Abstract
Endosomal trafficking is regulated by the recruitment of effector proteins to phosphatidylinositol 3-phosphate [PtdIns(3)P] on early endosomes. At the plasma membrane, phosphatidylinositol-(3,4)-bisphosphate [PtdIns(3,4)P2] binds the pleckstrin homology (PH) domain-containing proteins Akt and TAPP1. Type Iα inositol polyphosphate 4-phosphatase (4-phosphatase) dephosphorylates PtdIns(3,4)P2, forming PtdIns(3)P, but its subcellular localization is unknown. We report here in quiescent cells, the 4-phosphatase colocalized with early and recycling endosomes. On growth factor stimulation, 4-phosphatase endosomal localization persisted, but in addition the 4-phosphatase localized at the plasma membrane. Overexpression of the 4-phosphatase in serum-stimulated cells increased cellular PtdIns(3)P levels and prevented wortmannin-induced endosomal dilatation. Furthermore, mouse embryonic fibroblasts from homozygous Weeble mice, which have a mutation in the type I 4-phosphatase, exhibited dilated early endosomes. 4-Phosphatase translocation to the plasma membrane upon growth factor stimulation inhibited the recruitment of the TAPP1 PH domain. The 4-phosphatase contains C2 domains, which bound PtdIns(3,4)P2, and C2-domain-deletion mutants lost PtdIns(3,4)P2 4-phosphatase activity, did not localize to endosomes or inhibit TAPP1 PH domain membrane recruitment. The 4-phosphatase therefore both generates and terminates phosphoinositide 3-kinase signals at distinct subcellular locations.
INTRODUCTION
Phosphoinositides generated by phosphoinositide 3-kinases (PI 3-kinases) regulate cell survival, proliferation, migration, and vesicular trafficking (Cantley, 2002; Czech, 2003). In agonist-stimulated cells, phosphatidylinositol-3,4,5-trisphosphate [PtdIns(3,4,5)P3] is a transient plasma membrane (PM) signal, rapidly hydrolyzed to phosphatidylinositol-(3,4)-bisphosphate [PtdIns(3,4)P2] by the inositol polyphosphate 5-phosphatases (5-phosphatases) (Stephens et al., 1993; Mitchell et al., 2002). PtdIns(3,4)P2 is a more sustained signal lasting for up to 60 min, stimulated by B-cell activation, oxidative stress, or irreversible platelet aggregation (Banfic et al., 1998; Van der Kaay et al., 1999; Marshall et al., 2002).
PtdIns(3,4,5)P3 effectors include proteins with pleckstrin homology (PH) domains such as the serine/threonine kinases Akt and PDK1; the Tec family of tyrosine kinases; various adapter proteins; and exchange factors for small GTPases (Czech, 2003; Lemmon, 2003). PtdIns(3,4)P2 recruits and activates an overlapping and distinct subset of effector proteins relative to PtdIns(3,4,5)P3. For example, Akt, a key regulator of apoptotic and insulin signaling, contains a PH domain that in vitro binds both PtdIns(3,4,5)P3 and PtdIns(3,4)P2 with comparable affinity (Lemmon, 2003). Both PtdIns(3,4,5)P3 and PtdIns(3,4)P2 are required for the full in vivo activation of Akt (Scheid et al., 2002). The PH domain of the dual adaptor for phosphotyrosine and 3-phosphoinositides (DAPP1) binds both PtdIns(3,4,5)P2 and PtdIns(3,4)P3 (Ferguson et al., 2000), whereas the PX domain of p47phox binds both PtdIns(3,4)P2 and PtdIns(3)P (Kanai et al., 2001). Specific PtdIns(3,4)P2 binding proteins, the tandem PH-domain–containing proteins TAPP1 and TAPP2, have been characterized recently (Dowler et al., 2000).
PtdIns(3,4)P2 is hydrolyzed to form PtdIns(3)P by the inositol polyphosphate 4-phosphatases, but the intracellular site of this reaction remains to be determined. Furthermore, it is unknown whether the PtdIns(3)P generated by the 4-phosphatase contributes to a functional PtdIns(3)P pool at the PM or other intracellular membranes. Two human 4-phosphatase isoforms (type I and II) have been identified, sharing 37% amino acid identity. Both isoforms contain a catalytic “CX5R” motif found in both protein tyrosine phosphatases and dual specificity protein/lipid phosphatases. (Norris et al., 1995, 1997a). The type Iα 4-phosphatase regulates megakaryocyte cell growth, acting downstream of the transcription factor GATA-1. Overexpression of this 4-phosphatase in NIH 3T3 fibroblasts suppresses cellular proliferation, without affecting apoptosis (Vyas et al., 2000). A spontaneous mutant mouse (Weeble), which has a phenotype of neuronal loss in the cerebellum/hippocampus, severe ataxia, and neonatal death, has been identified and is associated with mutation in the type I 4-phosphatase gene (Nystuen et al., 2001).
In this study, we show that the type I 4-phosphatase localizes to endosomal membranes, generating PtdIns(3)P via hydrolysis of PtdIns(3,4)P2, thereby rescuing the phenotype of wortmannin-induced endosomal dilatation. After epidermal growth factor (EGF) stimulation, the 4-phosphatase translocates to the PM and inhibits the PM recruitment of the TAPP1 PH domain. The 4-phosphatase contains an N-terminal tandem C2 domain that is essential for catalytic activity, endosomal localization, and regulation of the recruitment of effectors with PtdIns(3,4)P2-specific PH domains to the PM. The 4-phosphatase hence functions in both generating PtdIns(3)P on endosomal membranes and terminating PtdIns(3,4)P2 signals at the PM.
MATERIALS AND METHODS
General Reagents and Vectors
Restriction and DNA-modifying enzymes were from New England Biolabs (Beverly, MA), Fermentas (Hanover, MD), or Promega (Madison, WI). Big dye terminator cycle sequencing was from PerkinElmer Life and Analytical Sciences (Boston, MA), synthetic peptides were from Chiron Mimotopes (Clayton, Australia), [γ-32P]ATP was from PerkinElmer Life and Analytical Sciences, phospholipids were from BIOMOL Research Laboratories (Plymouth Meeting, PA), oligonucleotides were from Monash University (Clayton, Australia), pTrcHisC vector was from Invitrogen (Carlsbad, CA), pGEX-KG was from American Type Culture Collection (Manassas, MD), and pEGFP-C2 was from BD Biosciences Clontech (Palo Alto, CA). pEFBOS-FLAG vector was from Dr. Willson (Walter and Eliza Hall Institute, Melbourne, Australia). Rab5 and Rab5Q79L cDNAs were kindly provided by Dr. R. Parton (University of Queensland, Brisbane, Australia). The PH domain of TAPP1 (aa 95–404) cDNA was from Dr. S. Dowler (MRC Protein Phosphorylation Unit, University of Dundee, Dundee, United Kingdom). Other reagents were from Sigma-Aldrich (St. Louis, MO).
Antibodies
Two New Zealand White rabbits were immunized with the 4-phosphatase type I peptides (1MTAREHSPRHGARARA16C and 174EEKSDQRPPVTRSVDTVNGR193C) conjugated via the terminal cysteine to diphtheria toxoid. Serum was purified on an immunopeptide-coupled thiopropyl-Sepharose 6B resin. Other antibodies or stains were obtained as indicated: Xpress antibody (Invitrogen); FLAG antibody (Sigma-Aldrich); hemagglutinin (HA) antibody (Silenus, Melbourne, Australia); green fluorescent protein (GFP) antibody, phalloidin, and Alexa Fluor-conjugated secondary antibodies (Molecular Probes, Eugene, OR); glutathione S-transferase (GST) antibody (Promega); β-tubulin, γ-adaptin, and β-COP, Rab11 antibody (Zymed Laboratories, South San Francisco, CA); Rab8 (BD Biosciences, San Jose, CA), LAMP-2 antibody (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA); and human early endosomal antigen (EEA)-1 antibody (gift from Dr. B. Hok-Toh, Monash University).
Purification of Recombinant 4-Phosphatase
Human 4-phosphatase type I sequence was PCR amplified and subcloned into the KpnI site of pTrcHisC (pTrcHisC-4ptase) or the HindIII site of pGEX-KG (pGEX-KG-4ptase), adding N-terminal hexa-His and GST tags in frame. Asp693 was PCR mutated to asparagine and ligated into the KpnI site of pTrcHisC (pTrcHis-D693N). N-terminal GST-tandem C2 domains were PCR amplified and subcloned into the pGEX-KG HindIII site. 4-Phosphatase (aa 305–939) was PCR amplified and subcloned into the KpnI site of pTrcHisC or into the HindIII site of pGEX-KG (pTrcHis-4ptaseΔC2AB and pGEX-KG-4ptaseΔC2AB). Constructs were transformed into BL21 Escherichia coli, incubated to an OD600 = 0.6, and protein was induced with isopropyl β-d-thiogalactopyranoside (1 mM final). Pelleted bacteria were resuspended in 50 mM Tris, pH 8, 1 M NaCl, protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) lysed by 3× sonication 30 s on ice, pelleted 19,000 × g (15 min) and purified by Ni2+ affinity chromatography. Ni-NTA resin (2 ml) (BD Biosciences, Palo Alto, CA), preequilibrated with lysis buffer, was added to the cleared bacterial lysate and rocked at 4°C 1 h, pelleted at 700 × g for 5 min, washed, proteins eluted with 10 ml of 75 mM imidazole, 50 mM Tris, pH 8, 1 M NaCl, and concentrated using a Vivaspin 10,000 molecular weight cut-off (Vivascience, Stonehouse, United Kingdom). Two milliliters of glutathione Sepharose resin, preequilibrated with phosphate-buffered saline (PBS), was added to the cleared bacterial lysate, rocked at 4°C for 1 h, pelleted at 500 × g for 5 min, washed with ice-cold PBS, and eluted with 10 mM glutathione, 50 mM Tris, pH 8.
