Abstract
B cell antigen receptor (BCR) association with lipid rafts, the actin cytoskeleton, and clathrin-coated pits influences B cell signaling and antigen presentation. Although all three cellular structures have been separately implicated in BCR internalization, the relationship between them has not been clearly defined. In this study, internalization pathways were characterized by specifically blocking each potential mechanism of internalization. BCR uptake was reduced by ∼70% in B cells conditionally deficient in clathrin heavy chain expression. Actin or raft antagonists were both able to block the residual, clathrin-independent BCR internalization. These agents also affected clathrin-dependent internalization, indicating that clathrin-coated pits, in concert with mechanisms dependent on rafts and actin, mediate the majority of BCR internalization. Clustering GM1 gangliosides enhanced clathrin-independent BCR internalization, and this required actin. Thus, although rafts or actin independently did not mediate BCR internalization, they apparently cooperate to promote some internalization even in the absence of clathrin. Simultaneous inhibition of all BCR uptake pathways resulted in sustained tyrosine phosphorylation and activation of the extracellular signal-regulated kinase (ERK), strongly suggesting that downstream BCR signaling can occur without receptor translocation to endosomes and that internalization leads to signal attenuation.
INTRODUCTION
The B cell immune response is initiated by antigen binding to the B cell antigen receptor (BCR). This results in signaling, entry of B cells into the cell cycle, and antigen internalization leading to its presentation to helper T-cells (Lanzavecchia, 1985; Reth and Wienands, 1997). Although BCR internalization is a well-defined event during antigen processing, several different membrane traffic pathways have been implicated in this process. Here, we address the relative contributions of three cellular mechanisms to BCR internalization and the relationship of BCR internalization to downstream receptor signaling.
Following engagement of the antigen receptor, the BCR can be observed in clathrin-coated pits and vesicles, suggesting a clathrin-mediated route of internalization (Salisbury et al., 1980; Brown and Song, 2001). The clathrin molecule has a triskelion or three-legged shape and is composed of three identical heavy chains, each with a tightly associated light chain. Clathrin triskelia, together with additional coat components, assemble into a polygonal lattice at the plasma membrane (Brodsky et al., 2001). The assembly of further triskelia accompanies invagination of the coated pit and transforms it into a clathrin-coated vesicle (CCV), which carries concentrated transmembrane receptors into the cell. In recent years, it has become increasingly apparent that internalization pathways are regulated by signaling cascades. For example, inhibitors or genetic deletions of protein tyrosine kinases prevent BCR internalization (Pure and Tardelli, 1992; Dykstra et al., 2001; Ma et al., 2001). Moreover, lipid rafts, which play a crucial role in the initiation and organization of signaling cascades, have been implicated in the control of clathrin-mediated internalization of the BCR (Stoddart et al., 2002). Here, we define rafts as cholesterolrich membrane microdomains, enriched in glycosphingolipids. These lipid microdomains vary in size depending on the aggregation of their contents, and they are domains with which acylated signaling molecules, such as SRC-family kinases, can associate. We previously presented evidence that lipid rafts spatially organize signaling cascades with clathrin for regulation of BCR internalization. An analysis of several different cell lines showed that BCR uptake occurred only when clathrin heavy chain (CHC) was associated with lipid rafts and tyrosine phosphorylated. These observations suggested the existence of a clathrin-mediated uptake pathway that is associated with rafts and regulated by receptors signaling in rafts.
In addition to a cooperative effort between rafts and clathrin, lipid rafts on their own may drive internalization of the BCR. However, the extent of raft involvement has been debated. It was originally reported that antigen-BCR complexes resident within plasma membrane lipid rafts can be internalized and delivered to endocytic compartments (Cheng et al., 1999). Whether this was direct or via raftassociated clathrin was not addressed. It was subsequently argued that lipid raft-mediated internalization cannot be a major pathway by which the bulk of antigen-BCR complexes gain access to the endocytic pathway, because the kinetics of BCR-mediated antigen internalization versus lipid raft-mediated cholera toxin B internalization are distinct (Putnam et al., 2003). Whether a cell uses a clathrinor raft-mediated pathway, membrane deformation is required for invagination and subsequent internalization. The actin cytoskeleton has been implicated in this process for both endocytic pathways. Actin has been found in association with rafts and caveolae, and actin-binding proteins, such as Hip 1R, link the clathrin coat to the actin cytoskeleton (Cheng et al., 1999; Chen and Brodsky, 2005). The BCR rapidly associates with the actin cytoskeleton following cross-linking (Hartwig et al., 1995), and actin polymerization appears necessary for scission and detachment of CCVs during BCR internalization (Brown and Song, 2001). Thus the actin cytoskeleton is implicated in BCR internalization in addition to its reported role in BCR signaling (Jugloff and Jongstra-Bilen, 1997).
Cross-linked cell surface receptors are ultimately degraded following uptake, so internalization eventually negatively regulates signaling. However, endocytosis can also play a positive role in receptor-mediated cellular activation. Some receptors continue to signal in endosomes, amplifying their impact after internalization and before degradation. For example, internalization of transforming growth factor-β, nerve growth factor, and epidermal growth factor receptors via the clathrin pathway is believed to trigger or amplify signaling from early endosomes (Grimes et al., 1996; Vieira et al., 1996; Howe et al., 2001; Hayes et al., 2002; Wang et al., 2002; Miaczynska et al., 2004). Thus, internalization can result in two different outcomes, depending on the surface receptors and context of their activation. Whether BCR internalization leads exclusively to inactivation of receptor signaling or is important for signal amplification has not been firmly established.
The recent development of a B cell line DKOS that is conditionally deficient in clathrin expression made it possible to investigate the relative contributions of different pathways to BCR internalization and their impact on BCR signaling. The hierarchy of internalization pathways was established by systematically studying the effects of raft and actin antagonists on clathrin-sufficient and -depleted B cells. The data suggest that the most extensive BCR internalization occurs when clathrin cooperates with rafts and actin. However, the BCR's promiscuous membrane associations enable it to exploit alternative, albeit less efficient, clathrin-independent routes of entry under certain cellular conditions. In cells where all pathways of BCR internalization were blocked, receptor activation, as measured by generalized tyrosine phosphorylation and ERK phosphorylation, was prolonged. This suggests that BCR endocytosis leads primarily to inactivation of receptor signaling.
