Abstract
Men who have sex with men (MSM) are disproportionately affected by HIV, accounting for two-thirds of HIV cases in the United States despite representing ~5% of the adult population. Delivery and use of existing and highly effective HIV prevention and treatment strategies remain suboptimal among MSM. To summarize the state of the science, we systematically review implementation determinants and strategies of HIV-related health interventions using implementation science frameworks. Research on implementation barriers has focused predominantly on characteristics of individual recipients (e.g., ethnicity, age, drug use) and less so on deliverers (e.g., nurses, physicians), with little focus on system-level factors. Similarly, most strategies target recipients to influence their uptake and adherence, rather than improving and supporting implementation systems. HIV implementation research is burgeoning; future research is needed to broaden the examination of barriers at the provider and system levels, as well as expand knowledge on how to match strategies to barriers—particularly to address stigma. Collaboration and coordination among federal, state, and local public health agencies; community-based organizations; health care providers; and scientists are important for successful implementation of HIV-related health innovations.
Keywords: implementation science, men who have sex with men, HIV, preexposure prophylaxis, HIV testing, linkage-to-care
INTRODUCTION
Over the past 40 years, treatment innovations have produced striking declines in deaths due to HIV/AIDS in the United States. However, dramatic disparities in infections and challenges in implementing effective prevention persist (Figure 1). New diagnoses of HIV rose exponentially in the early 1980s; major declines into the early 1990s were produced by community-led efforts at behavioral risk reduction (e.g., condom use, fewer partners) (Darrow 2021). The advent of antiretroviral therapy (ART) led to substantial declines in deaths but also created new Black–White racial disparities due to unequal access. The past decade has been characterized by the ascent of new biomedical interventions, such as pre-exposure prophylaxis (PrEP) to reduce infection and viral suppression by HIV treatment with ART to eliminate onward transmission (i.e., undetectable equals untransmittable). Despite these innovations, the rate of new cases has declined only modestly, particularly among men who have sex with men (MSM), and improvements in treatment have not produced dramatic shifts in mortality of the kind observed upon ART’s initial introduction. Put simply: Pills have gotten better and better, but cases among MSM have not fallen correspondingly. The challenge is implementation—the delivery and integration of these interventions into practice—and it is time for science to shift toward improving delivery of effective interventions to who, when, and where they are needed.
Figure 1.
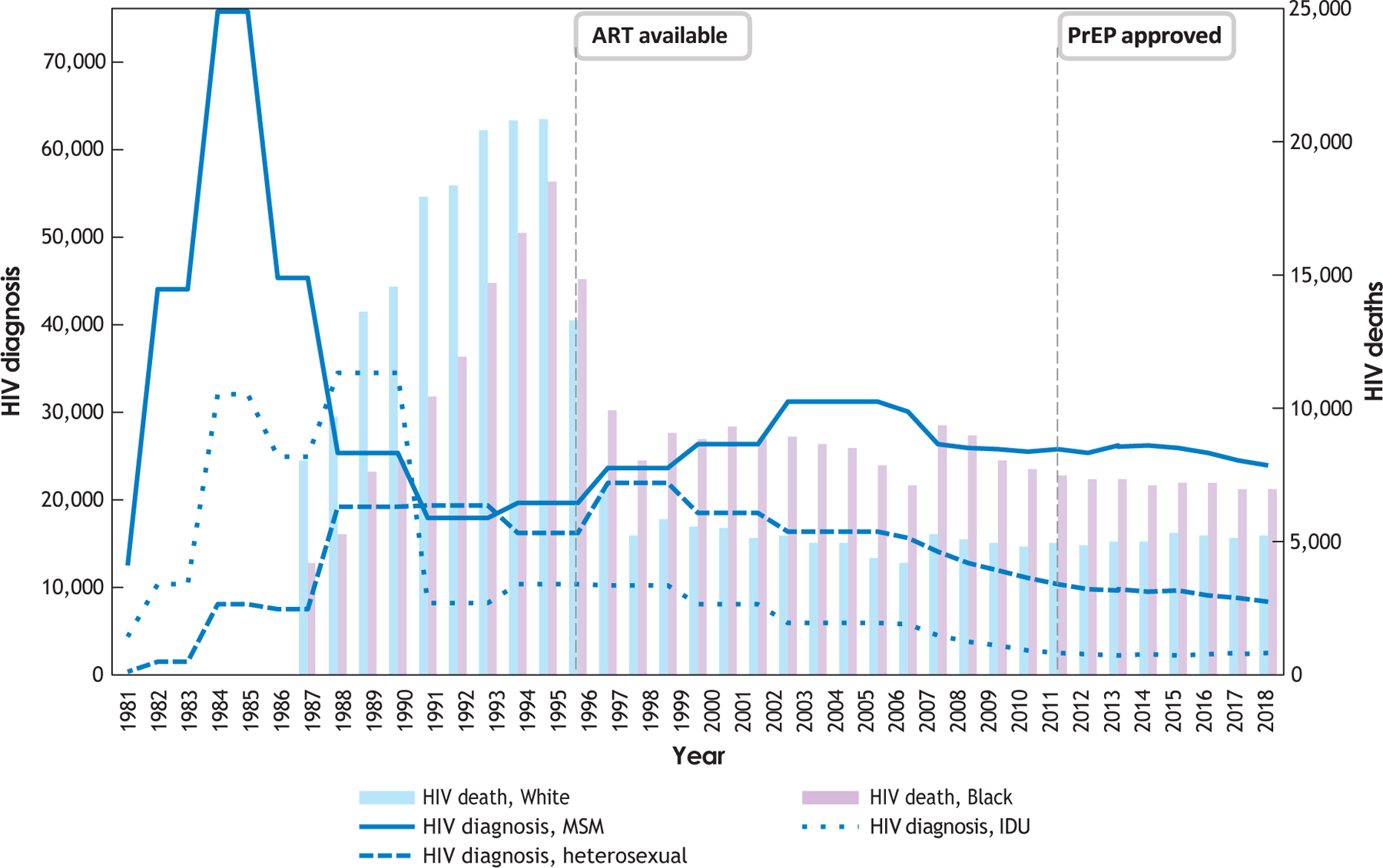
Total diagnoses and deaths due to HIV, 1981–2019. Abbreviations: ART, antiretroviral therapy; IDU, injection drug use; MSM, gay, bisexual, other men who have sex with men; PrEP, pre-exposure prophylaxis. Data for diagnoses, 1981–2007 (modeled), are from CDC (2021a). Data for total deaths, 1981–2007, are from CDC (1999, 2007). All data for the period 2008–2019 were obtained from https://gis.cdc.gov/grasp/nchhstpatlas/tables.html using the following variables: HIV diagnoses, HIV deaths, national, 2008–2019, white, Black/African American, cisgender male, male-to-male sexual contact, injection drug use.
Launched in 2019, the US Ending the HIV Epidemic initiative (EHE) set an ambitious goal to effectively end HIV transmission in the United States by 2030 through better implementation of effective tools already in hand: highly sensitive tests to diagnose HIV infection, interventions to prevent and treat infection, and cutting-edge technologies to identify outbreaks (Fauci et al. 2019). To achieve this goal, EHE specifically calls for implementation research to (a) identify how to best use new and existing HIV prevention tools; (b) adapt evidence-based interventions to local environments to maximize uptake, sustainability, and reach to priority populations; and (c) rapidly and effectively translate new discoveries into practice at the local level with fidelity. To summarize current implementation knowledge and highlight areas needing further research, we systematically review the implementation determinants and strategies for select HIV prevention interventions among MSM.
IMPLEMENTATION SCIENCE
Implementation science is the “scientific study of methods to promote the systematic uptake of research findings and other evidence-based practices into routine practice, and, hence, to improve the quality and effectiveness of health services” (Eccles & Mittman 2006). Implementation science has its roots in multiple disciplines, having risen in parallel out of social sciences, industry, and medicine since the mid-twentieth century before coalescing into a formal science of its own (Brownson et al. 2017). Psychologists and other behavioral scientists have significantly shaped and remain at the forefront of the field (Baumann et al. 2022, Nilsen 2015). For example, many of the leaders in the field, including editors of the leading journals Implementation Science and Implementation Research and Practice, were trained as psychologists (Baumann et al. 2022).
Implementation determinants, also referred to as barriers and facilitators, explain why implementation of an innovation (or intervention) may succeed or fail in a specific context. Determinants exist at multiple levels, ranging from individual to organizational and societal (Table 1). Given the vast number of possible determinants and the desire for generalizability, researchers have proposed numerous frameworks, of which the Consolidated Framework for Implementation Research (CFIR) has become the most widely cited (Damschroder et al. 2009). The updated CFIR 2.0 (Damschroder et al. 2022a) includes five domains: outer setting, inner setting, the innovation (or intervention), the implementation process, and characteristics of individuals, with multiple constructs and subconstructs within each domain (Table 1). These domains are applied to those who deliver or make decisions around delivery and sustainment of the intervention. However, both deliverers and recipients (e.g., patients or participants) are highlighted in the characteristics of individuals domain, which includes constructs based on the capability, opportunity, and motivation framework for understanding and supporting behavior change (COM-B; Michie et al. 2011). Because of its comprehensiveness and prominence in the field, we use CFIR as our guiding determinant framework for this review.
Table 1.
Number of measured determinants by CFIR 2.0 construct among articles related to implementation of PrEP, testing, and linkage-to-carea
| CFIR 2.0 domain and construct | Number of innovation determinants measuring HIV testing and linkage-to-care | Number of implementation determinants measuring HIV testing and linkage-to-care | Number of innovation determinants measuring PrEP | Number of implementation determinants measuring PrEP | Subtotal | |
|---|---|---|---|---|---|---|
| Characteristics of innovation | Innovation source | 0 | 0 | 1 | 0 | 1 |
| Evidence base | 0 | 0 | 32 | 28 | 60 | |
| Relative advantage | 0 | 0 | 42 | 10 | 52 | |
| Adaptability | 0 | 0 | 3 | 0 | 3 | |
| Trialability | 0 | 0 | 1 | 1 | 2 | |
| Complexity | 0 | 1 | 60 | 29 | 90 | |
| Design quality and packaging | 1 | 2 | 15 | 2 | 20 | |
| Cost | 0 | 6 | 64 | 18 | 88 | |
| Other intervention characteristic | 1 | 1 | 123 | 63 | 188 | |
| Subtotal | 2 | 10 | 341 | 151 | 504 | |
| Outer setting | Critical incidents | 0 | 0 | 0 | 0 | 0 |
| Local attitudes | 0 | 0 | 116 | 71 | 187 | |
| Local conditions | 0 | 0 | 67 | 13 | 80 | |
| Partnerships and connections | 0 | 0 | 38 | 9 | 47 | |
| Societal pressure | 0 | 0 | 0 | 0 | 0 | |
| Policies and laws | 1 | 5 | 4 | 18 | 28 | |
| Financing | 0 | 1 | 0 | 58 | 59 | |
| Structural or systemic oppression | 8 | 2 | 66 | 5 | 81 | |
| Market pressure: implementation determinant only | 0 | 0 | 0 | 2 | 2 | |
| Performance-measurement pressure: implementation determinant only | 0 | 0 | 0 | 0 | 0 | |
| Subtotal | 9 | 8 | 291 | 176 | 484 | |
| Inner setting | Structural characteristics | NA | 11 | NA | 25 | 36 |
| Relational connections | NA | 1 | NA | 0 | 1 | |
| Communication | NA | 3 | NA | 0 | 3 | |
| Culture | NA | 9 | NA | 12 | 21 | |
| Tension for change | NA | 1 | NA | 0 | 1 | |
| Compatibility | NA | 3 | NA | 22 | 25 | |
| Relative priority | NA | 0 | NA | 5 | 5 | |
| Incentive systems | NA | 5 | NA | 0 | 5 | |
| Mission alignment | NA | 0 | NA | 3 | 4 | |
| Available resources | NA | 1 | NA | 64 | 65 | |
| Subtotal | NA | 34 | NA | 131 | 165 | |
| Characteristics of individuals | High-level leaders | 0 | 0 | 0 | 1 | 1 |
| Midlevel leaders | 0 | 0 | 0 | 0 | 0 | |
| Opinion leaders | 0 | 0 | 0 | 0 | 0 | |
| Implementation facilitators | 0 | 0 | 0 | 0 | 0 | |
| Implementation leads | 0 | 0 | 0 | 0 | 0 | |
| Other implementation support | 1 | 0 | 0 | 8 | 9 | |
| Innovation deliverers: need | 0 | 2 | 4 | 1 | 7 | |
| Innovation deliverers: capability (self-efficacy) | 0 | 5 | 93 | 5 | 103 | |
| Innovation deliverers: opportunity | 0 | 2 | 48 | 4 | 54 | |
| Innovation deliverers: motivation | 0 | 5 | 139 | 0 | 144 | |
| Innovation deliverers: characteristics not associated with behavior | 11 | 2 | 0 | 3 | 16 | |
| Innovation recipients: need | 3 | 0 | 0 | 0 | 3 | |
| Innovation recipients: capability (self-efficacy) | 4 | 3 | 37 | 86 | 130 | |
| Innovation recipients: opportunity | 6 | 2 | 2 | 16 | 26 | |
| Innovation recipients: motivation | 24 | 9 | 14 | 60 | 107 | |
| Innovation recipients: characteristics not associated with behavior | 36 | 8 | 260 | 35 | 339 | |
| Subtotal | 86 | 38 | 592 | 224 | 940 | |
| Process | Teaming | 0 | 0 | 0 | 2 | 2 |
| Assessing for needs | 0 | 0 | 0 | 0 | 0 | |
| Assessing context | 0 | 0 | 0 | 0 | 0 | |
| Planning | 0 | 1 | 0 | 3 | 4 | |
| Tailoring strategies | 0 | 0 | 0 | 1 | 1 | |
| Engaging innovation deliverers | 0 | 1 | 0 | 2 | 3 | |
| Engaging innovation recipients | 0 | 8 | 0 | 4 | 12 | |
| Doing | 0 | 0 | 0 | 6 | 6 | |
| Reflecting and evaluating | 0 | 1 | 0 | 0 | 1 | |
| Adapting | 0 | 3 | 0 | 0 | 3 | |
| Subtotal | 0 | 14 | 0 | 18 | 32 | |
NA indicates that CFIR 2.0 does not include characteristics of innovation in the inner setting. Zeroes in the table represent domains and constructs that could be measured but did not appear in the existing literature.
