Abstract
Several Pseudomonas aeruginosa strains are internalized by epithelial cells in vitro and in vivo, but the host pathways usurped by the bacteria to enter nonphagocytic cells are not clearly understood. Here, we report that internalization of strain PAK into epithelial cells triggers and requires activation of phosphatidylinositol 3-kinase (PI3K) and protein kinase B/Akt (Akt). Incubation of Madin-Darby canine kidney (MDCK) or HeLa cells with the PI3K inhibitors LY294002 (LY) or wortmannin abrogated PAK uptake. Addition of the PI3K product phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] to polarized MDCK cells was sufficient to increase PAK internalization. PtdIns(3,4,5)P3 accumulated at the site of bacterial binding in an LY-dependent manner. Akt phosphorylation correlated with PAK invasion. The specific Akt phosphorylation inhibitor SH-5 inhibited PAK uptake; internalization also was inhibited by small interfering RNA-mediated depletion of Akt phosphorylation. Expression of constitutively active Akt was sufficient to restore invasion when PI3K signaling was inhibited. Together, these results demonstrate that the PI3K signaling pathway is necessary and sufficient for the P. aeruginosa entry and provide the first example of a bacterium that requires Akt for uptake into epithelial cells.
INTRODUCTION
Pseudomonas aeruginosa is one of the leading causes of nosocomial infections in humans (reviewed in Engel, 2003). This Gram negative opportunistic pathogen causes acute infections of the respiratory and urinary tract, skin, and eye in the setting of preexisting epithelial tissue damage and/or host immunocompromise. P. aeruginosa is also a cause of chronic lung infections and ultimately death in patients with cystic fibrosis.
Although usually considered an extracellular pathogen, ∼50% of clinical, laboratory, and environmental P. aeruginosa isolates demonstrate measurable internalization in vivo as well as in vitro (Chi et al., 1991; Fleiszig et al., 1994, 1995, 1997b, 1998; Hirakata et al., 1998; Grassmé et al., 2000). These two different phenotypes correlate with the differences in type III secreted effectors (reviewed in Engel, 2003). Both classes of strains are virulent in animal models of P. aeruginosa infection. The noninvasive, cytotoxic strains secrete ExoU (Hauser et al., 1998), a potent phospholipase (Sato et al., 2003), and ExoT, a bifunctional enzyme with N-terminal GAP activity toward Rho family GTPases (Krall et al., 2000; Kazmierczak and Engel, 2002) and C-terminal ADP ribosyltransferase (ADPRT) activity toward Crk (Sun and Barbieri, 2003). Both domains of ExoT contribute to its anti-internalization activity (Garrity-Ryan et al., 2000; Garrity-Ryan et al., 2004). The invasive strains are much less cytotoxic due to the loss of the ExoU gene (Allewelt et al., 2000). Interestingly, these strains secrete ExoT and a closely related protein ExoS that also possesses an N-terminal GAP domain whose substrates include Rho family GTPases (Goehring et al., 1999; Pederson et al., 1999) and a C-terminal ADPRT domain whose targets include Ras, Ral, Rabs, and Rho family GTPases (Bette-Bobillo et al., 1998; Ganesan et al., 1999; Riese et al., 2001; Fraylick et al., 2002). Why this class of strains is able to enter into nonphagocytic cells in the presence of two potential anti-internalization factors is still enigmatic. This contradictory phenotype can be partly explained by relatively inefficient translocation of ExoS and ExoT into host cells and the increased ability of these strains to survive intracellularly (Ha and Jin, 2001).
Much remains to be learned about the mechanism and role of P. aeruginosa invasion into epithelial cells. It has been suggested that invasion may permit the bacteria to penetrate the epithelial cell layer to reach the bloodstream and disseminate to distant organs or to escape recognition by the host immune system. Bacterial invasion also may benefit the host, as seen in respiratory cell shedding of infected cells (Pier et al., 1997). Likewise, the involvement of host signal transduction pathways in P. aeruginosa internalization is poorly understood. Several host cell receptors for P. aeruginosa internalization have been suggested, including aGM1 (de Bentzmann et al., 1996), fibronectin and the integrin α5β1 (Roger et al., 1999), and the cystic fibrosis transmembrane regulator (Pier et al., 1997). It has been shown that invasion results in tyrosine phosphorylation of several host proteins, including caveolin (Zaas et al., 2005) and may involve src-family tyrosine kinases (Evans et al., 1998; Esen et al., 2001). One candidate host phosphoprotein is phosphoinositide 3-kinase (PI3K) (Esen et al., 2001)
PI3Ks are a highly conserved subfamily of lipid kinases that catalyze the addition of a phosphate molecule specifically to the 3-position of the inositol ring of phosphoinositides to generate phosphatidylinositol-3-phosphate [PtdIns3P], phosphatidylinositol-3,4-bisphosphate [PtdIns(3,4)P2], and phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] (reviewed in Vanhaesebroeck and Alessi, 2000). These short-lived phospholipids modulate the actin cytoskeleton and function as scaffolds to which specific effectors that regulate membranes are recruited. Modification of phosphoinositides by kinases and phosphatases permits their precise temporal and spatial control, allowing them to tightly control local and transient cellular processes.
For class IA PI3Ks, activation and subsequent phosphorylation of membrane tyrosine kinase receptors recruit the p85 regulatory subunit of PI3K to the membrane by binding to its Src homology (SH)2 domain. On translocation to the membrane, the p110 catalytic subunit of PI3K is activated, leading to increased levels of 3-phosphoinositides, which recruit effector proteins to the plasma membrane by binding to a pleckstrin homology (PH) domain. PI3Ks modulate many cytoskeleton-based cellular processes, including adhesion, spreading, macropinocytosis, and phagocytosis. PI3K has been shown to be necessary for the invasion of epithelial cells by several bacteria, including Listeria monocytogenes (Ireton et al., 1996), Helicobacter pylori (Kwok et al., 2002), and Escherichia coli K1 (Reddy et al., 2000).
Both PtdIns(3,4)P2 and PtdIns(3,4,5)P3 have been shown to activate one of the main downstream targets of PI3K, the serine threonine protein kinase B (PKB), also known as Akt (Burgering and Coffer, 1995; Vanhaesebroeck and Alessi, 2000). On binding to phosphoinositides by its PH domain, Akt is recruited to the membrane where it is phosphorylated by PDK1 at threonine 473 and at serine 308, leading to activation of its kinase activity. The PI3K/Akt signaling pathway is involved in diverse processes such as vesicular trafficking, mitogenesis, and cell survival (Coffer et al., 1998). Although Akt has shown to be activated during the entry of several bacterial pathogens, in no case has it been shown to be required (Ireton et al., 1996; Steele-Mortimer et al., 2000; Coombes and Mahony, 2002; Martinez and Hultgren, 2002).