MATERIALS AND METHODS
Cell lines, antibodies and reagents
The DKOS cell line was derived by inactivation of both endogenous alleles of chicken clathrin heavy chain in the DT40 chicken B cell line and expression of the human clathrin cDNA under the control of a tetracycline-regulatable expression system (Tet-Off) (Wettey et al., 2002). B cells were maintained in RPMI 1640 with 10% fetal calf serum (FCS), 1% chicken serum, 10 mM HEPES, and 50 μM 2-ME. Chicken serum was purchased from Invitrogen (Carlsbad, CA) by both the University of California San Francisco and Cambridge laboratories, and cell growth properties varied depending on the serum batch (Supplemental Figure 2). Goat polyclonal antiserum specific for chicken immunoglobulin M (IgM) was from Bethyl Laboratories (Montgomery, TX). Conalbumin (ovotransferrin) iron complex was from Sigma (Saint Louis, MO), biotinylated with a ×5 molar excess of Molecular Probes (Eugene, OR) Biotin-XX succinimidyl ester and purified with Microspin G-25 columns from Amersham Biosciences (Piscataway, NJ). Fluorescein isothiocyanate (FITC)-conjugated mouse antibodies specific for goat IgG, Cy5-conjugated donkey anti-goat IgG, and R-phycoerythrin–conjugated streptavidin were from Jackson Laboratories (West Grove, PA). Mouse monoclonal antibodies (mAbs) specific for CHC were X22 (Brodsky, 1985) and TD.1 (Näthke et al., 1992). A polyclonal rabbit antiserum against the CHC22 C-terminal fragment (residues 1521–1640) was generated by contract laboratory Covance (Denver, PA) (Towler et al., 2004). The mouse mAb AC-40 specific for actin was from Sigma. The mouse mAb 4G10 specific for phosphotyrosines was from Dr. A. DeFranco (University of California, San Francisco, CA). Rabbit antibodies specific for phosphorylated and unphosphorylated p42/p44 mitogen-activated protein (MAP) kinase (ERK1, ERK2) were from Cell Signaling Technology (Beverly, MA) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Latrunculin B (Calbiochem, San Diego, CA), derived from Latrunculia magnifica, was dissolved in dimethyl sulfoxide (DMSO) to a stock solution of 2 mM. Nystatin was dissolved in methanol to a stock of 50 mg/ml.
FACS-based BCR internalization assay
B cells were grown with or without 0.5 μg/ml doxycycline for 96 h. For all experiments, cells were plated at 3 × 105 cells/ml, split 10-fold at 48 h, and assayed at 96 h. To prepare cells, they were first washed with cold phosphatebuffered saline (PBS). To depolymerize actin or disrupt lipid rafts, cells were pretreated for 30 min with latrunculin B (2 μM) diluted from stock dissolved in DMSO or nystatin (25 μg/ml) from stock dissolved in methanol, respectively. Cells were incubated with 10 μg/ml goat anti-chicken IgM or ∼40 μg/ml biotinylated conalbumin (white egg transferrin) at a concentration of ∼2 × 107 cells/ml for 30 min at 4°C. Cells were washed twice with cold PBS and incubated at 37°C for 0–20 min. Internalization was stopped by incubating the cells on ice and adding cold PBS plus 1% fetal calf serum. To detect receptors remaining on the cell surface, cells were stained on ice with FITC-labeled anti-goat Ig or Rhodamine Red-labeled streptavidin, washed twice with PBS plus 1% FCS, and then fixed in 1% paraformaldehyde. Flow cytometry was performed on a FACScan, and the data were analyzed with CellQuest software (BD Biosciences, San Jose, CA). Percent BCR uptake is calculated as 100 − [(MFI@37°C/MFI@4°C) × 100], where MFI@4 and 37°C is the mean fluorescent intensity before and after internalization.
Immunofluorescence microscopy
For colocalization with clathrin, B cells were incubated with mouse anti-chicken IgM (M4; 10 μg/ml) at 4°C for 30 min, washed, and incubated at 37°C for 0 or 10 min. To monitor effects of raft clustering, B cells were incubated with goat anti-chicken IgM (10 μg/ml) at 4°C for 30 min, washed, and incubated at 37°C for 0 or 10 min. CTB Alexa 594 (Molecular Probes) was either prebound with the anti-IgM and then cross-linked (37°C) or bound to cells after BCR cross-linking but before fixation (4°C). Following the stimulations, cells were fixed in 4% paraformaldehyde for 15 min and quenched with 50 mM NH4Cl for 10 min. Cells were allowed to settle on polylysinecoated coverslips for ∼40 min, permeabilized with 0.2% Triton X-100, washed, and blocked with PBS plus 1% fish gelatin (Sigma) for 30 min. BCR was detected using a FITC-conjugated anti-mouse IgG (for M4) or Cy5-conjugated anti-goat IgG (for polyclonal anti-IgM). To detect clathrin, permeabilized cells were stained for clathrin light chain (CLC) using a polyclonal rabbit serum, washed three times in PBS, and stained with Rhodamine Red-conjugate anti-rabbit IgG. Phalloidin Alexa 488 was used to detect polymerized actin. The cells were mounted with Vectashield-DAPI (Vector Laboratories, Burlingame, CA) to stain the nucleus. Cells were viewed using a DeltaVision Restoration Microscope (Applied Precision, Issaquah, Washington), and a single section through the middle of the cell is shown. In Supplemental Figure 1, cells were viewed with a Zeiss (Thornwood, NY) Axioplan epi-fluorescence microscope. Images were processed using Adobe (San Jose, CA) Photoshop software.
Immunoprecipitation and Western blotting
To monitor clathrin expression, cells were lysed in a 500 mM Tris lysis buffer, and CHC was immunoprecipitated as described previously (Stoddart et al., 2002). To detect phosphorylated proteins and ERK, cells were lysed in buffer containing 1% Triton X-100, 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 10 mM NaF, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitors (10 μg/ml pepstatin, aprotinin, and leupeptin) on ice for 30 min (∼107 cells/ml). Lysates and precipitated proteins were resolved on NuPage gradient gels, transferred onto Millipore Immobilon polyvinylidene difluoride membrane, and immunoblotted using antibodies specific for phosphotyrosine (4G10), CHC (TD.1), actin (AC-40), ERK, or phospho-ERK (rabbit serum), followed by incubation with secondary peroxidase-conjugated antibodies specific for primary antibody. Blots were visualized with enhanced chemiluminescence (Amersham Biosciences). Blots were stripped between exposures to different antibody probes.
