Abstract
Opioid use disorder (OUD) and opioid overdoses are public health emergencies. In 2021, 80,000 opioid overdose associated deaths were reported in the United States. Despite the availability of treatment strategies, including medications for opioid use disorder (MOUD) and naloxone, opioid overdoses continue to increase at an alarming rate. Opioid vaccines are a novel approach to combat the growing crisis with several candidates recently entering human clinical trials. In this study, we investigated Qβ bacteriophage virus-like particles (VLPs) as a vaccine platform for immunogenic display of oxycodone. A derivative of oxycodone was conjugated to pre-formed Qβ VLPs using a sulfhydryl-amine reactive heterobifunctional crosslinker with high loading of oxycodone. In mice, intramuscular immunization with Qβ-oxycodone elicited high-titer, high-avidity and long-lasting antibody responses. Qβ-oxycodone was also immunogenic after storage at ambient room temperature for over two weeks, demonstrating that the vaccine is highly thermostable. In mice, immunization with Qβ-oxycodone elicited antibodies that sequester oxycodone in the serum, an important mechanism for preventing the adverse effects of opioid activity. Finally, Qβ-oxycodone is immunogenic in nonhuman primates, eliciting serum oxycodone antibodies after intramuscular immunization of rhesus macaques. These data establish Qβ-oxycodone as a promising opioid vaccine candidate.
Keywords: virus-like particle, opioid, oxycodone, antibodies, vaccine
Introduction
Opioid use disorder (OUD) and opioid overdoses are public health crises of increasing urgency and with limited treatment options. Approximately 2.7 million people in the United States suffer from OUD and 80,000 opioid overdose deaths occurred in 2021[1]. The opioid epidemic is characterized by three waves in the population over the last several decades: i) prescription opioids, ii) heroin, and iii) fentanyl and fentanyl analogs[2]. Although fentanyl has recently taken the forefront of the opioid crisis, many individuals with chronic pain eventually misuse prescription opioids and progress to OUD, leading to a risk of adverse effects such as overdose[3–5]. Oxycodone is the most abused prescription opioid, and a leading cause of OUD and overdose[6]. Oxycodone, along with other exogenous opioids, acts primarily via binding μ opioid receptors in the central nervous system to elicit analgesia, euphoria, anti-nociception, and respiratory depression[7,8].
Medications for opioid use disorder (MOUD) are commonly utilized for OUD treatment and overdose management[9]. This class of medications includes methadone, a μ opioid receptor agonist, buprenorphine, a partial receptor agonist, and naloxone, a receptor antagonist[10]. Stigma associated with methadone treatment, and MOUD overall, often discourages patients from seeking treatment[11,12]. This poses a challenge to the widespread use of these medications. While methadone and buprenorphine are remarkably effective, these drugs also require regular, supervised administration by a medical professional, creating a barrier to patient accessibility[13]. Many individuals with OUD may also be experiencing housing insecurity or lack transportation, limiting their access to proper medical care[14]. Naloxone (i.e., Narcan®) acts by competitively binding opioid receptors, competing off bound molecules and simultaneously blocking the binding of additional opioid compounds[15]. The efficacy of this intervention for opioid overdose is dependent on bystander possession of naloxone, recognition of the signs of an overdose, and response in an appropriate, timely manner. Despite the available medications, OUD incidence and overdose continue to increase, highlighting the urgent need for novel interventions for both OUD and overdose.
Immunotherapies, such as monoclonal antibodies and vaccines, have recently been highlighted as promising approaches to address OUD and overdose[16,17]. Unlike other strategies, immunotherapies do not carry the potential for abuse and may offer long-lasting protection. Vaccines against nicotine, cocaine, and heroin have shown promising pre-clinical data, but no vaccines against drugs of abuse have been licensed for use in humans[18–21].
An effective opioid vaccine would ideally require the generation of durable high-titer and high-affinity serum antibodies that bind and sequester opioid drug targets in the blood. Antibodies bind the drug to form a complex that is, in turn, unable to cross the blood brain barrier[22]. Currently, the most advanced oxycodone vaccine candidates are conjugate vaccines in which oxycodone is linked to protein carriers such as keyhole limpet hemocyanin (KLH) or tetanus toxoid (TT)[23,24]. In pre-clinical studies, a KLH-oxycodone vaccine elicited antibody responses and blocked oxycodone activity in vivo[25]. Other approaches, including TT protein carrier-based opioid vaccines, have shown similar results in rodents and macaques[23,26,27]. However, these vaccines require four or more immunizations to achieve high-titer, protective antibody responses[25,27]. Since the target patient population for these vaccines may not have reliable access to health care and transportation, the need for many immunizations to reach protection may create a barrier to those seeking care. An optimal vaccine would elicit high-titer, long-lasting, durable and protective antibody responses in as few immunizations as possible.
Virus-like particles (VLPs) are non-infectious, spontaneously self-assembling, highly repetitive structures formed by the structural proteins of viruses that can serve as highly immunogenic stand-alone vaccines or vaccine platforms[28,29]. VLPs derived from the bacteriophage Qβ are readily expressed in E. coli, are highly thermostable, and have been used as a platform to produce vaccines for diverse infectious and chronic diseases by our group and others [30–32]. A Qβ VLP is composed of 180 copies of a single viral structural protein. Due to the repetitive nature of the Qβ VLPs, short peptides or small molecules can be displayed on their surface at high valency. Upon immunization, Qβ VLP based vaccines elicit high-titer and long-lasting antibodies, often after a single dose[33]. Qβ VLPs are compatible with GMP manufacturing and have a favorable safety profile in humans[34]. Indeed, a Qβ VLP-based vaccine targeting nicotine showed promising safety and immunogenicity in a clinical trial, although the vaccine showed limited clinical efficacy[35,36].
Here, we report our efforts to engineer a Qβ VLP based vaccine against oxycodone. Using a simple approach in which a derivative of oxycodone was conjugated to Qβ VLPs at high valency using a chemical crosslinker with high loading of oxycodone, we generated a Qβ-oxycodone (Qβ-OXY) vaccine. We show that this vaccine generates high-titer, high-avidity, and long-lasting antibody responses in rodents and non-human primates. Moreover, this vaccine retains immunogenicity after freeze-thaw cycles and storage at ambient room temperature. We also show that immunization with Qβ-oxycodone leads to a sequestering of drug in the serum after in vivo drug challenge in vaccinated mice. Together, these data establish the feasibility of Qβ-OXY as a novel intervention for opioid-use disorder.
Materials and Methods
Chemical synthesis of oxycodone-(Gly)4-Cys conjugate
Oxycodone-(Gly)4-Cys-NH2 (Oxycodone-(Gly)4-Cys) was custom synthesized at CellMosaic Inc. (Woburn, MA). Oxycodone was purchased from Cayman Chemical Company Inc. (Ann Arbor, MI) and modified at CellMosaic to introduce a carboxylic acid functional group. A five amino acid peptide (Gly-Gly-Gly-Gly-Cys; (Gly)4-Cys) with a protected C-terminal Cys residue was custom synthesized using the standard Fmoc Solid Phase Peptide Protocol. The N-terminus of the peptide contained a free amine group and the C-terminus of the peptide contained an amide. The peptide was first coupled to oxycodone acid as previously described [37]. After removal of the Cys protecting group, the conjugate was purified by standard C18 HPLC using a TFA system and lyophilized to dryness. The identity of the conjugate was confirmed by MALDI-TOF MS; calculated exact mass: 718.27 Da, obtained mass [M+H]+: 719.7 Da. Prior to conjugation the lyophilized hapten (oxycodone-(Gly)4-Cys) was reconstituted in deionized water.
Expression and purification of bacteriophage Qβ VLPs
Wild type Qβ bacteriophage VLPs were expressed from plasmid pETQCT and produced in E. coli using electrocompetent C41 cells (Sigma CMC0021) as previously described[38]. 1μL of 1:10 diluted pETQCT plasmid was combined with 150μL (~3 × 109 competent cells) and gently mixed by flicking the tube and incubating on ice for 5 min. The mixture was then transferred to an electroporation cuvette and electroporated using a Bio-Rad Electroporator (Bio-Rad, Hercules, CA, USA) using the setting “ECL1”. Cells were transferred to 1mL of LB broth and incubated at 37°C with shaking for 1hr prior to plating on LB agar plates containing kanamycin for incubation overnight at 37°C. A single isolated colony was transferred to LB broth with 50ug/mL kanamycin. The culture was allowed to grow at 37°C until the OD600 reached 0.6–0.8. Qβ coat protein expression was then induced with the addition of 0.5mM IPTG for 3 hours. Bacterial pellets were collected via centrifugation at 3750rpm for 30min at 4°C. Pellets were resuspended in isolation buffer (5.8g NaCl, 3.7g EDTA,7.9g Tris HCl in ddH2O), incubated for 1hr on ice followed by addition of 10% deoxycholate and sonication. Lysates were then incubated for one hour with 10mg/mL DNase and 2M MgCl2 at 37°C to remove DNA. Samples were then centrifuged, and supernatant incubated with 60% ammonium sulfate at 4°C overnight. Following centrifugation at 3,750rpm for 30min, supernatant was removed, and pellets resuspended in cold SCB buffer. Pellets were combined, centrifuged at 10,000rpm, and Qβ VLPs were purified by size exclusion chromatography using Sepharose column (Sepharose CL-4B). Qβ containing fractions were identified by agarose gel electrophoresis and SDS-PAGE, pooled, and then precipitated using 70% ammonium sulfate at 4°C. VLPs were pelleted by centrifugation, extensively dialyzed against PBS pH 7.4, and then residual endotoxin was depleted through five rounds of incubation with Triton X-114. Purified VLPs were stored at −20°C.
