coworkers22. Of these, only one (DNae014) appeared to be a precise match with the cell originally described as AX by Schnell and coworkers30 (Gregory Jefferis, pers. comm.), which is why we chose to adopt the new nomenclature32,33 (Eicher, Brooks, Stürner et al., in prep). DNae014 appears to be identical to the PS017_R neuron in the hemibrain connectome. Our confocal microscopy images of DNb01 matched only one DN in the two brain connectomes, which was already annotated as DNb01 in both the hemibrain and FlyWire datasets. Based on the results of our physiological and behavioral experiments (Figures 1–4), we propose that the DNae014 and DNb01 cells with somata on the left side of the brain form a functional unit that generates leftward saccades, with the right DNae014 and DNb01 cells forming a unit that generates rightward saccades. Currently, it remains possible that additional descending neurons are involved in these saccade generating units. Neurotransmitter predictions based on machine learning47 suggest that DNae014 is cholinergic and DNb01 is glutamatergic (Table S1), with the latter classification corroborated by in situ expression of VGlut20. These putative neurotransmitter types indicate that the two members of each unit collectively carry excitatory and inhibitory commands to opposite sides of the wing neuropil in the VNC. Recent analyses of the adult VNC in two new connectomes found that descending neurons connect to wing motor neurons both directly via monosynaptic inputs, and indirectly, via a large population of local premotor interneurons20,48. Because both inhibitory and excitatory premotor neurons exist, the valence of a DN’s neurotransmitter is not necessarily a clear predictor of its action on steering muscle activity during saccades.
Motivated by our hypothesis that each unilateral set of DNae014 and DNb01 cells functions as a saccade-generating unit (SGU), we performed a connectivity search for neurons that provide direct inputs to both cells in a SGU (Figure 5). Basing our analysis on the lowest synapse number threshold that maintained a bilaterally symmetric connectivity pattern (see Methods for details, Figure S3A), we identified 8 neuron types that provide direct input to the SGUs, (a diverse population we call SGUIs). Five of these SGUIs are pairs of neurons with unique morphology and three constitute small sets of cell types with nearly identical morphology and connectivity (Figure S3B). In addition to making direct synapses with the two members of each SGU, several SGUIs send a collateral that directly connects with other SGUI members on the ipsilateral or contralateral side (Figure 5). We further identified a class of intermediary neurons that form strong connections between members of contralateral SGUIs. Predictions of transmitter type based on machine learning suggest that the population of SGUIs and intermediary cells includes both excitatory (acetylcholine) and inhibitory (glutamate or GABA) neurons (Figures 5 and S4). We also ranked the top ten additional neuron types that form presynaptic connections with members of this putative saccade network. These inputs include neurons that likely carry sensory information from brain regions including the lobula (Lo), posterior lateral protocerebrum (PLP), accessory optic tubercle (AOTu), as well as the LAL and central complex (CX) (Figure 5I; Figure S5). A recent study also identified neuron types (PFL2 and PFL3) that carry information from the CX to DNs in the LAL49. In addition, DNb01 and several SGUIs make recurrent inhibitory connections to network members on the contralateral and ipsilateral sides (Figure S4A). These anatomical pathways suggest that external sensory information and internal processing likely influence the dynamics of the network that generates spontaneous saccades within the LAL.
Figure 5. VES041 neurons innervate all DNae014 and DNb01 cells, along with several of their upstream targets.

(A) Left saccade-generating unit (SGU) consisting of DNae014 (green) and DNb01 (magenta) cells (from FlyWire). (B) Schematized model for network generating left and right saccades. Bright colors or black indicate active neurons; pale colors or gray indicate inactive neurons. (C) Connectivity of putative members of the network that regulates directed saccades (rows) to SGU neurons DNae014 and DNb01 (columns; arrow indicates direction of information flow). Our analysis identified neurons that form input synapses to both SGU cells (SGUIs), neurons that form connections between ipsilateral and contralateral SGUIs (I to C SGUIs), and a neuron that connects to all four SGU cells (VES041). Connectivity data are averaged assuming symmetrical arrangement of left and right network members. (D) Morphology of the left VES041 cell in FlyWire. (E) Connections of VES041 cells (blue for left; grey for right) to both SGUs and bilateral pairs of SGUIs and intermediary neuron types. Colors match representations in Figure S4C–J. Line thickness is proportional to log of synapse count. See also Figures S3, S4, S5, Tables S1, S2, and S3.
One cell, VES041, stood out in our analysis due to its unique feature of providing direct monosynaptic input to the DNae014 and DNb01 cells of both SGUs as well as to several of their prominent upstream targets (Figures 5C, E and S4). The neurotransmitter for VES041 is predicted to be GABA, based on machine learning (Table S1). Thus, each of the paired VES041 cells could potentially inhibit all four cells that constitute the two SGUs, and thereby strongly suppress saccade rate. To test this, we generated two split-GAL4 lines that target VES041 neurons and used them to drive CsChrimson (see Methods; Figure 6A, Video S3). Presentation of 617 nm light strongly reduced spontaneous saccades in flies flying in the magnotether apparatus from 0.52 ± 0.22 Hz to 0.17 ± 0.10 Hz and from 0.71 ± 0.17 Hz to 0.09 ± 0.05 Hz in the two driver lines we tested (Figure 6). In contrast, presentation of 617 nm light slightly increased the spontaneous saccade in control flies from 0.41 ± 0.16 Hz to 0.68 ± 0.21 Hz (Figure 6E). The results are consistent with independent experiments conducted on rigidly tethered flies in which we classified saccades based on changes in wingstroke amplitude (Figure S6) during optogenetic activation of VES041. The simpler algorithm used for saccade classification from magnotether data, based on the actual angular velocity of the fly’s body, likely results in lower false negative and lower false positive rates compared to the method of classifying saccades performed by rigidly tethered flies. Nevertheless, despite the minor differences in the results from the activation experiments in the two different flight arenas, our findings were consistent across the two methods (Figures 6 and S6) and thus support our hypothesis that VES041 is a potent inhibitor of spontaneous saccades. The VES041 neuron receives its strongest input from the vest (VES), GNG, LAL, SPS, flange (FLA), inferior bridge (IB), and saddle (SAD) (Figures 6 and S6F), which suggests that this cell integrates both visual and olfactory information that are known to influence saccade rate.
Figure 6. Activation of GABAergic VES041 neurons suppresses spontaneous saccades.

