Abstract
Extracellular vesicles (EVs) are biological nanoparticles that promote intercellular communication by delivery of bioactive cargo over short and long distances. Short-distance communication takes place in the interstitium, while long-distance communication is thought to require transport through the blood circulation to reach distal sites. EV therapeutics are frequently injected systemically and diagnostic approaches often rely on the detection of organ-derived EVs in the blood. However, the mechanisms by which EVs enter and exit the circulation are poorly understood. Here, the lymphatic system and transport across the endothelial barrier through paracellular and transcellular routes are discussed as potential pathways for EV entry and exit to and from the blood circulatory system.
Keywords: Exosomes, Interendothelial junctions, Lymphatic system, Microvesicles, Transcytosis
MAIN
Extracellular vesicles (EVs) are cell-released nanoparticles that mediate intercellular communication over short and long distances. Numerous studies report the presence of organ-specific EVs in the blood,1 however, the mechanisms by which such EVs exit the interstitium remain largely unknown. Similarly, it has been demonstrated that systemically administered EVs can reach specific organs,2,3 although transport across the endothelial barrier remains elusive. The exponential growth of the EV field and the accelerated pace at which emerging therapeutic and diagnostic EV-based products are being developed,4–8 highlights a pressing need to understand EV transport in the body. Accordingly, it could be argued that EV transport phenomena are the most critical component of a desired function, as an incorrect spatial context is futile and potentially detrimental. This Perspective explores potential nanoscale processes that EVs use to enter and exit the blood circulation (Fig. 1).
Figure 1 -. Potential pathways of extracellular vesicle (EV) entry to and exit from the blood circulation.

a) General representation of endothelium types. It is worth noting that several types of endothelium can be found in some organs, such as the kidneys. b) EVs may cross the endothelial barrier through transcellular or paracellular transport routes. In the case of paracellular transport, EVs may pass through gaps between endothelial cells, which is likely to be a prominent transport route in organs with discontinuous vasculature, such as the liver (1). EVs may also modify interendothelial junctions to enable paracellular transport (2). In the case of transcellular transport, EVs may pass through intra-endothelial fenestrations (3) or use various transcytosis pathways (4). Binding to lipoproteins to hijack caveolae-mediated transcytosis may also be exploited (5). Exocytosis-independent mechanisms of transcellular transport are also plausible, resulting in multilayered EVs released through membrane budding (6). Finally, cell protrusions could potentially be exploited as an alternative route for EV transport across the endothelial barrier (7).
Most organs have capillaries with a non-fenestrated continuous endothelial lining, which only enables water, small solutes, and gasses to pass through. By contrast, organs such as the liver and spleen have a discontinuous endothelial lining, which allows macromolecules and nanosized particles to traverse between the interstitial space and the blood circulation (Fig. 1a). It is also common for various subtypes of endothelia to be present in the same organ. For example, the kidneys contain several endothelial cell types that possess distinct structures and roles.9,10 The kidney endothelial cells found in medium and large blood vessels form a non-fenestrated continuous layer, interconnected through intercellular junctions, and elongated in the direction of the blood flow. By contrast, glomerular endothelial cells possess numerous fenestrations and have a thick glycocalyx layer, which contributes to the filtration properties of the glomerular filtration barrier. The endothelial cells of peritubular capillaries are fenestrated and covered by a thin diaphragm made of glycoproteins, which facilitates the reabsorption and secretion of fluids and substances by neighboring tubular epithelial cells.9,10 Endothelial cell heterogeneity within and among organs is likely to have a substantial impact on the mechanisms by which EVs traverse the endothelial barrier, of which plausible routes will be discussed in this article.
PARACELLULAR TRANSPORT OF EXTRACELLULAR VESICLES
Transport across discontinuous endothelium
With pathological conditions, such as cancer and inflammation, interendothelial junctions in organs with continuous vasculature can become compromised, enabling paracellular transport of macromolecules and nanosized particles.11 This phenomenon has been exploited in the field of nanomedicine, where one of the main targeting mechanism for clinically approved nanoparticles relies on paracellular endothelial transport due to pathological vascular aberrations.11 Therefore, paracellular transport could represent a major mechanism by which EVs enter and exit the blood circulation during pathological conditions (Fig. 1b). Accordingly, the numerous observations that cancer patients have increased levels of circulating EVs,6 may be attributed to leaky tumor vasculature, in addition to the common explanation of elevated EV production by cancer cells. It is important to note that increased vascular permeability is consistently observed in preclinical tumor models, although substantial interpatient, intertumor, and intratumor heterogeneity in terms of leaky vasculature exists in the clinical oncology setting.12 This discrepancy is likely due to substantial differences in tumour growth and tumour-to-body weight ratios in animal models and patients, which affects vasculature morphology and function, consequently impacting macromolecular transport across the endothelial barrier. It is also important to note that the central paradigm of paracellular transport being responsible for leaky tumour vasculature has been questioned with new evidence suggesting a critical role of transcellular pathways.13
Transport across continuous endothelium
It is also possible that EVs have mechanisms of altering interendothelial junctions to enable paracellular transport across non-fenestrated continuous capillaries (Fig. 1b). In the case of immune cells, transendothelial migration through paracellular transport is an extensively studied phenomenon, involving the relocalization of structural proteins associated with endothelial tight and adherent junctions.14 Rolling adhesion between immune cells and endothelial cells results in the activation of multiple downstream signaling pathways within the latter, including production of reactive oxygen species and tyrosine phosphorylation of junctional components.14 These pathways induce cytoskeletal actin rearrangements that disturb binding interactions, enabling efficient leukocyte transendothelial migration across the vasculature.14 Several metastatic cancer cell lines appear to exploit similar mechanisms to enable EVs to cross the endothelial barrier. Such EVs have been shown to contain microRNAs (miRNAs)15 and proteins16 that destabilize the endothelial actin cytoskeleton or alter the expression and/or localization of junctional proteins, increasing vascular permeability.
