Abstract
Background and aims:
Oral nicotine pouches (ONPs) probably offer reduced harm compared with cigarettes, but independent data concerning their misuse liability are lacking. We compared nicotine delivery and craving relief from ONPs with different nicotine concentrations to cigarettes.
Design:
This was a single-blind, three-visit (≥ 48-hour washout), randomized-cross-over study. Participants were encouraged to complete all study visits in less than 1 month.
Setting:
The study took place in Rural/Appalachian Ohio.
Participants:
Participants comprised 30 adults who smoke cigarettes. Participants (meanage = 34.5) were 60% men and 90% White.
Intervention:
Participants who were ≥ 12-hour tobacco-abstinent used: (1) a 3-mg nicotine concentration ONP, (2) a 6-mg nicotine concentration ONP and (3) usual brand cigarette in separate visits. ONPs (wintergreen Zyn) were used for 30 minutes; cigarettes were puffed every 30 sec for 5 minutes.
Measurements:
Plasma nicotine and self-reported craving were assessed at t = 0, 5, 15, 30, 60 and 90 minutes. The primary outcome was plasma nicotine concentration at t = 30 minutes. A secondary outcome was craving relief at t = 5 minutes.
Findings:
At t = 30, mean [95% confidence interval (CI)] plasma nicotine was 9.5 ng/ml (95% CI = 7.1, 11.9 ng/ml) for the 3 mg nicotine ONP, 17.5 ng/ml (95% CI = 13.7, 21.3) for the 6 mg nicotine ONP and 11.4 ng/ml (95% CI = 9.2, 13.6 ng/ml) for the cigarette. Mean plasma nicotine at t = 30 minutes differed between the 3- and 6-mg nicotine ONPs (P = 0.001) and between the 6-mg nicotine ONP and cigarette (P = 0.002). Mean (95% CI) craving at t = 5 minutes was lower for the cigarette (mean = 1.00, 95% CI = 0.61, 1.39) than either the 3 mg (mean = 2.25, 95% CI = 1.68, 2.82; P < 0.0001) or 6 mg nicotine (mean = 2.19, 95% CI = 1.60, 2.79; P < 0.0001) ONP.
Conclusions:
Among adult smokers, using 6-mg nicotine concentration oral nicotine pouches (ONPs) was associated with greater plasma nicotine delivery at 30 minutes than 3-mg ONPs or cigarettes, but neither ONP relieved craving symptoms at 5 minutes as strongly as a cigarette. Accelerating the speed of nicotine delivery in ONPs might increase their misuse liability relative to cigarettes.
Keywords: Cigarettes, health disparities, misuse liability, nicotine, oral nicotine pouches, rural
INTRODUCTION
Oral nicotine pouches (ONPs) are small white pouches that contain nicotine but no tobacco leaf [1]. ONPs entered the US market as a consumer product in 2016, and sales have quickly skyrocketed [2, 3]. From August to December 2019, 126 million units of ONPs were sold; from January to March 2022, more than 808 million units of ONPs were sold [2, 3]. Leading ONP brands are owned by major tobacco companies, including Altria (On!), British American Tobacco (Velo), Swedish Match (Zyn) and Swisher (Rogue) [1, 2, 4, 5]. ONP marketing has predominantly targeted tobacco users in the United States, where they are often cross-promoted with cigarettes [5]. Advertisements for ONPs frequently make claims that they can be used anywhere (e.g. to avoid indoor air laws) and are spit-free, smoke-free alternatives to other tobacco products [5–7]. As they contain no tobacco leaf, some brands of ONPs advertise that they are ‘tobacco-free’ [7].
ONP uptake has thus far primarily occurred among adults and young people who use other forms of tobacco [8–10]. Because ONPs have fewer toxicants than other forms of tobacco [11], they might offer an opportunity for harm reduction if tobacco users completely switch to these products [12–15]. The subjective appeal of ONPs relative to cigarettes is one aspect of their misuse liability that could support switching from cigarettes to ONPs. In human laboratory studies, participants generally report moderate liking of study ONPs, but like them less than cigarettes [16–18]. Nicotine concentration of ONPs appears to affect product perceptions and liking. Regarding product perceptions, experimental evidence suggests that viewing product packaging that indicates 6 mg nicotine concentration (versus none or 3 mg) is associated with reduced perceptions of harm and addictiveness [19]. Regarding product liking, using ONPs with lower nicotine concentrations is associated with reduced liking and intentions to use the product again [17], as well as reduced plasma nicotine delivery [20]. These human laboratory studies have also reported that nicotine delivery from ONPs is most similar to other oral tobacco products, such as moist snuff, as maximum plasma nicotine concentration is reached 30–60 minutes after initiating product use (versus 5 minutes for a cigarette) [16–18, 21]. Additionally, most studies report that maximum plasma nicotine concentration is higher when smoking cigarettes than when using ONPs [16, 18], although using some brands of ONPs with higher nicotine concentrations (i.e. ≥ 6 mg/pouch) results in greater maximum plasma nicotine concentrations [17].
In the United States, the Food and Drug Administration (FDA) has authority to regulate ONPs, including by authorizing their marketing via the pre-market tobacco product application (PMTA) process. The FDA also has authority to define product standards that set a minimum or maximum nicotine concentration for ONPs, as long as such product standards are ‘appropriate for the protection of public health’ [22, 23]. The FDA has already authorized marketing of certain brands of ONPs following review of their PMTAs, noting that the reviewed brands of ONPs hold little appeal to youth and non-smokers and that ONPs contain fewer harmful and potentially harmful chemicals than cigarettes [24]. Additional regulations via product standards have not been proposed, although product standards that regulate the nicotine concentration of ONPs could affect their appeal. Of note, all human laboratory studies investigating the appeal and nicotine delivery of ONPs have been funded by ONP manufacturers, and independent research on this topic has not yet been published. From prior industry-funded research, nicotine concentration has emerged as a key factor that could support complete switching from other tobacco products by affecting product appeal and nicotine delivery [16–18], and is thus a potential target of regulation.
The goal of this randomized cross-over study was to evaluate differences in product appeal and plasma nicotine delivery associated with using ONPs of differing nicotine concentrations in a sample of adult people who smoke (PWS). Because of the disparately high prevalence of cigarette smoking in rural and Appalachian areas of the United States [25–28] and associated cancer disparities [29–32], we conducted this research among rural and Appalachian PWS to specifically assess the appeal of ONPs (versus cigarettes) within this group. We hypothesized that using ONPs with higher nicotine concentration would be associated with greater plasma nicotine delivery, reductions in craving and withdrawal symptoms and product appeal than ONPs with lower nicotine concentration.
METHODS
Design
We used a single-blind, three-visit, randomized cross-over design (Figure 1). There was a minimum 48-hour washout period between visits, and participants were encouraged to complete all visits within 1 month.
FIGURE 1.
