Abstract
To realize the full potential of emerging nucleic acid therapies, there is a need for effective delivery agents to transport cargo to cells of interest. Protein materials exhibit several unique properties, including biodegradability, biocompatibility, ease of functionalization via recombinant and chemical modifications, among other features, which establish a promising basis for therapeutic nucleic acid delivery systems. In this review, we highlight progress made in the use of non-viral protein-based nanoparticles for nucleic acid delivery in vitro and in vivo, while elaborating on key physicochemical properties that have enabled the use of these materials for nanoparticle formulation and drug delivery. To conclude, we comment on the prospects and unresolved challenges associated with the translation of protein-based nucleic acid delivery systems for therapeutic applications.
Keywords: Drug delivery, gene therapy, recombinant proteins, nanoparticles, polypeptides
Graphical Abstract:
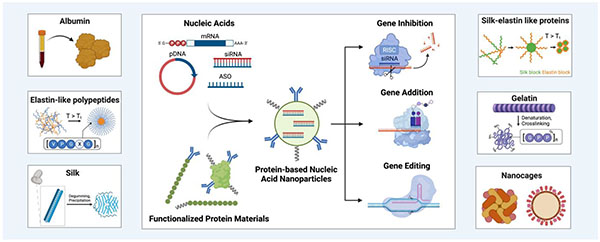
1. Introduction
Nucleic acid therapeutics have transformative potential across numerous areas of medicine. Approvals of the messenger RNA (mRNA) SARS-CoV-2 vaccines tozinameran (BioNTech/Pfizer)1 and elasomeran (Moderna)2 along with small interfering RNA (siRNA) therapies, such as inclisiran3 (Novartis/Alnylam Pharmaceuticals) by the EMA and FDA highlight the substantial impact this modality can have on patient care. To fully unlock the promise of nucleic acid therapies, there is a need for non-immunogenic, efficient delivery agents that overcome several in vivo barriers to transport nucleic acid cargo to cells of interest. While efforts in capsid engineering and envelope protein pseudotyping have yielded notable advances, existing viral vectors remain limited by stringent cargo capacities, immunogenicity, potential for oncogenic gene insertion, and manufacturing scaling challenges.4,5 Lipid nanoparticles have developed into the leading non-viral delivery vector, but the incorporation of active targeting domains, such as monoclonal antibodies (mAbs) and single chain variable fragments (scFvs) requires chemical conjugation that is challenging to scale, and the human relevance of reported passive targeting strategies remains unclear.6,7 Existing synthetic polymer and peptide-based (<50 residues) nanoparticle nucleic acid delivery systems often suffer from heterogeneous formulation characteristics, poor biocompatibility, and unfavorable pharmacokinetic performance that have limited the clinical progress of these vectors.8,9
Protein materials exhibit several unique properties that form a promising basis for the design of clinically effective therapeutic nucleic acid delivery systems. Both recombinant and native protein materials are derived from or inspired by natural sources, and therefore are generally biocompatible and biodegradable. Moreover, protein nanoparticles derived from human proteins, such as albumin, ferritin, and elastin, do not contain non-native sequences. Consequently, they are less likely to be immunogenic as compared to viral and other non-viral delivery vectors containing non-native components. While nanoparticles derived from native proteins may often display some degree of heterogeneous physicochemical properties, those produced from recombinant proteins typically exhibit a high level of homogeneity and tunability. Recombinant proteins also enable precise control of features that influence physicochemical properties, such as size and surface charge, the incorporation of targeting motifs with specific stoichiometry, as well as binding domains to facilitate the complexation and uptake of therapeutic cargo. The ability to include such domains biosynthetically eliminates the need for additional chemical modifications. Collectively, these properties have motivated the exploration of both native and recombinant protein-based nanoparticles for a variety of drug delivery applications, including nucleic acid therapeutics.
In this review, we highlight the progress of engineered protein-based nanoparticles for nucleic acid delivery in vitro and in vivo. We consider protein materials to be those composed of greater than 50 amino acids, with insights regarding peptide-based (<50 residues) nucleic acid delivery systems reviewed elsewhere.10–12 We specifically focus on the use of nonviral protein material; virus-like particles (VLPs) composed of viral proteins have been reported for nucleic acid delivery and progress in this area has been recently summarized.13–15 Additionally, computationally designed, atomically precise protein nanoparticles have attracted significant interest, but have not yet been used for nucleic acid delivery.16 After describing the physicochemical properties and reported nucleic acid delivery applications of notable non-viral protein materials, we describe examples of the use of protein materials as safe and effective drug delivery vehicles for a range of non-nucleic acid cargo in humans. To conclude, we comment on the prospects and unresolved challenges for translating protein-based nucleic acid delivery systems.
2. Unique Barriers to Effective Protein Nanoparticle-mediated Nucleic Acid Delivery
Protein nanoparticles face multiple barriers to reaching their intended target, dependent on the route by which they are administered (Fig. 1). In order to be effective as a nucleic acid complexation agent, protein materials must bind anionic nucleic acids with high affinity, generally accomplished by leveraging electrostatic attraction or covalent conjugation in the case of low molecular weight cargo, such as siRNA or antisense oligonucleotides. After intravenous administration, protein-nucleic acid complexes must resist degradation by plasma proteases and nucleases. Protein-based nanoparticles, specifically those constructed using intrinsically disordered, unfolded protein materials, such as elastin-like polypeptides (ELPs) are vulnerable to protease degradation. While this degradation mechanism is favorable for material clearance from the body, it could be unfavorable for nanoparticle performance. A study of ELP degradation in full plasma suggests that rate of degradation is slow, on the order of days, as compared to distribution and elimination half-lives of the material.17 Given that the self-assembly of protein nanoparticles is concentration dependent in many cases, these particles are also prone to disassembly following dilution in the bloodstream. To counter this effect, protein particles are often stabilized using amino-reactive crosslinkers like glutaraldehyde or genipin, as well as cysteine-based disulfide bridge crosslinking.
Figure 1. Barriers to successful protein nanoparticle-mediated nucleic acid delivery.
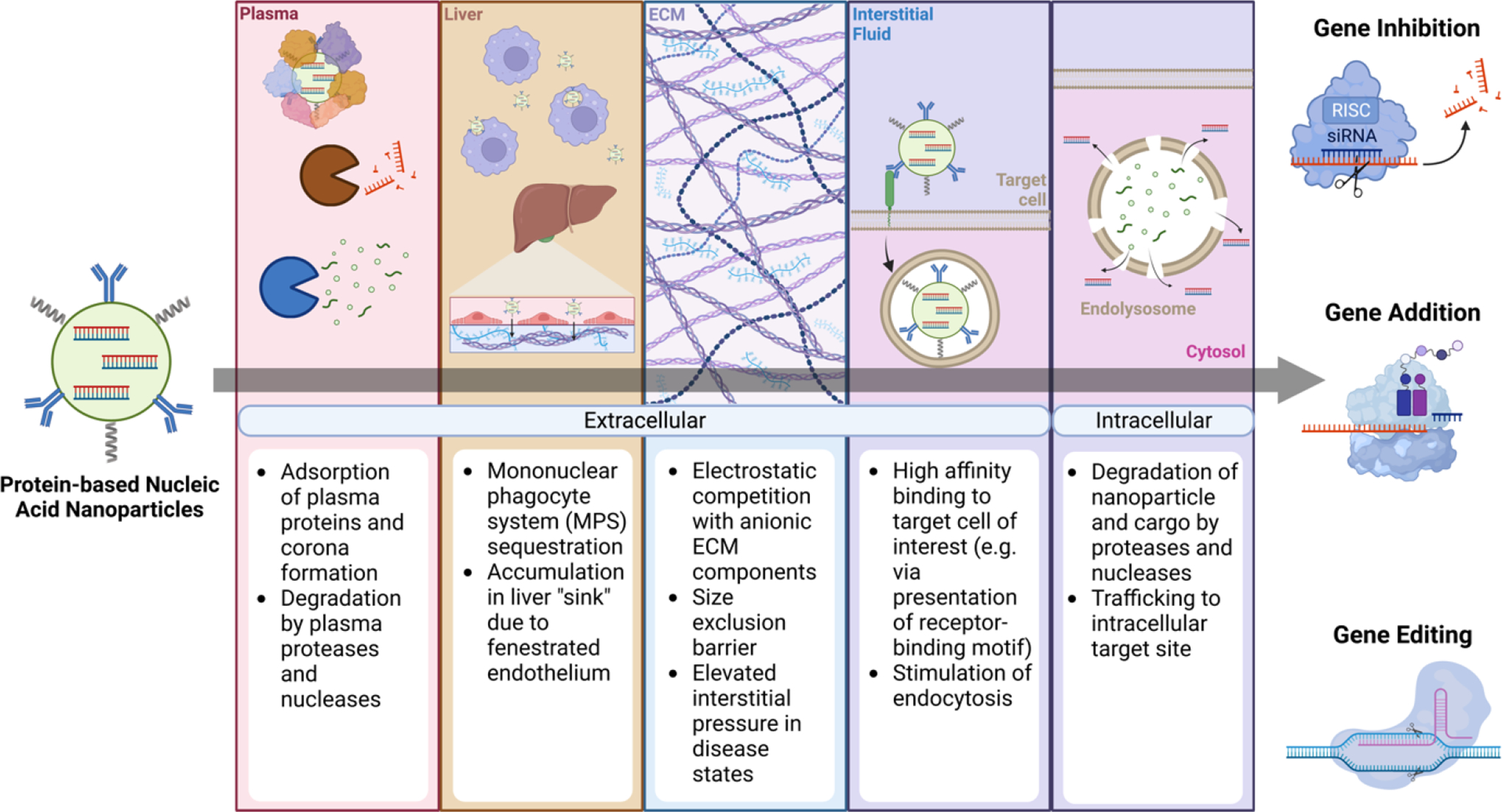
Particles traverse a number of extracellular and intracellular barriers on the path to effective delivery outcomes (e.g. gene inhibition, gene addition, or gene editing). Created with BioRender.
Critically, protein-nucleic acid nanoparticles must also evade sequestration by the mononuclear phagocyte system (MPS).18 The adsorption of opsonins, including complement proteins and antibodies, among other plasma constituents onto thenanoparticle surface leads to rapid clearance from the bloodstream19–23. Charge and hydrophobicity-mediated adsorption of plasma proteins with subsequent corona formation can also result in nanoparticle aggregation.24–27 These effects have been mitigated, in part, by incorporation of poly(ethylene glycol) (PEG) or other hydrophilic polymers, as well as through the inclusion of zwitterionic moieties.28–31 Recombinant incorporation of albumin binding domains has also been shown to improve the circulating half-life of protein nanoparticles, leveraging the long circulation time of serum albumin enabled by FcRN-mediated recycling.32,33
After navigating the bloodstream, protein-nucleic acid nanoparticles traverse the capillary endothelium to enter the target tissue prior to reaching cells of interest. While nanoparticles tend to accumulate in the liver due to high blood flow, the presence of fenestrated endothelium, and uptake by endothelial and phagocytic cells34,35, several strategies can be used to promote extrahepatic nanoparticle localization. Direct administration of nanoparticles to muscle via intramuscular injection, central nervous system (CNS) by intracranial, intranasal, or intrathecal administration, and the retina through intravitreal or subretinal routes can concentrate cargo delivery to these sites. Nonetheless, these approaches are suboptimal for many therapeutic indications due to their invasiveness and limited distribution. CNS nanoparticle localization can be promoted by ultrasound enhanced permeability across the blood-brain barrier (BBB), in addition to strategies that derivatize nanoparticles with ligands for endothelial apical transport receptors, which support transcytosis.36–39 Nanoparticle accumulation in certain tumors can be driven by an enhanced permeability and retention (EPR) effect attributed to the presence of a fenestrated vasculature and poor lymphatic drainage40–42. However, recent evidence suggests that both endothelial cell transcytosis along with significant nanoparticle uptake in perivascular tumor associated macrophages may be critical mediators of tumoral nanoparticle accumulation.43–47 Liver-, spleen-, and lung-specific accumulation of lipid nanoparticles in mice has been reported; this differential uptake is thought to be promoted by variations in protein corona composition dependent on the surface charge of the particles.48,49 Nonetheless, tissue selective extrahepatic nanoparticle delivery remains a significant challenge.
Once within the target tissue, protein-nucleic acid complexes are confronted by a dense fibrous network of extracellular matrix (ECM) proteins present in the interstitial space. The mesh-like organization of the ECM imposes a physical restriction on the diffusion of particles to the cell population of interest.50,51 Abundant proteoglycans in the ECM contain highly anionic glycosaminoglycans (GAG) that can compete with negatively charged nucleic acid cargo for binding to cationic groups present on the protein carrier, and repel anionic nanoparticles by electrostatic repulsion.52,53 Particle size and surface charge play an important role in dictating the effectiveness of transport with smaller, neutral particles less susceptible to limiting interactions.53,54 In addition, the ECM is subject to ongoing remodeling by metalloproteinases, elastases, and cathepsins, among other secreted proteases.55 Likewise, as mentioned above, protein-based materials are susceptible to degradation in the absence of shielding strategies. Furthermore, fluid homeostasis in the interstitium also plays an important role in nanoparticle transport. In healthy tissue, colloid osmotic and hydrostatic pressure in the microvasculature and interstitium are balanced, which affords a small net negative interstitial pressure that promotes the flow of fluid and molecules into the tissue.56 In contrast, the leaky vasculature and poor lymphatic drainage characteristic of tumors, opposes the influx of nanoparticles.57,58
Within the target tissue, protein-nucleic acid nanoparticles must bind to target cell membranes and stimulate internalization. Cationic protein materials or materials coformulated with a cationic agent are thought to associate with anionic proteoglycans and phospholipids present on the cell membrane in a non-specific manner.59–62 To promote particle binding to specific cells, active targeting strategies have been implemented that utilize ligands or binding moieties for receptors present on the surface of target cells. Such targeting domains have included full size monoclonal antibodies63–65, antibody fragments (e.g. Fabs, scFvs, nanobodies)66–69, synthetic binding proteins (e.g. DARPins, monobodies)70–72 and other peptide- and non-peptide ligands.73–76 The recombinant nature of many protein materials facilitates the direct incorporation of protein-based targeting domains, often with greater efficiency and control than chemical conjugation.77,78 Notably, protein corona formation may shield active targeting domains from recognition, limiting their ability to induce cell-specific delivery.47,79–82 The use of anti-fouling surface modifications along with plasma pre-equilibrated nanoparticles have been effective strategies for mitigating this effect.82–84
Following nanoparticle uptake, the endosome presents another barrier to the functional delivery of nucleic acid cargo. During endosomal maturation, the lumen becomes acidified through the action of V-ATPase proton pumps, and fusion occurs with lysosomes containing a variety of degradative enzymes.85 Without a mechanism to enable cytosol escape, conventional nucleic acid cargo is rapidly degraded. Nucleic acid binding to toll-like receptors (TLRs) on the endosomal membrane can also result in innate immune activation and limit cytosolic export.86 Chemically modified nucleotide structures such as locked nucleic acids have improved the resilience of RNA cargo to enzymatic degradation and have limited innate immune activation.87,88 Beyond these cargo-centered modifications, many protein-based nanoparticles incorporate membrane-disruptive cationic endosomolytic peptides to enable cargo delivery10,89; such peptides are thought to facilitate endosomal membrane disruption to permit cargo escape into the cytosol following cellular internalization. Protein-nucleic acid nanoparticles may also incorporate synthetic cationic polymer materials that can promote endosomal escape of cargo through a membrane disruption mechanism yet to be fully elucidated.90,91
Altogether, it is clear that protein-based nanoparticles must be designed to overcome several obstacles to effectively mediate intracellular nucleic acid delivery. In the following sections, we will highlight a range of engineered and naturally occurring protein materials used to surpass these barriers and enable effective nucleic acid cargo delivery in vitro and in vivo.
3. Polymeric Protein Materials
Analogous to chemically produced synthetic polymers, protein polymers are protein sequences containing multiple repeats of a monomeric unit. In contrast to synthetic polymers, the monomeric unit of a protein polymer is defined by a short amino acid motif, as opposed to a chemical monomer. Polymeric proteins like elastin-like polypeptides, silk, and gelatin do not exhibit defined tertiary structures, self-assemble through interactions between defined secondary structures, and are isolated from unique sources, in bulk from naturally occurring solid structures with chemical and mechanical post-processing or through recombinant expression. In this section, we review the use of polymeric protein materials as nucleic acid delivery vectors.
3.1. Elastin-like Polypeptides (ELPs)
Elastin-like polypeptides (ELPs) are engineered protein polymers that have been explored for a variety of biomedical applications.92 Elastin is a fibrous extracellular matrix protein that underlies the extensibility and elasticity of many mammalian tissues, including arteries, heart valves, lung, skin, and ligaments.93 The design of ELPs is inspired by the structure of tropoelastin, a soluble, non-crosslinked elastin precursor composed of alternating hydrophobic and hydrophilic domains.94 ELPs are comprised of a short repeating peptide motif of Val-Pro-Gly-X-Gly derived from the hydrophobic domain of tropoelastin, where X represents a guest residue that can be any amino acid except proline. ELPs exhibit lower critical solution temperature (LCST) phase behavior; below a specific transition temperature (Tt), ELPs are soluble in aqueous solution, while above Tt, ELPs reversibly phase separate to form insoluble coacervates.95 The transition temperature of ELPs decreases with increased polypeptide length, concentration, and guest residue hydrophobicity96,97 Additionally, the Tt is modulated by the pH and ionic strength of the aqueous environment.98,99 ELPs are produced recombinantly and can be purified by affinity purification or by inverse transition cycling (ITC)100,101, which leverages ELP temperature-dependent solubility to facilitate separation from contaminants.66,102 Collectively, these properties enable the design of ELP nanoparticles, including 1) multiblock micellar designs containing a phase separated hydrophobic block forming the particle core and a soluble hydrophilic block forming the corona (Fig. 2a), and 2) uniblock designs entirely based on phase separated ELP sequences. The hydrophobic core enables encapsulation and intracellular delivery of apolar small molecule drugs; in addition, the recombinant nature of the material facilitates direct incorporation of peptide drugs for extracellular presentation.106,107 Furthermore, ELPs can be modified to enable encapsulation and delivery of nucleic acid cargo, as described below (Table 1).
Figure 2. Elastin-like polypeptides (ELPs) for nucleic acid delivery.
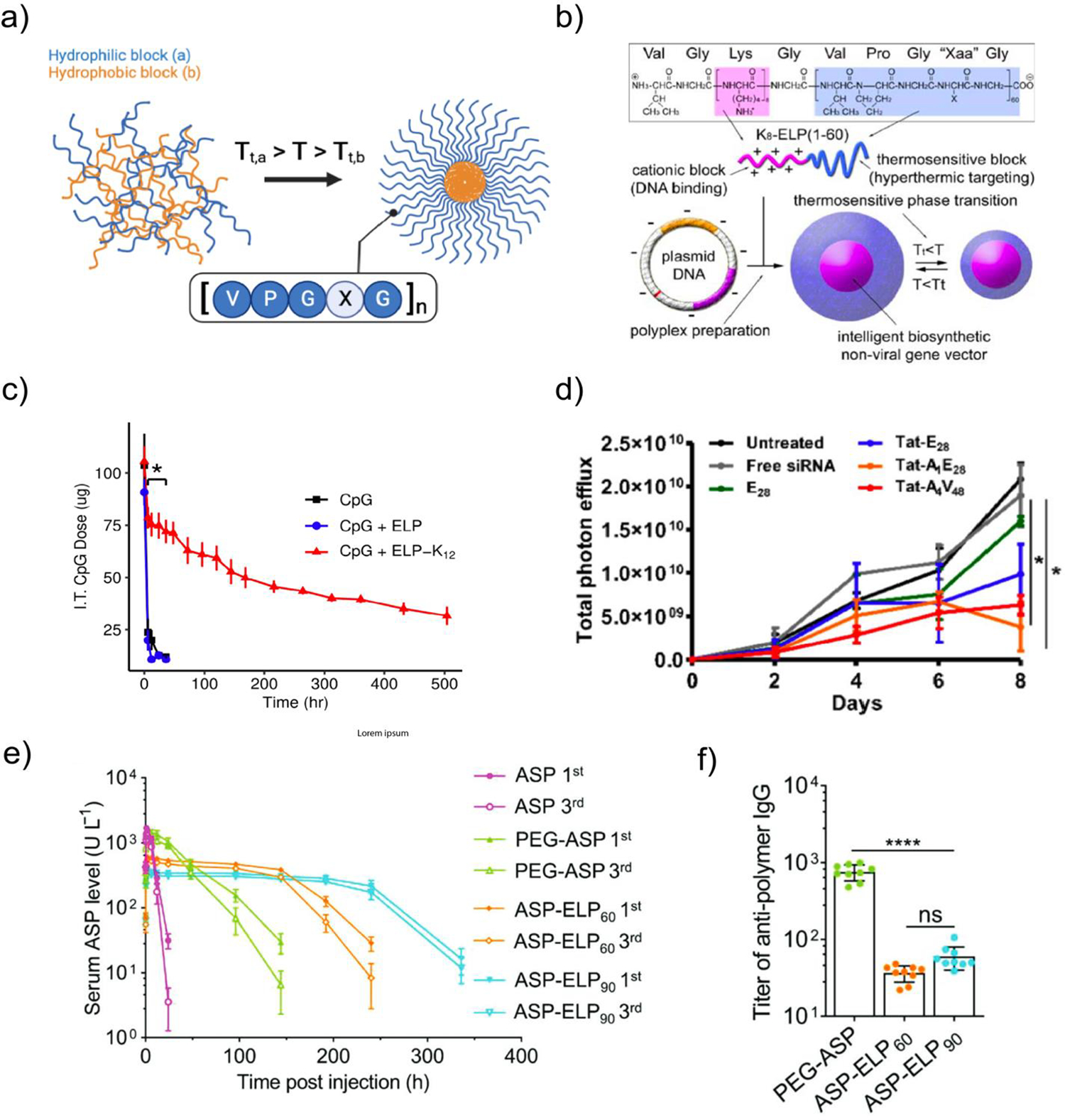
a) Temperature-induced ELP self-assembly for micelle formation. Tt = transition temperature for ELP block. Created with BioRender. b) Structure and thermal responsiveness of VGK8G-ELP60 used for plasmid DNA delivery. From Ref103. c) ELP-mediated intratumoral CpG retention following intratumoral administration. d) Quantification of 4T1-luc tumor bioluminescence intensities following daily administration of 0.25 mg/kg luciferase siRNA complexed with Tat-modified ELPs. From Ref104. e) Serum L-asparaginase (ASP) activity in unmodified ASP, PEGylated ASP, and ELP-modified ASP after intraperitoneal injections on day 0 (1st), day 14, and day 28 (3rd). No reduction in ASP activity was observed between the first and third injections in the ASP-ELP90 group. f) Anti-polymer (PEG or ELP) IgG titers after 3 ASP-polymer fusion administrations over 42 days. From Ref105
Table 1.
