Abstract
The elaboration of neuronal axons and dendrites is dependent on a functional cytoskeleton. Cytoskeletal components have been shown to play a major role in the maintenance of the nervous system through adulthood, and changes in neurofilaments and microtubule-associated proteins (MAPs) have been linked to a variety of neurodegenerative diseases. Here we show that Futsch, the fly homolog of MAP1B, is involved in progressive neurodegeneration. Although Futsch is widely expressed throughout the CNS, degeneration in futscholk primarily occurs in the olfactory system and mushroom bodies. Consistent with the predicted function of Futsch, we find abnormalities in the microtubule network and defects in axonal transport. Degeneration in the adult brain is preceded by learning deficits, revealing a neuronal dysfunction before detectable levels of cell death. Futsch is negatively regulated by the Drosophila Fragile X mental retardation gene, and a mutation in this gene delays the onset of neurodegeneration in futscholk. A similar effect is obtained by expression of either fly or bovine tau, suggesting a certain degree of functional redundancy of MAPs. The futscholk mutants exhibit several characteristics of human neurodegenerative diseases, providing an opportunity to study the role of MAPs in progressive neurodegeneration within an experimentally accessible, in vivo model system.
INTRODUCTION
Neuronal morphology is determined by the specialized neuronal cytoskeleton that provides the structural framework for distinct axonal and dendritic compartments. Integrity of the cytoskeleton is therefore a prerequisite for function and survival of neurons, and many neurodegenerative diseases are characterized by changes in specific cytoskeletal components (reviews see, McMurray, 2000; Brandt, 2001). In particular, abnormal modifications of microtubule-associated proteins (MAPs) have been linked to a variety of congenital and sporadic age-related neurological disorders. One such group, collectively named Tauopathies, are characterized by filamentous inclusions of hyperphosphorylated microtubule-associated protein tau (Heutink, 2000; Hutton et al., 2001; Lee et al., 2001; Johnson and Bailey, 2002) or mutated tau in frontal temporal dementia with parkinsonism linked to chromosome 17 (FTDP-17; Hutton et al., 1998; Poorkaj et al., 1998; Spillantini et al., 1998). Similarly, mutations that abnormally increase the number of microtubule binding domains in tau induce neurofibrillary tangles and neurodegeneration (Hutton et al., 1998; Spillantini et al., 1998). Given these severe neurological defects induced by modified forms of tau, mutations in mice inducing a loss of tau produced surprisingly subtle axonal defects, apparently due to functional redundancies with other MAPs (Harada et al., 1994). In Drosophila, overexpression of tau also induced severe axonal defects, neurodegeneration, and (in combination with hyperphosphorylation) a neurofibrillary pathology (Williams et al., 2000; Wittmann et al., 2001; Jackson et al., 2002). Flies lacking tau have no obvious morphological or behavioral defects (Doerflinger et al., 2003).
Besides tau, several other MAPs are expressed only in neurons, including MAP1A, MAP1B, and MAP2 (Matus, 1991; Schoenfeld and Obar, 1994). Although structural differences among these proteins exist, all of them can interact with tubulin, stabilize microtubules, and link microtubules with other components of the cytoskeleton. Cell culture experiments have suggested a role of the MAPs in axonal outgrowth during development and regeneration (Gordon-Weeks and Fischer, 2000) and a hypomorphic murine mutant of MAP1B is embryonic lethal or dies shortly after birth (Edelmann et al., 1996; Gonzalez-Billault et al., 2000). Another mouse MAP1B mutant that leaves the first 571 amino acids intact reveals a delay in nervous system development (Takei et al., 1997). These studies indicate that like tau, MAP1B may be particularly important for the formation and maintenance of a functional nervous system.
In Drosophila, a MAP1 homologue is encoded by the futsch gene (Hummel et al., 2000). Futsch is a protein of predicted 5327 amino acids with strong homology to vertebrate MAP1B at its N-and C-termini, plus a large middle portion that contains 66 direct repeats similar to repeats found in neurofilaments. Futsch is expressed in many neurons in the fly CNS, whereas in vitro experiments have revealed that it binds microtubules (Roos et al., 2000). Notably, a loss-of-function futsch allele produces defects in axonal and dendritic growth in the embryonic nervous system (Hummel et al., 2000), whereas hypomorphic viable alleles show disruption of synaptic microtubule organization, reduction in bouton number, and an increase in bouton size at the neuromuscular junction (Roos et al., 2000). These phenotypes are partially rescued by expression of a shortened Futsch protein containing only the conserved N-terminus. Together, these results suggest that Futsch is required to regulate microtubule architecture, thereby controlling growth cone function and synapse formation. Whether this protein also regulates neuronal structure and function in the postembryonic nervous system (as has been suggested for vertebrate MAP1B) has remained unexplored.
In this article, we show that three new viable alleles of futsch, called olk1–3, exhibit a progressive degeneration of the adult olfactory system, accompanied by deficits in learning and memory. In addition, we present evidence that these mutant proteins induce abnormalities in microtubule structure and function, and we show that futsch-associated defects can be ameliorated by compensatory expression of both fly and bovine tau. These results indicate that MAP1B homologues may play critical roles in the maintenance of the CNS throughout adult life.
MATERIALS AND METHODS
Drosophila Stocks
The three new olk alleles were isolated in a histological screen in the laboratory of M. Heisenberg. The futschN94 and dfmr1 deletion (5′Arh/TM6Tb) lines were kindly provided by C. Klämbt (University of Münster, Germany), UAS-dfmr1 by A. Bailey (University of California, Berkeley), UAS-bovine tau by S. Schneuwly (University of Regensburg, Germany), and UAS-dtau D. St. Johnston (University of Cambridge, Great Britain). Elav-GAL4 and UAS-GFP were provided by the Bloomington stock center and GAL4-GH146 by R. Stocker (University of Fribourg, Switzerland). Stocks were maintained and raised on standard cornmeal food (Guo et al., 1996) in a 14–10-h light-dark cycle at 25°C and 60% relative humidity.
Tissue Sections for Light and Electron Microscopy
Paraffin sections were performed as described by Jäger and Fischbach (1989) and observed under fluorescence light. For comparisons between fly strains, olk and aged-matched control flies were processed together on one slide to ensure identical histological processing. Plastic sections of fly heads were prepared for light and electron microscopy as described (Kretzschmar et al., 1997). For light microscopy, 2-μm serial sections were cut and stained with 1% toluidine blue plus 1% borax. For conventional electron microscopy, heads were fixed in 5% glutaraldehyde overnight. Subsequently, they were washed in phosphate-buffered saline (PBS), osmicated in 1% OsO4 in PBS for 1 h, washed again in PBS and distilled water, and dehydrated in graded ethanol and embedded in Durcopan (Fluka, Ronkonkoma, NY). Ultrathin sections were contrasted with uranyl acetate and lead citrate (Reynolds, 1963) and observed with a Jeol Jem-100CX II electron microscope (Peabody, MA). Silver stainings were performed after Blest (1961) as described by Heisenberg (1989).
Whole-mount Preparations
For visualizing GFP expression in adult brains, whole-mount preparations of the brain were dissected and fixed in 4% paraformaldehyde overnight, embedded in Gel Mount (Fisher Scientific, Pittsburgh, PA) and observed with an Olympus Fluoview confocal 300 microscope (Lake Success, NY).