RESULTS
BCR uptake by human clathrin in DKOS chicken B cells lacking endogenous clathrin
The observance of BCRs in clathrin-coated pits and vesicles by electron microscopy indicates that BCR can be internalized via a clathrin-dependent pathway (Salisbury et al., 1980; Brown and Song, 2001). However, the extent of BCR's dependence on clathrin for its internalization has not been conclusively determined. To test this dependence, BCR uptake in B cells conditionally deficient in clathrin expression was examined. The generation of these cells was previously described and was as follows (Wettey et al., 2002). Both alleles of the chicken clathrin heavy chain gene were inactivated in the chicken B cell lymphoma, DT40, and the ubiquitous human clathrin heavy chain cDNA was expressed under the control of a tetracycline-responsive promoter. These B cells were designated DKOS, and, following exposure to doxycycline, they lose expression of the transfected human CHC, so become clathrin-negative. To first establish that the clathrin pathway is a route of BCR internalization in DKOS cells and that human clathrin substitutes for endogenous clathrin, the localization of BCR with human clathrin was examined by immunofluorescence. The antigen-binding subunit of the BCR on DKOS cells is IgM (Buerstedde and Takeda, 1991). Thus, bound anti-IgM antibodies were used to cross-link the BCR and mimic antigen binding. After 5 min of cross-linking, several punctate clathrin “spots” colocalized with BCR at the membrane and inside the cell (Figure 1A). These results show that BCR can be internalized via clathrin-coated pits in DKOS cells. We previously demonstrated that BCR signaling at the plasma membrane results in clathrin modification by phosphorylation (Stoddart et al., 2002). Similar to what occurs in human and mouse B cell lines previously examined, BCR cross-linking resulted in tyrosine phosphorylation of the human CHC expressed in DKOS cells (Figure 1B). Together, these results suggest that BCR signaling in DKOS cells normally leads to CHC tyrosine phosphorylation and promotes internalization via clathrin-coated pits.
Figure 1.
Clathrin-mediated internalization in DKOS cells. (A) DKOS cells were treated with mouse anti-chicken IgM at 4°C, incubated at 37°C for 0 and 5 min, and then fixed and permeabilized. BCR was detected with an FITC-conjugated anti-mouse IgG and clathrin with polyclonal anti-CLC rabbit serum plus Rhodamine Red-conjugate anti-rabbit IgG. Immunofluorescent images of a central cell section showing the localization of the BCR (green), clathrin (red), and nucleus (blue). Yellow arrowheads denote colocalized BCR and CHC. Scale bar, 10 μM. (B) DKOS cells were untreated (-) or treated with 10 μg/ml goat anti-chicken IgM at 37°C for 0–20 min. CHC, immunoprecipitated with the mAb X22, was resolved by electrophoresis and immunoblotted for phosphotyrosine (pTyr) and CHC.
BCR internalization is inhibited upon conditional depletion of clathrin heavy chain
To determine whether BCR exclusively depends on a clathrin-mediated pathway for uptake, BCR internalization in the presence and absence of clathrin expression was examined. DKOS cells express the ubiquitous isoform of human CHC cDNA under the control of a tetracycline regulatable promoter and lack the ubiquitous isoform of chicken CHC (Wettey et al., 2002). Exposure of DKOS cells to the tetracycline derivative, doxycycline, for 96 h led to a >90% reduction in expression of the human CHC (Figure 2A). There is a second isoform of CHC, designated CHC22, that is present in humans and chickens, but it does not function in receptormediated endocytosis. CHC22 is expressed at high levels primarily in muscle tissue (Liu et al., 2001), where it appears to be involved in tissue-specific membrane organization (Towler et al., 2004). A low level of CHC22 is, however, expressed in DT40 cells, and, given its 85% sequence similarity to ubiquitous CHC, it was possible that CHC22 might compensate for loss of CHC. However, expression of CHC22 did not increase upon depletion of ubiquitous CHC (Figure 2A), suggesting that CHC22 was not responding to the absence of ubiquitous CHC expression. This is in keeping with its apparent lack of participation in conventional clathrin functions in nonmuscle cells (Liu et al., 2001).
Figure 2.
Conditional deletion of clathrin inhibits BCR internalization. DKOS cells were grown with (+) or without (−) 0.5 μg/ml doxycycline for 96 h. (A) Ubiquitous clathrin heavy chain (CHC), clathrin heavy chain isoform CHC22, and actin expression were determined by immunoblotting with anti-clathrin heavy chain mAb (TD.1), anti-CHC22 rabbit antiserum, and anti-actin mAb (AC-40). CHC** designates that this blot was overexposed to show residual CHC expression. (B) Cells were treated with 10 μg/ml goat anti-chicken IgM or biotinylated transferrin for 30 min at 4°C, washed, and warmed to 37°C for the times indicated. To detect receptors remaining on the cell surface, cells were stained on ice with FITC-labeled anti-goat Ig or Rhodamine Red-labeled streptavidin and analyzed by flow cytometry. The percent uptake represents the percentage of cells that no longer stain for surface BCR or TfR relative to staining at 4°C.
Internalization of the BCR and transferrin receptor (TfR) before and after clathrin depletion was next compared. Cells were treated with unlabeled goat anti-chicken IgM or biotinylated conalbumin, the chicken egg-white isoform of transferrin. Cells were washed to remove unbound ligand and then incubated at 37°C for 0–20 min. To monitor internalization, the B cell and transferrin receptors remaining on the cell surface were labeled with FITC-conjugated anti-goat antibody or streptavidin-PE, respectively, and then analyzed by flow cytometry (Figure 2B). In agreement with previous observations (Wettey et al., 2002), depletion of clathrin led to a 70–80% reduction in transferrin uptake. Ten minutes after BCR engagement, BCR internalization was inhibited ∼70%, confirming that the bulk of BCR internalization depends on clathrin expression. Some residual BCR uptake did, however, occur after conditional depletion of CHC, suggesting that there is also a minor clathrin-independent route for BCR uptake. Because CHC expression was not completely obliterated (Figure 2A), it remained possible that residual BCR uptake was mediated by a minor population of cells that continued to express high levels of clathrin. However, examination of internalized BCRs in doxycycline-treated cells revealed that there was no correlation between internalized BCR and uneven depletion of CHC (Figure S1). In contrast to the distinct punctate CHC staining in untreated cells (Figure S1A), CHC staining was diffuse and very dim for all doxycycline-treated cells (Figure S1B) (n > 500), indicating an absence of any lingering CHC-positive cells. These results suggest that residual BCR internalization was in fact clathrin-independent and not due to a few cells maintaining wild-type expression levels of CHC.