Recombinant protein polymer nanoparticles used for nucleic acid delivery.
| Material | Cargo | Cell line/Model | In vitro Efficacy | RoA | In vivo Efficacy | Ref |
|---|---|---|---|---|---|---|
| Elastin-like polypeptides (ELPs) | ||||||
| VGK8G-ELP60 Guest residues: V, A, G in 5:2:3 ratio | pEGFP | MCF-7 (human breast cancer) | Limited EGFP expression compared to BPEI control using 1 ⎧g DNA | N/A | N/A | 103 |
| p[Asp(DET)]-ELP90 Guest residues: V, A, G in 5:2:3 ratio | pGL4 | COS-7 (African green monkey kidney cells) | Effective, less effective than BPEI control | N/A | N/A | 116 |
| LAEL-ELP120-Penetratin Guest residues: K, I in 1:4 ratio | pLuc | C6 (Rat glioma cells) | Outperformed luciferase pDNA alone, less effective than PEI | N/A | N/A | 113 |
| 5TR1/s2.2-ELP72 Guest residues: K 5TR1, s2.2: MUC-1 targeting aptamers | pLuciferase, pPAP-S, pRicin | MCF-7 (xenograft in female BALB/c mice), SKRB (human breast cancer), HepG2 (human liver hepatocellular carcinoma), HFF-1 (human fibroblasts) | Aptamer functionalization improved efficiency. More effective than controls (Turbofect, Lipofectamine) in MUC-1+ cell lines | PT | Repeated administration (MCF-7 xenograft) of ELP-pRicin (d 0, 2, 4, 6, 9, 1 mg/kg) led to complete tumor growth suppression (evaluated d 23) | 108,109 |
| Tat-(AP1)4-ELP48 Guest residues: V AP1: IL-4 targeting ligand | siLuciferase, pEGFP | MDA-MB231 (human breast cancer), 4T1-luc (murine breast cancer, xenograft in female BALB/c mice), A549 (human lung adenocarcinoma) | siRNA: luciferase suppression comparable to Lipofectamine control pDNA: Enabled higher EGFP expression than Lipofectamine control in IL-4+ cell lines | IV | siRNA: Enhanced intratumoral siRNA accumulation, suppressed luciferase expression activity with repeated dosing (0.2 mg/kg daily for 6 d) | 104,115 |
| ELP12 or 24-K8 Guest residues: A, Y in 4:1 ratio | Yamanaka factor pDNA | HEK293T, Murine fibroblast-derived iPSCs | 0.004% fibroblast to iPSC reprogramming efficiency (0.0001–0.001% in other transfection systems) | N/A | N/A | 117 |
| ELP60-K12 Guest residues: V | CpG | 4T1 (xenograft in female BALB/c mice), murine macrophages | 2x increase in NO production in macrophages (signals TLR activation) | IT | Improved intratumoral retention of CpG follow intratumoral injection. 4 mg/kg CpG combined with 131I-ELP brachytherapy reduced tumor size | 111 |
| Silk-like peptides (SLPs) | ||||||
| Silk6mer-K30 | pEGFP | HEK293FT | Maximum efficiency ~15% (required lipofectamine co-transfection) | N/A | N/A | 118 |
| [RGD]11-Silk6mer-K30 | pLuciferase | HeLa, HEK293FT | Incorporation of RGD repeats greatly improved efficiency. Less effective than Lipofectamine. | N/A | N/A | 119 |
| Silk6mer-K30-[ppTG1]2 | pLuciferase | HEK293FT, MDA-MB-435 | ppTG1 dimer enabled transfection efficiencies comparable to Lipofectamine. | N/A | N/A | 120 |
| Silk6mer-K30-F3 | pLuciferase | MBA-MB-435,231, MCF10A | F3-functionalized Silk6mer greatly outperformed unfunctionalized construct. Less efficient than Lipofectamine. | IV | Complexes enabled luciferase expression in MDA-MB-231 tumors in mice | 121,122 |
Subscript indicates number of ELP pentapeptide repeats. EGFP, enhanced green fluorescent protein; BPEI, branched polyethyleneimine; GL4, luciferase reporter; iPSC, induced pluripotent stem cell; IT, intratumoral; IV, intravenous; Luc, luciferase; PAP-S, type I Pokeweed antiviral protein; PT, peritumoral; RoA, route of administration.
The first use of ELPs for nucleic acid delivery was reported in 2008.103 An ELP consisting of 60 pentapeptide units ([VPGXG]60; where X is Val, Ala, and Gly in a 5:2:3 ratio] was recombinantly modified with an oligolysine-containing cationic block (VGK8G) to facilitate electrostatic complexation and delivery of enhanced GFP (eGFP) plasmid DNA (pDNA) (Fig. 2b). The construct complexed eGFP pDNA into nanoparticles < 200 nm in diameter for all N/P ratios tested and enabled eGFP expression in vitro. Interestingly, complexation with pDNA greatly reduced the transition temperature of the ELP in an N/P-dependent manner, where lower N/P ratios resulted in lower Tt. This observation suggests that charge neutralization lowers the effective LCST, presumably by reducing the interaction of charged residues with water, consistent with the known effect of guest residue charge on ELP phase transition behavior.96 Similar effects have been noted in the studies described below.
A second lysine-containing ELP construct ([VPGKG]72) for targeted delivery of luciferase pDNA to breast cancer epithelial cells has also been reported.108 The ELP was functionalized with aptamers with high affinity for MUC1, a glycoprotein overexpressed in epithelial tumors, via electrostatic complexation. Incorporation of MUC1-targeted aptamer 5T1 led to a 5-fold improvement in transfection efficiency with delivery of PAP-S toxin pDNA as a suicide gene therapy. The same platform was also investigated for both in vitro and in vivo delivery of ricin pDNA, with repeated peritumoral injection suppressing tumor growth.109 In addition, oligolysine-containing ELPs have been explored for the delivery of plasmid-encoded Yamanaka factors for pluripotent stem cell induction110 and for intratumoral delivery of CpG DNA to promote antitumor immunity in a murine model of breast cancer (Fig. 2c).111 These early studies demonstrated the potential of ELPs to serve as a nucleic acid delivery vector.
To enhance the potency of ELPs for nucleic acid delivery, the incorporation of cell penetrating peptides (CPPs) and endosomolytic peptides have been explored. Piña et al constructed a lysine-containing ELP ({[VPGIG]2[VPGKG][VPGIG]2}24) containing a CPP, penetratin,112 and a fusogenic peptide, LAEL, which transitions from a random coil to an α-helix upon exposure to the low pH environment of the endosome, resulting in membrane association and disruption.113,114 More recently, ELPs modified with the cationic CPP Tat have been used for the delivery of siRNA and pDNA to cancer cells.104,115 To promote cell selectivity, ELPs were recombinantly functionalized with AP1, a targeting peptide for the IL-4 receptor which is highly expressed on many human tumors. The most effective construct (Tat-[AP1]4-[VPGVG]48) protected siRNA and DNA cargo from RNAse and DNAse mediated degradation in vitro. siRNA-mediated luciferase knockdown was observed, comparable to that of a lipid transfection reagent. ELPs without Tat were ineffective in both siRNA complexation and delivery. In luciferase+ tumor-bearing mice, Tat-AP1-[VPGVG]48-siRNA nanoparticles enhanced siRNA accumulation and suppressed luciferase expression following daily intravenous administration and enabled delivery of eGFP pDNA to multiple cancer cell lines (Fig 2d).104,115
Positively charged ELPs can be challenging to express and purify in high yield due to toxicity and high transition temperatures that complicates ITC purification. As an alternative, ELPs with neutrally charged guest residues that can be covalently modified with cationic moieties have been investigated. Chen et al generated a ELP diblock containing a chemically synthesized cationic poly[diethylenetriamine] (p[DET]) block.116 The ELP, consisting of a 90 pentapeptide repeat with Val, Ala, and Gly guest residues in a 5:2:3 ratio, was functionalized with a poly(aspartate) block via ring-opening polymerization of β-benzyl-L-aspartate N-carboxy-anhydride (BLA-NCA), followed by DET conjugation. The p[Asp(DET)]53-modified ELP complexed luciferase pDNA into 90 nm diameter nanoparticles with modest in vitro efficacy. Similarly, ELPs have been created with methionine guest residues ([VPGXG]40, X = Val, Met at a 3:1 ratio) that were thioalkyted to enable introduction of primary or secondary amine groups.123 Incorporation of the amine groups enabled pDNA complexation at low N/P ratios with efficacy observed at an N/P > 0.6. In a subsequent study, methionine-containing ELPs were covalently modified with cationic TMAEMA oligomers synthesized by reversible addition fragmentation chain transfer (RAFT) polymerization.124 ELP-g-p(TMAEMA)15 enabled internalization of FITC-labeled pDNA in HEK 293 cells. In the absence of covalent modification, hollow ELP spheres encapsulating cationic polymer-pDNA polyplexes have also been reported, demonstrating improved efficacy and safety in vitro and in vivo as compared to the polyplexes alone.125,126
Another key feature of ELP-based protein materials is their minimal immunogenicity, likely related to the high level of self-tolerance developed toward the native VPGXG motif in elastin. For example, recent research demonstrates that the fusion of an ELP with L-Asparaginase (ASP), as a treatment for hematologic malignancies, prolongs the half-life of this enzyme and diminishes its immunogenicity with a reduction in anti-ASP IgG and IgM levels by two orders of magnitude (Fig 2e).105 Significantly, in comparison to an FDA-approved PEGylated ASP, the IgG and IgM titers generated against the ELP protein polymer are an order of magnitude lower than those against PEG (Fig 2f), which is widely employed in the formulation of lipid nanoparticles. ELPs have demonstrated favorable safety and tolerability profiles as recombinant fusions with peptide therapeutics repeatedly administered to patients, substantiating the benign and redosable nature of the material (Table 4).
Table 4.
Assessment of protein-based drug delivery systems in humans.
| Drug | Description | ID | Phase | Indication | # pts | Outcome | Ref |
|---|---|---|---|---|---|---|---|
| Elastin-like polypeptides | |||||||
| PB1046 | Recombinant fusion of vasoactive intestinal peptide (VIP) and ELP | NCT03315507 | 1 | Pulmonary arterial hypertension | 3 | Dosed SC q1w for > 8 weeks, well tolerated. Improved disease in 1 pt | 164 |
| NCT02808585 | 2 | Heart failure w/reduced ejection fraction | 29 | Dosed SC q1w for 4 weeks up to 1.2 mg/kg, well tolerated. Significant reductions in diastolic BP vs placebo | 221 | ||
| PB0139 | Recombinant fusion of native insulin and ELP | NCT01835730 | 1 | T2D | 37 | Dose dependent decrease in FBG (range of −2 to −34 mg/dL). Well tolerated | 222 |
| PB1023 | Recombinant fusion of glucagon-like peptide 1 (GLP-1) and ELP | NCT01236404 | 1/2a | T2D | 80 | Dosed SC q1w for 4 weeks. Induced significant reductions in FBG in a dose-dependent manner | 223 |
| NCT01658501 | 2b | T2D | 593 | Dosed SC q1w for 4 weeks up to 1.35 mg/kg. Significant reductions in FBG at lowest tested dose (0.3 mg/kg) | 2241/ 2/2024 5:42:00 PM | ||
| Gelatin | |||||||
| Drug-loaded gelatin MPs | Cisplatin-conjugated gelatin microspheres bFGF-loaded gelatin microspheres | N/A | 1 | HCC | 19 | Transarterial chemoembolization using microspheres, ~48% nodule response rate | 225 |
| bFGF-loaded gelatin microspheres | N/A | 1 | Critical limb ischemia | 10 | IM injection of 200 μg bFGF-incorporated gelatin MPs enabled significant improvement in tissue oxygenation 24 weeks post treatment | 226 | |
| Albumin | |||||||
| Nab-paclitaxel | Paclitaxel loaded onto human albumin | NCT00046527 | 3 | Metastatic breast cancer | 460 | IV injections in 3-week treatment cycle. Albumin-loaded paclitaxel induced higher response rates than paclitaxel alone (33% vs 19%) | 227 |
| NCT00844649 | 3 | Pancreatic cancer | 861 | Gemcitabine + nab-paclitaxel improved survival rates vs gemcitabine alone | 228 | ||
| Silk | |||||||
| VX-103 | Antigen-loaded silk fibroin microneedle array | NCT06125717 | 1 | H1N1 influenza | 45 | Seroprotection rate of 92% at highest tested dose. Well tolerated. | 229 |
| Ferritin | |||||||
| H2HA-Ferittin | Ferritin nanoparticle presenting HA domain from H2N2 influenza virus | NCT03186781 | 1 | H2N2 influenza | 50 | Neutralizing antibody responses induced following IM administration | 2301/2/2024 5:42:00 PM |
bFGF, bovine fibroblast growth factor; FBG, fasting blood glucose; IM, intramuscular; IV, intravenous; q1w, once weekly; MP, microparticles; SC, subcutaneous.
3.2. Silk and Silk-like peptides (SLPs)
Silk and silk-like peptides are versatile biopolymers that have been widely investigated for drug delivery.127–131 Silk is produced by several arthropod species, including bees, wasps, silkworms, spiders, among others. The silk of the Bombyx mori (B. mori) domestic silkworm is commonly used in biomedical applications due to its abundance, ease of production, and clinical precedence.130,132 B. mori cocoon silk is predominantly composed of fibroin, which forms the core of silk fibers and provides robust mechanical properties, and sericin, which generates an outer adhesive coating. Silk fibroin (SF) is isolated from raw silk during processing and used for downstream applications. SF is comprised of a light chain (~28 kDa) and heavy chain (~391 kDa) linked via a disulfide bond. The fibroin heavy chain is arranged as an amphiphilic block copolymer containing alternating hydrophobic and short hydrophilic regions. The hydrophobic regions of SF are composed of Gly-X repeats, where X is Ala, Ser, Tyr, or Val, while the hydrophilic domains are nonrepetitive and generally anionic. Hydrogen bonding and van der Waals interactions between GAGAGS repeats in the hydrophobic block result in the formation of crystalline anti-parallel β-sheet structures that impart high strength, chemical resistance, and rigidity (Fig 3a).133–135 Self-assembling behavior enables the production of silk particles, in which SF is dissolved and reformed into particles by treatment with kosmotropic salts or organic solvents to induce β-sheet formation.136–138 Alternatively, to avoid the use of denaturating agents, mechanical methods are used to produce micro- to nano-scale particles.139,140 β-sheet formation within the hydrophobic regions of SF facilitates packing of nanoparticles and efficient loading of hydrophobic small molecules; positively charged small molecules can also be effectively encapsulated via electrostatic interactions with the anionic hydrophilic domains of SF. Additional modifications, as described below, enable effective nucleic acid complexation and delivery. Moreover, the biocompatibility of silk is supported by its extensive clinical history as a suture material, as well as recent applications in tissue engineering and drug delivery. Studies have indicated that purified Bombyx mori silk fibroin (BMSF) does not elicit allergic reactions, activate the adaptive immune response, or induce fibrous capsule formation, underscoring its low immunogenicity.143–145
Figure 3. Silk fibroin as a building block for nucleic acid delivery vectors.

a) Hydrophobicity pattern and self-assembly of B. mori silk fibroin (BMSF) heavy chain. Adapted from Ref141. b) Scheme depicting preparation of BMSF/PEI/Doxorubicin (DOX)/survivin siRNA nanoparticles. Adapted from Ref142.
As an anionic material, B. mori silk fibroin (BMSF) is often functionalized with cationic materials to enable effective nucleic acid complexation. For example, polyethyleneimine (PEI) has been widely leveraged in BMSF-based delivery systems as a cationic co-complexation agent. BMSF nanoparticles coated with 25 kDa branched PEI (bPEI25) effectively complexed and delivered eGFP pDNA in vitro, with lower toxicity than bPEI25 alone.146,147 Leveraging the presence of a hydrophobic core in BMSF nanoparticles, Norouzi et al utilized bPEI modified nanoparticles to co-complex doxorubicin and survivin siRNA, which in a co-delivery format were more effective in limiting in vivo tumor growth than either monotherapy alone (Fig 3b).142 In addition to PEI, oligochitosan has also been incorporated into BMSF nanoparticles which enhanced in vitro delivery of luciferase siRNA.148
BMSF does not inherently promote particle uptake due to its negative charge. In contrast, silk fibroin derived from Antheraea pernyi (A. pernyi) is rich in arginine-glycine-aspartate (RGD) motifs, which supports adhesion to multiple integrin receptors.149–152 A. pernyi fibroin (ASF)/PEI/pEGFP complexes enabled GFP protein expression in vitro, with transfection efficiency and reduced particle toxicity correlating with the ASF:PEI weight ratio153,154,155 These particles have also been used for delivery of pDNA encoding pro-angiogenic factors VEGF165 and Ang-1.155
SF nanoparticles derived from silk obtained from natural sources may contain impurities, display batch-to-batch variability in physicochemical properties, and are not readily amenable to the inclusion of additional functional domains. Alternatively, recombinant silk-like peptides (SLPs) have been used to form nanoparticles for nucleic acid delivery (Table 1). Numata et al created an SLP based on a consensus repeat sequence contained in the Nephila clavipes spider dragline protein MaSp1, which contains a β-sheet-forming poly(alanine) domain. SLPs integrating polylysine domains of various length enabled complexation of pDNA in an N/P ratio-dependent manner. A construct containing a 30-mer polylysine block facilitated optimal GFP pDNA delivery in vitro, though transfection efficiency was low (~15%).118 To enhance transfection, polylysine-containing SLPs have been functionalized with a poly(RGD) domain to improve nanoparticle internalization via receptor-mediated endocytosis (Fig 4a).119 To enable tumor cell targeting, SLP-polylysine constructs have been designed with the tumor homing peptide, F3, that binds nucleolin expressed on tumor associated endothelial cells, and CGKRK, which displays high affinity for heparan sulfates. F3-modified SLPs complexed luciferase pDNA and enabled luciferase expression in orthotopic MDA-MB-231 tumors following repeated intravenous injection.121,122 A modified SLP-polylysine construct, incorporating the cationic, membrane-disruptive α-helical peptide ppTG1 led to further improvement in luciferase pDNA transfection efficiency, comparable to Lipofectamine (Fig 4b). Notably, SLPs containing a ppTG1 dimer demonstrated 25-fold higher efficiency than those containing a monomeric version.120 SLPs modified with Tat have also been used for pDNA delivery in vitro.156
Figure 4. Recombinant silk-like peptides (SLPs) as a modular platform for nucleic acid delivery.
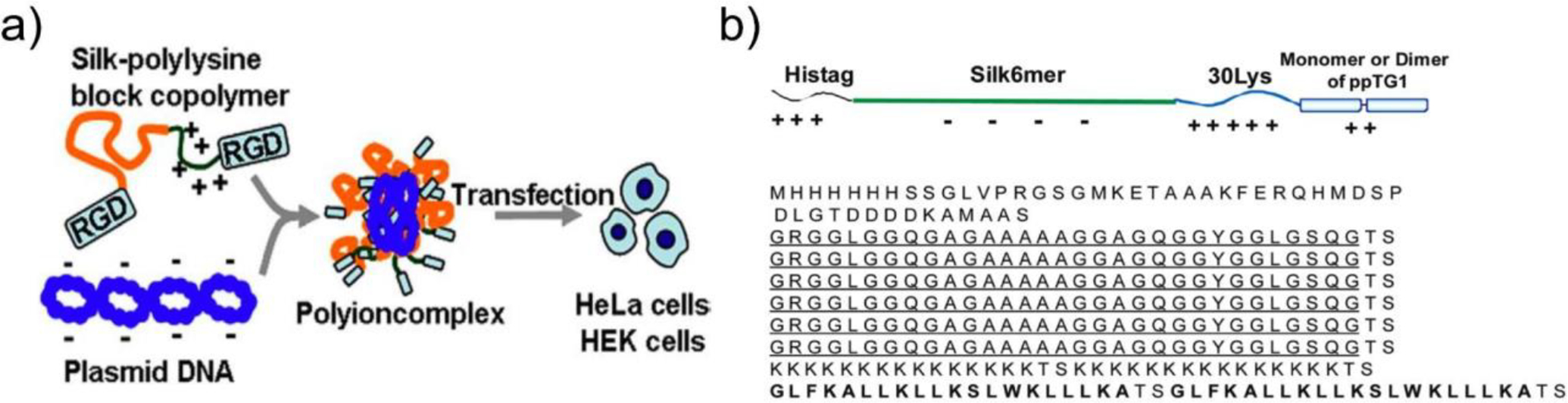
a) Schematic of procedure for complexation and delivery of pDNA with RGD- and polylysine (K30)-functionalized SLP. From Ref119. b) SLP construct functionalized with polylysine and cell penetrating peptide ppTG1. From Ref163.
3.3. Silk-elastin-like proteins (SELPs)
While the materials described above have been the most widely investigated as nucleic acid delivery vectors, several others have been explored due to their compelling physicochemical properties. For example, silk-elastin-like proteins (SELPs) have been utilized to form injectable hydrogels for the controlled release of gene delivery vectors. SELPs are recombinant block copolymers composed of repeating tandem units of silk fibroin-derived (GAGAGS) and ELP-derived (VPGXG) monomers. While the silk units spontaneously self-assemble into tightly packed β-sheets, which act as physical crosslinks, the ELP units display reversible stimulus-responsive assembly. Due to this hybrid composition, SELPs demonstrate an ability to transition from an aqueous solution to a hydrogel (sol-gel transition) at a temperature dictated by molecular weight, silk-elastin ratio, and elastin unit guest residue identities.131,157,158 Intratumorally administered SELPs designed to gel at a physiological temperature enabled sustained delivery of adenoviral vectors containing a thymidine kinase (Tk-1) transgene, enhancing Tk-1 expression and prolonging antitumor efficacy in mice bearing a head and neck squamous cell carcinoma.159,160 A similar approach has been used to tailor the release profile and improve the efficacy of adenoviral vectors loaded with a short hairpin RNA (shRNA) targeting the oncogene, C-met.161 To accelerate the vector release profile and hydrogel degradation rate, sequences susceptible to matrix metalloproteinases (MMP) were inserted, with those placed within closely packed silk units displaying slower rates of degradation as compared to those positioned in alternate locations.162
Elastin- and silk-based materials represent a promising basis for the development of cell-targeted nucleic acid delivery systems. In vitro and in vivo efficacy has been demonstrated for diverse nucleic acid cargo and clinical studies suggest that native silk and ELPs can be administered repeatedly and display minimal immunogenicity.143,164 However, to fully realize their potential, novel strategies will be needed to further improve in vivo stability and the potency of these nanoparticles.