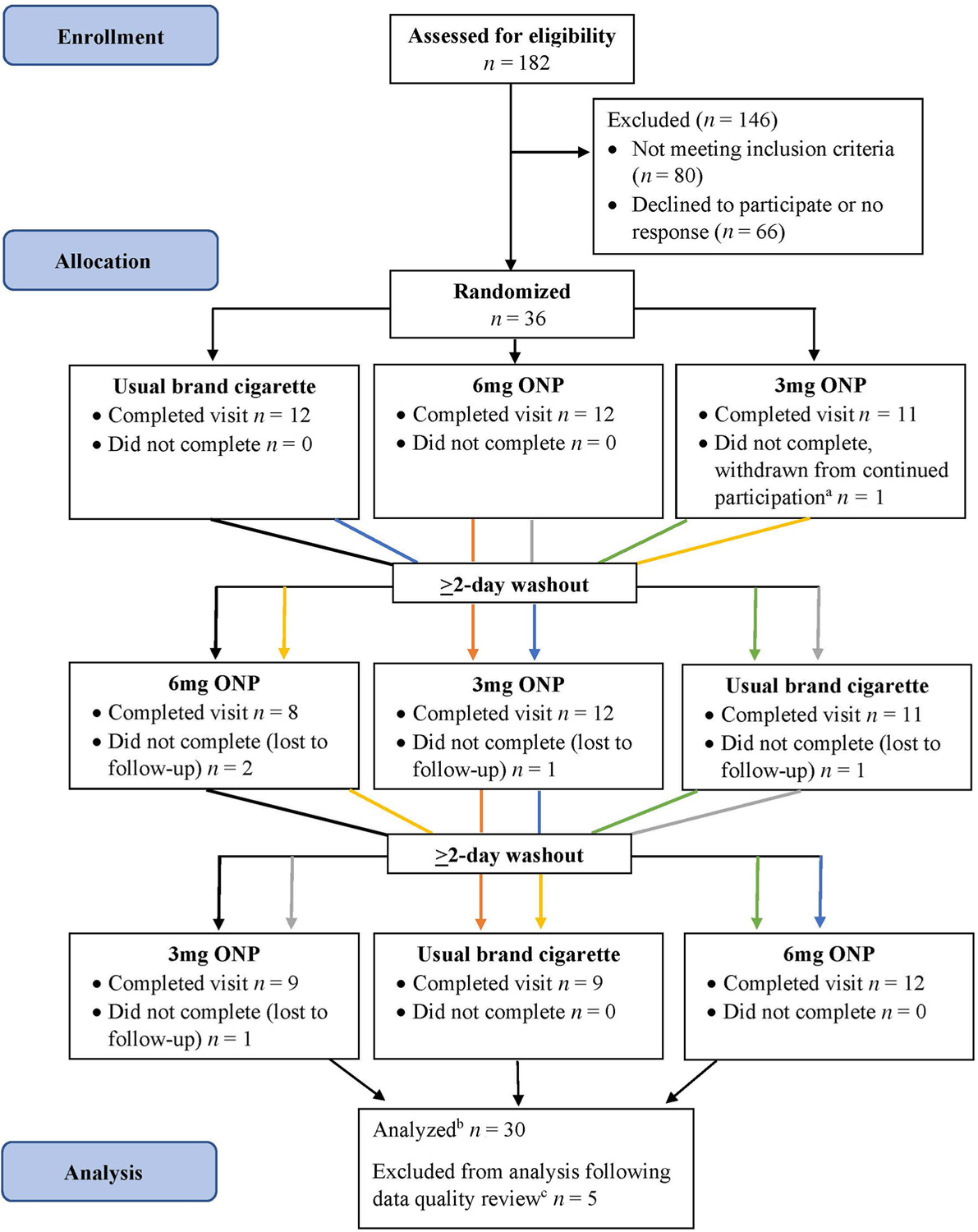
Randomized cross-over study flow chart, Ohio, 2022. aIV line could not be placed. bParticipants who were lost to follow-up but had complete data for the visits they completed were included in analyses. cThree participants were excluded due to inconsistent reporting on use of other tobacco products between the screener and clinic visit survey. Two participants (both completed visit 1 only) were excluded due to missing data in measures used for analysis. ONP = oral nicotine pouch.
Participants
Participants were adult PWS (n = 30) who lived in a rural (rural–urban commuting area code = 10) or Appalachian Ohio county (as designated by the Appalachian Regional Commission) and were recruited between May and September 2022. Participants were enrolled in aim 1 of a two-aim clinical trial evaluating misuse liability of ONPs (NCT05236894). We recruited participants using targeted social media advertisements and word-of-mouth. Interested individuals completed an on-line screening questionnaire, and study staff called potential participants to confirm eligibility over the telephone. Inclusion criteria were (1) ages ≥ 21 years; (2) residence in a rural or Appalachian Ohio county; (3) willing and able to abstain from all tobacco, nicotine and marijuana for ≥ 12 hours prior to study visits; and (4) smoke five or more cigarettes per day for longer than the past 30 days. Exclusion criteria were (1) use of other tobacco products for > 10 days of the past month; (2) currently trying to quit smoking or interested in quitting smoking in the next 3 months, as cigarette smoking as a study activity could hinder success of quit attempts; (3) pregnant, trying to become pregnant or breastfeeding; (4) uncontrolled mental health disorders or past-year hospitalization for a psychiatric condition; (5) cardiovascular health issues, including heart disease, chest pain or angina, uncontrolled diabetes or uncontrolled hypertension, irregular heart beat or abnormal heart rhythm and myocardial infarction or other cardiac event; (6) lung disease, including chronic obstructive pulmonary disorder, cystic fibrosis or uncontrolled asthma; (7) a history of problematic or difficult blood draws; and (8) use of illicit drugs (except for marijuana) in the past 30 days.
Procedures
Participants visited our clinic in Columbus, OH (1–3 hours’ travel from their home county) for three visits, using a different study product each time: (1) 3 mg nicotine concentration Zyn ONP, (2) 6 mg nicotine concentration Zyn ONP and (3) usual brand cigarette. All ONPs were wintergreen flavored. We selected Zyn wintergreen ONPs because wintergreen is a leading flavor [4, 33], and the pH of 3 versus 6 mg nicotine concentration Zyn wintergreen ONPs was similar [34]. Similar pH is important because pH affects the fraction of nicotine that is free (versus salt), with a greater fraction of free nicotine increasing the efficiency of nicotine transport across the oral mucosa [35]. Participants received one study product per visit, with visit order being randomized. The study research assistant randomized participants to one of six possible visit orders using the REDCap randomization function immediately after obtaining informed consent. Participants were blinded to visit order (and thus which ONP they were using at the ONP visits), but study research assistants were not blinded to product assignment.
We biochemically confirmed ≥ 12 hours combustible tobacco abstinence prior to product use at each visit using exhaled carbon monoxide < 10 parts per million (p.p.m.) and participant self-report. We also administered urine pregnancy tests prior to product use at each visit for all participants capable of pregnancy.
