Abstract
A variety of techniques exist to investigate retinal and choroidal vascular changes in experimental mouse models of human ocular diseases. While all have specific advantages, a method for evaluating the choroidal vasculature in pigmented mouse eyes has been more challenging especially for whole mount visualization and morphometric analysis. Here we report a simple, reliable technique involving bleaching pigment prior to immunostaining the vasculature in whole mounts of pigmented mouse choroids. Eyes from healthy adult pigmented C57BL/6J mice were used to establish the methodology. The retina and anterior segment were separated from the choroid. The choroid with retinal pigment epithelial cells (RPE) and sclera was soaked in 1% ethylenediaminetetraacetic acid (EDTA) to remove the RPE. Tissues were fixed in 2% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). Choroids were subjected to melanin bleaching with 10% hydrogen peroxide (H2O2) at 55°C for 90 minutes, washed in PBS and then immunostained with anti-podocalyxin antibody to label vascular endothelium followed by Cy3-AffiniPure donkey anti-goat IgG at 4°C overnight. Images of immunostained bleached choroids were captured using a Zeiss 710 confocal microscope. In addition to control eyes, this method was used to analyze the choroids from subretinal sodium iodate (NaIO3) RPE atrophy and laser-induced choroidal neovascularization (CNV) mouse models. The H2O2 pretreatment effectively bleached the melanin, resulting in a transparent choroid. Immunolabeling with podocalyxin antibody following bleaching provided excellent visualization of choroidal vasculature in the flat perspective. In control choroids, the choriocapillaris (CC) displayed different anatomical patterns in peripapillary (PP), mid peripheral (MP) and far peripheral (FP) choroid. Morphometric analysis of the vascular area (VA) revealed that the CC was most dense in the PP region (87.4±4.3% VA) and least dense in FP (79.9±6.7% VA). CC diameters also varied depending on location from 11.4 ±1.97 mm in PP to 15.1±3.15 mm in FP. In the NaIO3-injected eyes, CC density was significantly reduced in the RPE atrophic regions (50.7±5.8% VA in PP and 45.8±6.17% VA in MP) compared to the far peripheral non-atrophic regions (82.8±3.8% VA). CC diameters were significantly reduced in atrophic regions (6.35±1.02 mm in PP and 6.5±1.2 mm in MP) compared to non-atrophic regions (14.16±2.12 mm). In the laser-induced CNV model, CNV area was 0.26±0.09 mm2 and luminal diameters of CNV vessels were 4.7±0.9 mm. Immunostaining on bleached choroids with anti-podocalyxin antibody provides a simple and reliable tool for visualizing normal and pathologic choroidal vasculature in pigmented mouse eyes for quantitative morphometric analysis. This method will be beneficial for examining and evaluating the effects of various treatment modalities on the choroidal vasculature in mouse models of ocular diseases such as age-related macular degeneration, and degenerative genetic diseases.
Keywords: choroidal blood vessels, mouse, podocalyxin, sodium iodate, laser CNV, immunostaining
1. Introduction
There is increasing evidence that alterations in the choroidal vasculature play a key role in the early pathogenesis of age-related macular degeneration (AMD) (Seddon et al., 2016; Mullins et al., 2011; McLeod et al., 2009). The understanding of pathologic processes in the choroidal blood vessels requires an understanding of normal choroidal vascular structure and angioarchitecture. The choroidal vasculature has been extensively investigated in human and in experimental animals by various methods including histology, India ink injections, flat preparations, neoprene latex, and vascular corrosion casts, and alkaline phosphatase staining (Weiter and Ernest, 1974; Hayreh, 1975 and 1990; Yoneya and Tso, 1987; Bhutto and Amemiya, 2001; McLeod and Lutty, 1994; Jiao et al., 2020). Clinically, in vivo imaging includes conventional techniques using both fluorescein (FA) and indocyanine green angiography (ICGA) (Gelisken et al., 1998; Yannuzzi, 2011). In addition, optical coherence tomography angiography (OCTA) (de Carlo et al., 2015a, 2015b; Wong et al., 2011) and spectral domain optical coherence tomography (SD-OCT) are now routinely used in the clinic (Margolis and Spaide, 2009; Mrejen and Spaide, 2013; Ferrara et al., 2016; Koh et al., 2017; Wang et al., 2018). Although, the general distribution and pattern of the choroidal vasculature of primates and other species including rodents has been described, a reliable method for the qualitative and quantitative histological assessment of choroidal vasculature in pigmented mice would improve and benefit from the removal of melanin pigment. Here we describe a simple, reliable method for visualizing the choroidal vasculature in whole mounts of pigmented mouse eyes using a prebleaching technique compatible with immunostaining with anti-podocalyxin antibody. While the method was initially developed using normal pigmented mouse eyes (Bhutto et al., 2020), we subsequently compared the morphometric results with choroidal flat-mounts from a subretinal sodium iodate (NaIO3) retinal pigmented epithelial (RPE) atrophy model and a laser-induced choroidal neovascularization (CNV) model.
Currently, the NaIO3-induced RPE atrophy model and laser-induced CNV model are among the most widely used experimental mouse models of dry and wet AMD. The laser-induced CNV model, which leads to the growth of new blood vessels from the choroid into the subretinal space, mimics the main characteristic of the exudative form of human AMD (Tobe et al., 1998; Pennesi et al., 2012; Lambert et al., 2013) and provides an opportunity to explore the molecular mechanisms of CNV. The NaIO3-induced retinal degeneration model is universally used as a preclinical model of atrophic AMD and geographic atrophy (GA) (Machalinska et al., 2010; Jian et al., 2015; Chowers et al., 2017). NaIO3 is thought to directly affect the RPE cells with secondary effects on photoreceptors and the CC. The effects of systemic NaIO3 injection on retinal degeneration has been described in different mammalian species including swine, rabbit, rat and mouse with varying doses and routes of administration (Mones et al., 2016; Petrus-Reurer et al., 2017; Koh et al., 2019; Machalinska et al., 2010; Chowers et al., 2017). The systemic delivery, however, results in sporadic, widespread RPE degeneration. We recently reported a novel model in which a single 1μl subretinal injection of NaIO3 created a focal area of RPE atrophy which was followed by photoreceptor cell loss, subretinal glial membrane formation, and CC degeneration in Brown Norway pigmented rats (Bhutto et al., 2018). We report herein the choroidal vascular changes in the mice given subretinal NaIO3 injections at 3 weeks following injection. This timepoint was selected based on atrophic progression in the rat model which includes RPE loss by three days, extensive photoreceptor loss by seven days and choriocapillaris changes by 14 days post-injection (Bhutto et al, 2018).
The aim of this study was to develop a simple reliable method to stain choroidal vasculature in highly pigmented normal mouse eyes and to use the technique to perform semi-quantitative morphometric analysis of pathologic changes in choroidal vasculature in two murine models of AMD. Lastly, we compared the morphometric features in these models to previous results from human choroids with GA and neovascular AMD (nAMD).
