Abstract
Background
COVID-19 vaccines with alternative strain compositions are needed to provide broad protection against COVID-19 disease due to newly emergent SARS-CoV-2 variants of concern. The aim of the study was to describe the clinical efficacy and safety of the bivalent variant vaccine as a primary series during a period of Omicron circulation.
Methods
We conducted an international Phase 3, multi-stage, modified double-blind (those preparing the study interventions were not blinded), multi-armed efficacy study among adults aged ≥18 years at 54 centers in eight countries (Colombia, Mexico, Ghana, Kenya, Uganda, Ukraine, Nepal, and India). Participants were recruited from the community and randomized 1:1 using an interactive response technology system to receive two intramuscular injections 21 days apart of a bivalent SARS-CoV-2 recombinant protein vaccine (5 μg of ancestral (D614) and 5 μg of B.1.351 [beta] variant spike protein) with AS03-adjuvant or placebo. All participants, outcome assessors and laboratory staff performing assays were masked to group assignments. Participants were stratified by age (18–59 years/≥60 years) and baseline SARS-CoV-2 rapid serodiagnostic test positivity. Symptomatic COVID-19 was defined as laboratory-confirmed (nucleic acid amplification test or polymerase chain reaction [PCR] test) COVID-19 with COVID-19-like illness (CLI) symptoms. The primary efficacy endpoint was the prevention of symptomatic COVID-19 ≥14 days after the second injection (post dose 2 [PD2]). This trial is registered with ClinicalTrials.gov, NCT04904549, and is closed to recruitment.
Findings
13,002 participants were randomized to receive the first dose of the study vaccine (n=6512) or placebo (n=6490) between 19 Oct 2021 and 15 February 2022. Seventy-eight participants did not receive any injection. 12,924 participants (vaccine, n=6472; placebo, n=6,450 [two participants received an injection at V1 but it was not recorded whether they received the vaccine or the placebo]) received ≥1 study injection, of whom 7,542 (58·4%) were male and 9691 (75·0%) were SARS-CoV-2 non-naïve. Of these 12,924 participants, 11,543 (89·3%) received both study injections. The efficacy-evaluable population comprised 11,416 participants (vaccine, n=5736; placebo, n=5680). The median duration of follow-up was 85 days (Q1 50; Q3 95) PD1 and 58 days (Q1 29; Q3 70) PD2. Up to 15 March 2022, 121 symptomatic COVID-19 cases were reported (32 in the vaccine group and 89 in the placebo group) ≥14 days PD2 with a vaccine efficacy (VE) of 64·7 % (95% confidence interval [CI] 46·6; 77·2%). VE was 75·1% (95% CI 56·3; 86·6%) in non-naïve and 30·9% (95% CI −39·3; 66·7%) in naïve participants. Viral genome sequencing identified the infecting strain in 68 cases (Omicron [BA.1 and BA.2 subvariants]: 63; Delta: 4; Omicron and Delta: 1). Immediate unsolicited AEs were reported for <0.1% of the vaccine group and 0.1% for the placebo group. Immediate adverse reactions (ARs) ≤30 minutes after any injection were reported by <0.1% of participants for both groups. In the reactogenicity subset with available data, solicited reactions (SISRs and SSRs) ≤7 days after any injection occurred in 1,398 (57·8%) vaccine recipients and 983 (40.9%) placebo recipients. Grade 3 solicited reactions were reported by 196 (8·1%, 95% CI 7.0; 9.3) of vaccine recipients and 118 (4·9%, 95% CI 4.1; 5.9) of the placebo recipients within 7 days after any injection, with comparable frequency PD1 and PD2 in the vaccine group. The proportion of SAEs were 0.5% in the vaccine group and 0.4% in the placebo group. The proportion of AESIs and deaths were <0.1% in both study arms. No AESI, SAE or death was deemed to be treatment related. There were no reported cases of thrombosis with thrombocytopenia syndrome, myocarditis, pericarditis, Bell’s Palsy, or Guillain–Barré syndrome or other immune mediated diseases.
Interpretation
A bivalent vaccine conferred heterologous protection against symptomatic infection with contemporary Omicron (BA.1 and BA.2) in non-naïve adults 18–59 years of age.
Introduction
COVID-19 vaccines were originally developed using the Spike (S) sequence from the SARS-CoV-2 ancestral Wuhan-Hu-1 (D614) strain.1 However, currently available vaccines are less effective against COVID-19 disease due to new emergent SARS-CoV-2 variants of concern (VOCs; including Omicron [BA.1, BA.2, BA.4, and BA.5] variants and subvariants [BQ.1.1 and XBB]).2-8 Therefore, vaccines with variant strains have been subsequently developed to provide cross-protection against emerging variants. One strategy for variant vaccine composition is inclusion of the prevalent circulating strain, with mRNA Omicron-containing bivalent vaccines authorized as boosters based on demonstrated induction of antibodies to circulating Omicron variants.9,10 However, there are no data on whether an alternative non-Omicron variant vaccine provides cross-protective efficacy against Omicron variants.
Sanofi and GSK have developed a bivalent vaccine containing stabilized SARS-CoV-2 pre-fusion S proteins from both the ancestral D614 and the Beta (B.1.351) variant, with the GSK AS03 adjuvant system (CoV2 preS dTM-AS03 [D614 + B.1.351]). This bivalent vaccine is being evaluated as a two-injection primary series in previously unvaccinated individuals and as a booster vaccine in individuals with prior natural infection based on preclinical studies showing induction of cross-neutralizing antibody responses against a broad panel of VOCs.11 The first in human results supported the selection of the AS03 adjuvant system and a two-injection schedule.12 The Phase 2 results of two doses of the bivalent vaccine showed acceptable safety and reactogenicity, and robust immunogenicity in SARS-CoV2 naïve and non-naïve adults and supported progression to Phase 3 evaluation of the 10 μg antigen dose for primary vaccination and a 5 μg antigen dose for booster vaccination.13 Here, we describe the clinical efficacy and safety of the bivalent variant vaccine as a primary series during a period of Omicron circulation.
Methods
Study Design
This Phase 3, parallel, international, randomized, modified double-blind (those preparing the study interventions were not blinded), placebo-controlled study was designed as a multi-stage platform trial with two stages (NCT04904549). Stage 1 evaluated the efficacy and safety of the prototype vaccine, containing the ancestral D614 recombinant S protein (CoV2 preS dTM-AS03 [D614]) (manuscript in preparation). Stage 2, reported here, evaluated the efficacy and safety of a primary series of two injections of the bivalent vaccine, administered 21 days apart. Stage 2 was conducted in 54 clinical research centers across eight countries: Colombia, Ghana, India, Kenya, Mexico, Nepal, Uganda, and Ukraine (Supplementary Appendix Section 1.1). Participant enrollment started on 19 October 2021 and finished on 15 February 2022.
The study was conducted in compliance with the International Conference on Harmonization (ICH) guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. The protocol and amendments were approved by applicable Independent Ethics Committees/Institutional Review Boards and per local regulations (Supplementary Appendix 1.2). All participants provided written informed consent that was not subject to any conditions.
Participants
Adults aged ≥18 years who had not received a prior COVID-19 vaccine were eligible for inclusion; full details of the inclusion and exclusion criteria are reported in the Supplementary Appendix Section 1.3. Since approved/authorized COVID-19 vaccines were already available in some countries and regions where the study was conducted, investigators discussed their availability with participants, encouraged them to obtain the approved/authorized vaccine as applicable, and proceeded with enrollment only if, despite encouragement, the participant expressed no interest in seeking approved/authorized COVID-19 vaccines. Furthermore, participants were counselled at each opportunity about the availability and benefits of approved vaccines. Participants were allowed to receive an authorized vaccine outside the study protocol and were offered the option to continue in the study for safety and immunogenicity follow-ups. Participants potentially at high risk for severe COVID-19 (Supplementary Appendix Section 1.4) and other subpopulations at risk of COVID-19 infection, including ethnic and racial minorities, were included. At the time of enrollment, self-reported medical history, sex, ethnicity, and race were collected.
Randomization and masking
Eligible participants were randomized 1:1 to receive either the bivalent vaccine or placebo (saline). Randomization was conducted using an interactive response technology system (IRT). Stratified permuted sub-block randomization with a block size of eight (four vaccine and four placebo) were applied for study group randomization, where strata are age group (18–59 years and ≥ 60 years), baseline SARS-CoV-2 rapid serodiagnostic test positivity (positive/negative), and study site. Site staff entered identification and security information and confirmed a minimal amount of data in response to IRT prompts. The IRT then provided a group assignment and assigned a 12-digit participant number. All participants, outcome assessors, and laboratory staff performing assays were blinded to group assignments; only site staff involved in the preparation and administration of the vaccines were unblinded, but they were not involved in study outcome assessments.
Procedures
The recombinant protein antigen CoV2 preS dTM, stabilized in its prefusion form and produced using the baculovirus expression system technology, and the AS03 Adjuvant System (GSK Vaccines, Rixensart, Belgium) have been described previously.12-14 Each 0·5 mL injection of the bivalent vaccine formulation contained 5 μg of the ancestral D614 and 5 μg of the B.1.351 variant Spike protein antigen. The CoV2 preS dTM antigen and AS03 adjuvant were presented in two separate vials: a multi-dose vial containing AS03 (sufficient for ten injections) and a multidose vial containing the Spike protein antigen (ten doses of 5 μg of D614 + 5 μg B.1.351). An equal volume of the adjuvant emulsion was added to the vial containing the antigen and mixed prior to injection. At each vaccination participants in the vaccine group received one 0·5 mL injection containing the bivalent vaccine and participants in the placebo group received one 0·5 mL injection of 0·9% normal saline. Vaccinations were administered on study days 1 and 22 by intramuscular injection into the deltoid region by qualified and trained personnel.
Blood samples and nasopharyngeal swabs were collected before each vaccination to establish whether participants had previous or ongoing SARS-CoV-2 infection (naïve or non-naïve).