Participants reported baseline (visit 1) and nicotine withdrawal measures prior to product use (visits 1–3). Study product use was standardized. For ONP visits, participants were instructed to keep the ONP in place between their upper lip and gum for 30 minutes. For the cigarette visit, participants were instructed to take one puff of their usual brand cigarette every 30 sec for 5 minutes. All cigarette smoking sessions occurred in a negative pressure clinic room. Blood draws (3 ml) to measure plasma nicotine occurred at t = 0 (immediately prior to ONP placement or smoking), 5, 15, 30, 60 and 90 minutes via intravenous forearm catheter. Participants completed measures of withdrawal symptoms concurrent with each blood draw. Participants remained seated for the full 90 minutes. Following study product use, participants completed measures of product appeal. Participants were compensated $150 for each visit plus a $50 bonus for completing all sessions within 1 month. The Ohio State University’s Institutional Review Board approved all study procedures. Participants provided informed consent prior to completing study procedures.
Measures
Primary outcome measure
Plasma nicotine concentration at t = 30 minutes was the primary outcome measure.
Secondary outcome measures
Craving relief at t = 5 minutes, measured using the Minnesota Withdrawal Scale (MNWS) [36], was a secondary outcome. Other secondary outcome measures included plasma nicotine concentrations at t = 5, 15, 60 and 90 minutes and subjective measures of drug effects and liking and withdrawal symptoms. Subjective measures of drug effects and liking included pleasantness, desire/urge, need and wanting to use the study product again; interest and willingness to use the study product again; liking, enjoyment, pleasurableness and satisfaction from using the study product; and feeling any effects, good effects or bad effects from the study product [37, 38]. These variables were measured on a visual analogue scale with anchors from not at all to extremely or very. Withdrawal symptoms were measured using the Minnesota Withdrawal Scale (MNWS) [36]. The MNWS was analyzed in total and by the craving item at each time of assessment.
Independent variables
The independent variable of interest was study product: 3 mg nicotine ONP, 6 mg nicotine ONP and usual brand cigarette. Other independent variables used to characterize the sample included age (years), race, ethnicity, sex, gender, highest level of education, ever use of any oral tobacco product (i.e. snuff, chewing tobacco, snus or ONPs), number of cigarettes smoked per day and usual cigarette flavor (menthol versus tobacco).
Statistical analysis
Sample size
We calculated power with repeated-measures analysis of variance (ANOVA) based on plasma nicotine concentration at 30 minutes, using estimates from similar studies [17, 21]. At 30 minutes, assuming mean plasma nicotine levels of 15 ng/ml (6 mg nicotine ONP), 8 ng/ml (3 mg nicotine ONP) and 10 ng/ml (usual brand cigarette); a common standard deviation (SD) of 5 ng/ml; a within-subjects correlation of 0.2; and an alpha of 0.05, we would have > 95% power with a sample size of 20 participants. We aimed to accrue at least 30 participants to allow for attrition.
General statistical methods
We characterized the sample using descriptive statistics. We also inspected the distributions of dependent variables and log-transformed skewed variables (e.g. total MNWS score, plasma nicotine values) prior to analysis. We confirmed that independent variables and plasma nicotine concentration at t = 0 minutes were balanced by random visit order assignment using Fisher’s exact tests (categorical variables) or Kruskal–Wallis tests (continuous variables). All participants with complete data for at least one visit were included in analyses; n = 28 had complete data for all three visits, n = 29 had complete data for two visits and n = 30 had complete data for one visit. We adjusted for multiple comparisons for each longitudinal outcome using Holm’s procedure. All analyses were completed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Primary outcome
We used linear mixed-effect models with fixed effects for study product and a random participant intercept to compare plasma nicotine concentration at t = 30 minutes between the 3 mg nicotine ONP, 6 mg nicotine ONP and usual brand cigarette. We first conducted a global comparison across the three study products using the F-test. If the P-value for this global test was < 0.05, we then conducted three post-hoc tests to evaluate differences between study products with Tukey–Kramer adjustment of P-values.
Secondary outcomes
We used linear mixed-effect models with fixed effects for study product and a random participant intercept to compare (1) plasma nicotine concentration at t = 5, 15, 60 and 90 minutes; (2) subjective measures of drug effects and liking; (3) overall withdrawal symptoms measured at t = 5, 15, 30, 60 and 90 minutes; and (4) craving at t = 5, 15, 30, 60 and 90 minutes between the 3 mg nicotine ONP, 6 mg nicotine ONP and usual brand cigarette. We first conducted a global comparison across the three study products using the F-test. If the P-value for this global test was < 0.05, we then conducted three post-hoc tests to evaluate differences between study products with Tukey–Kramer adjustment of P-values.
RESULTS
Sample characteristics
Participants were aged 34.5 (SD = 11.1) years on average; 60% were men, 90% were White and 50% obtained education beyond a high school diploma (Table 1). Nearly two-thirds (63.3%) had ever used oral tobacco products, and participants smoked 16.8 (SD = 7.4) cigarettes per day on average.
TABLE 1.
Sample characteristics, rural and Appalachian Ohio, 2022.a
| n = 30 | |
|---|---|
| Age (mean, SD) | 34.5 (11.1) |
| Sex assigned at birth (n, %) | |
| Female | 11 (36.7) |
| Male | 19 (63.3) |
| Gender (n, %) | |
| Woman | 12 (40.0) |
| Man | 18 (60.0) |
| Race (n, %) | |
| White | 27 (90.0) |
| Black or African American | 2 (6.7) |
| More than one race | 1 (3.3) |
| Ethnicity (n, %) | |
| Hispanic or Latino/a | 0 (0.0) |
| Not Hispanic or Latino/a | 30 (100.0) |
| Highest level of education (n, %) | |
| < High school | 6 (20.0) |
| Graduated high school | 9 (30.0) |
| Currently attending college | 1 (3.3) |
| Some college but no degree | 8 (26.7) |
| Associate’s degree | 1 (3.3) |
| Bachelor’s degree | 4 (13.3) |
| Master’s degree | 1 (3.3) |
| Ever use of oral tobacco productsb (n, %) | |
| Yes | 19 (63.3) |
| No | 11 (36.7) |
| Cigarettes smoked per day (mean, SD) | 16.8 (7.4) |
| Usual cigarette flavor (n, %) | |
| Menthol | 11 (36.7) |
| Tobacco | 19 (63.3) |
| Random assignment at first visit (n, %) | |
| 3 mg nicotine ONP | 11 (36.7) |
| 6 mg nicotine ONP | 10 (33.3) |
| Usual brand cigarette | 9 (30.0) |
Abbreviations: ONP = oral nicotine pouch; SD = standard deviation.
Participants were healthy adults who smoke cigarettes who lived in a rural or Appalachian Ohio county. Study visits occurred at our clinic in Columbus, OH. Participants completed a three-visit, single-blind, randomized cross-over experiment in which they sampled oral nicotine pouches (3 versus 6 mg nicotine concentration) or used their usual brand cigarette.
Oral tobacco products included snuff, chew, snus and oral nicotine pouches.