To rule out the possibility that the observed inhibition of BCR uptake was due to compromised cell viability, cell growth was assessed. After 96 h of doxycycline treatment, corresponding to conditions of the internalization studies, cell growth was only slightly decreased when clathrin expression was suppressed (Figure 3A), and the percentage of cells in the sub-G0 or S + G2/M phase was similar for untreated and doxycycline-treated cells (Figure 3B). This is in slight contrast to the initial characterization of DKOS by Wettey et al. (2002). However, a side-by-side comparison of cell growth using the chicken serum used in these experiments with that previously used revealed that different serum lots were responsible for differences in cell viability following depletion of clathrin (Figure S2). Thus, under conditions used in these experiments, the inhibition of BCR internalization observed for doxycycline-treated cells can be attributed to repression of clathrin expression and not to cell death. These results conclusively demonstrate that clathrinmediated endocytosis is a major pathway of BCR uptake.
Figure 3.
DKOS viability is not compromised by CHC depletion in conditions used for the internalization assays.(A) DKOS cells were plated at 2 × 104 cells/ml with (CHC−) or without (CHC+) doxycycline and maintained for 5 d. A small sample was removed at each time point, and live cells were counted by trypan blue exclusion. (B) DKOS cells were plated at 3 × 105 cells/ml with or without doxycycline and split 10-fold at 48 h (condition used for internalization assays). Cells were fixed in 70% ethanol overnight at 4°C and then centrifuged and resuspended in propidium iodide/RNase A solution (Sigma 50 μg/ml and 5 mg/ml, respectively) at room temperature for >30 min. The percentage of cells in each phase of the cell cycle was assessed by flow cytometry.
The role of actin and lipid rafts in BCR-mediated endocytosis
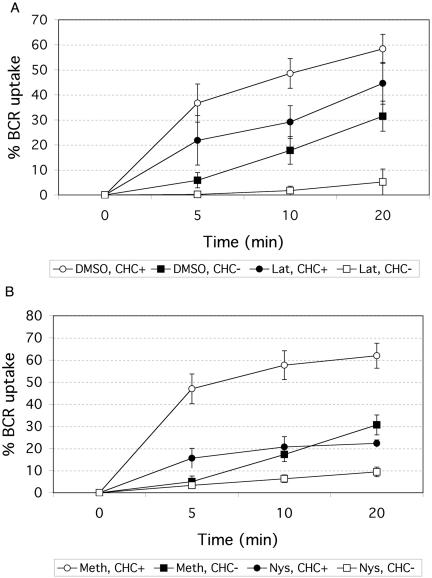
Because the BCR displays complex membrane associations, namely with lipid rafts, clathrin, and actin, we hypothesized that it could adapt its internalization route depending on physiological conditions. Thus, the internalization pathways of the BCR were examined by observing the effects of an actin antagonist (latrunculin) or a raft antagonist (nystatin) on B cells conditionally depleted of CHC. BCR internalization in clathrin-expressing DKOS was 50% inhibited after treatment with latrunculin, an actin-depolymerizing agent (Figure 4A). Clathrin depletion by doxycycline treatment resulted in a slightly greater inhibition. However, when both actin polymerization and clathrin expression were inhibited in DKOS cells, BCR internalization was completely blocked. This implicates the actin cytoskeleton in clathrin-independent internalization in addition to its known role in clathrindependent internalization of the BCR (Brown and Song, 2001).
Figure 4.
Pharmacological agents that disrupt actin or rafts inhibit BCR internalization. DKOS cells were grown with (CHC−) or without (CHC+) doxycycline. (A) Cells were treated for 30 min with 2 μM latrunculin B (Lat), stock dissolved in DMSO, or the equivalent concentration of DMSO alone. (B) Cells were treated for 30 min with 25 μg/ml nystatin (Nys) in 0.05% methanol (Meth) or 0.05% Meth alone. BCR internalization was monitored after each treatment as described in Figure 2.
It has been suggested that lipid rafts play a role in BCR-mediated internalization; however, the extent of raft involvement has been debated (Cheng et al., 1999; Putnam et al., 2003). To assess the involvement of lipid rafts in clathrindependent and clathrin-independent pathways of DKOS B cells, cells were treated with nystatin, a drug that binds cholesterol and has commonly been used to disrupt lipid rafts. Pretreatment with nystatin inhibited BCR internalization to a similar extent as clathrin depletion (∼66% inhibition) (Figure 4B), supporting our previous model that lipid rafts functionally interact with clathrin to regulate BCR internalization (Stoddart et al., 2002). When cells were treated with both nystatin and doxycycline, BCR internalization was reduced by >90%. This suggests that, for some internalizing BCRs, the raft pathway may be uncoupled from clathrin, and implicates lipid rafts in the clathrin-independent pathway. It also suggests that some BCR uptake is mediated by clathrin without raft participation. Although no one reagent is absolutely specific for disrupting rafts, nystatin at the concentration used appeared to disrupt lipid rafts without substantially affecting clathrin-coated pit formation, since its effect on TfR uptake (which has been shown to internalize exclusively through clathrin-coated pits) was negligible compared with its effect on BCR uptake (Figure S3). Thus, the nystatin disruption of rafts must uncouple rafts from clathrin in the cooperative pathways as well as interfere with the residual BCR internalization that occurs in the absence of clathrin expression. Taken together, our results support the following model. The most extensive BCR internalization occurs when BCR associates with lipid rafts, clathrin, and the actin cytoskeleton. Reduced BCR internalization can still proceed provided two of these three membrane components (rafts and actin, clathrin and actin, or clathrin and rafts) are able to cooperate with each other. However, if actin or rafts function independently (i.e., in the absence of any other components), they cannot mediate the internalization of BCRs on their own.