Immunohistochemistry
Paraffin sections and whole-mount brains were prepared as described above. The paraffin was removed with SafeClear (Fisher Scientific) and the sections were rehydrated in graded ethanol. The tissue samples were further processed as described by Buchner et al. (1989). The following antibodies were used: anti-tubulin (T9028, Sigma, St. Louis, MO, and DMI3A, Amersham Piscataway, NJ) 1:1000, anti-dfmr1 (Developmental Studies Hybridoma Bank, University of Iowa) 1:100, anti-futsch (22C10, Hybridoma supernatant obtained from A. Hofbauer, University of Regensburg) 1:100, Cy2- and Cy3-conjugated secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA).
Determination of Vacuole Size
For comparisons of olk flies expressing exogenous tau or deleted in one copy of dfmr 1 with the olk mutant alone, we photographed vacuoles in the region of the mechanosensory neuropil of the ventrolateral protocerebrum. For each preparation, pictures were taken and numbered (for double blind analysis) at the level of maximal vacuole size. The area of the vacuole was then calculated in Photoshop (Adobe, San Jose, CA) as total pixel number, which was subsequently converted into μm2. As controls, we used flies from the same cross that did not carry the elav-GAL4 insert and flies that had a balancer chromosome instead of the deletion chromosome.
Behavioral Assays
For behavioral experiments, we used 3–8-d-old males and females in mixed groups. Experimental flies were transferred to fresh food vials every 2 d. All behavioral experiments were performed either in dim red light or complete darkness at 80% relative humidity.
For reactivity to electric shock, groups of ∼100 flies were tested in a T-maze assay (slightly modified following Tully and Quinn, 1985), giving them 1 min to choose between an electrified (12 pulses of 130V, 1.3-s duration at 5-s intervals) and a nonelectrified tube, both equipped with copper wires. From each experiment we counted the number of flies that chose the electrified (Nshock) versus the nonelectrified tube (Nno shock) and calculated a response index as RIE = ([Nshock - Nno shock]/[Nshock + Nno shock]) × 100.
Spontaneous responses to odors were also tested in the T-maze assay, giving the flies 2 min to choose between two airstreams (750 ml/min), one scented with the test odor the other unscented (laboratory air). A reactivity index (RI) was calculated from the number of flies choosing the scented airstream (Nodor) versus the unscented one (Nair). RIO = ([Nodor - Nair]/[Nodor + Nair]) × 100.
Pavlovian training procedures in the T-maze apparatus were applied following the method of Tully and Quinn (1985). We modified the original apparatus to handle four groups of animals simultaneously. Aged flies were trained and tested in groups of ∼100. For electric shock-associated learning, flies were given 12 current pulses of 130 V, 1.3-s duration over the course of 1 min, during which the tube was scented (flow speed: 750 ml/min) with the first odor (CS+). After 45 s of fresh air, the tube was scented for an additional minute with the second odor but without electric shock (CS-), followed by another period of 45 s of fresh air. The memory test was initiated 100 s after the training period. Flies were placed between two air streams (750 ml/min), one scented with the formerly punished odor, the other with the nonpunished odor, and then were given 2 min to choose one of them. During a reciprocal experiment with a different group of flies, the first and second odors were exchanged. For each of the two experiments, we counted the number of flies choosing the punished (NCS+) and nonpunished odor (NCS-), respectively, and calculated the performance index as PI1/2 = ([NCS+ - NCS-]/[NCS+ + NCS-]) × 100. To rule out nonassociative effects, the PIs of the set of reciprocal experiments were averaged [PI = (PI1 + PI2)/2]. Test odorants were presented in 16 mm (3-octanol) and 5 mm (benzaldehyd) diameter cups placed in the air stream. Under these conditions naive flies showed no preference in the choice test between the two odors.
Transport Studies
Neuronal cell cultures were prepared from early pupae as described by (Kraft et al., 1998). Measurements of mitochondrial movements were performed on 24-h-old primary cultures, using Mitotracker Red CM-H2 × Ros (Molecular Probes, Eugene, OR). Cells were stained for 15 min and then observed with a Leica inverted microscope (Deerfield, IL). Images were taken every 2 s for 98 s. To perform an analysis of mitochondrial movement, we used the kymograph function in Metamorph Universal Imaging (West Chester, PA). For each movie frame, the brightest pixel within a 2-μm corridor along the axis of a dendrite (or axon) was displayed at the corresponding location along the neurite. The fluorescence patterns for all 50 movie frames were then displayed adjacent to one another. This produced graphs on which the x-axis represents time and the y-axis represents distance along the process. Mitochondria in the kymographs were grouped into the following three categories by double-blind scoring: (1) mitochondria that did not move, (2) mitochondria that moved (either forward or backward) for <2 μm, and (3) mitochondria that traveled more than 2 μm. At least 20 cells were used per genotype in four independent experiments. In total, 425 mitochondria were scored in wild-type and 551 in olk mutant cells.
RESULTS
olk Mutants Show Neural Degeneration in the Antennal Lobes and Mushroom Bodies of the Brain
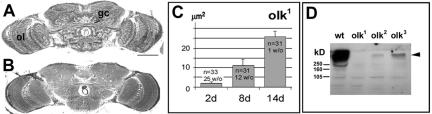
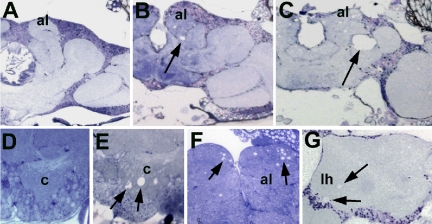
The Drosophila mutant omb-like (olk) was isolated in a screen for mutants with defects in the morphology of the adult brain. Histological sections of mutant brains lack the characteristic reduced silver staining associated with organized axonal bundles and fiber tracts (see Figure 2, A and B; this abnormality led to the mutant's name: as in the mutant optomotor-blind [omb], the giant fibers of the lobula plate are not discernible in silver stained or autofluorescent sections), especially in the case of large and medium-sized neural fibers. Three different alleles with this phenotype have been isolated. These mutations do not seem to interfere with normal development of the CNS, as no defects in brain morphology were visible in 1-d-old flies (Figure 1A and D). During adulthood, however, the brain revealed signs of progressive degeneration, manifested as increasing numbers of vacuoles in specific brain regions. The affected areas included the antennal lobes (which receive olfactory input) and the mushroom bodies, considered to be the seat of olfactory memory. Vacuoles could first be detected after 5 to 6 d of aging within the calyces, a substructure of the mushroom bodies (Figure 1E). After 10 d vacuoles were also detected in the antennal lobes (Figure 1, B and F), as well as in the region of the mechanosensory neuropil of the ventrolateral protocerebrum (arrow, Figure 1B; for a detailed description of the neuroanatomy of this region, see http://flybrain.neurobio.arizona.edu). Especially in this latter region, the vacuoles were seen to enlarge dramatically during subsequent aging (Figures 1, C, 20 d and 2C). In older flies, we also detected vacuoles in the region of the lateral horn (Figure 1G). Electron microscopic sections of the brains of these animals showed degeneration of both nerve fibers and neuronal cell bodies in these regions (Supplementary Figure 1). Swelling and lysis of the somata, whereas maintaining intact nuclei (arrow in Supplementary Figure 1C) suggested that neurons in these regions undergo necrotic cell death.
Figure 2.
