Abstract
Glycosylation of the flagellar core proteins (FlaBs) was detected in Treponema pallidum Nichols and in the type or reference strains of seven oral Treponema species. In several nonmotile strains of oral treponemes, the FlaBs were undetectable by both antibody and glycan staining. In contrast, a spontaneous low-motility variant of T. vincenti£i-related strain RitzA, OMZ 305A, lacked the flagellar sheath protein (FlaA) and the two glycan-staining FlaB bands of the wild type, but antibody labeling revealed a novel FlaB band with a lower relative molecular weight. A ca. 38-kDa component of isolated endoflagella of T. vincentii OMZ 800 was identified on Western blots as FlaA by monoclonal antibody (MAb) H9-2, which specifically labels the 37-kDa FlaA protein of T. pallidum. Glycan and H9-2 labeling patterns similar to those of T. pallidum were observed in whole-cell extracts of T. medium G7201 and of 10 strains classified as T. vincentii and as two T. vincentii-related taxons. These four groups were thus identified as cultivable pathogen (T. pallidum)-related oral spirochetes as defined by labeling with MAb H9-2. No H9-2 MAb-reactive component could be detected in T. amylovorum, T. denticola, T. maltophilum, T. pectinovorum, and the three subspecies of T. socranskii.
In patients with periodontal diseases, the microflora of subgingival plaque at affected sites is often dominated by highly heterogeneous populations of spirochetes, most of which, however, have never been cultured in vitro (5, 21). Because of their enormous increase in absolute and relative numbers associated with disease development and because of their fascinating viscosity-dependent motility, which is plausibly taken as a prerequisite for active tissue penetration, treponemes rank high on the list of suspected etiologic agents of periodontal diseases.
The type species of the genus Treponema is the causative agent of syphilis, Treponema pallidum. Riviere et al. (17) detected in plaque samples from sites of periodontitis and of acute necrotizing gingivitis a population of treponemes which were specifically labeled by monoclonal antibody (MAb) H9-2, previously characterized as reactive only with the definite pathogen T. pallidum (12); this was taken as suggestive evidence of a pathogenic role for these oral spirochetes in disease, and they were designated PROS (pathogen related oral spirochetes). Recently, we demonstrated the presence of MAb H9-2-reactive epitopes in whole cells and on Western blots of T. vincentii and related organisms and thereby identified them as cultivable PROS (6).
Motility of treponemes is due to the rotation of their endoflagella within the periplasmic space. The consequent rotation of the whole cell leads to directional movement only with the drag of a viscous environment. However, another spirochete with a similar motility apparatus, Spirochaeta aurantia, is capable of directed translocation in low-viscosity media (3). The major components of the endoflagella are flagellar core proteins, phylogenetically related to the flagellins of other eubacteria, and flagellar sheath proteins, which appear to be restricted to this group of organisms (4). In T. pallidum, all of these components have been well described at the DNA and protein levels (16). A single flaA gene codes for the flagellar sheath protein, which carries the MAb H9-2 epitope (12), and three flaB genes code for flagellar core proteins. For oral treponemes, however, the flagellar apparatus is much less well described, and these studies were concerned mainly with T. denticola (10).
Glycosylation of proteins is far less common in prokaryotes than in eukaryotes and has not hitherto been directly demonstrated in the genus Treponema (13). In other spirochetes, however, evidence of the glycosylation of flagellar proteins has been reported. By using labeling, as well as deglycosylation techniques, Li et al. (11) demonstrated glycosylation of FlaA in Serpulina hyodysenteriae. In contrast, two of three FlaBs of Spirochaeta aurantia endoflagellar preparations, but not FlaA, appear to be glycosylated on the basis of their reactivity with concanavalin A (3).
Here, I present a comparison of endoflagellar components from T. pallidum and cultured oral treponemes using MAb H9-2 and a specific stain for the detection of glycoproteins. In addition to wild-type oral treponemes, a number of spontaneous motility mutants were also investigated. The results indicate that the wild-type flagellar core proteins of all of these treponemes are glycosylated and that T. vincentii, two phenotypically distinct but T. vincentii-related oral treponemes, and T. medium (18) carry the H9-2-reactive epitope on the endoflagellar sheath protein. This defines them as cultivable PROS (17) and demonstrates the heterogeneity of this group.
MATERIALS AND METHODS
Bacteria.