2. Materials and methods
Ethical statement
All experiments were performed using adult C57BL/6J (pigmented) mice aged 8 to 10 weeks of both genders (The Jackson Laboratory, Bar Harbor, ME, USA). Mice were maintained on a standard 12-hour dark/12-hour light cycle with water and standard Chow diet (LabDiet Picolab Advanced; PMI Nutrition International, LLC., Arden Hills, MN) ad libitum. Animal husbandry and procedures were performed within the guidelines of the Johns Hopkins University Research Ethics Committee. All studies adhered to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Care and Use Committee of Johns Hopkins University.
2.1. Subretinal Sodium Iodate (NaIO3) Injection
To demonstrate the usefulness of podocalyxin immunostaining to study pathologic changes in choroidal vasculature, we first optimized the NaIO3 concentration of our rat subretinal NaIO3 model (Bhutto et al., 2018) in mice. We found that a single 50 mg/kg dose of NaIO3 was sufficient to generate rapid and well defined focal RPE loss. NaIO3 (#S4007–100G; Sigma-Aldrich, St. Louis, MO) was prepared fresh by dissolving in sterilized phosphate-buffered saline (PBS) to a concentration of 50 mg/kg. Mice were anesthetized by intraperitoneal (IP) injection of ketamine/xylazine (50mg/kg ketamine + 10mg/kg xylazine in 0.9% NaCl) mixture. One microliter of the NaIO3 was delivered into the subretinal space using a Pico-Injector (PLI-100, Harvard Apparatus, Holliston, MA) as previously described (Baba et al., 2010). Following the injection, Neomycin and Polymyxin B and Bacitracin Zinc Ophthalmic ointment (Bausch & Lomb, Inc., Bridgewater, NJ) was applied topically. Mice were sacrificed at 3 weeks post-injection of subretinal NaIO3 by intraperitoneal Ketamine/Xylazine overdose. Eyes having subretinal or vitreous hemorrhage were excluded from analysis.
2.2. Laser-induced CNV
Mice were anesthetized as described above. Pupils were dilated with 1% Tropicamide Ophthalmic solution (Akron Inc., Lake Forest, IL). After pupil dilation, lubricating eye drops (Alcon Laboratories, Inc., Fort Worth, TX) were applied to the cornea. The fundus was viewed with an imaging camera, and laser photocoagulation was induced using the image-guided laser system (Micron III, Phoenix Research Laboratories, Pleasanton, CA). CNV was established according to published protocols (Gong et al., 2015). Briefly, four laser burns were placed at equal distance from the optic nerve head in each eye by a green Argon laser pulse with a wavelength of 532 nm, a fixed spot diameter of 50 μm, duration of 70 ms, and power level of 300 mW. After laser photocoagulation, the eyes were gently rinsed with sterile saline to remove the lubricating eye drops and treated with an 0.5% erythromycin ophthalmic ointment (Bausch & Lomb, Inc., Bridgewater, NJ). Mice were sacrificed at 2 weeks after laser treatment by an overdose of intraperitoneal Ketamine/Xylazine.
2.3. Micron III Fundus Image Acquisition
Mice were anesthetized as described above and pupils were dilated with 1% Tropicamide Ophthalmic solution (Akron Inc., Lake Forest, IL). The animals were positioned on the Micron III (Phoenix Research Labs) stage and 2.5% Hypromellose Ophthalmic demulcent solution (Gonak TM; Akorn, Inc., Lake Forest, IL) was applied to the eye. The camera and eye position were adjusted ensuring correct alignment and focus on the optic nerve head plane using standard color fundus photography. Digital images of control mice and NalO3 mice at three weeks post-injection were captured as TIFFS at 1024 × 768 pixels resolution. Two weeks post laser treatment, fluorescein angiography was performed on CNV mice following an intraperitoneal injection (50uL) of 10% sodium fluorescein injection (100mg/mL; Altaire Pharmaceutical Inc., Aquebogue, NY). The time from injection to image capture was approximately 1 minute.
2.4. Tissue fixation and Melanin Bleaching
Mice were sacrificed by overdose (0.50mL) intraperitoneal injection of ketamine (50mg/kg) and xylazine (10mg/kg) mixture. After removing the cornea and lens, the anterior segment (iris and ciliary body) and retina were carefully separated from the RPE/choroid/sclera. The RPE/choroid/sclera complex was soaked in freshly prepared 1% EDTA (JT Baker grade, Avantor Performance Materials, LLC, PA, USA) in PBS for 2 hours at room temperature (RT) to facilitate removal of the RPE cells. If any adherent RPE cells remained, they were removed by manually pipetting the EDTA solution into the eyecup with a 25G blunt needle mounted on 1CC syringe. The RPE denuded choroids were then briefly washed with PBS, then fixed in 2% PFA in PBS at 4°C overnight (ON). The following day, tissues were washed three times in PBS containing 0.1% Triton X-100 at RT. The choroidal melanin in mouse eyes was bleached by incubating tissues in freshly prepared 10% hydrogen peroxide (H2O2; Sigma; H1009–100ML) diluted in PBS. Tissues were placed into microcentrifuge tubes with diluted H2O2 and incubated at 55°C for 90 minutes in a vacuum oven. After bleaching, the tissues were washed three times in PBS and twice in PBS w/0.1%Triton X-100 before proceeding with immunohistochemistry.
2.5. Immunolabeling of Choroidal Flat mounts with Podocalyxin
Immunohistochemical staining was performed on bleached whole choroids from normal control mice (n=3), subretinal NaIO3 injected mice (n=5) and laser induced CNV mice (n=3). Tissues were permeabilized in PBS containing 0.1% Triton X-100 (PBST), and then incubated in 2% normal donkey serum diluted in PBST with 1% bovine albumin serum (BSA) overnight at 4°C. Subsequently, the tissues were incubated with primary antibody (goat anti-mouse podocalyxin, R&D systems, Cat#AF1556; 1:100) ON at 4°C. After washing three times in PBS (20 min each), choroids were incubated with secondary antibody donkey anti-goat conjugated with Alexa Fluor 488 or Cy3 ON at 4°C. Tissues were then washed three times in PBS (20 min each) at 4°C, flat mounted in PBS on a glass slides and cover-slipped for confocal microscopy.
