Abstract
Previous studies in native tissues have produced conflicting data on the localization and metabolic fate of WT and ΔF508 cystic fibrosis transmembrane regulator (CFTR) in the lung. Combining immunocytochemical and biochemical studies utilizing new high-affinity CFTR mAbs with ion transport assays, we examined both 1) the cell type and region specific expression of CFTR in normal airways and 2) the metabolic fate of ΔF508 CFTR and associated ERM proteins in the cystic fibrosis lung. Studies of lungs from a large number of normal subjects revealed that WT CFTR protein localized to the apical membrane of ciliated cells within the superficial epithelium and gland ducts. In contrast, other cell types in the superficial, gland acinar, and alveolar epithelia expressed little WT CFTR protein. No ΔF508 CFTR mature protein or function could be detected in airway specimens freshly excised from a large number of ΔF508 homozygous subjects, despite an intact ERM complex. In sum, our data demonstrate that WT CFTR is predominantly expressed in ciliated cells, and ΔF508 CFTR pathogenesis in native tissues, like heterologous cells, reflects loss of normal protein processing.
INTRODUCTION
Cystic fibrosis (CF) is the most common lethal genetic disease in the Caucasian population, with >1000 mutations reported in the CFTR gene (www.genet.sickkids.on.ca/cftr). Functional studies have revealed that CFTR encodes a cAMP-regulated Cl– channel that is expressed in the apical plasma membrane (PM) of many epithelial tissues (Kartner et al., 1992). However, 15 years after the identification of the CFTR gene, the biological events that lead from mutations in CFTR to the failure of lung defense against inhaled bacteria that causes CF lung disease remain controversial. In part, this controversy reflects the relative paucity of information describing the expression and function of WT CFTR protein in the normal lung. Thus, a key starting point for investigations of the role of CFTR in lung defense is to identify which airway regions and cell types express CFTR.
A thin (∼20 μm) film of liquid covering the airway surfaces is responsible for much of the innate defense of the lung against inhaled noxious and infectious agents principally. This film of airway surface liquid (ASL) performs this function by mediating efficient mucus clearance, and recent studies suggest the volume of ASL is a major determinant of mucus clearance rates (Boucher, 2003). Both, the superficial epithelium (the epithelium lining the airway surfaces) and the submucosal glands regulate ASL volume, and CFTR has been reported to regulate ion and water flows in both regions (Boucher, 2003). However, the relative contribution of the superficial epithelium and submucosal glands to ASL volume homeostasis and thus to CF airway pathogenesis remains controversial (Verkman et al., 2003). Historically, submucosal glands, not the superficial epithelia, have been considered as the main site of CFTR expression in the lungs (Engelhardt et al., 1992). Submucosal glands are well developed in human airways and comprise a system of mucous (mucin-secreting) and serous (fluid-secreting) acinar tubules and conducting ducts that secrete a mixture of mucins and liquid into the lumen of large airways (Burkitt et al., 1996). An important study (Engelhardt et al., 1992) reported that CFTR expression in human lungs was highest in submucosal gland acini, suggesting that decreased gland output of fluid and antimicrobial factors might cause CF lung disease (Jiang and Engelhardt, 1998). However, compelling physiological evidence has recently accumulated suggesting that the superficial epithelium controls the volume of liquid on airway surfaces. In this view, CF pathogenesis results from the failure of the superficial epithelium to regulate ASL volume and mucus clearance (Boucher, 2003). A central question has been whether low/nondetectable levels of CFTR in the superficial epithelium can account for its role in CF disease and pathogenesis.
Similarly, there is no consensus on which airway cell types express CFTR in the superficial epithelium. The ciliated cell is believed to be involved in transepithelial ion transport. Some reports suggest that CFTR is expressed in ciliated cells within the surface epithelium (Puchelle et al., 1992), consistent with a role for CFTR in regulating ASL volume. However, others report CFTR is expressed in airway surface goblet cells (Barasch et al., 1991; Jacquot et al., 1993; Glick et al., 2001), suggesting a regulatory role for CFTR in mucin secretion rather than fluid transport. The accurate description of CFTR expression in the lung thus is important for understanding the function of CFTR in normal lung physiology and CF pathogenesis.
Other aspects of CF pathogenesis in the lung are also controversial. For example, the most common cause of CF in North America is deletion in CFTR of a codon for phenylalanine 508 (ΔF508). Studies in vitro in cellular models expressing heterologous or endogenous human CFTR have suggested that the molecular pathogenesis of ΔF508 CFTR is related to defective intracellular protein processing and trafficking (Cheng et al., 1990) rather than defective Cl– channel function (Li et al., 1993). The maturation and localization of ΔF508 CFTR protein in native human tissues, however, have been difficult to assess because of the limited sensitivity and specificity of CFTR antibodies and disease-related tissue damage. Early immunofluorescence studies described localization of WT but not ΔF508 CFTR to the apical PM of the sweat duct (Cohn et al., 1991; Kartner et al., 1992) and airway submucosal glands (Engelhardt et al., 1992; Puchelle et al., 1992). Functional studies also failed to detect a cAMP-dependent Cl– secretion in native CF airway and intestinal epithelia (Boucher et al., 1989; Veeze et al., 1991; Mall et al., 2004). However, the concept that the pathophysiology of ΔF508 CFTR in native epithelia reflects predominantly defective processing and protein mislocalization has been challenged. An immunohistochemical study detected CFTR in the apical PM at similar intensities in airway and intestinal epithelia from ΔF508 homozygous and normal subjects, suggesting that the maturation defect of ΔF508 CFTR is tissue-specific (Kalin et al., 1999). Similarly, recent functional studies concluded that residual CFTR-mediated Cl– secretion was present in rectal and nasal epithelia from a large subgroup of ΔF508 homozygous CF subjects (Bronsveld et al., 2000, 2001).
The controversy concerning ΔF508 CFTR trafficking to the apical PM has focused principally on the intrinsic folding properties of the mutant protein and its intracellular maturation (Jensen et al., 1995; Benharouga et al., 2002). However, it has recently been suggested that scaffolding proteins of the ezrin-radixin-moesin (ERM) family must be correctly localized at the PM for proper CFTR trafficking and function (Short et al., 1998; Mohler et al., 1999; Huang et al., 2003). Epithelial inflammation and remodeling have been reported to alter ERM protein expression (Laoukili et al., 2001), which could impair localization of ΔF508 protein to the apical PM. To date, there have been no colocalization studies of CFTR (WT or ΔF508) and ERM proteins in the human lung to test this notion.
In this study, we first sought to identify CFTR expression in normal airway regions and specific cell types within native epithelia freshly excised from normal individuals. Thus, we investigated in the normal lung CFTR expression in 1) the proximal airways that contain both superficial epithelia and glands; 2) the distal airway regions that contain only superficial epithelia; and 3) the individual cell types within the superficial epithelia. Next, we characterized whether functional ΔF508 CFTR reached the PM in airways resected from ΔF508 homozygous CF subjects with immunocytological and functional approaches and asked whether ERM proteins maintained their apical localization in the presence of persistent CF airways inflammation and infection. This characterization was made possible by the availability of highly sensitive/specific CFTR mAbs (Gentzsch et al., 2003; Mall et al., 2004) and an ample collection of normal and CF airway tissues.
MATERIALS AND METHODS
Subjects
Freshly excised nasal tissues were obtained from eight normal individuals (age range, 2–56 y) undergoing surgery for plastic reconstruction or sleep apnea syndromes and from 7 ΔF508 homozygous CF subjects (age range, 1 mo to 32 y) after polypectomy. Freshly excised bronchial, bronchiolar, and alveolar specimens were obtained from the resected lungs of 15 ΔF508 homozygous CF subjects (mean age, 26 ± 3 y; range, 9–38 y) undergoing lung transplant, and excess tissue from 26 healthy lung transplant donors (mean age, 37 ± 3 y; range, 6–57 y). The study was approved by the Institutional Review Board for Protection of Human Rights at the University of North Carolina at Chapel Hill and the University of Freiburg and written informed consents were given by subjects/parents of underage subjects.
Tissue Specimens
All the tissues were processed within 1 h of surgical excision. For immunofluorescence/LCM and ISH studies, tissues were quickly embedded in OCT (Sakura Finetek, Torrance, CA) and cryo-preserved at –80°C. For protein biochemistry studies, freshly excised nasal and bronchial specimens (∼0.25 cm2 in size) were scraped with a sterile scalpel to collect the superficial epithelial layer, which was rapidly frozen in liquid nitrogen and stored at –80°C. For Ussing chamber experiments, thin layers of nasal epithelium were dissected under a stereomicroscope (Mall et al., 2004). After Ussing chamber studies, tissues were embedded in OCT and cryo-preserved at –80°C.
In Situ Hybridization
Cryosections (∼8 μm thick) were processed as previously described (Kreda et al., 2001). Tissues were hybridized at 54°C for 18 h with CFTR sense or antisense riboprobes (1899–2623 bp) doubly labeled with 35S-UTP and 35S-CTP (∼107 cpm/ml). Exposing time to photographic emulsion were 30 d for nasal and bronchial tissues and 60 d for bronchioles and alveoli. A Nikon Microphot SA microscope (4× lens; Melville, NY) connected to a 3CC-Chilled Camera (Sony, Tokyo, Japan) was used to capture the images via Adobe Photoshop (San Jose, CA). A semiquantitative analysis of ISH signal intensity was performed in large airways using an arbitrary scale from 0 (background signal) to 4 (highest signal).
