Abstract
Intracellular trafficking and spatial dynamics of membrane receptors critically regulate receptor function. Using microscopic and subcellular fractionation analysis, we studied the localization of the murine G protein-coupled receptor G2A (muG2A). Evaluating green fluorescent protein-tagged, exogenously expressed as well as the endogenous muG2A, we observed that this receptor was spontaneously internalized and accumulated in endosomal compartments, whereas its surface expression was enhanced and stabilized by lysophosphatidylcholine (LPC) treatment. Monensin, a general inhibitor of recycling pathways, blocked LPC-regulated surface localization of muG2A as well as muG2A-dependent extracellular signal-regulated kinase (ERK) activation and cell migration induced by LPC treatment. Mutation of the conserved DRY motif (R→ A) enhanced the surface expression of muG2A, resulting in its resistance to monensin inhibition of ERK activation. Our data suggest that intracellular sequestration and surface expression regulated by LPC, rather than direct agonistic activity control the signaling responses of murine G2A toward LPC.
INTRODUCTION
G protein-coupled receptors (GPCRs) are seven-transmembrane proteins that transduce a variety of extracellular stimuli and mediate diverse biological processes such as cell growth, differentiation, apoptosis, and migration (Bockaert and Pin, 1999; Pierce et al., 2002). The general paradigm for GPCR activation involves agonist-induced conformational change of the receptor and coupling to heterotrimeric G protein-mediated signaling pathways (Cabrera-Vera et al., 2003).
Lysophospholipids such as lysophosphatidylcholine (LPC), sphingosylphosphoryl-choline (SPC), lysophosphatitic acid (LPA), and sphingosine 1-phosphate (S1P) regulate a wide array of biological processes (Moolenaar, 1999; Graler and Goetzl, 2002; Ishii et al., 2004). LPC is regularly produced from the cell membranes as a normal metabolic product of phosphatidylcholine (PC), the major phospholipid component in eukaryotic cells. LPC can be generated by hydrolysis of phosphatidylcholine catalyzed by phospholipase A2 (McKean et al., 1981). Alternatively, lecithin:cholesterol acyltransferase can transfer the sn-2 fatty acid of PC to free cholesterol in the plasma, generating cholesterol esters and LPC (Santamarina-Fojo et al., 2000). As a major lipid component of oxidized LDL, LPC displays inflammatory activity and is an important etiological factor in human systemic lupus erythematosus (SLE) (George et al., 1999) and atherosclerosis (Steinbrecher et al., 1990).
Murine G2A (muG2A) is a G protein-coupled receptor that is predominantly expressed in hematopoietic cells such as lymphocytes and myeloid cells and is transcriptionally up-regulated by proliferative stimuli, DNA damage, or stress (Weng et al., 1998). Potential roles of muG2A in peripheral tolerance and autoimmune control were revealed by gene knockout studies, where G2A null mice on a mixed genetic background developed a late onset autoimmune phenotype resembling the human autoimmune disease SLE (Le et al., 2001). Overexpression of muG2A in nonhematopoietic cell types induced various constitutive effects, including cell cycle arrest at G2/M, cytoskeleton rearrangement via activation of RhoA and growth inhibition (Weng et al., 1998; Kabarowski et al., 2000), and oncogenic transformation in some strains of NIH3T3 fibroblasts (Zohn et al., 2000). Overexpression of human G2A (huG2A) in HeLa cells resulted in the accumulation of inositol phosphate and cAMP as well as apoptotic responses (Lin and Ye, 2003).
Phylogenetic analysis shows that G2A belongs to a subfamily of GPCRs, including OGR1 (Xu et al., 2000), TDAG8 (Choi et al., 1996), and GPR4 (Heiber et al., 1995). Several lysophospholipids have been reported to be ligands for this GPCR family, such as SPC as a ligand for OGR1 (Xu et al., 2000) and psychosine as a ligand for TDAG8 (Im et al., 2001). LPC was reported as a direct ligand that binds and activates G2A (Kabarowski et al., 2001). Because we have not been able to repeat the original data provided by our collaborator's laboratory, claiming the direct binding of radioactive LPC to murine G2A, the authors have retracted this paper (Witte et al., 2005). LPC-induced cell migration responses dependent on G2A reported in (Kabarowski et al., 2001) have been repeated in other independent studies (Radu et al., 2004; Yang et al., 2005). This article reinvestigates the relationship of LPC to G2A intracellular localization and signaling.
A functional relationship between LPC and G2A has been documented by several independent studies. LPC was found to enhance cAMP production and to potentiate the apoptotic effects of huG2A in HeLa cells (Lin and Ye, 2003). LPC also antagonized the proton-dependent activity of huG2A at a pH lower than 7.2 (Murakami et al., 2004). Studies on the murine G2A homologue demonstrated that LPC induced muG2A-dependent extracellular signal-regulated kinase (ERK) activation (in Chinese hamster ovary [CHO] cells) and cell migration (in T lymphocytes and macrophages) (Kabarowski et al., 2001; Radu et al., 2004; Yang et al., 2005). These observations did not reveal the mechanism by which LPC and G2A communicate. It remains an open question as to whether LPC acts via directly binding to G2A or indirectly via another unknown pathway.
The intracellular trafficking and localization of GPCRs are regulated by various mechanisms and are critical for receptor signaling (Tan et al., 2004). Receptor mislocalization has been associated with human diseases. For example, aberrant membrane localization of the mutant rhodopsin and the vasopressin V2 receptor causes retinitis pigmentosa and nephrogenic diabetes insipidus, respectively (Tan et al., 2004). For most GPCRs, unbound receptors are localized on the cell surface. Agonist activation leads to receptor desensitization and internalization via arrestin/clathrin-mediated endocytosis (Ferguson, 2001). Internalized receptors are either degraded in lysosomes or resensitized and recycled back to the cell surface (von Zastrow, 2003).
Alternatively, many GPCRs are constitutively internalized in the absence of any ligands. Examples include the cholecystokinin receptor type A (Tarasova et al., 1997) and α1d-adrenergic receptor (McCune et al., 2000) or constitutively active mutant alleles of parathyroid hormone receptor (Ferrari and Bisello, 2001) and angiotensin II AT1A receptor (Miserey-Lenkei et al., 2002). In these cases, the constitutively active conformation of the receptor couples the receptor to arrestin- and clathrin-mediated endocytic pathways (Parnot et al., 2002; Seifert and Wenzel-Seifert, 2002). Inverse agonists can prevent the constitutive internalization by stabilizing the inactive conformation (Milligan, 2003; Prather, 2004). Other GPCRs, such as PAR1 (Shapiro et al., 1996), α1a-adrenergic receptor (Morris et al., 2004), and metabotropic glutamate receptors 1a and 5 (Dale et al., 2001; Fourgeaud et al., 2003) are spontaneously internalized in the absence of any receptor activity. An internal pool of receptors can thus be maintained and recycled to allow continuous signaling when agonists are overly present.
In light of multiple observations regarding LPC-induced responses of G2A (Lin and Ye, 2003; Murakami et al., 2004; Radu et al., 2004; Yang et al., 2005), we reexamined in this study how LPC might regulate the receptor intracellular trafficking. We now provide evidence that the surface expression of muG2A is enhanced and stabilized by LPC, and the surface redistribution of muG2A from the endosomal compartment is critical for LPC-induced signaling responses that lead to ERK activation and cell migration.
MATERIALS AND METHODS
Cell Culture
The wild-type (wt) DO11.10 mouse T-cell hybridoma and the G2AsiRNA DO11.10 clone stably expressing a muG2A-specific small interfering RNA (siRNA) were as described previously (Radu et al., 2004). All DO11.10 derivatives were maintained in RPMI 1640 medium supplemented with 2 mM l-glutamine and 5% charcoal dextran-treated fetal bovine serum (FBS) (Gemini, Irvine, CA). The tetracycline-regulated Swiss 3T3 clones expressing muG2A-green fluorescent protein (GFP) or LPA2R-GFP were maintained in DMEM supplemented with 2 mM l-glutamine, 10% FBS (Hyclone Laboratories, Logan, UT) and 1 μg/ml tetracycline. For the pulse-chase experiment, cell were washed with PBS to get rid of serum-borne LPC, and fresh DMEM containing 2% charcoal dextran treated FBS was added during the experiment.
