Abstract
Yersinia pestis, the causative agent of plague, harbors at least three plasmids necessary for full virulence of the organism, two of which are species specific. One of the Y. pestis-specific plasmids, pMT1, is thought to promote deep tissue invasion, resulting in more acute onset of symptoms and death. We determined the entire nucleotide sequence of Y. pestis KIM5 pMT1 and identified potential open reading frames (ORFs) encoded by the 100,990-bp molecule. Based on codon usage for known yersinial genes, homology with known proteins in the databases, and potential ribosome binding sites, we determined that 115 of the potential ORFs which we considered could encode polypeptides in Y. pestis. Five of these ORFs were genes previously identified as being necessary for production of the classic virulence factors, murine toxin (MT), and the fraction 1 (F1) capsule antigen. The regions of pMT1 encoding MT and F1 were surrounded by remnants of multiple transposition events and bacteriophage, respectively, suggesting horizontal gene transfer of these virulence factors. We identified seven new potential virulence factors that might interact with the mammalian host or flea vector. Forty-three of the remaining 115 putative ORFs did not display any significant homology with proteins in the current databases. Furthermore, DNA sequence analysis allowed the determination of the putative replication and partitioning regions of pMT1. We identified a single 2,450-bp region within pMT1 that could function as the origin of replication, including a RepA-like protein similar to RepFIB, RepHI1B, and P1 and P7 replicons. Plasmid partitioning function was located ca. 36 kb from the putative origin of replication and was most similar to the parABS bacteriophage P1 and P7 system. Y. pestis pMT1 encoded potential genes with a high degree of similarity to a wide variety of organisms, plasmids, and bacteriophage. Accordingly, our analysis of the pMT1 DNA sequence emphasized the mosaic nature of this large bacterial virulence plasmid and provided implications as to its evolution.
The facultative intracellular parasite Yersinia pestis harbors at least three plasmids, one of which is common to the enteropathogenic species Yersinia pseudotuberculosis and Yersinia enterocolitica (30, 72). The other two plasmids, designated pMT1 and pPCP1, are unique to Y. pestis (10) and are thought to promote the ability of this organism to penetrate deep tissues and to contribute to the acute infection associated with this species. In fact, the Y. pestis genome shares much homology with that of Y. pseudotuberculosis (2, 63), yet the infection caused by the latter organism is usually mild and self-limiting (15). Accordingly, a logical starting point to understanding the difference in the pathogenesis of Y. pestis and Y. pseudotuberculosis is to study the genes encoded on the plasmids unique to plague, pMT1 and pPCP1.
The 9.5-kb plasmid pPCP1 encodes a bacteriocin termed pesticin, a pesticin immunity protein, and a plasminogen activator (89). Loss of this plasmid increases the 50% lethal dose of the organism by a factor of 105 when the organism is injected subcutaneously in the mouse model of infection (90). The only characterized virulence determinant encoded by pPCP1, the plasminogen activator, has been implicated in deep tissue invasion by Y. pestis (11) and functions in the flea vector (58). These facts demonstrate that a plasmid, specifically harbored by Y. pestis, encodes a virulence factor necessary for the acute infection caused by the organism and that a single protein can influence the life cycle of the organism at multiple stages.
The largest extrachromosomal element present in Y. pestis was commonly called the cryptic plasmid until 1983. Protsenko et al. (73) demonstrated that the capsular protein fraction 1 (F1) and the murine toxin (MT) were both encoded by the ∼100-kb element now called pMT1. The genes for each of the proteins have been cloned from Y. pestis EV76 and sequenced previously (36, 37, 49). Data addressing the involvement of these proteins in plague pathogenesis are open to interpretation since the effect that mutational loss has on the 50% lethal dose depends on the animal model used in the study as well as the route of infection (8, 9). However, pMT1 does appear to contribute to the acute phase of plague infection, as evidenced by the fact that strains lacking the 100-kb plasmid demonstrate reduced morbidity (27, 80, 96).
Information pertaining to the genetic characterization of the pMT1 molecule is limited. The size of the plasmid has been found to vary from approximately 90 to 288 kb in size (31). Furthermore, pMT1 has been found to integrate at multiple sites into the chromosome of Y. pestis at high frequency (74), with speculation that the observed integration of pMT1 into the chromosome may have been due to IS100 homology between the two molecules. Both F1 and MT gene activation have been characterized in relation to environmental cues such as temperature and calcium (28). F1 capsule synthesis is maximal at 37°C in the absence of extracellular calcium while murine toxin expression is induced at 26°C. F1 expression is therefore maximum under conditions similar to those that induce the expression of one of the major virulence determinants of Y. pestis (91–93). In contrast, MT production is induced in an environment similar to that which Y. pestis would be expected to encounter in the flea vector. The presence of genes induced under these widely different conditions indicates the presence of at least two networks regulating expression of virulence determinants operating in plague.
DNA-sequencing technology has progressed to the point that large amounts of genetic material can be sequenced in a relatively short time. Several facts make pMT1 a good candidate for large-scale DNA sequencing. First, the plasmid is unique to Y. pestis. Second, some derivative of the ∼100-kb plasmid is always present in clinical isolates (31). Third, we already know that genes regulated by two different environmental stimuli that mimic different environments encountered during the life cycle of plague are present on this molecule. Here, we report and annotate the entire DNA sequence of the pMT1 plasmid derived from the Y. pestis laboratory strain KIM.
MATERIALS AND METHODS
Bacterial strains, media, and plasmid isolation.
Y. pestis KIM10+ is a strain that contains only pMT1 (71). Plasmid DNA was prepared by growing Y. pestis KIM10+ in heart infusion broth (Difco Laboratories, Detroit, Mich.) at 26 to 30°C followed by alkaline lysis and polyethylene glycol precipitation (4, 46). Purified pMT1 was used in preparing DNA libraries as described below.
pMT1 library construction and DNA sequencing.
Libraries of pMT1 were prepared by random shearing of plasmid DNA and size fractionation (62) and then cloned into the M13 Janus vector (12). Random phage clones were isolated, and their DNA was purified as described elsewhere (68). The DNA templates were subjected to dye-terminator sequencing by using the Prism cycle sequencing kit and ABI 377 automated sequencers (Applied Biosystems Division of Perkin Elmer, Foster City, Calif.). Sequences were assembled into contigs with the use of the Seqman II program (DNASTAR, Madison, Wis.). Suitable clones were selected for further sequencing from the opposite end to fill in coverage, resolve ambiguities, and close gaps (12). The final coverage was approximately eightfold.
DNA sequence analysis and annotation.
Open reading frames (ORFs) that were at least 50 amino acids in length were identified with GeneQuest (DNASTAR). Codon usage was assessed in the program by second- and third-order statistical comparison (6) with a matrix built from all available sequences for Yersinia species. Although this matrix was more useful than one built from Escherichia coli genes, it was necessarily constructed from a relatively small data set. Generally, the start codon (including GTG and TTG) farthest upstream was used to annotate the ORF (5). For the first pass, amino acid sequences were searched against the current GenPept database by using the BLOSUM62 matrix by the DeCypher II System (TimeLogic, Inc., Incline Village, Nev.). Subsequent searches of the Swiss Protein, E. coli, and nonredundant GenBank databases were obtained via the Internet with BLAST software (1) from the National Center for Biotechnology Information homepage (www.ncbi.nlm.gov/BLAST/). Pairwise protein alignments were done with the BLAST algorithm (1). Protein localization was predicted for relevant translated orf genes with the use of the PSORT program (66). The prediction of membrane-associated helices was done with the TMpred program (45). Where appropriate, multiple protein sequences were aligned by using the algorithm developed by Lipman et al. (55). These programs can be found as part of Pedros Molecular Biology Tools at Internet site http://zguw.ibb.waw.pl/pedros.htm.
GenBank nucleotide sequence accession number.
The annotated sequence was deposited in GenBank under accession no. AF074611.
RESULTS AND DISCUSSION
General overview.
