Abstract
It is believed that cell-mediated immunity alone can contain Mycobacterium tuberculosis, the pathogen responsible for tuberculosis. The induction of antibody, or of a mixed cell-mediated/humoral response, is associated with tuberculous disease. It is therefore important to determine the conditions of immunization with bacille Calmette Guérin (BCG), the attenuated strain of Mycobacterium bovis used to vaccinate humans against tuberculosis, that optimally induces an exclusive cell-mediated, Th1 response. Such a determination will then allow an assessment of whether the generation of such an exclusive Th1 response results in the generation of a Th1 imprint against mycobacteria. This Th1 imprint would ensure that the Th1 response is predominant following any challenge. We therefore tested the proposition that the dose of mycobacteria used for immunization generally determines the Th1/Th2 nature of the ensuing response. Our results demonstrate that relatively low doses lead to an almost exclusive cell-mediated, Th1 response, while higher doses induce a mixed Th1/Th2 response. Furthermore, the dependence on dose is independent of whether BCG is administered intravenously, subcutaneously, or intradermally. The implications of our findings to understanding how different classes of immunity are induced, to the epidemiology of tuberculosis, and to the design of effective vaccination strategies are discussed.
Tuberculosis results in the death of about 3 million people each year (36). The increasing prevalence of multidrug-resistant forms of the causative bacterium in infected patients has led to an acknowledgment that drug therapy, cumbersome under the best of circumstances, has intrinsic limitations (14). A standard and universally efficacious form of vaccination against Mycobacterium tuberculosis, should such vaccination prove feasible, would appear to be the ideal means of controlling this disease. However, the degree of protection afforded by vaccination with an attenuated form of Mycobacterium bovis, Bacille Calmette-Guérin (BCG), is notoriously variable (13). The last World Health Organization-sponsored trial, carried out between 1968 and 1971 and involving over a quarter of a million subjects, led to the conclusion that BCG vaccination, as performed, had no overall protective effect against tuberculosis. The relationship between protection achieved and the dose of BCG administered was unknown, and so the largest acceptable dose of BCG was administered in this trial (39).
Tuberculosis is one of several chronic diseases caused by intracellular parasites where protection and limited disease are correlated with a relatively exclusive cell-mediated attack (11, 38). These diseases include leprosy (41) and the leishmaniases (18, 31). The induction of antibody usually leads to chronic or progressive and fatal disease (11, 18, 31, 38, 41). This is because antigen-specific cells generated during a strong antibody response down-regulate the cell-mediated response required to contain these diseases, most probably at both the level of induction of the cell-mediated response and the level of counteracting the activity of cell-mediated effector T cells (22, 23, 32, 33, 37, 40).
We recently explored a vaccination strategy in a mouse model of one of these diseases, cutaneous leishmaniasis, caused by the protozoan Leishmania major. L. major either is contained or causes progressive disease when mice of different strains are infected with a substantial number of parasites. Resistance and susceptibility in these different strains are correlated with parasite-specific Th1 and Th2 responses, respectively (24). Our approach to vaccinating BALB/c mice, the prototypic susceptible strain, was based on older studies by others. It has been demonstrated with a variety of antigens, in different animal species, that the dose of antigen administered is crucial in determining the class of immunity induced. Low doses favor a cell-mediated response, and higher doses favor antibody production (16, 18, 21, 30, 35, 42). We showed that infection of susceptible BALB/c mice with low doses of L. major induces a stable, cell-mediated, Th1-like response that is exclusive of antibody production and that such mice do not suffer progressive disease. In contrast, infection with higher doses results in a transient cell-mediated response whose decline correlates with the production of antibody, the generation of Th2 cells, and progressive infection (7, 26a). Furthermore, we showed that low-dose-exposed mice become resistant to a high-dose challenge that causes progressive infection in immunologically naive BALB/c mice. This resistance to a high-dose challenge is associated with the induction of a stable, cell-mediated, Th1-like response (7, 26a). Thus, infection with low numbers of L. major not only favors cell-mediated immunity but causes an imprint on the immune system, ensuring a protective, cell-mediated, Th1 response upon subsequent infection. Low-dose infection thus constitutes effective vaccination.
The possibility that vaccination with relatively low doses of BCG provides better protection against tuberculosis than vaccination with the standard dose is intriguing, particularly in view of the use of the largest acceptable dose of BCG in the last World Health Organization-sponsored BCG trial referred to above. Our long-term plan is to test a strategy for achieving efficacious vaccination of people against tuberculosis (6). We explore in this report the validity of the proposition that infection with low numbers of BCG generates a relatively exclusive cell-mediated, Th1 response, independently of whether the route of infection is intravenous (i.v.), subcutaneous (s.c.), or intradermal.
MATERIALS AND METHODS
Mice.
BALB/c mice were obtained from the animal colony at the Department of Microbiology. Mice over 6 weeks of age were used and were of the same sex within each experiment.
Growth and enumeration of BCG and immunization of mice.
M. bovis BCG Montreal was kindly provided by Emil Skamene, McGill University. The mycobacteria were propagated in Dubos medium containing 0.5% bovine serum albumin and 0.05% Tween 80 (29). Bacteria were enumerated by the ability to form colonies (15), which can be counted 10 to 14 days after plating, and the number of bacteria is consequently given as CFU. Mice were immunized either i.v., s.c., or intradermally, as indicated.
Antigen preparation.
Bacteria were grown until they reached approximately 4 × 107/ml. They were then pelleted by spinning for 20 min at 8,000 × g, resuspended in 0.05% Tween 80 in saline, and washed two more times in this solution. The bacteria were then resuspended in 5 ml of ice-cold 0.05% Tween 80 in saline and sonicated for 14 cycles of 1 min each in a Branson Sonifier, model 450, according to the manufacturer’s instructions for disrupting mycobacteria. This sonicated suspension was used as antigen in the enzyme immunospot (ELISPOT) assay. In some cases the suspension was spun for 20 min at 8000 × g to remove particulate matter and the supernatant was collected. Protein concentration of the harvested supernatant was determined by the bicinchoninic acid protein assay reagent (Pierce, Rockford, Ill.), and the supernatant was stored at −70°C. This antigen preparation was used to stimulate the production of cytokines by spleen cells of BCG-immunized mice and in the measurement of BCG-specific delayed-type hypersensitivity (DTH) (see below).
