Summary
Complex behaviors depend on the precise developmental specification of neuronal circuits, but the relationship between genetic programs for neural development, circuit structure, and behavioral output is often unclear. The central complex (CX) is a conserved sensory-motor integration center in insects that governs many higher-order behaviors and largely derives from a small number of Type II neural stem cells. Here, we show that Imp, a conserved IGF-II mRNA-binding protein expressed in Type II neural stem cells, plays a role in specifying essential components of CX olfactory navigation circuitry. We show: (1) that multiple components of olfactory navigation circuitry arise from Type II neural stem cells. (2) Manipulating Imp expression in Type II neural stem cells alters the number and morphology of many of these circuit elements, with the most potent effects on neurons targeting the ventral layers of the fan-shaped body. (3) Imp regulates the specification of Tachykinin-expressing ventral fan-shaped body input neurons. (4) Imp is required in Type II neural stem cells for establishing proper morphology of the CX neuropil structures. (5) Loss of Imp in Type II neural stem cells abolishes upwind orientation to attractive odor while leaving locomotion and odor-evoked regulation of movement intact. Taken together, our work establishes that a temporally expressed gene can regulate the expression of a complex behavior by developmentally regulating the specification of multiple circuit components and provides a first step towards a developmental dissection of the CX and its roles in behavior.
Keywords: Neural stem cells, Type II lineages, Temporal patterning, RNA-Binding proteins, Neural identity, Neural cell fate, Central complex, Neural Circuits, Neuropeptides, Olfactory navigation
Graphical Abstract

In brief:
Hamid et al. describe the lineages of olfactory navigation circuit elements in Drosophila and show that stem cell specific RNA-binding protein, Imp, regulates the specification and morphology of multiple circuit elements, specifically in the central complex. Imp regulates upwind orientation in response to odor.
Introduction
Proper brain function relies on the development of intricate neural circuits that integrate and store information to generate meaningful behavior. Developmental defects can cause devastating malformations of brain circuitry in humans, with profound consequences for learning and behavior1,2. Conversely, modifications to developmental genetic programs can underlie the evolution of more elaborate brain structures and behaviors from simpler ancestral patterns3,4. However, the relationship between genetic programs for neural development, neural circuit structure, and behavioral capabilities is challenging to study in complex vertebrate brains.
In insects, a conserved brain structure known as the central complex (CX)5,6 has been implicated in many higher-order behaviors, such as locomotion and navigation6–21, action selection22–24, steering25,26, feeding27,28, courtship29, and sleep30–37. The CX comprises several conserved structures38–41 (Figure Figure1A), including the ellipsoid body (EB), which houses a global heading representation15, and the protocerebral bridge (PB), which broadcasts this signal to other parts of the CX42,43. The function of the fan-shaped body (FB) is more mysterious, although recent studies have implicated this region in controlling locomotion25, computing allocentric variables20,21, and specifying behavioral goals44,45. A scaffold of columnar neurons links the different regions of the CX. Columnar inputs to the fan-shaped body encode both heading information derived from the protocerebral bridge and optic flow or airflow information from the paired noduli (NO)20,46. The FB also receives many tangential inputs that carry information from various parts of the dorsal brain and encode non-spatial stimuli such as odors45, tastants28, and internal states32. Local fan-shaped body neurons known as h∆ cells integrate input from columnar and tangential inputs45,47. Patterned stimulation of a subset of local neurons can drive reproducible navigation in an allocentric direction, implying that these neurons may encode the animal's goals44,45. Transmission electron microscopy (TEM) reconstruction of the hemibrain connectome48 has identified 224 morphological and 262 connectivity types in the CX47, many of which are primarily conserved in other insects such as bees49. The near-crystalline structure of the CX, along with its role in complex behaviors, make it an ideal model for investigating the relationship between neural development, circuit structure, and behavior.
Figure1. Overview of central complex structure and development, and olfactory navigation behavioral and circuitry:
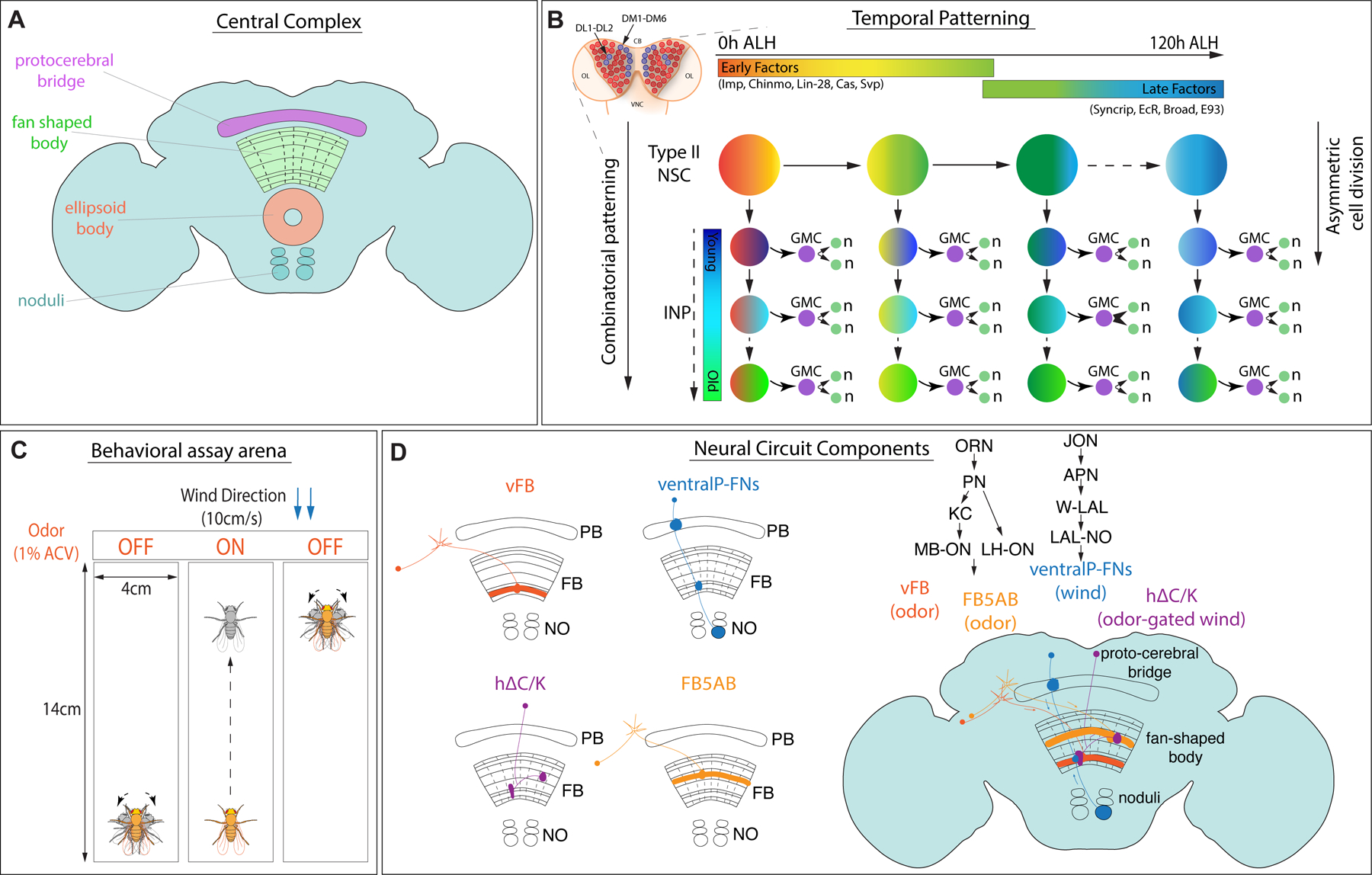
A. Drosophila adult brain central complex with labeled neuropils, protocerebral bridge (magenta), fan-shaped body (green), ellipsoid body (orange), and the paired noduli (blue). B. Type II NSCs in the larval brain (blue, 8 per lobe: DM1-6, and DL1-2; central brain-CB, ventral nerve cord-VNC, optic lobe-OL) divide asymmetrically, producing another NSC and an intermediate neural progenitor (INP). The INP divides and gives rise to another INP and a ganglion mother cell (GMC), which eventually forms two neurons and/or glia. Type II NSCs express early and late factors in a time-dependent manner starting from 0h ALH to 120h ALH referred to as early and late factors, respectively. In addition to temporal patterning in NSCs, INPs also express different factors in a birth order-dependent manner along with NSC factors and give rise to a combinatorial code. C. Schematic of the behavioral assay arena; walking flies are observed in a rectangular chamber (14cmX4cm) with constant flow of wind from one direction at 10cm/s. Odor is provided in 10s pulse (1% ACV) centered in a 70s trial. Flies tend to walk upwind in the presence of odor and show search behavior triggered by odor loss79. D. CX neurons involved in olfactory navigation investigated in this study. Long-field tangential neurons encode odor, and columnar ventral P-FNs encode wind. Local hΔC/K neurons integrate odor and wind45.
While multiple lineages give rise to the adult CX50–52, with anatomically distinct compartments53,54, the majority of adult Drosophila CX neurons arise from 8 bilateral larval neural stem cells known as Type II neural stem cells (based on their location: dorsomedial, DM1-6, and dorsolateral, DL1-2). In contrast to the more common Type I neural stem cells, which divide to self-renew and generate a pair of differentiated neural progeny, Type II neural stem cells divide asymmetrically to self-renew and produce an intermediate neural progenitor (INP)55,56. INPs further divide multiple times to generate 8–12 progeny 57–61 (Figure Figure1B). The dual division pattern of Type II neural stem cells is shared by the outer radial glia (oRG), stem cells that generate the primate and human cortex62–64. Intermediate progenitors have been reported in the human cortex as well65–67. Type II-like neural stem cells are also found in grasshoppers and beetles68–71, making them evolutionarily-conserved progenitors that generate higher-order brain centers. The CX columnar neurons arise from four bilateral Type II neural stem cells called DM1-4, while tangential neurons arise from mixed lineages, with a significant contribution from DL150,54.
To generate diverse classes of neuronal types over time, Type II neural stem cells express sets of temporally regulated transcription factors and RNA binding proteins (RBPs)72,73. In addition, INPs have been shown to express different factors in a birth-order-dependent manner that play a role in generating neural identity74–77. Using RNA-Seq methods, we and others have identified about a dozen temporally expressed genes in Type II neural stem cells72,73. These factors can be divided into two groups: early factors detected from 0-60h ALH (after larval hatching) and late factors from 60-120h ALH (Figure Figure1B). Early expressed factors include the RBPs Imp and Lin-28, and the transcription factors Chinmo, Seven-up, and Castor. Late factors include the RBP Syncrip and the transcription factors, Ecdysone receptor (EcR), Broad, and Ecdysone-induced protein 93 (E93)72. In addition, extrinsic hormonal signaling via timely expression of the receptor EcR at ~60h ALH induces an early-to-late gene transition in Type II neural stem cells72. Opposing gradients of Imp and Syncrip expression have previously been shown to regulate the number and morphology of neurons in the CX73. However, how these temporally expressed factors contribute to functional circuit formation, and behavior is unknown.
Here we use an innate goal-oriented behavior— olfactory navigation— and an associated neural circuit to gain insight into how developmental programs specify neural types and shape circuit structure to determine behavioral output. When a hungry fly encounters an attractive odor, it will turn and increase its velocity upwind78,79, a conserved behavior observed across many arthropods80 (Figure Figure1C). In a previous study, we implicated several CX neuron types in this behavior45 (Figure Figure1D, Table 1). The sensory cues required for this behavior— odor and wind direction— are encoded by different types of FB inputs. Tangential input neurons (vFB, mFB, dFB, FB5AB) innervate different layers of FB and encode odor45, while columnar input neurons (ventral P-FNs) encode wind direction46. A set of local FB neurons labeled by the line VT062617-GAL4 integrates these cues and is required for persistent upwind tracking in flies45. In that study, we identified this line as labeling h∆C neurons; however, single-cell clones revealed that this line also labels h∆K neurons which project to the EB (Figure S1A–C). We, therefore, refer to this population as h∆C/K. Here we show that all elements of this circuit are derived from Type II neural stem cells and that Imp is required for the specification of both tangential and columnar neurons targeting the ventral fan-shaped body. We find that different circuit elements show distinct requirements for Imp either in neural stem cells or post-mitotically. Imp knockdown in Type II neural stem cells shapes the gross morphology of the CX and the expression of neuropeptides within specific layers of the FB. Finally, we show that manipulating Imp expression in Type II neural stem cells profoundly impairs olfactory navigation behavior. Collectively, our findings demonstrate a novel role of Imp in the formation and function of an olfactory navigation circuit and trace the developmental origins of distinct components of a behavioral circuit.
Table 1:
Driver lines used for each neuron class
| GAL4/LexA line | Neuron class labeled |
|---|---|
| VT029515 | Ventral FB tangential inputs (vFB) |
| VT062617 | h∆C/K FB local neurons |
| 21D07 | FB5AB |
| 15E12/30E10 | Ventral P-FNs, FB column inputs |
| 65C03 | Dorsal FB tangential inputs (dFB) |
| 12D12 | Mid FB tangential input (mFB) |
| 25D01 | MBONs (MBON-12) |
| 55C09 | LH-ONs (LHAd1b2) |
| 38F04 | Descending neurons (DNp09) |
Results
Type II neural stem cells generate multiple components of an olfactory navigation circuit
The majority of the neurons populating the adult CX are derived from 16 larval Type II neural stem cells50 (schematics shown in Figure 2A). We first asked whether previously identified elements of an olfactory navigation circuit in the CX arise from these stem cells and determined their lineage. We used an intersectional genetic strategy to express GFP in specific neuron classes in a Type II specific manner (genetic scheme shown in Figure 2B). Wor-GAL4 is expressed in all neural stem cells, while Ase-GAL80, the repressor for GAL4, is expressed only in Type I neural stem cells, leaving GAL4 active in only Type II neural stem cells. Flippase, now expressed only in Type II neural stem cells, permanently flips out a stop sequence between LexAop and GFP, making Type II progeny eligible to express GFP under LexA control. A neuron class-specific LexA can then drive expression of GFP only if those neurons are derived from Type II neural stem cells.
