Abstract
The Ras family GTPase, R-Ras, elicits important integrin-dependent cellular behaviors such as adhesion, spreading and migration. While oncogenic Ras GTPases and R-Ras share extensive sequence homology, R-Ras induces a distinct set of cellular behaviors. To explore the structural basis for these differences, we asked whether the unique N-terminal 26 amino acid extension of R-Ras was responsible for R-Ras–specific signaling events. Using a 32D mouse myeloid cell line, we show that full-length R-Ras activates Rac and induces Rac-dependent cell spreading. In contrast, truncated R-Ras lacking its first 26 amino acids fails to activate Rac, resulting in reduced cell spreading. Truncated R-Ras also stimulates more β3 integrin-dependent cell migration than full-length R-Ras, suggesting that the N-terminus may negatively regulate cell movement. However, neither the subcellular localization of R-Ras nor its effects on cell adhesion are affected by the presence or absence of the N-terminus. These results indicate that the N-terminus of R-Ras positively regulates specific R-Ras functions such as Rac activation and cell spreading but negatively regulates R-Ras–mediated cell migration.
INTRODUCTION
The Ras family of small GTPases is essential for many highly complex cellular processes such as proliferation, differentiation, survival, and migration (Katz and McCormick, 1997). Unifying structural similarities among Ras family members include a highly conserved guanine nucleotide binding sequence and two “switch” regions that change conformation upon GDP release and subsequent GTP binding, a core effector loop that contacts target proteins involved in downstream signaling and one or more acylation sites at the C-terminus that facilitate membrane localization. Cycling between the inactive GDP-bound and the active GTP-bound conformations allows for precise regulation of G protein signaling.
R-Ras defines a Ras subfamily that also includes TC21/R-Ras2 and M-Ras/R-Ras3 (Reuther and Der, 2000). These three GTPases have highly divergent C-terminal sequences that distinguish them from other members of the Ras family, yet all three are highly homologous (∼50% identity) to the proto-oncogene, H-Ras, at key amino acid residues. For example, point mutations at amino acid positions 12 or 61 that activate H-Ras by lowering its intrinsic GTPase activity, also activate R-Ras at its corresponding residues (Saez et al., 1994). In addition, H-Ras and R-Ras share an identical 9 amino acid core effector domain. Consequently, H-Ras and R-Ras bind many of the same signaling proteins in vitro, such as phosphatidylinositol 3-kinase α (p110α PI3K; Marte et al., 1997), p101γ PI3K (Suire et al., 2002), c-Raf (Rey et al., 1994; Spaargaren et al., 1994), Nore1 (Vavvas et al., 1998; Oertli et al., 2000), the guanine nucleotide exchange factor (GEF) Ras-GRF (Gotoh et al., 1997), and the GTPase activating protein, Ras-GAP (Rey et al., 1994). Furthermore, R-Ras shares some common functions with oncogenic GTPases, including stimulation of cell proliferation (Yu and Feig, 2002) and transformation (Cox et al., 1994; Saez et al., 1994). Despite sharing common binding partners in vitro, however, R-Ras does not mimic all known effects of oncogenic Ras (Rey et al., 1994; Huff et al., 1997). For example, R-Ras does not inhibit integrin affinity modulation (Sethi et al., 1999), activate the mitogen-activated protein kinase (MAPK; Osada et al., 1999; Self et al., 2001), or become activated by mSOS, a ubiquitous GEF for Ras (Gotoh et al., 1997).
The first clue to the unique cellular functions of R-Ras came from the finding that expression of an activated mutant of R-Ras made cells highly adherent in an integrin-dependent manner (Zhang et al., 1996). These authors also showed that R-Ras increased the affinity of the prototype platelet integrin, αIIbβ3, for soluble ligand, suggesting that R-Ras regulates integrins through an “inside-out” signaling mechanism. R-Ras has also been implicated in cell spreading (Berrier et al., 2000), cell survival (Suzuki et al., 1997; Osada et al., 1999) and haptotactic migration (Keely et al., 1999; Suzuki et al., 2000).
Previous structural studies of R-Ras have focused on its core effector domain (Osada et al., 1999; Oertli et al., 2000) and C-terminal sequences (Wang et al., 2000; Hansen et al., 2002). Mutation of the effector domain diminishes R-Ras functions, but does not reveal how R-Ras achieves specificity in vivo (Osada et al., 1999; Oertli et al., 2000). In a recent study of H-Ras/R-Ras chimeras, the C-terminal 26 amino acid residues of R-Ras in the context of an H-Ras chimera blocked H-Ras effects on integrins (Hansen et al., 2002). These data are consistent with the idea that diverse C-terminal sequences within the hyper-variable region of Ras GTPases contribute to signaling specificity.
An unexplored region of great sequence diversity among Ras family GTPases is the N-terminus. R-Ras contains a unique 26 amino acid sequence at its N-terminus, whereas TC21 and M-Ras contain unique 12 and 13 amino acid sequences respectively. In addition to its novel sequence, the N-terminus of R-Ras is highly glycine- and proline-rich and contains a noncanonical class I SH3 binding motif. To address the importance of the N-terminus of R-Ras with respect to integrin-dependent cellular functions, we deleted the first 26 amino acids of activated R-Ras and asked how this deletion affected the ability of R-Ras to stimulate integrin-dependent events such as cell adhesion, spreading and migration.
MATERIALS AND METHODS
Cells, Media, and Reagents
Mouse 32D myeloid progenitor cells and WEHI helper cells were a generous gift of Dr. A. S. Baldwin, University of North Carolina (Reuther et al., 1998). Cells were cultured in suspension in 10-cm dishes in 32D media (RPMI 1640 containing 10% heat-inactivated fetal bovine serum [FBS] and 10% WEHI conditioned media). K562 cells, a generous gift of Dr. S. D. Blystone, were cultured in Iscove's media with 9% heat-inactivated FBS.
Purified, human vitronectin (Vn) from Chemicon (Temecula, CA) was used for cell adhesion and microscopy experiments. Purified human fibrinogen (Fg) was from Enzyme Research Laboratories (South Bend, IN). Dimer-specific anti-integrin αIIbβ3 antibody 1B5 was a generous gift from Dr. Susan Smyth, UNC-Chapel Hill (Smyth et al., 2000). Anti-integrin blocking antibodies to αv (clone RMV-7), β1 (clone Ha2/5), β3 (clone 2C9.G2), or control antibodies (clone G235–1 for β1; clone A19–3 for β3) were from BD Biosciences PharMingen (San Diego, CA). Anti-PP2A antibody was from BD Transduction Laboratories (Lexington, KY). The monoclonal anti-Rac antibody used was clone 23A8 (Upstate Biotechnology, Lake Placid, NY). The phospho-specific and total Akt antibodies were from Cell Signaling Technologies (Beverly, MA). All pharmacological protein inhibitors were from Calbiochem (La Jolla, CA).
Vectors
Myc-tagged R-Ras constructs were generated from existing plasmids kindly provided by Dr. Adrienne Cox (empty pCGN, pCGN Q87L, G38V, and G41A R-Ras vectors; University of North Carolina) and Dr. Alan Hall (S43N and WT alleles; University College, London). PCR products were generated using r-ras–specific oligonucleotides with BamHI (5′) and EcoRI (3′) sites, digested, and ligated into the mammalian expression vector pCMV-3b (Stratagene, La Jolla, CA). GFP-tagged R-Ras constructs were made by PCR using the pCMV R-Ras plasmids described above as templates to generate EcoRI-BamHI fragments, which were cloned into the pEGFP-C2 vector (Clontech, Palo Alto, CA). GFP-R-Ras proteins were functional because they stimulated cell adhesion to immobilized ligands. GFP-dynamin was a gift from Dr. JoAnn Trejo (University of North Carolina). Myc-tagged dominant-negative Rac and Cdc42 (both S17N) as well as activated Rac and Cdc42 (both Q61L) plasmids were a generous gift from Dr. Keith Burridge (University of North Carolina). The pGL-3 luciferase plasmid was obtained from Promega (Madison, WI). This plasmid continually transcribes the luc gene, resulting in constitutive luciferase production.
Deletion PCR was used to remove the first 26 codons of R-Ras. The template was pCMVR-Ras Q87L, which contains an activating point mutation at codon 87. The forward oligonucleotide sequence begins with an XbaI restriction endonuclease site 5′ of sequences in the r-ras open reading frame starting at codon 26: GCCGTCTAGAAGCGAGACACACAAGCTGGTG. The reverse oligonucleotide also begins with an XbaI site but anneals to the noncoding strand of the myc sequence in pCMV: GCCGTCTAGACAGATCCTCTTCAGAGATGAG. A linear 5-kb fragment was generated by PCR using Pfu polymerase (Stratagene) that was digested with XbaI. The digested fragment was ligated to itself to generate pCMV NΔ87L R-Ras. To confirm the absence of PCR-induced mutations, all pCMV constructs were sequenced with T3 and T7 oligonucleotides. All plasmids to be transfected into cells were purified using a low endotoxin protocol (Qiagen, Chatsworth, CA).
Electroporation
We found that transient transfection was necessary to preserve R-Ras phenotypes, because stable transfection methods resulted in decreased R-Ras expression over time (unpublished data). Cells (1–2 × 107) were added to a sterile 0.4-cm cuvette, incubated with plasmids, and electroporated using a Gene Pulser II apparatus at 260 V and 960 μF capacitance. Cells were immediately returned to 10 ml of warm media containing 20% serum and allowed to recover at 37°C for at least 16 h. All equipment was from BioRad (Richmond, CA).
