Abstract
We have identified a new protein kinase in Dictyostelium discoideum that carries the same conserved class of “α-kinase” catalytic domain as reported previously in myosin heavy chain kinases (MHCKs) in this amoeba but that has a completely novel domain organization. The protein contains an N-terminal von Willebrand factor A (vWFA)-like motif and is therefore named VwkA. Manipulation of VwkA expression level via high copy number plasmids (VwkA++ cells) or gene disruption (vwkA null cells) results in an array of cellular defects, including impaired growth and multinucleation in suspension culture, impaired development, and alterations in myosin II abundance and assembly. Despite sequence similarity to MHCKs, the purified protein failed to phosphorylate myosin II in vitro. Autophosphorylation activity, however, was enhanced by calcium/calmodulin, and the enzyme can be precipitated from cellular lysates with calmodulin-agarose, suggesting that VwkA may directly bind calmodulin. VwkA is cytosolic in distribution but enriched on the membranes of the contractile vacuole and Golgi-like structures in the cell. We propose that VwkA likely acts indirectly to influence myosin II abundance and assembly behavior and possibly has broader roles than previously characterized α kinases in this organism, which all seem to be MHCKs.
INTRODUCTION
Nearly all aspects of cell life are regulated by protein phosphorylation involving a large number of biochemical reactions and distinct signaling pathways. Existence of ∼500 genes encoding various protein kinases in the human genome further underscores their importance in cell life (Manning et al., 2002). Most of these conventional protein kinases belong to serine/threonine/tyrosine, a kinase superfamily whose members carry a conserved catalytic domain with recognizable sequence similarity in spite of having different targets in the cell (Hardie, 1994; Hanks and Hunter, 1995). In recent years, however, biochemical and molecular approaches have led to the identification of more divergent classes of eukaryotic protein kinases carrying a catalytic domain sequence with no sequence similarity with conventional protein kinase catalytic domains (Manning et al., 2002). One major class of unconventional protein kinases was first identified via representatives of myosin heavy chain kinases (MHCKs) in Dictyostelium discoideum (Futey et al., 1995; Côté et al., 1997) and via identification of mammalian eEF-2 kinase (eEF-2K) as an unconventional protein kinase (Ryazanov et al., 1997). This novel, conserved group of unconventional eukaryotic protein kinases has been termed the α-kinase family (Ryazanov et al., 1999; Drennan and Ryazanov, 2004).
Molecular cloning and biochemical characterization studies demonstrated that at least three Dictyostelium α-kinases genes encode enzymes that can specifically phosphorylate myosin II heavy chain (MHC) in vitro and in vivo, and were thus named as MHC kinase A (MHCK A), MHC kinase B (MHCK B) and MHC kinase C (MHCK C) (Futey et al., 1995; Clancy et al., 1997; Luo et al., 2001; Liang et al., 2002; Rico and Egelhoff, 2003). Although there are many open questions regarding the upstream regulation of Dictyostelium MHCKs, it is generally established that these enzymes play a direct role in myosin II phosphorylation and regulation of myosin II assembly dynamics in this organism (de la Roche et al., 2002).
The first recognized mammalian α-kinase, eEF-2K, is a calcium/calmodulin-activated enzyme that is an established regulator of translation, down-regulating protein synthesis via phosphorylation of elongation factor-2 (Ryazanov et al., 1997). Other human α-kinase genes include a series of cDNA clones that have been named based on tissue source, including Lymphocyte α-kinase, Heart α-kinase, and Muscle α-kinase (Ryazanov, 2002). A mouse α-kinase closely related to the human “muscle α-kinase,” named Midori, has been reported to be nuclear in localization and participates in gene expression control during murine heart development (Hosoda et al., 2001).
Two novel genes identified in humans and mouse were shown to carry an ion channel subdomain fused to an α-kinase catalytic domain and were termed channel kinases (ChaK1 and ChaK2) (Dorovkov et al., 2002), also referred to recently as transient receptor potential (TRP)M6 and TRPM7 (Montell et al., 2002). The channel portions of ChaK1 and ChaK2 are homologous to the TRP family of ion channels, and these novel kinase/ion channels have received considerable attention in recent years as divalent cation gates with possible roles in regulation of calcium and magnesium homeostasis, neuronal function, and control of anoxic cell death (Aarts et al., 2003; Montell, 2003; Drennan and Ryazanov, 2004; Chubanov et al., 2004).
There seems to be no genes encoding α-kinases in the genome of Drosophila, Arabidopsis, Saccharomyces cerevisiae, or Schizosaccharomyces pombe. However, DNA sequences that seem to encode α-kinase catalytic domains are present in Trypanosoma, Leishmania, Entamoeba, Neurospora, and Caenorhabditis elegans. With the characterization of the von Willebrand factor A (VwkA) α-kinase reported here, it is clear that Dictyostelium and the mammalian systems are notably similar in terms of carrying larger families of α-kinase genes than are present in the genomes of other organisms with completed genome databases. Also, both Dictyostelium and mammalian systems contain representatives displaying an array of domain organizations and likely divergent cellular regulation and functions. Here, we describe in depth the identification, cloning, and biochemical characterization of a novel D. discoideum α-kinase carrying an N-terminal vWFA-like motif and a domain organization distinct from the earlier characterized myosin heavy chain kinases in Dictyostelium.
vWFA motifs are named for the prototype member of the family, von Willebrand factor A, a platelet glycoprotein with critical roles in blood clotting. Mutations in von Willebrand factor result in human bleeding disorders (Matsushita et al., 1994; Ingerslev et al., 2004). The vWFA motif is present in a variety of cellular proteins involved in diverse cellular functions such as protein–protein interactions, transcription, DNA repair, ribosomal and membrane transport, and proteosomal functions (Whittaker and Hynes, 2002). This motif is also present in β-integrins and has been alternatively named as IntB motif (Takada et al., 1997). There are also vWFA motifs present in anthrax toxin receptor protein (Wigelsworth et al., 2004) and DICE1, a protein encoded by a gene located in a human tumor suppressor locus (Wieland et al., 1999).
To our knowledge, Dictyostelium VwkA is the first example of a vWFA-like domain present in the same polypeptide as a protein kinase catalytic domain. Notably, the Neurospora crassa genome contains a hypothetical protein with very similar domain organization, including a vWFA-like domain and an α-kinase catalytic domain (GenBank accession no. XP_323573). Given the widespread importance of vWFA domains in many settings, further understanding of this novel Dictyostelium enzyme is merited. Our analysis shows that Dictyostelium VwkA is enriched on the contractile vacuoles and Golgi-like structures in the cell. Manipulation of VwkA expression levels in vivo induced defects in myosin II assembly and abundance and defects in development and cytokinesis. Our data suggest that VwkA has roles in myosin II-related functions, although this interaction does not seem to occur via direct myosin II phosphorylation.
MATERIALS AND METHODS
Cell Culture and Other Materials
The D. discoideum cell line Ax2 was used as the parental line for all the experiments. Cells were cultured at 21°C in HL5 growth medium supplemented with penicillin-streptomycin (50 U/ml). G418 (Geneticin) and blasticidin, purchased from Calbiochem (San Diego, CA), added to medium in certain cases as indicated. PCR reagents and restriction enzymes were from Roche Diagnostics (Indianapolis, IN) and New England Biolabs (Beverly, MA), respectively. Radioisotope ([32P]γ-ATP) used for in vitro phosphorylation assays was from Amersham Biosciences (Piscataway, NJ). Starvation buffer consists of 20 mM 2-(N-morpholino)ethanesulfonic acid-KOH, pH 6.8, 2 mM MgCl2, and 0.2 mM CaCl2.
Cloning of vwkA cDNA
All DNA manipulations were carried out according to the standard molecular biology techniques (Sambrook et al., 1989). The D. discoideum genome data base was searched using MHCK B catalytic domain sequence (Clancy et al., 1997), and as a result, a DNA sequence (contig 4260) carrying a similar catalytic domain sequence was identified. The open reading frame (ORF) of the new gene was predicted using the DNAStar software package, and primers were designed for PCR amplification of full-length cDNA. Total RNA was isolated from 107 Ax2 cells grown in plates, by using TRIzol reagent (Invitrogen, Carlsbad, CA) following manufacturer's instructions. PCR amplification was performed in 50-μl reaction volume consisting of 5 μl of 10× reaction buffer, 4 μl of 25 mM MgCl2, 5 μl of 200 mM dNTPs, 2 μl of the above-mentioned cDNA, and 100 ng of each IMKA-S (5′-GTCGAGCTCGAATCAAAGTATGTTTTGTC-3′) and IMKA-AS (5′-CGATCTAGATTAATAAGAATATGAAATAGAAGAGG-3′) primers carrying SacI and XbaI cloning sites, respectively. Polymerase chain reactions also were performed using genomic DNA isolated and purified from Ax2 cells as described previously (Betapudi et al., 2004). All PCR amplifications were carried out using Expand high-fidelity PCR system (Roche Diagnostics) in PTC-100 Programmable Thermal Controller (MJ Research, Watertown, MA). The thermocycling program consisted of an initial polymerase activation step at 95°C for 2 min, 30 cycles of denaturation at 94°C for 1 min, annealing at 46°C for 1 min, and extension at 72°C for 4 min, followed by a final extension at 72°C for 10 min. The PCR product thus amplified was purified using QIAquick PCR purification kit (QIAGEN, Valencia, CA) and cloned in pTX-GFP plasmid vector (Levi et al., 2000) as SacI and XbaI fragment to create pTX-GFP-VwkA plasmid vector carrying N-terminal–tagged green fluorescent protein (GFP). To create C-terminal–tagged GFP plasmid vector, PCR amplification was performed again under similar reaction conditions with Hind-MHCKD (5′-CAGATCCAAGCTTAAAAAATGGAATCAAAGTATGTTTTGTCA-3′) and KpnI-MH-CKD5070 (5′-CGAGGTACCATAAGAATATGAAATAGAAGAGGAAGA-3′) primers carrying HindIII and XbaI cloning sites, respectively. The purified PCR product was swapped into pDXA-GFP2 (Levi et al., 2000) vector and then excised as SalI and XbaI fragment to clone into pTX-GFP vector to generate pTX-VwkA-GFP expression vector. The vwkA gene excised from pTX-GFP-VwkA expression vector by using SacI and XbaI was cloned into pTX-FLAG vector (Levi et al., 2000) to create pTX-FLAG-VwkA plasmid expression vector. The bacterial amplified and purified plasmid DNAs were subjected to sequencing before using for generating stable cell lines.
Reverse Transcription (RT)-PCR Analysis of vwkA Expression Pattern
At different time points during the development at room temperature, total RNA was isolated from Ax2 cells by using TRIzol reagent followed by synthesizing cDNA. Using synthesized cDNA as template, PCR amplifications were performed with VwkA-specific primers [vWFA-S(4229-55) [5′-CAACAGGTAAACAACGTAGTGAACGTG-3′] and vWFA-AS(4928-03) [5′-CCAGGTTTACCAAGGTTACAACTTCC-3′]. As controls, PCR was performed on the same template samples with primers specific to the developmentally induced gene Car2 (Saxe et al., 1993) and the constitutively expressed gene IG7 (Nagasaki et al., 2002), by using primers as follows: Car2(689-714) [5′CCAGTTGGTGGTTGGTGTTGGATTGG-3′] and Car23(1253-1229) [5′-CAGCATCAAGTGATAAAGAATCAGC-3′] and IG7-S[5′-TTACATTTATTAGACCCGAAACCAAGCG-3′] and IG7-AS[5′-TTCCCTTTAGACCTATGGACCTTAGCG-3′].