Fiber structure defects and progressive degeneration in olk1. (A) Silver staining reveals fibers and axon bundles in a wild-type head section. (B) In olk1, the fibers are much less prominent, revealing a defect in fiber structure within the mutant. (C) The mean size of vacuoles in the mechanosensory neuropil of olk1 increases with age (8 d, p < 0.1; 14 d, p < 0.01), whereas the number of flies that do not show vacuoles in this region (w/o) decreases with age. Histograms indicate mean ± SEM, and the number flies measured per condition is indicated. (D) Western blot using the anti-futsch antibody 22C10. The amount of Futsch protein (arrowhead) is significantly reduced in olk2 and olk3 mutants compared with wild-type controls. In olk1 flies, we could not detect any protein. Scale bar, (A and B) 50 μm.
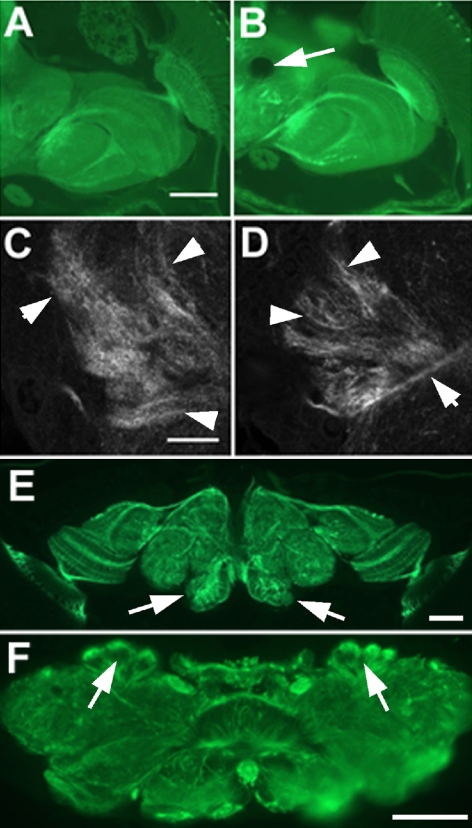
Figure 1.
Progressive degeneration in olk1. (A and D) Sections from the brain of a 1-d-old olk fly show no abnormalities compared with wild-type controls (unpublished data). (B and F) At 10 d into adult life, vacuoles have formed in the antennal lobes (al, arrows in F) and the mechanosensory neuropil of the lateral protocerebrum (arrow in B). (C) The lesion in the mechanosensory neuropil (arrow) enlarges dramatically with further aging (20 d). (E) The first vacuoles can be found in the calyx (c) of a 6-d-old mutant (arrows). (G) In 20-d-old olk flies, vacuoles can also be found in the region of the lateral horn (lh) of the protocerebrum (arrows). Sections are horizontal 1-μm plastic sections stained with toluidine blue. Scale bar, (A–C) 50 μm; (D and E) 10 μm; (F) 20 μm; (G) 25 μm.
olk Mutants Exhibit Selective Degeneration of Projection Neurons
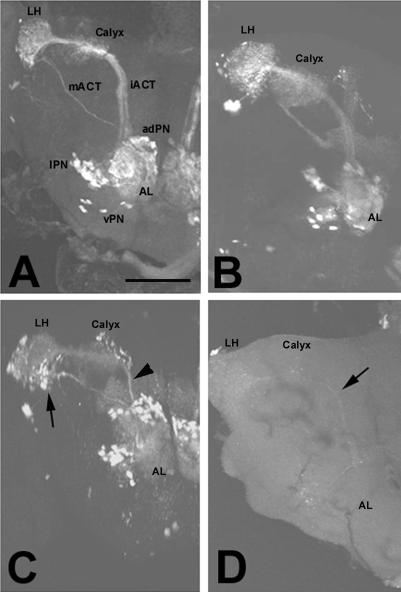
To assess which types of neurons are affected by this mutation, we crossed olk flies with various GAL4 lines that drive UAS-GFP in specific neuronal populations. One of these lines, GAL4-GH146 (Stocker et al., 1997), was used to visualize projection neurons (PNs), which connect the antennal lobes to the mushroom bodies, the center for olfactory memory (Heisenberg, 2003), and the lateral horn (Stocker, 2001; Jefferis et al., 2002; Marin et al., 2002; Wong et al., 2002). PNs have their dendrites in the antennal glomeruli, where they form synapses with axons from olfactory receptor neurons and local interneurons. The axons of the PNs run in at least two tracts, the larger inner antennocerebral tract (iACT) and the smaller medial antennocerebral tract (mACT; Figure 3A). In olk flies, the PNs initially appear to develop normally and make appropriate connections in their target areas (Figure 3B, 1 d old). In 10-d-old olk1 flies, however, GFP expression in the PN dendritic fields within the antennal lobes was noticeably weaker, and the inner antennocerebral tract appeared both thinner and more weakly stained (arrowhead, Figure 3C) than in wild-type animals of the same age. GFP labeling also appeared weaker in the lateral horn and calyx (Figure 3C), although the size of the terminal field in these regions appeared unchanged or possibly even slightly enlarged. In contrast, the medial antennocerebral tract (which bypasses the mushroom body calyx) and its terminal field seemed not to be affected at this age (arrow, Figure 3C). After 23 d, only a faint staining was still visible in the olk1 flies (arrow in Figure 3D), apparently because of the loss of all GFP-expressing neurons. These results suggest that the anterodorsal and lateral PNs, which send their axons through the inner anterocerebral tract, undergo axonopathy and cell death before the death of the more ventral neurons, which make projections directly to the lateral horn. In contrast to the PNs, GFP expression in the Kenyon cells of the mushroom bodies (using the D52H, 2384, and MB244–36y GAL4 line) or the ring neurons of the central complex (using the C232-GAL 4 line) did not reveal any comparable age-related abnormalities or death in these neighboring neuronal populations, indicating the observed changes in the PN neurons was not simply due to global deterioration of the CNS.
Figure 3.
Degeneration of projection neurons in olk1 mutants (visualized by GFP expression). (A) Wild-type, 10 d. The PNs send their dendrites into the antennal lobe (AL) and their axons into the calyx via the inner antennocerebral tract (iACT) and into the lateral horn (LH) via the medial antennocerebral tract (mACT). (B) In 1-d-old mutants, the PNs appear normal. (C) In a 10-d-old mutant, the staining of the dendritic field in the AL was noticeably weaker, and the iACT appeared both thinner and more weakly stained (arrowhead). The staining of the mACT (arrow) and its termination field and of the cell bodies of all three types of PNs was not noticeably altered at this age. (D) Further aging (23 d) resulted in severely reduced staining in all of these regions, suggesting that most or all of the PNs had undergone cell death. Images show whole-mounts using GH146-GAL4 and UAS-GFP. adPN, vPN, and lPN = anterodorsal, ventral, and lateral projection neurons, respectively. Scale bar, 50 μm.
olk Mutants Display Defects in Olfactory Memory
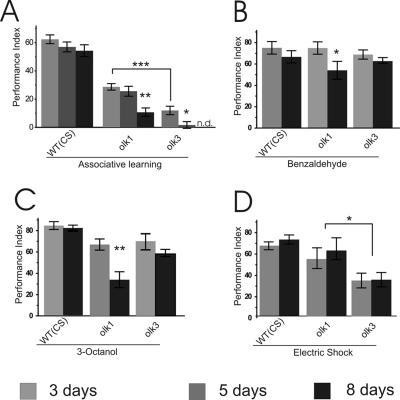
We next addressed whether PN degeneration is accompanied or preceded by behavioral changes. To investigate learning and memory defects in the mutant flies, we performed olfactory tests with three different age groups. Although young (3 d old) olk flies did not exhibit detectable signs of degeneration (unpublished data), they already displayed significant impairment in their olfactory learning compared with wild-type Canton-S control flies (Figure 4A). In olk1 flies, PI was reduced by ∼50% and in olk3 by ∼80% at this age. These results show that the affected neurons are measurably dysfunctional well before the onset of overt degeneration. By day 5, an age where first signs of degeneration become evident (Figure 1E), olfactory learning was completely disrupted in olk3 flies. In olk1 flies a more gradual age-dependent decline in the PI was observed, reaching a PI of 25% of control by day 5 and 10% by day 8, whereas aging in wild-type flies did not significantly change performance.