Immunoblotting
Mouse 32D cells were electroporated with 5 μg plasmid DNA as above, harvested with 3 ml of nonenzymatic cell dissociation solution (CDS, Specialty Media, Phillipsburg, NJ), washed once, and diluted to 3–4 × 106 cells/ml. Approximately 2 × 106 cells were then lysed in fresh RIPA buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, 2 mM NaF, and protease inhibitor cocktail) for 15 min on ice. The lysate was clarified by centrifugation and an equal number of cell equivalents was loaded for SDS-PAGE. Proteins were transferred to PVDF membranes by a semidry transfer method (Trans-Blot from BioRad). Blots were incubated with primary antibody for 1 h at a concentration of 1 μg/ml for anti-myc antibody (clone 9E10; Covance, Madison, WI), 0.4 μg/ml for anti-R-Ras (C-19; Santa Cruz Biotechnology, Santa Cruz, CA) and 0.25 μg/ml for anti-PP2A (protein phosphatase 2A; Transduction Laboratories). PP2A was used as a loading control since levels were not affected by expression of R-Ras. Bands were visualized with ECL (Amersham, Piscataway, NJ) or Western Lightning (Perkin Elmer-Cetus, Norwalk, CT). Horseradish peroxidase secondary antibodies were from Amersham.
NIH 3T3 cells were transfected with 10 μg DNA using Lipofectamine 2000 as per manufacturer's instructions. Lysates were obtained and processed as described above.
Indirect Immunofluorescence and Confocal Microscopy
For 32D cell microscopy, cells were electroporated with 5 μg of pCMV- or pCGN-based plasmids as indicated in the figure legends, washed once, and 8–10 × 106 cells were loaded onto coverslips coated with the indicated concentrations of extracellular matrix protein. Adhesion proceeded for 30–60 min at 37°C. Cells were fixed for 30 min at room temperature (RT) with 4% paraformaldehyde followed by permeabilization in 0.2% Triton X-100 in phosphate-buffered saline (PBS). The 9E10 anti-myc (1 μg/ml), anti-HA antibody (1 μg/ml; clone 12CA5; Boehringer Mannheim, Indianapolis, IN) or polyclonal anti-c-myc (0.5 μg/ml; Santa Cruz) primary antibodies were incubated with cells in 3% bovine serum albumin (BSA) for 1 h at RT. After 3–4 washes with PBS, goat anti-mouse IgG-Alexa 488 at 1 μg/ml was added simultaneously with phalloidin-Alexa 568 at 0.1 U/ml (1:2000) for 30 min at RT in the dark. Both secondary antibody and phalloidin were from Molecular Probes (Eugene, OR). When c-myc was detected in the absence of phalloidin, a goat anti-rabbit IgG-Alexa 568 secondary was used. Coverslips were inverted onto a drop of FluorSave antibleaching reagent (Calbiochem) and viewed after storage overnight at 4°C on an Olympus IX70 inverted confocal microscope (Lake Success, NY). Confocal images were captured using a 60× PlanApo lens and Fluoview software. K562 cells were processed as above except that cells adhered to 1 μg/ml fibronectin for 2 h and were electroporated with 15 μg of plasmid DNA. Epifluorescence cell morphology images were taken with a Roper HQ cooled CCD camera (Roper Scientific, Tucson, AZ) mounted on a Nikon TE300 inverted microscope (Melville, NY).
For scoring of filopodia-like structures, cells were fixed and stained as above and >100 R-Ras–expressing cells per transfection condition for each experiment from three independent experiments were scored. Scored cells had round cell bodies and 2–4 protrusions (indicated in the figure legends) that were approximately a cell length or longer.
For scoring Rac-like morphology, 5 μg of pCMV Rac Q61L (CA-Rac) or 2 μg of either pCGN NΔ87L R-Ras or pCGN Q87L R-Ras were transfected with 10 μg pCMV Rac S17N (DN-Rac), as indicated in Figure 8. Total plasmid mass for each transfection was 15 μg.
Figure 8.
Full-length R-Ras produces a Rac-like, spread morphology but truncated R-Ras fails to induce maximal spreading. (A) Cells were transfected as indicated and treated as in Figure 7, except that cells were plated on 0.5 μg/ml vitronectin (CA, constitutively active). An anti-HA antibody, 12CA5, was used to detect R-Ras and a polyclonal c-myc antibody was used to detect myc-tagged Rac. (B) Rac-like phenotypes of cells shown in A were scored by microscopically counting the percentage of randomly chosen transfected cells having the Rac phenotype defined as a round, cell area greater than 400 μm2. Phase micrographs were used for control cells not expressing epitope-tagged proteins. Each bar represents 190 cells for CA-Rac, 271 cells for pCGN, 307 cells for NΔ87L, 275 cells for NΔ87L+DN-Rac, 384 cells for Q87L, 374 cells for Q87L+DN-Rac, and 364 cells for DN-Rac alone. (C) The total cell area of empty vector control cells (n = 31), NΔ87L R-Ras–expressing cells (n = 25, *p < 0.00003 vs. Q87L), Q87L R-Ras–expressing cells (n = 15), and Q87L R-Ras + DN-Rac coexpressing cells (n = 8) was examined using ImagePro software.
Cell Adhesion Assay
The indicated concentrations of ECM protein were coated onto 96-well plates (100 μl) at 4°C overnight or for 3 h at 37°C. Plates were then blocked for 1 h in 1% BSA at RT and washed once before cell addition. Luciferase plasmid pGL-3 (4 μg) was present with all R-Ras or control pCMV plasmids (2 μg plasmid for antibody treatments or 5 μg for all other experiments) during electroporation. Approximately 17 h after electroporation, 32D cells were harvested with CDS as in Immunoblotting above, washed three times in RPMI (no phenol red)/1% BSA to remove serum and resuspended in 3 ml of the same media for adhesion to vitronectin. For adhesion to fibrinogen, cells were resuspended in adhesion buffer (20 mM HEPES, pH 7.4, 137 mM NaCl, 2.5 mM KCl, 5.5 mM glucose, 2 mM each CaCl2 and MgCl2 and 0.1% BSA). Cells were diluted to a density of 5 × 105 cells/ml. Antibodies or inhibitors were then added to cells as indicated in the figure legends. Cells (5 × 104/well) were loaded into plates for 40 min at 37°C in two groups, one for adhesion and one for measuring total luciferase activity. Wells containing cells designated for adhesion were washed three times with RPMI/1% BSA to remove nonadherent cells and the remaining cells were quantitated using a luciferase assay (Luc-Lite Plus; Packard Instruments, Meriden, CT). All cells expressing luciferase were detected with a TopCount NXT luminometer (Packard Instruments). The number of cells adhering to BSA was defined as nonspecific and was subtracted from the number of cells adhering to vitronectin or fibrinogen. Adhesion was expressed as the percentage of adherent cells per total cells loaded into the assay. Luciferase activity was linear beyond the total number of cells used in the assay. To further validate these assay conditions, we constructed a nonprenylated, activated R-Ras mutant that did not localize to the plasma membrane, because membrane localization is required for full biological function (Oertli et al., 2000). Compared with membrane-localized, activated R-Ras, the cytosolic R-Ras mutant did not stimulate adhesion to fibronectin above control levels (unpublished data).
Migration Assay
A 96-well migration plate (Neuroprobe, Gaithersburg, MD) containing a filter with 3-μm-diameter pores was coated with 1 μg/ml vitronectin on the underside of the filter and incubated at 4°C for 7 h. The coating solutions were then removed and the bottom chambers including the underside of the filters were washed once with 30 μl of RPMI containing 0.1% BSA. Prewarmed 32D medium (30 μl) was added to the bottom wells to establish a serum gradient for cells to be added on top of the filter.
After electroporation of 5 μg pCMV plasmid plus 4 μg pGL-3 luciferase plasmid, 32D cells were allowed to recover in 10-cm dishes for 7 h at 37°C. Cells were harvested using 3 ml of prewarmed CDS as in Immunoblotting and collected by mild centrifugation. Cells were washed in RPMI (no phenol red)/0.1% BSA three times to remove remnants of serum and counted on a hemocytometer. Cell number was adjusted to 2 × 106 cells/ml and 9–10 × 104 cells in 50 μl were loaded on top of each filter in triplicate. For inhibitor studies, 1 × 106 cells were treated with 0.37% dimethyl sulfoxide (DMSO) or 25 μM LY294002 for 30 min at 37°C and then loaded as above. Cells migrated for 15–18 h at 37°C. After migration, cells remaining on top of the filter were removed by aspiration. EDTA (3 mM, in 50 μl) was added to the top of the filter to loosen cells attached to the underside (30 min at 4°C). The top of the filter was washed once with 50 μl of RPMI/0.1% BSA, and cells were transferred to a new 96-well plate for detection of luciferase activity as described in Cell Adhesion Assay. An aliquot of total cells was treated with 30 μl serum to mimic migration conditions and to control for any proliferation effects during the course of the assay. Migration was expressed as the percentage of migrated cells per total cells loaded into the assay (1 × 105).