3.4. Gelatin
Gelatin, as a hydrolysis product of collagen, is considered GRAS (Generally Regarded as Safe) by the FDA and has a long history of use in food products, cosmetics, and clinical settings. Gelatin is a low-cost, readily available, biocompatible, and biodegradable material. Unlike collagen, which may display antigenicity due to its animal origin, denatured gelatin exhibits reduced immunogenicity. This decrease is linked to the reduction of antigen epitopes during the hydrolysis process. Additionally, gelatin does not produce harmful byproducts during enzymatic degradation, as collagen, being the most abundant protein in animals, undergoes well-tolerated degradation.165 Other merits of gelatin includes its versatile chemical and biological properties. Gelatin can be either positively charged (type A) or negatively charged (type B) depending on the pH of the hydrolysis conditions in which it is produced.166 It is also an amphiphilic polymer carrying charged hydrophilic and hydrophobic amino acids165,167, providing numerous chemical handles for bioconjugation. Furthermore, gelatin carries RGD motifs, which can be recognized by cell surface integrin receptors and promote cell attachment and internalization.165
Gelatin is characterized by repetitive Gly-X-Y tripeptide motifs, where the X and Y positions are occupied predominantly by proline and hydroxyproline. This specific motif plays a pivotal role in the formation of the triple-helical structure of gelatin, consisting of three polyproline-II type helices, which are stabilized by intermolecular hydrogen bonding and responsible for its sol-gel transition. Numerous methodologies have been developed to manipulate the bulk gelation behavior of gelatin, enabling the creation of nanoparticles. These techniques include desolvation168, nanoprecipitation169, and coacervation170, among others. It should be noted that nanoparticles generated by these approaches typically exceed 200 nanometers. Addressing this limitation, Gwon et al. recently introduced a freezing and thawing step following a two-step desolvation method, resulting in the production of nanoparticles < 100 nanometers.183 Gelatin may also be modified in a manner that promotes self-assembly into nanoparticles, including chemical modification with hydrophobic polymers184 or small molecules.185,186 Regardless of the manufacturing approach, gelatin nanoparticles typically require immediate crosslinking using agents such as glutaraldehyde and genipin to preserve their morphology and ensure sufficient stability for in vitro and in vivo applications.165
Both type A and type B gelatin have been used for nucleic acid delivery (Table 2). In contrast to positively charged type A gelatin, which forms electrostatic interactions with anionic nucleic acids, negatively charged type B is used to encapsulate nucleic acids through physical entrapment. Exploiting this approach, Amiji and colleagues have pioneered the development of a multi-compartmental nanoparticle system tailored for oral gene delivery. In this system, a core composed of type B gelatin serves as the carrier for nucleic acid cargo, enveloped within a protective shell constructed from poly-ε-caprolactone or a polymethacrylate-based material. The outer shell shields the core from degradation and facilitates intracellular release of cargo (Fig. 5a). Notably, these vehicles have proven effective for delivery of plasmids encoding IL-10171 and siRNA targeting TNF-α172 in murine models of dextran sulfate sodium (DSS)- and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis. Additionally, these nanoparticles have been applied for the delivery of siRNA targeting divalent metal transporter 1 (DMT1) to mitigate pathological iron absorption.173
Table 2.
Gelatin-based nanoparticles for nucleic acid delivery.
| Material | Cargo | Cell line/Model | In vitro Efficacy | RoA | In vivo Efficacy | Ref |
|---|---|---|---|---|---|---|
| Gelatin | ||||||
| Type-B gelatin nanoparticles embedded in PCL microparticles | IL-10 pDNA | TNBS-induced acute colitis model (Balb/c) | N/A | PO | ~10-fold increase in IL-10 protein production in intestinal lining vs empty controls via oral administration, resulting in rescue of acute colitis mouse model | 171 |
| siTNF-α | DSS-induced acute colitis model (Balb/c) | PO | Significant reduction of TNF-〈 expression at d10 post treatment (oral administration), resulting in partial rescue of colitis | 172 | ||
| Gelatin + Eudragit L100–55 | siDMT1 | CD-1 mice | N/A | PO | Oral administration of siDMT1-loaded NPs resulted in reduced DMT1 expression in duodenum, reduced Fe uptake | 173 |
| Type-A gelatin | IGF1, eGFP pDNA | Adult canine chondrocytes | Expression of both cargos observed following transfection | N/A | N/A | 174 |
| PEI-gelatin | siASS1 | HeLa | Protein expression reduction exceeded that enabled by Lipofectamine with optimized formulation, with less cytotoxicity | N/A | N/A | 175 |
| Type A Gelatin functionalized w/chondroitin or dextran sulfate | MUC5AC pDNA | HCE (human corneal epithelial cells), IOBA-NHC (epithelial cells) | Expression of MUC5AC ~20–50% that of positive control (JetPEI-RGD) | IO | Minimal expression of cargo observed in rabbit conjunctiva following ocular administration | 176 |
| Chitosan-gelatin-EGCG (CGE) | siTMEM44-AS1 | HGC-27, MKN-45 (5-FU resistant); xenograft tumor mouse model | ~80% knockdown of tumorigenic lncRNA target in both cell lines | IV | siRNA-loaded CGE nanoparticles + 5-FU prevented tumor growth, more effective than all controls | 177 |
| Gelatin-Lipofectamine coformulation | miR-506 mimic | MDA-MB-231, orthotopic TNBC mouse model | Nanoparticle-mediated miR-506 delivery slowed cell migration | IT | Intratumoral administration of NPs resulted in partially slowing of tumor growth (2 injections over 4 week model) | 178 |
| miR-4458 mimic, siCOL11 A1 | MCF-7 (human adenocarcinom a), xenograft mouse model | miR delivery slowed cell migration | IT | Single intratumoral administration of both miR-4458 and siCOL11A1-loaded NPs partially slowed tumor growth | 179 | |
| Thiolated gelatin | Polymeri zed siRFP | RFP+ B16-F10 | ~80% knockdown of RFP in RFP+ B16-F10, | IV | ~80% knockdown of RFP gene expression in B16-F10 xenograft following IV administration on d0, d, 2, d4 (measured on d5) | 180 |
| Thiolated PEG-gelatin | p53 pDNA | Panc-1 (human pancreatic adenocarcinom a) xenograft mouse model | N/A | IV | IV administration of p53 pDNA-loaded gelatin NPs resulted in 1000-fold increase in tumoral p53 expression, slowed growth | 181 |
| Anti-EGFR mAb functionalized gelatin | siKRAS-G12C | KRAS mutant NSCLC cell line | Target knockdown observed, resulting in ~10–15% reduction in downstream protein expression | N/A | N/A | 182 |
TNBS, 2,4,6-Trinitrobenzene sulfonic acid; DSS, dextran sulfate sodium; DMT1, Divalent metal transporter 1; IGF1, Insulin-like growth factor 1; ASS1, arginosuccinate synthase 1; EGCG, epigallocatechin gallate; lncRNA, long noncoding RNA; TNBC, triple negative breast cancer.
Figure 5. Gelatin as a basis for nucleic acid delivery vectors.
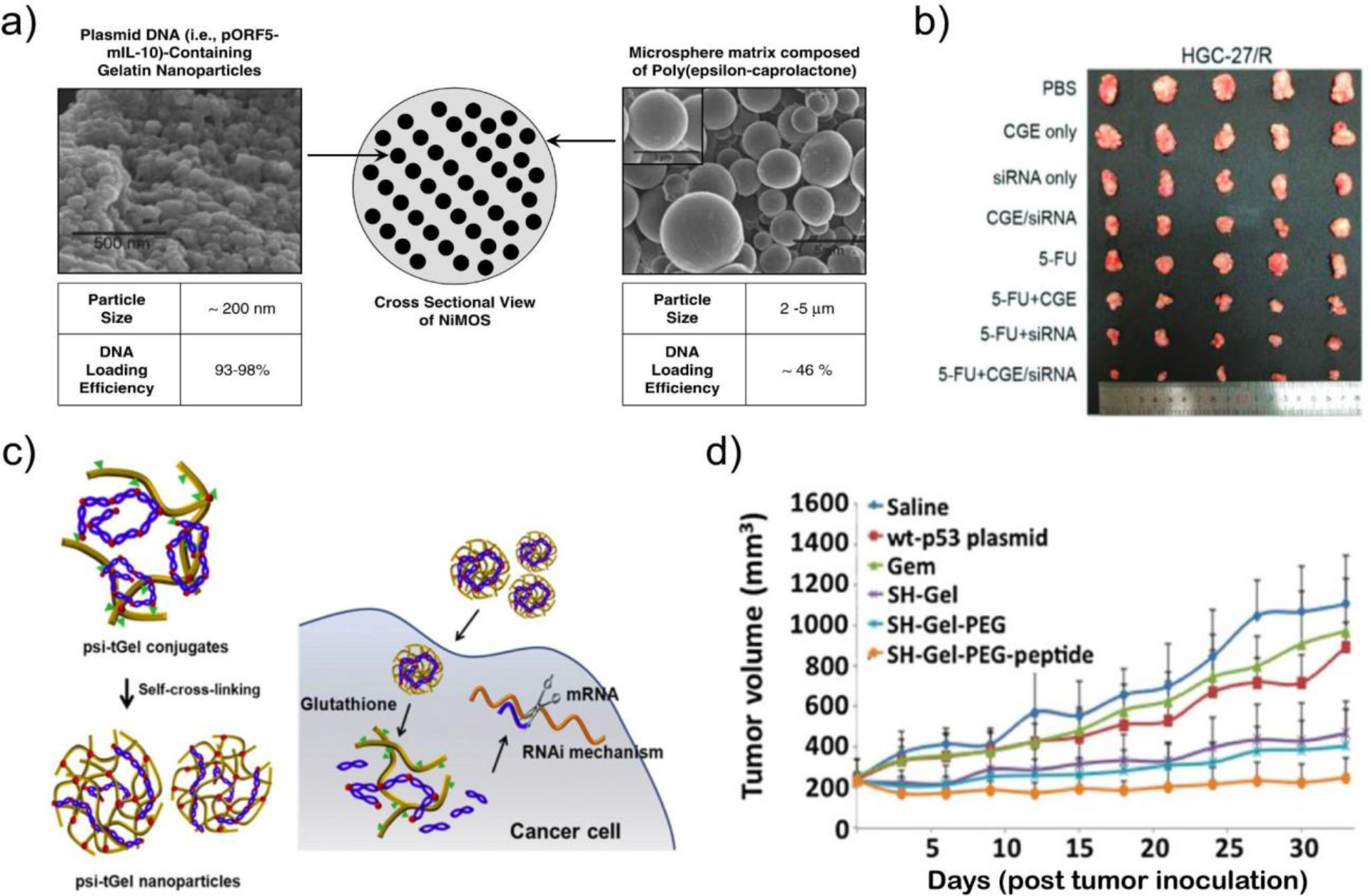
a) Scanning electron microscopy images of nanoparticles-in-microsphere oral system (NiMOS) development by Amiji et al. DNA cargo is encapsulated in gelatin nanoparticles that are subsequently loaded in poly(ε-caprolactone) microparticles for protection during gastric transit and to enable intestinal release. From Ref171 b) Images of HGC-27/R (human gastric cell) subcutaneous xenograft tumors in mice following treatment with 5-FU (chemotherapy) and chitosan-gelatin-epigallocatechin (CGE) nanoparticles formulated with siRNA against a resistance-promoting long noncoding RNA. From Ref177. c) Assembly and siRNA delivery mechanism of thiolated siRNA and gelatin coformulated into nanoparticles. From Ref180. d) Volumes of human adenocarcinoma xenograft tumors in mice following treatment with gemcitabine and EGFR-targeted, PEGylated gelatin nanoparticles loaded with pDNA-encoded tumor suppressor p53. From Ref181.
Similar to other protein polymer systems, gelatin can harness the advantages of an augmented positive charge through functionalization with cationic polymers, such as ethylenediamine174, PEI175, cholamine187, and spermine188. Zorzi et al. created a gelatin nanoparticle system comprised of positively charged type A gelatin that was further cationized with spermine, which exhibited efficient plasmid incorporation and transfection of ocular epithelial cells. Topical delivery of plasmid-encoded MUC5AC has also been demonstrated with potential for treatment of dry eye disease.176 In addition to N-hydroxysuccinimide (NHS) mediated coupling to produce branched cationic polymers, nanoparticles have been created by combining gelatin, chitosan, and epigallocatechin gallate in the presence of sodium tripolyphosphate. The resulting cationic particles were used for systemic delivery of siRNA targeting long non-coding RNA (lncRNA) TMEM44-AS1 with a pronounced tumor-suppressive effect (Fig. 5b).177 Liu et al. have recently reported complexing pDNA, siRNA, and miRNA with lipofectamine followed by loading within gelatin nanoparticles. Intratumoral injection led to a therapeutic response in mice bearing triple-negative and estrogen receptor-positive breast cancers.178,179
In addition to addressing charge and delivery efficiency, considerable attention has been directed toward enhancing particle stability, extending circulating half-life, and refining targeting specificity in nanoparticle systems. Traditional crosslinking methods, such as those involving glutaraldehyde, have raised concerns regarding toxicity and the elicitation of immune responses. Consequently, crosslinking strategies based on disulfide bond formation have emerged that lead to particle disassembly within the reducing environment of the intracellular space. Lee et al. observed a reduction in tumor expression of red fluorescent protein after intravenous administration of thiolated type B gelatin nanoparticles containing siRNA (Fig. 5c).180 Another well-established approach for further augmenting nanoparticle half-life and improving biodistribution is through PEGylation. Kaul and Amiji demonstrated that PEGylated gelatin particles exhibited higher levels of beta-galactosidase expression within tumors189.
While native RGD motifs and positive surface charge confers gelatin nanoparticles with a propensity for non-specific cell uptake, enhanced specificity can be achieved through surface modification with distinct targeting ligands. An array of targets and targeting moieties have been investigated. For example, Xu et al. have designed a comprehensive system in which thiolated and PEGylated gelatin nanoparticles are decorated with a peptide targeting the epidermal growth factor receptor (EGFR) for tumor treatment. Intravenous administration of particles loaded with plasmids encoding p53 effectively suppressed the growth of human pancreatic adenocarcinoma in a SCID mouse model (Fig. 5d).181 Moreover, EGFR antibodies have been affixed to gelatin nanoparticles to facilitate the co-delivery of siRNA targeting KRAS and a tyrosine kinase inhibitor, resulting in the inhibition of H23 non-small cell lung cancer cell growth in vitro.182 In another innovative approach, Zhao et al. engineered gelatin/silica nanoparticles derivatized with PEG, a fusogenic peptide (HA2), and a tumor-targeting aptamer (ARGO100) with a strong affinity for nucleolin expressed in cancer cells. These modified particles exhibited tumor accumulation following intravenous injection in A549 tumor-bearing mice in vivo.190 Lastly, Tian et al. have demonstrated the therapeutic potential of Tat-decorated gelatin-siloxane nanoparticles carrying a gene encoding the vasodilator calcitonin gene-related peptide (CGRP). This nanoparticle system exhibited notable therapeutic effects in a rat model of subarachnoid hemorrhage-induced cerebral vasospasm upon intracisternal injection.191
Interestingly, in a comparative study evaluating cationized atelocollagen, albumin, and gelatin for ocular gene delivery, gelatin cationized with spermine emerged as the most promising candidate. This choice struck an optimal balance between reduced toxicity, efficient encapsulation of pDNA and siRNA, and favorable functional outcomes in vitro.192 Similarly, casein, bovine serum albumin, and gelatin have each been evaluated for non-targeted and antibody-targeted siRNA delivery. As anticipated, antibody conjugated gelatin demonstrated the highest efficiency for intracellular siRNA release.193
In summary, gelatin nanoparticles have demonstrated commendable biocompatibility, stability, and efficiency for the delivery of therapeutic nucleic acids. Established methodologies have been devised for their production, crosslinking, PEGylation, and conjugation to targeting moieties. Notably, this system has exhibited promising therapeutic effects when delivering plasmid DNA and short RNAs in various in vivo animal models through systemic, local, and topical administration. Nonetheless, the limitations of gelatin nanoparticles include an inherent difficulty in controlling the molecular weight and composition of this biopolymer. Moreover, the introduction of various chemical modifications complicates the manufacturing process, introducing additional costs and variability through reaction and purification cycles.
4. Globular proteins
In contrast to polymeric protein materials, globular proteins do not contain repetitive sequences, exhibit defined tertiary structures, self-assemble through hydrophobic interactions enabled by their tertiary structures, and must be expressed recombinantly or isolated from human fluids (i.e. plasma). In this section, we review the use of globular proteins as nucleic acid delivery vectors.
4.1. Albumin
Albumin nanoparticles have been utilized as carriers for a wide variety of therapeutic agents, including nucleic acids. As the most abundant protein in human plasma, albumin is a small (MW ~67 kDa), water-soluble, anionic (pI 4.7) protein that is responsible for maintaining intravascular osmotic pressure and acts as a carrier for several classes of endogenous hormones, vitamins, and fatty acids, and exogenous small molecule drugs.194 Due to its lack of immunogenicity, human serum albumin (HSA) is commonly used in clinical therapeutics, though bovine serum albumin (BSA) shares significant (~80%) sequence homology with HSA and typically employed for preclinical formulation studies due to low cost and ease of sourcing.195 Albumin has been clinically approved as an excipient for multiple hydrophobic drugs, most notably Abraxane, which consists of paclitaxel co-formulated with albumin nanoparticles to improve tumor specificity and limit toxicity.209 Albumin nanocarriers traffic to tumors due to high affinity interactions with gp60 and SPARC (secreted protein acidic rich in cysteine) that are expressed on tumor endothelial cells.195,196 This tropism, along with an extended half-life (14–16 hours), biodegradability, and amenability to conjugation have motivated the investigation of albumin as a nucleic acid delivery vector (Table 3).
Table 3.
Globular protein-based delivery systems for nucleic acids.
| Material | Cargo | Cell line/Model | In vitro Efficacy | RoA | In vivo Efficacy | Ref |
|---|---|---|---|---|---|---|
| Albumin | ||||||
| PDMA-BSA | eGFP, luciferase pDNA | HEK293T, NIH/3T3, D1 (murine bone marrow cells) | Efficacy comparable to PEI at optimized BSA:DNA mass ratios | N/A | N/A | 196 |
| Ethylenediamine-functionalized BSA | siBcl2 | B16 (IV administered B16 cells for lung metastasis model in C57BL/6) | ~50% target knockdown in optimized conditions, comparable to Lipofectamine | IV | Particle accumulation in mouse lungs related to particle size (D ~5 μM). cBSA+siBcl2 particles enabled significant reduction in # of lung metastases vs controls | 197 |
| PEI-HSA | eGFP, luciferase pDNA | A549 | PEI-HSA nanoparticles performed comparably to Lipofectamine with less cytotoxicity | N/A | N/A | 198 |
| PEI-HSA | Luciferase pDNA | Mouse cerebellar granule cells | ~80% of treated wells showed luminescence following transfection | N/A | N/A | 199 |
| bPEI-HSA | siTurboGFP | HPMEC, MDA-MB-231 | ~90% knockdown in both cell types, comparable to Lipofectamine | N/A | N/A | 200 |
| PEI-BSA | Cas9 pDNA | MDA-MB-231 | Nanoparticles enabled efficient uptake of pDNA; editing efficiency not evaluated | N/A | N/A | 201 |
| BSA | siKRAS (G12S) | A549 | Significant target knockdown resulting in reduced cell viability | N/A | N/A | 202 |
| MSA (in situ) | Maleimide-anti-miRNA-21 ASO | U87 | Significant changes in miRNA-21 target gene expression | IV | In situ binding to endogenous circulating albumin resulted in prolonged circulating half life, increased tumor accumulation, and ~50% slowing in tumor growth | 203 |
| Heat shock proteins | ||||||
| HSP-Tat-RGD RGD: integrin targeting | siTERT | CT26 (murine colorectal carcinoma cells/xenograft in BALB/c nude mice) | RGD addition significantly reduced TERT expression; apoptosis rate ~1.5-fold higher than that of the Lipofectamine/si TERT control. | IV | Intravenous injection of HSP-Tat-RGD/siTERT (d 0, 2, 4, 6, 8) resulted in markedly reduced tumor volume (evaluated d 20) | 204 |
| Ferritin | ||||||
| Human heavy chain apoferritin | siInsR | Caco-2, Human PBMCs | Enabled ~85% knockdown of insulin receptor in Caco-2 cells, ~50% knockdown in activated human PBMCs | N/A | N/A | 205 |
| Pas-HumAfFT (humanized archaea ferritin); HumAfFT point mutation (M54C); PA (cationic polyamine) | siGAPDH | HeLa, HepG2 (human hepatoma), MCF-7(human breast cancer) | > 20% GAPDH silencing observed in HeLa, HepG, and MCF-7 cells. | N/A | N/A | 206 |
| Humanized A. flugidus ferritin | miRNA-145–5p | NB4 (human acute promyelocytic leukemia) | Granulocytic differentiation observed in cell morphology analysis, although not sufficient to achieve full differentiation | N/A | N/A | 207 |
| Poly(L-lysine) modified heavy chain apoferritin (4L-HFn) | siEGFR | HeLa, 4T1 (murine breast cancer cells/tumor model in female BALB/c mice) | Successfully downregulation of EGFR protein (>70%), but not as effective as Lipofectamine. | IV | Significant accumulation of 4L-HFn@siRNA in tumors from 2 to 10 h; tumor growth suppression effect following treatment every 2 d for 10 d | 208 |
ASO, antisense oligonucleotide; bPEI, branched poly(ethylenimine); BSA, bovine serum albumin; HPMEC, human pulmonary microvascular endothelial cells; HSP, heat shock protein; HSA, human serum albumin; lnsR, insulin receptor; MSA, Mouse serum albumin; PAMAM, polyamidoamine; PDMA, poly(N,N-dimethylacrylamide); PEI, poly(ethylenimine).
As with other non-cationic proteins, albumin has been functionalized with cationic polymers to facilitate electrostatic complexation with nucleic acids. Residues on the surface of albumin provide several chemical handles for the direct conjugation of polymers. Cationic bovine serum albumin (BSA)-based nanoparticles for plasmid DNA delivery have been synthesized through in situ polymerization of (dimethylamino)ethyl methacrylate (PDMA) on bromide-derivatized free amines (Figure 6a).196 Han et al and Chen et al pursued a similar approach, in which carboxylic acid groups were modified with ethylenediamine and branched PEI, respectively.197,198 Ethylenediamine-BSA particles were used to deliver siRNA targeting Bcl-2, reducing lung metastasis in a B16 melanoma model.197
Figure 6. Albumin-based nanoparticles as a non-immunogenic material for nucleic acid delivery vectors.