Figure 2. Lineage analysis of olfactory navigation circuit components:
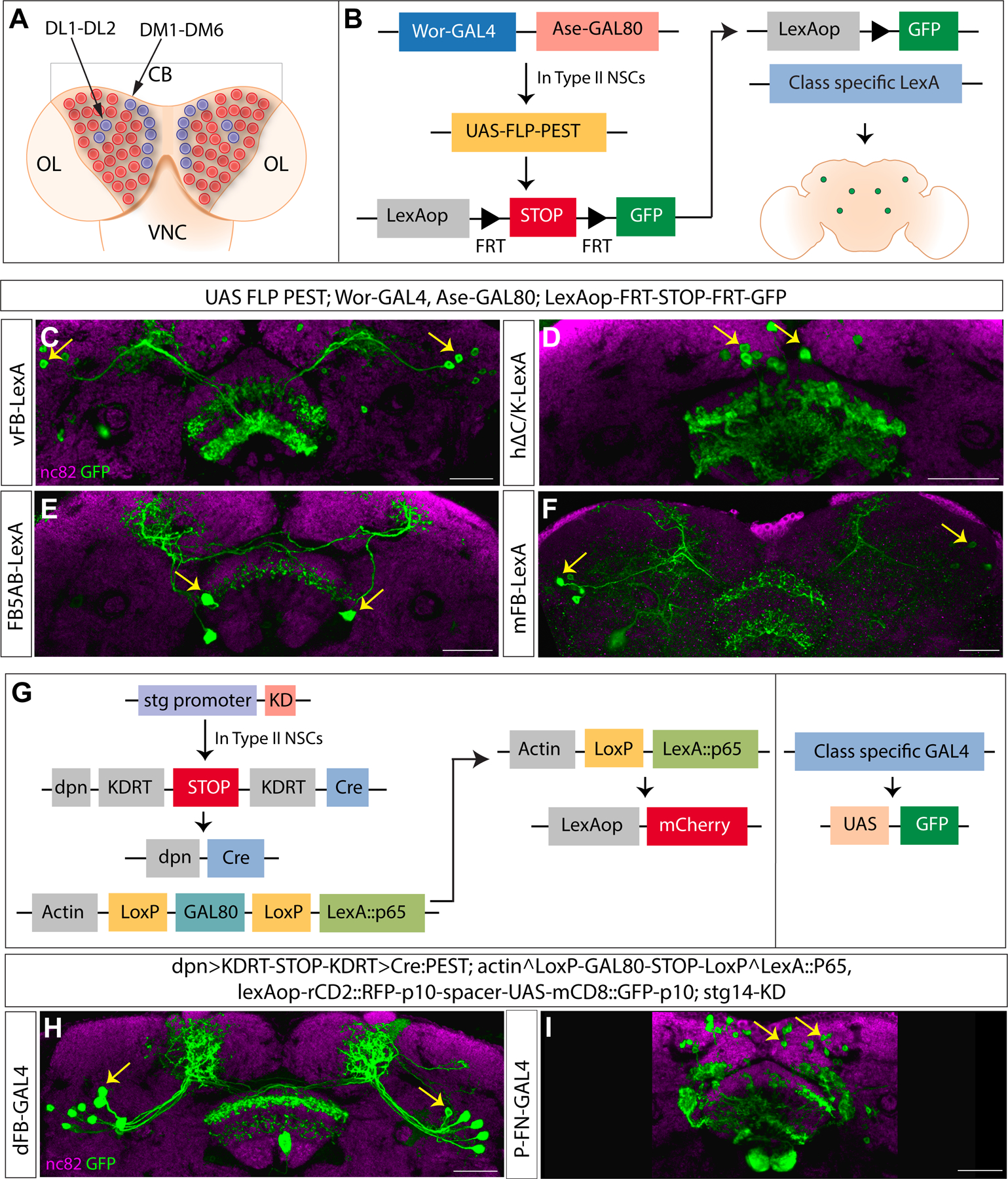
A. Schematic showing 8 Type II NSCs per larval brain lobe (blue) DM1-6 and DL1-2, among other NSCs (Type 0 and Type I) in red. Central brain-CB, ventral nerve cord-VNC, optic lobe-OL. B. Genetic scheme for Type II lineage analysis using Type II specific Flip-out. Ase-GAL80 active in Type I NSCs leaves Wor-GAL4 active only in Type II NSCs. Flippase downstream of a UAS sequence is activated in a Type II-specific manner and removes the STOP sequence between two FRT sites. This causes expression of reporter GFP in class-specific manner when crossed to a class-specific LexA driver line. C-F. Confocal images showing neuron types expressing reporter GFP in a Type II dependent manner. G. Genetic scheme for Type II lineage filtering using the CLIn technique. A Type II specific promoter fragment (stg) combined with KD recombinase removes a STOP sequence by a flip event and leaves Cre recombinase active in Type II NSCs. Cre generates Type II NSC clones positive for LexA::p65. GAL80 is active in the rest of the cells, and GAL4 expresses GFP as a reporter in class specific manner. We did not stain for mCherry here. H-I. Confocal images showing neuron types expressing reporter GFP in a Type II dependent manner. nc82 stain in magenta counterstains the brain, a stack of 2 slices was taken for visualizing the neuropil with nc82. GFP reporter is shown in green. Cell bodies are indicated by yellow arrows. The genotypes are shown at the top, and the abbreviated genotypes for neuron class are shown at the left. Scale bars correspond to 30μm. See also Figure S1, Table 1 and Video S1
Using this strategy, we found that several components of the CX olfactory navigation circuitry are derived from Type II neural stem cells. These include multiple tangential FB inputs that encode odor (vFB, mFB, and FB5AB), as well as local neurons that integrate odor and wind information (h∆C/K, Figure 2C–F). We used a different genetic strategy for two additional neuron classes to determine their lineage, as we had only GAL4 and not LexA lines for these classes at the time. This strategy is based on the Cell class-lineage intersection (CLIn) transgenic system81. Here multiple flip-out events mediated by Flippase, Cre, and KD recombinases restrict the clonal analysis to Type II neural stem cells. The Type II specific promoter Stg drives the expression of the reporter A-mCherry in a lineage-specific manner, and a class-specific GAL4 drives the expression of reporter B-GFP in a Type II dependent manner (genetic scheme shown in Figure 2G). This analysis showed that two additional components of olfactory navigation circuitry, an odor-encoding dorsal tangential input (dFB) and a wind-encoding columnar input (ventral P-FNs), also arise from Type II neural stem cells (Figure 2H–I). Taken together, our lineage analysis shows that multiple elements of an olfactory navigation circuit in the CX arise from Type II neural stem cells.
Mid-aged dorsolateral Type II neural stem cells produce odor-encoding ventral FB tangential input neurons
Our lineage analysis revealed that several tangential FB input neurons are derived from Type II neural stem cells. We decided to examine the lineage and birth time of one population of ventral tangential FB inputs (vFB) more closely. To do this, we used CLIn combined with a heat-sensitive Flippase, allowing us to determine the birth time of a specific neuronal class (complete genetic scheme shown in Figure 3A). Heat shock was given by shifting larvae to 37°C at different time points after larval hatching (ALH) (Figure 3B).
Figure 3. Birth-dating vFB long field tangential input neurons using cell class lineage intersection system:

A. Schematics describing the genetics of the CLIn system combined with a heat shock promoter for genetic birth-dating: Type II specific stg promoter fragment combined with KD recombinase removes a STOP sequence by a flip event, and an intact Flippase is reconstituted. This combined with heat-sensitive promoter removes STOP sequence and leaves Cre-recombinase active in Type II NSCs in a heat shock dependent manner. Cre in turn generates Type II NSC clones positive for LexA::p65. GAL80 is active in the rest of the cells, and GAL4 expresses GFP as a reporter in class-specific manner, while mCherry downstream of LexAop sequence is expressed as a reporter in a lineage-specific manner. B. Schematics representing heat shock given at different time points (ALH-after larval hatching), yellow arrow indicates heat shock, and GFP expression window is shown in green. C. Schematics showing the map of the progeny of the DL1 clone populating dorsal and ventral layers of the FB75 in the adult brain. D. Adult brain sample of 0h ALH DL1 clone of CLIn crossed to VT029515 GAL4 labeling vFB neurons, nc82 counterstains the brain, a stack of 2 slices was taken for visualizing the neuropil with nc82. DsRed in cyan labels the DL1 progeny, GFP in green labels vFB neurons. E-H. Adult brain samples of 0h, 24h, 48h, and 72h ALH clones, GFP in green labels vFB neurons. I. Quantification of number of cell bodies counted per hemibrain at indicated time points (n=data points indicated on the graph). J.17A12-GAL4 flipped vFB neurons, nc82 counterstains the brain, a stack of 2 slices was taken for visualizing the neuropil with nc82. GFP in green labels vFB neurons. K. Quantification of number cell bodies per hemibrain flipped by 17A12-GAL4.The genotypes are shown at the top, and the abbreviated genotypes for neuron class are shown at the left. Scale bars correspond to 30μm. See also Figure S1, Video S1 and S3
The progeny of DL1 neural stem cells have been earlier reported to innervate the FB in two distinct bundles, dorsal and ventral50 (schematics shown in Figure 3C). Single CLIn clonal analysis revealed that most vFB neurons are derived from the DL1 neural stem cell (number of cell bodies=7) (Figure 3D, Video S1), and a few are derived from DMs (number of cell bodies= 2, data not shown). Next, we performed genetic birth-dating by inducing flip-out events via temporal heat shock at different developmental time points in larvae: 0h, 24h, 48h, and 72h ALH. This genetic birth-dating revealed that most Type II derived vFB neurons are generated between 48h-72h ALH (Figure 3E–I), an interval spanning the early-to-late gene expression switch at 60h in all Type II neural stem cells72. The number of cell bodies counted in each sample varies; this could be due to variations in multiple flip-out events in the genetic technique. Combining our clonal analysis and genetic birth-dating assays, we conclude that vFB input neuron types are born from mid-aged DL1 neural stem cells. To confirm this result, we used an intersectional genetic strategy. From an independent screen, we identified that 17A12-GAL4 selectively labels DL1 and DL2 Type II neural stem cells during development (Figure S1D–E). We used this line to express Flippase and excise an intervening STOP cassette from LexAop-FRT-STOP-FRT-GFP only in DL1 and DL2 neural stem cells. We combined these transgenes with VT029515-LexA to label vFB tangential input neurons. Upon expression of Flippase by 17A12-GAL4, we observed 4–8 vFB neurons labeled (n=14 hemibrains) (Figure 3J–K). Variation in the number of neurons labeled might arise from variability in Flippase expression, and some vFB subtypes being derived from DM Type II neural stem cells. These findings support our hypothesis that most vFB neuron types are born from mid-aged DL1 Type II neural stem cells, while some are derived from other Type II neural stem cells.
Imp regulates the specification of odor-encoding ventral FB tangential input neurons
Early Type II neural stem cells express the transcription factors Chinmo, Castor, Seven up, and the conserved RBPs, Imp and Lin-28. To test our hypothesis that an early neural stem cell factor regulates the fate of vFB neurons, we focused on a conserved RBP, IGF-II mRNA-binding protein, Imp82. We asked if Imp expression during development determines the number and morphology of vFB neurons. We expressed either UAS-ImpRNAi (2 copies) or UAS-Imp in Type II neural stem cells throughout development using Pointed-GAL4 that labels all Type II neural stem cells, and assayed animals at adult stages. We combined this manipulation with a LexA-based GFP reporter to examine the number and morphology of vFB neurons (VT029515-LexA). We found that Imp manipulations profoundly affected the specification of vFB neurons. In control animals, we counted about 6–7 cell bodies per hemisphere (Figure 4A, A’, H). These neurons were completely absent in Type II>ImpRNAi flies (Figure 4B, B’, H). To test if Imp is sufficient for generating vFB neurons, we ectopically expressed Imp in Type II neural stem cells, Pointed-GAL4>UAS-Imp. In these animals, Imp is now expressed in young as well as late Type II neural stem cells83. We observed a ~3-fold increase in the number of vFB cell bodies with Imp overexpression. These ectopic neurons formed part of the same axon bundle and similarly innervated the ventral layer of the fan-shaped body (Figure 4C, C’, H). To determine whether the ectopic and endogenous vFB neurons have the same identity, we stained for a neuropeptide Tachykinin (TK), known to be expressed in the ventral layers of the FB84. We observed that the vFB arborization in the ventral layers of the FB colocalizes with TK immunofluorescence (Figure 4A’’). Interestingly, TK expression was lost in the ventral layers upon Imp knockdown, suggesting an essential role of Imp in specifying neurons with TK neuropeptide identity (Figure 4B’’). In contrast, Imp gain of function increased TK staining in the ventral layers, which co-localized with the innervations of the ectopic vFB neurons (Figure 4C’’). To confirm that vFB neurons express TK, we drove Flippase with TK-GAL4 and used vFB-LexA>LexAop-FRT-stop-FRT-GFP to express GFP only in vFB neurons that also express TK-GAL4. We observed 1–3 GFP-positive neurons per hemibrain (n=6), indicating that VT029515 are indeed TK positive (Figure 4D–D’’). Together, these results suggest that ectopic vFB neurons are morphologically and molecularly similar to normal vFB neurons, and Imp likely regulates TK neuropeptide expression in the FB. (Figure 4A’’–C’’).