Primary outcome measure: plasma nicotine delivery at t = 30 minutes
At t = 30 minutes, mean [95% confidence interval (CI)] plasma nicotine concentrations were 9.5 ng/ml (7.1, 11.9 ng/ml) for the 3 mg nicotine concentration ONP, 17.5 ng/ml (13.7, 21.3 ng/ml) for the 6 mg nicotine concentration ONP and 11.4 ng/ml (9.2, 13.6 ng/ml) for the cigarette. At t = 30 minutes, the mean plasma nicotine concentration differed significantly between the 3 and 6 mg nicotine concentration ONPs (P < 0.001) and between the 6 mg nicotine concentration ONP and cigarette (P < 0.001). Mean plasma nicotine concentration at t = 30 minutes did not differ between the 3 mg nicotine concentration ONP and cigarette (P = 0.40).
Secondary outcome measures
Craving relief at t = 5 minutes
Using the usual brand cigarette resulted in greater self-reported reductions in craving at 5 minutes of product use (mean = 1.00, 95% CI = 0.61, 1.39) than the 3 mg (mean = 2.25, 95% CI = 1.68, 2.82; P < 0.0001) or 6 mg nicotine concentration (mean = 2.19, 95% CI = 1.60, 2.79; P < 0.001) ONPs.
Plasma nicotine delivery
Relative to usual brand cigarette, using the 3 mg nicotine concentration ONP resulted in significantly lower plasma nicotine concentration at 5 and 15 minutes; using the 6 mg nicotine concentration ONP resulted in significantly lower plasma nicotine concentration at 5 minutes but significantly higher plasma nicotine concentration at 30 and 60 minutes (Table 2; Figure 2). Plasma nicotine concentration differed between ONPs, with the 6 mg ONP delivering significantly more nicotine than the 3 mg ONP from 5 to 90 minutes (Ps ≤ 0.004). Maximum plasma nicotine concentration was achieved earlier for usual brand cigarettes (t = 5 minutes) than for the ONPs (t = 30 minutes for both).
TABLE 2.
Primary and secondary outcomes of plasma nicotine delivery, withdrawal and craving relief and subjective drug effects and liking for oral nicotine pouches (3 and 6 mg nicotine concentration) and usual brand cigarettes in a sample of healthy adult PWS, rural and Appalachian Ohio, 2022.a
| 3 mg ONP n = 29 Mean (SD) | 6 mg ONP n = 29 Mean (SD) | Usual brand cigarette n = 29 Mean (SD) | Comparison across products P-value | 3 mg ONP versus cigarette P-valuec | 6 mg ONP versus cigarette P-valuec | 3 mg ONP versus 6 mg ONP P-valuec | |
|---|---|---|---|---|---|---|---|
| Primary outcome | |||||||
| Plasma nicotine at 30 mind | 9.5 (5.6) | 17.5 (9.8) | 11.4 (5.7) | < 0.001 | 0.40 | < 0.001 | < 0.001 |
| Secondary outcomes | |||||||
| Craving (minutes) | |||||||
| 5 | 2.3 (1.4) | 2.2 (1.5) | 1.0 (1.0) | < 0.001 | < 0.001 | < 0.001 | 0.45 |
| 15 | 1.7 (1.3) | 1.6 (1.4) | 1.3 (1.3) | 0.07 | – | – | – |
| 30 | 1.6 (1.4) | 1.3 (1.4) | 1.6 (1.4) | 0.27 | – | – | – |
| 60 | 2.1 (1.3) | 1.9 (1.5) | 2.0 (1.6) | 0.44 | – | – | – |
| 90 | 2.1 (1.4) | 2.2 (1.4) | 2.6 (1.3) | 0.39 | – | – | – |
| Plasma nicotined (minutes) | |||||||
| 5 | 5.5 (5.4) | 11.1 (11.7) | 27.8 (17.6) | < 0.001 | < 0.001 | < 0.001 | 0.004 |
| 15 | 8.1 (5.1) | 15.8 (10.4) | 14.5 (6.4) | < 0.001 | < 0.001 | 0.81 | 0.001 |
| 60 | 7.2 (4.6) | 13.4 (7.2) | 9.0 (5.1) | < 0.001 | 0.32 | < 0.001 | < 0.001 |
| 90 | 6.1 (3.8) | 11.1 (6.5) | 7.6 (4.4) | < 0.001 | 0.32 | 0.001 | 0.004 |
| Withdrawal symptomsd (minutes) | |||||||
| 5 | 7.0 (7.3) | 6.6 (6.2) | 5.4 (5.3) | 0.20 | – | – | – |
| 15 | 4.9 (5.6) | 4.6 (4.9) | 4.5 (5.0) | 0.35 | – | – | – |
| 30 | 4.8 (5.6) | 4.1 (5.3) | 4.9 (5.9) | 0.35 | – | – | – |
| 60 | 5.0 (5.7) | 4.2 (3.9) | 6.8 (7.9) | 0.13 | – | – | – |
| 90 | 5.6 (6.8) | 5.9 (6.6) | 7.8 (8.0) | 0.18 | – | – | – |
| Mean (SD) | Mean (SD) | Mean (SD) | P-value | P-valueb | P-valueb | P-valueb | |
| Pleasant to use again right nowe | 63.0 (21.7) | 58.4 (28.7) | 75.5 (25.9) | 0.007 | 0.07 | 0.006 | 0.69 |
| Desire/urge to use right nowe | 48.3 (25.5) | 48.7 (31.0) | 64.9 (26.5) | 0.005 | 0.01 | 0.01 | 0.99 |
| Need to use for reliefe | 47.0 (26.5) | 46.9 (23.7) | 66.0 (24.5) | < 0.001 | 0.002 | < 0.001 | 0.98 |
| Want to usee | 47.6 (24.2) | 51.8 (29.1) | 70.8 (25.5) | < 0.001 | < 0.001 | < 0.001 | 0.69 |
| Extent of likinge | 60.2 (24.4) | 60.1 (32.0) | 74.8 (26.0) | 0.002 | 0.007 | 0.007 | 0.99 |
| Extent of enjoymentf | 55.8 (25.1) | 57.3 (29.7) | 74.6 (24.9) | 0.002 | 0.004 | 0.008 | 0.96 |
| Extent of pleasurablenessf | 56.9 (27.3) | 55.3 (30.9) | 67.1 (26.6) | 0.02 | 0.07 | 0.03 | 0.90 |
| Extent of satisfactionf | 58.3 (24.1) | 63.5 (26.3) | 71.6 (26.0) | 0.008 | 0.006 | 0.11 | 0.46 |
| Interest in using again in futuref | 6.2 (2.7) | 6.1 (2.9) | 8.0 (2.3) | 0.001 | 0.006 | 0.002 | 0.95 |
| Willing to use againf | 7.7 (2.6) | 7.0 (2.8) | 8.2 (2.3) | 0.07 | – | – | – |
| Satisfactiond,g,h | 3.0 (1.4) | 2.9 (1.5) | 4.5 (1.3) | < 0.001 | < 0.001 | < 0.001 | 0.72 |
| Rewardg,i | 2.3 (1.4) | 2.3 (1.3) | 3.5 (1.4) | < 0.001 | < 0.001 | < 0.001 | 0.99 |
| Aversiond,g,j | 0.6 (1.0) | 1.0 (1.1) | 1.0 (1.2) | 0.08 | – | – | – |
| Enjoyed sensationg | 1.9 (1.5) | 2.3 (1.7) | 4.0 (1.6) | < 0.001 | < 0.001 | < 0.001 | 0.50 |
| Immediate reduction in cravingg | 3.0 (1.6) | 3.1 (1.7) | 4.3 (1.6) | 0.004 | 0.009 | 0.01 | 0.99 |
| Felt any effectsk | 42.2 (30.0) | 39.6 (32.4) | 44.8 (30.9) | 0.59 | – | – | – |
| Felt good effectsk | 45.9 (26.9) | 54.3 (27.1) | 62.5 (25.8) | 0.02 | 0.02 | 0.33 | 0.34 |
| Felt bad effectsd, k | 16.7 (22.9) | 23.8 (26.3) | 29.0 (26.8) | 0.07 | – | – | – |
Note: Bold type denotes statistical significance.
