Abstract
One in three adults in the United States has obesity; a chronic disease that is implicated in the etiology of at least 14 cancers. Cancer is the leading cause of death among U.S. Hispanic/Latino adults and the second most common cause of death, after cardiovascular disease, for Black adults. Our country’s legacy in overt discrimination (e.g., slavery, segregation) generated inequities across all spheres in which people function as defined by the socio-ecological model – biological, individual, community, structural– and two of the many areas in which it manifests today are the disproportionate burden of obesity and obesity-related cancers in populations of color. Inequities due to environmental, social, and economic factors may predispose individuals to poor lifestyle behaviors by hindering an individual’s opportunity to make healthy lifestyles choices. In this review, we examined the evidence on obesity and the lifestyle guidelines for cancer prevention in relation to cancer risk and outcomes for Black and Hispanic/Latino adults. We also discussed the role of structural and societal inequities on the ability of these two communities to adopt and maintain healthful lifestyle behaviors in accordance with the lifestyle guidelines for cancer prevention and control.
Keywords: Structural racism, Lifestyle guidelines, Obesity related cancers, Cancer health disparities, Black/African American, Hispanic/Latino, Neighborhood deprivation
Introduction
Obesity has been established in the etiology of at least 14 cancers (1–3) and is eclipsing other preventable causes like tobacco as the leading cause of cancer. (4) These obesity-related cancers include meningioma, multiple myeloma, esophagus (adenocarcinoma), thyroid, postmenopausal breast, gallbladder, stomach cardia, liver, pancreas, kidney (renal cell carcinoma), ovaries, uterus, colon and rectum (colorectal) (2), with mixed evidence for advanced/fatal prostate cancer. (5,6) There are important variations in the incidence rates of obesity-related cancers across racial and ethnic groups. (7–9) For cancers of the colon, rectum, kidney, liver, prostate, stomach, and uterus, Black and/or Hispanic/Latino adults have higher incidence rates relative to White adults. (10)
Cancer is the leading cause of death among U.S. Hispanic/Latino adults (11) and the second most common cause of death, following cardiovascular disease, for Black adults. (10,12) As of 2022, Black men had a 19% higher cancer mortality and Black women had a 12% higher risk of death from cancer, relative to White adults. (7) Similarly, Black and/or Hispanic/Latino communities also experienced a greater mortality rate for some obesity related cancers (e.g., breast, colon, rectum, kidney, liver, prostate, stomach, uterine, thyroid, pancreas, multiple myeloma) compared to White adults. (8) Furthermore, the increasing incidence and death rates of certain less common obesity-related cancers, such as multiple myeloma and cancers of the liver, stomach, thyroid, pancreas, and uterus among Black and Hispanic/Latino communities is noteworthy. (7–9)
Based on the 2017–2018 National Health and Nutrition Examination Survey (NHANES) estimates (13), the overall age-adjusted prevalence of obesity was 42% among all U.S. adults. When stratified by race, ethnicity, and gender, Black (56.9%) and Hispanic/Latino (43.7%) women experienced the greatest prevalence relative to White (39.8%) and Asian (17.2%) women. Among men, similar obesity prevalence (defined as BMI ≥ 30 kg/m2) were observed for Black (41.1%), Hispanics/Latino (45.7%), and White (44.7%) adults, but not Asian (17.5%) men. (13) However, there are differences in more extreme categories of obesity. For example, NHANES estimates from 2005–2014 showed differences in the age-adjusted prevalence of severe obesity (BMI ≥ 40 kg/m2) for racial and ethnic groups by gender: Black (All: 12.4%; Men: 7.2%; Women: 16.8%), Hispanics/Latino (All: 7.1; Men: 5.4%; Women: 8.7%), White (All: 7.6%; Men: 5.6%; Women: 9.7%), and Asian (All: 2.0%; gender breakdown not reported). (14) Recent estimates for trends in severe obesity have not been reported separately by gender. (13)
Studies of obesity among populations with and without a history of cancer paint a stark picture. Approximately 36% and 33% of all cancer survivors in the United States (U.S.) in 2019 were overweight (body mass index (BMI) ≥ 25 kg/m2) or had obesity (BMI ≥ 30 kg/m2), respectively. (15) The prevalence of obesity varies across race, ethnicity, and gender as well as by cancer history. (12,16) As obesity continues to rise among U.S. adults, the disproportionate burden in communities of color has been magnified. Between 1997 and 2014, trend analyses using data from the US National Health Interview Survey (NHIS) documented rapidly increasing obesity rates among individuals in the US with a history of cancer relative to those with no history of cancer, with the largest annual increases observed among Black adults, women, and breast and colorectal cancer survivors. (16) According to 2014 NHIS data, there was a significantly higher burden of obesity among Black and Hispanic/Latino cancer survivors compared to their counterparts with no history of cancer and even White adults with a history of cancer. (16) While the data are older, another analysis of the 2009 Behavioral Risk Factor Surveillance System (BRFSS) found a large difference in obesity prevalence among cancer survivors of Hispanic/Latino (47%) and Black (47%) ancestry compared to those of White (30%) ancestry. (17) Of note, a limitation of the cited obesity studies conducted among cancer survivors is that those survivors completing cross-sectional surveys are healthier overall, which may correlate with better energy balance (i.e., less obesity through better diet and more physical activity) such that in a more general sample of cancer survivors these behaviors may be worse. Nonetheless, a prospective analysis from the Framingham Heart Study among 6,197 adults followed for 24 years, showed that obesity and severe obesity were associated with up to a two-fold increase in all-cause mortality (BMI of 20 to <35: Hazard Ratio 1.2, 95% Confidence Interval (CI): 1.14–1.41; BMI of 35 to <40: HR 1.93, 95% CI: 1.68–2.20). A pooled analysis of 20 prospective cohorts, showed that adults with BMI of 40–60 have an estimated 6.5–13.7 years of life lost compared to adults of normal BMI (BMI of 40–44.9: 6.5 (95% CI: 5.7–7.3), BMI of 45–49.9: 8.9 (95% CI: 7.4–10.4), BMI of 50–54.9: 9.8 (95% CI: 7.4–12.2), and BMI of 55–59.9: 13.7 (95% CI: 10.5–16.9)). (18) Among cancer survivors, similar findings have been noted (19). Obesity has been linked to higher overall mortality (HR 1.14, 95% CI: 1.09–1.19), cancer specific mortality (HR: 1.17, 95% CI: 1.12–1.23), and cancer recurrence (HR 1.13, 95% CI 1.07–1.19) (19). Studies of cancer-free adults stratified by race and ethnicity document mixed findings for the link between BMI and mortality including null and inverse associations among Hispanic/Latino adults (20), and positive associations among Black and White adults. (21)
Materials and Methods
This narrative review aims to place the literature on energy balance (via adherence to the obesity, diet, and physical activity recommendations) and obesity related cancer risk and outcomes within the context of structural racism at the neighborhood level. While most research included in this review focuses on Black and Hispanic/Latino adults given the vast underrepresentation of racially minoritized groups in cancer research, literature in predominantly White cohorts was also discussed when relevant. We chose a narrative review format because of its inherit nature that enables us to “link together many studies on different topics, either for purposes of reinterpretation or interconnection…[as] a valuable theory building technique [that] may also serve hypothesis generating functions,” as described by Baumeister and Leary. (22,23) Therefore, formal guidelines for systematic reviews were not followed. (24)
In alignment with this goal, we identified studies via PubMed and Google Scholar searches using various combinations of the following terms: “Black/African American”, “Hispanic/Latino”, “cancer risk”, “cancer mortality”, “cancer outcomes”, “structural racism”, “neighborhood deprivation”, “neighborhood segregation”, “lifestyle behaviors”, “lifestyle interventions”, “cancer prevention guidelines,” and “cancer care” in January 2020. The search was repeated in November 2021 and in October 2022, with newly published studies added. Reference lists for all included studies were also cross checked for additional relevant studies.
Data Availability Statement
Data sharing is not applicable to this article as no data were created or analyzed in this study.