Plasticity of BCR internalization
Given the interaction of BCRs with both rafts and clathrin and the interplay of these interactions, we hypothesized that, under certain conditions, raft-mediated uptake may be able to compensate for the absence of clathrin. Sphingolipids, including gangliosides such as GM1, are proposed structural components of lipid rafts (Parton, 1994). Although rafts are thought to be heterogenous, the cholera toxin subunit B (CTB) is widely used as a marker for the microdomains containing GM1. Thus, the localization of CTB was compared with that of the BCR during internalization. First, DKOS cells with prebound anti-IgM were incubated at 37°C to induce internalization and then chilled and stained with red-labeled CTB (Figure 5A, 4°C). CTB remained on the cell surface for both CHC+ and CHC− cells, whereas BCR was internalized only in clathrin-expressing cells. If, however, cells were prebound with both anti-IgM and CTB before incubation at 37°C, the BCR colocalized with CTB in internalized vesicles in either clathrin-expressing or clathrin-deficient cells. (Figure 5A, 37°C). Interestingly, there was an increase in BCR internalization in the clathrin-deficient DKOS cells that were pretreated with CTB at 37°C compared with cells stained with CTB after BCR cross-linking (Figure 5A; compare d to e). Normally, in the absence of clathrin expression, only ∼10% of DKOS cells have completely internalized their BCRs, 10 min after cross-linking. This was observed by FACS (Figure 2B) and by immunofluorescence in at least eighty separate DKOS cells (n > 80). However, after treatment with CTB at 37°C, ∼80% of clathrin-deficient DKOS B cells (n > 200) exhibited internalized BCRs that colocalized with CTB (Figure 5A, d).
Figure 5.
Clustering GM1 gangliosides induces BCR internalization in clathrin-deficient DKOS cells. (A) DKOS cells were grown with or without doxycycline as indicated (CHC− and CHC+, respectively). Goat anti-chicken IgM was prebound to cells at 4°C. After a 30-min incubation, unbound antibody was removed by washing. Cells were placed at 37°C for 0 or 10 min to induce internalization. Cholera toxin B subunit (CTB)-Alexa 594 (red) was either prebound (b and d) with the anti-IgM and then cross-linked (37°C) or bound to cells after BCR cross-linking (a, c, and e) (4°C). Cells were permeabilized, fixed, and labeled with phalloidin Alexa 488 (green) or donkey anti-goat cy5 (blue) to stain actin and the BCR, respectively. (B) DKOS grown in doxycycline to deplete CHC were pretreated with DMSO or latrunculin B (Lat). CTB-Alexa 594 and anti-IgM were prebound on ice and then cross-linked at 37°C for 0 and 10 min. Cells were further processed as described in A. Scale bar, 10 μm.
The role of actin in CTB-mediated uptake of the BCR was then investigated. Phalloidin staining (green), which labels the actin filaments, did not colocalize with internalized receptors; rather, it remained on the cell surface (Figure 5A). Treatment of clathrin-deficient DKOS cells with latrunculin B caused actin depolymerization, resulting in the absence of phalloidin staining and reduced CTB-mediated internalization (Figure 5B). Less than 36% (n = 287) of latrunculin B-treated cells cointernalized CTB and BCR (Figure 5B, d). For untreated clathrin-deficient cells (with actin staining visible on the cell surface), cross-linking CTB led to the cointernalization of CTB with BCR in the majority of cells (∼78%, n = 382) (Figure 5B, b). Disruption of actin polymerization also completely blocked the nonclathrin-dependent BCR internalization in doxycycline-treated B cells that were not exposed to CTB (Figure 4A). CTB binds GM1 pentavalently (Janes et al., 1999), so clustering GM1 gangliosides could change the BCR internalization pathway by coclustering and/or by altering signaling to enhance BCR uptake in clathrin-depleted cells. Moreover, actin appears to play a role in this clathrin-independent internalization pathway whether or not the internalization is induced by CTB (Figures 4A and 5B). We speculate that binding of CTB mimics clustering of lipid raft domains and induces their internalization along with the BCR. This “raft”-dependent pathway may operate independently of clathrin, but actin polymerization appears to be required.
The experiments described above suggest that receptor clustering in rafts may in some instances provide an alternative route to clathrin-mediated BCR internalization. This plasticity in BCR internalization was demonstrated further by a spontaneous variant of DKOS cells, named DKOR. Like DKOS cells, exposure of DKOR cells to doxycycline led to a >90% reduction in CHC expression (Figure 6A). In striking contrast to DKOS cells, BCR internalization was not inhibited following clathrin depletion in DKOR cells (Figure 6B). This effect was specific to the BCR, since TfR internalization was still inhibited by the loss of clathrin expression in DKOR cells. These results suggest that TfR has a stricter dependence on clathrin for internalization than the BCR. Perhaps in contrast to the TfR, the BCR's promiscuous associations, namely with clathrin, rafts, and actin, enable it to adapt from a clathrin-dependent to an -independent pathway of internalization under certain cellular pressures.
Figure 6.
BCR internalization is not inhibited in a variant subclone, DKOR, upon conditional depletion of CHC. DKOR cells were grown with (+) or without (−) 0.5 μg/ml doxycycline for 96 h. (A) Ubiquitous clathrin heavy chain (CHC) and actin expression were determined by immunoblotting with anti-clathrin heavy chain mAb (TD.1) and anti-actin mAb (AC-40) in DKOR cells. CHC** designates that this blot was overexposed to reveal residual CHC expression. (B) DKOR cells were treated with 10 μg/ml goat anti-chicken IgM or biotinylated conalbumin (white egg transferrin) for 30 min at 4°C, washed, and warmed to 37°C for the times indicated. To detect receptors remaining on the cell surface, cells were stained on ice with FITC-labeled anti-goat Ig or Rhodamine Red-labeled streptavidin and analyzed by flow cytometry. The percent uptake represents the percentage of cells that no longer stain for surface BCR or TfR.