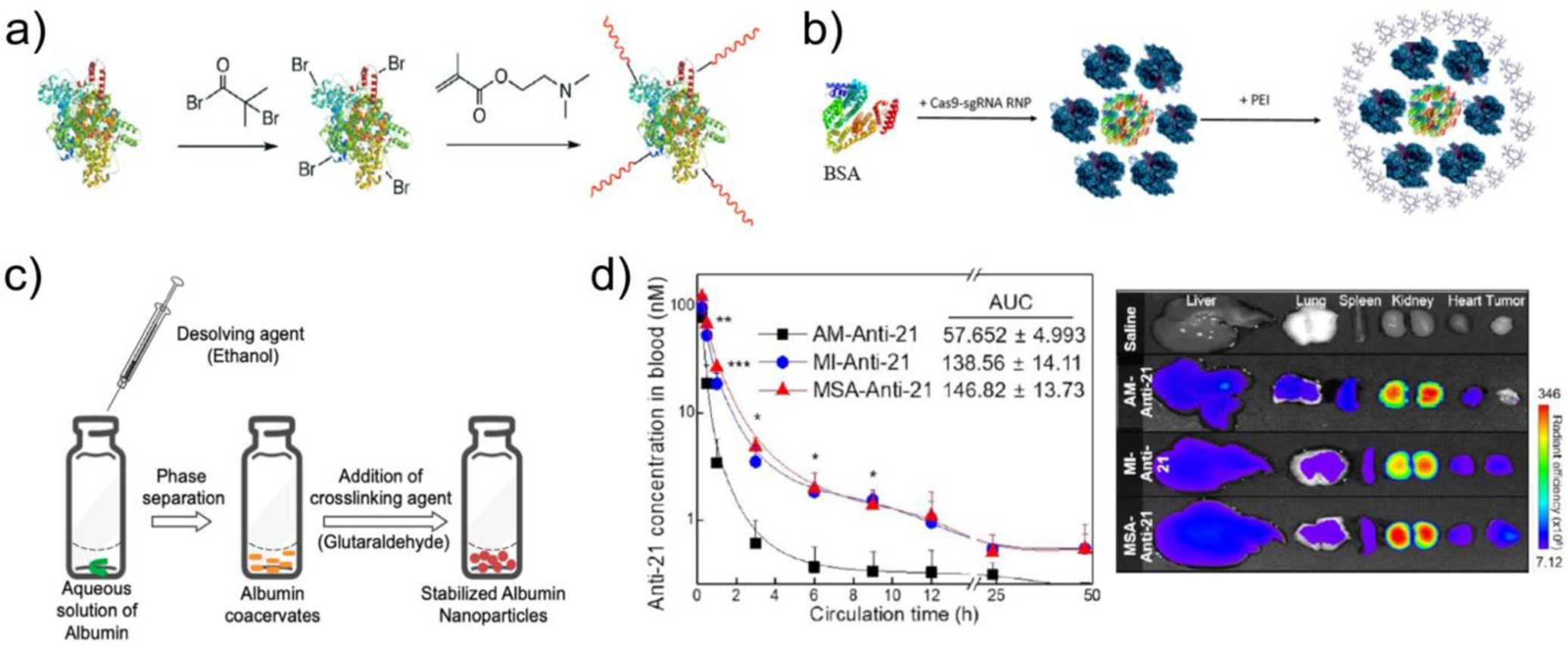
a) Synthesis of 2-(dimethylamino)ethyl methacrylate (DMA)-modified cationic BSA via in situ polymerization on bromide-functionalized free amines in BSA. Figure from Ref214. b) Schematic depicting co-formulation of BSA and PEI with Cas9 RNP. Figure from Ref201. c) Synthesis of albumin nanoparticles via desolvation and glutaraldehyde crosslinking. Nucleic acid (NA) cargo is combined in aqueous albumin solution for albumin-NA nanoparticle formation. Figure from Ref210. d) (Left) Plasma concentration and (right) organ distribution of an anti-miRNA-21 oligonucleotide following intravenous administration in tumor bearing mice. AM-Anti-21: unmodified oligonucleotide, MI-Anti-21: maleimide-functionalized oligonucleotide to facilitate in situ conjugation to endogenous circulating albumin via reaction with Cys34, MSA-Anti-21: maleimide-functionalized oligonucleotide pre-incubated with mouse serum albumin prior to administration.
Alternatively, albumin has been used in combination with cationic polymers for co-complexation and delivery of nucleic acids. Langiu et al combined HSA with linear PEI to encapsulate luciferase pDNA, enabling transfection of HeLa and mouse cerebellar granule cells.199 A similar formulation combining branched PEI with HSA demonstrated improved siRNA uptake and protein knockdown compared to bPEI alone.200 More recently, BSA-PEI nanoparticles effectively facilitated cellular uptake of plasmid and ribonucleoprotein (RNP) Cas9 (Figure 6b).201
Albumin nanoparticles have also been employed for nucleic acid delivery through co-desolvation and direct conjugation. Mehta et al prepared BSA-nucleic acid nanoparticles by desolvation of aqueous BSA and nucleic acids, followed by glutaraldehyde crosslinking.210 An optimized preparation method yielded an 85% encapsulation efficiency of pDNA with delivery of both GFP pDNA and KRAS-targeted siRNA to A549 lung cancer cells in vitro (Figure 6c). While direct conjugation of cargo to albumin results in markedly lower carrying capacity, Cys34 on the surface of HSA has been used as a chemical handle for conjugation to heavily-modified oligonucleotides, leveraging a monobromomaleimide linker to improve stability in serum.211 Cys34 can also be leveraged as an in situ conjugation handle. Kwak et al designed a maleimide-functionalized antisense oligonucleotide targeting (ASO) miRNA-21, a microRNA that suppresses tumor progression.203 When this ASO was administered intravenously in tumor bearing mice, covalent bonding to albumin facilitated a marked improvement in ASO circulating half-life and tumor growth suppression in vivo (Fig. 6d)
4.2. Protein Nanocages
Protein nanocages (NC) have recently gained interest as a delivery vehicle due to their unique material properties, as their cage-like structure enables monodisperse size, robust stability, innate biocompatibility and biodegradability, and ready functionalization by genetic or chemical means.204,212,213 NCs are defined by the self-assembly of a defined number of protein subunits into a hollow core-shell structure. Importantly, this precise organization facilitates both surface and internal presentation of distinct functional elements, such as receptor targeting ligands, antigenic proteins, and cargo binding motifs in specific amounts and geometries. Additionally, advances in recombinant protein production render NCs amenable to production and purification at scale. Altogether, these advantages have encouraged efforts to harness protein nanocages for nucleic acid delivery (Table 3). While existing efforts to engineer NCs as drug nanocarriers have included both viral and nonviral proteins, viral protein nanocages have been reviewed elsewhere.215 This review will focus on nonviral systems that avoid some of the key challenges of virus-derived carriers.
Among nonviral protein nanocages, human ferritin or apoferritin (demineralized ferritin) has been exploited for the delivery of nucleic acids, particularly siRNAs (Fig. 7a) Ferritin is a 24-mer heteropolymer that plays a pivotal role in the storage, transport, and detoxification of iron. In mammalian organisms, each of these subunits is encoded by a heavy (H) or light (L) chain, which integrate during a reversible self-assembly process to create a hollow, spherical structure with an inner diameter of ~8 nm and an outer diameter of ~12 nm.216,217 The resulting inner cavity provides ferritin with a unique storage ability. Due to an ability to be endocytosed through H-ferritin and TfR1 (transferrin 1 receptor) interactions, combined with tunable recombinant expression and intrinsic biocompatibility, ferritin nanocages represent an interesting opportunity for intracellular drug delivery. In addition, as a recombinantly expressed human protein, ferritin is expected to have minimal immunogenicity. Li et al demonstrated that unmodified human apoferritin can mediate delivery of a variety of siRNAs resulting in gene silencing in both tumor cells and human peripheral mononuclear blood cells (PBMCs) in vitro.205 However, the ferritin cavity naturally contains many negatively charged residues, inhibiting efficient encapsulation of siRNA molecules.206 To overcome this limitation, Pediconi et al functionalized human ferritin with positively charged cationic piperazine-based compounds (Pas) to physically entrap siRNA duplexes.206 PA-functionalized ferritin compounds were able to deliver GAPDH-targeted siRNA to HeLa, HepG2, and MCF-7 cancer cells with superior silencing as compared to traditional transfection methodologies (Fig 7b).206 Similarly, Huang et al synthesized a poly(L-lysine)-modified apoferritin nanocage that exhibited gene silencing in vitro and inhibited 4T1 tumor progression in vivo.208
Figure 7. Nonviral Protein Nanocages as an siRNA delivery platform.
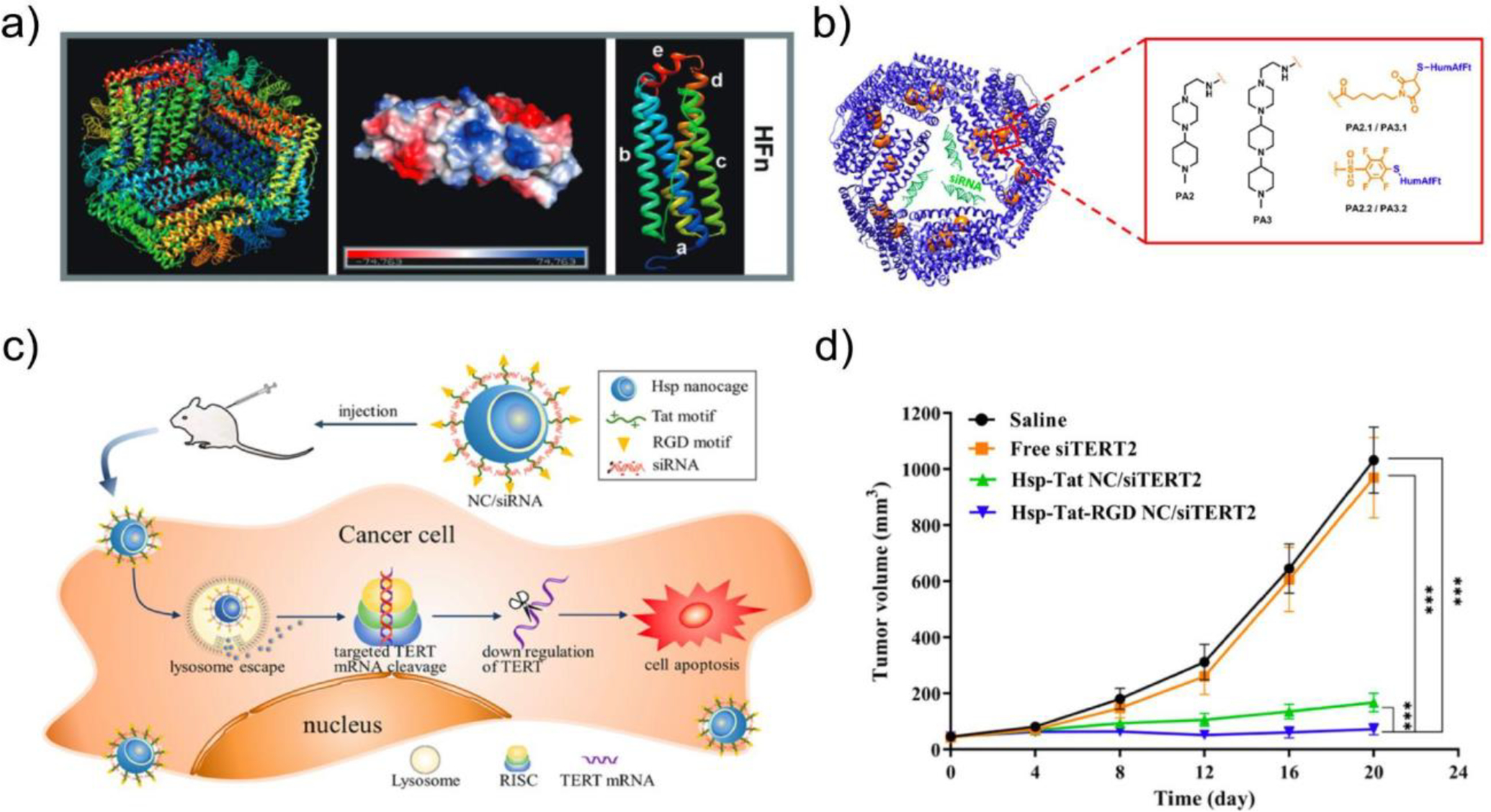
a) Wild-type human ferritin (PDB ID: 3AJO) with 24 monomers displayed in different colors (left), charge distribution of each subunit (middle), and the predicted structure of each subunit (right). From Ref218. b) Schematic of recombinant human ferritin (HFn) nanocages functionalized with cationic domains such as piperazine-based compounds (PAs) or poly(L-lysine) (4L) for improved siRNA encapsulation. Adapted from Ref206. c) Schematic of heat shock protein (HSP)-derived protein nanocages decorated with transactivator of transcription (Tat) (green) and arginine-glycine-aspartate (RGD) motifs (yellow), abbreviated HSP-Tat-RGD. d) In vivo antitumor efficacy of HSP-Tat-RGD-siTERT complexes delivered in CT26 xenograft mice, as demonstrated by reduced tumor volumes compared to saline and free siTERT2 controls. c) and d) adapted from Ref202.
Additional efforts to engineer nonviral protein nanocages for nucleic acid delivery have focused on heat shock proteins (HSPs). HSPs are a large family of protein chaperones involved in the first line of defense against a variety of cellular stressors, including heat shock, ionizing or ultraviolet radiation, and reactive oxygen species.219 These ubiquitously expressed proteins protect cell integrity and survival by various mechanisms, including genomic repair and the prevention of protein misfolding and aggregation.219 Much like ferritin, Hsp16.5, derived from Methanococcus jannaschii, is composed of 24 subunits, which self-assemble into a spherical nanocage. Guan et al engineered Hsp16.5 by introducing a cationic polyarginine motif at the C-terminus to enable condensation of siRNA within the nanocage.213 This delivery system protected condensed siRNA from degradation and enabled delivery to HeLa-EGFP cells with downregulation of GFP expression.213 Furthermore, HSP NCs decorated with Arg-Gly-Asp (RGD) and Tat peptide motifs were used to deliver telomerase reverse transcriptase (TERT)-targeting siRNA, inducing tumor cell apoptosis in vitro, and inhibiting the growth of tumor CT26 xenografts in vivo (Fig 7c–d).204 Together, these data demonstrate potential for Hsp-derived protein nanocages to serve as an efficient and biocompatible siRNA delivery system. Nonetheless, the carrying capacity of these natural protein scaffolds remains near the effective diameter of siRNAs (8–9 nm), precluding the application of nonviral nanocages to larger, protein-encoding nucleic acid cargo. Moreover, as bacterial-derived proteins, the immunogenicity of these nanoparticles requires further investigation.
In summary, diverse protein nanoparticles exhibit varying levels of in vitro and in vivo efficacy in delivering nucleic acid cargo. In numerous instances, their delivery efficiency rivals or even surpasses that of traditional lipid- or cationic polymer-based transfection agents (Tables 1 and 2). It is important to highlight that, in certain scenarios, these effects result from the modification of the protein material with cationic polymers, raising questions about the distinctive contribution of the protein component itself. The primary contribution of the protein component oftentimes lies in its ability to mitigate the cytotoxicity associated with cationic polymers while simultaneously enhancing delivery efficiency. This phenomenon is consistently observed in systems such as cationic ELPs103,220, silk139,140,151–153, gelatin191, and albumin199. This effect is believed to stem from a combination of reduction of surface positive charge, improvement of nanoparticle stability, and the enhancement of tissue targeting and cellular uptake, which is mediated via diverse interactions between the protein motifs and their native receptors.
5. Clinical Development
Preclinical data from the studies detailed above is part of a growing body of evidence supporting the clinical application of non-viral protein materials for nucleic acid delivery. At the time of publication of this review, none of the materials described have been used for nucleic acid delivery in humans. However, a proof-of-concept has been established for the use of many of these materials as safe and effective drug delivery vehicles for a range of non-nucleic acid cargo (Table 4). Leveraging the recombinant and thermoresponsive nature of elastin-like polypeptides, fusions of ELPs and therapeutic peptides have been created that form sustained release depots following subcutaneous administration, due to the coacervation of the ELPs. Slow egress of the ELP-peptide fusions from the depots into circulation leads to target engagement and therapeutic effects; the presence of the long ELP chain on the therapeutic peptide improves its circulating half-life and further prolongs its effects. ELP fusions to vasoactive intestinal peptide (VIP), insulin, and glucagon-like peptide 1 (GLP-1) were well tolerated and demonstrated sustained therapeutic benefit in pulmonary arterial hypertension and type 2 diabetes following weekly subcutaneous administration to patients in Phase 1/2 clinical studies (see Table 4).164,221–224 Repeated dose ELP fusions to hundreds of patients without adverse effects related to an anti-ELP immune response provides evidence of the minimal immunogenicity of this material, an important property for its use for nucleic acid delivery in humans.
Both gelatin and albumin have also been effectively used for therapeutic delivery in humans. Gelatin microspheres have been effectively used as a sustained release system for both proteins (bovine fibroblast growth hormone, in critical limb ischemia patients)226 and small molecules (cisplatin, in patients receiving transarterial chemoembolization of hepatocellular carcinoma nodules).225 Likely the most prominent example of a protein-based delivery vehicle used in humans is Abraxane (generic name nab-paclitaxel), an approved therapy consisting of hydrophobic chemotherapeutic paclitaxel co-formulated with albumin nanoparticles intended to 1) avoid the toxicities associated with alternative solvents such as polyethylated castor oil and 2) increase tumor exposure to the drug. In a Phase 3 trial directly comparing unformulated paclitaxel and nab-paclitaxel in metastatic breast cancer patients, nab-paclitaxel induced higher overall response rates (33% vs 19%), and lower rates of severe neutropenia (9% vs 22%).227 In a separate trial, nab-paclitaxel combined with gemcitabine enabled improved survival rates in late-stage pancreatic cancer patients compared to gemcitabine alone.228
Other protein materials have been utilized as part of vaccination delivery system in humans. Due to its mechanical strength, biodegradability, and biocompatibility, B. mori silk fibroin (BMSF)-derived thread has been used as the material basis for surgical repair mesh and sutures.231,232 More recently, BMSF has been used to create room temperature-stable, protein antigen-loaded microneedle arrays for minimally invasive vaccination. Sustained antigen release from the microneedles following application to the skin better recapitulates the natural course of exposure to an infectious agent; in a Phase 1 trial, 92% of patients who were vaccinated against H1N1 influenza through this approach produced a protective level of antibodies.229 Leveraging the self-assembly, multivalent structure, and high manufacturability of ferritin nanoparticles compared to traditional attenuated virus vaccines, Houser et al recombinantly produced a H2 hemagglutinin-ferritin nanoparticle vaccine (H2HA-Ferittin) that displayed eight copies of H2N2 influenza HA and produced neutralizing antibody responses in vaccinated patients.230 Collectively, these data provide some evidence for the clinical safety of the reviewed protein-based materials, setting the stage for the investigation of these materials as nucleic acid delivery vectors in humans.
6. Outlook and Unresolved Challenges
Protein-based nanoparticles have shown great promise as delivery vectors for multiple types of nucleic acid cargo. To realize the full potential of these materials, several unresolved challenges will need to be addressed (Fig. 8). Many of these challenges are ultimately related to the challenge of improving potency – the concentration or dose of nucleic acid cargo required to generate a desired therapeutic effect. As the leading non-viral nucleic acid delivery system, lipid nanoparticles (LNPs) have displayed greater potency when compared to protein-based systems. LNP-siRNA formulations incorporating the highly active ionizable lipid DLin-MC3-DMA achieved transthyretin (TTR) mRNA knockdown over 24 hours at a median effective dose (ED50) of 0.03 mg siRNA/kg in mice. In clinical studies, patisiran, a TTR-targeted siRNA drug formulated in a DLin-MC3-DMA based LNP, has been effectively at 0.3 mg/kg administered every 3 weeks.233,234 Other LNPs have demonstrated pDNA transfection efficiencies comparable or exceeding Lipofectamine in vitro.235,236 In contrast, protein nanoparticles evaluated as delivery platforms for siRNA and pDNA, particularly those not coformulated with synthetic polymers such as PEI, have largely failed to outperform Lipofectamine in vitro and require frequent multiple mg/kg dosing in vivo.104,109,119,122 While the advantages of protein nanoparticles remain evident, including reduced cytotoxicity, improved biodegradability, genetic programmability, and highly tunable physicochemical properties, those features do not outweigh the importance of achieving therapeutic delivery at scalable nucleic acid doses.
Figure 8. Emerging solutions to address key challenges faced by protein-based nucleic acid delivery vehicles.

If leveraged appropriately, innovations in the areas of endosomal escape, biostability, targeted delivery, and safety and manufacturing can come together as the missing pieces for constructing an ideal protein nanoparticle. Created with BioRender.
6.1. Endosomal escape
Entrapment in the endolysosomal system following cellular internalization is a key limitation that must be surmounted to enhance the potency of protein-based nucleic acid delivery systems. One approach to address this is through the use of cationic amphiphilic drugs (CADs), a class of molecules that accumulates in acidic lysosomal compartments, promotes phospholipidosis, and leads to lysosomal disruption.237,238 Chloroquine, a commonly used antimalarial medication, is a CAD that has been explored as an endosomolytic agent.239 Other CADs, such as desloratadine and loperamide, have demonstrated a remarkable ability to potentiate siRNA delivery by several types of nanocarriers.238 While CADs can be co-formulated into nanoparticles with nucleic acid cargo, more often these drugs have been administered separately, creating additional dosing complexities. Critically, CADs present a risk of toxicity, with chloroquine displaying immunosuppressive effects. Other agents with potentially better toxicity profiles are being investigated as alternatives.240,241
In contrast to small molecule endosomolytic agents, direct incorporation of membrane-disruptive endosomolytic peptides can improve nucleic acid cargo delivery without the need for a co-administered agent. While the precise mechanism of action is debated, interactions between positively-charged residues and negatively-charged phospholipids, coupled with membrane intercalation by hydrophobic peptide segments, appear to be critical aspects of peptide-mediated endosomolysis. It has been proposed that cationic endosomolytic peptides induce cargo-loaded vesicle budding from the parent endosome and eventual collapse.89,242,243 An alternative mechanism suggests that endosomolytic peptides may promote the fusion of intraluminal vesicles with the parent endosomal membrane, resulting in cargo release without requiring direct movement across the membrane.244–246 Recent studies have established that the amphiphilicity of endosomolytic peptides, a property of either the primary peptide sequence or emerging from its secondary structure, correlates with membrane disruptive potential.247,248 Tat, an arginine-rich, HIV-derived peptide249, penetratin and ppTG1, both cationic, amphiphilic α-helical peptides that are examples of secondary amphiphilic peptides,112,250 have been used to enable nucleic acid cargo complexation and mediate endosomal escape in protein nanoparticle systems.104,113,115,120,156 While significant progress has been made in improving the effectiveness of such peptides for nucleic acid delivery251, further exploration of membrane-disruptive peptides may well yield protein-nucleic acid nanoparticle designs with enhanced potency.