Results
The Role of Structural Racism
Our country’s legacy in overt discrimination (e.g. slavery, segregation) has generated inequities across all spheres of the socio-ecological model (25) in which people function – biological (i.e. biological /genetic pathways, biological responses), individual (i.e., individual risk behaviors, individual demographics), community (i.e., social relationships, social context), structural (i.e., neighborhood, built environment), and societal (i.e., institutional context, social conditions, policies). Two of the many areas in which it manifests today are the disproportionate burden of obesity and obesity-related cancers in communities of color. (26–29) Beyond individual level factors, factors at the community (30,31) and structural (31–38) levels may predispose individuals to poor lifestyle behaviors (39), often by hindering an individual’s opportunity to make healthy lifestyles choices. (40)
Another manifestation of structural racism regards access to medical care, utilization of healthcare, and the quality of that care, which may explain many of the poor health outcomes observed in populations of color. Limited access to quality medical care among communities of color has its roots in both segregation and medical apartheid. (41,42) Segregation redistributed medical resources and access to hospitals and physicians to predominantly White neighborhoods. Medical apartheid then exacerbated access by restricting care for Blacks to certain hospitals on the basis of race. (42) Today, medical apartheid manifests itself through payer-based systems that decrease access to high quality medical care at elite institutions based on insurance status and type of insurance. (41) The downstream consequences of intersecting marginalized identities—including, but not limited to, racial and ethnic identity and often lower socio-economic position—can serve as mechanisms by which structural discrimination results in increased disease risk and contributes to poor outcomes observed throughout the cancer continuum—diagnosis, treatment, and survivorship. Black and Hispanic/Latino adults are more likely to be under-insured, covered by Medicaid, or lack insurance. (43–46) A study of Medicaid patients found that patients were less likely to establish a new appointment at randomly selected cancer centers. (47) Data also suggest Black and Hispanic/Latino adults have less access to cancer screening (48,49), may be less likely to obtain timely referrals for cancer specialists (50,51) and cancer treatment (52), less likely to receive care at high quality cancer care facilities (e.g., accreditation, high hospital/provider volume) (47,53,54), and are more likely to experience fragmented care (55) given limited services at lower-quality facilities. In turn, these factors are associated with poorer cancer prognosis (43,46) at diagnosis and treatment delays. (56–59) The COVID pandemic further exacerbated access to timely diagnosis and cancer treatment among Black and Hispanic/Latino communities. (26,60) In addition to structural racism, individuals of Hispanic/Latino ancestry and immigrant populations face discrimination. Factors such as lack of policies on immigration, stigma, policing and fear due to documentation status, as well as language barriers overlay the larger structural discrimination, creating substantial barriers for Hispanic/Latino communities to access care and resources that enable healthful lifestyles (34,35), including access to cancer care at high quality cancer centers. (47)
Structural racism has led to a health aversive context concerning where people live and has been influenced by historic and more recent mechanisms of discrimination, such as legal and non-legal redlining across housing, education, retail, and public services. (61–65) These conditions have concentrated poverty in neighborhoods with a high density of Black and Hispanic/Latino individuals. In 2018, Black and Hispanic/Latino adults were more than twice as likely to live in poverty compared to their White counterparts (20.8%, 17.6%, and 8.1%, respectively). (66) Neighborhoods with high poverty are more likely to have low-quality built environments which in turn, is a major determinant of engagement in cancer preventive behaviors and cancer risk and outcomes. Structural racism at the neighborhood-level (e.g., historical redlining, neighborhood segregation, neighborhood deprivation) has been directly linked to adverse cancer risk and outcomes in Black and Hispanic/Latino communities across several obesity-related cancers. (67,68) A systematic review of 24 studies on neighborhood socioeconomic status (SES) and various outcomes along the cancer continuum suggest inconsistent relationships for Black and Hispanic/Latino adults compared to White adults. (69) Across studies, higher neighborhood SES was linked to lower overall cancer incidence in White adults, but not in Black or Hispanic/Latino adults. For cancer specific incidence, the authors reported that high neighborhood SES was linked to increased breast and colorectal cancer incidence, although a clear pattern of association between neighborhood SES and cancer survival was not seen primarily due to limited evidence. In a 2017 study incorporating data from three sources (the National Mortality Database, the 1979–2011 National Longitudinal Mortality Study (NLMS), and the Surveillance, Epidemiology, and End Results (SEER) cancer registry database), Singh and Jemal examined the link between temporal individual/neighborhood SES disparities between 1950 and 2013 and U.S. mortality, incidence and survival rate from overall cancers and major cancers. (70) For Black and White adults, residence in more affluent neighborhoods was associated with a 36% higher incidence rate of prostate cancer for men and a 47% higher incidence of female breast cancer. (70) Residence in neighborhoods with greater deprivation was linked to higher incidence of stomach, liver, and esophageal cancers. (70) The 5-year survival rates for adults in the most deprived vs. least deprived neighborhoods by race and ethnicity were: Black (46% vs. 61%), Hispanic (56% vs. 66%), and White (51% vs. 66%). (70) When examined by site, adults residing in the most vs. least deprived areas experienced worse survival for colorectal cancer (60% vs. 48.4%), prostate cancer (81% vs. 66%), and female breast cancer (81% vs. 62%). (70) In adjusted analysis, residence in neighborhoods with low SES (1st – 5th decile) was associated with a 15% to 29% increased risk of death from colorectal cancer for both men and women combined, a 26% to 57% higher risk of death from prostate cancer, and 35% to 68% higher risk of death from female breast cancer, relative to residence in the most affluent neighborhoods (10th decile). (70)
The link between high neighborhood SES and higher incidence of common obesity-related cancers like breast, prostate, and colorectal cancer provides some evidence of the protective role of increased neighborhood resources to engage in lifestyle and cancer screening recommendations. However, it is important to note that Black and Hispanic/Latino communities do not benefit from increased neighborhood SES to the same extent as White adults. For example, in their systematic review, Sorice et al. noted that their cancer incidence findings for minoritized groups were attenuated compared to that in White adults likely because of other factors related to structural racism and discrimination within the healthcare system (i.e., patient provider relationships, decision making and access to treatments, healthcare utilization), regardless of patient’s individual SES or area of residence. (69)
Structural racism may directly modulate biological and physiological pathways through continuous exposure to racism and discrimination, independent of factors at the neighborhood or individual level. The theories of weathering (71,72) and allostatic load (73–75) help us understand how the context in which Black and Hispanic/Latino people live, function, and receive their medical care impacts biological and physiological processes. For example, exposure to racism and discrimination has been shown to influence numerous biological processes (67,76,77) (e.g., systemic inflammation, metabolic and endocrine dysregulation, immune suppression, DNA methylation, oxidative stress, the microbiome) (Figure 1). In turn, these biological responses may contribute to a greater burden of chronic illnesses like hypertension, diabetes, and metabolic syndrome among people of color that increase risk of cancer development and prognosis or influence cancer development directly. (78,79) For example, studies have shown that the link between neighborhood deprivation and aggressive/ lethal breast (80) or prostate cancer (67) may be through modulation of immune and inflammatory pathways that enhance a tumor’s ability for local and distant metastasis.
Figure 1.
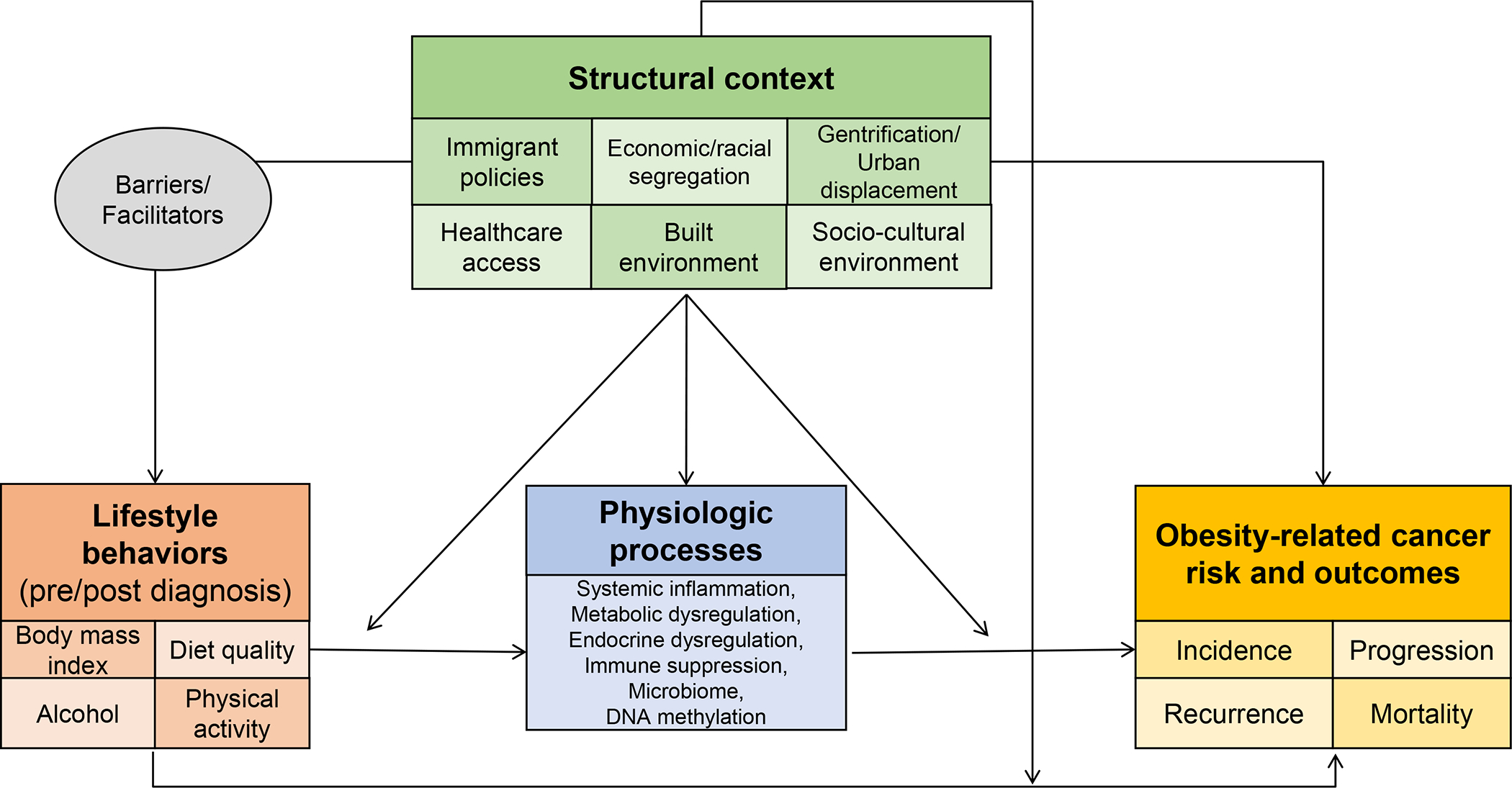
Conceptual framework to understand cancer health inequities in populations of color: the relationship between context, lifestyle behaviors, physiological processes and obesity-related cancer risk and outcomes.
Role of Structural Racism on Adherence to the Lifestyle Guidelines for Cancer Prevention and Control
The American Cancer Society (ACS) has been publishing lifestyle guidelines for cancer prevention since 1984. (81) The most recent iteration of the guidelines was published in 2020 (82) and recommended that individuals maintain a healthy weight throughout life, engage in at least 150–300 minutes of moderate to vigorous physical activity per week, limit sedentary behaviors (i.e. sitting, lying down, watching TV or screen based entertainments), increase intake of fruits, vegetables, and whole grains while limiting intake of red and processed meats, sugar-sweetened beverages, highly processed foods, refined grain products, and alcohol (Box 1). The World Cancer Research Fund/American Institute of Cancer Research’s (WCRF/AICR) Third Expert Report on Diet, Nutrition, Physical Activity and Cancer: A Global Perspective (83) highlighted independent associations between obesity, some diet components, physical activity and risk of certain cancers (Box 1). WCRF/AICR also developed ten cancer prevention recommendations, of which, three currently do not overlap with ACS’s guidelines, which include guidelines against use of supplements, breastfeeding among mothers, and recommendations for cancer survivors (https://www.aicr.org/cancer-prevention/). Wang et al. estimated that suboptimal diets were responsible for 3.04 million new cancer cases and 1.74 million cancer deaths, with racially minoritized and low-income groups having higher diet-attributable cancer burden than their counterparts. (84) Despite ample evidence that quality nutrition and physical activity may be important for cancer risk and outcomes, data suggest that Black and Hispanic/Latino adults may not be adherent to these recommendations, in part because quof structural racism at the neighborhood level.