Conjugation of haptens to Qβ VLPs and preparation of vaccine doses
Oxycodone-(Gly)4-Cys hapten was conjugated to the surface of Qβ VLPs using a heterobifunctional cross-linker, succinimidyl-6-([β-maleimidopropionamido]hexanoate) (SMPH) (Thermo Scientific 22363). Qβ VLPs were first incubated with SMPH at a 10:1 (SMPH: Qβ coat protein) molar ratio for 2 hours at room temperature to allow for the surface exposed amines (ε-amino group of lysine or N-terminal amines) on the Qβ VLP to react with the active succinimidyl ester of SMPH. Excess cross-linker was removed by ultrafiltration with an Amicon Ultra-4 centrifugal unit with a 100-kDa cutoff (Millipore Sigma UFC810024) at 4°C following manufacturer’s protocol. Oxycodone-(Gly)4-Cys was added to the Qβ-SMPH at a 10:1 (hapten: Qβ coat protein) molar ratio and incubated at 4°C overnight. The resulting complex is referred to as Qβ-oxycodone or Qβ-OXY. Conjugation of drug target onto Qβ was confirmed via SDS-PAGE using a 4–12% denaturing gel (Invitrogen NP0301BOX) followed by Coomassie staining. Oxycodone-(Gly)4-Cys was also conjugated to KLH using the bifunctional cross-linker SMPH and confirmed in the same manner.
Mouse immunization studies
All animal procedures were approved by the University of New Mexico Animal Care and Use Committee (Protocol #20–201045) under the National Research Council’s Guide for the Care and Use of Laboratory Animals. In dose response experiments, male BALB/c mice (8 weeks old; The Jackson Laboratory) received an intramuscular hindleg injection of 50μL Qβ-oxycodone (Qβ -OXY) at 10μg or 20μg doses. As controls, groups of mice received unconjugated Qβ (Qβ Control; 20μg), KLH-oxycodone (KLH OXY; 20μg) unconjugated KLH (KLH Control; 20μg) or PBS in one dose. For all other mouse immunization studies, male and female BALB/c mice (8 weeks old; The Jackson Laboratory) received vaccinations with Qβ-OXY or Qβ-control in 20μg/50μL doses every three weeks (Day 0, 21, 42 for mice receiving three vaccine doses; Day 0 and 21 for animals receiving two vaccine doses). Sera was collected by retro-orbital bleeds conducted under isoflurane anesthesia starting on day 3 after immunization followed by weekly collections in each vaccine group. Sera was harvested by sequential centrifugations at 10,000rpm for 10 min. The resulting sera was collected and then immediately frozen and stored at −20°C for subsequent analysis.
Immunogenicity studies in non-human primates
The non-human primate (NHP) studies used 3 male adult rhesus macaques (Macaca mulatta), between 3–7 years of age, from the breeding center of the California National Primate Research Center (CNPRC). All animals were negative for the simian immunodeficiency virus, type D retrovirus and simian T-cell lymphotropic virus type 1. The CNPRC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Animal care was performed in compliance with the 2011 Guide for the Care and Use of Laboratory Animals provided by the Institute for Laboratory Animal Research. The study was approved by the Institutional Animal Care and Use Committee of the University of California, Davis (Protocol# 21066). All procedures of animal husbandry and sample collections were performed according to established Standard Operating Procedures. For sample collections and immunizations, animals were sedated with ketamine anesthesia (10mg/kg body weight) administered by the intramuscular (IM) route. Each immunization consisted of a dose of 100 μg (in volume of 0.2 ml), injected in the right quadriceps muscle, during sedation. Animals received 3 doses, at weeks 0, 4, and 39 of the study.
Blood was collected via peripheral venipuncture. Complete blood counts (CBCs) were performed by the CNPRC Clinical Laboratories on EDTA-anticoagulated blood samples; electronic cell counts were performed on a Pentra 60C+ analyzer (ABX Diagnostics) and differential cell counts were determined manually using Giemsa/Wright-Giemsa staining. Blood tubes without coagulant were also collected for processing via centrifugation (900xg for 10 minutes) for serum. Serum chemistries were performed on a Beckman Coulter AU480 chemistry analyzer. Serum aliquots were stored at −70°C until further analysis.
ELISA to determine antibody titers
Serum antibody responses to oxycodone were measured by enzyme linked immunosorbent assay (ELISA). Ninety-six well plates (Immulon 3455) were coated for 2 hours at room temperature with 250 ng/well of oxycodone-BSA (MyBioSource MBS122031) in PBS. After blocking with 0.5% Milk (Quality Biologicals A614–1005) in PBS overnight at 4°C, diluted mouse sera (using four-fold dilutions from 1:40 to 1:655360) were added to each well and incubated at room temperature for 2 hours. After five washes using PBS, HRP-conjugated goat anti-mouse IgG (Jackson Immuno 115–035-003) was added at a 1:5000 dilution in blocking buffer and incubated for 1 hour at room temperature. Following five washes, 50μl substrate 3,3′,5,5′-tetramethylbenzidine (Millipore EM613544) (TMB) was added to each well then plates were incubated in the dark for 10 min while shaking. The reaction was stopped by addition of 50μl 1% hydrochloric acid (Acros AC12463–500l) to each well. The absorbance of each well at 450 nm was measured using a microplate reader (Fisher Scientific accuSkan FC). Endpoint dilution IgG is reported as the final serum dilution that generated A450 greater than twice that of background. Cross-reactivity to methadone, buprenorphine, and morphine was measured by ELISA using a similar protocol, but by using methadone-BSA (MyBioSource MBS122028) or buprenorphine-BSA (MyBioSource MBS122011) as coating antigens. For ELISAs on non-human primate (NHP) sera, HRP-conjugated goat anti-monkey IgG (ab112767) was used as a secondary antibody at a 1:4000 dilution and 0.5% PBST- Tween-20 (PBST) was used as the washing solution.
Measure of antibody avidity
Avidity was assessed utilizing a chaotropic urea-based avidity ELISA as previously described[32]. Briefly, this protocol followed a standard ELISA, as described above, except that wells were treated with 6 M urea for 10 min prior to the addition of the secondary antibody. Avidity Index (AI) was calculated as a ratio of ELISA A450 values of 6M urea treated wells/matched control wells treated with H2O.
Analysis of oxycodone drug concentrations in serum
Liquid Chromatography (LC) conditions were followed as previously described[39]. A Waters Corporation (Beverly, MA) Xevo G2-XS QTOF mass spectrometer in sensitivity mode coupled to an Acquity UPLC with a peptide BEH C18 column from Waters (130Å pore size, 1.7um particle size, 2.1 × 100mm) was used. UPLC analysis was done using a step gradient method with two solvents: 0.1% formic acid in water (Buffer A) and 0.1% formic acid in acetonitrile (Buffer B). The method starts with an isocratic hold of buffer B at 3% for 2 minutes, then ramps to 75% within 8 minutes (9% B/min) followed by a shallow increase from 75% to 100% within 9 minutes (2.78%B/min). After buffer B reaches 100% and holds for 1 minute, it ramps back from 100% to 3% for equilibrium within 13min (−7.46%B/min). Buffer B then holds at 3% for 1 minutes before the method stops. The flow rate is kept at 0.3 mL/min throughout the method. Full scan data was collected during the entire analysis, with additional MS/MS of m/z 316.1 from 5.2 to 7.5 minutes to confirm oxycodone fragments of m/z 298.1 and 241.1. Full scan data using m/z 316.1, 317.1, 298.1 was used for integration and referenced against the deuterated oxycodone standard at 322.1, 323.1, and 304.1[39]. Injection volume was 0.2 μL. Electrospray conditions were as follows: 3kV capillary, desolvation temperature 350°C, desolvation gas 600 L/hr, source temperature 150°C, source offset 80 and sampling cone 40V.