Mammalian Expression Plasmids
Wild-type 4-phosphatase cDNA was subcloned into pEGFP-C2 (KpnI site) and pEFBOS (MluI site) encoding GFP- and FLAG-tagged 4-phosphatase, respectively. The 4-phosphatase catalytic mutant C856S was PCR generated and cloned into pEGFP-C2 (KpnI site) and pEFBOS (MluI site). Various 4-phosphatase mutants or domains were PCR amplified and subcloned into the KpnI sites of pEGFP-C2, or pCGN and/or the MluI site of pEFBOS. All PCR product sequences were confirmed by Big-Dye Terminator DNA sequencing.
Cell Culture, Transient Transfections, and Microscopy
COS-1 and Chinese hamster ovary (CHO) cells were cultured in DMEM and Ham's-F-12, respectively, with 10% fetal calf serum (FCS) (CSL Biosciences, Parkville, Australia), 100 U/ml penicillin/streptomycin, and 2 mM glutamine. COS-1 cell transfections were performed by electroporation, using the Gene Pulser II System (Bio-Rad, Hercules, CA). For confocal microscopy, transfected cells were grown on coverslips and allowed to recover for 20–40 h. Nontransfected or transfected cells (24 h posttransfection) were serum-starved for 20 h and stimulated with 100 ng/ml EGF or grown in DMEM 10% FCS, or Ham's-F-12, or serum-starved for 1–2 h and incubated with nocodazole (30 mM for 45 min), or 100 nM wortmannin (for 30 min or 60 min), or brefeldin A, and fixed with 3% paraformaldehyde and permeabilized with 0.2% Triton X-100. Primary antibodies were incubated for 45 min at room temperature (RT) in the following dilutions: anti-EEA-1 (1:10,000); anti-FLAG and anti-HA (1:2000); anti-Xpress (1:500); anti-β-tubulin, anti-γ-adaptin, and anti-β-COP (1:50); anti-Rab11, anti-Rab8, and anti-LAMP2 (1:20); and anti-4-phosphatase (1:5). Antibodies were detected using Alexa-conjugated goat anti-mouse or mouse anti-rabbit secondary antibodies (1:800). Coverslips were washed three times with PBS and mounted onto glass slides by using SlowFade. Single plane or stacked images were obtained using a Leica TCS-NT Krypton or Fluroview confocal microscope.
For ultrastructural analysis of 4-phosphatase localization by using electron microscopy, CHO cells were transiently cotransfected with GFP-4-phosphatase and human transferrin receptor (hTfnR), incubated with 25 μg/ml Tfn-horseradish peroxidase (HRP), and Tfn-positive endosomes were labeled with the electron-dense peroxidase 3,3′-diaminobenzidine (DAB) reaction product. Ultrathin cryosections were incubated with GFP antibody followed by 10-nm gold-conjugated anti-mouse IgG secondary antibody.
Preparation of Cell Lysates
Cell lines grown to confluence were washed with PBS, lysed in 50 mM Tris, pH 8.0, 150 mM NaCl, 1% Triton X-100, 2 mM EDTA, 1 mM benzamidine, 2 mM phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, and 2 μg/ml aprotinin for 2 h at 4°C, and pelleted at 15,400 × g (10 min) to isolate the Triton-soluble fraction.
Lipid 4-Phosphatase Assays
PtdIns[32P-3,4]P2 was formed by incubating 56 μg of PtdIns(4)P with 20 μg of phosphatidylserine (PtdSer), 1 μg of recombinant PI 3-kinase (Layton et al., 1998), 50 μM ATP, 20 μl of [γ-32P]ATP (0.2 mCi, 3000 Ci/mM; MP Biomedicals, Irvine, CA), and 5 μl of 20× kinase buffer (400 mM Tris, pH 7.4, 100 mM MgCl2, and 10 mM EGTA) in 100 μl. Lipid products were extracted and suspended in 20 mM Tris, pH 7.4, 1 mM EGTA. Lipid 4-phosphatase assays (100 μl) were performed by adding PtdIns[32P-3,4]P2 to the recombinant wild-type or mutant 4-phosphatase in 50 mM 3-(N-morpholino)propanesulfonic acid, 10 mM EDTA, and 200 mM NaCl at 37°C for 30 min. Extracted lipids were analyzed by thin-layer chromatography (TLC) in chloroform/methanol/acetic acid/water [43:38:5:7 (vol/vol)]. Phosphoinositide products were excised from the TLC and counted using liquid scintillation counting.
Liposome Binding Assays
Phosphatidylethanolamine with PtdSer, PtdIns3P, PtdIns4P, PtdIns(3,4)P2, or PtdIns(4,5)P2 at a 5% molar ratio mixture was dried under N2 and resuspended in 20 mM HEPES, pH 7.2, 100 mM NaCl, and 500 μM EDTA; sonicated (5 min) to form liposomes; incubated with GST-fusion proteins in 50 μl of sample buffer for 10 min at RT; and pelleted at 100,000 × g for 30 min. The pellet was resuspended in 35 μl of SDS-PAGE reducing buffer, analyzed by 10% SDS-PAGE, and silver stained. Densitometry was performed using GelPro software (Media Cybernetics, Silver Spring, MD).
Determination of Phosphoinositide Levels in Cells Overexpressing 4-Phosphatase I
A T-Rex tetracycline-regulated system (Invitrogen) was used to create cell lines stably overexpressing 4-phosphatase-1 as described previously (Kisseleva et al., 2002), by using 293T-Rex cells (Invitrogen). Addition of 100 ng of tetracycline per milliliter to the growth medium induced the expression of the 4-phosphatase. Cells were labeled in 35-mm tissue culture dishes in 1 ml of medium containing 400 μCi of [32P]orthophosphate (PerkinElmer Life and Analytical Sciences) for 2–4 h at 37°C in a phosphate-free DMEM (Invitrogen) containing 5% FCS dialyzed against 0.85% NaCl. Cells were harvested, spun, and lysed by an addition of 1.8 ml of methanol/chloroform/8% HClO4 (20:10:1). Lipids were extracted by the addition of 500 μl of chloroform and 500 μl of 1% HClO4, and the lower organic phase was collected, washed twice with 1% HClO4, and evaporated. Deacylation was as described previously (Caldwell et al., 1991). Glycerophosphorylinositol derivatives were resolved on an anion-exchange column (PartiSphere Sax, 4.6 × 125 mm; Whatman, Maidstone, United Kingdom) by using a 0–1.25 M NaH2PO4, pH 4.5, gradient as described previously (Kisseleva et al., 2002). Radiolabeled deacylated phospholipids were detected by a β-RAM in-flow detection system (IN/US Systems, Tampa, FL). Data were normalized to the total 32P counts in PtdIns(4)P, which were stable in all cell lines regardless of construct expressed, accounting for 10% of [32P]orthophosphate incorporated.
Isolation of Mouse Embryonic Fibroblasts (MEFs)
MEFs were isolated from 12.5-d-old embryos from a heterozygous Weeble mouse mating (The Jackson Laboratory, Bar Harbor, ME). These cells were expanded for two passages before analysis by immunofluorescent confocal microscopy, which was performed as described above. Lysates from MEFs were immunoblotted using a previously described C-terminal 4-phosphatase antibody (Norris et al., 1997b). Genotyping of MEFs was performed as described previously (Nystuen et al., 2001).
RESULTS
4-Phosphatase Localizes to Intracellular Vesicles in Quiescent Cells
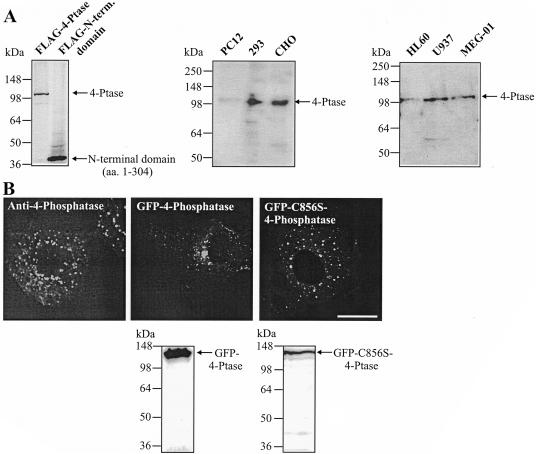
To investigate the intracellular localization of the type I 4-phosphatase, we developed antipeptide antibodies to two 4-phosphatase peptide sequences (aa 1–16 and 174–193), which showed no identity to any sequences in the protein databases. The antibody immunoblotted FLAG-4-phosphatase and the N-terminal domain (aa 1–304) to which the antibody was raised (Figure 1A). Moreover, this peptide affinity-purified antibody detected a 105-kDa polypeptide in human embryonic kidney 293, CHO, HL60 (myeloid), U937 (promonocytic), MEG-01 (megakaryocytic), and faintly in PC12 (neuroendocrine) cells, consistent with the endogenous enzyme (Figure 1A).
Figure 1.
4-Phosphatase intracellular localization. (A) Triton-soluble fraction of cell lysates from the indicated cell lines or COS-1 cells expressing FLAG-tagged wild-type 4-phosphatase, or the N-terminal 304 aa, were immunoblotted with affinity-purified 4-phosphatase antibody. (B) Serum-starved CHO cells were stained with affinity-purified 4-phosphatase antibody (left). COS-1 cells transiently expressing GFP-tagged wild-type (middle) or inactive 4-phosphataseC856S (right) were visualized by confocal microscopy, or lysates were immunoblotted with GFP antibody. Bar, 5 μm.