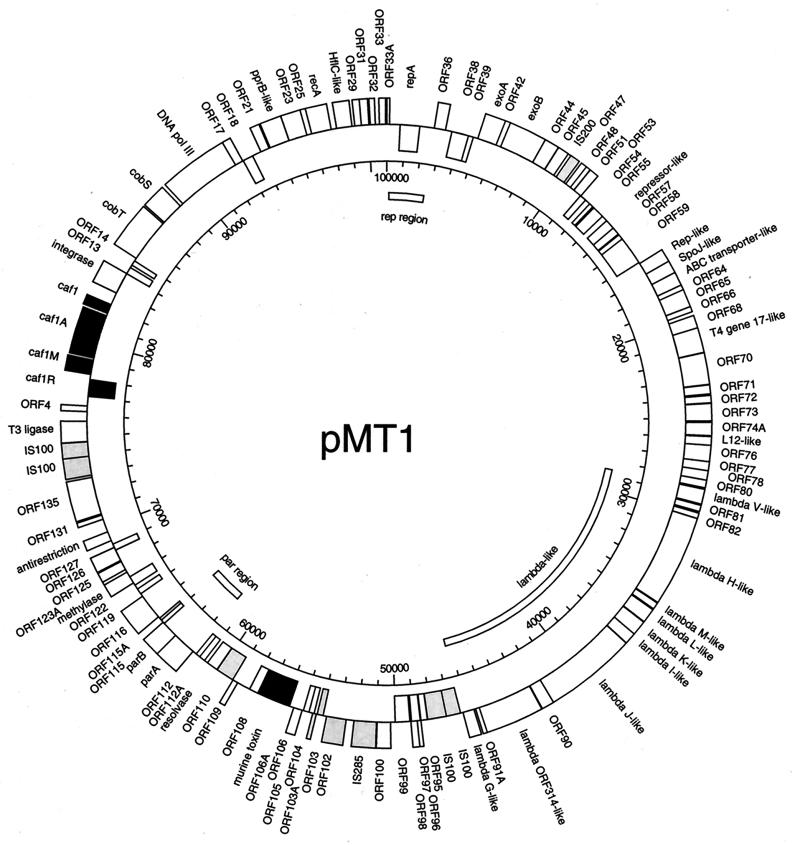
The fully assembled circular DNA sequence of pMT1 was 100,990 bp in length. An initial screening of the sequence with the DNASTAR program GeneQuest revealed 145 potential ORFs along the entire length of the plasmid. Each individual ORF was translated into the predicted protein, which then was used to search the various databases (GenBank, Swiss Protein, GenPept, and E. coli) for proteins with potentially significant homologies. The pertinent results of our searches are illustrated in Fig. 1 and summarized in Table 1. Several factors were taken into consideration for deciding whether a potential match was significant enough to report. In general, if the pMT1 ORF had significant similarity to known proteins in the database, we assigned the putative protein encoded by that ORF a like function. Homologies were considered to be significant when at least 25% of the amino acids were identical for at least 35% of the protein in the database. We decided on 25% identity to give a reasonable baseline, to which added conservative amino acid substitutions often result in higher similarity scores between protein molecules. However, in specific instances, we designated protein function as similar based on less than 25% identity. These instances are indicated in the text where relevant. The extent of homology with the database protein was set at 35% to allow for the possibility that protein domains might have different functions in different molecular contexts. We lowered our stringency when deciding whether a putative protein might function in pathogenesis. In these cases, when the region of homology included at least 20% amino acid identity with a protein that might interact with or substitute for the action of a host protein, we considered it a potential virulence factor. More weight was given to potential alignments when the homology between the Y. pestis ORF and the target protein sequence was located in a domain that had a known function in host physiology. Although these possibilities will require experimental confirmation, we felt it important given the fact that pMT1 is specifically harbored by Y. pestis and is thought to promote deep tissue spread of the organism. Finally, when the putative protein did not contain significant similarity to any known proteins, we analyzed the upstream DNA for ribosome binding sites (85) and also considered the known codon usage for Yersinia genes. After applying these criteria to the 145 potential ORFs initially identified on pMT1, we were left with 115 putative coding regions. Of these 115 putative ORFs, 38% had no regions of significant homology to any protein in the current databases and 7% had significant homology with previously described hypothetical proteins.
FIG. 1.
Map of the whole pMT1 plasmid. The outer circle shows ORFs with their orientation denoted by their positions: outside the ring indicates clockwise, and inside the ring indicates counterclockwise. Known virulence proteins are indicated by filled boxes; insertion-associated ORFs are indicated by shaded boxes. The scale is in basepairs. All ORF designations, except the previously known genes of the capsular antigen operon and the murine toxin (black square), are putative and derived from database matches. The map was derived from the annotated DNA sequence by the computer program GeneScene, under development at DNASTAR, and edited in Adobe Illustrator 7. Nomenclature of the ORFs is as indicated in Table 1.
TABLE 1.
ORFs identified in Y. pestis pMT1 DNA sequence by classificationa
| ORF class | Designation | Function or comments | Organism or element (gene if known) | Accession no. | Location (bp) |
|---|---|---|---|---|---|
| DNA metabolism | ORF1 | IS100 | Y. pestis IS100 (orfB) | U59875 | 73885–74661 |
| ORF2 | Ligase | Bacteriophage T3 | X05031 | 74680–75777 | |
| ORF12 | Integrase | Vibrio cholera | U39068 | 82931–84109 | |
| ORF16 | DNA Pol III | E. coli | M19334 | 88955–92479 | |
| ORF26 | RecA | Bacteroides fragilis | M63029 | 96910–97986 | |
| ORF34 | RepA | E. coli plasmid ColV | L01250 | Complement, 717–1781 | |
| ORF41 | exoA | Bacteriophage T4 | X01804 | 4968–6053 | |
| ORF43 | exoB | Bacteriophage T4 | X01804 | 6271–8199 | |
| ORF46 | IS200 | IS200 | U22457 | 9675–10184 | |
| ORF60 | Rep-like | Coxiella burnetti | L34077 | 16197–16895 | |
| ORF61 | SpoJ-like | Streptococcus pneumoniae | AF000658 | 16862–17563 | |
| ORF69 | Gene 17-like | Bacteriophage T4 | X52394 | 20457–21713 | |
| ORF93 | IS100 | Y. pestis IS100 (orfB) | U59875 | Complement, 46449–47231 | |
| ORF94 | IS100 | Y. pestis IS100 (orfA) | U59875 | Complement, 47228–48250 | |
| ORF101 | IS285 | Y. pestis IS285 (orf2) | X78303 | 51013–52221 | |
| ORF102 | Transposase | Enterobacter | U60777 | 52648–53712 | |
| ORF108 | Membrane endonuclease | E. coli plasmid-pKM101 (nuc) | U09868 | Complement, 57629–58117 | |
| ORF111 | Resolvase | Pseudomonas syringae (stbA) | L48985 | Complement, 60161–60781 | |
| ORF113 | ParA | Bacteriophage P1 | X02954 | 61767–63041 | |
| ORF114 | ParB | Bacteriophage P1 | K02380 | 63038–64009 | |
| ORF123 | Adenine-specific DNA methylase | E. coli pEC156 EcoVIII methylase | U48806 | 66648–67325 | |
| ORF128 | Antirestriction | E. coli | Z34467 | 69208–69714 | |
| ORF135 | DNA partitioning | Rhizobium meliloti (Orf1 and Orf2 of pRmeGR4a) | X69105 | 70730–72739 | |
| Shigella sonnei (psiB) | U82272 | ||||
| Streptococcus pneumoniae (spoOJ) | AF000658 | ||||
| ORF136 | IS100 | Y. pestis IS100 (orfA) | U59875 | 72863–73882 | |
| Protein metabolism | ORF28 | HflC-like | Vibrio parahaemolyticus | U09005 | 98281–99111 |
| ORF63 | ABC transporter/ATP binding | Archaeglobus fulgidus (AF1064) | AE001029 | 17500–18198 | |
| ORF75 | L12 ribosomal protein L12e | Haloferax volcanii | X58924 | 25927–26361 | |
| Gene regulation | ORF5 | Caf1R | Y. pestis (caf1R) | X61996 | Complement, 77118–78041 |
| ORF22 | PrpB-like | Pseudomonas putida | X80272 | 94557–95636 | |
| ORF56 | Repressor of flagellum synthesis | Salmonella abony (fljA) | D26167 | Complement, 13278–13841 | |
| Known virulence | ORF6 | Caf1M | Y. pestis (caf1M) | X61996 | 78318–79127 |
| ORF8 | Caf1A | Y. pestis (caf1A) | X61996 | 79152–81653 | |
| ORF9 | Caf1 | Y. pestis (caf1) | X61996 | 81734–82246 | |
| ORF107 | Murine toxin | Y. pestis (ymt) | X92727 | Complement, 55788–57551 | |
| Lambda-like | ORF80a | V major tail fiber | Bacteriophage lambda | P03733 | 28560–29303 |
| Intimin | E. coli O157:H7 (eae) | P43261 | |||
| ORF84 | H tail fiber | Bacteriophage lambda | AF007380 | 30041–34618 | |
| ORF85 | M minor tail fiber | Bacteriophage lambda | P03737 | 34660–34995 | |
| ORF86 | L minor tail fiber | Bacteriophage lambda | P03738 | 35052–35783 | |
| ORF87a | K tail assembly | Bacteriophage lambda | P03729 | 35815–36570 | |
| ORF88 | I tail assembly | Bacteriophage lambda | P03730 | 36561–37148 | |
| ORF89 | J host specificity | Bacteriophage lambda | P03749 | 37164–41801 | |
| ORF91 | Hypothetical protein ORF314 | Bacteriophage lambda | P03745 | 42469–45405 | |
| ORF92 | Tail fiber assembly | Bacteriophage lambda (tfa) | 225931 | 45707–46315 | |
| Hypothetical in databaseb | ORF15 | CobT | Pseudomonas denitrificans (cobT) | P29934 | 85075–87441 |
| ORF15a | CobS | Pseudomonas denitrificans (cobS) | P29933 | 87539–88771 | |
| ORF29 | Hypothetical | Bacteriophage P22 | X78401 | 99265–99636 | |
| ORF33a | Hypothetical regulatory | Bacteriophage P1 | 76816 | 100922–147 | |
| ORF38 | Hypothetical lipoprotein | Bacillus subtilis (orfK yzeA) | L16808, Z93102 | Complement, 3530–4552 | |
| ORF59 | Long hypothetical protein | Pyrococcus horikoshii (PHBW005) | AB009472 | Complement, 14573–16132 | |
| ORF73 | SRPI hypothetical protein | Synechococcus PCC7942 pANL | Q55032 | 24271–25146 | |
| ORF104 | Hypothetical protein | E. coli | U70214 | Complement, 54408–54803 | |
| ORF105 | Hypothetical protein | E. coli | U70214 | Complement, 54694–55002 | |
| ORF116 | Hypothetical | Sphingomonas S88 | U51197 | 64388–65785 | |
| ORF131 | Hypothetical | E. coli | AE000133 | 70427–70657 | |
| Fragmentsc | ORF23 | DNA polymerase | Lactococcus lactis | U78771 | 95646–96641 |
| ORF33 | Type II | Helicobacter pylori | AE000647 | 100590–100925 | |
| ORF99 | Hypothetical protein | Methanobacterium thermo-autotrophicum | AE000913 | Complement, 49210–50004 | |
| ORF103 | Hypothetical transposase | Salmonella typhimurium | Z29513 | Complement, 53911–54234 | |
| ORF103a | IS600 | Shigella sonnei | X05952 | 54281–54481 | |
| ORF106 | Hypothetical | Shigella flexneri | U97489 | 55073–55543 | |
| ORF106a | IS801 | Pseudomonas syringae | X57269 | 55589–55729 | |
| ORF110 | Hypothetical | Salmonella typhimurium | Z29513 | Complement, 59154–60140 | |
| ORF115a | SamB-like | Salmonella typhimurium | D90202 | 87539–88771 |
ORFs listed were assigned a putative function according to our criteria outlined in the general overview section of Results and Discussion. Classification then was based on these putative functions.