Measurement of DTH.
The expression of bacterium-specific DTH was assessed at different time points after infection by a passive transfer assay as described elsewhere (3). Briefly, 5 × 106 to 10 × 106 viable white, whole spleen cells either alone or with 10 μg of bacterial antigen were transferred s.c. to the footpads of each of three to five mice, and the 24-h swelling of the foot was measured in units of 10−2 millimeter. The antigen-dependent swelling is taken as a quantitative measure of DTH. For this assay, 10 μg of mycobacterial antigen was used, as this was considerably below the amount of antigen that, when given alone, produced a measurable swelling reaction at 24 h.
Analysis of mycobacterial antigen-dependent production of lymphokines by spleen cells from BCG-infected mice.
The RPMI culture medium used was supplemented with 7.5% fetal calf serum, 2.5% horse serum, and β-mercaptoethanol at a final concentration of 5 × 10−5 M and contained penicillin (100 U/ml) and streptomycin (100 μg/ml). Preliminary experiments showed that spleen cells from BCG-infected mice that expressed significant BCG-specific DTH by the passive transfer assay (see above) also proliferated in response to mycobacterial antigen. When spleen cells were plated at 5 million cells per well in a 24-well tray (Costar, Cambridge, Mass.) in 1.5 ml of medium, it was found that mycobacterial antigen-dependent proliferation of spleen cells from sensitized donors was maximal in the presence of 3.33 μg of antigen per ml. Spleen cells from unimmunized donors did not proliferate. The antigen-dependent production of interleukin-4 (IL-4) by sensitized spleen cells was optimal when the cells were cultured at 3 million viable white cells per well, and the supernatants were harvested at 48 h; the corresponding optimal density for the antigen-dependent production of gamma interferon (IFN-γ) was 6 million spleen cells per well. Supernatants harvested at 48 h of culture were stored at −70°C and later assessed for lymphokine content. As described in Results, the mycobacterial antigen at the concentration used did not interfere with the L. major antigen-dependent production of cytokines by spleen cells sensitized to L. major antigens. The mycobacterial antigen did not have, by this criterion, any inhibitory or immunomodulatory activity on cytokine production.
Lymphokine assays.
IFN-γ present in spleen cell culture supernatants was quantitated by using a viral cytopathic reduction assay employing the murine fibroblastic cell line L929 and endomyocarditis virus (12). The reduction in viral cytopathogenicity was invariably due to interferon, as assessed by its abrogation upon addition of the IFN-γ neutralizing monoclonal antibody XMG1.2 (8). The assay was standardized by reference to recombinant murine IFN-γ of known activity, as specified by the manufacturer (Genzyme). The IL-4-dependent cell line CT4.S (19) was used to quantitate IL-4. Proliferation of this cell line in response to either recombinant IL-4 (Genzyme) or IL-4 present in cell culture supernatants was determined by incorporation of [3H]thymidine. The dependency of proliferation on IL-4 was invariably confirmed by blocking proliferation upon addition of the IL-4 neutralizing monoclonal antibody 11B11 (28). All assays were done in triplicate. The production of IFN-γ and IL-4 is given as units per 106 cultured white spleen cells; 1 ng of IL-4 is equal to 10 U and 1 ng of IFN-γ is equal to 5 U according to our standard assay procedure.
ELISPOT assay for antigen-specific cells making IFN-γ or IL-4.
The ELISPOT assay was used to quantitate the number of antigen-specific spleen cells producing IFN-γ or IL-4 (10, 20). Ninety-six-well nitrocellulose-bottom culture plates (Polyfiltronics, Rockland, Mass.) were coated with purified anti-IFN-γ or anti-IL-4 antibodies (Pharmingen, San Diego, Calif.) by adding to each well 100 μl of antibody at 1.25 μg/ml in 65 mM bicarbonate buffer (pH 9.6) and incubating the mixture at 4°C overnight. The plates were blocked with 200 μl of RPMI medium containing 7.5% fetal calf serum for at least 1 h prior to addition of spleen cells. Spleen cells from experimental mice were plated in 100 μl of medium at two of three densities (106, 5 × 105, and 2.5 × 105 cells per well) in the presence of additional irradiated (1,500 R from a 60Co source) white spleen cells from unimmunized mice. The addition of this number of irradiated normal spleen cells was found to be necessary to ensure that the number of antigen-dependent spots observed was approximately proportional to the number of immunized spleen cells plated (31a). When required, antigen was added at a concentration of 3.33 μg per ml of medium. The number of spot-forming cells generated in the presence and absence of antigen by the spleen cells of each experimental mouse was assessed in triplicate. The seeded plates were placed in a 37°C incubator undisturbed for 8 h and then washed thoroughly with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBST). The appropriate biotinylated anticytokine antibody (Pharmingen) was added in 100 μl of PBST at a concentration of 1.25 μg/ml, and the plates were incubated overnight at 4°C. The plates were then washed once again with PBST. One hundred microliters of alkaline phosphatase-streptavidin (Jackson Immunoresearch Laboratories Inc., West Grove, Pa.) at a concentration of 0.2 μg/ml in PBST was added to each well and incubated at room temperature for 1.5 h. The plates were then washed with double-distilled H2O. The spots were developed by addition of a 1-in-50 dilution in 0.1 M Tris–0.1 M NaCl–0.05 M MgCl2 buffer (pH 9.5) of nitroblue tetrazolium chloride (8.75 mg/ml) and 5-bromo-4-chloro-3-indolyl phosphate, toluidine (9.4 mg/ml), in 67% (vol/vol) dimethyl sulfoxide as instructed by the manufacturer (Boehringer, Mannheim, Germany). Spots were counted in a dissecting microscope after the plates had dried. Only the number of antigen-specific cytokine-producing cells is reported. This number is calculated by assessing the number of spots obtained in the presence of antigen and subtracting the number obtained in the absence of antigen. In all cases, the average numbers of spots obtained without antigen in the wells were approximately 1 and <10 per 106 spleen cells in the IFN-γ and IL-4 ELISPOT assays, respectively.