Abbreviations: ONP = oral nicotine pouch; PWS = people who smoke; SD = standard deviation.
Participants completed three study visits in a randomized cross-over, single-blind experiment. Zyn wintergreen 3 and 6 mg nicotine concentration ONPs were used. The sample size for each study condition differs from the total sample size due to participant attrition.
P-values for pairwise comparisons with Tukey–Kramer adjustment.
P-values for pairwise comparisons with Holm’s adjustment.
Variable was log-transformed for analysis.
Response options ranged from 0 (not at all) to 100 (very).
Response options ranged from 0 (not at all) to 10 (very).
Items were assessed on a 7-point Likert scale ranging from 1 (not at all) to 7 (extremely).
Satisfaction is a composite measure of items assessing product satisfaction, taste, and enjoyment.
Reward is a composite measure of items assessing whether the product had a calming effect; made the participant feel more awake, less irritable and less hungry; and reduced hunger.
Aversion is a composite measure of items assessing whether the product made the participant feel dizzy or nauseous.
Response options ranged from 0 (not at all) to 100 (extremely)
FIGURE 2.

Plasma nicotine delivery associated with using 3 mg nicotine concentration oral nicotine pouches (ONPs), 6 mg nicotine concentration ONPs and usual brand cigarettes among adults who smoke cigarettes, Ohio, 2022. aFor ONP use sessions, participants used one ONP (Zyn brand) for 30 minutes while holding it in the same location between their upper lip and gum. For cigarette smoking sessions, participants took one puff from their usual brand cigarette every 30 s for 5 minutes. Error bars represent 95% confidence intervals. bStatistically significant difference between usual brand cigarette and 3 mg nicotine concentration ONP (P < 0.01). cStatistically significant difference between usual brand cigarette and 6 mg nicotine concentration ONP (P < 0.01). dStatistically significant difference between 3 mg nicotine concentration ONP and 6 mg nicotine concentration ONP (P < 0.01).
Subjective drug effects and liking
Overall, participants reported moderate appeal (e.g. pleasantness, wanting to use, extent of liking and enjoyment) of the study ONPs, and differences in product appeal were not observed between the two study ONPs (Table 2). Participants consistently reported both ONPs as less appealing than their usual brand cigarette.
Withdrawal relief
Use of each study product was associated with similar self-reported relief from overall withdrawal symptoms (Figure 3). Beyond differences in craving relief at t = 5 minutes, no other differences in craving relief were observed through the 90 minutes of follow-up.
FIGURE 3.
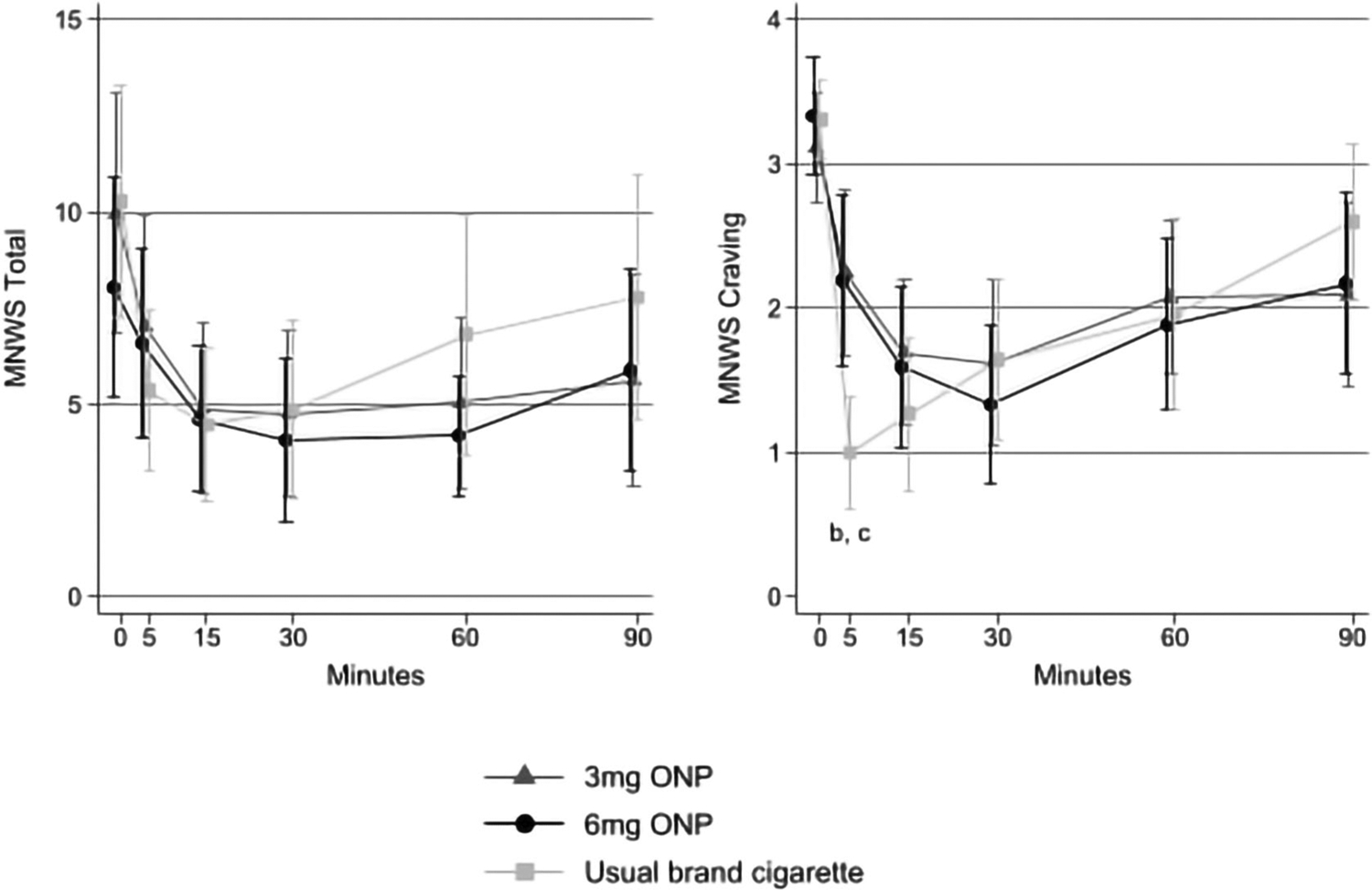
Withdrawal relief associated with using usual brand cigarettes, oral nicotine pouches (ONPs) with 3 mg nicotine concentration and ONPs with 6 mg nicotine concentration among adults who smoke cigarettes, Ohio, 2022. aWithdrawal measures were assessed immediately before ONP use or smoking (t = 0 minutes) and at t = 5, 15, 30, 60 and 90 minutes. Total Minnesota Withdrawal Scale (MNWS) scores were log-transformed for analysis. Error bars represent 95% confidence intervals. bStatistically significant difference between usual brand cigarette and 3 mg nicotine concentration ONP (P < 0.0001). cStatistically significant difference between usual brand cigarette and 6 mg nicotine concentration ONP (P < 0.0001).