The intracellular localization of endogenous 4-phosphatase was determined in serum-starved CHO cells by indirect immunofluorescence, by using anti-4-phosphatase antibodies, which labeled distinct punctate vesicles throughout the cytosol, reminiscent of endocytic organelles (Figure 1B, left). Preimmune serum was nonreactive (our unpublished data). GFP-tagged-wild-type or catalytically inactive 4-phosphataseC856S, which contains a C-S mutation in the CX5R catalytic site resulting in complete loss of enzyme activity (Norris et al., 1997a), was expressed in COS-1 cells (Figure 1B, middle and right, respectively). GFP-tagged 4-phosphatase localized in a perinuclear distribution, with punctate vesicular and faint cytosolic staining. A similar distribution was noted when the 4-phosphatase was expressed with a FLAG tag (our unpublished data). Cytosolic staining was more prominent in cells overexpressing the 4-phosphatase at high levels. The catalytically inactive mutant, whether expressed with an N-terminal GFP, or FLAG-tag (our unpublished data), demonstrated vesicular and faint cytosolic staining, with less prominent vesicle clustering noted in the perinuclear region than for the wild-type enzyme (Figure 1B). In control studies, GFP- and FLAG-tagged (our unpublished data) wild-type and catalytically inactive 4-phosphatase polypeptides were expressed without significant proteolysis (Figure 1B). The structural integrity of Golgi cisternae, endosomal compartments, and lysosomes is maintained by tethering of these organelles to microtubules emanating from the microtubule-organizing center (MTOC) (Matteoni and Kreis, 1987). On nocodozole treatment, the MTOC collapsed and microtubules dispersed (evident on costaining with anti-β-tubulin), correlating with cytosolic redistribution of 4-phosphatase–decorated vesicles (our unpublished data). In cells treated with dimethyl sulfoxide vehicle alone, 4-phosphatase perinuclear staining persisted, overlapping with the MTOC (our unpublished data). The 4-phosphatase therefore localizes to microtubule-tethered vesicles and its overexpression may promote vesicle clustering in the perinuclear region.
4-Phosphatase Colocalizes with Early and Recycling Endosomes in Quiescent Cells
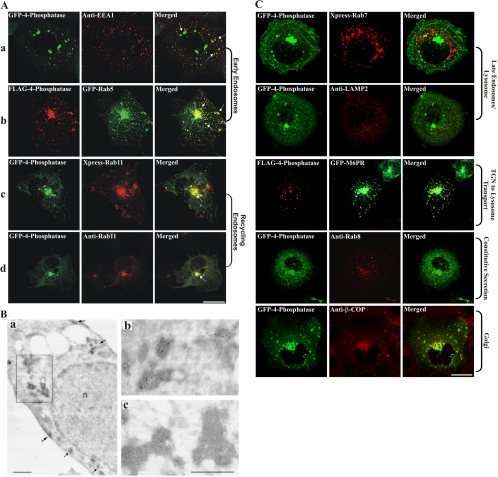
The subcellular localization of the 4-phosphatase was determined by colocalization of GFP- or FLAG-4-phosphatase with specific endocytic or secretory markers (Figure 2, A–C). A significant proportion of 4-phosphatase-positive vesicles colocalized with the EEA-1 (Figure 2A, a, arrows in merged image). FLAG-4-phosphatase also colocalized extensively with GFP-Rab5 (Figure 2A, b, arrows in merged image). Rab11 is a small GTPase that localizes to perinuclear recycling endosomes and the trans-Golgi network (Ren et al., 1998). GFP-4-phosphatase–decorated vesicles colocalized with both recombinant XPRESS-tagged and endogenous Rab11, most intensely in the perinuclear region (Figure 2A, c and d, arrow in merged images).
Figure 2.
4-Phosphatase colocalizes with endosomes. (A) COS-1 cells expressing GFP-4-phosphatase (green) or FLAG-4-phosphatase (row 2, red) were serum-starved overnight and colocalized with EEA-1 (a, red) or Rab11 (d, red) by using specific antibodies, or recombinant Xpress-Rab11 (c, red) or GFP-Rab5 (b, green), and visualized by confocal microscopy. Merged images are shown on the right. Arrows indicate colocalization. Cells were serum-starved overnight before fixation. Bar, 5 μm. (B) CHO cells expressing GFP-4-phosphatase and human TfnR were incubated with 25 μg/ml Tfn-HRP for 30 min at 37°C and subjected to DAB cytochemistry. Ultrathin cryosections were incubated with GFP antibody and gold particle-conjugated anti-mouse IgG secondary antibody. (a) Low-power electron micrograph with DAB-positive/gold-labeled tubulo-vesicular structures (see arrows and boxed region). (b) High-power electron micrograph of boxed region in a. (c) High-power electron micrograph of vesicle clusters by using an anti-tyrosine hydroxylase IgG1 isotype control, showing DAB-positive regions with no immunogold labeling. n, nucleus. Bar, 1 μm. (C) COS-1 cells were either cotransfected with wild-type 4-phosphatase and Xpress-Rab7, or GFP-M6PR and serum-starved overnight. 4-Phosphatase–expressing cells were costained for endogenous lysosome (anti-Lamp-2), Golgi (anti-β-COP), or secretory vesicle (anti-Rab8) markers. All cells were serum-starved overnight before fixation. Bar, 5 μm.
The colocalization of the 4-phosphatase with the hTfnR was performed at an ultrastructural level. The TfnR traverses the entire endocytic pathway from early/sorting to perinuclear recycling endosomes and back to PM (Maxfield and McGraw, 2004). CHO cells were transiently cotransfected with GFP-4-phosphatase and hTfnR, incubated with 25 μg/ml Tfn-HRP and Tfn-positive endosomes labeled with the electron-dense peroxidase DAB reaction product (Stoorvogel et al., 1996). Ultrathin cryosections were incubated with an anti-GFP antibody followed by 10-nm gold-conjugated anti-mouse IgG secondary antibody (Figure 2B). GFP-4-phosphatase–decorated clusters of tubulo-vesicular structures, ∼100 nm in diameter, which were DAB and hence Tfn positive. In addition 4-phosphatase-labeled vesicles were detected as densely staining DAB-positive vesicle clusters in the peri-Golgi region (Figure 2B, a, box, which is magnified in b). The 4-phosphatase–stained vesicles were not surrounded by an outer membrane and were hence distinct from multivesicular bodies (Figure 2B, b). The striking colocalization of the 4-phosphatase with Tfn-positive structures suggests it localizes to early and recycling endosomes. In control studies, gold particles were not detected using isotype IgG1 antibodies to tyrosine hydroxylase (Figure 2B, c).
No colocalization of the 4-phosphatase was demonstrated with Rab7 (late endosome/lysosome), LAMP-2 (lysosome), or mannose 6-phosphate receptor (M6PR) (Golgi to lysosome) (Figure 2C), or with the markers of the constitutive secretory pathway as shown by coexpression with GFP-tagged temperature-sensitive mutant of vesicular stomatitis virus glycoprotein (our unpublished data). Furthermore, the 4-phosphatase did not colocalize with endogenous Rab8, a marker of secretory vesicles (Figure 2C). 4-Phosphatase–expressing cells also were stained with antibodies to the Golgi-specific markers β-COP (Figure 2C, bottom), γ-adaptin and Rab6a (our unpublished data), but no colocalization was observed. Moreover, 4-phosphatase perinuclear localization remained unperturbed after brefeldin A-induced Golgi collapse (our unpublished data). Collectively, these studies demonstrate that in quiescent cells, the 4-phosphatase colocalizes most consistently with markers of early and recycling endosomal compartments.
4-Phosphatase Accumulates on Early and Recycling Endosomes upon Expression of Specific Rab GTPase Mutants
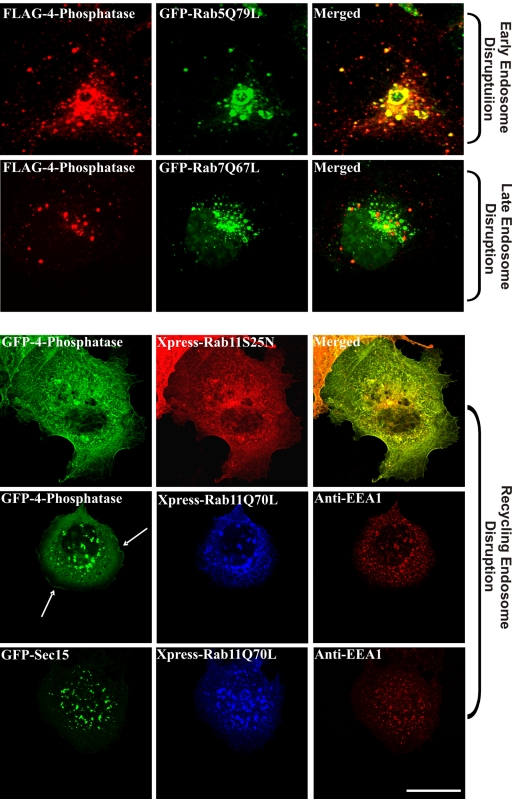
We examined 4-phosphatase localization upon coexpression with specific Rab GTPase mutants. Rab5 regulates clathrin-coated vesicle formation, endosome motility, and early endosome fusion (Smythe, 2002). Overexpression of the GTPase-deficient Rab5 mutant, Rab5Q79L, causes enlargement of early endosomes, resulting from enhanced homo- and heterotypic fusion (Barbieri et al., 1996). Under these conditions, the 4-phosphatase colocalized significantly with the enlarged Rab5Q79L-positive vesicles (Figure 3). Rab7 facilitates the correct aggregation and fusion of late endocytic structures and the biogenesis of lysosomes. Overexpression of the active GFP-Rab7Q67L causes enlargement and increased aggregation of late endocytic vesicles in the perinuclear region (Bucci et al., 2000). No significant colocalization between Rab7-decorated vesicles and the 4-phosphatase was apparent (Figure 3). Overexpression of Rab11Q70L, a dominant-positive GTPase-deficient mutant, increases the rate of Tfn uptake and accumulation of TfnR in early endosomes, as well as enhancing perinuclear recycling and inhibiting endosome to TGN trafficking (Wilcke et al., 2000). The GDP-locked mutant, Rab11S25N, localizes to the cytosol as GTP hydrolysis is required for recruitment of Rab11 and its effectors to endosomal membranes (Wilcke et al., 2000). In cells overexpressing Rab11S25N, the 4-phosphatase showed a more prominent cytosolic distribution with loss of endosomal localization (Figure 3). When coexpressed with Rab11Q70L, the 4-phosphatase colocalized with Rab11Q70L-positive vesicles (Figure 3). Interestingly, under these conditions the 4-phosphatase also localized to the PM in quiescent cells (Figure 3, row 4, arrow), suggesting that this constitutively active Rab11 mutant may promote the recycling of 4-phosphatase-decorated vesicles to the PM. In control studies, no change in the localization of EEA-1 (early endosomes) or Sec15 (recycling endosomes) (Zhang et al., 2004) was observed upon expression of Rab11Q70L (Figure 3, bottom two rows). This demonstrates that the 4-phosphatase may associate with and/or dissociate from early/recycling endosomes upon overexpression of specific Rab GTPase mutants.