An extract of T. pallidum subsp. pallidum Nichols, prepared as described by Hanff et al. (8), and an extract of T. medium G7201, prepared as described by Umemoto et al. (18), were generously provided by J. K. Howell and S. J. Norris (University of Texas, Houston) and T. Umemoto (Asahi University, Hozumi, Japan), respectively. The three reference strains (ATCC 700013 [= N9], ATCC 35580 [= LA-1], and RitzA) and seven clinical isolates (OMZ 800 through OMZ 806) of T. vincentii and T. vincentii-related taxons were grown in OMIZ-Pat medium without sugars but supplemented with 1% (vol/vol) human serum (Sigma H1388) and extracted as previously described (21). All other oral Treponema species were grown in OMIZ-Pat medium supplemented with 1% human serum (21). The clinical human strains, as well as numerous canine oral treponemes, were isolated by a limited-dilution technique as previously described (21, 22). The spontaneous low-motility mutants OMZ 447K, LABK4, and OMZ 305A were detected in soft agar cultures of their parents (T. socranskii subsp. socranskii ATCC 33536T, LA-1, and RitzA, respectively) as compact colonies with slightly fluffy surfaces, whereas the nonmotile mutants CDK and 33K2 (parents, T. denticola CD-1 and T. pectinovorum ATCC 33768T, respectively) formed compact colonies with smooth surfaces.
Isolation of endoflagella.
Cells of T. vincentii OMZ 800 were washed twice and resuspended in physiological saline. The suspension was mixed with an equal volume of 0.2% Triton X-100 in saline and incubated for 1 h at 35°C to remove the outer membrane. Following one wash in saline, Triton-treated cells were resuspended in 10 ml of saline and endoflagella were sheared off by squirting the suspension 20 times through a Falcon 10-ml pipette into a 15-ml centrifuge tube and then vortexing it for 3 min. After centrifugation (30 min at 4°C and 30,000 × g) to pellet cell bodies, the supernatant was removed and recentrifuged (1 h, 4°C, 100,000 × g). The final pellet, consisting mostly of endoflagella, was resuspended in saline and stored at −20°C. Before electrophoresis, 3 volumes of endoflagellar suspension was mixed with 1 volume of 4× Lämmli sample buffer and boiled for 5 min.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
Preparation of 10 and 12.5% polyacrylamide gels (5% cross-linking), transfer to nitrocellulose, staining for protein by using copper phthalocyanine 3,4′,4",4‴-tetrasulfonic acid sodium salt (CPTS) (1), immunolabeling (after CPTS destaining), and detection by alkaline phosphatase were done as previously described (21). MAb H9-2, which reacts specifically with the 37-kDa flagellar sheath protein of T. pallidum (12), was generously donated as hybridoma culture supernatant by S. A. Lukehart (University of Washington, Seattle). It was used at a 1:20 dilution and detected with an anti-mouse immunoglobulin G (whole molecule)-alkaline phosphatase conjugate (Sigma A 5153) at a 1:1,000 dilution.
Affinity purification of antibodies.
Patient sera diluted 1:50 were incubated with (CPTS-destained) strips of antigen cut from CPTS-stained preparative blots on nitrocellulose (target strips were identified by immunolabeling or glycan labeling of margin lanes), and specific antibodies were eluted with 4 M magnesium chloride (20).
DIG labeling and detection of glycans.
Oxidation of carbohydrate with periodate renders it amenable to specific covalent labeling with digoxigenin (DIG) hydrazide. The DIG label is then detected by an alkaline phosphatase conjugate of a specific anti-DIG antibody (9). Blots on nitrocellulose paper were stained for protein with CPTS (1), reference bands were marked with a pencil before removal of CPTS and staining with DIG-glycan kit no. 1142 372 in accordance with the manufacturer’s (Boehringer Mannheim) instructions. No reaction was seen when either the periodate oxidation or the DIG hydrazide coupling step was omitted from the procedure.
DIG-labeled lectins.
Additional experiments were performed with five different DIG-labeled lectins (from Galantus nivalis, Sambuccus nigra, Maackia amurensis, peanut, and Datura stramonium) to look for specific glycan structures (Boehringer Mannheim kit no. 1210 238); however, positive reactions were observed only with controls, not with treponemal extracts.