Surveillance for COVID-19-like illness (CLI) was both active and passive: participants were contacted once a week to determine whether they had any symptoms of a CLI (Supplementary Appendix Section 1.5) or if they had a positive COVID-19 test from another source at any time during the study. Participants were also instructed to contact the site if they experience symptoms of a COVID-19-like illness or if they have a positive COVID-19 test from any other source at any time during the study. In the event of CLI symptoms, nasopharyngeal and anterior nasal swabs were collected at the participant’s first visit after symptom onset and 2–4 days later for virological confirmation using NAAT. Further anterior nasal swabs were collected 7–9 days and 12–14 days after the first illness visit. If any specimen was found to be positive for SARS-CoV-2, the participant was asked to continue recording their daily COVID-19 symptoms until the end of their illness or for up to 30 days from symptom onset. If symptoms persisted for over 30 days, participants were asked to record the date the symptoms resolved. An independent adjudication committee reviewed potential cases to determine whether the case definitions for symptomatic and/or severe COVID-19 were met. Viral genomic sequencing was performed on respiratory samples from confirmed cases to identify the SARS-CoV-2 variant, as previously described.15
Outcomes
Participants were classified as naïve or non-naive at Day 1 and Day 22 or Day 1 or Day 22 by assessment of blood samples using Elecsys electrochemiluminescence immunoassays for detection of anti-S antibodies (Elecsys Anti-SARS-CoV-2 S assay; Roche, Indianapolis, IN, USA) on study Day 1 and for detection of anti-nucleocapsid antibodies (Elecsys Anti- SARS-CoV-2 N; Roche) on study Days 1 and 22; and detection of SARS-CoV-2 nucleic acids in nasopharyngeal swabs using nucleic-acid amplification tests (NAAT; Abbott RealTime SARS-CoV-2 assay; Abbott Molecular, Des Plaines, IL, USA) on study Days 1 and 22. Testing procedures and criteria for determination of prior SARS-CoV-2 infection are described in the (Supplementary Appendix Section 1.6).
The primary efficacy objective was to assess in all participants, regardless of prior infection, the clinical efficacy of the bivalent vaccine for prevention of symptomatic COVID-19 ≥14 days after the second injection (post dose 2 [PD2]). Secondary efficacy endpoints included the occurrence of symptomatic disease in naïve and non-naïve individuals; severe, moderate, or worse disease; or hospitalized COVID-19 ≥14 days PD2 in all participants and according to prior infection status. The impact of sex, age (18–59 years and ≥60 years), and high-risk medical conditions on the above outcomes was evaluated as pre-defined exploratory objectives. Other analyses included the occurrence of symptomatic or severe COVID-19 ≥14 days after the first injection. The occurrence of asymptomatic infection in SARS-CoV2 naïve participants was also reported. Definitions of COVID-19 efficacy outcomes are reported in Supplementary Appendix Section 1.7.
Participants were directed to report any adverse events (AEs) during their study visits or during any follow-up contact with the investigators. Safety data were collected from all participants receiving at least one injection of the study vaccine or placebo (Supplementary Appendix Section 1.8) throughout the duration of the study. Solicited injection site reactions (SISRs) and solicited systemic reactions (SSRs) occurring within 7 days after each vaccination and non-serious unsolicited AEs occurring within 21 days after each vaccination were collected in a subset of approximately 4,000 participants (the first 4,000 participants recruited [2,000 in each arm], as well as all participants ≥60 years of age).
Statistical Analyses
The data cut-off date for the analyses reported here was 15 March 2022, when the number of cases for the prespecified event-driven analysis was reached. Calculations for determining this sample size are reported in Supplementary Appendix Section 1.9. It was estimated that a sample of 10,886 participants would be required, with approximately 125 symptomatic COVID-19 cases needed to achieve 80% statistical power. The planned target for SARS-CoV-2 non-naïve participants was approximately 3,266 participants (1,633 per arm). The sample size of 7,620 SARS-CoV-2 naïve participants was powered independently to demonstrate the primary objective of vaccine efficacy (VE) against symptomatic COVID-19 in SARS-CoV-2 naïve adults. Given the global epidemiological situation where most of the population has already been infected, the primary population for the assessment of vaccine efficacy was changed from naïve participants to all participants who meet per-protocol defined criteria. The protocol was amended before the analysis was performed. The updated protocol includes both naïve and non-naïve individuals in the primary objective.
Therefore, sample size calculations were based on a primary endpoint that considered only naïve participants. The power of primary efficacy analysis was driven by the total number of symptomatic COVID-19 events. The incidence rate of symptomatic COVID-19 in Placebo was assumed as 2·25% illness rate per 2-months follow-up period. An attrition rate of 30% was expected, as, during the conduct of the study, a greater proportion of the cohort became eligible to receive locally available authorized COVID-19 vaccines. Because Omicron was the prevalent variant during case accrual and the expected VE against Omicron was expected to be lower than the original assumption of 70%, the expected true VE for symptomatic COVID-19 for was estimated at 60%. Therefore, a total of approximately 125 symptomatic COVID-19 events was required to achieve 80% power with one-sided type I error rate of 0·025, assuming no interim analysis. For interim analyses, the type I error rate was adjusted appropriately. If the planned interim analysis was skipped, or if the information fraction was different than planned (not within the range of 50% to 70% data), alpha splitting was adjusted based on the Lan-DeMets O’Brien-Fleming approximation spending function.
Descriptions of the analysis sets are reported in Supplementary Section 1.10. An independent data and safety monitoring board16 provided study oversight and reviewed unblinded data. Censoring occurred at random. Patients were censored if they had an early termination during the analysis period (the termination date was the censoring date); received another SARS-CoV-2 vaccine outside the protocol (the date of vaccination was the censoring date); had an event (either CDC-defined event or symptomatic COVID-19 event for CDC-defined endpoint; or symptomatic COVID-19 event for other endpoints (the start date of the event was the censoring date); or, if the participants does not meet any of the above, the cut-off date of the planned analysis was the censoring date.
The randomized group included all participants who were allocated to a treatment group, of whom those who received at least one study injection were included in the full analysis set (FAS). The primary efficacy analyses were conducted, as prespecified, on the modified full analysis set PD2 (mFAS-PD2), comprising participants who received both injections (excluding participants with onset of symptomatic COVID-19 between the first injection [post dose 1 (PD1)] and 14 days PD2) who did not meet any vaccine contraindications and did not discontinue the study within 14 days PD2. The modified full analysis set PD1 excluded participants in the FAS with the onset of symptomatic COVID-19 between the date of the first injection and 14 days after the first injection or those who discontinued from the study before 14 days after the first injection. Secondary efficacy analyses were conducted in subgroups further divided based on prior infection status PD1 and PD2. Results for participants in the mFAS-PD1 population are also included for comparison, as previous reports with COVID-19 vaccines have shown protection after a single injection.
For the primary endpoint, the point estimate of vaccine efficacy (VE) was calculated based on the incidence rate per 1000 person-years per group in the mFAS-PD2 population, regardless of prior infection status. The primary objective was met if the VE point estimate was >50% and the lower bound of the confidence interval (95% CI) was >30%.17 Survival analyses were also performed using Kaplan-Meier curves with 95% CI. As supportive analyses, survival analyses were also performed based on a Stratified Cox proportional hazards model (based on baseline strata age group, sex, high-risk medical condition, and prior SARS-CoV-2 infection status at the second injection for symptomatic COVID-19 after the second injection) to estimate the VE by one minus the hazard ratio with score based CI) were also performed. Sensitivity analyses against symptomatic COVID-19 was prespecified, with VE calculated by relative risk in the mFAS-PD2 and by the IRR of COVID-19 case occurrence in the per-protocol analysis set. Sensitivity analyses were also conducted assuming that unsequenced cases were due to the Omicron variant, which was the prevalent variant circulating at the time of the study. Safety outcomes were assessed in the safety analysis set (SafAS), comprising all randomized participants who received ≥1 injection of study vaccine or placebo. The reactogenicity safety analysis subset (RsafAS) comprised participants in the SafAS who received at least one study injection, were randomized into the reactogenicity subset and who reported reactogenicity data. The CI for the single proportions will be calculated using the exact binomial method (Clopper-Pearson method). Statistical analyses were performed using SAS® Version 9.4 or later. This study is registered with ClinicalTrials.gov, NCT04904549. Trial recruitment has now been completed.
Role of the funding source
The funders were involved in the study design, data analysis, data interpretation, writing of the report, and the decision to submit the paper for publication. GSK provided access to, and use of, the AS03 Adjuvant System.
Results
Between 19 October 2021 and 15 February 2022, 13,506 participants were randomized. Owing to the ongoing war in Ukraine, data completeness could not be confirmed for the four Ukrainian sites; therefore, none of the 504 participants from these sites were included in the main analyses, although sensitivity analyses including these data were performed.
In the current analysis, 13,002 participants were randomized to receive the study vaccine (n=6,512) or placebo (n=6,490) up to the cut-off date of 15 March 2022 (Supplementary Appendix Section 2.1). Of those, 414 participants (3·2%) discontinued the study, 89 of whom discontinued PD2 (Supplementary Appendix Section 2.2). 12,924 participants (6,472 in the vaccine group and 6,450 in the placebo group [two participants received an injection at V1 but it was not recorded whether they received the vaccine or the placebo]) were included in the FAS, of whom 12,809 (6,418 in the vaccine group and 6,391 in the placebo group) were included in the mFAS-PD1 and 11,416 were included in the mFS-PD2 (5,736 in the vaccine group and 5,680 in the placebo group). The participants in the main analysis sets are presented in Supplementary Appendix 2.3.