Reagents
Rhodamine-conjugated transferrin and Lysotracker Red DND-99 were from Molecular Probes (Eugene, OR). The muG2A-specific rabbit polyclonal antibodies were as described previously (Radu et al., 2004). The mouse monoclonal antibodies specific for mouse CD3zeta, Rab11b, and phosphorylated ERK; the rabbit polyclonal antibody for ERK2; and the goat polyclonal antibody for calnexin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse monoclonal antibody p115 was obtained from BD Biosciences (San Jose, CA). The Alexa-594 conjugated goat anti-mouse and donkey anti-goat secondary antibodies were obtained from Molecular Probes. Stromal cell-derived factor-1α (SDF1-α) (PeproTech, Rocky Hill, NJ) was dissolved in PBS to make a 20 μg/ml stock solution and stored at –20°C. Monensin (Calbiochem, San Diego, CA) was dissolved in ethanol to obtain 50 mM stock and stored at –20°C. The 14:0, 16:0, 18:0, and 18:1 forms of LPC (Avanti Polar Lipids, Alabaster, AL) were dissolved in methanol as 50 mM stock. Other lipids used in this study include lysophosphatidylethanolamine (LPE), SPC, LPA, S1P, and platelet-activating factor (PAF) (Avanti Polar Lipids). All lipid stocks were stored under nitrogen at –80°C in glass vials.
ERK Activation Assay
LPC-induced ERK activation was assayed as described previously (Kabarowski et al., 2001). Cells were washed in PBS and resuspended in serum-free medium (SFM) (pH 7.2) containing 0.1% fatty acid-free bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO). An aliquot was taken as time 0 and kept on ice. On addition of 10 μM LPC, cells were incubated at 37°C and 8% CO2 for indicated time before aliquots were taken and lysed in detergent buffer (10 mM Tris-HCl, pH 7.0, 100 mM NaCl, 1% Triton, and 5 mM EDTA) supplemented with protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). The level of phosphorylated-ERK and total ERK was examined by Western blotting.
Transmigration Assay
The transmigration assay was as described with modifications (Radu et al., 2004). Cells were washed with PBS, serum starved for 1 h, and recovered using Ficoll gradient (Ficoll-PaquePLUS; Amersham Biosciences, Piscataway, NJ). Cells (2 × 105) were resuspended in 100 μl of SFM (pH 7.2) containing 0.1% fatty acid-free BSA (Sigma-Aldrich), and added to the upper chamber of a 24-well plate with 5.0-μm pore size polycarbonate filters (Costar, Cambridge, MA). SFM containing chemotactic factors (10 μM LPC or 10 ng/ml SDF1-α) were added to the lower chamber in a 600-μl volume. After 2-h incubation at 37°C and 8% CO2, transmigrated cells were recovered from the lower chamber and counted. Data are presented as mean ± SE from three wells for each treatment. Typically, one of three repeated experiments is shown.
Subcellular Fractionation
Subcellular fractionation using sucrose gradient centrifugation was as described previously with modifications (Kassis and Sullivan, 1986; Liang et al., 2004). Cells (5 × 107) were washed with PBS (pH 7.4) and resuspended in 1 ml of ice-cold homogenizing buffer (50 mM Tris-HCl, pH 7.0, 5 mM EDTA, and 5% sucrose) supplemented with protease inhibitor cocktail (Roche Diagnostics). After 15-min incubation on ice, cells were homogenized with 30× strokes in a 1.5-ml Douncer, followed by 6× passage through a 27-gauage (0.41 × 32-mm) needle. Homogenates were spun at 300 × g for 5 min to get rid of cell debris and nuclei. The postnuclear supernatants were collected and spun at 80,000 rpm for 30 min to obtain the total membrane pellets, which were resuspended in 500 μl of homogenizing buffer. The total membrane fraction was layered on top of a discontinuous sucrose gradient that contains 1 ml of 15%, 1 ml of 30%, 1.5 ml of 35%, and 1 ml of 45% sucrose in buffer (50 mM Tris-HCl, pH 7.0, and 5 mM EDTA). The gradient was centrifuged in a Beckman SW55Ti rotor at 30,000 rpm for 1 h at 4°C. Fractions (400 μl) were collected from top of the gradient and analyzed by SDS-PAGE and Western blot.
Indirect Immunofluorescence, Confocal Fluorescence Microscopy, and Quantitative Analysis
Swiss 3T3 cells grown on polylysine-coated coverslips were fixed with 3% paraformaldehyde in PBS for 10 min at room temperature, washed with PBS, and permeabilized with 0.1% Triton-X 100 in PBS for 10 min. Cells were blocked with 10% serum in PBS for 30 min, incubated with primary antibodies for 1 h, washed with PBS, incubated with secondary antibodies for 1 h, and washed again with PBS. The coverslips were dried and mounted on slides with Slowfade (Molecular Probes).
Specimens were analyzed on a TCS SP2 Acusto-Optical beam splitter laser scanning confocal inverted microscope (Leica Microsystems, Exton, PA) equipped with a 63× oil immersion objective (HCX PL APO, 1.40 numerical aperture) as well as 488-nm (GFP), 534-nm (rhodamine and Lysotracker), and 594-nm (Alexa-594) laser lines. The detection range for each fluorophore was optimally set in separate channels using the Acusto-Optical beam splitter. Images of 50–100 cells from multiple random fields were acquired for each experimental condition. Serial optical sections were recorded at 1-μm intervals, and digital images were saved as 12-bit depth TIFF files. Images were imported into Image J version 1.32 for quantitative analysis. Background was determined as the mean pixel density of the region of interest and subtracted. For each cell, the intracellular region (IR) was defined by the cell contour omitting the plasma membrane region, whereas the total cellular region (CR) was defined as the plasma membrane region plus the intracellular region. The intracellular fluorescence (IF) was calculated as the mean pixel density times the pixel number from the IR. The total cellular fluorescence (TCF) was calculated as the mean pixel density times the pixel number from the CR. The percentage of intracellular receptors was calculated as IF/TCF × 100. The results were presented as mean ± SE.
RESULTS
Intracellular Localization of Murine G2A-GFP in Murine T-Cells
To study the intracellular localization of muG2A in a lymphoid cell type, we overexpressed a muG2A-GFP fusion protein in the T-cell hybridoma line DO11.10 by using a retroviral expression vector. The level of overexpression is ∼20-fold relative to endogenous receptor when analyzed by Western blotting (our unpublished data). It has been previously shown that this level of expression in this cell type does not cause major changes in cell growth or ability of the cells to respond to immune receptor activation (Radu et al., 2004).
LPA and LPC are closely related lysophospholipids. The LPA receptors belong to a highly conserved GPCR family (Graler and Goetzl, 2002). Like most GPCRs, the LPA1 receptor is localized at the cell surface in the absence of LPA and internalized upon ligand activation (Murph et al., 2003). Because DO11.10 cells express the endogenous LPA2, but not the LPA1 receptor (Radu et al., 2004), we examined the localization of the murine LPA2 receptor-GFP fusion protein in these cells.