Antibodies and Probes
CFTR mAbs were raised using the full-length human CFTR protein purified from BHK cells (Mall et al., 2004). Rabbit polyclonal antibodies against EBP50 (Mohler et al., 1999), MUC5AC, and InsP3-receptors (Mohler et al., 2004) were gifts from Dr. S. Milgram (UNC-CH), Dr. J Sheehan (UNC-CH), and Dr. P. Mohler (Duke University), respectively; the rabbit polyclonal antibody against AQP5 (Kreda et al., 2001) and the rat monoclonal antibody (mAb) against tubulin were from Chemicon (Temecula, CA); the mAb against ezrin (Mohler et al., 1999) was from BD Transduction Laboratories (San Diego, CA); the mAb against MUC5AC was from NeoMarkers (Fremont, CA); and mAb against βIV-tubulin from Sigma (St. Louis, MO); all secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). Phalloidin labeled with AlexaFluor dyes to identify the actin cytoskeleton, and concanavalin A fluorescently labeled to identify the endoplasmic reticulum (Mateo et al., 2003) were from Molecular Probes (Eugene, OR).
Immunofluorescence Studies
Thin frozen tissue sections (∼8 μm) were processed as previously described (Kreda et al., 2001; Mall et al., 2004). Serial tissue sections were incubated at 4°C overnight with CFTR mAbs 528, 769, or a nonimmune mouse IgG (all at 10 μg/ml). For immunodetection, sections were incubated with a Texas Red anti-mouse IgG Fab fragment alone, or plus AlexaFluor488 phalloidin diluted 1:200 and 1:150, respectively. Tissues were mounted in Vectashield containing DAPI to label nuclei (Vector Laboratories, Burlingame, CA). In some experiments, reducing (sodium borohydride) or oxidizing agents (hydrogen peroxide; both from Sigma) were used after the permeabilization step for epitope retrieval. Serial tissue sections were incubated with antibodies against MUC5AC to identify goblet cells, βIV-tubulin or tubulin to identify ciliated cells, AQP5 to identify serous cells in gland acini (Kreda et al., 2001), and ezrin and EBP50 to stain the scaffolding complex associated with CFTR (Short et al., 1998; Mohler et al., 1999). In some experiments, CFTR mAbs were coincubated with polyclonal antibodies against AQP5 and EBP50, or a rat mAb against tubulin, followed by fluorescent anti-mouse IgG Fab fragment and AlexaFluor 633 phalloidin plus either fluorescent anti-rabbit IgG, or anti-rat IgG, diluted 1:200, 1:150, 1:200, and 1:200 respectively.
LCM and Analysis of Immunofluorescence Data
Immunofluorescence studies were performed on 2–3 different tissue blocks per patient, and repeated at least three times for each tissue. For each experiment, two different CFTR mAbs were independently assayed and at least one assayed in duplicate on nonconsecutive sections. The investigators were blinded as to the identity and genotype of the tissues when scoring sections. Image acquisition and analysis were performed in a Leica SP2 AOBS confocal microscope with an Apochromat 40×, NA 1.25 Leica lens (Leica Microsystems, Heidelberg, Germany). Scanning of random areas of the superficial and glandular epithelia in the tissue sections was performed in the xy-axis, using three or four independent laser sources (364 nm UV, 488 nm Ar, 568 nm Kr, and 633 nm HeNe lasers) as required. Scanning parameters were set and utilized as a constant during data acquisition in different experiments. Images were processed using Leica (color encoding, magnification bar) and Adobe Photoshop software (RGB conversion, use of “sharpen filter” to increase contrast of the DIC images, overlay montage of confocal images, and labeling). Overlays of color to grayscale DIC images required a postoverlay correction of the background using “color balance” that did not affect data significance.
Ussing Chamber Experiments
Nasal epithelia were mounted in modified micro-Ussing chambers (Mall et al., 2004). The epithelium was bathed with Kreb's bicarbonate Ringer at 37°C, and studied under open circuit conditions with transepithelial resistance (Rte) and voltage (Vte) determined by applying intermittent (1 s) current pulses (ΔI = 0.5 μA). The equivalent short circuit current (Isc) was calculated according to Ohm's law (Isc = Vte/Rte). After tissues were equilibrated for 60 min, amiloride (10 μM, luminal) was added to block electrogenic Na+ absorption. cAMP-dependent Cl– secretion was measured as the plateau phase ∼15 min after addition of forskolin and 3-isobutyl-1-methylxanthine (IBMX; 1 μM and 100 μM, both surfaces).
Sequential Immunoprecipitation and Immunoblotting
The superficial epithelial layer from nasal and bronchial tissues from normal and CF subjects was processed as previously described (Mall et al., 2004).
Well-differentiated Human Bronchial Cultures
Cells from four normal and four ΔF508 homozygous specimens were grown on permeable supports (Transwell-Col filters, 12-mm diameter, 0.4-μm pore size, Corning Costar, Cambridge, MA) as previously described (Kreda et al., 2000). After 21 d, the cultures developed Rte > 300Ωcm2. CFTR Cl– channel function was measured with an EVOM (STX2 electrode, World Precision Instruments, Sarasota, FL) in open circuit configuration. Cultures were equilibrated on both surfaces with Kreb's bicarbonate Ringer at RT for 30 min, and then amiloride (100 μM) was added to the lumen, Rte and Vte recorded as a baseline (∼10 min), and the cAMP-induced response was recorded for ∼15 min after exposure to forskolin (10 μM; bilateral); Isc was calculated from Isc = Vte/Rte.
After functional studies, the human cultures were assayed for CFTR protein expression. For biochemical analyses, Transwell membranes were transferred into microcentrifuge tubes containing cold lysis buffer and subjected to Western blot analyses as above. For immunofluorescence and LCM analyses, cultures were fixed, permeabilized, and stained, and image acquisition and analysis performed as above. For LCM analyses of isolated cells, immunostained cells were gently scraped off the Transwell membranes, mounted under a coverslip, and analyzed by LCM xy scanning as before using a Leica PlanApo 63× NA 1.2 lens.
Statistics
Bioelectric measurements were performed on 2–4 nasal tissues per individual and 3–4 cultures per individual. Data were averaged to obtain a single value for each subject and shown as mean ± SEM. Student`s t test and Mann-Whitney Rank Sum Test were performed as appropriate; p < 0.05–0.01 was accepted to indicate statistical significance.
RESULTS
CFTR Expression in Normal Airways
Four regions of the respiratory tract of a large number of subjects were examined. The nasal mucosa (upper airways contain superficial epithelium and submucosal glands. The nasal mucosa is affected in CF and is the region of the human airways most frequently used for in vivo and ex vivo functional studies. The 3rd–6th generation bronchi represent the large (and proximal) airway region. Bronchi are characterized by superficial epithelium and submucosal glands and are also severely affected by CF lung disease. Small or distal airways (bronchioles) contain only superficial epithelium and are thought to be the initial site of disease in the CF lung (Davis, 1993). Finally, the distal lung region (respiratory bronchioles and alveoli) is lined by superficial epithelia, which in the alveolar region is of a different type than the airways and is thought not to be primarily affected in CF (Davis, 1993).
CFTR mRNA Expression
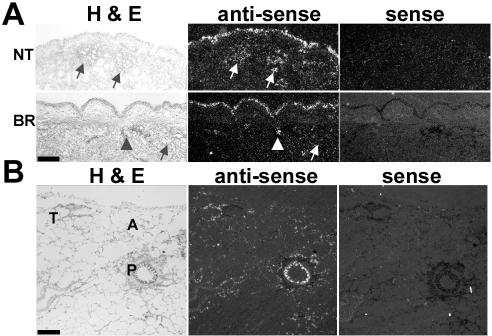
ISH analyses were performed to assess the expression of CFTR mRNA in normal airway epithelia. Nasal and bronchial airways required shorter exposure time (∼30 d) to the radiosensitive emulsion compared with bronchioles and distal lung (∼60 d), suggesting that the level of CFTR mRNA expression decreased distally in the human lung (Figure 1, A and B). In both, nasal (NT) and bronchial (BR) specimens the superficial epithelium, but not the submucosal gland acini, exhibited the most consistent and/or intense ISH signal (Figure 1A). In nasal epithelium, all the subjects (n = 4) exhibited a continuous ISH signal in the surface epithelium (3.25 ± 0.25 as scored by a semiquantitative index), whereas two of four subjects exhibited patchy hybridization signals in submucosal glands (1.25 ± 0.75). In all bronchial specimens (n = 5), the superficial epithelium (2.20 ± 0.48) and ciliated ducts of the glands (2.75 ± 0.49) exhibited a more continuous and robust hybridization signal than submucosal gland acini (0.70 ± 0.12). More distally, the intensity of the ISH signal was higher in the superficial epithelium of the proximal bronchioles than in the terminal bronchioles. In the alveoli, the signal was low and too diffuse and uneven to identify pneumocytes type I or II (Figure 1B).
Figure 1.
CFTR mRNA expression in normal human airways. Representative results from CFTR ISH analyses of (A) nasal turbinate (NT, n = 4) and bronchial (BR, n = 5) epithelia and (B) distal lung (n = 3). Bright field (H&E, left) and dark field (center) of a tissue section labeled with antisense probe, and dark field of a consecutive tissue section labeled with sense probe (right) are shown. Tissues were exposed to radiosensitive emulsion for 30 d (A) and 60 d (B). Both, nasal and bronchial specimens exhibited stronger and more consistent ISH signal in the surface epithelium than in submucosal gland acini. Some gland acini (arrows), ciliated ducts (arrowheads), proximal (P) and terminal (T) bronchioles, and alveoli (A) are identified; bar, 1 mm.