In the present work, we have addressed the involvement of PI3K and PKB/Akt in the entry of the invasive ExoS and ExoT producing P. aeruginosa strain PAK. Using comprehensive strategies, we demonstrate that PI3K and Akt are critical for PAK entry into nonphagocytic cells. To the best of our knowledge, this is the first example of a bacterial pathogen that requires Akt for entry.
MATERIALS AND METHODS
Binding and Internalization Assays
Madin-Darby canine kidney (MDCK) cells (1 × 106 cells/well; clone II obtained from Dr. Keith Mostov, University of California, San Francisco, San Francisco, CA) were cultured in minimal essential medium (MEM) containing 5% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA) in six-well culture plates or on 12-mm Transwell filters (0.4-μm pore size; Corning Glassworks, Corning, NY) and incubated for 24 h (day 1 MDCK cell monolayers) (unless otherwise indicated) at 37°C with 5% CO2. P. aeruginosa strain PAK (obtained from J. Mattick, University of Queensland, Brisbane, Australia) was routinely grown shaking overnight in Luria-Bertani broth at 37°C. These stationary phase bacteria were diluted in MEM-lite (Hauser et al., 1998) and added to the MDCK cells at a multiplicity of infection (MOI) of 30 unless otherwise indicated. Adhesion and internalization assays were performed as described previously (Kazmierczak et al., 2001).
Akt Immunoprecipitation
MDCK cells (4 × 106 cells) were seeded onto 10-cm plates for 24 h. The cells were washed and placed in serum-free MEM for ∼17 h. Stationary phase grown PAK were added for 1 h unless otherwise indicated. The infected monolayers were washed with cold phosphate-buffered saline (PBS) containing 1 mM sodium orthovanadate (Sigma-Aldrich, St. Louis, MO). Cells were lysed in modified radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, 1 mM sodium orthovanadate, 50 mM NaF, 0.1 mM okadaic acid (Sigma-Aldrich), 1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich), and proteinase inhibitor tablets (Complete; Roche Diagnostics, Indianapolis, IN) for 20 min. The cell lysates were centrifuged at 16,000 × g for 20 min. To preclear the cell lysate the supernatant was mixed with 20 μl of protein G-Sepharose (4 Fast Flow; Amersham Biosciences, Piscataway, NJ), and the protein content was determined using protein assay reagent (bicinchoninic acid; Pierce Chemical, Rockford, IL). The cleared lysate (300–400 μg of protein) was incubated with Akt antibody (Cell Signaling Technology, Beverly, MA) overnight at 4°C and incubated for 1 h with protein G-Sepharose. The immune complexes were washed three times with modified RIPA buffer without deoxycholate, eluted in SDS sample buffer, electrophoresed on 10% SDS-polyacrylamide gels, and transferred to polyvinylidene difluoride membranes. The membranes were blocked with PBS containing 0.05% Tween 20 (PBST) and 5% nonfat milk for 1 h at room temperature and then incubated overnight at 4°C with an antibody that recognizes Akt phosphorylated on serine 473 (Cell Signaling Technology). The membranes were washed with PBST and incubated with horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h at room temperature and developed using a enhanced chemiluminescence kit (Amersham Biosciences). Membranes were then stripped and reprobed with an antibody that recognizes all forms of Akt (Cell Signaling Technology). Primary antibodies were diluted 1/1000 and secondary antibodies 1/3000.
Inhibitor Experiments
MDCK (1 × 106 cells/well) and HeLa cells (3 × 105 cells/well) were grown in six-well plates in MEM supplemented with 5 or 10% FBS, respectively, for 24 h. Drug treatments were carried out in serum-free medium. Unless otherwise indicated, cells were preincubated for 1 h with MEM containing LY294002 (LY) (Sigma-Aldrich) or wortmannin (Sigma-Aldrich) or for 2 h with MEM containing the Akt inhibitor SH-5 (Calbiochem, San Diego, CA). Adhesion and invasion assays were performed as detailed above.
Small Interfering RNA (siRNA)-mediated Akt Depletion
Akt and control siRNA were purchased from Cell Signaling Technology. HeLa cells grown in 10-cm dishes to 50% confluence were transfected with 100 nM siRNA according to the manufacturer's instructions. Forty-eight hours after transfection, the standard adhesion and invasion assays were performed. In parallel, lysates were immunoblotted with Akt antibody to evaluate the efficiency of siRNA-mediated protein depletion.
Lipid Delivery
PtdIns(3,4,5)P3 was added to the cells via a shuttle system (Echelon, San Jose, CA). Long-chain (Di-C16) synthetic phosphoinositides were freshly prepared as a complex with histone (Weiner et al., 2002) and added to the apical domain of the monolayer of cells for 5 min. The lipid was removed by washing, and the cells were immediately infected with PAK. Standard adhesion and invasion assays were performed.
Immunofluorescence Analysis
MDCK cells (1 × 106 cells/transwell) stably transfected with the pleckstrin homology domain of Akt fused to green fluorescent protein (PH-Akt-GFP), a probe for PtdIns(3,4,5)P3 (Yu et al., 2003), were cultured on Transwell filters for 24 h. PAK was incubated for 5 min at 37°C with 20 μM Syto 59 (Molecular Probes, Eugene, OR), a red fluorescent stain that stains nucleic acids, and then washed and resuspended in MEM. The presence of this dye minimally affected invasion (our unpublished data). Syto 59-labeled bacteria were incubated with the MDCK cells (MOI of 500) for 30 min, washed three times with PBS, and fixed with 4% paraformaldehyde for 30 min at room temperature. Samples were examined with a Zeiss 510 LSM confocal microscope or with a Nikon TE2000 inverted microscope. Images were collected as TIFF files and analyzed with Adobe PhotoShop.
RESULTS
The PI3K Pathway Is Necessary for PAK Internalization into Epithelial Cells
To address the possibility that the PI3K signaling pathway is involved in the internalization of P. aeruginosa strain PAK, we assayed the effect of two structurally unrelated cell-permeable, low-molecular-weight inhibitors, LY and wortmannin, on bacterial entry into epithelial cells. These drugs inhibit PI3K activity by different mechanisms (Vlahos et al., 1994; Ui et al., 1995).