Studies have linked miRNA-mediated endothelial dysregulation to EV-promoted metastasis. For example, EV-associated miR-181c was shown to destabilize the continuous blood-brain barrier, promoting metastasis of brain-tropic cancer cells.17 Administration of brain-metastatic EVs in endothelial cell culture models resulted in the cytoplasmic internalization of tight junction proteins in conjunction with the remodeling of associated actin filaments (Fig. 2a).17 These effects were attributed to miR-181c-induced downregulation of phosphoinositide-dependent kinase-1 (PDPK1), a protein involved in the phosphorylation of cofilin,17 which disassembles actin filaments when dephosphorylated.18 Taken together, the impact of miRNA cargo on cytoskeletal actin dynamics extends beyond the entry and exit of EVs from the circulation to more wide-reaching effects on cross-endothelial transport of 100 times larger structures, that is, metastatic cancer cells.
Figure 2 – Mechanisms for cancer cell-derived EVs to cross the blood-brain barrier endothelium.
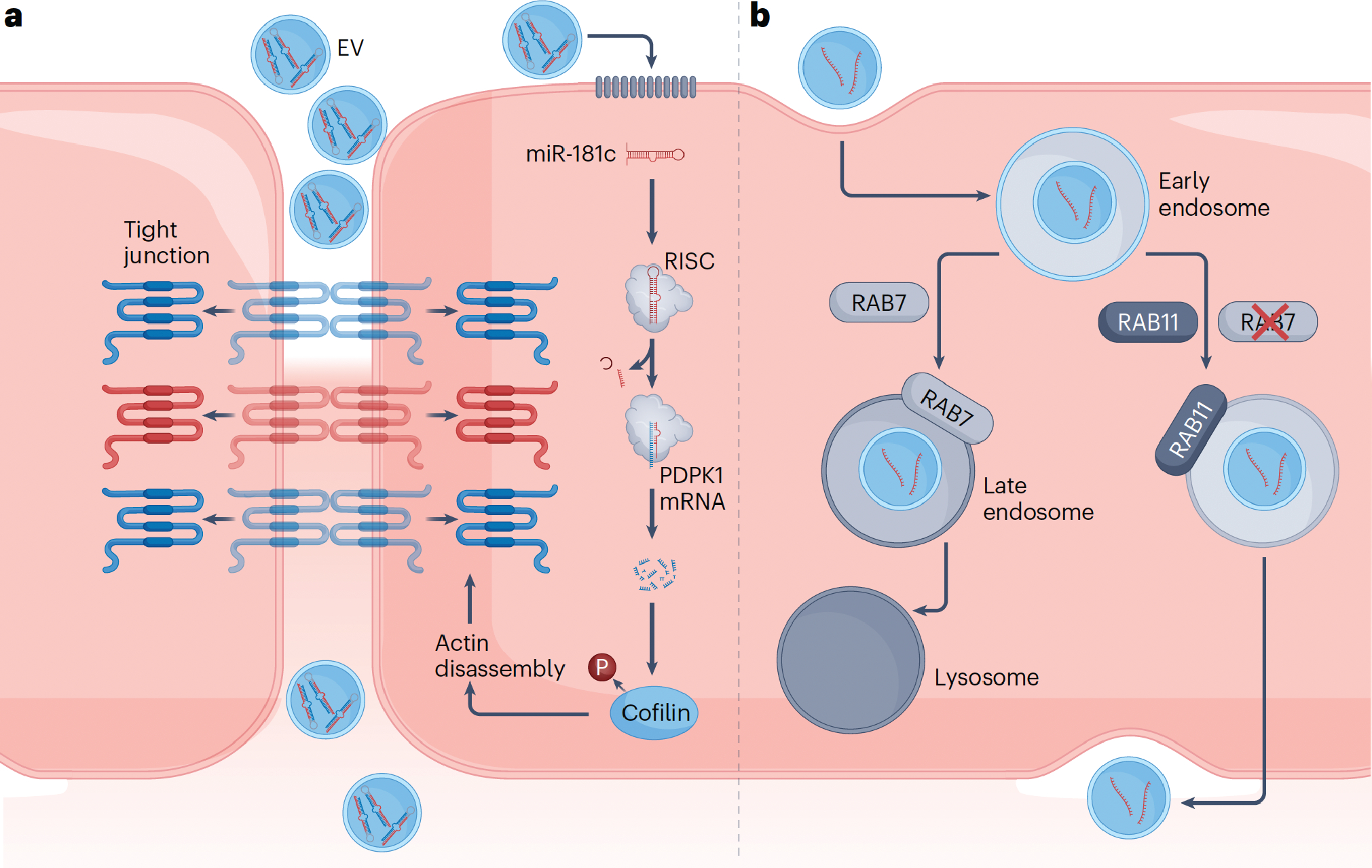
a) Cancer EV-associated miR181c modulates the integrity of the tight junction by downregulating the expression of PDPK1, a protein involved in the phosphorylation of cofilin.17 Increased cofilin dephosphorylation as a result of this process leads to eventual actin filament disassembly, displacing attached tight junction proteins from their transmembrane localities into the cytoplasm, causing increased vascular permeability for paracellular movement of EVs. b) Breast cancer-derived EVs have been found to suppress the RAB7-associated degradation pathway following endocytosis, redirecting themselves to the RAB11-associated recycling pathway.36 This enables subsequent transcytosis of the EVs through the basolateral membrane of the endothelial cells, into the interstitium. mRNA; messenger RNA; RISC, RNA-induced silencing complex; P, phosphate group; RAB, Ras-associated binding (protein).