Abbreviations: CFIR, Consolidated Framework for Implementation Research; PrEP, pre-exposure prophylaxis.
In implementation science, understanding contextual determinants is a precursor to selecting relevant implementation strategies, which are not included in CFIR. Implementation strategies are techniques to enhance adoption, implementation, and sustainability of a clinical program or practice. Whereas determinants are characteristics of a situation or context, strategies act upon individuals and entities in the delivery system to facilitate and support innovation implementation. Strategies can vary widely in terms of size and complexity and are frequently used in multifaceted bundles to target multiple determinants simultaneously (e.g., supporting clinicians while also providing education to patients) or in sequence over time (e.g., training clinicians before implementation and then providing feedback after implementation has begun) (Powell et al. 2012, 2015; Waltz et al. 2015). The most widely used taxonomy, Expert Recommendations for Implementing Change (ERIC), identified nine broad groups: engage consumers, use evaluative and iterative strategies, change infrastructure, adapt and tailor to context, develop stakeholder interrelationships, utilize financial strategies, support clinicians, provide interactive assistance, and train and educate stakeholders (Powell et al. 2015). For this review, we use ERIC to classify implementation strategies because of its ubiquity in the field, and we combine it with the Proctor et al. (2013) specification framework to specify the strategies toward replication.
While strategies act upon the deliverer or delivery system, adjunctive interventions support uptake, adherence, or continued engagement with the health intervention (J.D. Smith, D.H. Li, B. Keiser, B. Mustanski & N. Benbow, manuscript submitted) and therefore act upon the recipient (i.e., the patient or participant). Adjunctive interventions are often critical to the success of the overall implementation of an innovative intervention and are often developed by behavioral scientists. The differentiation between strategies, adjunctive interventions, and health interventions is a newer area of implementation science, and we do not include adjunctive interventions in our analysis but do discuss the concept in future directions.
POPULATIONS OF FOCUS
In the United States, MSM have a new HIV diagnosis rate 17 times greater than that of people who inject drugs and 144 times higher than that of heterosexuals. These disparities are growing (Crepaz et al. 2019) and are driven by biology (e.g., HIV is more transmissible via anal than vaginal sex), denser male–male sexual networks in comparison to male–female sexual networks, and higher numbers of sexual partners among MSM, alongside several implementation barriers (Mayer et al. 2021). We focus on MSM as a key population in the US HIV epidemic. Disparities are layered, with Black and Hispanic/Latino MSM having considerably higher new diagnosis rates than non-Hispanic White MSM. Drivers of Black–White HIV disparities are not caused by group differences in HIV risk behaviors but rather by differences in structural factors such as sexual network density and homophily, differences in health care outcomes, stigma, abuse, and incarceration (Goodreau et al. 2017, Mustanski et al. 2019a, Oster et al. 2011). Given these racial disparities, we approach this review from an intersectional perspective (L.R. Smith et al. 2022, Sullivan et al. 2021).
EFFECTIVE HIV INTERVENTIONS
Our analysis focuses on three key interventions: HIV PrEP, testing, and linkage-to-care. PrEP is an effective biomedical intervention that can prevent HIV acquisition (Fonner et al. 2016). The US Food and Drug Administration has approved three medications for use as PrEP: two daily oral medications and an injection given every 2 months. While all three forms of PrEP are highly effective, prevention depends strongly on adherence and sustained use during periods of risk exposure (Chou et al. 2019).
Rapid diagnosis is critical to improving the health of people living with HIV and reducing the risk of onward transmission. Testing late after HIV infection continues to fuel the epidemic, as undiagnosed and untreated individuals continue to spread HIV; indeed, 30.2% of HIV transmissions are attributable to individuals who are undiagnosed (Skarbinski et al. 2015). Therefore, the Centers for Disease Control and Prevention (CDC) recommend that MSM should test every 3–6 months. Linkage-to-care refers to accessing medical care after being diagnosed (Cohen et al. 2016). According to CDC data, of those who received an HIV diagnosis in 2019, 81% were linked to care within 1 month (Dep. Health Hum. Serv. 2022).
METHODS
Database Retrieval, Screening, and Extraction
The full protocol of our initial systematic review has been described elsewhere (Li et al. 2022, Merle et al. 2023). The protocol is registered with the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42021233089) and followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. All screening and extraction were completed using Covidence software (see https://www.covidence.org; for the PRISMA diagram of each study and full details of exclusion, see Figure 2). The search was conducted from November 2020 to January 2022. The extracted sample-level data were used to identify studies that included MSM as a priority population of focus. Included articles were separated into groups focused on determinants and implementation strategies.
Figure 2.
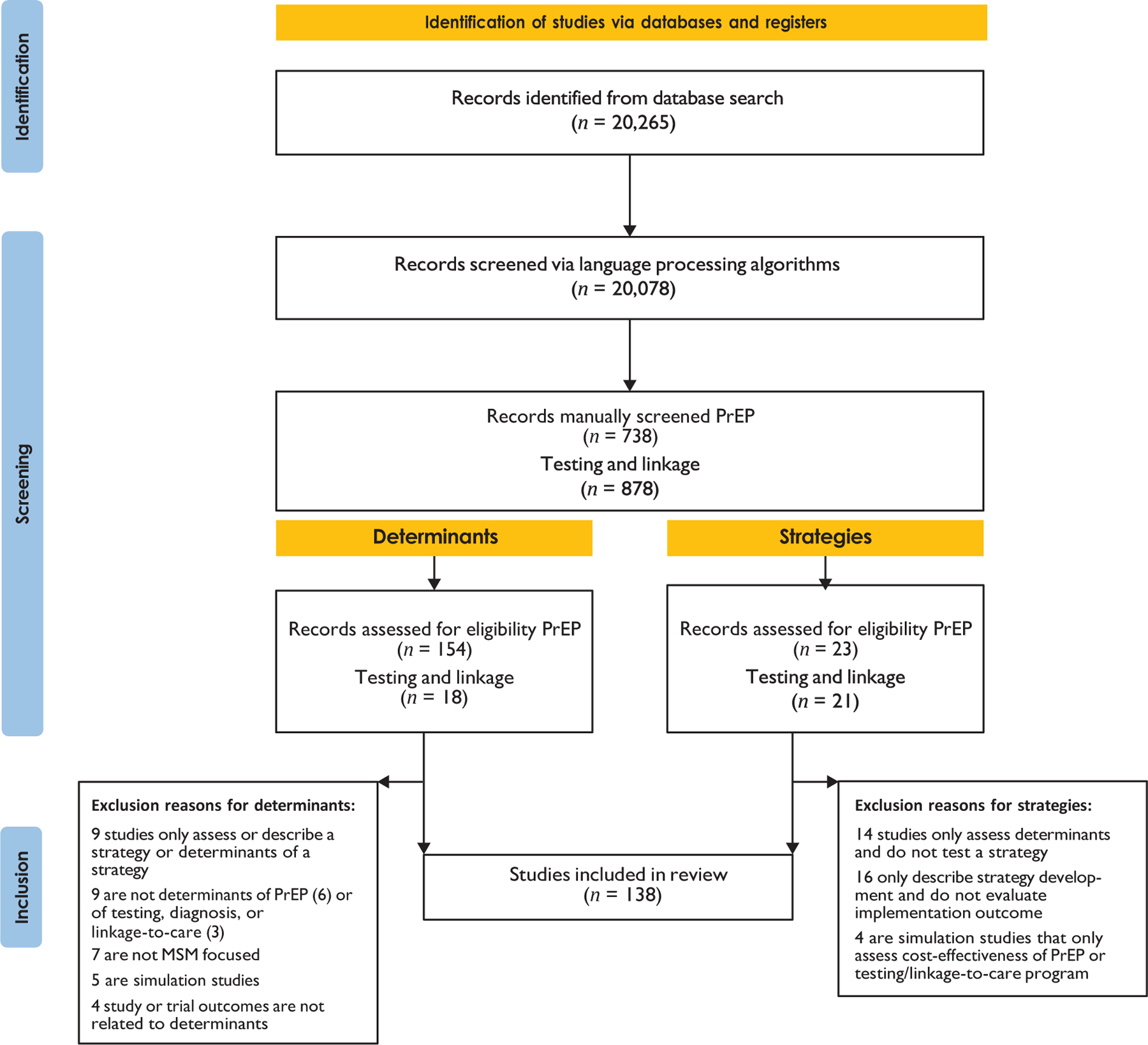
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 flow diagram for new systematic reviews that included searches of databases and registers only. Abbreviations: MSM, gay, bisexual, other men who have sex with men; PrEP, pre-exposure prophylaxis.
Determinant Coding
All papers identified as measuring implementation determinants associated with MSM were uploaded to MAXQDA and qualitatively coded using CFIR 2.0. A companion article to CFIR 2.0 was used to differentiate implementation determinants, which “capture setting-level barriers and facilitators that predict and/or explain… implementation outcomes” from deliverers, from innovation determinants, which “capture recipient-level characteristics and/or experiences with the innovation that predict and/or explain innovation outcomes” from recipients (Damschroder et al. 2022a). Therefore, under each domain, we coded whether the determinants described the deliverers or the recipients; we recognize that different frameworks are available for recipient-level determinants (Damschroder et al. 2022a,b), but we chose to use CFIR for its broad applicability across studies. Additional codes included determinant valence (barrier or facilitator), data collection method (quantitative, qualitative, mixed/multimethod), and HIV innovation (PrEP, testing, linkage-to-care). After the first round of coding, we processed all results and the conclusions sections from each included paper by using a lexical-type analysis technique in IRaMuTeQ (Ratinaud & Marchand 2012), which maps associations and connections between words across papers, using chi-square tests. The results of these analyses guided the mapping of themes across papers and the iterative construction of a second-round codebook, which was applied to all papers in MAXQDA.
Implementation Strategy Coding
We included studies that evaluated one or more implementation strategies. Overall, we assessed strategies in multiple ways. These included the components used within each strategy to effect change, the outcomes assessed, the study design, the extent of specification for future replicability, whether an underlying theory was used in the strategy development, and whether equity was considered. The following categories guided the coding: (a) strategy structure, which delineates the discrete strategies within multicomponent or bundled strategies used to effect implementation outcomes; (b) the ERIC taxonomy domain and code associated with each discrete strategy (Powell et al. 2015); (c) the measured implementation outcome(s) (Proctor et al. 2011); (d) the study design (Hwang et al. 2020); and (e) the degree of strategy specification provided (which allows for future replication), according to established standards (Proctor et al. 2013).
Additionally, informed by literature on assessing and conceptualizing equity in implementation research (Baumann & Cabassa 2020, Brownson et al. 2021, McNulty et al. 2019), we classified each study as (a) not having a health disparities or equity focus, (b) identifying a health disparity, (c) conducting an implementation trial among a priority population that experiences disparities, or (d) comparing the effectiveness of a strategy between a priority population and the general population. Our team coded each strategy on a continuum of effectiveness at influencing the intended implementation outcome: positive (significant improvements), mixed (at least one significant improvement and at least one not significant), null (no significant improvements), negative (worsening outcomes compared with the control), and NA (lack of comparison or baseline condition). Finally, we coded the underlying theory used to develop the strategy.