Confluent MDCK cells grown for 24 h (day 1 MDCK cell monolayers) were pretreated either with MEM containing dimethyl sulfoxide (DMSO) (control), MEM containing LY (2–100 μM), or MEM containing wortmannin (100 nM) for 1 h. The cells were then cocultivated for 1 h in the presence of drugs with stationary phase grown PAK, and standard bacterial adhesion and invasion assays were performed. Figure 1A shows that LY blocked PAK internalization in a dose-dependent manner. LY (50 μM; Figure 1) or 100 nM wortmannin (our unpublished data) reduced entry to ∼30% compared with control-treated cells, whereas 100 μM LY virtually abolished bacterial internalization. The inhibitory effect on invasion was not due to a decrease in adhesion. Rather, exposure for 1 h to 50 μM LY slightly increased adhesion (Figure 1B), although adhesion was not affected by shorter treatment times with LY (our unpublished data). Treatment with these inhibitors also blocked PAK uptake in HeLa cells (our unpublished data). Incubation of the bacteria with the drugs alone did not affect bacterial viability (our unpublished data). Likewise, exposure of the MDCK cells monolayers to the PI3K inhibitors did not results in cell death, as assayed by LDH release (our unpublished data). Together, these results suggest that PI3K activity is required for PAK internalization into epithelial cells
Figure 1.
Pharmacological inhibitors of PI3K block PAK internalization. Day 1 MDCK cells were pretreated with the indicated concentration of LY for 1 h. Standard bacterial invasion (A) or adhesion assays (B) were carried out in the presence of the indicated concentration of the drug. Shown are the results of least three independent experiments, each performed in triplicate. Error bars indicate SEM. *p < 0.01, **p < 0.001 compared with untreated cells, Student's two-tailed t test.
Apical Addition of a PI3K Product PtdIns(3,4,5)P3 Enhances the Internalization of PAK
PI3K phosphorylates the 3′-OH position of the inositol ring of phosphatidyl inositol to generate PtdIns3P, PtdIns(3,4)P2, and PtdIns(3,4,5)P3. PtdIns(4,5)P2 is the preferred substrate of class I PI3Ks. PtdIns(3,4)P2 is then generated from PtdIns(3,4,5)P3 via the action of 5′-inositol phosphatases. Both PtdIns(3,4)P2 and PtdIns(3,4,5)P3 have been shown to activate the downstream effector Akt (Vanhaesebroeck and Alessi, 2000).
Exogenous phosphoinositides complexed to a cationic carrier molecule such as histone can be delivered to mammalian cells and have been shown to cause the recruitment of endogenous PI3K to the host cell membrane. This results in the activation of a positive feedback loop that is sufficient to synthesize sufficient new endogenous PtdIns(3,4,5)P3 to elicit physiological responses, including phosphorylation of Akt (Ozaki et al., 2000; Weiner et al., 2002; our unpublished data). The apical surface of highly polarized MDCK cells (such as those plated as confluent monolayers and subsequently incubated for 3 d) is known to be devoid of detectable PtdIns(3,4,5)P3 (Watton and Downward, 1999). We used this technique (Ozaki et al., 2000) to determine whether the apical delivery of phosphoinositides was sufficient to enhance PAK invasion into polarized MDCK cells.
Three-day-old confluent MDCK cells grown as polarized monolayers were exposed apically to 30 μM PtdIns(3,4,5)P3 complexed with histone for 5 min. Under these conditions the PI3K pathway is activated (our unpublished data). The lipid was removed by washing, the MDCK cells were infected with PAK for 1 h, and standard adhesion and invasion assays were performed. Figure 2 shows that apical addition of PtdIns(3,4,5)P3 stimulated invasion of PAK approximately twofold above addition of histone alone (A) without affecting bacterial adhesion (B). Together, these results suggest that PtdIns(3,4,5)P3 is necessary and sufficient to promote PAK entry into MDCK cells.
Figure 2.
Exogenous addition of PIP(3,4,5)P3 is sufficient to promote bacterial invasion through the apical surface. PIP(3,4,5)P3 complexed with histone (30 μM) was added to the apical surface of filter-grown day 3 MDCK cells for 5 min. The lipid was removed by washing and standard invasion (A) and adhesion assays (B) were performed. Shown are the results of least three independent experiments, each performed in triplicate. Error bars indicate SEM. *p < 0.001 compared with the control cells, Student's two-tailed t test.
Products of PI3K Accumulate at the Site of Bacterial Binding and Entry
It is now recognized that specific protein domains bind to phosphoinositides. Fusions of these domains to fluorescent proteins such as GFP provide a useful tool to visualize the subcellular localization of specific phosphoinositides (Lemmon, 2003). We used the PH domain of Akt fused to GFP (PH-Akt-GFP) as a spatial probe to examine whether PtdIns(3,4,5)P3 accumulates at the site of bacterial entry (Servant et al., 2000). Day 1 MDCK cell monolayers expressing PH-Akt-GFP, a probe for PtdIns(3,4,5)P3, were cocultivated with Syto59-labeled PAK for 30 min. A relatively high MOI (500) was used to maximize the number of adherent bacteria. The cells were then fixed, and the samples were analyzed by confocal and conventional fluorescence microscopy. In uninfected cells, the fusion protein localized to the basolateral surface of MDCK cells (Figure 3E). On apical infection with PAK, PH-Akt-GFP accumulated at the apical surface of the cell, at the site where the bacteria bound (Figure 3, A and C). Interestingly, the bacteria were often observed to attach as aggregates.
Figure 3.
PH-Akt-GFP, a probe for PtdIns(3,4,5)P3, accumulates at the site of bacterial entry. Day 1 filter-grown MDCK cells stably transfected with PH-Akt-GFP (green) were infected for 30 min with Syto59-labeled PAK (red). The samples were fixed and analyzed by epifluorescence (A and B) and confocal microscopy (C–F). A and B, conventional fluorescence microscopy of bound bacteria in the absence (A) or presence (B) 50 μM LY. Bar, 1 μm. C–F, confocal (X-Z sections) of infected (C and D) or uninfected cells (E and F). Bar, 10 μm. In uninfected cells (E), PtdIns(3,4,5)P3 is localized to the basolateral membrane. In the presence of apically applied PAK, PtdIns(3,4,5)P3 accumulates at the apical site of bacteria binding (A, apical view; C, X-Z section). Treatment with 50 μM LY decreases the frequency of apical accumulation of PH-Akt-GFP at the site of bacterial binding to 30% of the control (B, D, and G). The graph in G shows the combined data for three different experiments. Error bars indicate SEM. *p < 0.01 compared with the control cells, Student's two-tailed t test.
