Abstract
Maintaining balance involves the combination of sensory signals from the visual, vestibular, proprioceptive, and auditory systems. However, physical and biological constraints ensure that these signals are perceived slightly asynchronously. The brain only recognizes them as simultaneous when they occur within a period of time called the temporal binding window (TBW). Aging can prolong the TBW, leading to temporal uncertainty during multisensory integration. This effect might contribute to imbalance in the elderly but has not been examined with respect to vestibular inputs. Here, we compared the vestibular-related TBW in 13 younger and 12 older subjects undergoing 0.5 Hz sinusoidal rotations about the earth-vertical axis. An alternating dichotic auditory stimulus was presented at the same frequency but with the phase varied to determine the temporal range over which the two stimuli were perceived as simultaneous at least 75% of the time, defined as the TBW. The mean TBW among younger subjects was 286 ms (SEM ± 56 ms) and among older subjects was 560 ms (SEM ± 52 ms). TBW was related to vestibular sensitivity among younger but not older subjects, suggesting that a prolonged TBW could be a mechanism for imbalance in the elderly person independent of changes in peripheral vestibular function.
Keywords: motion perception, reaction time, rotation, time factors, proprioception/physiology, sensory thresholds/physiology, vestibule, labyrinth/physiology
Maintaining balance requires the appropriate integration of multiple inputs including those from the vestibular, visual, and proprioceptive systems. 1 2 Multisensory integration improves perceptual performance, but requires the brain to merge related stimuli while avoiding the combination of unrelated inputs. 3 This process is complicated by the fact that sensory signals arising from the same event are usually perceived slightly asynchronously due to physical constraints (such as the speed of sound vs. light) and biological delays (such as processing time in the auditory vs. visual systems). The brain must therefore allow a period of time over which related inputs are interpreted and integrated as a single percept, while those outside the window are interpreted as belonging to unrelated events. The duration of this period is known as the temporal binding window (TBW). 4 5
A widened TBW has been associated with conditions such as autism, 6 7 8 dyslexia, 9 10 schizophrenia, 11 attention deficit hyperactivity disorder, 12 and even obesity. 13 It is also related to decreased performance among normal subjects in everyday situations. For example, a wider audiovisual TBW has been correlated with decreased performance in verbal and nonverbal problem-solving tasks. 14 By analogy, a widened TBW associated with balance-related inputs might lead to postural and gait instability.
Aging is associated with a widening of the TBW as determined using pairings of non-vestibular sensory modalities including auditory + visual, tactile + auditory, and visual + tactile stimuli. 15 16 17 18 19 Interestingly, some evidence indicates that older adults with a history of falling may have a prolonged TBW. 17 20 21 While this is generally assumed to result from changes in central processing, age-related sensory loss may also contribute to a widened TBW as shown in a study of auditory + visual stimuli. 19 Given these findings, and the important role of the vestibular system in maintaining balance, it is surprising that the temporal integration of balance-related stimuli in older people remains unexplored. Understanding the role that changes in the temporal perception of vestibular input play in the high incidence, morbidity, and mortality of falls in the elderly may allow for the development of new diagnostic and therapeutic measures to aid in fall prevention.
As a first step toward understanding the changes in the TBW of the vestibular system and its relation to aging and imbalance, we determined the TBW of vestibular + auditory cues in a healthy cohort of younger and older adults. We chose to use auditory cues because of our experience using them in related experiments, 22 23 recent findings that auditory cues may contribute to maintaining balance (see Lubetzky et al for review), 24 and some evidence that they may be optimally integrated with vestibular stimuli. 25 26 27 28 We compared these results to the peripheral vestibular sensitivity in each group and examined whether differences in TBW observed in the elderly population might correlate with their performance on a standard test of gait performance, the Timed Up and Go (TUG) test. 25
METHODS
Participants
This study was approved by the Washington University in St. Louis Human Studies Committee. Consent in accordance with the Declaration of Helsinki was obtained from all subjects. The subjects received compensation for their participation. The younger subjects (20–30 years of age) were recruited through convenience sampling, with most being undergraduate and graduate students affiliated with Washington University in St. Louis. All reported normal hearing and no balance disorders. One additional young subject, whose data were used to quantify the contribution of proprioception to rotational sensitivity and were not included in the experimental data collected from other subjects, had a history of gentamicin exposure with no detectable vestibular responses as measured via cervical vestibular evoked myogenic potentials, rotational chair testing, and caloric irrigations.
Older subjects (60 years and older) were recruited through the Washington University in St. Louis Psychology Department's Aging and Development Volunteer Pool. A history of auditory or vestibular disease, falling, or the use of medications known to affect balance function were used as exclusion criteria. Auditory thresholds were determined from 250 to 8,000 Hz using a calibrated audiometer (Model 10D; Beltone, Glenview, IL). All older subjects demonstrated normal cognition on the Short Blessed Test. 29
Experimental Procedure
Subjects were comfortably and securely seated in a custom-designed rotational chair consisting of a race car seat and four-point harness mounted on a motor (Kollmorgen Goldstar DDR D063M7; Danaher Motion, Radford, VA) rotating about an earth-vertical axis. The chair was padded to reduce vibratory input through the motor, and blocks of firm padding were placed between the knees as well as between the walls of the seat and the hips and shoulders to reduce motion of the body and possible related proprioceptive inputs. The head was strapped to a headrest with Reid's plane oriented 20 degrees nose-down to bring the horizontal semicircular canals into the plane of rotation. Testing occurred in a darkened booth with subjects blindfolded to prevent visual input. Rotational and auditory stimuli were generated using custom-written software in LabVIEW (v10; National Instruments, Austin, TX).