Adverse built and obesogenic environments are more common in neighborhoods with a high proportion of racial/ethnic minoritized groups and low-income areas (85–87), thereby limiting opportunities to choose and engage in healthy behaviors concordant with the lifestyle guidelines for cancer prevention and control. Disadvantaged neighborhoods have high rates of fast-food chains and stores (88), limited access to supermarkets/grocery stores with healthy food (89), and when available, fresh produce and higher quality foods are often unaffordable. (90,91) Low SES neighborhoods are more likely to lack safe, walkable, bikeable infrastructure for physical activity, resulting in built environments that deter residents from recreational physical activity and exercise. (92) These structural inequities in access at the neighborhood level likely exacerbate cancer disparities in Black and Hispanic/Latino communities. (93) Our group is the first to examine how social neighborhood environment was related to ACS guideline adherence (94–96); although two of these studies were limited by power to detect interactions. So far, we have found that neighborhood socioeconomic status was not an effect modifier of the relationship between ACS guideline adherence and obesity-related cancer outcomes among Black and Hispanic/Latino adults and that neighborhood segregation may not be directly related to overall adherence to the ACS scores (94,95), but may influence components of the guidelines independently and in opposing directions. (96)
In a large study of cancer-free Hispanic/Latino adults, researchers observed a direct association between residence in areas with greater racialized economic segregation (higher privilege (e.g., more White and more affluent)) and adherence to overall ACS guideline (Risk Ratio: 1.94, 95% CI: 1.01–3.70) and increased likelihood of meeting BMI recommendations moderately (RR: 1.75, 95% CI: 1.14–2.71) or highly (RR: 2.08; 95% CI: 1.18–3.67). (96) Hispanic/Latino residents of neighborhoods with greater racial/ethnic isolation (exposure segregation) had a lower likelihood of meeting the alcohol recommendations (moderate adherence RR: 0.86, 95% CI: 0.75–0.98; high adherence RR: 0.90, 95% CI: 0.78–1.04) and physical activity recommendations (high adherence RR: 0.73, 95% CI: 0.57–0.94), but a higher likelihood of meeting the dietary recommendations (moderate adherence RR: 1.07, 95% CI: 1.01–1.14)). (96) Other studies have reported that residence in segregated neighborhood environments was associated with lower odds of exercise and higher odds of obesity among Black (97,98) and/or Hispanic/Latino cancer-free adults. (97,99)
As a result, there is a documented low rate of guideline adherence in Black and Hispanic/Latino communities without a cancer history. Among Black and Hispanic/Latino cancer-free adults, studies estimate that only between 5.4% and 14% of individuals adhere to all the lifestyle cancer prevention guidelines and about 25% adherence to three and 40% adhere to four of the six recommendations (100–103), with several studies reporting low adherence to individual diet, physical activity and BMI recommendations (studies in Hispanic/Latino (104–106) and Black (103,107) adults). Our recent work in two large prospective cohorts has helped to further illuminate adherence to the ACS cancer prevention guidelines among cancer-free Black and Latino adults. Among 9,204 Hispanic/Latino older adults in the NIH-AARP Diet and Health Study, we documented that at start of the cohort just 12% of cancer-free adults had behaviors that were considered highly adherent with the ACS guidelines and when examined by components, few adults were meeting the individual recommendations (percent meeting recommendations for diet: 13%, alcohol: 31%, BMI: 31%, physical activity: 17%). (95) The proportion of cancer-free Black and Hispanic/Latino women at the start of Women’s Health Initiative who were considered highly adherent to the ACS guidelines was even lower, 8.7% out of 9,297 Black women and 8.3% out of 4,215 Hispanic/Latino women. McCullough et al conducted a cross-sectional analysis of diet quality measured by concordance with the 2020 ACS recommendations for cancer prevention score among 155,331 adults participating in the ACS’s Cancer Prevention Study-3 cohort, and found that Black adults had an increased risk of poor diet quality (HR: 1.16, 95% CI: 1.08–1.25) while Hispanic/Latino adults had a lower risk (HR: 0.0.84, 95% CI: 1.12–1.21), although only the relationship for Hispanic/Latinos remained significant after inclusion of additional covariates. (108) Adults with residence in rural areas (HR: 1.61, 95% CI: 1.48–1.75) and in food desert (HR: 1.17, 95% CI: 1.12–1.22) reported a higher risk of poor diet quality. (108) In analysis of 16,462 diverse adults, ages 18–74, enrolled in the Hispanic Health Community Study/Study of Latinos (HCHS/SOL), Pichardo et al. found that approximately 28% had overall lifestyle behaviors considered highly concordant with the ACS guidelines. (96) The higher reported proportion of adults adherent to the ACS guidelines in the HCHS/SOL cohort may be explained by the cohort’s younger age distribution, diversity in Hispanic/Latino background, the use of highly reliable and validated dietary scales that account for cultural and traditional foods found across Hispanic/Latino communities, as well as the use of objectively measured physical activity (for example, it is known that Hispanic/Latino adults are more likely to engage in non-recreatation moderate-vigorous physical activity stemming from higher rates of blue collar work- and commute/transportation- related physical activity than leisure time physical activity. (109)
Recommendations have also been developed for cancer survivors and include nutrition assessment at the time of diagnosis, as well as recommendations for long term survival (110). Recent recommendations also suggest incorporating physical activity at the time of diagnosis (111). Structural determinants of lifestyle guideline adherence among cancer survivors are likely similar to that of cancer-free individuals. Cancer survivors may experience additional barriers related to their chronic disease that prevent engagement in lifestyle behavior change including a lack of time, willpower, enjoyment from exercise, knowledge regarding disease specific side effects or on how to exercise, skills, equipment, and good physical health. (112,113) Perceptions, such as the belief of already doing enough exercise, as well as experiencing post treatment side effects (e.g. lymphedema, bone and joint pain, depression, self-motivation), have been reported as barriers. (114) Lack of encouragement from family and friends and lack of counseling from healthcare providers. (115–123) Support from friends, family and health professionals have been identified as facilitators to behavior change. (124–127) In a qualitative study of 26 Black and Hispanic/Latino female breast cancer survivors and 10 oncology health care providers, inconsistent sources of information, cancer information overload, the rapidly changing evidence, and gaps in the current evidence that contributed to non-cancer specific messaging about lifestyle behaviors were identified as major structural level barriers to behavior change after diagnosis. (123)
An analysis of the 2005–2010 NHIS found a high prevalence of non-adherence to the guidelines among Hispanic/Latino and Black adults who self-reported a history of cancer: for fruits (83% and 88%, respectively, were non-adherent), for vegetables (82.7% and 91.4%, respectively), for fiber (94% and 83%, respectively), for added sugars (63% and 69%, respectively), for physical activity (16% and 16%, respectively), and overweight or obesity (75% and 76%, respectively). (128) An analysis of the 2009 BRFSS survey data also found differences by race/ethnicity for adherence to individual components of the cancer prevention guidelines among cancer survivors. (17) Compared to White survivors, Black and Hispanic/Latino survivors had slightly higher adherence to the fruit and vegetable guidelines (26% vs. 27% and 29%, respectively), but significantly lower adherence to the physical activity (49% vs. 34% and 43%, respectively) and obesity (30% vs. 47% and 47%, respectively) guidelines. (17) In studies of smaller cohorts of Black and Hispanic/Latino cancer survivors enrolled in clinical trials or found through retrospective chart reviews, researchers have observed high adherence (78% to 95%) for individual recommendations on red and processed meat or alcohol, but low adherence to diet (32%), physical activity (21%) and BMI recommendations (25% to 70%). (106,107,128–130) In a recent study among U.S. Hispanic/Latino adults, Pichardo et al. reported ACS guideline adherence levels by cancer history status, with a higher proportion of adults without a history of cancer considered to have high (97.5%) vs. low (96.5%) adherence to the guidelines; while for cancer survivors the opposite was true, a greater proportion were classified as low (5.6%) vs. highly (2.5%) adherent to these guidelines (p<0.001). (96)
The Link between Adherence to Cancer Prevention Guidelines and Cancer Risk and Mortality
Additional research has evaluated adherence to the lifestyle guidelines in relation to cancer risk, cancer-related mortality, and all-cause mortality. Achieving a healthy BMI through overall healthy lifestyle choices—whether measured according to the ACS or the WCRF scoring systems— has been associated with significantly lower risk of developing cancer, as well as lower cancer-specific mortality and all-cause mortality. A meta-analysis of 12 studies in predominantly White populations found a 10%–61% reduction in overall cancer incidence and reductions in cancer mortality for cohorts of men and women combined (24% to 52%) and separately by gender (20–24% reduction in women and 24 to 30% reduction in men), who maintained an overall healthy lifestyle (i.e., higher guideline adherence) relative to those who did not. (131) For specific cancer sites, the authors observed reductions in risk of breast cancer (19% to 60%), endometrial cancer (23% to 60%) in women, and colorectal cancer for both men and women (27% to 52%) who maintained an overall healthy lifestyle (i.e., higher guideline adherence) relative to those who did not. (131) A 2015 study by Kabat et al. in the full NIH-AARP Diet and Health Study cohort (N= 566,401 adults) found that high ACS guideline adherence was associated with a 10% to 19% reduction in overall cancer risk and risk of 14 out of 25 specific cancer sites (esophagus, stomach, small intestine, colon, rectum, liver, gallbladder, pancreas, breast, endometrium, bladder, kidney, lung, and leukemia) compared to low guideline adherence. (132) A 2023 study by Pichardo and colleagues among 9,204 Hispanic/Latino adults enrolled in the NIH-AARP Diet and Health Study, found a 24% lower risk of developing an obesity-related cancer over a 10.5-year period among adults whose lifestyle behaviors were highly adherent with the ACS cancer prevention guidelines versus those with low guideline adherence (subdistribution hazard: 0.76, 95% CI: 0.58–0.996, P = 0.047, P trend = 0.039). (95)
In 2014, Thomson et al. evaluated 65,838 postmenopausal women in the Women’s Health Initiative (WHI) and found high ACS guideline adherence was associated with a 17% lower risk of overall cancer (HR: 0.83, 95% CI: 0.75–0.92), with the strongest associations (based on larger coefficient change) observed for Hispanic/Latino (47%) women compared to Black (33%), White (12%), or Asian (19%) women. (100) In another recent analysis among 9,301 Black and 4,221 Hispanic/Latino postmenopausal women in WHI with additional follow-up (up to 24 years), there was a strong association between high adherence to the ACS guidelines and obesity-related cancer risk. (94) Pichardo et al. found that high ACS guideline adherence was associated with a lower risk of obesity related cancers: 28–29% for Black women (HR: 0.72, 95% CI: 0.55–0.94) and 41–42% for Hispanic/Latino women (HR: 0.58, 95% CI: 0.36–0.93), as well as a lower risk of less common obesity-related cancers (cancers linked to obesity excluding breast, endometrial): 31% for Black and 63% for Hispanic/Latino women, P trend = 0.025. (94)