Non-cancer-derived EVs have also been found to modulate endothelial cell-to-cell junctions. For example, during inflammation, the surface of endothelial-derived EVs becomes enriched with adhesion molecules that mediate endothelial barrier disruption.19 However, it is unclear to what extent EV-mediated modifications of interendothelial junctions take place under pathological and physiological conditions. It is also worth noting that the glycocode is an essential component of leukocyte extravasation, as membrane-bound surface glycans mediate interactions with the endothelium and associated junctional complexes.20 However, glycans are a major overlooked component of EVs, and few studies have assessed the EV glycocode.21–25 An increased focus on EV glycomics could aid in understanding interactions that take place at the EV-endothelium interface.
LYMPHATIC TRANSPORT OF EXTRACELLULAR VESICLES
In organs with discontinuous vasculature, such as the liver, paracellular transport of nanosized cargo occurs through inter-endothelial gaps in the vasculature wall. In addition to having discontinuous vasculature, the liver is the largest internal organ. Therefore, it could be reasoned that the liver contributes to the majority of non-hematopoietic EVs in the blood. It is challenging to determine the organ-specific origin of EVs, as many markers overlap. A recent study based on long RNA sequencing, predicted that EVs of liver origin were the fourth most abundant population in the blood, excluding hematopoietic-derived EVs.26 Adipose tissue, muscle, and lung-derived EVs outnumbered those of liver origin,26 indicating that if these results are accurate, other mechanisms of entering the blood circulation may be more efficient than paracellular transport across the vascular endothelium. In fact, lymphatic transport represents an alternative pathway for eventual entry into the blood circulation.
The lymphatic endothelium has junctions that are opened in response to interstitial fluid pressure, making it easier for macromolecules to exit the interstitium through the lymphatic system compared to the non-fenestrated continuous vascular endothelial barrier. Similar to macromolecules, it is likely that EVs enter the lymphatic system through fluid pressure-regulated flap valves, which are known to allow the passage of nano and micrometre-sized particles (Fig. 3).27,28 Lymphatic vessels also express a myriad of receptors, such as integrins, that promote trafficking of cells throughout the lymphatic vessels. EVs, as a result of possessing cell-derived ligands on their surfaces, could potentially exploit receptor binding for lymphatic entry and transport. Given that lymph vessels are omnipresent, it is likely that lymphatic entry of EVs from the interstitium occurs across all organs, and could serve as the primary mechanism through which EVs enter the circulation in adipose tissue, muscle, lungs, and other organs with a non-fenestrated continuous endothelium.
Figure 3 -. Potential pathways of EV entry into the blood circulation through the lymphatic system.

EVs may exit the interstitium through fluid pressure-sensitive junctions (flap valves) in the lymphatic endothelium that create micrometre-sized gaps27,28 (1), subsequently entering the blood circulation, either by exiting through high endothelial venules (HEVs) in the lymph nodes (2) or through the lymphovenous junction that drains into the great veins (3).
The lymphatic system is connected with blood circulation through the lymphovenous junction and lymph nodes.29 On a daily basis, an average of 8–12 litres of lymph enters the blood; half through lymph nodes and half through the lymphovenous junction that empties into the great veins.29 In the case of the lymph nodes, specialized blood vessels termed high endothelial venules, form a vascular transport barrier for macromolecules. It is possible that EVs can cross this barrier through either paracellular or transcellular pathways, although evidence of either is currently lacking. However, in the case of the lymphovenous junction, the lymph enters directly into the blood circulation without passing a vascular endothelial barrier. Therefore, lymphatic transport from the tissue interstitium to the great veins could represent a major mechanism by which EVs enter the blood circulation (Fig. 3).
Cancer cell-derived EVs have been found in the lymphatic system, where they promote metastatic spread.30 In particular, cancer EVs can contain ‘don’t eat me’ surface proteins,31 which are likely to enable immunoevasion in the lymph. Transport in the lymphatic system may also occur in the opposite direction, as is the case for platelet-derived EVs, which have been found in the lymph in healthy and pathological settings.32 The prevention of retrograde flow of blood components into the lymph at the lymphovenous junction is thought to involve valves and platelet plugs.33 Beyond the context of EVs, many outstanding questions remain regarding the mechanisms governing lymph-blood separation at the lymphovenous junction.33
TRANSCELLULAR TRANSPORT OF EXTRACELLULAR VESICLES
In addition to the lymphatic system and paracellular transport through the vessel wall, other mechanisms exist for macromolecules to enter the blood circulation from the tissue interstitium. In tissues with fenestrated continuous vasculature, intra-endothelial fenestrations enable macromolecules to traverse the endothelium through channels within individual endothelial cells. In organs with a non-fenestrated continuous endothelial lining, proteins and other macromolecular are restricted from crossing the vascular endothelium through intra or inter-endothelial gaps. Consequently, a common mechanism by which macromolecules, such as lipoproteins and albumin, are transported across the endothelium relies on vesicle-facilitated cellular internalization and transcellular transport mediated by caveolae.34,35 Either receptor-bound or fluid-phase macromolecules within caveolae pits are internalized, and vesicles are transported across the cytoplasm to the opposite side of the endothelial cell where they are released extracellularly through fusion with the cell membrane.34 Therefore, it is possible that EVs use similar pathways of endothelial internalization and transcytosis.