Figure 4.
Progressive loss of olfactory learning ability in olk mutants. Associative and nonassociative olfactory behavior was tested at the age of 3, 5, and 8 d. (A) Performance of olfactory short-term memory progressively declines in olk mutant flies. Already by day 3, performance was significantly impaired (p < 0.001 for both olk1 and olk3 compared with wild-type Canton-S). Performance indices (PIs) were near zero by day 5 in olk3 (p < 0.05) and by day 8 in olk1 (p < 0.01). Day 8 was not measured in olk3 mutants (n.d.). (B–D) Response to the task-relevant stimuli was measured for the associated odorants (B and C) and the behavioral reinforcer electric shock (D). Olfactory acuity was also affected in an age-dependent manner in olk1 mutant flies (p < 0.05 for benzaldehyde and p < 0.01 for 3-octanol, respectively), whereas there was no progressive effect on acuity of response to electric shock. However, overall sensitivity was significantly reduced in olk3 mutants (p < 0.05). Histograms show means ± SEM of six experiments with 100 flies each.
This age-dependent effect on learning ability in olk mutants was accompanied by additional deficits in their response to task-relevant stimuli. In olk 1 mutants reactivity to the olfactory stimuli (benzaldehyde and octanol) was significantly reduced in 8-d-old flies (Figure 4, B and C), possibly the result of the onset of degeneration in their antennal lobes at this age. This decrement was not apparent in olk3 mutants. However, these alleles did show a significantly reduced response to electric shock by as early as day 3, an effect that was not influenced by aging (Figure 4D).
These results reveal that olfactory-associated behavioral defects precede overt degeneration of olfactory lobe–associated structures in the brain. This is obvious in young futscholk3 flies, which show a severe reduction of their performance index long before the onset of degeneration. Also an effect of aging on the associative learning phenotype is seen in this allele because the reactivity to odors and shock does not change significantly with age. In futscholk1, effects of aging on perception of the olfactory stimuli could add to the decrease of the learning index in aged flies. In young futscholk1 flies, however, learning is clearly affected because the PI is reduced to ∼50% without a significant change in olfactory perception.
olk Is Allelic to futsch (Drosophila MAP 1B Homolog)
The olk gene was localized to the X-chromosomal region 1E-2A by deletion mapping (Supplementary Figure 2, A and C). The futsch gene, which encodes the fly homolog of vertebrate MAP1B, has also been localized within the same region (2A2–3). We performed a complementation analysis of these genes, using the viable futschN94 allele (kindly provided by C. Klämbt). This analysis revealed that futsch does not complement olk, because the resulting flies showed a degenerative phenotype comparable to the olk alleles alone (see Supplementary Figure 2B). We further confirmed the identity of futsch and olk by Western blots using the monoclonal antibody (22C10), which recognizes the Futsch antigen. Futsch protein was undetectable in olk1 mutants and only very small amounts were found in olk2 and olk3 (Figure 2D). Subsequent genomic sequencing showed that olk1 is caused by the substitution of a guanine to a thymine at position 4783 of the futsch cDNA annotation (Gadfly). This mutation changes a codon for glutamic acid to a stop codon (GAA to UAA), which shortens the protein to approximately one-third of the predicted 5412 amino acids. Either this protein fragment cannot be detected in Western blots because it lacks the epitope recognized by 22C10 or the fragment is unstable and rapidly degraded. This truncated protein contains the highly conserved N-terminus and most likely retains a partial function. In contrast, futscholk2 and futscholk3 are most likely regulatory mutants, because we detected low amounts of the full-length protein in Western blots. The residual function and/or amounts of Futsch protein would explain the hypomorphic character of these alleles.
The Microtubule Scaffold Is Altered in Brains of olk Mutant Flies
As already noted, silver staining of olk mutant brains suggested that these animals exhibit defects in the organization of their neuronal cytoskeleton. To substantiate this observation, we stained brain sections with an antibody against tubulin. Surprisingly, overall antitubulin staining was noticeably more intense in 14-d-old olk1 flies than in age-matched wild-type flies (Figure 5, A and B; mean brightness per pixel 89.3 in olk1; 76.5 in wild type). Confocal microscopy of tubulin-stained brain sections revealed that this effect was due to a more densely localized pattern of staining in neurites of the mutant brains, including neurites in the calyx (Figure 5D), compared with the more dispersed distribution of tubulin in wild-type animals (Figure 5C). This difference was also apparent in the antennal lobes and optic neuropils, corresponding to the widespread expression of Futsch in the brain (Figure 5, E and F).
Figure 5.
Impact of the olk mutation on tubulin organization and Futsch expression. (A) Tubulin staining in a paraffin section of a 14-d-old wild-type fly. (B) Overall tubulin staining appeared stronger in an age-matched olk1 mutant animal. (C and D) Confocal microscopic images taken from the calyx of these sections from (C) wild-type and (D) olk1 animals reveal a more densely localized pattern of staining in the mutant (arrowheads). (E and F) Immunohistochemistry of wild-type brain sections using 22C10 (anti-Futsch). Futsch is widely distributed in the brain, with strong expression in the antennal lobes (arrows in E) and calyx (arrows in F). Scale bar, (A and B) 50 μm; (C and D) 10 μm; (E and F) 50 μm.
This altered pattern of tubulin staining in olk mutants might be due to structural differences in the microtubule network of CNS neurons and/or an upregulation of tubulin in the mutant antennal lobes. To investigate this issue in more detail, we performed electron microscopic studies on 14-d-old olk1 and wild-type flies. When we compared microtubule distributions in neuronal fibers within wild-type versus olk mutant brains, we observed a noticeable increase in microtubule number in the mutant animals, an alteration that was detectable both in longitudinal sections (Figure 6, A and B) and cross sections (Figure 6, C and D). Western blots from head homogenates of 1–4-d-old flies confirmed an increase in tubulin expression (139 ± 12%, see Supplementary Figure 3). This result strongly suggests that the more intense tubulin staining in the mutant brains (Figure 5B) is due to an increase in the overall amount of tubulin as well as its assembly into microtubules in olk mutant neurons. This upregulation would result in a greater number of microtubules in neurons but reduced microtubule spacing and this ultrastructural abnormality would also explain the more compact appearance of the microtubule distributions seen in immunostained brain sections. In addition, microtubules within the axons of olk1 brains often exhibited abnormalities in their organization, including uneven spacing, bending, and in some instances actually crossing of adjacent microtubules (Figure 6, E and F, arrowheads). These experiments confirm an important role of Futsch in the organization and dynamics of the cytoskeleton, not only during development (as previously reported, Roos et al., 2000) but also into adulthood.