2.6. Immunolabeling of human choroids with UEA lectin.
We have previously characterized the choroidal changes in human donor eyes with both GA and CNV (Edwards et al, 2023, McLeod et al, 2016, Seddon et al, 2016). For this study, we compared data from our archived human donor images to the two mouse models. Both the GA donor and the donor with CNV shown herein were 92 year old Caucasian males. Human donor eyes were processed as previously described (Edwards et al, 2023, McLeod et al, 2016, Seddon et al, 2016). Briefly, eyes were shipped on wet ice and retinas were removed after imaging eyes. RPE was removed by incubating the choroid in 1% EDTA. Choroids were fixed overnight in 2% PFA in 0.1M cocadylate buffer and then washed in TBS. Choroids were then blocked in 5% goat serum prepared in TBS containing 0.1% Triton X-100 and 1% BSA overnight and rabbit anti-IBA1 (1:500 dilution; Wako Chemicals USA, Inc., Richmond, VA, USA) for 3 days at 4°C. After three washes in TBS with 0.1% Triton X-100 (TBST), choroids were incubated in goat-anti rabbit-AlexaFluor647 (AF647; Invitrogen, Carlsbad, CA, USA) and Ulex europaeus agglutinin (UEA) lectin/fluorescein isothiocyanate (FITC)-conjugated (1:100 dilution; Sigma-Aldrich Corp., St. Louis, MO, USA) for 2 days at 4 °C. Choroids were imaged on a Zeiss 710 confocal microscope. For the present study, we are only showing the UEA lectin staining.
2.7. Confocal Microscopy
Flat mounts were imaged at 5X, 10X and 20X magnification with a Zeiss LSM 710 Confocal Laser Scanning Microscope (Carl Zeiss Microscopy, LLC, Thornwood, NY, USA). Tiled Z-stack images were obtained using the optimal settings defining the first and last Z position of the Z-stack using a frame size of X-1024 and Y-1024. The pinhole size was set at 1 airy unit (AU). To capture the entire choroid for quantification, 36 tiled Z-stacks (6×6 tile regions) with 10% overlap were obtained at 10X magnification, and then were combined into a maximum intensity projection and stitched together.
2.8. Image analysis for percent vascular area (%VA) and luminal diameters
Maximum intensity projection, 10X stitched images were exported from Zen Software as full resolution Tiff images and opened in Adobe Photoshop (CS6, Adobe Systems Incorporated, San Jose, CA, USA). Three 1204 × 1204 pixel dimension selections (equivalent to 0.5mm2) were made from peripapillary, mid peripheral and far peripheral regions in whole-mount choroid, as described previously (McLeod et al., 2009), and pasted into new image documents. For %VA determination, each image was adjusted using levels and thresholding and saved as a separate Tiff image for analysis in ImageJ (McLeod et al., 2016). The images were converted to binary and the calculated black and white pixel macro in ImageJ was used to perform %VA determinations. For CC diameter measurements, images were saved as RGB Tiffs and measured in ImageJ manually as described previously (McLeod et al., 2009).
2.9. Statistical analysis
Data are presented as the mean ± standard deviations (SDs). Statistical analyses were performed by a student’s t-test with Welch’s correction for two samples with unequal variances assumed using a GraphPad Prism 9 (version 9.5.1) software (GraphPad Software, Boston, MA, USA). The P value of 0.05 or less was considered statistically significant.
3. Results
3.1. Choroidal Vasculature of Normal Control Mice
Color fundus photos prior to sacrifice showed a normal spoke wheel appearance of retinal vessels and a uniform RPE in control eyes (Fig. 1A). Pretreatment of whole pigmented choroids with H2O2 prior to immunostaining resulted in complete bleaching of mouse choroidal melanocytes providing a transparent tissue with no apparent loss in tissue integrity (Fig. 1B, C). Immunostaining of bleached choroids with podocalyxin antibody resulted in uniform staining of the choroidal vasculature with the exception of the long posterior ciliary arteries (LPCA) which were only faintly stained (Fig. 1D). The choroidal vasculature of the normal mouse showed a similar morphological pattern as described in rat choroid (Bhutto and Amemiya, 2001). In the peripapillary (PP) region (Fig. 1E), the chorioocapillaris (CC) formed a dense freely interanatomosing honeycomb-like meshwork of capillaries. The intercapillary septa were rounded in shape and fairly uniform in size. In mid periphery (MP) (Fig. 1F), CC had a more linear arrangement with fewer interanastomosing connections and elongated intercapillary septa. In the far periphery (FP) (Fig. 1G), choroidal vessels had a palm-shaped pattern and consisted of arterioles, venules, and capillaries in the same plane. This arrangement allowed for CC connections between the arteriolar and venular components to be easily identified.
Figure 1:

Representative fundus photograph of a normal control mouse (A). Pigmented C57BL/6J mouse choroid before (B) and after 10% H2O2 bleaching (C). Flat mounted control mouse choroid immunolabeled with anti-podocalyxin antibody showing staining of the choroidal vasculature at low magnification (D). Morphology of the choriocapillaris (CC) in the peripapillary region (E) showing a dense freely interanastomosing honeycomb-like meshwork of capillaries. In the midperiphery (F), CC had a more linear arrangement with fewer interanastomosing connections and elongated intercapillary septa. In far periphery (G), choroidal vessels had a palm-shaped pattern and consisted of arterioles, venules and capillaries in the same plane. Scale bars = 1 mm in B–C, 500 μm in D and 50 μm in E–G.
3.2. NaIO3 Choroid
Funduscopic examination at 3 weeks post injection revealed widespread RPE degeneration and pigment clumping in the peripapillary and midperipheral regions of NalO3-injected eyes (Fig. 2A). As in the control, pretreatment with H2O2 resulted in complete bleaching of choroidal pigment without loss of morphology in NaIO3-injected choroids (Fig. 2B, C). CC degeneration was concomitant with overlying RPE atrophy. The average area of degenerative CC was 7.0 ± 1.56mm2 in size and was chiefly confined to the peripapillary and midperipheral choroid [Fig. 2D, E (arrowheads) and Fig. 3 (hand traced)]. The CC in these regions showed few interanastomosing connections, constriction of the remaining viable capillaries and increased visibility of underlying arteries and veins (Figs. 2F and 4). The far peripheral CC maintained a normal morphology (Figs. 2G, 3, 4I–L). Comparison of CC morphology in three distinct areas of NalO3 injected choroids are shown in Figure 4. In some NalO3 injected eyes, small CNV formations were observed at the injection site in midperipheral choroid (data not shown).
Figure 2:

Representative fundus photograph from a subretinal NalO3 injected mouse after 3 weeks showing extensive RPE degeneration and pigment clumping (A). Pigmented NaIO3-injected C57BL/6J mouse choroid before (B) and after 10% H2O2 bleaching (C). Flat mounted mouse choroid immunolabeled with anti-podocalyxin antibody three weeks after NalO3 injection showing the extent of CC degeneration (arrowheads) at low magnification (D). Higher magnification (E), shows the border of attenuated CC (arrowheads). CC degeneration in the bleb region (F) and unaffected CC outside the bleb in far periphery (G). Scale bars= 1 mm in B–C, 500 μm in A, 200 μm in D and 50 μm in E–G.
Figure 3:

Flat mounted choroids from a control mouse (A) and NalO3 injected mice (B–F) showing area of CC degeneration delineated in tracings. The average size of the degenerative area was 7.0±1.56mm2 and the pathology was generally confined to the peripapillary and midperipheral choroid. Scale bar = 500 μm for A–F.