To determine whether the localized accumulation of PtdIns(3,4,5)P3 required PI3K activity, we pretreated the MDCK cells expressing PH-Akt-GFP with 50 μM LY for 1 h. As shown in Figure 3, B and D, LY treatment inhibits this process. The effect of LY on the accumulation of PH-Akt-GFP in the vicinity of adherent bacteria was quantified by conventional fluorescence microscopy. As shown in the graph in Figure 3G, pretreatment with 50 μM LY decreased the frequency of PH-Akt-GFP colocalization with bacteria to 30% of control (p < 0.01). Exposure to LY did not alter the localization of the fusion protein in uninfected cells (Figure 3F). We conclude that PAK is able to activate PI3K and recruit PtdIns(3,4,5)P3 to the site of entry.
The PI3K/Akt Pathway Is Activated upon Infection with PAK
An important target of activated PI3K is the serine and threonine kinase Akt. Activation of PI3K by growth factors results in stimulation of Akt kinase activity, which correlates with phosphorylation of serine 473 and threonine 308 (Vanhaesebroeck and Alessi, 2000). Because activation of PI3K and subsequent phosphorylation of Akt has been implicated in the entry of a subset of intracellular pathogens (Reddy et al., 2000; Coombes and Mahony, 2002), the phosphorylation status of Akt upon PAK infection was addressed. Day 1 MDCK cell monolayers were infected with PAK (MOI of 200) for 60 min in serum-free media. As a positive control, MDCK cells were exposed to epidermal growth factor (EGF) (10 ng/ml) for 10 min. Total Akt was immunoprecipitated from soluble whole cell lysates and sequentially immunoblotted with a monoclonal anti-phospho AktSer473 antibody followed by a monoclonal antibody recognizing total Akt. As expected, EGF treatment robustly increased the amount of phosphorylated Akt (Figure 4A). Phosphorylation of Akt also was strongly increased upon infection with PAK in both a dose-dependent (Figure 4B) and time-dependent (Figure 4C) manner. Maximal phosphorylation was observed at 1 h postinfection (Figure 4C), which correlates well with the kinetics of PAK internalization. Activation of Akt by PAK was completely inhibited by pretreatment with 100 μM LY (Figure 4B). Lower concentrations of LY (50 μM) incompletely blocked invasion (Figure 1A) and Akt phosphorylation (our unpublished data). These results demonstrate that PAK induction of Akt phosphorylation was dependent upon PI3K activation.
Figure 4.

Akt is phosphorylated upon infection with PAK. Day 1 MDCK cells were infected with PAK; at the indicated times postinfection, host cell lysates prepared and immunoprecipitated with anti-Akt followed by immunoblotting with anti-Akt or an antibody specific for Akt phosphorylated on serine 473. (A) Levels of phosphorylated and total Akt (Takt) of untreated cells (U), cells infected with PAK (MOI of 200) for 1 h, or cells treated with 10 ng/ml EGF for 10 min. (B) Phosphorylation of AKT increases at increasing MOI and can be blocked by the PI3K inhibitor LY. (C) Relative to total AKT, an increase in phosphorylation of AKT is detected within 30 min of exposure to bacteria and peaks at 1 h. Gels are representative of at least three independent experiments.
Akt Activation Mediates PAK Entry
We used pharmacological inhibitors and siRNA-mediated protein depletion to determine whether Akt activation is necessary for PAK entry. Day 1 MDCK monolayers were pretreated for 2 h with 10 μM SH-5, a specific inhibitor of the serine/threonine kinase activity of Akt (Kozikowski et al., 2003), and then incubated with PAK in the presence of the drug. Standard adhesion and internalization assays were performed. Figure 5 demonstrates that SH-5 decreased bacterial internalization almost fivefold without affecting adherence. The inhibitor did not affect bacterial or host cell viability (our unpublished data).
Figure 5.
SH-5, a selective inhibitor of AKT activation, blocks PAK uptake. Day 1 MDCK cells were treated for 2 h with SH-5 (10 μM), an inhibitor that specifically blocks AKT phosphorylation, and standard adhesion (open bars) and internalization assays (closed bars) were performed. Results are representative of at least three independent experiments, each performed in triplicate. Error bars indicate SEM. *p < 0.001 compared with untreated cells, Student's two-tailed t test.
Because pharmacological inhibitors can have nonspecific effects, we confirmed these results by determining the effect of Akt depletion on PAK invasion. These experiments were performed in HeLa cells because siRNA can be efficiently introduced into these cells by transfection. Because Akt has known antiapoptotic effects, we first established that transfection of HeLa cells with a control or Akt siRNA did not affect cell viability, as determined by LDH release, and cell number, as measured by direct cell counting (our unpublished data). Immunoblot analysis of lysates from HeLa cells transfected with a control siRNA or the Akt siRNA for 48 h demonstrated that phosphorylated Akt and total Akt protein levels were diminished approximately fourfold (Figure 6A). Parallel samples transfected with siRNA (Akt or control) were cocultivated with PAK for 1 h and standard bacterial adhesion and invasion assays were performed (Figure 6B). Partial depletion of Akt diminished PAK entry into HeLa cells by 40% without having an effect on bacterial adherence. Although there have been numerous examples of microorganism entry that requires PI3K (Steele-Mortimer et al., 2000; Coombes and Mahony, 2002), to the best of our knowledge, this is the first time that Akt has been shown to mediate internalization of bacteria.
Figure 6.

siRNA-mediated AKT depletion abrogates PAK uptake. Akt protein was depleted in HeLa cells transfection with Akt siRNA. (A) Western blot of lysates transfected with a control siRNA or Akt siRNA to two of the three Akt isoforms for 48 h. Both total (TAkt) and phosphorylated Akt were effectively depleted. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is used as loading control. (B) Standard adhesion (open bars) and internalization assays (closed bars) were performed. Results correspond to at least three independent experiments, each performed in triplicate. Error bars indicate SEM. *p < 0.05 compared with control siRNA, Student's two-tailed t test.