Generation of Stable Cell Lines
Dictyostelium stable cell lines expressing GFP-tagged and FLAG-tagged VwkA fusion proteins were generated as described previously (Kolman et al., 1996) with a few modifications. Ax2 cells growing in plates were collected and electroporated in H-50 buffer (Betapudi et al., 2004) by using 10 μg of the above-mentioned plasmid expression vector DNA, and after growing overnight in growth medium, transformants were selected in the presence of 10 μg/ml G418. Individual colonies were picked after 5–7 d and then transferred to microtiter plate followed by further amplification in 10- and 15-cm culture plates. Total cell lysates made from each putative clone was tested for the expression of VwkA fusion proteins by performing Western blot analyses by using GFP and FLAG antibodies. Similarly, Ax2 stable cell lines expressing FLAG-MHCK C fusion protein also were created using pTX-FLAG-MHCK C plasmid vector DNA (Liang et al., 2002). Stable Dictyostelium cell lines expressing either GFP or FLAG proteins developed previously (Levi et al., 2000) were used as internal controls in all the experiments.
Gene Disruption in Dictyostelium
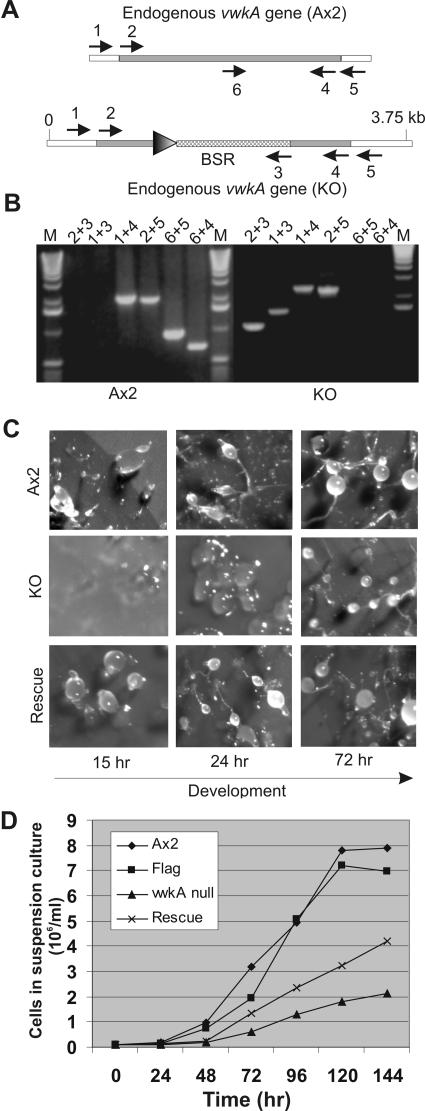
A strategy for the construction of plasmid vector carrying vwkA gene “knockout cassette” DNA used to disrupt gene is illustrated in Figure 6A. The disruption construct was assembled in the plasmid pBsr-Nsi. We created pBsr-Nsi from the previously described plasmid pBsrΔBam (Adachi et al., 1994) by restricting pBsrΔBam with the enzyme NsiI, treating with DNA polymerase to remove 3′ overhangs, and religating. These steps remove a duplicated segment of the actin 8 terminator that was flanked by NsiI sites in pBsrΔBam, producing a smaller vector that lacks any NsiI sites. A short DNA sequence of 502-base pair length was PCR amplified using MHCKD3238 (5′-CGAGGTACCAAAGCCATCCGGAAGAGTTAATG-3′) and MHCKD3740 (5′-CGATCTAGACTAACAAATTCTACAGTGGAACGAGAG-3′) from the N terminus of vwkA gene. The purified DNA fragment was cloned as KpnI and XbaI fragment in pBsr-Nsi plasmid vector to generate pBsr-Nsi-5′-VwkA vector. Similarly, another DNA fragment of 538-base pair length was PCR amplified using MHCKD4442 (5′-CAGATCCAAGCTTGCTTATTTAGGAATTGACGAACATGG-3′) and MHCKD4980 (5′-ACTCGAGGCGCCGAAGTAAAAGATGGAATGGTTAAACC-3′) primers from the C terminus of vwkA gene. The purified fragment was digested with HindIII and SfoI and cloned in pBsr-Nsi-5′-VwkA vector to create pBsr-Nsi-VwkA-KO plasmid vector. From this vector DNA, the entire knockout cassette carrying blasticidin resistance cartridge in the middle was PCR amplified using MHCKE248 (5-CAAAGCCATCCGGAAGAGTTAATG-3′) and MHCKE2426(5′-GAAGTAAAAGATGGAATGG-3′) primers lacking cloning sites. The amplified PCR DNA was purified and used for electroporation of Ax2 cells as described previously (Betapudi et al., 2004). Transformants were selected in the presence of 3 μg/ml blasticidin in the growth medium. Individual clones were isolated, amplified, and subjected to further analyses. Genomic DNA isolated from each clone was analyzed by genomic PCR by using various primers (1: 5′-GCATCTACAATTGTATTCAAAAGCCAACCTGATCTAC-3′, 2:5′-GATAATTAAACATAAAGACTGTTGC-3′, 3: 5′-GAGTTCTTCAATCGTAGTTTTGACTAACTTGCC-3′, 4: 5′-ACTCGAGGCGCCGAAGTAAAAGATGGAATGGTTAAACC-3′, 5: 5′-GGCAATCAAAAAATCCTTCAAATTACACC-3′, and 6: 5′-GCAGGATCCATGTTACAAACAAAATTTAAAATGCCATC-3′) as shown in Figure 6B. Immunoprecipitates were made from the putative vwkA null clones growing in plastic petri plates and were further subjected to Western blot analyses to confirm deletion of endogenous vwkA gene by using VwkA antiserum.
Figure 6.
Generation of vwkA null cells and evaluation of development. (A) Strategy for the deletion of vwkA gene. A gene-targeting cassette carrying a blasticidin selection marker (BSR; lower schematic image) was transfected into Ax2 cells, and clonal blasticidin-resistant cell lines were isolated. PCR analysis was performed with genomic DNA to identify candidate vwkA null lines, followed by extensive PCR analysis with indicated primers 1–6 as indicated by arrows. Sequences of these primers are given in Materials and Methods. PCR reactions were configured to discriminate between the wild-type locus in parental Ax2 cells (top schematic image) and the expected locus organization resulting from double crossover gene targeting to create a vwkA null (lower schematic). (B) Example of genomic PCR analysis of one vwkA null cell line. PCR amplifications performed as described in previously (Betapudi et al., 2004) were subjected to electrophoresis on 1.2% agarose gel. M indicates 1-kb DNA ladder marker. Ax2 lanes indicate PCR results obtained with parental Ax2 genomic DNA, KO indicates PCR results obtained with genomic DNA from a vwkA null line. PCR results confirm the lack of a complete vwkA gene in the vwkA null lines. For example, the vwkA null line genomic DNA fails to yield a product with primers 6 + 5 or 6 + 4, and with primers 1 + 4 or 2 + 5, the vwkA null displays a large product than Ax2, due to the replacement of the central part of the vwkA gene with the BSR cassette. (C) Developmental defects of vwkA null cells and rescued lines. The parental Ax2 cells, vwkA null cells, and vwkA null cells transfected with a FLAG-tagged VwkA expression construct (selected with G418 at a low concentration of 5 μg/ml) were grown in plastic petri plates and then collected and developed as in Figure 5. Images of the developing cells were taken at the indicated time points during development. (D) Suspension growth defects of vwkA null cells and rescued line. The parental Ax2 cells, vwkA null cells, and vwkA null cells transfected with a FLAG-tagged VwkA expression construct (selected with G418 at 5 μg/ml) were grown in plastic petri plates and then transferred to flasks containing HL5 for suspension growth. Cell densities were determined daily via hemocytometer counts. The “Flag” sample refers to growth of Ax2 cells transfected with the empty pTX-FLAG vector and grown in 5 μg/ml G418. The “rescue” sample refers to vwkA null cells transfected with the FLAG-VwkA construct and grown in 5 μg/ml G418.
Polyclonal Antiserum
A 652-base pair DNA sequence covering the entire catalytic domain from the predicted amino acid sequence of VwkA protein kinase corresponding to amino acids 373–600 was PCR amplified using MHCKD4313 (5′-GCAGGATCCATGTTACAAACAAAATTTAAAATGCCATC-3′) and MHCKD4965 (5′-CGAGGTACCGGAATGGTTAAACCTAATTTTTTACAATG-3′) primers carrying BamHI and KpnI cloning sites, respectively. The purified DNA fragment was cloned in pRSET-A expression vector (Novagen, Madison, WI) to generate pRSET-VwkA-Cat bacterial plasmid expression vector. The 30-kDa His-tagged fusion protein expressed and purified from bacteria was injected into New Zealand White rabbit at 4-wk intervals, and after 12–14 d serum was collected. Cleared serum was used at 1:1000 dilutions for Western blot analyses.
Preparation of Total Cell Lysates and Triton-insoluble Cytoskeletal Fractions
Total cell lysates to assess relative myosin II abundance were made either by directly lysing equal numbers of washed cells in SDS-PAGE sample buffer (heated to 95°C for 1 min) or via passage through 5-μm Nucleopore membranes as described previously (Theibert and Devreotes, 1986; Parent et al., 1998). Briefly, cells grown to nearly confluent in 15-cm plates were collected by centrifugation at 350 × g for 5 min followed by washing twice with ice-cold 50 mM TES buffer, pH 7. Cells were counted by hemocytometer, and 8 × 107 cells were pelleted and resuspended in 1 ml of ice-cold 10 mM TES buffer, pH 7, with 20 mM KCl and 2× protease inhibitors (Steimle et al., 2001a). The cell suspension was passed through Nuclepore Track-Etch membrane (Whatman, Clifton, NJ), which was clamped on the end of a 1-ml syringe. This treatment resulted in >95% lysis as assessed by microscopy. Bradford assays were performed on resulting cell lysates, and equal quantities of protein were subjected to SDS-PAGE by using 4–20% polyacrylamide gels. Gels were stained with Coomassie Blue to monitor proteins or subjected to Western blot analysis.
Triton X-100–insoluble cytoskeletal proteins and corresponding cytosolic fractions were isolated as described previously (Kolman et al., 1996). SDS-PAGE, Coomassie Blue staining, and densitometry were performed as described previously. The percentage of myosin II in the cytoskeleton was calculated for each individual sample by dividing the densitometric value for myosin heavy chain in the cytoskeletal pellet by the sum of the pellet and supernatant myosin heavy chain values for that sample, and multiplying this value by 100.