Figure 4:

Comparison of CC morphology in three distinct regions of choroid in a representative control eye and three NalO3 injected mouse eyes. Three separate images (0.5 mm2 in area) from each region of choroid (control eyes n=3 and NalO3 eyes n=5) were used for morphometric analysis. Compared to control choroids (A, E and I), there was marked uniformity of CC degeneration in peripapillary (B–D) and mid peripheral choroid (F–H) of NalO3 injected eyes while the far peripheral CC was unaffected (J–L). Scale bar = 20 μm in all.
3.3. Morphometric Analysis of Choroidal Vasculature in Normal and NaIO3 Atrophy Model
The %VA of normal control choroid was 87.4 ± 4.3% in the peripapillary region, 86.8± 7.7% in mid periphery, and 79.9 ± 6.7% in far periphery (Fig. 5A (blue bars)). In NaIO3 injected eyes, %VA was 50.7 ± 5.8% in the peripapillary region and 45.8 ± 6.17% in mid periphery (Fig. 7A (orange bars), p < 0.0001). These values represented a 42% decrease in peripapillary (p<0.0001) and 47% decrease in midperipheral (p<0.0001) %VA compared to controls. In the far periphery, the %VA was 82.8 ± 3.8% and similar to control eyes. In normal control choroid, CC diameters were 11.4 ±1.97 mm in the peripapillary region, 14.4 ± 2.52 mm in midperiphery and 15.1±3.15 mm in far periphery (Fig. 5B (blue bars)). In NaIO3 injected eyes, surviving attenuated CC diameters were reduced by 44.3% in peripapillary choroid (6.35 ± 1.02 mm, p<0.0001) and 54.3% in mid periphery (6.58 ± 1.2 mm, p < 0.0001). CC diameters in the far periphery were not statistically different (p=0.021) compared to controls (Fig. 5B [orange bars]). While CC degeneration in this mouse model was not as severe as we have reported in humans with long standing GA (Edwards et al., 2023), there are remarkable similarities in choroidal vascular pathology between the two (Fig. 6).
Figure 5:

Morphometric analysis of control (blue bars) versus NalO3 injected eyes (orange bars) showing percent vascular area (A) and choriocapillaris luminal diameters (B) in peripapillary, mid peripheral and far peripheral choroid. There was a statistically significant decrease in %VA in peripapillary (*p<0.0001) and midperipheral (*p<0.0001) choroid of NalO3 injected eyes compared to controls. CC luminal diameters in NalO3 injected eyes were reduced by 44.3% in peripapillary (*p<0.0001), and 54.3% in mid peripheral (*p<0.0001) choroid. Far peripheral %VA and CC luminal diameters were not significantly different between control and NalO3 injected eyes.
Figure 7:

Fundus photo (A) and fluorescein angiogram (B) of a representative laser induced CNV treated mouse eye. Lesion (arrows in A&B) shows scarring of retina/choroid and leakage of dye at the site of CNV.
Figure 6:

Comparison of choroidal vascular attenuation in the mouse NalO3 model immunostained with podocalyxin (A) and in a 92-year-old human with GA immunostained with Ulex europaeus agglutinin (UEA) lectin. The region of complete RPE atrophy (asterisks) and border of RPE atrophy are indicated (arrows). Scale bars = 100 μm.
3.4. Laser-induced CNV Model
Laser-treated pigmented mouse eyes showed focal scarring of the RPE (Fig. 7A) and characteristic fluorescein leakage from peripapillary CNV lesions one-minute post injection (Fig. 7B). Prebleached choroids typically showed pigmentary changes consisting of hyperpigmented central cores surrounded by hypopigmented peripheral borders. Podocalyxin immunostaining revealed CNV lesions (Fig. 8A–C) ranging in size from 0.16 to 0.32mm2 (mean = 0.26 ±0.09 mm2). The CNV was composed of several medium-sized vessels originating from the choroid, with branching capillaries extending peripherally (Fig. 8D–F). At their borders, they formed anastomotic and looping vessels, or they terminated in blind ends (Figs. 8G & H). Adjacent to CNV, the CC appeared normal morphologically (Fig. 8I). The diameters of CNV capillaries were quite thin (Fig. 9A–C) measuring 5.92±1.3mm (Fig. 9D). In comparison, the diameter of adjacent CC was 14.1 ± 2.8mm (Fig. 9D). In addition to neovascular lumen, we also observed single podocalyxin immunoreactive cells with long processes budding from and bridging well-formed capillaries within the CNV (Fig.9C). These cells appeared to represent proliferating and or migrating endothelial cells. Additionally, the density of the CC adjacent to the CNV was 83.36 ± 3.4% VA and only slightly reduced compared to CC density in normal control eyes. Compared to human donor eyes with nAMD from our archived tissue collection (Fig. 10), the average luminal diameters of CNV in human choroid are similar to that of adjacent CC (14.1 ± 2.8mm). Another distinguishing morphologic feature in human neovascular CNV is the reduced density of CC within the choroid adjacent to and in advance of the CNV (30% decrease). The laser induced CNV mouse model does not mimic this aspect of human nAMD.
Figure 8:

Whole bleached choroids of laser induced CNV mouse eyes immunostained with anti-podocalyxin antibody (A–C) showing CNV networks (arrows) adjacent to the optic nerve head. Higher magnification of CNV (D–F) shows thin diameter capillary networks (arrows) which have connections to the underlying choroidal vessels. Detail of laser induced CNV (arrowheads) and adjacent CC in “G”, at the edge of CNV (H) and adjacent CC (I). Blind ends of CNV at the neovascular front (arrow in H). Normal density and luminal diameters of CC in choroid nearest the CNV (I). Boxed areas in “G” represent higher magnification images shown in “H & I”. Scale bars = 500 μm in A, C & E, 100 μm in B, D, F, & G, and 50 μm in H & I .