RESULTS AND DISCUSSION
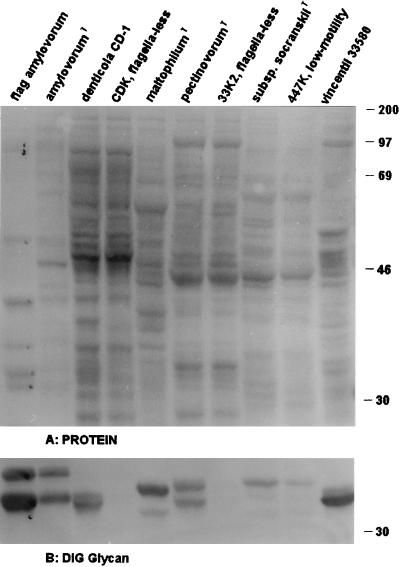
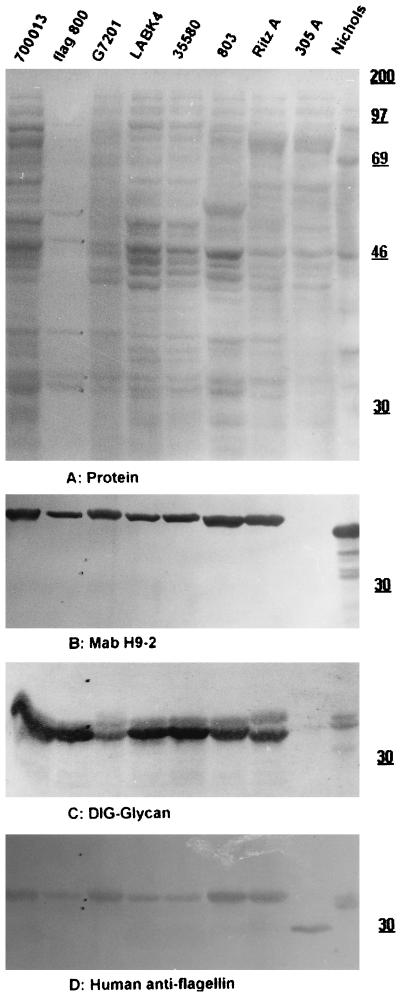
As shown in Fig. 1B and 2C, the flagellar core proteins of representatives of all of the oral treponemes identified to the species level, as well as T. pallidum strain Nichols, were stained after the periodate oxidation-DIG hydrazide labeling procedure for vicinal hydroxyls, which is used as a specific, although indirect, demonstration of protein glycosylation (9); no reaction was observed on the FlaA bands or any other regions of the blot. The difference in glycan staining between FlaA and FlaBs was confirmed by using purified endoflagella from T. vincentii OMZ 800 (Fig. 2C, lane 2), T. amylovorum, and several other as yet undescribed oral treponemes (data not shown). Depending on the treponemal species tested, between two and five bands in the molecular mass range expected for flagellar core proteins (28 to 35 kDa) were labeled in all strains (Fig. 1B and 2C and unpublished data), with a few interesting exceptions. No glycan-staining bands were detected in three groups of isolates: (i) strains lacking flagella (represented by one nonmotile mutant each of T. denticola CD-1 and T. pectinovorum ATCC 33768T [strains CDK and 33K2, respectively; Fig. 1B, lanes 4 and 7] and by two human and two canine primary oral isolates belonging to as yet undescribed species) (data not shown), (ii) a highly motile as yet undescribed canine isolate with six endoflagella per pole (data not shown), and (iii) strain OMZ 305A (a flagellated but low-motility mutant of T. vincentii-related strain RitzA). In this mutant, neither glycosylated FlaBs nor an H9-2 MAb-reactive FlaA component was detectable (Fig. 2C and B, lane 8 versus lane 7). Another low-motility mutant, strain LABK4, derived from T. vincentii ATCC 35580, showed the same glycan and H9-2 MAb labeling as its parent (Fig. 2C and B, lane 4 versus lane 5). Its impaired motility may be due to a reduced number (three or four, compared to five or six in the parent line [17a]) of endoflagella per pole. In contrast, a low-motility mutant of T. socranskii subsp. socranskii ATCC 35536T, OMZ 447K, showed the same glycan labeling as its parent (Fig. 1B, lane 9 versus lane 8), and no other biochemical character was found to correlate with the low motility of the mutant strain.
FIG. 1.