Analysis of antimycobacterial antibody by immunoblotting or by ELISA.
Blood from each healthy or infected mouse was collected by bleeding from the tail, and individual sera were harvested. Serum was examined for mycobacterium-specific immunoglobulin G1 (IgG1) and IgG2a antibodies by immunoblotting as described elsewhere (7), using 20 μg of mycobacterial antigen per lane. In some experiments, the IgG1 and IgG2a serum antibody titers were determined by an enzyme-linked immunosorbent assay (ELISA) (17). Immulon-4 96-well polystyrene plates were coated with BCG antigen at a concentration of 1 μg/well in 100 μl of PBS. Serum samples were diluted 1/100 in PBS containing 2% bovine serum albumin and 2% Tween 20 (assay diluent), and twofold serial dilutions were prepared in the ELISA plate. The plates were incubated at 37°C for 2 h and washed thoroughly with double-distilled H2O. Horseradish peroxidase-labeled rat anti-mouse monoclonal antibody against mouse IgG1 or IgG2a (Southern Biotechnology Associates, Birmingham, Ala.) was added to the wells at a dilution of 1/3,000 in assay diluent, 100 μl per well, and incubated at 37°C for 2 h. After washing, ABTS (2.2′-azino-di[3-ethylbenzthiazoline sulfonate [6]) substrate solution (Kirkegaard & Perry, Gaithersburg, Md.) was added to the wells, the plates were incubated for 20 min, readings were taken on a Bio-Rad (Hercules, Calif.) model 2550 EIA Reader. Readings were corrected for background, and the positive cutoff was set at twice the value for pooled normal mouse serum. The titer was considered to be the last dilution to give a positive result.
RESULTS
Intravenous infection of BALB/c mice with different numbers of BCG leads to qualitatively different kinds of immune response: low-dose infection results in an exclusive cell-mediated, Th1 response.
The dose of a nonreplicating antigen, or the number of replicating microorganisms, used to raise immunity is known in some cases to affect the class of immunity induced (7, 16, 18, 21, 26, 30, 35, 42). We wished to define conditions of immunization with viable BCG leading to a cell-mediated, Th1-like response exclusive of antibody production, as well as other conditions leading in the long term to responses with a significant antibody, Th2 component. In a preliminary experiment, we infected mice i.v. with different numbers of BCG CFU, ranging from 40 to 4 × 107, and monitored the mycobacterium-specific IgG1 and IgG2a antibodies present in sera at 3, 6, and 8 weeks postinfection by immunoblotting. We also assessed the induction of cell-mediated immunity by determining the ability of spleen cells from mice infected 10 weeks previously to transfer DTH to naive recipients. We found that infection with relatively low doses of BCG induced substantial DTH but undetectable antibody production. Very high numbers of CFU given i.v., i.e., 4 × 106 and 4 × 107, resulted in the production of substantial antibody, but the spleens of such mice expressed barely detectable DTH at 10 weeks postinfection.
We wished to characterize more fully the nature of the immune responses, including cytokine production by mycobacterium-specific cells, to different doses of BCG given i.v. We therefore injected mice i.v. with either saline or 40, 4 × 103, 4 × 105, or 4 × 107 CFU of BCG, and various parameters of the immune response were examined at 3, 6, and 10 weeks postinfection. First, we collected sera at these times and examined the mycobacterium-specific antibody present by Western blotting (Fig. 1). Second, three mice from each group were killed at 10 weeks postinfection, and the ability of their spleen cells to express DTH was assessed by the passive transfer assay (Fig. 2). Last, their spleen cells were also cultured with and without mycobacterial antigen, and the supernatants were harvested at 48 h and assessed for the presence of IFN-γ and IL-4. We had determined an optimal concentration of our preparation of mycobacterial antigen for causing the proliferation of mycobacterium-specific cells and optimal cell densities of sensitized spleen cells for the antigen-dependent production of IFN-γ and IL-4. We ensured that the concentration of the mycobacterial antigen chosen, 3.33 μg/ml, did not significantly affect the production by L. major-specific lymphocytes of IFN-γ and IL-4 in response to leishmania antigens. This demonstrated that the BCG antigen preparation neither inhibited nor enhanced the antigen-dependent production of IFN-γ and IL-4 that follows stimulation of sensitized lymphocytes with a nonmycobacterial antigen. The production of IFN-γ and IL-4 by mycobacterial antigen-sensitized spleen cells in the absence of mycobacterial antigen was below detection, and nonsensitized cells also did not produce detectable cytokines upon stimulation with mycobacterial antigen. We therefore report only the antigen-dependent production of cytokines by sensitized cells.
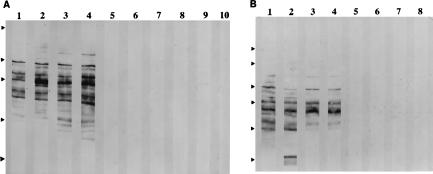
FIG. 1.