Figure 4. Imp is required for specifying and maintaining the identity of odor encoding vFB long field input neurons:
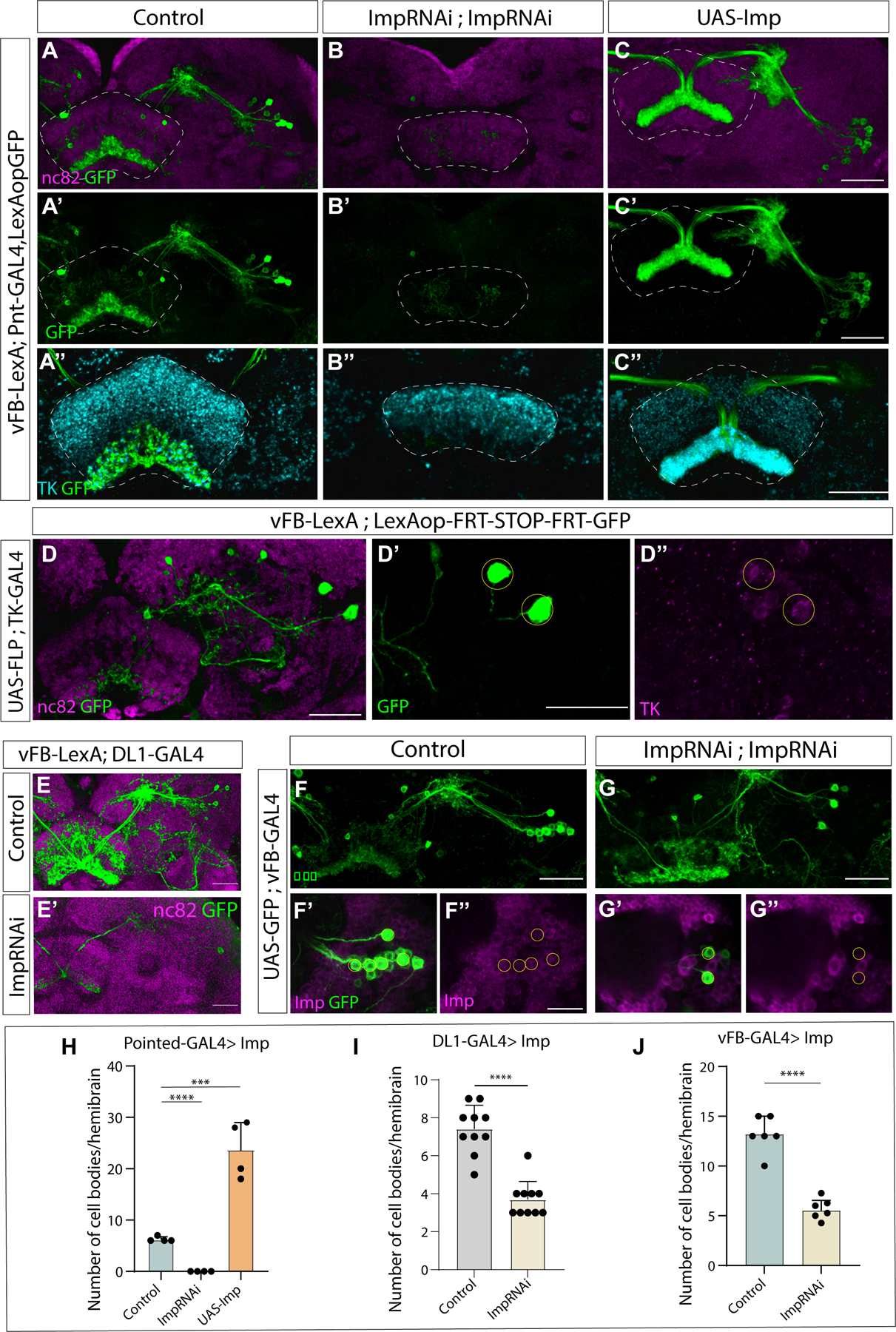
A-C’. Effects of Type II-specific knockdown (Pointed-GAL4>ImpRNAi; ImpRNAi) or overexpression (Pointed-GAL4>UAS-Imp) of Imp on vFB neurons. vFB neurons labeled by reporter GFP in control (A), Imp knockdown (B), and Imp overexpression (C), nc82 counterstains the brain, and GFP is shown in green. A’’-C’’. vFB neuron arbors shown by GFP expression in green, neuropeptide TK staining shown in cyan colocalizing with GFP for control (A’’), Imp knockdown, expression observed in dorsal layers of FB (B’’) and Imp overexpression, TK expressed in thick bundle colocalizing with GFP expression (C’’). D. vFB neurons labeled with GFP via conditional expression of GFP using TK-GAL4, nc82 counterstains the brain. Zoomed in view of vFB cell bodies, GFP in green (D’), and TK expression in magenta (D’’), (n=6). E-E’. Effects of DL1/2 specific knockdown of Imp on vFB neurons. vFB neurons labeled by reporter GFP are shown in green in control (E) and Imp knockdown (17A12-GAL4>ImpRNAi; ImpRNAi) (E’), nc82 counterstains the brain. F-G’’. Effects of post-mitotic knockdown of Imp on vFB neurons. vFB neurons labeled by reporter GFP are shown in green, Imp expression is shown in magenta in control (F-F’’) and Imp knockdown (vT029515-GAL4>ImpRNAi; ImpRNAi) (G-G’’). For F’-F’’ and G’-G’’, the Scale bar corresponds to 15μm.
Quantification of the number of cell bodies per hemibrain for Type II specific knockdown (H), DL1 specific knockdown (I), and post-mitotic knockdown (J). n=data points indicated on the graphs (Students t-test). The abbreviated genotypes are shown at the left and top, respectively. Scale bars correspond to 30μm (unless stated otherwise). The dashed outline shows FB in A-C’’ and cell bodies in D’-D’’ and F’’ and G’’. See also Figures S1, S2, S3, S4 and Table 1
Next, we used17A12-GAL4 to knockdown Imp specifically in DL1/DL2 Type II neural stem cells specifically, and as expected, we observed a significant decrease in vFB neuron number (Figure 4E–E’, I). These results suggest that Imp is required in Type II neural stem cells to specify the majority of the DL1-derived vFB neurons. Some vFB neurons that survive could be due to weak expression of 17A12-GAL4, and/or the surviving neurons are derived from DM Type II neural stem cells. Knockdown of the late NSC factor Syncrip did not affect vFB neuron numbers (Figure S2A–C). Knocking down Imp using a single ImpRNAi transgene resulted in a complete loss of vFB neurons (Figure S2D–F), confirming the essential role of Imp in the proper development of vFB tangential input neurons.
We observed that vFB neurons continue to express low levels of Imp in adulthood (Figure 4F–F’’). This led us to ask if Imp is required post-mitotically in these cells to maintain their identity. To address this question, we knocked down Imp post-mitotically in these neurons using VT029515-GAL4. We observed a decrease in cell body number in Imp knockdown compared to control, and Imp is undetectable in the small number of surviving neurons (Figure 4G–G’’ and J). We conclude that Imp is also required post-mitotically to maintain the identity of vFB neurons.
Distinct roles of Imp in regulating ventral and dorsal circuit elements
We next asked whether Imp also specifies other essential neuron types of olfactory navigation circuitry. Using a similar strategy to the one described above, we knocked down or overexpressed Imp during development using Pointed-GAL4, a broad driver for Type II neural stem cells, and assayed effects on each circuit element using LexA drivers and a GFP reporter in adult brains. Interestingly, we found that Imp knockdown in Type II neural stem cells caused a loss of wind-sensitive columnar neurons known as ventral P-FNs (here labeled by 30E10-LexA). However, unlike vFB neurons, the number of cell bodies did not change upon Imp overexpression (Figure 5A–C).
Figure 5. Imp regulates specification and morphology of other neural types in the olfactory navigation circuit:
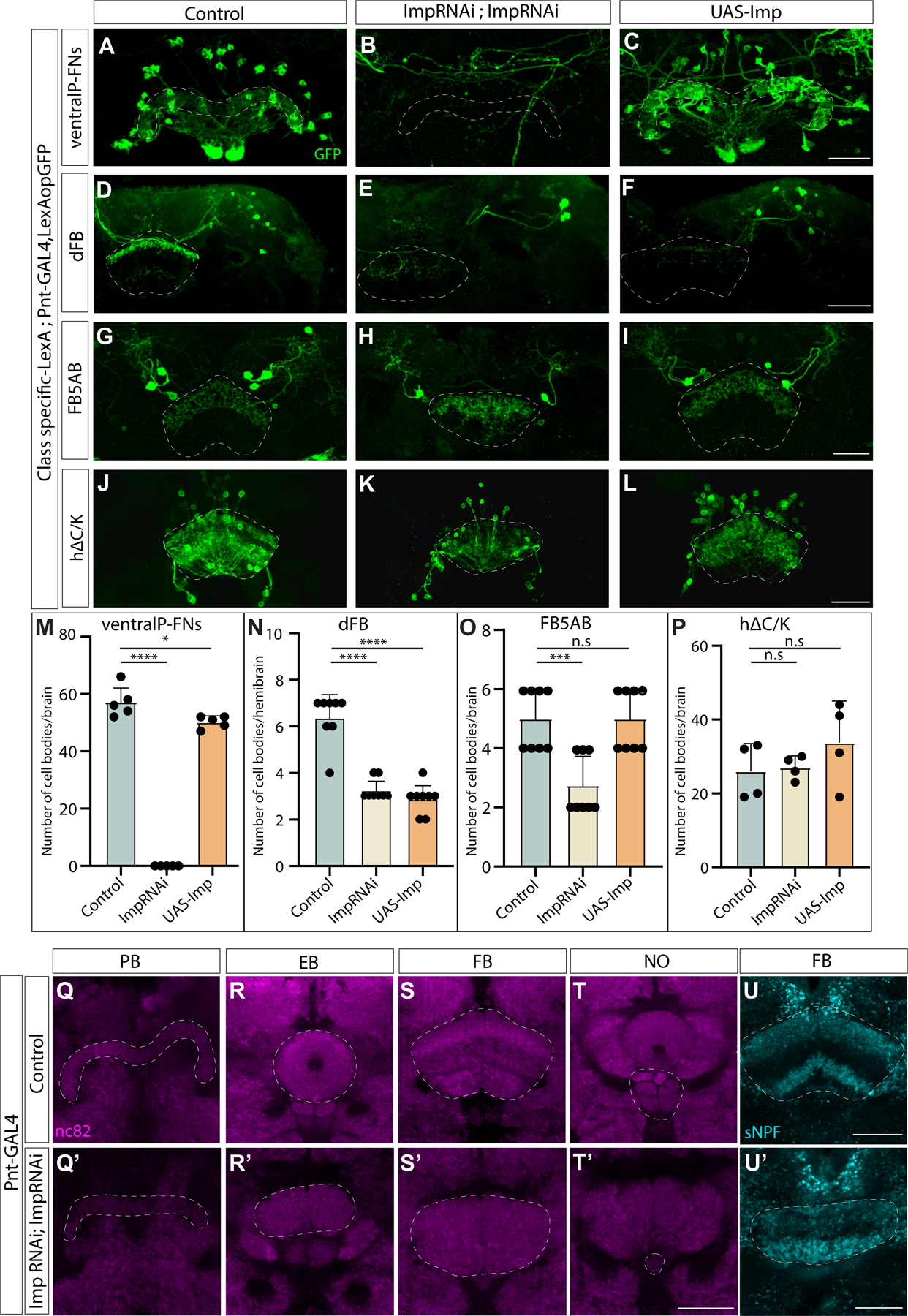
A-C. Ventral P-FN neurons labeled by reporter GFP shown in control (A), Imp knockdown (Pointed-GAL4>ImpRNAi; ImpRNAi) (B), and Imp overexpression (Pointed-GAL4>UAS-Imp) (C). D-F. dFB (65C03) neurons labeled by reporter GFP shown in control (D), Imp knockdown (Pointed-GAL4>ImpRNAi; ImpRNAi) with defective morphology of the neurites in FB (E), and Imp overexpression (Pointed-GAL4>UAS-Imp) (F). G-I. FB5AB input neurons labeled by reporter GFP in control (G), Imp knockdown (Pointed-GAL4>ImpRNAi; ImpRNAi) with defective morphology of the neurites in FB (H), and Imp overexpression (Pointed-GAL4>UAS-Imp) (I). J-L. hΔC/K neurons labeled by reporter GFP in control (J), neurites arborize multiple layers of FB with no clear demarcation in Imp knockdown (Pointed-GAL4>ImpRNAi; ImpRNAi) (K) and Imp overexpression (Pointed-GAL4>UAS-Imp) (L). GFP is shown in green, the abbreviated genotypes are shown at left and top. Scale bars correspond to 30μm. M-P. Quantification of number of cell bodies. Abbreviated neuron names are indicated at the top. n=data points indicated on the graphs. (Students t-test). Q-U’. The four neuropils of the CX: PB, NO, FB, and EB in control flies (Q-T), and Imp knockdown resulting in defective morphology of the CX neuropils (Q’-T’). nc82 stains the neuropils of the brain, all outlined using dashed lines, (n=22). U-U’. sNPF stains shown in cyan in control distributed in dorsal and ventral layers of FB distinctly (U), and Imp knockdown shows impaired distribution (U’). Scale bars correspond to 30μm. See also Figures S2, S4 and Videos S2 and S3
Since both dorsal and ventral FB tangential input neurons respond to odor45; next, we wanted to know whether Imp regulates the fate of both dorsal and ventral tangential inputs. We first focused on dFB odor input neurons, which are 6–8 in number per hemibrain, and project strongly to the dorsal layers and weakly to the ventral layers of the FB. We found that both Imp knockdown and overexpression decreased the number of tangential inputs labeled by 65C03-LexA (Figure 5D–F). In Imp knockdown flies, we observed only a single projection layer. Moving to the FB5AB input neurons, we found that 21D07-LexA labels 4–6 neurons projecting to dorsal FB per brain. We observed 2–4 cell bodies in Imp knockdown flies; this could be a decrease in dFB odor input neurons or loss of another subpopulation labeled by this LexA line. In Imp knockdown flies, the neurites of these neurons had severely impaired morphology. Imp overexpression did not alter the number of FB5AB tangential inputs, and the morphology was unaffected as well (Figure 5G–I). The number of local h∆C/K neurons labeled by VT062617-LexA did not change upon Imp knockdown or overexpression (Figure 5J–L). However, the morphology of h∆C/K neurons was altered in Imp knockdown. We observed that Imp knockdown caused axonal projections of h∆C/K to span multiple FB layers, and the division of dendrites and axons into ventral and dorsal layers was lost (Figure 5K). Together, these data suggest that Imp expression during development has the most potent effects on neurons innervating the ventral layers of the fan-shaped body, including both tangential inputs (vFB) and columnar inputs (ventral P-FNs). Manipulating Imp expression had distinct effects on each neuron type, suggesting that Imp plays multiple roles and acts via multiple mechanisms to regulate cell fate and morphology. These defects suggest that neurons still present in Imp loss-of-function may have altered connectivity.