Figure 3.
4-Phosphatase localization upon expression of Rab GTPase mutants. COS-1 cells were cotransfected with wild-type 4-phosphatase and Rab5Q79L, Rab7Q67L, dominant-negative Rab11 (Rab11S25N), or Rab11Q70L; serum-starved overnight; and visualized by confocal microscopy. Arrow shows 4-phosphatase PM localization upon coexpression with Rab11Q70L. Control studies in Rab11Q70L-expressing cells demonstrate unchanged EEA1 (early endosome) and Sec15 (recycling endosome) localization. Merged images are shown on the right in top three panels only. Bar, 5 μm.
4-Phosphatase Translocates to the Plasma Membrane upon EGF Stimulation
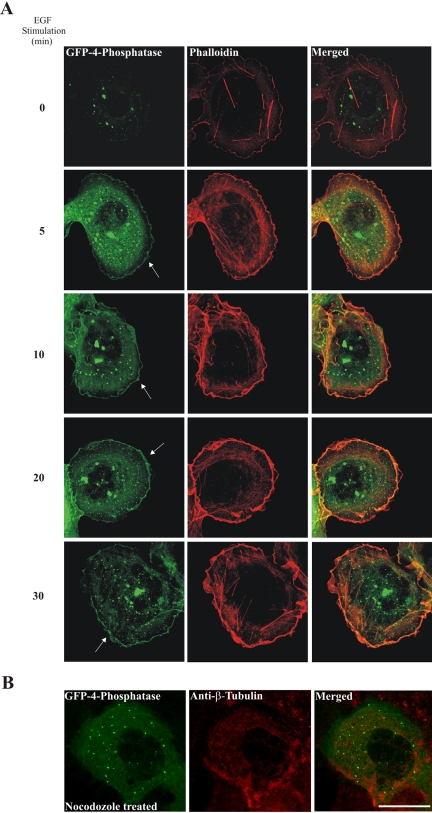
EGF treatment stimulates PtdIns(3,4)P2 synthesis at the PM (Cantley, 2002), and under these conditions, within 5 min, both the endogenous (our unpublished data) and the recombinant 4-phosphatase (Figure 4A, arrows) were detected at the PM persisting up to 30 min in the majority of cells (>300 cells scored from three independent transfections). In addition we noted 4-phosphatase endosomal localization persisted in EGF-stimulated cells. The 4-phosphatase colocalized with areas of intense actin staining at the leading edge of the EGF-stimulated cells (membrane ruffles), as shown by phalloidin staining (Figure 4A). Minimal colocalization of the 4-phosphatase was demonstrated with actin stress fibers, either in quiescent or stimulated cells. Notably, in cells overexpressing the recombinant 4-phosphatase, after EGF stimulation, no decrease in the number of membrane ruffles formed was detected relative to empty vector-expressing cells. Depolymerization of microtubules upon nocodozole treatment resulted in complete abrogation of 4-phosphatase translocation to the PM, indicating that 4-phosphatase PM translocation is microtubule dependent (Figure 4B).
Figure 4.
EGF stimulates 4-phosphatase plasma membrane translocation. COS-1 cells transiently expressing GFP-4-phosphatase (A) were serum-starved overnight, stimulated with 100 ng/ml EGF, fixed, counterstained with phalloidin, and visualized by confocal microscopy. Arrow indicates GFP-4-phosphatase plasma membrane localization. (B) COS-1 cells expressing GFP-4-phosphatase were serum-starved overnight and treated with 30 μM nocodazole for 45 min, washed, and stimulated with 100 ng/ml EGF for 10 min and counterstained with anti-β-tubulin monoclonal antibody. Merged images are shown on the right. Bar, 5 μm.
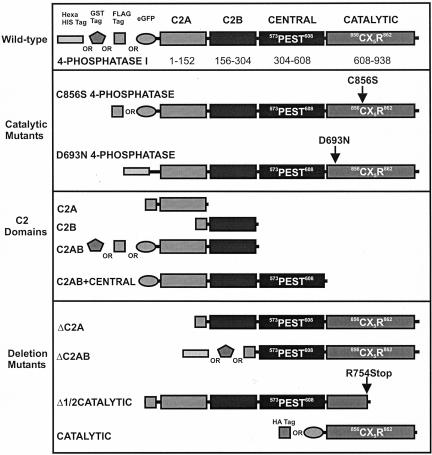
4-Phosphatase Contains an N-Terminal Tandem C2 Domain
The human type I 4-phosphatase sequence (GENPEPT [NP_004018]) was used as a probe in PSI-BLAST searches (Altschul et al., 1997) and showed significant sequence similarity (expect score 3 × 10–27) in the N terminus (residues 31–206) to the synaptotagmin tandem C2 domains (C2A/C2B) (pdb identifier 1DQV) (Bai and Chapman, 2004) and similarity to numerous other C2 domains (expect score 3 × 10–27) (Hurley and Meyer, 2001). The 4-phosphatase central domain (residues 305–608) seems unique, sharing no sequence similarity to other protein families. The C-terminal catalytic domain has been defined previously (Norris et al., 1997b). Based on these data and the structural analysis of the synaptotagmin tandem C2 domains, we propose that the N-terminal region of the human type I 4-phosphatase (residues 1–151) contains a C2 domain (C2A), and we predict that a second, tandem C2 domain (C2B) is located between residues 156–304 (Figure 5). The extent of sequence similarity is low (<15%) within the second putative C2 domain, and accurate assignment of domain boundaries based upon informatics data alone is difficult. A variety of 4-phosphatase constructs were developed to functionally assign individual regions that regulate enzyme activity and/or subcellular localization (Figure 5). Structural studies of PTEN and tyrosine phosphatases reveal that the catalytic CX5R motif forms a tight loop that binds the substrate's phosphate moiety and contains the catalytic cysteine responsible for the nucleophilic attack (Lee et al., 1999; Das et al., 2003). In addition, a conserved aspartic acid (Asp) located on a mobile loop N-terminal to the catalytic cysteine acts as a general acid essential for catalytic activity. We have identified a conserved candidate Asp residue in all 4-phosphatases, including the archeal SopB and SigD, as D693 (Figure 5), (Norris et al., 1998).
Figure 5.
Type I 4-phosphatase domain organization. 4-Phosphatase domains and summary of constructs which are NH2-terminally His6, GST, FLAG, HA, or GFP tagged. Shown also are the residues critical for catalysis, including D693 and C856.
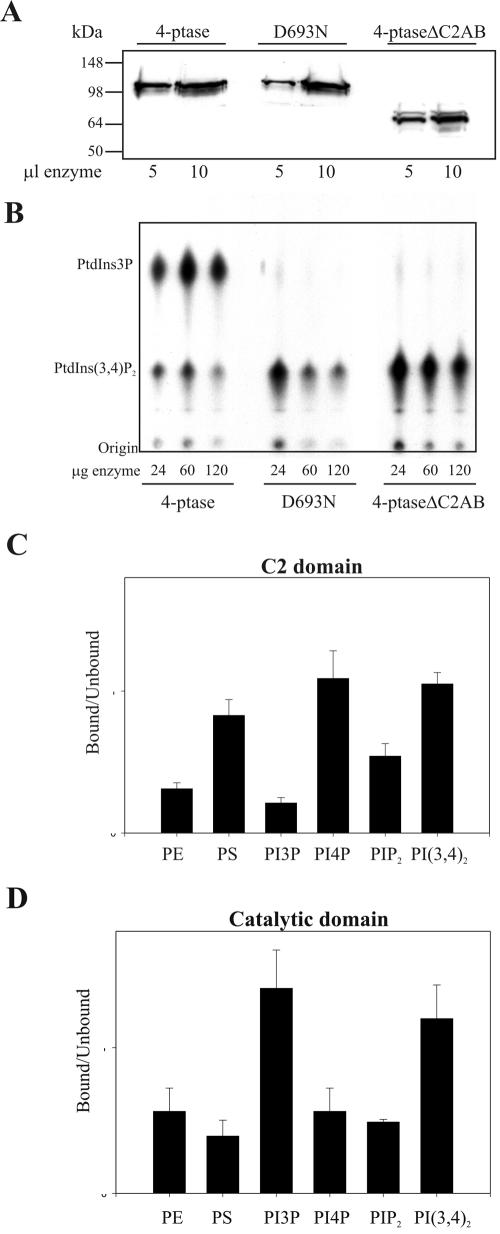
To characterize the role of the C2 domains and/or candidate catalytic Asp (D693) in PtdIns(3,4)P2 hydrolysis, we generated wild-type and mutant 4-phosphatases that lacked the N-terminal tandem C2 domain (ΔC2AB) or had a mutation in D693 (D693N) (Figure 5). His-tagged wild-type 4-phosphatase, D693N-4-phosphatase, and a mutant lacking the C2AB domain (ΔC2AB) were expressed and purified (Figure 6A). Recombinant wild-type 4-phosphatase dephosphorylated PtdIns[32P-3,4]P2 forming PtdIns[32P-3]P. However, mutation of the conserved Asp, D693N, resulted in complete loss of lipid phosphatase activity at all enzyme concentrations tested (Figure 6B). Despite the presence of this essential catalytic Asp and the Cx5R motif, characteristic of tyrosine phosphatases, the 4-phosphatase exhibited no tyrosine phosphatase activity (our unpublished data). Recombinant 4-phosphatase which lacked the tandem C2 domain (4-phosphataseΔC2AB) showed little PtdIns(3,4)P2 4-phosphatase activity, suggesting that this domain may significantly regulate either substrate binding and/or substrate orientation with respect to the catalytic domain (Figure 6B).
Figure 6.