Extracellular vesicle uptake by endothelial cells
Studies have shown that endothelial cells can internalize EVs through clathrin-dependent endocytosis, caveolin-mediated uptake, macropinocytosis, and lipid raft-mediated internalization.36–39 Phagocytosis has also been observed to facilitate EV uptake in endothelial cells.40 The presence of phosphatidylserine in the outer leaflet of EV membranes appears to facilitate phagocytic and macropinocytic uptake by immune cells,41,42 and increases endothelial cell internalization of EVs.43 Similar to synthetic nanoparticles,44,45 it is likely that the internalization mechanisms are also dependent on the physical properties of EVs. EV biogenesis has resulted in three distinct classifications: exosomes (~30–150 nm), microvesicles (~100–1000 nm), and apoptotic bodies (~500–2000 nm). The significant overlap in size and absence of subtype-specific surface markers amongst these categories make it challenging to distinguish between vesicle types.46,47 This intrinsic heterogeneity of EVs could potentially play a role in the cellular internalization profiles, as has been observed in the case of synthetic nanoparticles. For example, larger nanoparticles tend to be internalized through phagocytosis or macropinocytosis, whereas smaller ones are endocytosed by other mechanisms.48
Multiple internalization mechanisms can be engaged by endothelial cells when taking up EVs. Protein and glycan interactions between the two interfaces have been shown to be cardinal in EV uptake, with certain ligands on EV surfaces complementing membrane receptors on target cells and triggering internalization.38,39 For example, tetraspanins play a pivotal role in endothelial cell uptake. EVs overexpressing the tetraspanin 8-cluster of differentiation (CD)49d were more readily internalized, and CD106/vascular adhesion molecule 1 (VCAM-1) on endothelial cells was speculated to mediate enhanced uptake.49 In the context of brain endothelial cells, EVs expressing lymphocyte function-associated antigen 1 (LFA-1) displayed enhanced internalization through binding to intercellular adhesion molecule 1 (ICAM-1).50 Membrane-bound C-type lectin receptors on endothelial cells have been shown to mediate the internalization of macrophage-derived EVs.50 Heparan sulfate proteoglycans on endothelial surfaces also play a role in EV internalization.51 Heparan sulfate proteoglycans are ubiquitous on the luminal face of the endothelium, being the most common endothelial cell-surface glycosaminoglycans, comprising between 50–90% of this glycan population.52 Several ligands commonly found on EV surfaces, such as fibronectin,53–55 are known to interact with heparan sulfate proteoglycans.56 Additionally, treatment of EVs with integrin blocking peptides, reduced uptake in endothelial cells.40
Extracellular vesicle transcytosis in endothelial cells
Following uptake by cells, vesicles can be subject to various fates: fusion with endosomes and subsequent recycling of lipid and lipid-associated biomolecules to the plasma membrane or other organelles, transport to the lysosomal compartment, or transcytosis into the extracellular space. Cancer-derived EVs can engage in transcytosis to cross the endothelium of the blood-brain barrier (Fig. 1b).36 In such cases, EVs downregulate the expression of genes responsible for endocytic degradation, diverting to a recycling pathway. Specifically, a study showed that brain-targeting EVs from metastatic breast cancer cells engage in caveolin-independent uptake mechanisms and increase innately low levels of transcytosis (Fig. 2b).36 Studies assessing the intracellular fate of the endocytosed EVs revealed co-localization with Ras-associated binding protein (RAB)11 and vesicle associated membrane protein 3 (VAMP3), markers for recycling endosomes and/or exocytosis.36 The transcytosis pathway was further confirmed by fusion of the endosomes containing the EVs with receptors on the basolateral membrane. Taken together, EVs from metastatic cancer cells can be transported from the luminal (blood-facing) to abluminal (brain interstitium-facing) side of the endothelium by circumventing intracellular degradation pathways and increasing the physiologically low levels of EV transcytosis at the blood-brain barrier. It is possible that similar mechanisms are employed by EVs when crossing non-fenestrated continuous endothelium encountered in other organs, such as the medium and large blood vessels in the kidneys. Adsorptive-mediated transcytosis is another mechanism proposed for blood-brain barrier crossing of EVs.57,58 This mechanism relies on cationic moieties, which augment vesicular trafficking resulting in transcytotic release.59 It is possible that cationic components are incorporated during biogenesis or after EV release as part of a biomolecular corona, enabling endothelial crossing through electrostatic interactions. Adsorptive-mediated transcytosis occurs with other macromolecular agents such as viruses, which are thought to co-opt EV trafficking mechanisms to cross endothelial barriers.58,60–62
An ability to block transcytosis in specific endothelial cells would be a valuable tool to assess EV entry and exit into the circulation. In terms of the blood-brain barrier, major facilitator superfamily domain containing protein-2a (MFSD2A) was shown to inhibit transcytosis.63 MFSD2A specifically hinders caveolae-mediated transcytosis64 by facilitating the movement of unsaturated phospholipids from the outer to the inner layer of the endothelial cell membrane.65 Consequently, the altered composition of the membrane lipids prevents the formation of caveolae.64 It is likely that multiple other proteins specific to the central nervous system regulate transcytosis, and future studies are necessary to understand general and EV-specific mechanisms of transcytosis across the blood-brain barrier and other endothelial barriers.