Consistent with these findings, a 2016 study by Warren Andersen et al. using data from the Southern Community Cohort Study (SCCS), of 25,509 majority Black participants without chronic disease at baseline, who were of low socio-economic status, found that participants meeting three or four vs. zero ACS guidelines had 7%−45% lower cancer risk (HR: 0.70, 95% CI: 0.51–0.97, HR: 0.55, 95% CI: 0.31–0.99, respectively). (107) The stronger cancer risk reduction in Black and Hispanic/Latino individuals suggests that lifestyle interventions focused on increasing guideline adherence have the potential to modify cancer risk more robustly in this population. However, randomized lifestyle trials that evaluate intervention efficacy among Black and Hispanic/Latino cancer survivors are limited (to be discussed later). (133)
Several studies examining mortality have found mixed results. In a study by Kabat et al. inverse associations between guideline adherence and all-cause mortality (men HR: 0.74, 95% CI: 0.72–0.76); women HR 0.67, 95% CI: 0.65–0.70) and cancer-specific mortality (men HR: 0.75, 95% CI: 0.70–0.80); women HR 0.76, 95% CI: 0.70–0.83) in non-racially stratified analyses were reported. (132) Similarly, in the WHI study by Thomson et al, pre-diagnosis high guideline adherence was associated with a reduced risk of all-cause mortality (Black: −44%; Hispanic/Latino: −39%; White: −27% [referent]; women), with a non-significant reduction in cancer-specific mortality (Black: −23%; Latina: −40%; White: −19% [referent] women). (100) On the contrary, in a recent analysis by Pichardo et al., no associations were observed between pre-diagnosis guideline adherence and all-cause mortality for Black women (moderate adherence HR: 1.17, 95% CI: 0.91–1.52; high adherence HR: 0.86, 95% CI: 0.53–1.39, P trend = 0.801) or Latina women (moderate adherence HR: 1.04, 95% CI: 0.64–1.70; high adherence HR: 0.81, 95% CI: 0.32–2.06, P trend = 0.833). (94) These two analyses in WHI are difficult to compare directly as they used different statistical methods and mortality outcomes (cause-specific versus all-cause) and the most recent paper had considerably longer follow-up time. Additionally, studies examining longitudinal behavior change as well as post-diagnosis behavior may be important to fully understand associations between lifestyle factors and mortality outcomes in cancer survivors.
Lifestyle Interventions to Improve Adoption and Maintenance Healthy Behaviors
Many lifestyle interventions to promote weight loss, behavior change, and improvements in obesity related co-morbidities, have been conducted in cancer-free populations of Black and Hispanic/Latino adults. However, there are significant limitations across many of these studies that may prevent success of the intervention itself or long-term adoption of the behavior changes elicited. Reviews of these interventions highlight a range of barriers that may limit the success of the weight loss program, such as focusing on just one level of the socioecological model (134,135), not incorporating a randomized study design (135,136), having small sample sizes or limited to power (135–137), the duration of the intervention not being sufficient to enable behavior change (135,137), questionable role of cultural adaptations (135,138,139), and even in well-designed trials, racially minoritized groups tend to lose less weight than their non-minority counterparts. (135,140) A large gap remains in terms of access to lifestyle interventions among communities of color. In a large systematic review of 124 studies by the U.S. Preventive Services Task Force to identify interventions for weight loss (behavior based (n=80), maintenance (n=9), or medication based (n=35)), just 11 specifically focused on racial minority groups, highlighting the vast under representation and inequities that persist for minority communities to access and engage in weight loss programming. (141) Despite the extensive limitations in the current body of work on lifestyle behavior interventions, the majority of researchers agree on the need to conduct multi-systems and multi-level programming to target obesity in medically at risk minoritized communities. (134,135,142–144)
Among cancer survivors, the oncology community has targeted healthy lifestyle via instituting comprehensive survivorship care, provider-based counseling, and increasing access to lifestyle interventions and programs after diagnosis. There are many diet and physical activity interventions among cancer survivors that have been effective in improving body weight, physical activity, and dietary intake and quality (145,146) as well as cancer outcomes. (147,148) Helping survivors of color adopt and maintain healthy lifestyles requires intervening across the multidimensional forces that influence their lives after a cancer diagnosis and ensuring that interventions address barriers and leverage facilitators for behavior change that have been identified. There is a rapidly growing body of lifestyle intervention literature in Black and Hispanic/Latino cancer survivors. (133) However, most of these interventions focused on providing lifestyle counseling and limited resources are often not available to participants beyond the study’s duration. Because lifestyle interventions often focus on individual level changes and may not account for various forms of systematic or structural discrimination, it is not surprising that few interventions observe long-term maintenance of behavior changes. Understanding what factors, beyond those at the individual level, contribute to survivors’ ability to engage and maintain behavior change goals long-term will be critical to eliminate cancer inequities. (149)
Discussion
There is a need for research and interventions that place energy balance and obesity related cancer risk and outcomes within a socio-ecological model framework—biological, individual, community, structural, and societal (25) and in the context of structural racism. (26) There is mounting evidence on the role of structural racism at the neighborhood level on inequities with regards to obesity (150) and cancer (93) outcomes. As neighborhood environments change due to mobility of racial/ethnic groups from one area to another (also known as gentrification or urban displacement), area level poverty and income inequality also change (151) and may potentially influence cancer risk and outcomes over time. The links between gentrification, energy balance, and cancer remain unexamined, and there are notable limitations in the existing body of literature on segregation and cancer. Studies examining direct, moderating, and mediating effects of social neighborhood context are warranted. There is a need for qualitative studies to understand the role that changing neighborhood dynamics and gentrification may have on access to affordable and healthy diet and food security. This information can help identify critical areas for intervention and develop multi-level based programming, that incorporates multiple social determinants of health (152) at various levels of the socioecological model (25) to enable allocation of resources to those who are most at risk of not meeting the lifestyle guidelines, to design interventions focused on mitigating the negative consequences of rapidly changing neighborhood dynamics, and inform local policies targeting neighborhood development and revitalization projects.
Racial/ethnic disparities are evident at every stage of the cancer continuum—etiology, prevention, detection, diagnosis, treatment survivorship—which may in part be due to the complex interplay with biological, individual, community, structural and societal consequences of discrimination. To eliminate cancer disparities deeply rooted in long-standing structural inequities, there is an imperative need for high quality care and research that considers the historical, social, cultural, and economic context that racially minoritized groups experience daily.
Box 1.
Lifestyle Recommendations for Cancer Prevention
| The 2020 ACS Guidelines on Diet and Physical Activity For Cancer Prevention 83 |
|
|
| 1. Achieve and maintain a healthy weight throughout life. Keep your weight within a healthy range and avoid weight gain in adult life. |
| 2. Be physically active. |
| a. Get at least 150–300 minutes of moderate intensity or 75 minutes of vigorous intensity activity each week (or a combination of these). Getting to or exceeding the upper limit of 300 minutes is ideal. |
| b. Limit sedentary behavior such as sitting, lying down, watching TV, or other forms of screen-based entertainment. |
| 3. Follow a healthy eating pattern at all ages. |
| A healthy eating pattern includes: |
| a. Foods that are high in nutrients in amounts that help you get to and stay at a healthy body weight. |
| b. A variety of vegetables—dark green, red and orange, fiber-rich legumes (beans and peas) and others. |
| c. Fruits, especially whole fruits in a variety of colors. |
| d. Whole grains. |
| A healthy eating pattern limits or does not include: |
| e. Red and processed meats |
| f. Sugar-sweetened beverages |
| g. Highly processed foods and refined grain products. |
| h. It is best not to drink alcohol. People who do choose to drink alcohol should have no more than 1 drink per day for women and 2 drinks per day for men. |
| The 2018 WCRF/AICR Cancer Prevention Recommendation 84 |
|
|
| 1. Be a healthy weight. Keep your diet within the healthy range and avoid weight gain in adult life. |
| 2. Be physically active. We recommend being physically active as part everyday life—walk more and sit less. |
| 3. Eat a better diet. Make wholegrains, vegetables, fruits, and beans as part of your usual diet.” |
| 4. Limit fast foods. Limit consumption of ‘fast foods’ and other processed foods high in fat, starches, or sugars. |
| 5. Limit red and processed meat. Eat no more than moderate amounts of red meat, such as beef, pork, and lamb. |
| 6. Cut down on sugary drinks. Limit sugar-sweetened drinks, drink mostly water and unsweetened drinks. |
| 7. Limit alcohol consumption. For cancer prevention, it’s best not to drink alcohol. |
| 8. Do not use supplements for cancer prevention. Aim to meet nutritional needs through diet alone |
| 9. Breastfeed your baby, if you can. Breastfeeding is good for mother and baby. |
| 10. After a cancer diagnosis, follow our recommendations, if you are able to.” |
Acknowledgements
Funding:
Research reported in this publication was supported by the National Institutes of Health – National Cancer Institute, Award (MSP and MI, Award # R01CA207753). DAE was supported by the Yale Clinical and Translational Science Award (UL1 TR001863). The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Abbreviations
- CDC
Centers for Disease Control and Prevention
- BMI
Body Mass Index
- ICD-O
The International Classification of Diseases for Oncology
- NHANES
National Health and Nutrition Examination Survey
- NHIS
National Health Interview Survey
- BRFSS
Behavioral Risk Factor Surveillance System
- WCRF/AICR
World Cancer Research Fund/American Institute of Cancer Research
- WHI
Women’s Health Initiative
- SCCS
Southern Community Cohort Study
Footnotes
Disclosures: The authors report no conflicts of interest.