A total of 12,924 participants received ≥1 study injection (SafAS), for whom demographic characteristics are reported based on first visit samples (Table 1). 4,851 participants were included in the RsafAS (2,433 in the vaccine group and 2,418 in the placebo group). Participant demographics were comparable across treatment groups. The mean (SD) age was 36·1 (12·9) years and 58·4% were male (Table 1). High-risk medical conditions were present in 4,165 (32·2%) of participants (Table 1 and Supplementary Appendix Section 2.4). 9,691 (75·0%) of participants had evidence of prior infection (non-naïve) at enrollment (vaccine, n=4,860; placebo, n=4,831).
Table 1: Demographics and clinical characteristics at baseline in the participants who received at least one injection (SafAS).
*Two participants received a vaccine at V1 but whether they received the vaccine or the placebo is unknown. Therefore, there is a difference of 2 participants in the total number of participants of the SafAS.
†One of the 2 participants who had missing information about the vaccine/placebo was American Indian or Alaska Native.
| Vaccine group (N=6472) |
Placebo group (N=6450) |
Total (N=12,924*) |
|
|---|---|---|---|
| Sex, n (%) | |||
| Male | 3789 (58·5) | 3751 (58·2) | 7542 (58·4) |
| Female | 2683 (41·5) | 2699 (41·8) | 5382 (41·6) |
| Age, years | |||
| Mean (SD) | 36·1 (13·0) | 36·0 (12·9) | 36·1 (12·9) |
| Age categories, n (%) | |||
| 18-59 years | 6078 (93·9) | 6067 (94·1) | 12,147 (94·0) |
| ≥60 years | 394 (6·1) | 383 (5·9) | 777 (6·0) |
| BMI (kg/m2), mean (SD) | 23·8 (4·61) | 23·8 (4·41) | 23·8 (4·51) |
| Race, n (%) | |||
| American Indian or Alaskan native | 408 (6·3) | 402 (6·2) | 811† (6·3) |
| Asian | 2562 (39·6) | 2567 (39·8) | 5129 (39·7) |
| Black or African American | 2873 (44·4) | 2854 (44·2) | 5727 (44·3) |
| White | 36 (0·6) | 38 (0·6) | 74 (0·6) |
| Multiracial | 5 (<0·1) | 6 (<0·1) | 11 (<0·1) |
| Not reported | 95 (1·5) | 82 (1·3) | 177 (1·4) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 1056 (16·3) | 1051 (16·3) | 2109† (16·3) |
| Not Hispanic or Latino | 5381 (83·1) | 5372 (83·3) | 10,753 (83·2) |
| Not reported | 15 (0·2) | 13 (0·2) | 28 (0·2) |
| Country, n (%) | |||
| Mexico | 495 (7·6) | 493 (7·6) | 989 (7·7) |
| Colombia | 537 (8·3) | 532 (8·2) | 1070 (8·3) |
| India | 1661 (25·7) | 1672 (25·9) | 3333 (25·8) |
| Uganda | 212 (3·3) | 206 (3·2) | 418 (3·2) |
| Ghana | 597 (9·2) | 598 (9·3) | 1195 (9·2) |
| Kenya | 2066 (31·9) | 2052 (31·8) | 4118 (31·9) |
| Nepal | 904 (14·0) | 897 (13·9) | 1801 (13·9) |
| Prior SARS-CoV-2 infection, n (%) | |||
| Naïve at Day 1 | 588 (9·1) | 588 (9·1) | 1176 (9·1) |
| Non-naïve at Day 1 | 4860 (75·1) | 4831 (74·9) | 9693 (75·0) |
| Undetermined at Day 1 | 1024 (15·8) | 1031 (16·0) | 2055 (15·9) |
| Naïve at Day 22 | 333 (5·1) | 350 (5·4) | 683 (4·3) |
| Non-naïve at Day 22 | 5478 (84·6) | 5486 (85·1) | 10,966 (84·8) |
| Undetermined at Day 22 | 661 (10·2) | 614 (9·5) | 1275 (9·9) |
| High-risk medical condition | |||
| Yes | 2095 (32·4) | 2070 (32·1) | 4165 (32·2) |
| No | 4377 (67·6) | 4380 (67·9) | 8759 (67·8) |
For the other participant, the race was unknown although the ethnicity was Hispanic or Latino.
BMI, body mass index; Q1, quartile 1; Q3, quartile 3; SD, standard deviation.
In both treatment groups, the longest duration of follow-up was 148 days (median 85 days [Q1 50; Q3 95]) PD1 and 118 days (median 58 days [Q1 29; Q3 70]) PD2 (Supplementary Appendix Sections 2.5 and 2.6). The proportion of participants with ≥2 months’ follow-up at the data cut-off date was 67·4% (8,706/12,924) PD1 and 47·2% (5,453/11,543) PD2. The vast majority of the cases in all countries were due to Omicron BA.1 and BA.2 (Supplementary Appendix Section 2.7).
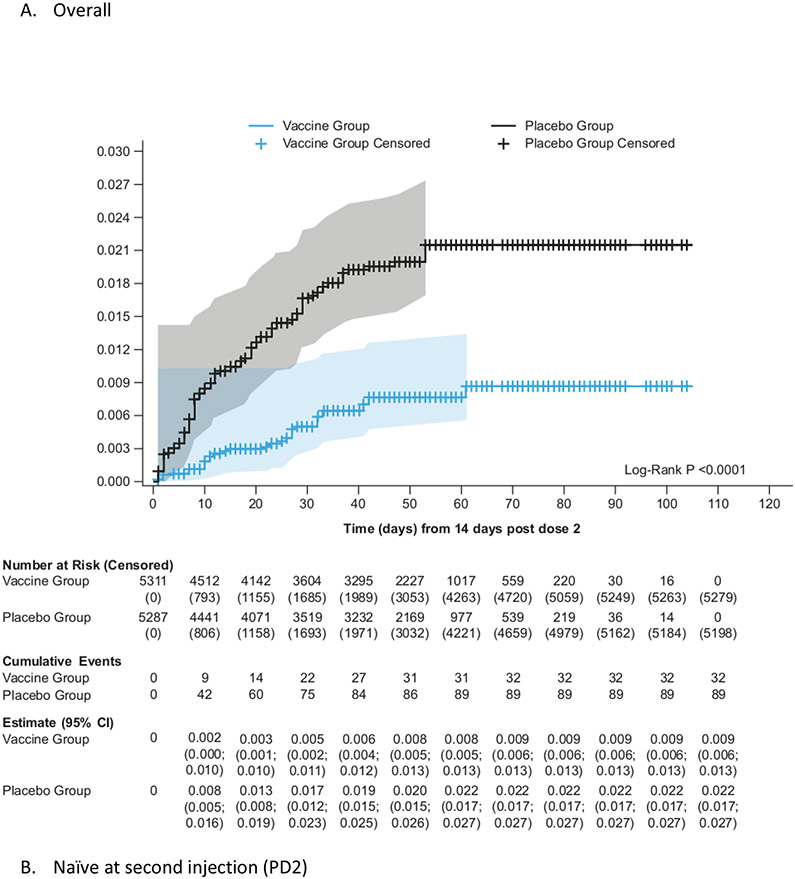
The mFAS-PD2 set comprised 11,416 participants (5,736 [50·2%] in the vaccine group; 5,680 [49·8%] in the placebo group). 121 symptomatic COVID-19 episodes were reported ≥14 days PD2 (32 in the vaccine vs 89 in the placebo group), with an overall VE of 64·7% (95% CI 46·6; 77·2%), which met the primary efficacy endpoint (Figure 1). In the sensitivity analysis, the VE based on the RR of symptomatic COVID-19 case occurrence in the mFAS-PD2 was 64.2% (95% 45.8; 76.9 [32 cases in the vaccine group; 89 cases in the placebo group). In the per-protocol analysis set, VE calculated by the IRR of symptomatic COVID-19 case occurrence was 63.4% (95% CI 44.5; 76.4 [32 cases in the vaccine group; 86 cases in the placebo group]). Similar results for the primary endpoint were reported in a sensitivity analysis that included the Ukrainian participants (VE 64·0%, 95% CI 45·9; 76·6 [Supplementary Appendix Section 2.8]). The cumulative incidence rate of symptomatic COVID-19 was higher in the placebo group than in the vaccine group starting from 14 days after the second dose (Figure 2; Supplementary Appendix Section 2.9). The results of the survival analysis based on the Stratified Cox proportional model are reported in Supplementary Appendix Section 2.10.
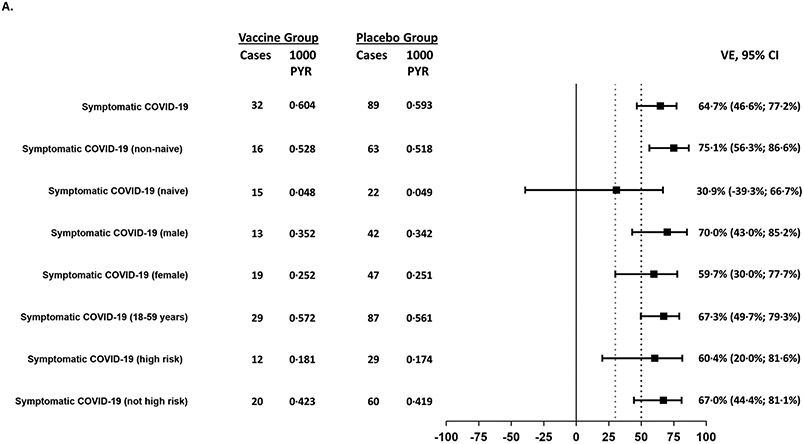
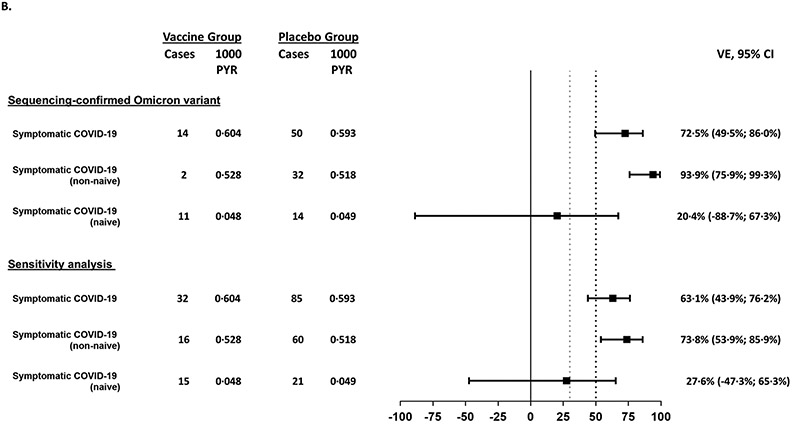
Figure 1:
Forest plots for efficacy outcomes against symptomatic disease in all participants and subgroups caused by (A) all variants and (B) for the Omicron variant
A. Efficacy outcomes overall and by subgroups for the mFAS-PD2 analysis subset. The success criterion for demonstration of efficacy was defined as a point estimate >50% (black dotted line) and a lower bound confidence interval >30% (grey dotted line).