Imp regulates CX neuropil morphology and neuropeptide distribution
Our previous results show that Imp loss-of-function influences the morphology of the neurite projections for many of the elements within a CX olfactory navigation circuit. To test if the adult CX is more broadly altered by Imp knockdown in Type II neural stem cells, we compared neuropil (nc82) stains in knockdown and control flies. We found that Imp knockdown results in severely defective CX morphology. The PB is fragmented, the EB is only partially formed, the NO is almost entirely absent, and the FB is smaller, with no clear demarcation of layers (Figure 5Q–T’, Video S2 and S3). This phenotype is consistent in all Pointed-GAL4>ImpRNAi flies in Figures 4 and 5 (n=22). The FB phenotype is reminiscent of cortical layer deformations observed in Imp1 knockout mice85. Knockdown of Imp in DL1/DL2 Type II neural stem cell only using 17A12-GAL4 does not alter CX morphology and architecture. (Figure S3).
Next, we wanted to examine the role of Imp in more broadly defining the neuropeptide distribution of FB layers. Previously we described the expression pattern of the neuropeptide TK in the CX (Figure 4). We then examined the expression of short neuropeptide F (sNPF), which is reported to be strongly expressed in FB ventral layers 1–2 and dorsal layers 6–784. In Imp knockdown flies, sNPF appears to be reduced in the dorsal layers (Figure 5U,U’). Together these results suggest that both the overall morphology of distinct neuropils and neuropeptide distribution of the CX are dependent on Imp expression in Type II neural stem cells during development.
Imp is required in Type II neural stem cells for upwind orientation during olfactory navigation behavior
Given the profound effects of Type II-specific Imp expression on olfactory navigation circuitry, we sought to understand the impact of Imp on adult olfactory navigation behavior. We used Pointed-GAL4 to knockdown Imp in Type II neural stem cells, as in our anatomical experiments, using two copies of ImpRNAi. We then measured behavioral responses to a 10-second pulse of attractive odor (1% apple cider vinegar) using a previously described wind tunnel paradigm79.
Knocking down Imp in all Type II neural stem cells significantly impaired olfactory navigation behavior. In control flies (Pointed-GAL4>mCherryRNAi), odor evoked an increase in upwind velocity, an increase in groundspeed, and a decrease in angular velocity, while odor loss evoked an increase in turning (Figure 6A, C), as observed previously79. Odor also increased the probability of movement, and this effect outlasted the stimulus by tens of seconds. In Imp knockdown flies (Figure 6B, C), upwind velocity, ground speed, and angular velocity responses to odor were dramatically reduced. However, an odor-evoked increase in the probability of movement was still observed, arguing that the olfactory system is still functional and can influence motor behavior. To better understand these behavioral deficits, we decomposed upwind velocity during odor into two components: upwind orientation, and ground speed. Upwind orientation was strikingly decreased in Imp knockdown flies (Figure 6D). In contrast, the distribution of groundspeeds during odor (for moving flies) was not substantially different for Imp knockdown flies versus controls (Figure 6E). The trajectories of Imp knockdown flies also showed pronounced zig-zagging (Figure 6B) highlighted by a greater average angular velocity (Figure 6C) and a greater probability of making large amplitude turns (Figure 6F). This suggests that in the absence of Imp, flies make more larger turns and have difficulty maintaining a stable heading.
Figure 6. Imp regulates the upwind orientation during olfactory navigation:
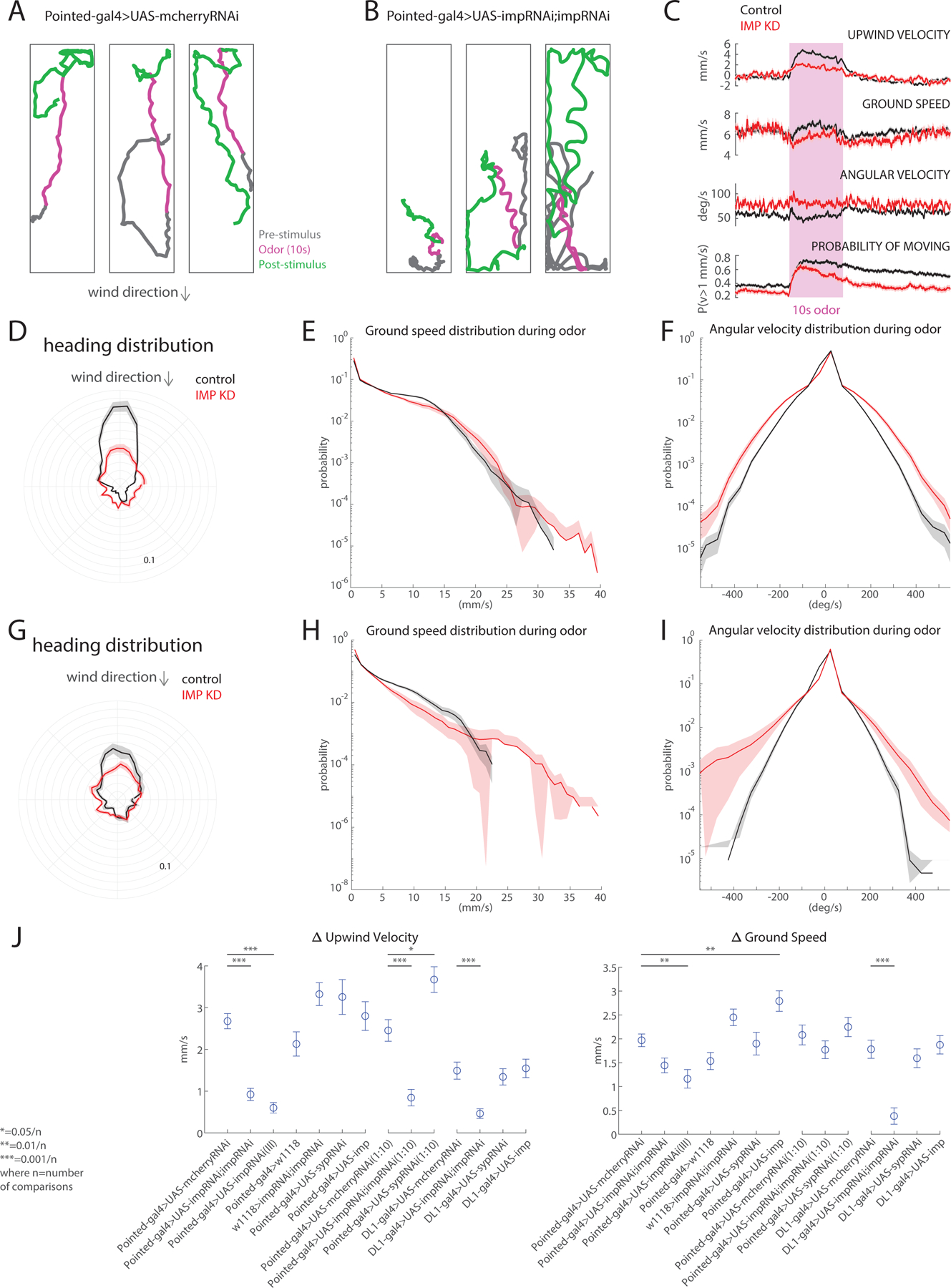
A. Representative walking trajectories of three different control flies (Pointed-GAL4>UASmCherryRNAi) presented with a 10s odor pulse (1% ACV, magenta) centered in a 70s trial with 10cm/s wind. Flies demonstrate upwind movement during odor and local search after odor offset. Wind direction indicated above. B. Representative walking trajectories of three different Imp knockdown flies (Pointed-GAL4>UAS-ImpRNAi;UAS-ImpRNAi) in the same wind and odor conditions as Figure A. Trajectories reveal lack of upwind movement during odor and increased turning behavior throughout the entirety of trial. C. Comparison of movement parameters between control (black, n = 86 flies) and Imp knockdown flies (red, n = 61 flies) calculated as an average across flies (mean±SEM; see Methods). Pink-shaded area: 10-second odor stimulation period (1% ACV). In the presence of odor, Imp knockdown flies show decreased upwind velocity comparable to control flies. Increased turning behavior noted in Figure B can be observed quantitatively through the increased angular velocity throughout the duration of trials. D. Heading distribution during odor for both control (black) and Imp knockdown (red) flies (wind direction indicated above). Imp knockdown flies exhibit decreased upwind orientation. E. Histogram showing ground speed distributions during odor for both control (black) and Imp knockdown (red). Distributions appear similar, suggesting that general movement is not impaired by Imp knockdown. F. Histogram showing angular velocity distributions for both control (black) and Imp knockdown flies(red) during odor. Imp knockdown flies favor larger angular velocities, even in the presence of an attractive stimulus. G. Heading distribution during odor for both control (black) and knockdown of Imp (red) in DL1/2 Type II NSCs (wind direction indicated by above arrow). H. Histogram showing ground speed distribution during odor for both control (black) and Imp knockdown (red) flies. Imp knockdown flies tend towards lower ground speeds than control counterparts. I. Histogram showing angular velocity distribution during odor for both control (black) and Imp knockdown (red) flies. Widening of distribution indicates that Imp knockdown flies favor larger angular velocities. J. Change in upwind velocity and ground speed, calculated as the difference in the mean value during the first 5 seconds of odor and the 5 seconds preceding odor, across various control and experimental genotypes. Knockdown of Imp results in significant decreases in upwind velocity change when compared to respective controls, across multiple odor strengths and driver lines (Pointed-GAL4>mCherryRNAi compared to pointed>double ImpRNAi p = 5.906x10^-11, Pointed-GAL4>mCherryRNAi compared to Pointed-GAL4>ImpRNAi(III) p=1.14768x10^-9, Pointed-GAL4>mCherryRNAi (1:10 AVC) compared to Pointed-GAL4>double ImpRNAi (10:1) p=1.2293x10^-4, DL1>mCherry compared to DL1>double ImpRNAi p=9.922x10^-6). Knockdown of late transcription factor Syncrip (Syp) results in a significant increase in upwind velocity when using a higher odor concentration (1:10 ACV, Pointed-GAL4>mCherryRNAi compared to Pointed-GAL4>SypRNAi (10:1) p=0.0042). Knockdown of Imp using a single copy of the RNAi results in a significant decrease in ground speed (Pointed-GAL4>mCherryRNAi compared to Pointed-GAL4>ImpRNAi (III) p=0.0015), as does knockdown in DL1/DL2 Type II NSCs (17A12-GAL4> ImpRNAi;ImpRNAi ,two-sample t-test; p =2.2281x10^-7). Upregulation of Imp results in increased ground speed but no change to upwind velocity (Pointed-GAL4>mCherryRNAi compared to Pointed-GAL4>UAS-Imp p=7.7113x10^-4). All comparisons were completed using an unpaired t-test with Bonferroni correction for multiple comparisons. See also Figures 4, S1 and S2
As Imp knockdown in Type II neural stem cells has broad effects on CX morphology (Figure 5Q–T’’), we next performed a series of control experiments to determine the specificity of behavioral deficits. We first asked whether we could also see behavioral deficits when knocking down Imp only in the DL1/DL2 Type II neural stem cells. This manipulation decreases the number of vFB neurons (Figure 4E,E’), while leaving overall CX morphology intact (Figure S3). This manipulation also reduced both upwind orientation and average groundspeed during odor (Figure 6G–H), suggesting that a narrower manipulation also impacts olfactory behavior. We also asked if we could see behavioral deficits when knocking down a different late-expressed RBP, Syncrip, which regulates Imp expression in Type II neural stem cells. Knockdown of Syncrip in Type II neural stem cells was previously shown to produce similar anatomical phenotypes to Imp overexpression73; however, we found that it did not affect vFB neuron number (Figure S2A–C). Knockdown of Syncrip produced no change in upwind velocity in response to 1% ACV but produced an increase in upwind velocity in response to 10% ACV (Figure 6J), consistent with the idea that Imp and Syncrip produce opposing anatomical effects. Finally, we repeated our experiments using a single ImpRNAi transgene which also results in a complete loss of vFB neurons (Figure S2D–F) and using a higher concentration of odor (10% vs 1% apple cider vinegar). In both cases we observed similar effects of Imp knockdown on behavior (Figure 6J). We saw no change in upwind velocity modulation in parental strains (Pointed-GAL4 or ImpRNAi) crossed to wild-type.
To further determine whether the behavioral phenotypes we observed arise from CX deficits, we examined the effects of Imp knockdown on elements of olfactory navigation circuitry up- and down-stream of the CX. We examined three neuron types that have previously been implicated in olfactory navigation or locomotion. Lateral horn output neurons known as LHAD1b286 and Mushroom Body output neurons known as MBON-1287 both respond to odor and drive strong upwind movement45. Descending neurons known as DNp09 are known to drive changes in forward velocity and turning when activated88. Knocking down Imp in Type II neural stem cells had no effect on any of these neuron types. The neuron number and morphology of lateral horn output neurons (LH-ONs) labeled by 55C09-LexA, mushroom body output neurons (MBONs) labeled by 25D01-LexA, and descending neurons (DNs) labeled by 38F04-LexA were unaltered (Figure S4, Table 1). nc82 stains also show that MB morphology is normal in Type II>ImpRNAi flies (Video S2 and S3). Although not fully conclusive, these data support the idea that the behavioral phenotype we observe arises majorly from CX deficits.
Discussion
During development, neural stem cells generate diverse neuron types that assemble into distinct neural circuits enabling complex behaviors. Extensive work in genetic model systems has provided an overview of conserved temporal programs that govern the formation of diverse neuronal cell types 62,64,89–91. Although much is now known about the stem cell-specific molecular cues that determine cell type identity, understanding how these molecular cues shape the expression of complex behaviors is still in its infancy92–94. The insect CX provides an ideal model for dissecting the relationship between developmental processes and behavioral complexity. The majority of Drosophila CX neurons are derived from a few Type II neural stem cells, which follow a division program similar to that of cortical progenitors57,58,61–64. The CX has been implicated in a number of behaviors, including olfactory navigation45, menotaxis23,43, sleep30–32 and path integration in Drosophila95–97, and species-specific behaviors such as long-distance migration in monarch butterflies98, and allocentric dispersion in dung beetles99. For these reasons, the insect CX provides an ideal model for dissecting the relationship between developmental processes and behavioral complexity.