The TBW is usually measured by providing two instantaneous suprathreshold stimuli such as a flash and beep. 5 This is not possible when measuring TBWs involving vestibular stimuli, as motion cannot be applied suddenly without powerful equipment, risk of injury to experimental subjects, and the likelihood that proprioceptive or other sensory inputs will become significant enough to confound interpretation of the data. If slower-onset vestibular stimuli are used to avoid these problems, however, measurement of the TBW is affected by the elapsed time between the onset of movement and when it exceeds the detection threshold 22 23 which is approximately 1 to 2 degree/s at the rotational frequency of 0.5 Hz.
Given the limitations on providing abrupt vestibular stimuli, we employed a slower, repetitive stimulus instead. The chair was continuously rotated about an earth-vertical axis along a sinusoidal trajectory at a frequency of 0.5 Hz at a peak velocity of 12 degree/s An auditory stimulus (5 ms white noise burst, 80 dB A SPL) was delivered through sound-dampening headphones (frequency response: 10–20,000 Hz; MDR-7506, Sony, Japan) once per second. The auditory stimulus alternated dichotically, so that the left ear was presented with a sound when the subject was facing more toward the right and the right ear when the subject was facing more toward the left ( Fig. 1 ). Prior to the beginning of testing, each subject was asked to verify that the auditory stimulus and chair rotations were clearly perceptible to ensure that the stimuli were suprathreshold. Machine sound from the rotating chair was slight, and a minimal effect was ensured by the sound-dampening headphones and its very poor temporal resolution provided by its slow variability over the course of the rotation.
Figure 1.
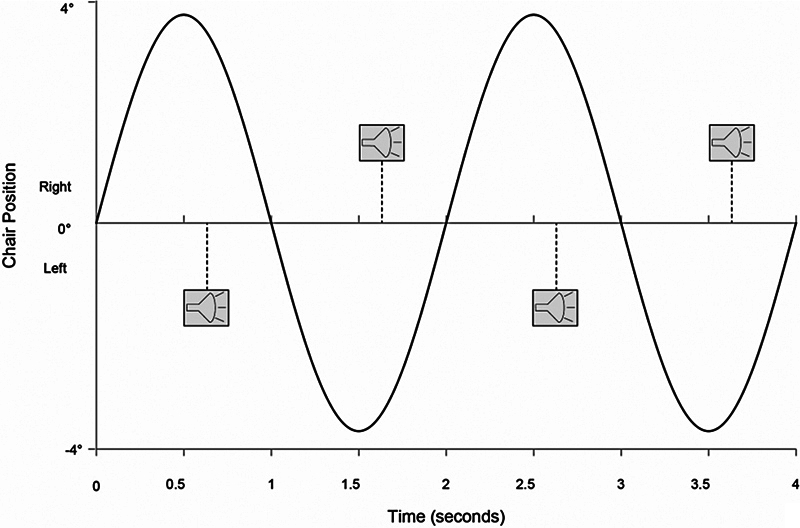
Stimulus paradigm. Sinusoidal line represents the chair's position (negative values represent position to the left of center, positive values to the right). Dashed vertical lines represent auditory stimuli presented dichotically, with symbols above the horizontal line indicating sound presented in the right ear and those below the line indicating sounds presented in the left ear. In this example, the auditory stimulus occurs at a stimulus onset asynchrony of +125 ms following the time the chair's position reaches its extremes.
The stimuli were defined as synchronous when, at the moment the chair was at its maximal leftward position (where rotational velocity was 0 degree/s), the auditory stimulus was perceived to occur in the right ear, and when at the moment the chair was at its maximal rightward position, the auditory stimulus was perceived in the left ear. This was designed to imitate, at least abstractly, a stationary beeping sound source placed directly in front of the observer when the chair was at neutral position. Stimulus onset asynchrony (SOA) was defined as the difference in time between when the chair reached its maximal position and when the auditory stimulus was presented. The SOA was denoted as a negative value when the auditory stimulus preceded the chair reaching its maximal point of rotation and positive when the auditory stimulus occurred following the chair reaching its maximal point of rotation.
Each subject's TBW was determined through a series of 180 trials using 21 SOAs over the range of −695 to 639 ms. The intervals between the first three and last three SOAs were 111 ms, and between each of the other SOAs was 56 ms. The first three and last three SOAs were tested five times, and the others were tested ten times. This allowed a detailed impression to be gained of the subject's response in the most relevant areas while minimizing the time required for completing the experiment. The range of SOAs to be tested was determined from the results of preliminary testing of the equipment in healthy individuals. The relative timing of the vestibular and auditory stimuli was verified to be accurate to within 5 ms using Spike2 data logging equipment (CED, Cambridge, UK).
Data were collected in a single-interval, two-alternative forced-choice simultaneity judgment (SJ) task using a method of constant stimuli. A maximum of 20 rotational cycles were provided in each trial. The number of repetitions varied among subjects, with older subjects typically using between 5 and 15 and younger subjects between 5 and 10. When a subject arrived at a conclusion, he or she reported whether the stimuli seemed “synchronous” or “not synchronous,” with subjects allowed to terminate each trial once they were confident of their response. While testing was in progress, the chair was constantly rotating back and forth, with phase shifts occurring to the auditory stimulus following each subject response. There was no period between phase shifts when only the vestibular stimulus was delivered.
Prior to testing the subjects were given thorough instructions of the task and what constituted stimuli that were synchronous versus asynchronous. Comprehension of the task was further verified in the rotational chair. This was accomplished by exposing subjects to several SOAs (0, 500, and 1,000 ms) that were almost universally perceived as synchronous and asynchronous and then asking subjects to determine whether the auditory and vestibular stimuli were synchronous. Feedback was given during this portion and all subjects were able to distinguish between synchronous and asynchronous stimuli prior to beginning testing. During testing, feedback was not given to the subjects concerning the accuracy of their responses. Subjects were given frequent breaks during testing. During breaks, the chair was stopped completely. Prior to resuming testing, the subjects were reminded of what constituted a properly aligned stimulus pair.