(A) Split-GAL4 lines constructed to target VES041 (green, GFP). (B) Optogenetic activation of VES041 in a magnotether arena using a 623 nm LED ring light (orange annulus in B, orange patches in C). (C) Top: empty vector control fly, with right and left saccades (dots above and below the panel) identified using angular velocity of body orientation (grey trace). Middle and bottom: activation of VES041 drivers 1 and 2 (angular velocity in blue). (D) Raster plots represent spontaneous saccades (black dots), during exposure to 623 nm light (orange patch). Each set of three rows are the presentations for an individual fly. Left: empty vector split-GAL4 line driving CsChrimson; middle and right: VES041 split-GAL4 lines 1 and 2 driving CsChrimson. Gray histograms indicate mean saccade rate across populations. (E) Individual saccade rates during light-off and light-on epochs for control and the two VES041 drivers; Each line represents an individual fly (n = 20, 24, and 23 flies). (F) Individual differences in saccade rate from the light-off to light-on condition for the same groups as in E. (** = p<0.01; *** = p<0.001; n.s. = not significant, Kruskal-Wallis test followed by post-hoc comparisons with Tukey’s HSD method). See also Figure S6, Table S1, and Video S3.
DISCUSSION
We investigated the role of two pairs of DNs in the generation of body saccades in flying flies. Our results suggest a model in which two neurons, DNae014 and DNb01, form two saccade-generating units (SGUs). Although, we could not record from DNae014 and DNb01 simultaneously, every rapid change in wing motion accompanied a transient change in the activity of either cell when recorded separately. This one-to-one correspondence supports the hypothesis that unilateral cells from DNae014 and DNb01 function together as single command units that carry both excitatory and inhibitory inputs to the premotor circuits in the VNC to execute flight saccades. In the brain, we used the DNae014 and DNb01 cells to identify a mosaic of excitatory and inhibitory neurons that innervate neuropils associated with steering and multi-modal sensory integration. One pair of large inhibitory interneurons, VES041, emerged as a likely candidate to suppress the execution of spontaneous turns because each cell makes strong inhibitory connections with all members of both the left and right SGUs. Optogenetic activation of VES041 strongly suppresses saccades, which suggests that it may play a regulatory role in natural behaviors such as long-distance dispersal or upwind surges in response to an attractive odor. Although we have not yet succeeded in performing a viable double ablation experiment (i.e. silence or ablate both DNae014 and DNb01 in the same individuals) the results of VES041 activation serve as a rough proxy for simultaneous silencing of both DNae014 and DNb01 (Figures 6 and S6). Although the inhibition of saccades by activation of VES041 is parsimoniously explained by its GABAergic inputs to the DNae014 and DNb01 neurons, this tentative transmitter classification is based solely on machine learning, and must be verified more using complementary techniques.
While the high fidelity of the activity of both cells to the changes in wing motion is itself compelling (Figure 1), this phenomenon might result from strong ascending proprioceptive feedback or efferent copy. However, brief activation of either single DNae014 or single DNb01 cells reliably evoke syndirectional turns (Figure 3). Further, ablating either cell type results in unusually long sequences of straight flight interspersed by brief bursts of small amplitude saccades (Figure 4). The induction of long inter-saccade intervals provides intriguing evidence that these cells contribute directly to temporal patterning of the spontaneous saccades and do not simply relay upstream commands (Figures 1–4). However, ablating either cell alone does not abolish all spontaneous turns, even though the persisting saccades are slower. This partial loss-of-function effect might indicate that the motor commands resulting from either DNae014 or DNb01 activate sub-components of the complete motor program that are alone sufficient to generate partial, slower saccades (Figure 4J), or alternatively, that the fly’s nervous system contains as-of-yet unidentified parallel pathways for generating spontaneous turns. These alternative hypotheses are not mutually exclusive and, because our ability to cluster saccades according to type based on behavioral measurements is limited, not currently distinguishable.
Unilateral activation of both DNae014 and DNb01 evoked changes in wing motion similar to syndirectional saccades (Figure 3), consistent with the results from our functional imaging experiments (Figure 1). However, saccades evoked by DNb01 activation were smaller in amplitude and longer in time course than those evoked by DNae014 activation. These differences in magnitude of the motor response could be due to systematic differences in CsChrimson expression levels or cell-specific sensitivity to optogenetic activation, and do not necessarily reflect inherent differences between the respective DNs. Nevertheless, when considered along with the difference in putative neurotransmitter types between DNae014 and DNb01, these nuanced differences in effects on wing motion suggest that the two cells in a SGU drive different components of the saccade motor program within the VNC.
The activity levels between the left and right DNae014 cells followed an inverse, highly non-linear relationship, analogous to ‘flip-flop’ components in digital electronic circuits (Figure 1J), and reminiscent of neurons identified in the steering behavior of male silkmoths37. Further, the DNb01 cells synapse directly onto contralateral DNae014 cells. This is a simple reciprocal inhibitory motif consistent with a network responsible for binary turning to either the left or the right (Table S2), analogous with the arrangement of Mauthner cells in fish50. In addition, DNb01 and several SGUIs make recurrent inhibitory connections to network members on the contralateral and ipsilateral sides (Table S2, Figure S5A). These anatomical pathways suggest that external sensory information and internal processing likely influence the dynamics of the network that generates spontaneous saccades within the LAL.
In summary, our results suggest that DNae014 and DNb01 form two saccade-generating units that are inhibited by VES041. DNae014 and DNb01 cells are probably members of a quite ancient network that is centered in the paired LALs of the brain and widespread among insects and other arthropods39,51. In particular, comparative anatomical findings suggest these two pairs of DNs are homologous to ‘flip-flop’ neurons responsible for the rapid turns exhibited during plume tracking behavior in the silkmoth, Bombyx mori37,52. Future experiments are needed to identify the specific sensory cues and their anatomical pathways that influence VES041 activity, and thus determine its specific role in regulating this ecologically important behavioral state transition in flying insects.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to Michael H. Dickinson (flyman@caltech.edu).