Similar to EVs, lipoproteins are biological nanoparticles that enter and exit the circulation, serving as long-distance delivery systems for endogenous biomolecules and exogenous drugs.66 Notably, lipoproteins are six orders of magnitude more abundant in the blood than EVs,67 suggesting that EVs are likely to encounter numerous lipoproteins in the interstitial fluid and circulation. Proteomic studies have shown that the protein components of lipoproteins, that is, apolipoproteins, are bound to the EV surface.68 Other studies have demonstrated that EVs can interact and bind to lipoproteins when mixed together.69,70 Evidence that EVs bind to lipoproteins under physiological conditions has been reported,71 and a preprint has supported these findings.72 Notably, a recent study indicated that EVs and lipoproteins can form complexes that remain intact upon cellular internalization and release.73 Therefore, it is possible that EVs form complexes with lipoproteins to hijack caveolae-mediated endothelial transcytosis pathways to traverse between interstitial spaces and the blood (Fig. 1b). Notably, EVs from a brain metastatic variant displayed enhanced interactions with low-density lipoprotein compared to a regular breast cancer cell line.70 Further research is necessary to assess whether pro-metastatic EVs exploit lipoprotein interactions to promote transport across the endothelial barrier.
Exocytosis-independent transcellular transport
It is also possible that EVs cross the endothelial barrier through exocytosis-independent transcellular mechanisms involving endocytosis followed by membrane budding (Fig. 1b). The close proximity of membrane-bound organelles to the cell membrane can result in multilayered EVs upon membrane budding,74 such as those containing mitochondria.75 Similarly, it was speculated that endocytosed EVs in close proximity to the membrane could be released through membrane budding, resulting in the formation of multilayered EVs.74 However, it is unknown whether endocytosis, structural preservation, and release through membrane budding, could serve as a mechanism of transcellular transport in general, and specifically, across the endothelial barrier.
CELL EXTENSION-MEDIATED TRANSPORT OF EXTRACELLULAR VESICLES
Cells produce various extensions, such as microvilli, filopodia, cilia, retraction fibres, and apoptopodia, all of which can release EVs.76 Therefore, an additional speculative mechanism for entry of EVs into the blood circulation is through cellular extensions that interface with the vasculature. For example, cell extensions from osteocytes (dendritic processes)77 and astrocytes (endfeet processes)78 form tight connections with the vasculature (Fig. 1b).
OUTLOOK
Taken together, there are various possible mechanisms by which EVs traffic between the interstitium, the lymphatic system, and the blood circulation. Although individual examples of paracellular and transcellular transport have been described, a comprehensive understanding of EV entry and exit routes to and from the blood circulation in physiological and pathological contexts are lacking. This article highlights the pressing need to study EV transport across the vascular barrier, which is critical for understanding intercellular communication beyond the local interstitium, as well as for leveraging EV biology for mechanistic, diagnostic, and therapeutic uses.
In the case of therapeutics, an understanding of EV transport could aid in selecting the most efficient administration route for a given application. Subcutaneous, intraperitoneal, and intramuscular administration routes are likely to enhance lymphatic transport, as demonstrated for synthetic nanoparticles.79–81 However, a poor understanding of EV fate following lymphatic drainage limits the exploitation of administration routes for improved transport and therapeutic effects. In addition, the impact of physical EV properties (such as their size) on interactions with the vascular barrier remains largely unknown, preventing the rational design of ideal therapeutics. Small EVs (approximately <100 nm) and large EVs (approximately >200 nm) have demonstrated varying distribution and accumulation profiles when administered in animal models. For example, a systematic review of biodistribution studies demonstrated that an elevated deposition of small EVs was observed in the liver and kidneys (first hour) and lungs and spleen (2–12 hours), whereas large EVs demonstrated increased accumulation within the lungs (first hour) and liver (2–12 hours).82 However, EV transport phenomena and vascular interactions that mediate distinct biodistribution profiles remain elusive.83 An understanding of EV transport is also important for diagnostic applications. For example, depending on the importance of lymphatic transport, diagnostic capabilities could be improved by sampling EVs from lymph nodes and screening for disease-associated proteins,84 nucleic acids,85 and lipids.86,87 Taken together, the lymph presents a promising avenue for diagnostic and therapeutic purposes, due to the likelihood of it being a primary route for EV trafficking.
ACKNOWLEDGEMENTS
Partial funding for this work was provided by The University of Queensland, Australia (JW), the National Institute on Aging, National Institutes of Health, United States under award number R01AG076537 (JW), and The Medical Research Future Fund, Australia under award number MRF2019485 (JW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the organizations and funding agencies. Figures were made in ©BioRender - biorender.com.
CONFLICT OF INTEREST
The authors report no conflicts of interest. Dr. Joy Wolfram is listed on an extracellular vesicle grant from Ionis Pharmaceuticals, but the article topic is not a primary focus of the grant, and the grant funding did not contribute to this work.