Homology above our criteria with proteins in the database that have not been assigned a function.
ORFs that appear to be remnants of larger proteins in the database.
We noted an approximately 24-kb inversion when our pMT1 sequence was compared to the sequence recently submitted by Hu et al. (45a) (accession no. AF053947). The two IS100 elements which form 1,954-bp inverted repeats (only one base difference) are 24,440 bp apart. Coordinates of the IS100 elements are bp 46382 and 48337 and bp 72777 and 74730. It was not possible to deduce from the sequence whether this difference was due to misassembly of individual sequence reads at the areas of homology or the result of in vivo recombination in one of the two sequenced plasmids. Therefore, to confirm our physical map with the DNA sequence, we performed SphI and HindIII restriction digests of our plasmid DNA and compared the fragment sizes with those predicted from the two sequence arrangements. Diagnostic fragments were obtained, confirming that the sequence assembly is correct for the molecule sequenced by us.
Potential virulence factors.
An important reason for performing large sequencing projects is to aid the discovery of new virulence factors which might be used as vaccine candidates or as targets for therapeutic drugs. Since Y. pestis is a facultative intracellular parasite and pMT1 is thought to enhance deep tissue spread of the organism, we took note of several ORFs that had limited homology with proteins that might function during various stages of the organism’s life cycle. These proteins are listed in Table 2. Although many of these homologies do not meet our criteria for general ORF homologies, we felt that a more relaxed standard should be applied to protein homologies in order to aid future research pertaining to plague pathogenesis.
TABLE 2.
ORFs that may be potential virulence factors
| ORF | Location (bp) | Homologous protein (target) | % Homologya | Accession no. | Refer-ence(s) |
|---|---|---|---|---|---|
| ORF4 | 76298–76603 | C-type natriuretic peptide from Squalus acanthias | 43/30 | P41319 | 83 |
| ORF17 | 92476–92919 | Delta insecticidal protein from Bacillus thuringiensis | 40/18 | P05628 | 35 |
| ORF18 | Complement, 92949–93512 | RTXb toxin of Actinobacillus pleuropneumoniae | 21/11 | D16582 | 32, 65 |
| ORF21 | 94015–94448 | Laminin of Homo sapiens | 23/5 | Q16787 | 79, 95 |
| Paramysin-related protein of Onchocerca gibsoni | 21/18 | U20609 | 25, 99 | ||
| ORF72 | 23873–24244 | Major myristoylated alanine-rich protein kinase C substrate | 24/32 | P29966 | 41 |
| ORF74a | 25221–25883 | Bacteriophage lambda V protein | 40/41 | P03733 | 81 |
| Citrobacter freundii intimin | 30/10 | Q07591 | 82 |
Percentage of identical amino acids compared with the percentage of the total target protein sequence.
RTX represents repeats in the structural toxin.
Potential evolution of the F1 capsule and murine toxin coding regions.
The coding region for the F1 capsule protein and accessory factors was located between genes that encode proteins with a high degree of identity to phage integrases and ligases as well as IS100. ORF2 was similar to bacteriophage T3 ligase, and ORF12 showed a high degree of identity to a Vibrio cholera prophage integrase. The genes for these two phage-like proteins also flank orf4, which may encode a potential vasorelaxant (Fig. 1 and Table 2). Thus, the F1 capsule-coding region and one potential virulence factor may have originated by a recombination event initiated through mobile DNA metabolism.
Although the region of pMT1 that encodes the F1 capsular protein has little homology with the E. coli genome at the nucleotide level, we found a region from bp 77738 to 77780 within the caf1R gene that was 88% identical to the E. coli afrR locus (Table 3). The AfrR protein is a pilus expression transcriptional regulator (GeneBank accession no. L08467 [98]). The similarity extended for 42 bp, which would precisely encode the amino acids FYDSQQTFTREFKK. The deduced peptide sequence lies within the region of homology between the Caf1R and AfrR proteins as well as many other transcriptional regulators in the E. coli AraC family. These findings support the idea that the Y. pestis protein is a member of the AraC family (49) of transcriptional regulators and suggest a conserved block of nucleotides involved in the functional evolution of these cis-acting regulatory elements. Furthermore, the fact that the Y. pestis caf1R gene encodes a region identical to a small region of an E. coli pilus regulatory gene suggests that the plague capsule operon may have originated as an adhesin. This possibility is supported by the fact that the two other genes necessary for F1 expression are similar to pilin chaperones (36) and membrane anchor proteins (49).
TABLE 3.
Nucleotide identities between Y. pestis pMT1 and DNA sequences in the nonredundant GenBank databasea
| Position (bp) in pMT1 | Homologous DNA source (% identity)b | GenBank accession no. |
|---|---|---|
| 77738–77780 | E. coli afrR (88) | L08467 |
| 44273–44300 | Bacteriophage K3 gene 37 (93) | X00615 |
| 45200–45395 | Bacteriophage lambda (87) | J02459 |
| 45347–45395 | Salmonella typhimurium pagJ (88) | AF013776 |
| 53973–53997 | E. coli hlyR (96) | X07565 |
| 54023–54219 | Salmonella typhimurium plasmid partitioning (88.5) | M97752 |
| 59329–59486 | Salmonella typhimurium plasmid partitioning (88) | M97752 |
| 59891–60117 | Salmonella typhimurium plasmid partitioning (79) | M97752 |
| 60886–60970 | Salmonella typhimurium plasmid partitioning (89) | M97752 |
| 54411–55559 | E. coli chromosomal DNA (90) | U70214 |
| 54418–55559 | Shigella flexneri RT (90) | U97489 |
| 55347–55399 | Pseudomonas putida (85) | AB004059 |
| 58641–58691 | Salmonella enteritidis virR (92) | D14491 |
| 58641–58691 | Salmonella choleraesuis pMBA1 (92) | X54148 |
| 58641–58691 | Salmonella dublin pSDL2 (92) | M58505 |
| 58675–58745 | Salmonella pSC101 (84) | X01654 |
| 61836–62386 | Bacteriophage P7 par region (78) | X17529 |
| 61661–61934 | E. coli F plasmid RepF1B (89) | M26308 |
| 58627–58662 | Neisseria gonorrhoeae pFA7 (89) | X01654 |
| 58627–58662 | Klebsiella pneumoniae pLST1000 (89) | X64367 |
| 58627–58662 | E. coli pRK2 (89) | U05773 |
| 69212–69714 | E. coli antirestriction protein (92) | Z34467 |
| 72557–72630 | E. coli F plasmid psiB (96) | X12462 |
| 72558–72630 | Shigella sonnei plasmid ColIb-P9 psiB (82) | U82272 |
Identities of 78% or greater for a continuous group of 25 nucleotides were considered significant.
In some cases, nucleotide identity between pMT1 and the target DNA sequence varied over the length of the homologous region. In these instances, the percent identity was averaged for all homologous regions.
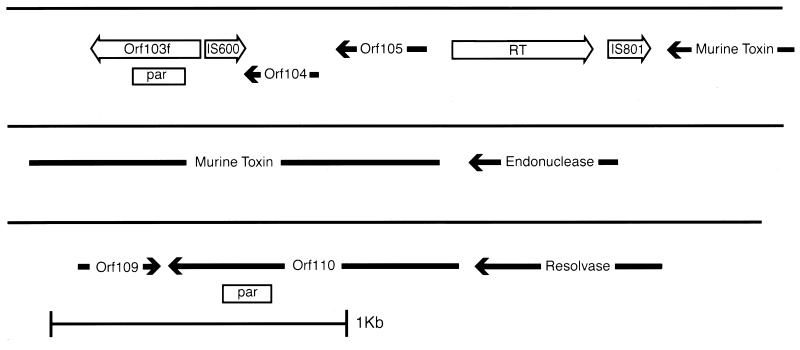
The molecular evolution of the region surrounding the other known virulence factor of Y. pestis, MT, may have occurred through several abortive or imprecise transposition events from another plasmid or bacteriophage. Several features found within the pMT1 DNA sequence suggest this possibility. First, the MT-coding locus, ymt, is flanked by a partial gene duplication event with several incomplete transposon sequences and ORFs (Fig. 2). A striking example of an incomplete ORF was found after ORF110 was compared with ORF103. The ORF110 peptide was predicted to be composed of 312 amino acids and was found to be 78% identical over its entire length to a group of repeated hypothetical proteins in the E. coli (13) and Salmonella typhimurium (GenBank accession no. Z29513) genomes designated the YadD family. Second, there is a partial repetition of DNA that encodes the S. typhimurium parA and parB loci (17). The par repetition was noted by the fact that nucleotides 54023 through 54219 are an imperfect partial duplication of bp 59329 through 59486 (Fig. 2). To the right of the second copy of the Salmonella par region, we identified an ORF that had 38% identity with a resolvase encoded by Pseudomonas syringae (40). To the right of the putative resolvase, we located a small 85-bp region (nucleotides 60886 to 60970) that was 89% identical to the Salmonella plasmid-partitioning sequences. Third, we located two fragments of transposons in this region. The first remnant was found as a partial ORF from bp 54281 through 54481 and would be predicted to encode amino acids that were 69% similar to residues 75 to 116 of the Shigella sonnei IS600 hypothetical 31-kDa protein (57). The second partial ORF was encoded by nucleotides 55589 to 55729 and was predicted to encode a polypeptide that was 88% similar to IS801 transposase amino acids 260 to 277 (78). The fourth piece of evidence that the MT gene was acquired by Y. pestis through an illegitimate recombination event from another replicon is the presence of DNA homology with other known plasmids (Table 3). Taken together, these observations strongly suggest that Y. pestis acquired the MT-coding sequences and possibly other virulence factors through recombination events that originated from mobile genetic elements. However, no one single event can explain the molecular architecture that we observed.