At three-weeks post-second immunization, mice (n=12, male and female Balb/c) were challenged with a subcutaneous injection of 2mg/kg oxycodone. At 30min post injection, mice were placed under heavy isoflurane anesthesia and complete sedation was validated by a firm pinch of the foot pad without generating a pain response. Animals were decapitated using a rodent guillotine and trunk blood was immediately drained. Sera was isolated by centrifugation and immediately frozen at −80°C. Drug was extracted from serum samples using a 10:1 mixture of ice-cold 1% formic acid in methanol followed by centrifugation. Isolated samples were analyzed on the Xevo G2 XS Quadrupole Time-of-Flight Mass Spectrometer with UPLC sample introduction/separation following the parameters as described above.
Results
Generation of oxycodone (Gly)4-Cys haptens and conjugation to Qβ VLPs
To allow for chemical conjugation to the Qβ VLPs, oxycodone was modified with a short amino acid sequence consisting of four glycines and a cysteine [(Gly)4-Cys-NH2] with C-terminal amidation. (Figure 1a). The terminal cysteine provides a reactive sulfhydryl group for conjugation using a crosslinker and the glycines serve as a short peptide linker to extend the drug away from the surface of the Qβ VLP. C18 HPLC analysis indicated the resulting oxycodone-(Gly)4-Cys-NH2 (oxycodone-(Gly)4-Cys) hapten had a purity of >99.9% and an observed mass of 718 Da. The oxycodone hapten was linked to Qβ VLPs using the bifunctional crosslinker SMPH (shown schematically in Figure 1b). Successful conjugation of oxycodone-(Gly)4-Cys to Qβ was confirmed by a slight increase in the molecular weight of the Qβ coat protein upon SDS-PAGE analysis (Figure 1c). Notably, there is no unconjugated Qβ coat protein at the 14kDa band, indicating that each of the 180 coat protein subunits on a Qβ VLP was modified by at least one oxycodone molecule. Based on the distance traveled by the conjugate from the Qβ coat protein (origin) and the total distance between the Qβ coat protein (14kDa) and the 17kDa reference standard in the SDS-PAGE, we calculated there are around 1.2 oxycodone molecules per Qβ coat protein representing approximately 216 oxycodone molecules per VLP.
Figure 1. Conjugation of oxycodone-(Gly)4-Cys to Qβ VLPs.
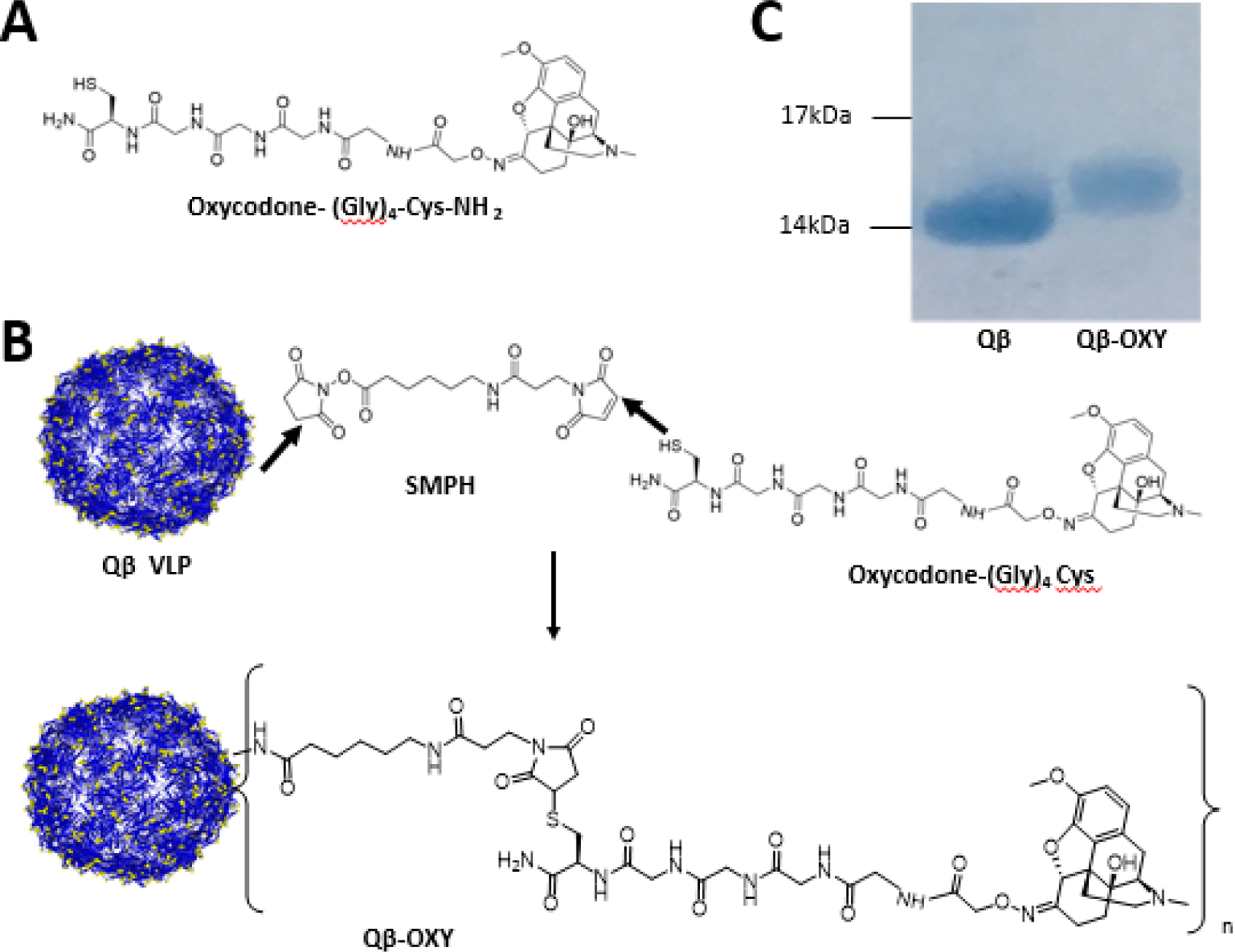
A synthetic derivative of oxycodone was created with a peptide linker composed of four glycine residues and a terminal cysteine residue with c-terminal amidation [(Gly)4-Cys-NH2]; simplified as oxycodone-(Gly)4Cys). (A). Oxycodone-(Gly)4-Cys was then chemically conjugated to the surface amines of Qβ VLPs to generate the Qβ-OXY vaccine via a heterobifunctional crosslinker SMPH (B). Conjugation of Oxycodone-(Gly)4-Cys to Qβ coat protein was confirmed by slower migration of the conjugate (Qβ-OXY) by SDS-PAGE and Coomassie staining compared to unconjugated Qβ coat protein. Average 1.2 oxycodone molecules were loaded onto each unit of Qβ coat protein based on the gel shift assay. Molecular weights: Qβ coat protein (14kDa), oxycodone-(Gly)4-Cys (718Da), addition of linker and oxycodone (984 Da).
Qβ-oxycodone elicits high-titer and durable antibodies to oxycodone.
To evaluate the immunogenicity of Qβ-oxycodone, male Balb/c mice received a single intramuscular immunization Qβ-oxycodone (Qβ-OXY) at 10 μg and 20 μg doses of VLPs. As a comparison, we also immunized mice with oxycodone conjugated to keyhole limpet hemocyanin (KLH), a commonly used carrier protein. Control groups of mice were immunized once with unconjugated Qβ VLPs, unconjugated KLH, or PBS. As is shown in Figure 2a, immunization with Qβ-OXY elicited strong anti-oxycodone IgG titers that were detectable within three days after immunization. Qβ-OXY elicited high-titer antibody responses at each of the doses evaluated, but the highest dose (20 μg) elicited the most durable antibody responses over the course of this study (116 days post-immunization). In contrast, mice immunized with a similar dose of KLH-oxycodone (KLH-OXY) elicited ~100-fold lower titer anti-oxycodone antibody levels and these antibodies were less durable, declining to background levels by 56 days post-immunization.
Figure 2. Qβ-OXY elicits high-titer, high-avidity, long-lasting antibodies.