Akt Is Sufficient to Restore Invasion in the Absence PI3K Signaling
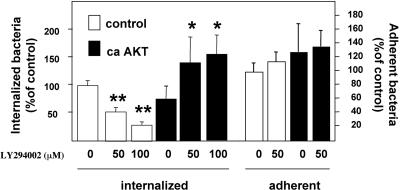
To determine whether Akt is sufficient to promote invasion of PAK, we used an MDCK cell line that expresses a constitutively active (CA) allele of Akt that lacks the pleckstrin homology domain and whose phosphorylation sites (serine 473 and threonine 308) have been changed to aspartic acid (Stokoe et al., 1997). Figure 7 shows the PAK adhesion and invasion assays performed with MDCK cells stably transfected with CA Akt. In the absence of LY, invasion was not augmented by expression of CA Akt. However, expression of CA Akt could overcome the LY-mediated inhibition of invasion. Together, these experiments suggest that CA Akt is sufficient to allow efficient PAK internalization in the presence of a PI3K inhibitor.
Figure 7.
Akt is sufficient to restore invasion in the absence of PI3K signaling. Invasion and adhesion assays were performed in MDCK cells stably transfected with CA Akt or with vector alone (control). Cells were exposed to carrier or to 50 and 100 μM LY. Shown are the combined results from five independent experiments, each performed in triplicate. Error bars indicate SEM. *p < 0.05 comparing the CA Akt cell line treated with LY to control cells treated with LY, Student's two-tailed t test. **p < 0.01 comparing the control cells treated with LY to control cells treated with carrier alone, Student's t test.
DISCUSSION
Microbial pathogens have developed diverse strategies to adapt and survive within their specific hosts. Intracellular pathogens commonly hijack host signaling pathways for invasion, intracellular motility, and intracellular replication and survival. In all cases, the infection process requires a precise subversion of the cellular machinery to establish a replicative or survival niche. Although microbial exploitation of the host cytoskeleton and associated proteins has been well described, we are just beginning to appreciate that lipids and lipid metabolism also are targeted by pathogenic organisms (reviewed in Pizarro-Cerda and Cossart, 2004). In particular, phosphoinositides, short-lived lipids whose production at specific membrane locations in the cell enables the tightly controlled recruitment or activation of diverse cellular effectors involved in cell motility or phagocytosis, are modulated by microorganisms. Modification of phosphoinositides by kinases and phosphatases allows the precise control of their temporal and spatial distribution.
The carefully orchestrated sequential localized synthesis of phosphoinositides is particularly well established for phagocytosis. PtdIns(4,5)P2 and the enzyme required for its synthesis accumulates in pseudopods during extension of the phagocytic cup and coincides with actin enrichment (Botelho et al., 2000), suggesting that localized production of PtdIns(4,5)P2 is required for the phagocytic process. As the phagosome seals, PtdIns(4,5)P2 and actin disappear, and PtdIns(3,4,5)P5 accumulates locally, only to disappear concomitantly with the sealing of the phagosomal vacuole (Cox et al., 1999; Marshall et al., 2001).
Modulation of host phosphoinositides and activation of Akt are increasingly appreciated in microbial entry. For example, InlA- and InlB-mediated entry of Listeria monocytogenes (Ireton et al., 1996), FimH-mediated entry of uropathogenic E. coli (Martinez et al., 2000), and the entry of C. pneumoniae (Coombes and Mahony, 2002) require PI3K and result in the activation of Akt. Enteropathogenic E. coli are able to avoid uptake into cultured macrophages by inhibiting the activation of PI3K and Akt (Celli et al., 2001). Through the action of SigD, an inositol phosphatase, Salmonella typhimurium entry activates PI3K and Akt, although engagement of this pathway is not required for its entry (Steele-Mortimer et al., 2000). However, in none of these cases has activation of Akt been shown to be necessary for entry. Interestingly, both PI3K and Akt are necessary for the entry of the protozoan parasite Trypanosoma cruzi into nonphagocytic and phagocytic cells (Wilkowsky et al., 2001).
In this work, we have investigated the entry pathway of P. aeruginosa strain PAK and now demonstrate that for postexponential phase grown bacteria, activation of PI3K and Akt is both necessary and sufficient for bacterial entry into cultured epithelial cells. Pharmacological inhibitors of PI3K and Akt, as well as depletion of Akt, blocked invasion. The effect of Akt depletion on bacterial entry was only partial; this finding could be explained by the persistence of residual Akt activity or by the requirement for other downstream targets of PI3K in PAK uptake. Addition of PtdIns(3,4,5)P3 complexed to a cationic carrier to polarized MDCK cells was sufficient to augment PAK internalization. Accumulation of PtdIns(3,4,5)P3, normally found at the basolateral surface, at the site of apically entering bacterial was observed, suggesting that P. aerguinosa entry involves recruitment, either by translocation from the basolateral surface and/or by new synthesis, of PtdIns(3,4,5)P3. Because LY blocked both the accumulation of PtdIns(3,4,5)P3 and bacterial entry, these results suggest that PAK-mediated activation of PI3K and subsequent localized accumulation of PtdIns(3,4,5)P3 is necessary for bacterial invasion. Expression of CA Akt in the presence of a PI3K inhibitor was sufficient to restore bacterial entry. To the best of our knowledge, this is the first time that Akt has been shown to mediate internalization of a bacterium.
It is interesting that addition of PtdIns(3,4,5)P3 only augmented PAK entry into highly polarized MDCK cells but not into incompletely polarized confluent monolayers (our unpublished data). Although the increase in PAK internalization was modest, it is remarkable that any effect was seen, given that the exogenous phosphoinositides were present only for a brief period. There are numerous suggestions in the literature that P. aeruginosa entry into cultured epithelial cells occurs preferentially at the basolateral surface in polarized monolayers or is enhanced in less well polarized or wounded epithelium (Yamaguchi and Yamada, 1991; Zahm et al., 1991; Tsang et al., 1994; de Bentzmann et al., 1996; Fleiszig et al., 1997a, 1998; Kazmierczak et al., 2004). It is possible that apical localization PtdIns(3,4,5)P3 in incompletely polarized cells accounts for their increased susceptibility to P. aeruginosa invasion; we are currently investigating the molecular basis for the differing effect of PtdIns(3,4,5)P3 on highly polarized versus incompletely polarized cells.