FIG. 2.
Map of Y. pestis pMT1 region encoding the MT virulence determinant. The region from ca. bp 53500 to 61300 is shown and is represented by the solid line. ORFs that were predicted to be intact are shown as solid arrows. ORFs that appear only partial and were judged to be remnants of intact coding regions are shown as open arrows. The open boxes labeled par designate regions with high nucleotide homology with the S. typhimurium par locus (17). RT, reverse transcriptase-like partial ORF.
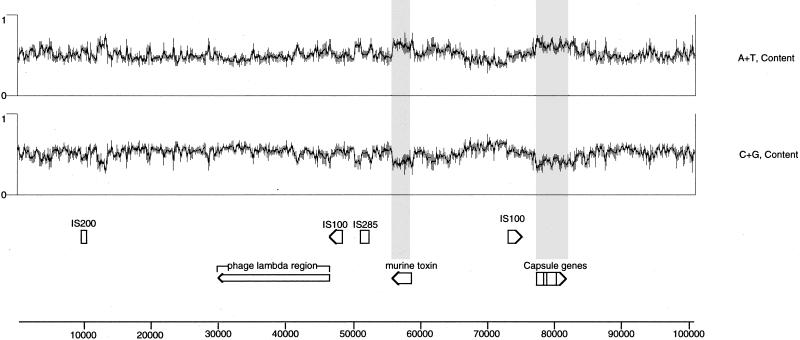
If Y. pestis pMT1 is a mosaic of different genetic elements or regions, we reasoned that a base composition analysis of the plasmid might indicate and confirm potential regions acquired by horizontal gene transfer. In agreement with the hypothesis that the regions of the Y. pestis plasmid containing the F1 capsule genes and the gene encoding the MT originated by transfer of mobile genetic elements, we noted that the regions of pMT1 which contained these genes had a guanine-plus-cytosine (G+C) content significantly different from those of surrounding regions of DNA (Fig. 3). Specifically, the DNA encoding the caf1R, caf1M, caf1A, and caf1 genes (bp 3067 through 8195) had a G+C content of 39.2% and the ymt locus (bp 82727 through 84490) had a G+C content of 38.1%. These base compositions can be contrasted with the overall 50.1% G+C content of the entire pMT1 molecule. Our observation that the two known virulence factors encoded by pMT1 have G+C contents significantly different from that of the surrounding DNA may be similar to one of the criteria currently used to define pathogenicity islands (39). The differing base composition of these known virulence genes relative to that of surrounding pMT1 DNA does suggest, in a limited way, that plasmid-borne virulence genes may be analogous to chromosomal pathogenicity islands. The observation that virulence genes generally have a base composition different from that of the flanking DNA has been observed for the hemolysin genes present on the E. coli virulence plasmid pO157 (14) and within the pathogenicity island of this organism which encodes the locus for enterocyte effacement (70). If the trend of virulence genes, or blocks of genes, to have a G+C content different from that of the surrounding genetic material is genuine, the region located to the right of IS200 on pMT1, as shown in Fig. 3, may warrant further investigation. Database searches with putative ORF48 through ORF58 translated from pMT1 sequences in this area did not reveal any homology with known or potential virulence factors. In fact, only ORF56 displayed any significant homology with any of the proteins in the current databases.
FIG. 3.
Base composition of pMT1. The plots showing A+T and G+C content were derived by DNASTAR’s GeneQuest program, which also displays selected ORFs and other annotated features to the correct scale. The shaded bars mark the previously known virulence genes to highlight the low G+C composition of these regions. Note a small region to the right of IS200 that also shows low G+C content (discussed in Results and Discussion). The scale at the left is equivalent to 0 to 100% for each plot. The scale below the figure shows the plasmid genome in base pairs.
Insertion sequence elements.
The MT plasmid appeared to be a chimera of many types of mobile genetic elements including bacteriophage, plasmids, insertion sequences, and one defective bacterial reverse transcriptase which may be a remnant of a retron (87, 94). Insertion sequences are known to promote illegitimate recombination events and promote genetic plasticity. With this fact in mind, we noted that pMT1 encoded several IS elements as well as what appeared to be nonfunctional remnants of mobile genetic elements. IS elements were considered to be an allele of a previously published mobile element when the DNA sequence was at least 85% identical to entries in the current GenBank database. An element was considered to be an incomplete copy when the identity covered less than 80% of the known length of functional elements in the database. Using these criteria, we noted four apparently complete IS elements: one copy of IS200 (3, 86) from bp 9576 to 10292, two copies of IS100 (31, 59) from bp 46383 to 48336 and 72777 to 74730, and one copy of IS285 (31) from bp 50872 to 55256.
Our analysis revealed one potential new IS element located at bp 52465 to 53758. These coordinates mark the beginning and end of a direct repeat sequence, GATGATAA, that flanks a putative transposase which we designated ORF102. ORF102 had the greatest identity, 40% over 96% of the target protein, with a putative transposase previously found in Enterobacter aerogenes (88) as well as 36% identity over 96% of a putative transposase previously described in Yersinia enterocolitica (77). We analyzed the region surrounding ORF102 for DNA sequence features previously found in IS1328 of Y. enterocolitica. The only nucleotide feature that we found was an exact match of a GGAGG potential ribosome binding site that was 8 bp upstream of the ORF102 ATG translation initiation codon. We did not find any inverted repeat sequences of 5 bp or longer in contrast to the 6-bp inverted repeats described for Y. enterocolitica IS1328. This result was not particularly surprising given the amount of sequence divergence at the protein and DNA levels. Accordingly, we decided to identify this potential IS with a new designation, IS1618.
The Y. pestis MT plasmid included a 1,150-bp region that was 93% identical to the E. coli chromosome (5) at the DNA level. We identified a putative protein coding sequence within this homologous region, designated ORF106, that was 91% identical over 32% of a previously described Shigella flexneri reverse transcriptase (RT)-like protein (76). The full-length S. flexneri RT is 431 amino acids compared to 151 residues for ORF106. Given the high degree of identity of ORF106 with the amino terminus of a bacterial RT, we speculate that the pMT1 ORF may be a remnant of a bacterial retron element (94). However, the pMT1 DNA sequence immediately upstream of ORF106 lacks any inverted repeat sequences or potential RNA structures that have been associated with bacterial retron elements (44, 52, 94). The fact that ORF106 includes such high identity to a bacterial RT but none of the other features associated with retrons suggests that this region of DNA may have been acquired by Y. pestis pMT1 through a mechanism other than retrotransposition. Furthermore, the high level of conservation of the DNA sequence in this area of pMT1 suggests that these sequences were acquired recently in the evolution of this plasmid.
Partial lambdoid prophage.
ORF80a through ORF92 show a high degree of similarity to lambda (81) and phage BF23 (67) proteins (Fig. 1). The amount of amino acid identity ranged from 31% over 54% of lambda protein J to 61% over 91% of lambda protein G. Further support for the lambdoid origin of this region of pMT1 was found in the gene order of several of the ORFs. Specifically, ORF84, ORF85, ORF86, ORF87a, ORF88, and ORF89 are in the same order as the genes encoding lambda proteins H, M, L, K, I, and J. The gene that encodes one of the lambda-like proteins, ORF80a, appears to have been partially duplicated to generate orf74a. The partial duplication event is indicated by our observation that ORF74a is 45% identical over 47% of ORF80a. We analyzed the DNA surrounding ORF80a and ORF74a for repeat elements that may have been involved in this duplication but without success. Accordingly, a mechanism for the partial duplication and rearrangement of these sequences encoded by pMT1 was not evident in the DNA sequence. It is interesting that ORF74a may encode a potential virulence factor (Table 2).
Other mobile genetic elements.
Some regions of pMT1 obviously arose from either plasmid or phage gene transfer and recombination events. The DNA sequence data that suggest an origin of known virulence factors in relation to putative mobile genetic events have already been presented. However, in general, it was difficult to determine exactly where the potential prophage or other plasmid molecules began and ended. To the best of our ability, the most that can be determined about the origins of various regions of Y. pestis pMT1 is the mosaic nature of the entire molecule. As an example, the DNA sequence between ORF12 and ORF43 includes putative gene products with a high degree of homology to E. coli DNA polymerase III (ORF16), Bacteroides fragilis RecA (ORF26), and the gene 47 polypeptide of bacteriophage T4 (ORF42). Given a mosaic genetic element, the most reliable means of determining the origin of specific regions of pMT1 is comparison of nucleotide sequences. Table 3 lists all significant nucleotide homologies found between pMT1 and the current nonredundant GenBank databases excluding previously entered Y. pestis sequences or known insertion sequences. Two important features were noted from this analysis. First, most of the significant nucleotide homologies occur in a segment containing 30% of the pMT1 sequence from bp 44272 to 72640. The high conservation of DNA sequence in this region could indicate either that these sequences were acquired relatively late in pMT1 evolution or that the functions encoded by these sequences are very important in plasmid maintenance and function. In fact, most of the nucleotide homology was found with genes required for plasmid maintenance, such as partitioning during cell division. Second, the majority of conserved DNA sequence was similar to that of large plasmids known to be involved in the pathogenesis of several enteric organisms. Taken together, these observations indicate an evolutionary linkage among enteric virulence plasmids.
In support of a link between the evolution of plasmids found in enteric organisms, we noted that ORF123, a putative DNA methylase, had 91% identity to ORFL7074 found on E. coli pO157 (14). Furthermore, Krause et al. (51) have reported a small region of homology between the Y. pseudotuberculosis virulence plasmid and a Salmonella dublin virulence plasmid. We found a 51-bp region (nucleotides 58641 to 58691) that was 92% identical to the common sequence found on the S. dublin plasmid (51). The 51-bp region of homology encoded by pMT1 was located upstream of S. dublin ORF1 (51) and did not include the inverted repeats reported by these investigators. The possible implications of this nucleotide homology remains to be elucidated.