Protein Purification and Pull-Downs
Ax2 stable cell lines expressing FLAG-tagged VwkA fusion protein were grown in 15-cm plates, and 5 × 107 cells were collected by centrifugation at 350 × g for 5 min at 4°C followed by washing with ice-cold 50 mM TES buffer, pH 7. The cell pellet was lysed in 400 μl of lysis buffer (50 mM TES buffer, pH 7, 5 mM EDTA, 200 mM NaCl, 1 mM dithiothreitol [DTT], 4× PIC I, PIC II, and 50 μg/ml phenylmethylsulfonyl fluoride protease inhibitors; Steimle et al., 2001a) and 1% Triton X-100. The cell suspension was vortexed for 5 s and then incubated on ice for 5 min. Clear supernatant obtained by centrifugation at high speed in cold condition (10 min in Microfuge, ∼10,000 × g) was mixed with anti-FLAG-IgG beads (Sigma-Aldrich, St. Louis, MO) and kept on rotating shaker for 1 h at 4°C and then beads were collected by centrifugation at high speed for 30 s. The protein-bound beads were subjected to thorough washing with ice-cold washing buffer (50 mM TES buffer, pH 7, consisting of 200 mM NaCl, 1 mM EDTA, and 1 mM DTT). After removing excess washing buffer, protein beads were incubated in 20 μl of elution buffer carrying 10 mM TES buffer, pH 7, 0.1 mM EDTA, 1 mM DTT, and 200 μg/ml competing FLAG peptide (Sigma-Aldrich) for 10 min at 4°C to elute FLAG-tagged VwkA protein. Supernatant was carefully removed and elution was performed again with additional 10 μl of elution buffer. Both the eluants were pooled and stored as 3-μl aliquots at –80°C until used for kinase assays. Under identical conditions, control protein purification was performed from the parental Ax2 cells (negative kinase activity control) and Ax2 stable cell lines expressing FLAG-tagged MHCK C protein kinase (positive protein kinase activity control).
CaM-agarose pull-down tests were performed with lysates generated with the same lysis solution as for FLAG-immunoprecipitations. For these tests, 108 cells were lysed in a 500-μl volume. A 250-μl portion of this lysates was brought to 450 μl with 50 mM TES, pH 7.0, and adjusted to contain a final concentration of either 10 mM EGTA or 10 mM CaCl2. CaM-agarose (20 μl; Sigma-Aldrich) was added to each sample, followed by incubation for 1 h at 4°C. CaM-agarose beads were washed twice via centrifugation, with buffer containing 50 mM TES, pH 7.0, and 50 mM NaCl and also containing either 10 mM EGTA or 10 mM CaCl2, as in initial incubation. Beads were then boiled in SDS-PAGE sample buffer and solubilized protein from the beads was subjected to SDS-PAGE and Western blot analysis.
Kinase Assays
In vitro phosphorylation reactions using the above-mentioned purified FLAG-tagged fusion proteins were performed as described previously (Liang et al., 2002) with a few modifications. In a typical 20-μl phosphorylation reaction mixture, 20 mM TES buffer, pH 7, 2 mM MgCl2, 0.5 mM ATP, 1 μCi of [32P]γ-ATP, and 1 μM purified Dictyostelium myosin II (Steimle et al., 2001a) were incubated at 21°C for 20 min. The reactions were stopped by adding 2× SDS sample buffer followed by boiling at 95°C for 2 min and then subjected to SDS-electrophoresis on 4–20% polyacrylamide gels. Phosphorylation of proteins was analyzed by performing autoradiography using dried acrylamide gels. Similarly, calcium calmodulin (CaM) assays were performed with 50 mM HEPES buffer, pH 7.4, with 4 mM MgCl2, 5 mM DTT, 100 μM CaCl2, 1 mM CaM, 1 μM myosin II, 1 mM ATP, 1 μCi of [32P]γ-ATP, and VwkA-immunoprecipitate.
Western Blot Analysis
Western blot analyses were performed as described previously (Betapudi et al., 2004) with some modifications. Cells (1 × 107) growing in 10-cm plates were collected by centrifugation at 350 × g for 5 min at 4°C and washed twice with the ice-cold 50 mM TES buffer, pH 7. The cell pellet was then resuspended in 50 mM TES buffer containing protease inhibitors (Steimle et al., 2001a). An equal volume of prewarmed sample buffer carrying 1 mM 2-mercaptoethanol was added and boiled at 95°C for 1 min. Lysate samples (10 μl) were subjected to electrophoresis on 10% SDS-acrylamide gel and then transferred to polyvinylidene difluoride membranes using Trans-Blot SD Semidry Transfer Cell (Bio-Rad, Hercules, CA) and probed with polyclonal rabbit antiserum raised as described above. Signal was detected with an alkaline phosphatase-linked goat anti-rabbit antibody using CDP-star reagent (Tropix, Bedford, MA) chemiluminescence detection system.
Microscopic Studies
Cells (3 × 106) growing in suspension culture or in plastic Petri plates approximately for 72 h were collected and seeded in glass-bottomed microscope chambers (Nalge Nunc, Naperville, IL). Chambers were kept at room temperature to settle cells for 10 min, and then growth medium was removed carefully. Cells were washed twice with ice-cold starvation buffer. Cells were fixed by adding 1 ml of ice-cold methanol (–10°C) and incubated for 5 min. Methanol was removed, and 1 ml of 1× Tris-buffered saline (TBS) was added and incubated for 2 min at room temperature. After repeating TBS washing, cell nuclei were stained by adding 1 ml of freshly diluted 4,6-diamidino-2-phenylindole (DAPI) solution (20 μg/ml) and incubated for 5 min at room temperature. DAPI solution was removed followed by washing cells twice with distilled water at room temperature. After adding 1 ml of distilled water, cells were visualized by inverted light microscope for stained nuclei. To visualize subcellular localization of GFP-VwkA, cells growing in plastic petri plates were collected and seeded in glass-bottomed chambers as described above. Cells were incubated in growth medium for 1 h to settle at room temperature. Growth medium was removed followed by washing cells carefully with starvation buffer. Localization studies in live cells were performed in starvation buffer using confocal microscopy performed on a Zeiss LSM510. To examine contractile vacuoles in the live cells, starvation buffer was replaced by 1 ml of FM2-10 (Molecular Probes, Eugene, OR) solution (10 μg/ml) made in water. A series of images were collected immediately after adding the dye solution to the chamber.
RESULTS
Identification and Cloning of a Novel α-Kinase cDNA
With the resources of the D. discoideum genome project (www.dbase.org; Eichinger and Noegel, 2003), we identified additional sequences with high identity to the α-kinase catalytic domains of MHCK A, B, and C. In particular, a genomic DNA sequence corresponding to annotated locus DDB0216405 exhibited a significant sequence homology with the earlier identified α-kinases in Dictyostelium and other organisms. We performed RT-PCR, subcloning, and DNA sequence analysis to further characterize this locus. From these analyses, we identified an expressed sequence with an ORF of 1875 base pairs with no introns (deposited in GenBank, accession no. AY672633). The full-length cDNA is predicted to encode a polypeptide of 625 amino acids with a calculated molecular mass of 69 kDa.
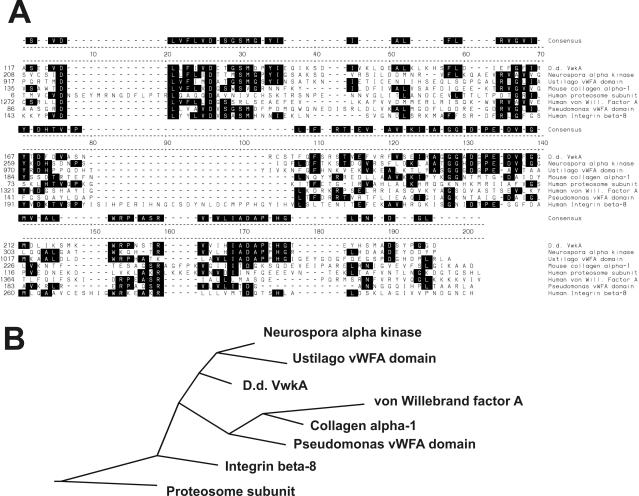
GenBank BLAST and MOTIF analyses revealed that the unconventional catalytic domain (corresponding to amino acid residues 398–592) of this protein is coupled to an N-terminal domain bearing a vWFA-like motif (corresponding to amino acid sequences 121–233), and we therefore refer to this gene as vwkA and the encoded protein as VwkA or vWF kinase. The vWFA motif is a structural fold that is present in a wide range of proteins that mediate protein–protein interactions (for review, see Whittaker and Hynes, 2002), including both β and α integrins, and this motif is also commonly referred to as a β-integrin motif. Analysis of the Conserved Domain Database at National Center for Biotechnology Information (Marchler-Bauer et al., 2003) demonstrates these motifs as closely related. ClustalW sequence alignments further demonstrate the similarity of this segment of VwkA to both integrins and vWFA family members (Figure 1A). Phylogenetic tree analysis (Figure 1B) reveals the domain in VwkA to be most closely related to sequences present in several fungal species such as another hypothetical α-kinase identified as an open reading frame in the N. crassa genome (discussed further below), and hypothetical protein domains present in the Ustilago genome. ClustalW analysis reports both human von Willebrand factor and β-integrins as slightly less similar. To our knowledge this is the first protein kinase coupled to vWFA-like domain described in any organism to date. Although several other proteins in the Dictyostelium genome database seem to encode vWFA domains, none seems to be coupled to protein kinase domains.
Figure 1.
The vWFA/β-integrin motif of VwkA. (A) ClustalW alignment of the vWFA domain of VwkA with other vWFA domain family members. Consensus residues are defined as residues identical in at least three of the sequences and are shaded. (B) Phylogenetic tree based upon ClustalW alignment. Alignment and tree produced with the DNAStar program MegAlign. GenBank accession numbers for included sequences are as follows: Neurospora α-kinase (discussed later in paper), XP_323573; Ustilago vWFA domain containing protein, EAK82057; mouse collagen α-1, Q60847; human proteosome 26S subunit Rpn10, P55036; human von Willebrand factor A (motif A1), P04275; Pseudomonas vWFA domain protein, NP-744179; and human β-integrin 8, P26012.
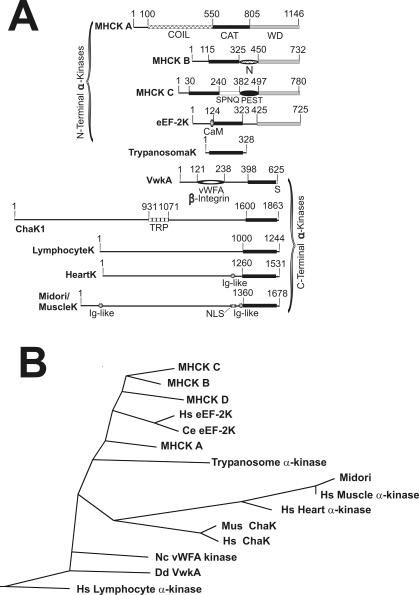
Although the catalytic domain of VwkA displays strong identity to the previously characterized Dictyostelium α-kinases (MHCK A, MHCK B, and MHCK C), there is a notable difference in the position of the catalytic domains relative to the other enzymes. Whereas Dictyostelium MHCK A, B, and C all carry a C-terminal WD-repeat domain distal to their α-kinase catalytic domain, in VwkA the α-kinase catalytic domain is positioned at the extreme C terminus of the polypeptide (Figure 2A). Notably, the C-terminal catalytic domain position also is observed in the majority of the mammalian α-kinases (Figure 2A). This domain organization suggests that VwkA may in fact be more closely related in phylogeny to mammalian α-kinases than to the other Dictyostelium family members represented by MHCK A, B, and C. To further explore this possibility, phylogenetic analysis was performed via alignments of the catalytic domain segments of multiple members of the family, by using the ClustalW algorithm. This analysis revealed two distinct phylogenetic branches to the α-kinase family (Figure 2B). Dictyostelium MHCK A, B, C, and the metazoan eEF-2Ks form one branch of the family, which we refer to here as the N-terminal subbranch. The Dictyostelium VwkA kinase falls outside of this group, more closely related to the mammalian ChaK and other C-terminal catalytic domain members, which we refer to here as the C-terminal subbranch (Figure 2, A and B). Notably, the phylogenetic branches revealed by catalytic domain similarity follow closely to the domain organization in each subbranch, with all members of the C-terminal subbranch bearing the catalytic domain at the extreme C terminus of the polypeptide, and all members of the N-terminal subbranch carrying the catalytic domain upstream of another domain either demonstrated (Steimle et al., 2001a) or implicated (Pavur et al., 2000) in substrate targeting. The presence of both N-terminal and C-terminal subbranch kinase members in Dictyostelium and in mammals suggests that α-kinases are an ancient family of enzymes and that the divergence of these two subbranches predates the evolutionary divergence of Dictyostelium and metazoan ancestors.