Protein and glycan patterns of the type and reference strains of six species of oral treponemes and of three of their motility mutants. Western blots on nitrocellulose after SDS-12.5% PAGE were stained for protein with CPTS (A); after destaining, the same blot was stained for glycan (B) (only the area comprising FlaBs and FlaA, marked in lane 1, is shown; no glycan labeling was detected in other areas of the blot). Lanes 1 to 10 (left to right), respectively: isolated endoflagella of T. amylovorum ATCC 700288T, T. amylovorum ATCC 700288T, T. denticola CD-1, T. denticola motility mutant CDK, T. maltophilum ATCC 51939T, T. pectinovorum ATCC 33768T, T. pectinovorum motility mutant 33K2, T. socranskii subsp. socranskii ATCC 35536T, T. socranskii subsp. socranskii motility mutant 447K, and T. vincentii ATCC 35580. The positions of rainbow molecular size standards (Amersham) are indicated in kilodaltons on the right.
FIG. 2.
Protein, glycan, and antigen patterns of T. pallidum, cultured PROS, and isolated endoflagella of T. vincentii. Western blots on nitrocellulose after SDS–12% PAGE were stained for protein with CPTS (A). At this stage, the bands of endoflagellar components in lane 2 were marked by pencil to permit accurate reference after removal of the CPTS stain (depending on the intensity of the subsequent glycan labeling or immunolabeling, some of these reference dots may be obscured in panels B to D). After CPTS destaining, the same blot was immunolabeled with MAb H9-2 (B). Parallel blots were stained for glycan (C) or immunolabeled with antibodies affinity purified on the flagellar core protein region of OMZ 803 (D). Lanes 1 to 9 (left to right), respectively: strain ATCC 700013, endoflagella of T. vincentii OMZ 800, T. medium G7201, low-motility mutant LABK4, ATCC 35580 (the parent of LABK4), OMZ 803, RitzA (the parent of OMZ 305A), OMZ 305A, and T. pallidum Nichols. Panels B to D were cut to include only the three bands corresponding to FlaA and the two FlaBs marked in lane 2. The values on the right are molecular masses in kilodaltons.
The present data confirm the suggestion by Norris et al. (16) that FlaBs of T. pallidum are glycosylated; this was based on a difference between the calculated and observed molecular masses of T. pallidum FlaBs and on the metabolic labeling with glucosamine of components with relative molecular masses similar to those of FlaBs (14). A difference in electrophoretic mobility, and thus apparent molecular weight, between glycosylated and unglycosylated FlaBs may have been detected with the low-motility mutant OMZ 305A: increased amounts of a single, low-molecular-weight flagellin (Fig. 2D, lane 8 versus lane 7) appear concomitant with the virtual disappearance of the two bands of glycosylated flagellins of the parent strain RitzA (Fig. 2C, lane 8 versus lane 7). However, in the absence of amino acid sequence data, this remains speculative.
The demonstration of glycans on treponemal FlaBs should stimulate efforts to elucidate the chemical structure of these posttranslational substitutions. Qualitative experiments to this end with five different DIG-labeled lectins (see Materials and Methods) and the same spectrum of oral spirochetes as shown in Fig. 1 gave negative results. Current efforts toward a structural analysis of FlaB glycans concentrate on T. amylovorum because its two widely spaced FlaBs (Fig. 1B, lane 1) are relatively easy to isolate. This may be important to distinguish heterogeneity of glycans on a single FlaB protein from unequal glycosylation of different FlaBs.
Sequence data of the regulatory region of FlaBs indicate the absence of a signal sequence (16) and therefore suggest that flagellar core assembly in treponemes follows the same route as in gram-negative bacteria, where the unit proteins traverse the membranes through the core channel of the growing flagellum and are added at the growing tip (15). The increase in molecular diameter of FlaB due to the attachment of glycan moieties, however, may preclude transport through the narrow flagellar core channel. Glycosylation of FlaBs may thus occur in the periplasmic space on the polymerized flagellar core, possibly with the aid of the endoflagellar sheath protein.
Glycosylation of endoflagellar proteins is not essential for the motility of spirochetes in general: FlaA glycosylation has only been detected in S. hyodysenteriae (11), and FlaB glycosylation has only been detected in treponemes (this report) and possibly S. aurantia (3). No glycosylated flagellar components were detected in Borrelia burgdorferi, Brachyspira aalborgii, Serpulina pilosicoli, and one canine oral treponeme with six endoflagella per pole (data not shown). The reduced motility of the mutant OMZ 305A cannot be directly attributed to loss of glycosylation, since this mutant also lacks FlaA. Therefore, the importance of FlaB glycosylation remains unclear but may include roles in the assembly of FlaA on the core, in the reduction of rotation-inhibiting interactions with the periplasmic gel, and in the transport and stability of flagellar components.