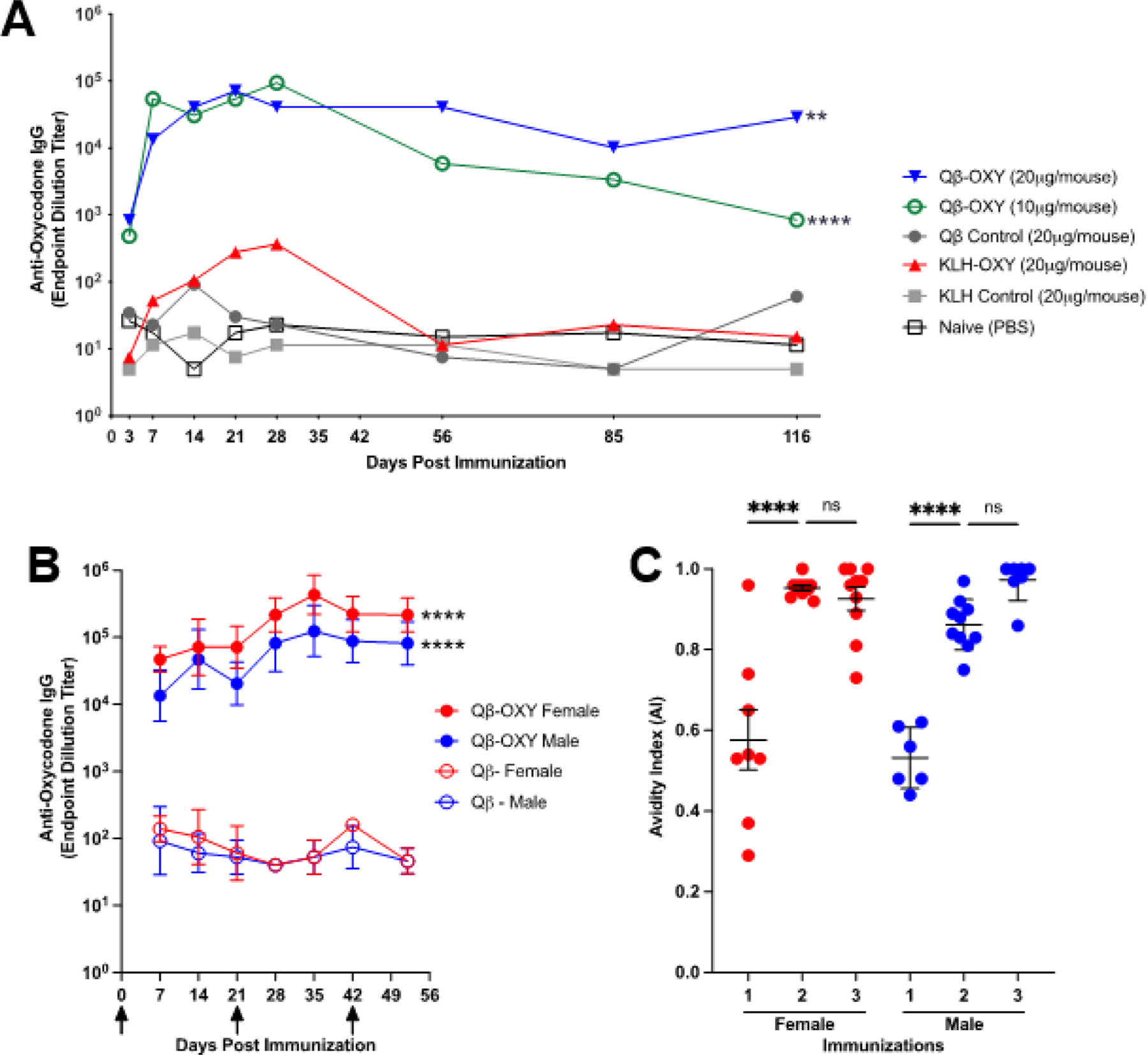
(A) In a pilot experiment, mice (n=5, Balb/c, Male) were immunized on day 0 with Qβ-OXY (10μg or 20μg), unconjugated Qβ control (20μg), unconjugated KLH control (20μg), KLH-OXY (20ug) or PBS control. Blood was collected at various times post-immunization and assessed for antioxycodone IgG by ELISA, with geometric mean titer reported. ** p<0.005, ****p<0.0001 by Sidak’s multiple comparisons test, compared to Qβ control. (B) Male and female BALB/c mice (n=10) received three immunizations (20μg/50μL, days 0, 21, and 42 indicated by arrows) of QβOXY or unconjugated Qβ control. Blood was collected at various times post-immunization and assessed for anti-oxycodone IgG by ELISA, with geometric mean and geometric SD reported. ****p<0.0001 by Sidak’s multiple comparisons test, compared to appropriate Qβ control (C) Urea-based avidity ELISAs were performed to assess avidity of elicited antibodies after each immunization with Qβ-OXY. Line represents mean avidity index (AI) for each group with error bars indicating SEM. Avidity index (AI) = (A4506M Urea)/(A450H2O) **p<0.01, ****p<0.0001, Brown-Forsythe and Welch’s ANOVA test, GraphPad Prism.
To evaluate the effects of boosting and sex on immunogenicity, male and female mice were immunized three times with 20 μg Qβ-OXY or Qβ control delivered three weeks apart (Day 0, 21, 42). Qβ-OXY rapidly elicited high titer antibodies in male and female mice (Figure 2b). Titers increased over time, including half a log increase after the delivery of a second dose.
Two doses of Qβ-oxycodone results in higher avidity antibodies to oxycodone
Although the delivery of a third dose of Qβ-OXY did not significantly increase antibody titers (Figure 2b) relative to two doses, we hypothesized that boosting would increase antibody avidity. We measured the Avidity Index of antibodies induced after each dose of vaccine using a chaotrope-based ELISA assay. For both male and female mice, the delivery of a second dose of vaccine significantly increased antibody avidity (Figure 2c). However, a third dose resulted in only a slight increase in avidity and no significant increase in antibody titer, indicating that two immunizations are sufficient to elicit optimal antibody responses in male and female mice.
Cross-reactivity with off-target opioids is minimal
One concern for potential treatment with opioid vaccine is cross-reactivity with other opioids and therapeutics for OUD. To address this, we measured the cross-reactivity of Qβ-OXY elicited antibodies against buprenorphine and methadone by ELISA (the structures of these molecules are shown in Figure 3a). We found that antibodies induced by Qβ-OXY have some cross-reactivity to buprenorphine (titers were ~100-fold lower than to oxycodone; Figure 3b), but that these antibodies were low avidity (Figure 3c). In contrast, there was only minimal cross reactivity to methadone (Figure 3b). Thus, Qβ-OXY elicits highly specific antibody responses that are not strongly cross-reactive.
Figure 3. Cross-reactivity of Qβ-OXY elicited antibodies with off-target opioids.
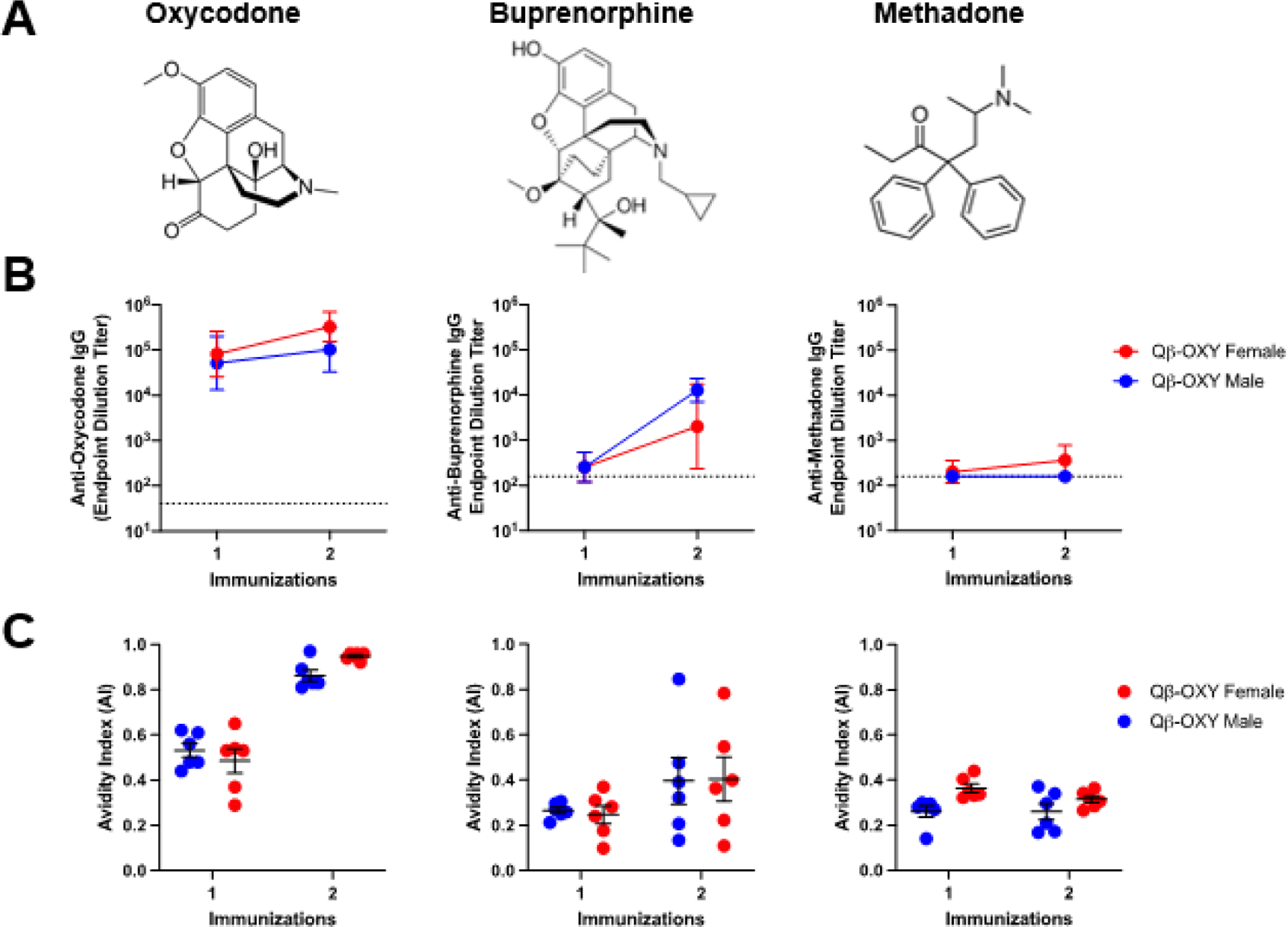
Serum from male and female BALB/c mice (n=6) were collected after 1 and 2 immunizations with Qβ-OXY and assessed for binding to buprenorphine and methadone. (A) Oxycodone, buprenorphine, and methadone were used to coat ELISA plates and (B) serum endpoint dilution IgG titers were determined (geometric mean titer and geometric SD are indicated by line and error bars). Dotted line indicates Qβ control serum IgG titer to each target antigen. (C) A urea-based avidity ELISA was used to assess avidity of Qβ-OXY elicited antibodies to each target. Avidity index (AI) is calculated as the ratio of absorbance values for urea treated wells compared to water treated control wells. AI= (A4506M Urea)/(A450H2O). Data points show mean titer with error bars indicating SEM.