We note that the most of the bacteria were bound as aggregates; these clusters of bacteria were commonly associated with PtdIns(3,4,5)P3. These bacterial aggregates may represent pack swarming, a bacterial chemotaxic response to host cell factors released upon damage by the bacterial type III secretion apparatus (Dacheux et al., 2001).
How might activation of the PI3K and Akt pathway lead to PAK internalization? Both PI3K and Akt have been shown to modify the cytoskeleton dynamics, to be involved in the regulation of membrane traffic, and to have direct roles in macropinocytosis, a pathway used by some pathogenic bacteria for cellular entry (Ojcius et al., 1998; Zenni et al., 2000). The regulatory subunit P85 of PI3K contains an amino-terminal SH3 domain and two SH2 domains that allow the p85 subunit to recruit multiple intracellular signaling molecules at the same time, possibly to the site of bacterial entry. Several proteins involved in modulating the actin cytoskeleton have PH domains, such as guanine exchange factors for Rho family GTPases. The downstream targets of Akt are numerous and affect diverse host cell processes including cell survival, transcription, metabolic, and host defense pathways (reviewed in Vanhaesebroeck and Alessi, 2000). Of interest, Akt also has been shown to stimulate Rab5-dependent endocytosis. The identification of other PI3K effectors and the specific targets of Akt involved in PAK internalization are under study.
One possible category of PI3K effectors is the Rho family GTPases, although the relationship of Rho family GTPases to PI3K and Akt activation is complex. Ras and Rho family GTPases seem to act both upstream and downstream of PI3K and Akt (Nobes and Hall, 1995; Ren and Schwartz, 1998; Weiner et al., 2002). For entry of strain PA103, we have previously shown a requirement for Rho family GTPases that is antagonized by the type III translocated effector ExoT (Kazmierczak et al., 2001; Kazmierczak et al., 2004); we are currently investigating the role of Rho family GTPases in PAK entry. Interestingly, Exo S, a type III secretion-dependent effector translocated into host cells by invasive P. aeruginosa strains, is able to inactivate Ras via ADP ribosylation (Ganesan et al., 1998). ExoS also has been shown to abrogate EGF-mediated activation of Akt-(Henriksson et al., 2000) and Rab5-mediated endocytosis (Barbieri et al., 2001). It will be interesting to determine whether PAK stimulates Rab5-mediated endocytosis.
In summary, we provide the first example of a bacterial pathogen that exploits and requires the PI3K and Akt pathway for internalization. Future work will be directed to determining how the bacteria activate this pathway and what downstream targets of Akt are involved.
Acknowledgments
We thank Drs. David Stokoe and Emma Shtivelman and members of the Engel and Mostov laboratories for advice and encouragement. We thank Dr. Emma Shtivelman for the cell lines expressing constitutively active Akt and Dr. Orion Weiner for help with the PtdIns(3,4,5)P3 lipid transfer experiments. This work was supported by National Institutes of Health grants HL-55980 (to K. M. and J.N.E.) and AI-42806 (to J.N.E.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-08-0717) on March 16, 2005.
References
- Allewelt, M., Coleman, F. T., Grout, M., Priebe, G. P., and Pier, G. B. (2000). Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 68, 3998-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri, A. M., Sha, Q., Bette-Bobillo, P., Stahl, P. D., and Vidal, M. (2001). ADP-ribosylation of Rab5 by ExoS of Pseudomonas aeruginosa affects endocytosis. Infect. Immun. 69, 5329-5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bette-Bobillo, P., Giro, P., Sainte-Marie, J., and Vidal, M. (1998). Exoenzyme S from P. aeruginosa ADP ribosylates rab4 and inhibits transferrin recycling in SLO-permeabilized reticulocytes. Biochem. Biophys. Res. Commun. 244, 336-341. [DOI] [PubMed] [Google Scholar]
- Botelho, R. J., Teruel, M., Dierckman, R., Anderson, R., Wells, A., York, J. D., Meyer, T., and Grinstein, S. (2000). Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 151, 1353-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgering, B. M., and Coffer, P. J. (1995). Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376, 599-602. [DOI] [PubMed] [Google Scholar]
- Celli, J., Olivier, M., and Finlay, B. B. (2001). Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways. EMBO J. 20, 1245-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, E., Mehl, T., Nunn, D., and Lory, S. (1991). Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect. Immun. 59, 822-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffer, P. J., Jin, J., and Woodgett, J. R. (1998). Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 335, 1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes, B. K., and Mahony, J. B. (2002). Identification of MEK- and phosphoinositide 3-kinase-dependent signalling as essential events during Chlamydia pneumoniae invasion of HEp2 cells. Cell Microbiol. 4, 447-460. [DOI] [PubMed] [Google Scholar]
- Cox, D., Tseng, C. C., Bjekic, G., and Greenberg, S. (1999). A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 274, 1240-1247. [DOI] [PubMed] [Google Scholar]
- Dacheux, D., Goure, J., Chabert, J., Usson, Y., and Attree, I. (2001). Poreforming activity of type III system-secreted proteins leads to oncosis of Pseudomonas aeruginosa-infected macrophages. Mol. Microbiol. 40, 76-85. [DOI] [PubMed] [Google Scholar]
- de Bentzmann, S., Roger, P., Dupuit, F., Bajolet-Laudinat, O., Fuchey, C., Plotkowski, M. C., and Puchelle, E. (1996). Asialo GM1 is a receptor for Pseudomonas aeruginosa adherence to regenerating respiratory epithelial cells. Infect. Immun. 64, 1582-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bentzmann, S., Roger, P., and Puchelle, E. (1996). Pseudomonas aeruginosa adherence to remodelling respiratory epithelium. Eur. Resp. J. 10, 2145-2150. [DOI] [PubMed] [Google Scholar]
- Engel, J. N. (2003). Molecular Pathogenesis of acute Pseudomonas aeruginosa infections. In: Severe Infections Caused by Pseudomonas aeruginosa, ed. A. Hauser and J. Rello, New York: Kluwer Academic/Plenum Press, 201-230.