Internalization is not a prerequisite for BCR signaling
Recently, analyses of other cell surface receptors have suggested that internalization to endosomes is important to maintain or amplify signaling (Grimes et al., 1996; Vieira et al., 1996; Howe et al., 2001; Wang et al., 2002; Di Guglielmo et al., 2003). Whether BCR internalization plays an active role in the propagation of signals has not been firmly established. The ability to completely block BCR internalization in DKOS cells and eliminate the potential for endosomal signaling provides a direct means to address this question. To determine whether BCR internalization through the clathrin pathway was required to activate signal transduction pathways, two indicators of signaling were measured following clathrin depletion with and without actin depolymerization (Figure 7). DKOS cells were stimulated with anti-BCR antibodies for 0, 10, and 30 min. Signaling after BCR cross-linking, as measured by inducible protein tyrosine phosphorylation, was not diminished in clathrin-negative DKOS cells compared with clathrin-positive cells. Nor was it diminished when internalization was completely blocked by depletion of clathrin and depolymerization of actin. In fact, overall tyrosine phosphorylation at the 30-min time point was increased after administration of doxycycline and latrunculin B. The fact that signaling was not diminished and was, rather, increased by an absence of BCR internalization suggests that endosomal signaling is not important in maintaining or amplifying cellular responses in B cells.
Figure 7.
Inhibition of BCR endocytosis does not block BCR-stimulated signaling. DKOS cells were grown with (CHC−) or without (CHC+) doxycycline for 96 h and pretreated with DMSO or latrunculin B. B cells were either not stimulated (−) or treated with 10 μg/ml goat anti-chicken IgM at 37°C for 10 and 30 min. Ten micrograms of lysate was resolved by electrophoresis and immunoblotted for phosphotyrosine (pTyr), CHC, unphosphorylated and phosphorylated ERK1 and ERK2, and actin. Only the ERK2 isoform is detected in DKOS cells.
It remained possible that prolonged plasma membrane signaling, resulting from the retention of receptors on the cell surface, simply masked any effects caused by the absence of endosomal signaling. To address this possibility, a second signaling readout, the MAP kinase cascade, was examined. It has been reported that various inhibitors of endocytosis can inhibit the activation of the p42/p44 MAP kinases (also named ERK1 and ERK2) (Kholodenko, 2003), indicating a potential role for endosomal signaling in this cascade. Lysates of DKOS cells subjected to inhibition of BCR internalization, as described above, were immunoblotted with antiphospho-ERK antibodies to detect activated ERK. A large increase in phosphorylated ERK2 was detected after the BCR was cross-linking for 10 and 30 min. (Only the ERK2 isoform is expressed in DKOS cells.) This increase in phosphorylated ERK2 was not diminished in the absence of clathrin expression nor by the disruption of the actin cytoskeleton. Thus, the absence of BCR internalization did not prevent ERK activation but, rather, prolonged it. One possible explanation for the slightly increased phospho-tyrosine and phospho-ERK in cells that were treated with latrunculin, but not stimulated by antibody ligand, is that the absence of actin polymerization may sustain association of BCR with raft components and thereby enhance signaling, similar to the results reported for the high-affinity receptor for IgE (FcεR) (Holowka et al., 2000). This may occur if actin polymerization is required for keeping receptors out of rafts unless cross-linked. Together, these results support the hypothesis that BCR signaling is initiated at the plasma membrane and that trafficking through endosomes does not play an active role in amplification or propagation of signals in response to BCR cross-linking. Thus, with regard to signaling, the role of BCR internalization appears to be attenuation.
DISCUSSION
The BCR has been shown to associate with the actin cytoskeleton, lipid rafts, and clathrin following activation, indicating the potential for several internalization routes. Although for years it was generally assumed that the BCR internalized via clathrin-coated pits (Salisbury et al., 1980; Brown and Song, 2001), more recent data put forth the idea that rafts may be the conduit to the cell's interior (Cheng et al., 1999; Putnam et al., 2003). In addition, experiments have suggested that actin is a major player in the internalization of BCRs (Brown and Song, 2001). This study is the first to selectively block each potential mechanism in order to dissect the internalization pathways utilized by the BCR following activation. Our results suggest the following pathways of BCR internalization. The most extensive BCR internalization occurs when clathrin cooperates with lipid rafts and the actin cytoskeleton. This likely represents the major pool of internalizing receptors. However, the BCR displays some plasticity, since it can also utilize additional, albeit less efficient, pathways of internalization. Reduced BCR internalization was observed when B cells were separately depleted of clathrin, treated with a raft antagonist, or treated with an actin antagonist. This suggests that two out of the three membrane traffic mechanisms (rafts and actin, clathrin and actin, or clathrin and rafts) can cooperate to mediate some internalization. However, rafts or actin on their own do not appear sufficient for BCR internalization, since depletion of clathrin combined with raft or actin antagonists completely abrogated BCR internalization. The enhanced and prolonged activation of ERK, in conditions that prevent BCR internalization, suggested that signaling in endocytic compartments does not maintain or amplify B cell responses and that internalization is the first step to attenuation of BCR signaling. The dual function of the BCR for signaling and antigen delivery could account for this behavior that distinguishes it from other signaling receptors that continue to signal from endosomes. We propose that delivery of the BCR to an endocytic compartment involved in antigen processing explains its lack of sustained endosomal signaling.