Machine learning has emerged as a powerful tool for accelerating the discovery of membrane active peptide sequences.252–254 In contrast to in vitro screening based on rational design, in silico screening enables the exploration of much larger spans of physicochemical space encompassing potentially active peptides in a far less resource-intensive manner. Many machine learning models designed to support cell penetrating and endosomolytic peptide discovery have been trained using the CPPsite database, which contains structural information for over 1800 CPPs and associated cell uptake efficiency.252,255–257 As opposed to using this non-standardized database for training, some groups have generated datasets derived from peptide library screens labeled with nucleic cargo delivery efficacy as a basis for predictive model generation.258–260 For example, data from an in vitro delivery screen of 600 CPP-phosphorodiamidate morpholino oligomer (PMO) conjugates, which enable EGFP expression in treated cells was applied to train a convolutional neural network for the prediction of peptide efficacy. This model was leveraged for the identification of de novo sequences with the discovery of peptides displaying two fold greater activity than the initial lead peptide.259 These studies highlight the potential of in silico design of potent endosomolytic peptides, which in turn can be incorporated into protein-nucleic acid nanoparticle systems to achieve enhanced potency.
Beyond endosomolytic peptides, membrane disruptive proteins are emerging as another means to improve endosomal escape of nucleic acid cargo. Goldbach et al created membrane disruptive, pH-responsive helical bundle proteins designed to lyse membranes via direct intercalation within a narrow range of pH (6.0–5.5).261 Naturally occurring proteins have also been investigated, including Perfringolysin O (PFO), Listeriolysin O (LFO), and Phospholipase A2 (PLA2), which are pathogenic proteins that enable pore formation in lipid membranes (PFO and LFO) or hydrolyze phospholipids found in membranes (PLA2). These proteins have been used as fusions with receptor targeting and RNA-binding protein or synthetic polymer domains to enable delivery of siRNA and DNA cargo to cell lines in vitro.251,262–264 Given that these modules are proteins, they are amenable to direct incorporation into the recombinant protein nanoparticle systems described above, including elastin-like polypeptides, silk-like polypeptides, and protein nanocages and may be worthy of further exploration. However, there are two important limitations that must be addressed for membrane disruptive proteins to be used as endosomolytic agents in humans: 1) immunogenicity, as the variants of these proteins used are typically derived from pathogens or venomous animals, and 2) membrane selectivity, as undesired activity on the cell membrane can result in significant toxicity. The use of human pore-forming proteins and phospholipase homologues may represent less immunogenic alternatives worthy of further exploration.265,266 Emerging in silico protein design tools may be useful for reengineering these proteins to improve endosomal membrane specificity, either via reducing activity at neutral pH or tuning activity towards anionic lipids specifically enriched in endosomal membrane.267,268
6.2. Improving biostability
Sustained colloidal stability prior to use, together with resistance to protein aggregation following drug administration ensure that particles can be stored for an extended period and achieve effective biodistribution to target tissues. Studies that assess nanoparticle stability have generally been lacking and many reports have been limited to investigations under reduced serum transfection conditions suggestive of limitations in particle stability or uptake in physiologic conditions. Anti-fouling surface modifications have been explored as a means to prevent nanoparticle aggregation, improve particle biostability, enhance circulating half-life, and thereby improve the potency of in vivo cargo delivery.
Coating nanoparticles with hydrophilic polymer chains, such as PEG, promotes surface hydration, creates a steric barrier that discourages nanoparticle aggregation, and establishes an entropic penalty for protein adsorption to the nanoparticle surface.22,27,28 Silk fibroin nanoparticles bearing PEG5000 displayed improved stability, limited particle aggregation, and maintained particle size over 24 hours under physiologic conditions.269 Similarly, gelatin nanoparticles modified with a PEG-poly(2-dimethyl amino ethyl methacrylate-co-itaconic acid) copolymer (PEG-P(DMAEA-co-IAc)) have been used to co-encapsulate and deliver doxorobucin and betanin to breast cancer cells.270 Other synthetic and natural-occurring anti-fouling polymers explored have included poly(glycerol)271,272, poly(acrylamide)273,274, and hyaluronic acid.275
While feasible, polymer conjugation to recombinant proteins is challenging to implement and scale, and the widespread use of PEG has resulted in the emergence of anti-PEG immunity and reports of an associated accelerated blood clearance (ABC) phenomenon.28,276 Zwitterionic polypeptides have emerged as an alternative strategy for incorporating anti-fouling domains into recombinant protein nanoparticles with formation of a well-hydrated, compact surface that prevents protein adsorption.277,278 Poly(glutamate-lysine) (poly(EK)) peptides have demonstrated robust anti-fouling properties in multiple nanoparticle systems, resulting in improved circulation times and biodistribution.31,279–282 Importantly, zwitterionic polypeptides can be easily incorporated into recombinant protein nanoparticle systems without the need for chemical conjugation, and with precise control of size and valency. Thorough pharmacokinetic studies are needed to establish the benefit of zwitterionic and other anti-fouling modifications on nanoparticle half-life and biodistribution. Improvements in circulating half-life mediated by zwitterion functionalization can ultimately increase target cell transfection efficiency in vivo, while reducing non-specific uptake.
6.3. Extrahepatic and extratumoral delivery
The majority of approved and late-stage nanoparticle drug delivery systems are intended to promote hepatic and tumoral drug accumulation following intravenous administration.283,284 A majority of protein nanoparticle nucleic acid delivery vectors have also been directed towards liver and oncologic applications. As noted, liver and tumors contain a fenestrated vasculature, a shared characteristic that favors nanoparticle drug delivery.35,40 However, many indications require delivery to tissues and cells, such as myocytes and neural cells for the treatment of inherited muscular dystrophies and neurodegenerative diseases, respectively, that harbor significant barriers to particle transport.285,286 Both trafficking to the tissue of interest and internalization by target cells remain critical factors that dictate cell-specific cargo delivery. In turn, enhancing target tissue or cell tropism will reduce the amount of nucleic acid cargo required to achieve a therapeutic effect, thereby improving potency.
Recombinant protein-based nanoparticles can easily incorporate receptor targeting domains, including peptide motifs and antibody fragments that promote internalization via receptor-mediated endocytosis. As described, this strategy has been pursued in ELP and SLP-based nucleic acid delivery systems, leveraging peptides targeting surface receptors that are upregulated in a variety of cancer types.104,109,119 Likewise, antibody variable fragments derived from camelid single-chain antibodies (nanobodies) have been used to decorate protein nanoparticles and promote targeted internalization.77,78 Costa et al. designed diblock ELPs modified with a nanobody targeting EGFR overexpressed in many cancers, which led to a significant improvement in uptake in EGFR+ cell lines.77 In addition to active targeting, passive targeting can be achieved by altering surface chemistry to modulate the composition of the corona and particle trafficking. This underexplored approach can potentially be utilized to generate protein nucleic acid delivery vectors with unique tropisms.48,287,288
6.4. Safety and Manufacturing
For protein nanoparticle delivery systems to be clinically viable, there will be a need for scalable production, as well as analytical methods to ensure that these materials meet medical grade quality and safety standards. Immunogenicity is an important feature to consider in assessing the safety profile of protein materials. Many protein materials derived from or inspired by human proteins, such as ELPs, nanocage proteins, and human serum albumin have demonstrated low immunogenicity in settings relevant to their use as nucleic acid delivery vectors. Other proteins with high homology to human proteins, such as bovine serum albumin, gelatin, and collagen, as well as silk are capable of inducing an allergic response in rare cases, but have typically demonstrated low immunogenicity.292–294
Of greater concern are xenogeneic proteins and other molecules that may be present in material preparations, which may be residual from material source organisms, including E. coli for recombinant proteins, silkworms or spiders for silk fibroin, or bovine or porcine sources for collagen, gelatin, and albumin. Non-mammalian-derived endosomolytic and cell-penetrating peptides incorporated to facilitate cytosolic penetration of cargo may represent another source of potential immune stimulation, though there is some evidence suggesting that certain CPPs are non-immunogenic.295 The purification of protein materials by affinity chromatography or acidic or thermal extraction, along with the removal of inflammatory contaminants like endotoxin should minimize the effect of xenogeneic contaminants. In addition, in silico predictions of peptide antigenicity can be leveraged in the design of cell penetrating and endosomolytic peptides to select for sequences displaying minimal risk of immunogenicity.296–298
Recombinant protein materials to be used as excipients for nucleic acid delivery can be produced at scale using existing biomanufacturing methods. E. coli expression systems have been favored in the laboratory setting due to low cost, high yields, and well-established protocols.299–301 While E. coli do not easily generate human-like glycosylation or phosphorylation features, such post-translational modifications are typically not necessary for the assembly of protein nanoparticles, making E. coli an ideal vector for scaled recombinant protein material production if paired with high fidelity purification methods. Recently, continuous manufacturing has been explored as a strategy to improve yields and quality, reduce operational costs, and increase efficiency by reducing the pauses and transfers associated with batch manufacturing.299,302 Downstream of protein production and purification, the quality of protein materials used for nucleic acid delivery should be assessed using quality control tests focused on purity (e.g. SDS-PAGE, and liquid chromatography methods), homogeneity (e.g. DLS or other light scattering methods, size exclusion chromatography), and identity (e.g. mass spectrometry).303,304
7. Conclusions
Addressing the longstanding delivery challenges associated with nucleic acid therapies is critical to unlocking the full potential of this modality to transform disease outcomes. As highlighted in this review, protein-based nanoparticles are well-positioned to serve as a vehicle that both combines many desirable features of existing nucleic acid delivery systems and overcomes their limitations. Recombinant protein materials exhibit the genetic programmability of existing viral vector systems with improved robustness to modification, enabling highly selective incorporation of peptide-based cell receptor targeting domains and membrane active domains to mediating cargo endosomal escape. Moreover, in contrast to viral delivery systems, engineered protein materials also demonstrate a level of scalability, resilience to harsh, denaturing purification conditions, and cargo accommodation capacity comparable to existing non-viral delivery systems. More broadly, protein materials are biodegradable and can be designed with limited immunogenic risk, and therefore, suitable for many clinical applications requiring repeated nucleic acid delivery. As issues of potency and biostability are addressed, protein nanoparticles are poised to mature into a promising new class of nucleic acid delivery vectors for the treatment of human disease.
Acknowledgements
This research was supported by the NIH (UG3AI15055, UH3AI150551). F.E. is supported by the Harvard/MIT MD-PhD program (5T32GM007753-42), and the Ruth L. Kirschstein NRSA F31 Fellowship (F31HL167533). M.W. is supported by the Harvard/MIT MD-PhD program (5T32GM144273-02).
Elliot L. Chaikof reports financial support was provided by National Institutes of Health. Elliot L. Chaikof has patent protein-based delivery system pending to Beth Israel Deaconess Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Author Statement
Feyisayo Eweje: Conceptualization, Investigation, Writing – Original Draft, Writing – Review & Editing, Michelle Walsh: Investigation, Writing – Original Draft, Writing – Review & Editing, Kiran Ahmad: Investigation, Writing – Original Draft, Writing – Review & Editing Vanessa Ibrahim: Investigation, Writing – Review & Editing, Assma Alrefai: Investigation, Jiaxuan Chen: Investigation, Supervision, Writing – Original Draft, Writing – Review & Editing, Elliot Chaikof: Supervision, Writing – Review & Editing.
Competing Interests
F.E., J.C., and E.C. are named as inventors in a provisional patent application related to a protein-based nanoparticle delivery system (US Provisional Application No. 63/514,222).
References
- 1.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2021;384:403–16. 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. New England Journal of Medicine 2020;382:1520–30. 10.1056/NEJMoa1913805. [DOI] [PubMed] [Google Scholar]
- 4.Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. Viral vector platforms within the gene therapy landscape. Sig Transduct Target Ther 2021;6:1–24. 10.1038/s41392-021-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Z, Anselmo AC, Mitragotri S. Viral vector-based gene therapies in the clinic. Bioeng Transl Med 2021;7:e10258. 10.1002/btm2.10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater 2021;6:1078–94. 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das R, Kanjilal P, Medeiros J, Thayumanavan S. What’s Next after Lipid Nanoparticles? A Perspective on Enablers of Nucleic Acid Therapeutics. Bioconjugate Chem 2022. 10.1021/acs.bioconjchem.2c00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarvirdipour S, Skowicki M, Schoenenberger C-A, Palivan CG. Peptide-Assisted Nucleic Acid Delivery Systems on the Rise. International Journal of Molecular Sciences 2021;22:9092. 10.3390/ijms22169092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Berg AIS, Yun C-O, Schiffelers RM, Hennink WE. Polymeric delivery systems for nucleic acid therapeutics: Approaching the clinic. J Control Release 2021;331:121–41. 10.1016/j.jconrel.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Boisguérin P, Konate K, Josse E, Vivès E, Deshayes S. Peptide-Based Nanoparticles for Therapeutic Nucleic Acid Delivery. Biomedicines 2021;9:583. 10.3390/biomedicines9050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varanko A, Saha S, Chilkoti A. Recent trends in protein and peptide-based biomaterials for advanced drug delivery. Adv Drug Deliv Rev 2020;156:133–87. 10.1016/j.addr.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Spruijt E, Miserez A, Langer R. Peptide-based liquid droplets as emerging delivery vehicles. Nat Rev Mater 2023;8:139–41. 10.1038/s41578-022-00528-8. [DOI] [Google Scholar]
- 13.He J, Yu L, Lin X, Liu X, Zhang Y, Yang F, et al. Virus-like Particles as Nanocarriers for Intracellular Delivery of Biomolecules and Compounds. Viruses 2022;14:1905. 10.3390/v14091905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segel M, Lash B, Song J, Ladha A, Liu CC, Jin X, et al. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 2021;373:882–9. 10.1126/science.abg6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu B, Javidi-Parsijani P, Makani V, Mehraein-Ghomi F, Sarhan WM, Sun D, et al. Delivering SaCas9 mRNA by lentivirus-like bionanoparticles for transient expression and efficient genome editing. Nucleic Acids Research 2019;47:e44. 10.1093/nar/gkz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olshefsky A, Richardson C, Pun SH, King NP. Engineering Self-Assembling Protein Nanoparticles for Therapeutic Delivery. Bioconjugate Chem 2022;33:2018–34. 10.1021/acs.bioconjchem.2c00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuna M, Mahdi F, Chade AR, Bidwell GL. Molecular Size Modulates Pharmacokinetics, Biodistribution, and Renal Deposition of the Drug Delivery Biopolymer Elastin-like Polypeptide. Sci Rep 2018;8:7923. 10.1038/s41598-018-24897-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vegh P, Fletcher J, Dixon D, Haniffa M. Mononuclear Phagocyte System. eLS. John Wiley & Sons, Ltd; 2017. p. 1–8. [Google Scholar]
- 19.Papini E, Tavano R, Mancin F. Opsonins and Dysopsonins of Nanoparticles: Facts, Concepts, and Methodological Guidelines. Frontiers in Immunology 2020;11:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015;10:487–510. 10.1016/j.nantod.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmoudi M, Lynch I, Ejtehadi MR, Monopoli MP, Bombelli FB, Laurent S. Protein−Nanoparticle Interactions: Opportunities and Challenges. Chem Rev 2011;111:5610–37. 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 22.Bertrand N, Grenier P, Mahmoudi M, Lima EM, Appel EA, Dormont F, et al. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat Commun 2017;8:777. 10.1038/s41467-017-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm 2006;307:93–102. 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Safi M, Courtois J, Seigneuret M, Conjeaud H, Berret J-F. The effects of aggregation and protein corona on the cellular internalization of iron oxide nanoparticles. Biomaterials 2011;32:9353–63. 10.1016/j.biomaterials.2011.08.048. [DOI] [PubMed] [Google Scholar]
- 25.Li X, He E, Xia B, Liu Y, Zhang P, Cao X, et al. Protein corona-induced aggregation of differently sized nanoplastics: impacts of protein type and concentration. Environ Sci: Nano 2021;8:1560–70. 10.1039/D1EN00115A. [DOI] [Google Scholar]
- 26.Cukalevski R, Ferreira SA, Dunning CJ, Berggård T, Cedervall T. IgG and fibrinogen driven nanoparticle aggregation. Nano Res 2015;8:2733–43. 10.1007/s12274-015-0780-4. [DOI] [Google Scholar]
- 27.Walkey CD, Olsen JB, Guo H, Emili A, Chan WCW. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. J Am Chem Soc 2012;134:2139–47. 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 28.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 2016;99:28–51. 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoang Thi TT, Pilkington EH, Nguyen DH, Lee JS, Park KD, Truong NP. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers (Basel) 2020;12:298. 10.3390/polym12020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He M, Gao K, Zhou L, Jiao Z, Wu M, Cao J, et al. Zwitterionic materials for antifouling membrane surface construction. Acta Biomaterialia 2016;40:142–52. 10.1016/j.actbio.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 31.Liu EJ, Sinclair A, Keefe AJ, Nannenga BL, Coyle BL, Baneyx F, et al. EKylation: Addition of an Alternating-Charge Peptide Stabilizes Proteins. Biomacromolecules 2015;16:3357–61. 10.1021/acs.biomac.5b01031. [DOI] [PubMed] [Google Scholar]
- 32.Sleep D, Cameron J, Evans LR. Albumin as a versatile platform for drug half-life extension. Biochimica et Biophysica Acta (BBA) - General Subjects 2013;1830:5526–34. 10.1016/j.bbagen.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Zhang C, Li Z, Yin S, Wang Q, Guo F, et al. Extending Half Life of H-Ferritin Nanoparticle by Fusing Albumin Binding Domain for Doxorubicin Encapsulation. Biomacromolecules 2018;19:773–81. 10.1021/acs.biomac.7b01545. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y-N, Poon W, Tavares AJ, McGilvray ID, Chan WCW. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J Control Release 2016;240:332–48. 10.1016/j.jconrel.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Ngo W, Ahmed S, Blackadar C, Bussin B, Ji Q, Mladjenovic SM, et al. Why nanoparticles prefer liver macrophage cell uptake in vivo. Advanced Drug Delivery Reviews 2022;185:114238. 10.1016/j.addr.2022.114238. [DOI] [PubMed] [Google Scholar]
- 36.Ceña V, Játiva P. Nanoparticle crossing of blood–brain barrier: a road to new therapeutic approaches to central nervous system diseases. Nanomedicine 2018;13:1513–6. 10.2217/nnm-2018-0139. [DOI] [PubMed] [Google Scholar]
- 37.Chen K-T, Wei K-C, Liu H-L. Theranostic Strategy of Focused Ultrasound Induced Blood-Brain Barrier Opening for CNS Disease Treatment. Frontiers in Pharmacology 2019;10:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu D, Cheng Y, Cai R, Wen-wen Wang B, Cui H, Liu M, et al. The enhancement of siPLK1 penetration across BBB and its anti glioblastoma activity in vivo by magnet and transferrin co-modified nanoparticle. Nanomedicine: Nanotechnology, Biology and Medicine 2018;14:991–1003. 10.1016/j.nano.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Ohta S, Kikuchi E, Ishijima A, Azuma T, Sakuma I, Ito T. Investigating the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood–brain barrier opening. Sci Rep 2020;10:18220. 10.1038/s41598-020-75253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J Pers Med 2021;11:771. 10.3390/jpm11080771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaughan HJ, Green JJ, Tzeng SY. Cancer-Targeting Nanoparticles for Combinatorial Nucleic Acid Delivery. Adv Mater 2020;32:e1901081. 10.1002/adma.201901081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater 2016;1:1–12. 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- 43.Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, et al. The entry of nanoparticles into solid tumours. Nat Mater 2020;19:566–75. 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- 44.Kingston BR, Lin ZP, Ouyang B, MacMillan P, Ngai J, Syed AM, et al. Specific Endothelial Cells Govern Nanoparticle Entry into Solid Tumors. ACS Nano 2021;15:14080–94. 10.1021/acsnano.1c04510. [DOI] [PubMed] [Google Scholar]