REFERENCES
- 1.Alberro A, Iparraguirre L, Fernandes A & Otaegui D Extracellular Vesicles in Blood: Sources, Effects, and Applications. Int J Mol Sci 22, doi: 10.3390/ijms22158163 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witwer KW & Wolfram J Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nature Reviews Materials 6, 103–106, doi: 10.1038/s41578-020-00277-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busatto S, Pham A, Suh A, Shapiro S & Wolfram J Organotropic drug delivery: Synthetic nanoparticles and extracellular vesicles. Biomed. Microdevices 21, 46, doi: 10.1007/s10544-019-0396-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beetler DJ et al. Extracellular vesicles as personalized medicine. Mol. Aspects Med, 101155, doi: 10.1016/j.mam.2022.101155 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker S et al. Extracellular vesicle-based drug delivery systems for cancer treatment Theranostics In press, doi: 10.7150/thno.37097 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu T, Wolfram J & Srivastava S Extracellular Vesicles in Cancer Detection: Hopes and Hypes. Trends Cancer, doi: 10.1016/j.trecan.2020.09.003 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Iannotta D, Yang M, Celia C, Di Marzio L & Wolfram J Extracellular vesicle therapeutics from plasma and adipose tissue. Nano Today In press, doi: j.nanotod.2021.101159 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghodasara A, Raza A, Wolfram J, Salomon C & Popat A Clinical Translation of Extracellular Vesicles. Advanced healthcare materials n/a, 2301010, doi: 10.1002/adhm.202301010. [DOI] [PubMed] [Google Scholar]
- 9.Dumas SJ et al. Phenotypic diversity and metabolic specialization of renal endothelial cells. Nature Reviews Nephrology 17, 441–464, doi: 10.1038/s41581-021-00411-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jourde-Chiche N et al. Endothelium structure and function in kidney health and disease. Nat Rev Nephrol 15, 87–108, doi: 10.1038/s41581-018-0098-z (2019). [DOI] [PubMed] [Google Scholar]
- 11.Wolfram J & Ferrari M Clinical cancer nanomedicine. Nano Today 25, 85–89 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhakar U et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 73, 2412–2417, doi: 10.1158/0008-5472.CAN-12-4561 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sindhwani S et al. The entry of nanoparticles into solid tumours. Nature materials 19, 566–575, doi: 10.1038/s41563-019-0566-2 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Lessey-Morillon EC et al. The RhoA guanine nucleotide exchange factor, LARG, mediates ICAM-1-dependent mechanotransduction in endothelial cells to stimulate transendothelial migration. J. Immunol 192, 3390–3398, doi: 10.4049/jimmunol.1302525 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng Z et al. Cancer-derived exosomal miR-25–3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nature communications 9, 5395, doi: 10.1038/s41467-018-07810-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treps L, Perret R, Edmond S, Ricard D & Gavard J Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. J Extracell Vesicles 6, 1359479, doi: 10.1080/20013078.2017.1359479 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tominaga N et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun 6, 6716–6716, doi: 10.1038/ncomms7716 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De La Cruz EM How cofilin severs an actin filament. Biophys Rev 1, 51–59, doi: 10.1007/s12551-009-0008-5 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjee V et al. Endothelial microvesicles carrying Src-rich cargo impair adherens junction integrity and cytoskeleton homeostasis. Cardiovasc. Res 116, 1525–1538, doi: 10.1093/cvr/cvz238 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sperandio M, Gleissner CA & Ley K Glycosylation in immune cell trafficking. Immunol. Rev 230, 97–113, doi: 10.1111/j.1600-065X.2009.00795.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goncalves JP, Deliwala VJ, Kolarich D, Souza-Fonseca-Guimaraes F & Wolfram J The cancer cell-derived extracellular vesicle glycocode in immunoevasion. Trends Immunol, doi: 10.1016/j.it.2022.09.004 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Yang M et al. Extracellular vesicle glucose transporter-1 and glycan features in monocyte-endothelial inflammatory interactions. Nanomedicine, 102515, doi: 10.1016/j.nano.2022.102515 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Walker SA et al. Glycan Node Analysis of Plasma-Derived Extracellular Vesicles. Cells 9, 1946, doi: 10.3390/cells9091946 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams C et al. Glycosylation of extracellular vesicles: current knowledge, tools and clinical perspectives. J Extracell Vesicles 7, 1442985, doi: 10.1080/20013078.2018.1442985 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pendiuk Goncalves J et al. Glycan Node Analysis Detects Varying Glycosaminoglycan Levels in Melanoma-Derived Extracellular Vesicles. Int J Mol Sci 24, doi: 10.3390/ijms24108506 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y et al. EV-origin: Enumerating the tissue-cellular origin of circulating extracellular vesicles using exLR profile. Comput Struct Biotechnol J 18, 2851–2859, doi: 10.1016/j.csbj.2020.10.002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baluk P et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204, 2349–2362, doi: 10.1084/jem.20062596 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trzewik J, Mallipattu SK, Artmann GM, Delano FA & Schmid-Schönbein GW Evidence for a second valve system in lymphatics: endothelial microvalves. Faseb j 15, 1711–1717, doi: 10.1096/fj.01-0067com (2001). [DOI] [PubMed] [Google Scholar]