Qβ-oxycodone vaccine elicited antibodies sequester oxycodone in the serum upon in vivo drug challenge
The ability of opioids to exert their effects is dependent on binding and activation of opioid receptors in the CNS[42,43]. Sequestration of opioids in the blood and limiting the amount of drug that makes it into the brain is the key mechanism of action of opioid vaccines[22,44]. In order to determine if antibodies elicited Qβ-OXY function by the same mechanism, immunized and control animals were challenged with 2mg/kg subcutaneous oxycodone three weeks following a second immunization. At 30min post-injection, animals were decapitated, and trunk blood harvested. Serum oxycodone levels were measured by UPLC-MS (Figure 4). We observed a significant increase in oxycodone drug retained in the serum of Qβ-OXY immunized mice compared to control animals immunized with Qβ-control (Figure 4, p = <0.0001), showing that vaccine elicited antibodies are capable of binding oxycodone drug in vivo and sequestering it in the blood.
Figure 4. Qβ-OXY elicited responses sequester oxycodone in the serum upon in vivo drug challenge in mice.
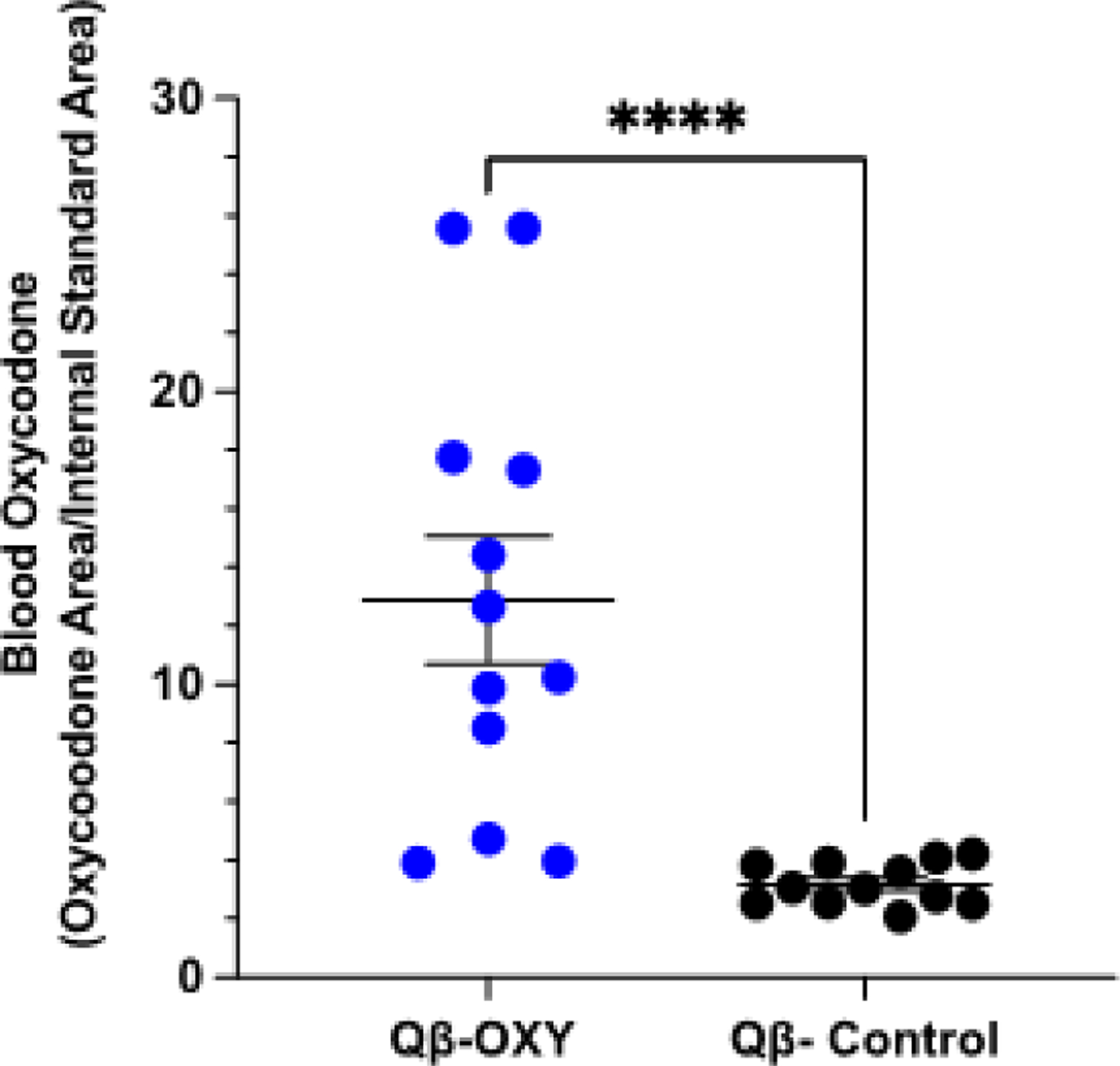
At three-weeks post-second immunization, BALB/c mice (n=12, M/F) were challenged with 2mg/kg subcutaneous oxycodone. 30 min post-injection, truck blood was harvested via decapitation to assess drug serum amount. UPLC-MS based analysis of drug in the serum was conducted for Qβ-OXY and Qβ control immunized animals. Reported values are expressed as the ratio of the peak area of oxycodone compared to the peak area of d6-oxycodone internal standard. ****p<0.0005, Kolmogorov-Smirnov test.
Qβ-oxycodone is thermostable and retains immunogenicity after temperature changes
Patient populations most in need of opioid vaccines may reside in resource-poor areas where it is difficult to maintain a cold-chain, making the storage and delivery conditions of vaccine an important consideration. We assessed the thermostability of Qβ-OXY by subjecting the vaccine to two freeze-thaw cycles followed by storage for two weeks at room temperature. Mice were then given a single immunization and serum anti-oxycodone antibody titers were measured at various times through 70 days post-immunization. Endpoint dilution titers were similar to that of animals immunized with vaccine that was maintained at −20°C prior to injection (Figure 2), showing that these vaccines can be stored for several weeks at room temperature without affecting immunogenicity (Figure 5).
Figure 5. Qβ-OXY remains immunogenic after storage at room temperature.