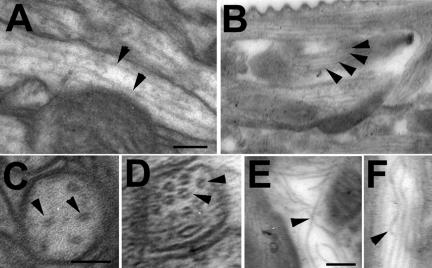
Figure 6.
Disorganized microtubule in the antennal lobes of olk1. (A) Microtubules (arrowheads) in a 14-d-old wild-type antennal lobe are straight and evenly spaced. (B) In an age-matched olk1 mutant, a noticeable increase in the number of microtubules was apparent (arrowheads), leading to reduced spacing between microtubules. This difference in density could also be seen in cross sections from wild-type (C) and olk1 mutant flies (D). (E and F) In addition, microtubules in the mutant were seen to bend, touch, or cross each other (arrowheads), a phenotype not observed in wild-type brains. Scale bar, 0.05 μm.
Mitochondrial Transport Is Reduced in olk Neurons
To assess the possible consequences of the alterations seen in the microtubule network of olk1 flies, we examined various aspects of outgrowth and transport in primary neuronal cultures from wild-type and mutant brains. Neurons derived from olk1 flies did not show any obvious defects in general outgrowth in vitro, developing a neuritic network indistinguishable to that of wild-type neurons (Figure 7, A and B). To determine whether olk mutations induce defects in cellular transport, we also used time-lapse imaging to analyze the movements of mitochondria within the growing neurites (movies in Supplementary Figure 4). Mitochondria were grouped into three categories: mitochondria that did not change their position during the entire observation time, mitochondria that did not exhibit net changes in their position but wobbled back and forth (covering not more than 2 μm in distance), and mitochondria that moved >2 μm either retrograde or anterograde within the neurites. Although the number of mitochondria undergoing small wobbling movements was not significantly different between wild-type and olk1 neurons, we detected significant differences in the two other categories of motion (Figure 7C). In olk1 neurons, almost twice as many mitochondria showed no changes in their positions (33 vs. 18% in wild-type, p < 0.05), whereas the number of actively moving mitochondria was reduced by half (18 vs. 37% in wild-type, p < 0.01). These results indicate that the abnormalities seen in the microtubule network of olk1 mutant flies corresponds with measurable disruptions in mitochondrial transport within the axons and dendrites of neuronal cells.
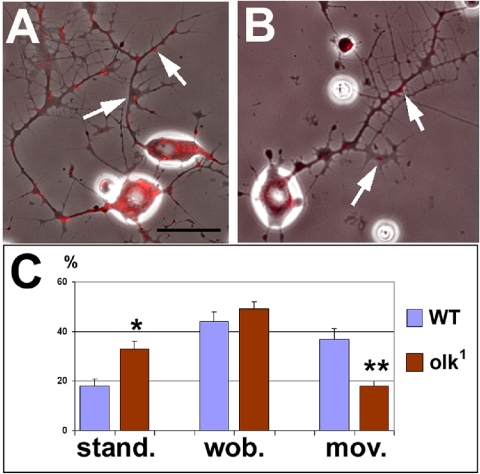
Figure 7.
olk mutations affect mitochondrial transport. Neurons cultured for 24 h from (A) wild-type and (B) olk1 pupae show similar patterns of outgrowth and branching. (C) To analyze axonal transport, we labeled mitochondria (red, arrows in A and B) and observed their movement for 98 s with pictures taken every 2 s. During this interval, significantly more mitochondria in olk1 did not change their position (stand.) and fewer mitochondria showed active movement within the neurites (mov.). The number of mitochondria exhibiting small back-and-forth movements (<2 μm; wob.) was not significantly changed in olk1 neurons (movies can be seen in Supplementary Figure 3). Histograms show means ± SEMs. (stand. Phosphate-buffered saline < 0.05, mov. Phosphate-buffered saline < 0.01). Scale bar, (A and B) 10 μm.
olk Interacts with the Fragile X Mental Retardation Gene
Futsch is negatively regulated by the Drosophila Fragile X mental retardation gene (dfmr1; Zhang et al., 2001). Mutations in dfmr1 show a similar synaptic phenotype as futsch in the larval neuromuscular junction, whereas the combined mutations of both genes restores normal synaptic structure (Zhang et al., 2001). DFMR1 is highly expressed in the larval mushroom bodies (Schenck et al., 2002) and in most, if not all, neurons in the adult brain (Morales et al., 2002). Using an anti-dfmr antibody (red) to immunostain PNs expressing GFP (via the GAL4-GH146/UAS-GFP construct; green), we showed that these neurons also specifically express the DFMR1 protein (Figure 8, A and B). To investigate whether mutations in dfmr1 suppresses the degenerative phenotype of olk, we crossed our olk1 mutants with flies carrying a homozygous lethal genomic deletion in dfmr1 (kindly provided by C. Klämbt, University Münster). When we compared olk1 flies carrying one copy of the dfmr1 deletion with control flies coming out of the same cross, we discovered a dramatic suppression of the olk degenerative phenotype. The size of vacuoles in the mechanosensory neuropil of the ventrolateral protocerebrum was reduced by approximately one-third at day 14, compared with olk1 mutants of the same age (Figure 8C).
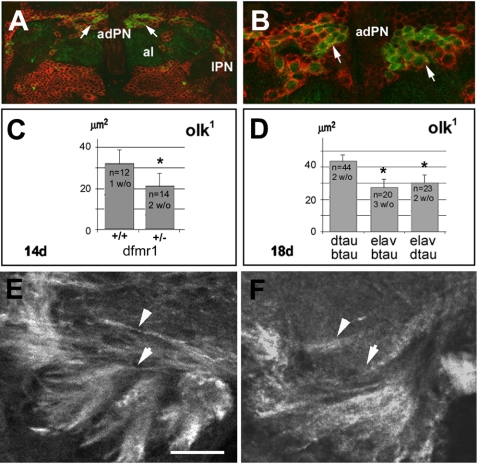
Figure 8.
Interaction of olk with dfmr1 and tau. (A) Double staining using an anti-DFMR1 antibody (red) and GFP staining of PNs (green). (B) A higher magnification view shows that the anterodorsal PNs (adPN; arrows) express DFMR1. Al, antennal lobes; lPN, lateral projection neurons. (C) The average size of vacuoles in the mechanosensory neuropil of the lateral protocerebrum was significantly reduced in day 14 olk1 flies carrying the dfmr1 deletion (+/-), compared with age-matched olk1 flies from the same cross but carrying a balancer chromosome (+/+). Average vacuole size was reduced by approximately one-third compared with control olk flies (p < 0.01). (D) Eighteen-day-old olk1 flies that also expressed either fly tau (dtau) or bovine tau (btau) in all neurons (driven by the ELAV promoter) had significantly fewer vacuoles than in olk1 flies (p < 0.05). Histograms show means ± SEM, and the number of flies measured in each strain are indicated. (E and F) Anti-tubulin immunostaining of brain sections from 14-d-old olk1 flies without the dfmr1 deficiency (E) and with the dfmr1 deficiency (F). Removing one copy of dfmr1 restored the overall pattern of tubulin staining pattern toward the more diffuse pattern seen in wild-type flies (compare with Figure 5). Scale bar, (A) 15 μm; (B) 6 μm; (E and F) 10 μm.