Ethics statement: N/A
References
- 1.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body Fatness and Cancer — Viewpoint of the IARC Working Group. New England Journal of Medicine 2016;375(8):794–8 doi 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, Agurs-Collins T, et al. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity - United States, 2005–2014. MMWR Morbidity and mortality weekly report 2017;66(39):1052–8 doi 10.15585/mmwr.mm6639e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobbins M, Decorby K, Choi BCK. The Association between Obesity and Cancer Risk: A Meta-Analysis of Observational Studies from 1985 to 2011. ISRN Preventive Medicine 2013;2013:1–16 doi 10.5402/2013/680536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, et al. American Society of Clinical Oncology Position Statement on Obesity and Cancer. Journal of Clinical Oncology 2014;32(31):3568–74 doi 10.1200/jco.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrington WE, Schenk JM, Etzioni R, Arnold KB, Neuhouser ML, Thompson IM, Jr., et al. Difference in Association of Obesity With Prostate Cancer Risk Between US African American and Non-Hispanic White Men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA Oncol 2015;1(3):342–9 doi 10.1001/jamaoncol.2015.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pichardo MS, Smith CJ, Dorsey TH, Loffredo CA, Ambs S. Association of Anthropometric Measures with Prostate Cancer among African American Men in the NCI-Maryland Prostate Cancer Case-Control Study. Cancer Epidemiol Biomarkers Prev 2018;27(8):936–44 doi 10.1158/1055-9965.EPI-18-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giaquinto AN, Miller KD, Tossas KY, Winn RA, Jemal A, Siegel RL. Cancer statistics for African American/Black People 2022. CA: A Cancer Journal for Clinicians 2022. doi 10.3322/caac.21718. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians 2021;71(1):7–33 doi 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 9.Miller KD, Ortiz AP, Pinheiro PS, Bandi P, Minihan A, Fuchs HE, et al. Cancer statistics for the US Hispanic/Latino population, 2021. CA: A Cancer Journal for Clinicians 2021;71(6):466–87 doi 10.3322/caac.21695. [DOI] [PubMed] [Google Scholar]
- 10.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69(1):7–34 doi 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 11.Miller KD, Goding Sauer A, Ortiz AP, Fedewa SA, Pinheiro PS, Tortolero-Luna G, et al. Cancer Statistics for Hispanics/Latinos, 2018. CA Cancer J Clin 2018. doi 10.3322/caac.21494. [DOI] [PubMed] [Google Scholar]
- 12.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 13.Hales CM CM, Fryar CD, Ogden CL.,. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. Hyattsville, MD:: National Center for Health Statistics.; 2020. [Google Scholar]
- 14.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. Jama 2016;315(21) doi 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute. 2021 November 11. Cancer Survivors and Weight. In Cancer Trends Progress Report. <https://progressreport.cancer.gov/after/weight>. Accessed 2021 November 11.
- 16.Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY. Trends in Obesity Prevalence in Adults With a History of Cancer: Results From the US National Health Interview Survey, 1997 to 2014. J Clin Oncol 2016;34(26):3133–40 doi 10.1200/jco.2016.66.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nayak P, Paxton RJ, Holmes H, Thanh Nguyen H, Elting LS. Racial and ethnic differences in health behaviors among cancer survivors. Am J Prev Med 2015;48(6):729–36 doi 10.1016/j.amepre.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Kitahara CM, Flint AJ, Berrington de Gonzalez A, Bernstein L, Brotzman M, MacInnis RJ, et al. Association between class III obesity (BMI of 40–59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med 2014;11(7):e1001673 doi 10.1371/journal.pmed.1001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrelli F, Cortellini A, Indini A, Tomasello G, Ghidini M, Nigro O, et al. Association of Obesity With Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-analysis0JAMA Netw Open 2021;4(3):e213520 doi 10.1001/jamanetworkopen.2021.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontaine KR, McCubrey R, Mehta T, Pajewski NM, Keith SW, Bangalore SS, et al. Body mass index and mortality rate among Hispanic adults: a pooled analysis of multiple epidemiologic data sets. International Journal of Obesity 2011;36(8):1121–6 doi 10.1038/ijo.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel AV, Hildebrand JS, Gapstur SM. Body mass index and all-cause mortality in a large prospective cohort of white and black U.S. Adults. PLoS One 2014;9(10):e109153 doi 10.1371/journal.pone.0109153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumeister RF. Writing a Literature Review. The Portable Mentor2013. p 119–32. [Google Scholar]
- 23.Baumeister RF, Leary MR. Writing Narrative Literature Reviews. Review of General Psychology 1997;1(3):311–20 doi 10.1037/1089-2680.1.3.311. [DOI] [Google Scholar]
- 24.Siddaway AP, Wood AM, Hedges LV. How to Do a Systematic Review: A Best Practice Guide for Conducting and Reporting Narrative Reviews, Meta-Analyses, and Meta-Syntheses. Annual Review of Psychology 2019;70(1):747–70 doi 10.1146/annurev-psych-010418-102803. [DOI] [PubMed] [Google Scholar]
- 25.Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, et al. Approaching Health Disparities From a Population Perspective: The National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health 2008;98(9):1608–15 doi 10.2105/ajph.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Best AL, Roberson ML, Plascak JJ, Peterson CE, Rogers CR, Hastert TA, et al. Structural Racism and Cancer: Calls to Action for Cancer Researchers to Address Racial/Ethnic Cancer Inequity in the United States. Cancer Epidemiol Biomarkers Prev 2022;31(6):1243–6 doi 10.1158/1055-9965.EPI-21-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang ET, Yang J, Alfaro-Velcamp T, So SK, Glaser SL, Gomez SL. Disparities in liver cancer incidence by nativity, acculturation, and socioeconomic status in California Hispanics and Asians. Cancer Epidemiol Biomarkers Prev 2010;19(12):3106–18 doi 10.1158/1055-9965.EPI-10-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeRouen MC, Schupp CW, Koo J, Yang J, Hertz A, Shariff-Marco S, et al. Impact of individual and neighborhood factors on disparities in prostate cancer survival. Cancer Epidemiol 2018;53:1–11 doi 10.1016/j.canep.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez SL, Shariff-Marco S, DeRouen M, Keegan TH, Yen IH, Mujahid M, et al. The impact of neighborhood social and built environment factors across the cancer continuum: Current research, methodological considerations, and future directions. Cancer 2015;121(14):2314–30 doi 10.1002/cncr.29345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conroy SM, Shariff-Marco S, Yang J, Hertz A, Cockburn M, Shvetsov YB, et al. Characterizing the neighborhood obesogenic environment in the Multiethnic Cohort: a multi-level infrastructure for cancer health disparities research. Cancer Causes Control 2018;29(1):167–83 doi 10.1007/s10552-017-0980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lian M, Schootman M, Yun S. Geographic variation and effect of area-level poverty rate on colorectal cancer screening. BMC Public Health 2008;8(1) doi 10.1186/1471-2458-8-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dean LT, Gehlert S, Neuhouser ML, Oh A, Zanetti K, Goodman M, et al. Social factors matter in cancer risk and survivorship. Cancer Causes Control 2018;29(7):611–8 doi 10.1007/s10552-018-1043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanez B, Thompson EH, Stanton AL. Quality of life among Latina breast cancer patients: a systematic review of the literature. Journal of Cancer Survivorship 2011;5(2):191–207 doi 10.1007/s11764-011-0171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pruitt SL, Lee SJC, Tiro JA, Xuan L, Ruiz JM, Inrig S. Residential racial segregation and mortality among black, white, and Hispanic urban breast cancer patients in Texas, 1995 to 2009. Cancer 2015;121(11):1845–55 doi 10.1002/cncr.29282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller AM, Ashing KT, Modeste NN, Herring RP, Sealy DA. Contextual factors influencing health-related quality of life in African American and Latina breast cancer survivors. J Cancer Surviv 2015;9(3):441–9 doi 10.1007/s11764-014-0420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Von Behren J, Abrahão R, Goldberg D, Gomez SL, Setiawan VW, Cheng I. The influence of neighborhood socioeconomic status and ethnic enclave on endometrial cancer mortality among Hispanics and Asian Americans/Pacific Islanders in California. Cancer Causes & Control 2018;29(9):875–81 doi 10.1007/s10552-018-1063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanez B, McGinty HL, Buitrago D, Ramirez AG, Penedo FJ. Cancer Outcomes in Hispanics/Latinos in the United States: An Integrative Review and Conceptual Model of Determinants of Health. J Lat Psychol 2016;4(2):114–29 doi 10.1037/lat0000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pruitt S, Tiro J, Xuan L, Lee S. Hispanic and Immigrant Paradoxes in U.S. Breast Cancer Mortality: Impact of Neighborhood Poverty and Hispanic Density. International Journal of Environmental Research and Public Health; 2016;13(12) doi 10.3390/ijerph13121238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang CY, Tseng M. Ethnic density and cancer: A review of the evidence. Cancer 2018;124(9):1877–903 doi 10.1002/cncr.31177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roux AVD, Mair C. Neighborhoods and health. Ann Ny Acad Sci 2010;1186:125–45 doi 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- 41.Golub M, Calman N, Ruddock C, Agarwal N, Davis JH, Foley RL, et al. A Community Mobilizes to End Medical Apartheid. Progress in Community Health Partnerships: Research, Education, and Action 2011;5(3):317–25 doi 10.1353/cpr.2011.0041. [DOI] [PubMed] [Google Scholar]
- 42.Washington HA. Medical apartheid: The dark history of medical experimentation on Black Americans from colonial times to the present. Doubleday Books; 2006. [Google Scholar]