- 29.Breslin JW et al. Lymphatic Vessel Network Structure and Physiology. Compr Physiol 9, 207–299, doi: 10.1002/cphy.c180015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D et al. CD97 promotion of gastric carcinoma lymphatic metastasis is exosome dependent. Gastric Cancer 19, 754–766, doi: 10.1007/s10120-015-0523-y (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu A et al. Exosomal CD47 Plays an Essential Role in Immune Evasion in Ovarian Cancer. Mol. Cancer Res 19, 1583–1595, doi: 10.1158/1541-7786.MCR-20-0956 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Tessandier N et al. Platelets Disseminate Extracellular Vesicles in Lymph in Rheumatoid Arthritis. Arterioscler. Thromb. Vasc. Biol 40, 929–942, doi: 10.1161/ATVBAHA.119.313698 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welsh JD, Kahn ML & Sweet DT Lymphovenous hemostasis and the role of platelets in regulating lymphatic flow and lymphatic vessel maturation. Blood 128, 1169–1173, doi: 10.1182/blood-2016-04-636415 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Mehta D & Malik AB Signaling mechanisms regulating endothelial permeability. Physiol Rev 86, 279–367, doi: 10.1152/physrev.00012.2005 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Fernández-Hernando C et al. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab 10, 48–54, doi: 10.1016/j.cmet.2009.06.003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morad G et al. Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis. ACS Nano 13, 13853–13865, doi: 10.1021/acsnano.9b04397 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen CC et al. Elucidation of Exosome Migration Across the Blood–Brain Barrier Model In Vitro. Cell Mol Bioeng 9, 509–529, doi: 10.1007/s12195-016-0458-3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonda A, Kabagwira J, Senthil GN & Wall NR Internalization of Exosomes through Receptor-Mediated Endocytosis. Mol Cancer Res 17, 337–347, doi: 10.1158/1541-7786.MCR-18-0891 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Mulcahy LA, Pink RC & Carter DRF Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3, 24641–n/a, doi: 10.3402/jev.v3.24641 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng Y et al. The Blocking of Integrin-Mediated Interactions with Maternal Endothelial Cells Reversed the Endothelial Cell Dysfunction Induced by EVs, Derived from Preeclamptic Placentae. Int J Mol Sci 23, doi: 10.3390/ijms232113115 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fomina AF, Deerinck TJ, Ellisman MH & Cahalan MD Regulation of membrane trafficking and subcellular organization of endocytic compartments revealed with FM1–43 in resting and activated human T cells. Exp Cell Res 291, 150–166, doi: 10.1016/S0014-4827(03)00372-0 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morelli AE et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104, 3257–3266, doi: 10.1182/blood-2004-03-0824 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Wei X et al. Surface Phosphatidylserine Is Responsible for the Internalization on Microvesicles Derived from Hypoxia-Induced Human Bone Marrow Mesenchymal Stem Cells into Human Endothelial Cells. PLoS One 11, e0147360, doi: 10.1371/journal.pone.0147360 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He C, Hu Y, Yin L, Tang C & Yin C Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 31, 3657–3666, doi: 10.1016/j.biomaterials.2010.01.065 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Lu F, Wu SH, Hung Y & Mou CY Size Effect on Cell Uptake in Well‐Suspended, Uniform Mesoporous Silica Nanoparticles. Small 5, 1408–1413, doi: 10.1002/smll.200900005 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Théry C et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7, 1535750, doi: 10.1080/20013078.2018.1535750 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gould SJ & Raposo G As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles 2, 20389–n/a, doi: 10.3402/jev.v2i0.20389 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sousa de Almeida M et al. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev 50, 5397–5434, doi: 10.1039/d0cs01127d (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nazarenko I et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res 70, 1668–1678, doi: 10.1158/0008-5472.CAN-09-2470 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Yuan D et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 142, 1–12, doi: 10.1016/j.biomaterials.2017.07.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joshi BS & Zuhorn IS Heparan sulfate proteoglycan-mediated dynamin-dependent transport of neural stem cell exosomes in an in vitro blood-brain barrier model. Eur. J. Neurosci 53, 706–719, doi: 10.1111/ejn.14974 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ihrcke NS, Wrenshall LE, Lindman BJ & Platt JL Role of heparan sulfate in immune system-blood vessel interactions. Immunol Today 14, 500–505, doi: 10.1016/0167-5699(93)90265-M (1993). [DOI] [PubMed] [Google Scholar]
- 53.Chanda D et al. Fibronectin on the Surface of Extracellular Vesicles Mediates Fibroblast Invasion. Am J Respir Cell Mol Biol 60, 279–288, doi: 10.1165/rcmb.2018-0062OC (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Purushothaman A et al. Fibronectin on the Surface of Myeloma Cell-derived Exosomes Mediates Exosome-Cell Interactions. J Biol Chem 291, 1652–1663, doi: 10.1074/jbc.M115.686295 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng Z et al. Tumor Cell Cross Talk with Tumor-Associated Leukocytes Leads to Induction of Tumor Exosomal Fibronectin and Promotes Tumor Progression. The American Journal of Pathology 180, 390–398, doi: 10.1016/j.ajpath.2011.09.023 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Mertens G, Cassiman JJ, Van den Berghe H, Vermylen J & David G Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein characterization and antithrombin III binding properties. J Biol Chem 267, 20435–20443 (1992). [PubMed] [Google Scholar]