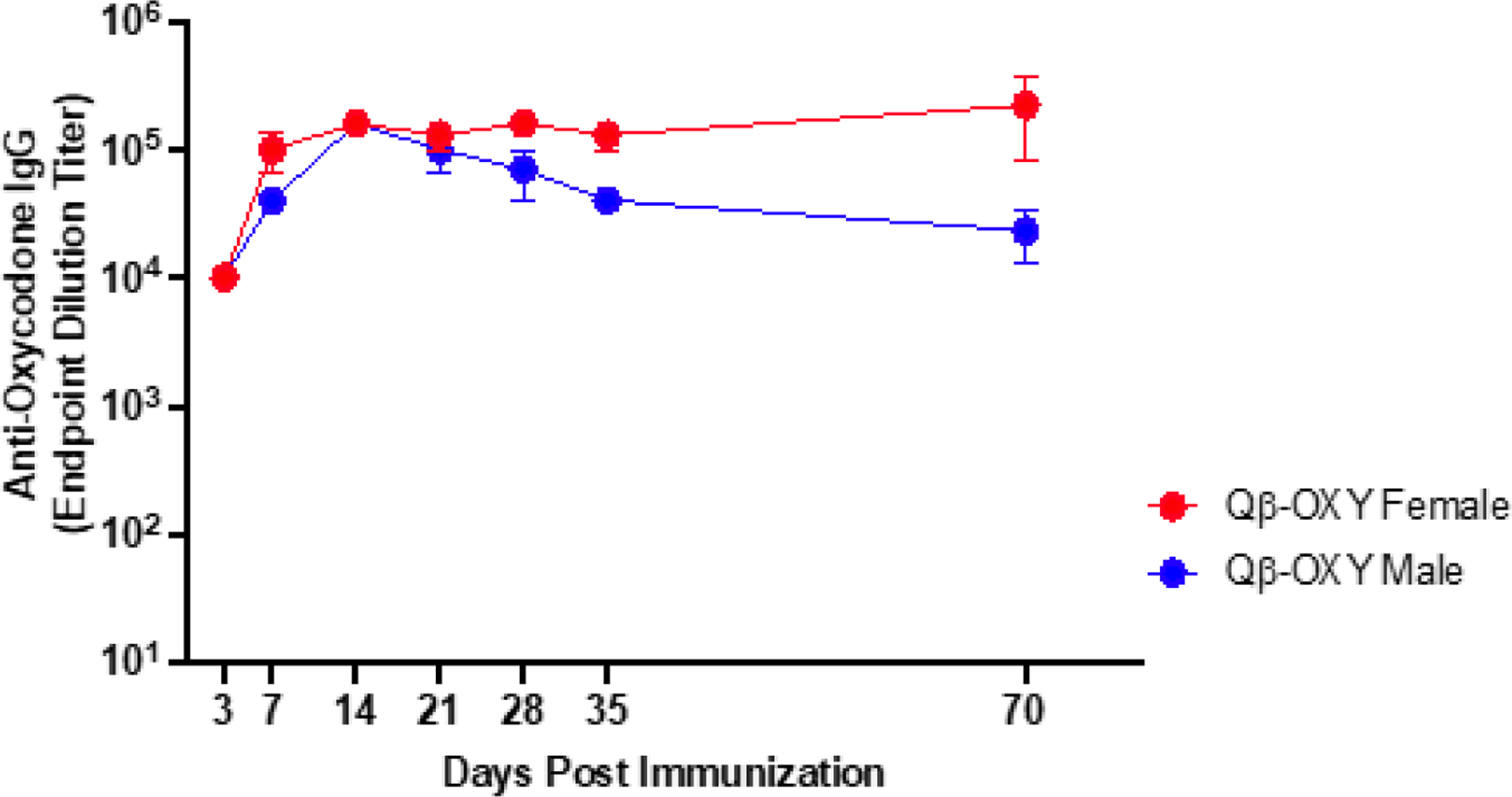
Mice (n=4, BALB/c M/F) received a single intramuscular immunization with Qβ-OXY that had been previously placed through two freeze-thaw cycles and maintained at room temperature for two weeks. Serum was collected at various times post-immunization and anti-oxycodone IgG titers were determined by ELISA. Geometric mean titer with geometric SD are reported.
Qβ-oxycodone is immunogenic in non-human primates
To study the immunogenicity of Qβ-OXY in a non-human primate model, rhesus macaques (n=3, males) were immunized with 100μg of Qβ-OXY on days 0, 21, and 273. A single immunization with Qβ-OXY elicited anti-oxycodone antibodies that were detectable as early as 7 days after immunization (Figure 6). Boosting at day 21 increased end-point dilution antibody titers to ~104. Titers then dropped to ~103 and remained steady. Due to the waning antibody responses, a third dose was delivered on day 273, after which end point dilution antibody titers increased to >105. Avidity significantly improved between one and two doses, with Avidity Index (AI) ~1 at 2 weeks post-second dose (*p=0.037). Based on regular complete blood counts (CBC), serum chemistry screening panels, and daily clinical monitoring of the animals, there was no evidence of any local or systemic adverse effects after each dose. Together, these data extend the safety and immunogenicity of Qβ-OXY into a non-human primate model and supports its feasibility as a future treatment option for patients.
Figure 6. Qβ-oxycodone is immunogenic in nonhuman primates.
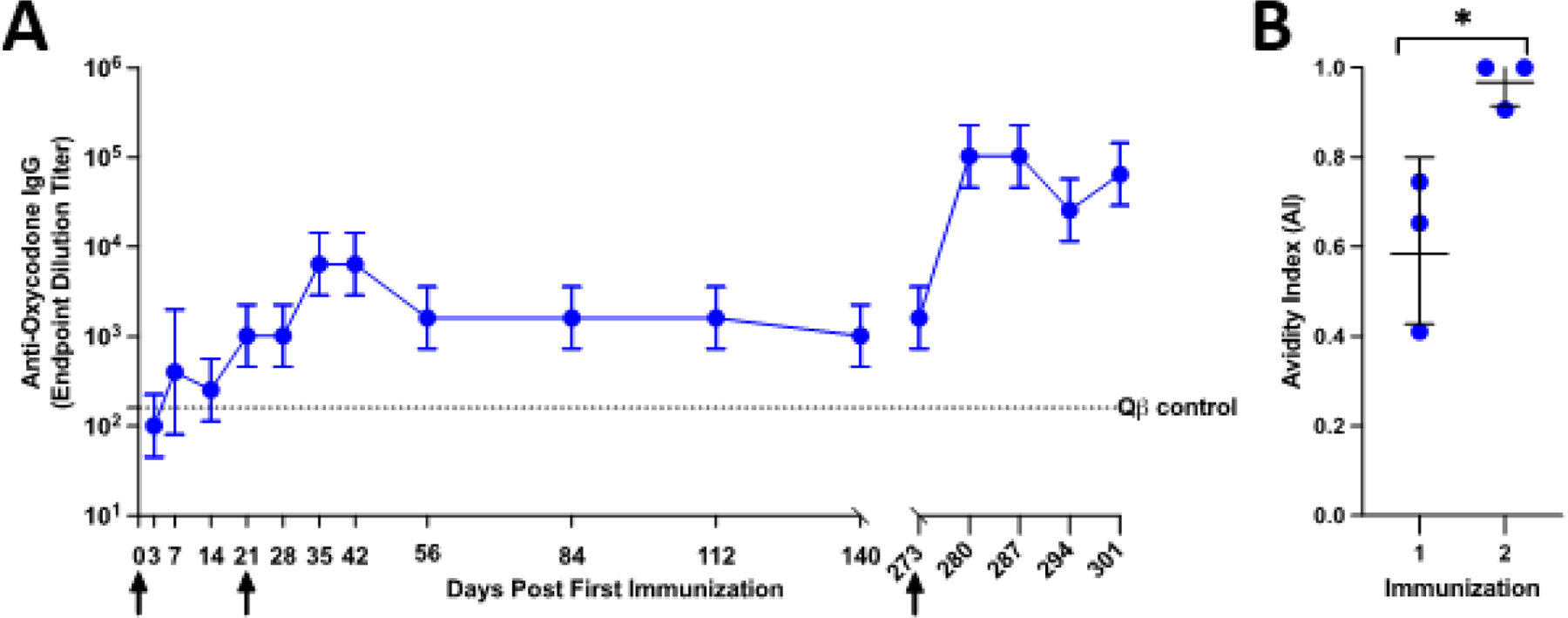
Male rhesus macaques (n=3) were immunized with Qβ-OXY at day 0, day 21, and day 273. Serum was collected at various times post immunization and assessed for anti-oxycodone IgG by ELISA. Arrows indicate when each immunization was administered. Data points represent geometric mean titer with geometric SD shown by error bars. Dotted line shows anti-oxycodone IgG end point dilution titer for historical Qβ immunized control rhesus macaque. (B) Avidity of IgG elicited by Qβ-OXY after 1 and 2 immunizations was determined by avidity urea-based avidity ELISA. Avidity index (AI) is the calculated as the ratio of absorbance values in urea treated wells compared to water treated control wells(AI= A4506M Urea)/(A450H2O). *p<0.05 paired t-test, p=0.0349
Discussion
Vaccines offer a new strategy to help combat the growing opioid crisis. Previous studies have investigated the feasibility of opioid vaccines using traditional protein carrier-based approaches, establishing immunogenicity and protection in animal models[21–25]. In this study, we demonstrate the successful generation of a Qβ VLP based vaccine candidate targeting oxycodone. We used a SMPH and a free-sulfhydryl on the linker to attach oxycodone to Qβ VLPs, a strategy we have previously used with heterologous peptides [31,32], and others have used a similar strategy to link opioids to carrier proteins [40,41]. We show that an oxycodone-(Gly)4-Cys hapten is conjugated onto the Qβ VLP with high efficiency and generates high-titer antibody responses in mice, with endpoint dilution IgG titers that remain above 104 for up to 112 days after a single dose. The addition of a boost vaccination delivered on day 21 modestly increased antibody titers, but significantly improved antibody avidity. One potential concern is that antibodies against oxycodone may cross-react with buprenorphine or methadone. We investigated the specificity of antibody responses and showed that antibodies induced by Qβ-OXY only weakly cross-react with buprenorphine and fail to bind to methadone. We show that Qβ-OXY elicited antibodies that retain oxycodone in the serum, compared to control immunized animals. To further demonstrate the promise of Qβ-OXY, we investigated its immunogenicity in a nonhuman primate model and show that after three immunizations, Qβ-OXY demonstrates high immunogenicity and no signs of toxicity in rhesus macaques.