Figure 2.
(A) Schematic representation of α-kinase family member domain organization. The N-terminal and C-terminal kinase subfamilies are indicated by brackets. WD refers to the WD repeat domain (gray box), and coil refers to segments with strongly predicted coiled-coil character (cross-filled box). N refers to polyasparagine-rich sequence; SPNQ refers to serine, asparagines, proline, and glutamic acid-rich sequence; and S refers to a serine-rich sequence. A possible PEST domain in MHCK C (proline, glutamic acid, serine, and threonine-rich) is indicated, and CaM refers to Ca+2-calmodulin binding motif. Distorted circle in VwkA indicates vWFA/β-integrin motif. NLS refers to nuclear localization signal, Ig-like refers to immunoglobulin-like, and TRP indicates transient receptor potential ion channel domain. The amino acid numbers shown for each correspond to the following accession numbers: MHCK A (A55532), MHCK B (AAB50136), MHCK C (AAC31918), human eEF2K (NP_037434), mouse Chak1 (AFF73131), human LymphocyteK (AAK94675) human HeartK (NP_443179), mouse Midori/MuscleK (NP_473426), and Trypanosome kinase (AC079815). (B) Phylogenetic tree of α-kinase catalytic domains. ClustalW alignments and phylogenetic tree were performed with the DNAStar software package. Protein sequence sources listed in A, but alignments also included the D. discoideum genomic sequence for MHCK D (our unpublished data and from genome project), and an N. crassa sequence for an α-kinase (Nc vWFA kinase; XP_328675) related in domain organization to Dictyostelium VwkA.
Impaired Growth and Multinucleation of VwkA-overexpressing Cells in Suspension Culture
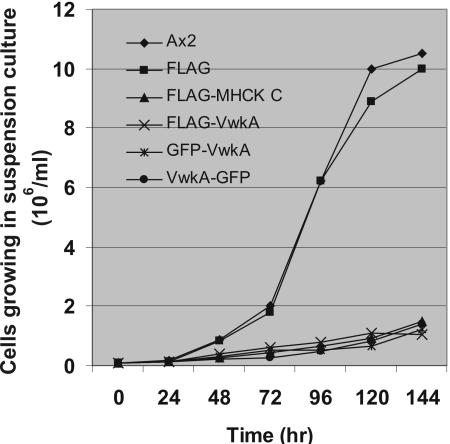
To investigate the effect of VwkA overexpression on cell growth, we performed suspension culture growth curve measurements by using stable cell lines expressing FLAG-tagged-VwkA, GFP-tagged-VwkA (as either a N-terminal or C-terminal fusions), parental Ax2 cells, or Ax2 cells expressing the empty FLAG vector pTX-FLAG as a control. The growth curve kinetics demonstrates that overexpression of VwkA leads to impaired cell proliferation in suspension culture (Figure 3). These results are reminiscent of early work showing that when cells lacking myosin II are grown in suspension culture, they become large and multinucleated due to incomplete cytokinesis, and eventually lyse (De Lozanne and Spudich, 1987; Manstein et al., 1989). However, cells without myosin II are viable when attached to petri dish surfaces, where they divide by a nonmyosin II-mediated cell division mechanism (Gerisch and Weber, 2000; Weber et al., 2000). These results are also reminiscent of our previous observations made with myosin heavy chain kinase-overexpressing cells in suspension culture (Kolman et al., 1996; Rico and Egelhoff, 2003). For example, purified myosin II can be phosphorylated by MHCK C in vitro driving filament disassembly (Liang et al., 2002), and MHCK C-overexpressing cells fail to grow in suspension culture due to defective cytokinesis, a consequence of myosin filament disassembly in vivo due to hyperphosphorylation of myosin II by MHCK C (Liang et al., 2002) and Figure 3.
Figure 3.
Cell growth kinetics of VwkA-overexpressing cell lines in suspension culture. Cells were collected from petri dish culture and transferred to flasks rotating at 200 rpm at 22°C in HL5 medium at an initial density of 1 × 105 cells/ml. Cell lines evaluated were parental Ax2, cells expressing empty FLAG-tag vector (FLAG), FLAG-tagged VwkA (FLAG-VwkA), FLAG-tagged MHCK C (FLAG-MHCK-C), N-terminal GFP-tagged VwkA (GFP-VwkA), and C-terminal tagged VwkA (VwkA-GFP). All of the stable cells expressing fusion proteins or FLAG-vector were grown in the HL5 with G418 at 10 μg/ml. Cell density was determined by hemocytometer counts every 24 h.
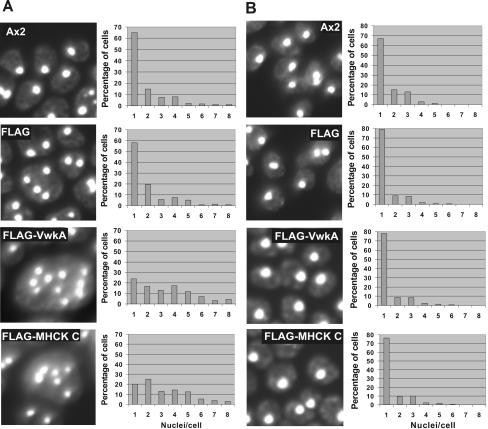
To further investigate whether overexpression of VwkA leads to cytokinesis defects when cells are grown in suspension culture, nuclear staining with DAPI was performed to determine whether cells were becoming multinucleated. Cells were transferred from petri dish cultures into growth medium in 50-ml conical flasks (1 × 105 cells/ml initially) and grown for 72 h at room temperature with continuous agitation (200 rpm). These cells were fixed and then stained with DAPI to observe nuclei via fluorescence microscopy. Cells from multiple random fields of view were scored for degree of multinucleation (Figure 4A). This analysis revealed substantial multinucleation in cell lines overexpressing VwkA, indicating that overexpression impairs cytokinesis in suspension culture. Multinucleation of cells also was observed in myosin heavy chain kinase C-overexpressing cells (Figure 4A).
Figure 4.
Nuclear staining of cells. (A) DAPI staining of cells growing in suspension culture. Cells growing in suspension culture were transferred to glass-bottomed microscope chambers, allowed to attach for 10 min, and then fixed and DAPI stained as described in Materials and Methods. (B) DAPI staining of cells growing in plastic petri plates. Cells growing in plastic petri plates to near confluent were collected, fixed, and stained as described in Materials and Methods. Cells were scored with respect to nuclei/cell as presented. Each graph represents score of 300 cells from several fields of view.
As noted above, myosin II is essential for Dictyostelium cells to grow in suspension culture, but it is not essential for growth when Dictyostelium cells are allowed to attach to petri dish surfaces. As a further test of whether VwkA cytokinesis defects might be myosin II related, cells overexpressing VwkA were assessed for multinucleation when growing as surface attached cultures. As also was observed for the control cell lines that overexpress MHCK C, in the surfaced-attached setting no significant multinucleation was observed in VwkA-overexpressing cell lines (Figure 4B). These results suggest that VwkA may be involved in the regulation of myosin II function during cytokinesis.
VwkA Expression and Developmental Defects
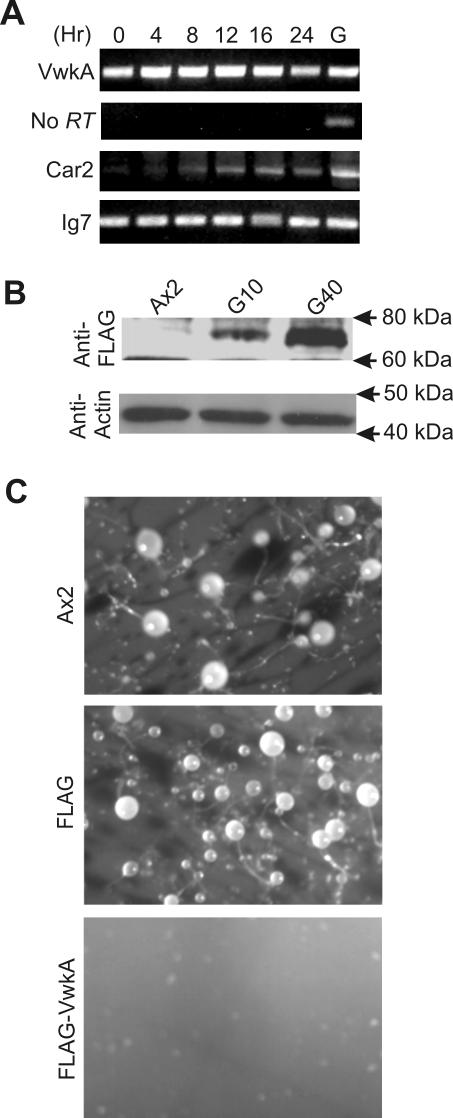
On nutrient depletion, Dictyostelium cells enter a multicellular developmental program leading to the formation of multicellular fruiting bodies and dormant spores. Earlier studies showed that myosin II is essential for the developmental program and for the morphological changes that accompany it (De Lozanne and Spudich, 1987; Knecht and Loomis, 1987). To assess potential roles of VwkA during the developmental program, we first evaluated gene expression levels via RT-PCR by using total RNA isolated from various stages of parental Ax2 Dictyostelium cells during development. As shown in Figure 5A, the vwkA transcript is expressed throughout all tested developmental stages. To assess the effects of VwkA overexpression on development, stably transfected clonal cell lines were passed repeatedly in elevated levels of the selected marker antibiotics (G418, 40 μg/ml) to increase expression levels. As described previously for MHCK constructs (Liang et al., 2002), this culture method significantly increased VwkA expression levels (Figure 5B). When harvested from cultures grown in HL5 medium containing 10 μg/ml G418, VwkA-overexpressing cells were still capable of completing development with relatively normal kinetics (our unpublished data). However, when grown in G418 at 40 μg/ml, VwkA-overexpressing cells failed to develop beyond the mound stage (Figure 5C). In contrast, cells expressing empty FLAG-vector developed robustly, even if grown in G418 at 40 μg/ml (Figure 5C).
Figure 5.