Figure 9:
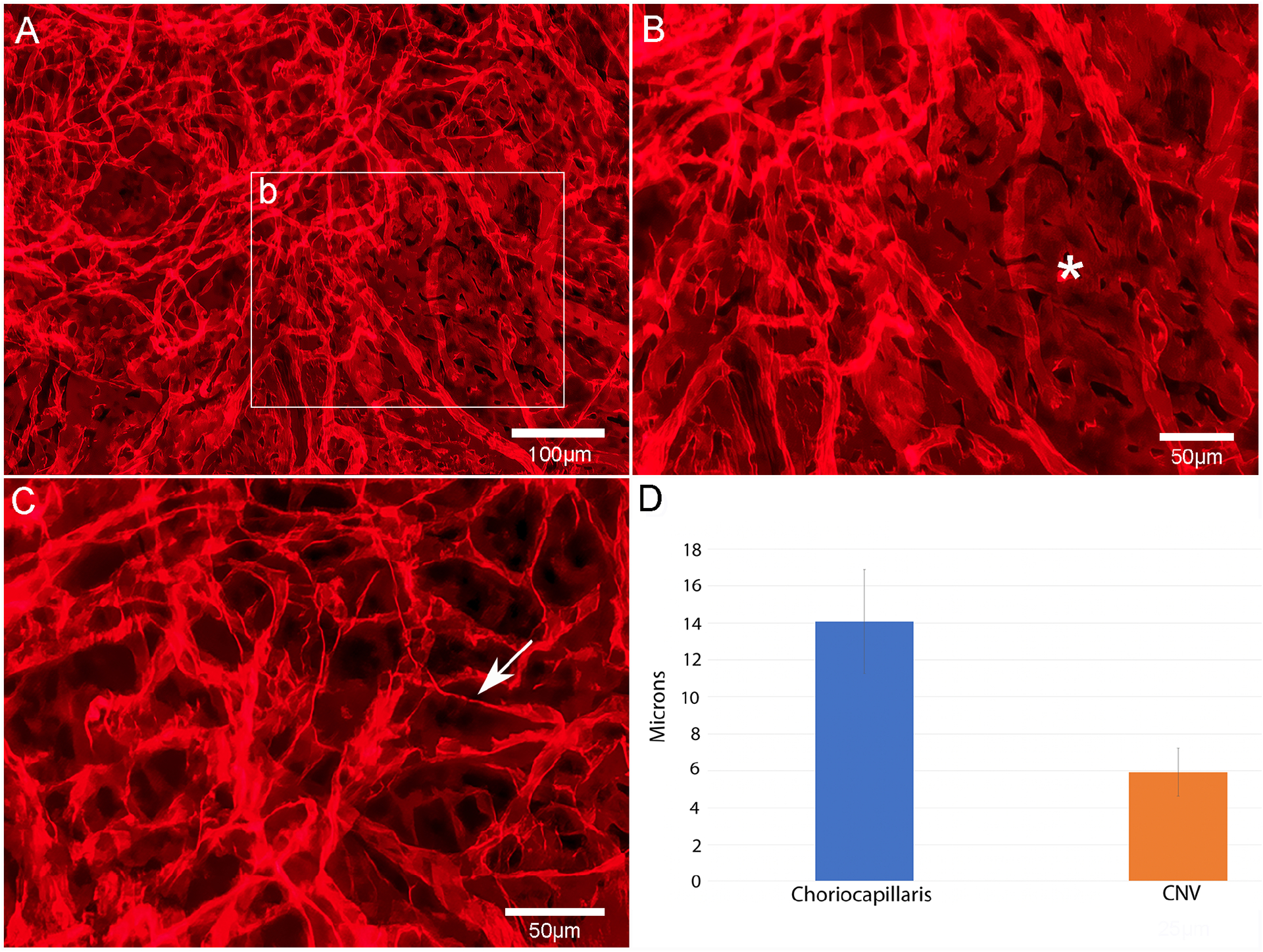
Edge of podocalyxin stained laser induced CNV shows thin diameter capillaries of neovascularization (A), broad diameter lumen of CC adjacent to CNV (asterisk in B) and individual cells within the CNV with fine processes (arrow) representative of proliferating endothelial cells (C). Morphometric measurements of luminal diameters in CC vs CNV (D). Scale bars= 100 μm in A & 50 μm in B & C.
Figure 10:

Comparison of CNV (arrowheads) in the mouse laser-induced CNV model immunostained with podocalyxin (A) and in a 92-year-old Caucasian male with neovascular AMD immunostained with Ulex europaeus agglutinin (UEA) lectin (B). Note the attenuated CC in advance of CNV in the human choroid (asterisks). Scale bars = 100 μm in A & 1mm in B.
4. Discussion
In the present study, we developed a simple, reliable, and rapid method to bleach highly pigmented whole mouse choroids prior to immunostaining. Since melanin can obscure and interfere with histopathological assessments of choroidal vascular morphology and pathology it is advantageous to bleach it. Choroids prepared with this method were compatible with subsequent podocalyxin immunohistochemical staining and analyses. As we have previously shown (Bhutto et al., 2020), the podocalyxin antibody staining provided excellent labeling of all components of choroidal vasculature in pigmented mouse eyes. An exception to this was the LPCA which were only weakly stained. Weak labeling of LPCA was also reported recently by another group using the same podocalyxin antibody (Zaitoun et al., 2022). The technique reported herein provides flat mounts that can be imaged using confocal microscopy without melanin interference and used for subsequent high-resolution morphometric analysis of choroidal vascular anatomy and pathology.
Podocalyxin is broadly expressed on the luminal face of most blood vessels in adult vertebrates, yet its function on these cells is poorly defined. Podocalyxin was initially identified in kidney glomeruli, where it is not only abundant but also essential for kidney development (Kerjaschki et al., 1984; Doyonnas et al., 2001). In addition, it is found in all three germ layers during embryogenesis, as well as hematopoietic progenitors, megakaryocytes and platelets, vascular endothelial, mesothelial cells lining organs, and a subset of neurons (Doyonnas et al., 2001; McNagny et al., 1997; Meittinen et al., 1999; Horvat et al., 1986; Vitureira et al., 2005). Podocalyxin is most closely related to the hematopoietic stem cell marker CD34 and the recently discovered membrane-associated mucins, sialomucin (CD164) and endoglycan (Doyonnas et al., 2001; Sassetti et al., 2000; Nielsen et al., 2002). These mucins have similar protein and gene structures and partially overlapping expression patterns (Nielsen et al., 2002; Furness et al., 2006).
Following our initial reports of podocalyxin immunostaining of pigmented mouse choroidal vasculature (Bhutto et al., 2020), Zaitoun et al. (2022) reported a detailed description of the mouse choroidal vasculature with RPE attached in several strains using anti-podocalyxin antibody. They also described a method for double/triple staining the choroidal blood vessels, mast cells, and macrophages in both pigmented and albino mouse choroids with post immunohistochemistry melanin bleaching. While they successfully used anti-alpha-SMA and anti-podocalyxin together to label arteries and other vessels within the vascular tree in post bleached choroids, we were unsuccessful using other antibodies commonly used in vascular biology after bleaching. This may be due to inactivation of other antigens during exposure to H2O2 prior to immunohistochemistry. One extra step to the technique reported herein was performed that was not reported by Zaitoun and colleagues, removing of RPE from the choroid before bleaching and flat mount immunostaining. This step provided us with enhanced and more succinct podocalyxin immunostaining of the choroidal vasculature.