Coding Procedures
Due to the complexity of coding, each article was coded in Excel by two independent coders, and consensus was reached for each code. If consensus could not be reached, a third reviewer adjudicated.
Analyses
Analysis of variance (ANOVA) testing was conducted to test the hypothesis that a greater number of strategies used in a study would be associated with higher effectiveness.
RESULTS
Determinants
Our analysis included 134 papers, most (n = 126) focused on PrEP and the rest (n = 8) focused on HIV testing and linkage-to-care. Table 1 presents the distribution of determinants by CFIR 2.0 constructs. Most papers on PrEP reported recipient-focused determinants, particularly in the characteristics of individuals domain, followed by innovation and outer setting. Among determinants of intervention deliverers, the most common domains were characteristics of individuals, outer setting, and innovation (in order). Among the subset of papers on testing/linkage-to-care, recipient-focused determinants were, again, mostly in the characteristics of individuals domain, while deliverer determinants were mostly in the characteristics of individuals and inner setting domains. Process determinants were the least frequently measured, in part due to extensive conceptual overlap with implementation strategies (i.e., training, planning, adapting), described below, so we do not include them in this article. In the following sections, we highlight some of the specific constructs found in each of the five CFIR domains and describe their relationship to deliverers or recipients.
Characteristics of individuals.
As described above, the characteristics of individuals domain is grounded in COM-B. Constructs including need, capability, opportunity, and motivation were evaluated for a range of roles, from high-level leaders to recipients. We found limited information on the types of individuals evaluated and therefore simply reported on two predominant and encompassing groups—deliverers and recipients.
PrEP deliverers.
Among studies of PrEP, most deliverer determinants were related to motivation (n = 139) and capability (n = 98). For instance, clinician (i.e., physician, nurse, medical assistant) beliefs that PrEP was better suited for general primary care settings and feeling uncomfortable prescribing PrEP were a barrier to implementation in other systems.
Determinants associated with deliverers’ perceived opportunities included training (n = 52). For example, clinicians in non-HIV clinics were also less likely to have access to or to have read the CDC’s guidelines on PrEP (Doblecki-Lewis & Jones 2016, Shaeer et al. 2014). Additional barriers were measured in other implementation support (n = 8): perceptions of individuals who support the implementation team to implement the innovation. For instance, some nonclinical personnel did not feel as though discussions of PrEP were suited for them in their current capacity (Hakre et al. 2016). Notably, no determinants assessed the perceptions of opinion leaders or implementation facilitators. While papers often delved into recipients’ perceptions of providers’ motivations and attitudes, there is a noticeable gap in addressing the providers’ own perspectives and perceptions within the context of PrEP implementation for MSM.
Testing/linkage-to-care deliverers.
The most common HIV testing/linkage-to-care construct was individual-level demographics that serve as proxies for larger, structural, or social barriers, coded as characteristics otherwise not associated with behavior (n = 13). The second- and third-most-reported testing/linkage-to-care constructs were capability (n = 5) and motivation (n = 5). Deliverers’ perceived capability (or self-efficacy) to provide testing and connect someone to care was a facilitator. A lack of knowledge regarding how to provide testing in different clinical settings was a barrier (e.g., delivery in non-HIV-specific clinics, such as pharmacies or dental clinics). Determinants about motivation focused more on deliverers’ rationale to support or provide testing in their setting. For example, clinicians at a non-HIV-specific clinic stated that they would be more willing to implement testing in their setting if they had access to outcomes from other testing sites (Parish & Santella 2018).
Recipients.
Determinants often came from studies that were focused on specific populations, like Black MSM (41%), Latino MSM (21%), MSM who inject drugs (17%), young MSM (8%), and transgender MSM (i.e., men who were assigned female at birth and who have sex with men; 7%). Capability relates to recipients’ psychological proficiency (or self-efficacy) to know where to access PrEP, how to use PrEP, and what PrEP is (the most common construct in this domain). Across studies, MSM participants identified a lack of confidence in their ability to adhere to oral daily PrEP. Lelutiu-Weinberger & Golub (2016) found that this concern was greater for Black and Latino MSM, but Rolle et al. (2017) found that White men were more likely than Black MSM to cite this lack of confidence as a reason they did not intend to use PrEP. Other factors related to concerns regarding recipients’ ability to take PrEP daily, such as remembering to take a pill, having pills available when away from home, and HIV pill stigma, were associated with oral PrEP (John et al. 2018). Another aspect of capability, awareness, differed across subpopulations of MSM. White and Northeastern MSM are more aware of PrEP than Black and Southern MSM. Latino MSM, MSM with lower levels of educational attainment, and low-income MSM are less likely to be aware of PrEP than other MSM (Garcia & Harris 2017). Cisgender MSM have greater awareness of PrEP than transgender men.
The next most common construct was motivation, including automatic (emotions and impulses arising from associative learning and/or innate dispositions) and reflexive (evaluations and plans) motivation. Automatic motivation included apathy, fear of HIV, fear of disease, trust, and ambivalence. For example, Black MSM are less likely to trust doctors when recommended to take PrEP. Fear, worry, and anxiety were particularly prominent as determinants related to automatic motivation for both testing and PrEP. For some, a fear of disclosure or learning their own status was a barrier to testing. For others, fear and worry facilitated testing, returning for results, and PrEP uptake as mechanisms to relieve those feelings. This facilitative role of PrEP functioned through a sense of relief and peace of mind among individuals who use drugs, engage in casual sex, and/or engage in condomless sex, as well as individuals in serodiscordant relationships. Many studies found that MSM fear discussing sex with a provider, how much they would have to divulge, or that their results would be disclosed to others if they tested positive for HIV. While these findings held among all MSM, rates of such fears were higher among Black and Latino MSM. Additional reflexive motivations that hindered intent to uptake PrEP included low sexual activity, which Rolle et al. (2017) identified as more common among Black MSM. Participants who felt otherwise healthy did not desire to take a pill that they perceived to have a potentially high toxicity.
Multiple determinants were associated with recipients’ perceived physical and social opportunities. For example, availability of testing or PrEP outside of a doctor’s office was a facilitator, and access to support or counseling (including text-based) increased PrEP uptake. However, barriers to PrEP and testing included a lack of access to transportation, lower rates of educational attainment, lower income, lack of access to insurance, and not having a primary care provider. Facilitators related to social opportunity included access to community, connections, and social support (e.g., LGBTQ groups). MSM were more likely to use and adhere to PrEP if they had supportive parents or partners. Higher rates of caballerismo, or Latino MSM’s self-perceptions of “chivalry, familial ties, and emotional connectedness,” significantly increased PrEP awareness, use, and adherence (Rivera et al. 2021).
As found in other domains, multiple types of stigma in the opportunity construct were also key barriers to PrEP and testing. For example, higher rates of anticipated stigma of PrEP were associated with lower uptake. Stigmatizing labels were attached not only to PrEP users but to the pills themselves, with participants referring to PrEP as the “bareback pill,” “slut pill,” or “recreational pill” (Dubov et al. 2018). Internalized stigma led to some MSM being afraid to acknowledge that they have sex with men. MSM also experienced external stigma from other MSM and providers; deliverers perceived youth who want PrEP as naïve and promiscuous or as fearing that PrEP would increase promiscuity. Stigma decreased testing, PrEP use, and adherence (Dubov et al. 2018), and in one study only half of MSM reported that they were proud to be on PrEP (Siegler et al. 2020).
Least examined were determinants related to the recipients’ need, that is, whether recipients and/or deliverers believed MSM recipients truly needed PrEP. For example, some MSM felt that PrEP is only for individuals who engage in “very high risk” sex.
Commonly identified characteristics not otherwise associated with behavior included race, income, education, age, and relationship status as proxies for larger structural issues of racism and classism. Racial disparities in PrEP awareness, uptake, and adherence were prevalent (Ojile et al. 2017, Terndrup et al. 2019, Tripathi et al. 2012). Some studies reported income as a barrier to retention in PrEP care, PrEP awareness, intent to continue use of PrEP, willingness to use PrEP, and history of testing for HIV, while other studies did not. It is possible that discrepancies between studies that found associations and those that did not are due to differences in how income was defined across studies. In addition, education, age, and relationship status shaped innovation access, uptake, and adherence. Facilitators of PrEP use included being single and being in a serodiscordant relationship. However, being in a relationship was a barrier to testing, as was younger age for MSM. While Maksut et al. (2021) found no association between age and PrEP awareness, Whitfield et al. (2020) found that PrEP use increased with age. Finally, education was a determinant of PrEP use, particularly increased education as a facilitator or PrEP use, though increases in educational attainment led to decreased desire for long-acting injectable PrEP.
Characteristics of innovation.
The domain characteristics of innovation assesses the qualities of the innovation itself—such as complexity, cost, trialability, and evidence—that may influence its use. Most of the determinants identified in this domain were for PrEP, specifically cost (n = 82), complexity (n = 89), design (n = 17), other characteristics (n = 186), relative advantage (n = 52), and evidence base (n = 60). The design of PrEP medications stands out as a facilitator, as do the frequency and adherence of injectable PrEP in particular.
Cost-related determinants were often identified are barriers for recipients and deliverers. In particular, price hindered young MSM’s capacity to access PrEP, given privacy concerns related to parents’ insurance or ineligibility for assistance programs (D’Angelo et al. 2021, Greene et al. 2017). Some deliverers also refrained from offering PrEP due to the cost of the drug for recipients. The evidence base for PrEP was a relevant determinant for recipients and deliverers alike. Studies found deliverers and recipients to be skeptical of PrEP and highlighted the need for better dissemination of evidence. Innovation recipients were more likely to question injectable PrEP’s effectiveness compared with that of oral PrEP. MSM desired PrEP advertisements with greater clarity on the number and frequency of necessary appointments and HIV/STI (sexually transmitted infection) screening, the reality that PrEP does not protect against other STIs, and the possibility of side effects. However, John et al. (2018) found that frequency of appointments and HIV/STI screening was not a major concern for most MSM.
Outer setting.
Outer setting determinants are sociocultural, political, and environmental factors that affect implementation. No studies identified determinants related to critical incidents (e.g., disruption due to a natural disaster or mass shortage of supplies) or societal pressure (e.g., mass media campaigns, advocacy groups); however, the studies were conducted prior to the COVID-19 pandemic. Outer setting determinants were most often identified as barriers—for example, how racism impedes equitable uptake of PrEP for Black and Latino MSM. The most frequently measured determinants related to local attitudes (n = 187) and local conditions (n = 80), followed by systemic and structural oppression (n = 139), financing (n = 58), partnerships and connections among organizations and health care agencies (n = 47), policies and laws (n = 22), and market pressure (i.e., to compete with growing supply and demand; n = 2).
Local attitudes were a facilitator of PrEP in some contexts. However, numerous types of stigmas (anticipated, internal, and experienced) about sexual orientation, HIV, and PrEP itself were counterbalancing barriers. In addition, structural racism, medical mistrust, access, and poverty were barriers to PrEP awareness, willingness, uptake, and adherence. Cahill et al. (2017), for instance, conducted focus groups with MSM and highlighted the roles of medical mistrust and distrust of HIV researchers and government officials as barriers to PrEP uptake among Black MSM, while nearly all Black MSM participants in the study also experienced housing instability. Finally, access played a significant role in PrEP delivery and receipt, with nearly a quarter of MSM having to travel 30 min or more—and 10% having to travel 60 min or more—to access PrEP (Siegler et al. 2018).
Inner setting.
Determinants related to the inner setting, the specific context where the innovation is implemented, were reported mostly in studies of PrEP and described only deliverers. The most identified constructs were available resources (n = 64), structural characteristics (n = 25), compatibility (n = 22), and culture (n = 12).
Clinical protocol and effective workflows were important facilitators for PrEP and testing, mainly for deliverers but also in terms of respecting recipients’ time. While culture was the least identified construct within the inner setting, it included some of the richest data. For instance, Krakower et al. (2012) found that providers adopted a passive approach to PrEP provision, such that they relied on patients’ requests for PrEP instead of routinely assessing whether patients would benefit from its use. Other cultural barriers included lack of on-site psychological support and/or sensitivity to patients’ emotional needs (Brant et al. 2020).