Materials availability
All new fly lines generated for this paper are listed in the key resources table.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| All-trans-retinal | Sigma-Aldrich | CAS: 116-31-4 |
| Triton X-100 | Sigma-Aldrich | X100 |
| 32% Paraformaldehyde | Electron Microscopy Sciences | 15714-S |
| Vectashield Plus | Vectorlabs | H-1900 |
| Antibodies | ||
| Mouse mAb anti-Bruchpilot (nc82) | Developmental Studies Hybridoma Bank | nc82; RRID: AB_2314866 |
| Rabbit polyclonal anti-DsRED | ThermoFisher Scientific | Cat #: PA1-986; RRID: AB_560939 |
| Chicken anti-GFP | ThermoFisher Scientific | Cat #: A10262; RRID: AB_2534023 |
| Cy5 goat anti-mouse | Jackson ImmunoResearch | Cat #: 115-175-166, RRID: AB_2338714 |
| Alexa Fluor 488 goat anti-chicken | ThermoFisher Scientific | Cat #: A32931; RRID: AB_2762843 |
| Alexa Fluor 546 goat anti-rabbit | ThermoFisher Scientific | Cat #: A-11035; RRID: AB_2534093 |
| Deposited Data | ||
| Raw and analyzed data | This paper | Doi: 10.17632/wx3v67wb3w.1 |
| Experimental Models: Organisms/Strains | ||
| D. melanogaster: w[*]; ; P{20XUAS-IVS-CsChrimson.mVenus}attP2 | Bloomington Drosophila Stock Center | RRID: BDSC_55136 |
| D. melanogaster: w[1118]; ; 20XUAS-GtACR1-EYFPVK00005 | Gift from A. Mauss | N/A |
| D. melanogaster: w[1118]; P{20XUAS-IVS-jGCaMP7f}su(Hw)attP5; | Bloomington Drosophila Stock Center | RRID: BDSC_80906 |
| D. melanogaster: ; ; P{w[+mC]=UAS-tdTom.S}3 | Gift from D. Anderson | N/A |
| D. melanogaster: UAS-OpGCaMP7f; UAS-tdTomato | Constructed from above two lines | N/A |
| D. melanogaster: w[1118]; P{y[+t7.7] w[+mC]=VT025718-p65.AD}attP40/CyO; + | Bloomington Drosophila Stock Center | RRID: BDSC_72300 |
| D. melanogaster: w[1118]; + ; P{y[+t7.7] w[+mC]=R56G08-GAL4.DBD}attP2 | Bloomington Drosophila Stock Center | RRID: BDSC_69592 |
| D. melanogaster: VT025718.AD; R56G08.DBD | Constructed from above two lines | N/A |
| D. melanogaster: ;20XUAS-SPARC2-I-Syn21-CsChrimson::tdTomato-3.1; | Bloomington Drosophila Stock Center | RRID: BDSC_84144 |
| D. melanogaster: ;20XUAS-SPARC2-S-Syn21-CsChrimson::tdTomato-3.1; | Bloomington Drosophila Stock Center | RRID: BDSC_84145 |
| D. melanogaster: P{nSyb-IVS-PhiC31}attP18; S/CyO; Pri/TM6B | Bloomington Drosophila Stock Center | RRID: BDSC_84151 |
|
D. melanogaster: nSyb-IVS-PhiC31; VT025718.AD/CyO; R56G08.DBD/TM6B |
Constructed from above and DNae014 split driver. | N/A |
| D. melanogaster: nSyb-IVS-PhiC31; VT013121.AD/CyO; R45H03.DBD/TM6B | Constructed from two above and DNb01 split driver. | N/A |
|
D. melanogaster: SS02383 VT013121.AD; R45H03.DBD |
Gift from G. Card, W. Korff, S. Namiki | RRID: BDSC_75888 |
| D. melanogaster: UAS-hid, UAS-reaper;; | Gift from D. Allan | N/A |
| D. melanogaster: w[1118]; P{y[+t7.7] w[+mC]=R64G09-p65.AD}attP40; MKRS/TM6B, Tb[1] | Bloomington Drosophila Stock Center | RRID: BDSC_71205 |
| D. melanogaster: w[1118]; P{y[+t7.7] w[+mC]=VT044519-GAL4.DBD}attP2 | Bloomington Drosophila Stock Center | RRID: BDSC_75123 |
| D. melanogaster: R64G09.AD; VT044519.DBD | Constructed from above two lines | N/A |
| D. melanogaster: w[1118] P{y[+t7.7] w[+mC]=10xUAS-IVS-myr::smGdP-HA}attP18 P{y[+t7.7] w[+mC]=13xLexAop2-IVS-myr::smGdP-V5}su(Hw)attP8; sna[Sco]/CyO; P{y[+t7.7] w[+mC]=R31E10-GAL4.DBD}attP2 | Bloomington Drosophila Stock Center | RRID: BDSC_70311 |
| D. melanogaster: R64G09.AD; R31E10.DBD | Constructed from above line and RRID: BDSC_71205 | N/A |
| D. melanogaster: y[1] w[*] P{y[+t7.7] w[+mC]=UAS-myrGFP.QUAS-mtdTomato-3xHA}su(Hw)attP8 | Bloomington Drosophila Stock Center | RRID: BDSC_77479 |
| Software and Algorithms | ||
| Kinefly wing tracking software | [62] | https://github.com/ssafarik/Kinefly |
| Magnotether fly tracking software | W. Dickson | https://github.com/willdickson/find_fly_angle |
| Python | https://www.python.org | RRID: SCR_008394 |
| Matplotlib | https://matplotlib.org | RRID: SCR_008624 |
| FIJI | https://fiji.sc/ | RRID:SCR_002285 |
| Other | ||
| UV-activated cement | Henkel | Loctite® 3972TM |
| Minutien pins | Fine Science Tools | 26002-10 |
| Neodymium ring magnet | Grainger | 10E804 |
| Rod magnet | Magcraft | NSN0750/N40 |
| Digital camera | Basler | acA640-120 gm |
| Digital camera | FLIR | BFS-U3-16S2M-CS |
| IR light source LED | Thorlabs | M850F2 |
| IR LED ring | W. Dickson | https://github.com/willdickson/magneto_ir_led_ring |
| IR light source driver | Thorlabs | LEDD1B |
| Panel controller | IO Rodeo | Panels display controller unit |
| Indigo, Skelton Exotic Sangria, and Cyan transmission filters. | Roscolux | Roscolux numbers 59, 39, and 4390. |
Data and code availability
All data have been deposited on Mendeley at https://doi.org/10.17632/wx3v67wb3w.1 and are publicly available as of the date of publication. All new fly lines generated for this paper are mentioned in the manuscript. All original code for data analysis has been deposited on Mendeley at: https://doi.org/10.17632/wx3v67wb3w.1 and is publicly available as of the date of publication. All original code used for the Kinefly wing tracking and magnotether body tracking software is publicly available on Github at: https://github.com/ssafarik/Kinefly and https://github.com/willdickson/find_fly_angle, respectively. Any additional information required to reanalyze the data reported in this paper is available upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All experiments were conducted using 2-to-5-day-old adult female Drosophila melanogaster. To specifically target DNae014 cells, we constructed a split-GAL4 line using the hemidrivers VT025718-GAL4.AD to drive the activation domain, and R56G08-GAL4.DBD to drive the DNA-binding domain, resulting in split-GAL4[VT025718.AD; R56G08.DBD]22,54–56 (Video S1). Similarly, we created two split-GAL4 drivers that target VES041 cells, split-GAL4[R64G09.AD; VT044519.DBD] and split-GAL4[R64G09.AD; R31E10.DBD]. Candidate hemi-drivers followed from screening databases of standardized MCFO confocal scans of expression patterns of GAL4 drivers57,58. All hemidrivers were obtained from the Bloomington Drosophila Stock Center59. For simplicity, we refer to these specific drivers by their primary targeted neurons, while acknowledging the presence of off-target cells (Videos S1–S3). We used the split-GAL4 driver SS02383 to target DNb01 cells22. We list off-target cells in the central brain for every driver line in the captions of all Supplemental Videos. For functional imaging experiments, we crossed males of either the DNae014 split driver or the DNb01 split driver to w+;UAS-tdTomato;UAS-GCaMP7f female flies60. For stochastic optogenetic activation experiments, we crossed males from the respective split-GAL4 lines combined with nSyb-PhiC31 (X) with virgin females carrying the intermediate or sparse variants of 20XUAS-SPARC-Syn21-CsChrimson::tdTomato-3.142. For VES041 optogenetic activation experiments, we crossed males from the two VES041 split drivers with females carrying 20XUAS-CsChrimson-mVenus on the third chromosome. For temporal silencing experiments, we drove expression of the light-gated GtACR1 channel by crossing males of the DNae014 split driver with females carrying 20XUAS-GtACR1-EYFP on the third chromosome43. For genetic ablation experiments, we drove expression of the pre-apoptotic genes reaper and hid by using female offspring from crosses between males from each of the DNae014 and DNb01 split drivers with females carrying UAS-rpr, hid on the first chromosome44. We reared the progeny of experimental crosses on standard cornmeal fly food, supplemented with additional yeast. For optogenetic activation experiments, larvae were reared in food supplemented with 0.2 mM all trans-Retinal (ATR) (Sigma-Aldrich). For the first two days following eclosion, adult flies were transferred to bottles with standard cornmeal fly food supplemented with 0.4 mM ATR.