Lineage-specific developmental plan of the CX neuron types
Lineage-based architecture plays an essential role in generating complex brain structures and circuits. In the mammalian cerebral cortex, lineage is known to regulate neuron connectivity, where excitatory neurons originating from the same progenitors process related information and connect with each other, and inhibitory neurons are also known to organize in a lineage-dependent manner100,101. In the Drosophila larval ventral nerve cord, different lineages were reported to assemble with the sequential addition of temporal cohorts where circuit output neurons are born before circuit input neurons102. In the CX, local and columnar neurons primarily arise from the dorsomedial lineages (DM1-4), while long-field tangential input neurons arise from a variety of lineages50,54. One prominent cluster primarily arises from the dorsolateral Type II lineage (DL1), and generates neurons that fasciculate in two distinct bundles, one innervating the dorsal and the other ventral layers of the FB. However, many other Type I lineages also generate long-field tangential inputs, including AOTU, LALv1, and SIPp150,54.
Here, we show that the major components of a previously described olfactory navigation circuit in the CX are all derived from Type II neural stem cells (Figure 2). While many odor-encoding input neurons (vFB) are born from DL1 neural stem cells, a few are derived from other DMs (Figure 3). Our clonal analysis revealed that although tangential input neurons look morphologically similar, they can be derived from different lineages. Based on our genetic birth-dating results, we found that the neurons that innervate the ventral layers and provide odor input to the navigation circuits are born between 48-72h ALH (Figure 3). Our findings align with birth-dating studies on the entire DL1 lineage, which revealed that the ventral projecting neurons are born before 72h ALH, and that the dorsal projecting neurons are born throughout larval development73. Our studies indicate that components of a functional circuit associated with a behavior in CX are derived from multiple lineages and assembled over time. It will be intriguing to investigate whether other elements of the circuit are born at the same or different times. Whether other insects share a similar lineage-based circuit architecture and assembly will be interesting to pursue.
Role of Imp in specifying an olfactory navigation circuit
Imp has previously been shown to be important in neuronal development and specification. In mice, loss of Imp leads to deformities in the posterior brain, neuroepithelial orientation defects, and cellular packing deficiency with poorly defined barriers between cortical layers/zones85, similar to the overall defective morphology and lamination defects we observe in the CX. Temporal gradients of two RBPs, Imp and Syncrip, have been shown to determine neural identity in the insect mushroom body, antennal lobe, and CX73,103. In mushroom body neuroblasts, high Imp/low Syncrip levels early in development promote the specification of early-born γ Kenyon cells, while low Imp/high Syncrip levels late in development promote the specification of late-born α/β Kenyon cells. In mushroom body neuroblasts, Imp was shown to regulate fate of neuronal subtypes partly via regulating the translation of transcription factor Chinmo.103–105, suggesting that Imp governs fate by regulating the expression of other temporal transcription factors. In the antennal lobe, high Imp/low Syncrip leads to an increased number of late-born neurons from the antennal lobe antero-dorsal 1 (ALad1) lineage at the expense of early-born ones103. In the Type II lineages, DM1 and DL1 Imp/Syncrip levels control the number of several cell types73. However, the effects of these RBPs on multiple components of a functional circuit have not been previously characterized.
Here, focusing on an olfactory navigation circuit within the CX, we found the strongest effects of Imp on neurons targeting the ventral FB— ventral tangential inputs and ventral P-FNs. Curiously, Imp levels had distinct effects on each circuit element. In some cases (such as vFB neurons), knockdown of Imp caused a reduction in neuron number, while overexpression caused an increase in neuronal number (Figure 4), supporting existing models that Imp expression promotes the specification of neurons normally born in a particular temporal window73,103. However, for other neurons we observed diverse effects of knockdown and overexpression (Figure, 5). These observations suggest that precise levels of Imp are required to specify the correct number of these neurons, and Imp might be acting differently in Type II neural stem cells and in combination with other neural stem cell factors or INP factors to give rise to different cell types. Imp may play multiple roles and act via different molecular mechanisms in the neural stem cells to specify distinct neuron types from distinct lineages. We further show that the expression of Imp post mitotically in vFB neurons maintains their identity (Figure 4G). Moreover, we find that manipulating Imp levels in Type II neural stem cells affects the morphology of CX neuropils and distributions of neuropeptides TK and sNPF, throughout the FB (Figure 4 and 5). These studies provide the first insights into the relationship between developmental timing and establishing neuropeptidergic identity in CX.
An open question is how Imp regulates neural fate and the function of multiple circuit elements at the molecular level. Imp was previously shown to regulate fate specification by regulating the translation of transcription factor Chinmo104–106. Previous studies have shown that Chinmo is persistently expressed in Type II neural stem cells that are mutant for Syncrip and thus maintain high Imp expression throughout development, and this manipulation resulted in the formation of extra early-born neuron types72,73. This suggests the underlying mechanism to be the regulation of the expression of other transcription factors. However, we cannot rule out the possibility of Imp regulating cell fate directly. RBPs are known to play essential roles in regulating temporal gene expression by affecting the stability and translation of mRNA103. In the ventral cord motor neuron lineage, LinA/15, Imp, and Syncrip regulate axon-muscle connectivity by regulating various transcription factors post-transcriptionally107. Previous work has also shown a role for Imp in the timing of neural stem cell quiescence and proliferation83,108. Since many other neural cell types that arise from Type II neural stem cells are not affected upon Imp knockdown, we conclude that the phenotypes we observe are less likely to be associated with quiescence or cell proliferation. RBPs can regulate gene expression by creating liquid phase separation granules. Imp has intrinsically disordered domains and is known to promote phase separation in mushroom body neurons109. Whether Imp makes phase-separated granules in Type II neural stem cells and if that has a role in fate specification is not known. Future studies will elaborate on the different molecular mechanisms underlying the effect of Imp on multiple circuit elements.
Developmental dissection of CX-mediated behavior
In this study, we showed that knockdown of the early-expressed RBP Imp in Type II neural stem cell lineages largely abolishes upwind orientation in response to odor. We also observed a weaker phenotype when we knocked down Imp, specifically in DL1/DL2 Type II neural stem cells, arguing that tangential inputs to the CX have a specific role in generating this behavior (Figure 6). We did not observe any behavioral deficit with Syncrip knockdown in Type II neural stem cells, even though this manipulation has previously been shown to significantly impact neuronal specification in the CX73. Our control experiments suggest that these behavioral deficits are most likely due to alterations to CX structure. However, we cannot be sure that they arise from the changes in circuit structure that we have characterized here, and we cannot rule out a role for Type II derived neurons outside the CX in the more severe phenotype. Current approaches to CX circuit dissection in fruit flies have emphasized the use of highly-specific GAL4 and Split-GAL4 lines, which target specific subsets of neurons110,111. Silencing of highly specific CX lines often produces subtle phenotypes. For example, silencing of compass neurons eliminates menotaxis (orientation to a visual stimulus at an angular offset) but not visual fixation per se23,112, while silencing of h∆C/K neurons impairs the persistence of upwind tracking behavior during odor, but not the initial upwind turn45. Here we have shown that developmental manipulation of small groups of stem cells can produce a more striking phenotype, providing a complementary approach to dissecting neural circuits with highly specific driver lines. Future experiments manipulating other temporally expressed transcription factors or intersecting their manipulation with the temporal transcription factor code in INPs, should allow us to make more precise manipulations of CX development.
STAR Methods
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Mubarak Hussain Syed (FlyGuy@unm.edu).
Materials availability
This study did not generate new unique reagents. Requests of fly stocks should be directed to and will be fulfilled by the lead contact, Mubarak Hussain Syed (FlyGuy@unm.edu).
Data and code availability
All data generated in this study has been made publicly available at https://osf.io/nsrf8/
This paper does not report original code.
Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request.
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
For all experiments, we used Drosophila melanogaster. All the fly stocks were maintained at 25°C on standard cornmeal-agar medium and a 12h light-dark cycle. The RNAi and overexpression experimental crosses were raised at 29°C. Adult flies dissected were 3–7 days old. For behavioral experiments, female flies were collected at least 1 day after eclosion and then placed in vials that were situated in custom-made cardboard boxes at room temperature, with a 12-hour light-dark cycle, for at least two days to acclimate to ambient temperature and ensure correct circadian rhythm for the experimental time of day. All experiments were conducted within 4 hours after the flies’ apparent “dawn” (ZT0-ZT4). The flies were then starved for 24 hr in an empty transparent polystyrene vial that contained a Kimwipe that was soaked in distilled water to humidify the air. By the day of the behavioral experiments, the flies had an average age of 3 to 7 days. Flies were anesthetized over ice for approximately 1 minute before being loaded into wind tunnels and allowed at least 5 minutes to recover before starting the first trial. The exact genotypes for each figure panel are listed in the table below. Parental strains and RRIDs are listed in the Key resources table.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Chicken anti-GFP Antibody, Polyclonal | Aves Lab | Cat#GFP-1010 RRID:AB_2307313 |
| Mouse anti-Brp (nc82) Antibody, Monoclonal | DSHB | Cat#AB_2314866 RRID:AB_2314866 |
| Rabbit anti-DsRed Antibody, Polyclonal | Takar | Cat#632496 RRID:AB_10013483 |
| Rabbit anti-Tachykinin Antibody | Jan Veenstra Lab | N/A |
| Rabbit anti-sNPF Antibody | Jan Veenstra Lab | N/A |
| Donkey Anti-Chicken IgY Antibody (Alexa Fluor 488) | Jackson ImmunoResearch | Cat#703-545-155 RRID:AB_2340375 |
| Donkey Anti-Mouse IgG Antibody (Alexa Fluor 647) | Jackson ImmunoResearch | Cat#715-605-151 RRID:AB_2340863 |
| Goat Anti-Rabbit IgG (H+L) Antibody (Alexa Fluor 555) | Invitrogen | Cat#A-21429 RRID:AB_2535850 |
| Normal Donkey Serum | Jackson ImmunoResearch | Cat#017-000-121 RRID:AB_2337258 |
| Normal Goat Serum | Jackson ImmunoResearch | Cat#005-000-121 RRID:AB_2336990 |
| Chemicals, peptides, and recombinant proteins | ||
| Schneider’s Insect medium | Sigma-Aldrich | Cat#S0146 |
| 16% Paraformaldehyde (PFA) | Electron Microscopy Sciences | Cat#15710 |
| TritonX-100 | Sigma-Aldrich | Cat#T8787 |
| DPX mounting medium | Sigma-Aldrich | Cat#06522 |
| Deposited data | ||
| Raw and analyzed data This study https://osf.io/nsrf8/ | ||
| Experimental models: Organisms/strains | ||
| VT029515-LexA | Nagel Lab | N/A |
| R21D07-LexA | BDSC | RRID:BDSC_54637 |
| R12D12-LexA | BDSC | RRID:BDSC_52446 |
| VT062617-LexA | Nagel Lab | N/A |
| 15E12-GAL4 | BDSC | RRID:BDSC_48608 |
| 65C03-GAL4 | BDSC | RRID:BDSC_41290 |
| VT029515-GAL4 | VDRC | N/A |
| 17A12-GAL4 | BDSC | RRID:BDSC_45815 |
| w [1118]; P{y[+t7.7] w[+mC]=R9D11-CD4-tdTom}attP2/TM6B, Tb[1] | BDSC | RRID:BDSC_35847 |
| VT062617-GAL4 | VDRC | N/A |
| Pointed-GAL4 (Chromosome 3) | Yuh-Nung Jan | N/A |
| LexAopmCD8GFP (Chromosome 3) | BDSC | RRID:BDSC_32207 |
| Wor-GAL4, Ase-GAL80 (Chromosome 2) | Chris Doe Lab | |
| UAS-FLP-PEST (Chromosome X) | BDSC | RRID:BDSC_55807 |
| UAS-FLP-D5 (Chromosome 2) | Rubin Lab, JRC | N/A |
| mCherry RNAi (Chromosome 3) | BDSC | RRID:BDSC_35785 |
| LexAop-FRT-STOP-FRT-GFP (Chromosome 3) | BDSC | RRID:BDSC_57588 |
| UAS-FLP-PEST; Wor-GAL4, Ase-GAL80; LexAop-FRT-stop-FRT-GFP | Syed Lab | N/A |
| dpn>KDRT-stop-KDRT>Cre:PEST; actin^LoxP-GAL80-stop-LoxP^LexA::P65, LexAop-rCD2::RFP-p10-spacer-UAS-mCD8::GFP-p10; stg14-KD | Tzumin Lee Lab | N/A |
| Hs-ATG>KOT>FLP, dpn>FRT-stop-FRT>Cre:PEST; actin^LoxP-GAL80-stopLoxP^LexA::P65, LexAop-rCD2::RFP-p10-spacer-UAS-mCD8::GFP-p10; stg14-KD | Tzumin Lee Lab | N/A |
| VT029515-LexA; Pointed-GAL4, LexAopGFP | This study | N/A |
| 30E10-LexA; Pointed-GAL4, LexAopGFP | This study | N/A |
| 65C03-LexA; Pointed-GAL4, LexAopGFP | This study | N/A |
| 21D07-LexA, LexAopmCherryHA; Pointed-GAL4 | This study | N/A |
| VT062617-LexA; Pointed-GAL4, LexAopGFP | This study | N/A |
| UAS-ImpRNAi; UAS-ImpRNAi (Chromosome 2 and 3) | Syed Lab | RRID:BDSC_38219 and RRID:BDSC_34977 |
| Pin/CyO; UAS-Imp | Tzumin Lee Lab | N/A |
| w1118 | BDSC | 5905 |
| UAS-ImpRNAi (Chromosome 3) | BDSC | RRID:BDSC_34977 |
| UAS-SyncripRNAi | BDSC | RRID:BDSC_56972 |
| Software and Algorithms | ||
| ImajeJ | Open source | 2.9.0/1.53t |
| Adobe Illustrator | Adobe Systems | (v27.2) |
| Adobe Photoshop | Adobe Systems | (v24.1.1) |
| Prism 9 | GraphPad | (v9.5.1) |
| Zotero | Open source | (v6.0.26) |
| NI LabVIEW | National Instruments | Version 17.0 |
| MATLAB 2021b | MATLAB | 9.11.0.1769968 (R2021b) |
METHOD DETAILS
Immunostaining
Adult fly brains were dissected in Schneider’s Insect medium (Sigma-Aldrich), and then fixed at room temperature for 23 min in 4% paraformaldehyde (EMS) in PBT buffer. 10X PBS buffer stock contained NaCl: 1.37 M, KCl: 27 mM, Na2HPO4: 100 mM, KH2PO4: 18 mM with a pH of 7.4. Working 1X PBT had an addition of 0.5% TritonX-100 (Sigma-Aldrich). Fixing was followed by washing at room temperature in PBT to remove PFA. The samples were blocked at room temperature for 40 min in PBT containing 2.5% normal goat serum (Jackson ImmunoResearch) and 2.5% normal donkey serum (Jackson ImmunoResearch). Adult brains were then incubated for 48hr at 4°C in the primary antibody solution. Primary antibody staining was followed by washing in PBS-T and then the adult brains were incubated for 2hr at room temperature in the secondary antibody solution. The brains were then washed in PBT. The primary and secondary antibody solutions were prepared in the blocking solution (prep described above).