DISCUSSION
We compared the product appeal and nicotine delivery of 3 and 6 mg nicotine concentration ONPs with usual brand cigarettes in a sample of rural and Appalachian PWS. Overall, participants found ONPs to be moderately appealing, but less appealing than their usual brand cigarette. ONPs and cigarettes performed similarly with respect to withdrawal relief, although using usual brand cigarettes demonstrated greater reductions in craving after 5 minutes of use. We identified differences in plasma nicotine delivery associated with the product used, with use of the 6 mg nicotine concentration ONPs resulting in greater plasma nicotine levels than the 3 mg nicotine concentration ONPs from 5 to 90 minutes and greater plasma nicotine levels than usual brand cigarettes at 30 and 60 minutes. Results suggest that while 6 mg nicotine concentration ONPs are probably better substitutes for cigarettes than 3 mg nicotine concentration ONPs, neither of the ONPs we tested appear to match the misuse liability of cigarettes. Thus, rural and Appalachian PWS might not find the ONPs that we tested to be acceptable reduced-harm substitutes for cigarettes.
Our findings that ONPs at both nicotine concentrations held moderate appeal—but less appeal than cigarettes—agree with the prior industry-funded research [16–18, 20]. We expected that participants would find the 6 mg nicotine concentration ONP more appealing than the 3 mg ONP, but we identified no differences in subjective measures of appeal between the ONPs as was found in a similar study [20]. These results are similar to other research that found no differences in product appeal according to flavor of ONP [16], but contrast with a study indicating that ONPs with lower nicotine concentrations were rated as less appealing by PWS [17]. Our results might differ from this prior study’s results for several reasons. First, the prior study [17] included a sampling period in which participants tried two kinds of ONPs for 30 minutes to become familiar with the products. Following this sampling period participants used five ONPs for 60 minutes each, and measures of product appeal were assessed following these longer periods of use. Because participants had greater exposure to a variety of ONPs, it is possible that they were more attuned to differences associated with nicotine concentration than our participants. Secondly, the prior study tested ONPs with varying nicotine concentrations across different brands. ONP brands vary in free nicotine fraction [34], so it is possible that nicotine concentration was not the only variable affecting product appeal.
Regarding the plasma nicotine delivery findings, neither ONP matched the maximum plasma nicotine concentration of participants’ usual brand cigarettes. We also found that time of maximum nicotine concentration was later for ONPs (t = 30 minutes) than for usual brand cigarettes (t = 5 minutes), and that smoking resulted in a greater reduction in craving after 5 minutes of use. Together, these findings are an indication that the ONPs we tested do not match the misuse liability of cigarettes, as rapid delivery of the desired dose of nicotine is key to the reinforcing experience of cigarette smoking [39]. Unsurprisingly, the plasma nicotine delivery of the ONPs we tested more closely matched that of other forms of smokeless tobacco, such as moist snuff—the use of which is also associated with maximum plasma nicotine concentrations occurring after 30–60 minutes of product use [21, 40]. Given that we observed consistently higher plasma nicotine delivery of the 6 mg ONP than the 3 mg ONP, it is possible that 6 mg ONPs could more closely match the misuse liability of moist snuff, as has been reported in industry-funded research [21].
We conducted this study of ONPs’ misuse liability relative to cigarettes among rural and Appalachian PWS because the prevalence of cigarette smoking remains high in these regions—particularly in Ohio [25–28, 41], where our study was conducted. For example, the prevalence of cigarette smoking in rural Appalachian Ohio was 27% among men and 26% among women in 2019; in non-Appalachian metro areas of Ohio the prevalence of cigarette smoking was 23% among men and 21% among women [41]. These disparities in cigarette smoking, in combination with other factors including higher poverty rates and reduced access to specialized health care [42, 43], contribute to cancer disparities for people living in rural and Appalachian regions of the United States [29–32]. With the stubbornly high prevalence of tobacco use in these regions, approaches that involve switching from a higher-risk to a lower-risk tobacco product, such as ONPs [11], could translate to a reduced cancer risk. However, our results indicate that the ONPs we tested in this study are unlikely to be strong substitutes for cigarettes in this population.
Our results are subject to the following limitations. First, our focus upon rural and Appalachian adults in this study resulted in a sample that had little racial or ethnic diversity, and results might not generalize to more diverse or urban samples. Secondly, we only tested two nicotine concentrations of ONPs (3 and 6 mg/pouch), one flavor (wintergreen) and one brand (Zyn), but nicotine concentrations of some brands exceed 10 mg/pouch. It is possible that we would have identified differences in product appeal between ONPs if we had used an ONP with a higher nicotine concentration that more closely matched a cigarette’s nicotine delivery. Indeed, recent industry-funded research identified no difference in product appeal between > 10 mg nicotine concentration ONPs and usual brand cigarettes [18]. Thirdly, although we selected commercially available ONPs with similar fractions of free nicotine [34] we did not confirm the fraction of free nicotine, and thus it is possible differences in free nicotine between pouches contributed to our findings. Finally, ONP flavor was held constant to minimize differences between conditions. While this improved internal validity, it limited generalizability of results to other ONPs of different flavors. Relatedly, we could not explore whether participants’ usual cigarette flavor (menthol versus tobacco) modified the effects of ONP nicotine concentration on the product appeal outcomes due to small cell sizes.