QUANTIFICATION AND STATISTICAL ANALYSIS
All experiments were analyzed with custom software written in Python. Sample sizes refer to the number of individuals tested. Variance across individuals was assessed by either standard deviation of individual means or boot-strapped, 95% confidence intervals (CI) for the mean of the individual means (Figures 4C, F, H, J, 6E, and S2D, and Figures 1M, 2B–D, 3C, G–I, and 5I). Statistical differences between peak responses in the activity of DNs and loom directions were determined with a 2-way ANOVA with cell type and loom direction as the main effects and individual as a random effect (Figure 2D). Statistical differences between controls and DN groups were assessed using Kruskal-Wallis tests with Tukey’s HSD method for post-hoc comparisons. For VES041 activation in the magnotether, paired samples t-tests, we used a Kruskal-Wallis test followed by post-hoc comparisons with Tukey’s HSD method to evaluate differences among the three genetic lines during CsChrimson activation (Figure 6E). Significance levels are indicated as ns (not significant), * (p < 0.05), ** (p < 0.01), or *** (p < 0.001) (Figures 2D, 4C, F, H, I, and K, and 6E).
METHODS DETAILS
Anatomy.
The central nervous system of 3-to-5-day-old female adult progeny were dissected at room temperature (~20°C) in 1X PBS (Sigma) and fixed for 25 min in 4% paraformaldehyde diluted in 1X PBS, and washed 3 times for 15 min in 1X PBS and subsequently 5 times for 15 min in 0.3% PBS-T (1X PBS with 0.3% Triton-X). Samples were then blocked in 7.5% normal goat serum diluted in 0.3% PBS-T for at least 1 hour. The samples were incubated with primary antibodies (mouse nc82 supernatant at 1:30, rabbit polyclonal anti-DsRED at 1:2000 or chicken anti-GFP 1:1000, diluted in blocking solution) for 4 hours at 20°C and 3 days at 4°C, followed by incubation with secondary antibodies (Cy5 goat anti-mouse at 1:300, and Alexa Fluor 546 goat anti-rabbit at 1:1000 or Alexa Fluor 488 goat anti-chicken at 1:1000, diluted in blocking solution) for 4 hours at 20°C and 3 days at 4°C. Samples were imaged using a confocal microscope (Zeiss LSM 880) with a 40X water objective. Optical sections were taken at 1-μm intervals with resolutions of 512X512 or 1024X1024 pixels. The samples were mounted on microscope slides with spacers using Vectashield Plus (VectorLabs).
Functional imaging.
Tethered, flying flies were imaged using a galvanometric scan mirror-based 2-photon microscope (Thorlabs) equipped with a Nikon CFI apochromatic, near-infrared objective water-immersion lens (40x mag., 0.8 N.A., 3.5 mm W.D.), as previously described 30,60. GCaMP7f and tdTomato fluorescence signals were imaged in the arbors of the left and right DNae014 or DNb01 cells in the SPS neuropil in the brain. Imaging parameters varied depending on the preparation; we either acquired 72 × 36 μm images with 128 × 64 pixel resolution at 13.1 Hz, or in a few cases, 36 × 36 μm images with 96 × 96 pixel resolution at 10.6 Hz (Figure S1). To correct for brain motion in the x-y plane, we registered both channels for each frame using a cross-correlation between each tdTomato image and the trial-averaged image61. A field of view (FOV) centered along the bilateral axis was selected based on tdTomato expression. Regions of interest (ROIs) to capture signals of the left and right cells were automatically determined based on GCaMP7f fluorescence pixel variance and covariance with turning behavior, refining signal-to-background ratios and reducing cross talk between contralateral DNb01 cell clusters (see below for details). To account for motion in the z-axis, we normalized GCaMP7f fluorescence to tdTomato fluorescence. To find the baseline fluorescence (F0), we subtracted the background fluorescence, defined as the 20% dimmest pixels of the entire FOV, from the mean of the lowest 5% fluorescence values in a trial. To standardize neuronal activity, we normalized baseline-subtracted fluorescence, Ft, to the maximum observed for each individual fly in each ROI: ΔF/F = (Ft − F0) / (Ft − F0) max. Neural activity during spontaneous flight turns was recorded in 90 s trials in darkness (i.e. the microscope was enclosed in a light-tight enclosure and the visual arena was turned off). To measure responses to looming, files were presented with an expanding stimulus created using a 96 × 32 pixel arena that covered 216° of azimuth and ~74° of elevation62. Looming stimuli simulated a 0.15 m diameter disk approaching at 1.5 ms−1 from a starting position of 1.5 m away from the fly; each trial ended when the stimulus subtended an angle of 60°. Looming stimuli were presented every 6 seconds from one of five directions in pseudo-random order. To reduce light pollution, we shifted the spectral peak of the visual stimuli from 470 nm to 450 nm by covering the arena panels with transmission filters (one sheet of Roscolux no. 59 Indigo, two sheets of no. 39 Skelton Exotic Sangria, and two sheets of no. 4390 Cyan). We tracked left and right wingstroke amplitudes with a machine vision system, Kinefly, as described before63.
Functional Imaging, regions of interest.