To determine whether dfmr1 similarly affects the microtubule phenotype seen in olk mutants, we immunostained sections of fly brains from both strains with anti-tubulin antibodies. In contrast to the abnormally condensed pattern of tubulin staining seen in the calyx of olk1 mutants (Figure 8E), olk flies carrying the dfmr1 deletion showed a noticeable reversion to a more diffuse pattern of staining seen in wild-type animals (Figure 8F). These results substantiate the hypothesis that futsch and dfmr1 interact in CNS neurons, demonstrating that the degenerative phenotype of the olk mutants (hypomorphic alleles of futsch) can be suppressed by a reduction of the negative regulator dfmr1.
The synaptic defects in dfmr1 mutants can be attributed to an upregulation of futsch expression in the absence of dfmr1 (Zhang et al., 2001). Thus, both underexpression and overexpression of functional Futsch protein may result in similar (though subtly different) abnormalities in synaptic function. By this model, we would predict that an increase in Dfmr1 function might be sufficient to create an olk-like phenotype. We tried to test this hypothesis by overexpressing Dfmr1, but unfortunately both pan-neuronal expression and expression specifically in the PNs induced lethality during development (as has also been reported when dfmr1 was overexpressed in the eye and wing; Wan et al., 2000).
olk Mutations Can Also Be Partially Rescued by Fly and Bovine tau Expression
Experiments in vertebrates have suggested that different members of the MAP family may have overlapping functions (DiTella et al., 1996; Teng et al., 2001). In flies, previous reports have shown that endogenous tau MAP protein is normally highly expressed in the eye but not in the brain (Heidary and Fortini, 2001). To investigate the possibility of similar compensatory or redundant functions in Drosophila, we expressed either fly or bovine tau (driven by the neuronal-specific GAL4-ELAV) in olk1 mutant flies. In comparison with olk flies carrying the UAS-tau constructs but lacking the GAL4-ELAV driver, flies expressing either Drosophila or vertebrate tau showed an ∼25% reduction in the size of vacuoles that developed in the mechanosensory neuropil (Figure 8D). However, we could not detect an effect of exogenously expressed tau on the microtubule phenotype induced by our futscholk mutants (as seen at the light microscopic level). This suggests that the neurodegenerative effects of defective Futsch protein may include additional functions or result from more subtle effects on microtubule. The suppression of the degenerative phenotype of olk suggests that ectopic tau expression can partially substitute for the loss of Futsch in the projection neurons. Thus, different members of the MAP family share overlapping functional activity that has been evolutionarily conserved.
DISCUSSION
Our characterization of the olk1–3 mutants revealed that they are hypomorphic alleles of the futsch gene, which encodes a protein with substantial sequence similarity to vertebrate MAP1B.
In the embryo, futsch is required for axonal pathfinding, and transgenic rescue experiments have shown that a short fragment of the protein (containing the first 618 aa) partially restores embryonic viability (Hummel et al., 2000). Similarly in mice, the N-terminus of MAP1B is vital for the function of this protein, because animals expressing only the first 571 aa are also viable (Takei et al., 1997). However, these results are still controversial, as other groups reported that expression of this truncated MAP1B does not rescue lethality (Edelmann et al., 1996; Gonzalez-Billault et al., 2000). Our characterization of futscholk1 has shown that the N-terminal third of Futsch is sufficient to support normal development into adulthood. However, the progressive degeneration seen in adults in all three olk alleles demonstrates that the structural and functional maintenance of the nervous system requires additional domains of the protein and/or increased protein levels.
Our experiments have shown that the PNs of the olfactory system undergo cell death in futscholk mutants, apparently due to necrotic cell death. The first signs of degeneration appear within the calyx regions, well before any signs of cell death, indicating that axonal degeneration precedes neuronal death of the PNs. Moreover, our results support the conclusion that defects in the cytoskeleton of these neurons lead to a progressive disruption in their axonal and dendritic integrity, ultimately culminating in the death of the neurons.
In vertebrates, mitral cells are analogous to insect PNs, connecting the olfactory bulb with the olfactory cortex (Marin et al., 2002). Innervation of the olfactory bulb continues to be modified throughout adult life, corresponding with the persistent expression of juvenile MAP isoforms in the mitral cells (Viereck et al., 1989). Interestingly, olfactory dysfunctions have been described as one of the first signs of both Parkinson's and Alzheimer's disease (Kovacs et al., 2001; Hawkes, 2003), and mitral cell degeneration is a common feature in Alzheimer patients (Kovacs et al., 1999). Our results suggest that as in humans, the olfactory centers of the brain in Drosophila are particularly susceptible to disruptions in MAP proteins, an effect that corresponds with the need of these neurons for continuous, experience-dependent remodeling throughout adult life.
The role of Futsch in regulating tubulin assembly in neurons was confirmed by the marked changes seen in the organization and distribution of microtubules in the futscholk mutants. We suggest that the lack of functional Futsch protein results in a destabilization of the cytoskeleton, which induces a compensatory upregulation of tubulin expression. A similar defect has been shown in mice lacking both MAP1B and MAP2, in which the spacing of microtubules was significantly diminished (Teng et al., 2001). Alternatively, the futscholk mutations might disrupt the dynamics of tubulin assembly/disassembly, resulting in an abnormal accumulation of microtubules.
In primary neuronal cultures prepared from the futscholk mutants, we observed a significant reduction in mitochondrial movement compared with wild-type neurons, which most likely reflects a general reduction in axonal and dendritic transport due to disruptions in the microtubule network. Recently, it was shown that regenerating neurons in MAP1B-deficient mice exhibited defects in axonal branching and growth cone motility, also apparently due to a disruption of normal cytoskeletal dynamics (Bouquet et al., 2004). Our results show that mutations in futscholk affect the ability of neurons to execute normal aspects of transport and motility required to maintain them through adult life.
Alterations in the microtubule network were detected throughout the brains of futscholk mutants, concordant with the widespread expression of Futsch in most neurons. Neurodegeneration, however, was restricted to the olfactory system. The specificity of this degenerative phenotype could be due in part to the relatively long axons and extensive termination fields of the PNs, which might render them more sensitive to partial reductions in Futsch function. Similar observations of cell-specific sensitivities have been made in a number of human diseases. For example Huntingtin protein (Tobin and Signer, 2000), several spinocerebellar ataxia proteins (Ross, 2002), and the Amyloid Precursor Protein (Selkoe, 2001) are expressed in many neurons, but only specific subsets of cells are affected and undergo cell death in the associated diseases.
Alternatively, cell type-specific expression of different MAPs might account for the sensitivities of specific brain regions to defects in a particular MAP. In vertebrates, several MAPs have been shown to have overlapping functions: MAP2 can substitute MAP1B in dendrites, whereas tau may substitute for MAP1B in axons (DiTella et al., 1996; Takei et al., 2000; Teng et al., 2001). A similar redundancy in Drosophila MAPs might preclude more widespread neurodegeneration in the futscholk mutants. Notably, suppression of the futscholk phenotype by ectopic expression of tau showed that another MAP can partially substitute for Futsch. Two other MAPs have also been described in Drosophila (Pereira et al., 1992, Kellogg et al., 1995) but their expression patterns in the adult brain are not yet known. Thus, the potential role of functional compensation by endogenously expressed MAP proteins in Drosophila merits further investigation.