Unisensory Vestibular Thresholds
To assess the impact of unisensory vestibular perceptual performance on the TBW, the detection threshold and discrimination threshold (just-noticeable difference for a 60-degree/s reference stimulus) were determined with a previously described procedure. 30 Briefly, thresholds were found using a three-down one-up, two-interval, forced choice staircase task. The task consisted of a reference vestibular stimulus and a test vestibular stimulus. The reference stimulus was no-movement for determining the detection threshold and 60 degree/s at 0.5 Hz for determining the discrimination threshold. The test vestibular stimulus for both conditions was always at a higher rotational velocity than the reference vestibular stimulus (both at 0.5 Hz). Subjects reported in which of the two intervals they perceived that they were moving most rapidly. These intervals were indicated by two separate auditory tones delivered diotically through headphones. Each vestibular stimulus interval lasted 5 seconds with a 1-second ramp up or down in between the two stimuli. The order of the test and reference stimulus varied randomly. A total of 11 reversals were required prior to the termination of the staircase task, with the average of the last 6 reversals used to determine each subject's 79% accuracy threshold. Eight younger and seven older participants had their detection thresholds measured; six younger and seven older participants had their discrimination thresholds measured.
Data Analysis
The fraction of synchronous responses from the continuous vestibular + auditory paradigm was plotted at all SOAs tested for each subject. These were graphed as a psychometric curve and the total area under the curve was calculated using the trapezoidal rule. The SOA that bisected the area under the curve into two equal halves was used to separate the psychometric curve into two distinct portions. These were each fit independently using a cumulative binomial distribution ( glmfit binomial logit function, MATLAB r2010a; The Mathworks Inc, Natick, MA) and analyzed using a method similar to other studies. 31 32 The resulting sigmoidal curves were used to determine the SOA for each curve at which the subjects had a 75% probability of reporting that the stimuli were synchronous. The TBW was defined as the elapsed time between these two SOAs. In cases where a 100% simultaneity response was not achieved for any of the SOAs tested, the 75% of the maximum fraction reported as synchronous at any of the SOAs tested was used.
In addition to the TBW, another important measure of timing in studies of multisensory integration is the PSS, or “point of subjective simultaneity.” This is defined as the temporal offset of presentation for two stimuli where subjects are most likely to judge them as simultaneous. For example, typically auditory + visual stimulus pairs require the auditory source to be presented after the visual in order for the two to seem simultaneous. 33 34 35 36 37 This may be due to relative processing times in each stimulus modality (e.g., the visual system is known to process information more slowly than the auditory system). 5 Typically, the PSS is measured at the peak of the psychometric curve used for determining the TBW. Here, this was not feasible because the psychometric curves were often not Gaussian. Instead, we defined the PSS as the midpoint between the SOAs defining the limits of the TBW. In cases where the curve was Gaussian, this would be equivalent to the peak of the curve.
Balance Testing
The TUG test was used as a dynamic task to detect balance deficits among older subjects. 25 38 39 The test begins with the subject seated in a chair, rising and walking 3 m, then walking back to the chair and sitting. An elapsed time of 13.5 seconds is commonly used as a threshold for discriminating between people with an increased risk of falling and those without. 25
All data analysis was performed using SPSS 19.0 (IBM). A χ 2 test was used to assess differences in gender and hand preference between the groups. Continuous data were evaluated using the Mann–Whitney U -test. Correlations were evaluated using Pearson's r .
RESULTS
Subjects included 13 younger (average age = 23.8, age range = 21–26, 6 males) and 12 older (average age = 69.7, age range = 63–89, 7 males) participants. There was no significant difference in gender or handedness between older and younger subjects ( χ 2 = 0.371, p = 0.543; χ 2 = 2.564, p = 0.109, respectively). All older subjects scored within the normal range for the Short Blessed Test. The pure-tone average from audiometric testing of older subjects was 24 ± 4 dB (mean ± SEM). No experimental subjects reported difficulty sensing either the vestibular or auditory stimulus. The control subject with no measurable horizontal canal response reported that he could not detect any movement during testing. He was unable to perceive any asynchronous responses at any of the SOA tested indicating that proprioceptive cues, such as those related to motor vibration, were unlikely to be major factors in the data collected in this paradigm.
The psychometric curves of all subjects are shown in Fig. 2 (A, B) . Few of the curves approximated the roughly normal distribution commonly observed in psychophysical experiments. The younger subjects in general had narrower curves indicating that they felt the two stimuli were synchronous over a limited range of SOAs. The older subjects had wider curves indicating they were confident that the stimuli were synchronous over a much wider range. In both groups, some subjects did not perceive the stimuli to be synchronous for all trials at any of the SOAs tested.
Figure 2.
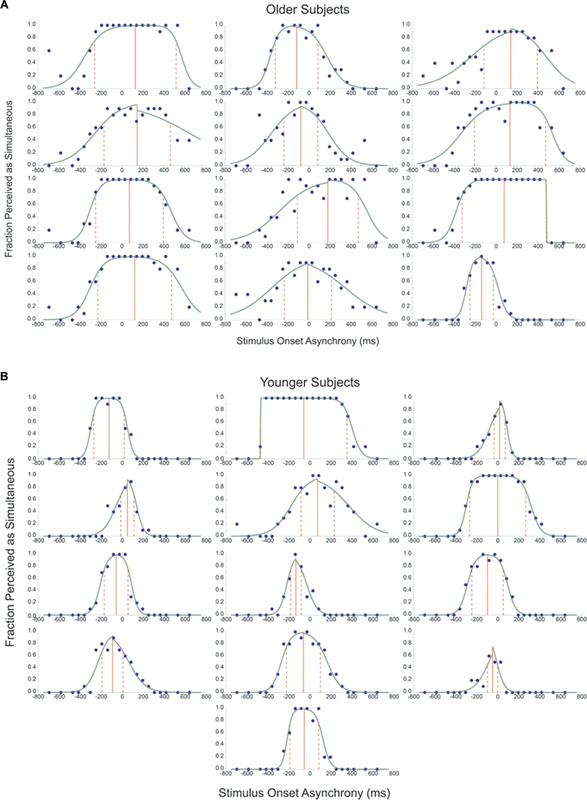
Psychometric curves from older ( A ) and younger ( B ) subjects. The x -axis represents the stimulus onset asynchrony in milliseconds, and the y -axis represents the proportion of times the stimuli were perceived as simultaneous. The green lines represent the psychometric curves and the closed circles represent each subject's actual response. The temporal binding window is contained between the set of red dashed lines, with the solid red line representing the point of subjective simultaneity.