We refined the imaging ROIs for functional imaging via an automated process that used the covariance between wingstroke amplitude and pixel variance in GCaMP7f fluorescence, which we confirmed via direct inspection of cell anatomy (Figure S1). Initially, we manually assigned ROIs to capture activity of left and right cells based on visual assessment of tdTomato and GCaMP7f intensities. For the tdTomato signal, we determined the maximum intensity projection in the red channel across all images for each flight trial. For the GCaMP7f signal, we determined the standard deviation for every pixel across each flight trial. We then set a threshold for GCaMP7f intensity within the interrogation areas to identify active pixels and created a binary mask for the left and right cells (Figure S1B). These manually constructed ROIs were used to calculate normalized fluorescence signals (ΔF/F) as described earlier. Relationships between neural activity and behavior were determined using ΔF/F based on manual ROIs through cross-correlation with wingstroke amplitude (Figure S1C). The ΔF/F signal in the manually assigned ROI correlated positively with contralateral wingstroke amplitude, and contralateral minus ipsilateral wingstroke amplitude, for both DNae014 and DNb01. To refined single-cell ROIs, we constructed pixel correlograms, based on time-varying fluorescence within a small set of pixels assessed to belong to a single cell, or, for automatic assignment of DNae014 and DNb01 morphology, we cross-correlated the contralateral minus ipsilateral wingstroke amplitude with all pixel fluorescence values from the GCaMP7f channel in a given flight trial. Top ranked pixels from the resulting correlograms were thresholded and binarized to create ROIs for the left and right cells. These ROIs were visually validated against cellular anatomy (Figure S1E). Automatic ROIs were used to obtain single cell GCaMP7f ΔF/F separately for DNae014 and DNb01 trials, following the same procedures described earlier.
Saccade detection.
Fictive saccades were detected using a threshold-based classifier30 operating on the temporal derivative of the bilateral difference in wingstroke amplitude. We used a threshold of twice the measured standard deviation of the derivative for both the functional imaging setup and the rigid tether arena. To avoid tracking errors, we excluded derivative values exceeding 420 ° s−1 and cases in which stroke amplitude fell below 60°. To avoid false positives, caused by rapid changes in wingstroke angles at the end of saccades, we implemented a 0.25 s refractory period following each saccade as in earlier studies64. Wingstroke amplitude signals were smoothed using a Kalman filter that approximated a 4th-order Butterworth with a low-pass cut-off at 6 Hz. Saccades were classified similarly for magnotether data, using the time derivative of the body angle and a threshold of 1.5 standard deviations (Figure 4E). Peak saccade speeds in the magnotether were detected by identifying extrema in body angle velocity after applying a Savitsky-Golay filter (< 9 Hz). We normalized peak saccade speed to expected peak speed for a given saccade magnitude, based on the measured relationship between saccade speed and saccade magnitude for control flies. Specifically, we normalized all saccade data to the orthogonal-distance regression (ODR)65 between peak speed and magnitude for all saccades measured in empty-vector control flies (Figure 4I). We used an 2nd-order polynomial in the ODR because it matched the curvilinear relationship better than a simple line.
Optogenetic activation experiments.
Tethered flies were positioned for imaging with a digital camera and backlit with infrared light. Prior to CsChrimson activation, we presented flies with an 8 s visual starfield pattern oscillating sinusoidally about the vertical axis through two full cycles of 216° in amplitude. Wingstroke amplitudes were tracked using Kinefly software63. For optogenetic activation in the rigid tether setup, we guided a fiber optic from a 617 nm LED source (M617F2, Thorlabs) through a microscope objective, adjusted to create a 0.5 mm spot on the posterior side of the head (Figures 3D and S6), with a power density of ~0.2 mW mm−2. For optogenetic activation in the magnotether, we delivered 623 nm light via a custom-built LED ring (Figure 6B). For unilateral activation experiments of either DNae014 or DNb01 (Figure 3D–I) we elicited 6 responses to a 0.25 s light pulse at 10 s intervals. Bilateral CsChrimson activation experiments of VES041 involved three 15 s periods of excitation surrounded by 15 s periods of no excitation. If a fly stopped flying, we collected additional trials. For each fly, the three first presentations with continuous flight were included for further analysis.
Unilateral genetic targeting of CsChrimson.
To stochastically target the optogenetic effector CsChrimson tagged with tdTomato within the split-GAL4 driver lines (for either DNae014 or DNb01), we employed Sparse Predictive Activity through Recombinase Competition (SPARC)42. We crossed parental lines containing pan-neuronal phi-C31 and the split-GAL4 of interest with the appropriate SPARC line (20XUAS-SPARC2-I-Syn21-CsChrimson::tdTomato-3.1 for intermediate labeling or (20XUAS-SPARC2-D-Syn21-CsChrimson::tdTomato-3.1 for dense labeling). The resulting flies expressed CsChrimson and a fluorescent marker, tdTomato, in either the left cell, the right cell, both cells, or no cells. Expression patterns were manually scored using fluorescence microscopy after behavioral measurements, and a subset of flies underwent immunostaining to obtain representative confocal microscopy images (Figure 3E). All experiments were performed in a blinded fashion, with expression patterns naïvely scored behavioral data collection. These experiments were performed on intact, rigidly tethered flies.
Connectomics.
We identified the DNae014 and DNb01 cells in publicly available connectomes32–34. Comparison to fly brain connectomes indicated at least five DNs in the subclass of DNa neurons22 that roughly resembled DNae014, but one neuron matched the morphology of the cell in our split-GAL4 line most closely (Gregory Jefferis and Marta Costa, pers. comm.). The hemibrain dataset contains two neurons labeled DNb01, with one matching the expression pattern of the cell we refer to a DNb01 based on the description of Namiki at coworkers22, which is under control of the split-GAL4 driver we used in our experiments, SS02383. We conducted a connectivity analysis based on both the FlyWire connectome based on the FAFB EM dataset, and the hemibrain connectome datasets32–35,66 (see flywire.ai). Neurons in FlyWire were considered of the same type following labeling in the hemibrain and/or due to similarity in both anatomy and connectivity (see Figure S3A). We identified saccade-generating unit input neuron types (SGUIs) that innervated both unilateral DNae014 and DNb01 cells with combined connection strengths above a threshold where the product of synapse counts onto DNae014 and DNb01 cells exceeded the square of 75. This threshold was objectively determined as the lowest threshold at which the set of SGUIs to the left SGU were identical to the symmetrical set of SGUIs to the right SGU (Figure S3B, C). Putative saccade network neurons were labeled according to the FAFB or hemibrain connectomes (Table S3). To assess interconnectivity between identified neurons, we included direct connections that contained over 37 synapses (Figures S3 and 5), as well as indirect connections between left and right SGUIs that contained over 75 synapses. For these interneurons connecting left and right SGUIs, we applied a higher threshold to exclude more likely false positive connections due to high local innervation density. Neurotransmitter types were predicted using a machine-learning synapse classification algorithm based on confidence scores and synapse cleft width47,67,68.
Supplementary Material
As in all following supplemental videos, the optical sections appear temporally from posterior to anterior in the brain, and from dorsal to ventral in the ventral nerve cord. GFP expression is shown in green; nc82 staining in magenta. Notable off-targets cells in the brain include sparse, but strongly expressing ascending neurons innervating the gnathal ganglion and saddle, sparsely expressing projection neurons from the optic lobe, and a weakly expressing descending neuron with a cell body adjacent to DNae014.