Futsch is negatively regulated by the Drosophila Fragile X mental retardation (dfmr) gene. The neurodegeneration and learning deficiencies seen in futscholk mutants are caused by a decrease in Futsch function, and a deletion in dfmr1 partially suppressed this phenotype. We hypothesize that this effect is due to a reduction in dfmr-mediated inhibition of Futsch expression, resulting in a net increase in Futsch activity. Disease-associated mutations in the human FraX gene also result in transcriptional silencing and loss of FraX function (Jin and Warren, 2003). In combination with our results, these observations suggest that the functional relationship between MAP1B and FraX-related proteins has been maintained across phylogenetic lines, whereby the function of FraX proteins is fundamentally linked to the regulation of MAP1B/Futsch expression.
Altered MAP protein expression and disruptions in the cytoskeleton are associated with a number of neurodegenerative diseases, but whether defective MAPs actually cause neurodegenerative phenotypes is still in dispute (Brandt, 2001). As already noted, the overlapping or complementary functions of different vertebrate MAPs has hindered an analysis of their importance to neuronal viability in the adult brain. In this regard, the relative simplicity of cytoskeletal-associated proteins in Drosophila provides a means of decyphering their authentic roles in the nervous system. In this article, we have shown that Futsch, the MAP1B orthologue of Drosophila, plays an essential role in the maintenance of the adult nervous system and that defects in this protein result in progressive behavioral and neurodegenerative defects as well as a reduced survival rate (see Supplementary Figure 5). Thus, the futscholk mutants reveal that also other MAPs besides tau can be involved in neurodegeneration. Although MAP1B has yet to be implicated in a human neurodegenerative disease, mutations in gigaxonin, a binding partner of MAP1B, result in axonal degeneration and cell death in humans (Ding et al., 2002). Besides providing new insight into the endogenous function of MAP-related proteins in vivo, these results demonstrate the potential value of a model systems approach for investigating the molecular mechanisms underlying neurodegenerative disorders in more complex organisms, including humans.
Acknowledgments
Special thanks are due to Jennifer Holliday, Jennifer Friedman, and Laura Reese for technical assistance and to Stefanie Käch-Petrie for many helpful discussions and her vital support using the imaging techniques. We are also grateful to Burkhard Poeck and Philip Copenhaver for critical reading of the manuscript. The work was supported by the Alzheimer Forschung Initiative e. V. (01801), the Medical Research Foundation of Oregon and the National Institutes of Health (R01 NS047663–01).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-11-1004) on March 16, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Blest, A. D. (1961). Some modifications of Holme's silver method for insect central nervous systems. Q. J. Microsc. Sci. 102, 413. [Google Scholar]
- Bouquet, C., Soares, S., von Boxberg, Y., Ravaille-Veron, M., Propst, F., and Nothias, F. (2004). Microtubule-associated protein 1B controls directionality of growth cone migration and axonal branching in regeneration of adult dorsal root ganglia neurons. J. Neurosci. 24, 7204-7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, R. (2001). Cytoskeletal mechanisms of neuronal degeneration. Cell Tissue Res. 305, 255-265. [DOI] [PubMed] [Google Scholar]
- Buchner, S., Buchner, E., and Hofbauer, A. (1989). Immunohistochemistry of the brain. In: Drosophila: A Laboratory Manual. ed. M. Ashburner, Cold Spring Harbor, NY: Cold Spring Harbor Press, 271-273.
- Ding, J., Liu, J. J., Kowal, A. S., Nardine, T., Bhattacharya, P., Lee, A., and Yang, Y. (2002). Microtubule-associated protein 1B: a neuronal binding partner for gigaxonin. J. Cell Biol. 158, 427-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiTella, M. C., Feiguin, F., Carri, N., Kosik, K. S., and Caceres, A. (1996). MAP-1B/TAU functional redundancy during laminin-enhanced axonal growth. J. Cell Sci. 109(Pt 2), 467–477. [DOI] [PubMed] [Google Scholar]
- Doerflinger, H., Benton, R., Shulman, J. M., and St Johnston, D. (2003). The role of PAR-1 in regulating the polarised microtubule cytoskeleton in the Drosophila follicular epithelium. Development 130, 3965-3975. [DOI] [PubMed] [Google Scholar]
- Edelmann, W., Zervas, M., Costello, P., Roback, L., Fischer, I., Hammarback, J. A., Cowan, N., Davies, P., Wainer, B., and Kucherlapati, R. (1996). Neuronal abnormalities in microtubule-associated protein 1B mutant mice. Proc. Natl. Acad. Sci. USA 93, 1270-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Billault, C., Demandt, E., Wandosell, F., Torres, M., Bonaldo, P., Stoykova, A., Chowdhury, K., Gruss, P., Avila, J., and Sanchez, M. P. (2000). Perinatal lethality of microtubule-associated protein 1B-deficient mice expressing alternative isoforms of the protein at low levels. Mol. Cell Neurosci. 16, 408-421. [DOI] [PubMed] [Google Scholar]
- Gordon-Weeks, P. R., and Fischer, I. (2000). MAP1B expression and microtubule stability in growing and regenerating axons. Microsc. Res. Tech. 48, 63-74. [DOI] [PubMed] [Google Scholar]
- Guo, A., Li, L., Xia, S. Z., Feng, C. H., Wolf, R., and Heisenberg, M. (1996). Conditioned visual flight orientation in Drosophila: dependence on age, practice, and diet. Learn Mem. 3, 49-59. [DOI] [PubMed] [Google Scholar]
- Harada, A., Oguchi, K., Okabe, S., Kuno, J., Terada, S., Ohshima, T., Sato-Yoshitake, R., Takei, Y., Noda, T., and Hirokawa, N. (1994). Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature 369, 488-491. [DOI] [PubMed] [Google Scholar]
- Hawkes, C. (2003). Olfaction in neurodegenerative disorder. Mov. Disord. 18, 364-372. [DOI] [PubMed] [Google Scholar]
- Heidary, G., and Fortini, M. E. (2001). Identification and characterization of the Drosophila tau homolog. Mech. Dev. 108, 171-178. [DOI] [PubMed] [Google Scholar]
- Heisenberg, M. (1989). Silverstaining the central nervous system. In: Drosophila: A Laboratory Manual, ed. M. Ashburner, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 277-278.
- Heisenberg, M. (2003). Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4, 266-275. [DOI] [PubMed] [Google Scholar]
- Heutink, P. (2000). Untangling tau-related dementia. Hum. Mol. Genet. 9, 979-986. [DOI] [PubMed] [Google Scholar]
- Hummel, T., Krukkert, K., Roos, J., Davis, G., and Klambt, C. (2000). Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron 26, 357-370. [DOI] [PubMed] [Google Scholar]
- Hutton, M. et al. (1998). Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702-705. [DOI] [PubMed] [Google Scholar]
- Hutton, M., Lewis, J., Dickson, D., Yen, S. H., and McGowan, E. (2001). Analysis of tauopathies with transgenic mice. Trends Mol. Med. 7, 467-470. [DOI] [PubMed] [Google Scholar]
- Jackson, G. R., Wiedau-Pazos, M., Sang, T. K., Wagle, N., Brown, C. A., Massachi, S., and Geschwind, D. H. (2002). Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron 34, 509-519. [DOI] [PubMed] [Google Scholar]
- Jäger, R. J., and Fischbach, K. F. (1989). Mass histology of adult heads. In: Drosophila: A Laboratory Manual, ed. M. Ashburner, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 254-259.