Following the antibody staining, brains were mounted on the Poly-lysine-coated cover slips, serially dehydrated in alcohol with concentrations 30%, 50%, 75%, 95% and 3 rounds of 100% to replace PBT. The brains were then cleared in Xylene with 3 rounds of 5 min each and then mounted in DPX mounting medium, dibutyl phthalate in xylene (Sigma-Aldrich #06522). The samples were allowed to set in DPX for 3 days before imaging.
Microscopy
Images were acquired using a Zeiss LSM780 and LSM980 confocal microscope, analyzed using ImageJ, and processed using ImageJ and Photoshop. Cell body numbers were calculated manually using a cell counter in ImageJ, and the statistical analysis and graphs were made using GraphPad Prism. Two-tailed student t-tests were used to compare control with loss of function and gain of function respectively for cell body numbers. Asterisks denote levels of significant differences *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Birth-dating using Cell class lineage intersection system
Males from neuron class specific GAL4 lines were mated to females from the CLIn line81. Egg collection was done for 3 to 4 hr intervals on apple juice containing agar plates. Hatched larvae (0–3 hr old) were manually collected and raised in standard fly food at 25°C. Heat shock was applied to the hatched larvae for 12–15 min for lineage analysis and 40 min for birth-dating at the time point 0 hr, 24 hr, 48 hr and 72 hr ALH. F1 adult flies of age 3–7 days were dissected.
Behavioral Apparatus
All behavioral experiments were performed in miniature wind tunnels79. Flies were constrained to walk in a shallow acrylic arena with a constant laminar wind at 10 cm/second. The wind tunnels were backlit using IR LEDS (850 nm, Environmental Lights) and movement was recorded using a camera placed below the chamber (Basler acA1920-155um). A 10 s pulse of odor (either 1% or 10% apple cider vinegar) was presented through Lee valves connected to the wind tunnel with polyethylene tubing. Within each 70 s trial, flies experienced 30 s of clean wind, 10 s of odor with wind, and then 30 s of clean wind again. All stimuli were controlled through a NIDAQ board. There are roughly 5 seconds between the end of one trial and the start of the next. Position (x,y) and orientation data were computed and collected in real time using a double-thresholding algorithm as described previously using NI LabVIEW).
QUANTIFICATION AND STATISTICAL ANALYSIS
Analysis of Behavioral Data
All analysis was completed using MATLAB (Mathworks, Natick, MA). Calculation of trajectory and basic movement parameters seen in Fig. 7. A–C (ground speed, orientation, etc.) were completed using analysis code described in Alvarez-Salvado et al, 2018.
Orientation histograms
To compute orientation histograms, we pooled trials across all flies, and separated orientation data from the period before odor presentation, the period during the 10 seconds of odor, and the period following odor offset. We binned orientation data from the first 5 seconds of each of these periods into 36 bins of width 10 degrees. Error bars were created using jackknife resampling across flies and represent standard error calculated as:
where is standard deviation and n is the number of samples. The resultant data, in polar coordinates, was then converted to Cartesian coordinates for ease of plotting and creation of error bars.
Histograms of ground speed and angular velocity
To compute groundspeed and angular velocity histograms, we again pooled data across flies. We binned values of ground speed and angular velocity into bins of width 1mm/s and 50 deg/s respectively (Fig. 6. E/H and F/I) Histograms of both parameters were normalized to probability. Error bars represent standard error and were created using jackknife resampling with bias correction across all flies. Angular velocity of stationary flies (ground speed< 1mm/s) were excluded from analysis.
Summary plots of change in forward velocity and ground speed
Iterating through all trials for a single fly, we calculated the difference between the mean upwind velocity in the first 5 seconds of odor presentation and the mean upwind velocity in the 5 seconds preceding odor presentation. We then calculated the mean change in upwind velocity between these two periods for a single fly by averaging across all trials. For data representation, we then calculated the mean value across all flies, as well as the standard error. Significance was calculated using the mean value of each fly as a data point in an unpaired t test with Bonferroni correction for multiple comparisons using the equations:
where and are sample means, 𝑛1and 𝑛2 are sample sizes, is the population variance, and and are the sample variances. The same logic was used for calculating change in ground speed and performing statistical testing (Fig. 6J). Significance is marked with asterisks in the figure and the associated p-values can be found below.
Upwind Velocity
| Comparison | p-Value |
|---|---|
| Pointed-GAL4>UAS-mCherryRNAi compared to Pointed-GAL4>UAS-ImpRNAi;ImpRNAi | p = 5.906x10^-11 |
| Pointed-GAL4>UAS-mCherryRNAi compared to Pointed-GAL4>UAS-ImpRNAi (III) | p = 1.1476x10^-9 |
| Pointed-GAL4>UAS-mCherryRNAi (1:10) compared to Pointed-GAL4>ImpRNAi;ImpRNAi (1:10) | p = 1.2293x10^-4 |
| Pointed-GAL4>UAS-mCherryRNAi (1:10) compared to Pointed-GAL4>UAS-SypRNAi(1:10) | p = 0.0042 |
| DL1-GAL4>UAS-mCherryRNAi compared to DL1-GAL4>UAS-ImpRNAi;ImpRNAi | p = 9.922x10^-6 |
Ground Speed
| Comparison | p-Value |
|---|---|
| Pointed-GAL4>UAS-mCherryRNAi compared to Pointed-GAL4>UAS-ImpRNAi (III) | p= 0.0015 |
| Pointed-GAL4>UAS-mCherryRNAi compared to Pointed-GAL4>UAS-Imp | p= 7.7113x10^-4 |
| DL1-GAL4>UAS-mCherryRNAi compared to DL1-GAL4>UAS-ImpRNAi;ImpRNAi | p = 2.2281x10^-7 |
Supplementary Material
| Fly cross | Figure Panel | |
| UAS-FLP-PEST; Wor-GAL4, Ase-GAL80; LexAop-FRT-stop-FRT-GFP crossed to VT029515-LexA | Fig 2C | |
| UAS-FLP-PEST; Wor-GAL4, Ase-GAL80; LexAop-FRT-stop-FRT-GFP crossed to VT062617-LexA | Fig 2D | |
| UAS-FLP-PEST; Wor-GAL4, Ase-GAL80; LexAop-FRT-stop-FRT-GFP crossed to R21D07-LexA | Fig 2E | |
| UAS-FLP-PEST; Wor-GAL4, Ase-GAL80; LexAop-FRT-stop-FRT-GFP crossed to R12D12-LexA | Fig 2F | |
| dpn>KDRT-stop-KDRT>Cre:PEST; actin^LoxP-GAL80-stop-LoxP^LexA::P65, LexAop-rCD2::RFP-p10-spacer-UAS-mCD8::GFP-p10; stg14-KD crossed to 65C03-GAL4 | Fig 2H | |
| dpn>KDRT-stop-KDRT>Cre:PEST; actin^LoxP-GAL80-stop-LoxP^LexA::P65, LexAop-rCD2::RFP-p10-spacer-UAS-mCD8::GFP-p10; stg14-KD crossed to 15E12-GAL4 | Fig 2I | |
| Hs-ATG>KOT>FLP, dpn>FRT-stop-FRT>Cre:PEST; actin^LoxP-GAL80-stopLoxP^LexA::P65, LexAop-rCD2::RFP-p10-spacer-UAS-mCD8::GFP-p10; stg14-KD crossed to VT029515-GAL4 | Fig 3D–H | |
| UAS-FLP; 17A12-GAL4 crossed to VT029515-LexA; LexAop-FRT-stop-FRT-GFP | Fig 3J | |
| VT029515-LexA; Pointed -GAL4, LexAopGFP | Fig 4A–A’’ | |
| VT029515-LexA; Pointed -GAL4, LexAopGFP crossed to UAS-ImpRNAi; UAS-ImpRNAi | Fig 4B–B’’ | |
| VT029515-LexA; Pointed-GAL4, LexAopGFP crossed to Pin/CyO; UAS-Imp | Fig 4C–C’’ | |
| UAS-FLP; TK-GAL4 crossed to VT029515-LexA; LexAop-FRT-stop-FRT-GFP | Fig 4D–D’’ | |
| VT029515-LexA; 17A12-GAL4, LexAopGFP | Fig 4E | |
| VT029515-LexA; 17A12-GAL4, LexAopGFP crossed to UAS-ImpRNAi; UAS-ImpRNAi | Fig 4E’ | |
| UAS-GFP; VT029515-GAL4 | Fig 4F–F’’ | |
| UAS-GFP; VT029515-GAL4 crossed to UAS-ImpRNAi; UAS-ImpRNAi | Fig 4G–G’’ | |
| 30E10-LexA; Pointed-GAL4, LexAopGFP | Fig 5A | |
| 30E10-LexA; Pointed-GAL4, LexAopGFP crossed to UAS-ImpRNAi; UAS-ImpRNAi | Fig 5B | |
| 30E10-LexA; Pointed -GAL4, LexAopGFP crossed to Pin/CyO; UAS-Imp | Fig 5C | |
| 65C03-LexA; Pointed-GAL4, LexAopGFP | Fig 5D | |
| 65C03-LexA; Pointed-GAL4, LexAopGFP crossed to UAS-ImpRNAi; UAS-ImpRNAi | Fig 5E | |
| 65C03-LexA; Pointed-GAL4, LexAopGFP crossed to Pin/CyO; UAS-Imp | Fig 5F | |
| 21D07-LexA, LexAopmCherryHA; Pointed -GAL4 | Fig 5G | |
| 21D07-LexA, LexAopmCherryHA; Pointed GAL4 crossed to UAS-ImpRNAi; UAS-ImpRNAi | Fig 5H | |
| 21D07-LexA, LexAopmCherryHA; Pointed-GAL4 crossed to Pin/CyO; UAS-Imp | Fig 5I | |
| VT062617-LexA; Pointed-GAL4, LexAopGFP | Fig 5J | |
| VT062617-LexA; Pointed-GAL4, LexAopGFP crossed to UAS-ImpRNAi; UAS-ImpRNAi | Fig 5K | |
| VT062617-LexA; Pointed -GAL4, LexAopGFP crossed to Pin/CyO; UAS-Imp | Fig 5L | |
| VT029515-LexA; Pointed-GAL4, LexAopGFP | Fig 5Q–U | |
| VT029515-LexA; Pointed-GAL4, LexAopGFP crossed to UAS-ImpRNAi; UAS-ImpRNAi | Fig 5Q’–U’ | |
| Pointed-GAL4/UAS-mCherryRNAi | Figure 6A, C–F | |
| UAS-ImpRNAi/+; Pointed-GAL4/UAS-ImpRNAi | Figure 6B, C–F | |
| 17A12-GAL4/UAS-mCherryRNAi | Figure 6G–I | |
| UAS-ImpRNAi/+; 17A12-GAL4/UAS-ImpRNAi | Figure 6G–I | |
| Pointed-GAL4/UAS-ImpRNAi | Figure 6J | |
| Pointed-GAL4/w1118 | Figure 6J | |
| Pointed-GAL4 UAS-SypRNAi | Figure 6J | |
| Pin/+;UAS-Imp/+; Pointed-GAL4/UAS-Imp | Figure 6J | |
| 17A12-GAL4/UAS-mCherryRNAi | Figure 6J | |
| 1UAS-ImpRNAi/+; 7A12-GAL4; UAS-ImpRNAi | Figure 6J | |
| 17A12-GAL4/UAS-SypRNAi | Figure 6J | |
| Pin/+; 17A12-GAL4 /UAS-Imp | Figure 6J | |
Highlights:
Drosophila olfactory navigation circuit components are derived from Type II NSCs
The dorsolateral Type II NSC cell generates ventral-FB odor-encoding input neurons
Imp governs specification and identity of the olfactory navigation circuit elements
Loss of Imp in Type II NSCs impairs upwind orientation during olfactory navigation
Acknowledgments
The authors would like to thank Chris Doe and Doe lab members for their valuable feedback and discussions. We thank Gonzalo Morales Chaya for the 17A12GAL4 expression image and Adil Wani for the 17A12GAL4, LexAopmCD8GFP fly. We thank Chris Doe, Raees Andrabi, and Dena Goldblatt for providing critical feedback on the manuscript. We are grateful to Chris Doe, Claude Desplan, Gerry Rubin, Jan Veenstra and Tzumin Lee for sharing the reagents. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. The monoclonal nc82 antibody was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. We thank UNM Biology cell biology core for providing confocal microscopy facility. We thank the Advanced Imaging Core facility at Janelia Research Campus used for the experiments conducted during the revision of this manuscript. This research was supported in part by NSF Grant No. PHY-1748958 and the Gordon and Betty Moore Foundation Grant No. 2919.02. A.N.C was supported by NIH MARC T34GM008751 and A.G was supported by NIH URISE T34 GM145428. The research was supported by NSF 2014217, NIH R01DC017979, and RF1NS127129 to K.I.N and University of New Mexico College of Arts & Sciences Office of Research, Sloan Research Fellowship, and National Science Foundation CAREER Award IOS-2047020 to MHS.
Footnotes
Inclusion and diversity
One or more of the authors of this paper self-identify as an underrepresented ethnic minority in their field of research or within their geographical location.
Declaration of interests
The authors declare no competing interests.
Supplemental video titles and legends
Video S1. Lineage identification of vFB neurons. Related to Figure 3.