Flow Cytometry
For detection of αIIbβ3 integrin, 32D cells were electroporated with 5 μg of pCGN plasmids, washed three times in cold PBS/1% BSA, resuspended in 2 ml of the same buffer, and incubated with 3 μg/ml either hamster IgG control, anti-β3 integrin (clone 2C9.G2; PharMingen, San Diego, CA) or anti-αIIbβ3 antibody 1B5 for 30 min on ice. Cells were washed once and incubated with 10 μg/ml FITC-conjugated goat anti-mouse immunoglobulin (Ig) F(ab′)2 (Biosource International, Camarillo, CA) for 30 min on ice in the dark. This secondary antibody cross-reacts with hamster Ig isotypes. Cells were washed again and resuspended in 500 μl, and fluorescence was detected by a FACScan flow cytometer (Becton-Dickinson, Mountain View, CA).
For fibrinogen binding, 32D cells were electroporated with 6 μg of the indicated GFP plasmids. After 18–19 h, cells were washed three times with RPMI/0.1% bovine serum albumin, left untreated, or pretreated with 500 μM MnCl2, which was used to activate integrins. All cells were incubated with Alexa 546–conjugated fibrinogen (Molecular Probes) at 90–100 μg/ml for 30 min at RT in the dark. Cells were washed once, resuspended in 500 μl of the above media, and analyzed with a FACScan as above.
Rac Activation Assay
Mouse 32D cells (1–5 × 107) were electroporated with 5 μg of empty pCGN vector, pCGN NΔ87L R-Ras or pCGN Q87L R-Ras and allowed to recover for 19 h. Cells were washed twice with RPMI/0.1% BSA, washed twice more with RPMI alone, and plated onto 60-mm dishes precoated with 10 μg/ml vitronectin for 1 h at 37°C. For PI3K inhibition, 25 μM LY294002 or 0.1% DMSO was added to cells for 15 min at 37°C before plating. Unbound cells were removed by aspiration and the remaining adherent cells were lysed in lysis buffer (25 mM HEPES, pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% deoxycholate, 10% glycerol, 10 mM MgCl2, 50 μM Na3VO4, and protease inhibitor cocktail from CalBiochem) for 25 min on ice. The lysate was clarified and total protein quantitated using the BioRad reagent. Rac activation was determined as previously described (Wennerberg et al., 2002) by the addition of GST-Pak1 PBD (p21 binding domain; gift of Dr. Keith Burridge) to 225–375 μg of total protein for 30 min at 4°C. The beads were washed with 10 volumes of lysis buffer three times, resuspended in 40 μl Laemmli sample buffer, and subjected to SDS-PAGE for immunoblotting. Blots were probed for Rac with the 23A8 monoclonal anti-Rac antibody (1 μg/ml; Upstate) in 5% milk/TBST overnight at 4°C. To detect HA-tagged R-Ras in the whole cell lysate, the blot was stripped and reprobed with 0.25 μg/ml the 12CA5 anti-HA antibody.
Statistics
Data were analyzed with the Student's one-tailed t test using independent comparisons. Error bars represent SEM.
RESULTS
Rationale of Mutant Design
R-Ras promotes distinct biological effects that require integrins. To identify sequences within R-Ras that specifically affect its ability to stimulate integrin-dependent cellular responses, we analyzed the primary sequences of Ras family members for sizable regions of nonhomology to identify candidate domains unique to R-Ras. We targeted the first 26 amino acids of R-Ras since this region represents the largest contiguous R-Ras sequence having no homology to other Ras family members and contains the sequence “PGDP,” a potential SH3 domain-binding motif (Figure 1A). Our strategy was to delete the N-terminal 26 amino acids of constitutively active (Q87L or G38V) R-Ras (Figure 1B). The resulting mutants were N-terminally tagged with myc or hemagglutinin (HA) epitopes to avoid disruption of the C-terminal CAAX box and named NΔ87L R-Ras or NΔ38V R-Ras.
Figure 1.
R-Ras contains a unique N-terminus that does not alter protein expression. (A) Shown is a primary amino acid sequence alignment of Ras family members most closely related to R-Ras. The initiator methionine of R-Ras is labeled as 1. Conserved regions are bolded, including the invariant glutamine at position 87. Amino acids that define a putative SH3-binding domain in the N-terminus of R-Ras are underlined. (B) A schematic representation of the full-length versus the truncation mutant of R-Ras lacking its first 26 amino acids is depicted. (C) Whole-cell lysates were prepared from mouse 32D cells transiently transfected (see Materials and Methods) with empty pCMV vector (lane 1), NΔ87L R-Ras (lane 2), or Q87L R-Ras (lane 3). Myc-tagged R-Ras proteins were detected with the polyclonal anti-R-Ras antibody, C19. Bottom numbers indicate ratios of R-Ras expression normalized to protein phosphatase 2A levels, which served as a loading control. Myc-tagged R-Ras proteins were also detected in 32D and human K562 cell lysates with the 9E10 anti-myc monoclonal antibody (unpublished data).
Expression Level of NΔ87L R-Ras
Because mutations in key residues can alter protein expression, we evaluated NΔ87L R-Ras expression in two different cell lines. Both myc-tagged NΔ87L and Q87L R-Ras were transiently transfected (see Materials and Methods) into 32D mouse myeloid cells and detected with an anti-R-Ras polyclonal antibody in whole cell lysates (Figure 1C). N-terminally truncated NΔ87L R-Ras exhibited an increased electrophoretic mobility compared with full-length Q87L R-Ras. Both NΔ87L R-Ras and Q87L R-Ras levels were equivalent when normalized to levels of the abundant cytosolic protein phosphatase, PP2A. Similar results were obtained with expression of NΔ87L R-Ras and Q87L R-Ras in K562 cells, a human erythroleukemic cell line (unpublished data).
Effect of NΔ87L R-Ras on Cell Adhesion
We and others (Zhang et al., 1996) have observed that overexpressed, activated R-Ras induces robust adhesion of K562 and 32D cell lines to extracellular matrix proteins (ECM). We therefore asked whether deletion of the N-terminus of R-Ras affected adhesion of 32D cells using a luciferase reporter assay, validated as described in Materials and Methods. Deletion of the N-terminus of R-Ras had little effect on 32D cell adhesion to vitronectin (Figure 2A), indicating that the N-terminus of R-Ras is not required for R-Ras-stimulated cell adhesion. As before, NΔ87L R-Ras protein expression was equivalent to that of full-length Q87L R-Ras (Figure 2A, inset). Moreover, adhesion of Q87L R-Ras-transfected cells to vitronectin was β3 integrin-dependent because an anti-β3 but not an anti-β1 integrin antibody dramatically inhibited R-Ras–mediated adhesion (Figure 2B).
Figure 2.
The N-terminus of R-Ras is not critical for integrin-dependent cell adhesion to vitronectin. (A) Mouse 32D cells were transiently cotransfected with empty pCMV vector, pCMV NΔ87L R-Ras, or pCMV Q87L R-Ras plus pGL-3, a luciferase reporter plasmid. Cells (50,000) were loaded into a 96-well plate coated with the indicated concentrations of vitronectin. Cells were incubated for 40 min, washed, and assayed for luciferase activity (see Materials and Methods). Cells not used for adhesion were lysed to determine expression levels of truncated and full-length R-Ras (Figure 2A, inset). (B) 32D cells were cotransfected with pCMV Q87L R-Ras and pGL-3 and pretreated with the indicated integrin blocking antibodies at the indicated concentrations for 15 min at RT. Cells were loaded onto wells coated with 0.5 μg/ml vitronectin in the presence of antibodies and assayed for adhesion as in A. Means of triplicate samples ± SEM from a representative experiment are shown for both A (n >5 experiments) and B (two experiments).
Regulation of αIIbβ3 Integrin on 32D Cells by R-Ras
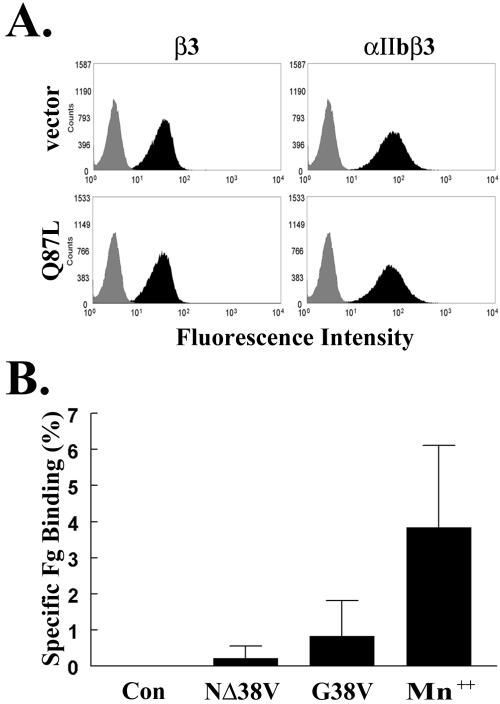
Although R-Ras-induced cell adhesion to vitronectin was sensitive to an anti-β3 antibody (Figure 2B) and the αvβ3 integrin is known to be present on the surface of 32D cells, we addressed the possibility that the lineage-restricted integrin, αIIbβ3 (Parise, 1999), might also be present. Flow cytometry with 1B5, an αIIbβ3 heterodimer-specific antibody (Smyth et al., 2000), indicated that αIIbβ3 was expressed on the surface of nearly 100% of 32D cells. Integrin levels remained unchanged upon expression of activated R-Ras (Figure 3A). The αIIbβ3 integrin was functional, since Q87L R-Ras promoted 32D cell adhesion to immobilized fibrinogen in a 1B5-sensitive manner (unpublished data).