CFTR Immunolocalization
CFTR expression was also investigated by immunolocalization and laser confocal microscopy (LCM) techniques with recently described mAbs against human CFTR (Gentzsch etal., 2003; Mall et al., 2004). Briefly, these mAbs recognize human WT and ΔF508 CFTR heterologously expressed in epithelial MTE18 and BHK cells with high specificity and with 10–100 times higher sensitivity than previously published antibodies (Cohn et al., 1991; Kalin et al., 1999) in biochemical and immunocytochemical assays (see Figure 2 in Mall et al., 2004). The mAbs used in this study react with different and nonoverlapping epitopes within the R-domain (570 and 217, biochemical studies) and NBD2 domain (596, 528, and 729, biochemical and immunocytochemical studies) of the CFTR molecule.
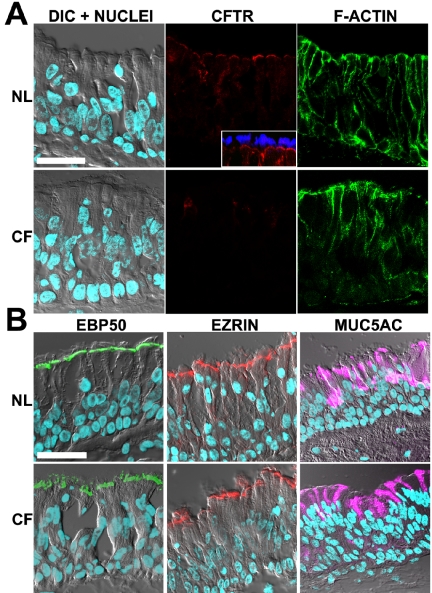
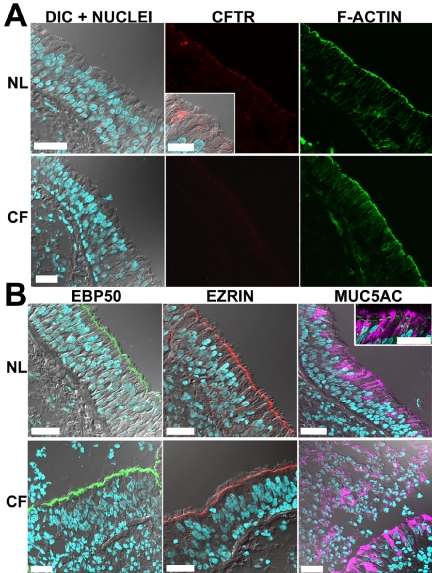
CFTR Immunolocalization in Superficial Epithelium of Proximal Airways In nasal and bronchial airways, the superficial epithelium is tall and pseudostratified and is comprised mainly of ciliated (fluid-secreting) cells. It also contains variable numbers of intercalated goblet (mucin-secreting) cells (Burkitt et al., 1996), which typically secrete MUC5AC mucin (Perez-Vilar et al., 2003). CFTR immunostaining/LCM studies revealed a consistent and clear CFTR signal in the apical PM of surface columnar cells in the superficial epithelium of nasal and bronchial airways (see Figures 2A and 3A). Fluorescent phalloidin was used to identify the cortical actin adjacent to the apical PM and as a test of epithelial structural integrity. CFTR immunostained cells were identified as ciliated cells by morphology (see differential interference contrast [DIC] plane) and by costaining with tubulin antibodies in LCM analyses (Figure 2A, inset). CFTR staining of the apical PM was observed in every ciliated cell and was present in all the subjects analyzed (n = 8 and n = 26 for nasal and bronchial specimens, respectively). Intracellular CFTR staining was low in most ciliated cells (e.g., associated with the endoplasmic reticulum, which was identified using antibodies against InsP3-receptors and/or concanavalin A, unpublished data). Of note, many of the bronchial specimens had a few lumenal cells that were intensely stained by CFTR mAbs (Figure 3A, inset). These cells appeared to be ciliated and were scattered throughout the surface epithelium and ciliated ducts.
Figure 2.
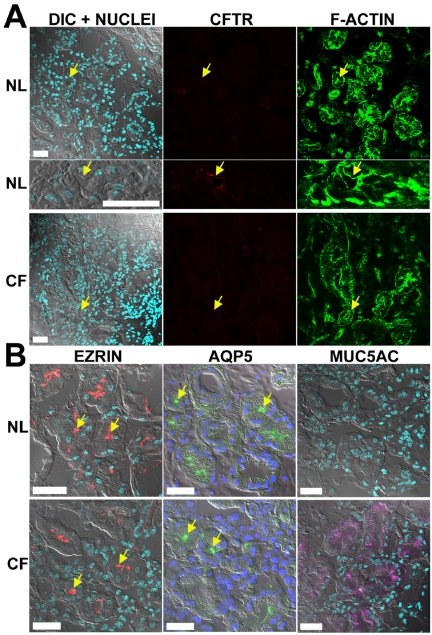
CFTR localization in nasal epithelium. Representative localization of CFTR, ERM proteins, and MUC5AC in freshly excised nasal tissues; normal (NL; n = 8) and ΔF508 homozygous CF (CF; n = 7) by immunofluorescence and LCM. (A) Confocal images acquired in four channels with independent laser sources: left, overlay of DIC and nuclei staining (DAPI, blue); center, CFTR (mAb 528, red); right, actin cytoskeleton (fluorescent phalloidin, green). Inset: costaining of CFTR (red) and cilia (tubulin, blue) in normal nasal tissue. CFTR is expressed in the apical PM of all ciliated cells of normal but not CF tissues. (B) Typical immunolocalization results for left, EBP50 (green); center, ezrin (red); right, MUC5AC (purple) in normal and CF tissues; images are presented as overlays of confocal planes for DIC, DAPI, and antibody stainings. Bar, 40 μm.
Figure 3.
CFTR localization in bronchial epithelium. Results in freshly excised bronchial specimens (normal n = 26; ΔF508 homozygous CF; n = 15) are presented as in Figure 2. (A) CFTR is expressed in the apical PM of all ciliated cells of normal but not CF bronchi; note in normal, a ciliated cell strongly expressing CFTR, which is also shown magnified in a different focal plane (inset; bar, 20 μm). (B) Typical immunolocalization results for EBP50, ezrin, and MUC5AC displayed as in Figure 2. Inset: costaining of CFTR (green) and MUC5AC (purple) in normal bronchial tissue. Bar, 40 μm.
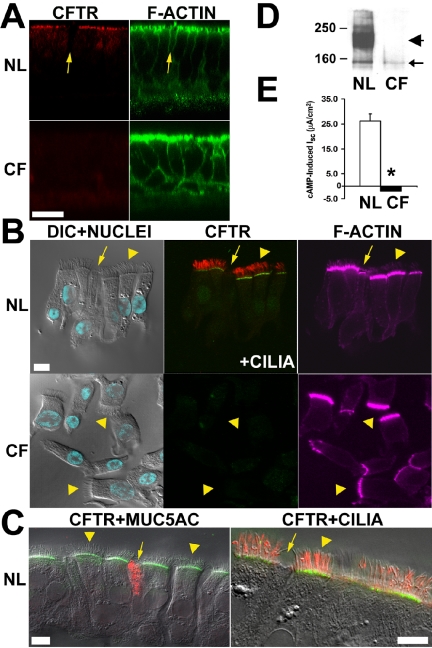
Importantly, CFTR-specific apical membrane-associated staining was not observed in other cell types, including goblet cells identified with MUC5AC antibodies (Figures 2B and 3B, inset), or basal cells. These results were confirmed by high resolution LCM analyses of well-differentiated bronchial cultures derived from some of the tissue specimens included in the study. These cultures developed into a pseudostratified epithelium and clearly revealed that CFTR was localized exclusively at the apical PM of ciliated cells but not in goblet cells or any other cell type (see Figure 9C).
Figure 9.
CFTR protein expression and function in normal versus ΔF508 homozygous bronchial epithelial cultures. Well-differentiated cultures (normal, n = 4; CF, n = 4) were subjected to protein expression and Cl– transport function analyses. (A) LCM xz images of CFTR (red) and actin cytoskeleton (green) localization on normal and CF cultures; arrow denotes a non-ciliated cell; bar, 20 μm. (B) LCM xy images for stained cells isolated from normal (CFTR, green; cilia, red) and CF (CFTR, green) cultures (note the cortical actin cap in the goblet cell pointed by the arrow). (C) LCM xy images for costained CFTR (green) and either MUC5AC (red) or tubulin (red) cells isolated from normal cultures. Ciliated (arrowheads) and goblet (arrows) cells are indicated; bar, 8 μm. CFTR is expressed in the apical PM of normal but not CF ciliated cells, nor in goblet cells of either group. (D) Representative immunoblot of normal and CF cultures; labels as in Figure 8. (E) CFTR Cl– transport function in normal and CF cultures; ΔIsc data presented as mean ± SEM (p < 0.01) in response to forskolin/IBMX.
The normal bronchial specimens showed variable degrees of acute inflammation and goblet cell hyperplasia, which is expected since lung donors were mechanically ventilated and evaluated by bronchoscopy before lung harvest (Dr. S. Randell, UNC-CH, personal communication). Of note, in a few normal subjects, sections exhibiting intense inflammation and epithelial hyperplasia displayed intermediate and/or poorly differentiated cells with intracellular CFTR staining (unpublished data).