- 43.Ko NY, Hong S, Winn RA, Calip GS. Association of Insurance Status and Racial Disparities With the Detection of Early-Stage Breast Cancer. JAMA Oncology 2020;6(3) doi 10.1001/jamaoncol.2019.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thamer M, Richard C, Casebeer AW, Ray NF. Health insurance coverage among foreign-born US residents: the impact of race, ethnicity, and length of residence. Am J Public Health 1997;87(1):96–102 doi 10.2105/ajph.87.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Racial Sohn H. and Ethnic Disparities in Health Insurance Coverage: Dynamics of Gaining and Losing Coverage Over the Life-Course. Population Research and Policy Review 2016;36(2):181–201 doi 10.1007/s11113-016-9416-y.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The Relation between Health Insurance Coverage and Clinical Outcomes among Women with Breast Cancer. New England Journal of Medicine 1993;329(5):326–31 doi 10.1056/nejm199307293290507. [DOI] [PubMed] [Google Scholar]
- 47.Alio AP, Wharton MJ, Fiscella K. Structural Racism and Inequities in Access to Medicaid-Funded Quality Cancer Care in the United States. JAMA Netw Open 2022;5(7):e2222220 doi 10.1001/jamanetworkopen.2022.22220. [DOI] [PubMed] [Google Scholar]
- 48.Dailey AB, Kasl SV, Holford TR, Calvocoressi L, Jones BA. Neighborhood-level socioeconomic predictors of nonadherence to mammography screening guidelines. Cancer Epidemiol Biomarkers Prev 2007;16(11):2293–303 doi 10.1158/1055-9965.epi-06-1076. [DOI] [PubMed] [Google Scholar]
- 49.Hughes AE, Tiro JA, Balasubramanian BA, Skinner CS, Pruitt SL. Social Disadvantage, Healthcare Utilization, and Colorectal Cancer Screening: Leveraging Longitudinal Patient Address and Health Records Data. Cancer Epidemiol Biomarkers Prev 2018;27(12):1424–32 doi 10.1158/1055-9965.EPI-18-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy MM, Simons JP, Ng SC, McDade TP, Smith JK, Shah SA, et al. Racial differences in cancer specialist consultation, treatment, and outcomes for locoregional pancreatic adenocarcinoma. Annals of surgical oncology 2009;16(11):2968–77 doi 10.1245/s10434-009-0656-5. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez M, Winicki N, Kadivar A, Alvarez S, Zhang Y, Maguire S, et al. Racial and ethnic variation in referral times for thoracic oncologic surgery in a major metropolitan area. J Thorac Cardiovasc Surg 2023;165(2):482–94 e1 doi 10.1016/j.jtcvs.2022.05.036. [DOI] [PubMed] [Google Scholar]
- 52.Hutten RJ, Weil CR, Gaffney DK, Kokeny K, Lloyd S, Rogers CR, et al. Racial and Ethnic Health Disparities in Delay to Initiation of Intensity-Modulated Radiotherapy. JCO Oncol Pract 2022;18(10):e1694–e703 doi 10.1200/OP.22.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang LC, Ma Y, Ngo JV, Rhoads KF. What factors influence minority use of National Cancer Institute-designated cancer centers? Cancer 2014;120(3):399–407 doi 10.1002/cncr.28413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wheeler SB, Carpenter WR, Peppercorn J, Schenck AP, Weinberger M, Biddle AK. Structural/organizational characteristics of health services partly explain racial variation in timeliness of radiation therapy among elderly breast cancer patients. Breast Cancer Research and Treatment 2012;133(1):333–45 doi 10.1007/s10549-012-1955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rhoads KF, Patel MI, Ma Y, Schmidt LA. How do integrated health care systems address racial and ethnic disparities in colon cancer? J Clin Oncol 2015;33(8):854–60 doi 10.1200/JCO.2014.56.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gallups SF, Connolly MC, Bender CM, Rosenzweig MQ. Predictors of Adherence and Treatment Delays among African American Women Recommended to Receive Breast Cancer Chemotherapy. Women’s Health Issues 2018;28(6):553–8 doi 10.1016/j.whi.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Molina Y, Silva A, Rauscher GH. Racial/Ethnic Disparities in Time to a Breast Cancer Diagnosis. Med Care 2015;53(10):872–8 doi 10.1097/mlr.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wasif N, Etzioni D, Habermann EB, Mathur A, Pockaj BA, Gray RJ, et al. Racial and Socioeconomic Differences in the Use of High-Volume Commission on Cancer-Accredited Hospitals for Cancer Surgery in the United States. Annals of surgical oncology 2018;25(5):1116–25 doi 10.1245/s10434-018-6374-0. [DOI] [PubMed] [Google Scholar]
- 59.Keating NL, Kouri E, He Y, Weeks JC, Winer EP. Racial Differences in Definitive Breast Cancer Therapy in Older Women: Are They Explained by the Hospitals Where Patients Undergo Surgery? Med Care 2009;47(7):765–73. [DOI] [PubMed] [Google Scholar]
- 60.Patel MI, Ferguson JM, Castro E, Pereira-Estremera CD, Armaiz-Pena GN, Duron Y, et al. Racial and Ethnic Disparities in Cancer Care During the COVID-19 Pandemic. JAMA Netw Open 2022;5(7):e2222009 doi 10.1001/jamanetworkopen.2022.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beyer KM, Zhou Y, Matthews K, Bemanian A, Laud PW, Nattinger AB. New spatially continuous indices of redlining and racial bias in mortgage lending: links to survival after breast cancer diagnosis and implications for health disparities research. Health Place 2016;40:34–43 doi 10.1016/j.healthplace.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 62.White K, Borrell LN. Racial/ethnic residential segregation: framing the context of health risk and health disparities. Health Place 2011;17(2):438–48 doi 10.1016/j.healthplace.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Massey DS, Rothwell J, Domina T. The Changing Bases of Segregation in the United States. Ann Am Acad Polit Ss 2009;626:74–90 doi 10.1177/0002716209343558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams DR, Collins C. Racial residential segregation: A fundamental cause of racial disparities in health. Public Health Reports 2001;116(5):404–16 doi DOI 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwate NOA, Loh JM, White K, Saldana N. Retail Redlining in New York City: Racialized Access to Day-to-Day Retail Resources. Journal of Urban Health-Bulletin of the New York Academy of Medicine 2013;90(4):632–52 doi 10.1007/s11524-012-9725-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jessica Semega MK, Creamer John, and Mohanty Abinash. Income and Poverty in the United States: 2018. U.S. Census Bureau; 2019. P60–266 p. [Google Scholar]
- 67.Pichardo MS, Minas TZ, Pichardo CM, Bailey-Whyte M, Tang W, Dorsey TH, et al. Association of Neighborhood Deprivation With Prostate Cancer and Immune Markers in African American and European American Men. JAMA Netw Open 2023;6(1):e2251745 doi 10.1001/jamanetworkopen.2022.51745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Plascak JJ, Beyer K, Xu X, Stroup AM, Jacob G, Llanos AAM. Association Between Residence in Historically Redlined Districts Indicative of Structural Racism and Racial and Ethnic Disparities in Breast Cancer Outcomes. JAMA Netw Open 2022;5(7):e2220908 doi 10.1001/jamanetworkopen.2022.20908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sorice KA, Fang CY, Wiese D, Ortiz A, Chen Y, Henry KA, et al. Systematic review of neighborhood socioeconomic indices studied across the cancer control continuum. Cancer medicine 2022;11(10):2125–44 doi 10.1002/cam4.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. Journal of Environmental and Public Health 2017;2017:1–19 doi 10.1155/2017/2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health 2006;96(5):826–33 doi 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ellen IG, Mijanovich T, Dillman K-N. Neighborhood Effects on Health: Exploring the Links and Assessing the Evidence. Journal of Urban Affairs 2016;23(3–4):391–408 doi 10.1111/0735-2166.00096. [DOI] [Google Scholar]
- 73.McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci U S A 2012;109 Suppl 2:17180–5 doi 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peters A, McEwen BS. Introduction for the allostatic load special issue. Physiol Behav 2012;106(1):1–4 doi 10.1016/j.physbeh.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 75.McEwen BS. Stressed or stressed out: what is the difference? J Psychiatry Neurosci 2005;30(5):315–8. [PMC free article] [PubMed] [Google Scholar]
- 76.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 2014;10(8):455–65 doi 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giurgescu C, Nowak AL, Gillespie S, Nolan TS, Anderson CM, Ford JL, et al. Neighborhood Environment and DNA Methylation: Implications for Cardiovascular Disease Risk. Journal of Urban Health-Bulletin of the New York Academy of Medicine 2019;96:23–34 doi 10.1007/s11524-018-00341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gallagher EJ, Fei K, Feldman SM, Port E, Friedman NB, Boolbol SK, et al. Insulin resistance contributes to racial disparities in breast cancer prognosis in US women. Breast Cancer Research 2020;22(1) doi 10.1186/s13058-020-01281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khambaty T, Schneiderman N, Llabre MM, Elfassy T, Moncrieft AE, Daviglus M, et al. Elucidating the Multidimensionality of Socioeconomic Status in Relation to Metabolic Syndrome in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). International Journal of Behavioral Medicine 2020;27(2):188–99 doi 10.1007/s12529-020-09847-y.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saini G, Ogden A, McCullough LE, Torres M, Rida P, Aneja R. Disadvantaged neighborhoods and racial disparity in breast cancer outcomes: the biological link. Cancer Causes & Control 2019;30(7):677–86 doi 10.1007/s10552-019-01180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nutrition Weinhouse S. and cancer: cause and prevention. An American Cancer Society special report. CA Cancer J Clin 1984;34(2):121–6 doi 10.3322/canjclin.34.2.121. [DOI] [PubMed] [Google Scholar]
- 82.Rock CL, Thomson C, Gansler T, Gapstur SM, McCullough ML, Patel AV, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA: A Cancer Journal for Clinicians 2020;70(4):245–71 doi 10.3322/caac.21591. [DOI] [PubMed] [Google Scholar]