Figure 3.
Integrin αIIbβ3 is present on 32D cells but R-Ras does not increase β3 integrin affinity for ligand. (A) Mouse 32D cells were cotransfected with empty pCGN control vector or pCGN Q87L R-Ras plus pGL-3. Cells were analyzed by flow cytometry using control hamster IgG (gray histograms), the anti-β3 antibody, 2C9.G2 (black histograms) or the anti-αIIbβ3 antibody, 1B5 (black histograms). (B) As a positive control, mouse 32D cells were treated with Mn2+ to activate integrins or transfected with the negative control GFP-tagged dynamin 2 (Con), GFP-NΔ38V R-Ras (NΔ38V) or GFP-G38V R-Ras (G38V). Alexa 546–conjugated fibrinogen binding to GFP-transfected cells was assessed by flow cytometry. Specific binding was determined by subtracting nonspecific fibrinogen binding occurring in the presence of an inhibitory β3 integrin antibody, 2C9.G2, from total fibrinogen binding. Results were expressed as the mean percentage ± SEM of transfected cells specifically binding fibrinogen from four experiments.
Because the mechanism by which R-Ras regulates β3 integrin avidity is unknown, we asked whether R-Ras increased integrin affinity by measuring soluble fibrinogen binding to 32D cells. Cells expressing activated GFP-G38V R-Ras or GFP-NΔ38V R-Ras did not bind increased levels of soluble fibrinogen relative to control cells (Figure 3B, p > 0.4), suggesting that β3 integrins on 32D cells do not undergo “inside-out” affinity modulation in response to activated R-Ras.
Mechanisms of NΔ87L R-Ras–mediated Cell Adhesion
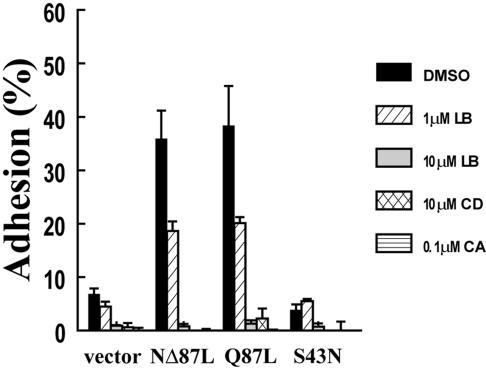
Because R-Ras does not appear to enhance integrin affinity for ligand, we further investigated the mechanism of R-Ras–stimulated cell adhesion by treating R-Ras–overexpressing 32D cells with various pharmacological inhibitors of proteins known to affect integrin-mediated adhesion. NΔ87L R-Ras- and Q87L R-Ras–mediated adhesion to fibronectin or vitronectin was not affected by phosphatidylinositol 3-kinase (PI3K) inhibitors such as wortmannin (0.1–10 μM) or LY294002 (1–20 μM; unpublished data), consistent with previous data showing that PI3K is not an essential downstream R-Ras effector for cell adhesion (Osada et al., 1999; Kinashi et al., 2000). Inhibitors of protein kinase C (bisindolylmaleiamide), Src-family kinases (PP2), tyrosine kinases (genistein), VEGF receptors, calpain (calpeptin), and p70 S6 kinase (rapamycin) also did not affect G38V R-Ras–dependent cell adhesion to fibronectin (unpublished data). However, filamentous actin (F-actin) poisons such as latrunculin B and cytochalasin D, which prevent actin filament elongation, as well as the serine, threonine phosphatase inhibitor calyculin A completely blocked NΔ87L R-Ras– and Q87L R-Ras–dependent cell adhesion to vitronectin (Figure 4). Finally, as a negative control, we observed that dominant-negative R-Ras (S43N) failed to induce adhesion to vitronectin (Figure 4). These results indicate that R-Ras signaling requires an intact actin cytoskeleton and predict that R-Ras influences actin filament formation and/or protein phosphatase activity in a manner that does not require the N-terminus of R-Ras.
Figure 4.
Inhibition of actin polymerization and phosphatase activity block R-Ras–dependent cell adhesion. Mouse 32D cells were cotransfected with empty pCGN control vector, pCGN NΔ87L R-Ras, pCGN Q87L R-Ras, or pCGN S43N R-Ras plus pGL-3 and assayed for cell adhesion as described in Figure 2A. Cells were pretreated with the indicated concentrations of latrunculin B (LB), cytochalasin D (CD), calyculin A (CA), or 0.1% DMSO control for 30 min at 37°C before adhesion to vitronectin. Data represent triplicate means of a representative experiment from four independent trials.
Colocalization of NΔ87L R-Ras with Filamentous Actin at the Plasma Membrane
The requirement of the actin cytoskeleton for R-Ras–mediated adhesion (Figure 4) and the known localization of R-Ras to the plasma membrane via C-terminal lipid modifications (Ohba et al., 2000), suggest that R-Ras may colocalize with F-actin at dynamic plasma membrane regions. Before comparing R-Ras localization relative to F-actin, we first assessed the impact of the 26 amino acid deletion on R-Ras sorting to the plasma membrane. Both Q87L R-Ras and NΔ87L R-Ras targeted to the plasma membrane compared with control cells (Figure 5, e and i). This was confirmed in live cells with GFP-tagged R-Ras (unpublished data), similar to that reported by Ohba et al. (2000). When coexpressed in the same cell, the two proteins colocalized exactly at the plasma membrane in most cells examined, especially in dorsal focal planes (unpublished data), suggesting that truncation of the R-Ras N-terminus does not preclude lipid insertion into the plasma membrane and affect GTPase localization.
Figure 5.
Activated R-Ras colocalizes with actin in filopodia and membrane ruffles. Mouse 32D cells were transfected with pCGN vector alone (one cell shown in a–d), pCGN Q87L R-Ras (one cell shown in e–h), or pCGN NΔ87L R-Ras (one cell shown in i–l). An anti-HA antibody, 12CA5, was used to detect R-Ras (e and i) and Alexa 568-phalloidin was used to detect F-actin (b, f, and j). “Merge” signifies an overlay of two single focal plane images ∼0.15-μm-thick collected by z-series scanning, whereas “smash” refers to an overlay of all slices in the z-series and thus reveals additional data. Images of single focal planes depict dorsal sections of the cells. Arrowheads in panel g and k point to membrane ruffles at the cell periphery. The arrow in panel h indicates an actin “rib” or filopod associated with a lamellipod.
We next determined whether R-Ras was present in regions of the plasma membrane that contained F-actin and found that in most transfected cells, both Q87L R-Ras and NΔ87L R-Ras colocalized with F-actin in ribbon-like membrane ruffles (Figure 5, g–h, and k–l). Ruffles were usually concentrated at the cell periphery in dorsal focal planes, but could also be seen in more central locations above the cell body. Q87L R-Ras also colocalized with F-actin in filopodia in a fraction of polarized cells (Figure 5h). In contrast, neither protein was abundant in actin-rich lamellipodia (Figure 5, h and l). Control cells displayed well-polarized F-actin upon adhesion to vitronectin, but remained generally round in shape (Figure 5, a–d). Both R-Ras mutants, therefore, show very similar localization patterns to actin-rich membrane structures in 32D cells.
Generation of a Protrusive Morphology by NΔ87L R-Ras
Although colocalization of R-Ras with actin was independent of the N-terminus, we observed during the course of these studies that NΔ87L R-Ras generated cell morphologies quite different from Q87L R-Ras–transfected and vector control cells. These morphological differences became more pronounced with higher expression levels of NΔ87L R-Ras. To investigate these morphological differences in more detail, we determined the number of transfected cells having defined morphologies and the molecular mechanisms underlying these differences.
Q87L R-Ras induced a flattened, asymmetric cell morphology (Figure 6A, d–f), with some cells having polarized actin filaments (Figure 6A, e). In contrast, NΔ87L R-Ras stimulated the formation of long, thin extensions (Figure 6A, g–l). These extensions radiated from random positions along a round cell body proximal to the cell-matrix interface and resembled actin microspikes (Kozma et al., 1995). Nearly 60% of all NΔ87L R-Ras–transfected cells displayed two or more extensions (Figure 6B) and 33% displayed four or more extensions, which were often highly branched (Figure 6C). In Q87L R-Ras–expressing cells, extensions were observed at a significantly lower frequency and were less branched (37%, Figure 6B; 14%, Figure 6C).
Figure 6.
The absence of the N-terminus of R-Ras produces a Rac-dependent dendritic morphology. Mouse 32D cells were transfected as in Figure 2A (without pGL-3) and adhered to coverslips. (A) Myc-tagged R-Ras was visualized with the 9E10 monoclonal antibody (mAb) and filamentous actin was stained with Alexa 568-phalloidin. Shown are a representative nonspreading control cell (a–c) and a representative cell expressing Q87L R-Ras (d–f). The bottom six panels depict two cells expressing NΔ87L R-Ras (first cell, g–i; second cell, j–l). The merged image is an overlay of two single focal planes near the ventral surface of the cells. (B) The filopodia-like morphology of the 32D cells having two or more protrusions (A, cells g–l) was scored microscopically by an observer who was unaware of sample identity. Data represent means from three independent experiments (*p = 0.007). (C) The percentage of cells containing four or more protrusions was scored in cells transfected with empty vector (n = 347), NΔ87L R-Ras (n = 959) or Q87L R-Ras (n = 1002), NΔ87L R-Ras plus DN-Rac (n = 442) or DN-Rac alone (n = 255). Data represent means from three independent experiments (*p < 0.03 for NΔ87L vs. Q87L and p < 0.02 for NΔ87L vs. NΔ87L+DN Rac).