CFTR Immunolocalization in Submucosal Glands of Upper/Proximal Airways Human submucosal glands consist of acini of serous (fluid-secreting) and mucous (mucin-secreting) cells and conducting ducts lined by an epithelium that transitions from simple to pseudostratified (similar to the superficial epithelium) as the ducts progress toward the airway lumen. It has been widely accepted that CFTR channel activity is involved in volume homeostasis of gland secretions and that CFTR protein is predominantly localized in gland acini (Engelhardt et al., 1992; Jiang and Engelhardt, 1998). Surprisingly, this study revealed that CFTR immunostaining signal in the gland acini was not as strong and consistent as in the superficial epithelium (Figures 4B and 5A). Nasal specimens from only four of eight subjects showed specific CFTR immunostaining in the submucosal gland acini. One specimen showed specific CFTR staining in all the acinar serous cells in a tissue section (Figure 4A), whereas the other three specimens exhibited only a few positive serous acini. CFTR staining was localized exclusively in the apical PM of serous cells, as identified by morphology (DIC and actin staining confocal images) and by costaining with aquaporin5 (AQP5; Kreda et al., 2001; Figure 5B). AQP5 is highly expressed in human submucosal glands (Kreda et al., 2001), and studies in the AQP5 knockout mouse indicate that AQP5 deficiency in the apical membrane of the serous glandular epithelium is associated with decreased transepithelial water permeability (Song and Verkman, 2001).
Figure 4.
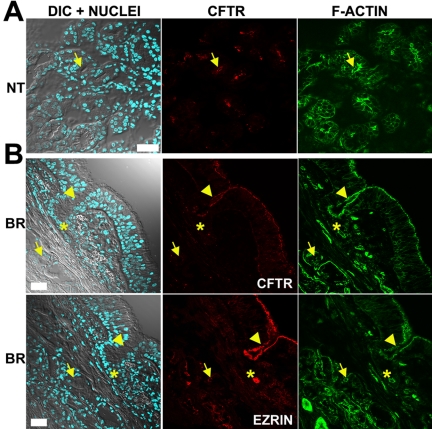
CFTR localization in nasal and bronchial submucosal glands. Immunolocalization results in normal submucosal glands displayed as in Figure 2. (A) Representative CFTR nasal (NT) immunolocalization results for 4 of 8 subjects. (B) Representative bronchial (BR) immunolocalization results of CFTR and ezrin in sequential sections for 20 of 26 subjects. Some gland acini (arrows), ciliated ducts (arrowheads), and collecting ducts (*) are indicated. Bar, 40 μm.
Figure 5.
CFTR expression in bronchial submucosal gland acini. (A) CFTR immunolocalization results in bronchial submucosal gland acini displayed as in Figure 2; top panel, negative CFTR staining in normal acini (n = 20/26); middle panel, positive CFTR staining in normal acini (n = 6/26); bottom panel, absence of staining in CF acini (n = 15). (B) Typical immunolocalization results displayed as in Figure 2 for ezrin, AQP5 (green), and MUC5AC. The lumen of some serous glands is indicated by arrows. Bar, 40 μm.
In the bronchial specimens, CFTR immunostaining was not detected in submucosal gland acini of most normal bronchial tissues (20 of 26 subjects; Figures 4B and 5A). In contrast, the CFTR staining signal was stronger and consistent in the apical PM of ciliated cells lining the ciliated ducts localized in close proximity to (CFTR) negative acini in all the specimens (Figure 4B). Serial sections stained with ezrin or EBP50 antibodies revealed intense apical staining of both ciliated ducts and acini of all normal specimens irrespective of CFTR staining (Figures 4B and 5B), suggesting that the minimal CFTR signal in gland acini could not be attributed to poor tissue preservation. Specific, but moderate CFTR immunostaining was observed only in a few submucosal serous acini of 6 normal subjects (6 of 26 subjects, Figure 5A). Identical results were obtained using the two new CFTR mAbs, CFTR antibodies from other laboratories, and epitope retrieval techniques. Importantly, AQP5 staining decorated the apical PM of serous cells in all nasal and bronchial specimens, despite the absence of a CFTR signal (Figure 5B), indicating that the PM of serous cells was intact and protein localization for other channels was not abnormal.
In sum, in nasal and bronchial airways, the pattern of CFTR immunolocalization signals (Figures 2, 3, 4, 5) was consistent with the pattern of CFTR mRNA expression (Figure 1A), suggesting that CFTR was predominantly expressed in the superficial epithelium rather than in the gland acini. Moreover, these results indicate unequivocally that CFTR was localized to the apical PM of ciliated (fluid-secreting) cells lining the superficial and gland ductal epithelia.
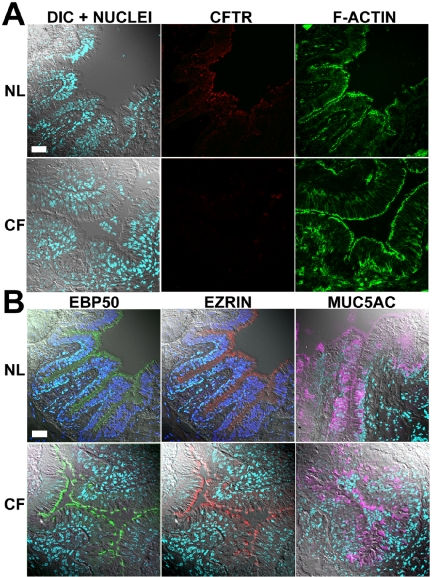
CFTR Immunolocalization in Small Airways Proximal bronchioles are lined with ciliated and goblet cells organized in a pseudostratified epithelium. As in proximal airways, the CFTR immunostaining signal was clear and consistent in the apical PM of every ciliated cell of the bronchiolar epithelium (7 normal subjects, Figure 6A). CFTR was not detected in Clara cells (note, Clara cells were absent in the human proximal bronchioles as previously reported by Boers et al. 1999), nor in the PM of goblet cells identified with MUC5AC antibodies (Figure 6B).
Figure 6.
CFTR localization in proximal bronchiolar epithelium. Representative immunolocalization results in normal (n = 7) and ΔF508 homozygous CF (n = 9) bronchioles displayed as in Figure 2 for (A) CFTR and (B) EBP50, ezrin, and MUC5AC. Bar, 40 μm.
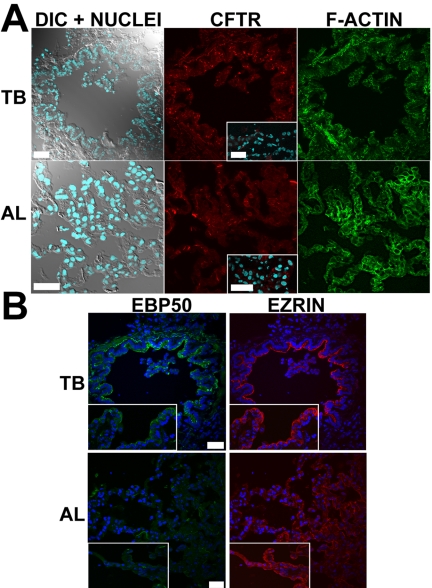
CFTR immunostaining of terminal bronchioles and alveoli was indistinguishable from the IgG control signal in all the specimens, despite well-preserved cellular structures including the PM as demonstrated by actin cytoskeleton staining (Figure 7A). High levels of autofluorescence emitted in most of the visible spectrum in distal lung tissues, may have masked a CFTR-specific signal, as previously suggested (Engelhardt et al., 1994). In contrast, staining of EBP50 and ezrin was very intense in the apical domain of terminal bronchiolar epithelial cells (see below, Figure 7B).
Figure 7.
CFTR localization in terminal bronchioles and alveoli. Representative immunolocalization results in terminal bronchioles (TB) and alveoli (AL) of normal lungs for (A) CFTR and (B) EBP50 and ezrin. Insets: immunolocalization results in ΔF508 homozygous CF lungs. Bar, 40 μm.
ΔF508 CFTR Expression, Maturation, and Function in CF Airways
We performed immunolocalization studies in CF airways excised at time of transplantation to investigate the fate of ΔF508 CFTR in native airways. Because of the potential for insensitivity of immunolocalization, we supplemented these studies with biochemical studies of ΔF508 CFTR protein maturation and bioelectric studies of function in freshly excised CF compared with normal tissues. Finally, to control for nonspecific effects of in situ inflammation on ΔF508 CFTR processing (Dupuit et al., 1995), we repeated key studies in cell culture.
CFTR Immunolocalization in ΔF508 Homozygous CF Airway Epithelia
In proximal airways, nasal (7 subjects) and bronchial (15 subjects) CF specimens exhibited epithelial structures and preservation comparable to normal tissues (nasal tissues, 8 subjects; bronchial tissues, 26 subjects) as examined by H&E and actin cytoskeletal staining and confocal DIC planes (Figures 2A and 3A). In contrast to the consistent and clear CFTR immunostaining signal in the apical PM of surface ciliated cells observed in all normal subjects, we failed to detect specific CFTR immunostaining in any cell type in the CF nasal and bronchial superficial epithelia of any CF subjects (Figures 2A and 3A).