- Esen, M., Grassme, H., Riethmuller, J., Riehle, A., Fassbender, K., and Gulbins, E. (2001). Invasion of human epithelial cells by Pseudomonas aeruginosa involves src-like tyrosine kinases p60Src and p59Fyn. Infect. Immun. 69, 281-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, D. J., Frank, D. W., Finck-Barbançon, V., Wu, C., and Fleiszig, S. M. (1998). Pseudomonas aeruginosa invasion and cytotoxicity are independent events, both of which involve protein tyrosine kinase activity. Infect. Immun. 66, 1453-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig, S. M., Evans, D. J., Do, N., Vallas, V., Shin, S., and Mostov, K. (1997a). Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect. Immun. 65, 2861-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig, S. M., Vallas, V., Jun, C., Mok, L., Balkovetz, D., Roth, M., and Mostov, K. (1998). Susceptibility of epithelial cells to Pseudomonas aeruginosa invasion and cytotoxicity is upregulated by hepatocyte growth factor. Infect. Immun. 66, 3443-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig, S. M., Zaidi, T. S., Fletcher, E. L., Preston, M. J., and Pier, G. B. (1994). Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect. Immun. 62, 3485-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig, S. M., Zaidi, T. S., and Pier, G. B. (1995). Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect. Immun. 63, 4072-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig, S.M.J., Wiener-Kronish, J. P., Miyazaki, H., Vallas, V., Mostov, K., Kanada, D., Sawa, T., Yen, T.S.B., and Frank, D. (1997b). Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect. Immun. 65, 579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraylick, J. E., Riese, M. J., Vincent, T. S., Barbieri, J. T., and Olson, J. C. (2002). ADP-ribosylation and functional effects of Pseudomonas exoenzyme S on cellular RalA. Biochemistry 41, 9680-9687. [DOI] [PubMed] [Google Scholar]
- Ganesan, A. K., Frank, D. W., Misra, R. P., Schmidt, G., and Barbieri, J. T. (1998). Pseudomonas aeruginosa exoenzyme S ADP-ribosylates Ras at multiple sites. J. Biol. Chem. 273, 7332-7337. [DOI] [PubMed] [Google Scholar]
- Ganesan, A. K., Vincent, T. S., Olson, J. C., and Barbieri, J. T. (1999). Pseudomonas aeruginosa exoenzyme S disrupts Ras-mediated signal transduction by inhibiting guanine nucleotide exchange factor-catalyzed nucleotide exchange. J. Biol. Chem. 274, 21823-21829. [DOI] [PubMed] [Google Scholar]
- Garrity-Ryan, L., Kazmierczak, B., Kowal, R., Commolli, J., Hauser, A., and Engel, J. (2000). The arginine finger domain of ExoT is required for actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun. 68, 7100-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity-Ryan, L., et al. (2004). The ADP ribosyltransferase domain of Pseudomonas aeruginosa ExoT contributes to its biological activities. Infect. Immun. 72, 546-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring, U. M., Schmidt, G., Pederson, K. J., Aktories, K., and Barbieri, J. T. (1999). The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J. Biol. Chem. 274, 36369-36372. [DOI] [PubMed] [Google Scholar]
- Grassmé, H., Kirschnek, S., Riethmueller, J., Andrea Riehle, von Kürthy, G., Lang, F., Weller, M., and GulbinS, E. (2000). CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science 290, 527-530. [DOI] [PubMed] [Google Scholar]
- Ha, U., and Jin, S. (2001). Growth phase-dependent invasion of Pseudomonas aeruginosa and its survival within HeLa cells. Infect. Immun. 69, 4398-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, A. R., Kang, P. J., and Engel, J. (1998). PepA, a novel secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 27, 807-818. [DOI] [PubMed] [Google Scholar]
- Henriksson, M. L., Rosqvist, R., Telepnev, M., Wolf-Watz, H., and Hallberg, B. (2000). Ras effector pathway activation by EGF is inhibited in vivo by exoenzyme S ADP-ribosylation of Ras. Biochem. J. 347, 217-222. [PMC free article] [PubMed] [Google Scholar]
- Hirakata, Y., et al. (1998). Adherence to and penetration of human intestinal Caco-2 epithelial cell monolayers by Pseudomonas aeruginosa. Infect. Immun. 66, 1748-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton, K., Payrastre, B., Chap, H., Ogawa, W., Sakaue, H., Kasuga, M., and Cossart, P. (1996). A role for phosphoinositide 3-kinase in bacterial invasion. Science 274, 780-782 [correction published in Science (1997) 275, 464]. [DOI] [PubMed] [Google Scholar]
- Kazmierczak, B., and Engel, J. (2002). Pseudomonas aeruginosa ExoT acts in vivo as a GTPase activating protein for RhoA, Rac1, and Cdc42. Infect. Immun. 70, 2198-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak, B. I., Jou, T.-S., Mostov, K., and Engel, J. (2001). Rho-GTPase activity modulates Pseudomonas aeruginosa internalization by epithelial cells. Cell Microbiol. 3, 85-98. [DOI] [PubMed] [Google Scholar]
- Kazmierczak, B. I., Mostov, K., and Engel, J. N. (2004). Epithelial cell polarity alters Rho-GTPase responses to Pseudomonas aeruginosa. Mol. Biol. Cell 15, 411-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozikowski, A. P., Sun, H., Brognard, J., and Dennis, P. A. (2003). Novel PI analogues selectively block activation of the pro-survival serine/threonine kinase Akt. J. Am. Chem. Soc. 125, 1144-1145. [DOI] [PubMed] [Google Scholar]
- Krall, R., Schmidt, G., Aktories, K., and Barbieri, J. T. (2000). Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect. Immun. 68, 6066-6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok, T., Backert, S., Schwarz, H., Berger, J., and Meyer, T. F. (2002). Specific entry of Helicobacter pylori into cultured gastric epithelial cells via a zipper-like mechanism. Infect. Immun. 70, 2108-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon, M. A. (2003). Phosphoinositide recognition domains. Traffic 4, 201-213. [DOI] [PubMed] [Google Scholar]
- Marshall, J. G., Booth, J. W., Stambolic, V., Mak, T., Balla, T., Schreiber, A. D., Meyer, T., and Grinstein, S. (2001). Restricted accumulation of phosphatidylinositol 3-kinase products in a plasmalemmal subdomain during Fc gamma receptor-mediated phagocytosis. J. Cell Biol. 153, 1369-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, J. J., and Hultgren, S. J. (2002). Requirement of Rho-family GTPases in the invasion of Type 1-piliated uropathogenic Escherichia coli. Cell Microbiol. 4, 19-28. [DOI] [PubMed] [Google Scholar]