Because the small Rho GTPase, Rac, can induce an elongated morphology similar to that described above (Sahai and Marshall, 2003) and because Rac can act downstream of R-Ras during cell spreading (Berrier et al., 2000), we asked whether the NΔ87L R-Ras–induced protrusive phenotype requires Rac. Dominant-negative Rac (DN-Rac) blocked the formation of these protrusive structures in NΔ87L R-Ras–expressing cells by 81%, suggesting that NΔ87L R-Ras requires Rac-GTP to produce an elongated phenotype (Figure 6C).
Effects of NΔ87L R-Ras on Rac Activation
The findings that R-Ras colocalizes with actin structures (Figure 5) and NΔ87L R-Ras induces a Rac-dependent protrusive phenotype (Figure 6) suggest that R-Ras acts locally to regulate Rac activity. However, it is not known whether R-Ras directly activates Rac to facilitate actin remodeling or whether Rac contributes to R-Ras function via a parallel but independent pathway. We therefore performed Rac “pull-down” assays (Wennerberg et al., 2002) to determine whether R-Ras increased Rac activity. Full-length Q87L R-Ras boosted Rac activation in cells plated on vitronectin by nearly threefold compared with control cells (Figure 7, A and B). In contrast, NΔ87L R-Ras had no effect on Rac activation (Figure 7, A–D). These results indicate that the N-terminus of R-Ras is required for Rac activation and that the Rac-dependency of the NΔ87L R-Ras–mediated protrusive phenotype observed above (Figure 6) may reflect a requirement for basal as opposed to stimulated Rac-GTP levels.
Figure 7.
Full-length but not truncated R-Ras stimulates Rac activation in a PI3K-dependent manner in 32D cells. Mouse 32D cells were transfected as in Figure 6, washed four times, and adhered to 60-mm dishes coated with 10 μg/ml vitronectin for 1 h. Adherent cells were lysed and recombinant GST-Pak1 PBD was used to precipitate GTP-bound Rac. R-Ras expression levels were determined in whole-cell lysates by reprobing the same blot using an anti-HA mAb (bottom panel). (A) Lane 1, empty vector control; lane 2, NΔ87L R-Ras; and lane 3, Q87L R-Ras. (B) Relative Rac activation was calculated by dividing Rac-GTP by total Rac present in whole cell lysates (*p < 0.002 for Q87L vs. NΔ87L). Shown is the mean ± SEM of three independent pull-down experiments (control n = 2). (C) Cells were transfected as in A and treated with 0.12% DMSO or 25 μM LY294002 for 30 min at 37°C before plating for 1 h as above. An example of four independent experiments is shown. Lane 1, vector control + DMSO; lane 2, NΔ87L R-Ras + DMSO; lane 3, NΔ87L R-Ras + LY; lane 4, Q87L R-Ras + DMSO; and lane 5, Q87L R-Ras + LY. (D) Inhibition of Rac activation by LY294002 was calculated as in B. Data represent the mean ± SEM of four independent experiments (*p < 0.03 for Q87L vs. Q87L + LY).
Because PI3K generates lipids that stimulate Rac guanine nucleotide exchange factor (GEF) activity and Rac GTP loading (Nimnual et al., 1998; Han et al., 1998), we next asked whether PI3K contributed to R-Ras–stimulated Rac activation (Marte et al., 1997). The PI3K inhibitor, LY294002, inhibited full-length R-Ras–induced Rac-GTP loading by 72% (p < 0.03; Figure 7, C and D), but failed to block the low level of Rac activation present in cells expressing either empty vector or NΔ87L R-Ras, consistent with the inability of NΔ87L R-Ras to activate Rac. These results demonstrate that full-length R-Ras requires PI3K for complete Rac activation.
Effects of NΔ87L R-Ras and Rho GTPases on Cell Spreading
To independently confirm that the N-terminus of R-Ras is important for Rac activation, we compared the morphological phenotypes of 32D cells expressing activated Rac (Q61L) or the closely related Rho GTPase, Cdc42 (Q61L), to full-length Q87L R-Ras or truncated NΔ87L R-Ras. In these experiments, less R-Ras plasmid was transfected to minimize the protrusive phenotype described in Figures 5 and 6 and favor a rounder, more traditional Rac-like phenotype. The 32 cells expressing activated Rac displayed a morphology resembling that of fibroblasts expressing activated Rac (Hall, 1998): large, round, highly spread cells with abundant membrane ruffles (Figure 8A). Similarly, cells expressing full-length Q87L R-Ras showed a spread morphology nearly identical to cells expressing activated Rac (Figure 8A). We defined this Rac-like morphology as round, spread cells having a cell area greater than 400 μm2 and found that 50% of Q87L R-Ras–expressing cells displayed this phenotype (Figure 8B). These cells had a mean cell area of 1077 μm2, which is nearly threefold greater than the mean cell area of 389 μm2 for control cells (Figure 8C).
In contrast, cells expressing truncated NΔ87L R-Ras displayed a spreading defect characterized by fewer large cells and decreased cell area compared with cells expressing full-length R-Ras or Rac (Figure 8A). Only 27% of NΔ87L R-Ras–expressing cells showed a Rac-like morphology as defined above (Figure 8B) and their mean cell area was 609 μm2, nearly twofold less than Q87L-expressing cells (Figure 8C). Thus, the N-terminus of R-Ras is important for the ability of R-Ras to promote a highly spread morphology, similar to that of activated Rac. Although Cdc42 is proposed to be upstream of Rac in fibroblasts (Nobes and Hall, 1995), 32D cells expressing activated Cdc42 did not produce a phenotype similar to that of Rac, nor did these cells resemble fibroblasts microinjected with activated Cdc42 (Hall, 1998; unpublished data).
We then asked whether these phenotypes required Rac by cotransfecting dominant-negative Rac (DN-Rac) with full-length or truncated R-Ras. DN-Rac blocked 71% of Q87L R-Ras–induced Rac-like morphology but only 57% of NΔ87L R-Ras–induced morphology (Figure 8B). Because DN-Rac should block both basal and R-Ras–mediated Rac activation, the partial inhibition of NΔ87L R-Ras–induced morphology by DN-Rac presumably represents a Rac-independent mode of cell spreading. DN-Rac reduced the mean cell area of Q87L R-Ras–expressing cells below control levels to 326 μm2 (Figure 8C), demonstrating that Rac is required for R-Ras–induced cell spreading.
Effects of NΔ87L R-Ras on PI3K Activation
To explore why NΔ87L R-Ras failed to activate Rac, we tested the ability of NΔ87L R-Ras to activate PI3K via phosphorylation of a common PI3K effector, Akt. Like full-length R-Ras, NΔ87L R-Ras enhanced Akt phosphorylation compared with a vector alone control (Figure 9), revealing that the N-terminus of R-Ras is not required for PI3K activation in 3T3 cells. Akt phosphorylation was dependent on PI3K since LY294002 inhibited >90% of R-Ras–induced Akt phosphorylation. These data demonstrate that the inability of NΔ87L R-Ras to activate Rac does not stem from defective initiation of PI3K signaling.
Figure 9.
NΔ87L R-Ras and Q87L R-Ras activate PI3K in 3T3 cells. NIH 3T3 cells were transfected with empty pCMV vector (lanes 1 and 2), pCGN NΔ87L R-Ras (lanes 3 and 4) or pCGN Q87L (lanes 5 and 6). Cells were treated with 0.37% DMSO (odd lanes) or 25 μM LY294002 (even lanes) for 30 min and lysed. Whole cell lysates were subjected to SDS-PAGE and phosphorylation of Akt on serine 473 was detected with a polyclonal antibody. Phosphorylated Akt was used as a reporter of PI3K activation. The phospho-Akt blot was stripped and reprobed for HA-tagged R-Ras using the 12CA5 mAb. Total Akt was detected with a distinct polyclonal antibody to assess protein loading.
Effects of NΔ87L R-Ras on Cell Migration
Previous data by Keely et al. (1999) showed that activated R-Ras stimulates haptotaxis, or cell movement in the direction of an ECM gradient. Because we observed that mouse 32D cells do not undergo efficient haptotaxis, we tested the ability of NΔ87L R-Ras or Q87L R-Ras to stimulate 32D cell migration in the presence of a serum gradient through 3 μm pores (see Materials and Methods). NΔ87L R-Ras–expressing 32D cells migrated almost three times more than control cells (Figure 10A; 11 ± 1.9% for NΔ87L R-Ras vs. 4.2 ± 1.2% for control cells) and 26% more than Q87L R-Ras–expressing cells (Figure 10A; 11 ± 1.9% for NΔ87L R-Ras vs. 8.1 ± 0.97% for Q87L R-Ras, p < 0.04), implicating the N-terminus of R-Ras as a negative regulator of cell migration.
Figure 10.