This driver line (SS02383) was generated at the Janelia Research Campus. GFP expression is shown in green; nc82 staining in magenta. A notable off-target cell in the brain is a neuron in the pars intercerebralis that innervates the ocellar ganglion.
CsChrimson-Venus expression is shown in green; nc82 staining in magenta. The image stacks for the two driver lines we created are shown sequentially in the video. Notable off-target cells in the brain for line 1 include sparse neurons innervating the superior medial protocerebrum, a small cluster of somata ventral to the antennal lobes, and a cluster of interneurons with somata in the ventrolateral cell body rind that innervate the antennal mechanosensory and motor neuropils as well as the saddle. Notable off-target cells in the brain for line 2 include a pair of interneurons with somata in the ventrolateral cell body rind that innervate the saddle.
Highlights.
The activity of two pairs of descending neurons drives flight turns called saccades
Couplets of one excitatory and one inhibitory descending cell form functional units
An array of excitatory and inhibitory neurons provides input to the saccade network
VES041 inhibits saccade network, possibly regulating state transitions during flight
ACKNOWLEDGMENTS
We thank members of our lab, including Ainul Huda, Will Dickson, Johan Melis, Francesca Ponce, and Matthew Clark. Additionally, we are grateful to Shigehiro Namiki, Sasha Rayshubskiy, Rachel Wilson, and Anna Ahn for helpful contributions and discussions, and to Shigehiro Namiki, Wyatt Korff, and Gwyneth Card for sharing the driver lines used in the functional imaging screen. We thank Sasha Rayshubskiy for suggesting the presence of DNae014 in the GAL4 line VT025718. We thank the Princeton FlyWire team and members of the Allen Institute for Brain Science for development and maintenance of FlyWire (supported by BRAIN Initiative grants MH117815 and NS126935 to Murthy and Seung). We also acknowledge members of the Princeton FlyWire team and the FlyWire consortium for neuron proofreading and annotation, specifically J. Dolorosa, D. Sapkal, S. Fang, and Z. Vohra from the Murthy, Seung, and Jefferis labs. This research was supported by NIH grants U19NS104655 and 1R21NS106471-01A1.
Footnotes
DECLARATION OF INTEREST
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Berg HC (2000). Motile behavior of bacteria. Physics Today 53, 24–29. 10.1063/1.882934. [DOI] [Google Scholar]
- 2.Collett TS, and Land MF (1975). Visual control of flight behaviour in the hoverfly, Syritta pipiens L. J. Comp. Physiol. 99, 1–66. 10.1007/BF01464710. [DOI] [Google Scholar]
- 3.Cellini B, Salem W, and Mongeau J-M (2021). Mechanisms of punctuated vision in fly flight. Curr. Biol. 31, 4009–4024.e3. 10.1016/j.cub.2021.06.080. [DOI] [PubMed] [Google Scholar]
- 4.Land MF (1999). Motion and vision: why animals move their eyes. J. Comp. Physiol. A 185, 341–352. 10.1007/s003590050393. [DOI] [PubMed] [Google Scholar]
- 5.Schilstra C, and van Hateren JH (1998). Stabilizing gaze in flying blowflies. Nature 395, 654–654. 10.1038/27114. [DOI] [PubMed] [Google Scholar]
- 6.Heisenberg M, and Wolf R (1984). Vision in Drosophila. Genetics of microbehaviour. (Springer Verlag; ). [Google Scholar]
- 7.van Breugel F, Jewell R, and Houle J (2022). Active anemosensing hypothesis: how flying insects could estimate ambient wind direction through sensory integration and active movement. J. R. Soc. Inter. 19, 20220258. 10.1098/rsif.2022.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stupski SD, and Breugel F van (2023). Wind gates search states in free flight. Preprint at bioRxiv, 10.1101/2023.11.30.569086 10.1101/2023.11.30.569086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Götz KG (1987). Course-control, metabolism and wing interference during ultralong tethered flight in Drosophila melanogaster. J. Exp. Biol. 128, 35–46. 10.1242/jeb.128.1.35. [DOI] [Google Scholar]
- 10.Giraldo YM, Leitch KJ, Ros IG, Warren TL, Weir PT, and Dickinson MH (2018). Sun navigation requires compass neurons in Drosophila. Curr. Biol. 28, 2845–2852. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren TL, Weir PT, and Dickinson MH (2018). Flying Drosophila melanogaster maintain arbitrary but stable headings relative to the angle of polarized light. J. Exp. Biol. 221, jeb177550. 10.1242/jeb.177550. [DOI] [PubMed] [Google Scholar]
- 12.Weir PT, and Dickinson MH (2012). Flying Drosophila orient to sky polarization. Curr. Biol. 22, 21–27. 10.1016/j.cub.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Jundi B, Foster JJ, Khaldy L, Byrne MJ, Dacke M, and Baird E (2016). A snapshot-based mechanism for celestial orientation. Curr. Biol. 26, 1456–1462. [DOI] [PubMed] [Google Scholar]
- 14.Coyne JA, Boussy IA, Prout T, Bryant SH, Jones JS, and Moore JA (1982). Long-distance migration of Drosophila. Am. Nat. 119, 589–595. 10.1086/283936. [DOI] [Google Scholar]
- 15.Leitch KJ, Ponce FV, Dickson WB, Breugel F van, and Dickinson, M.H. (2021). The long-distance flight behavior of Drosophila supports an agent-based model for wind-assisted dispersal in insects. PNAS 118, e2013342118. 10.1073/pnas.2013342118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrows WM (1907). The reactions of the Pomace fly, Drosophila ampelophila loew, to odorous substances. J. Exp. Zool. 4, 515–537. 10.1002/jez.1400040403. [DOI] [Google Scholar]
- 17.Budick SA, and Dickinson MH (2006). Free-flight responses of Drosophila melanogaster to attractive odors. J. Exp. Biol. 209, 3001–3017. 10.1242/jeb.02305. [DOI] [PubMed] [Google Scholar]
- 18.van Breugel F, and Dickinson MH (2014). Plume-tracking behavior of flying Drosophila emerges from a set of distinct sensory-motor reflexes. Curr. Biol. 24, 274–286. 10.1016/j.cub.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Heinrich R (2002). Impact of descending brain neurons on the control of stridulation, walking, and flight in orthoptera. Microscopy Research and Technique 56, 292–301. 10.1002/jemt.10033. [DOI] [PubMed] [Google Scholar]
- 20.Cheong HSJ, Eichler K, Stuerner T, Asinof SK, Champion AS, Marin EC, Oram TB, Sumathipala M, Venkatasubramanian L, Namiki S, et al. (2023). Transforming descending input into behavior: The organization of premotor circuits in the Drosophila male adult nerve cord connectome. Preprint at bioRxiv, 10.1101/2023.06.07.543976 10.1101/2023.06.07.543976. [DOI] [Google Scholar]