Outcomes with too few cases to reliably calculate vaccine efficacy (severe COVID-19, moderate or worse COVID-19, hospitalization, and symptomatic COVID-19 in participants aged ≥60 years) are not shown.
B. Vaccine efficacy is shown for all sequence-confirmed Omicron cases and for the sensitivity analysis, which included sequence confirmed cases and cases for which there were no sequencing results, assuming that the latter group were caused by the Omicron variant as this was the variant that was responsible for most of the symptomatic COVID-19 cases at the time of the study. The success criterion for demonstration of efficacy was defined as a point estimate >50% (black dotted line) and a lower bound confidence interval >30% (grey dotted line). Owing to the low number of cases due to the Delta variant, these are not shown in the Forest plot.
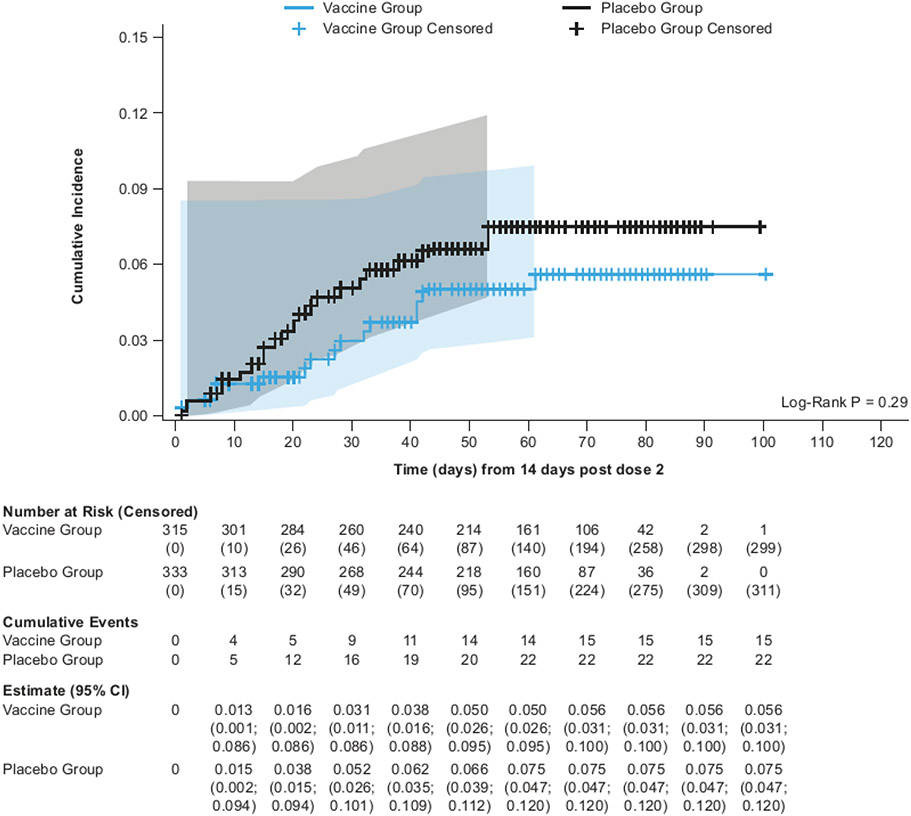
Figure 2:
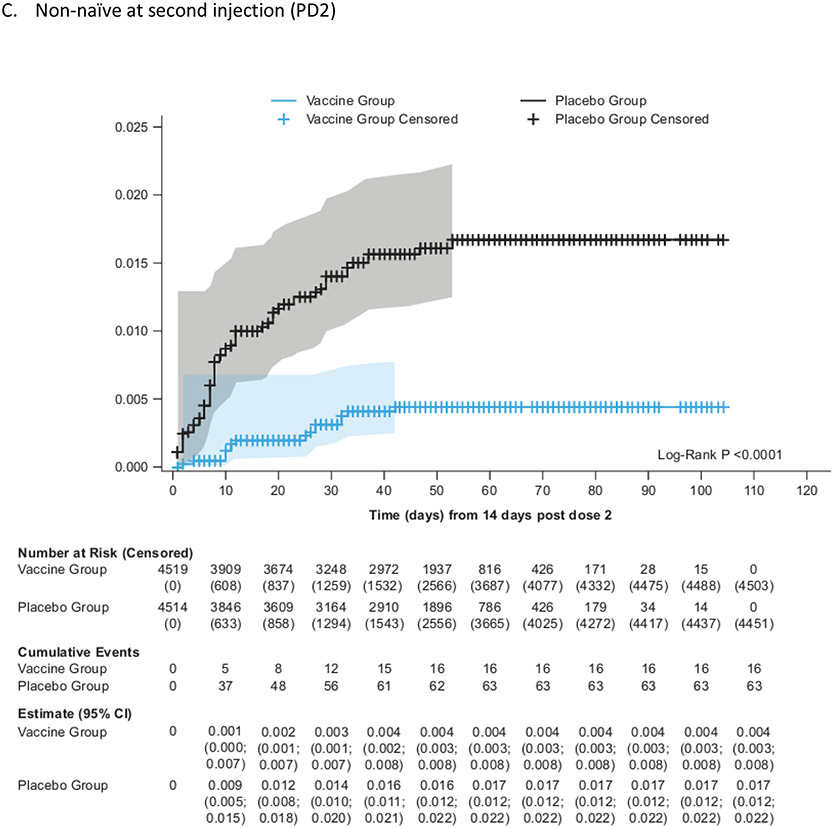
Kaplan-Meier cumulative incidence of symptomatic COVID-19 in the mFAS-PD2 population (overall, naïve and non-naïve populations)
Five participants (three vaccine recipients, two placebo recipients) reported severe COVID-19, and 12 participants reported moderate or worse symptomatic COVID-19 (five vaccine recipients, seven placebo recipients) occurring from 14 days PD2 in mFAS-PD2 participants. Two placebo recipients in the mFAS-PD2 were hospitalized with COVID-19. No deaths associated with COVID-19 were reported.
VE against symptomatic COVID-19 infection in non-naïve participants was 75·1% (95% CI: 56·3%; 86·6%), while in naïve participants the point estimate for VE was 30·9% (95% CI −39·3%; 66·7%) (Figure 1). The cumulative incidence was higher in the placebo group than in the vaccine group starting from 14 days PD2 in non-naïve participants (Figure 2; Supplementary Appendix Section 2.9). The number at risk decreases rapidly after 60 days PD2 + 14 days as the majority of participants had their second vaccination after 1 January 2022, so were censored before this time point. The overall VE against symptomatic COVID-19 was 60·3% (95% CI 47·1%; 70·5%) PD1 (Supplementary Appendix Section 2.11). The higher cumulative incidence in the placebo group started within 14 days PD1 in naïve, non-naïve, and all participants in the mFAS-PD1 population (Supplementary Appendix 2.12).
Efficacy results against symptomatic disease in all participants and subgroups are shown in Figure 1 and Supplementary Appendix Section 2.13. Outcomes with too few cases to reliably calculate vaccine efficacy (severe COVID-19, moderate or worse COVID-19, hospitalization, and symptomatic COVID-19 in participants aged ≥60 years) are not shown. Efficacy against asymptomatic SARS-CoV-2 infection (assessed in naïve participants only) was 1·2% (95% CI −31·0; 25·5) with 100 cases in the vaccine group and 107 cases in the placebo group (Supplementary Appendix Section 2.14). VE was higher for males (70·0%, 95% CI 43S0; 85·2) compared with females (59·7%, 95% CI 30·0; 77·7) (Figure 1).
Of the 121 adjudicated cases, the causative viral strain was sequenced in 68 cases (56·0%), with the majority (63/68) corresponding to the BA.1 and BA.2 subvariants of Omicron and the others corresponding to Delta (4/68). One participant had mixed infection with the Omicron and Delta variants and was included in the analysis for both variants. Results for the other 53 adjudicated cases (approximately 44%) were not available for several reasons: cases being diagnosed using a local test for which no specimen was available (n=8), the viral load threshold was too low for detection (n=12), the laboratory did not produce a valid result (n=32), or the sample was not tested (n=1). Of the 53 cases with undiagnosed variants, 18 out of 32 (56%) were in the vaccine group and 35 out of 89 (39%) were in the placebo group.
Among the 68 sequenced cases, 64 were Omicron (14 in the vaccine recipients and 50 in the placebo recipients), with the Omicron-specific VE estimated as 72·5% (95% CI: 49.5; 86·0) in all participants (Figure 1), 93.9% (95% CI 49.5; 86.0) in non-naïve participants and 20.4% (95% CI −88.7; 67.3) in naïve participants. Kaplan-Meier analyses showed higher cumulative incidence in the placebo group compared with the vaccine group 14 days PD2 (Supplementary Appendix Section 2.15). There were no Delta-related COVID-19 cases in the vaccine group versus five cases in the placebo group.