Phosphoinositide binding of 4-phosphatase domains. (A) Purified His6-tagged wild-type, catalytically inactive 4-phosphatase (D493N), or 4-phosphatase lacking the C2AB domain (4-phosphatase ΔC2AB) were immunoblotted with anti-His6 antibodies. (B) Hydrolysis of PtdIns[32P-3,4]P2 by the recombinant wild-type 4-phosphatase (24–120 μg) or the indicated mutants. Extracted lipids were analyzed by TLC and autoradiography and compared with the migration of known standards. (C and D) Liposomes containing phosphatidylethanolamine/PtdSer and PtdIns3P, PtdIns4P, PtdIns(3,4)P2, or PtdIns(4,5)P2 (500 μM) were mixed with GST-C2 domains (C) or GST-catalytic domain (D), and binding studies were performed as described under Materials and Methods. Data are the mean and SE of four assays.
4-Phosphatase C2AB Domain Binds to Phosphoinositides In Vitro
We investigated the potential role of the tandem C2 domain in PtdIns(3,4)P2 binding, given that the 4-phosphatase C2 domain deletion mutants lose catalytic activity. Liposomal binding studies were undertaken to examine the interaction of the C2AB domains versus the catalytic domain with PtdIns(3,4)P2 and the 4-phosphatase reaction product PtdIns(3)P. GST-C2AB bound PtdIns(3,4)P2 and to a lesser extent phosphatidylserine, but failed to bind PtdIns(3)P, although it could bind other monophosphorylated phosphoinositides such as PtdIns(4)P (Figure 6C). A 4-phosphatase mutant that contains the catalytic domain, but not the C2AB domain (ΔC2AB), bound both PtdIns(3,4)P2 and PtdIns (3)P (Figure 6D). In control studies, GST did not bind any phospholipids (our unpublished data). Thus, both the C2AB domain and the catalytic domain facilitate PtdIns(3,4)P2 binding, whereas the catalytic domain also binds the product of 4-phosphatase PtdIns(3,4)P2 hydrolysis, PtdIns(3)P. C2AB domain binding to PtdIns(3,4)P2 may contribute to 4-phosphatase substrate orientation with respect to residue D693 and thus facilitate PtdIns(3,4)P2 D4 phosphate hydrolysis.
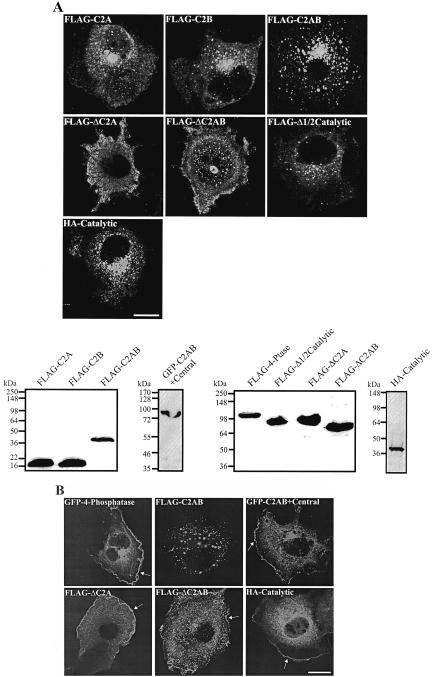
4-Phosphatase C2AB Domain Mediates Endosomal Localization
We investigated the subcellular targeting of the 4-phosphatase by specific domains. The individual C2A or C2B domains localized diffusely in the cytosol (Figures 7A and 5, for constructs). However, the C2AB domain showed distinct vesicular staining with little cytosolic distribution. These vesicles colocalized with Rab11 (our unpublished data). 4-phosphatase mutants lacking the C2A, or the C2AB domain (ΔC2A and ΔC2AB, respectively) showed predominantly cytosolic staining. A 4-phosphatase mutant lacking the C-terminal half of the catalytic domain (Δ1/2 catalytic) localized to endosomal vesicles and the cytosol like the intact wild-type 4-phosphatase but without the prominent perinuclear vesicle clustering. The catalytic domain localized diffusely in the cytosol and the perinuclear region, but endosomal vesicles were not as clearly delineated. Hence, the C2 domains play a significant role in directing the 4-phosphatase to endosomes. Immunoblot analysis showed intact expression of all mutants (Figure 7A).
Figure 7.
4-Phosphatase endosomal targeting by the C2 domain. COS-1 cells expressing the indicated constructs were serum-starved overnight (A) and stimulated with 100 ng/ml EGF for 10 min (B), fixed, and stained with HA or FLAG antibodies, and visualized by confocal microscopy. Arrows in B indicate PM localization. Triton-soluble extracts of transfected cell lysates were immunoblotted with either FLAG, HA, or GFP antibodies (A). Molecular weight markers are shown on the left.
We also investigated which 4-phosphatase domain directs PM localization. After EGF stimulation (10 min), the isolated C2AB domain was not detected at the PM; however, the addition of the central domain to the C2AB domain (C2AB + central) resulted in prominent PM localization, suggesting that this central domain contains critical motifs that facilitate PM targeting (Figure 7B, arrow). Mutant 4-phosphatase lacking either the C2A domain (ΔC2A) or both the N-terminal C2 domains (ΔC2AB) showed PM recruitment (Figure 7B, arrow). The isolated 4-phosphatase catalytic domain also localized to the PM on EGF stimulation (Figure 7B, arrow). Therefore, 4-phosphatase PM relocalization is facilitated by the catalytic and central but not the C2AB domains.
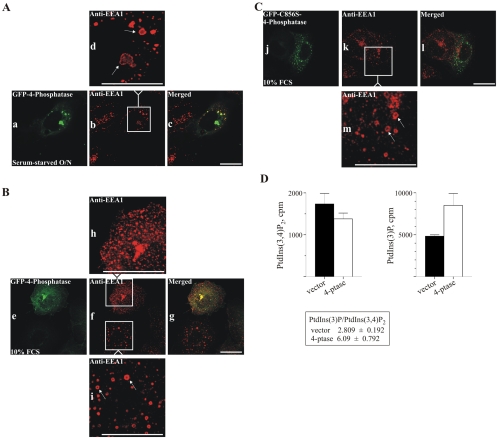
Expression of the Catalytically Active 4-Phosphatase Rescues the Wortmannin-induced Dilatation of Early Endosomes
The studies reported here have revealed a previously unrecognized localization of the 4-phosphatase to endosomes. PtdIns(3)P is synthesized on early endosomes by the wortmannin-sensitive PI3-kinase hVPS34, which is recruited to endosomal membranes by Rab5GTP (Christoforidis et al., 1999). Several studies have proposed that PtdIns(3)P regulates early endosomal fusion, because inhibition of PI3-kinase activity by using the inhibitor wortmannin is associated with early endosomal dilatation and inhibition of in vitro early endosomal fusion (Jones and Clague, 1995; Li et al., 1995; Jones et al., 1998). EEA-1 localizes to early endosomal membranes, by an interaction between its FYVE domain, PtdIns(3)P, and Rab5-GTP (Stenmark et al., 1996). Treatment of cells with 100 nM wortmannin blocks PtdIns(3)P synthesis on early endosomes, leading to EEA-1 dissociation (Patki et al., 1997), and the early endosomes become dilated, looking like large, hollow rings (Simonsen et al., 1998). PtdIns(3,4)P2 accumulates both at the plasma membrane and on intracellular membranes, including tubulo-vesicular structures, the endoplasmic reticulum, and multivesicular bodies upon agonist stimulation (Watt et al., 2004). We reasoned that overexpression of the 4-phosphatase may catalyze the hydrolysis of PtdIns(3,4)P2, forming PtdIns(3)P on endosomal membranes. The effect of 4-phosphatase overexpression on wortmannin-induced endosomal dilatation was therefore examined. To this end, COS-1 cells, serum-starved or grown in serum, the latter being the conditions under which PtdIns(3,4)P2 levels increase (Figure 8, A and B, respectively), transiently expressing the wild-type or inactive 4-phosphataseC856S were treated with 100 nM wortmannin (30 min) and counterstained with EEA-1 antibody. Serum-starved, wortmannin-treated cells developed dilated early endosomes in vector and inactive 4-phosphatase–expressing cells (our unpublished data) and also upon overexpression of the wild-type 4-phosphatase, as shown by the appearance of dilated “EEA-1–stained rings” (Figure 8A, a–c, magnified in d). Although EEA-1 dissociates from endosomal membranes upon wortmannin treatment, we found endosomal EEA-1 staining was detected on dilated endosomes, consistent with reports that wortmannin-induced EEA-1 disassociation is incomplete in some cell lines (Chen and Wang, 2001; Tuma et al., 2001b). Notably, when grown in serum, wortmannin-treated cells transiently overexpressing GFP-4-phosphatase demonstrated normal-sized EEA-1–decorated endosomes (Figure 8B, magnified in h), whereas adjacent nontransfected cells showed endosomal dilatation. (Figure 8B, magnified in i). Significantly, in cells grown in serum, expression of the catalytically inactive 4-phosphataseC856S did not rescue the wortmannin-induced dilatation of endosomes, and EEA-1–decorated endosomes looked dilated, similar to nontransfected cells (Figure 8C, magnified in m). No colocalization of the inactive 4-phosphatase C856S with dilated endosomes was evident. Therefore, under serum-stimulated conditions resulting in increased PtdIns(3,4)P2 synthesis, the 4-phosphatase may generate a PtdIns(3)P pool on early endosomes, via the hydrolysis of its cognate substrate PtdIns(3,4)P2. Furthermore, given that wortmannin inhibits hVPS34-mediated synthesis of PtdIns(3)P on early endosomes, the PtdIns(3,4)P2 pool hydrolyzed by the 4-phosphatase in wortmannin-treated cells may be synthesized by a wortmannin-resistant PI3-kinase, such as the class II C2α PI 3-kinase (Domin et al., 2000).
Figure 8.