Implementation Strategies
Out of 78 articles identified via extraction, 44 were included in the strategy review (23 PrEP, 21 testing/linkage-to-care). Across all articles, the study designs included seven observational studies, 26 within-site designs, 10 between-site designs, and one simulation (Hwang et al. 2020). Regarding the stage of research (Smith et al. 2020a), 19 were pilot trials, 22 tested/trialed implementation strategies, and three were tests to compare strategies (Hwang et al. 2020). Two studies included elements across more than one stage, but none self-identified as hybrid effectiveness–implementation studies (Curran et al. 2022). A total of 37 bundled/blended strategies (i.e., packages of discrete strategies delivered together) were included across studies (18 PrEP, 19 testing/linkage-to-care); within those bundles, 175 discrete strategies were evaluated. On average, each study used 4 discrete strategies (minimum, 1; maximum, 11).
ERIC taxonomy coding.
We coded 175 discrete strategies to the nine ERIC domains (Powell et al. 2015). Of the 73 possible strategies listed in ERIC, 33 (45%) were used. Common domains were engage consumers (n = 95), followed by train and educate stakeholders (n = 25), develop stakeholder interrelationships (n = 16), and change infrastructure (n = 12). Infrequent domains were use evaluative and integrative strategies (n = 3) and provide interactive assistance (n = 3). Regarding strategies, the most commonly used were: intervene with patients/consumers to enhance uptake and adherence (n = 49), prepare patients/consumers to be active participants (n = 26), and involve patients/consumers and families in the implementation effort (n = 14)—all focused on recipients. The next most common strategies, change service sites (n = 10) and conduct ongoing training (n = 8), focus on the deliverers. We added a further strategy that was not captured by the ERIC taxonomy: integrating HIV testing within an existing service. We created a new ERIC code (10.1) for this strategy because it fell outside the nine ERIC domains. Supplemental Table 1 provides the domain and the name, number, and definition of each strategy coded in this study.
Strategy specification.
Proctor et al. (2013) provide strategy specification reporting guidelines that encourage authors to describe the actor, action, action target, temporality, dose, and justification of each strategy for replicability and comparability. For each element, we rated our confidence in the presence of this information on a four-point scale (where 3 indicates completely confident and 0 indicates not present or undeterminable). We used confidence rather than presence/absence given that these elements were rarely explicitly labeled using the Proctor et al. guidelines. Overall, strategies were well specified, though lower overall for the action category and for PrEP studies. Average ratings were 2.13 for actor [mean (M) = 1.68 for PrEP; M = 2.54 for testing/linkage-to-care], 2.04 for action (M = 1.96 for PrEP; M = 2.13 for testing/linkage-to-care), 2.41 for temporality (M = 2.34 for PrEP; M = 2.48 for testing/linkage-to-care), and 2.40 for dose (M = 2.26 for PrEP; M = 2.54 for testing/linkage-to-care). Notably, recipients were the action target for 41 (91%) of strategies. In addition, most studies (n = 43; 95%) provided a justification for the strategies chosen.
Theory underlying strategies.
Although most studies justified their strategies, the underlying theory was mentioned in only nine articles (20%). Multiple studies (Landovitz et al. 2017, Liu et al. 2019, Mitchell et al. 2018) described the adoption of the information–motivation–behavioral skills model of health behavior (Fisher & Fisher 2002). Another study (Desrosiers et al. 2019) developed its strategy using the self-determination theory of human motivation (Ng et al. 2012), where conditions supporting an individual’s psychological needs foster motivation to engage in a healthy behavior. Social cognitive theory (Bandura 1986) was used to embed the Many Men, Many Voices (3MV) intervention into a PrEP delivery study (Hosek et al. 2013a) along with the strategies used by Alonzo et al. (2016) and Rhodes (2004). Other theories mentioned were the transtheoretical model (Prochaska & DiClemente 1982), social support (Clark et al. 1999), self-regulation theory (Baumeister & Heatherton 1996), developmental learning theory (Schank & Berman 2002), and the Pedagogy of the Oppressed (Freire 1970).
Health equity.
We also captured whether studies focused on health disparities or health equity. Most commonly, studies (n = 15) conducted an implementation trial among a priority population or a population with an identified health disparity (n = 11). Four studies compared the effectiveness of a strategy between a priority population and the general population, and 14 did not focus on health disparities/equity.
Implementation outcomes.
A total of 102 implementation outcomes were coded. Most outcomes were at the patient level (n = 82), with fewer at the provider level (n = 20) (Table 2). The most frequently studied outcome was the acceptability, feasibility, or appropriateness of the strategy (19 were patient level and 9 provider level), followed by patient-level PrEP adherence (n = 16), patient-level adoption/uptake of HIV testing or linkage-to-care (n = 11), and patient-level reach of HIV testing (n = 9). The most-studied provider-level outcomes were implementation costs (n = 6) and intervention fidelity (n = 5). At the provider level, no studies captured knowledge or awareness of PrEP and sustainment/ability. Other notable outcomes beyond our a priori categories included patient-level engagement with the implementation strategy and positivity rates.
Table 2.
Implementation strategy characteristics
| Reference | Innovation | Strategy name | Unique ERIC code(s) includeda | Research stage | Effectiveness rating |
|---|---|---|---|---|---|
| Amico et al. (2019) | PrEP | iNSC training | 2.1, 5.1, 5.2, 5.3, 5.4, 5.6 | Test/trial strategies | Positive |
| Burns et al. (2022) | PrEP | Meet Me Where I Am training | 5.1, 5.4, 5.7 | Test/trial strategies | NA |
| Chai et al. (2021) | PrEP | Digital pill systems | 7.2 | Pilot strategies | NA |
| Chen & Dowdy (2014) | PrEP | Decision analysis model | 4.12, 6.1 | Simulation | NA |
| Clement et al. (2019) | PrEP | PrEP model | 1.2, 4.5, 4.6, 4.17, 5.3, 6.5, 7.3, 7.2, 8.6 | Test/trial strategies | Mixed |
| Colson et al. (2020) | PrEP | enPrEP | 2.3, 5.1, 7.1, 7.2, 8.4 | Compare strategies | Negative |
| Desrosiers et al. (2019) | PrEP | PrEP counseling center | 7.2 | Pilot strategies | Mixed |
| Egan et al. (2020) | PrEP | Epi-PrEP | 7.2, 7.3 | Pilot strategies | Positive |
| Fuchs et al. (2018) | PrEP | iText | 7.1, 7.2 | Pilot strategies | Mixed |
| Havens et al. (2019) | PrEP | Pharmacist-led HIV screening and PrEP program | 7.1, 7.2, 7.3 | Pilot strategies | Mixed |
| Hosek et al. (2017) | PrEP | Comprehensive HIV prevention package | 7.2 | Pilot strategies | Mixed |
| Hosek et al. (2013b) | PrEP | 3MV | 4.10, 4.11, 7.2 | Test/trial strategies | Positive |
| Hosek et al. (2013a) | PrEP | 3MV | 7.2 | Pilot strategies | Mixed |
| King et al. (2014) | PrEP | PrEP counseling | 5.3, 7.3 | Test/trial strategies | Negative |
| Klein et al. (2017) | PrEP | Real Talk | 7.3 | Pilot strategies | Null |
| Koss et al. (2018) | PrEP | Wisepill wireless adherence monitoring device | 7.2 | Pilot strategies | Mixed |
| Lalley-Chareczko et al. (2018) | PrEP | PrEP administration, intervention, and augmented adherence monitoring | 7.3, 6.1, 10.1 | Test/trial strategies | Positive |
| Landovitz et al. (2017) | PrEP | PATH-PrEP | 7.2, 7.3, 6.1 | Test/trial strategies | Positive |
| Liu et al. (2019) | PrEP | PrEPmate | 7.2, 7.3, 7.5 | Pilot strategies | Positive |
| Mayer et al. (2021) | PrEP | Life-Steps for PrEP (nurse-led) | 5.4, 6.1, 7.2, 7.3 | Pilot strategies | Mixed |
| Mitchell et al. (2018) | PrEP | eHealth Contingency Management (mSmart) | 7.2, 7.3 | Pilot strategies | Positive |
| Moitra et al. (2020) | PrEP | PrEP motivational interview | 7.2 | Simulation | Mixed |
| Phillips et al. (2020) | PrEP | PrEP4Love | 7.2 | Test/trial strategies | Mixed |
| Alonzo et al. (2016) | Testing | Hola en Grupos | 7.2 | Test/trial strategies | NA |
| Avoundjian et al. (2019) | Testing | HIV testing integrated into syphillis partner services | 9.2, 9.5, 10.1 | Test/trial strategies | NA |
| Blank et al. (2005) | Testing | Hot Shot! Healthy Men’s Night Out program | 4.10, 4.17, 7.2, 7.5, 9.5 | Test/trial strategies | NA |
| Catania et al. (2020) | Testing | Oral self-implemented HIV testing training | 7.2, 7.3 | Pilot strategies | Positive |
| Daskalakis et al. (2009) | Testing | Bathhouse-based testing | 9.5 | Test/trial strategies | Positive |
| Frye et al. (2021) | Testing | TRUST | 7.2, 7.3 | More than one stage | Positive |
| Grusky et al. (2010) | Testing | MTU | 9.5 | Compare strategies | NA |
| Halkitis et al. (2011) b | Testing | SNS | 4.10, 5.2, 7.1, 8.4 | Compare strategies | Positive |
| Halkitis et al. (2011) | Testing | Alternate venue testing | 1.7, 3.4, 7.2, 9.5 | Test/trial strategies | NA |
| Hirshfield et al. (2012) | Testing | Prevention digital media | 7.1 | Pilot strategies | Mixed |
| Jenkins Hall et al. (2017) | Testing | Mobile apps for prevention and testing, health educator | 4.1, 7.5 | Test/trial strategies | NA |
| Lightfoot et al. (2018) | Testing | Project T | 4.1, 5.1, 5.4, 7.1 | Pilot strategies | Positive |
| Maksut et al. (2016) | Testing | Self-administered at-home HIV testing with web-based counseling | 7.2, 7.3, 9.5 | Pilot strategies | Positive |
| Marlin et al. (2014) | Testing | Commercial voucher program | 4.5, 6.3, 7.5 | Test/trial strategies | Mixed |
| Martínez-Donate et al. (2010) | Testing | Hombres Sanos digital campaign | 7.1, 7.5 | Pilot strategies | Mixed |
| McCree et al. (2013) | Testing | SNS for testing | 3.1, 4.1, 5.1, 8.4 | Test/trial strategies | Mixed |
| McGoy et al. (2018) | Testing | SNS program | 1.5, 2.2, 4.1, 8.4 | Test/trial strategies | Mixed |
| Qureshi et al. (2018) | Testing | HIV and STI educational session | 7.1, 7.2 | Pilot strategies | Positive |
| Rhodes (2004) | Testing | Online public chat room with health educator | 7.3 | Test/trial strategies | Positive |
| Shrestha et al. (2020). | Testing | HIV self-testing | 7.5, 9.2, 9.5 | Test/trial strategies | Positive |
| Shrestha et al. (2010) | Testing | SNS to identify new HIV diagnoses | 5.1, 7.2, 8.4 | Test/trial strategies | Positive |
| Washington et al. (2017) | Testing | TIM | 7.3 | Test/trial strategies | Positive |
For the ERIC codes, as well as their names and descriptions, see Supplemental Table 1. Some strategies had multiple instances of the same ERIC code. Only the unique ERIC codes are listed here for each strategy.
Two strategies were compared for the Halkitis et al. (2011) study, which is why there are 45 rows instead of 44 (which would match the total strategies noted in the text).
Abbreviations: ERIC, Expert Recommendations for Implementing Change; iSNC, Integrated Next Step Counseling; MTU, mobile testing unit; NA, not applicable; PrEP, pre-exposure prophylaxis; SNS, social network strategy; STI, sexually transmitted infection; TIM, Testing Intervention Model; 3MV, adapted Many Men, Many Voices.
Strategy effectiveness.
Regarding effectiveness, 18 strategies were rated as having a positive effect (n = 7 on PrEP; n = 11 on testing/linkage-to-care), 15 had a mixed effect (n = 10 on PrEP; n = 5 on testing/linkage-to-care), 2 had a negative effect (PrEP), and 1 had a null effect (PrEP) (Table 3). Nine studies were rated NA (n = 2 on PrEP; n = 7 on testing/linkage-to-care) due to a lack of comparison. Table 2 displays the ERIC codes, research stage, and associated outcomes of the strategies rated as having a positive effect (n = 17), and Supplemental Table 1 briefly describes the strategies provided by the studies’ authors along with our ERIC codes. One-way ANOVA tests found no significant differences between the number of discrete strategies within a blended strategy and the strategy’s effectiveness, indicating that, assuming all else equal, including more discrete strategies within a bundled package did not predict a higher effectiveness rating (for details, see Supplemental Table 2).
Table 3.