- 45.Miller MA, Chandra R, Cuccarese MF, Pfirschke C, Engblom C, Stapleton S, et al. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Science Translational Medicine 2017;9:eaal0225. 10.1126/scitranslmed.aal0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumoto Y, Nichols JW, Toh K, Nomoto T, Cabral H, Miura Y, et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nature Nanotech 2016;11:533–8. 10.1038/nnano.2015.342. [DOI] [PubMed] [Google Scholar]
- 47.Dai Q, Wilhelm S, Ding D, Syed AM, Sindhwani S, Zhang Y, et al. Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano 2018;12:8423–35. 10.1021/acsnano.8b03900. [DOI] [PubMed] [Google Scholar]
- 48.Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat Nanotechnol 2020;15:313–20. 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dilliard SA, Cheng Q, Siegwart DJ. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proceedings of the National Academy of Sciences 2021;118:e2109256118. 10.1073/pnas.2109256118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karamanos NK, Theocharis AD, Piperigkou Z, Manou D, Passi A, Skandalis SS, et al. A guide to the composition and functions of the extracellular matrix. The FEBS Journal 2021;288:6850–912. 10.1111/febs.15776. [DOI] [PubMed] [Google Scholar]
- 51.Engin AB, Nikitovic D, Neagu M, Henrich-Noack P, Docea AO, Shtilman MI, et al. Mechanistic understanding of nanoparticles’ interactions with extracellular matrix: the cell and immune system. Particle and Fibre Toxicology 2017;14:22. 10.1186/s12989-017-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burke RS, Pun SH. Extracellular Barriers to in Vivo PEI and PEGylated PEI Polyplex-Mediated Gene Delivery to the Liver. Bioconjugate Chem 2008;19:693–704. 10.1021/bc700388u. [DOI] [PubMed] [Google Scholar]
- 53.Le Goas M, Testard F, Taché O, Debou N, Cambien B, Carrot G, et al. How Do Surface Properties of Nanoparticles Influence Their Diffusion in the Extracellular Matrix? A Model Study in Matrigel Using Polymer-Grafted Nanoparticles. Langmuir 2020;36:10460–70. 10.1021/acs.langmuir.0c01624. [DOI] [PubMed] [Google Scholar]
- 54.Stylianopoulos T, Poh M-Z, Insin N, Bawendi MG, Fukumura D, Munn LL, et al. Diffusion of Particles in the Extracellular Matrix: The Effect of Repulsive Electrostatic Interactions. Biophysical Journal 2010;99:1342–9. 10.1016/j.bpj.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ricard-Blum S, Vallet SD. Proteases decode the extracellular matrix cryptome. Biochimie 2016;122:300–13. 10.1016/j.biochi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 56.Stewart RH. A Modern View of the Interstitial Space in Health and Disease. Frontiers in Veterinary Science 2020;7:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heldin C-H, Rubin K, Pietras K, Östman A. High interstitial fluid pressure — an obstacle in cancer therapy. Nat Rev Cancer 2004;4:806–13. 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 58.Böckelmann LC, Schumacher U. Targeting tumor interstitial fluid pressure: will it yield novel successful therapies for solid tumors? Expert Opinion on Therapeutic Targets 2019;23:1005–14. 10.1080/14728222.2019.1702974. [DOI] [PubMed] [Google Scholar]
- 59.Xia T, Kovochich M, Liong M, Meng H, Kabehie S, Zink JI, et al. Polyethyleneimine Coating Enhances the Cellular Uptake of Mesoporous Silica Nanoparticles and Allows Safe Delivery of siRNA and DNA Constructs. ACS Nano 2009;3:3273–86. 10.1021/nn900918w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sokolova V, Kozlova D, Knuschke T, Buer J, Westendorf AM, Epple M. Mechanism of the uptake of cationic and anionic calcium phosphate nanoparticles by cells. Acta Biomater 2013;9:7527–35. 10.1016/j.actbio.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 61.Pang H-B, Braun GB, Ruoslahti E. Neuropilin-1 and heparan sulfate proteoglycans cooperate in cellular uptake of nanoparticles functionalized by cationic cell-penetrating peptides. Sci Adv 2015;1:e1500821. 10.1126/sciadv.1500821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zarska M, Novotny F, Havel F, Sramek M, Babelova A, Benada O, et al. Two-Step Mechanism of Cellular Uptake of Cationic Gold Nanoparticles Modified by (16-Mercaptohexadecyl)trimethylammonium Bromide. Bioconjugate Chem 2016;27:2558–74. 10.1021/acs.bioconjchem.6b00491. [DOI] [PubMed] [Google Scholar]
- 63.Kedmi R, Veiga N, Ramishetti S, Goldsmith M, Rosenblum D, Dammes N, et al. A modular platform for targeted RNAi therapeutics. Nature Nanotech 2018;13:214–9. 10.1038/s41565-017-0043-5. [DOI] [PubMed] [Google Scholar]
- 64.Rurik JG, Tombácz I, Yadegari A, Méndez Fernández PO, Shewale SV, Li L, et al. CAR T cells produced in vivo to treat cardiac injury. Science 2022;375:91–6. 10.1126/science.abm0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan DPY, Deleavey GF, Owen SC, Damha MJ, Shoichet MS. Click conjugated polymeric immuno-nanoparticles for targeted siRNA and antisense oligonucleotide delivery. Biomaterials 2013;34:8408–15. 10.1016/j.biomaterials.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 66.Kim W, Haller C, Dai E, Wang X, Hagemeyer CE, Liu DR, et al. Targeted antithrombotic protein micelles. Angew Chem Int Ed Engl 2015;54:1461–5. 10.1002/anie.201408529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y, Zhu X, Zhang X, Liu B, Huang L. Nanoparticles Modified With Tumor-targeting scFv Deliver siRNA and miRNA for Cancer Therapy. Molecular Therapy 2010;18:1650–6. 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sabrina Yang Y-S, Moynihan KD, Bekdemir A, Dichwalkar TM, Noh MM, Watson N, et al. Targeting small molecule drugs to T cells with antibody-directed cell-penetrating gold nanoparticles. Biomaterials Science 2019;7:113–24. 10.1039/C8BM01208C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van de Broek B, Devoogdt N, D’Hollander A, Gijs H-L, Jans K, Lagae L, et al. Specific Cell Targeting with Nanobody Conjugated Branched Gold Nanoparticles for Photothermal Therapy. ACS Nano 2011;5:4319–28. 10.1021/nn1023363. [DOI] [PubMed] [Google Scholar]
- 70.Komuro H, Aminova S, Lauro K, Woldring D, Harada M. Design and Evaluation of Engineered Extracellular Vesicle (EV)-Based Targeting for EGFR-Overexpressing Tumor Cells Using Monobody Display. Bioengineering 2022;9:56. 10.3390/bioengineering9020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deyev S, Proshkina G, Ryabova A, Tavanti F, Menziani MC, Eidelshtein G, et al. Synthesis, Characterization, and Selective Delivery of DARPin-Gold Nanoparticle Conjugates to Cancer Cells. Bioconjug Chem 2017;28:2569–74. 10.1021/acs.bioconjchem.7b00410. [DOI] [PubMed] [Google Scholar]
- 72.Winkler J, Martin-Killias P, Plückthun A, Zangemeister-Wittke U. EpCAM-targeted delivery of nanocomplexed siRNA to tumor cells with designed ankyrin repeat proteins. Molecular Cancer Therapeutics 2009;8:2674–83. 10.1158/1535-7163.MCT-09-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones SK, Lizzio V, Merkel OM. Folate Receptor Targeted Delivery of siRNA and Paclitaxel to Ovarian Cancer Cells via Folate Conjugated Triblock Copolymer to Overcome TLR4 Driven Chemotherapy Resistance. Biomacromolecules 2016;17:76–87. 10.1021/acs.biomac.5b01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tao W, Yurdagul A, Kong N, Li W, Wang X, Doran AC, et al. siRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Science Translational Medicine 2020;12:eaay1063. 10.1126/scitranslmed.aay1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther 2010;18:1357–64. 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proceedings of the National Academy of Sciences 2006;103:6315–20. 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Costa SA, Mozhdehi D, Dzuricky MJ, Isaacs FJ, Brustad EM, Chilkoti A. Active Targeting of Cancer Cells by Nanobody Decorated Polypeptide Micelle with Bio-orthogonally Conjugated Drug. Nano Lett 2019;19:247–54. 10.1021/acs.nanolett.8b03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pille J, van Lith SAM, van Hest JCM, Leenders WPJ. Self-Assembling VHH-Elastin-Like Peptides for Photodynamic Nanomedicine. Biomacromolecules 2017;18:1302–10. 10.1021/acs.biomac.7b00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mirshafiee V, Mahmoudi M, Lou K, Cheng J, Kraft ML. Protein corona significantly reduces active targeting yield. Chemical Communications 2013;49:2557–9. 10.1039/C3CC37307J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barui AK, Oh JY, Jana B, Kim C, Ryu J-H. Cancer-Targeted Nanomedicine: Overcoming the Barrier of the Protein Corona. Advanced Therapeutics 2020;3:1900124. 10.1002/adtp.201900124. [DOI] [Google Scholar]
- 81.Xiao W, Wang Y, Zhang H, Liu Y, Xie R, He X, et al. The protein corona hampers the transcytosis of transferrin-modified nanoparticles through blood–brain barrier and attenuates their targeting ability to brain tumor. Biomaterials 2021;274:120888. 10.1016/j.biomaterials.2021.120888. [DOI] [PubMed] [Google Scholar]
- 82.Su G, Jiang H, Xu B, Yu Y, Chen X. Effects of Protein Corona on Active and Passive Targeting of Cyclic RGD Peptide-Functionalized PEGylation Nanoparticles. Mol Pharmaceutics 2018;15:5019–30. 10.1021/acs.molpharmaceut.8b00612. [DOI] [PubMed] [Google Scholar]
- 83.Oh JY, Kim HS, Palanikumar L, Go EM, Jana B, Park SA, et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat Commun 2018;9:4548. 10.1038/s41467-018-06979-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stordy B, Zhang Y, Sepahi Z, Khatami MH, Kim PM, Chan WCW. Conjugating Ligands to an Equilibrated Nanoparticle Protein Corona Enables Cell Targeting in Serum. Chem Mater 2022;34:6868–82. 10.1021/acs.chemmater.2c01168. [DOI] [Google Scholar]
- 85.Huotari J, Helenius A. Endosome maturation. EMBO J 2011;30:3481–500. 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bishani A, Chernolovskaya EL. Activation of Innate Immunity by Therapeutic Nucleic Acids. Int J Mol Sci 2021;22:13360. 10.3390/ijms222413360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Veedu RN, Wengel J. Locked Nucleic Acids: Promising Nucleic Acid Analogs for Therapeutic Applications. Chemistry & Biodiversity 2010;7:536–42. 10.1002/cbdv.200900343. [DOI] [PubMed] [Google Scholar]
- 88.Kormann MSD, Hasenpusch G, Aneja MK, Nica G, Flemmer AW, Herber-Jonat S, et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat Biotechnol 2011;29:154–7. 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- 89.Sahni A, Qian Z, Pei D. Cell-Penetrating Peptides Escape the Endosome by Inducing Vesicle Budding and Collapse. ACS Chem Biol 2020;15:2485–92. 10.1021/acschembio.0c00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bus T, Traeger A, Schubert US. The great escape: how cationic polyplexes overcome the endosomal barrier. J Mater Chem B 2018;6:6904–18. 10.1039/C8TB00967H. [DOI] [PubMed] [Google Scholar]
- 91.Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL. The Possible “Proton Sponge ” Effect of Polyethylenimine (PEI) Does Not Include Change in Lysosomal pH. Molecular Therapy 2013;21:149–57. 10.1038/mt.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Varanko AK, Su JC, Chilkoti A. Elastin-Like Polypeptides for Biomedical Applications. Annu Rev Biomed Eng 2020;22:343–69. 10.1146/annurev-bioeng-092419-061127. [DOI] [PubMed] [Google Scholar]
- 93.Reichheld SE, Muiznieks LD, Lu R, Sharpe S, Keeley FW. Sequence variants of human tropoelastin affecting assembly, structural characteristics and functional properties of polymeric elastin in health and disease. Matrix Biol 2019;84:68–80. 10.1016/j.matbio.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 94.Ozsvar J, Yang C, Cain SA, Baldock C, Tarakanova A, Weiss AS. Tropoelastin and Elastin Assembly. Frontiers in Bioengineering and Biotechnology 2021;9:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li NK, García Quiroz F, Hall CK, Chilkoti A, Yingling YG. Molecular description of the LCST behavior of an elastin-like polypeptide. Biomacromolecules 2014;15:3522–30. 10.1021/bm500658w. [DOI] [PubMed] [Google Scholar]
- 96.Urry DW, Luan CH, Parker TM, Gowda DC, Prasad KU, Reid MC, et al. Temperature of polypeptide inverse temperature transition depends on mean residue hydrophobicity. J Am Chem Soc 1991;113:4346–8. 10.1021/ja00011a057. [DOI] [Google Scholar]
- 97.Dreher MR, Simnick AJ, Fischer K, Smith RJ, Patel A, Schmidt M, et al. Temperature Triggered Self-Assembly of Polypeptides into Multivalent Spherical Micelles. J Am Chem Soc 2008;130:687–94. 10.1021/ja0764862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.MacKay JA, Callahan DJ, FitzGerald KN, Chilkoti A. Quantitative Model of the Phase Behavior of Recombinant pH-Responsive Elastin-Like Polypeptides. Biomacromolecules 2010;11:2873–9. 10.1021/bm100571j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cho Y, Zhang Y, Christensen T, Sagle LB, Chilkoti A, Cremer PS. Effects of Hofmeister Anions on the Phase Transition Temperature of Elastin-like Polypeptides. J Phys Chem B 2008;112:13765–71. 10.1021/jp8062977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat Biotechnol 1999;17:1112–5. 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- 101.Christensen T, Trabbic-Carlson K, Liu W, Chilkoti A. Purification of recombinant proteins from Escherichia coli at low expression levels by inverse transition cycling. Anal Biochem 2007;360:166–8. 10.1016/j.ab.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim W, Xiao J, Chaikof EL. Recombinant Amphiphilic Protein Micelles for Drug Delivery. Langmuir 2011;27:14329–34. 10.1021/la202864x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen T-HH, Bae Y, Furgeson DY. Intelligent Biosynthetic Nanobiomaterials (IBNs) for Hyperthermic Gene Delivery. Pharm Res 2008;25:683–91. 10.1007/s11095-007-9382-5. [DOI] [PubMed] [Google Scholar]
- 104.Yi A, Sim D, Lee Y-J, Sarangthem V, Park R-W. Development of elastin-like polypeptide for targeted specific gene delivery in vivo. Journal of Nanobiotechnology 2020;18:15. 10.1186/s12951-020-0574-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang S, Sun Y, Zhang L, Zhang F, Gao W. Thermoresponsive Polypeptide Fused L-Asparaginase with Mitigated Immunogenicity and Enhanced Efficacy in Treating Hematologic Malignancies. Advanced Science 2023;10:2300469. 10.1002/advs.202300469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jenkins IC, Milligan JJ, Chilkoti A. Genetically Encoded Elastin-Like Polypeptides for Drug Delivery. Advanced Healthcare Materials 2021;10:2100209. 10.1002/adhm.202100209. [DOI] [PubMed] [Google Scholar]
- 107.Milligan JJ, Saha S, Jenkins IC, Chilkoti A. Genetically encoded elastin-like polypeptide nanoparticles for drug delivery. Curr Opin Biotechnol 2022;74:146–53. 10.1016/j.copbio.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 108.Piña MJ, Girotti A, Santos M, Rodríguez-Cabello JC, Arias FJ. Biocompatible ELR-Based Polyplexes Coated with MUC1 Specific Aptamers and Targeted for Breast Cancer Gene Therapy. Mol Pharmaceutics 2016;13:795–808. 10.1021/acs.molpharmaceut.5b00712. [DOI] [PubMed] [Google Scholar]
- 109.Piña MJ, Girotti A, Serrano S, Muñoz R, Rodríguez-Cabello JC, Arias FJ. A double safety lock tumor-specific device for suicide gene therapy in breast cancer. Cancer Letters 2020;470:43–53. 10.1016/j.canlet.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 110.Lee chang hyun, Ingrole R, Gill H. Generation of induced pluripotent stem cells using elastin like polypeptides as a non-viral gene delivery system. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2019;1866:. 10.1016/j.bbadis.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 111.Kelly G, Milligan JJ, Mastria EM, Kim S, Zelenetz SR, Dobbins J, et al. Intratumoral delivery of brachytherapy and immunotherapy by a thermally triggered polypeptide depot. Journal of Controlled Release 2022;343:267–76. 10.1016/j.jconrel.2022.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. Journal of Biological Chemistry 1994;269:10444–50. 10.1016/S0021-9258(17)34080-2. [DOI] [PubMed] [Google Scholar]
- 113.Piña MJ, Alex SM, Arias FJ, Santos M, Rodriguez-Cabello JC, Ramesan RM, et al. Elastin-like recombinamers with acquired functionalities for gene-delivery applications. Journal of Biomedical Materials Research Part A 2015;103:3166–78. 10.1002/jbm.a.35455. [DOI] [PubMed] [Google Scholar]
- 114.Ohmori N, Niidome T, Wada A, Hirayama T, Hatakeyama T, Aoyagi H. The enhancing effect of anionic alpha-helical peptide on cationic peptide-mediating transfection systems. Biochem Biophys Res Commun 1997;235:726–9. 10.1006/bbrc.1997.6880. [DOI] [PubMed] [Google Scholar]
- 115.Yi A, Sim D, Lee S-B, Sarangthem V, Park R-W. Application of bioengineered elastin-like polypeptide-based system for targeted gene delivery in tumor cells. Biomaterials and Biosystems 2022;6:100050. 10.1016/j.bbiosy.2022.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen T-HH, Bae Y, Furgeson DY, Kwon GS. Biodegradable hybrid recombinant block copolymers for non-viral gene transfection. Int J Pharm 2012;427:105–12. 10.1016/j.ijpharm.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 117.Lee CH, Ingrole RSJ, Gill HS. Generation of induced pluripotent stem cells using elastin like polypeptides as a non-viral gene delivery system. Biochim Biophys Acta Mol Basis Dis 2020;1866:165405. 10.1016/j.bbadis.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 118.Numata K, Subramanian B, Currie HA, Kaplan DL. Bioengineered silk protein-based gene delivery systems. Biomaterials 2009;30:5775–84. 10.1016/j.biomaterials.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Numata K, Hamasaki J, Subramanian B, Kaplan DL. Gene delivery mediated by recombinant silk proteins containing cationic and cell binding motifs. Journal of Controlled Release 2010;146:136–43. 10.1016/j.jconrel.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Numata K, Kaplan DL. Silk-Based Gene Carriers with Cell Membrane Destabilizing Peptides. Biomacromolecules 2010;11:3189–95. 10.1021/bm101055m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Numata K, Reagan MR, Goldstein RH, Rosenblatt M, Kaplan DL. Spider Silk-Based Gene Carriers for Tumor Cell-Specific Delivery. Bioconjugate Chem 2011;22:1605–10. 10.1021/bc200170u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Numata K, Mieszawska-Czajkowska AJ, Kvenvold LA, Kaplan DL. Silk-Based Nanocomplexes with Tumor-Homing Peptides for Tumor-Specific Gene Delivery. Macromolecular Bioscience 2012;12:75–82. 10.1002/mabi.201100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bravo-Anaya LM, Garbay B, Nando-Rodríguez JLE, Carvajal Ramos F, Ibarboure E, Bathany K, et al. Nucleic acids complexation with cationic elastin-like polypeptides: Stoichiometry and stability of nano-assemblies. Journal of Colloid and Interface Science 2019;557:777–92. 10.1016/j.jcis.2019.09.054. [DOI] [PubMed] [Google Scholar]
- 124.Mónica Bravo-Anaya L, Rosselgong J, Gricelda Fernández-Solís K, Xiao Y, Vax A, Ibarboure E, et al. Coupling of RAFT polymerization and chemoselective post-modifications of elastin-like polypeptides for the synthesis of gene delivery hybrid vectors. Polymer Chemistry 2021;12:226–41. 10.1039/D0PY01293A. [DOI] [Google Scholar]
- 125.Dash BC, Mahor S, Carroll O, Mathew A, Wang W, Woodhouse KA, et al. Tunable elastin-like polypeptide hollow sphere as a high payload and controlled delivery gene depot. Journal of Controlled Release 2011;152:382–92. 10.1016/j.jconrel.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 126.Dash BC, Thomas D, Monaghan M, Carroll O, Chen X, Woodhouse K, et al. An injectable elastin-based gene delivery platform for dose-dependent modulation of angiogenesis and inflammation for critical limb ischemia. Biomaterials 2015;65:126–39. 10.1016/j.biomaterials.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 127.Hofmann S, Wong Po Foo CT, Rossetti F, Textor M, Vunjak-Novakovic G, Kaplan DL, et al. Silk fibroin as an organic polymer for controlled drug delivery. Journal of Controlled Release 2006;111:219–27. 10.1016/j.jconrel.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 128.Mottaghitalab F, Farokhi M, Shokrgozar MA, Atyabi F, Hosseinkhani H. Silk fibroin nanoparticle as a novel drug delivery system. Journal of Controlled Release 2015;206:161–76. 10.1016/j.jconrel.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 129.Xiao L, Lu G, Lu Q, Kaplan DL. Direct Formation of Silk Nanoparticles for Drug Delivery. ACS Biomater Sci Eng 2016;2:2050–7. 10.1021/acsbiomaterials.6b00457. [DOI] [PubMed] [Google Scholar]
- 130.Nguyen TP, Nguyen QV, Nguyen V-H, Le T-H, Huynh VQN, Vo D-VN, et al. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers (Basel) 2019;11:1933. 10.3390/polym11121933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chambre L, Martín-Moldes Z, Parker RN, Kaplan DL. Bioengineered elastin- and silk-biomaterials for drug and gene delivery. Adv Drug Deliv Rev 2020;160:186–98. 10.1016/j.addr.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pereira RFP, Silva MM, de Zea Bermudez V. Bombyx mori Silk Fibers: An Outstanding Family of Materials. Macromolecular Materials and Engineering 2015;300:1171–98. 10.1002/mame.201400276. [DOI] [Google Scholar]
- 133.Qi Y, Wang H, Wei K, Yang Y, Zheng R-Y, Kim IS, et al. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int J Mol Sci 2017;18:237. 10.3390/ijms18030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koh L-D, Cheng Y, Teng C-P, Khin Y-W, Loh X-J, Tee S-Y, et al. Structures, mechanical properties and applications of silk fibroin materials. Progress in Polymer Science 2015;46:86–110. 10.1016/j.progpolymsci.2015.02.001. [DOI] [Google Scholar]