- 21.Hsu CT, and Bhandawat V (2016). Organization of descending neurons in Drosophila melanogaster. Sci. Rep. 6, 20259. 10.1038/srep20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Namiki S, Dickinson MH, Wong AM, Korff W, and Card GM (2018). The functional organization of descending sensory-motor pathways in Drosophila. eLife 7, e34272. 10.7554/eLife.34272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohatsu S, Koganezawa M, and Yamamoto D (2011). Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 69, 498–508. 10.1016/j.neuron.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 24.von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, and Dickson BJ (2011). Neuronal control of Drosophila courtship song. Neuron 69, 509–522. 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Bidaye SS, Machacek C, Wu Y, and Dickson BJ (2014). Neuronal control of Drosophila walking direction. Science 344, 97–101. 10.1126/science.1249964. [DOI] [PubMed] [Google Scholar]
- 26.Rayshubskiy A, Holtz SL, D’Alessandro I, Li AA, Vanderbeck QX, Haber IS, Gibb PW, and Wilson RI (2020). Neural circuit mechanisms for steering control in walking Drosophila. Preprint at bioRxiv, 10.1101/2020.04.04.024703 10.1101/2020.04.04.024703. [DOI] [Google Scholar]
- 27.von Reyn CR, Breads P, Peek MY, Zheng GZ, Williamson WR, Yee AL, Leonardo A, and Card GM (2014). A spike-timing mechanism for action selection. Nat. Neurosci. 17, 962–970. 10.1038/nn.3741. [DOI] [PubMed] [Google Scholar]
- 28.Wyman RJ, Thomas JB, Salkoff L, and King DG (1984). The Drosophila giant fiber system. In Neural Mechanisms of Startle Behavior, Eaton RC, ed. (Springer US; ), pp. 133–161. 10.1007/978-1-4899-2286-1_5. [DOI] [Google Scholar]
- 29.Ache JM, Namiki S, Lee A, Branson K, and Card GM (2019). State-dependent decoupling of sensory and motor circuits underlies behavioral flexibility in Drosophila. Nat. Neurosci. 22, 1132–1139. 10.1038/s41593-019-0413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnell B, Ros IG, and Dickinson MH (2017). A descending neuron correlated with the rapid steering maneuvers of flying Drosophila. Curr. Biol. 27, 1200–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferris BD, Green J, and Maimon G (2018). Abolishment of spontaneous flight turns in visually responsive Drosophila. Curr. Biol. 28, 170–180.e5. 10.1016/j.cub.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorkenwald S, Matsliah A, Sterling AR, Schlegel P, Yu S, McKellar CE, Lin A, Costa M, Eichler K, Yin Y, et al. (2023). Neuronal wiring diagram of an adult brain. Preprint at bioRxiv, 10.1101/2023.06.27.546656 10.1101/2023.06.27.546656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlegel P, Yin Y, Bates AS, Dorkenwald S, Eichler K, Brooks P, Han DS, Gkantia M, Santos M. dos, Munnelly EJ, et al. (2023). A consensus cell type atlas from multiple connectomes reveals principles of circuit stereotypy and variation. Preprint at bioRxiv, 10.1101/2023.06.27.546055 10.1101/2023.06.27.546055. [DOI] [Google Scholar]
- 34.Scheffer LK, Xu CS, Januszewski M, Lu Z, Takemura S, Hayworth KJ, Huang GB, Shinomiya K, Maitlin-Shepard J, Berg S, et al. (2020). A connectome and analysis of the adult Drosophila central brain. eLife 9, e57443. 10.7554/eLife.57443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorkenwald S, McKellar CE, Macrina T, Kemnitz N, Lee K, Lu R, Wu J, Popovych S, Mitchell E, Nehoran B, et al. (2022). FlyWire: online community for whole-brain connectomics. Nat. Methods 19, 119–128. 10.1038/s41592-021-01330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luan H, Diao F, Scott RL, and White BH (2020). The Drosophila split Gal4 system for neural circuit mapping. Front. Neural Circuit. 14, 603397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olberg RM (1983). Pheromone-triggered flip-flopping interneurons in the ventral nerve cord of the silkworm moth, Bombyx mori. J. Comp. Physiol. 152, 297–307. 10.1007/BF00606236. [DOI] [Google Scholar]
- 38.Homberg U (1994). Flight-correlated activity changes in neurons of the lateral accessory lobes in the brain of the locust Schistocerca gregaria. J. of Comp. Physiol. A 175, 597–610. 10.1007/BF00199481. [DOI] [Google Scholar]
- 39.Steinbeck F, Adden A, and Graham P (2020). Connecting brain to behaviour: a role for general purpose steering circuits in insect orientation? J. Exp. Biol. 223, jeb212332. 10.1242/jeb.212332. [DOI] [PubMed] [Google Scholar]
- 40.Kim H, Park H, Lee J, and Kim AJ (2023). A visuomotor circuit for evasive flight turns in Drosophila. Curr. Biol. 33, 321–335. 10.1016/j.cub.2022.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Lima SQ, and Miesenböck G (2005). Remote control of behavior through genetically targeted photostimulation of neurons. Cell 121, 141–152. 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Isaacman-Beck J, Paik KC, Wienecke CFR, Yang HH, Fisher YE, Wang IE, Ishida IG, Maimon G, Wilson RI, and Clandinin TR (2020). SPARC enables genetic manipulation of precise proportions of cells. Nat. Neurosci. 23, 1168–1175. 10.1038/s41593-020-0668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Govorunova EG, Sineshchekov OA, Janz R, Liu X, and Spudich JL (2015). Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science 349, 647–650. 10.1126/science.aaa7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goyal L, McCall K, Agapite J, Hartwieg E, and Steller H (2000). Induction of apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function. EMBO J. 19, 589–597. 10.1093/emboj/19.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bender JA, and Dickinson MH (2006). Visual stimulation of saccades in magnetically tethered Drosophila. J. Exp. Biol. 209, 3170–3182. 10.1242/jeb.02369. [DOI] [PubMed] [Google Scholar]
- 46.Censi A, Straw AD, Sayaman RW, Murray RM, and Dickinson MH (2013). Discriminating external and internal causes for heading changes infreely flying Drosophila. PLOS Comp. Biol. 9, e1002891. 10.1371/journal.pcbi.1002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buhmann J, Sheridan A, Malin-Mayor C, Schlegel P, Gerhard S, Kazimiers T, Krause R, Nguyen TM, Heinrich L, Lee W-CA, et al. (2021). Automatic detection of synaptic partners in a whole-brain Drosophila electron microscopy data set. Nat. Meth. 18, 771–774. 10.1038/s41592-021-01183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lesser E, Azevedo AW, Phelps JS, Elabbady L, Cook A, Mark B, Kuroda S, Sustar A, Moussa A, Dallmann CJ, et al. (2023). Synaptic architecture of leg and wing motor control networks in Drosophila. Preprint at bioRxiv, 10.1101/2023.05.30.542725 10.1101/2023.05.30.542725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hulse BK, Haberkern H, Franconville R, Turner-Evans D, Takemura S, Wolff T, Noorman M, Dreher M, Dan C, Parekh R, et al. (2021). A connectome of the Drosophila central complex reveals network motifs suitable for flexible navigation and context-dependent action selection. eLife 10, e66039. 10.7554/eLife.66039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korn H, and Faber DS (2005). The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron 47, 13–28. 10.1016/j.neuron.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 51.Strausfeld NJ, and Hirth F (2013). Deep homology of arthropod central complex and vertebrate basal ganglia. Science 340, 157–161. 10.1126/science.1231828. [DOI] [PubMed] [Google Scholar]