4-Phosphatase rescues the wortmannin-induced dilatation of early endosomes. COS-1 cells transiently expressing GFP-wild type (A and B) or catalytically inactive (C856S) 4-phosphatase (C) were treated with 100 nM wortmannin for 30 min after serum starvation overnight (A) or growth in serum (B and C) and were fixed and counterstained with EEA-1 antibody. White box in b is magnified in d; in f, upper box is magnified in h; in f, lower box magnified in i; and box in k is magnified in m. Arrows indicate endosomal enlargement. Bar, 5 μm. (D) Cells stably overexpressing the 4-phosphatase or empty vector were grown in serum and labeled for 2 h with [32P]orthophosphate. Deacylated lipids were analyzed by SAX HPLC. 32P counts (cpm) derived from PtdIns(3,4)P2 or PtdIns(3)P for vector cells showing mean and SE of three experiments (black bars); cells overexpressing type I 4-phosphatase showing mean and SE of four experiments (open bars). PtdIns(3,4)P2 to PtdIns-3P ratios in vector versus type I 4-phosphatase–overexpressing cells are shown in boxed area.
We investigated whether the 4-phosphatase regulates the total cellular levels of PtdIns(3)P by using stable cell lines overexpressing this enzyme, generated using the T-Rex tetracycline-regulated system (Kisseleva et al., 2002). These studies were performed on cells grown in serum, conditions under which we demonstrated overexpression of the 4-phosphatase rescued the wortmannin-induced dilatation of early endosomes. Deacylated 32P-labeled phosphoinositides were analyzed by anion-exchange high-performance liquid chromatography (HPLC). The levels of PtdIns(3,4)P2 and PtdIns(3)P are as shown in Figure 8D. In cells stably transfected with type I 4-phosphatase, PtdIns(3,4)P2 was reduced by 20% compared with the empty vector cells, accompanied by a 1.76-fold increase in PtdIns(3)P. In vector cells, the PtdIns(3)P/PtdIns(3,4)P2 ratio was 2.8 (Figure 8D), whereas in cells overexpressing the 4-phosphatase, this ratio increased to 6. In control studies, the levels of PtdIns(4)P and PtdIns(4,5)P2 were comparable in vector and 4-phosphatase–overexpressing cells (our unpublished data). Therefore, under conditions of serum stimulation, the total intracellular levels of PtdIns(3)P are increased upon 4-phosphatase overexpression.
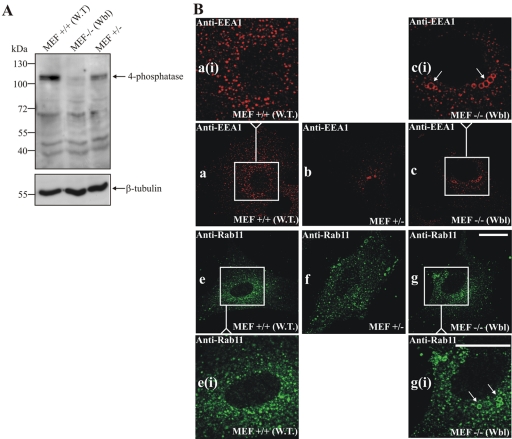
To further reveal whether the 4-phosphatase generates a functional PtdIns (3)P pool, we examined the endosomes of MEFs derived from homozygous Weeble mice (Nystuen et al., 2001). The Weeble mutation was localized by genetic mapping to the 4-phosphatase type I gene. Deletion of a single nucleotide 744G in the 4-phosphatase sequence, results in a frame shift, creating a stop codon at aa 263. As anticipated, 4-phosphatase type I immunoblot analysis of cell lysates from wild-type (WT), heterozygous, and homozygous Weeble MEFs revealed the absence of the 105-kDa 4-phosphatase in Weeble –/– MEF lysates (Figure 9A). Wild-type MEFs grown in serum demonstrated punctate EEA-1 staining of endosomes (Figure 9B, a and ai). In contrast +/– MEFs from hetereozygotes (Figure 9B, b), and more notably homozygous –/– Weeble MEFs showed significant enlargement of endosomes with the appearance of dilated EEA-1–decorated rings (Figure 9B, c and ci, arrows). Furthermore, Rab11-decorated endosomes also demonstrated dilatation in Weeble –/– MEFs (Figure 9B, g and gi, arrows) that was not detected in the wild-type MEFs (Figure 9B, e and ei). These studies strongly indicate that the 4-phosphatase generates a functional PtdIns(3)P pool on endosomes.
Figure 9.
Type I 4-phosphatase –/– MEFs show endosomal dilatation. (A) Detergent-soluble fractions of MEFs were immunoblotted using 4-phosphatase (C-terminal) anti-peptide antibodies or β-tubulin antibodies as a loading control. (B) MEFs, +/+ wild-type (WT), +/– heterozygotes, or homozygous –/– Weeble (Wbl) were stained with EEA-1 or Rab11 antibodies. The EEA-1–stained boxed area in a is shown enlarged in ai, the boxed area in c is shown enlarged in ci. Rab11-stained boxed area in e is shown enlarged in ei; boxed area in g is shown enlarged in gi. Arrows show endosomal dilatation. Bar, 5 μm.
4-Phosphatase Regulates the PM Recruitment of the TAPP1 PH Domain
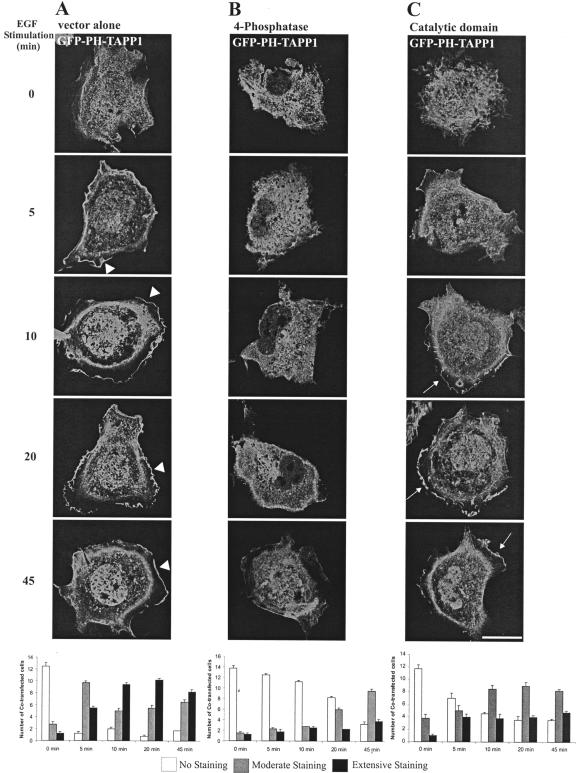
The 4-phosphatase regulation of the recruitment of PtdIns(3,4)P2-specific effectors to the PM has not been reported previously. To address this question, we examined the effect of overexpression of the intact 4-phosphatase versus the isolated catalytic domain on the PM recruitment of the PH-domain of TAPP1 (GFP-PH-TAPP1), upon EGF stimulation (Figure 10). Cotransfected cells from three independent transfections were scored for extensive, moderate or absent PM GFP-PH-TAPP1. In serum-starved cells expressing empty FLAG-vector and GFP-PH-TAPP1, GFP fluorescence was detected diffusely throughout the cytoplasm and the nucleus (Figure 10A). On EGF stimulation, GFP-PH-TAPP1 PM staining was evident within 5 min, persisting up to 45 min (Marshall et al., 2002) (Figure 10A, arrowheads). After 10 min EGF stimulation, the majority of cells showed extensive PM staining. By 45 min, the intensity of PM staining had decreased. Significantly, upon coexpression of GFP-PH-TAPP1 with wild-type FLAG-4-phosphatase, no PM GFP fluorescence was detected after 5- to 10-min EGF stimulation (Figure 10B). In some cells, faint PM fluorescence was evident by 20 and 45 min EGF stimulation, but this was significantly reduced relative to the empty vector-expressing cells. As described here, the 4-phosphatase mutants lacking C2 domains can bind PtdIns(3,4)P2, but they do not possess PtdIns(3,4)P2 4-phosphatase activity (Figure 6). The isolated catalytic domain which lacks the C2 domains (HA-catalytic) was coexpressed with GFP-PH-TAPP1 (Figure 10C). After 10-min EGF stimulation, moderate-to-extensive GFP-PH-TAPP1 fluorescence was detected at the PM in the majority of cells (Figure 10C, arrows). Therefore, the C2AB domain is essential for 4-phosphatase catalytic activity and thereby regulates the PM recruitment of PtdIns(3,4)P2-specific PH domains.
Figure 10.
4-Phosphatase regulates the PM recruitment of a PtdIns(3,4)P2-specific effector. COS-1 cells transiently expressing GFP-TAPP1 PH domain and FLAG empty vector (A) or FLAG-4-phosphatase (B) or HA-4-phosphatase catalytic domain (aa 608–938) (C). Cells were serum-starved overnight and stimulated with 100 ng/ml EGF and visualized by confocal microscopy. Cells were scored for TAPP1 PM localization. Data are expressed as the mean number of cells ± SEM (n ≥ 3) showing no (white bars), moderate (gray bars), or extensive (black bars) GFP-PH-TAPP1 PM staining (20 cells scored for each of three independent transfections).
DISCUSSION
The results of this study have revealed that the type I 4-phosphatase localizes to early and recycling endosomes and generates an endosomal pool of PtdIns(3)P, via hydrolysis of PtdIns(3,4)P2. After EGF-stimulated PI 3-kinase activation, the 4-phosphatase translocates to membrane ruffles, the site of PtdIns(3,4,5)P3 and PtdIns(3,4)P2 synthesis, where the enzyme inhibits the PM recruitment of the PtdIns(3,4)P2-specific TAPP1 PH domain. The 4-phosphatase C2 domains are critical for the enzyme's endosomal targeting, PtdIns(3,4)P2 4-phosphatase catalytic activity, and in agonist-stimulated cells, 4-phosphatase-mediated inhibition of PM recruitment of TAPP1 PH domain. Collectively, these studies demonstrate a functional role for the 4-phosphatase in regulating PI 3-kinase signaling events, both in the endosomal pathway and at the PM.