Evolution of pMT1 could have been facilitated by either conjugational (50) or transductional (100) movement of genetic material. The Y. pestis murine toxin plasmid has been shown to integrate into the chromosome and to promote transfer of genetic markers to a recipient strain (74). With these facts in mind, we looked for genes that might promote or be associated with transfer of pMT1 between bacterial cells. We took particular note of ORF128 since it displayed 93% identity over 95% of the predicted sequence of an E. coli antirestriction protein (21). Antirestriction proteins are thought to function during conjugation to inhibit cleavage of the donor DNA before methylation can occur and are usually located near the origin of transfer (oriT) of the plasmid. In fact, we searched the area downstream from orf128 between bp 69714 and 70397 for features found in F-like plasmid oriT sequences (33, 38, 53, 97) without success. An expanded search of the entire pMT1 sequence did not reveal any other portion of the plasmid that might function as oriT. Since the plasmid has evolved as part of the Y. pestis genetic material, it is possible that any oriT that may have been present on pMT1 has been lost or has become nonfunctional due to mutation. Further experimentation will be required to determine if the plasmid encodes an oriT and where it is located.
Replication and partition functions.
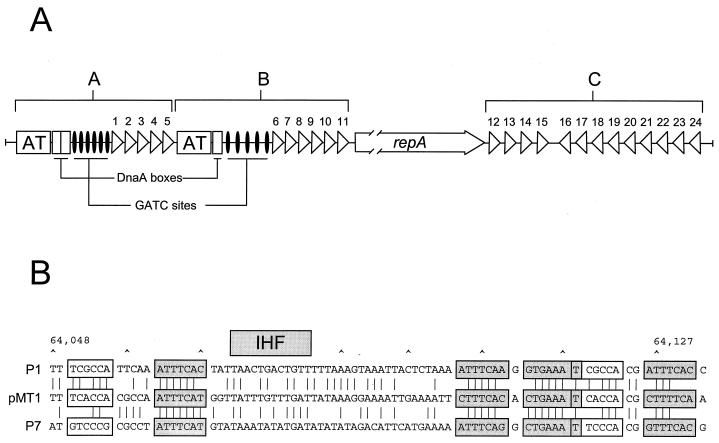
DNA sequence analysis revealed a single potential plasmid replication region (bp 151 to 2601) consisting of a structural gene (repA) and additional sequence elements characteristic of plasmid replicons that employ an iteron-based replication initiation and control mechanism. The ∼32-kDa predicted repA gene product (ORF34) showed a high degree of similarity to a number of plasmid replication initiation proteins, including those associated with the RepFIB (62% identity), P1 (47% identity), P7 (47% identity), and RepHI1B (40% identity) replicons. Upstream and downstream of the repA locus we found two sets of 19-bp direct DNA repeats (iterons). The location and orientation of these repeats and their orientation relative to repA are presented in Fig. 4A. The consensus sequence for these 19-bp repeats (5′-AACCACTGTAGAGAGTAAA-3′) is most similar to the 17-bp direct repeats associated with the RepHI1B replicon (5′-ATCCACTATACCGGGTA-3′), matching at 12 of the 17 possible positions (34). The direct repeats of iteron-containing plasmids have been shown to provide specific multiple binding sites for the plasmid Rep protein, which is an essential step in the initiation of plasmid replication. In addition to their role in plasmid replication, Rep proteins of iteron-carrying plasmids are involved in the regulation of their own synthesis, i.e., autoregulation (19), and in the control of plasmid copy number (18).
FIG. 4.
Organization of the pMT1 replicon. (A) The pMT1 replicon (bp 151 to 2601) consists of a single repA gene (open arrow) flanked by sets of repeated elements (iterons) indicated by triangles and numbered 1 to 24. The region upstream from repA contains a number of sites present in many bacterial origin sequences including DnaA-binding sites, GATC Dam methylase recognition sites and two A-T-rich regions. The iterons and additional sequence elements are organized into two potentially functional origins of replication (regions A and B). Downstream of repA are 13 additional repeats (region C) that by analogy with other iteron-based replicons, are involved in plasmid-copy-number control. (B) Alignment of the pMT1 (bp 64048 to 64113) and P1 and P7 parS partition sites. The hexamer motifs (open boxes), heptamer motifs (shaded boxes), and the IHF recognition sites are shown. Base identities are indicated by lines.
Additional sequence elements characteristic of origins of replication are present in the sequence upstream from the repA coding region. These include three DnaA binding sites, two A-T-rich regions, and 11 Dam methylase (GATC) recognition sequences (Fig. 4A). These elements are organized into two separate potentially functional origins of replication (regions A and B). Binding of the Rep protein to iterons at the replication origin has been shown to cause bending and/or melting of the adjacent A-T-rich region (18, 64). The Rep protein, in concert with the host DnaA protein, then functions to recruit host replication proteins into the open replication initiation complex (64).
The region downstream of the RepA coding region (region C) contains an additional 13 direct repeats (Fig. 4A). The downstream cluster of repeats in a number of iteron-containing plasmids has been shown to be nonessential for plasmid replication but plays an essential role in plasmid-copy-number control (18, 69). Furthermore, incompatibility of iteron-containing plasmids is primarily a function of the iterons and the binding specificity of the replication initiation protein for these DNA sequences (18). However, neither the copy number nor the incompatibility characteristics of a plasmid can be empirically determined from the sequence or genetic organization of these repeats.
The partitioning system of pMT1 (bp 61661 to 64161) is located approximately 36-kb (Fig. 1) from the origin of replication and appears to closely resemble the parABS system of bacteriophages P1 (18) and P7 (56). The pMT1 parA gene is predicted to encode an ∼44.9-kDa product (ORF113) that shows 90% identity and 95% similarity to the P7 ParA protein and 57% identity and 75% similarity to the P1 ParA protein. The pMT1 parB locus is predicted to encode an ∼36.4-kDa product (ORF114) that is 67% identical and 83% similar to the P7 ParB protein and 44% identical and 62% similar to the P1 ParB protein. The P1 and P7 ParA proteins are ATPases whose activities are stimulated in vitro by ParB (23). ParB is a DNA binding protein that recognizes the cis-acting parS site, which lies immediately downstream of parB (22, 43). The genetic organization, spacing, and specific sequence motifs of the pMT1 parS site are similar to those found at the P1 and P7 parS sites. Figure 4B shows an alignment of the pMT1 parS site with both the P1 and P7 parS sites. Two repeated elements, a hexamer box (open box) and a heptamer box (shaded box), have been shown to be required for proper ParB binding and partitioning activity (22). The relatively well-conserved heptamer motifs represent individual binding sites for ParB. The hexamer motifs, which differ somewhat between the P1 and P7 parS sites, are responsible for the species specificity of the ParB-parS interaction (42). Interestingly, the P1 parS site, which does not function with P7 Par proteins, can be converted to a completely functional P7 parS site by exchanging just 5 bases located within the two hexamer motifs (42, 75) shown in Fig. 4B. Also, in the P1 and P7 systems, ParB interacts cooperatively with the integration host factor (IHF) for parS binding (22). We noted a putative IHF binding site within the putative pMT1 parS site (Fig. 4B). The amino acid and nucleotide sequence similarities between the pMT1, P1, and P7 parABS partitioning components suggest that the pMT1 system functions in a manner analogous to that of the P1 and P7 systems.
Metabolic genes.
In order to survive, obviously a pathogen must be able to scavenge or to synthesize required cellular precursors. We found two potential anabolic genes encoded by pMT1. ORF15 is highly similar (28% identity over 42% of the target protein) to the Pseudomonas denitrificans cobT gene product, and ORF15a is similar (42% identity over 75% of the target protein) to the P. denitrificans CobS protein (16). Interestingly, cobT and cobS are linked to each other yet are not located with other cob gene clusters in P. denitrificans (16). Furthermore, the two genes are arranged in reverse order compared to their relative locations in P. denitrificans. The products of the cobT and cobS genes are involved in conversion of flavin mononucleotide to vitamin B12. In E. coli and S. typhimurium there are at least 28 genes necessary for the synthesis of this vitamin cofactor which are linked on the chromosome (47). To our knowledge, this is the first example of genes necessary for vitamin B12 synthesis being located on a plasmid. We find this fact very intriguing given the fact that Y. pestis is a facultative intracellular parasite. It remains to be determined if these other loci are present on the Y. pestis chromosome or if the organism scavenges the precursors necessary for the CobT and CobS homologs encoded on pMT1 for use as a substrate in vitamin synthesis.
Gene mosaics.
Shapiro (84) and Boyd et al. (7) have noted that the mosaic nature of genetic organization can be seen at multiple levels. Our examination of the molecular structure of pMT1 supports the view that evolution occurs by a natural genetic engineering process as well as by classical mechanisms of genetic drift and selection. Specific examples of these processes have already been presented. Briefly, ORF74a may be an example of a bacteriophage protein that has undergone classic gene duplication and has evolved by selection of a more important function in pathogenesis. The highly conserved group of 42 nucleotides found in the E. coli afrR locus and the Y. pestis caf1R regulatory elements may represent DNA that encodes a functional domain which in nature can generate different regulatory proteins in the araC family. The regions of pMT1 that encode groups of genes with significant identity to bacteriophage lambda genes and the regions of highly conserved nucleotide sequences (Table 3) indicate the overall mosaic structure of this molecule.