In CF bronchial tissues, morphological differences associated with chronic infection were present in most of the CF specimens, e.g., goblet cell hyperplasia, consistent with lungs from CF subjects undergoing lung transplantation. Accordingly, in most cases, more than one bronchial specimen from each of the 15 ΔF508 homozygous CF patients was analyzed for CFTR immunolocalization and bronchial areas with clearly identifiable epithelial morphology (e.g., presence of ciliated cells) were utilized in the study. Epithelial areas presenting advanced metaplasia and/or tissue damage were not included in the comparative analyses between both groups. In most CF bronchial specimens, metaplasic tissues showed an absence of CFTR expression in the apical PM of superficial cells, but in a few CF subjects, intracellular CFTR staining was observed in some intermediate and/or nondifferentiated cells (unpublished data). Staining for MUC5AC revealed that the number and distribution of goblet cells varied according to the degree of inflammation in CF specimens. CF nasal tissues displayed moderate signs of epithelial inflammation and goblet cell hyperplasia (Figure 2B), whereas bronchial tissues showed extensive goblet cell hyperplasia (Figure 3B). Interestingly, MUC5AC staining was also observed in the “mucus plaques” lining the surface of many bronchial CF specimens (Figure 3B).
Submucosal glands in all CF subjects also displayed an absence of specific CFTR immunostaining signal in any gland cell type (Figure 5A) despite intense staining for AQP5 in the apical domain of acinar serous cells (Figures 5B). Speculation has been raised about AQP5 role in submucosal gland secretory functions and whether AQP5 expression could be altered in CF (Verkman et al., 2003). In this study, the intensity of AQP5 staining in serous cells was not different in CF versus normal tissues, suggesting that the expression levels of AQP5 protein are not altered in CF. Interestingly, although not detected in normal glands, MUC5AC immunostaining was observed in scattered gland mucous cells of CF submucosal glands (Figure 5B).
In the CF proximal (Figure 6A) and terminal bronchioles and alveoli (Figure 7A, insets), no specific CFTR staining was detected in any epithelial cell type (9 subjects) despite well-preserved epithelial cell integrity as demonstrated by actin cytoskeleton staining and DIC images (Figures 6A and 7A). MUC5AC staining indicated the presence of goblet cells in proximal bronchioles and was again observed in the “mucus plaques” lining the surface of CF proximal bronchioles (Figure 6B).
In sum, CFTR immunolocalization studies indicate that ΔF508 homozygous CF subjects exhibited undetectable levels of CFTR protein in the apical PM of epithelial cells throughout all the airway regions.
cAMP-dependent Cl– Secretion in ΔF508 Homozygous CF Nasal Tissues
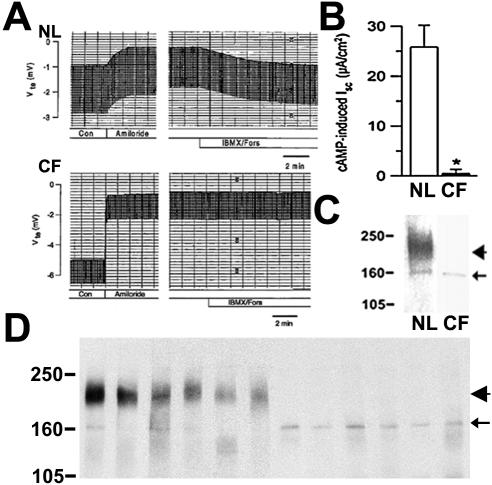
CFTR Cl– channel activity was examined in freshly isolated nasal tissues that were also subjected to CFTR protein localization (see above) and maturation studies (see below). Both indices of Na+ transport, i.e., basal and amiloride-sensitive equivalent short circuit current (Isc), were significantly increased in nasal epithelia from five ΔF508 homozygous CF subjects compared with seven normal specimens. Importantly, after blockade of Na+ transport, forskolin/IBMX induced a significant Cl– secretory response in normal (31.0 ± 8.1 μA.cm2) but not ΔF508 homozygous CF nasal tissues (0.5 ± 0.8 μA.cm2; Figure 8, A and B). These data indicate that CFTR-mediated Cl– secretion is absent in ΔF508 homozygous CF nasal epithelia.
Figure 8.
CFTR function and protein expression in nasal and bronchial epithelia from normal and ΔF508 homozygous subjects. CFTR Cl– channel activity in response to forskolin/IBMX was examined in freshly excised nasal tissues from normal (n = 6) and CF (n = 5) subjects in modified Ussing chambers. (A) Representative transepithelial voltage (Vte; mV) traces for normal and CF tissues. (B) cAMP induced short circuit current (Isc; μA/cm2) data for each group, mean ± SEM, p < 0.05. (C) Representative immunoblot of tissues studied in A. (D) Representative immunoprecipitation/immunoblot of freshly excised bronchial tissues studied in Figure 3 (normal, n = 10; CF, n = 10). Position of mol wt markers in KDa, CFTR mature (band C, arrowhead) and core-glycosylated (band B, arrow) proteins are indicated.
Biochemical Analyses of Freshly Excised CF versus Normal Epithelia
Nasal and bronchial tissues utilized in immunolocalization (and functional) studies were also subjected to Western blot analysis to investigate CFTR protein maturation. CFTR is a glycoprotein that separates into two well-defined bands in a denaturing gel. The slower band is the fully glycosylated mature protein or band “C” (180–190 kDa), which corresponds to the CFTR protein localized in the PM and membrane compartments in transit to the PM. The faster band or band “B” (∼160 kDa) corresponds to the core-glycosylated immature protein that has not progressed beyond the endoplasmic reticulum. Both mature (band C) and immature (band B) CFTR protein bands were observed in tissues from normal individuals (nasal, 3 subjects; bronchial, 10 subjects). In contrast, CF tissues from ΔF508 homozygous CF individuals (nasal, 3 subjects; bronchial, 10 subjects) exhibited only band B, i.e., the immature protein form of CFTR (Figure 8, C and D). These results indicate that CF tissues express undetectable levels of mature protein in epithelial cells.
Interestingly, both normal and CF tissues exhibited only a low level of core-glycosylated CFTR protein (Figure 8, C and D), which corresponded to the absence of specific intracellular immunostaining of ciliated cells in normal and CF airways (Figure 3A). In contrast, heterologous overexpression systems often display a higher level of core-glycosylated CFTR protein and intracellular immunostaining in cells expressing either WT or ΔF508 CFTR (Mall et al., 2004). This phenomenon probably reflects either higher protein synthetic rates in heterologous cells or that core-glycosylated CFTR protein may be processed more efficiently into mature protein and/or disappear faster in native tissues.
Bronchial Epithelial Cultures
CFTR expression/function studies were performed in well-differentiated bronchial cultures derived from some specimens included in the immunolocalization study. Immunofluorescence and high-resolution LCM studies of normal cultures revealed that CFTR was expressed at the apical PM of columnar cells in this pseudostratified epithelium (Figure 9A). CFTR localization was identified exclusively in the apical PM of ciliated cells but not in goblet cells or any other cell type (Figures 9, B and C). Similar studies revealed that CF cultures also exhibited a pseudostratified epithelium, but CFTR was not detected in the apical PM of any cell type (Figures 9, A and B). Normal cultures exhibited mature CFTR protein (180–190 kDa) by Western blot, whereas ΔF508 homozygous cultures exhibited only immature CFTR protein (∼160 kDa; Figure 9D).
CFTR Cl– channel activity was measured in bronchial cultures as forskolin (cAMP)-dependent Cl– secretion after amiloride pretreatment. In normal cultures, forskolin induced a large secretory current (ΔIsc = 26.1 ± 2.9 μA/cm2, range 14.2–38.1 μA/cm2, 4 subjects). In contrast, forskolin failed to induce Cl– secretion in any CF culture (Isc =–1.9 ± 0.5 μA/cm2, range –0.3 to –4.0 μA/cm2, 4 subjects, p < 0.01; Figure 9E).
In sum, ΔF508 homozygous CF bronchial cultures produced identical results to those in native bronchial tissue. There were undetectable levels of mature CFTR protein in the apical PM of epithelial culture cells, and CFTR-mediated Cl– secretion was absent in ΔF508 homozygous CF airway cultures.
Expression of ERM Proteins in Native Airway Epithelia from Normal and ΔF508 Homozygous CF Individuals
We focused on EBP50 and ezrin localization as these are the most widely studied ERM proteins relevant to positioning CFTR within the PM (Short et al., 1998; Mohler et al., 1999).
Nasal, Bronchial, and Bronchiolar Superficial Epithelia
The distribution and intensity of EBP50 and ezrin immunofluorescence was indistinguishable in normal and CF specimens from nasal (Figure 2B), bronchial (Figure 3B), and proximal bronchiolar (Figure 6B) regions. Both proteins colocalized to the apical region of columnar cells, overlapping mostly with the cortical actin staining signal and to a high contrast line observed in the DIC plane characteristic of microvilli. The highest intensity of EBP50 and ezrin staining was in the microvilli of ciliated cells.
Airway Submucosal Glands
Ezrin and EBP50 antibodies produced intense apical staining in ciliated and collecting ducts and in acini of the submucosal glands in all normal and CF nasal and bronchial specimens (Figures 4B and 5B).
Terminal Bronchioles and Alveoli
EBP50 and ezrin colocalized to the apical PM of all epithelial cuboidal cells of the terminal bronchioles and alveolar epithelial cells in both normal and CF specimens (Figure 7B). In both, normal and CF specimens the intensity of the staining signals were similar in all airway regions for ezrin but appeared to be decreased in the alveoli for EBP50.
DISCUSSION
There has been little agreement about the localization of CFTR in native airway epithelia. (Engelhardt et al., 1992, 1194; Puchelle et al., 1992; Kalin et al., 1999; Penque et al., 2000). With the availability of very sensitive anti-CFTR mAbs (Mall et al., 2004) and LCM technologies, we initiated a comprehensive study to explore two major issues controversial in CF research: 1) the cell type specific localization of CFTR in the normal lung; and 2) the localization of ΔF508 CFTR in native airway epithelium. With respect to this latter issue, we also sought to test whether absence of ΔF508 CFTR in the apical PM reflected an abnormal metabolic fate of ΔF508 CFTR within the cell or abnormalities in the scaffolding protein complex that associates with CFTR in the PM that may be disrupted by the chronic inflammation associated with CF. A summary of the data are presented in Table 1.