Previous analyses done in this laboratory by whole-cell immunofluorescence analysis and Western blotting of a wide range of cultured spirochetes probed with MAb H9-2 demonstrated a reactive antigen of ca. 38 kDa exclusively in some T. vincentii-related oral spirochetes (6). These findings were confirmed and extended in the present investigation. In T. pallidum, the H9-2-reactive epitope is borne by the 37-kDa FlaA protein (12). The purified endoflagella from T. vincentii OMZ 800 in Fig. 2B, lane 2, show that the PROS-defining ca. 38-kDa component copurifies with endoflagella and is likely the FlaA protein of these oral treponemes. The comparative search for glycosylated and/or H9-2-reactive components included T. pallidum subsp. pallidum and representatives of all seven published species of cultivable oral spirochetes (T. amylovorum, T. denticola, T. maltophilum, T. medium, T. pectinovorum, T. socranskii (three subspecies), and T. vincentii), as well as numerous other oral treponemes (20a). An H9-2 MAb-reactive band with a relative molecular mass of ca. 38 kDa (i.e., larger than the 37-kDa band of T. pallidum) was detected in T. medium and in all T. vincentii-related strains (Fig. 2B, lanes 1 to 7 versus lane 9). The only other reaction with MAb H9-2 was observed in some canine treponeme isolates, where a ca. 80-kDa band was labeled (data not shown).
Endoflagella lie within the periplasmic space and are exposed only if the outer membrane barrier, and thus cellular vitality, has already been impaired (6, 7). Therefore, antibody responses to endoflagellar epitopes are likely to be more useful for diagnostic purposes than for host defense, although a protective role of antibodies with respect to a cloned endoflagellar protein of S. hyodysenteriae has been reported (2). The functional roles of the endoflagellar sheath in toto and of specific regions of the FlaA protein are largely unknown. However, the basic function of endoflagella in the motility of all treponemes is unlikely to be influenced by variations in those regions of the FlaA protein responsible for differences in reactivity to specific antibodies between different Treponema species (19). That not only T. vincentii-related treponemes but also the saccharolytic species T. medium carries an H9-2-reactive epitope (Fig. 2B) supports an earlier suggestion that PROS is a heterogeneous group (6). The special relationship between PROS and T. pallidum suggested on the basis of the single H9-2-reactive epitope phenotype is not apparent from the phylogenetic distances between different treponemes (21). To permit more direct conclusions regarding the potential role of PROS in the etiology of periodontal diseases, extensive epidemiological studies using DNA probes are under way.
ACKNOWLEDGMENTS
I thank J. K. Howell, S. A. Lukehart, S. J. Norris, and T. Umemoto for their generous gifts of cell extracts and antibodies, C. Weiss and V. Zängerle for expert technical assistance, B. Guggenheim and S. Shapiro for critical review of the manuscript, and B. Guggenheim for long-term support.
REFERENCES
- 1.Bickar D, Reid P D. A high-affinity protein stain for Western blots, tissue prints, and electrophoretic gels. Anal Biochem. 1992;203:109–115. doi: 10.1016/0003-2697(92)90049-d. [DOI] [PubMed] [Google Scholar]
- 2.Boyden D A, Albert F G, Robinson C S. Cloning and characterization of Treponema hyodysenteriae antigens and protection in a CF-1 mouse model by immunization with a cloned endoflagellar antigen. Infect Immun. 1989;57:3808–3815. doi: 10.1128/iai.57.12.3808-3815.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahamsha B, Greenberg E P. A biochemical and cytological analysis of the complex periplasmic flagella from Spirochaeta aurantia. J Bacteriol. 1988;170:4023–4032. doi: 10.1128/jb.170.9.4023-4032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charon N W, Greenberg E P, Koopman M B H, Limberger R J. Spirochete chemotaxis, motility, and the structure of the spirochetal periplasmic flagella. Res Microbiol. 1992;143:597–603. doi: 10.1016/0923-2508(92)90117-7. [DOI] [PubMed] [Google Scholar]