Development of VwkA-overexpressing cells. (A) RT-PCR analysis of vwkA gene expression during development of Ax2 cells. Ax2 cells growing in suspension culture were collected by centrifugation followed by washing with starvation buffer. The cell pellet was resuspended in starvation buffer at 2 × 108 cells/ml and layered on presoaked Whatman filter paper in starvation buffer placed on top of agarose plates made in starvation buffer. RNA was isolated at indicated times. RT-PCR was performed with vwkA-specific primers in the presence of reverse transcriptase (top) or as a control, without reverse transcriptase (second panel from top). G represents control PCR performed with genomic DNA as template. PCR mixtures were subjected to electrophoresis on 1% agarose gel. As referenced in Materials and Methods, Car2 is a control developmentally expressed gene, and Ig7 is a control constitutively expressed gene. (B) Analysis of FLAG-VwkA overexpression levels. Total cell lysates were subjected to Western blot analyses by using FLAG antibodies. These cell lysates also were subjected to Western blot analysis by using actin antibodies as a loading control. Left lane, parental Ax2 cells; middle lane, FLAG-VwkA cells growing in medium with G-418 at 10 μg/ml; and right lane, FLAG-VwkA cells growing in medium with G418 at 40 μg/ml. (C) Development of FLAG-VwkA–overexpressing cells. Parental Ax2 and stable transfected cell lines expressing empty FLAG vector or FLAG-VwkA fusion protein were grown in the presence of G418 at 40 μg/ml and then subjected to development as described above. Images were taken after 72 h of development.
vwkA Gene Disruption Defects
To further investigate its role during development, the vwkA gene was disrupted in the parental Ax2 cell line via gene targeting with a blasticidin resistance cassette (Figure 6A). Gene disruption cell lines were identified and confirmed by genomic PCR as shown in Figure 6B. These null cells were monitored during development. In contrast to behavior reported earlier for MHCK A, MHCK B, and MHCK C gene-targeted cell lines (which generally develop normally), fruiting body formation was delayed by 36–48 h during the development of vwkA null cells (Figure 6C). A delay in development was observed with three independent vwkA gene disruption cell lines. We also generally observed in the disruption lines that stalks and spores were thin and smaller than those of the parental Ax2 cells. Interestingly, similar observations were observed in the AP-1 clathrin-adaptor protein null cell development in Dictyostelium (Lefkir et al., 2003). These results suggest that VwkA may have physiological functions that are different from those of MHCK A, MHCK B, and MHCK C during development of D. discoideum.
We further tested the ability of plasmid expressed FLAG-tagged VwkA to rescue this defect. Given the fact that high-level expression of FLAG-VwkA can block development (Figure 5), vwkA null cells were transfected with the pTX-FLAG-VwkA plasmid, and tranformants were selected with G418 at 5 μg/ml to bias for low protein expression level. These rescued cell lines were tested for developmental kinetics in parallel to Ax2 and the vwkA null lines. For three independent vwkA null lines, this rescue experiment resulted in a restoration of development to rates similar to that of Ax2 cells (Figure 6C, example of one rescued line).
The vwkA null cell lines and the cells rescued by transfection of the FLAG-VwkA construct (at 5 μg/ml G418) were assessed for ability to perform cytokinesis in a suspension culture setting. This analysis revealed a severe defect in suspension culture growth in the vwkA null cells (Figure 6D), similar to that of the VwkA overexpression cell lines shown earlier in Figure 3. The vwkA null cells also displayed a severe suspension culture multinucleation defect like that of the overexpression cell line (our unpublished data). This suspension growth defect was partially complemented when the FLAG-VwkA construct was transfected into the vwkA null background (Figure 6D). The suspension growth behavior of these cell lines and that of the FLAG-VwkA overexpression lines presented in Figure 3, suggest that either absence of VwkA, or its overexpression can result in defects in cytokinesis.
VwkA Involvement in the Regulation of Myosin II Expression and Assembly
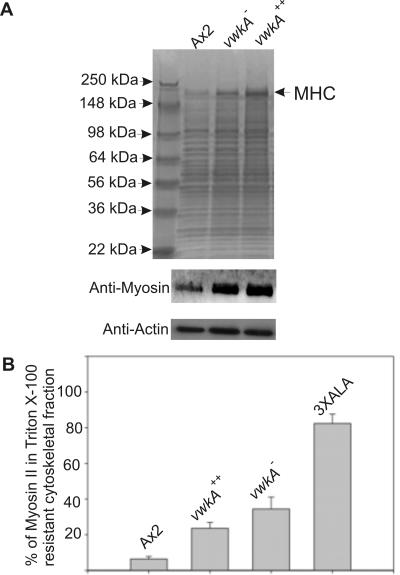
To test for possible roles of VwkA in the regulation of myosin II expression and assembly control, we made total cell extracts from VwkA-overexpressing cells (VwkA++) and vwkA null cells growing in petri dishes. These extracts were subjected to SDS-gel electrophoresis and stained with Coomassie Blue to visualize myosin II protein bands. As shown in Figure 7A, myosin II levels seem to be increased upon VwkA overexpression as well as by vwkA deletion. This is further confirmed by Western blot analysis performed with myosin II antibodies as shown in Figure 7A. This increase in MHC abundance was observed both in lysates prepared by passage through Nucleopore membranes (see Materials and Methods), and when lysates were prepared by directly boiling cells in SDS-PAGE sample buffer. To our knowledge, there are no reports to date in any system showing elevation of total nonmuscle myosin II levels in cells upon either overexpression or deletion of other genes, although there are studies in mammalian cells showing that sodium butyrate and trichostatin A, known inhibitors of histone deacetylase, can induce expression of myosin IIC in mammalian cells (Buxton et al., 2003). Altered expression of VwkA in Dictyostelium thus seems to be connected via some unknown regulatory mechanism to control of MHC protein levels in the cell. However, total level of actin, another essential cytoskeletal protein, was unaffected by changes in VwkA expression levels in the cells (Figure 7A).
Figure 7.
Effect of VwkA expression level on myosin II expression and assembly. (A) SDS-PAGE analysis of total cell extracts. Total cell extracts were made from VwkA++, vwkA null, and parental Ax2 cells grown in plastic petri plates by passage through Nucleopore membranes. Equal quantities of total protein from each sample were subjected to SDS-PAGE, followed by Coomassie Blue staining. Arrow indicates MHC band, further confirmed by performing Western blot analysis by using anti-myosin II antibody My4 (Flicker et al., 1985; Peltz et al., 1985) as shown in second panel. The myosin II Western blot was stripped and reprobed with anti-actin antisera to confirm equal loading of lysates samples. (B) Myosin II levels in Triton X-100–insoluble cytoskeletal ghosts. Triton X-100 lysates were fractionated via centrifugation, and percentage of MHC present in the cytoskeletal ghost was quantified via SDS-PAGE, Coomassie staining, and densitometry. Error bars represent SEM, and n = 8 independent samples for each bar.
We also evaluated the effects of altered VwkA expression levels on myosin II assembly levels in vivo. Triton X-100–resistant cytoskeletal ghosts were isolated as described previously (Kolman et al., 1996). In earlier studies, it was found that overexpression of MHCK A reduced myosin II assembly into such ghosts and that disruption of MHCK A increased myosin II assembly levels, consistent with the idea that MHCK A phosphorylates MHC in vivo, driving filament disassembly (Kolman et al., 1996). Earlier studies also demonstrated that mutation of the three mapped MHCK target sites in the myosin tail, creating a nonphosphorylatable “3x ALA” mutant myosin II, resulted in gross overassembly of myosin in the cytoskeletal ghost assay (Egelhoff et al., 1993), due to inability of any MHCKs to phosphorylate these target sites in the myosin tail. To assess whether VwkA might act in a similar manner, we isolated cytoskeletal ghosts and evaluated myosin II assembly levels via SDS-PAGE, Coomassie staining, and densitometry. These results revealed a significant overassembly of myosin II upon deletion of vwkA, relative to the parental cell line (Figure 7B). This behavior is similar to that observed earlier upon disruption of Dictyostelium MHCK A (Kolman et al., 1996) and MHCK B (Rico and Egelhoff, 2003), possibly consistent with a role for VwkA in phosphorylation of myosin II in vivo. As observed in the earlier studies, 3x ALA myosin displayed a severe overassembly defect (Figure 7B). However, in contrast to earlier studies of verified MHCKs, myosin II assembly behavior in VwkA++ cells gave an unexpected result. With the established MHCKs (MHCK A, B, and C), overexpression reduces assembly levels in this assay (Kolman et al., 1996; Rico and Egelhoff, 2003). For VwkA, however, we consistently observed increased myosin II association with cytoskeletal ghosts upon overexpression (Figure 7B). These results conflict with the simple hypothesis that VwkA acts primarily as an MHCK to regulate myosin II filament assembly and further suggest that VwkA may have roles in the cell related to myosin II function but distinct from the simple phosphorylation of MHC.
FLAG-VwkA Purification and Myosin II Phosphorylation In Vitro
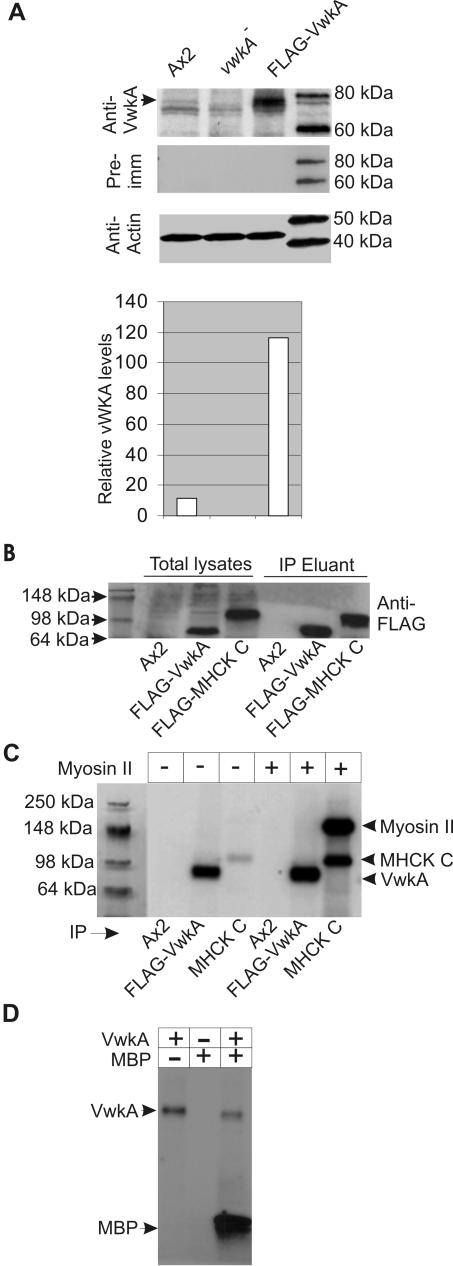
Previously identified and characterized α-kinase family members in D. discoideum (MHCK A, MHCK B, and MHCK C) were found to phosphorylate myosin II efficiently in vitro and drive myosin II filament disassembly (Côté and McCrea, 1987; Steimle et al., 2001a; Liang et al., 2002; Rico and Egelhoff, 2003). To evaluate whether VwkA can act as a myosin II heavy chain kinase in vitro in a similar manner, we pursued biochemical purification and phosphorylation analysis with FLAG-tagged VwkA protein. Western blot analysis performed with peptide-based anti-VwkA antisera suggested that endogenous VwkA is expressed at low levels (Figure 8A; additional analysis not shown). We therefore used an immunoprecipitation approach to isolate the more highly expressed FLAG-VwkA (Figure 8, A and B). FLAG-MHCK C also was isolated in parallel as a positive control for the biochemical analysis, and parallel immunoprecipitation was performed with untransfected Ax2 cells as a negative control (Figure 8B). The immunoprecipitated FLAG fusion proteins were eluted from the anti-FLAG antibody beads by competing with FLAG peptides, and eluants were evaluated for yield by Western blot analysis (Figure 8B, right). The kinase assays performed using this eluted FLAG-VwkA protein failed to phosphorylate myosin II in vitro (Figure 8C). As reported previously (Liang et al., 2002), immunoprecipitated FLAG-MHCK C phosphorylated myosin II very efficiently under the same conditions (Figure 8C). It is noteworthy that the FLAG-VwkA seems to undergo autophosphorylation robustly in these assay conditions (Figure 8C), confirming that the immunoprecipitated material is biochemically active.