Future research is needed to inform regulations on ONPs that would best position them as a reduced harm option for PWS. Studies that evaluate ONPs with higher nicotine concentrations, and that control the fraction of free nicotine, would be useful to inform regulations on nicotine concentration that support switching from cigarettes (or other tobacco products) to ONPs. In addition, exploration of the role of free nicotine in ONPs’ misuse liability, holding nicotine concentration constant, would inform regulations that could bolster the effects of nicotine concentration regulations by limiting the industry’s ability to appeal to youth by making milder products with lower free nicotine fractions [44]. Exploration of the role of synthetic nicotine in the misuse liability of ONPs, again in the context of total nicotine concentration and fraction of free nicotine, would further strengthen regulatory approaches. For example, regulating a total fraction of the R-nicotine stereoisomer present in many synthetic nicotine products [45] could be a potential target to position ONPs as appealing only to adults who already use tobacco products.
In conclusion, we identified no differences in product appeal related to nicotine concentration in ONPs, but we did identify differences in nicotine delivery, with 6 mg nicotine concentration ONPs delivering greater nicotine than 3 mg ONPs. Participants reported that their usual brand cigarette was more appealing than either ONP and felt greater reductions in craving after 5 minutes of use from their usual brand cigarette than from the ONPs. Altogether, results suggest that the 3 and 6 mg nicotine concentration ONPs that we tested probably do not match the misuse liability of cigarettes, and thus they might not be strong reduced-harm substitutes for rural and Appalachian PWS. Future research that evaluates higher nicotine concentrations, fractions of free nicotine and flavors in ONPs would be useful to determine whether specific varieties of ONPs could reasonably be used to help adults reduce their cancer risks from cigarette smoking. Such studies would also inform the FDA’s review of ONPs as part of the PMTA process and potential product standards concerning ONPs’ nicotine concentration, fraction of free nicotine and flavors. Additionally, exploring the misuse liability of ONPs relative to moist snuff, which has similar plasma nicotine pharmacokinetics and is also used frequently in Appalachia and rural areas of the United States, could be useful to promote switching from moist snuff to ONPs [21, 46]. Finally, if ONPs gain approval to be marketed as modified-risk tobacco products (MRTPs) in the United States, evaluation of how MRTP claims might affect the appeal of ONPs among PWS would be useful.
ACKNOWLEDGEMENTS
This study was funded by the Addiction Innovation Fund at The Ohio State University’s College of Public Health. This research was also partially supported by The Ohio State University Comprehensive Cancer Center—The James and the National Cancer Institute of the National Institutes of Health (grant number P30CA016058). BKH was supported by grant number K01DA055696 from the National Institute on Drug Abuse. BKH, AH, TLW, and DM were supported by grant number U54CA287392 from the National Cancer Institute. The sponsors had no role in the study design; in the collection, analysis or interpretation of the data; in the writing of the report; or in the decision to submit the manuscript for publication.
Funding information
National Cancer Institute, Grant/Award Number: U54CA287392; National Institute on Drug Abuse, Grant/Award Number: K01DA055696; The Ohio State University Addiction Innovation Fund; The Ohio State University Comprehensive Cancer Center–The James; National Cancer Institute of the National Institutes of Health
Footnotes
DECLARATION OF INTERESTS
None.
CLINICAL TRIAL REGISTRATION
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Robichaud MO, Seidenberg AB, Byron MJ. Tobacco companies introduce ‘tobacco-free’ nicotine pouches. Tob Control. 2020;29:e145–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marynak KL, Wang X, Borowiecki M, Kim Y, Tynan MA, Emery S, et al. Nicotine pouch unit sales in the US, 2016–2020. JAMA. 2021; 326:566–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majmundar A, Okitondo C, Xue A, Asare S, Bandi P, Nargis N. Nicotine pouch sales trends in the US by volume and nicotine concentration levels from 2019 to 2022. JAMA Netw Open. 2022;5: e2242235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delnevo CD, Hrywna M, Miller EJ, Wackowski OA. Examining market trends in smokeless tobacco sales in the United States: 2011–2019. Nicotine Tob Res. 2020;23:1420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talbot EM, Giovenco DP, Grana R, Hrywna M, Ganz O. Crosspromotion of nicotine pouches by leading cigarette brands. Tob Control. 2021;32:528–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czaplicki L, Patel M, Rahman B, Yoon S, Schillo B, Rose SW. Oral nicotine marketing claims in direct-mail advertising. Tob Control. 2021; 31:663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan Z, Henriksen L, Vallone D, Rath JM, Evans WD, Romm KF, et al. Nicotine pouch marketing strategies in the USA: an analysis of Zyn, On! and Velo. Tob Control. 2022;1–10. 10.1136/tc-2022-057360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harlow AF, Vogel EA, Tackett AP, Cho J, Han DH, Wong M, et al. Adolescent use of flavored non-tobacco oral nicotine products. Pediatrics. 2022;150:e2022056586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Borland R, Cummings KM, Gravely S, Quah ACK, Fong GT, et al. Patterns of non-cigarette tobacco and nicotine use among current cigarette smokers and recent quitters: findings from the 2020 ITC four country smoking and vaping survey. Nicotine Tob Res. 2021;23: 1611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hrywna M, Gonsalves NJ, Delnevo CD, Wackowski OA. Nicotine pouch product awareness, interest and ever use among US adults who smoke, 2021. Tob Control. 2022;1–4. 10.1136/tobaccocontrol-2021-057156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azzopardi D, Liu C, Murphy J. Chemical characterization of tobacco-free ‘modern’ oral nicotine pouches and their position on the toxicant and risk continuums. Drug Chem Toxicol. 2022;45:2246–54. [DOI] [PubMed] [Google Scholar]
- 12.Arnold MJ, Nollen NL, Mayo MS, Ahluwalia JS, Leavens EL, Zhang G, et al. Harm reduction associated with dual use of cigarettes and e-cigarettes in Black and Latino smokers: secondary analyses from a randomized controlled e-cigarette switching trial. Nicotine Tob Res. 2021;23:1972–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller-Holt J, Baskerville-Abraham I, Sakimura M, Fukushima T, Puglisi A, Gafner J. In vitro evaluation of mutagenic, cytotoxic, genotoxic and oral irritation potential of nicotine pouch products. Toxicol Rep. 2022;9:1316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azzopardi D, Haswell LE, Frosina J, McEwan M, Gale N, Thissen J, et al. Assessment of biomarkers of exposure and potential harm, and physiological and subjective health measures in exclusive users of nicotine pouches and current, former and never smokers. Biomarkers. 2023;28:118–29. [DOI] [PubMed] [Google Scholar]
- 15.Back S, Masser AE, Rutqvist LE, Lindholm J. Harmful and potentially harmful constituents (HPHCs) in two novel nicotine pouch products in comparison with regular smokeless tobacco products and pharmaceutical nicotine replacement therapy products (NRTs). BMC Chem. 2023;17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rensch J, Liu J, Wang J, Vansickel A, Edmiston J, Sarkar M. Nicotine pharmacokinetics and subjective response among adult smokers using different flavors of on!® nicotine pouches compared to combustible cigarettes. Psychopharmacology. 2021;238:3325–34. [DOI] [PubMed] [Google Scholar]