There has been a tendency to classify receptor internalization pathways as “clathrin-dependent” or “-independent”. Our studies show that rigid classifications do not apply to receptors with complex membrane associations, such as the BCR. We previously proposed the existence of a clathrinmediated pathway for BCR uptake that is physically associated with receptors that signal in rafts and that depends on signal-induced CHC phosphorylation. We published biochemical evidence for this (Stoddart et al., 2002), and morphological evidence for clathrin in the vicinity of rafts has been observed for the FcεR (Wilson et al., 2000). The new data presented here showing 70% inhibition in BCR internalization following clathrin depletion suggests that clathrin-mediated internalization is a major pathway for BCR uptake. Raft integrity was shown to be equally important for BCR uptake, correlating with our model that clathrin-mediated BCR internalization is associated with signaling in rafts. In addition, we observed that the BCR displayed an ability to adapt its internalization pathway under certain conditions. This was demonstrated by the induction of BCR internalization in clathrin-deficient DKOS cells following the clustering of glycosphingolipids, which are enriched in lipid rafts. These experiments suggest that raft-dependent internalization may in some instances compensate for clathrinmediated endocytosis or, in cases of receptor clustering in rafts, even provide an alternate route of receptor internalization. The BCR's inherent adaptability was also demonstrated by a spontaneous variant of DKOS, named DKOR, that lost its dependence on clathrin for BCR-mediated uptake. This effect was specific to the BCR, since TfR internalization was still inhibited by the loss of clathrin expression in DKOR cells. We speculate that the intimate relationship between rafts and clathrin that normally regulates BCR internalization allows the cell to adapt its internalization pathways under certain cellular conditions. Receptors such as the TfR that localize strongly to clathrin-coated pits in the absence of CHC phosphorylation and never to raft domains (Lamaze et al., 2001; Stoddart et al., 2002) likely do not have access to such alternative routes of internalization. Rather, this sort of plasticity might be expected of receptors that signal in rafts, but can be internalized by clathrin-mediated routes, such as the epidermal growth factor receptor, the T-cell receptor, and the FcεR (Wilde et al., 1999; Wilson et al., 2000; Stoddart et al., 2002; Crotzer et al., 2004). The ability of these receptors to move from one membrane domain to another and to regulate that movement reflects their complexity of function, such that internalization can lead to receptor signaling, degradation, and/or recycling depending on the requirements of the stimulated cell. Such plasticity was also recently reported for the TGF-β receptor and likely reflects a similar diversity of internalization fates in response to cellular conditions (Di Guglielmo et al., 2003).
It is becoming ever more apparent that the processes of receptor signaling and internalization are interdependent in that each can have a significant influence on the other. It is well documented that signaling induces modification of proteins involved in internalization, including clathrin (Wilde and Brodsky, 1996; Slepnev et al., 1998; Wilde et al., 1999; Stoddart et al., 2002; Crotzer et al., 2004). The recent observations that various inhibitors of endocytosis inhibit MAP kinase activation emphasizes the converse interplay between endocytic and signaling systems (Grimes et al., 1996; Howe et al., 2001; Di Guglielmo et al., 2003; Kholodenko, 2003). With regard to the latter observations, it has been proposed that complexes between receptors and signaling partners may be prolonged or even promoted by their colocalization in endosomes (Kholodenko, 2003). However, for some receptors, endocytosis does not appear to be essential for the activation of MAP kinase cascades (Leof, 2000). Our data suggest that BCR internalization falls into this latter category and further imply that endocytosis of the BCR serves to dampen signaling that is initiated at the plasma membrane. The second major function of the BCR is to internalize antigen for delivery to appropriate processing compartments. This functionally important targeting to degradative compartments may explain why BCR signaling primarily occurs at the plasma membrane and does not rely on further residence in endosomes for amplification. Thus, BCR differs from growth factor receptors that signal in endosomes and progress further along the endocytic pathway only for their down-regulation.
In conclusion, our results demonstrate that clathrin-mediated endocytosis in conjunction with raft signaling and actin polymerization is the major pathway of BCR internalization. Moreover, our results suggest that the BCR's ability to associate with membrane microdomains also allows it to exploit a lipid raft- and actin-dependent route of internalization under certain cellular conditions. Whether this alternative internalization pathway occurs preferably at synapse junctions, where raft clustering is promoted, remains an open question. Unlike some other growth factor receptors, internalization and trafficking to endosomes is not required to amplify BCR signaling, and we propose that this reflects the dual function of the BCR for both signaling and antigen delivery.
Acknowledgments
We thank A. DeFranco for the phospho-tyrosine specific antibody (4G10) and M. LeBeau (University of Chicago, Chicago, IL) for use of the Zeiss epifluorescence microscope. This work was supported by National Institutes of Health grant GM38093 to F. M. Brodsky and by a postdoctoral fellowship from the Lymphoma Research Foundation to A. Stoddart.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–01–0025) on February 16, 2005.
Abbreviations used: BCR, B cell antigen receptor; CHC, clathrin heavy chain; CCV, clathrin-coated vesicle; CTB, cholera toxin B; DMSO, dimethyl sulfoxide; ERK, extracellular signal-regulated kinase; FITC, fluorescein isothiocyanate; FCS, fetal calf serum; Ig, immunoglobulin; MAP, mitogen-activated protein; PBS, phosphatebuffered saline; TfR, transferrin receptor.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Brodsky, F. M. (1985). Clathrin structure characterized with monoclonal antibodies. I. Analysis of multiple antigenic sites. J. Cell Biol. 101, 2047–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky, F. M., Chen, C. Y., Knuehl, C., Towler, M. C., and Wakeham, D. E. (2001). Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17, 517–568. [DOI] [PubMed] [Google Scholar]
- Brown, B. K., and Song, W. (2001). The actin cytoskeleton is required for the trafficking of the B cell antigen receptor to the late endosomes. Traffic 2, 414–427. [DOI] [PubMed] [Google Scholar]
- Buerstedde, J. M., and Takeda, S. (1991). Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell 67, 179–188. [DOI] [PubMed] [Google Scholar]
- Chen, C-Y. and Brodsky, F. M. (2005). Huntingtin-interacting protein 1 (Hip 1) and Hip 1-related protein (Hip 1R) bind the conserved sequences of clathrin light chains and thereby influence clathrin assembly in vitro and actin distribution in vivo. J. Biol. Chem. 280, 6109–6117. [DOI] [PubMed] [Google Scholar]