Figure 8.
Immunopurification of FLAG-VwkA and protein kinase activity. (A) Western blot analysis of FLAG-VwkA overexpression. Total cell lysates were subjected to SDS-PAGE and Western blot analysis with anti-VwkA peptide antisera. Ax2 lysates reveal a weak but reproducible band at ∼75 kDa (arrow), which is absent in vwkA null cell lysates. FLAG-VwkA–overexpressing cells (grown in G418 at 10 μg/ml) display a robust signal at this position. Western blot samples also were probed with anti-actin antibodies as a loading control. Densitometric analysis of Western blots indicates ∼11-fold overexpression of FLAG-VwkA relative to Ax2. (B) Western blot analysis of immunoprecipitates. Western blot analyses were performed with anti-FLAG antibodies and either Ax2 lysates (negative control), FLAG-VwkA lysates, or FLAG-MHCK-C lysates. The right three lanes indicate signal from material that was immunopurified and eluted from beads with competing FLAG peptide. (C) Autoradiogram of in vitro phosphorylation assays with immunopurified FLAG-VwkA using myosin II as a substrate. Reactions were performed in 20-μl volume containing 1 μM myosin II purified from Dictyostelium and incubated at 22°C for 20 min in a mixture containing 2 mM MgCl2 and 0.5 mM [32P]γ-ATP (1 μCi). Reaction mixtures were stopped by addition of SDS-sample buffer and subjected to SDS-PAGE. Dried gels were exposed to x-ray films. (D) VwkA phosphorylates MBP efficiently. In vitro phosphorylation assay was performed as described in C for myosin II except using 5 μM MBP instead of myosin II in the reaction mixture.
To ensure that the FLAG-VwkA was in fact biochemically active in vitro, we performed phosphorylation assays using myelin basic protein (MBP), a common substrate for many protein kinases under in vitro conditions. These assays revealed robust in vitro phosphorylation of MBP by FLAG-VwkA, indicating that the purified fusion protein is not lacking kinase activity (Figure 8D).
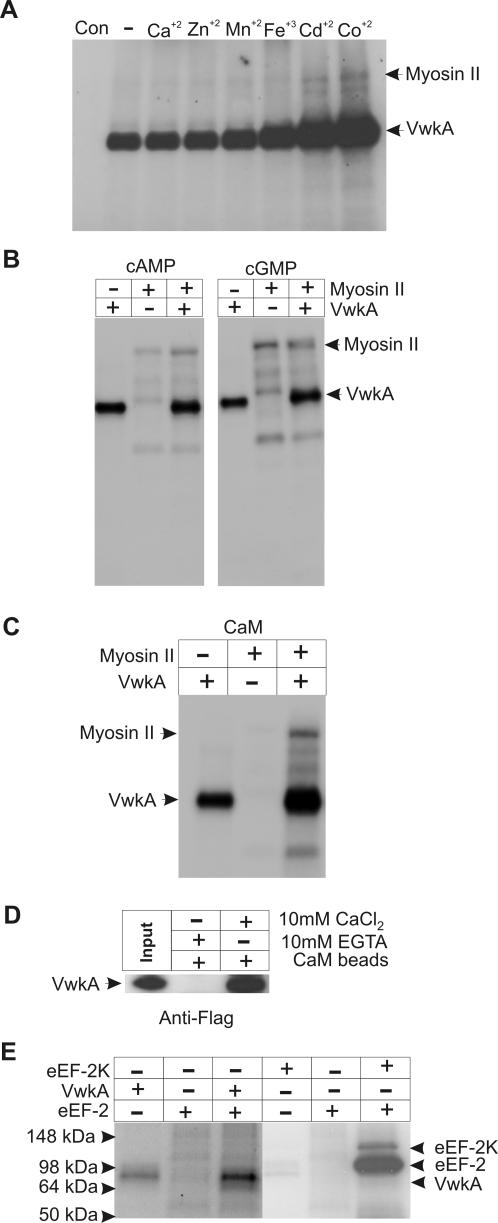
A series of additional tests were performed to see whether specific activators might be necessary to stimulate significant MHC kinase activity by the purified FLAG-VwkA protein. These tests included assessment of various divalent metal ions, cGMP, and cAMP. Although several of the divalent cations enhanced autophosphorylation, none of these compounds significantly enhanced VwkA kinase activity toward Dictyostelium myosin II (Figure 9, A and B). We further tested whether CaM affected VwkA kinase activity (Figure 9C). Although CaM did not significantly increase activity toward myosin II, it did produce an increase in autophosphorylation in the presence of myosin II. As a further test of a potential role for CaM in VwkA function, we tested whether calmodulin-agarose beads would pull-down FLAG-VwkA from total cell lysates in a calcium-dependent manner. FLAG-VwkA could be quantitatively recovered from crude lysates via binding to calmodulin-agarose, and this recovery was calcium dependent (Figure 9D). This behavior suggests the possibility that VwkA may directly bind to and be activated by CaM in the presence of calcium.
Figure 9.
Evaluation of VwkA kinase substrates and activation. (A) Effect of metal ions on phosphorylation of myosin II by FLAG-VwkA. Phosphorylation assays performed as in previous figure but with addition of 50 μM CaCl2, ZnCl2, MnCl2, Fe2Cl3, CdCl2, or CoCl2, as indicated, with myosin II as a substrate. (B) Effect of cAMP and cGMP on in vitro phosphorylation of myosin II by FLAG-VwkA. Assays performed as in previous figure, with addition of 0.5 mM cAMP or 0.5 mM cGMP, as indicated, with myosin II as substrate. (C) In vitro phosphorylation of myosin II in the presence of CaM. Phosphorylation assays were carried out using the purified FLAG-VwkA protein in the presence of calcium/calmodulin. (D) FLAG-VwkA is precipitated from crude cell lysates by CaM-agarose. Total lysates from FLAG-VwkA cells were incubated with CaM-agarose beads in the presence of either EGTA or CaCl2. Beads were washed twice in the same solution and then boiled in SDS-PAGE sample buffer, and solubilized proteins were subjected to SDS-PAGE and Western blot analysis by using FLAG antibodies. Left lane indicates signal from equivalent amount of input total cell lysate. (E) eEF-2 is not a substrate for VwkA in vitro. In vitro phosphorylation assays were performed with either mammalian eEF-2K or FLAG-VwkA and purified mammalian eEF-2 as substrate as described by Ryazanov et al. (1997). The eEF-2 and eEF-2 kinase samples were generously provided by Dr. Alexy Ryazanov.
Given the presence of a conserved eEF-2 homologue in Dictyostelium (accession no. A34347), and the absence of a recognizable eEF-2 kinase homologue in the Dictyostelium genome, we further tested the ability of FLAG-VwkA to phosphorylate mammalian eEF-2. In reactions performed with FLAG-VwkA and purified mammalian eEF-2 (Ryazanov et al., 1997), no phosphorylation of eEF-2 was observed. However, under similar kinase assay conditions the purified mammalian eEF-2K efficiently phosphorylates eEF-2 in vitro (Figure 9E), suggesting that VwkA is not an eEF-2 kinase in D. discoideum. It also should be noted that the mapped target sites in mammalian eEF-2 for mammalian eEF-2 kinase are two threonine residues at positions 56 and 58 (Redpath et al., 1993). Alignment analysis reveals that these residues are replaced in Dictyostelium eEF-2 by nonphosphorylatable methionine and cysteine, respectively. Although the Dictyostelium eEF-2 displays nearby serine and tyrosine residues, the lack of local sequence conservation also argues that Dictyostelium eEF-2 may not be targeted for phosphorylation via a pathway conserved with mammalian systems. However, definitive studies to determine whether VwkA can phosphorylate Dictyostelium eEF-2 will require biochemical analysis performed with eEF-2 purified from Dictyostelium cells.
Intracellular Localization of GFP-VwkA
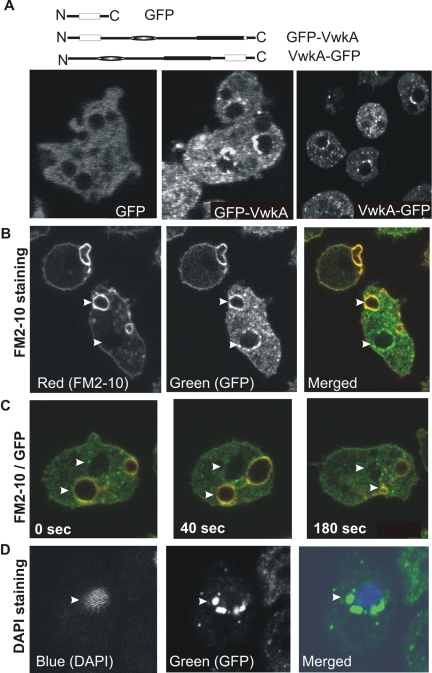
Earlier studies showed that GFP-tagged MHCK A and MHCK C are enriched in the cell cortex, whereas MHCK B is mostly distributed throughout the cytoplasm of nonmigrating cells (Liang et al., 2002; Nagasaki et al., 2002). The low endogenous expression levels of VwkA precluded immunocytochemical localization analysis, so we developed stable cell lines expressing N-terminal GFP-tagged VwkA (GFP-VwkA) and C-terminal GFP-tagged VwkA (VwkA-GFP) to allow dynamic localization studies to be performed on live cells (Figure 10A). Both constructs were evaluated in parallel to control for possible interference of the GFP moiety with possible N or C-terminal domain functions in vivo. Intracellular localization studies of these cells reveal that both GFP fusions to VwkA localize throughout the cytoplasm, with a punctate distribution that is frequently enriched adjacent to large spherical structures (Figure 10A). Some of the cells seem to have more than one organelle surrounded by GFP-VwkA fusion protein.
Figure 10.
Analysis of VwkA subcellular localization. (A) Schematic organization of GFP constructs for imaging. Cells expressing empty GFP vector displayed diffuse GFP fluorescence throughout the cytosol, whereas cells expressing the GFP-VwkA fusion (GFP at N terminus) and the VwkA-GFP (GFP at C terminus) both displayed punctate fluorescence throughout the cytosol in confocal sections. Cell lines growing in plastic petri plates were collected by centrifugation at low speed, washed with starvation buffer, and seeded into glass-bottomed microscopic chambers for live cell imaging via confocal microscopy. Images were collected using a 100× objective on a Zeiss LSM510. (B) Staining of contractile vacuoles with FM2-10 dye: GFP-VwkA cells seeded into glass bottom microscopic chambers as described above were stained with FM2-10 dye and images were collected starting 2 min after addition of dye by exciting at 488 nm. Time-lapse series of images were collected in parallel with red and green emission filters to obtain FM2-10 and GFP emission patterns, respectively. See Supplemental Movie files VwkA-GFP-FM-1.mov and VwkA-GFP-FM-2.mov for additional examples. (C) Contractions of organelles localized with GFP-VwkA. Time-lapse series of GFP-VwkA cell stained with FM2-10, dual image of GFP and FM2–10 signal. Series displays association of GFP signal adjacent to a noncontractile structure (top arrowhead; identified as the nucleus in data below) and GFP signal colocalizing with a contractile vacuole (bottom arrowhead). (D) DAPI staining of cells expressing VwkA-GFP: Cells expressing VwkA-GFP were stained with DAPI as described in text. See Supplemental Movie file VwkA-GFP-DAPI.mov for z-section of fixed cells displaying additional examples.