Table 1.
Summary of the data for the analyses of protein expression and function of WT and ΔF508 CFTR
| Normal | ΔF508/ΔF508 | |
|---|---|---|
| Apical CFTR expressiona | ||
| Superficial epithelium | ||
| Nasal | 8/8 | 0/7 |
| Bronchial | 26/26 | 0/15 |
| Prox. bronchiolar | 7/7 | 0/9 |
| Term. bronchiolar | ND/7 | ND/9 |
| Nasal subm. gland | ||
| Acinus | 4/8 | - |
| Ciliated duct | 8/8 | - |
| Bronchial subm. gland | ||
| Acinus | 6/26 | 0/15 |
| Ciliated duct | 26/26 | 0/15 |
| Bronchial cultures | 4/4 | 0/4 |
| CFTR protein maturationb | ||
| Nasal tissue | 3/3 | 0/3 |
| Bronchial tissue | 10/10 | 0/10 |
| Bronchial culture | 4/4 | 0/4 |
| CFTR Channel functionc | ||
| Nasal tissue | 7/7 | 0/5 |
| Bronchial culture | 4/4 | 0/4 |
Data are expressed as a fraction of the number of positive subjects vs. the total number of subjects examined. ND, nondetectable specific signal above background fluorescence emission.
Expression of CFTR protein at the apical PM of ciliated cells (surface epithelium and ciliated ducts) and serous cells (submucosal glands) identified by immunofluorescence/LCM analysis
Presence of mature protein identified by Western blot.
CFTR Cl- channel activity determined as cAMP-dependent short circuit current in the presence of amiloride.
Normal Airway Superficial Epithelia
CFTR mRNA expression was detected throughout the surface epithelium of nasal, tracheal (unpublished data), bronchial, and proximal bronchiolar tissues from normal individuals. The level of the mRNA expression appeared to decrease distally through the airways, consistent with previous PCR data (Trapnell et al., 1991) and functional studies (Knowles et al., 1981).
CFTR protein, as detected by immunolocalization, was first reported in a few unidentified columnar cells within the superficial epithelium (Engelhardt et al., 1992, 1994) and more recently in a restricted population of columnar cells (Penque et al., 2000; Carvalho-Oliveira et al., 2004). We identified the ciliated cell as the only cell type expressing CFTR in nasal, bronchial, and proximal bronchiolar surface epithelia by high resolution LCM and coimmunostaining with antibodies against cellular markers (e.g., tubulin). CFTR protein was expressed at the apical PM of all ciliated cells in a continuous pattern along the airway surfaces, consistent with its mRNA distribution.
Interestingly, we found a few ciliated cells scattered along the surface epithelium of bronchi and proximal bronchioles with very high levels of CFTR protein expression (Figure 3A, inset). These “hot cells” may correspond to the small population of cells immunostained by CFTR antibodies with less sensitivity (Engelhardt et al., 1992) than ours.
In terminal and respiratory bronchioles and alveoli, there is absence of specific CFTR immunostaining signal, which mirrors the low level of expression suggested by ISH as previously reported (Engelhardt et al., 1994). Because there are no early pathological changes in distal lung associated with CF, we speculate that the low levels of CFTR expression reflect a less important role for CFTR than other Cl– channels in fluid homeostasis in this pulmonary region.
Function of Normal Superficial epithelia in Mucus/Fluid Balance
Ciliated but not goblet (mucin-secreting) cells are predicted to be involved in airway epithelial ion and water transport (Cotton et al., 1987). This study clearly demonstrated that CFTR protein is expressed in all ciliated cells within the surface epithelium and is consistent with a significant role for CFTR in airway fluid balance. Other studies, however, have reported CFTR expression in mucous cells by immunohistochemistry (Jacquot et al., 1993; Kalin et al., 1999) and CFTR regulation of mucin sialylation and sulfation and granule exocytosis (Barasch et al., 1991; Glick et al., 2001). However, consistent with our data that CFTR is not expressed in goblet cells, mucin granule secretion rates have been reported not to differ in CF versus normal epithelium (Lethem et al., 1993), and there is little evidence for abnormal modifications of mucins in CF in the absence of infection/inflammation (Roussel, 2001). Thus, we conclude that CFTR does not directly regulate mucin composition or expression, but rather CFTR exhibits a dual function as mediator of Cl– secretion and regulator of ENaC-mediated Na+ transport that maintains ASL volume homeostasis.
Submucosal Glands of Normal Airways
A previous study (Engelhardt et al., 1992), indicating that gland acinar serous cells were the predominant site for CFTR expression in the human large airways, argued for a dominant role of submucosal glands in ASL volume regulation and CF pathogenesis (Jiang and Engelhardt, 1998). Surprisingly, we found little or no CFTR in gland acinar cells by immunofluorescence/LCM analyses. In our study, only 4/8 normal nasal turbinates showed apical immunostaining of serous gland acinar cells, whereas only 6/26 bronchial tissues showed some level of apical immunostaining in serous gland acinar cells (Figures 4 and 5). Consistent with the immunolocalization data, our ISH studies indicated that CFTR mRNA expression is routine in the surface epithelium and ciliated ducts but scattered in the gland acini of large airways (Figure 1A). The explanation as why there is a difference in the pattern of CFTR expression in the submucosal glands among normal subjects remains obscure, and further investigation is required to understand the implication of this finding. However, it is noteworthy that a previous report also described that CFTR immunostaining signal within the submucosal gland acini was variable, i.e., only in 2 of 4 normal subjects (Kalin et al., 1999).
Function of Glands in Airway Fluid Balance
Our data showing minimal, variable expression of CFTR in serous acinar cells suggest a more minor role for CFTR in airway gland acinar fluid secretion (volume production). Thus, these observations conflict with previous reports suggesting CFTR is the predominant mediator of gland acinar secretion, based on high CFTR immunolocalization signals in acinar serous cells (Engelhardt et al., 1992), studies of agonist regulation of porcine and human gland secretion rates (Joo et al., 2002; Ballard and Inglis, 2004), and studies of CalU-3 cells, which have been used as a model system of acinar serous cells (Shen et al., 1994).
Our observations also appear to conflict with ex vivo studies of CF gland preparations that have shown decreased agonist-stimulated glandular secretion rates and a more viscous (increased mucin concentration) secretion, implying that CFTR is involved in regulating glandular fluid volume (Jayaraman et al., 2001; Joo et al., 2002). However, in every functional ex vivo study of normal and CF tissues, all the glandular components (e.g., acini and ducts) and adjacent gland tissue/cells (e.g., muscle cells) were exposed to the pharmacological stimulus, and glandular secretions were analyzed at the duct entrance onto the airway surface (Joo et al., 2002; Verkman et al., 2003; Ballard and Inglis, 2004). Therefore, only the final secretion product was analyzed. In this context, we found CFTR expressed in all ciliated cells of ciliated ducts, and in scattered cells of the collecting ducts (unpublished data). Therefore, these data support a role of CFTR regulating glandular secretion homeostasis, but indicate that this CFTR function predominates in the submucosal ducts rather than the serous acini.
Expression of ΔF508 CFTR in CF Airway Epithelia
Immunofluorescence and LCM analyses of nasal (n = 7), bronchial (n = 15), and bronchiolar and alveolar (n = 9) CF specimens revealed a complete absence of CFTR immunostaining signal in the apical PM of ciliated cells or any other cell type in these epithelia. The absence of staining could not be attributed to poor reactivity of the mAbs against ΔF508 CFTR (Mall et al., 2004) or to tissue damage because stainings of actin cytoskeleton, ezrin, and EBP50 were robust in the CF tissues and not different from normal specimens.
Consistent with the immunolocalization data, biochemical analyses of excised superficial mucosa from the nasal and bronchial CF tissues indicated that ΔF508 CFTR protein did not progress beyond the endoplasmic reticulum, as suggested by the absence of mature CFTR protein and the presence of only the immature, core-glycosylated CFTR protein B band (Figure 8).
A functional assessment of CFTR channel activity is likely more sensitive than protein immunodetection. Therefore, we assayed for Cl– channel function in ΔF508 CF and normal freshly excised nasal tissues. Normal nasal tissues displayed a large CFTR-mediated Cl– secretion. In contrast, there was a complete absence of cAMP-dependent–mediated Cl– secretion in ΔF508 homozygous subjects (n = 5; Figure 8). Comparable data were obtained in cultured normal and CF airway epithelia (Figure 9). Thus, we conclude there is no functional or biochemical evidence for ΔF508 CFTR reaching the apical PM of human airway epithelium in vivo.
There are several possible reasons why our data and conclusions differ from some previous reports. First, our mAbs exhibit very high affinity for CFTR and hence may be less cross-reactive with other apical PM proteins in the CF lung compared with CFTR antibodies (Claass et al., 2000) used in previous studies (Kalin et al., 1999; Penque et al., 2000; Carvalho-Oliveira et al., 2004). Second, we applied multiple, complementary techniques to the same specimens. Finally, the ability to culture epithelial cells from aliquots of the same freshly excised specimens used for localization and protein studies provided an alternative strategy to minimize the effects of inflammation on CFTR expression (Dupuit et al., 1995).