- 5.Choi B K, Paster B J, Dewhirst F E, Göbel U B. Diversity of cultivable and uncultivable oral spirochetes from a patient with severe destructive periodontitis. Infect Immun. 1994;62:1889–1895. doi: 10.1128/iai.62.5.1889-1895.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi B K, Wyss C, Göbel U B. Phylogenetic analysis of pathogen-related oral spirochetes. J Clin Microbiol. 1996;34:1922–1925. doi: 10.1128/jcm.34.8.1922-1925.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox D L, Akins D R, Porcella S F, Norgard M V, Radolf J D. Treponema pallidum in gel microdroplets: a novel strategy for investigation of treponemal molecular structure. Mol Microbiol. 1995;15:1151–1164. doi: 10.1111/j.1365-2958.1995.tb02288.x. [DOI] [PubMed] [Google Scholar]
- 8.Hanff P A, Norris S J, Lovett M A, Miller J N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984;11:275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Haselbeck A, Hösel W. Immunological detection of glycoproteins on blots based on labeling with digoxigenin. In: Hounsell E F, editor. Glycoprotein analysis in biomedicine. Totowa, N.J: Humana Press Inc.; 1993. pp. 161–173. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Ruby J, Charon N, Kuramitsu H. Gene inactivation in the oral spirochete Treponema denticola: construction of an flgE mutant. J Bacteriol. 1996;178:3664–3667. doi: 10.1128/jb.178.12.3664-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Dumas F, Dubreuil D, Jaques M. A species-specific periplasmic flagellar protein of Serpulina (Treponema) hyodysenteriae. J Bacteriol. 1993;175:8000–8007. doi: 10.1128/jb.175.24.8000-8007.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukehart S A, Tam M R, Hom J, Baker-Zander S A, Holmes K K, Nowinski R C. Characterization of monoclonal antibodies to Treponema pallidum. J Immunol. 1985;134:585–592. [PubMed] [Google Scholar]
- 13.Messner P. Bacterial glycoproteins. Glycoconjugate J. 1997;14:3–11. doi: 10.1023/a:1018551228663. [DOI] [PubMed] [Google Scholar]
- 14.Moskophidis M, Müller F. Identification of glycosylated protein antigens of Treponema pallidum and Treponema phagedenis. Zentralbl Bakteriol Hyg A. 1985;259:468–476. doi: 10.1016/s0176-6724(85)80078-x. [DOI] [PubMed] [Google Scholar]
- 15.Namba K, Yamashita I, Vonderviszt F. Structure of the core and central channel of bacterial flagella. Nature. 1989;342:648–654. doi: 10.1038/342648a0. [DOI] [PubMed] [Google Scholar]
- 16.Norris S J the Treponema pallidum Polypeptide Research Group. Polypeptides of Treponema pallidum: progress toward understanding their structural, functional, and immunological roles. Microbiol Rev. 1993;57:750–779. doi: 10.1128/mr.57.3.750-779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riviere G R, Wagoner M A, Baker-Zander S A, Weisz K S, Adams D F, Simonson L, Lukehart S A. Identification of spirochetes related to Treponema pallidum in necrotizing ulcerative gingivitis and chronic periodontitis. N Engl J Med. 1991;325:539–543. doi: 10.1056/NEJM199108223250803. [DOI] [PubMed] [Google Scholar]
- 17a.Schüpbach, P. and C. Wyss. Unpublished data.
- 18.Umemoto T, Nakazawa F, Hoshino E, Okada K, Fukunaga M, Namikawa I. Treponema medium sp. nov., isolated from human subgingival dental plaque. Int J Syst Bacteriol. 1997;47:67–72. doi: 10.1099/00207713-47-1-67. [DOI] [PubMed] [Google Scholar]
- 19.Umemoto T, Wadood A, Nakamura Y, Nakatani Y, Namikawa I. Antigenic behaviors of two axial flagellar proteins detected in Treponema denticola. Microbiol Immunol. 1993;37:159–163. doi: 10.1111/j.1348-0421.1993.tb03194.x. [DOI] [PubMed] [Google Scholar]
- 20.Wyss C. Selected low-cohesion variants of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus lack distinct antigens recognized by human antibodies. Arch Microbiol. 1989;151:133–136. doi: 10.1007/BF00414427. [DOI] [PubMed] [Google Scholar]
- 20a.Wyss, C. Unpublished data.
- 21.Wyss C, Choi B K, Schüpbach P, Guggenheim B, Göbel U B. Treponema amylovorum sp. nov., a saccharolytic spirochete of medium size isolated from an advanced human periodontal lesion. Int J Syst Bacteriol. 1997;47:842–845. doi: 10.1099/00207713-47-3-842. [DOI] [PubMed] [Google Scholar]
- 22.Wyss C, Choi B K, Schüpbach P, Guggenheim B, Göbel U B. Treponema maltophilum sp. nov., a small oral spirochete isolated from human periodontal lesions. Int J Syst Bacteriol. 1996;46:745–752. doi: 10.1099/00207713-46-3-745. [DOI] [PubMed] [Google Scholar]