- 83.World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective-The Third Expert Report. 2018. [Google Scholar]
- 84.Wang L, Du M, Cudhea F, Griecci C, Michaud DS, Mozaffarian D, et al. Disparities in Health and Economic Burdens of Cancer Attributable to Suboptimal Diet in the United States, 2015–2018. Am J Public Health 2021;111(11):2008–18 doi 10.2105/ajph.2021.306475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Corral I, Landrine H, Hall MB, Bess JJ, Mills KR, Efird JT. Residential Segregation and Overweight/Obesity Among African-American Adults: A Critical Review. Front Public Health 2015;3:169 doi 10.3389/fpubh.2015.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corral I, Landrine H, Hao Y, Zhao L, Mellerson JL, Cooper DL. Residential segregation, health behavior and overweight/obesity among a national sample of African American adults. J Health Psychol 2012;17(3):371–8 doi 10.1177/1359105311417191. [DOI] [PubMed] [Google Scholar]
- 87.Corral I, Landrine H, Zhao L. Residential segregation and obesity among a national sample of Hispanic adults. J Health Psychol 2014;19(4):503–8 doi 10.1177/1359105312474912. [DOI] [PubMed] [Google Scholar]
- 88.Kwate NO. Fried chicken and fresh apples: racial segregation as a fundamental cause of fast food density in black neighborhoods. Health Place 2008;14(1):32–44 doi 10.1016/j.healthplace.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 89.Gouri Suresh SS, Schauder SA. Income Segregation and Access to Healthy Food. Am J Prev Med 2020;59(2):e31–e8 doi 10.1016/j.amepre.2020.02.009.. [DOI] [PubMed] [Google Scholar]
- 90.Gao Y, Hickson DA, Talegawkar S, Norwood AF, Tucker KL, Sims M, et al. Influence of individual life course and neighbourhood socioeconomic position on dietary intake in African Americans: the Jackson Heart Study. BMJ Open 2019;9(3):e025237 doi 10.1136/bmjopen-2018-025237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Camacho-Rivera M, Rosenbaum E, Yama C, Chambers E. Low-Income Housing Rental Assistance, Perceptions of Neighborhood Food Environment, and Dietary Patterns among Latino Adults: the AHOME Study. J Racial Ethn Health Disparities 2017;4(3):346–53 doi 10.1007/s40615-016-0234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Child ST, Kaczynski AT, Fair ML, Stowe EW, Hughey SM, Boeckermann L, et al. ‘We need a safe, walkable way to connect our sisters and brothers’: a qualitative study of opportunities and challenges for neighborhood-based physical activity among residents of low-income African-American communities. Ethn Health 2019;24(4):353–64 doi 10.1080/13557858.2017.1351923. [DOI] [PubMed] [Google Scholar]
- 93.Landrine H, Corral I, Lee JGL, Efird JT, Hall MB, Bess JJ. Residential Segregation and Racial Cancer Disparities: A Systematic Review. J Racial Ethn Health Disparities 2017;4(6):1195–205 doi 10.1007/s40615-016-0326-9. [DOI] [PubMed] [Google Scholar]
- 94.Pichardo MS, Esserman D, Ferrucci LM, Molina Y, Chlebowski RT, Pan K, et al. Adherence to the American Cancer Society Guidelines on nutrition and physical activity for cancer prevention and obesity-related cancer risk and mortality in Black and Latina Women’s Health Initiative participants. Cancer 2022;128(20):3630–40 doi 10.1002/cncr.34428. [DOI] [PubMed] [Google Scholar]
- 95.Pichardo MS, Irwin ML, Esserman D, Molina Y, Ferrucci LM. A competing risk analysis of adherence to the American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention and obesity-related cancer risk in Hispanic/Latino adults in the NIH-AARP Diet and Health Study. International journal of cancer 2022;151(11):1902–12 doi 10.1002/ijc.34200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pichardo MS, Pichardo CM, Talavera GA, Gallo LC, Castaneda SF, Sotres-Alvarez D, et al. Neighborhood segregation and cancer prevention guideline adherence in US Hispanic/Latino adults: Results from the HCHS/SOL. Front Oncol 2022;12:1024572 doi 10.3389/fonc.2022.1024572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu CY, Woo A, Hawkins C, Iman S. The Impacts of Residential Segregation on Obesity. J Phys Act Health 2018;15(11):834–9 doi 10.1123/jpah.2017-0352. [DOI] [PubMed] [Google Scholar]
- 98.Lopez R Black-white residential segregation and physical activity. Ethn Dis 2006;16(2):495–502. [PubMed] [Google Scholar]
- 99.Mellerson J, Landrine H, Hao Y, Corral I, Zhao L, Cooper DL. Residential segregation and exercise among a national sample of Hispanic adults. Health Place 2010;16(3):613–5 doi 10.1016/j.healthplace.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 100.Thomson CA, McCullough ML, Wertheim BC, Chlebowski RT, Martinez ME, Stefanick ML, et al. Nutrition and physical activity cancer prevention guidelines, cancer risk, and mortality in the women’s health initiative. Cancer prevention research (Philadelphia, Pa) 2014;7(1):42–53 doi 10.1158/1940-6207.CAPR-13-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nomura SJ, Dash C, Rosenberg L, Yu J, Palmer JR, Adams-Campbell LL. Is adherence to diet, physical activity, and body weight cancer prevention recommendations associated with colorectal cancer incidence in African American women? Cancer Causes Control 2016;27(7):869–79 doi 10.1007/s10552-016-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nomura SJ, Dash C, Rosenberg L, Yu J, Palmer JR, Adams-Campbell LL. Adherence to diet, physical activity and body weight recommendations and breast cancer incidence in the Black Women’s Health Study. International journal of cancer 2016;139(12):2738–52 doi 10.1002/ijc.30410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Onyeaghala G, Lintelmann AK, Joshu CE, Lutsey PL, Folsom AR, Robien K, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research cancer prevention guidelines and colorectal cancer incidence among African Americans and whites: The Atherosclerosis Risk in Communities study. Cancer 2019;126(5):1041–50 doi 10.1002/cncr.32616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Siega-Riz AM, Pace ND, Butera NM, Van Horn L, Daviglus ML, Harnack L, et al. How Well Do U.S. Hispanics Adhere to the Dietary Guidelines for Americans? Results from the Hispanic Community Health Study/Study of Latinos. Health Equity 2019;3(1):319–27 doi 10.1089/heq.2018.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vásquez PM, Durazo-Arvizu RA, Marquez DX, Argos M, Lamar M, Odoms-Young A, et al. Moderate-vigorous physical activity and health-related quality of life among Hispanic/Latino adults in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Journal of Patient-Reported Outcomes 2019;3(1) doi 10.1186/s41687-019-0129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arredondo EM, Sotres-Alvarez D, Stoutenberg M, Davis SM, Crespo NC, Carnethon MR, et al. Physical Activity Levels in U.S. Latino/Hispanic Adults: Results From the Hispanic Community Health Study/Study of Latinos. Am J Prev Med 2016;50(4):500–8 doi 10.1016/j.amepre.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Warren Andersen S, Blot WJ, Shu XO, Sonderman JS, Steinwandel MD, Hargreaves MK, et al. Adherence to Cancer Prevention Guidelines and Cancer Risk in Low-Income and African American Populations. Cancer Epidemiol Biomarkers Prev 2016;25(5):846–53 doi 10.1158/1055-9965.EPI-15-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McCullough ML, Chantaprasopsuk S, Islami F, Rees-Punia E, Um CY, Wang Y, et al. Association of Socioeconomic and Geographic Factors With Diet Quality in US Adults. JAMA Network Open 2022;5(6) doi 10.1001/jamanetworkopen.2022.16406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Slattery ML, Sweeney C, Edwards S, Herrick J, Murtaugh M, Baumgartner K, et al. Physical activity patterns and obesity in Hispanic and non-Hispanic white women. Med Sci Sports Exerc 2006;38(1):33–41. [DOI] [PubMed] [Google Scholar]
- 110.Rock CL, Thomson CA, Sullivan KR, Howe CL, Kushi LH, Caan BJ, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin 2022;72(3):230–62 doi 10.3322/caac.21719. [DOI] [PubMed] [Google Scholar]
- 111.Ligibel JA, Bohlke K, May AM, Clinton SK, Demark-Wahnefried W, Gilchrist SC, et al. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. Journal of Clinical Oncology 2022. doi 10.1200/jco.22.00687. [DOI] [PubMed] [Google Scholar]
- 112.Van Royen K, Verstraeten R, Andrade S, Ochoa-Avilés A, Donoso S, Maes L, et al. Factors Affecting Physical Activity in Ecuadorian Adolescents: A Focus Group Study. Journal of Physical Activity and Health 2015;12(3):340–8 doi 10.1123/jpah.2013-0288. [DOI] [PubMed] [Google Scholar]
- 113.Marquez DX, McAuley E. Social Cognitive Correlates of Leisure Time Physical Activity Among Latinos. Journal of Behavioral Medicine 2006;29(3):281–9 doi 10.1007/s10865-006-9055-6.. [DOI] [PubMed] [Google Scholar]
- 114.Larsen BA, Pekmezi D, Marquez B, Benitez TJ, Marcus BH. Physical Activity in Latinas: Social and Environmental Influences. Women’s Health 2013;9(2):201–10 doi 10.2217/whe.13.9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lynch BM, Owen N, Hawkes AL, Aitken JF. Perceived barriers to physical activity for colorectal cancer survivors. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 2010;18(6):729–34 doi 10.1007/s00520-009-0705-4. [DOI] [PubMed] [Google Scholar]
- 116.Ottenbacher AJ, Day RS, Taylor WC, Sharma SV, Sloane R, Snyder DC, et al. Exercise among breast and prostate cancer survivors—what are their barriers? Journal of Cancer Survivorship 2011;5(4):413–9 doi 10.1007/s11764-011-0184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spector D, Battaglini C, Groff D. Perceived exercise barriers and facilitators among ethnically diverse breast cancer survivors. Oncol Nurs Forum 2013;40(5):472–80 doi 10.1188/13.Onf.472-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oyekanmi G, Paxton RJ. Barriers to physical activity among African American breast cancer survivors. Psycho-oncology 2014;23(11):1314–7 doi 10.1002/pon.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Black KZ, Johnson L-S, Samuel-Hodge CD, Gupta L, Sundaresan A, Nicholson WK. Perceived barriers and preferred components for physical activity interventions in African-American survivors of breast or endometrial cancer with type 2 diabetes: the S.U.C.C.E.S.S. framework. Supportive Care in Cancer 2017;26(1):231–40 doi 10.1007/s00520-017-3839-9. [DOI] [PubMed] [Google Scholar]