To extend the theme of mosaic nature of genetic material, we noted an example of a single gene that appears to be composed of domains derived from other individual protein molecules. ORF135 shows greater than 20% identity to at least four different proteins in the current databases. Beginning at the amino terminus, residues 37 through 201 were 26% identical to a S. pneumoniae SpoJ-like protein (20). SpoJ is a chromosomal partitioning protein originally identified in Bacillus subtilis (54). The next region of homology within ORF135, predicted amino acids 135 to 310, displayed 38% identity to Rhizobium meliloti ORF1 (60). This third region of homology, residues 309 through 602, was 23% identical to another R. meliloti protein, designated ORF2 (61). The percent identity between ORF2 and ORF135 was below our general cutoff; however, given the high degree of identity to R. meliloti ORF1, we decided that the homology was genuine. Both ORF1 and ORF2 of R. meliloti are necessary for stable replication of plasmid DNA and are thought to function in partitioning of the replicon into daughter cells. The last region of homology within ORF135 occurred in both the nucleotide and protein sequences. As shown in Table 3, bp 72558 to 72585 was homologous to E. coli psiB and bp 72558 to 72630 was homologous to S. sonnei psiB. ORF135 residues 575 to 646 were 65% identical to S. sonnei PsiB (48). The PsiB-like proteins are thought to be responsible for inhibition of the cellular SOS response during bacterial conjugation (29). Interestingly, the region of protein identity, residues 610 to 634, would encode a polypeptide of only approximately 8-kDa compared to the 15-kDa molecular mass for the S. sonnei PsiB protein. Therefore, the PsiB-like region of ORF135 may define a small domain which, in the context of the large protein, could perform the same function as PsiB. Alternatively, the PsiB region of ORF135 may not function in SOS inhibition but rather has evolved to some other function in the current molecular context.
Although it is not obvious from the DNA or protein sequence how ORF135 might have evolved, the homologies strongly suggest that this putative protein is involved in plasmid partitioning. The molecular structure of ORF135 could have arisen either by mutation of an ancestral protein or by domain splicing similar to exon shuffling in eucaryotes (26). The fact that some of the regions of homology overlap each other would argue for mutation of an ancestral protein. Specifically, region 1 of ORF135, which has homology with SpoJ-like proteins, overlaps region 2, which has homology with R. meliloti ORF1 by 66 amino acids. Similarly, region 3, which has identity with R. meliloti ORF2, overlaps the PsiB region by 27 residues. Only the second and third regions of homology, R. meliloti ORF1 and ORF2, respectively, appear to have no appreciable overlap (two amino acids). Taken together, our analysis of ORF135 suggests a combination of mutation and potential splicing or recombination of independent genes to create the pMT1 putative polypeptide. In this scenario a protein similar to SpoJ-R. meliloti ORF1 would have been recombined in frame with a protein similar to R. meliloti ORF2-PsiB. The molecular mechanism for such natural genetic engineering in prokaryotes is not clear; however, directly repeated sequences have been suggested as one possibility (24). We found many directly repeated sequences within ORF135, although none could explain the potential joining of the two proteins as proposed above.
Summary.
The nucleotide sequence of Y. pestis pMT1 has provided a wealth of new information. Our analysis has allowed us to identify several genes to target for further study in order to access their possible roles in pathogenesis. Deciphering the potential roles of these proteins improves our understanding not only of disease but also of host physiology. As more complete virulence plasmid DNA sequences become available we will begin to understand the mosaic nature of these molecules and what new combinations we might expect in the future. Detailed molecular analysis of the structure of virulence plasmids will impact our ability to predict the emergence of bacterial pathogens as well as to detect their presence. Although much of our specific analysis requires confirmation of protein function, it does confirm and expand our knowledge about pathogen evolution.
ACKNOWLEDGMENTS
We thank R. D. Perry for supplying pMT1 purified DNA for nucleotide sequencing. J. Norris-Thomas is thanked for her help in manuscript preparation. We thank N. J. Perna, N. Saunders, and B. Larson for helpful comments during preparation of the manuscript. We also thank G. Plunkett III for constructing the codon usage matrix for Yersinia and N. J. Perna for help with database searches. We also thank D. J. Rose and the technical staff of the Wisconsin Genome Project for sequencing and also G. Peyrot for editing the aligned project.
This work was supported by Public Health Service grant P01 HG10428 (F.R.B.) and the United States Army Medical Research and Materiel Command (L.E.L.).
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bercovier H, Mollaret H H, Alonso J M, Brault J, Fanning G R, Steigerwalt A G, Brenner D J. Intra- and interspecies relatedness of Yersinia pestis by deoxyribonucleic acid hybridization and its relationship to Yersinia pseudotuberculosis. Curr Microbiol. 1980;4:225–229. [Google Scholar]
- 3.Beuzon C R, Casadesus J. Conserved structure of IS200 elements in Salmonella. Nucleic Acids Res. 1997;25:1355–1361. doi: 10.1093/nar/25.7.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirpatrick H A, Goeden M A, Rose D J, Mau R, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 6.Borodovsky M, McIninch J. Genmark: parallel gene recognition for both DNA strands. Comput Chem. 1993;17:123–133. [Google Scholar]
- 7.Boyd E F, Hill C W, Rich S M, Hartl D L. Mosaic structure of plasmids from natural populations of Escherichia coli. Genetics. 1996;143:1091–1100. doi: 10.1093/genetics/143.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brubaker R R. The genus Yersinia: biochemistry and genetics of virulence. Curr Top Microbiol. 1972;57:111–158. doi: 10.1007/978-3-642-65297-4_4. [DOI] [PubMed] [Google Scholar]
- 9.Brubaker R R. The Vwa+ virulence factor of yersinae: the molecular basis of the attendant nutritional requirement for Ca++ Rev Infect Dis. 1983;5:S748–S758. doi: 10.1093/clinids/5.supplement_4.s748. [DOI] [PubMed] [Google Scholar]
- 10.Brubaker R R. Factors promoting acute and chronic diseases caused by yersiniae. Clin Microbiol Rev. 1991;4:309–324. doi: 10.1128/cmr.4.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brubaker R R, Beesley E D, Surgalla M J. Pasturella pestis: role of pesticin I and iron in experimental plague. Science. 1965;149:422–424. doi: 10.1126/science.149.3682.422. [DOI] [PubMed] [Google Scholar]
- 12.Burland V, Daniels D L, Plunkett III G, Blattner F R. Genome sequencing on both strands: the Janus strategy. Nucleic Acids Res. 1993;21:3385–3390. doi: 10.1093/nar/21.15.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burland V, Plunkett III G, Sofia H J, Daniels D L, Blattner F R. Analysis of the Escherichia coli genome VI: DNA sequence of the region from 92.8 through 100 minutes. Nucleic Acids Res. 1995;23:2105–2119. doi: 10.1093/nar/23.12.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burland, V., Y. Shao, N. T. Perna, G. Plunkett III, H. J. Sofia, and F. R. Blattner. The complete DNA sequence and analysis of the large virulence plasmid of Escherichia coli O157:H7. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 15.Butler T. Plague and other yersinia infections. In: Greenbugh III W B, Merigan T C, editors. Current topics in infectious disease. New York, N.Y: Plenum Press; 1983. pp. 111–159. [Google Scholar]
- 16.Cameron B, Guilhot C, Blanche F, Cauchois L, Rouyez M C, Rigault S, Levy-Schil S, Crouzet J. Genetic and sequence analysis of the Pseudomonas denitrificans DNA fragment containing two cob genes. J Bacteriol. 1991;173:6058–6065. doi: 10.1128/jb.173.19.6058-6065.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerin H, Hackett J. The parVP region of the Salmonella typhimurium virulence plasmid pSLT contains four loci required for incompatibility and partition. Plasmid. 1993;30:30–38. doi: 10.1006/plas.1993.1031. [DOI] [PubMed] [Google Scholar]
- 18.Chattoraj D K, Schneider T D. Replication control of plasmid P1 and its host chromosome: the common ground. Prog Nucleic Acid Res Mol Biol. 1997;57:145–186. doi: 10.1016/s0079-6603(08)60280-9. [DOI] [PubMed] [Google Scholar]
- 19.Chattoraj D K, Snyder K M, Abeles A L. P1 plasmid replication: multiple functions of RepA protein at the origin. Proc Natl Acad Sci USA. 1985;82:2588–2592. doi: 10.1073/pnas.82.9.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Q, Campbell E A, Naughton A M, Johnson S, Masure H R. The com locus controls genetic transformation in Streptococcus pneumoniae. Mol Microbiol. 1997;23:683–692. doi: 10.1046/j.1365-2958.1997.2481617.x. [DOI] [PubMed] [Google Scholar]
- 21.Chilley P M, Wilkins B M. Distribution of the ardA family of antirestriction genes on conjugative plasmids. Microbiology. 1995;141:2157–2164. doi: 10.1099/13500872-141-9-2157. [DOI] [PubMed] [Google Scholar]