The VE against symptomatic COVID-19 caused by the Omicron or undefined variants (sensitivity analyses) was 63·1% (95% CI 43·9; 76·2%) in all participants, 73·8% (95% CI 53·9; 85·9) in non-naïve participants and 27.6% (95% CI −47.3; 65·3) in naïve participants (Supplementary Appendix Section 2.16).
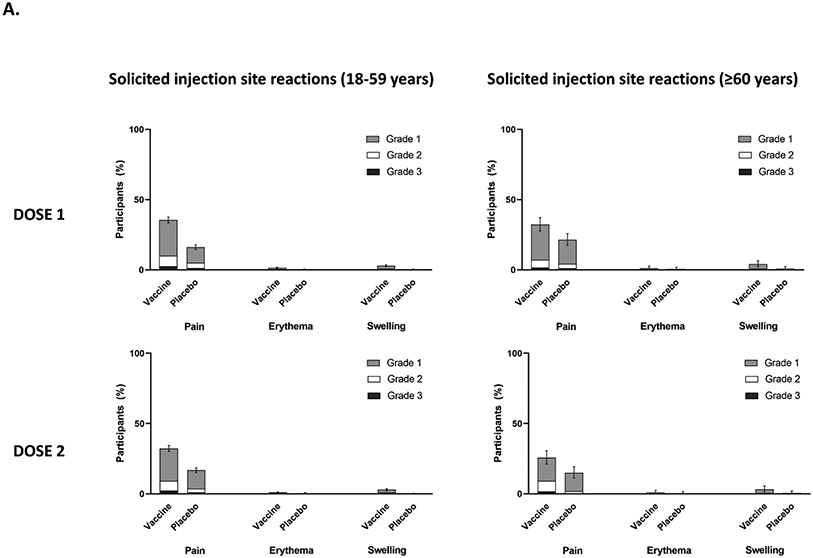
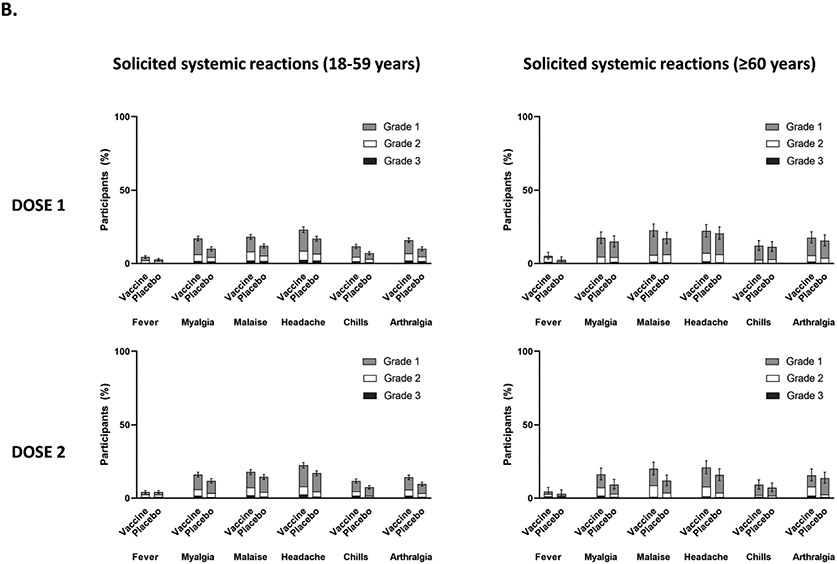
A summary of safety outcomes in participants who received at least one injection of vaccine or placebo (SafAS population) is reported in Table 2 and Supplementary Appendix Sections 2.17 and 2.18. For both the vaccine and placebo groups, immediate unsolicited AEs and adverse reactions (ARs) ≤30 minutes after any injection were reported by 0.1%. Of the 4,823 participants in the reactogenicity subset with available data, solicited reactions (SISRs and SSRs) ≤7 days after any injection occurred in 1,398 (57·8%) vaccine recipients and 983 (40.9%) placebo recipients (Figure 3). Grade 3 solicited reactions were reported by 196 (8·1%, 95% CI 7.0; 9.3) of vaccine recipients and 118 (4·9%, 95% CI 4.1; 5.9) of the placebo recipients within 7 days after any injection, with comparable frequency PD1 and PD2 in the vaccine group (Table 2; Supplementary Appendix Section 2.17). The proportion of MAAEs reported was similar in the vaccine (5·7%, 95% CI 5.1; 6.2) and placebo (6.0%, 95% CI 5.4; 6.6) groups. The proportion of AESIs, SAEs and deaths were <1% in both study arms; no AESI, SAE or death was deemed to be treatment related. There were no reported cases of thrombosis with thrombocytopenia syndrome, myocarditis, pericarditis, Bell’s Palsy, or Guillain–Barré syndrome or other immune mediated diseases.
Table 2: Summary of safety outcomes in participants who received at least one injection (SafAS).
| Population | Vaccine (N=6472) |
Placebo (N=6450) |
||
|---|---|---|---|---|
| n/M | % (95% CI) | n/M | % (95% CI) | |
| Participants experiencing at least one of the following within 30 minutes after any injection | ||||
| SafAS | ||||
| Immediate unsolicited AE | 4/6472 | <0·1 (0.0–0·2) | 7/6450 | 0·1 (0.0–0·2) |
| Immediate unsolicited AR | 4/6472 | <0·1 (0.0–0·2) | 6/6450 | <0·1 (0.0–0·2) |
| Participants experiencing at least one solicited reaction within 7 days after an injection | ||||
| RSafAS | ||||
| Solicited reaction | 1398/2420 | 57·8 (55·8–59·7) |
983/2403 | 40·9 (38·9–42·9) |
| Grade 3 solicited reaction | 196/2420 | 8·1 (7·0–9·3) |
118/2403 | 4·9 (4·1–5·9) |
| Solicited injection site reaction | 1130/2419 | 46·7 (44·7–48·7) |
645/2403 | 26·8 (25·1–28·7) |
| Grade 3 solicited injection site reaction | 98/2419 | 4.1 (3·3–4·9) |
43/2403 | 1·8 (1·3–2·4) |
| Solicited systemic reaction | 1100/2420 | 45·5 (43·5–47·5) |
823/2403 | 34·2 (32·4–36·2) |
| Grade 3 solicited systemic reaction | 172/2420 | 7·1 (6·1–8·2) |
109/2403 | 4·5 (3·7–5·4) |
| Participants experiencing at least one of the following up to analysis cut-off date | ||||
| SafAS | ||||
| AE leading to study termination | 5/6472 | <0·1 (0.0–0·2) |
5/6450 | <0·1 (0.0–0·2) |
| SAE | 30/6472 | 0·5 (0·3–0·7) |
26/6450 | 0·4 (0·3–0 ·6) |
| Related SAE | 0/6472 | 0.0 (0.0–0·1) |
0/6450 | 0.0 (0.0–0·1) |
| Death* | 4/6472 | <0·1 (0.0–0 ·2) |
6/6450 | <0·1 (0.0–0·2) |
| AESI | 1/6472 | <0·1 (0.0–0·1) |
1/6450 | <0.1 (0.0–0·1) |
| Related AESI | 0/6472 | 0.0 (0.0–0·1) |
0/6450 | 0.0 (0.0–0·1) |
| MAAE | 366/6472 | 5·7 (5·1–6·2) |
385/6450 | 6·0 (5·4–6·6) |
| Related MAAE | 11/6472 | 0·2 (0·1–0·3) |
7/6450 | 0·1 (0.0–0·2) |
| COVID-19-associated MAAE | 67/6472 | 1·0 (0·8–1·3) |
86/6450 | 1·3 (1·1–1·6) |
| Virologically confirmed SARS-CoV-2 infection and/or symptomatic COVID-19 (regardless of adjudication)** | 928/6472 | 14·3 (13·5–15·2) |
1181/6450 | 18·3 (17·4–19·3) |
M: Number of participants with available data for the relevant endpoint (for solicited AEs) and for corresponding subgroup for unsolicited AEs. n: number of participants experiencing the endpoint listed. The denominator for the reactogenicity subset was 4823 (i.e., the first 2000 participants recruited to each trial arm and all participants ≥60 years of age).
Four deaths in the vaccine group due to angioedema (after carbimazole and propranolol administration), acute respiratory distress syndrome (negative Covid-19 test), chronic kidney disease, and gunshot wound. Six deaths in the placebo group due to hepatic failure, inguinal hernia, desmoid fibromatosis tumor, esophageal carcinoma, hemorrhagic enterocolitis , and septic shock . None of the deaths were considered related to the treatment.
Cases collected for safety purposes; not necessarily laboratory-confirmed.
AE, adverse event; AESI, adverse events of special interest; AR, adverse reaction; CI, confidence interval; MAAE, medically attended adverse event; RSafAS, reactogenicity safety analysis set; SAE, serious adverse event; SafAS: safety analysis set.
Figure 3:
(A) Proportion of participants with solicited injection site reactions within 7 days of each study injection in participants aged 18–59 years and participants aged ≥60 years; (B) the proportion of participants with solicited systemic reactions within 7 days of each study injection in participants aged 18–59 years and participants aged ≥60 years. Error bars are the 95% CI for any solicited reaction.
Discussion
Current bivalent COVID-19 vaccines gained authorization based on immunogenicity data; however, as real-world data is prone to bias related to patient behavior and characteristics, the need for clinical trials remains.18 This article reports on a trial conducted to assess efficacy of a variant COVID-19 vaccine. In this Phase 3 study evaluating a bivalent vaccine containing both ancestral (D614) and beta (B.1.351) variant spike protein as a primary series during the period of predominant Omicron (BA.1 and BA.2) circulation, the primary objective of demonstrating efficacy against symptomatic COVID-19 of >50·0%, with a lower bound of the 95% confidence interval >30·0%, in all participants was met.