Sodium iodate injection has been extensively used as a preclinical model of RPE degeneration and geographic atrophy (GA) (Machalinska et al., 2010; Lambert et al., 2013; Jian et al., 2015; Chowers et al., 2017). NaIO3 is reported to affect primarily the RPE cells with subsequent damage to photoreceptors (Chowers et al., 2017), additional retinal structures (Kiuchi et al., 2002) and CC atrophy (Korte et al., 1984; Bhutto et al., 2018). Different delivery routes and various doses of NaIO3 have been reported to induce RPE changes in mammalian species. The most common administration methods used are intravenous and intraperitoneal. However, these methods provide immediate absorption of injected NaIO3 into the systemic circulation, and result in widespread patchy degeneration of RPE cells with no evidence of CC degeneration (Hosoda et al., 1993; Enzmann et al., 2006; Harris et al., 2006; Yang et al., 2014). To the best of our knowledge, no report has been published describing choroidal vascular changes in flat perspective in NaIO3 mouse model. We chose to use the subretinal administration method that we recently reported in the rat, in which a single subretinal injection causes attenuation in a succinct, well demarcated area (Bhutto et al, 2018). However, the dose to produce this effect in pigmented mouse eyes was 10 times higher than in pigmented and albino rat eyes. The dose required for the mouse subretinal NaIO3 is like that given systemically in mice (Hosoda et al., 1993; Enzmann et al., 2006; Harris et al., 2006; Yang et al., 2014; Enzbrenner et al., 2021; Yang et al., 2023) so we speculate the increased tolerance is a species difference between rats and mice.
The viability of CC depends on an intact, functioning RPE. Similarly, the RPE are reliant on CC, reflecting a mutualistic symbiosis between the two structures (McLeod et al., 2009). The RPE atrophy and CC loss in the mouse reported herein were contained within well-defined borders surrounded by intact RPE. In the regions with intact RPE, vascular density and luminal CC diameters were similar morphologically to control eyes. As observed in human eyes with GA, there is good correlation between RPE atrophy and CC degeneration (Korte et al., 1984; Lutty et al., 1999; McLeod et al., 2009; Seddon et al., 2016). Moreover, the remarkable similarities in choroidal vascular pathology between this model and human GA, make it a useful platform to test the effects of RPE/stem cell transplantation on the rescue/recovery of choroidal vasculature in patients with GA.
The mouse model of laser induced CNV has been a mainstay for nAMD research. This in vivo model relies on laser injury to perforate Bruch’s membrane, resulting in subretinal blood vessel recruitment from the choroid. By reproducing one of the key features of nAMD, this assay has served as the backbone for testing antiangiogenic therapies. The simple technique of using the laser, the reproducibility of CNV if the proper laser settings are used, the short time-course of CNV to develop are all merits of this model. In this study we used the laser model to examine the immunostaining of CNV with podocalyxin and to perform morphometric analysis to quantify the neovascular response. CNV vessels were stained intensely with podocalyxin. We speculate that the increased intensity of CNV vessels compared to normal choroidal endothelial cells, is due to their smaller size. Podocalyxin staining also revealed fine fiber-like processes from the endothelial cells in the laser-induced CNV model. We have never seen such processes or seen them reported in human CNV specimens with UEA lectin. It is possible that podocalyxin staining, which unfortunately does not work in human tissue, uniquely labels these fine processes. Therefore, we do not want to put too much emphasis on this difference between the mouse model and human CNV.
In most published reports, the quantitative morphometric assessment of laser-induced CNV lesions is achieved by either tissue sections immunostained with primary anti-endothelial cell antibodies or through analysis of flat-mounted choroids using lectin fluorescent conjugated antibodies to label the CNV. However, the anatomical connections of CNV to feeder vessels and the viability of adjacent choriocapillaris cannot be adequately visualized and analyzed in flat mounts because RPE is usually left intact and melanin pigmentation in choroid obscures vascular details. The podocalyxin staining of whole mounts successfully labelled CNV in the laser-induced model and bleaching allowed for CNV area to be accurately measured. Moreover, we visualized and quantified luminal diameters in the CNV and adjacent choroid. We observed that the diameter of CNV lumen was much smaller than that observed in adjacent untreated CC. This contrasts with human nAMD where luminal diameters of CNV are similar to that of adjacent choriocapillaris. Additionally, another distinguishing morphologic feature in human CNV is the reduced density of CC within the choroid adjacent to and in advance of the CNV (30% decrease). The laser induced CNV mouse model does not mimic this aspect of the human nAMD. Therefore, caution should be taken when using the laser CNV model to model nAMD.
5. Conclusion
We have described a reliable method for bleaching melanin and choroidal vessel visualization in pigmented mice, which has been challenging. Podocalyxin antibody staining provided consistent labeling of choroidal vasculature in the flat perspective in normal and pathological conditions. We can measure changes in CC diameter and density using this technique. The staining also labels CNV. Since many genetic models are generated in pigmented mice, the techniques presented herein provide a simple and reliable method to perform quantitative morphological analysis of choroidal vascular changes in these models. This method will be beneficial evaluating the effects of various treatment modalities on the choroidal vasculature of murine models of both atrophic and nAMD.
Highlights.
In pigmented mice, whole mount visualization and morphometric analysis of choroid vasculature are challenging tasks.
We report a simple, reliable technique involving bleaching pigment prior to immunostaining the vasculature in whole mounts of pigmented mouse choroids.
Immunostaining on bleached choroid with anti-podocalyxin antibody provides a simple and reliable tool for visualizing normal and pathologic choroidal vasculature in pigmented mouse eyes for quantitative morphometric analysis.
This method will be beneficial for examining and evaluating the effects of various treatment modalities on the choroidal vasculature in mouse models of ocular diseases.
Acknowledgments
We dedicate this work to our late mentor, colleague and friend, Prof. Gerard A. Lutty, for his outstanding contributions to the field of ocular vascular biology and for always being available to share his knowledge and guide us in our studies and beyond. This study was funded by National Institutes of Health (NIH) Grants RO1-EY016151 (GL & ME), P30-EY001765 (Wilmer Core), RPB Unrestricted Grant (Wilmer Eye Institute, The Johns Hopkins University).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare no relevant conflicts of interest.
Data availability
Data will be made available on request.