- 17.McEwan M, Azzopardi D, Gale N, Camacho OM, Hardie G, Fearon IM, et al. A randomised study to investigate the nicotine pharmacokinetics of oral nicotine pouches and a combustible cigarette. Eur J Drug Metab Pharmacokinet. 2022;47:211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman F, McDermott S, Rudd K, Taverner V, Stevenson M, Chaudhary N, et al. A randomised, open-label, cross-over clinical study to evaluate the pharmacokinetic, pharmacodynamic and safety and tolerability profiles of tobacco-free oral nicotine pouches relative to cigarettes. Psychopharmacology. 2022;239:2931–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mays D, Long L, Alalwan MA, Wagener TL, Shang C, Roberts ME, et al. The effects of oral nicotine pouch packaging features on adult tobacco users’ and non-users’ product perceptions. Int J Environ Res Public Health. 2023;20:3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Rensch J, Wang J, Jin X, Vansickel A, Edmiston J, et al. Nicotine pharmacokinetics and subjective responses after using nicotine pouches with different nicotine levels compared to combustible cigarettes and moist smokeless tobacco in adult tobacco users. Psychopharmacology. 2022;239:2863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lunell E, Fagerström K, Hughes J, Pendrill R. Pharmacokinetic comparison of a novel non-tobacco-based nicotine pouch (ZYN) with conventional, tobacco-based Swedish snus and American moist snuff. Nicotine Tob Res. 2020;22:1757–63. [DOI] [PubMed] [Google Scholar]
- 22.111th Congress, US Government Printing Office [organization]. H.R. 1256—111th Congress. Family Smoking Prevention and Tobacco Control Act 2009 [Internet]. Available at: https://www.gpo.gov/fdsys/pkg/PLAW-111publ31/pdf/PLAW-111publ31.pdf. Accessed 12 June 2018. [Google Scholar]
- 23.US Food and Drug Administration (FDA) [organization]. Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products [Internet]. 2016. Available at: https://www.federalregister.gov/documents/2016/05/10/2016-10685/deeming-tobacco-products-to-be-subject-to-the-federal-food-drug-and-cosmetic-act-as-amended-by-the. Accessed 12 June 2018. [PubMed]
- 24.US Food and Drug Administration (FDA) [organization]. FDA Permits Marketing of New Oral Tobacco Products through Premarket Tobacco Product Application Pathway [Internet]. 2021. Available at: https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-new-oral-tobacco-products-through-premarket-tobacco-product-application. Accessed 18 July 2023.
- 25.Cardarelli K, Westneat S, Dunfee M, May B, Schoenberg N, Browning S. Persistent disparities in smoking among rural Appalachians: evidence from the Mountain Air Project. BMC Public Health. 2021;21:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doogan NJ, Roberts ME, Wewers ME, Stanton CA, Keith DR, Gaalema DE, et al. A growing geographic disparity: rural and urban cigarette smoking trends in the United States. Prev Med. 2017;104: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziller EC, Lenardson JD, Paluso NC, Talbot JA, Daley A. Rural–urban differences in the decline of adolescent cigarette smoking. Am J Public Health. 2019;109:771–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coughlin LN, Bonar EE, Bohnert KM, Jannausch M, Walton MA, Blow FC, et al. Changes in urban and rural cigarette smoking and cannabis use from 2007 to 2017 in adults in the United States. Drug Alcohol Depend. 2019;205:107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henley SJ, Anderson RN, Thomas CC, Massetti GM, Peaker B, Richardson LC. Invasive cancer incidence, 2004–2013, and deaths, 2006–2015, in nonmetropolitan and metropolitan counties—United States. Morb Mortal Wkly Rep Surveill Summ. 2017;66:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackley D, Behringer B, Zheng S. Cancer mortality rates in Appalachia: descriptive epidemiology and an approach to explaining differences in outcomes. J Community Health. 2012;37:804–13. [DOI] [PubMed] [Google Scholar]
- 31.Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017;26:992–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zahnd WE, James AS, Jenkins WD, Izadi SR, Fogleman AJ, Steward DE, et al. Rural–urban differences in cancer incidence and trends in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27:1265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaiha SM, Lin C, Lempert LK, Halpern-Felsher B. Use, marketing, and appeal of oral nicotine products among adolescents, young adults, and adults. Addict Behav. 2023;140:107632. [DOI] [PubMed] [Google Scholar]
- 34.Stanfill S, Tran H, Tyx R, Fernandez C, Zhu W, Marynak K, et al. Characterization of total and unprotonated (free) nicotine content of nicotine pouch products. Nicotine Tob Res. 2021;23:1590–6. [DOI] [PubMed] [Google Scholar]
- 35.Benowitz NL. The central role of pH in the clinical pharmacology of nicotine: implications for abuse liability, cigarette harm reduction and FDA regulation. Clin Pharmacol Ther. 2022;111:1004–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes JR. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289. [DOI] [PubMed] [Google Scholar]
- 37.Leavens EL, Driskill LM, Molina N, Eissenberg T, Shihadeh A, Brett EI, et al. Comparison of a preferred versus non-preferred waterpipe tobacco flavour: subjective experience, smoking behaviour and toxicant exposure. Tob Control. 2018;27:319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arger CA, Heil SH, Sigmon SC, Tidey JW, Stitzer ML, Gaalema DE, et al. Preliminary validity of the modified Cigarette Evaluation Questionnaire in predicting the reinforcing effects of cigarettes that vary in nicotine content. Exp Clin Psychopharmacol. 2017;25:473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362: 2295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickworth W, Rosenberry ZR, Gold W, Koszowski B. Nicotine absorption from smokeless tobacco modified to adjust pH. J Addict Res Ther. 2014;5:1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts M, Doogan N, Ferketich A, Crane D, Applegate M, Defiore-Hyrmer J et al. A profile of substance use in Ohio: 2020 update. Available at: http://grc.osu.edu/sites/default/files/inlinefiles/2019_OMAS_Substance_Use_Chartbook.pdf. Accessed 28 December 2020.
- 42.Herb J, Holmes M, Stitzenberg K. Trends in rural–urban disparities among surgical specialties treating cancer, 2004–2017. J Rural Health. 2022;38:838–44. [DOI] [PubMed] [Google Scholar]
- 43.Shrider EA, Kollar M, Chen F, Semega J. Income and poverty in the United States: 2020 current population reports [Internet]. Washington, DC: United States Census Bureau. 2021. Report no.: P60–273. Available at: https://www.census.gov/content/dam/Census/library/publications/2021/demo/p60-273.pdf. Accessed 12 January 2023. [Google Scholar]
- 44.Connolly GN. The marketing of nicotine addiction by one oral snuff manufacturer. Tob Control. 1995;4:73–9. [Google Scholar]
- 45.Jordt SE. Synthetic nicotine has arrived. Tob Control. 2021;32(e1): e113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts ME, Doogan NJ, Stanton CA, Quisenberry AJ, Villanti AC, Gaalema DE, et al. Rural versus urban use of traditional and emerging tobacco products in the United States, 2013–2014. Am J Public Health. 2017;107:1554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