Analysis by western blotting of sera from mice infected i.v. with different numbers of BCG at various times postinfection for antimycobacterial IgG1 and IgG2a antibodies. The odd- and even-numbered lanes were developed to reveal antimycobacterial IgG1 and IgG2a antibodies, respectively. No antibodies were detected in sera collected 3 weeks postinfection. (A) Antibodies from mice 6 weeks postinfection. Lanes 1 to 4, from two mice infected with 4 × 107 BCG; lanes 5 to 10, from three mice infected with 4 × 105 BCG. The relative positions of the molecular weight markers are indicated by arrowheads at the left; from the top of the blot, the markers are maltose-binding protein–paramyosin (83 kDa), glutamic dehydrogenase (62 kDa), aldolase (47.5 kDa), triosephosphate isomerase (32.5 kDa), and β-lactoglobulin A (25 kDa). (B) Antibodies from a pool of sera from three mice infected with the same number of BCG and collected at week 10 postinfection. Lanes 1 and 2, 4 × 107 BCG; lanes 3 and 4, 4 × 105 BCG; lanes 5 and 6, 4 × 103; lanes 7 and 8, 40 BCG. The markers are the same as for panel A, but with the addition of maltose-binding protein–β-galactosidase (17s kDa). No antibody is detectable in mice immunized with the two lower doses at later times up to 3.5 months.
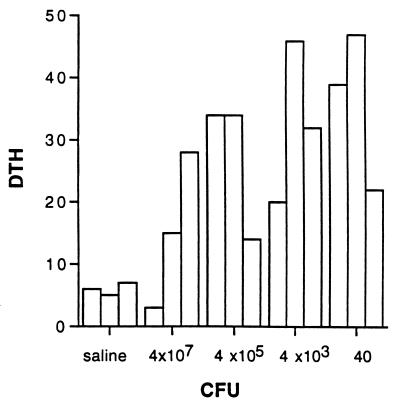
FIG. 2.
DTH expressed by spleen cells of mice infected i.v. with the indicated number of BCG CFU 10 weeks postinfection, measured by the passive transfer assay as described in Materials and Methods. The units of DTH are 10−2 mm of antigen-dependent footpad swelling.
The data shown in Fig. 1 to 3 are representative of two separate and independent experiments. Figure 1 demonstrates that only those mice which have been immunized with the relatively high doses of 4 × 107 and 4 × 105 BCG develop a significant antibody response to BCG antigens in the Western blot assay. Both the IgG1 and IgG2a subclasses of antibody are involved in this response. Lower doses of BCG do not induce a detectable antibody response but result in substantial cell-mediated immunity in the form of DTH-mediating cells, as demonstrated in Fig. 2. The observations recorded in Fig. 3 show that i.v. infection with few BCG CFU (40 and 4 × 103) results in the generation of mycobacterium-specific spleen cells able to produce substantial IFN-γ but not IL-4 when stimulated in vitro with mycobacterial antigen, while infection with the highest dose (4 × 107 CFU) results in the production of substantial amounts of IL-4 upon stimulation of spleen cells with bacterial antigen at 10 weeks postinfection. One measure of the relative size of the Th1 and Th2 components of the response is the ratio of IFN-γ to IL-4 produced upon antigen stimulation. High and low ratios indicate responses dominated by the Th1 and Th2 components of the immune response, respectively. This ratio is between 100 and 1,000 for spleen cells derived from mice infected 10 weeks previously with 4 × 105 or fewer mycobacteria, whereas the corresponding ratios are about 10, 1, and 0.1 for the three mice infected with 4 × 107 mycobacteria.
FIG. 3.
Mycobacterial antigen-dependent production of IFN-γ and IL-4 (units per 106 spleen cells) from mice infected i.v. with the indicated number of BCG at the indicated times postinfection. Open bars represent production of IFN-γ, and hatched bars represent production of IL-4. Asterisks indicate <6 U for IFN-γ and 0.4 U for IL-4. The ratio of IFN-γ to IL-4 produced at 10 weeks postinfection is also shown.
We also used the ELISPOT assay to determine the number of antigen-specific cells producing IFN-γ or IL-4 in spleens taken from mice following i.v. infection with BCG. This assay had been established recently in our laboratory and was shown to be more sensitive in detecting Th1 and Th2 responses than the bioassays used to measure cytokines. The results of such an experiment are shown in Table 1. The low dose of 200 BCG CFU induces an exclusive Th1 response, whereas the considerably higher dose of 2 × 106 CFU induced a mixed response. The low dose does not induce significant antibody, whereas the high dose resulted in a potent humoral response. It should be noted that we sometimes detect a very low mycobacterium-specific antibody response in older mice which have not been deliberately immunized. Our findings suggest that this is a response to the presence of mycobacteria in the environment (unpublished observations).
TABLE 1.
Immune responses of mice 11 weeks after immunization by the i.v. route with different numbers of viable BCG
| Treatment | Mouse | No. of cytokine-producing cellsa/106 spleen cells
|
Serum antibody titerb
|
||
|---|---|---|---|---|---|
| IFN-γ | IL-4 | IgG1 | IgG2a | ||
| Saline only | C1 | 4 | 2 | 200 | — |
| C2 | 2 | 6 | — | 400 | |
| C3 | 2 | 0 | — | 200 | |
| C4 | 4 | 8 | — | 200 | |
| BCG CFU | |||||
| ∼2 × 102 (low dose) | L1 | 292 | 4 | — | — |
| L2 | 98 | 0 | — | — | |
| L3 | 101 | 6 | 100 | 100 | |
| L4 | 188 | 0 | 200 | — | |
| L5 | 98 | 14 | 200 | 100 | |
| ∼2 × 106 (high dose) | H1 | 246 | 222 | 8 × 104 | 3.2 × 105 |
| H2 | 212 | 278 | 4 × 104 | 1.6 × 105 | |
| H3 | 332 | 302 | 8 × 104 | 3.2 × 105 | |
| H4 | 326 | 300 | 4 × 104 | 3.2 × 105 | |
| H5 | 398 | 370 | 8 × 104 | 3.2 × 105 | |
As determined by antigen-dependent ELISPOT assay.
Defined as the last dilution to give a positive result when tested by ELISA. All sera from mice before immunization had titers of <100 (—).
There is a similar dependency of the Th1/Th2 nature of the immune response on dose of BCG following subcutaneous infection of BALB/c mice.