CLIn analysis reveals that most of the vFB neurons labeled in green (GFP) are derived from a single DL1 Type II neural stem cell. The lineages of DL1 clone, labeled in red (mCherry) pseudo-colored in cyan, and the marker nc82 in magenta labels neuropil structures. The Z stacks from the anterior to posterior of the stained brain were exported as a movie. Note the projection pattern of the vFB neurons to the ventral layers of the FB outlines with dashed boundaries.
Video S2. Control CX neuropil structures. Related to Figure 5.
The neuropil marker nc82 in magenta labels all the neuropil structures. From anterior to posterior, the movie displays the morphology of adult neuropil structures. Note the morphology of the mushroom body and major CX neuropil structures EB, FB, NO, and PB.
Video S3. Deformed CX neuropil structures. Related to Figure 5.
Upon Type II neural stem cell specific knockdown of Imp, the CX neuropil structures EB, FB, NO, and PB are deformed. Note the normal mushroom body morphology.
References:
- 1.Rubenstein JL (2010). Three hypotheses for developmental defects that may underlie some forms of autism spectrum disorder. Current Opinion in Neurology 23, 118. 10.1097/WCO.0b013e328336eb13. [DOI] [PubMed] [Google Scholar]
- 2.Guerrini R, Dobyns WB, and Barkovich AJ (2008). Abnormal development of the human cerebral cortex: genetics, functional consequences and treatment options. Trends in Neurosciences 31, 154–162. 10.1016/j.tins.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert SL, Dobyns WB, and Lahn BT (2005). Genetic links between brain development and brain evolution. Nat Rev Genet 6, 581–590. 10.1038/nrg1634. [DOI] [PubMed] [Google Scholar]
- 4.Farris SM, and Sinakevitch I (2003). Development and evolution of the insect mushroom bodies: towards the understanding of conserved developmental mechanisms in a higher brain center. Arthropod Structure & Development 32, 79–101. 10.1016/S1467-8039(03)00009-4. [DOI] [PubMed] [Google Scholar]
- 5.Strausfeld NJ (1976). Atlas of an insect brain
- 6.Honkanen A, Adden A, Freitas J. da S., and Heinze S (2019). The insect central complex and the neural basis of navigational strategies. Journal of Experimental Biology 222. 10.1242/jeb.188854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homberg U (1985). Interneurones of the central complex in the bee brain (Apis mellifera, L.). Journal of Insect Physiology 31, 251–264. 10.1016/0022-1910(85)90127-1. [DOI] [Google Scholar]
- 8.Strauss R, and Heisenberg M (1993). A higher control center of locomotor behavior in the Drosophila brain. J. Neurosci 13, 1852–1861. 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Homberg U (2004). In search of the sky compass in the insect brain. Naturwissenschaften 91, 199–208. 10.1007/s00114-004-0525-9. [DOI] [PubMed] [Google Scholar]
- 10.Wessnitzer J, and Webb B (2006). Multimodal sensory integration in insects—towards insect brain control architectures. Bioinspir. Biomim 1, 63. 10.1088/1748-3182/1/3/001. [DOI] [PubMed] [Google Scholar]
- 11.Homberg U (2008). Evolution of the central complex in the arthropod brain with respect to the visual system. Arthropod Structure & Development 37, 347–362. 10.1016/j.asd.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Strausfeld NJ (2009). Brain organization and the origin of insects: an assessment. Proc. Biol. Sci 276, 1929–1937. 10.1098/rspb.2008.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ofstad TA, Zuker CS, and Reiser MB (2011). Visual place learning in Drosophila melanogaster. Nature 474, 204–207. 10.1038/nature10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeiffer K, and Homberg U (2014). Organization and Functional Roles of the Central Complex in the Insect Brain. Annu. Rev. Entomol 59, 165–184. 10.1146/annurev-ento-011613-162031. [DOI] [PubMed] [Google Scholar]
- 15.Seelig JD, and Jayaraman V (2015). Neural dynamics for landmark orientation and angular path integration. Nature 521, 186–191. 10.1038/nature14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner-Evans D, Wegener S, Rouault H, Franconville R, Wolff T, Seelig JD, Druckmann S, and Jayaraman V (2017). Angular velocity integration in a fly heading circuit. eLife 6, e23496. 10.7554/eLife.23496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green J, Adachi A, Shah KK, Hirokawa JD, Magani PS, and Maimon G (2017). A neural circuit architecture for angular integration in Drosophila. Nature 546, 101–106. 10.1038/nature22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner-Evans DB, Jensen KT, Ali S, Paterson T, Sheridan A, Ray RP, Wolff T, Lauritzen JS, Rubin GM, Bock DD, et al. (2020). The Neuroanatomical Ultrastructure and Function of a Biological Ring Attractor. Neuron 108, 145–163.e10. 10.1016/j.neuron.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behbahani AH, Palmer EH, Corfas RA, and Dickinson MH (2021). Drosophila re-zero their path integrator at the center of a fictive food patch. Current Biology 31, 4534–4546.e5. 10.1016/j.cub.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyu C, Abbott LF, and Maimon G (2022). Building an allocentric travelling direction signal via vector computation. Nature 601, 92–97. 10.1038/s41586-021-04067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu J, Behbahani AH, Hamburg L, Westeinde EA, Dawson PM, Lyu C, Maimon G, Dickinson MH, Druckmann S, and Wilson RI (2022). Transforming representations of movement from body- to world-centric space. Nature 601, 98–104. 10.1038/s41586-021-04191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuser K, Triphan T, Mronz M, Poeck B, and Strauss R (2008). Analysis of a spatial orientation memory in Drosophila. Nature 453, 1244–1247. 10.1038/nature07003. [DOI] [PubMed] [Google Scholar]
- 23.Giraldo YM, Leitch KJ, Ros IG, Warren TL, Weir PT, and Dickinson MH (2018). Sun Navigation Requires Compass Neurons in Drosophila. Current Biology 28, 2845–2852.e4. 10.1016/j.cub.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dan Chuntao, Kappagantula Ramya, Hulse Brad K., Jayaraman Vivek, and Hermundstad Ann M. (2022). Flexible control of behavioral variability mediated by an internal representation of head direction. bioRxiv, 2021.08.18.456004. 10.1101/2021.08.18.456004. [DOI]
- 25.Martin JP, Guo P, Mu L, Harley CM, and Ritzmann RE (2015). Central-Complex Control of Movement in the Freely Walking Cockroach. Current Biology 25, 2795–2803. 10.1016/j.cub.2015.09.044. [DOI] [PubMed] [Google Scholar]
- 26.Rayshubskiy Aleksandr, Holtz Stephen L., Isabel D’Alessandro Anna A. Li, Vanderbeck Quinn X., Haber Isabel S., Gibb Peter W., and Wilson Rachel I. (2020). Neural circuit mechanisms for steering control in walking Drosophila. bioRxiv, 2020.04.04.024703. 10.1101/2020.04.04.024703. [DOI]
- 27.Musso P-Y, Junca P, and Gordon MD (2021). A neural circuit linking two sugar sensors regulates satiety-dependent fructose drive in Drosophila. Science Advances 7, eabj0186. 10.1126/sciadv.abj0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sareen PF, McCurdy LY, and Nitabach MN (2021). A neuronal ensemble encoding adaptive choice during sensory conflict in Drosophila. Nat Commun 12, 4131. 10.1038/s41467-021-24423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishimoto H, and Kamikouchi A (2020). A Feedforward Circuit Regulates Action Selection of Pre-mating Courtship Behavior in Female Drosophila. Current Biology 30, 396–407.e4. 10.1016/j.cub.2019.11.065. [DOI] [PubMed] [Google Scholar]
- 30.Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, and Shaw PJ (2011). Inducing Sleep by Remote Control Facilitates Memory Consolidation in Drosophila. Science 332, 1571–1576. 10.1126/science.1202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donlea JM, Pimentel D, and Miesenböck G (2014). Neuronal Machinery of Sleep Homeostasis in Drosophila. Neuron 81, 860–872. 10.1016/j.neuron.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donlea JM, Pimentel D, Talbot CB, Kempf A, Omoto JJ, Hartenstein V, and Miesenböck G (2018). Recurrent Circuitry for Balancing Sleep Need and Sleep. Neuron 97, 378–389.e4. 10.1016/j.neuron.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu S, Liu Q, Tabuchi M, and Wu MN (2016). Sleep Drive Is Encoded by Neural Plastic Changes in a Dedicated Circuit. Cell 165, 1347–1360. 10.1016/j.cell.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubowy C, and Sehgal A (2017). Circadian Rhythms and Sleep in Drosophila melanogaster. Genetics 205, 1373–1397. 10.1534/genetics.115.185157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kempf A, Song SM, Talbot CB, and Miesenböck G (2019). A potassium channel β-subunit couples mitochondrial electron transport to sleep. Nature 568, 230–234. 10.1038/s41586-019-1034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakravarti Dilley L, Szuperak M, Gong NN, Williams CE, Saldana RL, Garbe DS, Syed MH, Jain R, and Kayser MS (2020). Identification of a molecular basis for the juvenile sleep state. eLife 9, e52676. 10.7554/eLife.52676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shafer OT, and Keene AC (2021). The Regulation of Drosophila Sleep. Curr Biol 31, R38–R49. 10.1016/j.cub.2020.10.082. [DOI] [PubMed] [Google Scholar]
- 38.Hanesch U, Fischbach K-F, and Heisenberg M (1989). Neuronal architecture of the central complex in Drosophila melanogaster. Cell Tissue Res 257, 343–366. 10.1007/BF00261838. [DOI] [Google Scholar]
- 39.Ito K, Shinomiya K, Ito M, Armstrong JD, Boyan G, Hartenstein V, Harzsch S, Heisenberg M, Homberg U, Jenett A, et al. (2014). A systematic nomenclature for the insect brain. Neuron 81, 755–765. [DOI] [PubMed] [Google Scholar]
- 40.Wolff T, Iyer NA, and Rubin GM (2015). Neuroarchitecture and neuroanatomy of the Drosophila central complex: A GAL4-based dissection of protocerebral bridge neurons and circuits: Drosophila Central Complex Anatomy and Neurons. J. Comp. Neurol 523, 997–1037. 10.1002/cne.23705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolff T, and Rubin GM (2018). Neuroarchitecture of the Drosophila central complex: A catalog of nodulus and asymmetrical body neurons and a revision of the protocerebral bridge catalog. J Comp Neurol 526, 2585–2611. 10.1002/cne.24512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franconville R, Beron C, and Jayaraman V (2018). Building a functional connectome of the Drosophila central complex. eLife 7, e37017. 10.7554/eLife.37017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green J, and Maimon G (2018). Building a heading signal from anatomically defined neuron types in the Drosophila central complex. Current Opinion in Neurobiology 52, 156–164. 10.1016/j.conb.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mussells Pires Peter, Abbott LF, and Maimon Gaby (2022). Converting an allocentric goal into an egocentric steering signal. bioRxiv, 2022.11.10.516026. 10.1101/2022.11.10.516026. [DOI] [PMC free article] [PubMed]
- 45.Matheson AMM, Lanz AJ, Medina AM, Licata AM, Currier TA, Syed MH, and Nagel KI (2022). A neural circuit for wind-guided olfactory navigation. Nat Commun 13, 4613. 10.1038/s41467-022-32247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Currier TA, Matheson AM, and Nagel KI (2020). Encoding and control of orientation to airflow by a set of Drosophila fan-shaped body neurons. eLife 9, e61510. 10.7554/eLife.61510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hulse BK, Haberkern H, Franconville R, Turner-Evans DB, Takemura S, Wolff T, Noorman M, Dreher M, Dan C, Parekh R, et al. (2021). A connectome of the Drosophila central complex reveals network motifs suitable for flexible navigation and context-dependent action selection. eLife 10, e66039. 10.7554/eLife.66039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheffer LK, Xu CS, Januszewski M, Lu Z, Takemura S-Y, Hayworth KJ, Huang GB, Shinomiya K, Maitlin-Shepard J, Berg S, et al. (2020). A connectome and analysis of the adult Drosophila central brain. eLife 9. 10.7554/eLife.57443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sayre ME, Templin R, Chavez J, Kempenaers J, and Heinze S (2021). A projectome of the bumblebee central complex. eLife 10, e68911. 10.7554/eLife.68911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang JS, Awasaki T, Yu H-H, He Y, Ding P, Kao J-C, and Lee T (2013). Diverse neuronal lineages make stereotyped contributions to the Drosophila locomotor control center, the central complex. J Comp Neurol 521, 2645-Spc1. 10.1002/cne.23339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu H-H, Awasaki T, Schroeder MD, Long F, Yang JS, He Y, Ding P, Kao J-C, Wu GY-Y, Peng H, et al. (2013). Clonal development and organization of the adult Drosophila central brain. Current biology : CB 23, 633–643. 10.1016/j.cub.2013.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito M, Masuda N, Shinomiya K, Endo K, and Ito K (2013). Systematic analysis of neural projections reveals clonal composition of the Drosophila brain. Current biology : CB 23, 644–655. 10.1016/j.cub.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 53.Pereanu W, Kumar A, Jennett A, Reichert H, and Hartenstein V (2010). Development-based compartmentalization of the Drosophila central brain. Journal of Comparative Neurology 518, 2996–3023. 10.1002/cne.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kandimalla P, Omoto JJ, Hong EJ, and Hartenstein V (2023). Lineages to circuits: the developmental and evolutionary architecture of information channels into the central complex. J Comp Physiol A 10.1007/s00359-023-01616-y. [DOI] [PMC free article] [PubMed]
- 55.Homem CCF, and Knoblich JA (2012). Drosophila neuroblasts: a model for stem cell biology. Development 139, 4297–4310. 10.1242/dev.080515. [DOI] [PubMed] [Google Scholar]
- 56.Hamid A, Gutierrez A, Munroe J, and Syed MH (2022). The Drivers of Diversity: Integrated genetic and hormonal cues regulate neural diversity. Seminars in Cell & Developmental Biology 10.1016/j.semcdb.2022.07.007. [DOI] [PubMed]