One potential concern in the development of opioid vaccines is that vaccine induced antibodies against the target opioid may cross react with current medications for opioid use disorder (MOUD) such as methadone and buprenorphine. Our data suggests that immunization with Qβ-oxycodone does not generate antibodies with significant cross-reactivity to off target opioids. However, there were low-titer, low-avidity cross-reactive responses with buprenorphine. This may be a concern when looking into future clinical applications of this Qβ-OXY candidate. Other studies have examined cross-reactivity via both ELISA and in vivo drug challenges with off-target opioids[46,47]. In the future, we plan to evaluate if animals vaccinated with Qβ-OXY display reduced sensitivity to buprenorphine and methadone in vivo as well as investigating potential cross-reactivity with naloxone to ensure that immunization will not alter the response to current standard treatments for OUD and opioid overdose.
Previous vaccine efforts have shown that protection from the adverse effects of opioid activity is based on the ability of vaccine-elicited antibodies to sequester drug in the serum, thereby limiting the concentration of free drug that crosses the blood-brain barrier to access opioid receptors in the CNS[46,47]. We show that Qβ-OXY generated antibodies retain significantly more oxycodone in the serum compared to what was observed in control animals. This finding is consistent with previous studies of other opioid vaccines[40,45]. Although this is convincing evidence that our vaccine will be effective in protecting animals from oxycodone activity in vivo, this must be confirmed with additional studies. In the future, we plan to investigate additional in vivo measures of efficacy, including protection from oxycodone-induced anti-nociception and oxycodone-induced respiratory depression.
Cold-chain requirements for vaccines can limit their implementation in resource deprived areas. Moreover, loss of the cold-chain can lead to reduced efficacy of vaccines that require maintenance and a specific temperature during transport and storage. In the effort to generate a vaccine candidate that can be delivered to vulnerable patient populations, the generation of a thermostable opioid vaccine is desirable. In addition to the immunogenicity and protective capacity of Qβ-OXY, the thermostability studies support this vaccine strategy as having potential for translation to the clinic.
In our experiments, we investigated titers of serum anti-oxycodone IgG. The primary mechanism of action for drug vaccines is drug sequestration in the blood, where IgG is the predominating antibody isotype. However, recent studies investigating a fentanyl conjugate vaccine delivered via multiple routes of administration showed that IgA levels were correlated to protection from fentanyl challenge in mouse models[48]. Additionally, a recent vaccine investigating protection from cocaine use found that IgA rather than IgG mediates protective effects[49]. In future studies, we will investigate if IgA plays a role in mediating the protective capacities of our Qβ-OXY vaccine. If IgA is an important driving factor of protection, we can promote an IgA response via mucosal route of vaccine administration as well as the use of adjuvants.
Previously reports of opioid vaccines used exogenous adjuvant as a component of vaccine candidates. This includes adjuvants such as alum and dmLT[25,27,48]. In our Qβ-OXY studies, we have not investigated the addition of exogenous adjuvant due to the endogenous adjuvating properties of the VLP itself which encapsidates RNA. This ssRNA acts as a TLR ligand, with the ability to boost T helper (TH) responses and increase immunogenicity[28]. However, due to the promising results of other opioid vaccines which use exogenous adjuvant, we may employ the use of adjuvants in future studies. Potential adjuvants that may be employed include alum, Advax, or mastoparan-7 which have been utilized in other vaccines for drugs of abuse or other Qβ VLP vaccines in our lab[32,50].
In conclusion, we show the successful generation of a novel oxycodone vaccine using a Qβ VLP based approach. We establish this Qβ-OXY vaccine as immunogenic in both mice and non-human primate models. Moreover, we demonstrate the high-avidity and durable responses generated by this vaccine candidate as well as the ability of vaccine-elicited antibodies to sequester drug in the serum upon challenge with oxycodone. Together, these data establish Qβ-OXY as a promising novel opioid vaccine candidate.
Acknowledgments:
We thank Jennifer K. Watanabe, Jodie L. Usachenko, Ramya Immareddy, and the staff of CNPRC Colony Management and Research Services, Clinical Laboratories, Anatomic and Clinical Pathology and Veterinary staff for their expert technical assistance.
Funding Sources:
This work was supported by NIH/NIDA/NHLBI (R01 HL131696-03S1) to BC, NIH/NCATS (KL2 TR001448) to KMF, and a CNPRC pilot research grant to KMF and award P51OD011107 from the Office of Research Infrastructure Program, Office of The Director, National Institutes of Health to the California National Primate Research Center. NIH/NIDA (F31DA059236) to IGR. This project was supported in part by the Dedicated Health Research Funds from The University of New Mexico, School of Medicine. Funding sources had no involvement in the study design; the collection, analysis, or interpretation of data; the writing of the manuscript; or in the decision to submit the article for publication.
Bryce Chackerian reports financial support was provided by National Heart Lung and Blood Institute. Kathryn M. Frietze reports financial support was provided by National Center for Advancing Translational Sciences. Kathryn M. Frietze reports financial support was provided by Office of Research Infrastructure Program, Office of The Director, National Institutes of Health to the California National Primate Research Center. Isabella G. Romano reports financial support was provided by National Institute of Drug Abuse. Bryce Chackerian reports a relationship with Metaphore Biotechnologies, Inc. that includes: equity or stocks. Kathryn M. Frietze has patent Virus-like particle vaccines for opioid drugs pending to None. Bryce Chackerian has patent Virus-like particle vaccines for opioid drugs. pending to None. Susan B. Core has patent Virus-like particle vaccines for opioid drugs pending to None. Naomi R. Lee has patent Virus-like particle vaccines for opioid drugs pending to None. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: B.C., K.M.F, S.B.C., and N.R.L. are inventors on a patent describing the Qβ-oxycodone vaccine. B.C. has an equity stake in Metaphore Biotechnologies.
Reference List
- [1].CDC, National Center for Health Statistics, Office of Communication. U.S. Overdose Deaths In 2021 Increased Half as Much as in 2020 – But Are Still Up 15%. CdcGov n.d https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm.
- [2].Gardner EA, McGrath SA, Dowling D, Bai D. The Opioid Crisis: Prevalence and Markets of Opioids. Forensic Sci Rev 2022;34:43–70. [PubMed] [Google Scholar]
- [3].Weiner SG, El Ibrahimi S, Hendricks MA, Hallvik SE, Hildebran C, Fischer MA, et al. Factors Associated With Opioid Overdose After an Initial Opioid Prescription. JAMA Netw Open 2022;5:e2145691. 10.1001/jamanetworkopen.2021.45691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vowles KE, Schmidt ZS, Ford CG. Opioid and Alcohol Misuse in Veterans with Chronic Pain: A Risk Screening Study. J Pain 2022:S1526–5900(22)00347–9. 10.1016/j.jpain.2022.06.003. [DOI] [PubMed] [Google Scholar]
- [5].Commonly Used Terms | CDC’s Response to the Opioid Overdose Epidemic | CDC n.d https://www.cdc.gov/opioids/basics/terms.html (accessed August 25, 2022).