Effective strategy characteristics and outcomesa
| Reference | Strategy name(s) | Unique ERIC domains | Research stage | Patient-level outcomes | Provider-level outcomes |
|---|---|---|---|---|---|
| Amico et al. (2019) | iNSC training | 2, 5 | Test/trial strategies | PrEP feasibility and adherence | Strategy acceptability, appropriateness, and feasibility; strategy fidelity |
| Egan et al. (2020) | Epi-PrEP | 7 | Pilot strategies | PrEP adherence and sustainment | NA |
| Hosek et al. (2013b) | 3MV | 4, 7 | Test/trial strategies | Strategy acceptability, appropriateness, feasibility | Strategy fidelity |
| Lalley-Chareczko et al. (2018) | PrEP administration, intervention, and augmented adherence monitoring | 6, 7, 10 | Test/trial strategies | PrEP fidelity and sustainment | NA |
| Landovitz et al. (2017) | PATH-PrEP | 6, 7 | Test/trial strategies | PrEP acceptability, appropriateness, adoption, adherence | NA |
| Liu et al. (2019) | PrEPmate | 7 | Pilot strategies | PrEP adherence, reach; strategy acceptability, appropriateness, feasibility | NA |
| Mitchell et al. (2018) | eHealth Contingency Management (mSmart) | 7 | Pilot strategies | PrEP adherence; strategy acceptability, appropriateness, feasibility | NA |
| Catania et al. (2020) | Oral self-implemented HIV testing training | 7 | Pilot strategies | Testing fidelity | Strategy fidelity |
| Daskalakis et al. (2009) | Bathhouse-based testing | 9 | Test/trial strategies | Testing reach; strategy acceptability, appropriateness, feasibility | Strategy acceptability, appropriateness, feasibility |
| Frye et al. (2021) | TRUST | 7 | More than one stage | Testing uptake, adherence, sustainment; strategy acceptability, appropriateness, feasibility | NA |
| Halkitis et al. (2011) | SNS | 4, 5, 7, 8 | Compare strategies | Testing reach | NA |
| Lightfoot et al. (2018) | Project T | 4, 5, 7 | Pilot strategies | Testing adoption/uptake | Strategy acceptability, appropriateness, feasibility |
| Maksut et al. (2016) | Self-administered at-home HIV testing with web-based counseling | 7, 9 | Pilot strategies | Testing adoption/uptake; strategy acceptability, appropriateness, feasibility | NA |
| Qureshi et al. (2018) | HIV and STI educational session | 7 | Pilot strategies | Testing adoption/uptake | NA |
| Rhodes (2004) | Online public chat room with health educator | 7 | Test/trial strategies | Strategy acceptability, appropriateness, feasibility | NA |
| Shrestha et al. (2020) | HIV self-testing | 7, 9 | Test/trial strategies | Testing reach | Costs |
| Shrestha et al. (2010) | SNS to identify new HIV diagnoses | 5, 7, 8 | Test/trial strategies | Testing adoption/uptake; strategy acceptability, appropriateness, feasibility | Costs; testing adoption/uptake |
| Washington et al. (2017) | TIM | 7 | Test/trial strategies | Testing knowledge/awareness, adoption/uptake, reach; strategy acceptability, appropriateness, feasibility | NA |
The table includes only the strategies rated positive. For additional descriptions of the strategies, see Supplemental Table 2.
Abbreviations: ERIC, Expert Recommendations for Implementing Change; iSNC, Integrated Next Step Counseling; NA, not applicable; PrEP, pre-exposure prophylaxis; SNS, social network strategy; STI, sexually transmitted infection; TIM, Testing Intervention Model; 3MV, adapted Many Men, Many Voices.
DISCUSSION
We conducted this systematic review to better understand existing implementation research on PrEP and HIV testing/linkage-to-care interventions with MSM. Among the determinants we identified, nearly two-thirds described recipient-level characteristics regarding the use of PrEP or HIV testing/linkage-to-care; a smaller proportion were implementation determinants, which capture delivery-level barriers and facilitators that affect implementation. Most determinants focused on characteristics of individuals for PrEP and HIV testing/linkage-to-care. For deliverers, capability and motivation were key barriers; for recipients, stigma, mistrust, fear, intersections with racism and other oppressions, and access were important constructs. However, there are likely important gaps in knowledge here—no studies, for example, reported on administrative leadership or opinion leaders. While addressing capability, motivation, and opportunities is important for behavior change, the overwhelming focus on characteristics of innovation deliverers and recipients reveals a dearth of barriers related to other domains. Within the innovation domain, the characteristics of PrEP itself stood out as a facilitator, while costs and level of evidence were a concern for deliverers and recipients. Within the outer setting, stigma again emerged as an important barrier to recipients in terms of local attitudes; however, there were few studies of policies, critical incidents (such as COVID-19), or market pressures, all of which may be more relevant to delivery systems. Finally, the inner setting domain focused on deliverer determinants, highlighting the importance of resources, workflows, and culture.
Unsurprisingly, among the discrete implementation strategies identified, more than half were at the recipient level (i.e., directed at MSM), and the majority were measured using recipient-level outcomes (e.g., PrEP acceptability). Only 33 of 73 potential strategies were reported—mostly education of either recipients or stakeholders, with a few focused on changing infrastructure. While most studies used an underlying theory to select their strategy, there may be a gap in addressing the most important barriers, particularly at the delivery level. In terms of effectiveness, most studies with a positive result used a combination of strategies, including patient engagement and stakeholder education; however, these were also the most common strategies overall.
Below we discuss gaps, future directions, and resources to advance HIV implementation science, including areas where the field of psychology can further contribute to implementation science in HIV, as well as integrating implementation into the development and testing of psychology-based interventions (Pyra et al. 2022).
Literature Gaps and Future Directions
Our review has highlighted that most research was on PrEP, likely reflecting the advent of implementation science for HIV at the same time PrEP was being rolled out. We observed many common determinants across PrEP and HIV testing/linkage-to-care, particularly around capacity and motivation of providers; capacity, motivation, and opportunity of recipients, with an emphasis on stigma; evidence and cost of the innovation; and inner setting resources. Therefore, these determinants likely apply to emerging innovations carried out in similar settings. Effective system-level implementation strategies and adjunctive interventions to address common determinants can facilitate effective scale-up of new innovations (e.g., long-acting PrEP/ART) and scale-out (Aarons et al. 2017) to nontraditional settings such as pharmacy and syringe service programs or new delivery conceptualizations (e.g., status-neutral, rapid initiation).
Our literature review has identified limited research on young MSM, particularly among adolescents under the age of 18. Adolescent MSM have a high HIV incidence (Balaji et al. 2018, Garofalo et al. 2016, Mustanski et al. 2019b), poor HIV status awareness due to low rates of testing (CDC 2021b, Mustanski et al. 2020), and lower rates of viral suppression (CDC 2022), indicating a need for better services across the HIV prevention and care continuum. Furthermore, implementation determinants (e.g., parental permission and support) and strategies (e.g., school-based) are likely to differ for teens and therefore merit new implementation research.
Only 37 of the 73 ERIC strategies were present in the literature included, again with a focus on recipients over deliverers/systems. Expanding the use of strategies, particularly for deliverers and larger delivery systems, is an area of needed growth. We also note that the ERIC taxonomy was a product of experts in mental health (Powell et al. 2015); while we believe many of these strategies are relevant to HIV (with some adaptation), there may be value in replicating the taxonomy’s development with HIV subject matter experts to create an HIV-specific version of ERIC. In the meantime, the tools described below can help researchers and implementers select and test appropriate strategies based on the determinants identified in their context.
Most of the studies we evaluated were at an early stage on the implementation science continuum, with a focus on understanding determinants. Rigorous research designs to test and compare strategies occur later on the continuum, as do hybrid effectiveness–implementation studies that simultaneously test and evaluate the innovation and the implementation strategies. Many of the strategy studies were piloting or exploratory and had outcomes related to acceptability, appropriateness, and feasibility of the strategy. Some authors do not consider such outcomes to be formal implementation science outcomes but instead conceptualize them as perceptual or antecedent outcomes (Damschroder et al. 2022a, Reilly et al. 2020). More research is needed to include formal, summative implementation outcomes, such as adoption, reach, and sustainment (Smith & Hasan 2020). This aligns with a recent mapping review by Smith et al. (2020a), who found that around half of HIV implementation studies funded by the US National Institutes of Health (NIH) were implementation preparation studies, defined as studies conducted in preparation for a formal prospective evaluation or test of implementation strategies. Getting to testing of strategies is critical, and better methods of selecting strategies for context are needed to make it happen. This could be a fruitful area for psychologists advancing newer study designs to test strategies on the basis of appropriate theories (Curran et al. 2022, Hwang et al. 2020).
Adjunctive Interventions
A significant proportion of articles studied adjunctive interventions to support clients so that they can benefit from intervention effects. For instance, many of the implementation strategies coded using the engage consumers domain of ERIC may be better conceptualized as adjunctive interventions (Smith et al. 2023). Adjunctive interventions act upon the recipient to influence innovation-level outcomes (uptake, adherence). For example, they often aim to increase patients’ adherence to daily oral PrEP and their ability to accurately complete an at-home HIV rapid test. This distinction is important for two reasons. First, implementation strategies need to be identified to support delivery of the adjunctive intervention. Second, implementation of the adjunctive intervention alone will not affect the endpoint health outcome without main innovation (PrEP or testing/linkage-to-care).
Of the 44 articles involving MSM, 17 exclusively examined adjunctive interventions, 20 studied a mix of strategies and adjunctive interventions, and only 7 focused on strategies that solely targeted the delivery system. More accurately defining and studying adjunctive interventions—rather than the currently inconsistent conceptualization of them as implementation strategies or health interventions—would advance our understanding of unique challenges to implementation and the strategies required. Psychologists have played a substantial role in the development of many evidence-based adjunctive interventions and can help lead the development of the next generation (e.g., mHealth).
TOOLS AND RESOURCES
Tailoring Strategies to Determinants
Researchers and practitioners newer to implementation science are often comfortable assessing barriers and facilitators but struggle with selecting or developing appropriate strategies associated with those determinants. As we continue to synthesize literature around determinants and strategies, it is critical that we also build knowledge of which strategies effectively address which barriers to achieve which outcomes and then translate that knowledge for others to use. Experts in the broader implementation science field have attempted to match determinants to specific strategies through consensus (Waltz et al. 2019) but have found little consistency in recommendations linking the 39 barriers from CFIR 1.0 to the 47 ERIC strategies. This is an important area for psychologists to explore and develop additional theories and methods to improve matching of strategies to barriers. By narrowing the focus to HIV contexts, we may identify more homogeneity in published evidence. To this end, we are developing a tool that can recommend HIV implementation strategies given known contextual factors or identify determinants targeted by a given strategy type. We are also developing a tool to facilitate specification of the relationships among determinants, strategies, and outcomes using the implementation research logic model (Smith et al. 2020b).
High-Quality and Comparable Measurement and Reporting Across Studies
The development of high-quality generalizable knowledge through research synthesis is contingent on the comparability of findings across studies. As observed in this review, standardization of determinant measurement, specification of strategies, and assessment of outcomes would greatly facilitate the development of the evidence base—a noted problem in implementation science (Martinez et al. 2014, Rabin et al. 2012). To that end, researchers have created a crosswalk of HIV implementation science outcomes that guides researchers in operationalizing outcomes for their respective projects and also serves as a data collection tool for harmonization across the 200-plus EHE projects funded by NIH (Mustanski et al. 2022, Queiroz et al. 2022). Relatedly, we found a need for better justification of the strategies selected and tested—that is, better use of theory and, although sometimes scant, empirical evidence. Adherence to reporting and specification standards could be enhanced with new methods for data collection on strategy use and modifications over time, developed by J.D. Smith et al. (2022) and others (Rabin et al. 2018).
These issues are critical for the synthesis of findings and replication of implementation research that would lead to generalizable findings. Without such efforts toward generalizability and improved rigor, the potential of this field to more efficiently and effectively translate effective innovations into practice could be unrealized.
Sharing Data
Implementation researchers need to efficiently identify evidence on determinants and strategies germane to their context. A novel example is the use of interactive visualization dashboards, such as the HIV Implementation Review Dashboard (see https://hivimpsci.northwestern.edu/dashboard), which allows users to examine more than 1,900 determinants of PrEP implementation from 239 peer-reviewed articles from a systematic review (Li et al. 2022), coded by CFIR, population, region, and so forth. This tool was created to allow researchers to quickly take stock of evidence and complete their own analysis of subsets of the literature [e.g., the in-depth analysis of determinants of uptake and adherence to PrEP in transgender communities by Zamantakis et al. (2022)]. Implementers can also quickly find evidence for determinants (and, soon, strategies) for their population and context. A similar dashboard has been created for international HIV implementation science (see https://idig.science/LIVE).