References
- Baba T, Bhutto IA, Merges C, Grebe R, Emmert D, McLeod DS, Armstrong D, Lutty GA, 2010. A rat model for choroidal neovascularization using subretinal lipid hydroperoxide injection. Am. J. Pathol 176(6), 3085–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutto IA, Amemiya T, 2001. Microvascular Architecture of the Rat Choroid: Corrosion Cast Study. Anat. Rec 264, 63–71. [DOI] [PubMed] [Google Scholar]
- Bhutto IA, Tiwari A, Thomson BR, Edwards MM, Lutty GA, 2020. Visualization of choroidal vasculature in pigmented mouse eyes. Invest Ophthalmol Vis Sci. 61, 2235. [Google Scholar]
- Bhutto IA, Ogura S, Baldeosingh R, McLeod DS, Lutty GA, Edwards MM, 2018. An acute injury model for the phenotypic characteristics of geographic atrophy. Invest. Ophthalmol. Vis. Sci 59(4), AMD143–AMD151. 10.1167/iovs.18-24245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowers G, Cohen M, Marks-Ohana D, Stika S, Eijzenberg A, Banin E, Obolensky A, 2017. Course of sodium iodate-induced retinal degeneration in albino and pigmented mice. Invest. Ophthalmol. Vis. Sci 58(4), 2239–2249. doi: 10.1167/iovs.16-21255 [DOI] [PubMed] [Google Scholar]
- de Carlo TE, Romano A, Waheed NK, Duker JS, 2015a. A review of optical coherence tomography angiography (OCTA). Int. J. Ret. Vitreous 1:5. doi: 10.1186/s40942-015-0005-8. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carlo TE, Bonini Filho MA, Chin AT, Adhi M, Ferrara D, Baumal CR, Witkin AJ, Reichel E, Duker JS, Waheed NK, 2015b. Spectral-domain optical coherence atrophy angiography of choroidal neovascularization. Ophthalmology. 122(6), 1228–38. doi: 10.1016/j.ophtha.2015.01.029. Epub 2015 Mar 17. [DOI] [PubMed] [Google Scholar]
- Doyonnas R, Kershaw DB, Duhme C, Merkens H, Chelliah S, McNagny KM, 2001. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J. Exp. Med 194(1), 13–27. doi: 10.1084/jem.194.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M, McLeod DS, Shen M, Grebe R, Sunness JS, Bhutto IA, McDonnell E, Pado AM, Gregori G, Rosenfeld PJ, Lutty GA, 2023. Clinicopathologic findings in three siblings with geographic atrophy. Invest. Ophthalmol. Vis. Sci 64(3), 2. doi: 10.1167/iovs.64.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzbrenner A, Zulliger R, Biber J, Quintela Pousa AM, Schafer N, Stuki C, Giroud N, Berrera M, Kortvely E, Schmucki R, Badi L, Grosche A, Pauly D, Enzmann V, 2021. Sodium iodate-induced degeneration results in local complement changes and inflammatory processes in murine retina. Int. J. Mol. Sci 22(17), 9218. doi: 10.3390/ijms22179218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzmann V, Row BW, Yamauchi Y, Kheirandish L, Gozal D, Kaplan HJ, McCall MA, 2006. Behavioral and anatomical abnormalities in a sodium iodate-induced model of retinal pigment epithelium degeneration. Exp. Eye. Res 82(3), 441–448. doi: 10.1016/j.exer.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Ferrara D, Waheed NK, Duker JS, 2016. Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog. Retin. Eye. Res 52, 130–155. doi: 10.1016/j.preteyeres.2015.10.002. Epub 2015 Oct 23. [DOI] [PubMed] [Google Scholar]
- Furness SG, McNagny K, 2006. Beyond mere markers: functions for CD34 family of sialomucins in hematopoiesis. Immunol. Res 34(1), 13–32. doi: 10.1385/IR:34:1:13. [DOI] [PubMed] [Google Scholar]
- Gelisken F, Inhoffen W, Schneider U, Stroman G, Kreissig I, 1998. Indocyanine Green Angiography in Classic Choroidal Neovascularization. Jpn. J. Ophthalmol 42, 300–303. [DOI] [PubMed] [Google Scholar]
- Gong Y, Li J, Sun Y, Fu Z, Liu C, Evans L, Tian K, Saba N, Fredrick T, Morss P, Chen J, Smith LEH, 2015. Optimization of an Image-Guided Laser-Induced Choroidal Neovascularization Model in Mice. Plos. One 10(7), e0132643. doi: 10.1371/journal.pone.0132643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JR, Brown GAJ, Jorgensen M, Kaushal S, Ellis EA, Grant MB, Scott EW, 2006. Bone Marrow–Derived Cells Home to and Regenerate Retinal Pigment Epithelium after Injury. Invest. Ophthalmol. Vis. Sci 47(5), 2108–2113. doi: 10.1167/iovs.05-0928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, 1975. Segmental nature of the choroidal vasculature. Br. J. Ophthalmol 59(11), 631–648. doi: 10.1136/bjo.59.11.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, 1990. In vivo choroidal circulation and its watershed zones. Eye (Lond). 4(Pt 2), 273–289. doi: 10.1038/eye.1990.39. [DOI] [PubMed] [Google Scholar]
- Horvat R, Hovorka A, Dekan G, Poczewski H, Herjaschki D, 1986. Endothelial cell membranes contain podocalyxin - the major sialoprotein of visceral glomerular epithelial cells. J. Cell. Biol 102(2), 484–491. doi: 10.1083/jcb.102.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda L, Adachi-Usami E, Mizota A, Hanawa T, Kimura T, 1993. Early effects of sodium iodate injection on ERG in mice. Acta. Ophthalmol 71, 616–622. [DOI] [PubMed] [Google Scholar]
- Jian Q, Tao Z, Li Y, Yin ZQ, 2015. Acute retinal injury and the relationship between nerve growth factor, Notch1 transcription and short-lived dedifferentiation transient changes of mammalian Muller cells. Vision. Res 110(Pt A), 107–117. doi: 10.1016/j.visres.2015.01.030. Epub 2015 Mar 27. [DOI] [PubMed] [Google Scholar]
- Jiao C, Adler K, Liu X, Sun W, Mullins RF, Sohn EH, 2020. Visualization of mouse choroidal and retinal vasculature using fluorescent tomato lectin perfusion. Transl. Vis. Sci. Technol 9(1), 1. doi: 10.1167/tvst.9.1.1. eCollection 2020 Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D, Sharkey DJ, Farquhar MG, 1984. Identification and characterization of podocalyxin--the major sialoprotein of the renal glomerular epithelial cell. J. Cell. Biol 98(4), 1591–1596. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi K, Yoshizawa K, Shikata N, Moriguchi K, Tsubura A, 2002. Morphologic characteristics of retinal degeneration induced by sodium iodate in mice. Curr. Eye. Res 25(6), 373–379. doi: 10.1076/ceyr.25.6.373.14227. [DOI] [PubMed] [Google Scholar]
- Koh AE, Alsaeedi HA, Rashid MA, Lam C, Harun MHN, Mohd Saleh MF, Luu CD, Kumar SS, Ng MH, Isa HM, Leow SN, Then KY, Bastion MC, Ali Khan MS, Mok PL, 2019. Retinal degeneration rat model: A study on the structural and functional changes in the retina following injection of sodium iodate. J. Photochem. Photobiol. B, Biol 196, 111514. 10.1016/j.jphotobiol.2019.111514 [DOI] [PubMed] [Google Scholar]
- Koh LHL, Agrawal R, Khandelwal N, Charan LS, Chhablani J, 2017. Choroidal vascular changes in age-related macular degeneration. Acta. Ophthalmol 95(7), e597–e601. doi: 10.1111/aos.13399. Epub 2017 Apr 9. [DOI] [PubMed] [Google Scholar]
- Korte GE, Reppucci V, Henkind P, 1984. RPE destruction causes choriocapillary atrophy. Invest. Ophthalmol. Vis. Sci 25(10), 1135–1145. [PubMed] [Google Scholar]