- 135.Nguyen AT, Huang Q-L, Yang Z, Lin N, Xu G, Liu XY. Crystal Networks in Silk Fibrous Materials: From Hierarchical Structure to Ultra Performance. Small 2015;11:1039–54. 10.1002/smll.201402985. [DOI] [PubMed] [Google Scholar]
- 136.Lammel A, Hu X, Park S-H, Kaplan DL, Scheibel T. Controlling silk fibroin particle features for drug delivery. Biomaterials 2010;31:4583–91. 10.1016/j.biomaterials.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yin Z, Wu F, Zheng Z, Kaplan DL, Kundu SC, Lu S. Self-Assembling Silk-Based Nanofibers with Hierarchical Structures. ACS Biomater Sci Eng 2017;3:2617–27. 10.1021/acsbiomaterials.7b00442. [DOI] [PubMed] [Google Scholar]
- 138.Liu Y, Ling S, Wang S, Chen X, Shao Z. Thixotropic silk nanofibril-based hydrogel with extracellular matrix-like structure. Biomater Sci 2014;2:1338–42. 10.1039/C4BM00214H. [DOI] [PubMed] [Google Scholar]
- 139.Bhardwaj N, Rajkhowa R, Wang X, Devi D. Milled non-mulberry silk fibroin microparticles as biomaterial for biomedical applications. Int J Biol Macromol 2015;81:31–40. 10.1016/j.ijbiomac.2015.07.049. [DOI] [PubMed] [Google Scholar]
- 140.Ling S, Li C, Jin K, Kaplan DL, Buehler MJ. Liquid Exfoliated Natural Silk Nanofibrils: Applications in Optical and Electrical Devices. Advanced Materials 2016;28:7783–90. 10.1002/adma.201601783. [DOI] [PubMed] [Google Scholar]
- 141.Jin H-J, Kaplan DL. Mechanism of silk processing in insects and spiders. Nature 2003;424:1057–61. 10.1038/nature01809. [DOI] [PubMed] [Google Scholar]
- 142.Norouzi P, Motasadizadeh H, Atyabi F, Dinarvand R, Gholami M, Farokhi M, et al. Combination Therapy of Breast Cancer by Codelivery of Doxorubicin and Survivin siRNA Using Polyethylenimine Modified Silk Fibroin Nanoparticles. ACS Biomater Sci Eng 2021;7:1074–87. 10.1021/acsbiomaterials.0c01511. [DOI] [PubMed] [Google Scholar]
- 143.Thurber AE, Omenetto FG, Kaplan DL. In Vivo Bioresponses to Silk Proteins. Biomaterials 2015;71:145–57. 10.1016/j.biomaterials.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dewair M, Baur X, Ziegler K. Use of immunoblot technique for detection of human IgE and IgG antibodies to individual silk proteins. Journal of Allergy and Clinical Immunology 1985;76:537–42. 10.1016/0091-6749(85)90772-9. [DOI] [PubMed] [Google Scholar]
- 145.Zeplin PH, Maksimovikj NC, Jordan MC, Nickel J, Lang G, Leimer AH, et al. Spider Silk Coatings as a Bioshield to Reduce Periprosthetic Fibrous Capsule Formation. Advanced Functional Materials 2014;24:2658–66. 10.1002/adfm.201302813. [DOI] [Google Scholar]
- 146.Li L, Puhl S, Meinel L, Germershaus O. Silk fibroin layer-by-layer microcapsules for localized gene delivery. Biomaterials 2014;35:7929–39. 10.1016/j.biomaterials.2014.05.062. [DOI] [PubMed] [Google Scholar]
- 147.Niu L, Chen G, Feng Y, Liu X, Pan P, Huang L, et al. Polyethylenimine-Modified Bombyx mori Silk Fibroin as a Delivery Carrier of the ING4-IL-24 Coexpression Plasmid. Polymers 2021;13:3592. 10.3390/polym13203592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shahbazi B, Taghipour M, Rahmani H, Sadrjavadi K, Fattahi A. Preparation and characterization of silk fibroin/oligochitosan nanoparticles for siRNA delivery. Colloids and Surfaces B: Biointerfaces 2015;136:867–77. 10.1016/j.colsurfb.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 149.Sezutsu H, Yukuhiro K. Dynamic Rearrangement Within the Antheraea pernyi Silk Fibroin Gene Is Associated with Four Types of Repetitive Units. J Mol Evol 2000;51:329–38. 10.1007/s002390010095. [DOI] [PubMed] [Google Scholar]
- 150.Kang Z, Wang Y, Xu J, Song G, Ding M, Zhao H, et al. An RGD-Containing Peptide Derived from Wild Silkworm Silk Fibroin Promotes Cell Adhesion and Spreading. Polymers 2018;10:1193. 10.3390/polym10111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bellis SL. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011;32:4205–10. 10.1016/j.biomaterials.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Silva SS, Kundu B, Lu S, Reis RL, Kundu SC. Chinese Oak Tasar Silkworm Antheraea pernyi Silk Proteins: Current Strategies and Future Perspectives for Biomedical Applications. Macromolecular Bioscience 2019;19:1800252. 10.1002/mabi.201800252. [DOI] [PubMed] [Google Scholar]
- 153.Sponner A, Schlott B, Vollrath F, Unger E, Grosse F, Weisshart K. Characterization of the Protein Components of Nephila clavipes Dragline Silk. Biochemistry 2005;44:4727–36. 10.1021/bi047671k. [DOI] [PubMed] [Google Scholar]
- 154.Liu Y, You R, Liu G, Li X, Sheng W, Yang J, et al. Antheraea pernyi Silk Fibroin-Coated PEI/DNA Complexes for Targeted Gene Delivery in HEK 293 and HCT 116 Cells. International Journal of Molecular Sciences 2014;15:7049–63. 10.3390/ijms15057049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ma C, Lv L, Liu Y, Yu Y, You R, Yang J, et al. $\less$i$\greater$Antheraea pernyi$\less$/i$\greater$ silk fibroin for targeted gene delivery of VEGF165-Ang-1 with PEI. Biomed Mater 2014;9:035015. 10.1088/1748-6041/9/3/035015. [DOI] [PubMed] [Google Scholar]
- 156.Yigit S, Tokareva O, Varone A, Georgakoudi I, Kaplan DL. Bioengineered Silk Gene Delivery System for Nuclear Targeting. Macromolecular Bioscience 2014;14:1291–8. 10.1002/mabi.201400113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xia X-X, Xu Q, Hu X, Qin G, Kaplan DL. Tunable Self-Assembly of Genetically Engineered Silk–Elastin-like Protein Polymers. Biomacromolecules 2011;12:3844–50. 10.1021/bm201165h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huang W, Rollett A, Kaplan DL. Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics. Expert Opin Drug Deliv 2015;12:779–91. 10.1517/17425247.2015.989830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Greish K, Araki K, Li D, O’Malley BW, Dandu R, Frandsen J, et al. Silk-Elastinlike Protein Polymer Hydrogels for Localized Adenoviral Gene Therapy of Head and Neck Tumors. Biomacromolecules 2009;10:2183–8. 10.1021/bm900356j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Price R, Gustafson J, Greish K, Cappello J, McGill L, Ghandehari H. Comparison of silk-elastinlike protein polymer hydrogel and poloxamer in matrix-mediated gene delivery. International Journal of Pharmaceutics 2012;427:97–104. 10.1016/j.ijpharm.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jung S-H, Choi J-W, Yun C-O, Yhee JY, Price R, Kim SH, et al. Sustained local delivery of oncolytic short hairpin RNA adenoviruses for treatment of head and neck cancer. The Journal of Gene Medicine 2014;16:143–52. 10.1002/jgm.2770. [DOI] [PubMed] [Google Scholar]
- 162.Price R, Poursaid A, Cappello J, Ghandehari H. In vivo evaluation of matrix metalloproteinase responsive silk–elastinlike protein polymers for cancer gene therapy. Journal of Controlled Release 2015;213:96–102. 10.1016/j.jconrel.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Numata K, Kaplan DL. Silk-Based Gene Carriers with Cell Membrane Destabilizing Peptides. Biomacromolecules 2010;11:3189–95. 10.1021/bm101055m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Paul S, Benza RL, Chakinala M, Correa P, Ghosh K, Lee J, et al. Safety, Tolerability, and Changes in Six-Minute Walk Test After Open-Label Subcutaneous PB1046, A Sustained-Release Analogue for Vasoactive Intestinal Peptide (VIP), in Pulmonary Arterial Hypertension (PAH) 2021. [Google Scholar]
- 165.Elzoghby AO. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. Journal of Controlled Release 2013;172:1075–91. 10.1016/j.jconrel.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 166.Hosseinkhani H, Abedini F, Ou K-L, Domb AJ. Polymers in gene therapy technology. Polymers for Advanced Technologies 2015;26:198–211. 10.1002/pat.3432. [DOI] [Google Scholar]
- 167.Madkhali O, Mekhail G, Wettig SD. Modified gelatin nanoparticles for gene delivery. International Journal of Pharmaceutics 2019;554:224–34. 10.1016/j.ijpharm.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 168.Hassani Besheli N, Martens M, Macías-Sánchez E, Olijve J, Yang F, Sommerdijk N, et al. Unraveling the Formation of Gelatin Nanospheres by Means of Desolvation. Nano Lett 2023. 10.1021/acs.nanolett.3c03459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee EJ, Khan SA, Park JK, Lim K-H. Studies on the characteristics of drug-loaded gelatin nanoparticles prepared by nanoprecipitation. Bioprocess Biosyst Eng 2012;35:297–307. 10.1007/s00449-011-0591-2. [DOI] [PubMed] [Google Scholar]
- 170.Lu Z, Yeh T-K, Tsai M, Au JL-S, Wientjes MG. Paclitaxel-loaded gelatin nanoparticles for intravesical bladder cancer therapy. Clin Cancer Res 2004;10:7677–84. 10.1158/1078-0432.CCR-04-1443. [DOI] [PubMed] [Google Scholar]
- 171.Bhavsar MD, Amiji MM. Oral IL-10 gene delivery in a microsphere-based formulation for local transfection and therapeutic efficacy in inflammatory bowel disease. Gene Ther 2008;15:1200–9. 10.1038/gt.2008.67. [DOI] [PubMed] [Google Scholar]
- 172.Kriegel C, Amiji M. Oral TNF-α Gene Silencing using a Polymeric Microsphere-Based Delivery System for the Treatment of Inflammatory Bowel Disease. J Control Release 2011;150:77–86. 10.1016/j.jconrel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fan Y, Dhaliwal HK, Menon AV, Chang J, Choi JE, Amiji MM, et al. Site-specific intestinal DMT1 silencing to mitigate iron absorption using pH-sensitive multi-compartmental nanoparticulate oral delivery system. Nanomedicine: Nanotechnology, Biology and Medicine 2019;22:102091. 10.1016/j.nano.2019.102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xu X, Capito RM, Spector M. Delivery of plasmid IGF-1 to chondrocytes via cationized gelatin nanoparticles. Journal of Biomedical Materials Research Part A 2008;84A:73–83. 10.1002/jbm.a.31372. [DOI] [PubMed] [Google Scholar]
- 175.Mimi H, Ho KM, Siu YS, Wu A, Li P. Polyethyleneimine-Based Core-Shell Nanogels: A Promising siRNA Carrier for Argininosuccinate Synthetase mRNA Knockdown in HeLa Cells. Journal of Controlled Release 2012;158:123–30. 10.1016/j.jconrel.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 176.Konat Zorzi G, Contreras-Ruiz L, Párraga JE, López-García A, Romero Bello R, Diebold Y, et al. Expression of MUC5AC in Ocular Surface Epithelial Cells Using Cationized Gelatin Nanoparticles. Mol Pharmaceutics 2011;8:1783–8. 10.1021/mp200155t. [DOI] [PubMed] [Google Scholar]
- 177.Zhou M, Dong J, Huang J, Ye W, Zheng Z, Huang K, et al. Chitosan-Gelatin-EGCG Nanoparticle-Meditated LncRNA TMEM44-AS1 Silencing to Activate the P53 Signaling Pathway for the Synergistic Reversal of 5-FU Resistance in Gastric Cancer. Advanced Science 2022;9:2105077. 10.1002/advs.202105077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu X, Liu W, Chen Q, Liu J, Yang C, Zhang G, et al. miR-506-loaded gelatin nanospheres target PENK and inactivate the ERK/Fos signaling pathway to suppress triple-negative breast cancer aggressiveness. Molecular Carcinogenesis 2021;60:538–55. 10.1002/mc.23310. [DOI] [PubMed] [Google Scholar]
- 179.Liu J, Yang C-Q, Chen Q, Yu T-Y, Zhang S-L, Guo W-H, et al. MiR-4458-loaded gelatin nanospheres target COL11A1 for DDR2/SRC signaling pathway inactivation to suppress the progression of estrogen receptor-positive breast cancer. Biomater Sci 2022;10:4596–611. 10.1039/D2BM00543C. [DOI] [PubMed] [Google Scholar]
- 180.Lee SJ, Yhee JY, Kim SH, Kwon IC, Kim K. Biocompatible gelatin nanoparticles for tumor-targeted delivery of polymerized siRNA in tumor-bearing mice. Journal of Controlled Release 2013;172:358–66. 10.1016/j.jconrel.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 181.Xu J, Singh A, Amiji MM. Redox-responsive targeted gelatin nanoparticles for delivery of combination wt-p53 expressing plasmid DNA and gemcitabine in the treatment of pancreatic cancer. BMC Cancer 2014;14:75. 10.1186/1471-2407-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Srikar R, Suresh D, Zambre A, Taylor K, Chapman S, Leevy M, et al. Targeted nanoconjugate co-delivering siRNA and tyrosine kinase inhibitor to KRAS mutant NSCLC dissociates GAB1-SHP2 post oncogene knockdown. Sci Rep 2016;6:30245. 10.1038/srep30245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gwon Y, Kim W, Park S, Hong S, Kim J. A Freezing and Thawing Method for Fabrication of Small Gelatin Nanoparticles with Stable Size Distributions for Biomedical Applications. Tissue Eng Regen Med 2021;19:301–7. 10.1007/s13770-021-00380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Won YW, Yoon SM, Sonn CH, Lee KM, Kim YH. Nano self-assembly of recombinant human gelatin conjugated with α-tocopheryl succinate for Hsp90 inhibitor, 17-AAG, delivery. ACS Nano 2011;5:3839–48. 10.1021/nn200173u. [DOI] [PubMed] [Google Scholar]
- 185.Chen Y-C, Yu S-H, Tsai G-J, Tang D-W, Mi F-L, Peng Y-P. Novel Technology for the Preparation of Self-Assembled Catechin/Gelatin Nanoparticles and Their Characterization. J Agric Food Chem 2010;58:6728–34. 10.1021/jf1005116. [DOI] [PubMed] [Google Scholar]
- 186.Li Z, Gu L. Effects of Mass Ratio, pH, Temperature, and Reaction Time on Fabrication of Partially Purified Pomegranate Ellagitannin−Gelatin Nanoparticles. J Agric Food Chem 2011;59:4225–31. 10.1021/jf200024d. [DOI] [PubMed] [Google Scholar]
- 187.Geh KJ, Hubert M, Winter G. Optimisation of one-step desolvation and scale-up of gelatine nanoparticle production. Journal of Microencapsulation 2016;33:595–604. 10.1080/02652048.2016.1228706. [DOI] [PubMed] [Google Scholar]
- 188.Zorzi GK, Párraga JE, Seijo B, Sánchez A. Hybrid Nanoparticle Design Based on Cationized Gelatin and the Polyanions Dextran Sulfate and Chondroitin Sulfate for Ocular Gene Therapy. Macromolecular Bioscience 2011;11:905–13. 10.1002/mabi.201100005. [DOI] [PubMed] [Google Scholar]
- 189.KAUL G, AMIJI M. TUMOR-TARGETED GENE DELIVERY USING POLY(ETHYLENE GLYCOL)-MODIFIED GELATIN NANOPARTICLES: IN VITRO AND IN VIVO STUDIES. Pharm Res 2005;22:951–61. 10.1007/s11095-005-4590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhao X, Wang J, Tao S, Ye T, Kong X, Ren L. In Vivo Bio-distribution and Efficient Tumor Targeting of Gelatin/Silica Nanoparticles for Gene Delivery. Nanoscale Res Lett 2016;11:195. 10.1186/s11671-016-1409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tian X-H, Wang Z-G, Meng H, Wang Y-H, Feng W, Wei F, et al. Tat peptide-decorated gelatin-siloxane nanoparticles for delivery of CGRP transgene in treatment of cerebral vasospasm. Int J Nanomedicine 2013;8:865–76. 10.2147/IJN.S39951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zorzi GK, Párraga JE, Seijo B, Sanchez A. Comparison of different cationized proteins as biomaterials for nanoparticle-based ocular gene delivery. Colloids Surf B Biointerfaces 2015;135:533–41. 10.1016/j.colsurfb.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 193.Suresh D, Jenkins B, Zambre A, Upendran A, Kannan R. Systematic Evaluation of Protein-Based Nanoparticles for Stable Delivery of Small Interfering RNA. Journal of Biomedical Nanotechnology 2020;16:1169–81. 10.1166/jbn.2020.2953. [DOI] [PubMed] [Google Scholar]
- 194.Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. Journal of Controlled Release 2012;157:168–82. 10.1016/j.jconrel.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 195.Spada A, Emami J, Tuszynski JA, Lavasanifar A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol Pharmaceutics 2021;18:1862–94. 10.1021/acs.molpharmaceut.1c00046. [DOI] [PubMed] [Google Scholar]
- 196.Zhang J, Lei Y, Dhaliwal A, Ng QK, Du J, Yan M, et al. Protein−Polymer Nanoparticles for Nonviral Gene Delivery. Biomacromolecules 2011;12:1006–14. 10.1021/bm101354a. [DOI] [PubMed] [Google Scholar]
- 197.Han J, Wang Q, Zhang Z, Gong T, Sun X. Cationic Bovine Serum Albumin Based Self-Assembled Nanoparticles as siRNA Delivery Vector for Treating Lung Metastatic Cancer. Small 2014;10:524–35. 10.1002/smll.201301992. [DOI] [PubMed] [Google Scholar]
- 198.Chen J-L, Peng S-W, Ko W-H, Wu C-J, Li T-W, Yeh M-K, et al. An efficient and low toxic human serum albumin conjugated polyethylenimine nano-sized complex for gene delivery. J Nanopart Res 2014;16:2593. 10.1007/s11051-014-2593-x. [DOI] [Google Scholar]
- 199.Langiu M, Dadparvar M, Kreuter J, Ruonala MO. Human serum albumin-based nanoparticle-mediated in vitro gene delivery. PLoS One 2014;9:e107603. 10.1371/journal.pone.0107603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nicolì E, Syga MI, Bosetti M, Shastri VP. Enhanced Gene Silencing through Human Serum Albumin-Mediated Delivery of Polyethylenimine-siRNA Polyplexes. PLOS ONE 2015;10:e0122581. 10.1371/journal.pone.0122581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rahimi H, Zaboli KA, Thekkiniath J, Mousavi SH, Johari B, Hashemi MR, et al. BSA-PEI Nanoparticle Mediated Efficient Delivery of CRISPR/Cas9 into MDA-MB-231 Cells. Mol Biotechnol 2022. 10.1007/s12033-022-00514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mehta A, Dalle Vedove E, Isert L, Merkel OM. Targeting KRAS Mutant Lung Cancer Cells with siRNA-Loaded Bovine Serum Albumin Nanoparticles. Pharm Res 2019;36:133. 10.1007/s11095-019-2665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kwak G, Kim H, Park J, Kim EH, Jang H, Han G, et al. A Trojan-Horse Strategy by In Situ Piggybacking onto Endogenous Albumin for Tumor-Specific Neutralization of Oncogenic MicroRNA. ACS Nano 2021;15:11369–84. 10.1021/acsnano.1c00799. [DOI] [PubMed] [Google Scholar]
- 204.Wang H, Liu N, Yang F, Hu N, Wang M, Cui M, et al. Bioengineered Protein Nanocage by Small Heat Shock Proteins Delivering mTERT siRNA for Enhanced Colorectal Cancer Suppression. ACS Appl Bio Mater 2022;5:1330–40. 10.1021/acsabm.1c01221. [DOI] [PubMed] [Google Scholar]
- 205.Li L, Muñoz-Culla M, Carmona U, Lopez MP, Yang F, Trigueros C, et al. Ferritin-mediated siRNA delivery and gene silencing in human tumor and primary cells. Biomaterials 2016;98:143–51. 10.1016/j.biomaterials.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 206.Pediconi N, Ghirga F, Del Plato C, Peruzzi G, Athanassopoulos CM, Mori M, et al. Design and Synthesis of Piperazine-Based Compounds Conjugated to Humanized Ferritin as Delivery System of siRNA in Cancer Cells. Bioconjugate Chem 2021;32:1105–16. 10.1021/acs.bioconjchem.1c00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Palombarini F, Masciarelli S, Incocciati A, Liccardo F, Di Fabio E, Iazzetti A, et al. Self-assembling ferritin-dendrimer nanoparticles for targeted delivery of nucleic acids to myeloid leukemia cells. Journal of Nanobiotechnology 2021;19:172. 10.1186/s12951-021-00921-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Huang H, Yuan S, Ma Z, Ji P, Ma X, Wu Z, et al. Genetic recombination of poly(L-lysine) functionalized apoferritin nanocages that resemble viral capsid nanometer-sized platforms for gene therapy. Biomater Sci 2020;8:1759–70. 10.1039/C9BM01822K. [DOI] [PubMed] [Google Scholar]
- 209.Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int J Nanomedicine 2009;4:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mehta A, Dalle Vedove E, Isert L, Merkel OM. Targeting KRAS Mutant Lung Cancer Cells with siRNA-Loaded Bovine Serum Albumin Nanoparticles. Pharm Res 2019;36:133. 10.1007/s11095-019-2665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dinesen A, Winther A, Wall A, Märcher A, Palmfeldt J, Chudasama V, et al. Albumin Biomolecular Drug Designs Stabilized through Improved Thiol Conjugation and a Modular Locked Nucleic Acid Functionalized Assembly. Bioconjugate Chem 2022;33:333–42. 10.1021/acs.bioconjchem.1c00561. [DOI] [PubMed] [Google Scholar]
- 212.Bhaskar S, Lim S. Engineering protein nanocages as carriers for biomedical applications. NPG Asia Mater 2017;9:e371–e371. 10.1038/am.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Guan X, Chang Y, Sun J, Song J, Xie Y. Engineered Hsp Protein Nanocages for siRNA Delivery. Macromol Biosci 2018;18:1800013. 10.1002/mabi.201800013. [DOI] [PubMed] [Google Scholar]
- 214.Zhang J, Lei Y, Dhaliwal A, Ng QK, Du J, Yan M, et al. Protein−Polymer Nanoparticles for Nonviral Gene Delivery. Biomacromolecules 2011;12:1006–14. 10.1021/bm101354a. [DOI] [PubMed] [Google Scholar]
- 215.He J, Yu L, Lin X, Liu X, Zhang Y, Yang F, et al. Virus-like Particles as Nanocarriers for Intracellular Delivery of Biomolecules and Compounds. Viruses 2022;14:1905. 10.3390/v14091905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhang C, Zhang X, Zhao G. Ferritin Nanocage: A Versatile Nanocarrier Utilized in the Field of Food, Nutrition, and Medicine. Nanomaterials (Basel) 2020;10:1894. 10.3390/nano10091894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Truffi M, Fiandra L, Sorrentino L, Monieri M, Corsi F, Mazzucchelli S. Ferritin nanocages: A biological platform for drug delivery, imaging and theranostics in cancer. Pharmacological Research 2016;107:57–65. 10.1016/j.phrs.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 218.Yuan Z, Wang B, Teng Y, Ho W, Hu B, Boakye-Yiadom KO, et al. Rational design of engineered H-ferritin nanoparticles with improved siRNA delivery efficacy across an in vitro model of the mouse BBB. Nanoscale 2022;14:6449–64. 10.1039/D1NR07880A. [DOI] [PubMed] [Google Scholar]
- 219.Dubrez L, Causse S, Borges Bonan N, Dumétier B, Garrido C. Heat-shock proteins: chaperoning DNA repair. Oncogene 2020;39:516–29. 10.1038/s41388-019-1016-y. [DOI] [PubMed] [Google Scholar]
- 220.Bravo-Anaya LM, Garbay B, Nando-Rodríguez JLE, Carvajal Ramos F, Ibarboure E, Bathany K, et al. Nucleic acids complexation with cationic elastin-like polypeptides: Stoichiometry and stability of nano-assemblies. Journal of Colloid and Interface Science 2019;557:777–92. 10.1016/j.jcis.2019.09.054. [DOI] [PubMed] [Google Scholar]
- 221.PhaseBio Pharmaceuticals Inc. Randomized, Double-blind, Placebo-controlled, Multiple-Dose, Study to Assess the Safety, Tolerability, PK and PD After 4 Weeks of Once Weekly Sc. Inj. of PB1046 in Adults With Stable HFrEF, and in Subjects With Cardiac Dysfunction Secondary to DMD. clinicaltrials.gov; 2022.