Younger subjects had a TBW width of 286 ± 56 ms (mean ± SEM) with a median of 274 ms, and older subjects had a TBW width of 560 ± 52 ms with a median of 606 ms. The difference in width between the TBW of the younger and older groups was significant ( p = 0.002). A significant age-related difference was also found for the PSS ( p = 0.030). Older subjects had a PSS of 54 ± 32 ms (with a median of 99 ms) and younger subjects had a PSS of −46 ± 18 ms (with a median of −58 ms), indicating that older people were most likely to feel like the stimuli were synchronous when the auditory stimulus occurred slightly after the maximal rotation, whereas younger people reported the opposite. The TBW and PSS for the group as a whole showed a positive correlation (Pearson's r = 0.609, p = 0.001). Pure-tone average auditory thresholds did not correlate significantly with the TBW (Pearson's r = 0.085, p = 0.793) or PSS (Pearson's r = 0.1, p = 0.758) among older subjects.
Five younger and three older adults participated in three additional testing sessions each to define test–retest reliability and determine if any learning effect took place while undergoing multiple tests ( Fig. 3 ). These participants included an older subject with one of the narrowest TBWs in their cohort and a younger subject with one of the widest TBWs in their cohort. The TBW and PSS showed strong reliability, with neither evidence of a learning effect nor significant variability among sessions (Cronbach's α = 0.982 and 0.946, respectively; Fig. 4 ).
Figure 3.
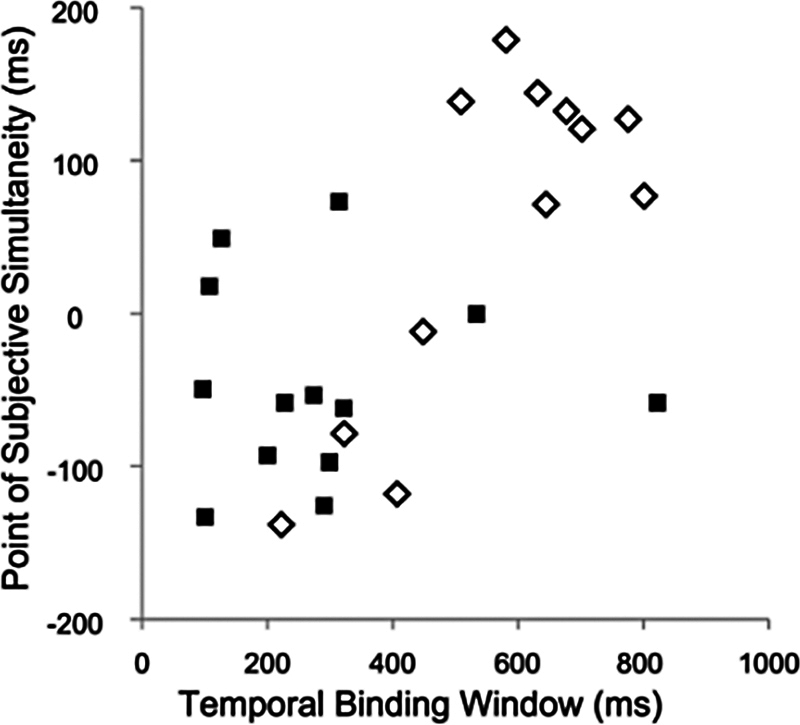
Relationship of temporal binding window to the point of subjective simultaneity. Older subjects represented by open diamonds, younger subjects by closed squares.
Figure 4.
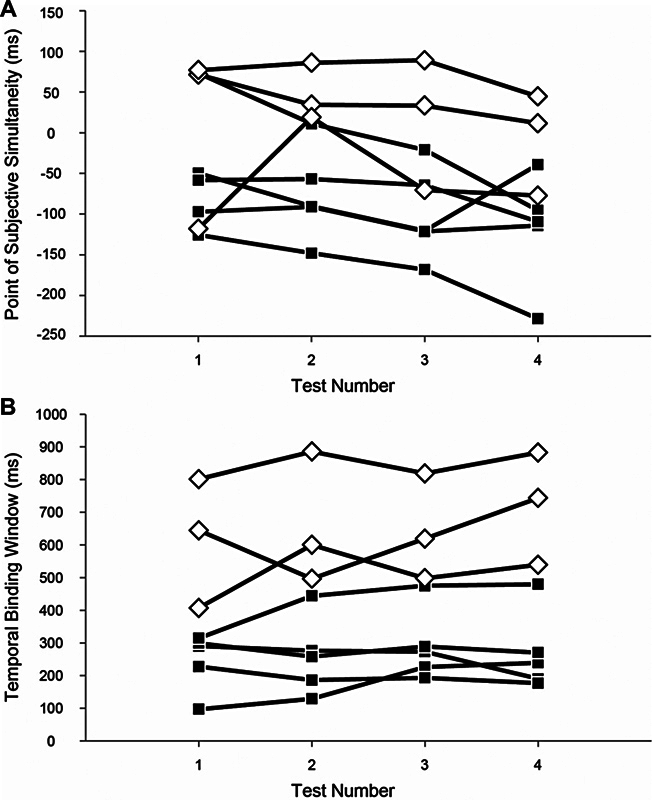
Reliability analysis. The point of subjective simultaneity ( A ) and the temporal binding window ( B ) of younger (closed squares) and older (open diamonds) subjects. Time intervals of ∼4 days between testing sessions.