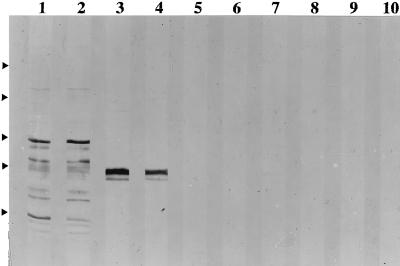
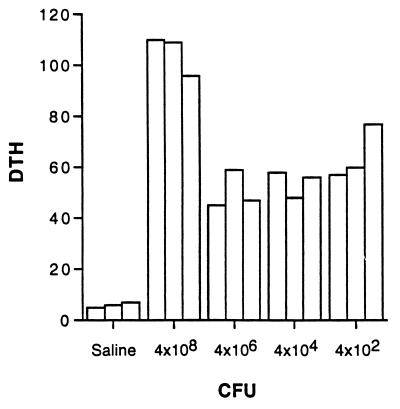
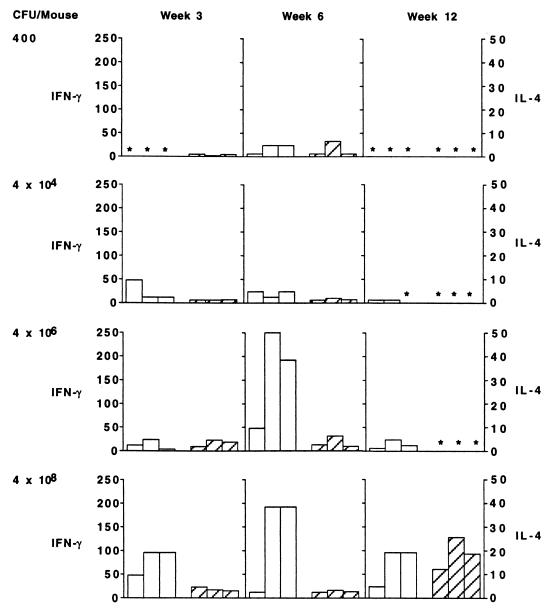
We examined, in two different experiments, the nature of the immune response following s.c. infection with different numbers of BCG CFU. Figure 4 summarizes our observations on antibody production from one experiment. Infection with both 4 × 106 and 4 × 108 CFU led to detectable antibody production, whereas infection with 4 × 104 and 4 × 102 mycobacteria did not, even when sera were collected 12 weeks postinfection. All doses led to the induction of cell-mediated immunity as indicated by the substantial expression of DTH by spleen cells harvested 12 weeks postinfection, (Fig. 5). The production of IFN-γ and IL-4 by spleen cells, harvested at different times following s.c. infection, in response to stimulation by bacterial antigen is shown in Fig. 6. The most consistent and substantial production of IL-4 occurs in mice infected with the highest dose at the latest time examined, i.e., week 12 postinfection. Overall, these observations show the same trend as seen for mice injected i.v. with different doses of BCG; i.e., low doses induce DTH and antigen-specific IFN-γ production, but significant IL-4 and specific antibody production is induced only at higher doses of infection. A dose of BCG given i.v. appears to be a more effective immunogen than the same dose given s.c. This finding is not surprising and is consistent with other observations in the literature (see Discussion).
FIG. 4.
Analysis of sera from mice infected s.c. with different numbers of BCG at 6 weeks postinfection for antimycobacterial IgG1 and IgG2a antibodies. Sera were pooled from three mice similarly injected. The pattern for sera collected 6 weeks postinfection is similar to but stronger than the pattern for sera collected at 3 weeks and similar to that seen for sera collected at week 12. The odd- and even-numbered lanes were developed to reveal antimycobacterial IgG1 and IgG2a antibodies, respectively. Sera were from mice injected with 4 × 108 (lanes 1 and 2), 4 × 106 (lanes 3 and 4), 4 × 104 (lanes 5 and 6), and 4 × 102 (lanes 7 and 8) BCG CFU and with saline (lanes 9 and 10). The relative positions of the molecular weight markers are indicated by the arrowheads at the left; from the top of the blot, the markers are maltose-binding protein–β-galactosidase (175 kDa), maltose-binding protein–paramyosin (83 kDa), glutamic dehydrogenase (62 kDa), aldolase (47.5 kDa), and triosephosphate isomerase (32.5 kDa).
FIG. 5.
DTH expressed by spleen cells of mice infected s.c. with the indicated number of BCG CFU 12 weeks postinfection, measured by the passive transfer assay as described in Materials and Methods. The units of DTH are 10−2 mm of antigen-dependent footpad swelling.
FIG. 6.
Mycobacterial antigen-dependent production of IFN-γ and IL-4 (units per 106 spleen cells) from mice infected s.c. with the indicated number of BCG at the indicated times postinfection. Open bars represent IFN-γ production, and hatched bars represent IL-4 production. Asterisks indicate <6 U for IFN-γ and 0.4 U for IL-4.
There is also a similar dependency of the Th1/Th2 nature of the immune response on dose of BCG following intradermal infection of BALB/c mice.
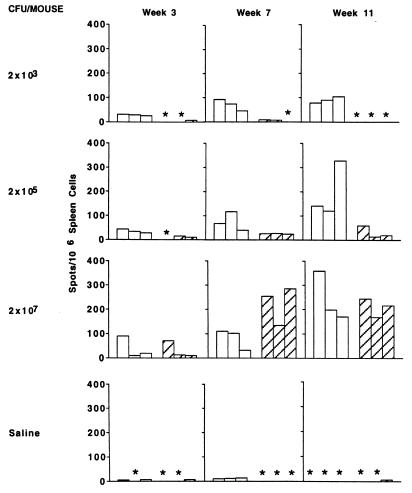
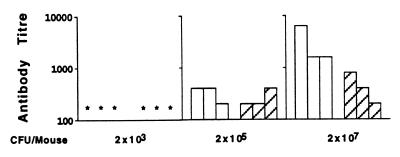
We were particularly interested in determining how the dose of BCG affected the Th1/Th2 nature of the immune response following intradermal immunization, as this is the route by which BCG is administered to humans for vaccination. The same pattern concerning the dependence of the Th1/Th2 nature of the response upon antigen dose as seen following i.v. and s.c. immunization is found following intradermal immunization (Fig. 7 and 8). Low doses induce a virtually exclusive Th1 response, with undetectable antibody production, whereas higher doses generate substantial numbers of IL-4-producing cells and lead to substantial antibody production as quantitatively assessed by ELISA. The data presented in Fig. 7 and 8 are representative of three independent experiments performed with the intradermal route of injection. All experiments gave similar results.