- 222.Rosenstock J, Del Prato S. Basal weekly insulins: the way of the future! Metabolism 2022;126:154924. 10.1016/j.metabol.2021.154924. [DOI] [PubMed] [Google Scholar]
- 223.PhaseBio Pharmaceuticals Inc. Phase 1/2a, Randomized, Double-Blind Placebo-Controlled, Study to Assess the Safety, Tolerability, Pharmacokinetics and Pharmacodynamic Response of PB1023 Injection Following Single and Multiple Ascending Subcutaneous Doses in Adult Subjects With Type 2 Diabetes Mellitus (T2DM). clinicaltrials.gov; 2013.
- 224.PhaseBio Pharmaceuticals Inc. Phase 2b Multicenter, Randomized, Double-Blind, Placebo- and Active-Controlled, Parallel-Group Study to Assess the Pharmacodynamic Response and Safety of Three Dose Levels of (PB1023) Injection Following 20 Weeks of Once-Weekly Subcutaneous Dosing in Adult Subjects With Inadequately Treated Type 2 Diabetes Mellitus. clinicaltrials.gov; 2015.
- 225.Toyama T, Nitta N, Ohta S, Tanaka T, Nagatani Y, Takahashi M, et al. Clinical trial of cisplatin-conjugated gelatin microspheres for patients with hepatocellular carcinoma. Jpn J Radiol 2012;30:62–8. 10.1007/s11604-011-0010-2. [DOI] [PubMed] [Google Scholar]
- 226.Kumagai M, Marui A, Tabata Y, Takeda T, Yamamoto M, Yonezawa A, et al. Safety and efficacy of sustained release of basic fibroblast growth factor using gelatin hydrogel in patients with critical limb ischemia. Heart Vessels 2016;31:713–21. 10.1007/s00380-015-0677-x. [DOI] [PubMed] [Google Scholar]
- 227.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared With Polyethylated Castor Oil–Based Paclitaxel in Women With Breast Cancer. Journal of Clinical Oncology 2016. 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 228.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. New England Journal of Medicine 2013;369:1691–703. 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Vaxess Technologies. A Phase 1, Randomized, Rater and Subject Blinded Placebo Controlled Study to Evaluate the Safety, Reactogenicity, Tolerability and Immunogenicity of a Standard and a Fractional Dose of H1 Influenza Vaccine Delivered by VX-103 (a MIMIX Microneedle Patch (MAP) System) in Healthy Adults ≥18–39 Years of Age. clinicaltrials.gov; 2023. [DOI] [PMC free article] [PubMed]
- 230.Houser KV, Chen GL, Carter C, Crank MC, Nguyen TA, Burgos Florez MC, et al. Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: a phase 1 trial. Nat Med 2022;28:383–91. 10.1038/s41591-021-01660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Holland C, Numata K, Rnjak-Kovacina J, Seib FP. The Biomedical Use of Silk: Past, Present, Future. Advanced Healthcare Materials 2019;8:1800465. 10.1002/adhm.201800465. [DOI] [PubMed] [Google Scholar]
- 232.van Turnhout AAWM, Franke CJJ, Vriens-Nieuwenhuis EJC, van der Sluis WB. The use of SERI™ Surgical Scaffolds in direct-to-implant reconstruction after skin-sparing mastectomy: A retrospective study on surgical outcomes and a systematic review of current literature. Journal of Plastic, Reconstructive & Aesthetic Surgery 2018;71:644–50. 10.1016/j.bjps.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 233.Jayaraman M, Ansell SM, Mui BL, Tam YK, Chen J, Du X, et al. Maximizing the Potency of siRNA Lipid Nanoparticles for Hepatic Gene Silencing In Vivo**. Angewandte Chemie International Edition 2012;51:8529–33. 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang X, Goel V, Robbie GJ. Pharmacokinetics of Patisiran, the First Approved RNA Interference Therapy in Patients With Hereditary Transthyretin-Mediated Amyloidosis. J Clin Pharmacol 2020;60:573–85. 10.1002/jcph.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kulkarni JA, Myhre JL, Chen S, Tam YYC, Danescu A, Richman JM, et al. Design of lipid nanoparticles for in vitro and in vivo delivery of plasmid DNA. Nanomedicine: Nanotechnology, Biology and Medicine 2017;13:1377–87. 10.1016/j.nano.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 236.Cui L, Renzi S, Quagliarini E, Digiacomo L, Amenitsch H, Masuelli L, et al. Efficient Delivery of DNA Using Lipid Nanoparticles. Pharmaceutics 2022;14:1698. 10.3390/pharmaceutics14081698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Joris F, De Backer L, Van de Vyver T, Bastiancich C, De Smedt SC, Raemdonck K. Repurposing cationic amphiphilic drugs as adjuvants to induce lysosomal siRNA escape in nanogel transfected cells. Journal of Controlled Release 2018;269:266–76. 10.1016/j.jconrel.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 238.Van de Vyver T, Bogaert B, De Backer L, Joris F, Guagliardo R, Van Hoeck J, et al. Cationic Amphiphilic Drugs Boost the Lysosomal Escape of Small Nucleic Acid Therapeutics in a Nanocarrier-Dependent Manner. ACS Nano 2020;14:4774–91. 10.1021/acsnano.0c00666. [DOI] [PubMed] [Google Scholar]
- 239.Hajimolaali M, Mohammadian H, Torabi A, Shirini A, Khalife Shal M, Barazandeh Nezhad H, et al. Application of chloroquine as an endosomal escape enhancing agent: new frontiers for an old drug. Expert Opinion on Drug Delivery 2021;18:877–89. 10.1080/17425247.2021.1873272. [DOI] [PubMed] [Google Scholar]
- 240.Cho H, Cho Y-Y, Bae YH, Kang HC. Nucleotides as Nontoxic Endogenous Endosomolytic Agents in Drug Delivery. Advanced Healthcare Materials 2014;3:1007–14. 10.1002/adhm.201400008. [DOI] [PubMed] [Google Scholar]
- 241.Ma Y, Fenton OS. A Unified Strategy to Improve Lipid Nanoparticle Mediated mRNA Delivery Using Adenosine Triphosphate. J Am Chem Soc 2023. 10.1021/jacs.3c05574. [DOI] [PubMed] [Google Scholar]
- 242.Pei D, Buyanova M. Overcoming Endosomal Entrapment in Drug Delivery. Bioconjugate Chem 2019;30:273–83. 10.1021/acs.bioconjchem.8b00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhukovsky MA, Filograna A, Luini A, Corda D, Valente C. Protein Amphipathic Helix Insertion: A Mechanism to Induce Membrane Fission. Frontiers in Cell and Developmental Biology 2019;7:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Brock DJ, Kondow-McConaghy HM, Hager EC, Pellois J-P. Endosomal Escape and Cytosolic Penetration of Macromolecules Mediated by Synthetic Delivery Agents. Bioconjugate Chem 2019;30:293–304. 10.1021/acs.bioconjchem.8b00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Brock DJ, Kondow-McConaghy H, Allen J, Brkljača Z, Kustigian L, Jiang M, et al. Mechanism of Cell Penetration by Permeabilization of Late Endosomes: Interplay between a Multivalent TAT Peptide and Bis(monoacylglycero)phosphate. Cell Chemical Biology 2020;27:1296–1307.e5. 10.1016/j.chembiol.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kabelka I, Pachler M, Prévost S, Letofsky-Papst I, Lohner K, Pabst G, et al. Magainin 2 and PGLa in Bacterial Membrane Mimics II: Membrane Fusion and Sponge Phase Formation. Biophysical Journal 2020;118:612–23. 10.1016/j.bpj.2019.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Del’Guidice T, Lepetit-Stoffaes J-P, Bordeleau L-J, Roberge J, Théberge V, Lauvaux C, et al. Membrane permeabilizing amphiphilic peptide delivers recombinant transcription factor and CRISPR-Cas9/Cpf1 ribonucleoproteins in hard-to-modify cells. PLOS ONE 2018;13:e0195558. 10.1371/journal.pone.0195558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Allen J, Pellois J-P. Hydrophobicity is a key determinant in the activity of arginine-rich cell penetrating peptides. Sci Rep 2022;12:15981. 10.1038/s41598-022-20425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Vivès E, Brodin P, Lebleu B. A Truncated HIV-1 Tat Protein Basic Domain Rapidly Translocates through the Plasma Membrane and Accumulates in the Cell Nucleus *. Journal of Biological Chemistry 1997;272:16010–7. 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 250.Rittner K, Benavente A, Bompard-Sorlet A, Heitz F, Divita G, Brasseur R, et al. New Basic Membrane-Destabilizing Peptides for Plasmid-Based Gene Delivery in Vitro and in Vivo. Molecular Therapy 2002;5:104–14. 10.1006/mthe.2002.0523. [DOI] [PubMed] [Google Scholar]
- 251.Klipp A, Burger M, Leroux J-C. Get out or die trying: Peptide- and protein-based endosomal escape of RNA therapeutics. Advanced Drug Delivery Reviews 2023;200:115047. 10.1016/j.addr.2023.115047. [DOI] [PubMed] [Google Scholar]
- 252.Porosk L, Gaidutšik I, Langel Ü. Approaches for the discovery of new cell-penetrating peptides. Expert Opinion on Drug Discovery 2021;16:553–65. 10.1080/17460441.2021.1851187. [DOI] [PubMed] [Google Scholar]
- 253.Plisson F, Ramírez-Sánchez O, Martínez-Hernández C. Machine learning-guided discovery and design of non-hemolytic peptides. Sci Rep 2020;10:16581. 10.1038/s41598-020-73644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lee EY, Wong GCL, Ferguson AL. Machine learning-enabled discovery and design of membrane-active peptides. Bioorganic & Medicinal Chemistry 2018;26:2708–18. 10.1016/j.bmc.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Agrawal P, Bhalla S, Usmani SS, Singh S, Chaudhary K, Raghava GPS, et al. CPPsite 2.0: a repository of experimentally validated cell-penetrating peptides. Nucleic Acids Research 2016;44:D1098–103. 10.1093/nar/gkv1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Manavalan B, Patra MC. MLCPP 2.0: An Updated Cell-penetrating Peptides and Their Uptake Efficiency Predictor. Journal of Molecular Biology 2022;434:167604. 10.1016/j.jmb.2022.167604. [DOI] [PubMed] [Google Scholar]
- 257.de Oliveira ECL, Santana K, Josino L, Lima e Lima AH, de Souza de Sales Júnior C. Predicting cell-penetrating peptides using machine learning algorithms and navigating in their chemical space. Sci Rep 2021;11:7628. 10.1038/s41598-021-87134-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wolfe JM, Fadzen CM, Choo Z-N, Holden RL, Yao M, Hanson GJ, et al. Machine Learning To Predict Cell-Penetrating Peptides for Antisense Delivery. ACS Cent Sci 2018;4:512–20. 10.1021/acscentsci.8b00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Schissel CK, Mohapatra S, Wolfe JM, Fadzen CM, Bellovoda K, Wu C-L, et al. Deep learning to design nuclear-targeting abiotic miniproteins. Nat Chem 2021;13:992–1000. 10.1038/s41557-021-00766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.López-Vidal EM, Schissel CK, Mohapatra S, Bellovoda K, Wu C-L, Wood JA, et al. Deep Learning Enables Discovery of a Short Nuclear Targeting Peptide for Efficient Delivery of Antisense Oligomers. JACS Au 2021;1:2009–20. 10.1021/jacsau.1c00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Goldbach N, Benna I, Wicky BIM, Croft JT, Carter L, Bera AK, et al. De novo design of monomeric helical bundles for pH-controlled membrane lysis. Protein Science n.d.;n/a:e4769. 10.1002/pro.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Liu DV, Yang NJ, Wittrup KD. A Nonpolycationic Fully Proteinaceous Multiagent System for Potent Targeted Delivery of siRNA. Molecular Therapy - Nucleic Acids 2014;3:e162. 10.1038/mtna.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yang NJ, Kauke MJ, Sun F, Yang LF, Maass KF, Traxlmayr MW, et al. Cytosolic delivery of siRNA by ultra-high affinity dsRNA binding proteins. Nucleic Acids Research 2017;45:7602–14. 10.1093/nar/gkx546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kim NH, Provoda C, Lee K-D. Design and Characterization of Novel Recombinant Listeriolysin O–Protamine Fusion Proteins for Enhanced Gene Delivery. Mol Pharmaceutics 2015;12:342–50. 10.1021/mp5004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol 2015;15:388–400. 10.1038/nri3839. [DOI] [PubMed] [Google Scholar]
- 266.Vasquez AM, Mouchlis VD, Dennis EA. Review of four major distinct types of human phospholipase A2. Advances in Biological Regulation 2018;67:212–8. 10.1016/j.jbior.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Watson JL, Juergens D, Bennett NR, Trippe BL, Yim J, Eisenach HE, et al. De novo design of protein structure and function with RFdiffusion. Nature 2023;620:1089–100. 10.1038/s41586-023-06415-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Huang J, Xie X, Zheng Z, Ye L, Wang P, Xu L, et al. De Novo Computational Design of a Lipase with Hydrolysis Activity towards Middle-Chained Fatty Acid Esters. International Journal of Molecular Sciences 2023;24:8581. 10.3390/ijms24108581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wongpinyochit T, Uhlmann P, Urquhart AJ, Seib FP. PEGylated Silk Nanoparticles for Anticancer Drug Delivery. Biomacromolecules 2015;16:3712–22. 10.1021/acs.biomac.5b01003. [DOI] [PubMed] [Google Scholar]
- 270.Amjadi S, Hamishehkar H, Ghorbani M. A novel smart PEGylated gelatin nanoparticle for codelivery of doxorubicin and betanin: A strategy for enhancing the therapeutic efficacy of chemotherapy. Materials Science and Engineering: C 2019;97:833–41. 10.1016/j.msec.2018.12.104. [DOI] [PubMed] [Google Scholar]
- 271.Panja P, Das P, Mandal K, Jana NR. Hyperbranched Polyglycerol Grafting on the Surface of Silica-Coated Nanoparticles for High Colloidal Stability and Low Nonspecific Interaction. ACS Sustainable Chem Eng 2017;5:4879–89. 10.1021/acssuschemeng.7b00292. [DOI] [Google Scholar]
- 272.Zou Y, Ito S, Yoshino F, Suzuki Y, Zhao L, Komatsu N. Polyglycerol Grafting Shields Nanoparticles from Protein Corona Formation to Avoid Macrophage Uptake. ACS Nano 2020;14:7216–26. 10.1021/acsnano.0c02289. [DOI] [PubMed] [Google Scholar]
- 273.Liu Q, Singh A, Lalani R, Liu L. Ultralow Fouling Polyacrylamide on Gold Surfaces via Surface-Initiated Atom Transfer Radical Polymerization. Biomacromolecules 2012;13:1086–92. 10.1021/bm201814p. [DOI] [PubMed] [Google Scholar]
- 274.Qin X, Chen K, Cao L, Zhang Y, Li L, Guo X. Antifouling performance of nano-sized spherical poly(N-hydroxyethyl acrylamide) brush. Colloids and Surfaces B: Biointerfaces 2017;155:408–14. 10.1016/j.colsurfb.2017.04.048. [DOI] [PubMed] [Google Scholar]
- 275.Karakocak BB, Liang J, Biswas P, Ravi N. Hyaluronate coating enhances the delivery and biocompatibility of gold nanoparticles. Carbohydrate Polymers 2018;186:243–51. 10.1016/j.carbpol.2018.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Yang Q, Lai SK. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2015;7:655–77. 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Chen S, Cao Z, Jiang S. Ultra-low fouling peptide surfaces derived from natural amino acids. Biomaterials 2009;30:5892–6. 10.1016/j.biomaterials.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 278.Schlenoff JB. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir 2014;30:9625–36. 10.1021/la500057j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Nowinski AK, White AD, Keefe AJ, Jiang S. Biologically Inspired Stealth Peptide-Capped Gold Nanoparticles. Langmuir 2014;30:1864–70. 10.1021/la404980g. [DOI] [PubMed] [Google Scholar]
- 280.Banskota S, Yousefpour P, Kirmani N, Li X, Chilkoti A. Long circulating genetically encoded intrinsically disordered zwitterionic polypeptides for drug delivery. Biomaterials 2019;192:475–85. 10.1016/j.biomaterials.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Banskota S, Saha S, Bhattacharya J, Kirmani N, Yousefpour P, Dzuricky M, et al. Genetically Encoded Stealth Nanoparticles of a Zwitterionic Polypeptide-Paclitaxel Conjugate Have a Wider Therapeutic Window than Abraxane in Multiple Tumor Models. Nano Lett 2020;20:2396–409. 10.1021/acs.nanolett.9b05094. [DOI] [PubMed] [Google Scholar]
- 282.Yuan Z, Li B, Niu L, Tang C, McMullen P, Jain P, et al. Zwitterionic Peptide Cloak Mimics Protein Surfaces for Protein Protection. Angewandte Chemie International Edition 2020;59:22378–81. 10.1002/anie.202004995. [DOI] [PubMed] [Google Scholar]
- 283.Anselmo AC, Mitragotri S. Nanoparticles in the clinic: An update. Bioengineering & Translational Medicine 2019;4:e10143. 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kulkarni JA, Witzigmann D, Thomson SB, Chen S, Leavitt BR, Cullis PR, et al. The current landscape of nucleic acid therapeutics. Nat Nanotechnol 2021;16:630–43. 10.1038/s41565-021-00898-0. [DOI] [PubMed] [Google Scholar]
- 285.Asher DR, Thapa K, Dharia SD, Khan N, Potter RA, Rodino-Klapac LR, et al. Clinical development on the frontier: gene therapy for duchenne muscular dystrophy. Expert Opinion on Biological Therapy 2020;20:263–74. 10.1080/14712598.2020.1725469. [DOI] [PubMed] [Google Scholar]
- 286.Puhl DL, D’Amato AR, Gilbert RJ. Challenges of gene delivery to the central nervous system and the growing use of biomaterial vectors. Brain Research Bulletin 2019;150:216–30. 10.1016/j.brainresbull.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhong Z, Fang S, Li Y, Huang Y, Zhang Y, Chen H, et al. Quantitative Analysis of Protein Corona on Precoated Protein Nanoparticles and Determined Nanoparticles with Ultralow Protein Corona and Efficient Targeting in Vivo. ACS Appl Mater Interfaces 2021;13:56812–24. 10.1021/acsami.1c12008. [DOI] [PubMed] [Google Scholar]
- 288.Pustulka SM, Ling K, Pish SL, Champion JA. Protein Nanoparticle Charge and Hydrophobicity Govern Protein Corona and Macrophage Uptake. ACS Appl Mater Interfaces 2020;12:48284–95. 10.1021/acsami.0c12341. [DOI] [PubMed] [Google Scholar]
- 289.Nouri FS, Wang X, Chen X, Hatefi A. Reducing the Visibility of the Vector/DNA Nanocomplexes to the Immune System by Elastin-Like Peptides. Pharm Res 2015;32:3018–28. 10.1007/s11095-015-1683-5. [DOI] [PubMed] [Google Scholar]
- 290.Abiri N, Pang J, Ou J, Shi B, Wang X, Zhang S, et al. Assessment of the immunogenicity of residual host cell protein impurities of OsrHSA. PLOS ONE 2018;13:e0193339. 10.1371/journal.pone.0193339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wang Z, Gao H, Zhang Y, Liu G, Niu G, Chen X. Functional ferritin nanoparticles for biomedical applications. Front Chem Sci Eng 2017;11:633–46. 10.1007/s11705-017-1620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Long Y, Cheng X, Tang Q, Chen L. The antigenicity of silk-based biomaterials: sources, influential factors and applications. Journal of Materials Chemistry B 2021;9:8365–77. 10.1039/D1TB00752A. [DOI] [PubMed] [Google Scholar]
- 293.Alves AL, Costa-Gouveia J, Vieira de Castro J, Sotelo CG, Vázquez JA, Pérez-Martín RI, et al. Study of the immunologic response of marine-derived collagen and gelatin extracts for tissue engineering applications. Acta Biomaterialia 2022;141:123–31. 10.1016/j.actbio.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 294.Lynn A k., Yannas I v., Bonfield W. Antigenicity and immunogenicity of collagen. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2004;71B:343–54. 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 295.Suhorutsenko J, Oskolkov N, Arukuusk P, Kurrikoff K, Eriste E, Copolovici D-M, et al. Cell-Penetrating Peptides, PepFects, Show No Evidence of Toxicity and Immunogenicity In Vitro and In Vivo. Bioconjugate Chem 2011;22:2255–62. 10.1021/bc200293d. [DOI] [PubMed] [Google Scholar]
- 296.Gupta S, Madhu MK, Sharma AK, Sharma VK. ProInflam: a webserver for the prediction of proinflammatory antigenicity of peptides and proteins. Journal of Translational Medicine 2016;14:178. 10.1186/s12967-016-0928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Soria-Guerra RE, Nieto-Gomez R, Govea-Alonso DO, Rosales-Mendoza S. An overview of bioinformatics tools for epitope prediction: Implications on vaccine development. Journal of Biomedical Informatics 2015;53:405–14. 10.1016/j.jbi.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 298.Yao B, Zhang L, Liang S, Zhang C. SVMTriP: A Method to Predict Antigenic Epitopes Using Support Vector Machine to Integrate Tri-Peptide Similarity and Propensity. PLOS ONE 2012;7:e45152. 10.1371/journal.pone.0045152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Tripathi NK, Shrivastava A. Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Frontiers in Bioengineering and Biotechnology 2019;7:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Rosano GL, Ceccarelli EA. Recombinant protein expression in Escherichia coli: advances and challenges. Frontiers in Microbiology 2014;5:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Rosano GL, Morales ES, Ceccarelli EA. New tools for recombinant protein production in Escherichia coli: A 5-year update. Protein Science 2019;28:1412–22. 10.1002/pro.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Patil R, Walther J. Continuous Manufacturing of Recombinant Therapeutic Proteins: Upstream and Downstream Technologies. In: Kiss B, Gottschalk U, Pohlscheidt M, editors. New Bioprocessing Strategies: Development and Manufacturing of Recombinant Antibodies and Proteins. Cham: Springer International Publishing; 2018. p. 277–322. [DOI] [PubMed] [Google Scholar]
- 303.Oliveira C, Domingues L. Guidelines to reach high-quality purified recombinant proteins. Appl Microbiol Biotechnol 2018;102:81–92. 10.1007/s00253-017-8623-8. [DOI] [PubMed] [Google Scholar]
- 304.de Marco A, Berrow N, Lebendiker M, Garcia-Alai M, Knauer SH, Lopez-Mendez B, et al. Quality control of protein reagents for the improvement of research data reproducibility. Nat Commun 2021;12:2795. 10.1038/s41467-021-23167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