We considered the possibility that the widened TBW in older adults was due to elevated vestibular perceptual thresholds. To evaluate that possibility, the vestibular detection threshold and just noticeable difference were measured in a subset of older and younger subjects. In younger subjects, the mean ( ± SEM) detection threshold was 0.77 ( ± 0.14) degree/s and the average just noticeable difference was 4.3 ( ± 1.2) degree/s. In the older subjects, the average detection threshold was 0.67 ( ± 0.09) degree/s and the average just noticeable difference was 5.8 ( ± 0.8) degree/s. There was no statistically significant difference between these groups for detection threshold ( U = 26, p = 0.867) or just noticeable difference ( U = 11, p = 0.181).
Detection thresholds showed no significant correlation with TBW (Pearson's r = 0.328, p = 0.233) or PSS (Pearson's r = 0.169, p = 0.547) in the group as a whole. Similarly, discrimination (just noticeable difference) thresholds showed no significant correlation with the TBW (Pearson's r = −0.244, p = 0.422) or PSS (Pearson's r = −0.338, p = 0.259) overall. When the younger subjects were analyzed separately, the relationship between the detection threshold and TBW approached significance (Pearson's r = 0.676, p = 0.066). This was mainly due to a shift of the rightmost edge of the TBW (the SOA where the psychometric curve crossed the 75% threshold). This showed a significant correlation with the detection threshold in younger subjects only (Pearson's r = 0.835, p = 0.01). Linear regression was performed to determine the extent by which differences in the TBW of younger subjects could be explained by altered thresholds. Using this method, a subject with 1 degree/s greater detection threshold would be expected to display a shift of the rightmost SOA of their TBW by 314 ms (95% CI: 107–521 ms) with their overall TBW increasing by 415 ms (95% CI: −37 to 867 ms). The leftmost SOA of the TBW did not approach significance (95% CI: −427 to 225 ms). The discrimination and detection thresholds showed no significant correlation in the group as a whole (Pearson's r = − 0.616, p = 0.193).
PTA thresholds did not correlate significantly with the TBW (Pearson's r = 0.085, p = 0.793) or PSS (Pearson's r = 0.1, p = 0.758). No correlation between hearing threshold and vestibular detection threshold (Pearson's r = 0.537, p = 0.214) or discrimination (just noticeable difference) thresholds (Pearson's r = 0.545, p = 0.205) was found.
The older subjects had a mean ( ± SD) TUG time of 10.9 ( ± 1.7) seconds overall with only one subject having a score of over 13.5 seconds. The results of the TUG test did not have a significant correlation with the TBW, PSS, detection threshold, or discrimination threshold (for all combinations, Pearson's r ranged from −0.246 to 0.381 and p ranged from 0.222 to 0.966).
The control subject with no measurable horizontal canal response reported that he could not detect any movement during testing. He was unable to perceive any asynchronous responses at any of the SOA tested indicating that proprioceptive cues, such as those related to motor vibration, were unlikely to be major factors in the data collected in this paradigm.
DISCUSSION
The results presented here indicate that healthy older individuals have a significantly wider vestibular + auditory TBW than younger adults and show that the perception of vestibular stimuli relative to auditory stimuli is delayed in older compared with younger adults. The decreased ability to distinguish between temporally discrete vestibular + auditory inputs among the elderly has implications for understanding mechanisms of sensory integration and emphasizes the possibility that a widened TBW may be a mechanism for imbalance in the elderly.
A few previous studies using other stimulus pairings have found that aging widens the TBW and changes the PSS, as seen here. 15 16 17 40 For example, Poliakoff et al (2006) found in an experiment investigating visual + tactile stimulus pairs that the TBW went from ∼200 to 300 ms. They also reported that the PSS changed by ∼40 ms. 16 These changes are significantly less than we found here, both in absolute time as well as percentages. Further investigation will be required to determine why the effect of aging on the temporal relationships of stimulus pairs involving vestibular stimuli are relatively greater than pairings of other types of stimuli.
Mechanisms
One possible reason for changes in the TBW and PSS seen with aging is slower processing speed in older adults relative to younger people. This slowing is a generally accepted principle associated with aging 41 and has been implicated as the reason that reaction times tend to be longer in older people than in younger controls. 42 43 This explanation would suggest that the PSS is determined at least in part by a subject's reaction time to each stimulus. While presumably processing speed in both auditory and vestibular channels was lengthened with aging, the PSS was greater among older adults (i.e., the vestibular signal needed to be presented earlier with respect to the auditory stimulus in older vs. younger adults). This seems to indicate that age-related delays affect the two sensory channels unequally, with vestibular perception being slowed more than auditory perception.
Reaction times and PSS measure the difference between when a stimulus occurs and when it is perceived (with reaction times also being influenced by the duration of the motor processes involved in indicating a response). In contrast, TBW can be thought to quantify the precision of that measurement. It is possible that the widened TBW in our older subjects might be related to changes in processing speed (and PSS, as stated earlier) rather than an independent effect. For example, as the reaction time increases, its standard deviation could naturally increase (maintaining a constant coefficient of variation) leading to greater uncertainty and a wider TBW. Some data from a study of auditory reaction times in subjects concurrently undergoing vestibular stimulation may support this possibility. In that article, the standard deviation of reaction times among older people performing a complicated auditory reaction time test was higher than for younger people. 44
In addition to considering processing speed in auditory and vestibular circuits separately, parameters governing the circuits responsible for integrating the two stimuli must also be considered in interpreting our results. This is not necessarily a simple task, as these circuits may form complicated interactions among different sets of stimuli. For example, vestibular and visual input may be mutually inhibitory, rather than simply combining to form a unified percept. 45 Some evidence suggests that these circuits change with age. In animals, the number of neurons sensitive to multisensory inputs and the ability to integrate temporally discrete stimuli increases with maturation. 46 N-methyl-D-aspartate (NMDA) receptors have been implicated in both neurocellular plasticity and multisensory integration 47 48 and the level of NMDA receptors have been shown to decrease with aging and could provide another mechanism for changes in multisensory integration seen in aging. 49 GABA has also been shown to block multimodal integration while displaying no effect on unisensory perception. 50 Current evidence indicates that the levels of GABA change minimally with aging, which combined with a decrease in NMDA receptors, could be a contributing factor to the changes in sensory integration observed with aging. 51
Theoretically, poorer vestibular thresholds make it difficult to determine whether a stimulus is occurring, which of course also makes it difficult to know when it occurred. Indeed, we found that detection thresholds were correlated to the TBW in young subjects here as had been reported in a different population of patients previously. 52 Among the older subjects, however, no association was observed. One possible explanation is that factors associated with aging widen the TBW sufficiently to obscure the smaller effect of peripheral sensitivity seen in younger people. 53 Although none of the subjects in this study demonstrated symptoms of vestibular dysfunction, characterization of horizontal canal responses in subjects of future studies may help clarify this point.