Equity Considerations
Health equity is a central focus of EHE and a growing area of emphasis in implementation science (Brownson et al. 2021, Schlechter et al. 2021), so we sought to code for it in our review. There are several ways to conceptualize implementation research addressing health inequities. We took the broad perspective of the overall study including or focusing exclusively on populations with known health disparities (McNulty et al. 2019). As this area of implementation science matures, we hope to be able to understand more-nuanced aspects of health equity, such as implementation strategies selected and used specifically to address barriers that caused the disparities in the first place. For instance, we hope to start seeing studies of equitable implementation, meaning the use of strategies that address underlying structural causes of the disparities, such as the disinvestment in communities of color that has resulted in scarce and underresourced health care systems (Smith et al. 2021).
Numerous studies identified determinants driving HIV-related inequalities; likewise, many strategy studies focused on priority populations or identifying additional health disparities. However, only a handful of studies examined or targeted implementation determinants related to structural racism or heterosexism, which are known drivers of HIV risk among young Black and Latino MSM (Babel et al. 2021, Mustanski et al. 2019a). There is a need for strategies and adjunctive interventions to address stigma (especially intersectional stigma) that are developed to address the deliverer, recipient, and system/community levels, another area where psychologists can help advance the field (Taggart et al. 2022). Furthermore, it is unclear whether the most effective strategies are being used to address inequity-driving determinants, given that few studies examined determinants among health leaders or used structural interventions, or whether all strategies have been identified to address the many determinants found in our review. Most studies focused on implementation determinants and strategies in settings where HIV testing and PrEP are traditionally offered, but the large percentage of people undiagnosed with HIV and the low percentage of people on PrEP among racial/ethnic minorities and young MSM call for studies of implementation in nontraditional settings that could maximize reach to those subpopulations that are less likely to access traditional health care services.
Limitations
Our review of determinants and strategies was grounded in CFIR 2.0 and ERIC, respectively. While they are commonly used in the field, some factors we know to be salient to HIV intervention delivery and uptake (e.g., structural oppression, adjunctive interventions) are not captured. Our study focused on MSM, and a previous scoping review conducted by our team (Zamantakis et al. 2022) highlighted the recurring difficulty of disaggregating results in studies lumping cisgender MSM and transgender women/transfeminine individuals. Transgender women more often experience determinants shaped by how contextual factors, institutions, and individuals interact with their gender and racial identities. As such, we discourage the lumping together of cisgender MSM and transgender women in future HIV implementation science and encourage greater attention to strategies for effective implementation of HIV services for transgender women.
CONCLUSION
Ending the HIV epidemic in the United States will require implementation strategies tailored to priority populations, such as subgroups of MSM (i.e., youth, those living in the South), as generalized approaches will be insufficient to address their specific barriers. The increased federal HIV funding tied to EHE is essential to support prevention, testing, and treatment programs as well as research into new innovations and implementation strategies. Access to these programs needs to be expanded, and programs should work to reduce structural barriers such as cost and transportation issues. Many forms of stigma came up in our review as essential barriers—addressing intersectional stigma is an active area of HIV research (Dale et al. 2022), and we look forward to seeing strategies for implementing the resulting interventions.
While most research to date has focused on recipients, understanding barriers at the deliverer and delivery system levels, as well as testing the appropriate strategies targeting deliverers, is another important and growing area. Concerning outcomes, as the field matures and strategies are formally tested and trialed, we hope to see more summative implementation outcomes, such as reach, adoption, and sustainment of interventions for MSM, as opposed to the preponderance of studies on acceptability and appropriateness of the intervention that pay little attention to the strategies and their effects. Finally, the ongoing collaboration and coordination among federal, state, and local public health agencies; community-based organizations; health care providers; and scientists that have been enabled by EHE (Purcell et al. 2022) will be critical to ensuring that scientific innovations can rapidly reach the people who most need them.
Supplementary Material
RELATED RESOURCES.
HIV Implementation Literature Review Dashboard, version 1.0 (https://hivimpsci.northwestern.edu/dashboard). This interactive dashboard allows users to extract the articles included in this sytematic review by key characteristics such as population of focus, setting, and CFIR domains.
Implementation Outcomes Crosswalk (https://hivimpsci.northwestern.edu/implementation-outcomes-crosswalk). The Crosswalk supports the identifcation of implementation outcomes for research studies ranging from exploratory to sustainment.
Implementation Science Navigation (https://hivimpsci.northwestern.edu/is-navigation). This online HIV implementation science curriculum has versions tailored to researchers, practioners, and funders.
JAIDS Special Issue on Harnessing Implementation Science to Inform Strategies for Ending the HIV Epidemic in the United States (https://journals.lww.com/jaids/toc/2022/06001). This supplemental issue of JAIDS focuses on implemenntation science to help end the US HIV epidemic.
SUMMARY POINTS.
Implementation science is needed to advance efforts to end the HIV epidemic.
Most studies on pre-exposure prophylaxis (PrEP) uptake and use focused on non-Latino, White, cisgender men who have sex with men (MSM), with very few attending to the needs of Latino, Spanish-speaking, and/or transgender MSM.
Common PrEP determinants include difficulty integrating PrEP into daily routines; limited access to LGBTQ-affirming care; cost (barrier); evidence base (barrier); stigma (barrier); structural racism and poverty (barrier); inner setting culture (facilitator); provider capability (barrier); provider stigma (barrier); differences in PrEP awareness and access across race/ethnicity, income, and geography; patient-level stigma (barrier); and planning for implementation (facilitator).
Common testing/diagnosis/linkage-to-care determinants include ease of integrating testing into daily clinical routines (facilitator); provider knowledge (barrier); provider motivation (barrier); availability of testing outside of a doctor’s office (facilitator); time and transportation (diagnosis barrier); structural, provider, and patient stigma (barrier); patient fear and worry (barrier); being in a relationship (barrier); and being a younger MSM (barrier).
There is much less of a focus on MSM populations in HIV testing/diagnosis/linkage-to-care implementation-related research compared with the PrEP literature.
Few papers attend to determinants within the inner setting (i.e., clinics or other settings where the innovation is being implemented) or the process of implementation.
Many interventions characterized in the literature as implementation strategies may be better conceptualized as adjunctive interventions.
There is a need for structural and system-level interventions and implementation strategies.
FUTURE ISSUES.
We identify many common determinants across PrEP and HIV testing/linkage-to-care, suggesting that effective system-level implementation strategies and adjunctive interventions can facilitate effective scale-up of new innovations and scale-out to nontraditional settings.
There is limited research on young MSM, particularly adolescents under 18, who have a high HIV incidence, poor HIV status awareness due to low testing rates, and lower rates of viral suppression.
Only 37 of the 73 ERIC strategies were coded, indicating significant room for new research to develop strategies in other domains, particularly those that address determinants in the inner and outer settings and at the policy level.
Better methods for selecting strategies for context are needed to move beyond early stages of implementation science and into testing of strategies.
Adjunctive interventions require their own implementation strategies to optimize delivery, but additional implementation strategies will be needed to ensure effective scale-up of innovations.
Developing tools that can recommend HIV implementation strategies given known contextual factors or identify determinants targeted by a given strategy type could help researchers and practitioners who are newer to implementation science.
The HIV implementation science literature continues to grow, and reporting results using standardized methods such as the Implementation Research Logic Model can help advance the field.
ACKNOWLEDGMENTS
The writing of this review was made possible through a supplement grant to the Third Coast Center for AIDS Research, a National Institutes of Health–funded center (P30 AI117943). J.L.M.’s time was supported by a training grant from the National Library of Medicine (T15LM007124). J.P.Z.’s and a.z.’s time was supported by a training grant from the National Institute of Mental Health (T32MH130325). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsors had no involvement in the conduct of the research or the preparation of the article. We appreciate the data shared by Dr. Karin Bosh and her team, which allowed us to create some of the images used in this review. We appreciate the work of Jasmine S. Deskins, Ana Michaela Pachicano, Melissa Mongrella, Juan Villamar, Ginger McKay, and Brennan Keiser, who helped review, code, and analyze data. We appreciate the work of Samantha Vera Levya, who helped with formatting, citations, and other work necessary for the completion of this review.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Aarons GA, Sklar M, Mustanski B, Benbow N, Brown CH. 2017. “Scaling-out” evidence-based interventions to new populations or new health care delivery systems. Implement. Sci 12:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo J, Mann L, Tanner AE, Sun CJ, Painter TM, et al. 2016. Reducing HIV risk among Hispanic/Latino men who have sex with men: qualitative analysis of behavior change intentions by participants in a small-group intervention. J. AIDS Clin. Res 7:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico KR, Miller J, Balthazar C, Serrano PA, Brothers J, et al. 2019. Integrated Next Step Counseling (iNSC) for sexual health and PrEP use among young men who have sex with men: implementation and observations from ATN110/113. AIDS Behav 23:1812–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoundjian T, Stewart J, Peyton D, Lewis C, Johnson K, et al. 2019. Integrating human immunodeficiency virus testing into syphilis partner services in Mississippi to improve human immunodeficiency virus case finding. Sex. Transm. Dis 46:240–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babel RA, Wang P, Alessi EJ, Raymond HF, Wei C. 2021. Stigma, HIV risk, and access to HIV prevention and treatment services among men who have sex with men (MSM) in the United States: a scoping review. AIDS Behav 25:3574–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji AB, An Q, Smith JC, Newcomb ME, Mustanski B, et al. 2018. High human immunodeficiency virus incidence and prevalence and associated factors among adolescent sexual minority males—3 cities, 2015. Clin. Infect. Dis 66:936–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A 1986. Social Foundations of Thought and Action: A Social Cognitive Theory Englewood Cliffs, NJ: Prentice Hall [Google Scholar]
- Baumann AA, Cabassa LJ. 2020. Reframing implementation science to address inequities in healthcare delivery. BMC Health Serv. Res 20:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann AA, Woodward EN, Singh RS, Adsul P, Shelton RC. 2022. Assessing researchers’ capabilities, opportunities, and motivation to conduct equity-oriented dissemination and implementation research: an exploratory cross-sectional study. BMC Health Serv. Res 22:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Heatherton TF. 1996. Self-regulation failure: an overview. Psychol. Inq 7:1–15 [Google Scholar]
- Blank S, Gallagher K, Washburn K, Rogers M. 2005. Reaching out to boys at bars: utilizing community partnerships to employ a wellness strategy for syphilis control among men who have sex with men in New York City. Sex. Transm. Dis 32(Suppl.):65–72 [DOI] [PubMed] [Google Scholar]
- Brant AR, Dhillon P, Hull S, Coleman M, Ye PP, Lotke PS, et al. 2020. Integrating HIV pre-exposure prophylaxis into family planning care: a RE-AIM framework evaluation. AIDS Patient Care STDs 34:259–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownson RC, Colditz GA, Proctor EK. 2017. Dissemination and Implementation Research in Health: Translating Science to Practice Oxford, UK: Oxford Univ. Press [Google Scholar]
- Brownson RC, Kumanyika SK, Kreuter MW, Haire-Joshu D. 2021. Implementation science should give higher priority to health equity. Implement. Sci 16:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns PA, Omondi AA, Monger M, Ward L, Washington R, et al. 2022. Meet me where I am: an evaluation of HIV patient navigation intervention to increase update of PrEP among black men who have sex with men in the Deep South. J. Racial Ethn. Health Dispar 9:103–16 [DOI] [PubMed] [Google Scholar]
- Cahill S, Taylor SW, Elsesser SA, Mena L, Hickson D, Mayer KH. 2017. Stigma, medical mistrust, and perceived racism may affect PrEP awareness and uptake in black compared to white gay and bisexual men in Jackson, Mississippi and Boston, Massachusetts. AIDS Care 29:1351–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania JA, Dolcini MM, Harper G, Fortenberry D, Singh RR, et al. 2020. Oral HIV self-implemented testing: performance fidelity among African American MSM. AIDS Behav 24:395–403 [DOI] [PubMed] [Google Scholar]