- Lambert V, Lecomte J, Hansen S, Blacher S, Gonzalez ML, Struman I, Sounni NE, Rozet E, de Tullio P, Foidart JM, Rakic JM, Noel A, 2013. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc 8(11), 2197–2211. doi: 10.1038/nprot.2013.135. Epub 2013 Oct 17. [DOI] [PubMed] [Google Scholar]
- Lutty G, Grunwald J, Majji AB, Uyama M, Yoneya S, 1999. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol. Vis 5, 35. [PubMed] [Google Scholar]
- Machalinska A, Lubinski W, Klos P, Kawa M, Baumert B, Penkala K, Grzegrzolka R, Karczewicz D, Wiszniewska B, Machalinski B, 2010. Sodium iodate selectively injuries the posterior pole of the retina in a dose-dependent manner: morphological and electrophysiological study. Neurochem. Res 35(11), 1819–1827. doi: 10.1007/s11064-010-0248-6. Epub 2010 Aug 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R, Spaide RF, 2009. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am. J. Ophthalmol 147(5), 811–815. doi: 10.1016/j.ajo.2008.12.008. Epub 2009 Feb 20. [DOI] [PubMed] [Google Scholar]
- McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA, 2009. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 50(10), 4982–4991. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DS, Lutty GA, 1994. High-Resolution Histologic Analysis of the Human Choroidal Vasculature. Invest. Ophthalmol. Vis. Sci 35(11), 3799–3811. [PubMed] [Google Scholar]
- McLeod DS, Bhutto I, Edwards MM, Silver RE, Seddon JM, Lutty GA, 2016. Distribution and quantification of choroidal macrophages in human eyes with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 57(14), 5843–5855. doi: 10.1167/iovs.16-20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNagny KM, Pettersson I, Rossi F, Flamme I, Shevchenko A, Mann M, Garf T, 1997. Thrombomucin, a novel cell surface protein that defines thrombocytes and multipotent hematopoietic progenitors. J. Cell. Biol 138(6), 1395–1407. doi: 10.1083/jcb.138.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen A, Solin ML, Reivinen J, Juvonen E, Vaisanen R, Holthofer H, 1999. Podocalyxin in rat platelets and megakaryocytes. Am. J. Pathol 154(3), 813–822. doi: 10.1016/S0002-9440(10)65328-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mones J, Leiva M, Pena T, Martinez G, Biarnes M, Garcia M, Serrano A, Fernandez E, 2016. A Swine model of selective geographic atrophy of outer retinal layers mimicking atrophic AMD: a phase I escalating dose of subretinal sodium iodate. Invest. Ophthalmol. Vis. Sci 57(10), 3974–83. Doi: 10.1167/iovs.16-19355. [DOI] [PubMed] [Google Scholar]
- Mrejen S, Spaide RF, 2013. Optical coherence tomography: Imaging of the choroid and beyond. Surv. Ophthalmol 58(5), 387–429. doi: 10.1016/j.survophthal.2012.12.001. Epub 2013 Aug 2. [DOI] [PubMed] [Google Scholar]
- Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J, 2011. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 52(3), 1606–1612. doi: 10.1167/iovs.10-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JS, Doyonnas R, McNagny KM, 2002. Avian models to study the transcriptional control of hematopoietic lineage commitment and to identify lineage-specific genes. Cells. Tissues. Organs 171(1), 44–63. doi: 10.1159/000057691. [DOI] [PubMed] [Google Scholar]
- Pennesi ME, Neuringer M, Courtney RJ, 2012. Animal models of age-related macular degeneration. Mol Aspects Med. 33(4), 487–509. 10.1016/j.mam.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrus-Reurer S, Bartuma H, Aronsson M, Westman S, Lanner F, Andre H, Kvanta A, 2017. Integration of subretinal suspension transplants of human embryonic stem cell-derived retinal pigment epithelial cells in a large-eyed model of geographic atrophy. Invest. Ophthalmol. Vis. Sci 58(2), 1314–1322. doi: 10.1167/iovs.16-20738 [DOI] [PubMed] [Google Scholar]
- Sassetti C, Van Zante A, Rosen SD, 2000. Identification of endoglycan, a member of the CD34/podocalyxin family of sialomucins. J. Biol. Chem 275(12), 9001–9010. doi: 10.1074/jbc.275.12.9001. [DOI] [PubMed] [Google Scholar]
- Seddon JM, McLeod DS, Bhutto IA, Villalonga MB, Silver RE, Wenick AS, Edwards MM, Lutty GA, 2016. Histopathological insights into choroidal vascular loss in clinically documented cases of age-related macular degeneration. JAMA. Ophthalmol 134(11), 1272–1280. doi: 10.1001/jamaophthalmol.2016.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, Vinores SA, Basilico C, Campochiaro PA, 1998. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am. J. Pathol 153(5), 1641–1646. 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitureira N, McNagny K, Soriano E, Burgaya F, 2005. Pattern of expression of the podocalyxin gene in the mouse brain during development. Gene. Expr. Patterns 5(3), 349–354. doi: 10.1016/j.modgep.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Wang JC, Lains I, Silverman RF, Sobrin L, Vavvas DG, Miller JW, Miller JB, 2018. Visualization of choriocapillaris and choroidal vasculature in healthy eyes with enface swept-source optical coherence tomography versus angiography. Trans. Vis. Sci. Technol 7(6), 25. doi: 10.1167/tvst.7.6.25. eCollection 2018 Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiter JJ, Ernest JT, 1974. Anatomy of the choroidal vasculature. Am. J. Ophthalmol 78(4), 583–590. doi: 10.1016/s0002-9394(14)76294-4. [DOI] [PubMed] [Google Scholar]
- Wong IY, Koizumi H, Lai WW, 2011. Enhanced depth imaging optical coherence tomography. Ophthalmic. Surg. Lasers. Imaging 42 Suppl, S75–84. doi: 10.3928/15428877-20110627-07. [DOI] [PubMed] [Google Scholar]
- Yang X, Chung Jin-Y., Rai U, Esumi N, 2023. SIRT6 overexpression in the nucleus protects mouse retinal pigment epithelium from oxidative stress. Life. Sci. Alliance 6(7), e202201448. doi: 10.26508/lsa.202201448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ng TK, Ye C, Yip YW, Law K, Chan SO, Pang CP, 2014. Assessing sodium iodate-induced outer retinal changes in rats using confocal scanning laser ophthalmoscopy and optical coherence tomography. Invest. Ophthalmol. Vis. Sci 55, 1696–1705. 10.1167/iovs.13-12477 [DOI] [PubMed] [Google Scholar]
- Yannuzzi LA, 2011. Indocyanine green angiography: a perspective on use in the clinical setting. Am. J. Ophthalmol 151(5), 745–751.e1. [DOI] [PubMed] [Google Scholar]
- Yoneya S, Tso MO, 1987. Angioarchitecture of the human choroid. Arch. Ophthalmol 105(5), 681–687. doi: 10.1001/archopht.1987.01060050099046. [DOI] [PubMed] [Google Scholar]
- Zaitoun IS, Song YS, Zaitoun HB, Sorenson CM, Sheibani N, 2022. Assessment of choroidal vasculature and innate immune cells in the eyes of albino and pigmented mice. Cells. 11(20), 3329, 10.3390/cells11203329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