Implications for Imbalance
Is the prolonged vestibular + auditory TBW observed in older people related to balance function? After all, older adults tend to benefit more from the integration of multiple sensory inputs than younger adults do, while at the same time they show a greater susceptibility to interference from irrelevant stimuli and impaired reweighting of sensory inputs. 2 16 54 In line with this thinking, previous work by others indicated that a prolonged TBW involving auditory + visual stimuli correlates with an increased chance of falling. 17 Despite the increased TBW in older adults, however, we did not observe abnormal results on the TUG tests consistent with worsening balance function. One explanation for the apparent difference with previous work might be because the TUG test was originally designed to identify differences between fallers and non-fallers. Subclinical differences within a relatively healthy population without a history of falling, such as used here, may be less easily detected. Another potential reason we failed to find a link is the fact that we used an auditory stimulus, which is not a stimulus modality traditionally considered to contribute to maintaining balance. 55 This explanation seems less convincing given a wealth of recent studies finding that auditory cues do in fact serve as relevant balance-related cues. 24 56 Furthermore, previous studies have shown that prolonged TBW in one modality has been linked to poorer performance in seemingly unrelated tasks. 9 57 58 59 60 Finally, the TUG evaluates a combination of both sensory and motor performance, but the TBW relies strictly on sensory function.
Further Observations
It is interesting to note that, while most older subjects demonstrated typical age-related changes in PSS and TBW, several appeared to maintain youthful values. Similar effects have been reported before when measuring timing in older subjects (Poliakoff et al., 2006). 16 The environmental, genetic, or other underpinnings to this effect remain to be investigated and may provide important insights into healthy aging.
The PSS and TBW characterizing the perception of two sensory signals can be altered with training 31 61 62 63 64 as can the TBW between a motor event, such as a finger tap, and its sensory consequence, such as a flash or tone. 65 Along the same lines, experienced musicians demonstrate a relatively short TBW for auditory + visual stimuli compared with controls, suggesting that the TBW may be narrowed simply through experience with a task or hobby rather than requiring a repetitive laboratory training regimen as typically used in other studies. 60 66 Whether similar mechanisms could be found for TBWs involving vestibular inputs in the elderly and whether they would have any practical impact on reducing falls is unknown.
Funding Statement
FUNDING/ACKNOWLEDGMENTS This work was supported by NIH R01DC017425 and VA RR&D I50RX002361 (M.E.H., T.E.H.); the Doris Duke Charitable Foundation (A.K.M.); VA RR&D C9230C (M.E.H., T.E.H.), and NIH T35DC008765 (S.B.S.). This material is the result of work supported with resources and the use of facilities at the VA Rehabilitation Research and Development (RR&D) National Center for Rehabilitative Auditory Research (NCRAR) (Center Award #C2361C/I50 RX002361) at the VA Portland Health Care System in Portland, Oregon.
Footnotes
CONFLICTS OF INTEREST None of the authors have conflicts to declare.
References
- 1.Green A M, Angelaki D E. Multisensory integration: resolving sensory ambiguities to build novel representations. Curr Opin Neurobiol. 2010;20(03):353–360. doi: 10.1016/j.conb.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeka J J, Allison L K, Kiemel T. The dynamics of visual reweighting in healthy and fall-prone older adults. J Mot Behav. 2010;42(04):197–208. doi: 10.1080/00222895.2010.481693. [DOI] [PubMed] [Google Scholar]
- 3.Ernst M O, Bülthoff H H. Merging the senses into a robust percept. Trends Cogn Sci. 2004;8(04):162–169. doi: 10.1016/j.tics.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Spence C, Squire S. Multisensory integration: maintaining the perception of synchrony. Curr Biol. 2003;13(13):R519–R521. doi: 10.1016/s0960-9822(03)00445-7. [DOI] [PubMed] [Google Scholar]
- 5.Vroomen J, Keetels M. Perception of intersensory synchrony: a tutorial review. Atten Percept Psychophys. 2010;72(04):871–884. doi: 10.3758/APP.72.4.871. [DOI] [PubMed] [Google Scholar]
- 6.Yaguchi A, Hidaka S. Distinct autistic traits are differentially associated with the width of the multisensory temporal binding window. Multisens Res. 2018;31(06):523–536. doi: 10.1163/22134808-00002612. [DOI] [PubMed] [Google Scholar]
- 7.Zhou H Y, Cai X L, Weigl M, Bang P, Cheung E FC, Chan R CK. Multisensory temporal binding window in autism spectrum disorders and schizophrenia spectrum disorders: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;86:66–76. doi: 10.1016/j.neubiorev.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson R A, Siemann J K, Schneider B C et al. Multisensory temporal integration in autism spectrum disorders. J Neurosci. 2014;34(03):691–697. doi: 10.1523/JNEUROSCI.3615-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hairston W D, Burdette J H, Flowers D L, Wood F B, Wallace M T.Altered temporal profile of visual-auditory multisensory interactions in dyslexia Exp Brain Res 2005166(3-4):474–480. [DOI] [PubMed] [Google Scholar]