- Jefferis, G. S., Marin, E. C., Watts, R. J., and Luo, L. (2002). Development of neuronal connectivity in Drosophila antennal lobes and mushroom bodies. Curr. Opin. Neurobiol. 12, 80-86. [DOI] [PubMed] [Google Scholar]
- Jin, P., and Warren, S. T. (2003). New insights into fragile X syndrome: from molecules to neurobehaviors. Trends Biochem. Sci. 28, 152-158. [DOI] [PubMed] [Google Scholar]
- Johnson, G. V., and Bailey, C. D. (2002). Tau, where are we now? J. Alzheimers Dis. 4, 375-398. [DOI] [PubMed] [Google Scholar]
- Kellogg, D. R., Oegema, K., Raff, J., Schneider, K., and Alberts, B. M. (1995). CP60, a microtubule-associated protein that is localized to the centrosome in a cell cycle-specific manner. Mol. Biol. Cell 6, 1673-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs, T., Cairns, N. J., and Lantos, P. L. (1999). beta-amyloid deposition and neurofibrillary tangle formation in the olfactory bulb in ageing and Alzheimer's disease. Neuropathol. Appl. Neurobiol. 25, 481-491. [DOI] [PubMed] [Google Scholar]
- Kovacs, T., Cairns, N. J., and Lantos, P. L. (2001). Olfactory centres in Alzheimer's disease: olfactory bulb is involved in early Braak's stages. Neuroreport 12, 285-288. [DOI] [PubMed] [Google Scholar]
- Kraft, R., Levine, R. B., and Restifo, L. L. (1998). The steroid hormone 20-hydroxyecdysone enhances neurite growth of Drosophila mushroom body neurons isolated during metamorphosis. J. Neurosci. 18, 8886-8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar, D., Hasan, G., Sharma, S., Heisenberg, M., and Benzer, S. (1997). The swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J. Neurosci. 17, 7425-7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, V. M., Goedert, M., and Trojanowski, J. Q. (2001). Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24, 1121-1159. [DOI] [PubMed] [Google Scholar]
- Marin, E. C., Jefferis, G. S., Komiyama, T., Zhu, H., and Luo, L. (2002). Representation of the glomerular olfactory map in the Drosophila brain. Cell 109, 243-255. [DOI] [PubMed] [Google Scholar]
- Matus, A. (1991). Microtubule-associated proteins and neuronal morphogenesis. J. Cell Sci. 15(Suppl), 61-67. [DOI] [PubMed] [Google Scholar]
- McMurray, C. T. (2000). Neurodegeneration: diseases of the cytoskeleton? Cell Death Differ. 7, 861-865. [DOI] [PubMed] [Google Scholar]
- Morales, J., Hiesinger, P. R., Schroeder, A. J., Kume, K., Verstreken, P., Jackson, F. R., Nelson, D. L., and Hassan, B. A. (2002). Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron 34, 961-972. [DOI] [PubMed] [Google Scholar]
- Pereira, A., Doshen, J., Tanaka, E., and Goldstein, L. S. (1992). Genetic analysis of a Drosophila microtubule-associated protein. J. Cell Biol. 116, 377-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorkaj, P., Bird, T. D., Wijsman, E., Nemens, E., Garruto, R. M., Anderson, L., Andreadis, A., Wiederholt, W. C., Raskind, M., and Schellenberg, G. D. (1998). Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 43, 815-825. [DOI] [PubMed] [Google Scholar]
- Reynolds, E. S. (1963). The use of lead citrate at high pH as an electron-opaque stain in electron microskopy. J. Cell Biol. 17, 208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos, J., Hummel, T., Ng, N., Klambt, C., and Davis, G. W. (2000). Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron 26, 371-382. [DOI] [PubMed] [Google Scholar]
- Ross, C. A. (2002). Polyglutamine pathogenesis: emergence of unifying mechanisms for Huntington's disease and related disorders. Neuron 35, 819-822. [DOI] [PubMed] [Google Scholar]
- Schenck, A., Van de Bor, V., Bardoni, B., and Giangrande, A. (2002). Novel features of dFMR1, the Drosophila orthologue of the fragile X mental retardation protein. Neurobiol. Dis. 11, 53-63. [DOI] [PubMed] [Google Scholar]
- Schoenfeld, T. A., and Obar, R. A. (1994). Diverse distribution and function of fibrous microtubule-associated proteins in the nervous system. Int. Rev. Cytol. 151, 67-137. [DOI] [PubMed] [Google Scholar]
- Selkoe, D. J. (2001). Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 81, 741-766. [DOI] [PubMed] [Google Scholar]
- Spillantini, M. G., Murrell, J. R., Goedert, M., Farlow, M. R., Klug, A., and Ghetti, B. (1998). Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc. Natl. Acad. Sci. USA 95, 7737-7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker, R. F. (2001). Drosophila as a focus in olfactory research: mapping of olfactory sensilla by fine structure, odor specificity, odorant receptor expression, and central connectivity. Microsc. Res. Tech. 55, 284-296. [DOI] [PubMed] [Google Scholar]
- Stocker, R. F., Heimbeck, G., Gendre, N., and de Belle, J. S. (1997). Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J. Neurobiol. 32, 443-456. [DOI] [PubMed] [Google Scholar]
- Takei, Y., Kondo, S., Harada, A., Inomata, S., Noda, T., and Hirokawa, N. (1997). Delayed development of nervous system in mice homozygous for disrupted microtubule-associated protein 1B (MAP1B) gene. J. Cell Biol. 137, 1615-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei, Y., Teng, J., Harada, A., and Hirokawa, N. (2000). Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J. Cell Biol. 150, 989-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, J., Takei, Y., Harada, A., Nakata, T., Chen, J., and Hirokawa, N. (2001). Synergistic effects of MAP2 and MAP1B knockout in neuronal migration, dendritic outgrowth, and microtubule organization. J. Cell Biol. 155, 65-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin, A. J., and Signer, E. R. (2000). Huntington's disease: the challenge for cell biologists. Trends Cell Biol. 10, 531-536. [DOI] [PubMed] [Google Scholar]
- Tully, T., and Quinn, W. G. (1985). Classical conditioning and retention in normal and mutant Drosophila melanogaster. J. Comp. Physiol. [A] 157, 263-277. [DOI] [PubMed] [Google Scholar]
- Viereck, C., Tucker, R. P., and Matus, A. (1989). The adult rat olfactory system expresses microtubule-associated proteins found in the developing brain. J. Neurosci. 9, 3547-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, L., Dockendorff, T. C., Jongens, T. A., and Dreyfuss, G. (2000). Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol. Cell. Biol. 20, 8536-8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, D. W., Tyrer, M., and Shepherd, D. (2000). Tau and tau reporters disrupt central projections of sensory neurons in Drosophila. J. Comp. Neurol. 428, 630-640. [DOI] [PubMed] [Google Scholar]
- Wittmann, C. W., Wszolek, M. F., Shulman, J. M., Salvaterra, P. M., Lewis, J., Hutton, M., and Feany, M. B. (2001). Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293, 711-714. [DOI] [PubMed] [Google Scholar]
- Wong, A. M., Wang, J. W., and Axel, R. (2002). Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell 109, 229-241. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. Q., Bailey, A. M., Matthies, H. J., Renden, R. B., Smith, M. A., Speese, S. D., Rubin, G. M., and Broadie, K. (2001). Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell 107, 591-603. [DOI] [PubMed] [Google Scholar]