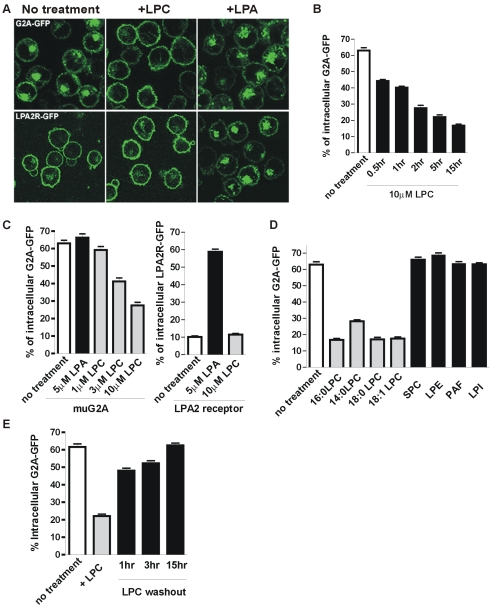
Cells were grown in media (pH 7.2) supplemented with charcoal dextran-stripped FBS. We observed a surface localization of the LPA2 receptor in the absence of exogenous LPA (Figure 1). We found that a significant fraction of muG2A-GFP was localized in intracellular vesicles that also contained internalized transferrin. Treatment with hypertonic sucrose, which inhibits the clathrin-mediated endocytosis (Heuser and Anderson, 1989), enhanced the surface expression of muG2A-GFP, but it had no effect on the LPA2 receptor. This result suggests that in this T-cell line, muG2A is constitutively internalized and the prominent intracellular localization might result from a slower recycling rate.
Figure 1.
Intracellular localization of murine G2A and the LPA2 receptor in DO11.10 T hybridoma cells. DO11.10 cells overexpressing muG2A-GFP or LPA2 receptor-GFP were either untreated or treated with hypertonic sucrose medium (pH 7.2) containing 0.45 M sucrose for 6 h, labeled with rhodamine-labeled transferrin for 40 min, and analyzed using confocal microscopy.
Intracellular Localization of Murine G2A-GFP in Murine Fibroblasts
Cells with extensive cytoplasm and extended morphology are optimal for protein localization studies. Because lymphocytes lack such features, we chose a fibroblast cell line (Swiss3T3) to further characterize the intracellular trafficking of muG2A. Similar to what has been observed in DO11.10 cells, the majority of the fusion protein was localized on intracellular vesicles, but it accumulated at the cell surface upon treatment with hypertonic sucrose (Figure 2A).
Figure 2.
Intracellular localization of murine G2A and the LPA2 receptor in Swiss 3T3 fibroblasts. (A) Swiss3T3 cells overexpressing muG2A-GFP were either untreated or treated with hypertonic sucrose medium (pH 7.2) for 6 h, labeled with rhodamine-labeled transferrin for 40 min, and analyzed using confocal microscopy. Alternatively, cells were transfected with plasmids expressing either wt or K44A mutant dynamin2. After 48 h, cells were labeled with rhodamine-labeled transferrin and analyzed using confocal microscopy. (B) Colocalization of G2A-GFP with various intracellular marker proteins. Cells were stained by rhodamine-labeled transferrin and Lysotracker Red or labeled using antibodies recognizing the endosome marker EEA1, the endoplasmic reticulum marker calnexin, and the Golgi marker p115. Insets show magnified region where G2A-GFP colocalizes with transferrin and EEA1. Bar, 20 μm.
We examined the effect of the dominant negative mutant of dynamin2 (K44A), which inhibits both clathrin-dependent and -independent endocytic pathways (Damke et al., 1994; Conner and Schmid, 2003). When transiently overexpressed in Swiss 3T3 cells for 48 h, the K44A mutant of dynamin2 resulted in surface accumulation of muG2A-GFP, whereas the wt dynamin2 had no effect. The uptake of rhodamine-labeled transferrin was examined to confirm the inhibition of endocytic pathways by mutant dynamin2.
The colocalization of muG2A-GFP with various intracellular markers was examined (Figure 2B). muG2A-GFP did not colocalize with the ER marker protein calnexin, the Golgi marker protein P115, or the late endosome/lysosomes that were stained by Lysotracker. Significant colocalization, however, could be seen with the endosomal compartment that was identified either by rhodamine-labeled transferrin or by early endosomal marker early endosome antigen 1 (EEA1). These data suggest that the intracellular muG2A is localized on endosomal vesicles.
Spatial Dynamics of Murine G2A-GFP in Response to LPC
To monitor the spatial dynamics of newly synthesized muG2A, we established a tetracycline-regulated expression system, in which the expression of the muG2A-GFP fusion protein is suppressed by tetracycline but rapidly turned on upon its removal.
As shown in Figure 3, tetracycline in the medium (pH 7.2) prevented the expression of muG2A-GFP and LPA2 receptor-GFP in Swiss3T3 cells. A “pulse” period was initiated by removal of tetracycline to allow protein expression. The localization of muG2A-GFP or LPA2R-GFP was examined after 5-h incubation in medium supplemented with 2% charcoal-treated serum, which contains low concentration of LPC. Newly synthesized muG2A-GFP and LPA2R-GFP were found to be largely at the cell surface. A “chase” phase was started by the addition of 10 μg/ml cycloheximide, a protein synthesis inhibitor. The majority of muG2A-GFP accumulated in intracellular vesicles at the end of the 5-h chase, whereas LPA2R-GFP was still maintained at the cell surface. This result indicates that muG2A is constitutively internalized from the plasma membrane into an intracellular receptor pool.
Figure 3.
Spatial dynamics of muG2A-GFP in Swiss 3T3 cells. Swiss3T3 cells were engineered to stably express muG2A-GFP or LPA2 receptor-GFP under a tetracycline (Tet)-regulated promoter. Cells were initially grown in presence of 1 μg/ml tetracycline to turn off the gene expression. The pulse phase was started by removal of tetracycline. The regular FBS in the growth medium (pH 7.2) was also replaced with 2% charcoal dextran treated FBS, which contains low level of serum-borne LPC. After 5-h pulse, the chase phase (5 h) was initiated by addition of 10 μg/ml cycloheximide (CHM) to block further protein synthesis. The efficiency of cycloheximide was confirmed when added at the beginning. 10 μM LPC or 5 μM LPA was added to the cells at the end of the chase period, and cells were further incubated for 60 min before being analyzed by confocal microscopy. Representative images are shown. Bar, 20 μm.
Because LPC induces G2A-dependent signaling responses, such as ERK activation and cell migration, we examined the effect of LPC on muG2A localization. Cells were treated with 10 μM LPC or 5 μM LPA at the end of the chase period. As shown in Figure 3, LPC but not LPA treatment resulted in the movement of intracellular muG2A-GFP to the cell surface. LPA but not LPC induced internalization of the LPA2 receptor.
Similar receptor behavior was observed in DO11.10 cells and confirmed by quantitative analysis of confocal images (Figure 4). For each treatment, we analyzed >50 cells from multiple random fields. The percentage of intracellular receptor was shown as mean values with standard deviations. About 65% of receptors were initially localized on intracellular vesicles. Treatment with LPC at pH 7.2 decreased the intracellular receptor level down to ∼20% in a time-dependent and dose-dependent manner (Figure 4, B and C). A comparable concentration of LPA did not have any effect on muG2A but caused ∼60% of the LPA2 receptor to be internalized. Prolonged treatment with LPC rendered the majority (>80%) of the muG2A-GFP to the cell surface without significant change of protein expression level (Figure 4B; our unpublished data). To exclude the possibility that the enhanced surface receptor level arises from new protein synthesis, we treated cells with 10 μg/ml cycloheximide together with 10 μM LPC for 2 h. Cycloheximide had no effect on muG2A localization (our unpublished data). We also have compared the localization of huG2A and muG2A in DO11.10 cells. In the absence of added LPC, the level of endosomal huG2A was lower (∼45%) compared with that of muG2A (∼65%) (Supplemental Figure 1). Similar to muG2A, LPC treatment enhanced the surface expression of huG2A.
Figure 4.
LPC treatment enhances the surface expression of muG2A-GFP. (A) DO11.10 cells overexpressing muG2A-GFP or LPA2R-GFP were treated with either 10 μM LPC for 2 h or 5 μM LPA for 30 min at pH 7.2 before the microscopic analysis. Representative images are shown. For quantitative analysis, multiple fields of images were acquired, and the intracellular receptor level was quantified and presented as mean ± SE from typically 50–100 cells. The time course and dose dependence of LPC-mediated muG2A surface expression (B and C) and the effects of other lysophospholipids (D) are shown. (E) LPC-pretreated cells were washed and incubated in medium (pH 7.2) without added LPC. Aliquots of cells were taken at indicated time and the reinternalized receptors were examined.