Expression of Scaffolding Proteins Ezrin and EBP50 in Airway Epithelia
We localized ezrin and EBP50 to the apical PM of human airway epithelial cells (Short et al., 1998; Mohler et al., 1999; Ingraffea et al., 2002; Kulaksiz et al., 2002). Both proteins colocalized to the microvillus domain of epithelial cells in nasal, tracheal, bronchial, and bronchiolar superficial epithelia and were expressed at equivalent levels in CF and normal epithelia. Ezrin and EBP50 staining were readily detected in the apical PM of all ciliated cells but nondetectable in goblet cell apical membranes. In gland regions, ezrin and EBP50 were found to be coexpressed at high levels in the apical PM of cells in the collecting and acinar-serous ducts. Ezrin was also sometimes observed expressed alone in basolateral PMs of columnar cells, in PMs of basal cells, and in the apical PM of alveolar cells. These findings are in agreement with previous reports indicating that some epithelial cells express one but not both proteins (Ingraffea et al., 2002).
In sum, there were no evident changes in the pattern of expression of these ERM proteins in CF tissues, suggesting that ERM protein localization was not affected by Pseudomonas infection and inflammation (in disagreement with Laoukili et al., 2001; Maresso et al., 2004), or a defect in ΔF508 CFTR trafficking. Importantly, failure of ΔF508 CFTR to localize in the apical PM of CF cells could not be attributed to faulty ERM protein expression.
CONCLUSIONS
There is an evolving consensus that CF pathogenesis reflects ASL volume depletion. To explain this deficiency, one hypothesis has focused on the superficial epithelium as initiating and maintaining the ASL volume deficit (Boucher, 2003), whereas another hypothesis has focused on defective gland acinar secretory function (Verkman et al., 2003). Our data demonstrate that the predominant site of CFTR expression is the superficial epithelium of all airway regions including bronchiolar epithelium that is the site of early disease, suggesting a key role for the superficial epithelium in the initiation of ASL volume depletion. Defective gland secretion may well exacerbate ASL volume depletion, but our data suggest that this deficit may reflect defective CFTR expression/function in gland ductal rather than acinar epithelial cells. Therefore, therapeutic approaches might profitably focus on reestablishing CFTR function in the superficial epithelium.
In this context, speculation has been raised in the gene therapy literature as to which cell types and what fraction of cells in the superficial epithelium of the CF airway must be transduced with WT CFTR to restore normal ion-liquid transport (Johnson et al., 1992). Our data demonstrate that normal airway epithelia express CFTR in every ciliated cell and hence suggest that a goal for CF gene therapy is to transduce WT CFTR in all ciliated cells. In contrast, it may not be necessary to transduce submucosal gland serous cells.
Two competing hypotheses describe the metabolic fate of ΔF508 CFTR in affected epithelium in vivo. One maintains that ΔF508 CFTR protein reaches the PM in airway and intestinal epithelial cells in a tissue-specific manner to produce some Cl– channel function in CF subjects (Kalin et al., 1999; Bronsveld et al., 2000). The alternative hypothesis maintains that regardless of the tissue, ΔF508 CFTR protein does not fully mature and escape the endoplasmic reticulum quality control mechanisms, and hence, reach the PM to express Cl– channel activity (Mall et al., 2004). If the first hypothesis is correct, effective therapeutic agents should address mainly deficient channel activity of ΔF508 CFTR. If the second hypothesis is correct, enhancement of maturation and apical PM localization of ΔF508 CFTR is the therapeutic goal. The data presented here support the second hypothesis.
Finally, much speculation exists whether proteins that associate with CFTR in functional multiprotein complexes are deficient in the CF epithelia. This study demonstrates that two relevant scaffolding proteins are expressed and correctly localized in all airway regions of the CF lung, at levels similar to those observed in normal airway epithelia. Therefore, therapeutic approaches that attempt to reestablish CFTR function in airway epithelial cells appear plausible.
Acknowledgments
The authors gratefully thank the CF and non-CF volunteers for their participation in this study; Drs. S Milgram and J Sheehan (UNC-CH) for their generous gifts of EBP50 and MUC5AC antibodies, respectively; K. Burns and T. Eldred (UNC-CH) for their expert technical assistance; Dr. S. Randell, L. Fulchner, and R. Lampe for their help in tissue and cell procurement; and Lisa Brown for the editing of the manuscript. This study was supported by grants from the Cystic Fibrosis Foundation and the Mary Lynn Richardson Fund (S.M.K.), the Mukoviszidose e.V. and the Deutsche Forschungsgemeinschaft (M.M.), NIH grants DK51870 (J.R.R.) and HL34322 and HL60280 (R.C.B.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–11–1010) on February 16, 2005.
Abbreviations used: ASL, airway surface liquid; AQP5, aquaporin 5; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; ΔF508; ΔF, phenylalanine508 deletion; DIC, differential interference contrast; ERM, ezrin-radixin-moesin; EBP50, ERM binding protein50; Isc, short circuit current; IBMX, 3-isobutyl-1-methylxanthine; ISH, in situ hybridization; LCM, laser confocal microscopy; NL, normal; PM, plasma membrane; Rte, transepithelial resistance; Vte, transepithelial voltage.
References
- Ballard, S. T. and Inglis, S. K. (2004). Liquid secretion properties of airway submucosal glands. J. Physiol. (Lond.) 556, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasch, J., Kiss, B., Prince, A., Saiman, L., Gruenert, D., and Al-Awqati, Q. (1991). Defective acidification of intracellular organelles in cystic fibrosis. Nature 352, 70–73. [DOI] [PubMed] [Google Scholar]
- Benharouga, M., Sharma, M., and Lukacs, G. L. (2002). CFTR folding and maturation in cells. Methods Mol. Med. 70, 229–243. [DOI] [PubMed] [Google Scholar]
- Boers, J. E., Ambergen, A. W., and Thunnissen, F. B. (1999). Number and proliferation of clara cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 159, 1585–1591. [DOI] [PubMed] [Google Scholar]
- Boucher, R. C. (2003). Regulation of airway surface liquid volume by human airway epithelia. Pfluegers Arch. 445, 495–498. [DOI] [PubMed] [Google Scholar]
- Boucher, R. C., Cheng, E.H.C., Paradiso, A. M., Stutts, M. J., Knowles, M. R., and Earp, H. S. (1989). Chloride secretory response of cystic fibrosis human airway epithelia: Preservation of calcium but not protein kinase C- and A-dependent mechanisms. J. Clin. Invest. 84, 1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronsveld, I. et al. (2001). Chloride conductance and genetic background modulate the cystic fibrosis phenotype of Delta F508 homozygous twins and siblings. J. Clin. Invest. 108, 1705–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronsveld, I. et al. (2000). Residual chloride secretion in intestinal tissue of deltaF508 homozygous twins and siblings with cystic fibrosis. The European CF Twin and Sibling Study Consortium. Gastroenterology 119, 32–40. [DOI] [PubMed] [Google Scholar]
- Burkitt, H. G., Young, B., and Heath, J. W. (1996). Respiratory system. In: Wheater's Functional Histology, New York: Churchill Livingston, 220–234.
- Carvalho-Oliveira, I., Efthymiadou, A., Malho, R., Nogueira, P., Tzetis, M., Kanavakis, E., Amaral, M. D., and Penque, D. (2004). CFTR localization in native airway cells and cell lines expressing wild-type or F508del-CFTR by a panel of different antibodies. J. Histochem. Cytochem. 52, 193–203. [DOI] [PubMed] [Google Scholar]
- Cheng, S. H., Gregory, R. J., Marshall, J., Paul, S., Souza, D. W., White, G. A., O`Riordan, C., and Smith, A. E. (1990). Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63, 827–834. [DOI] [PubMed] [Google Scholar]
- Claass, A., Sommer, M., de Jonge, H., Kalin, N., and Tummler, B. (2000). Applicability of different antibodies for immunohistochemical localization of CFTR in sweat glands from healthy controls and from patients with cystic fibrosis. J. Histochem. Cytochem. 48, 831–837. [DOI] [PubMed] [Google Scholar]
- Cohn, J. A., Melhus, O., Page, L. J., Dittrich, K. L., and Vigna, S. R. (1991). CFTR: development of high-affinity antibodies and localization in sweat gland. Biochem. Biophys. Res. Commun. 181, 36–43. [DOI] [PubMed] [Google Scholar]
- Cotton, C. U., Stutts, M. J., Knowles, M. R., Gatzy, J. T., and Boucher, R. C. (1987). Abnormal apical cell membrane in cystic fibrosis respiratory epithelium. An in vitro electrophysiologic analysis. J. Clin. Invest. 79, 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, P. B. (1993). Pathophysiology of the lung disease in cystic fibrosis. In: Cystic Fibrosis (Lung Biology in Health and Disease, Vol. 64), ed. P. B. Davis, New York: Marcel Dekker, 193–218.