- 52.Kanzaki R (1998). Coordination of wing motion and walking suggests common control of zigzag motor program in a male silkworm moth. J. Comp. Physiol. A 182, 267–276. 10.1007/s003590050177. [DOI] [Google Scholar]
- 53.Muijres FT, Elzinga MJ, Melis JM, and Dickinson MH (2014). Flies evade looming targets by executing rapid visually directed banked turns. Science 344, 172–177. [DOI] [PubMed] [Google Scholar]
- 54.Dionne H, Hibbard KL, Cavallaro A, Kao J-C, and Rubin GM (2018). Genetic reagents for making split-GAL4 lines in Drosophila. Genetics 209, 31–35. 10.1534/genetics.118.300682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pfeiffer BD, Ngo T-TB, Hibbard KL, Murphy C, Jenett A, Truman JW, and Rubin GM (2010). Refinement of tools for targeted gene expression in Drosophila. Genetics 186, 735–755. 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tirian L, and Dickson BJ (2017). The VT GAL4, LexA, and split-GAL4 driver line collections for targeted expression in the Drosophila nervous system. Preprint at bioRxiv, 10.1101/198648 10.1101/198648. [DOI] [Google Scholar]
- 57.Clements J, Goina C, Hubbard PM, Kawase T, Olbris DJ, Otsuna H, Svirskas R, and Rokicki K (2022). NeuronBridge: an intuitive web application for neuronal morphology search across large data sets. Preprint at bioRxiv, 10.1101/2022.07.20.500311 10.1101/2022.07.20.500311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nern A, Pfeiffer BD, and Rubin GM (2015). Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. PNAS 112, E2967–E2976. 10.1073/pnas.1506763112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cook KR, Parks AL, Jacobus LM, Kaufman TC, and Matthews K (2010). New research resources at the Bloomington Drosophila Stock Center. Fly 4, 88–91. 10.4161/fly.4.1.11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Namiki S, Ros IG, Morrow C, Rowell WJ, Card GM, Korff W, and Dickinson MH (2022). A population of descending neurons that regulates the flight motor of Drosophila. Curr. Biol. 32, 1189–1196.e6. 10.1016/j.cub.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guizar-Sicairos M, Thurman ST, and Fienup JR (2008). Efficient subpixel image registration algorithms. Opt. Lett., OL 33, 156–158. 10.1364/OL.33.000156. [DOI] [PubMed] [Google Scholar]
- 62.Reiser MB, and Dickinson MH (2008). A modular display system for insect behavioral neuroscience. J. Neurosci. Meth. 167, 127–139. 10.1016/j.jneumeth.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 63.Suver MP, Huda A, Iwasaki N, Safarik S, and Dickinson MH (2016). An array of descending visual interneurons encoding self-motion in Drosophila. J. Neurosci. 36, 11768–11780. 10.1523/JNEUROSCI.2277-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim AJ, Fitzgerald JK, and Maimon G (2015). Cellular evidence for efference copy in Drosophila visuomotor processing. Nat. Neurosci. 18, 1247–1255. 10.1038/nn.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boggs PT, Spiegelman CH, Donaldson JR, and Schnabel RB (1988). A computational examination of orthogonal distance regression. J. Econometrics 38, 169–201. 10.1016/0304-4076(88)90032-2. [DOI] [Google Scholar]
- 66.Zheng Z, Lauritzen JS, Perlman E, Robinson CG, Nichols M, Milkie D, Torrens O, Price J, Fisher CB, Sharifi N, et al. (2018). A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell 174, 730–743.e22. 10.1016/j.cell.2018.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eckstein N, Bates AS, Du M, Hartenstein V, Jefferis GSXE, and Funke J (2020). Neurotransmitter classification from electron microscopy images at synaptic sites in Drosophila. bioRxiv, 2020.06.12.148775. 10.1101/2020.06.12.148775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heinrich L, Funke J, Pape C, Nunez-Iglesias J, and Saalfeld S (2018). Synaptic Cleft Segmentation in Non-isotropic Volume Electron Microscopy of the Complete Drosophila Brain. In Medical Image Computing and Computer Assisted Intervention – MICCAI 2018 Lecture Notes in Computer Science., Frangi AF, Schnabel JA, Davatzikos C, Alberola-López C, and Fichtinger G, eds. (Springer International Publishing; ), pp. 317–325. 10.1007/978-3-030-00934-2_36. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As in all following supplemental videos, the optical sections appear temporally from posterior to anterior in the brain, and from dorsal to ventral in the ventral nerve cord. GFP expression is shown in green; nc82 staining in magenta. Notable off-targets cells in the brain include sparse, but strongly expressing ascending neurons innervating the gnathal ganglion and saddle, sparsely expressing projection neurons from the optic lobe, and a weakly expressing descending neuron with a cell body adjacent to DNae014.
This driver line (SS02383) was generated at the Janelia Research Campus. GFP expression is shown in green; nc82 staining in magenta. A notable off-target cell in the brain is a neuron in the pars intercerebralis that innervates the ocellar ganglion.
CsChrimson-Venus expression is shown in green; nc82 staining in magenta. The image stacks for the two driver lines we created are shown sequentially in the video. Notable off-target cells in the brain for line 1 include sparse neurons innervating the superior medial protocerebrum, a small cluster of somata ventral to the antennal lobes, and a cluster of interneurons with somata in the ventrolateral cell body rind that innervate the antennal mechanosensory and motor neuropils as well as the saddle. Notable off-target cells in the brain for line 2 include a pair of interneurons with somata in the ventrolateral cell body rind that innervate the saddle.
Data Availability Statement
All data have been deposited on Mendeley at https://doi.org/10.17632/wx3v67wb3w.1 and are publicly available as of the date of publication. All new fly lines generated for this paper are mentioned in the manuscript. All original code for data analysis has been deposited on Mendeley at: https://doi.org/10.17632/wx3v67wb3w.1 and is publicly available as of the date of publication. All original code used for the Kinefly wing tracking and magnotether body tracking software is publicly available on Github at: https://github.com/ssafarik/Kinefly and https://github.com/willdickson/find_fly_angle, respectively. Any additional information required to reanalyze the data reported in this paper is available upon request.