The spatiotemporal dynamics of membrane trafficking events are governed by reversible recruitment of effector proteins to restricted intracellular membranes decorated with phosphoinositide signals such as PtdIns(3)P and PtdIns(3,4)P2 (Cantley, 2002; Czech, 2003). Molecular mechanisms mediating the concentration of such lipid signaling molecules to specific microdomains of intracellular compartmental membranes are in turn regulated by the subcellular targeting of the specific kinases and/or phosphatases that respectively synthesize or degrade these phosphoinositides. Consistent with the contention that PtdIns(3)P and/or PtdIns(3,4)P2 signals are turned over at high rates in restricted locations, we have shown here that the type I 4-phosphatase localizes to endosomal membranes, colocalizing with early and/or recycling endosomes. Although the 4-phosphatase failed to demonstrate complete colocalization with early endosomal markers such as EEA-1 or the recycling endosomal marker Rab11, extensive colocalization with these markers was shown upon coexpression with Rab5Q79L or Rab11Q70L. Furthermore, the lack of complete colocalization between 4-phosphatase and the endosomal marker EEA-1, is consistent with recent studies showing that PtdIns(3)P exists on endosomal membrane microdomains, associated with specific binding proteins. For example, Hrs, which binds PtdIns(3)P, does not show complete colocalization with EEA-1 (Gillooly et al., 2003). Although cells overexpressing the 4-phosphatase showed some perinuclear staining, little evidence was demonstrated that this enzyme localizes to the Golgi, because the enzyme did not consistently colocalize with Golgi markers.
PtdIns(3)P promotes the association of EEA-1 with early endosomes (Stenmark et al., 1996). The PI 3-kinase inhibitor wortmannin inhibits the synthesis of PtdIns(3)P, resulting in enlarged endosomes. (Simonsen et al., 2001). Because wortmannin inhibits both class I and III PI 3-kinases, which synthesize PtdIns(3)P, PtdIns(3,4)P2, PtdIns(3,4,5)P3, and PtdIns(3,5)P2, dissection of the trafficking pathways regulated by each specific phosphoinositide has not been directly evaluated. We have provided evidence here that 4-phosphatase hydrolysis of PtdIns(3,4)P2 generates a functional pool of PtdIns(3)P. In agonist-stimulated cells, conditions resulting in PtdIns(3,4)P2 synthesis, cells overexpressing the 4-phosphatase showed increased total cellular levels of PtdIns(3)P. Also, serum-stimulated but not serum-starved cells overexpressing the 4-phosphatase were resistant to wortmannin-induced endosomal dilatation. Finally, MEFs from 4-phosphatase–deficient mice exhibited endosomal enlargement, reminiscent of that seen in wortmannin-treated cells.
Recent studies using antibodies to class I and III PI 3-kinases demonstrated that the wortmannin-sensitive PI 3-kinases cannot account for all the endosomal PtdIns(3)P, suggesting that the wortmannin-insensitive class II PI 3-kinases may synthesize a PtdIns(3)P pool that maintains membrane transport (Tuma et al., 2001a). The studies reported here support this hypothesis and furthermore indicate that a functional PtdIns(3)P pool can be generated via 4-phosphatase hydrolysis of PtdIns(3,4)P2. In addition, it is an intriguing possibility that hVPS34 and the 4-phosphatase may generate distinct endosomal PtdIns(3)P pools. The functional analysis of endosomal trafficking in Weeble –/– MEFs may provide further evidence of the specific roles PtdIns(3,4)P2 and the 4-phosphatase-generated PtdIns(3)P play in membrane transport and is the subject of ongoing laboratory investigation. The intracellular localization or function of the type II 4-phosphatase has not been reported, but given we have shown defects in endosomal morphology in Weeble MEFs, these two 4-phosphatases are likely to play nonredundant roles in distinct subcellular locations.
We have shown here that the 4-phosphatase tandem C2 domain binds PtdIns(3,4)P2 and plays a significant role in directing 4-phosphatase to endosomes in quiescent cells. However, we cannot exclude the possibility that the C2AB domain mediates 4-phosphatase endosomal association via protein–protein rather than protein–phosphoinositide interaction, as has been shown for other C2 domains (Hurley and Meyer, 2001; Bai and Chapman, 2004). The C2AB domain also was critical for 4-phosphatase catalytic activity, as shown by in vitro enzyme assays. In addition, the wild-type 4-phosphatase, but not mutants lacking the C2AB domain, inhibited the PtdIns(3,4)P2-dependent translocation of PH domain of TAPP1 to the PM, consistent with a significant functional role for the C2 domains in facilitating either PtdIns(3,4)P2 binding and/or orientation with respect to the catalytic domain. Studies of the lipid phosphatase PTEN, which also uses PtdIns(3,4)P2 as a substrate and contains an N-terminal C2 domain, have revealed that both its phosphatase domain and C2 domain are involved in electrostatic membrane binding (Das et al., 2003). Like PTEN, we have shown that the 4-phosphatase catalytic domain and the C2 domains both facilitate membrane binding, the C2 domain being necessary and sufficient for endosomal membrane association and the catalytic domain mediating the enzyme's association with the PM.
PtdIns(3,4)P2 functions as a signaling molecule in growth factor-stimulated pathways and also is generated independently of PtdIns(3,4,5)P3 in response to oxidative stress (Van der Kaay et al., 1999). The major metabolic pathway for PtdIns(3,4)P2 synthesis in growth factor-stimulated cells is via 5-phosphatase hydrolysis of PtdIns(3,4,5)P3. It is noteworthy that several PtdIns(3,4,5)P3 5-phosphatases localize to membrane ruffles in stimulated cells (Dyson et al., 2001; Gurung et al., 2003). In addition, the TAPP1 PH domain specifically binds PtdIns(3,4)P2 and also localizes to membrane ruffles, suggesting that this is the site of PtdIns(3,4)P2 synthesis (Marshall et al., 2002). We have shown that upon EGF-stimulation, the 4-phosphatase translocates to membrane ruffles, where the enzyme regulates the PM recruitment of the PH domain of TAPP1. The targeting of 4-phosphatase to endosomes in quiescent cells, away from the PM, and its delayed recruitment to membrane ruffles upon agonist stimulation may represent a molecular mechanism mediating delayed and sustained PtdIns(3,4)P2 signaling at the PM.
A widely accepted concept in the field is that the lipid phosphatases act to terminate PI 3-kinase signals. However, with this study we have presented a paradigm shift, showing that the 4-phosphatase also can function in signal generation, forming PtdIns(3)P. In addition, in agonist-stimulated cells, the 4-phosphatase terminates PI 3-kinase signals at the PM via hydrolysis of PtdIns(3,4)P2 and thereby abrogates the recruitment of its effector proteins to this site. Therefore, the activity and the subcellular targeting of the 4-phosphatase are critical to its function in both signal generation and termination at specific subcellular sites.
Acknowledgments
We thank Drs. Jenny Dyson and Rajendra Gurung for help with preparing figures, Dr. Arne Nystuen for providing a Weeble mouse breeding pair and Dr. Dario Alessi for providing the GFP-TAPP1 construct. This work was funded from a grant from the National Health and Medical Research Council of Australia (to C.A.M.) and National Institutes of Health grant HL-55672 (to P.W.M.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–09–0799) on February 16, 2005.
Abbreviations used: EEA, early endosomal antigen; 4-phosphatase, inositol polyphosphate 4-phosphatase; 5-phosphatase, inositol polyphosphate 5-phosphatase; MTOC, microtubule organizing center; PtdIns(3,4)P2, phosphatidylinositol (3,4)-bisphosphate; PtdIns3-P, phosphatidylinositol 3-phosphate; PI 3-kinase, phosphoinositide 3-kinase; PM, plasma membrane; TAPP1, tandem PH domain-containing protein-1.