Previously a panel of structurally related lysophospholipids were tested in the radioactive LPC binding assay (Kabarowski et al., 2001). Among various forms of LPC, 16:0, 18:0, and 18:1 LPC were claimed to have similar affinity for muG2A, whereas 14:0 LPC failed to compete against 16:0 LPC for receptor binding. SPC also was claimed to have intermediate affinity for the receptor, leading to the conclusion that LPC and SPC are direct ligands for G2A with high and low affinity, respectively. Because those data have not been successfully reproduced by independent studies or our laboratory's recent attempts, we reevaluated the specificity of the interaction between muG2A and these lipids, by using the LPC-mediated receptor localization assay (Figure 4D). We found that addition of 16:0, 18:0, and 18:1 LPC (10 μM) resulted in similar surface redistribution of muG2A, whereas 14:0 LPC showed significant but lower activity. In contrast, addition of 10 μM SPC had no effect. These results are contradictory to the report by Kabarowski et al. (2001) regarding the binding affinity of 14:0 LPC and SPC. However, they are consistent with another independent study, showing that 14:0 LPC was capable of inducing muG2A-dependent cell migration with 50% efficiency compared with 16:0, 18:0, and 18:1 LPC (Yang et al., 2005). In addition, SPC was not able to induce G2A-dependent cell migration (Kabarowski et al., 2001).
Lysophospholipids contain a large polar head group and a single acyl chain. Such structural characteristics facilitate their insertion into lipid membranes, resulting in the alteration of spontaneous curvature of the lipid monolayer, as well as the conformation and function of membrane proteins (Lundbaek and Andersen, 1994). To rule out the possibility that LPC-induced muG2A redistribution might result from its general effect on membrane deformation, we examined other lysophospholipids, including 10 μM LPE, 10 μM PAF, and 10 μM lysophosphatidylinositol. None of these lysophospholipids affected muG2A localization in the absence and presence of added LPC (Figure 4D). Additional lysophospholipids, such as lysoPAF, showed weak activity in activating G2A-dependent migration and ERK activation as well as inducing G2A relocalization to the cell surface (Yang and Witte; our unpublished data). It is under investigation as to whether these lipids act via the same mechanism as LPC.
To further test the hypothesis that muG2A is continuously internalized in the absence of LPC, we examined the reinternalization of muG2A-GFP upon removal of LPC. Cells were treated with 10 μM LPC at pH 7.2 for 6 h before being washed and resuspended in serum-free medium. Significant reinternalization occurred after 1 h of LPC washout (Figure 4E).
The Spatial Redistribution of Endogenous Murine G2A Monitored by Subcellular Fractionation
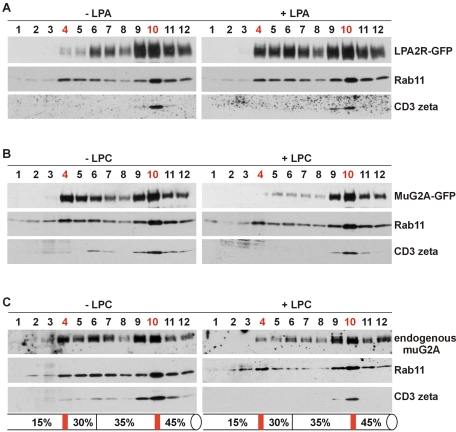
Subcellular fractionation is an alternative approach to study GPCR intracellular localization. On a discontinuous sucrose gradient, surface receptors such as β-adrenergic receptor, are enriched at the interface of 30 and 45% sucrose (30/45% fraction). An agonist, such as isoproterenol, leads to receptor internalization and accumulation at the interface of 15 and 30% sucrose (15/30% fraction), where smaller endosomal vesicles reside (Krueger et al., 1997; Liang et al., 2004).
We used the similar fractionation technique to resolve the intracellular and surface localized muG2A. DO11.10 cells overexpressing muG2A-GFP or LPA2R-GFP were either untreated or treated with LPC or LPA, respectively. Total membrane fractions were then prepared and analyzed. CD3zeta is a subunit of the T-cell receptor TCR/CD3 complex (Exley et al., 1991). Rab11 is a small G protein that is involved in the endosomal recycling pathway and colocalizes with the transferrin receptor on pericentriolar recycling endosomes (Ullrich et al., 1996; Schimmoller et al., 1998). These two proteins were first examined as marker proteins for plasma membrane and endosomal vesicles, respectively (Figure 5A). CD3zeta was predominantly enriched at the 30/45% interface, whereas Rab11b was fractionated to both 15/30 and 30/45% interfaces. This result indicates a partial separation of endosomal vesicles from the plasma membrane vesicles. Namely, the 15/30% fraction was enriched with endosomal vesicles, whereas the 30/45% fraction contained a mixture of both types of membranes.
Figure 5.
Subcellular fractionation confirms LPC-regulated surface redistribution of both overexpressed muG2A-GFP fusion protein and endogenous muG2A. (A) DO11.10 cells overexpressing LPA2R-GFP were either untreated or treated with 5 μM LPA for 30 min at pH 7.2 at 37°C. Cells were fractionated by discontinuous sucrose gradient and fractions were analyzed using antibodies specific for GFP. DO11.10 cells overexpressing muG2A-GFP (B) or the wt cells (C) were either untreated or treated with 10 μM LPC for 2 h at pH 7.2 before the fractionation analysis.
We then examined the localization of LPA2R-GFP on the gradient. In the absence of LPA, LPA2R-GFP was mostly enriched at the 30/45% fraction. Treatment with LPA (5 μM for 30 min) enhanced the amount of LPA2R-GFP at the 15/30% fraction. This result is indicative of receptor internalization from the cell surface to the endosomal compartment. However, due to the cofractionation of endosomal and plasma vesicles at the 30/45% interface, the amount of internalized receptors would be greatly underestimated if quantified using this result. Nevertheless, the enhancement of LPA2R-GFP at the endosomal fraction in response to LPA is consistent with and complementary to the microscopic data.
The localization pattern of overexpressed muG2A-GFP on the gradient, however, was different from that of LPA2R-GFP. A significant portion of muG2A-GFP accumulated at the 15/30% interface in the absence of LPC (Figure 5B). LPC treatment (10 μM for 2 h) reduced the amount of G2A at the lower density fractions and resulted in a predominant peak at the 30/45% interface. A similar result was observed for endogenously expressed muG2A, monitored using an antibody specific for the C-terminal region of the receptor (Radu et al., 2004) (Figure 5C). Thus, the behavior of the moderately overexpressed muG2A-GFP fusion protein reflects that of the endogenous muG2A. Together, these results indicate that LPC triggers the relocalization of G2A from the endosomal compartment to the cell surface.
Blockade of Intracellular Recycling Pathways Alters Murine G2A Trafficking and Signaling
We hypothesized that LPC-regulated surface expression enhancement of murine G2A might be an initial step during receptor responses and that a blockade of this pathway could abolish muG2A-dependent signaling responses to LPC. We tested the effect of monensin, a proton ionophore that disrupts the recycling pathway (Mollenhauer et al., 1990). As shown in Figure 6, A and B, pretreatment with monensin (25 μM for 1 h) effectively blocked LPC-triggered surface relocation of muG2A, as demonstrated by both microscopic and subcellular fractionation analysis.
Figure 6.
Blockade of general recycling pathways via monensin inhibits LPC-mediated muG2A redistribution to the cell surface. DO11.10 cells overexpressing muG2A-GFP were either untreated or pretreated with 50 μM monensin for 1 h at 37°C in serum-free medium (pH 7.2). Viable cells were recovered using Ficoll gradient and treated with 10 μM LPC for 2 h before being analyzed by confocal microscopy (A) and subcellular fractionation (B). Representative images are shown. Bar, 20 μm.