- Dupuit, F., Kalin, N., Brezillon, S., Hinnrasky, J., Tummler, B., and Puchelle, E. (1995). CFTR and differentiation markers expression in non-CF and delta F 508 homozygous CF nasal epithelium. J. Clin. Invest. 96, 1601–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt, J. F., Yankaskas, J. R., Ernst, S. A., Yang, Y., Marino, C. R., Boucher, R. C., Cohn, J. A., and Wilson, J. M. (1992). Submucosal glands are the predominant site of CFTR expression in human bronchus. Nat. Genet. 2, 240–247. [DOI] [PubMed] [Google Scholar]
- Engelhardt, J. F., Zepeda, M., Cohn, J. A., Yankaskas, J. R., and Wilson, J. M. (1994). Expression of the cystic fibrosis gene in adult human lung. J. Clin. Invest. 93, 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzsch, M., Cui, L., Mengos, A., Chang, X. B., Chen, J. H., and Riordan, J. R. (2003). The PDZ-binding chloride channel ClC-3B localizes to the Golgi and associates with cystic;Fibrosis transmembrane conductance regulator-interacting PDZ proteins. J. Biol. Chem. 278, 6440–6449. [DOI] [PubMed] [Google Scholar]
- Glick, M. C., Kothari, V. A., Liu, A., Stoykova, L. I., and Scanlin, T. F. (2001). Activity of fucosyltransferases and altered glycosylation in cystic fibrosis airway epithelial cells. Biochimie 83, 743–747. [DOI] [PubMed] [Google Scholar]
- Huang, T. et al. (2003). Foxj1 is required for apical localization of ezrin in airway epithelial cells. J. Cell Sci. 116, 4935–4945. [DOI] [PubMed] [Google Scholar]
- Ingraffea, J., Reczek, D., and Bretscher, A. (2002). Distinct cell type-specific expression of scaffolding proteins EBP50 and E3KARP: EBP50 is generally expressed with ezrin in specific epithelia, whereas E3KARP is not. Eur. J. Cell Biol. 81, 61–68. [DOI] [PubMed] [Google Scholar]
- Jacquot, J. et al. (1993). Localization of the cystic fibrosis transmembrane conductance regulator in airway secretory glands. Eur. Respir. J. 6, 169–176. [PubMed] [Google Scholar]
- Jayaraman, S., Joo, N. S., Reitz, B., Wine, J. J., and Verkman, A. S. (2001). Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na+] and pH but elevated viscosity. Proc. Natl. Acad. Sci. USA 98, 8119–8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, T. J., Loo, M. A., Pind, S., Williams, D. B., Goldberg, A. L., and Riordan, J. R. (1995). Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 83, 129–135. [DOI] [PubMed] [Google Scholar]
- Jiang, Q. and Engelhardt, J. F. (1998). Cellular heterogeneity of CFTR expression and function in the lung: implications for gene therapy of cystic fibrosis. Eur. J. Hum. Genet. 6, 12–31. [DOI] [PubMed] [Google Scholar]
- Johnson, L. G., Olsen, J. C., Sarkadi, B., Moore, K. L., Swanstrom, R., and Boucher, R. C. (1992). Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat. Genet. 2, 21–25. [DOI] [PubMed] [Google Scholar]
- Joo, N. S., Irokawa, T., Wu, J. V., Robbins, R. C., Whyte, R. I., and Wine, J. J. (2002). Absent secretion to vasoactive intestinal peptide in cystic fibrosis airway glands. J. Biol. Chem. 277, 50710–50715. [DOI] [PubMed] [Google Scholar]
- Kalin, N., Claabeta, A., Sommer, M., Puchelle, E., and Tummler, B. (1999). DeltaF508 CFTR protein expression in tissues from patients with cystic fibrosis. J. Clin. Invest. 103, 1379–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartner, N., Augustinas, O., Jensen, T. J., Naismith, A. L., and Riordan, J. R. (1992). Mislocalization of DF508 CFTR in cystic fibrosis sweat gland. Nat. Genet. 1, 321–327. [DOI] [PubMed] [Google Scholar]
- Knowles, M., Gatzy, J., and Boucher, R. (1981). Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N. Engl. J. Med. 305, 1489–1495. [DOI] [PubMed] [Google Scholar]
- Kreda, S. M., Gynn, M. C., Fenstermacher, D. A., Boucher, R. C., and Gabriel, S. E. (2001). Expression and localization of epithelial aquaporins in the adult human lung. Am. J. Respir. Cell. Mol. Biol. 24, 224–234. [DOI] [PubMed] [Google Scholar]
- Kreda, S. M., Pickles, R. J., Lazarowski, E. R., and Boucher, R. C. (2000). G-protein-coupled receptors as targets for gene transfer vectors using natural small-molecule ligands. Nat. Biotechnol. 18, 635–640. [DOI] [PubMed] [Google Scholar]
- Kulaksiz, H., Schmid, A., Hoenscheid, M., Ramaswamy, A., and Cetin, Y. (2002). Clara cell impact in air-side activation of CFTR in small pulmonary airways. Proc. Natl. Acad. Sci. USA 99, 6796–6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoukili, J. et al. (2001). IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J. Clin. Invest. 108, 1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lethem, M. I., Dowell, M. L., Van Scott, M., Yankaskas, J. R., Egan, T., Boucher, R. C., and Davis, C. W. (1993). Nucleotide regulation of goblet cells in human airway epithelial explants: normal exocytosis in cystic fibrosis. Am. J. Respir. Cell. Mol. Biol. 9, 315–322. [DOI] [PubMed] [Google Scholar]
- Li, C., Ramjeesingh, M., Reyes, E., Jensen, T., Chang, X., Rommens, J. M., and Bear, C. E. (1993). The cystic fibrosis mutation (DeltaF508) does not influence the chloride channel activity of CFTR. Nat. Genet. 3, 311–316. [DOI] [PubMed] [Google Scholar]
- Mall, M., Kreda, S. M., Mengos, A., Jensen, T. J., Hirtz, S., Seydewitz, H. H., Yankaskas, J., Kunzelmann, K., Riordan, J. R., and Boucher, R. C. (2004). The DeltaF508 mutation results in loss of CFTR. function and mature protein in native human colon. Gastroenterology 126, 32–41. [DOI] [PubMed] [Google Scholar]
- Maresso, A. W., Baldwin, M. R., and Barbieri, J. T. (2004). ERM proteins are high affinity targets for ADP-ribosylation by P. aeruginosa ExoS. J. Biol. Chem. 279, 38402–38408. [DOI] [PubMed] [Google Scholar]
- Mateo, J., Kreda, S., Henry, C. E., Harden, T. K., and Boyer, J. L. (2003). Requirement of Cys399 for processing of the human ecto-ATPase (NTPDase2) and its implications for determination of the activities of splice variants of the enzyme. J. Biol. Chem. 278, 39960–39968. [DOI] [PubMed] [Google Scholar]
- Mohler, P. J., Davis, J. Q., Davis, L. H., Hoffman, J. A., Michaely, P., and Bennett, V. (2004). Inositol 1,4,5-trisphosphate receptor localization and stability in neonatal cardiomyocytes requires interaction with ankyrin-B. J. Biol. Chem. 279, 12980–12987. [DOI] [PubMed] [Google Scholar]
- Mohler, P. J., Kreda, S. M., Boucher, R. C., Sudol, M., Stutts, M. J., and Milgram, S. L. (1999). Yes-associated protein 65 localizes p62(c-Yes) to the apical compartment of airway epithelia by association with EBP50. J. Cell Biol. 147, 879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penque, D., Mendes, F., Beck, S., Farinha, C., Pacheco, P., Nogueira, P., Lavinha, J., Malho, R., and Amaral, M. D. (2000). Cystic fibrosis F508del patients have apically localized CFTR in a reduced number of airway cells. Lab. Invest. 80, 857–868. [DOI] [PubMed] [Google Scholar]
- Perez-Vilar, J., Sheehan, J. K., and Randell, S. H. (2003). Making more MUCS. Am. J. Respir. Cell. Mol. Biol. 28, 267–270. [DOI] [PubMed] [Google Scholar]
- Puchelle, E., Gaillard, D., Ploton, D., Hinnrasky, J., Fuchey, C., Boutterin, M.-C., Jacquot, J., Dreyer, D., Pavirani, A., and Dalemans, W. (1992).. Differential localization of the cystic fibrosis transmembrane conductance regulator in normal and cystic fibrosis airway epithelium. Am. J. Respir. Cell. Mol. Biol. 7, 485–491. [DOI] [PubMed] [Google Scholar]
- Roussel, P. (2001). Airway glycoconjugates and cystic fibrosis. Glycoconj. J. 18, 645–647. [DOI] [PubMed] [Google Scholar]
- Shen, B. Q., Finkbeiner, W. E., Wine, J. J., Mrsny, R. J., and Widdicombe, J. H. (1994). Calu-3, a human airway epithelial cell line that shows cAMP-dependent Cl– secretion. Am. J. Physiol. 266, L493–L501. [DOI] [PubMed] [Google Scholar]
- Short, D. B., Trotter, K. W., Reczek, D., Kreda, S. M., Bretscher, A., Boucher, R. C., Stutts, M. J., and Milgram, S. L. (1998). An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J. Biol. Chem. 273, 19797–19801. [DOI] [PubMed] [Google Scholar]
- Song, Y. and Verkman, A. S. (2001). Aquaporin-5 dependent fluid secretion in airway submucosal glands. J. Biol. Chem. 276, 41288–41292. [DOI] [PubMed] [Google Scholar]
- Trapnell, B. C., Chu, C., Paakko, P. K., Banks, T. C., Yoshimura, K., Ferrans, V. J., Chernick, M. S., and Crystal, R. G. (1991). Expression of the cystic fibrosis transmembrane conductance regulator gene in the respiratory tract of normal individuals with cystic fibrosis. Proc. Natl. Acad. Sci. USA 88, 6565–6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeze, H. J., Sinaasappel, M., Bijman, J., Bouquet, J., and de Jonge, H. R. (1991). Ion transport abnormalities in rectal suction biopsies from children with cystic fibrosis. Gastroenterology 101, 398–403. [DOI] [PubMed] [Google Scholar]
- Verkman, A. S., Song, Y., and Thiagarajah, J. R. (2003). Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am. J. Physiol. Cell Physiol. 284, C2–C15. [DOI] [PubMed] [Google Scholar]