- Martinez, J. J., Mulvey, M. A., Schilling, J. D., Pinkner, J. S., and Hultgren, S. J. (2000). Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19, 2803-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes, C. D., and Hall, A. (1995). Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53-62. [DOI] [PubMed] [Google Scholar]
- Ojcius, D. M., Bravo de Alba, Y., Kanellopoulos, J. M., Hawkins, R. A., Kelly, K. A., Rank, R. G., and Dautry-Varsat, A. (1998). Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia-specific T cells. J. Immunol. 160, 1297-1303. [PubMed] [Google Scholar]
- Ozaki, S., DeWald, D. B., Shope, J. C., Chen, J., and Prestwich, G. D. (2000). Intracellular delivery of phosphoinositides and inositol phosphates using polyamine carriers. Proc. Natl. Acad. Sci. USA 97, 11286-11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson, K. J., Vallis, A. J., Aktories, K., Frank, D. W., and Barbieri, J. T. (1999). The amino-terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP-binding proteins. Mol. Microbiol. 32, 393-401. [DOI] [PubMed] [Google Scholar]
- Pier, G. B., Grout, M., and Zaidi, T. S. (1997). Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc. Nat. Acad. Sci. USA 94, 12088-12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Cerda, J., and Cossart, P. (2004). Subversion of phosphoinositide metabolism by intracellular bacterial pathogens. Nat. Cell Biol. 6, 1026-1033. [DOI] [PubMed] [Google Scholar]
- Reddy, M. A., Prasadarao, N. V., Wass, C. A., and Kim, K. S. (2000). Phosphatidylinositol 3-kinase activation and interaction with focal adhesion kinase in Escherichia coli K1 invasion of human brain microvascular endothelial cells. J. Biol. Chem. 275, 36769-36774. [DOI] [PubMed] [Google Scholar]
- Ren, X. D., and Schwartz, M. A. (1998). Regulation of inositol lipid kinases by Rho and Rac. Curr. Opin. Genet. Dev. 8, 63-67. [DOI] [PubMed] [Google Scholar]
- Riese, M. J., Wittinghofer, A., and Barbieri, J. T. (2001). ADP ribosylation of Arg41 of Rap by ExoS inhibits the ability of Rap to interact with its guanine nucleotide exchange factor, C3G. Biochemistry 40, 3289-3294. [DOI] [PubMed] [Google Scholar]
- Roger, P., Puchelle, E., Bajolet-Laudinat, O., Tournier, J. M., Debordeaux, C., Plotkowski, M. C., Cohen, J. H., Sheppard, D., and de Bentzmann, S. (1999). Fibronectin and α5β1 integrin mediate binding of Pseudomonas aeruginosa to repairing airway epithelium. Eur. Respir. J. 13, 1301-1309. [PubMed] [Google Scholar]
- Sato, H., et al. (2003). The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 22, 2959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant, G., Weiner, O. D., Herzmark, P., Balla, T., Sedat, J. W., and Bourne, H. R. (2000). Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287, 1037-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele-Mortimer, O., Knodler, L. A., Marcus, S. L., Scheid, M. P., Goh, B., Pfeifer, C. G., Duronio, V., and Finlay, B. B. (2000). Activation of Akt/protein kinase B in epithelial cells by the Salmonella typhimurium effector sigD. J. Biol. Chem. 275, 37718-37724. [DOI] [PubMed] [Google Scholar]
- Stokoe, D., Stephens, L. R., Copeland, T., Gaffney, P. R., Reese, C. B., Painter, G. F., Holmes, A. B., McCormick, F., and Hawkins, P. T. (1997). Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277, 567-570. [DOI] [PubMed] [Google Scholar]
- Sun, J., and Barbieri, J. T. (2003). Pseudomonas aeruginosa ExoT ADP-ribosylates CT10-regulator of kinase (Crk) proteins. J. Biol. Chem. 278, 32794-32800. [DOI] [PubMed] [Google Scholar]
- Tsang, K.W.T., Rutman, A., Tanaka, E., Lundt, V., Dewar, A., Cole, P. J., and Wilson, R. (1994). Interaction of Pseudomonas aeruginosa with human respiratory mucosa in vitro. Eur. Respir. J. 7, 1746-1753. [DOI] [PubMed] [Google Scholar]
- Ui, M., Okada, T., Hazeki, K., and Hazeki, O. (1995). Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem. Sci. 20, 303-307. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck, B., and Alessi, D. R. (2000). The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346, 561-576. [PMC free article] [PubMed] [Google Scholar]
- Vlahos, C. J., Matter, W. F., Hui, K. Y., and Brown, R. F. (1994). A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 269, 5241-5248. [PubMed] [Google Scholar]
- Watton, S. J., and Downward, J. (1999). Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr. Biol. 9, 433-436. [DOI] [PubMed] [Google Scholar]
- Weiner, O. D., Neilsen, P. O., Prestwich, G. D., Kirschner, M. W., Cantley, L. C., and Bourne, H. R. (2002). A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 4, 509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkowsky, S. E., Barbieri, M. A., Stahl, P., and Isola, E. L. (2001). Trypanosoma cruzi: phosphatidylinositol 3-kinase and protein kinase B activation is associated with parasite invasion. Exp. Cell Res. 264, 211-218. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, T., and Yamada, H. (1991). Role of mechanical injury on airway surface in the pathogenesis of Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 144, 1147-1152. [DOI] [PubMed] [Google Scholar]
- Yu, W., O'Brien, L. E., Wang, F., Bourne, H., Mostov, K. E., and Zegers, M. M. (2003). Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial structures. Mol. Biol. Cell 14, 748-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaas, D., Duncan, M., Li, G., Wright, J. R., and Abraham, S. N. (2005). Pseudomonas invasion of type I pneumocytes is dependent on the expression and phosphorylation of caveolin-2. J. Biol. Chem. 280, 4864-4872. [DOI] [PubMed] [Google Scholar]
- Zahm, J. M., Chevillard, M., and Puchelle, E. (1991). Wound repair of human surface respiratory epithelium. Am. J. Respir. Cell. Mol. Biol. 5, 242-248. [DOI] [PubMed] [Google Scholar]
- Zenni, M. K., Giardina, P. C., Harvey, H. A., Shao, J., Ketterer, M. R., Lubaroff, D. M., Williams, R. D., and Apicella, M. A. (2000). Macropinocytosis as a mechanism of entry into primary human urethral epithelial cells by Neisseria gonorrhoeae. Infect. Immun. 68, 1696-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]