Previous studies on muG2A have established that LPC-induced cell migratory responses are dependent on G2A (Radu et al., 2004). It was shown that the majority of endogenous G2A was knocked down in a DO11.10 cell line expressing a G2A-specific siRNA (DO11.10 G2AsiRNA). These cells failed to migrate toward an LPC gradient. However, when reconstituted with a mutant form of muG2A that is resistant to siRNA due to a silent mutation in the siRNA target region (Radu et al., 2004), LPC could induce cell migration (Figure 7A). Monensin (50 μM for 1 h) blocked LPC-dependent cell migration, but it did not affect chemokine SDF1-α–mediated cell migration. To rule out the potential artifact caused by overexpression and GFP tagging, we analyzed the effect of monensin on wild-type DO11.10 cells that express endogenous muG2A. Consistently, LPC but not SDF1-α–induced cell migration was inhibited by monensin (Figure 7B).
Figure 7.
LPC-induced cell migration via muG2A is blocked by monensin. (A) DO11.10 G2AsiRNA cells, as well as cells reconstituted with an siRNA-resistant form of muG2A fused to RFP, were either untreated or pretreated with 50 μM monensin for 1 h at pH 7.2/37°C. Cells (2 × 105) were added to the upper chamber of a 24-well plate. LPC (10 μM) or 20 ng/ml SDF1-α was added to the lower chamber as chemoattractant. Transmigrated cells were recovered after 2 h and counted. Data are presented as mean ± SE. (B) Wild-type DO11.10 cells were treated and analyzed as in A.
When heterologously overexpressed in CHO cells, muG2A mediated LPC-induced ERK activation (Kabarowski et al., 2001). We used DO11.10 G2AsiRNA cells reconstituted with muG2A fused to a monomeric red fluorescent protein (RFP) (Campbell et al., 2002) to further analyze such a response. Exogenous 10 μM LPC induced ERK activation only in muG2A-RFP-reconstituted cells (Figure 8A). This process was inhibited by monensin treatment (10 μM for 30 min), whereas SDF1-α–dependent ERK activation was unaffected. Similar inhibition was observed in monensin treated DO11.10 wild-type cells (Figure 8B). Together, these data indicate that LPC-regulated surface expression of muG2A is critical for signaling responses that lead to ERK activation and cell migration.
Figure 8.
LPC-induced ERK activation via muG2A is inhibited by monensin. (A) DO11.10 G2AsiRNA cells as well as muG2A-RFP reconstituted cells were either untreated or pretreated with 10 μM monensin for 30 min at pH 7.2/37°C. Cells were spun and resuspended in fresh monensin medium containing either 10 μM LPC or 10 ng/ml SDF1-α. Aliquots of cells were taken at indicated time points and cell lysates were prepared. The level of phosphorylated ERK and total ERK were analyzed using Western blot. (B) Wild-type DO11.10 cells were treated and analyzed as in A.
We noted that the kinetics of LPC-induced ERK activation (15–30 min; Figure 8) seemed to be faster than the relocalization process of G2A (1–2 h; Figure 4). It is conceivable that G2A-residing vesicles might consist of heterogeneous populations even in individual cells. A subpopulation of vesicles might deliver G2A to the cell surface via faster kinetics to mediate early ERK activation. Continuous delivery of intracellular G2A may be achieved by slower recycling over a much longer period. These latter receptors may not be required for ERK activation but instead be involved in other downstream signaling pathways such as cell migration. Such differential kinetics might facilitate the sequential coupling of G2A to multiple signaling pathways.
Mutation at the Arginine Residue of the DRY Motif Alters Receptor Trafficking
Many GPCRs contain a highly conserved E/DRY motif in the cytoplasmic loop at the end of the third transmembrane domain, which plays critical roles in regulating receptor conformation and activities (Savarese and Fraser, 1992; Oliveira et al., 1994). We mutated the arginine residue to alanine (DRY to DAY) and examined the localization of the DAY muG2A-GFP in DO11.10 cells and Swiss 3T3 cells.
A higher surface expression of this mutant in the absence of exogenous LPC and a lower localization to endosomal vesicles were observed, as shown by both microscopic and subcellular fractionation analysis (Figure 9, A and B). Treatment with LPC did not change the percentage of receptor at the cell surface. A small portion of the mutant receptor showed a perinuclear reticular localization that had not been seen in the wt receptor. Although the nature of this subfraction is unclear, it might represent the biosynthetic intermediates of the mutant protein.
Figure 9.
The R-A mutation of the DRY motif (DAY muG2A) resulted in constitutive surface expression as well as monensin-insensitive signaling responses. Localization of DAY muG2A-GFP expressed in DO11.10 cells and Swiss3T3 cells were analyzed by microscopy (A) and by subcellular fractionation analysis (B) as described in text. (C) Similar ERK activation in response to 10 μM LPC at pH 7.2 was observed in DO11.10 G2AsiRNA cells reconstituted with wt or DAY muG2A-RFP. (D) LPC-induced ERK activation is resistant to monensin treatment (10 μM, 30 min at pH 7.2/37°C) in DO11.10 G2AsiRNA cells overexpressing the DAY muG2A mutant.
A similar level of ERK activation was induced by LPC in G2AsiRNA cells reconstituted with either wt or DAY muG2A as RFP fusion proteins (Figure 9C). Unlike wt muG2A, however, the mutant receptor was resistant to monensin effects for blocking ERK activation (Figure 9D). This result suggests that the DAY muG2A mutant bypasses the surface redistribution step regulated by LPC and achieves a more prominent surface localization in the absence of LPC. The monensin resistance of the DAY muG2A mutant supports the hypothesis that LPC-regulated surface expression of muG2A is an important initial step in receptor activation.
DISCUSSION
Mechanisms Regulating the Activity of Murine G2A Receptor
Multiple mechanisms have been reported to regulate the activity and signaling of muG2A. Its expression level varies between different tissues and cell lines and is transcriptionally up-regulated by specific stimuli (i.e., stress or DNA damaging reagents) (Weng et al., 1998). LPC induces G2A-dependent ERK activation and cell migration. The level of LPC is regulated by PLA2 that catalyzes PC hydrolysis during LPC biosynthesis (McKean et al., 1981) and also by lysophospholipase D (autotaxin) that converts LPC into LPA (Xie and Meier, 2004).
Using a combination of microscopic and biochemical analyses, we have demonstrated that muG2A is spontaneously internalized via dynamin-dependent endocytosis, and its surface expression is enhanced by LPC. The possible mechanisms for LPC-triggered muG2A redistribution include slower receptor internalization, accelerated recycling, or regulated exocytosis. In addition, other signaling adaptor molecules such as β-arrestin and spinophilin (Kohout and Lefkowitz, 2003; Wang et al., 2004), or other regulatory mechanisms such as receptor oligomerization (George et al., 2002) might be involved in the spatial regulation of muG2A.
Recent studies demonstrate that members of this GPCR family can be activated by extracellular protons (Ludwig et al., 2003; Murakami et al., 2004). However, we did not observe any effects of pH on muG2A localization in the absence or presence of LPC (our unpublished data). In addition, our recent studies reproduced the pH-dependent activation of OGR1, GPR4, and TDAG8 but showed that muG2A does not behave as a proton-sensing receptor, and huG2A is a much weaker pH sensor (Radu et al., 2005).