- 57.Bello BC, Izergina N, Caussinus E, and Reichert H (2008). Amplification of neural stem cell proliferation by intermediate progenitor cells in Drosophila brain development. Neural Dev 3, 5. 10.1186/1749-8104-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boone JQ, and Doe CQ (2008). Identification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev Neurobiol 68, 1185–1195. 10.1002/dneu.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bowman SK, Rolland V, Betschinger J, Kinsey KA, Emery G, and Knoblich JA (2008). The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev Cell 14, 535–546. 10.1016/j.devcel.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Izergina N, Balmer J, Bello B, and Reichert H (2009). Postembryonic development of transit amplifying neuroblast lineages in the Drosophila brain. Neural Development 4, 44. 10.1186/1749-8104-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang Y, and Reichert H (2013). Analysis of neural stem cell self-renewal and differentiation by transgenic RNAi in Drosophila. Arch Biochem Biophys 534, 38–43. 10.1016/j.abb.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Oberst P, Agirman G, and Jabaudon D (2019). Principles of progenitor temporal patterning in the developing invertebrate and vertebrate nervous system. Current Opinion in Neurobiology 56, 185–193. 10.1016/j.conb.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Florio M, and Huttner WB (2014). Neural progenitors, neurogenesis and the evolution of the neocortex. Development 141, 2182–2194. 10.1242/dev.090571. [DOI] [PubMed] [Google Scholar]
- 64.Lodato S, and Arlotta P (2015). Generating Neuronal Diversity in the Mammalian Cerebral Cortex. Annual Review of Cell and Developmental Biology 31, 699–720. 10.1146/annurev-cellbio-100814-125353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hansen DV, Lui JH, Parker PRL, and Kriegstein AR (2010). Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561. 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 66.Lui JH, Hansen DV, and Kriegstein AR (2011). Development and Evolution of the Human Neocortex. Cell 146, 18–36. 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pebworth M-P, Ross J, Andrews M, Bhaduri A, and Kriegstein AR (2021). Human intermediate progenitor diversity during cortical development. Proc Natl Acad Sci U S A 118, e2019415118. 10.1073/pnas.2019415118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams JLD, and Boyan GS (2008). Building the central complex of the grasshopper Schistocerca gregaria: axons pioneering the w, x, y, z tracts project onto the primary commissural fascicle of the brain. Arthropod Structure & Development 37, 129–140. 10.1016/j.asd.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 69.Boyan GS, and Reichert H (2011). Mechanisms for complexity in the brain: generating the insect central complex. Trends in Neurosciences 34, 247–257. 10.1016/j.tins.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 70.Boyan G, Liu Y, Khalsa SK, and Hartenstein V (2017). A conserved plan for wiring up the fan-shaped body in the grasshopper and Drosophila. Dev Genes Evol 227, 253–269. 10.1007/s00427-017-0587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He B, Buescher M, Farnworth MS, Strobl F, Stelzer EH, Koniszewski ND, Muehlen D, and Bucher G (2019). An ancestral apical brain region contributes to the central complex under the control of foxQ2 in the beetle Tribolium. eLife 8, e49065. 10.7554/eLife.49065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Syed MH, Mark B, and Doe CQ (2017). Steroid hormone induction of temporal gene expression in Drosophila brain neuroblasts generates neuronal and glial diversity. eLife 6, e26287. 10.7554/eLife.26287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ren Q, Yang C-P, Liu Z, Sugino K, Mok K, He Y, Ito M, Nern A, Otsuna H, and Lee T (2017). Stem Cell-Intrinsic, Seven-up-Triggered Temporal Factor Gradients Diversify Intermediate Neural Progenitors. Curr Biol 27, 1303–1313. 10.1016/j.cub.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y-C, Yang JS, Johnston R, Ren Q, Lee Y-J, Luan H, Brody T, Odenwald WF, and Lee T (2014). Drosophila intermediate neural progenitors produce lineage-dependent related series of diverse neurons. Development 141, 253–258. 10.1242/dev.103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdusselamoglu MD, Eroglu E, Burkard TR, and Knoblich JA (2019). The transcription factor odd-paired regulates temporal identity in transit-amplifying neural progenitors via an incoherent feed-forward loop. eLife 8, e46566. 10.7554/eLife.46566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang JLY, Hakes AE, Krautz R, Suzuki T, Contreras EG, Fox PM, and Brand AH (2021). NanoDam identifies novel temporal transcription factors conserved between the Drosophila central brain and visual system (Developmental Biology) 10.1101/2021.06.07.447332. [DOI] [PMC free article] [PubMed]
- 77.Bayraktar OA, and Doe CQ (2013). Combinatorial temporal patterning in progenitors expands neural diversity. Nature 498, 449–455. 10.1038/nature12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Demir M, Kadakia N, Anderson HD, Clark DA, and Emonet T (2020). Walking Drosophila navigate complex plumes using stochastic decisions biased by the timing of odor encounters. eLife 9, e57524. 10.7554/eLife.57524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Álvarez-Salvado E, Licata AM, Connor EG, McHugh MK, King BM, Stavropoulos N, Victor JD, Crimaldi JP, and Nagel KI (2018). Elementary sensory-motor transformations underlying olfactory navigation in walking fruit-flies. eLife 7, e37815. 10.7554/eLife.37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steele TJ, Lanz AJ, and Nagel KI (2023). Olfactory navigation in arthropods. J Comp Physiol A 10.1007/s00359-022-01611-9. [DOI] [PMC free article] [PubMed]
- 81.Ren Q, Awasaki T, Huang Y-F, Liu Z, and Lee T (2016). Cell Class-Lineage Analysis Reveals Sexually Dimorphic Lineage Compositions in the Drosophila Brain. Current Biology 26, 2583–2593. 10.1016/j.cub.2016.07.086. [DOI] [PubMed] [Google Scholar]
- 82.Degrauwe N, Suvà M-L, Janiszewska M, Riggi N, and Stamenkovic I (2016). IMPs: an RNA-binding protein family that provides a link between stem cell maintenance in normal development and cancer. Genes Dev 30, 2459–2474. 10.1101/gad.287540.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Munroe JA, Syed MH, and Doe CQ (2022). Imp is required for timely exit from quiescence in Drosophila type II neuroblasts. PLOS ONE 17, e0272177. 10.1371/journal.pone.0272177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kahsai L, and Winther ÅME (2011). Chemical neuroanatomy of the Drosophila central complex: Distribution of multiple neuropeptides in relation to neurotransmitters. J. Comp. Neurol 519, 290–315. 10.1002/cne.22520. [DOI] [PubMed] [Google Scholar]
- 85.Núñez L, Buxbaum AR, Katz ZB, Lopez-Jones M, Nwokafor C, Czaplinski K, Pan F, Rosenberg J, Monday HR, and Singer RH (2022). Tagged actin mRNA dysregulation in IGF2BP1−/− mice. Proceedings of the National Academy of Sciences 119, e2208465119. 10.1073/pnas.2208465119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dolan M-J, Frechter S, Bates AS, Dan C, Huoviala P, Roberts RJ, Schlegel P, Dhawan S, Tabano R, Dionne H, et al. (2019). Neurogenetic dissection of the Drosophila lateral horn reveals major outputs, diverse behavioural functions, and interactions with the mushroom body. eLife 8, e43079. 10.7554/eLife.43079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aso Y, Sitaraman D, Ichinose T, Kaun KR, Vogt K, Belliart-Guérin G, Plaçais P-Y, Robie AA, Yamagata N, Schnaitmann C, et al. (2014). Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. eLife 3, e04580. 10.7554/eLife.04580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bidaye SS, Laturney M, Chang AK, Liu Y, Bockemühl T, Büschges A, and Scott K (2020). Two Brain Pathways Initiate Distinct Forward Walking Programs in Drosophila. Neuron 108, 469–485.e8. 10.1016/j.neuron.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kohwi M, and Doe CQ (2013). Temporal Fate Specification and Neural Progenitor Competence During Development. Nat Rev Neurosci 14, 823–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun H, and Hobert O (2023). Temporal transitions in the postembryonic nervous system of the nematode Caenorhabditis elegans: Recent insights and open questions. Seminars in Cell & Developmental Biology 142, 67–80. 10.1016/j.semcdb.2022.05.029. [DOI] [PubMed] [Google Scholar]
- 91.El-Danaf RN, Rajesh R, and Desplan C (2023). Temporal regulation of neural diversity in Drosophila and vertebrates. Seminars in Cell & Developmental Biology 142, 13–22. 10.1016/j.semcdb.2022.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Al-Khindi T, Sherman MB, Kodama T, Gopal P, Pan Z, Kiraly JK, Zhang H, Goff LA, du Lac S, and Kolodkin AL (2022). The transcription factor Tbx5 regulates direction-selective retinal ganglion cell development and image stabilization. Current Biology 32, 4286–4298.e5. 10.1016/j.cub.2022.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahmed Maria, Rajagopalan Adithya E., Pan Yijie, Li Ye, Williams Donnell L., Pedersen Erik A., Thakral Manav, Previero Angelica, Close Kari C., Christoforou Christina P., et al. (2023). Hacking brain development to test models of sensory coding. bioRxiv, 2023.01.25.525425. 10.1101/2023.01.25.525425. [DOI]
- 94.Goldblatt D, Huang S, Greaney MR, Hamling KR, Voleti V, Perez-Campos C, Patel KB, Li W, Hillman EMC, Bagnall MW, et al. (2023). Neuronal birthdate reveals topography in a vestibular brainstem circuit for gaze stabilization. Current Biology 33, 1265–1281.e7. 10.1016/j.cub.2023.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stone T, Webb B, Adden A, Weddig NB, Honkanen A, Templin R, Wcislo W, Scimeca L, Warrant E, and Heinze S (2017). An Anatomically Constrained Model for Path Integration in the Bee Brain. Current Biology 27, 3069–3085.e11. 10.1016/j.cub.2017.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yilmaz A, Gagnon Y, Byrne M, Baird E, and Dacke M (2022). Cold-induced anesthesia impairs path integration memory in dung beetles. Current Biology 32, 438–444.e3. 10.1016/j.cub.2021.10.067. [DOI] [PubMed] [Google Scholar]
- 97.Pisokas I, Rössler W, Webb B, Zeil J, and Narendra A (2022). Anesthesia disrupts distance, but not direction, of path integration memory. Current Biology 32, 445–452.e4. 10.1016/j.cub.2021.11.039. [DOI] [PubMed] [Google Scholar]
- 98.Beetz MJ, Kraus C, Franzke M, Dreyer D, Strube-Bloss MF, Rössler W, Warrant EJ, Merlin C, and el Jundi B (2022). Flight-induced compass representation in the monarch butterfly heading network. Current Biology 32, 338–349.e5. 10.1016/j.cub.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 99.el Jundi B, Baird E, Byrne MJ, and Dacke M (2019). The brain behind straight-line orientation in dung beetles. Journal of Experimental Biology 222, jeb192450. 10.1242/jeb.192450. [DOI] [PubMed] [Google Scholar]
- 100.Lin Y, Zhang X-J, Yang J, Li S, Li L, Lv X, Ma J, and Shi S-H (2023). Developmental neuronal origin regulates neocortical map formation. Cell Rep 42, 112170. 10.1016/j.celrep.2023.112170. [DOI] [PubMed] [Google Scholar]
- 101.Gao P, Sultan KT, Zhang X-J, and Shi S-H (2013). Lineage-dependent circuit assembly in the neocortex. Development 140, 2645–2655. 10.1242/dev.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y, Wreden CC, Levy M, Meng JL, Marshall ZD, MacLean J, and Heckscher E (2022). Sequential addition of neuronal stem cell temporal cohorts generates a feed-forward circuit in the Drosophila larval nerve cord. eLife 11, e79276. 10.7554/eLife.79276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Z, Yang C-P, Sugino K, Fu C-C, Liu L-Y, Yao X, Lee LP, and Lee T (2015). Opposing intrinsic temporal gradients guide neural stem cell production of varied neuronal fates. Science 350, 317–320. 10.1126/science.aad1886. [DOI] [PubMed] [Google Scholar]
- 104.Marchetti G, and Tavosanis G (2017). Steroid Hormone Ecdysone Signaling Specifies Mushroom Body Neuron Sequential Fate via Chinmo. Curr Biol 27, 3017–3024.e4. 10.1016/j.cub.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 105.Rossi AM, and Desplan C (2020). Extrinsic activin signaling cooperates with an intrinsic temporal program to increase mushroom body neuronal diversity. eLife 9, e58880. 10.7554/eLife.58880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Z, Yang C-P, Sugino K, Fu C-C, Liu L-Y, Yao X, Lee LP, and Lee T (2015). Opposing intrinsic temporal gradients guide neural stem cell production of varied neuronal fates. Science 350, 317–320. 10.1126/science.aad1886. [DOI] [PubMed] [Google Scholar]
- 107.Guan W, Bellemin S, Bouchet M, Venkatasubramanian L, Guillermin C, Laurençon A, Kabir C, Darnas A, Godin C, Urdy S, et al. (2022). Post-transcriptional regulation of transcription factor codes in immature neurons drives neuronal diversity. Cell Reports 39, 110992. 10.1016/j.celrep.2022.110992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Samuels TJ, Järvelin AI, Ish-Horowicz D, and Davis I (2020). Imp/IGF2BP levels modulate individual neural stem cell growth and division through myc mRNA stability. eLife 10.7554/eLife.51529. [DOI] [PMC free article] [PubMed]
- 109.Vijayakumar J, Perrois C, Heim M, Bousset L, Alberti S, and Besse F (2019). The prion-like domain of Drosophila Imp promotes axonal transport of RNP granules in vivo. Nat Commun 10, 2593. 10.1038/s41467-019-10554-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pfeiffer BD, Ngo T-TB, Hibbard KL, Murphy C, Jenett A, Truman JW, and Rubin GM (2010). Refinement of Tools for Targeted Gene Expression in Drosophila. Genetics 186, 735–755. 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dionne H, Hibbard KL, Cavallaro A, Kao J-C, and Rubin GM (2018). Genetic Reagents for Making Split-GAL4 Lines in Drosophila. Genetics 209, 31–35. 10.1534/genetics.118.300682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Green J, Vijayan V, Mussells Pires P, Adachi A, and Maimon G (2019). A neural heading estimate is compared with an internal goal to guide oriented navigation. Nat Neurosci 22, 1460–1468. 10.1038/s41593-019-0444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in this study has been made publicly available at https://osf.io/nsrf8/
This paper does not report original code.
Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request.