Removal of the R-Ras N-terminus increases cell migration in response to serum. (A) Mouse 32D cells were transfected as in Figure 2A. The underside of a 96-well plate embedded with 3-μm pores was coated with 1 μg/ml vitronectin. Serum-containing media was placed in the bottom chamber and 1 × 105 cells were loaded on top of the chamber. Migration was expressed as the percentage of migrated luciferase-expressing cells (**p = 0.001 for NΔ87L R-Ras vs. vector control and *p < 0.006 for Q87L R-Ras vs. vector control; p < 0.04 for NΔ87L R-Ras vs. Q87L R-Ras). Data represent triplicate means from five experiments ± SEM (B) Cells were transfected as above but were pretreated with an anti-integrin β3 antibody (clone 2C9.G2) or control hamster IgG for 30 min at 37°C. Percent inhibition of migration was calculated according to the formula: (migrationIgG - migrationβ3)/migrationIgG× 100 and represents means from three independent experiments ± SEM. (C) Cells were transfected as above and treated with 0.37% DMSO or 25 μM LY294002 for 30 min at 37°C. Cells were allowed to migrate in the presence of these reagents. Percent inhibition was calculated according to the formula: (migrationDMSO - migrationLY)/migrationDMSO× 100. Data represent means from three independent experiments ± SEM (*p = 0.02 for DMSO vs. LY294002). (D) Cells were transfected with pCGN R-Ras plasmid alone or pCGN R-Ras plus pCMV DN-Rac as indicated such that the amount of DNA totaled 12 μg for each condition. Cell migration was performed as above, except that a migration index was calculated by normalizing migration values to R-Ras levels determined by densitometry of anti-HA immunoblots. Control cells are not shown because the data cannot be normalized to R-Ras expression levels. Data represent means ± SEM from two separate experiments. (E) Checkerboard analysis was performed to determine if migration to serum was chemotactic. Mouse 32D cells were transfected as in Figure 6 and resuspended in 0, 0.5, 10, or 20% serum-containing media (abscissa) and allowed to migrate toward 0.5% serum-containing media in the bottom chamber. Shown is a representative experiment from two independent trials.
To test the mechanism of R-Ras–stimulated cell migration, we blocked the function of proteins putatively involved in this process such as β3 integrins, PI3K and Rac. Migration of 32D cells was partially dependent on β3 integrins, because an anti-β3 antibody reduced migration by 42% for vector control cells, 51% for NΔ87L R-Ras cells and 54% for Q87L R-Ras cells (Figure 10B). Although Q87L R-Ras–mediated migration was partially blocked by the selective PI3K inhibitor, LY294002 (44%, p = 0.02), NΔ87L R-Ras–mediated migration was not significantly affected (Figure 10C). Unexpectedly, DN-Rac did not significantly inhibit Q87L R-Ras– or NΔ87L R-Ras–mediated migration (mean inhibition of 13%; Figure 10D). Taken together, these data indicate the importance of integrin-mediated adhesion and/or signaling during cell migration but also point toward a PI3K- and Rac-independent pathway stimulated by NΔ87L R-Ras, distinct from pathways initiated by Q87L R-Ras.
To discriminate between chemokinesis, which is random cell movement, and chemotaxis, which is directed movement toward a chemoattractant gradient (Parent and Devreotes, 1999; Rickert et al., 2000), we compared the movement of cells in the presence and absence of a serum gradient (Figure 10E). As cells were exposed to increasing serum concentrations above the transwell, which destroys the gradient, the percentage of Q87L R-Ras–expressing 32D cells moving to the bottom chamber increased compared with vector-transfected cells. This result indicated that R-Ras amplifies a chemokinetic rather than chemotactic form of cell movement.
DISCUSSION
The first study to focus on the role of the N-terminus of R-Ras was conducted by Lowe and Goeddel (Lowe et al., 1987). They found that a 26 amino acid truncation mutant behaved like full-length R-Ras, weakly causing cell transformation and growth in soft agar compared with robust effects induced by H-Ras. The function of the divergent R-Ras N-terminus was therefore unknown and attention was shifted to more C-terminal sequences. Since that time, R-Ras has been shown to dramatically affect integrin-dependent cellular processes such as adhesion, spreading, migration, and invasion (Zhang et al., 1996; Keely et al., 1999; Berrier et al., 2000). Two recent studies have shown that the R-Ras N-terminus is involved in neither protein targeting to focal adhesions (Furuhjelm and Peranen, 2003) nor in the rescue of chimeric integrin activation from H-Ras–induced integrin suppression (Hansen et al., 2002). In the present study, we truncated the first 26 amino acids of R-Ras in the context of an activating point mutation (NΔ87L R-Ras) to address the role of the unique N-terminus relative to distinct integrin-dependent events. Although the presence or absence of the R-Ras N-terminus does not affect the membrane localization of R-Ras or its ability to induce integrin-dependent cell adhesion, the N-terminus negatively regulates R-Ras–induced cell movement and positively regulates Rac activation.
Regulation of Cell Adhesion by R-Ras
Multiple studies have demonstrated a clear role for R-Ras in integrin-dependent cell adhesion (Zhang et al., 1996; Osada et al., 1999; Kinashi et al., 2000). Even though we found that R-Ras could dramatically increase cell adhesion to immobilized substrates, deletion of the N-terminus of R-Ras had little effect on this event. Although the pathway by which R-Ras induces cell adhesion is still incomplete, we provide evidence that F-actin and protein phosphatases such as PP2A are required. Because actin filaments and phosphatases are also necessary for basal 32D cell adhesion, overexpression of GTP-bound R-Ras likely amplifies an endogenous signaling pathway that normally regulates actin organization and/or phosphatase activity during integrin-dependent adhesion events. Proteins that increase membrane fluidity or contact integrins directly such as talin are also good candidates for R-Ras effectors (Calderwood, 2004).
Two possible modes of integrin avidity regulation, namely affinity modulation of individual integrins and organization of multiple low-affinity interactions, each result in enhanced cell adhesion (Keely et al., 1998). R-Ras has been proposed to increase the affinity of the platelet integrin αIIbβ3 expressed in suspended Chinese hamster ovary cells (Zhang et al., 1996) by a mechanism called “inside-out signaling” (reviewed in Parise, 1999). Our data as well as results from other investigators show that neither full-length (Sethi et al., 1999) nor truncated R-Ras (this study) significantly increase binding of soluble agonists to suspension cells. This implies that R-Ras is unlikely to induce cell adhesion via affinity modulation of integrins in 32D cells. However, the fact that R-Ras did increase αIIbβ3 integrin binding to immobilized fibrinogen demonstrates its functionality in a myeloid cell line. Thus, we favor a model in which R-Ras signaling in 32D cells directly increases cell adhesion by a mechanism largely independent of integrin affinity modulation.
Colocalization of R-Ras with Actin
We find that both NΔ87L R-Ras and Q87L R-Ras colocalize with F-actin in dynamic plasma membrane structures such as peripheral membrane ruffles and filopodia. Ruffles are thought to be important for cellular events involving uptake of fluids or microorganisms (Ridley et al., 1992). Consistent with this localization, activated R-Ras has been shown to enhance phagocytosis of opsonized red blood cells in a macrophage-like cell line (Caron et al., 2000). Filopodia are putative environmental sensors that extend beyond the periphery of the cell and interpret extracellular stimuli, guiding directed cell movement (Small et al., 2002; Svitkina et al., 2003). Therefore, R-Ras may be acting locally on the actin cytoskeleton to influence cell adhesion and other cellular processes that require navigation such as leukocyte chemotaxis, neuronal migration, or embryonic tissue patterning.
In contrast to colocalization with actin in membrane ruffles and filopodia, both truncated and full-length R-Ras were largely excluded from the branched actin network present in lamellipodia, indicating that activated R-Ras primarily associates with a subset of actin structures within the cell. By localizing to the plasma membrane and certain actin-rich structures, however, NΔ87L R-Ras behaved like the parental Q87L R-Ras protein and other Ras family members, reinforcing the idea that sequences in the hypervariable region (HVR) of Ras GTPases are responsible for intracellular targeting (Choy et al., 1999; Bondeva et al., 2002).
Rac Activation by R-Ras
The small GTPase Rac has been implicated downstream of R-Ras signaling during cell spreading, a step thought to precede cell migration in fibroblasts (Berrier et al., 2000; Borisy and Svitkina, 2000). We show here that full-length Q87L R-Ras but not NΔ87L R-Ras greatly enhances Rac activation in adherent 32D cells. Furthermore, when activated Rac is coexpressed with NΔ87L R-Ras in the same cell, a round, Rac-like morphology replaces the protrusive NΔ87L R-Ras morphology, which is consistent with the inability of NΔ87L R-Ras to activate Rac (unpublished data).
We investigated the mechanism of full-length R-Ras–dependent Rac activation by testing the lipid kinase, PI3K. PI3K is perfectly positioned to link R-Ras and Rac since it is a well-recognized in vivo effector of R-Ras (Marte et al., 1997) as well as an upstream activator of many Rac guanine nucleotide exchange factors (Han et al., 1998; Nimnual et al., 1998; Welch et al., 2003). The PI3K inhibitor, LY294002, blocked R-Ras–dependent Rac activation almost completely in 32D cells, indicating that full-length R-Ras does indeed stimulate Rac activation through PI3K but may utilize additional effectors as well. However, in 3T3 cells, both full-length and truncated R-Ras stimulated comparable levels of Akt phosphorylation, indicating that both forms of R-Ras can activate PI3K in vivo. The molecular explanation as to why NΔ87L R-Ras activates PI3K but does not increase Rac GTP loading is presently unknown but may be due to the differences in cell type and/or kinetics of the Akt phosphorylation versus Rac activation assays. Alternatively, the uncoupling of PI3K activity from Rac activation may imply that an additional undefined signaling input is necessary for Rac activation in vivo.