Potential Roles of the DRY Motif in Regulating Receptor Localization and Signaling
The conserved DRY motif of many GPCRs has been implicated in regulating receptor conformation and signaling. Mutations of the conserved arginine often results in a loss of receptor activity by decreasing the G protein coupling, as observed for rhodopsin (Acharya and Karnik, 1996) and m1 muscarinic acetylcholine receptor (Zhu et al., 1994). Other effects also have been seen in different receptors. For example, the R→ A mutation only weakly affected the Gi-coupling of the CB2 cannabinoid receptor (Rhee et al., 2000), whereas in the A3 adenosine receptor it resulted in a constitutive activity (Chen et al., 2001). In the histamine H2 receptor, this mutation resulted in a highly unstable receptor with enhanced agonist affinity but decreased G protein coupling (Alewijnse et al., 2000). In the α1b-adrenergic receptor, various substitutions of arginine resulted in constitutive activity, impaired activity, or complete loss of activity, indicating that the DRY motif might help the receptor to assume multiple conformations that lead to different activities (Scheer et al., 2000). In addition, the DRY motif of CXCR4 was dispensable for its Gi signaling (Roland et al., 2003).
The arginine in the DRY motif also has been shown to regulate receptor trafficking. The R→ H mutation in the vasopressin receptor V2 and angiotensin receptor AT1aR, and the R→ E mutation in α1b-adrenergic receptor enhanced the binding affinity of β-arrestin to the receptor. This resulted in a loss-of-function phenotype due to receptor constitutive internalization and localization in endocytic vesicles in the absence of agonists (Wilbanks et al., 2002). Our studies provide another example in which the DRY motif plays a role in regulating receptor trafficking. The DAY mutant of muG2A acquires enhanced surface-expression and is no longer spatially regulated like the wt receptor.
Control of Receptor Response Threshold
Proper localization and turnover of receptors at the cell surface are key determinants for appropriate receptor activities (Edwards et al., 2000). The surface expression of many membrane receptors is regulated. For example, tyrosine kinases have been implicated in stabilizing the surface expression of the T-cell coreceptor CD4 (Pelchen-Matthews et al., 1992) and the erythropoietin receptor (Huang et al., 2001). Smoothened (Smo) is a membrane receptor that is normally sequestered in intracellular compartments, but it is mobilized to the cell surface by Hedgehog (Hh) signaling (Zhu et al., 2003).
Intracellular sequestration and regulated surface expression could be key mechanisms to control receptor activities. A well studied example is the mobilization of intracellular glucose transporter 4 (Glut4) via insulin-triggered exocytosis of Glut4 vesicles (Kanzaki and Pessin, 2003). Such regulation not only ensures efficient responses toward fluctuating glucose levels but also prevents chronic responses that might be either wasteful or pathological.
Similar to glucose, LPC is ubiquitously present in most tissues and in plasma (Croset et al., 2000). The prevalent nature of LPC presents a unique challenge for cells to regulate its signaling. Our studies on murine G2A indicate that LPC-dependent signaling via muG2A could be fine-tuned by receptor spatial control. We hypothesize that physiological levels of LPC might set the sensitivity threshold of muG2A by maintaining a basal level of surface expression. Elevated LPC levels (i.e., under inflammatory conditions) would mobilize the intracellular receptor pool to reach higher surface density for the initiation of signaling pathways.
Currently it is unclear whether LPC acts as a free lipid or needs to be “presented” by an unknown protein carrier for signaling. In either case, the concentration of total LPC in plasma is probably not indicative of how much LPC is capable of signaling because LPC remains mostly bound to serum proteins such as albumin (Croset et al., 2000). Nevertheless, when DO11.10 cells were treated with fresh FBS, intracellular G2A also was relocalized to cell surface (our unpublished data), indicating that active LPC or another regulator is present in serum. The physiological implication based on this observation would be that when G2A expressing cells (i.e., lymphocytes and monocytes) circulate in the blood, they are exposed to active LPC, which could set the balance of intracellular versus cell surface G2A and thus the signaling threshold of G2A before cells enter a local tissue site.
One also might speculate that by stabilizing muG2A at the cell surface, the LPC concentration gradient could help to establish a polarized distribution of muG2A, similar to that of chemokine receptors (Nieto et al., 1997), which may in turn regulate cell migration or other downstream responses.
In conclusion, the study presented here defines a unique spatial regulation of muG2A mediated by LPC. Regardless of whether LPC exerts its effects directly through binding to G2A or indirectly via other unknown pathway, further understanding of intracellular receptor trafficking should help to elucidate the complex role of protons and LPC in G2A signaling.
Acknowledgments
We thank Matthew Au for creating the tetracycline-regulated muG2A-expressing Swiss3T3 cell line. We also thank Shirley Quan and Donghui Cheng for technical assistance; Barbara Anderson for preparation of the manuscript; and Dr. Bill Wickner, Amar Nijagal, Shuling Guo, and Chengyi Shu for critically reviewing the manuscript. Fluorescent microscopy was performed at the CNSI Advanced Light Microscopy/Spectroscopy Shared Facility at UCLA, directed by Dr. Shimon Weiss, Department of Chemistry and Biochemistry. We gratefully acknowledge support from the Plum Foundation. L. W. is a Fellow of the Leukemia and Lymphoma Society. C.G.R is a Fellow of the Cancer Research Institute. O.N.W. is an Investigator of the Howard Hughes Medical Institute.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–12–1044) on February 23, 2005.
Abbreviations used: LPA, lysophosphatitic acid; LPC, lysophosphatidylcholine; S1P, sphingosine 1-phosphate; SPC, sphingosylphosphoryl-choline.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Acharya, S., and Karnik, S. S. (1996). Modulation of GDP release from transducin by the conserved Glu134-Arg135 sequence in rhodopsin. J. Biol. Chem. 271, 25406–25411. [DOI] [PubMed] [Google Scholar]
- Alewijnse, A. E., Timmerman, H., Jacobs, E. H., Smit, M. J., Roovers, E., Cotecchia, S., and Leurs, R. (2000). The effect of mutations in the DRY motif on the constitutive activity and structural instability of the histamine H(2) receptor. Mol. Pharmacol. 57, 890–898. [PubMed] [Google Scholar]
- Bockaert, J., and Pin, J. P. (1999). Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 18, 1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Vera, T. M., Vanhauwe, J., Thomas, T. O., Medkova, M., Preininger, A., Mazzoni, M. R., and Hamm, H. E. (2003). Insights into G protein structure, function, and regulation. Endocr. Rev. 24, 765–781. [DOI] [PubMed] [Google Scholar]
- Campbell, R. E., Tour, O., Palmer, A. E., Steinbach, P. A., Baird, G. S., Zacharias, D. A., and Tsien, R. Y. (2002). A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 7877–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, A., Gao, Z. G., Barak, D., Liang, B. T., and Jacobson, K. A. (2001). Constitutive activation of A(3) adenosine receptors by site-directed mutagenesis. Biochem. Biophys. Res. Commun. 284, 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. W., Lee, S. Y., and Choi, Y. (1996). Identification of a putative G protein-coupled receptor induced during activation-induced apoptosis of T cells. Cell Immunol. 168, 78–84. [DOI] [PubMed] [Google Scholar]