- [6].Vearrier D, Grundmann O. Clinical Pharmacology, Toxicity, and Abuse Potential of Opioids. J Clin Pharmacol 2021;61 Suppl 2:S70–88. 10.1002/jcph.1923. [DOI] [PubMed] [Google Scholar]
- [7].Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the muopioid-receptor gene. Nature 1996;383:819–23. 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- [8].James A, Williams J. Basic Opioid Pharmacology — An Update. British Journal of Pain 2020;14:115–21. 10.1177/2049463720911986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Volkow ND, Wargo EM. Overdose Prevention Through Medical Treatment of Opioid Use Disorders. Ann Intern Med 2018;169:190. 10.7326/M18-1397. [DOI] [PubMed] [Google Scholar]
- [10].Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-Assisted Therapies — Tackling the Opioid-Overdose Epidemic. N Engl J Med 2014;370:2063–6. 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- [11].Dickson-Gomez J, Spector A, Weeks M, Galletly C, McDonald M, Green Montaque HD. “You’re Not Supposed to be on it Forever”: Medications to Treat Opioid Use Disorder (MOUD) Related Stigma Among Drug Treatment Providers and People who Use Opioids. Subst Abuse 2022;16:117822182211038. 10.1177/11782218221103859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Madden EF, Prevedel S, Light T, Sulzer SH. Intervention Stigma toward Medications for Opioid Use Disorder: A Systematic Review. Subst Use Misuse 2021;56:2181–201. 10.1080/10826084.2021.1975749. [DOI] [PubMed] [Google Scholar]
- [13].Sullivan LE, Fiellin DA. Narrative Review: Buprenorphine for Opioid-Dependent Patients in Office Practice. Ann Intern Med 2008;148:662. 10.7326/0003-4819-148-9-200805060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yamamoto A, Needleman J, Gelberg L, Kominski G, Shoptaw S, Tsugawa Y. Association between homelessness and opioid overdose and opioid-related hospital admissions/emergency department visits. Social Science & Medicine 2019;242:112585. 10.1016/j.socscimed.2019.112585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bateman JT, Saunders SE, Levitt ES. Understanding and countering opioid-induced respiratory depression. British J Pharmacology 2021:bph.15580. 10.1111/bph.15580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Volkow ND, Collins FS. The Role of Science in Addressing the Opioid Crisis. N Engl J Med 2017;377:391–4. 10.1056/NEJMsr1706626. [DOI] [PubMed] [Google Scholar]
- [17].Collins FS, Koroshetz WJ, Volkow ND. Helping to End Addiction Over the Long-term: The Research Plan for the NIH HEAL Initiative. JAMA 2018;320:129. 10.1001/jama.2018.8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther 2011;89:392–9. 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature 1974;252:708–10. 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- [20].Kosten TR, Domingo CB, Shorter D, Orson F, Green C, Somoza E, et al. Vaccine for cocaine dependence: A randomized double-blind placebo-controlled efficacy trial. Drug and Alcohol Dependence 2014;140:42–7. 10.1016/j.drugalcdep.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bloom BT, Bushell M-J. Vaccines against Drug Abuse—Are We There Yet? Vaccines 2022;10:860. 10.3390/vaccines10060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shen XY, Orson FM, Kosten TR. Vaccines against drug abuse. Clin Pharmacol Ther 2012;91:60–70. 10.1038/clpt.2011.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kimishima A, Wenthur CJ, Zhou B, Janda KD. An Advance in Prescription Opioid Vaccines: Overdose Mortality Reduction and Extraordinary Alteration of Drug Half-Life. ACS Chem Biol 2017;12:36–40. 10.1021/acschembio.6b00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Raleigh MD, Accetturo C, Pravetoni M. Combining a Candidate Vaccine for Opioid Use Disorders with Extended-Release Naltrexone Increases Protection against Oxycodone-Induced Behavioral Effects and Toxicity. J Pharmacol Exp Ther 2020;374:392–403. 10.1124/jpet.120.000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hamid FA, Marker CL, Raleigh MD, Khaimraj A, Winston S, Pentel PR, et al. Pre-clinical safety and toxicology profile of a candidate vaccine to treat oxycodone use disorder. Vaccine 2022;40:3244–52. 10.1016/j.vaccine.2022.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nguyen JD, Hwang CS, Grant Y, Janda KD, Taffe MA. Prophylactic vaccination protects against the development of oxycodone self-administration. Neuropharmacology 2018;138:292–303. 10.1016/j.neuropharm.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tenney RD, Blake S, Bremer PT, Zhou B, Hwang CS, Poklis JL, et al. Vaccine blunts fentanyl potency in male rhesus monkeys. Neuropharmacology 2019;158:107730. 10.1016/j.neuropharm.2019.107730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Frietze KM, Peabody DS, Chackerian B. Engineering virus-like particles as vaccine platforms. Current Opinion in Virology 2016;18:44–9. 10.1016/j.coviro.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chackerian B, Lowy DR, Schiller JT. Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. J Clin Invest 2001;108:415–23. 10.1172/JCI11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Collar AL, Linville AC, Core SB, Frietze KM. Epitope-Based Vaccines against the Chlamydia trachomatis Major Outer Membrane Protein Variable Domain 4 Elicit Protection in Mice. Vaccines 2022;10:875. 10.3390/vaccines10060875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Warner NL, Frietze KM. Development of Bacteriophage Virus-Like Particle Vaccines Displaying Conserved Epitopes of Dengue Virus Non-Structural Protein 1. Vaccines 2021;9:726. 10.3390/vaccines9070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jelínková L, Jhun H, Eaton A, Petrovsky N, Zavala F, Chackerian B. An epitope-based malaria vaccine targeting the junctional region of circumsporozoite protein. Npj Vaccines 2021;6:13. 10.1038/s41541-020-00274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Peabody DS, Peabody J, Bradfute SB, Chackerian B. RNA Phage VLP-Based Vaccine Platforms. Pharmaceuticals 2021;14:764. 10.3390/ph14080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nooraei S, Bahrulolum H, Hoseini ZS, Katalani C, Hajizade A, Easton AJ, et al. Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J Nanobiotechnol 2021;19:59. 10.1186/s12951-021-00806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One 2008;3:e2547. 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, et al. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. Eur J Immunol 2005;35:2031–40. 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- [37].Pravetoni M, Le Naour M, Tucker AM, Harmon TM, Hawley TM, Portoghese PS, et al. Reduced Antinociception of Opioids in Rats and Mice by Vaccination with Immunogens Containing Oxycodone and Hydrocodone Haptens. J Med Chem 2013;56:915–23. 10.1021/jm3013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Crossey E, Amar MJA, Sampson M, Peabody J, Schiller JT, Chackerian B, et al. A cholesterol-lowering VLP vaccine that targets PCSK9. Vaccine 2015;33:5747–55. 10.1016/j.vaccine.2015.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nakhla D, Hussien L, Nasef N, Hassan H, Abdallah I. Sensitive UPLC–MS/MS Method for Oxycodone Quantification in Serum and Brain Tissue Homogenates: Application to an Interaction Study in Rats. Journal of Advanced Pharmacy Research 2020;4:83–93. 10.21608/aprh.2020.31331.1109. [DOI] [Google Scholar]
- [40].Raleigh MD, Laudenbach M, Baruffaldi F, Peterson SJ, Roslawski MJ, Birnbaum AK, et al. Opioid Dose- and Route-Dependent Efficacy of Oxycodone and Heroin Vaccines in Rats. J Pharmacol Exp Ther 2018;365:346–53. 10.1124/jpet.117.247049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Raleigh MD, Pentel PR, LeSage MG. Pharmacokinetic Correlates of the Effects of a Heroin Vaccine on Heroin Self-Administration in Rats. PLoS ONE 2014;9:e115696. 10.1371/journal.pone.0115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Baldo BA, Rose MA. Mechanisms of opioid-induced respiratory depression. Arch Toxicol 2022;96:2247–60. 10.1007/s00204-022-03300-7. [DOI] [PubMed] [Google Scholar]
- [43].Listos J, Łupina M, Talarek S, Mazur A, Orzelska-Górka J, Kotlińska J. The Mechanisms Involved in Morphine Addiction: An Overview. IJMS 2019;20:4302. 10.3390/ijms20174302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Raleigh MD. Pharmacological mechanisms underlying the efficacy of antibodies generated by a vaccine to treat oxycodone use disorder. Neuropharmacology n.d;195. 10.1016/j.neuropharm.2021.108653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pravetoni M, Comer SD. Development of vaccines to treat opioid use disorders and reduce incidence of overdose. Neuropharmacology 2019;158:107662. 10.1016/j.neuropharm.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pravetoni M, Le Naour M, Harmon TM, Tucker AM, Portoghese PS, Pentel PR. An oxycodone conjugate vaccine elicits drug-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J Pharmacol Exp Ther 2012;341:225–32. 10.1124/jpet.111.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Raleigh MD, King SJ, Baruffaldi F, Saykao A, Hamid FA, Winston S, et al. Pharmacological mechanisms underlying the efficacy of antibodies generated by a vaccine to treat oxycodone use disorder. Neuropharmacology 2021;195:108653. 10.1016/j.neuropharm.2021.108653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Stone AE, Scheuermann SE, Haile CN, Cuny GD, Velasquez ML, Linhuber JP, et al. Fentanyl conjugate vaccine by injected or mucosal delivery with dmLT or LTA1 adjuvants implicates IgA in protection from drug challenge. NPJ Vaccines 2021;6:69. 10.1038/s41541-021-00329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kosten TR, Haile CN, Domingo CB, Norton EB. Anti-Cocaine IgA Rather Than IgG Mediates Vaccine Protection from Cocaine Use. Pharmaceutics 2022;14:2368. 10.3390/pharmaceutics14112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].St. John AL, Choi HW, Walker QD, Blough B, Kuhn CM, Abraham SN, et al. Novel mucosal adjuvant, mastoparan-7, improves cocaine vaccine efficacy. Npj Vaccines 2020;5:12. 10.1038/s41541-020-0161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