Consistent with Rac activation, full-length R-Ras promotes a spread morphology nearly identical to that of activated Rac in 32D cells adhering to vitronectin in the absence of other stimuli. This phenotype requires Rac, as DN-Rac almost completely inhibited Q87L R-Ras–stimulated spreading. However, cells expressing truncated R-Ras display a spreading defect characterized by a decreased cell area, suggesting an inability of these cells to protrude membranes. Because truncated R-Ras stimulates integrin-dependent cell adhesion in 32D cells, the spreading defect likely does not result from suboptimal integrin binding to immobilized ligand. Alternatively, this spreading defect may result from inefficient “outside-in” signaling generated by ligand occupied integrins. Kwong et al. (2003) have shown that R-Ras enhances “outside-in” signals downstream of integrin ligation, resulting in increased focal adhesion formation in breast epithelial cells. Because integrins are known to regulate Rac activation upon adhesion to extracellular matrix ligands (Price et al., 1998), it is tempting to speculate that R-Ras, via its N-terminal domain, may activate Rac as part of a postadhesion integrin signaling pathway. This pathway may be cell type–specific, however, as activated R-Ras has recently been reported to reduce rather than increase levels of activated Rac in epithelial cells (Wozniak et al., 2005).
Regulation of Cell Migration by R-Ras
R-Ras is known to induce haptotaxis in breast epithelia and skeletal myoblasts (Keely et al., 1999; Holly et al., 2000; Suzuki et al., 2000). We extend this observation further by showing that Q87L R-Ras potentiates chemokinesis or random cell movement driven by the presence of serum. Interestingly, NΔ87L R-Ras induces significantly more cell movement than full-length Q87L R-Ras and control cells under these conditions, suggesting a negative regulatory role for the N-terminus. Wozniak et al. (2005) also find evidence of a role for R-Ras in chemokinesis; depletion of endogenous R-Ras levels via RNAi in breast epithelial cells results in a dramatic loss of cell motility on collagen. These data suggest that R-Ras may be fundamentally important for cell movement and further implicate R-Ras in the regulation of cytoskeletal dynamics. Moreover, chemokinetic movement may be an important prerequisite for metastatic spread and invasion of tumor cells.
To provide further evidence of a negative regulatory role for the R-Ras N-terminus during chemokinesis, we constructed a chimera comprised of the R-Ras N-terminus fused to the N-terminus of the entire TC21 polypeptide, a close relative of R-Ras. Contrary to our prediction, this chimera did not exhibit decreased cell migration levels compared with TC21 (unpublished data), indicating that the N-terminus of R-Ras is not a modular domain but more likely a region that acts in a sequence specific manner to regulate protein activity. However, a replacement of the short N-terminus of TC21 might have yielded different results.
Cell migration often requires PI3K, which is a common effector for many Ras GTPases (Sasaki et al., 2000; Ridley, 2001). Pharmacological inhibition of PI3K with LY294002 blocks full-length R-Ras–mediated chemokinesis by 44%, but blocks truncated R-Ras–mediated chemokinesis by only 25%. This confirms that truncated R-Ras does not rely heavily on PI3K for cell migration, which is consistent with its inability to activate Rac.
The elongated, protrusive morphology of NΔ87L R-Ras–expressing cells partially explains why these cells migrate more efficiently than Q87L R-Ras–expressing cells. Rac can generate similar elongated cell morphologies in colon and squamous carcinoma cells in combination with growth factors in matrigel (Sahai and Marshall, 2003). Because DN-Rac blocked the ability of truncated R-Ras to promote an elongated, dendritic phenotype but only blocked migration by roughly 10%, we conclude that an elongated cell shape accounts for a fraction of the total migration response under these conditions. This phenotype may be more relevant for other types of migratory conditions, however, such as invasion through soft agar or chemotaxis.
The apparent paradox that NΔ87L R-Ras induces more cell migration than full-length Q87L R-Ras but fails to fully activate Rac, may be explained by two observations. First, DN-Rac only slightly reduced R-Ras–dependent migration, suggesting that Rac activation is not crucial for this process. Second, rac1-/- mouse macrophages migrate normally despite defects in cell spreading, similar to cells expressing NΔ87L R-Ras (Wells et al., 2004). These data emphasize the importance of Rac-independent signaling pathways leading to cell migration.
In summary, the N-terminus of R-Ras is necessary for the efficient dissemination of R-Ras signals that govern cell shape and migration capacity. Specifically, the unique N-terminus of R-Ras positively regulates Rac activation and cell spreading, but slows R-Ras–mediated cell migration. It is therefore possible that the N-terminus of R-Ras in the context of the entire GTPase favors the binding of currently undefined, yet important effectors involved in integrin-dependent cell morphology and migration.
Acknowledgments
We thank Drs. Al Baldwin and Scott Blystone for their generous gifts of cell lines, Dr. Susan Smyth for the 1B5 antibody, and Drs. JoAnn Trejo, Adrienne Cox, Keith Burridge (all from the University of North Carolina, Chapel Hill, NC), and Dr. Alan Hall (University College, London) for generous donation of plasmids. This work was supported by National Institutes of Health 2-P01-HL06350 and 2-P01-HL45100 (L.V.P.) and Lineberger Comprehensive Cancer Center postdoctoral training grant CA09156 (S.P.H.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-12-0917) on March 16, 2005.
References
- Berrier, A. L., Mastrangelo, A. M., Downward, J., Ginsberg, M., and LaFlamme, S. E. (2000). Activated R-ras, Rac1, PI 3-kinase and PKCepsilon can each restore cell spreading inhibited by isolated integrin beta1 cytoplasmic domains. J. Cell Biol. 151, 1549-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondeva, T., Balla, A., Varnai, P., and Balla, T. (2002). Structural determinants of Ras-Raf interaction analyzed in live cells. Mol. Biol. Cell 13, 2323-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy, G. G., and Svitkina, T. M. (2000). Actin machinery: pushing the envelope. Curr. Opin. Cell Biol. 12, 104-112. [DOI] [PubMed] [Google Scholar]
- Calderwood, D. A. (2004). Integrin activation. J. Cell Sci. 117, 657-666. [DOI] [PubMed] [Google Scholar]
- Caron, E., Self, A. J., and Hall, A. (2000). The GTPase Rap1 controls functional activation of macrophage integrin alphaMbeta2 by LPS and other inflammatory mediators. Curr. Biol. 10, 974-978. [DOI] [PubMed] [Google Scholar]
- Choy, E., Chiu, V. K., Silletti, J., Feoktistov, M., Morimoto, T., Michaelson, D., Ivanov, I. E., and Philips, M. R. (1999). Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell 98, 69-80. [DOI] [PubMed] [Google Scholar]
- Cox, A. D., Brtva, T. R., Lowe, D. G., and Der, C. J. (1994). R-Ras induces malignant, but not morphologic, transformation of NIH3T3 cells. Oncogene 9, 3281-3288. [PubMed] [Google Scholar]
- Furuhjelm, J., and Peranen, J. (2003). The C-terminal end of R-Ras contains a focal adhesion targeting signal. J. Cell Sci. 116, 3729-3738. [DOI] [PubMed] [Google Scholar]
- Gotoh, T., Niino, Y., Tokuda, M., Hatase, O., Nakamura, S., Matsuda, M., and Hattori, S. (1997). Activation of R-Ras by Ras-guanine nucleotide-releasing factor. J. Biol. Chem. 272, 18602-18607. [DOI] [PubMed] [Google Scholar]
- Hall, A. (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509-514. [DOI] [PubMed] [Google Scholar]
- Han, J., Luby-Phelps, K., Das, B., Shu, X., Xia, Y., Mosteller, R. D., Krishna, U. M., Falck, J. R., White, M. A., and Broek, D. (1998). Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 279, 558-560. [DOI] [PubMed] [Google Scholar]
- Hansen, M., Rusyn, E. V., Hughes, P. E., Ginsberg, M. H., Cox, A. D., and Willumsen, B. M. (2002). R-Ras C-terminal sequences are sufficient to confer R-Ras specificity to H-Ras. Oncogene 21, 4448-4461. [DOI] [PubMed] [Google Scholar]
- Holly, S. P., Larson, M. K., and Parise, L. V. (2000). Multiple roles of integrins in cell motility. Exp. Cell Res. 261, 69-74. [DOI] [PubMed] [Google Scholar]
- Huff, S. Y., Quilliam, L. A., Cox, A. D., and Der, C. J. (1997). R-Ras is regulated by activators and effectors distinct from those that control Ras function. Oncogene 14, 133-143. [DOI] [PubMed] [Google Scholar]