FIG. 7.
Numbers of BCG-specific cytokine-producing cells/106 spleen cells, measured by ELISPOT assay in mice immunized intradermally with different numbers of BCG CFU at the indicated times postinfection. Open and hatched bars represent the numbers of IFN-γ spots and IL-4 spots, respectively. Control mice received saline only. Asterisks indicate <5 spots per 106 spleen cells.
FIG. 8.
Serum antibody (IgG1 and IgG2a) titers of mice 11 weeks after intradermal immunization with various doses of BCG. Open bars represent IgG1 titers, and hatched bars represent IgG2a titers. An asterisk indicates an antibody titer of 100 or less. Negligible titers were observed at 3 weeks postinoculation. Only immunization with the highest dose induced significant antibody at 7 weeks postinfection. Sera of mice were tested up to 11 months postinfection, and no BCG specific antibody was detected in the sera of mice which received 2 × 103 BCG during this time.
DISCUSSION
The studies described here support the proposition that the dose of BCG is crucial in determining the Th1/Th2 nature of the immune response, with low doses favoring a predominantly cell-mediated, Th1 response, independently of whether the BCG is given i.v., s.c., or intradermally.
We were surprised to find that infection with very low numbers of BCG, the lowest numbers tried (40 CFU i.v., 400 CFU s.c., and 2,000 CFU intradermally), induced detectable immune responses shortly after infection. These findings support the plausibility of low-dose vaccination in that (i) these doses of BCG are much below those used in most murine studies and (ii) the corresponding responses are predominantly of the cell-mediated, Th1 type. They are also of interest from two other perspectives. It seems to be generally accepted that an environmental factor favoring the spread of tuberculosis is crowded living conditions. Most individuals infected with M. tuberculosis do not become ill (13). It is to be expected, within the context of the findings reported here and of the view that only cell-mediated responses are protective, that the dose of infection is an important factor in determining whether disease develops, with infection with higher numbers of bacilli leading to disease. It is natural to suppose that crowded living conditions will lead more often to the substantial infection required to establish disease. Second, it might be anticipated that the effective dose of mycobacteria, as perceived by the immune system of a mouse or of a human, would depend not only on the number of bacilli that infect an individual but also on the rate at which the bacilli divide. It is interesting from this perspective that a ranking in mice of the relative virulence of different mycobacterial strains able to cause tuberculosis has been correlated with their in vivo rate of replication (27). This again is understandable if high-dose infection is essential for the generation of an antimycobacterial response with a significant antibody, Th2 component, and hence for disease development.
Comparison of the observations recorded in Fig. 1 to 6 suggests that BCG is a more effective immunogen when given i.v. than when given s.c., since a dose of 4 × 105 CFU given i.v. could induce antibody but a dose of 4 × 106 CFU given s.c. induces barely detectable antibody. The greater effectiveness of antigen given i.v. than of that given s.c. is not surprising, as it is expected that in the former case the antigen will reach secondary lymphoid organs more effectively. Our observations are consistent with others reported in the literature showing that antigen given i.v. is more effective as an immunogen than antigen given s.c. (21, 42).
Our observations also bear on the nature of the decision criterion determining whether antigen, in the form of a nonreplicating substance or of an infectious agent, induces a cell-mediated, Th1 response or an antibody, Th2 response (9). Any valid description of this decision criterion must account for the conditions of immunization known to affect the Th1/Th2 nature of the ensuing response. There are similar patterns of antigen-dose dependence for the generation of cell-mediated, Th1 responses and antibody, Th2 responses in many different systems (7, 16, 21, 25, 26, 30, 35, 42), and such a dependence needs to be explained. One hypothetical description of the decision criterion, called the threshold hypothesis (2, 4, 5), predicts that the antigen dose dependence of Th1 and of Th2 responses reported here will generally hold, and so our observations can be regarded as providing indirect support for this hypothesis. This dose dependence, if generally true, appears to limit the validity of some suggestions as to the nature of the criterion determining whether antigen induces a Th1 or Th2 response. For example, the constitutive cytokine environment may be different in different strains of mice, thus favoring either Th1 or Th2 responses (reviewed in reference 9). Such an explanation for different Th responses in different strains of mice may be partially valid but cannot be an overriding factor, as it does not explain how different doses of the same antigen can induce responses of different Th phenotype in the same mouse strain. Another suggestion is that the type of APC that induces Th cells may be crucial in determining what kind of immune response ensues (reviewed in reference 9). We find it striking that the same kind of dependency of the Th1/Th2 nature of the response on BCG dose can be seen when infection is i.v., s.c., or intradermal. One might anticipate that such routes of infection would result in uptake by different types of APC. Our results suggest that it is important, in experimentally determining whether antigen presented by a given type of APC intrinsically favors the generation of Th1 or Th2 responses, to examine the nature of the Th response following immunization with different amounts of antigen presented by a given type of APC. It may be that a given type of APC has the potential to induce both Th1 and Th2 responses and that the antigen dose determines which response predominates under a given set of circumstances.