- CDC (Cent. Dis. Control Prev.). 1999. U.S. HIV and AIDS cases reported through December 1999. HIV Surveill. Suppl. Rep 11(2). https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-1999-vol-11-2.pdf [Google Scholar]
- CDC (Cent. Dis. Control Prev.). 2007. Cases of HIV infection and AIDS in the United States and dependent areas, 2007. HIV Surveill. Suppl. Rep 19. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2007-vol-19.pdf [Google Scholar]
- CDC (Cent. Dis. Control Prev.). 2021a. Estimated annual number of HIV infections—United States, 1981–2019. Morb. Mortal. Wkly. Rep 70:801–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Cent. Dis. Control Prev.). 2021b. Estimated HIV incidence and prevalence in the United States, 2015–2019. HIV Surveill. Suppl. Rep 26(1). https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-26-1.pdf [Google Scholar]
- CDC (Cent. Dis. Control Prev.). 2022. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2020. HIV Surveill. Rep 33. https://stacks.cdc.gov/view/cdc/121127/cdc_121127_DS1.pdf [Google Scholar]
- Chai PR, Goodman G, Bustamante M, Mendez L, Mohamed Y, et al. 2021. Design and delivery of real-time adherence data to men who have sex with men using antiretroviral pre-exposure prophylaxis via an ingestible electronic sensor. AIDS Behav 25:1661–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Dowdy DW. 2014. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLOS ONE 9:e108742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Evans C, Hoverman A, Sun C, Dana T, et al. 2019. Preexposure prophylaxis for the prevention of HIV Infection: evidence report and systematic review for the US Preventive Services Task Force. JAMA 321:2214–30 [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, Williams DR. 1999. Racism as a stressor for African Americans: a biopsychosocial model. Am. Psychol 54:805–16 [DOI] [PubMed] [Google Scholar]
- Clement ME, Johnston BE, Eagle C, Taylor D, Rosengren AL, et al. 2019. Advancing the HIV pre-exposure prophylaxis continuum: a collaboration between a public health department and a federally qualified health center in the southern United States. AIDS Patient Care STDs 33:366–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. 2016. Antiretroviral therapy for the prevention of HIV-1 transmission. N. Engl. J. Med 375:830–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson PW, Franks J, Wu Y, Winterhalter FS, Knox J, et al. 2020. Adherence to pre-exposure prophylaxis in black men who have sex with men and transgender women in a community setting in Harlem, NY. AIDS Behav 24:3436–55 [DOI] [PubMed] [Google Scholar]
- Crepaz N, Hess KL, Purcell DW, Hall HI. 2019. Estimating national rates of HIV infection among MSM, persons who inject drugs, and heterosexuals in the United States. AIDS 33:701–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran GM, Landes SJ, McBain SA, Pyne JM, Smith JD, et al. 2022. Reflections on 10 years of effectiveness–implementation hybrid studies. Front. Health Serv 2:1053496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo AB, Lopez-Rios J, Flynn AW, Holloway IW, Pantalone DW, Grov C. 2021. Insurance- and medical provider–related barriers and facilitators to staying on PrEP: results from a qualitative study. Transl. Behav. Med 11:573–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale SK, Ayala G, Logie CH, Bowleg L. 2022. Addressing HIV-related intersectional stigma and discrimination to improve public health outcomes: an AJPH supplement. Am. J. Public Health 112(Suppl.):335–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. 2009. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement. Sci 4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damschroder LJ, Reardon CM, Opra Widerquist MA, Lowery JC. 2022a. Conceptualizing outcomes for use with the Consolidated Framework for Implementation Research (CFIR): the CFIR Outcomes Addendum. Implement. Sci 17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damschroder LJ, Reardon CM, Opra Widerquist MA, Lowery JC. 2022b. The updated Consolidated Framework for Implementation Research based on user feedback. Implement. Sci 17:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis D, Silvera R, Bernstein K, Stein D, Hagerty R, et al. 2009. Implementation of HIV testing at 2 New York City bathhouses: from pilot to clinical service. Clin. Infect. Dis 48:1609–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow WW. 2021. The first 40 years of AIDS: promising programs, limited success. AIDS Behav 25:3449–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dep. Health Hum. Serv. 2022. HIV care continuum Policies & Issues Fact Sheet, Dep. Health Hum. Serv., Washington, DC. https://www.hiv.gov/federal-response/policies-issues/hiv-aids-care-continuum [Google Scholar]
- Desrosiers A, Levy M, Dright A, Zumer M, Jallah N, et al. 2019. A randomized controlled pilot study of a culturally-tailored counseling intervention to increase uptake of HIV pre-exposure prophylaxis among young Black men who have sex with men in Washington, DC. AIDS Behav 23:105–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblecki-Lewis S, Jones D. 2016. Community federally qualified health centers as homes for HIV preexposure prophylaxis: perspectives from South Florida. J. Int. Assoc. Provid. AIDS Care 15:522–28 [DOI] [PubMed] [Google Scholar]
- Dubov A, Galbo P Jr., Altice FL, Fraenkel L. 2018. Stigma and shame experiences by MSM who take PrEP for HIV prevention: a qualitative study. Am. J. Men’s Health 12:1843–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles MP, Mittman BS. 2006. Welcome to Implementation Science. Implement. Sci 1:1 [Google Scholar]
- Egan JE, Ho K, Stall R, Drucker MT, Tappin R, et al. 2020. Feasibility of short-term PrEP uptake for men who have sex with men with episodic periods of increased HIV risk. J. Acquir. Immune Defic. Syndr 84:508–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. 2019. Ending the HIV epidemic: a plan for the United States. JAMA 321:844–45 [DOI] [PubMed] [Google Scholar]
- Fisher JD, Fisher WA. 2002. The information–motivation–behavioral skills model. In Emerging Theories in Health Promotion Practice and Research: Strategies for Improving Public Health, ed. DiClemente RJ, Crosby RA, Kegler MC, pp. 40–70. San Francisco, CA: Jossey-Bass [Google Scholar]
- Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O’Reilly KR, et al. 2016. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS 30:1973–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire P 1970. Pedagogy of the Oppressed New York: Penguin [Google Scholar]
- Frye V, Nandi V, Paige MQ, McCrossin J, Lucy D, et al. 2021. TRUST: assessing the efficacy of an intervention to increase HIV self-testing among young Black men who have sex with men (MSM) and transwomen. AIDS Behav 25:1219–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs JD, Stojanovski K, Vittinghoff E, McMahan VM, Hosek SG, et al. 2018. A mobile health strategy to support adherence to antiretroviral preexposure prophylaxis. AIDS Patient Care STDs 32:104–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Harris AL. 2017. PrEP awareness and decision-making for Latino MSM in San Antonio, Texas. PLOS ONE 12:e0184014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo R, Hotton AL, Kuhns LM, Gratzer B, Mustanski B. 2016. Incidence of HIV infection and sexually transmitted infections and related risk factors among very young men who have sex with men. J. Acquir. Immune Defic. Syndr 72:79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodreau SM, Rosenberg ES, Jenness SM, Luisi N, Stansfield SE, et al. 2017. Sources of racial disparities in HIV prevalence in men who have sex with men in Atlanta, GA, USA: a modelling study. Lancet HIV 4:e311–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene GJ, Swann G, Fought AJ, Carballo-Dieguez A, Hope TJ, et al. 2017. Preferences for long-acting pre-exposure prophylaxis (PrEP), daily oral PrEP, or condoms for HIV prevention among U.S. men who have sex with men. AIDS Behav 21:1336–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusky O, Roberts KJ, Swanson AN, Rhoades H, Lam M. 2010. Staff strategies for improving HIV detection using mobile HIV rapid testing. Behav. Med 35:101–11 [DOI] [PubMed] [Google Scholar]
- Hakre S, Blaylock JM, Dawson P, Beckett C, Garges EC, et al. 2016. Knowledge, attitudes, and beliefs about HIV pre-exposure prophylaxis among US Air Force health care providers. Medicine 95:e4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkitis PN, Kupprat SA, McCree DH, Simons SM, Jabouin R, et al. 2011. Evaluation of the relative effectiveness of three HIV testing strategies targeting African American men who have sex with men (MSM) in New York City. Ann. Behav. Med 42:361–69 [DOI] [PubMed] [Google Scholar]
- Havens JP, Scarsi KK, Sayles H, Klepser DG, Swindells S, Bares SH. 2019. Acceptability and feasibility of a pharmacist-led HIV pre-exposure prophylaxis (PrEP) program in the Midwestern United States. Open Forum Infect. Dis 6(10):ofz365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield S, Chiasson MA, Joseph H, Scheinmann R, Johnson WD, et al. 2012. An online randomized controlled trial evaluating HIV prevention digital media interventions for men who have sex with men. PLOS ONE 7:e46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosek SG, Green KR, Siberry G, Lally M, Balthazar C, et al. 2013a. Integrating behavioral HIV interventions into biomedical prevention trials with youth: lessons from Chicago’s Project PrEPare. J. HIV AIDS Soc. Serv 12:333–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosek SG, Landovitz RJ, Kapogiannis B, Siberry GK, Rudy B, et al. 2017. Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatr 171:1063–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosek SG, Siberry G, Bell M, Lally M, Kapogiannis B, et al. 2013b. The acceptability and feasibility of an HIV preexposure prophylaxis (PrEP) trial with young men who have sex with men. J. Acquir. Immune Defic. Syndr 62:447–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Birken SA, Melvin CL, Rohweder CL, Smith JD. 2020. Designs and methods for implementation research: advancing the mission of the CTSA program. J. Clin. Transl. Sci 4:159–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins Hall W, Sun CJ, Tanner AE, Mann L, Stowers J, Rhodes SD. 2017. HIV-prevention opportunities with GPS-based social and sexual networking applications for men who have sex with men. AIDS Educ. Prev 29:38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SA, Whitfield THF, Rendina HJ, Parsons JT, Grov C. 2018. Will gay and bisexual men taking oral pre-exposure prophylaxis (PrEP) switch to long-acting injectable PrEP should it become available? AIDS Behav 22:1184–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HL, Keller SB, Giancola MA, Rodriguez DA, Chau JJ, et al. 2014. Pre-exposure prophylaxis accessibility research and evaluation (PrEPARE study). AIDS Behav 18:1722–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CH, Kuhn T, Huxley D, Kennel J, Withers E, Lomonaco CG. 2017. Preliminary findings of a technology-delivered sexual health promotion program for black men who have sex with men: quasi-experimental outcome study. JMIR Public Health Surveill 3:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss CA, Hosek SG, Bacchetti P, Anderson PL, Liu AY, et al. 2018. Comparison of measures of adherence to human immunodeficiency virus preexposure prophylaxis among adolescent and young men who have sex with men in the United States. Clin. Infect. Dis 66:213–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakower DS, Mimiaga MJ, Rosenberger JG, Novak DS, Mitty JA, et al. 2012. Limited awareness and low immediate uptake of pre-exposure prophylaxis among men who have sex with men using an internet social networking site. PLOS ONE 7:e33119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley-Chareczko L, Clark D, Conyngham C, Zuppa A, Moorthy G, et al. 2018. Delivery of TDF/FTC for pre-exposure prophylaxis to prevent HIV-1 acquisition in young adult men who have sex with men and transgender women of color using a urine adherence assay. J. Acquir. Immune Defic. Syndr 79:173–78 [DOI] [PubMed] [Google Scholar]
- Landovitz RJ, Beymer M, Kofron R, Amico KR, Psaros C, et al. 2017. Plasma tenofovir levels to support adherence to TDF/FTC preexposure prophylaxis for HIV prevention in MSM in Los Angeles, California. J. Acquir. Immune Defic. Syndr 76:501–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelutiu-Weinberger C, Golub SA. 2016. Enhancing PrEP access for Black and Latino men who have sex with men. J. Acquir. Immune Defic. Syndr 73:547–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DH, Benbow N, Keiser B, Mongrella M, Ortiz K, et al. 2022. Determinants of implementation for HIV pre-exposure prophylaxis based on an updated consolidated framework for implementation research: a systematic review. J. Acquir. Immune Defic. Syndr 90(Suppl. 1):235–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot MA, Campbell CK, Moss N, Treves-Kagan S, Agnew E, et al. 2018. Using a social network strategy to distribute HIV self-test kits to African American and Latino MSM. J. Acquir. Immune Defic. Syndr 79:38–45 [DOI] [PubMed] [Google Scholar]