Time-lapse movies revealed that some of these circular-shaped bodies in the cell seemed to undergo contraction and expansion. This suggested that these circular bodies surrounded by GFP-VwkA might be contractile vacuoles, dynamic organelles involved in osmoregulation (Gerisch et al., 2002). To explore this localization further, live GFP-VwkA–expressing cells were stained with FM2-10, a lipophylic styryl fluorescent dye that preferentially labels contractile vacuoles in living Dictyostelium cells (Heuser et al., 1993; Gerisch et al., 2002). As shown in the Figure 10B, one of the circular shaped bodies is stained with FM2-10 dye, indicating that VwkA is localized to contractile vacuole in the cells (Figure 10B, top arrowhead). Time-lapse image series reveal the dynamic expansion and contraction of these structures (Figure 10C, bottom arrowhead). The contractile nature of these structures is particularly apparent when time-lapse movies derived from confocal microscopy images are evaluated (as presented in supplemental movie files VwkA-GFP-FM-1.mov and VwkA-GFP-FM-2.mov, where GFP is visible in the green channel and the FM2-10 signal is in red, with overlapping signals generating a yellow signal).
However, clustered, punctate VwkA-GFP staining also was frequently observed adjacent to another spherical structure that was not contractile vacuole and was not stained by FM2-10 dye (Figure 10B, bottom arrowhead, and C, top arrowhead). This pattern suggests that VwkA-GFP fusions localize not only to contractile vacuoles but also to another distinct circular organelle in the cell. This separate organelle displayed no contraction and expansion (Figure 10C). In addition to these distinct punctate localizations in the cell, GFP-VwkA also occurred as a punctuate signal dispersed throughout the cytoplasm (Figure 10). As a further step to characterize in vivo localization of VwkA, nuclei were stained with DAPI in VwkA-GFP cells. These studies demonstrate that the other circular organelle surrounded by punctate VwkA-GFP was the nucleus (Figure 10D and Supplemental Movie file VwkA-GFP-DAPI.mov). This clustered perinuclear localization is highly reminiscent of Golgi staining as reported for other Dictyostelium proteins (Schneider et al., 2000).
It is noteworthy that at least one other vWFA domain-carrying protein, Sec23 in S. cerevisiae, has a role in Golgi function and vesicle trafficking (Hicke et al., 1992; Whittaker and Hynes, 2002). This localization behavior further suggests that VwkA likely has broader roles in the cell than do previously identified α-kinases in Dictyostelium. Although all other Dictyostelium α-kinases studied to date seem to be dedicated MHC kinases that directly affect myosin II assembly, both the localization and biochemical properties of VwkA suggest broader roles that influence myosin II behavior but may involve other aspects of cellular physiology, perhaps even Golgi and contractile vacuole function.
DISCUSSION
Previous studies have demonstrated the presence of a family of unconventional kinases in D. discoideum, whose members display distinctive localization patterns during cell migration and cytokinesis. The thoroughly analyzed members to date include MHCK A, MHCK B, and MHCK C, and at both the biochemical level and in vivo all three seem to be involved in the control of myosin II filament assembly and localization within the cell (Steimle et al., 2001b; Liang et al., 2002; Nagasaki et al., 2002; Rico and Egelhoff, 2003). We have identified a fourth member of this family in Dictyostelium with similar domain organization that we refer to as MHCK D (our unpublished data). Our current study focused on VwkA, which is distinct from the other four MHCK proteins in its domain organization because it lacks a WD repeat domain and is the only family member containing a von Willebrand Factor A motif. Our studies were initiated with the working hypothesis that this protein, as a conserved member of the small family of α-kinases in Dictyostelium, also would act as an MHC kinase. Although initial cellular phenotypes were consistent with this hypothesis (growth kinetics, multinucleation, and developmental defects upon VwkA overexpression), several other cellular phenotypes were distinctly different from those of cells overexpressing MHCK A, B, or C. Specifically, cytokinesis defects in vwkA null cells, the enhanced abundance of MHC levels in cells overexpressing or lacking VwkA, and the apparent overassembly of myosin II in both VwkA++ cells and vwkA null cells did not fit that hypothesis. We therefore performed extensive biochemical analysis with immunopurified FLAG-VwkA in an attempt to find any conditions under which VwkA would efficiently phosphorylate myosin II. Although FLAG-VwkA autophosphorylates robustly in vitro, and efficiently phosphorylates MBP, we were not able to detect efficient MHC phosphorylation under any conditions. We currently believe that the cellular substrate(s) of VwkA is therefore likely to be a protein or proteins other than myosin II.
The localization of VwkA-GFP fusions to membrane organelle compartments, including the contractile vacuole system and a perinuclear Golgi-like compartment further suggested that VwkA may have cellular roles distinct and perhaps more broad than those identified for MHCK A, B, and C. One possibility is that VwkA plays a role in membrane dynamics or contractile activity of the contractile vacuole system. Although myosin II in mammalian systems is most widely associated with cortical cytoskeletal functions such as cell migration and cytokinesis, it is intriguing that several reports have implicated myosin II in Golgi function and trafficking in higher systems (DeGiorgis et al., 2002; Duran et al., 2003; Wollert et al., 2003). Further studies to determine whether myosin II in Dictyostelium may have more direct roles in vesicle trafficking, Golgi organization, or membrane dynamics in other settings would be worthwhile.
The contractile vacuole system in Dictyostelium is a reticulated membrane system consisting of interconnected tubules that collect water when cells are in hypoosmotic solutions, and then form discrete swollen cisternae or contractile vacuoles that fuse with the plasma membrane to expel the collected water (Becker et al., 1999; Gerisch et al., 2002). We performed kinetic analysis of average contractile vacuole lifetime in vwkA null and VwkA++ cells in starvation buffer, but we did not observe clear differences in overall vacuole lifetime relative to parental Ax2 cells (our unpublished data). Although this initial population scoring did not reveal gross differences in contractile vacuole lifetime, the subcellular localization pattern of GFP-VwkA suggest that this protein may influence osmotic behavior of the cell at the contractile vacuole level, a possibility that merits further study.
VwkA overexpression or gene disruption clearly impacts other aspects of cell function, with inhibition of cytokinesis in suspension culture being particularly striking. Myosin II phosphorylation analysis in vitro and Triton-insoluble cytoskeleton analysis both suggest that VwkA is not acting directly to disassemble myosin filaments but rather acts at some other regulatory level. It is possible that the apparent overassembly of myosin II filaments is a result of mass action, with the elevated abundance of MHC in these cell lines directly inducing a greater percentage of the pool to reside in filament form.
It is intriguing that several studies focused on regulators of Rho family GTPases in Dictyostelium have revealed multifaceted roles for these GTPase signaling systems, with pathway manipulations leading to both cytokinesis and contractile vacuole system defects (Knetsch et al., 2001; Rivero et al., 2002). Further studies are clearly merited to explore possible connections between VwkA and other cytokinesis-related signaling pathways and to establish the role of calmodulin in regulation of VwkA cellular functions. Preliminary studies performed with constructs that overexpress only the vWFA domain of VwkA or that express a kinase-dead form of VwkA suggest that the vWFA domain rather than kinase activity is critical for the developmental defects and cytokinesis defects reported in this work (Betapudi and Egelhoff, unpublished data). Future studies to identify interacting partners for this vWFA domain may help illuminate the signaling pathways that interact with VwkA.
Our current hypothesis is that the suspension culture defects in cytokinesis incurred upon VwkA overexpression may relate either to altered membrane trafficking/fusion events during cytokinesis, by analogy to the roles of the Dictyostelium BEACH proteins LvsA and LvsB (De Lozanne, 2003), or that VwkA overexpression acts via other proteins (perhaps Rho family GTPases or cGMP pathways) to alter myosin filament assembly control in a manner that blocks normal contractile ring formation and contraction. Further studies are needed to test these hypotheses.
Acknowledgments
We thank Margaret Clarke and Arturo De Lozanne for helpful suggestions on contractile vacuole imaging. Purified mammalian eEF-2 and eEF-2 kinase were generous gifts from Dr. Alexy Ryazanov. This work was supported by National Institutes of Health grant GM-50009 (to T.T.E.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–07–0639) on February 23, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Aarts, M., Iihara, K., Wei, W. L., Xiong, Z. G., Arundine, M., Cerwinski, W., MacDonald, J. F., and Tymianski, M. (2003). A key role for TRPM7 channels in anoxic neuronal death. Cell 115, 863–877. [DOI] [PubMed] [Google Scholar]
- Adachi, H., Hasebe, T., Yoshinaga, K., Ohta, T., and Sutoh, K. (1994). Isolation of Dictyostelium discoideum cytokinesis mutants by restriction enzyme-mediated integration of the blasticidin S resistance marker. Biochem. Biophys. Res. Commun. 205, 1808–1814. [DOI] [PubMed] [Google Scholar]
- Becker, M., Matzner, M., and Gerisch, G. (1999). Drainin required for membrane fusion of the contractile vacuole in Dictyostelium is the prototype of a protein family also represented in man. EMBO J. 18, 3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betapudi, V., Shoebotham, K., and Egelhoff, T. T. (2004). Generation of double gene disruptions in Dictyostelium discoideum using a single antibiotic marker selection. Biotechniques 36, 106–112. [DOI] [PubMed] [Google Scholar]
- Buxton, D. B., Golomb, E., and Adelstein, R. S. (2003). Induction of nonmuscle myosin heavy chain II-C by butyrate in RAW 264.7 mouse macrophages. J. Biol. Chem. 278, 15449–15455. [DOI] [PubMed] [Google Scholar]
- Chubanov, V., Waldegger, S., Schnitzler, M., Vitzthum, H., Sassen, M. C., Seyberth, H. W., Konrad, M., Gudermann, T. (2004). Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc. Natl. Acad. Sci. USA 101, 2894–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy, C. E., Mendoza, M. G., Naismith, T. V., Kolman, M. F., and Egelhoff, T. T. (1997). Identification of a protein kinase from Dictyostelium with homology to the novel catalytic domain of myosin heavy chain kinase A. J. Biol. Chem. 272, 11812–11815. [DOI] [PubMed] [Google Scholar]
- Côté, G. P., Luo, X., Murphy, M. B., and Egelhoff, T. T. (1997). Mapping of the novel protein kinase catalytic domain of Dictyostelium myosin II heavy chain kinase A. J. Biol. Chem. 272, 6846–6849. [DOI] [PubMed] [Google Scholar]