Our results appear to reflect the general rule that for those antigens able to induce both Th1 and Th2 responses, the Th1/Th2 nature of the response depends on the dose of the antigen used for immunization, with lower doses favoring the Th1 component. Instances of this rule were first described after employing protein antigens and examining the immune response in terms of DTH and antibody production (7, 35) and later extended to xenogeneic erythrocytes (21, 42). Killed mycobacteria have been used in attempts to vaccinate against a pathogenic mycobacterial challenge, with diverse results (34). Given the diversity of the systems used diverse factors are likely to be responsible for the lack of consistent findings. We anticipate that mycobacterial dose will be one of these diverse factors. Indeed, studies with heat-killed Mycobacterium vaccae show that immunization with low doses generates a predominantly Th1 response, correlated with increased resistance, whereas higher doses induce a mixed Th1/Th2 response, resulting in increased pathology upon challenge with virulent M. tuberculosis (16). These observations suggest that dead mycobacteria do not inevitably induce Th2-predominant responses, but that the Th1/Th2 nature of the response depends on several conditions of immunization, including antigen dose.
The observations reported here and elsewhere (7, 26a) involve the slowly replicating intracellular microorganisms L. major and BCG and an examination of the antigen-dependent generation of Th1 and Th2 cells. L. major and BCG are highly complex from a chemical point of view, containing, for example, many distinct proteins. These proteins are presumably present in very different amounts. One could imagine a scenario in which the Th1/Th2 nature of the immune response to each individual component was separately determined by the immune system, dependent on a variety of factors, including perhaps its concentration. Such independence of responses would lead to mixed Th1/Th2 responses to a mixture of the microorganism’s antigens under most conditions. Because we find a tendency for responses to be exclusive, our observations support the idea that the Th1/Th2 nature of the response to different components of these microorganisms is not independently but coordinately regulated. This phenomenon has been referred to as coherence (1). It is essential to our vaccination strategy, as it means that we can ensure a protective, Th1 response to protective antigens, without defining their nature, by ensuring a Th1 response to the majority of the components of the microorganism.
These studies define conditions under which predominantly Th1 responses are generated against mycobacteria. Our current studies are testing the hypothesis that the generation of such responses can establish a Th1 imprint upon the immune system and that such an imprint results in resistance to a pathogenic challenge of virulent mycobacteria.
ACKNOWLEDGMENTS
This work was supported by grants MT-12924 and MT-14121 from the Medical Research Council of Canada.
REFERENCES
- 1.Bretscher P A. Quantitative considerations in the design of vaccination strategies against pathogens uniquely susceptible to cell-mediated attack. In: Kaufmann S H E, editor. Concepts in vaccine development. Berlin, Germany: Walter de Gruyter; 1996. pp. 187–204. [Google Scholar]
- 2.Bretscher P A. Hypothesis. On the control between cell-mediated, IgM and IgG immunity. Cell Immunol. 1974;13:171–195. doi: 10.1016/0008-8749(74)90237-8. [DOI] [PubMed] [Google Scholar]
- 3.Bretscher P A. In vitro induction of delayed-type hypersensitivity. Eur J Immunol. 1979;9:311–316. doi: 10.1002/eji.1830090412. [DOI] [PubMed] [Google Scholar]
- 4.Bretscher P A. The regulatory functions of CD4+ and CD8+ T-cell subsets in immune class regulation. Res Immunol. 1991;142:45–49. doi: 10.1016/0923-2494(91)90011-7. [DOI] [PubMed] [Google Scholar]
- 5.Bretscher P A. Significance and mechanisms of cellular regulation of the immune response. Fed Proc. 1981;40:1473–1478. [PubMed] [Google Scholar]
- 6.Bretscher P A. A strategy to improve the efficacy of vaccination against tuberculosis and leprosy. Immunol Today. 1992;13:342–345. doi: 10.1016/0167-5699(92)90168-7. [DOI] [PubMed] [Google Scholar]
- 7.Bretscher P A, Wei G, Menon J N, Beilefeldt-Ohmann H. Establishment of stable cell mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science. 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 8.Cherwinski H M, Schumacher J H, Brown K D, Mosmann R R. Two types of helper T cell clones. III. Further differences in lymphokine synthesis between TH1 and TH2 clones revealed by RNA hybridization, functionally monospecific bioassays and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffman R L, Mosmann T R. CD4+ T cell subsets: regulation of differentiation and function. Res Immunol. 1991;142:7–8. doi: 10.1016/0923-2494(91)90002-z. [DOI] [PubMed] [Google Scholar]
- 10.Czerkinsky C, Andersson G, Ekre H P, Nilsson L K, Klareskog L, Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- 11.Ellner J J. Tuberculosis. In: Kelley W N, editor. Textbook of internal medicine. J. B. Philadelphia, Pa: Lippincott; 1989. pp. 647–664. [Google Scholar]
- 12.Familletti P C, Rubinstein S, Pestka S. A convenient and rapid cytopathic effect inhibition assay for interferon. Methods Enzymol. 1981;78:387–394. doi: 10.1016/0076-6879(81)78146-1. [DOI] [PubMed] [Google Scholar]
- 13.Fine P E M. BCG vaccination against tuberculosis and leprosy. Br Med Bull. 1988;44:691–704. doi: 10.1093/oxfordjournals.bmb.a072277. [DOI] [PubMed] [Google Scholar]
- 14.Frieden T R, Sterling T, Paldos-Meider A, Kilburn T O, Cauthen G M, Dooley S W. The emergence of drug-resistant tuberculosis in New York City. N Engl J Med. 1993;328:521–526. doi: 10.1056/NEJM199302253280801. [DOI] [PubMed] [Google Scholar]
- 15.Gros P, Skamene E, Forget A. Genetic control of natural resistance to Mycobacterium bovis (BCG) in mice. J Immunol. 1981;127:2417–2421. [PubMed] [Google Scholar]