- Cheng, P. C., Dykstra, M. L., Mitchell, R. N., and Pierce, S. K. (1999). A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J. Exp. Med. 190, 1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotzer, V. L., Mabardy, A. S., Weiss, A., and Brodsky, F. M. (2004). T cell receptor engagement leads to phosphorylation of clathrin heavy chain during receptor internalization. J. Exp. Med. 199, 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guglielmo, G. M., Le Roy, C., Goodfellow, A. F., and Wrana, J. L. (2003). Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 5, 410–421. [DOI] [PubMed] [Google Scholar]
- Dykstra, M. L., Longnecker, R., and Pierce, S. K. (2001). Epstein-Barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity 14, 57–67. [DOI] [PubMed] [Google Scholar]
- Grimes, M. L., Zhou, J., Beattie, E. C., Yuen, E. C., Hall, D. E., Valletta, J. S., Topp, K. S., LaVail, J. H., Bunnett, N. W., and Mobley, W. C. (1996). Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J. Neurosci. 16, 7950–7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig, J. H., Jugloff, L. S., De Groot, N. J., Grupp, S. A., and Jongstra-Bilen, J. (1995). The ligand-induced membrane IgM association with the cytoskeletal matrix of B cells is not mediated through the Ig alpha beta heterodimer. J. Immunol. 155, 3769–3779. [PubMed] [Google Scholar]
- Hayes, S., Chawla, A., and Corvera, S. (2002). TGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J. Cell Biol. 158, 1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowka, D., Sheets, E. D., and Baird, B. (2000). Interactions between Fc(epsilon)RI and lipid raft components are regulated by the actin cytoskeleton. J. Cell Sci. 113 (Pt 6), 1009–1019. [DOI] [PubMed] [Google Scholar]
- Howe, C. L., Valletta, J. S., Rusnak, A. S., and Mobley, W. C. (2001). NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron 32, 801–814. [DOI] [PubMed] [Google Scholar]
- Janes, P. W., Ley, S. C., and Magee, A. I. (1999). Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 147, 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugloff, L. S., and Jongstra-Bilen, J. (1997). Cross-linking of the IgM receptor induces rapid translocation of IgM-associated Ig alpha, Lyn, and Syk tyrosine kinases to the membrane skeleton. J. Immunol. 159, 1096–1106. [PubMed] [Google Scholar]
- Kholodenko, B. N. (2003). Four-dimensional organization of protein kinase signaling cascades: the roles of diffusion, endocytosis and molecular motors. J. Exp. Biol. 206, 2073–2082. [DOI] [PubMed] [Google Scholar]
- Lamaze, C., Dujeancourt, A., Baba, T., Lo, C. G., Benmerah, A., and DautryVarsat, A. (2001). Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol. Cell 7, 661–671. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia, A. (1985). Antigen-specific interaction between T and B cells. Nature 314, 537–539. [DOI] [PubMed] [Google Scholar]
- Leof, E. B. (2000). Growth factor receptor signalling: location, location, location. Trends Cell Biol. 10, 343–348. [DOI] [PubMed] [Google Scholar]
- Liu, S.-H., Towler, M. C., Chen, E., Chen, C.-Y., Song, W., Apodaca, G., and Brodsky, F. M. (2001). A novel clathrin homolog that co-distributes with cytoskeletal components functions in the trans-Golgi network. EMBO J. 20, 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, H., Yankee, T. M., Hu, J., Asai, D. J., Harrison, M. L., and Geahlen, R. L. (2001). Visualization of Syk-antigen receptor interactions using green fluorescent protein: differential roles for Syk and Lyn in the regulation of receptor capping and internalization. J. Immunol. 166, 1507–1516. [DOI] [PubMed] [Google Scholar]
- Miaczynska, M., Christoforidis, S., Giner, A., Shevchenko, A., UttenweilerJoseph, S., Habermann, B., Wilm, M., Parton, R. G., and Zerial, M. (2004). APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell 116, 445–456. [DOI] [PubMed] [Google Scholar]
- Näthke, I., Heuser, J., Lupas, A., Stock, J., Turck, C. W., and Brodsky, F. M. (1992). Folding and trimerization of clathrin subunits at the triskelion hub. Cell 68, 899–910. [DOI] [PubMed] [Google Scholar]
- Parton, R. G. (1994). Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J. Histochem. Cytochem. 42, 155–166. [DOI] [PubMed] [Google Scholar]
- Pure, E., and Tardelli, L. (1992). Tyrosine phosphorylation is required for ligand-induced internalization of the antigen receptor on B lymphocytes. Proc. Natl. Acad. Sci. USA 89, 114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam, M. A., Moquin, A. E., Merrihew, M., Outcalt, C., Sorge, E., Caballero, A., Gondre-Lewis, T. A., and Drake, J. R. (2003). Lipid raft-independent B cell receptor-mediated antigen internalization and intracellular trafficking. J. Immunol. 170, 905–912. [DOI] [PubMed] [Google Scholar]
- Reth, M., and Wienands, J. (1997). Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol. 15, 453–479. [DOI] [PubMed] [Google Scholar]
- Salisbury, J. L., Condeelis, J. S., and Satir, P. (1980). Role of coated vesicles, microfilaments, and calmodulin in receptor-mediated endocytosis by cultured B lymphoblastoid cells. J. Cell Biol. 87, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepnev, V. I., Ochoa, G.-C., Butler, M. H., Grabs, D., and De Camilli, P. (1998). Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science 281, 821–824. [DOI] [PubMed] [Google Scholar]
- Stoddart, A., Dykstra, M. L., Brown, B. K., Song, W., Pierce, S. K., and Brodsky, F. M. (2002). Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity 17, 451–462. [DOI] [PubMed] [Google Scholar]
- Towler, M. C., Gleeson, P. A., Hoshino, S., Rahkila, P., Manalo, V., Ohkoshi, N., Ordahl, C., Parton, R. G., and Brodsky, F. M. (2004). Clathrin isoform CHC22, a component of neuromuscular and myotendinous junctions, binds sorting nexin 5 and has increased expression during myogenesis and muscle regeneration. Mol. Biol. Cell 15, 3181–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, A. V., Lamaze, C., and Schmid, S. L. (1996). Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274, 2086–2089. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Pennock, S., Chen, X., and Wang, Z. (2002). Endosomal signaling of EGF receptor stimulates signal transduction pathways leading to cell survival. Mol. Cell. Biol. 22, 7279–7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettey, F. R., Hawkins, S. F., Stewart, A., Luzio, J. P., Howard, J. C., and Jackson, A. P. (2002). Controlled elimination of clathrin heavy-chain expression in DT40 lymphocytes. Science 297, 1521–1525. [DOI] [PubMed] [Google Scholar]
- Wilde, A., Beattie, E. C., Lem, L., Riethof, D. A., Liu, S.-H., Mobley, W. C., Soriano, P., and Brodsky, F. M. (1999). EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell 96, 677–687. [DOI] [PubMed] [Google Scholar]
- Wilde, A., and Brodsky, F. M. (1996). In vivo phosphorylation of adaptors regulates their interaction with clathrin. J. Cell Biol. 135, 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, B. S., Pfeiffer, J. R., and Oliver, J. M. (2000). Observing FcepsilonRI signaling from the inside of the mast cell membrane. J. Cell Biol. 149, 1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]