- Côté, G. P., and McCrea, S. M. (1987). Selective removal of the carboxyl-terminal tail end of the Dictyostelium myosin II heavy chain by chymotrypsin. J. Biol. Chem. 262, 13033–13038. [PubMed] [Google Scholar]
- de la Roche, M. A., Smith, J. L., Betapudi, V., and Egelhoff, T. T. (2002). Signaling pathways regulating Dictyostelium myosin II. J. Muscle Res. Cell Motil. 23, 703–718. [DOI] [PubMed] [Google Scholar]
- De Lozanne, A. (2003). The role of BEACH proteins in Dictyostelium. Traffic 4, 6–12. [DOI] [PubMed] [Google Scholar]
- De Lozanne, A., and Spudich, J. A. (1987). Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science 236, 1086–1091. [DOI] [PubMed] [Google Scholar]
- DeGiorgis, J. A., Reese, T. S., and Bearer, E. L. (2002). Association of a nonmuscle myosin II with axoplasmic organelles. Mol. Biol. Cell 13, 1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorovkov, M. V., Pavur, K. S., Petrov, A. N., and Ryazanov, A. G. (2002). Regulation of elongation factor-2 kinase by pH. Biochemistry 41, 13444–13450. [DOI] [PubMed] [Google Scholar]
- Drennan, D., and Ryazanov, A. G. (2004). Alpha-kinases: analysis of the family and comparison with conventional protein kinases. Prog. Biophys. Mol. Biol. 85, 1–32. [DOI] [PubMed] [Google Scholar]
- Duran, J. M., Valderrama, F., Castel, S., Magdalena, J., Tomas, M., Hosoya, H., Renau-Piqueras, J., Malhotra, V., and Egea, G. (2003). Myosin motors and not actin comets are mediators of the actin-based Golgi-to-endoplasmic reticulum protein transport. Mol. Biol. Cell 14, 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhoff, T. T., Lee, R. J., and Spudich, J. A. (1993). Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell 75, 363–371. [DOI] [PubMed] [Google Scholar]
- Eichinger, L., and Noegel, A. A. (2003). Crawling into a new era-the Dictyostelium genome project. EMBO J. 22, 1941–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicker, P. F., Peltz, G., Sheetz, M. P., Parham, P., and Spudich, J. A. (1985). Site-specific inhibition of myosin-mediated motility in vitro by monoclonal antibodies. J. Cell Biol. 100, 1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futey, L. M., Medley, Q. G., Côté, G. P., and Egelhoff, T. T. (1995). Structural analysis of myosin heavy chain kinase A from Dictyostelium. Evidence for a highly divergent protein kinase domain, an amino-terminal coiled-coil domain, and a domain homologous to the β-subunit of heterotrimeric G proteins. J. Biol. Chem. 270, 523–529. [DOI] [PubMed] [Google Scholar]
- Gerisch, G., Heuser, J., and Clarke, M. (2002). Tubular-vesicular transformation in the contractile vacuole system of Dictyostelium. Cell Biol. Int. 26, 845–852. [DOI] [PubMed] [Google Scholar]
- Gerisch, G., and Weber, I. (2000). Cytokinesis without myosin II. Curr. Opin. Cell Biol. 12, 126–132. [DOI] [PubMed] [Google Scholar]
- Hanks, S. K., and Hunter, T. (1995). Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9, 576–596. [PubMed] [Google Scholar]
- Hardie, D. G. (1994). An emerging role for protein kinases: the response to nutritional and environmental stress. Cell Signal. 6, 813–821. [DOI] [PubMed] [Google Scholar]
- Heuser, J., Zhu, Q., and Clarke, M. (1993). Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. J. Cell Biol. 121, 1311–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke, L., Yoshihisa, T., and Schekman, R. (1992). Sec23p and a novel 105-kDa protein function as a multimeric complex to promote vesicle budding and protein transport from the endoplasmic reticulum. Mol. Biol. Cell 3, 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda, T., Monzen, K., Hiroi, Y., Oka, T., Takimoto, E., Yazaki, Y., Nagai, R., and Komuro, I. (2001). A novel myocyte-specific gene Midori promotes the differentiation of P19CL6 cells into cardiomyocytes. J. Biol. Chem. 276, 35978–35989. [DOI] [PubMed] [Google Scholar]
- Ingerslev, J., Hvitfeldt, P. L., and Sorensen, B. (2004). Current treatment of von Willebrand's disease. Hamostaseologie 24, 56–64. [DOI] [PubMed] [Google Scholar]
- Knecht, D. A., and Loomis, W. F. (1987). Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science 236, 1081–1086. [DOI] [PubMed] [Google Scholar]
- Knetsch, M. L., Schafers, N., Horstmann, H., and Manstein, D. J. (2001). The Dictyostelium Bcr/Abr-related protein DRG regulates both Rac- and Rab-dependent pathways. EMBO J. 20, 1620–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolman, M. F., Futey, L. M., and Egelhoff, T. T. (1996). Dictyostelium myosin heavy chain kinase A regulates myosin localization during growth and development. J. Cell Biol. 132, 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkir, Y., de Chassey, B., Dubois, A., Bogdanovic, A., Brady, R. J., Destaing, O., Bruckert, F., O'Halloran, T. J., Cosson, P., and Letourneur, F. (2003). The AP-1 clathrin-adaptor is required for lysosomal enzymes sorting and biogenesis of the contractile vacuole complex in Dictyostelium cells. Mol. Biol. Cell 14, 1835–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi, S., Polyakov, M., and Egelhoff, T. T. (2000). Green fluorescent protein and epitope tag fusion vectors for Dictyostelium discoideum. Plasmid 44, 231–238. [DOI] [PubMed] [Google Scholar]
- Liang, W., Licate, L., Warrick, H., Spudich, J., and Egelhoff, T. (2002). Differential localization in cells of myosin II heavy chain kinases during cytokinesis and polarized migration. BMC Cell Biol. 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, X., Crawley, S. W., Steimle, P. A., Egelhoff, T. T., and Côté, G. P. (2001). Specific phosphorylation of threonine by the Dictyostelium myosin II heavy chain kinase family. J. Biol. Chem. 276, 17836–17843. [DOI] [PubMed] [Google Scholar]
- Manning, G., Whyte, D. B., Martinez, R., Hunter, T., and Sudarsanam, S. (2002). The protein kinase complement of the human genome. Science 298, 1912–1934. [DOI] [PubMed] [Google Scholar]
- Manstein, D. J., Titus, M. A., De Lozanne, A., and Spudich, J. A. (1989). Gene replacement in Dictyostelium: generation of myosin null mutants. EMBO J. 8, 923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer, A., et al. (2003). CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 31, 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita, T., Dong, Z., and Sadler, J. E. (1994). von Willebrand's factor and von Willebrand's disease. Curr. Opin. Hematol. 1, 362–368. [PubMed] [Google Scholar]
- Montell, C. (2003). Mg2+ homeostasis: the Mg2+nificent TRPM chanzymes. Curr. Biol. 13, R799–R801. [DOI] [PubMed] [Google Scholar]
- Montell, C., et al. (2002). A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell 9, 229–231. [DOI] [PubMed] [Google Scholar]
- Nagasaki, A., Itoh, G., Yumura, S., and Uyeda, T. Q. (2002). Novel myosin heavy chain kinase involved in disassembly of myosin II filaments and efficient cleavage in mitotic Dictyostelium cells. Mol. Biol. Cell 13, 4333–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent, C. A., Blacklock, B. J., Froehlich, W. M., Murphy, D. B., and Devreotes, P. N. (1998). G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95, 81–91. [DOI] [PubMed] [Google Scholar]
- Pavur, K. S., Petrov, A. N., and Ryazanov, A. G. (2000). Mapping the functional domains of elongation factor-2 kinase. Biochemistry 39, 12216–12224. [DOI] [PubMed] [Google Scholar]
- Peltz, G., Spudich, J. A., and Parham, P. (1985). Monoclonal antibodies against seven sites on the head and tail of Dictyostelium myosin. J. Cell Biol. 100, 1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath, N. T., Price, N. T., Severinov, K. V., and Proud, C. G. (1993). Regulation of elongation factor-2 by multisite phosphorylation. Eur. J. Biochem. 213, 689–699. [DOI] [PubMed] [Google Scholar]
- Rico, M., and Egelhoff, T. T. (2003). Myosin heavy chain kinase B participates in the regulation of myosin assembly into the cytoskeleton. J. Cell. Biochem. 88, 521–532. [DOI] [PubMed] [Google Scholar]
- Rivero, F., Illenberger, D., Somesh, B. P., Dislich, H., Adam, N., and Meyer, A. K. (2002). Defects in cytokinesis, actin reorganization and the contractile vacuole in cells deficient in RhoGDI. EMBO J. 21, 4539–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryazanov, A. G. (2002). Elongation factor-2 kinase and its newly discovered relatives. FEBS Lett. 514, 26–29. [DOI] [PubMed] [Google Scholar]
- Ryazanov, A. G., Pavur, K. S., and Dorovkov, M. V. (1999). Alpha-kinases: a new class of protein kinases with a novel catalytic domain. Curr. Biol. 9, R43–R45. [DOI] [PubMed] [Google Scholar]
- Ryazanov, A. G., et al. (1997). Identification of a new class of protein kinases represented by eukaryotic elongation factor-2 kinase. Proc. Natl. Acad. Sci. USA 94, 4884–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989). Molecular Cloning. A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Saxe, C. L., Ginsburg, G. T., Louis, J. M., Johnson, R., Devreotes, P. N., and Kimmel, A. R. (1993). CAR2, a prestalk cAMP receptor required for normal tip formation and late development of Dictyostelium discoideum. Genes Dev. 7, 262–272. [DOI] [PubMed] [Google Scholar]
- Schneider, N., Schwartz, J. M., Kohler, J., Becker, M., Schwarz, H., and Gerisch, G. (2000). Golvesin-GFP fusions as distinct markers for Golgi and post-Golgi vesicles in Dictyostelium cells. Biol. Cell 92, 495–511. [DOI] [PubMed] [Google Scholar]
- Steimle, P. A., Naismith, T., Licate, L., and Egelhoff, T. T. (2001a). WD repeat domains target Dictyostelium myosin heavy chain kinases by binding directly to myosin filaments. J. Biol. Chem. 276, 6853–6860. [DOI] [PubMed] [Google Scholar]
- Steimle, P. A., Yumura, S., Côté, G. P., Medley, Q. G., Polyakov, M. V., Leppert, B., and Egelhoff, T. T. (2001b). Recruitment of a myosin heavy chain kinase to actin-rich protrusions in Dictyostelium. Curr. Biol. 11, 708–713. [DOI] [PubMed] [Google Scholar]
- Takada, Y., Kamata, T., Irie, A., Puzon-McLaughlin, W., and Zhang, X. P. (1997). Structural basis of integrin-mediated signal transduction. Matrix Biol. 16, 143–151. [DOI] [PubMed] [Google Scholar]
- Theibert, A., and Devreotes, P. N. (1986). Surface receptor-mediated activation of adenylate cyclase in Dictyostelium. Regulation by guanine nucleotides in wild-type cells and aggregation deficient mutants. J. Biol. Chem. 261, 15121–15125. [PubMed] [Google Scholar]
- Weber, I., Neujahr, R., Du, A., Kohler, J., Faix, J., and Gerisch, G. (2000). Two-step positioning of a cleavage furrow by cortexillin and myosin II. Curr. Biol. 10, 501–506. [DOI] [PubMed] [Google Scholar]
- Whittaker, C. A., and Hynes, R. O. (2002). Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol. Biol. Cell 13, 3369–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland, I., Arden, K. C., Michels, D., Klein-Hitpass, L., Bohm, M., Viars, C. S., and Weidle, U. H. (1999). Isolation of DICE 1, a gene frequently affected by LOH and downregulated in lung carcinomas. Oncogene 18, 4530–4537. [DOI] [PubMed] [Google Scholar]
- Wigelsworth, D. J., Krantz, B. A., Christensen, K. A., Lacy, D. B., Juris, S. J., and Collier, R. J. (2004). Binding stoichiometry and kinetics of the interaction of a human anthrax toxin receptor, CMG2, with protective antigen. J. Biol. Chem. 279, 23349–23356. [DOI] [PubMed] [Google Scholar]
- Wollert, T., DePina, A. S., DeSelm, C. J., and Langford, G. M. (2003). Rho-kinase is required for myosin-II-mediated vesicle transport during M-phase in extracts of clam oocytes. Biol. Bull. 205, 195–197. [DOI] [PubMed] [Google Scholar]