- 10.Meilleur A, Foster N EV, Coll S M, Brambati S M, Hyde K L. Unisensory and multisensory temporal processing in autism and dyslexia: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2020;116:44–63. doi: 10.1016/j.neubiorev.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson R A, Park S, Cochran C et al. The associations between multisensory temporal processing and symptoms of schizophrenia. Schizophr Res. 2017;179:97–103. doi: 10.1016/j.schres.2016.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panagiotidi M, Overton P G, Stafford T. Multisensory integration and ADHD-like traits: Evidence for an abnormal temporal integration window in ADHD. Acta Psychol (Amst) 2017;181:10–17. doi: 10.1016/j.actpsy.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Scarpina F, Migliorati D, Marzullo P, Mauro A, Scacchi M, Costantini M. Altered multisensory temporal integration in obesity. Sci Rep. 2016;6(01):28382. doi: 10.1038/srep28382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zmigrod L, Zmigrod S. On the temporal precision of thought: individual differences in the multisensory temporal binding window predict performance on verbal and nonverbal problem solving tasks. Multisens Res. 2016;29:679–701. [Google Scholar]
- 15.Dinnerstein A J, Zlotogura P. Intermodal perception of temporal order and motor skills: effects of age. Percept Mot Skills. 1968;26(03):987–1000. doi: 10.2466/pms.1968.26.3.987. [DOI] [PubMed] [Google Scholar]
- 16.Poliakoff E, Shore D I, Lowe C, Spence C. Visuotactile temporal order judgments in ageing. Neurosci Lett. 2006;396(03):207–211. doi: 10.1016/j.neulet.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 17.Setti A, Burke K E, Kenny R A, Newell F N. Is inefficient multisensory processing associated with falls in older people? Exp Brain Res. 2011;209(03):375–384. doi: 10.1007/s00221-011-2560-z. [DOI] [PubMed] [Google Scholar]
- 18.Bedard G, Barnett-Cowan M. Impaired timing of audiovisual events in the elderly. Exp Brain Res. 2016;234(01):331–340. doi: 10.1007/s00221-015-4466-7. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson R A, Baum S H, Krueger J, Newhouse P A, Wallace M T. Links between temporal acuity and multisensory integration across life span. J Exp Psychol Hum Percept Perform. 2018;44(01):106–116. doi: 10.1037/xhp0000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lupo J, Barnett-Cowan M. Impaired perceived timing of falls in the elderly. Gait Posture. 2018;59:40–45. doi: 10.1016/j.gaitpost.2017.09.037. [DOI] [PubMed] [Google Scholar]
- 21.Stapleton J, Setti A, Doheny E P, Kenny R A, Newell F N. A standing posture is associated with increased susceptibility to the sound-induced flash illusion in fall-prone older adults. Exp Brain Res. 2014;232(02):423–434. doi: 10.1007/s00221-013-3750-7. [DOI] [PubMed] [Google Scholar]
- 22.Sanders M C, Chang N YN, Hiss M M, Uchanski R M, Hullar T E.Temporal binding of auditory and rotational stimuli Exp Brain Res 2011210(3-4):539–547. [DOI] [PubMed] [Google Scholar]
- 23.Chang N YN, Uchanski R M, Hullar T E. Temporal integration of auditory and vestibular stimuli. Laryngoscope. 2012;122(06):1379–1384. doi: 10.1002/lary.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubetzky A V, Gospodarek M, Arie L, Kelly J, Roginska A, Cosetti M. Auditory input and postural control in adults: a narrative review. JAMA Otolaryngol Head Neck Surg. 2020;146(05):480–487. doi: 10.1001/jamaoto.2020.0032. [DOI] [PubMed] [Google Scholar]
- 25.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80(09):896–903. [PubMed] [Google Scholar]
- 26.Marme-Karelse A M, Bles W. Circular vection and human posture, II. Does the auditory system play a role? Agressologie. 1977;18(06):329–333. [PubMed] [Google Scholar]
- 27.Lewald J, Karnath H O. Vestibular influence on human auditory space perception. J Neurophysiol. 2000;84(02):1107–1111. doi: 10.1152/jn.2000.84.2.1107. [DOI] [PubMed] [Google Scholar]
- 28.Shayman C S, Peterka R J, Gallun F J, Oh Y, Chang N N, Hullar T E. Frequency-dependent integration of auditory and vestibular cues for self-motion perception. J Neurophysiol. 2020;123(03):936–944. doi: 10.1152/jn.00307.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140(06):734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 30.Mallery R M, Olomu O U, Uchanski R M, Militchin V A, Hullar T E. Human discrimination of rotational velocities. Exp Brain Res. 2010;204(01):11–20. doi: 10.1007/s00221-010-2288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powers A R, III, Hillock A R, Wallace M T. Perceptual training narrows the temporal window of multisensory binding. J Neurosci. 2009;29(39):12265–12274. doi: 10.1523/JNEUROSCI.3501-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hillock A R, Powers A R, Wallace M T. Binding of sights and sounds: age-related changes in multisensory temporal processing. Neuropsychologia. 2011;49(03):461–467. doi: 10.1016/j.neuropsychologia.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirsh I J, Fraisse P. [Simultaneous character and succession of heterogenous stimuli] Annee Psychol. 1964;64:1–19. [PubMed] [Google Scholar]
- 34.Dixon N F, Spitz L. The detection of auditory visual desynchrony. Perception. 1980;9(06):719–721. doi: 10.1068/p090719. [DOI] [PubMed] [Google Scholar]
- 35.Slutsky D A, Recanzone G H. Temporal and spatial dependency of the ventriloquism effect. Neuroreport. 2001;12(01):7–10. doi: 10.1097/00001756-200101220-00009. [DOI] [PubMed] [Google Scholar]