- Conner, S. D., and Schmid, S. L. (2003). Regulated portals of entry into the cell. Nature 422, 37–44. [DOI] [PubMed] [Google Scholar]
- Croset, M., Brossard, N., Polette, A., and Lagarde, M. (2000). Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem. J. 345, 61–67. [PMC free article] [PubMed] [Google Scholar]
- Dale, L. B., Bhattacharya, M., Seachrist, J. L., Anborgh, P. H., and Ferguson, S. S. (2001). Agonist-stimulated and tonic internalization of metabotropic glutamate receptor 1a in human embryonic kidney 293 cells: agonist-stimulated endocytosis is β-arrestin1 isoform-specific. Mol. Pharmacol. 60, 1243–1253. [DOI] [PubMed] [Google Scholar]
- Damke, H., Baba, T., Warnock, D. E., and Schmid, S. L. (1994). Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127, 915–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, S. W., Tan, C. M., and Limbird, L. E. (2000). Localization of G-protein-coupled receptors in health and disease. Trends Pharmacol. Sci. 21, 304–308. [DOI] [PubMed] [Google Scholar]
- Exley, M., Terhorst, C., and Wileman, T. (1991). Structure, assembly and intracellular transport of the T cell receptor for antigen. Semin. Immunol. 3, 283–297. [PubMed] [Google Scholar]
- Ferguson, S. S. (2001). Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 53, 1–24. [PubMed] [Google Scholar]
- Ferrari, S. L., and Bisello, A. (2001). Cellular distribution of constitutively active mutant parathyroid hormone (PTH)/PTH-related protein receptors and regulation of cyclic adenosine 3′,5′-monophosphate signaling by β-arrestin2. Mol. Endocrinol. 15, 149–163. [DOI] [PubMed] [Google Scholar]
- Fourgeaud, L., Bessis, A. S., Rossignol, F., Pin, J. P., Olivo-Marin, J. C., and Hemar, A. (2003). The metabotropic glutamate receptor mGluR5 is endocytosed by a clathrin-independent pathway. J. Biol. Chem. 278, 12222–12230. [DOI] [PubMed] [Google Scholar]
- George, J., Harats, D., Gilburd, B., Levy, Y., Langevitz, P., and Shoenfeld, Y. (1999). Atherosclerosis-related markers in systemic lupus erythematosus patients: the role of humoral immunity in enhanced atherogenesis. Lupus 8, 220–226. [DOI] [PubMed] [Google Scholar]
- George, S. R., O'Dowd, B. F., and Lee, S. P. (2002). G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat. Rev. Drug. Discov. 1, 808–820. [DOI] [PubMed] [Google Scholar]
- Graler, M. H., and Goetzl, E. J. (2002). Lysophospholipids and their G protein-coupled receptors in inflammation and immunity. Biochim. Biophys. Acta 1582, 168–174. [DOI] [PubMed] [Google Scholar]
- Heiber, M., et al. (1995). Isolation of three novel human genes encoding G protein-coupled receptors. DNA Cell Biol. 14, 25–35. [DOI] [PubMed] [Google Scholar]
- Heuser, J. E., and Anderson, R. G. (1989). Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, L. J., Constantinescu, S. N., and Lodish, H. F. (2001). The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol. Cell 8, 1327–1338. [DOI] [PubMed] [Google Scholar]
- Im, D. S., Heise, C. E., Nguyen, T., O'Dowd, B. F., and Lynch, K. R. (2001). Identification of a molecular target of psychosine and its role in globoid cell formation. J. Cell Biol. 153, 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii, I., Fukushima, N., Ye, X., and Chun, J. (2004). Lysophospholipid receptors: signaling and biology. Annu. Rev. Biochem. 73, 321–354. [DOI] [PubMed] [Google Scholar]
- Kabarowski, J. H., Feramisco, J. D., Le, L. Q., Gu, J. L., Luoh, S. W., Simon, M. I., and Witte, O. N. (2000). Direct genetic demonstration of Gα13 coupling to the orphan G protein-coupled receptor G2A leading to RhoA-dependent actin rearrangement. Proc. Natl. Acad. Sci. USA 97, 12109–12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabarowski, J. H., Zhu, K., Le, L. Q., Witte, O. N., and Xu, Y. (2001). Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science 293, 702–705. [DOI] [PubMed] [Google Scholar]
- Kanzaki, M., and Pessin, J. E. (2003). Insulin signaling: GLUT4 vesicles exit via the exocyst. Curr. Biol. 13, R574–R576. [DOI] [PubMed] [Google Scholar]
- Kassis, S., and Sullivan, M. (1986). Desensitization of the mammalian β-adrenergic receptor: analysis of receptor redistribution on nonlinear sucrose gradients. J. Cyclic Nucleotide Protein Phosphor. Res. 11, 35–46. [PubMed] [Google Scholar]
- Kohout, T. A., and Lefkowitz, R. J. (2003). Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol. Pharmacol. 63, 9–18. [DOI] [PubMed] [Google Scholar]
- Krueger, K. M., Daaka, Y., Pitcher, J. A., and Lefkowitz, R. J. (1997). The role of sequestration in G protein-coupled receptor resensitization. Regulation of β2-adrenergic receptor dephosphorylation by vesicular acidification. J. Biol. Chem. 272, 5–8. [DOI] [PubMed] [Google Scholar]
- Le, L. Q., Kabarowski, J. H., Weng, Z., Satterthwaite, A. B., Harvill, E. T., Jensen, E. R., Miller, J. F., and Witte, O. N. (2001). Mice lacking the orphan G protein-coupled receptor G2A develop a late-onset autoimmune syndrome. Immunity 14, 561–571. [DOI] [PubMed] [Google Scholar]
- Liang, W., Curran, P. K., Hoang, Q., Moreland, R. T., and Fishman, P. H. (2004). Differences in endosomal targeting of human (β)1- and (β)2-adrenergic receptors following clathrin-mediated endocytosis. J. Cell Sci. 117, 723–734. [DOI] [PubMed] [Google Scholar]
- Lin, P., and Ye, R. D. (2003). The lysophospholipid receptor G2A activates a specific combination of G proteins and promotes apoptosis. J. Biol. Chem. 278, 14379–14386. [DOI] [PubMed] [Google Scholar]
- Ludwig, M. G., Vanek, M., Guerini, D., Gasser, J. A., Jones, C. E., Junker, U., Hofstetter, H., Wolf, R. M., and Seuwen, K. (2003). Proton-sensing G-protein-coupled receptors. Nature 425, 93–98. [DOI] [PubMed] [Google Scholar]
- Lundbaek, J. A., and Andersen, O. S. (1994). Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J. Gen. Physiol. 104, 645–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune, D. F., Edelmann, S. E., Olges, J. R., Post, G. R., Waldrop, B. A., Waugh, D. J., Perez, D. M., and Piascik, M. T. (2000). Regulation of the cellular localization and signaling properties of the α(1B)- and α(1D)-adrenoceptors by agonists and inverse agonists. Mol. Pharmacol. 57, 659–666. [DOI] [PubMed] [Google Scholar]
- McKean, M. L., Smith, J. B., and Silver, M. J. (1981). Formation of lysophosphatidylcholine by human platelets in response to thrombin. Support for the phospholipase A2 pathway for the liberation of arachidonic acid. J. Biol. Chem. 256, 1522–1524. [PubMed] [Google Scholar]
- Milligan, G. (2003). Constitutive activity and inverse agonists of G protein-coupled receptors: a current perspective. Mol. Pharmacol. 64, 1271–1276. [DOI] [PubMed] [Google Scholar]
- Miserey-Lenkei, S., Parnot, C., Bardin, S., Corvol, P., and Clauser, E. (2002). Constitutive internalization of constitutively active angiotensin II AT(1A) receptor mutants is blocked by inverse agonists. J. Biol. Chem. 277, 5891–5901. [DOI] [PubMed] [Google Scholar]
- Mollenhauer, H. H., Morre, D. J., and Rowe, L. D. (1990). Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim. Biophys. Acta 1031, 225–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar, W. H. (1999). Bioactive lysophospholipids and their G protein-coupled receptors. Exp. Cell Res. 253, 230–238. [DOI] [PubMed] [Google Scholar]