The epidemiological context for this efficacy trial is markedly different from those conducted at the outset of the pandemic.19,20 A large proportion of participants had serological evidence of previous infection, which is relevant in the current climate where the population is largely non-naïve, due to previous infection and/or vaccination.21-23 Thus, the VE against symptomatic COVID-19 in non-naïve participants of 75·0% observed in this study starting 14 days PD1 is of particular relevance. This also suggests the potential use of the vaccine as a booster dose at this stage of the pandemic when most of the population have already been exposed to the virus or have been vaccinated. The use of the bivalent vaccine as a booster in BNT162b2-primed adults (18–55 years) has resulted in significantly higher anti-D614G or -B.1.351 PsVN titers post-booster than anti-D614G titers post-primary vaccination in controls, with anti-D614G and anti-B.1.351 ratios of 2.34 (1.84; 2.96) and 1.39 (1.09; 1.77), respectively, for the bivalent vaccine. Furthermore, the booster elicited cross-neutralizing antibodies against Omicron BA.2 (for BNT162b2 and mRNA-1273 primed adults) and Omicron BA.1 (in BNT162b2-primed participants).24 VE was not observed in naïve individuals, albeit the number of participants in this sub-group was limited. This is in line with a study by Anderson et al. 2021, which showed that one dose of the mRNA vaccine, BNT162b2, elicits stronger antibody responses in individuals previously exposed to COVID-19 compared with two doses of BNT162b2 in those without prior infection.25 Notwithstanding this point, In the current situation where high numbers of people have been vaccinated and the virus is still circulating, we should also consider the possibility that many people may also be developing ‘hybrid’ immunity to SARS-CoV-2, whereby immunity is formed by the combination of vaccination and infection.26 Although the data on hybrid immunity are currently limited, the consensus opinion is that it confers greater protection than obtained from either infection or vaccination alone.
The use of placebo as a comparator is the most scientifically rigorous way to assess the absolute efficacy of the vaccine candidate. This enabled us to perform safety comparisons and also maintain the blinding of the randomization, allowing unbiased evaluation of clinical outcomes related to COVID-19 illness and SARS-CoV-2 infection by intervention group. Such an approach has been recommended by a WHO-organised consultation and validated in a real-world setting.27,28 The regions and subgroups analysed in this article were where trials of head-to-head comparisons to demonstrate non-inferior efficacy with currently authorized COVID-19 vaccines would be very large and operationally challenging to conduct in a timely manner. During the surveillance period, the two major variants circulating were the Omicron BA.1 and BA.2 subvariants and, to a lesser extent, Delta, with no cases of more contemporary Omicron BA.4 and BA.5 subvariants. Thus, the data reported here are the first assessment of clinical efficacy of a COVID-19 variant vaccine against the Omicron variant. Since sequencing results were unavailable in approximately 44·0% of the cases in the mFAS-PD2, we conducted sensitivity analyses that assumed these cases were caused by Omicron variants, based on the temporal distribution of variants in the countries, and VE greater than our objective threshold of 50·0% was also demonstrated.
The ability of three doses of the prototype vaccines BNT162b2, ChAdOx1 nCOV-19 and mRNA-1273 to protect against symptomatic disease has been shown to be lower for the Omicron variant (65.5%, 48.9% and 75.1%, respectively) than the Delta variant (90.9%, 82.8% and 94.5%, respectively), with effectiveness waning rapidly 20–25 weeks post second dose.29 By contrast, we demonstrated efficacy against Omicron with two doses of a Beta-containing variant vaccine as a primary series.
A BNT162b2 or mRNA-1273 booster after a primary course substantially increased protection, but that protection waned over time.30 Variant-updated COVID-19 vaccines and booster vaccines incorporating Omicron subvariants are currently under development or are authorized for use. Their use has been endorsed by global regulators, provided that novel COVID-19 booster vaccines containing alternative variants still confer adequate protection against Omicron and other VOCs. Our Beta strain-containing vaccine confers protection against Omicron BA.1 and BA.2 variants that are not a component of the vaccine, thus providing clinical evidence that cross-protection may be conferred without a variant-chasing approach.24
In further support of the broad cross-protection, it is clear that the protection provided by the original vaccines against new strains is insufficient; therefore, new formulations for booster vaccines were authorized for use in place of the original vaccines. While the exact mechanism of cross-protection is unknown, it may be related to the B.1.351 component of the bivalent vaccine; however, there is no evidence directly supporting that inclusion of the beta variant improves cross-protection and it is possible that the prototypic strain alone may also contribute to the protective efficacy measured against omicron variants.31-34 Substitutions in the Beta variant spike at positions K417N, E484K, N501Y may provide new antibody epitopes that are well-positioned to provide cross-neutralizing immunogenicity against a wide array of variants including contemporary circulating strains.13 The results of this study in Omicron-confirmed cases suggests the potential for a Beta variant-containing vaccine to be used as a part of a booster program. A beta variant-containing vaccine (VidPrevtyn Beta) has now been recommended by the European Medicines Agency and in the UK as a booster in adults previously vaccinated with a mRNA or adenoviral vector COVID-19 vaccine. Results from a booster study in individuals previously primed with the CoV2 preS dTM-AS03 (D614) vaccine or with other approved mRNA and adenovirus-vectored vaccines confirmed that a booster with a CoV2 preS dTM-AS03 (B.1.351, Beta) vaccine delivered an immune response comparable to that of the bivalent (ancestral + Beta variant) booster.24 Additionally, SCTV01C, a bivalent protein vaccine based on the spike protein sequences of SARS-CoV-2 Alpha and Beta variants, induced potent cross-strain neutralizing antibody responses to non-vaccine variants, Delta and Omicron, when used as a booster in adults previously vaccinated with two doses (primary series) of mRNA vaccine in a Phase1/2 RCT.35
The VE was numerically higher in the male participants than the female participants, which may be the result of higher male enrolment in countries such as India (2237/3246 [68.9%]) combined with higher seropositivity rates than in other countries (3153/3246 [97.1%]). The number of severe COVID-19 cases or hospitalizations was limited; however, all hospitalized cases were observed in the placebo group. The few severe and hospitalized cases may have been due to the Omicron variant leading to milder COVID-19 disease versus other variants, particularly as most participants had already experienced a prior SARS-CoV-2 infection.36 Additionally, most participants in this study were younger adults aged 18–59 years with lower risk of severe COVID-19 than older people.37,38 Of note, the VE in participants aged 18–59 years with risk factors for severe COVID-19 was similar to that in the same age group without risk factors. We will continue to monitor the incidence of participants with moderate or severe disease as part of the long-term follow-up on the performance of the vaccine.
The bivalent vaccine showed an acceptable reactogenicity profile in this study; after both doses, AEs were mostly mild to moderate and transient, regardless of participant age or prior infection. Injection-site and systemic reactions were each reported by less than half of participants in the reactogenicity subset. These rates may indicate potentially less reactogenicity compared with mRNA-based licensed vaccines (at least one injection-site reaction in 68.5% after dose 1 and in 72.9% after dose 2 or at least one systemic reaction in 50.6% after dose 1 and 69.5% after dose 2 within 2 weeks of vaccination39), although these vaccines have not been evaluated together in the context of a single trial. No cases of myocarditis, pericarditis or thrombosis with thrombocytopenia syndrome, which have previously been reported after vaccination with other vaccines, were reported during the observed 2–3 months of safety follow-up.40,41 However, as these events are extremely rare (411 cases of myocarditis and/or pericarditis per 15,148,369 individuals aged 18-64 years;42 and 15.1 cases of thrombosis with thrombocytopenia syndrome per million doses43), we would not expect to observe these with the sample size included in this study. Immunogenicity data are currently not available; these data will be published when available.
Our study has limitations. Due to the limited number (approximately 6%) of adults aged ≥60 years enrolled in the trial, VE could not be accurately estimated in this age group (only three cases were reported in the vaccine group and two in the placebo group). This was most likely due to the roll-out of vaccines authorized for emergency use in this age category that were available at the time of the study. We acknowledge that the overall risk in the 18–59 years age group with medical issues is significantly lower than older populations, and not having data on the ≥60 years age group makes it difficult to generalize the potential for clinical benefit in the most at-risk populations. The limited number of hospitalized and severe cases prevented any conclusions for VE against these outcomes, and contrasts with efficacy data for previously developed vaccines on severe disease; however, these data were obtained from a COVID-19 naïve population prior to the emergence VOCs.44 To address this, we continue to monitor and report data on moderate and severe disease and on hospitalizations. The short duration of follow-up (median length of follow up PD2 was 58 days) also precluded conclusions on the durability of the vaccine’s protection and long-term safety, which we also continue to monitor. While sequencing was attempted on all viral strains isolated for the primary endpoint, results were only available in approximately 56.0% of cases. We observed a higher rate of missing sequence data in the vaccine group (56·0%) compared with the placebo group (39·0%). One explanation for this observation is the potential impact of the vaccine on reducing viral load. Although the higher rate of missing data in the vaccine group may bias the variant-specific efficacy estimates, sensitivity analyses confirmed efficacy against Omicron.
In conclusion, our results demonstrate the clinical efficacy of a Beta variant-containing vaccine to protect against different SARS-CoV-2 variants, including Omicron (BA.1 and BA.2), and an acceptable safety profile in adults <60 years old. These data provide clinical evidence for a vaccination strategy to develop vaccines with an antigen from a non-predominant strain to confer cross-protection against newly emergent variants. This is particularly relevant in the current climate where, although more than 50 vaccines have been approved worldwide, the addition of new vaccines to the current armamentarium will extend the options to facilitate protection across different regions, healthcare settings and populations in the context of the ongoing pandemic, regardless of prior infection, and with the threat of rapidly evolving SARS-CoV-2 virus strains.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed from database inception up to 20 December 2022, with no language restrictions, for studies reporting the efficacy or effectiveness of vaccines against emergent SARS-CoV-2 variants, including Omicron, using the search terms ‘vaccine’, ‘efficacy OR effectiveness’, ‘SARS-CoV-2’, ‘Omicron OR variant of concern OR emerging variant’ and ‘clinical trial’, and for those reporting data from updated or bivalent vaccine candidates using the search terms, ‘updated vaccine OR bivalent OR adapted vaccine’, ‘SARS-CoV-2’, ‘Omicron OR variant of concern OR emerging variant’ AND ‘clinical trial’. Among the observational cohort studies retrieved, first generation COVID-19 vaccines were shown to be less effective against new emergent SARS-CoV-2 variants of concern including Omicron (BA.1, BA.2, BA.4 and BA.5 variants). Vaccines with variant strains have been developed to provide cross-protection against emerging variants when used as boosters; however, there are no data on these vaccines when used as a primary series.