- 22.Davis M A, Austin S J. Recognition of the P1 plasmid centromere analog involves binding of the ParB protein and is modified by a specific host factor. EMBO J. 1988;7:1881–1888. doi: 10.1002/j.1460-2075.1988.tb03021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis M A, Martin K A, Austin S J. Biochemical activities of the ParA partition protein of the P1 plasmid. Mol Microbiol. 1992;6:1141–1147. doi: 10.1111/j.1365-2958.1992.tb01552.x. [DOI] [PubMed] [Google Scholar]
- 24.De Chateau M, Bjorck L. Identification of interdomain sequences promoting the intronless evolution of a bacterial protein family. Proc Natl Acad Sci USA. 1996;93:8490–8495. doi: 10.1073/pnas.93.16.8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donelson J E, Duke B O L, Moser D, Zeng W, Erondu N E, Lucius R, Renz A, Karam M, Flores G Z. Construction of Onchocerca volvulus cDNA libraries and partial characterization of the cDNA for a major antigen. Mol Biochem Parasitol. 1988;31:241–250. doi: 10.1016/0166-6851(88)90154-5. [DOI] [PubMed] [Google Scholar]
- 26.Doolittle R F. The multiplicity of domains in proteins. Annu Rev Biochem. 1995;64:287–314. doi: 10.1146/annurev.bi.64.070195.001443. [DOI] [PubMed] [Google Scholar]
- 27.Drozdov I G, Anisimov A P, Samoilova S V, Yezhov I N, Yeremin S A, Karlyshev A V, Krasilniova V M, Kravchenko V I. Virulent non-capsulate Yersinia pestis variants constructed by insertion mutagenesis. J Med Microbiol. 1995;42:264–268. doi: 10.1099/00222615-42-4-264. [DOI] [PubMed] [Google Scholar]
- 28.Du Y, Galyov E, Forsberg A. Genetic analysis of virulence determinants unique to Yersinia pestis. Contrib Microbiol Immunol. 1995;13:321–324. [PubMed] [Google Scholar]
- 29.Dutreix M, Backman A, Celerier J, Bagdasarian M M, Sommer S, Bailone A, Devoret R, Bagdasatian M. Identification of psiB genes of plasmids F and R6-5. Molecular basis for psiB enhanced expression in plasmid R6-5. Nucleic Acids Res. 1988;16:10669–10679. doi: 10.1093/nar/16.22.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferber D M, Brubaker R R. Plasmids in Yersinia pestis. Infect Immun. 1981;31:839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filippov A A, Solodovnikov N S, Kookleva L M, Protsenko O A. Plasmid content in Yersinia pestis strains of different origin. FEMS Microbiol Lett. 1990;67:45–48. doi: 10.1016/0378-1097(90)90165-m. [DOI] [PubMed] [Google Scholar]
- 32.Frey J, Meier R, Gygi D, Nicolet J. Nucleotide sequence of the hemolysin I gene from Actinobacillus pleuropneumoniae. Infect Immun. 1991;59:3026–3032. doi: 10.1128/iai.59.9.3026-3032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frost L S, Ippen-Ihler K, Skurry R A. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol Rev. 1994;58:162–210. doi: 10.1128/mr.58.2.162-210.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabant P, Chahdi A O, Couturier M. Nucleotide sequence and replication characteristics of RepHI1B: a replicon specific to the IncHI1 plasmids. Plasmid. 1994;31:111–120. doi: 10.1006/plas.1994.1012. [DOI] [PubMed] [Google Scholar]
- 35.Galjart N J, Sivasubramanian N, Federici B A. Plasmid localization, cloning and sequence of the gene encoding a 27.3-kilodalton cytolytic protein from Bacillus thuringiensis subspecies morrisoni (PG14) Curr Microbiol. 1987;16:171–177. [Google Scholar]
- 36.Galyov E E, Karlyshev A V, Chernoskaya T V, Dolgikh D A, Smirnov O Y, Volkovoy K I, Abramov V M, Zav’yalov V P. Expression of the envelope antigen F1 of Yersinia pestis is mediated by the product of caf1M gene having homology with the chaperone protein PapD of Escherichia coli. FEBS Lett. 1991;286:79–82. doi: 10.1016/0014-5793(91)80945-y. [DOI] [PubMed] [Google Scholar]
- 37.Galyov E E, Smirnov O Y, Karlishev A V, Volkovoy K I, Denesyuk A I, Nazimov I V, Rubtsov K S, Dalvadyanz V M, Zav’yalov V P. Nucleotide sequence of the Yersinia pestis gene encoding F1 antigen and the primary structure of the protein. Putative T and B cell epitopes. FEBS Lett. 1990;277:230–232. doi: 10.1016/0014-5793(90)80852-a. [DOI] [PubMed] [Google Scholar]
- 38.Goodrich J A, Schwartz M L, McClure W R. Searching for and predicting the activity of sites for DNA binding proteins: compilation and analysis of the binding sites for Escherichia coli integration host factor (IHF) Nucleic Acids Res. 1990;18:4993–5000. doi: 10.1093/nar/18.17.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hacker J, Blum-Oehler G, Muhldorfer I, Tschape H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 40.Hanekamp T, Kobayashi D, Hayes S, Stayton M M. Avirulence gene D of Pseudomonas syringae pv. tomato may have undergone horizontal gene transfer. FEBS Lett. 1997;415:40–44. doi: 10.1016/s0014-5793(97)01089-2. [DOI] [PubMed] [Google Scholar]
- 41.Harlan D M, Graff J M, Stumpo D J, Eddy R L, Jr, Stows T B, Boyle J M, Blackshear P J. The human myristoylated alanine-rich C kinase substrate (MARCKS) gene. Analysis of its gene product, promoter and chromosomal localization. J Biol Chem. 1991;266:14399–14405. [PubMed] [Google Scholar]
- 42.Hayes F, Austin S J. Specificity determinants of the P1 and P7 centromere analogs. Proc Natl Acad Sci USA. 1993;90:9228–9232. doi: 10.1073/pnas.90.19.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes F, Austin S J. Topological scanning of the P1 plasmid partition site. J Mol Biol. 1994;243:190–198. doi: 10.1006/jmbi.1994.1646. [DOI] [PubMed] [Google Scholar]
- 44.Herzer P J, Inouye S, Inouye M. Retron-Ec107 is inserted into the Escherichia coli genome by replacing a palindromic 34bp intergenic sequence. Mol Microbiol. 1992;6:345–354. doi: 10.1111/j.1365-2958.1992.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 45.Hofman K, Stoffel W. Tmbase—a database of membrane spanning protein segmants. Biol Chem. 1993;347:166–172. [Google Scholar]
- 45a.Hu P, Elliott J, McCready P, Skowronski E, Garnes J, Kobayashi A, Brubaker R R, Garcia E. Structural organization of virulence-associated plasmids of Yersinia pestis. J Bacteriol. 1998;180:5192–5202. doi: 10.1128/jb.180.19.5192-5202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Humphreys G O, Willshaw G A, Anderson E S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975;383:457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- 47.Jeter R, Escalante-Semerena J C, Roof D, Olivera B, Roth J. Synthesis and use of vitamin B12. In: Neidhardt F C, Ingraham J L, Magasanik B, Low K B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 551–556. [Google Scholar]
- 48.Jones A J, Barth P T, Wilkins B M. Zygotic induction of plasmid ssb and psiB genes following conjugative transfer of IncI1 plasmid ColIb-P9. Mol Microbiol. 1992;6:605–613. doi: 10.1111/j.1365-2958.1992.tb01507.x. [DOI] [PubMed] [Google Scholar]
- 49.Karlyshev A V, Galyov E E, Abramov V M, Zav’yalov V P. Caf1R gene and its role in the regulation of capsule formation of Y. pestis. FEBS Lett. 1992;305:37–40. doi: 10.1016/0014-5793(92)80650-6. [DOI] [PubMed] [Google Scholar]
- 50.Koltsova E G, Suchkov Y G, Lebedeva S A. Transmission of a bacteriocinogenic factor in Pasturella pestis. Sov Genet. 1975;7:507–510. [PubMed] [Google Scholar]
- 51.Krause M, Harwood J, Fierer J, Guiney D. Genetic analysis of homology between the virulence plasmids of Salmonella dublin and Yersinia pseudotuberculosis. Infect Immun. 1991;59:1860–1863. doi: 10.1128/iai.59.5.1860-1863.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lampson B C, Inouye S, Inouye M. msDNA of bacteria. Prog Nucleic Acid Res Mol Biol. 1991;40:1–24. doi: 10.1016/s0079-6603(08)60838-7. [DOI] [PubMed] [Google Scholar]
- 53.Lanka E, Wilkins B M. DNA processing reactions in bacterial conjugation. Annu Rev Biochem. 1995;64:141–169. doi: 10.1146/annurev.bi.64.070195.001041. [DOI] [PubMed] [Google Scholar]
- 54.Lewis P J, Errington J. Direct evidence for active segregation of oriC regions on the Bacillus subtilis chromosome and co-localization with the SpoOJ partitioning protein. Mol Microbiol. 1997;25:945–954. doi: 10.1111/j.1365-2958.1997.mmi530.x. [DOI] [PubMed] [Google Scholar]
- 55.Lipman D, Altschul S, Kececioglu J. A tool for multiple sequence alignment. Proc Natl Acad Sci USA. 1989;86:4412–4415. doi: 10.1073/pnas.86.12.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ludtke D N, Austin S J. The plasmid-maintenance functions of the P7 prophage. Plasmid. 1987;18:93–98. doi: 10.1016/0147-619x(87)90083-7. [DOI] [PubMed] [Google Scholar]
- 57.Matsutani S, Ohtsubo H, Maeda Y, Ohtsubo E. Isolation and characterization of IS elements repeated in the bacterial chromosome. J Mol Biol. 1987;196:445–455. doi: 10.1016/0022-2836(87)90023-4. [DOI] [PubMed] [Google Scholar]
- 58.McDonough K A, Barnes A M, Quan T J, Montenieri J, Falkow S. Mutation in the pla gene of Yersinia pestis alters the course of plague bacillus-flea (Siphonaptera: ceratophyllidae) interaction. J Med Entomol. 1993;30:772–780. doi: 10.1093/jmedent/30.4.772. [DOI] [PubMed] [Google Scholar]
- 59.McDonough K A, Hare J M. Homology with a repeated Yersinia pestis DNA sequence IS100 correlates with pesticin sensitivity in Yersinia pseudotuberculosis. J Bacteriol. 1997;179:2081–2085. doi: 10.1128/jb.179.6.2081-2085.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mercado-Blanco J, Olivares J. A protein involved in stabilization of a large non-symbiotic plasmid of Rhizobium meliloti shows homology to eukaryotic cytoskeletal proteins and DNA binding proteins. Gene. 1994;139:133–134. doi: 10.1016/0378-1119(94)90536-3. [DOI] [PubMed] [Google Scholar]