- Morris, D. P., Price, R. R., Smith, M. P., Lei, B., and Schwinn, D. A. (2004). Cellular trafficking of human α1a-adrenergic receptors is continuous and primarily agonist-independent. Mol. Pharmacol. 66, 843–854. [DOI] [PubMed] [Google Scholar]
- Murakami, N., Yokomizo, T., Okuno, T., and Shimizu, T. (2004). G2A is a proton-sensing G-protein coupled receptor antagonized by lysophosphatidylcholine. J. Biol. Chem. [DOI] [PubMed]
- Murph, M. M., Scaccia, L. A., Volpicelli, L. A., and Radhakrishna, H. (2003). Agonist-induced endocytosis of lysophosphatidic acid-coupled LPA1/EDG-2 receptors via a dynamin2- and Rab5-dependent pathway. J. Cell Sci. 116, 1969–1980. [DOI] [PubMed] [Google Scholar]
- Nieto, M., Frade, J. M., Sancho, D., Mellado, M., Martinez, A. C., and Sanchez-Madrid, F. (1997). Polarization of chemokine receptors to the leading edge during lymphocyte chemotaxis. J. Exp. Med. 186, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, L., Paiva, A. C., Sander, C., and Vriend, G. (1994). A common step for signal transduction in G protein-coupled receptors. Trends Pharmacol. Sci. 15, 170–172. [DOI] [PubMed] [Google Scholar]
- Parnot, C., Miserey-Lenkei, S., Bardin, S., Corvol, P., and Clauser, E. (2002). Lessons from constitutively active mutants of G protein-coupled receptors. Trends Endocrinol. Metab. 13, 336–343. [DOI] [PubMed] [Google Scholar]
- Pelchen-Matthews, A., Boulet, I., Littman, D. R., Fagard, R., and Marsh, M. (1992). The protein tyrosine kinase p56lck inhibits CD4 endocytosis by preventing entry of CD4 into coated pits. J. Cell Biol. 117, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, K. L., Premont, R. T., and Lefkowitz, R. J. (2002). Seven-transmembrane receptors. Nat. Rev. Mol. Cell. Biol. 3, 639–650. [DOI] [PubMed] [Google Scholar]
- Prather, P. L. (2004). Inverse agonists: tools to reveal ligand-specific conformations of G protein-coupled receptors. Sci. STKE 2004, pe1. [DOI] [PubMed] [Google Scholar]
- Radu, C. G., Nijagal, A., McLaughlin, J., Wang, L., and Witte, O. N. (2005). Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc. Natl. Acad. Sci. USA 102, 1632–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu, C. G., Yang, L. V., Riedinger, M., Au, M., and Witte, O. N. (2004). T cell chemotaxis to lysophosphatidylcholine through the G2A receptor. Proc. Natl. Acad. Sci. USA 101, 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee, M. H., Nevo, I., Levy, R., and Vogel, Z. (2000). Role of the highly conserved Asp-Arg-Tyr motif in signal transduction of the CB2 cannabinoid receptor. FEBS Lett. 466, 300–304. [DOI] [PubMed] [Google Scholar]
- Roland, J., Murphy, B. J., Ahr, B., Robert-Hebmann, V., Delauzun, V., Nye, K. E., Devaux, C., and Biard-Piechaczyk, M. (2003). Role of the intracellular domains of CXCR4 in SDF-1-mediated signaling. Blood 101, 399–406. [DOI] [PubMed] [Google Scholar]
- Santamarina-Fojo, S., Lambert, G., Hoeg, J. M., and Brewer, H. B., Jr. (2000). Lecithin-cholesterol acyltransferase: role in lipoprotein metabolism, reverse cholesterol transport and atherosclerosis. Curr. Opin. Lipidol. 11, 267–275. [DOI] [PubMed] [Google Scholar]
- Savarese, T. M., and Fraser, C. M. (1992). In vitro mutagenesis and the search for structure-function relationships among G protein-coupled receptors. Biochem. J. 283 (Pt 1), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer, A., Costa, T., Fanelli, F., De Benedetti, P. G., Mhaouty-Kodja, S., Abuin, L., Nenniger-Tosato, M., and Cotecchia, S. (2000). Mutational analysis of the highly conserved arginine within the Glu/Asp-Arg-Tyr motif of the α(1b)-adrenergic receptor: effects on receptor isomerization and activation. Mol. Pharmacol. 57, 219–231. [PubMed] [Google Scholar]
- Schimmoller, F., Simon, I., and Pfeffer, S. R. (1998). Rab GTPases, directors of vesicle docking. J. Biol. Chem. 273, 22161–22164. [DOI] [PubMed] [Google Scholar]
- Seifert, R., and Wenzel-Seifert, K. (2002). Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 366, 381–416. [DOI] [PubMed] [Google Scholar]
- Shapiro, M. J., Trejo, J., Zeng, D., and Coughlin, S. R. (1996). Role of the thrombin receptor`s cytoplasmic tail in intracellular trafficking. Distinct determinants for agonist-triggered versus tonic internalization and intracellular localization. J. Biol. Chem. 271, 32874–32880. [DOI] [PubMed] [Google Scholar]
- Steinbrecher, U. P., Zhang, H. F., and Lougheed, M. (1990). Role of oxidatively modified LDL in atherosclerosis. Free Radic. Biol. Med. 9, 155–168. [DOI] [PubMed] [Google Scholar]
- Tan, C. M., Brady, A. E., Nickols, H. H., Wang, Q., and Limbird, L. E. (2004). Membrane trafficking of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 44, 559–609. [DOI] [PubMed] [Google Scholar]
- Tarasova, N. I., Stauber, R. H., Choi, J. K., Hudson, E. A., Czerwinski, G., Miller, J. L., Pavlakis, G. N., Michejda, C. J., and Wank, S. A. (1997). Visualization of G protein-coupled receptor trafficking with the aid of the green fluorescent protein. Endocytosis and recycling of cholecystokinin receptor type A. J. Biol. Chem. 272, 14817–14824. [DOI] [PubMed] [Google Scholar]
- Ullrich, O., Reinsch, S., Urbe, S., Zerial, M., and Parton, R. G. (1996). Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 135, 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow, M. (2003). Mechanisms regulating membrane trafficking of G protein-coupled receptors in the endocytic pathway. Life Sci. 74, 217–224. [DOI] [PubMed] [Google Scholar]
- Wang, Q., Zhao, J., Brady, A. E., Feng, J., Allen, P. B., Lefkowitz, R. J., Greengard, P., and Limbird, L. E. (2004). Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science 304, 1940–1944. [DOI] [PubMed] [Google Scholar]
- Weng, Z., Fluckiger, A. C., Nisitani, S., Wahl, M. I., Le, L. Q., Hunter, C. A., Fernal, A. A., Le Beau, M. M., and Witte, O. N. (1998). A DNA damage and stress inducible G protein-coupled receptor blocks cells in G2/M. Proc. Natl. Acad. Sci. USA 95, 12334–12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbanks, A. M., Laporte, S. A., Bohn, L. M., Barak, L. S., and Caron, M. G. (2002). Apparent loss-of-function mutant GPCRs revealed as constitutively desensitized receptors. Biochemistry 41, 11981–11989. [DOI] [PubMed] [Google Scholar]
- Witte, O. N., Kabarowski, J. H., Xu, Y., Le, L. Q., and Zhu, K. (2005). Retraction. Science 307, 206. [DOI] [PubMed] [Google Scholar]
- Xie, Y., and Meier, K. E. (2004). Lysophospholipase D and its role in LPA production. Cell Signal 16, 975–981. [DOI] [PubMed] [Google Scholar]
- Xu, Y., Zhu, K., Hong, G., Wu, W., Baudhuin, L. M., Xiao, Y., and Damron, D. S. (2000). Sphingosylphosphorylcholine is a ligand for ovarian cancer G-protein-coupled receptor 1. Nat. Cell Biol. 2, 261–267. [DOI] [PubMed] [Google Scholar]
- Yang, L. V., Radu, C. G., Wang, L., Riedinger, M., and Witte, O. N. (2005). Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood 105, 1127–1134. [DOI] [PubMed] [Google Scholar]
- Zhu, A. J., Zheng, L., Suyama, K., and Scott, M. P. (2003). Altered localization of Drosophila Smoothened protein activates Hedgehog signal transduction. Genes Dev. 17, 1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, S. Z., Wang, S. Z., Hu, J., and el-Fakahany, E. E. (1994). An arginine residue conserved in most G protein-coupled receptors is essential for the function of the m1 muscarinic receptor. Mol. Pharmacol. 45, 517–523. [PubMed] [Google Scholar]
- Zohn, I. E., Klinger, M., Karp, X., Kirk, H., Symons, M., Chrzanowska-Wodnicka, M., Der, C. J., and Kay, R. J. (2000). G2A is an oncogenic G protein-coupled receptor. Oncogene 19, 3866–3877. [DOI] [PubMed] [Google Scholar]