- Liu AY, Vittinghoff E, von Felten P, Rivet Amico K, Anderson PL, et al. 2019. Randomized controlled trial of a mobile health intervention to promote retention and adherence to preexposure prophylaxis among young people at risk for human immunodeficiency virus: the EPIC study. Clin. Infect. Dis 68:2010–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksut JL, Eaton LA, Driver R, Knowles CM, Watson RJ. 2021. Factors associated with awareness and use of pre-exposure prophylaxis (PrEP) among Black men who have sex with men with a recent STI diagnosis. Behav. Med 47:161–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksut JL, Eaton LA, Siembida EJ, Driffin DD, Baldwin R. 2016. A test of concept study of at-home, self-administered HIV testing with web-based peer counseling via video chat for men who have sex with men. JMIR Public Health Surveill 2:e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin RW, Young SD, Bristow CC, Wilson G, Rodriguez J, et al. 2014. Piloting an HIV self-test kit voucher program to raise serostatus awareness of high-risk African Americans, Los Angeles. BMC Public Health 14:1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez RG, Lewis CC, Weiner BJ. 2014. Instrumentation issues in implementation science. Implement. Sci 9:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Donate AP, Zellner JA, Sañudo F, Fernandez-Cerdeño A, Hovell MF, et al. 2010. Hombres sanos: evaluation of a social marketing campaign for heterosexually identified Latino men who have sex with men and women. Am. J. Public Health 100:2532–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KH, Nelson L, Hightow-Weidman L, Mimiaga MJ, Mena L, et al. 2021. The persistent and evolving HIV epidemic in American men who have sex with men. Lancet 397:1116–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCree DH, Millett G, Baytop C, Royal S, Ellen J, et al. 2013. Lessons learned from use of social network strategy in HIV testing programs targeting African American men who have sex with men. Am. J. Public Health 103:1851–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGoy SL, Pettit AC, Morrison M, Alexander LR, Johnson P, et al. 2018. Use of social network strategy among young black men who have sex with men for HIV testing, linkage to care, and reengagement in care, Tennessee, 2013–2016. Public Health Rep 133(2 Suppl.):43S–51S [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty M, Smith JD, Villamar J, Burnett-Zeigler I, Vermeer W, et al. 2019. Implementation research methodologies for achieving scientific equity and health equity. Ethn. Dis 29:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle JL, Li D, Keiser B, Zamantakis A, Quieroz A, et al. 2023. Categorizing implementation determinants and strategies within the HIV implementation literature: a global systematic review protocol. BMJ Open 13:e070216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie S, van Stralen MM, West R. 2011. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement. Sci 6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JT, LeGrand S, Hightow-Weidman LB, McKellar MS, Kashuba AD, et al. 2018. Smartphone-based contingency management intervention to improve pre-exposure prophylaxis adherence: pilot trial. JMIR mHealth uHealth 6:e10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra E, van den Berg JJ, Sowemimo-Coker G, Chau S, Nunn A, Chan PA. 2020. Open pilot trial of a brief motivational interviewing-based HIV pre-exposure prophylaxis intervention for men who have sex with men: preliminary effects, and evidence of feasibility and acceptability. AIDS Care 32:406–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustanski B, Morgan E, D’Aquila R, Birkett M, Janulis P, Newcomb ME. 2019a. Individual and network factors associated with racial disparities in HIV among young men who have sex with men: results from the RADAR Cohort Study. J. Acquir. Immune Defic. Syndr 80:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustanski B, Moskowitz DA, Moran KO, Rendina HJ, Newcomb ME, Macapagal K. 2020. Factors associated with HIV testing in teenage men who have sex with men. Pediatrics 145:e20192322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustanski B, Ryan DT, Newcomb ME, D’Aquila RT, Matson M. 2019b. Very high HIV incidence and associated risk factors in a longitudinal cohort study of diverse adolescent and young adult men who have sex with men and transgender women. AIDS Behav 24:1966–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustanski B, Smith JD, Keiser B, Li DH, Benbow N. 2022. Supporting the growth of domestic HIV implementation research in the united states through coordination, consultation, and collaboration: how we got here and where we are headed. J. Acquir. Immune Defic. Syndr 90(Suppl. 1):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JY, Ntoumanis N, Thogersen-Ntoumani C, Deci EL, Ryan RM, et al. 2012. Self-determination theory applied to health contexts: a meta-analysis. Perspect. Psychol. Sci 7:325–40 [DOI] [PubMed] [Google Scholar]
- Nilsen P 2015. Making sense of implementation theories, models and frameworks. Implement. Sci 10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojile N, Sweet D, Kallail KJ. 2017. A preliminary study of the attitudes and barriers of family physicians to prescribing HIV preexposure prophylaxis. Kan. J. Med 10(2):40. [PMC free article] [PubMed] [Google Scholar]
- Oster AM, Wiegand RE, Sionean C, Miles IJ, Thomas PE, et al. 2011. Understanding disparities in HIV infection between black and white MSM in the United States. AIDS 25:1103–12 [DOI] [PubMed] [Google Scholar]
- Parish CL, Santella AJ. 2018. A qualitative study of rapid HIV testing and lesbian, gay, bisexual, transgender, and queer competency in the oral health setting: practices and attitudes of New York State dental directors. Oral Health Prev. Dent 16:333–38 [DOI] [PubMed] [Google Scholar]
- Phillips G II, McCuskey DJ, Felt D, Raman AB, Hayford CS, et al. 2020. Geospatial perspectives on health: the PrEP4Love campaign and the role of local context in health promotion messaging. Soc. Sci. Med 265:113497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell BJ, McMillen JC, Proctor EK, Carpenter CR, Griffey RT, et al. 2012. A compilation of strategies for implementing clinical innovations in health and mental health. Med. Care Res. Rev 69:123–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, et al. 2015. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement. Sci 10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. 1982. Transtheoretical therapy: toward a more integrative model of change. Psychother. Theory Res. Pract 19:276–88 [Google Scholar]
- Proctor EK, Powell BJ, McMillen JC. 2013. Implementation strategies: recommendations for specifying and reporting. Implement. Sci 8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor EK, Silmere H, Raghavan R, Hovmand P, Aarons G, et al. 2011. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm. Policy Ment. Health 38:65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell DW, Namkung Lee A, Dempsey A, Gordon C. 2022. Enhanced federal collaborations in implementation science and research of HIV prevention and treatment. J. Acquir. Immune Defic. Syndr 90(Suppl. 1):17–22 [DOI] [PubMed] [Google Scholar]
- Pyra M, Motley D, Bouris A. 2022. Moving toward equity: fostering transdisciplinary research between the social and behavioral sciences and implementation science to end the HIV epidemic. Curr. Opin. HIV AIDS 17:89–99 [DOI] [PubMed] [Google Scholar]
- Queiroz A, Mongrella M, Keiser B, Li DH, Benbow N, Mustanski B. 2022. Profile of the portfolio of NIH-funded HIV implementation research projects to inform ending the HIV epidemic strategies. J. Acquir. Immune Defic. Syndr 90(Suppl. 1):23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi N, Javanbakht M, Tadesse M, Malek M, Cox G. 2018. Risk-based HIV testing at Los Angeles County Men’s Central Jail. J. Correct. Health Care 24309–19 [DOI] [PubMed] [Google Scholar]
- Rabin BA, McCreight M, Battaglia C, Ayele R, Burke RE, et al. 2018. Systematic, multimethod assessment of adaptations across four diverse health systems interventions. Front. Public Health 6:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin BA, Purcell P, Naveed S, Moser RP, Henton MD, et al. 2012. Advancing the application, quality and harmonization of implementation science measures. Implement. Sci 7:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratinaud P, Marchand P. 2012. Application de la méthode ALCESTE aux «gros» corpus et stabilité des «mondes lexicaux»: analyse du «CableGate» avec IRAMUTEQ. J. Int. Anal. Stat. Données Text 11:835–44 [Google Scholar]
- Reilly KL, Kennedy S, Porter G, Estabrooks P. 2020. Comparing, contrasting, and integrating dissemination and implementation outcomes included in the RE-AIM and implementation outcomes frameworks. Front. Public Health 8:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SD. 2004. Hookups or health promotion? An exploratory study of a chat room–based HIV prevention intervention for men who have sex with men. AIDS Educ. Prev 16:315–27 [DOI] [PubMed] [Google Scholar]
- Rivera DB, Brady JP, Blashill AJ. 2021. Traditional machismo, caballerismo, and the pre-exposure prophylaxis (PrEP) cascade among a sample of Latino sexual minority men. J. Sex Res 58:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolle C-P, Rosenberg ES, Luisi N, Grey J, Sanchez T, et al. 2017. Willingness to use pre-exposure prophylaxis among Black and White men who have sex with men in Atlanta, Georgia. Int. J. STD AIDS 28:849–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank RC, Berman TR. 2002. The pervasive role of stories in knowledge and action. In Narrative Impact: Social and Cognitive Foundations, ed. Green MC, Strange JJ, Brock TC, pp. 287–313. Mahwah, NJ: Erlbaum [Google Scholar]
- Schlechter CR, Del Fiol G, Lam CY, Fernandez ME, Greene T, et al. 2021. Application of community–engaged dissemination and implementation science to improve health equity. Prev. Med. Rep 24:101620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaeer KM, Sherman EM, Shafiq S, Hardigan P. 2014. Exploratory survey of Florida pharmacists’ experience, knowledge, and perception of HIV pre-exposure prophylaxis. J. Am. Pharm. Assoc 54:610–17 [DOI] [PubMed] [Google Scholar]
- Shrestha RK, Chavez PR, Noble M, Sansom SL, Sullivan PS, et al. 2020. Estimating the costs and cost-effectiveness of HIV self-testing among men who have sex with men, United States. J. Int. AIDS Soc 23:e25445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha RK, Sansom SL, Kimbrough L, Hutchinson AB, Daltry D, et al. 2010. Cost-effectiveness of using social networks to identify undiagnosed HIV infection among minority populations. J. Public Health Manag. Pract 16:457–64 [DOI] [PubMed] [Google Scholar]
- Siegler AJ, Mouhanna F, Giler RM, Weiss K, Pembleton E, et al. 2018. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis–to–need ratio in the fourth quarter of 2017, United States. Ann. Epidemiol 28:841–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler AJ, Wiatrek S, Mouhanna F, Amico KR, Dominguez K, et al. 2020. Validation of the HIV pre-exposure prophylaxis stigma scale: performance of Likert and semantic differential scale versions. AIDS Behav 24:2637–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarbinski J, Rosenberg E, Paz-Bailey G, Hall HI, Rose CE, et al. 2015. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern. Med 175:588–96 [DOI] [PubMed] [Google Scholar]
- Smith JD, Davis P, Kho AN. 2021. Community-driven health solutions on Chicago’s South Side. Stanford Soc. Innov. Rev 19:A27–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Hasan M. 2020. Quantitative approaches for the evaluation of implementation research studies. Psychiatr. Res 283:112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Li DH, Hirschhorn LR, Gallo C, McNulty M, et al. 2020a. Landscape of HIV implementation research funded by the National Institutes of Health: a mapping review of project abstracts. AIDS Behav 24:1903–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Li DH, Rafferty MR. 2020b. The implementation research logic model: a method for planning, executing, reporting, and synthesizing implementation projects. Implement. Sci 15:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Merle JL, Webster KA, Cahue S, Penedo FJ, Garcia SF. 2022. Tracking dynamic changes in implementation strategies over time within a hybrid type 2 trial of an electronic patient-reported oncology symptom and needs monitoring program. Front. Health Serv 2:983217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LR, Patel VV, Tsai AC, Mittal ML, Quinn K, et al. 2022. Integrating intersectional and syndemic frameworks for ending the US HIV epidemic. Am. J. Public Health 112(Suppl. 4):340–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PS, Johnson AS, Pembleton ES, Stephenson R, Justice AC, et al. 2021. Epidemiology of HIV in the USA: epidemic burden, inequities, contexts, and responses. Lancet 397:1095–106 [DOI] [PubMed] [Google Scholar]
- Taggart T, Rendina HJ, Boone CA, Burns P, Carter J, et al. 2022. Stigmatizing spaces and places as axes of intersectional stigma among sexual minority men in HIV prevention research. Am. J. Public Health 112(Suppl. 4):371–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terndrup C, Streed CG, Tiberio P, Black M, Davis J, et al. 2019. A cross-sectional survey of internal medicine resident knowledge, attitudes, behaviors, and experiences regarding pre-exposure prophylaxis for HIV infection. J. Gen. Int. Med 34:1258–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Whiteside YO, Duffus WA. 2013. Perceptions and attitudes about preexposure prophylaxis among seronegative partners and the potential of sexual disinhibition. South. Med. J 106:558–64 [DOI] [PubMed] [Google Scholar]
- Washington TA, Applewhite S, Glenn W. 2017. Using Facebook as a platform to direct young black men who have sex with men to a video-based HIV testing intervention: a feasibility study. Urban Soc. Work 1:36–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz TJ, Powell BJ, Fernandez ME, Abadie B, Damschroder LJ. 2019. Choosing implementation strategies to address contextual barriers: diversity in recommendations and future directions. Implement. Sci 14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz TJ, Powell BJ, Matthieu MM, Damschroder LJ, Chinman MJ, et al. 2015. Use of concept mapping to characterize relationships among implementation strategies and assess their feasibility and importance: results from the Expert Recommendations for Implementing Change (ERIC) study. Implement. Sci 10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield THF, Parsons JT, Rendina HJ. 2020. Rates of pre-exposure prophylaxis use and discontinuation among a large U.S. national sample of sexual minority men and adolescents. Arch. Sex. Behav 49:103–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamantakis A, Li DH, Benbow N, Smith JD, Mustanski B. 2022. Determinants of pre-exposure prophylaxis (PrEP) implementation in transgender populations: a qualitative scoping review. AIDS Behav 27:1600–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