- 61.Mercado-Blanco J, Olivares J. The large non-symbiotic plasmid pRmeGR4a of Rhizobium meliloti GR4 encodes a protein involved in replication that has homology with the RepC protein of Agrobacterium plasmids. Plasmid. 1994;32:75–79. doi: 10.1006/plas.1994.1046. [DOI] [PubMed] [Google Scholar]
- 62.Millon, J., H. A. Kirkpatrick, H. L. Kijenski, C. A. Bloch, C. K. Rode, G. F. Mayhew, D. J. Rose, G. Plunkett III, V. Burland, and F. R. Blattner. Subdivision of the Escherichia coli K-12 genome for sequencing: manipulation and DNA sequence of transposable elements introducing unique restriction sites. Submitted for publication. [DOI] [PubMed]
- 63.Moore R L, Brubaker R R. Hybridization of deoxyribonucleic acid sequences of Yersinia enterocolitica and other selected members of Enterobacteriaceae. Int J Syst Bacteriol. 1975;25:336–339. [Google Scholar]
- 64.Mukhopadhyay G, Carr K M, Kaguni J M, Chattoraj D K. Open-complex formation by the host initiator, DnaA, at the origin of P1 plasmid replication. EMBO J. 1993;12:4547–4554. doi: 10.1002/j.1460-2075.1993.tb06143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagai S, Yagihashi T, Ishihama A. DNA sequence of an allelic variant of the Actinobacillus pleuropneumoniae-RTX-toxin I (ApxIA) from serotype 10. Microb Pathog. 1993;15:485–495. doi: 10.1006/mpat.1993.1096. [DOI] [PubMed] [Google Scholar]
- 66.Nakai K, Kanehisa M. Expert system for predicting protein localization sites in Gram-negative bacteria. Proteins Struct Funct Genet. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 67.Nakayama S, Kaneko T, Ishimaru H, Moriwaki H, Mizobuchi K. Cloning, sequencing and expression of bacteriophage BF23 late genes 24 and 25 encoding tail proteins. J Bacteriol. 1994;176:7280–7290. doi: 10.1128/jb.176.23.7280-7290.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olsen C H, Blattner F R, Daniels D L. Simultaneous preparation of up to 768 single-stranded DNAs for use as templates in DNA sequencing. Methods. 1991;3:27–32. [Google Scholar]
- 69.Pal S K, Mason R J, Chattoraj D K. P1 plasmid replication. Role of initiator titration in copy number control. J Mol Biol. 1986;20:275–285. doi: 10.1016/0022-2836(86)90364-5. [DOI] [PubMed] [Google Scholar]
- 70.Perna N T, Mayhew G F, Posfai G, Elliot S, Donnenberg M S, Kaper J B, Blattner F R. Molecular evolution of the pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1998;66:3810–3817. doi: 10.1128/iai.66.8.3810-3817.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perry R D, Pendrak M L, Schuetze P. Identification and cloning of the hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol. 1990;172:5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Portnoy D A, Martinez R J. Role of a plasmid in the pathogenicity of Yersinia species. Curr Top Microbiol Immunol. 1985;118:29–51. doi: 10.1007/978-3-642-70586-1_3. [DOI] [PubMed] [Google Scholar]
- 73.Protsenko O A, Anisimov P I, Mosarov O T, Donnov N P, Popov Y A, Kokushkin A M. Detection and characterization of Yersinia pestis plasmids determining pesticin I, fraction I antigen and mouse toxin synthesis. Genetika. 1983;19:1081–1090. [PubMed] [Google Scholar]
- 74.Protsenko O A, Filippov A A, Kutyrev V V. Integration of the plasmid encoding the synthesis of capsular antigen and murine toxin into Yersinia pestis chromosome. Microb Pathog. 1991;11:123–128. doi: 10.1016/0882-4010(91)90005-u. [DOI] [PubMed] [Google Scholar]
- 75.Radnedge L, Davis M A, Austin S J. P1 and P7 plasmid partition: ParB protein bound to its partition site makes a separate discriminator contact with the DNA that determines species specificity. EMBO J. 1996;15:1155–1162. [PMC free article] [PubMed] [Google Scholar]
- 76.Rajakumar K, Sasakawa C, Adler B. Shigella flexneri she pathogenicity island which encodes a homolog of the immunoglobulin A protease-like family of proteins. Infect Immun. 1997;65:4606–4614. doi: 10.1128/iai.65.11.4606-4614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rakin A, Heesemann J. Virulence-associated fyuA/irp2 gene cluster of Yersinia enterocolitica biotype 1B carries a novel insertion sequence IS1328. FEMS Microbiol Lett. 1995;129:287–292. doi: 10.1111/j.1574-6968.1995.tb07594.x. [DOI] [PubMed] [Google Scholar]
- 78.Romantschuk M, Richter G Y, Mikhopadhyay P, Mills D. IS801, an insertion sequence element isolated from Pseudomonas syringae pathovar phaseolicola. Mol Microbiol. 1991;5:617–622. doi: 10.1111/j.1365-2958.1991.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 79.Ryan M C, Tizard R, VanDevanter D R, Carter W G. Cloning of the Lam3 gene encoding the alpha 3 chain of the adhesive ligand epiligrin: expression in wound repair. J Biol Chem. 1994;269:22779–22787. [PubMed] [Google Scholar]
- 80.Samoilova S V, Somoilova L V, Yezhov I N, Drozdov I G, Anisimov A P. Virulence of pPST+ and pPST− strains of Yersinia pestis for guinea pigs. J Med Microbiol. 1996;45:440–444. doi: 10.1099/00222615-45-6-440. [DOI] [PubMed] [Google Scholar]
- 81.Sanger F, Coulson A R, Hong G F, Hill D F, Peterson G B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982;162:729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- 82.Schauer D B, Falkow S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect Immun. 1993;61:2486–2492. doi: 10.1128/iai.61.6.2486-2492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schofield J P, Jones D S, Forrest J N., Jr Identification of C-type natriuretic peptide in heart of spiny dogfish shark (Squalus acanthias) Am J Physiol. 1991;261:F734–F739. doi: 10.1152/ajprenal.1991.261.4.F734. [DOI] [PubMed] [Google Scholar]
- 84.Shapiro J A. Natural genetic engineering in evolution. Genetika. 1992;86:99–111. doi: 10.1007/BF00133714. [DOI] [PubMed] [Google Scholar]
- 85.Shine, J., and L. Dalgarno. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc. Natl. Acad. Sci. USA 71:1342–1346. [DOI] [PMC free article] [PubMed]
- 86.Simonet M, Riot B, Fortineau N, Berche P. Invasin production by Yersinia pestis is abolished by insertion of an IS200-like element within the inv gene. Infect Immun. 1996;64:375–379. doi: 10.1128/iai.64.1.375-379.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singer M F. Unusual reverse transcriptases. J Biol Chem. 1995;270:24623–24626. doi: 10.1074/jbc.270.42.24623. [DOI] [PubMed] [Google Scholar]
- 88.Smith C A, Pinkney M, Guiney D G, Thomas C M. The ancestral IncP replication system consisted of contiguous oriV and trfA segments as deduced from a comparison of the nucleotide sequences of diverse IncP plasmids. J Gen Microbiol. 1993;139:1761–1766. doi: 10.1099/00221287-139-8-1761. [DOI] [PubMed] [Google Scholar]
- 89.Sodeinde O A, Goguen J D. Genetic analysis of the 9.5-kilobase virulence plasmid of Yersinia pestis. Infect Immun. 1988;56:2743–2748. doi: 10.1128/iai.56.10.2743-2748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sodeinde O A, Subrahmanyam Y V B K, Stark K, Quan T, Bao Y, Goguen J D. A surface protease and the invasive character of plague. Science. 1992;258:1004–1007. doi: 10.1126/science.1439793. [DOI] [PubMed] [Google Scholar]
- 91.Straley S C. The plasmid-encoded outer-membrane proteins of Yersinia pestis. Rev Infect Dis. 1988;10:S323–S326. doi: 10.1093/cid/10.supplement_2.s323. [DOI] [PubMed] [Google Scholar]
- 92.Straley S C. The low-Ca+2 response virulence regulon of human-pathogenic yersiniae. Microb Pathog. 1991;10:87–91. doi: 10.1016/0882-4010(91)90069-m. [DOI] [PubMed] [Google Scholar]
- 93.Straley S C, Brubaker R R. Cytoplasmic and membrane proteins of yersiniae cultivated under conditions simulating mammalian intracellular environment. Proc Natl Acad Sci USA. 1981;78:1224–1228. doi: 10.1073/pnas.78.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Temin H M. Reverse transcriptases: retrons in bacteria. Nature. 1989;339:254–255. doi: 10.1038/339254a0. [DOI] [PubMed] [Google Scholar]
- 95.Vidal F, Baudoin C, Miquel C, Galliano M F, Christiano A M, Uitto J, Ortonne J P, Meneguzzi G. Cloning of the laminin alpha 3 chain gene (LAMA3) and identification of a homozygous deletion in a patient with Herlitz junctional epidermolysis bullosa. Genomics. 1995;30:273–280. doi: 10.1006/geno.1995.9877. [DOI] [PubMed] [Google Scholar]
- 96.Welkos S L, Davis K M, Pitt L M, Worsham P L, Friedlander A M. Studies on the contribution of the F1 capsule-associated plasmid pFra to the virulence of Yersinia pestis. Contrib Microbiol Immunol. 1995;13:299–305. [PubMed] [Google Scholar]
- 97.Willetts N, Skurry R. Structure and function of the F factor and mechanism of conjugation. In: Neidhardt F C, Ingraham J L, Magasanik B, Low K B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1,110–1,133. [Google Scholar]
- 98.Wolf M K, Boedeker E C. Cloning of the genes for AF/R1 pili from rabbit enteroadherent Escherichia coli RDEC-1 and DNA sequence of the major structural subunit. Infect Immun. 1990;58:1124–1128. doi: 10.1128/iai.58.4.1124-1128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang D, Miller D J. Characterization of a novel non-muscle myosin-related protein from Onchocerca gobsoni. Int J Parasitol. 1995;25:1385–1391. doi: 10.1016/0020-7519(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 100.Zitman D, Ben-Gurion R. Transduction of Pasturella pestis. Virology. 1972;47:513–516. doi: 10.1016/0042-6822(72)90291-7. [DOI] [PubMed] [Google Scholar]