- 120.Burse NR, Bhuiyan N, Mama SK, Schmitz KH. Physical activity barriers and resources among black women with a history of breast and endometrial cancer: a systematic review. Journal of Cancer Survivorship 2020. doi 10.1007/s11764-020-00873-z.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Williams F, Imm KR, Colditz GA, Housten AJ, Yang L, Gilbert KL, et al. Physician role in physical activity for African-American males undergoing radical prostatectomy for prostate cancer. Supportive Care in Cancer 2016;25(4):1151–8 doi 10.1007/s00520-016-3505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marquez DX, Aguinaga S, Campa J, Pinsker EC, Bustamante EE, Hernandez R. A Qualitative Exploration of Factors Associated With Walking and Physical Activity in Community-Dwelling Older Latino Adults. Journal of applied gerontology : the official journal of the Southern Gerontological Society 2016;35(6):664–77 doi 10.1177/0733464814533819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pichardo MS, Irwin ML, Sanft T, Ferrucci LM, Ginader A, Nguyen TH, et al. A qualitative study identifying challenges resulting from complex evidence on lifestyle factors and cancer: perspectives from Black and Latina cancer survivors and healthcare providers. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 2023;31(2):111 doi 10.1007/s00520-022-07539-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stolley MR, Sharp LK, Wells AM, Simon N, Schiffer L. Health Behaviors and Breast Cancer: Experiences of Urban African American Women. Health Educ Behav 2006;33(5):604–24 doi 10.1177/1090198106290845. [DOI] [PubMed] [Google Scholar]
- 125.Er V, Lane JA, Martin RM, Persad R, Chinegwundoh F, Njoku V, et al. Barriers and facilitators to healthy lifestyle and acceptability of a dietary and physical activity intervention among African Caribbean prostate cancer survivors in the UK: a qualitative study. BMJ Open 2017;7(10) doi 10.1136/bmjopen-2017-017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Husain M, Nolan TS, Foy K, Reinbolt R, Grenade C, Lustberg M. An overview of the unique challenges facing African-American breast cancer survivors. Supportive Care in Cancer 2018;27(3):729–43 doi 10.1007/s00520-018-4545-y. [DOI] [PubMed] [Google Scholar]
- 127.Ashing-Giwa K, Tapp C, Brown S, Fulcher G, Smith J, Mitchell E, et al. Are survivorship care plans responsive to African-American breast cancer survivors?: voices of survivors and advocates. Journal of Cancer Survivorship 2013;7(3):283–91 doi 10.1007/s11764-013-0270-1. [DOI] [PubMed] [Google Scholar]
- 128.Byrd DA, Agurs-Collins T, Berrigan D, Lee R, Thompson FE. Racial and Ethnic Differences in Dietary Intake, Physical Activity, and Body Mass Index (BMI) Among Cancer Survivors: 2005 and 2010 National Health Interview Surveys (NHIS). J Racial Ethn Health Disparities 2017;4(6):1138–46 doi 10.1007/s40615-016-0319-8. [DOI] [PubMed] [Google Scholar]
- 129.Springfield S, Odoms-Young A, Tussing-Humphreys L, Freels S, Stolley M. Adherence to American Cancer Society and American Institute of Cancer Research dietary guidelines in overweight African American breast cancer survivors. J Cancer Surviv 2019;13(2):257–68 doi 10.1007/s11764-019-00748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Palacios C, Daniel CR, Tirado-Gomez M, Gonzalez-Mercado V, Vallejo L, Lozada J, et al. Dietary Patterns in Puerto Rican and Mexican-American Breast Cancer Survivors: A Pilot Study. J Immigr Minor Health 2017;19(2):341–8 doi 10.1007/s10903-016-0398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kohler LN, Garcia DO, Harris RB, Oren E, Roe DJ, Jacobs ET. Adherence to Diet and Physical Activity Cancer Prevention Guidelines and Cancer Outcomes: A Systematic Review. Cancer Epidemiol Biomarkers Prev 2016;25(7):1018–28 doi 10.1158/1055-9965.EPI-16-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kabat GC, Matthews CE, Kamensky V, Hollenbeck AR, Rohan TE. Adherence to cancer prevention guidelines and cancer incidence, cancer mortality, and total mortality: a prospective cohort study. Am J Clin Nutr 2015;101(3):558–69 doi 10.3945/ajcn.114.094854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pichardo MS, Sanft T, Ferrucci LM, Romero-Ramos YM, Cartmel B, Harrigan M, et al. Diet and physical activity interventions in Black and Latina women with breast cancer: A scoping review. Front Oncol 2023;13(1079293):1079293 doi 10.3389/fonc.2023.1079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Coughlin SS, Smith SA. Community-Based Participatory Research to Promote Healthy Diet and Nutrition and Prevent and Control Obesity Among African-Americans: a Literature Review. J Racial Ethn Health Disparities 2017;4(2):259–68 doi 10.1007/s40615-016-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fitzgibbon ML, Tussing-Humphreys LM, Porter JS, Martin IK, Odoms-Young A, Sharp LK. Weight loss and African-American women: a systematic review of the behavioural weight loss intervention literature. Obes Rev 2012;13(3):193–213 doi 10.1111/j.1467-789X.2011.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Smith SA, Ansa B. A systematic review of lifestyle interventions for chronic diseases in rural communities. Journal of the Georgia Public Health Association 2016;5(4):304–13 doi 10.21663/jgpha.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Osei-Assibey G, Kyrou I, Adi Y, Kumar S, Matyka K. Dietary and lifestyle interventions for weight management in adults from minority ethnic/non-White groups: a systematic review. Obes Rev 2010;11(11):769–76 doi 10.1111/j.1467-789X.2009.00695.x. [DOI] [PubMed] [Google Scholar]
- 138.McCurley JL, Gutierrez AP, Gallo LC. Diabetes Prevention in U.S. Hispanic Adults: A Systematic Review of Culturally Tailored Interventions. Am J Prev Med 2017;52(4):519–29 doi 10.1016/j.amepre.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wadi NM, Asantewa-Ampaduh S, Rivas C, Goff LM. Culturally tailored lifestyle interventions for the prevention and management of type 2 diabetes in adults of Black African ancestry: a systematic review of tailoring methods and their effectiveness. Public Health Nutr 2022;25(2):422–36 doi 10.1017/s1368980021003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang J, Xia Y, Sun Y, Guo Y, Shi Z, Cristina do Vale Moreira N, et al. Effect of lifestyle intervention on HbA1c levels in overweight and obese adults with type 2 diabetes across ethnicities: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2023;199:110662 doi 10.1016/j.diabres.2023.110662. [DOI] [PubMed] [Google Scholar]
- 141.LeBlanc EL, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: An Updated Systematic Review for the US Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018. [PubMed] [Google Scholar]
- 142.Enyioha C, Hall M, Voisin C, Jonas D. Effectiveness of Mobile Phone and Web-Based Interventions for Diabetes and Obesity Among African American and Hispanic Adults in the United States: Systematic Review. JMIR Public Health Surveill 2022;8(2):e25890 doi 10.2196/25890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Williamson DF, Vinicor F, Bowman BA. Primary prevention of type 2 diabetes mellitus by lifestyle intervention: implications for health policy. Ann Intern Med 2004;140(11):951–7 doi 10.7326/0003-4819-140-11-200406010-00036. [DOI] [PubMed] [Google Scholar]
- 144.Venditti EM. Behavioral lifestyle interventions for the primary prevention of type 2 diabetes and translation to Hispanic/Latino communities in the United States and Mexico. Nutr Rev 2017;75(suppl 1):85–93 doi 10.1093/nutrit/nuw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Anderson C, Harrigan M, George SM, Ferrucci LM, Sanft T, Irwin ML, et al. Changes in diet quality in a randomized weight loss trial in breast cancer survivors: the lifestyle, exercise, and nutrition (LEAN) study. NPJ Breast Cancer 2016;2:16026 doi 10.1038/npjbcancer.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Y, et al. Randomized Trial Comparing Telephone Versus In-Person Weight Loss Counseling on Body Composition and Circulating Biomarkers in Women Treated for Breast Cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. J Clin Oncol 2016;34(7):669–76 doi 10.1200/JCO.2015.61.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chlebowski RT, Blackburn GL, Hoy MK, Thomson CA, Giuliano AE, McAndrew P, et al. Survival analyses from the Women’s Intervention Nutrition Study (WINS) evaluating dietary fat reduction and breast cancer outcome. Journal of Clinical Oncology 2008;26(15_suppl):522– doi 10.1200/jco.2008.26.15_suppl.522. [DOI] [Google Scholar]
- 148.Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 2007;298(3):289–98 doi 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pichardo MS, Irwin ML, Sanft T, Ferrucci LM, Ginader A, Nguyen TH, et al. A qualitative study identifying challenges resulting from complex evidence on lifestyle factors and cancer: perspectives from Black and Latina cancer survivors and healthcare providers. Supportive Care in Cancer 2023;31(2) doi 10.1007/s00520-022-07539-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kershaw KN, Pender AE. Racial/Ethnic Residential Segregation, Obesity, and Diabetes Mellitus. Curr Diab Rep 2016;16(11):108–27 doi 10.1007/s11892-016-0800-0. [DOI] [PubMed] [Google Scholar]
- 151.Schnake-Mahl AS, Jahn JL, Subramanian SV, Waters MC, Arcaya M. Gentrification, Neighborhood Change, and Population Health: a Systematic Review. Journal of urban health : bulletin of the New York Academy of Medicine 2020;97(1):1–25 doi 10.1007/s11524-019-00400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alcaraz KI, Wiedt TL, Daniels EC, Yabroff KR, Guerra CE, Wender RC. Understanding and addressing social determinants to advance cancer health equity in the United States: A blueprint for practice, research, and policy. CA Cancer J Clin 2020;70(1):31–46 doi 10.3322/caac.21586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data were created or analyzed in this study.