- 16.Hernandez-Pando R, Pavon L, Arriaga K, Orozco H, Madrid-Marina V, Rook G A W. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect Immun. 1997;65:3317–3327. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornbeck P. Antibodies. In: Colligan J E, Kruisbeck A, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 2.1.2–2.1.20. [Google Scholar]
- 18.Howard J G. Immunological regulation and control of experimental leishmaniasis. Int Rev Exp Pathol. 1988;28:79–116. [PubMed] [Google Scholar]
- 19.Hu-Li J, Ohara J, Watson C V, Tsang W, Paul W E. Derivation of a T cell line that is highly responsive to IL4 and IL2 (CT.4R) and of an hyporesponsive mutant of that cell line (CT.4S) J Immunol. 1989;142:800–807. [PubMed] [Google Scholar]
- 20.Klinman D M, Nutman T B. ELISPOT assay to detect cytokine-secreting murine and human cells. In: Colligan J E, Kruisbeck A, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons Inc.; 1994. pp. 6.16.1–6.19.6. [Google Scholar]
- 21.Lagrange P H, Mackaness G B, Miller T E. Influence of dose and route of antigen injections on the immunological induction of T cells. J Exp Med. 1974;139:528–542. doi: 10.1084/jem.139.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehn M, Weiser W Y, Engelhorn S, Gillis S, Remold H G. IL4 inhibits hydrogen peroxide production and anti-leishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989;143:3020–3024. [PubMed] [Google Scholar]
- 23.Liew F Y, Howard J G. Immunologic regulation of experimental cutaneous leishmaniasis. V. Characterization of effector and specific effector T cells. J Immunol. 1982;128:1917–1922. [PubMed] [Google Scholar]
- 24.Locksley R M, Scott P. Helper T-cell subsets in mouse leishmaniasis: induction, expansion and effector function. Immunol Today. 1991;12(3):A58–A61. doi: 10.1016/S0167-5699(05)80017-9. [DOI] [PubMed] [Google Scholar]
- 25.Menacci A, Spaccapelo R, delSero G, Enssle K-H, Cassone A, Bistoni F, Romani L. CD4+ T-helper-cell responses in mice with low-level Candida albicans infections. Infect Immun. 1996;64:4907–4914. doi: 10.1128/iai.64.12.4907-4914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menon J N, Bretscher P A. Characterization of the immunological memory state generated in mice “susceptible” to Leishmania major following exposure to low doses of L. major and resulting in resistance to a normally pathogenic challenge. Eur J Immunol. 1996;26:243–249. doi: 10.1002/eji.1830260138. [DOI] [PubMed] [Google Scholar]
- 26a.Menon, J. N., and P. A. Bretscher. Parasite dose determines the Th1/Th2 nature of the response to Leishmania major independently of infection route, strain of host or of parasite. Eur. J. Immunol., in press. [DOI] [PubMed]
- 27.North R J, Izzo A A. Mycobacterial virulence. Virulent strains of Mycobacterium tuberculosis have faster in vivo doubling times and are better equipped to resist the growth inhibiting functions of specific immunity. J Exp Med. 1993;177:1723–1733. doi: 10.1084/jem.177.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohara J, Paul W E. Production of a monoclonal antibody to and molecular characterisation of B-cell stimulatory factor-1. Nature. 1985;315:333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- 29.Orme I M, Collins F M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983;158:74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parish C R. The relationship between humoral and cell-mediated immunity. Transplant Rev. 1972;13:35–66. doi: 10.1111/j.1600-065x.1972.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 31.Pearson R D, Wheeler D A, Harrison L H, Kay H D. The immunobiology of leishmaniasis. Rev Infect Dis. 1983;5:907–927. doi: 10.1093/clinids/5.5.907. [DOI] [PubMed] [Google Scholar]
- 31a.Power, C. A., et al. Unpublished data.
- 32.Ramshaw I A, Bretscher P A, Parish C R. Regulation of the immune response. I. Suppression of delayed-type hypersensitivity by T cells from mice expressing humoral immunity. Eur J Immunol. 1976;6:674–679. doi: 10.1002/eji.1830061003. [DOI] [PubMed] [Google Scholar]
- 33.Ramshaw I A, McKenzie I F C, Bretscher P A, Parish C R. Discrimination of suppressor T cells of humoral and cell-mediated immunity by anti-Ly and anti-Ia sera. Cell Immunol. 1976;31:364. doi: 10.1016/0008-8749(77)90038-7. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal S R. Vaccines other than BCG used against tuberculosis. In: Rosenthal S R, editor. BCG vaccine: tuberculosis-cancer. Littleton, Mass: PSG Publishing Inc.; 1980. pp. 14–34. [Google Scholar]
- 35.Salvin S B. Occurrence of DTH during the development of Arthus type sensitivity. J Exp Med. 1958;107:109–124. doi: 10.1084/jem.107.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schurr E, Malo D, Radzioch D, Buschman E, Morgan K, Gros P, Skamene E. Genetic control of innate resistance to mycobacterial infections. Immunol Today. 1991;12(3):A42–A45. doi: 10.1016/S0167-5699(05)80012-X. [DOI] [PubMed] [Google Scholar]
- 37.Sher A, Gazzinelli R T, Oswald I P, Clerici M, Kullberg M, Pearce E J, Berzovsky J A, Mosman T R, James S L, Morse H C, Shearer G M. Role of T-cell derived cytokines in the down regulation of immune responses in parasitic and retroviral infections. Immunol Rev. 1992;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 38.Surcel H M, Troye-Blomberg M, Paulie S, Andersson G, Moreno C, Pasvol G, Ivanyi J. Th1/Th2 profiles in tuberculosis, based upon proliferation and the cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994;81:171–176. [PMC free article] [PubMed] [Google Scholar]
- 39.ten Dam H G. Research on BCG vaccination. Adv Tuberc Res. 1984;21:79–106. [PubMed] [Google Scholar]
- 40.Titus R G, Sherry B, Cerami A. Tumor necrosis factor plays a protective role in experimental murine leishmaniasis. J Exp Med. 1989;170:2097–2104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turk J L, Bryceson A D M. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]
- 42.Wortis H H, Taylor R B, Dresser D W. Antibody production studied by means of the LHG assay. I. The splenic response of CBA mice to sheep erythrocytes. Immunology. 1966;11:663–669. [PMC free article] [PubMed] [Google Scholar]