Sanofi and GSK have developed a bivalent vaccine containing stabilized SARS-CoV-2 pre-fusion S proteins from both the ancestral D614 and the Beta (B.1.351) variant, with the GSK AS03 adjuvant system (CoV2 preS dTM-AS03 [D614 + B.1.351]).
Added value of this study
These are the first data to be published suggesting that a primary series with a Beta variant vaccine provides cross-protective efficacy against Omicron variants. We show an overall VE of 64·7% (95% CI 46·6; 77·2%) against symptomatic COVID-19 in the epidemiological context of Omicron BA1 and BA2 circulation. Genomic sequencing was available for approximately 56·2% of cases, with BA.1 and BA.2 subvariants of Omicron identified as the causative strains in the majority of cases. In additional sensitivity analyses, the VE against symptomatic COVID-19 caused by Omicron or undefined variants was 63·1% (95% CI 43·9; 76·2%). In non-naïve participants, the VE against symptomatic COVID-19 caused by Omicron or undefined variants was 73·8% (95% CI 53·9; 85·9).
Implications of all the available evidence
To our knowledge, this is the first international Phase 3 study to demonstrate the clinical efficacy of a beta variant-containing vaccine to protect against different SARS-CoV-2 variants, including Omicron (BA.1 and BA.2), in non-naïve individuals. These results suggest that AS03-adjuvant vaccines developed with an antigen that is not present in the prevalent circulating strain may confer cross-protection in the current context of widely circulating Omicron subvariants. Therefore, this might warrant further investigation in the future in light of the expected highly variable and unpredictable viral epidemiology. This is relevant in the current situation where approximately 50 vaccines have been approved worldwide, but around a third of the global population have still not been vaccinated.
Acknowledgments
The authors thank all participants, investigators, and study site personnel who took part in this study, including: those from the Ghana Health Service Kintampo Health Research Centre; Kwame Ayisi Boateng, Anthony Afum-Adjei Awuah; Melvin Agbogbatey; Ebenezer Ahenkan, and Esther Daley Matey from the Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana; Claudia Pimentel from the Instituto Nacional de Pediatría, México; Victor Mudhune Otieno, Grace Mugure Mboya, Zipporah Nyamoita Buko, and Taraz Samandari from the KEMRI CGHR Kisumu Kenya; Andrea Accini Valencia, Melissa Accini Valencia, Andrea de Moya, and Yineth Conrado, from the IPS Cenatro Cientifico S.A.A, Colombia; Kenneth K Ngure, Eddah Mbugua, Stanley Ndwiga, Stephen Maina Gakuo, Philip Mwangi, David Chege, Jacinta Nyokabi, and Mercy Nyawira, from the PHRD-Thika KEMRI Clinic, Thika, Kenya; Nichlous Ssebudde, Mary Grace Nalubega, Jenifer Alaba, Emmanuel Obonyo, Raymondo Oola, Harriet Tino, Geoffrey Magombe, Vanon Kyehayo, Ritah Norah Nalybwama, Rebecca Asiimire, Denis Wokorac, Agnes Alimo, and Abigail Link, from the SICRA site at Lira Regional Referral Hospital, Uganda; Korutaro Violet, Elyanu Peter James, Baguma Allan, Kekitiinwa Adeodata, Sekabira Rogers, Ssebunnya Billy, and Ashaba Justus from the Baylor College of Medicine Children’s Foundation-Uganda; Maricianah Onono, Imeldah Wakhungu, Kevin Onyango, Dismas Congo, Samya Rashid, Florence Ondiek, George Otieno, Job Ouma, Donnavane Ondego, Maqline Juma, Penina Amboka, Perez Odhiambo, Mildred Obare, Teresia Otieno, Caren Awinja, and Lizzie Kabete, from the Kenya Medical Research Institute – Centre for Microbiological Research, Research Care and Training Program; Lorena Buitrago from the Centro de Atencion e Investigacion Medica S.A.S. – Caimed S.A.S – sede Bogotá; Patrick Ansah, Oscar Bangre, Michael Bandasua Kaburise and Francis Broni, from the Navrongo Health Research Centre, Ghana; Shelly Ramirez, Gail Broder, Liz Breisemeister, Jim Kublin, David Benkeser, and John Hural, from the Fred Hutch Cancer Center, Seattle, WA, USA; Seyram Kaali, Samuel Harrison, Prince Agyapong, Felicia Serwah, Cynthia Bema, Elvis Eilson, Afia Korkor Opare Yeboah, Dennis Adu-Gyasi, Elisha Adeniji, Owusu Boahen, and Zakariah Buwah from the Research and Development Division, Ghana Health Service, Kintampo North Municipality, Kintampo; Oumou Maiga-Ascofare from the Kumasi Center for Collaborative Research in Tropical Medicine, Kumasi. The authors would also like to thank the Ministry of Health & Population, Government of Nepal for providing swift approval to conduct this trial in Nepal. The authors acknowledge Steven Goodrick PhD of inScience Communications, Springer Healthcare Ltd, London, UK, for providing medical writing assistance with the preparation of this manuscript, funded by Sanofi. The authors also thank Hanson Geevarghese PhD for providing editorial assistance and manuscript coordination on behalf of Sanofi. Funding was provided by Sanofi and by federal funds from the Biomedical Advanced Research and Development Authority, part of the office of the Administration for Strategic Preparedness and Response at the U.S. Department of Health and Human Services under contract number HHSO100201600005I, and in collaboration with the U.S. Department of Defense Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense under contract number W15QKN-16-9-1002. The views presented here are those of the authors and do not purport to represent those of the Department of the Army. This work was done in collaboration with GSK, who provided access to, and use of, the AS03 Adjuvant System.
The NIAID provides grant funding to the HIV Vaccine Trials Network (HVTN) Leadership and Operations Center (UM1 AI 68614HVTN), the Statistics and Data Management Center (UM1 AI 68635), the HVTN Laboratory Center (UM1 AI 68618), the HIV Prevention Trials Network Leadership and Operations Center (UM1 AI 68619), the AIDS Clinical Trials Group Leadership and Operations Center (UM1 AI 68636), and the Infectious Diseases Clinical Research Consortium leadership group 5 (UM1 AI 148684-03).
Declaration of interests
GHD, MIB, BF, M-HG, MC, JA, CAG, RMC, SG, SSr SS are Sanofi employees. MIB, BF, M-HG, JA, CAG, RMC, SSr hold stock or stock options in Sanofi. SG, RMC, SSr hold patents pending on COVID-19 vaccine. The Center for Vaccine Development and Global Health (CVD) receives grants from Pfizer to conduct clinical trials of COVID-19 vaccines; KMNreceives no salary support for this grant. KMN receives grants from NIH to participate in overall organization of COVID vaccine trials and for participation in vaccine trials. RMC has received institutional funding from BARDA for the present study; has received support for attending meetings and/or travel from Sanofi; and holds patents planned, issued or pending from Sanofi. M-HG has received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Sanofi. NR has received institutional funding from the National Institutes of Health; and institutional grants or contracts from Merck, Sanofi, Quidel, Pfizer and Lilly. SRW has received institutional funding from Sanofi and the National Institute of Allergy and Immunology/National Institutes of Health; and institutional grants or contracts from Janssen Vaccines/Johnson & Johnson, Moderna Tx, Vir Biotechnology and Worcester HIV Vaccine; has participated on data safety monitoring or advisory boards for Janssen Vaccines/Johnson & Johnson; and his spouse holds stock/stock options in Regeneron Pharmaceuticals. NG has received institutional funding from Sanofi, GSK and the National Institute of Allergy and Immunology/National Institutes of Health; and is in receipt of grants or contracts from the NIH/NIAID/DAIDS. MA and TT are employees of the NIAID, which funded aspects of the current study. LS, MAC and MK are employees of GSK and own shares in the GSK group of companies. MJ and JJK have received institutional support from Sanofi and the NIAID/NIH with respect to this study. MLR has received institutional support/contracts for the present manuscript from WRAIR IPA and the US Medical Research and Development Command. MA is an employee of the NIAID, which funded aspects of the current study; The NIAID provides grant funding to the HIV Vaccine Trials Network (HVTN) Leadership and Operations Center (UM1 AI 68614HVTN), the Statistics and Data Management Center (UM1 AI 68635), the HVTN Laboratory Center (UM1 AI 68618), the HIV Prevention Trials Network Leadership and Operations Center (UM1 AI 68619), the AIDS Clinical Trials Group Leadership and Operations Center (UM1 AI 68636), and the Infectious Diseases Clinical Research Consortium leadership group 5 (UM1 AI 148684-03). SSa was a Sanofi employee at the time of study conduct; and holds patents planned, issued of pending on COVID-19 vaccines. AC, JA KPA, ASB, TB, DD, MKJ, HK, RM, NM, HR, SMVM, FS, JT, TAW, SG have no interests to declare.