- 57.Matsumoto J et al. Transmission of alpha-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol Commun 5, 71, doi: 10.1186/s40478-017-0470-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banks WA et al. Transport of extracellular vesicles across the blood-brain barrier: Brain pharmacokinetics and effects of inflammation. Int J Mol Sci 21, 1–21, doi: 10.3390/ijms21124407 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hervé F, Ghinea N & Scherrmann J-M CNS Delivery Via Adsorptive Transcytosis. AAPS J 10, 455–472, doi: 10.1208/s12248-008-9055-2 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banks WA, Kastin AJ, Brennan JM & Vallance KL Adsorptive Endocytosis of HIV-1gp120 by Blood–Brain Barrier Is Enhanced by Lipopolysaccharide. Exp Neurol 156, 165–171, doi: 10.1006/exnr.1998.7011 (1999). [DOI] [PubMed] [Google Scholar]
- 61.Wurdinger T et al. Extracellular Vesicles and Their Convergence with Viral Pathways. Adv Virol 2012, 767694–767612, doi: 10.1155/2012/767694 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banks WA et al. Transport of human immunodeficiency virus type 1 pseudoviruses across the blood-brain barrier: role of envelope proteins and adsorptive endocytosis. J Virol 75, 4681–4691, doi: 10.1128/jvi.75.10.4681-4691.2001 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ben-Zvi A et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509, 507–511, doi: 10.1038/nature13324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andreone BJ et al. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 94, 581–594.e585, doi: 10.1016/j.neuron.2017.03.043 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen LN et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509, 503–506, doi: 10.1038/nature13241 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Busatto S et al. Lipoprotein-based drug delivery. Adv Drug Deliv Rev 159, 377–390, doi: 10.1016/j.addr.2020.08.003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simonsen JB What Are We Looking At? Extracellular Vesicles, Lipoproteins, or Both? Circ. Res 121, 920–922, doi: 10.1161/CIRCRESAHA.117.311767 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Toth EA et al. Formation of a protein corona on the surface of extracellular vesicles in blood plasma. J Extracell Vesicles 10, e12140, doi: 10.1002/jev2.12140 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sodar BW et al. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci. Rep 6, 24316, doi: 10.1038/srep24316 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Busatto S et al. Brain metastases-derived extracellular vesicles induce binding and aggregation of low-density lipoprotein. J Nanobiotechnology 18, 162, doi: 10.1186/s12951-020-00722-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Busatto S et al. Considerations for extracellular vesicle and lipoprotein interactions in cell culture assays. J Extracell Vesicles 11, e12202, doi: 10.1002/jev2.12202 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lozano-Andrés E et al. Physical association of low density lipoprotein particles and extracellular vesicles unveiled by single particle analysis. bioRxiv, 2022.2008.2031.506022, doi: 10.1101/2022.08.31.506022 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pham M-T et al. Endosomal egress and intercellular transmission of hepatic ApoE-containing lipoproteins and its exploitation by the hepatitis C virus. PLoS Pathog 19, e1011052–e1011052, doi: 10.1371/journal.ppat.1011052 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Broad K et al. Unraveling multilayered extracellular vesicles: Speculation on cause. J Extracell Vesicles 12, e12309, doi: 10.1002/jev2.12309 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Phinney DG et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nature communications 6, 8472, doi: 10.1038/ncomms9472 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dixson AC, Dawson TR, Di Vizio D & Weaver AM Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nature Reviews Molecular Cell Biology, doi: 10.1038/s41580-023-00576-0 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dallas SL, Prideaux M & Bonewald LF The osteocyte: an endocrine cell … and more. Endocr. Rev 34, 658–690, doi: 10.1210/er.2012-1026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abbott NJ, Ronnback L & Hansson E Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci 7, 41–53, doi: 10.1038/nrn1824 (2006). [DOI] [PubMed] [Google Scholar]
- 79.Xie Y, Bagby TR, Cohen MS & Forrest ML Drug delivery to the lymphatic system: importance in future cancer diagnosis and therapies. Expert Opin Drug Deliv 6, 785–792, doi: 10.1517/17425240903085128 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parker RJ, Hartman KD & Sieber SM Lymphatic absorption and tissue disposition of liposome-entrapped [14C]adriamycin following intraperitoneal administration to rats. Cancer Res 41, 1311–1317 (1981). [PubMed] [Google Scholar]
- 81.Fujimoto Y, Okuhata Y, Tyngi S, Namba Y & Oku N Magnetic resonance lymphography of profundus lymph nodes with liposomal gadolinium-diethylenetriamine pentaacetic acid. Biol Pharm Bull 23, 97–100, doi: 10.1248/bpb.23.97 (2000). [DOI] [PubMed] [Google Scholar]
- 82.Kang M, Jordan V, Blenkiron C & Chamley LW Biodistribution of extracellular vesicles following administration into animals: A systematic review. J Extracell Vesicles 10, e12085–n/a, doi: 10.1002/jev2.12085 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amruta A, Iannotta D, Cheetham SW, Lammers T & Wolfram J Vasculature organotropism in drug delivery. Adv Drug Deliv Rev 201, 115054, doi: 10.1016/j.addr.2023.115054 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li C et al. The role of Exosomal miRNAs in cancer. J Transl Med 20, 6, doi: 10.1186/s12967-021-03215-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crowl JT, Gray EE, Pestal K, Volkman HE & Stetson DB Intracellular Nucleic Acid Detection in Autoimmunity. Annu Rev Immunol 35, 313–336, doi: 10.1146/annurev-immunol-051116-052331 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Snaebjornsson MT, Janaki-Raman S & Schulze A Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab 31, 62–76, doi: 10.1016/j.cmet.2019.11.010 (2020). [DOI] [PubMed] [Google Scholar]
- 87.Lei K et al. Cancer-cell stiffening via cholesterol depletion enhances adoptive T-cell immunotherapy. Nat Biomed Eng 5, 1411–1425, doi: 10.1038/s41551-021-00826-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