References
- Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D. J. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, J., and Chapman, E. R. (2004). The C2 domains of synaptotagmin-partners in exocytosis. Trends Biochem. Sci. 29, 143–151. [DOI] [PubMed] [Google Scholar]
- Banfic, H., Tang, X., Batty, I. H., Downes, C. P., Chen, C., and Rittenhouse, S. E. (1998). A novel integrin-activated pathway forms PKB/Akt-stimulatory phosphatidylinositol 3,4-bisphosphate via phosphatidylinositol 3-phosphate in platelets. J. Biol. Chem. 273, 13–16. [DOI] [PubMed] [Google Scholar]
- Barbieri, M. A., Li, G., Mayorga, L. S., and Stahl, P. D. (1996). Characterization of Rab 5, Q79L-stimulated endosome fusion. Arch. Biochem. Biophys. 326, 64–72. [DOI] [PubMed] [Google Scholar]
- Bucci, C., Thomsen, P., Nicoziani, P., McCarthy, J., and van Deurs, B. (2000). Rab 7, a key to lysosome biogenesis. Mol. Biol. Cell 11, 467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell, K. K., Lips, D. L., Bansal, V. S., and Majerus, P. W. (1991). Isolation and characterization of two 3-phosphatases that hydrolyze both phosphatidylinositol 3-phosphate and inositol 1,3-bisphosphate. J. Biol. Chem. 266, 18378–18386. [PubMed] [Google Scholar]
- Cantley, L. C. (2002). The phosphoinositide 3-kinase pathway. Science 296, 1655–1657. [DOI] [PubMed] [Google Scholar]
- Chen, X., and Wang, Z. (2001). Regulation of intracellular trafficking of the EGF receptor by Rab5 in the absence of phosphatidylinositol 3-kinase activity. EMBO Rep. 2, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis, S., Miaczynska, M., Ashman, K., Wilm, M., Zhao, L., Yip, S. C., Waterfield, M. D., Backer, J. M., and Zerial, M. (1999). Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat. Cell Biol. 1, 249–252. [DOI] [PubMed] [Google Scholar]
- Czech, M. P. (2003). Dynamics of phosphoinositides in membrane retrieval and insertion. Annu. Rev. Physiol. 65, 791–815. [DOI] [PubMed] [Google Scholar]
- Das, S., Dixon, J. E., and Cho, W. (2003). Membrane-binding and activation mechanism of PTEN. Proc. Natl. Acad. Sci. USA 100, 7491–7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domin, J., Gaidarov, I., Smith, M. E., Keen, J. H., and Waterfield, M. D. (2000). The class II phosphoinositide 3-kinase PI3K-C2alpha is concentrated in the trans-Golgi network and present in clathrin-coated vesicles. J. Biol. Chem. 275, 11943–11950. [DOI] [PubMed] [Google Scholar]
- Dowler, S., Currie, R. A., Campbell, D. G., Deak, M., Kular, G., Downes, C. P., and Alessi, D. R. (2000). Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem. J. 351, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson, J. M., O`Malley, C. J., Becanovic, J., Munday, A. D., Berndt, M. C., Coghill, I. D., Nandurkar, H. H., Ooms, L. M., and Mitchell, C. A. (2001). The SH2-containing inositol polyphosphate 5-phosphatase, SHIP-2, binds filamin and regulates submembranous actin. J. Cell Biol. 155, 1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, K. M., Kavran, J. M., Sankaran, V. G., Fournier, E., Isakoff, S. J., Skolnik, E. Y., and Lemmon, M. A. (2000). Structural basis for discrimination of 3-phosphoinositides by pleckstrin homology domains. Mol. Cell 6, 373–384. [DOI] [PubMed] [Google Scholar]
- Gillooly, D. J., Raiborg, C., and Stenmark, H. (2003). Phosphatidylinositol 3-phosphate is found in microdomains of early endosomes. Histochem. Cell Biol. 120, 445–453. [DOI] [PubMed] [Google Scholar]
- Gurung, R., Tan, A., Ooms, L. M., McGrath, M. J., Huysmans, R. D., Munday, A. D., Prescott, M., Whisstock, J. C., and Mitchell, C. A. (2003). Identification of a novel domain in two mammalian inositol-polyphosphate 5-phosphatases that mediates membrane ruffle localization. The inositol 5-phosphatase skip localizes to the endoplasmic reticulum and translocates to membrane ruffles following EGF stimulation. J. Biol. Chem. 278, 11376–11385. [DOI] [PubMed] [Google Scholar]
- Hurley, J. H., and Meyer, T. (2001). Subcellular targeting by membrane lipids. Curr. Opin. Cell Biol. 13, 146–152. [DOI] [PubMed] [Google Scholar]
- Jones, A. T., and Clague, M. J. (1995). Phosphatidylinositol 3-kinase activity is required for early endosome fusion. Biochem. J. 311, 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A. T., Mills, I. G., Scheidig, A. J., Alexandrov, K., and Clague, M. J. (1998). Inhibition of endosome fusion by wortmannin persists in the presence of activated Rab5. Mol. Biol. Cell 9, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai, F., Liu, H., Field, S. J., Akbary, H., Matsuo, T., Brown, G. E., Cantley, L. C., and Yaffe, M. B. (2001). The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat. Cell Biol. 3, 675–678. [DOI] [PubMed] [Google Scholar]
- Kisseleva, M. V., Cao, L., and Majerus, P. W. (2002). Phosphoinositide-specific inositol polyphosphate 5-phosphatase IV inhibits Akt/protein kinase B phosphorylation and leads to apoptotic cell death. J. Biol. Chem. 277, 6266–6272. [DOI] [PubMed] [Google Scholar]
- Layton, M. J., Harpur, A. G., Panayotou, G., Bastiaens, P. I., and Waterfield, M. D. (1998). Binding of a diphosphotyrosine-containing peptide that mimics activated platelet-derived growth factor receptor beta induces oligomerization of phosphatidylinositol 3-kinase. J. Biol. Chem. 273, 33379–33385. [DOI] [PubMed] [Google Scholar]
- Lee, J. O., Yang, H., Georgescu, M. M., Di Cristofano, A., Maehama, T., Shi, Y., Dixon, J. E., Pandolfi, P., and Pavletich, N. P. (1999). Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99, 323–334. [DOI] [PubMed] [Google Scholar]
- Lemmon, M. A. (2003). Phosphoinositide recognition domains. Traffic 4, 201–213. [DOI] [PubMed] [Google Scholar]
- Li, G., D'Souza-Schorey, C., Barbieri, M. A., Roberts, R. L., Klippel, A., Williams, L. T., and Stahl, P. D. (1995). Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc. Natl. Acad. Sci. USA 92, 10207–10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, A. J., Krahn, A. K., Ma, K., Duronio, V., and Hou, S. (2002). TAPP1 and TAPP2 are targets of phosphatidylinositol 3-kinase signaling in B cells: sustained plasma membrane recruitment triggered by the B-cell antigen receptor. Mol. Cell. Biol. 22, 5479–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoni, R., and Kreis, T. E. (1987). Translocation and clustering of endosomes and lysosomes depends on microtubules. J. Cell Biol. 105, 1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield, F. R., and McGraw, T. E. (2004). Endocytic recycling. Nat. Rev. Mol. Cell. Biol. 5, 121–132. [DOI] [PubMed] [Google Scholar]
- Mitchell, C. A., Gurung, R., Kong, A. M., Dyson, J. M., Tan, A., and Ooms, L. M. (2002). Inositol polyphosphate 5-phosphatases: lipid phosphatases with flair. IUBMB Life 53, 25–36. [DOI] [PubMed] [Google Scholar]
- Norris, F. A., Atkins, R. C., and Majerus, P. W. (1997a). The cDNA cloning and characterization of inositol polyphosphate 4-phosphatase type II. Evidence for conserved alternative splicing in the 4-phosphatase family. J. Biol. Chem. 272, 23859–23864. [DOI] [PubMed] [Google Scholar]
- Norris, F. A., Atkins, R. C., and Majerus, P. W. (1997b). Inositol polyphosphate 4-phosphatase is inactivated by calpain-mediated proteolysis in stimulated human platelets. J. Biol. Chem. 272, 10987–10989. [DOI] [PubMed] [Google Scholar]
- Norris, F. A., Auethavekiat, V., and Majerus, P. W. (1995). The isolation and characterization of cDNA encoding human and rat brain inositol polyphosphate 4-phosphatase. J. Biol. Chem. 270, 16128–16133. [DOI] [PubMed] [Google Scholar]
- Norris, F. A., Wilson, M. P., Wallis, T. S., Galyov, E. E., and Majerus, P. W. (1998). SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc. Natl. Acad. Sci. USA 95, 14057–14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystuen, A., Legare, M. E., Shultz, L. D., and Frankel, W. N. (2001). A null mutation in inositol polyphosphate 4-phosphatase type I causes selective neuronal loss in weeble mutant mice. Neuron 32, 203–212. [DOI] [PubMed] [Google Scholar]
- Patki, V., Virbasius, J., Lane, W. S., Toh, B. H., Shpetner, H. S., and Corvera, S. (1997). Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA 94, 7326–7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, M., Xu, G., Zeng, J., De Lemos-Chiarandini, C., Adesnik, M., and Sabatini, D. D. (1998). Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc. Natl. Acad. Sci. USA 95, 6187–6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid, M. P., Huber, M., Damen, J. E., Hughes, M., Kang, V., Neilsen, P., Prestwich, G. D., Krystal, G., and Duronio, V. (2002). Phosphatidylinositol (3,4,5)P3 is essential but not sufficient for protein kinase B (PKB) activation; phosphatidylinositol (3,4)P2 is required for PKB phosphorylation at Ser-473, studies using cells from SH2-containing inositol-5-phosphatase knockout mice. J. Biol. Chem. 277, 9027–9035. [DOI] [PubMed] [Google Scholar]
- Simonsen, A., Lippe, R., Christoforidis, S., Gaullier, J. M., Brech, A., Callaghan, J., Toh, B. H., Murphy, C., Zerial, M., and Stenmark, H. (1998). EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394, 494–498. [DOI] [PubMed] [Google Scholar]
- Simonsen, A., Wurmser, A. E., Emr, S. D., and Stenmark, H. (2001). The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol. 13, 485–492. [DOI] [PubMed] [Google Scholar]
- Smythe, E. (2002). Direct interactions between rab GTPases and cargo. Mol. Cell 9, 205–206. [DOI] [PubMed] [Google Scholar]
- Stenmark, H., Aasland, R., Toh, B. H., and D'Arrigo, A. (1996). Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J. Biol. Chem. 271, 24048–24054. [DOI] [PubMed] [Google Scholar]
- Stephens, L. R., Jackson, T. R., and Hawkins, P. T. (1993). Agonist-stimulated synthesis of phosphatidylinositol(3,4,5)-trisphosphate: a new intracellular signalling system? Biochim. Biophys. Acta 1179, 27–75. [DOI] [PubMed] [Google Scholar]
- Stoorvogel, W., Oorschot, V., and Geuze, H. J. (1996). A novel class of clathrin-coated vesicles budding from endosomes. J. Cell Biol. 132, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma, P. L., Nyasae, L. K., Backer, J. M., and Hubbard, A. L. (2001a). Vps34p differentially regulates endocytosis from the apical and basolateral domains in polarized hepatic cells. J. Cell Biol. 154, 1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma, P. L., Nyasae, L. K., Backer, J. M., and Hubbard, A. L. (2001b). Vps34p differentially regulates endocytosis from the apical and basolateral domains in polarized hepatic cells. J. Cell Biol. 154, 1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kaay, J., Beck, M., Gray, A., and Downes, C. P. (1999). Distinct phosphatidylinositol 3-kinase lipid products accumulate upon oxidative and osmotic stress and lead to different cellular responses. J. Biol. Chem. 274, 35963–35968. [DOI] [PubMed] [Google Scholar]
- Vyas, P., Norris, F. A., Joseph, R., Majerns, P. W., and Orkin, S. H. (2000). Inositol polyphosphate 4-phosphatase type I regulates cell growth downstream of transcription factor GATA-1. Proc. Natl. Acad. Sci. USA 97, 13696–13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt, S. A., Kimber, W. A., Fleming, I. N., Leslie, N. R., Downes, C. P., and Lucocq, J. M. (2004). Detection of novel intracellular agonist responsive pools of phosphatidylinositol 3,4-bisphosphate using the TAPP1 pleckstrin homology domain in immunoelectron microscopy. Biochem. J. 377, 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcke, M., Johannes, L., Galli, T., Mayau, V., Goud, B., and Salamero, J. (2000). Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J. Cell Biol. 151, 1207–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. M., Ellis, S., Sriratana, A., Mitchell, C. A., and Rowe, T. (2004). Sec15 is an effector for the Rab11 GTPase in mammalian cells. J. Biol. Chem. 279, 43027–43034. [DOI] [PubMed] [Google Scholar]