†. VAT00008 Study Team
Karina Abalos, Jose Accini, Naveena Aloysia, John Humphrey Amuasi, Nana Akosua Ansah, David Benkeser, Aude Berge, Hanna Beyko, Oleksandra Bilotkach, Thomas Breuer, Alberto Cadena Bonfanti, Elisabeth Bukusi, Richard Canter, Jaime Augusto Carrillo, Danaya Chansinghakul, Florence Coux, Chandan Das, Santa Kumar Das, Louis Devlin, Luis Espinoza, Michael Fay, Dean Follmann, Carina Frago, Agnes Garinga, Peter B Gilbert, Claudia Gonzalez, Maria Angelica Granados, Lea Guillery, Ying Huang, Kathy Hudzina, Manish Jain, Piush Kanodia, Nitin Khandelwal, Cissy Kityo Mutuluuza, Francis Kiweewa, Noah Kiwanuka, Chalit Kosolsak, Darshna Kukian, Jitendra Singh Kushwaha, Thelma Laot, Eduardo Lopez-Medina, Hugo Macareno Arroyo, Kishorchandra Mandaliya, Stephanie Mamod, Somnath Mangarule, Javier Martínez, Scott McClelland, Lisa Menard, Sandra Mendoza, Satyajit Mohapatra, Catherine Moreau, Nelly Mugo, Videlis Nduba, Fernando Noriega, Patricia Nahirya Ntege, Brenda Okech, Maria Otero, Samuel Gurrion Ouma, Janet Oyieko, Mercedes Paredes, Erwin Pardo, Svitlana Postol, David Pekala, Penny Peng, Marie-Laure Py, Enrique Rivas, Rafael Rivero, Edith Rodriguez, Mansoor Saleh, Pedro Sánchez, Nessryne Sater, Jinen Shah, Rajeev Shrestha, Abraham Siika, Chandramani Singh, Veer Bahadur Singh, Dipesh Tamrakar, Fernanda Tavares Da-Silva, Lucas Otieno Tina, Hector Velasquez, Deo Wabwire, Anne Wajja, Elodie Zaworski, Nianxian Zhang.
Footnotes
Data sharing
Qualified researchers can request access to participant-level data and related study documents, including the clinical study report, study protocol with any amendments, blank case report forms, statistical analysis plan, and dataset specifications. Participant-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://vivli.org/.
References
- 1.Cai Y, Zhang J, Xiao T, et al. Structural basis for enhanced infectivity and immune evasion of SARS-CoV-2 variants. Science 2021; 373(6555): 642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emary KRW, Golubchik T, Aley PK, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet 2021; 397(10282): 1351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med 2021; 384(20): 1885–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shinde V, Bhikha S, Hoosain Z, et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med 2021; 384(20): 1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai D, Khan AR, Soneja M, et al. Effectiveness of an inactivated virus-based SARS-CoV-2 vaccine, BBV152, in India: a test-negative, case-control study. The Lancet Infectious diseases 2022; 22(3): 349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchan SA, Chung H, Brown KA, et al. Effectiveness of COVID-19 vaccines against Omicron or Delta symptomatic infection and severe outcomes. medRxiv 2022: 2021.12.30.21268565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collie S, Champion J, Moultrie H, Bekker L-G, Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. The New England journal of medicine 2021; 386(5): 494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imai M, Ito M, Kiso M, et al. Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB. New England Journal of Medicine 2022; 388(1): 89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalkias S, Harper C, Vrbicky K, et al. A Bivalent Omicron-Containing Booster Vaccine against Covid-19. N Engl J Med 2022; 387(14): 1279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin DY, Xu Y, Gu Y, et al. Effectiveness of Bivalent Boosters against Severe Omicron Infection. N Engl J Med 2023; 388(8): 764–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavot V, Berry C, Kishko M, et al. Protein-based SARS-CoV-2 spike vaccine booster increases cross-neutralization against SARS-CoV-2 variants of concern in non-human primates. Nature communications 2022; 13(1): 1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goepfert PA, Fu B, Chabanon AL, et al. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo-controlled, phase 1-2, dose-ranging study. Lancet Infect Dis 2021; 21(9): 1257–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sridhar S, Joaquin A, Bonaparte MI, et al. Safety and immunogenicity of an AS03-adjuvanted SARS-CoV-2 recombinant protein vaccine (CoV2 preS dTM) in healthy adults: interim findings from a phase 2, randomised, dose-finding, multicentre study. Lancet Infect Dis 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garçon N, Vaughn DW, Didierlaurent AM. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert review of vaccines 2012; 11(3): 349–66. [DOI] [PubMed] [Google Scholar]
- 15.Shrestha L, Lin MJ, Xie H, et al. Clinical Performance Characteristics of the Swift Normalase Amplicon Panel for Sensitive Recovery of Severe Acute Respiratory Syndrome Coronavirus 2 Genomes. The Journal of molecular diagnostics : JMD 2022; 24(9): 963–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joffe S, Babiker A, Ellenberg SS, et al. Data and Safety Monitoring of COVID-19 Vaccine Clinical Trials. The Journal of infectious diseases 2021; 224(12): 1995–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FDA. Development and Licensure of Vaccines to Prevent COVID-19: Guidance for Industry. 2020. https://www.fda.gov/media/139638/download (accessed 19 May 2023). [Google Scholar]
- 18.Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med 2011; 26(5): 546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2021; 384(5): 403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. The New England journal of medicine 2020; 383(27): 2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke K, Jones J, Deng Y, et al. Seroprevalence of Infection-Induced SARS-CoV-2 Antibodies — United States, September 2021–February 2022. MMWR Morb Mortal Wkly Rep 2022; 71: 606–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones JM, Stone M, Sulaeman H, et al. Estimated US Infection- and Vaccine-Induced SARS-CoV-2 Seroprevalence Based on Blood Donations, July 2020-May 2021. Jama 2021; 326(14): 1400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finch D, Tinson A. The continuing impact of COVID-19 opn health and inequalities: a year on from out COVID-19 impact enquiry. 2022. https://www.health.org.uk/publications/long-reads/the-continuing-impact-of-covid-19-on-health-and-inequalities (accessed 19 May 2023). [Google Scholar]
- 24.de Bruyn G, Wang J, Purvis A, et al. Safety and immunogenicity of a variant-adapted SARS-CoV-2 recombinant protein vaccine with AS03 adjuvant as a booster in adults primed with authorized vaccines. medRxiv 2022: 2022.12.02.22282931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson M, Stec M, Rewane A, Landay A, Cloherty G, Moy J. SARS-CoV-2 Antibody Responses in Infection-Naive or Previously Infected Individuals After 1 and 2 Doses of the BNT162b2 Vaccine. JAMA Netw Open 2021; 4(8): e2119741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GAVI. Hybrid immunity: a combination of vaccination and prior infection probably offers the best protection against COVID. 2022; available at: https://www.gavi.org/vaccineswork/hybrid-immunity-combination-vaccination-and-prior-infection-probably-offers-best. Accessed 2 June 2023. [Google Scholar]
- 27.Krause pR, Feming TR, Longini IM, et al. Placebo-Controlled Trials of Covid-19 Vaccines - Why We Still Need Them. N Engl J Med 2021;384:e2. [DOI] [PubMed] [Google Scholar]
- 28.Sisa I, Noblecilla E, Orozco F. Rationale to continue approving placebo-controlled COVID-19 vaccine trials in LMICs. Lancet 2021;397:878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. Jama 2022; 327(7): 639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams K, Rhoads JP, Surie D, et al. Vaccine effectiveness of primary series and booster doses against covid-19 associated hospital admissions in the United States: living test negative design study. Bmj 2022; 379: e072065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau JJ, Cheng AM, Leung K, et al. Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population. Nature Medicine 2023;29:348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsang NNY, So HC, Cowling BJ, et al. Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and sym. ptomatic infection of SARS-CoV-2 omicron BA.2 in Hong Kong: a prospective cohort studyLancet Infect Dis 2023;23:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glatman-Freedman A, Hershkovitz Y, Dichtiar R, et al. Effectiveness of BNT162b2 Vaccine against Omicron Variant Infection among Children 5–11 Years of Age, Israel. Emerging Infectious Diseases 2023;29:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou Y, Huang D, Jiang, et al. The Vaccine Efficacy Against the SARS-CoV-2 Omicron: A Systemic Review and Meta-Analysis. Front Public Health 2022;10: 940956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hannawi S, Saifeldin L, Abuquta A, et al. Safety and immunogenicity of a bivalent SARS-CoV-2 protein booster vaccine, SCTV01C, in adults previously vaccinated with mRNA vaccine: A randomized, double-blind, placebo-controlled phase 1/2 clinical trial. EBioMedicine 2022; 87: 104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meo SA, Meo AS, Al-Jassir FF, Klonoff DC. Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. European review for medical and pharmacological sciences 2021; 25(24): 8012–8. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, Wu Y, He Y, et al. Age-Related risk factors and complications of patients with COVID-19: a population-based retrospective study. Front Med (Lausanne) 2021; 8: 757459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol 2020; 35(12): 1123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapin-Bardales J, Myers T, Gee J, et al. Reactogenicity within 2 weeks after mRNA COVID-19 vaccines: Findings from the CDC v-safe surveillance system. Vaccine 2021;39:7066–7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Husby A, Køber L. COVID-19 mRNA vaccination and myocarditis or pericarditis. Lancet (London, England) 2022; 399(10342): 2168–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hafeez MU, Ikram M, Shafiq Z, et al. COVID-19 vaccine-associated thrombosis with thrombocytopenia syndrome (TTS): a systematic review and post hoc analysis. Clin Appl Thromb Hemost 2021; 27: 10760296211048815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong HL, Hu M, Zhou CK, et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet 2022; 399(10342): 2191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MHRA. Coronavirus vaccine – weekly summary of Yellow Card reporting (Updated 14 October 2021).. 2021. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting. [Google Scholar]
- 44.McIntyre PB, Aggarwal R, Jani I, et al. COVID-19 vaccine strategies must focus on severe disease and global equity. Lancet 2022; 399(10322): 406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers can request access to participant-level data and related study documents, including the clinical study report, study protocol with any amendments, blank case report forms, statistical analysis plan, and dataset specifications. Participant-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://vivli.org/.