- Katz, M. E., and McCormick, F. (1997). Signal transduction from multiple Ras effectors. Curr. Opin. Genet. Dev. 7, 75-79. [DOI] [PubMed] [Google Scholar]
- Keely, P., Parise, L., and Juliano, R. (1998). Integrins and GTPases in tumour cell growth, motility and invasion. Trends Cell Biol. 8, 101-106. [DOI] [PubMed] [Google Scholar]
- Keely, P. J., Rusyn, E. V., Cox, A. D., and Parise, L. V. (1999). R-Ras signals through specific integrin alpha cytoplasmic domains to promote migration and invasion of breast epithelial cells. J. Cell Biol. 145, 1077-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinashi, T., Katagiri, K., Watanabe, S., Vanhaesebroeck, B., Downward, J., and Takatsu, K. (2000). Distinct mechanisms of alpha 5beta 1 integrin activation by Ha-Ras and R-Ras. J. Biol. Chem. 275, 22590-22596. [DOI] [PubMed] [Google Scholar]
- Kozma, R., Ahmed, S., Best, A., and Lim, L. (1995). The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell Biol. 15, 1942-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong, L., Wozniak, M. A., Collins, A. S., Wilson, S. D., and Keely, P. J. (2003). R-Ras promotes focal adhesion formation through focal adhesion kinase and p130(Cas) by a novel mechanism that differs from integrins. Mol. Cell. Biol. 23, 933-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, D. G., Capon, D. J., Delwart, E., Sakaguchi, A. Y., Naylor, S. L., and Goeddel, D. V. (1987). Structure of the human and murine R-ras genes, novel genes closely related to ras proto-oncogenes. Cell 48, 137-146. [DOI] [PubMed] [Google Scholar]
- Marte, B. M., Rodriguez-Viciana, P., Wennstrom, S., Warne, P. H., and Downward, J. (1997). R-Ras can activate the phosphoinositide 3-kinase but not the MAP kinase arm of the Ras effector pathways. Curr. Biol. 7, 63-70. [DOI] [PubMed] [Google Scholar]
- Nimnual, A. S., Yatsula, B. A., and Bar-Sagi, D. (1998). Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science 279, 560-563. [DOI] [PubMed] [Google Scholar]
- Nobes, C. D., and Hall, A. (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53-62. [DOI] [PubMed] [Google Scholar]
- Oertli, B., Han, J., Marte, B. M., Sethi, T., Downward, J., Ginsberg, M., and Hughes, P. E. (2000). The effector loop and prenylation site of R-Ras are involved in the regulation of integrin function. Oncogene 19, 4961-4969. [DOI] [PubMed] [Google Scholar]
- Ohba, Y., Mochizuki, N., Yamashita, S., Chan, A. M., Schrader, J. W., Hattori, S., Nagashima, K., and Matsuda, M. (2000). Regulatory proteins of R-Ras, TC21/R-Ras2, and M-Ras/R-Ras3. J. Biol. Chem. 275, 20020-20026. [DOI] [PubMed] [Google Scholar]
- Osada, M., Tolkacheva, T., Li, W., Chan, T. O., Tsichlis, P. N., Saez, R., Kimmelman, A. C., and Chan, A. M. (1999). Differential roles of Akt, Rac, and Ral in R-Ras-mediated cellular transformation, adhesion, and survival. Mol. Cell. Biol. 19, 6333-6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent, C. A., and Devreotes, P. N. (1999). A cell's sense of direction. Science 284, 765-770. [DOI] [PubMed] [Google Scholar]
- Parise, L. V. (1999). Integrin alpha(IIb)beta(3) signaling in platelet adhesion and aggregation. Curr. Opin. Cell Biol. 11, 597-601. [DOI] [PubMed] [Google Scholar]
- Price, L. S., Leng, J., Schwartz, M. A., and Bokoch, G. M. (1998). Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell 9, 1863-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuther, G. W., and Der, C. J. (2000). The Ras branch of small GTPases: Ras family members don't fall far from the tree. Curr. Opin. Cell Biol. 12, 157-165. [DOI] [PubMed] [Google Scholar]
- Reuther, J. Y., Reuther, G. W., Cortez, D., Pendergast, A. M., and Baldwin, A. S., Jr. (1998). A requirement for NF-kappaB activation in Bcr-Abl-mediated transformation. Genes Dev. 12, 968-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey, I., Taylor-Harris, P., van Erp, H., and Hall, A. (1994). R-ras interacts with rasGAP, neurofibromin and c-raf but does not regulate cell growth or differentiation. Oncogene 9, 685-692. [PubMed] [Google Scholar]
- Rickert, P., Weiner, O. D., Wang, F., Bourne, H. R., and Servant, G. (2000). Leukocytes navigate by compass: roles of PI3Kgamma and its lipid products. Trends Cell Biol. 10, 466-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A. J. (2001). Rho proteins, PI 3-kinases, and monocyte/macrophage motility. FEBS Lett. 498, 168-171. [DOI] [PubMed] [Google Scholar]
- Ridley, A. J., Paterson, H. F., Johnston, C. L., Diekmann, D., and Hall, A. (1992). The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401-410. [DOI] [PubMed] [Google Scholar]
- Saez, R., Chan, A. M., Miki, T., and Aaronson, S. A. (1994). Oncogenic activation of human R-ras by point mutations analogous to those of prototype H-ras oncogenes. Oncogene 9, 2977-2982. [PubMed] [Google Scholar]
- Sahai, E., and Marshall, C. J. (2003). Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat. Cell Biol. 5, 711-719. [DOI] [PubMed] [Google Scholar]
- Sasaki, T. et al. (2000). Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science 287, 1040-1046. [DOI] [PubMed] [Google Scholar]
- Self, A. J., Caron, E., Paterson, H. F., and Hall, A. (2001). Analysis of R-Ras signalling pathways. J. Cell Sci. 114, 1357-1366. [DOI] [PubMed] [Google Scholar]
- Sethi, T., Ginsberg, M. H., Downward, J., and Hughes, P. E. (1999). The small GTP-binding protein R-Ras can influence integrin activation by antagonizing a Ras/Raf-initiated integrin suppression pathway. Mol. Biol. Cell 10, 1799-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, J. V., Stradal, T., Vignal, E., and Rottner, K. (2002). The lamellipodium: where motility begins. Trends Cell Biol. 12, 112-120. [DOI] [PubMed] [Google Scholar]
- Smyth, S. S., Tsakiris, D. A., Scudder, L. E., and Coller, B. S. (2000). Structure and function of murine alphaIIbbeta3 (GPIIb/IIIa): studies using monoclonal antibodies and beta3-null mice. Thromb. Haemost. 84, 1103-1108. [PubMed] [Google Scholar]
- Spaargaren, M., Martin, G. A., McCormick, F., Fernandez-Sarabia, M. J., and Bischoff, J. R. (1994). The Ras-related protein R-ras interacts directly with Raf-1 in a GTP-dependent manner. Biochem. J. 300(Pt 2), 303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suire, S., Hawkins, P., and Stephens, L. (2002). Activation of phosphoinositide 3-kinase gamma by Ras. Curr. Biol. 12, 1068-1075. [DOI] [PubMed] [Google Scholar]
- Suzuki, J., Kaziro, Y., and Koide, H. (1997). An activated mutant of R-Ras inhibits cell death caused by cytokine deprivation in BaF3 cells in the presence of IGF-I. Oncogene 15, 1689-1697. [DOI] [PubMed] [Google Scholar]
- Suzuki, J., Kaziro, Y., and Koide, H. (2000). Positive regulation of skeletal myogenesis by R-Ras. Oncogene 19, 1138-1146. [DOI] [PubMed] [Google Scholar]
- Svitkina, T. M., Bulanova, E. A., Chaga, O. Y., Vignjevic, D. M., Kojima, S., Vasiliev, J. M., and Borisy, G. G. (2003). Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol. 160, 409-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavvas, D., Li, X., Avruch, J., and Zhang, X. F. (1998). Identification of Nore1 as a potential Ras effector. J. Biol. Chem. 273, 5439-5442. [DOI] [PubMed] [Google Scholar]
- Wang, B., Zou, J. X., Ek-Rylander, B., and Ruoslahti, E. (2000). R-Ras contains a proline-rich site that binds to SH3 domains and is required for integrin activation by R-Ras. J. Biol. Chem. 275, 5222-5227. [DOI] [PubMed] [Google Scholar]
- Welch, H. C., Coadwell, W. J., Stephens, L. R., and Hawkins, P. T. (2003). Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett. 546, 93-97. [DOI] [PubMed] [Google Scholar]
- Wells, C. M., Walmsley, M., Ooi, S., Tybulewicz, V., and Ridley, A. J. (2004). Rac1-deficient macrophages exhibit defects in cell spreading and membrane ruffling but not migration. J. Cell Sci. 117, 1259-1268. [DOI] [PubMed] [Google Scholar]
- Wennerberg, K., Ellerbroek, S. M., Liu, R. Y., Karnoub, A. E., Burridge, K., and Der, C. J. (2002). RhoG signals in parallel with Rac1 and Cdc42. J. Biol. Chem. 277, 47810-47817. [DOI] [PubMed] [Google Scholar]
- Wozniak, M. A., Kwong, L., Chodniewicz, D., Klemke, R. L., and Keely, P. J. (2005). R-Ras controls membrane protrusion and cell migration through the spatial regulation of Rac and Rho. Mol. Biol. Cell 16, 84-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y., and Feig, L. A. (2002). Involvement of R-Ras and Ral GTPases in estrogen-independent proliferation of breast cancer cells. Oncogene 21, 7557-7568. [DOI] [PubMed] [Google Scholar]
- Zhang, Z., Vuori, K., Wang, H., Reed, J. C., and Ruoslahti, E. (1996). Integrin activation by R-ras. Cell 85, 61-69. [DOI] [PubMed] [Google Scholar]