- 36.Lewald J, Guski R. Cross-modal perceptual integration of spatially and temporally disparate auditory and visual stimuli. Brain Res Cogn Brain Res. 2003;16(03):468–478. doi: 10.1016/s0926-6410(03)00074-0. [DOI] [PubMed] [Google Scholar]
- 37.Zampini M, Guest S, Shore D I, Spence C. Audio-visual simultaneity judgments. Percept Psychophys. 2005;67(03):531–544. doi: 10.3758/BF03193329. [DOI] [PubMed] [Google Scholar]
- 38.Bohannon R W. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther. 2006;29(02):64–68. doi: 10.1519/00139143-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Kristensen M T, Foss N B, Kehlet H. Timed “up & go” test as a predictor of falls within 6 months after hip fracture surgery. Phys Ther. 2007;87(01):24–30. doi: 10.2522/ptj.20050271. [DOI] [PubMed] [Google Scholar]
- 40.Virsu V, Lahti-Nuuttila P, Laasonen M. Crossmodal temporal processing acuity impairment aggravates with age in developmental dyslexia. Neurosci Lett. 2003;336(03):151–154. doi: 10.1016/s0304-3940(02)01253-3. [DOI] [PubMed] [Google Scholar]
- 41.Diederich A, Colonius H. Elsevier; 2009. Crossmodal interaction in speeded responses: time window of integration model; pp. 119–135. [DOI] [PubMed] [Google Scholar]
- 42.Salthouse T A, Somberg B L. Isolating the age deficit in speeded performance. J Gerontol. 1982;37(01):59–63. doi: 10.1093/geronj/37.1.59. [DOI] [PubMed] [Google Scholar]
- 43.Kail R, Salthouse T A.Processing speed as a mental capacity Acta Psychol (Amst) 199486(2-3):199–225. [DOI] [PubMed] [Google Scholar]
- 44.Furman J M, Müller M LTM, Redfern M S, Jennings J R. Visual-vestibular stimulation interferes with information processing in young and older humans. Exp Brain Res. 2003;152(03):383–392. doi: 10.1007/s00221-003-1560-z. [DOI] [PubMed] [Google Scholar]
- 45.Brandt T, Bartenstein P, Janek A, Dieterich M.Reciprocal inhibitory visual-vestibular interaction. Visual motion stimulation deactivates the parieto-insular vestibular cortex Brain 1998121(Pt 9):1749–1758. [DOI] [PubMed] [Google Scholar]
- 46.Wallace M T, Stein B E. Development of multisensory neurons and multisensory integration in cat superior colliculus. J Neurosci. 1997;17(07):2429–2444. doi: 10.1523/JNEUROSCI.17-07-02429.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Binns K E, Salt T E. Importance of NMDA receptors for multimodal integration in the deep layers of the cat superior colliculus. J Neurophysiol. 1996;75(02):920–930. doi: 10.1152/jn.1996.75.2.920. [DOI] [PubMed] [Google Scholar]
- 48.Huang L, Pallas S L. NMDA antagonists in the superior colliculus prevent developmental plasticity but not visual transmission or map compression. J Neurophysiol. 2001;86(03):1179–1194. doi: 10.1152/jn.2001.86.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newcomer J W, Farber N B, Olney J W. NMDA receptor function, memory, and brain aging. Dialogues Clin Neurosci. 2000;2(03):219–232. doi: 10.31887/DCNS.2000.2.3/jnewcomer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirokawa J, Bosch M, Sakata S, Sakurai Y, Yamamori T. Functional role of the secondary visual cortex in multisensory facilitation in rats. Neuroscience. 2008;153(04):1402–1417. doi: 10.1016/j.neuroscience.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Rissman R A, Bennett D A, Armstrong D M.Subregional analysis of GABA(A) receptor subunit mRNAs in the hippocampus of older persons with and without cognitive impairment J Chem Neuroanat 200428(1-2):17–25. [DOI] [PubMed] [Google Scholar]
- 52.Shayman C S, Seo J H, Oh Y, Lewis R F, Peterka R J, Hullar T E. Relationship between vestibular sensitivity and multisensory temporal integration. J Neurophysiol. 2018;120(04):1572–1577. doi: 10.1152/jn.00379.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brooks C J, Chan Y M, Anderson A J, McKendrick A M. Audiovisual temporal perception in aging: the role of multisensory integration and age-related sensory loss. Front Hum Neurosci. 2018;12:192. doi: 10.3389/fnhum.2018.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hugenschmidt C E, Mozolic J L, Tan H, Kraft R A, Laurienti P J.Age-related increase in cross-sensory noise in resting and steady-state cerebral perfusion Brain Topogr 200921(3-4):241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angelaki D E, Cullen K E. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci. 2008;31(01):125–150. doi: 10.1146/annurev.neuro.31.060407.125555. [DOI] [PubMed] [Google Scholar]
- 56.Stevens M N, Barbour D L, Gronski M P, Hullar T E.Auditory contributions to maintaining balance J Vestib Res 201626(5-6):433–438. [DOI] [PubMed] [Google Scholar]
- 57.Foucher J R, Lacambre M, Pham B T, Giersch A, Elliott M A.Low time resolution in schizophrenia Lengthened windows of simultaneity for visual, auditory and bimodal stimuli Schizophr Res 200797(1-3):118–127. [DOI] [PubMed] [Google Scholar]
- 58.Başkent D, Bazo D. Audiovisual asynchrony detection and speech intelligibility in noise with moderate to severe sensorineural hearing impairment. Ear Hear. 2011;32(05):582–592. doi: 10.1097/AUD.0b013e31820fca23. [DOI] [PubMed] [Google Scholar]
- 59.Foss-Feig J H, Kwakye L D, Cascio C J et al. An extended multisensory temporal binding window in autism spectrum disorders. Exp Brain Res. 2010;203(02):381–389. doi: 10.1007/s00221-010-2240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee H, Noppeney U. Long-term music training tunes how the brain temporally binds signals from multiple senses. Proc Natl Acad Sci U S A. 2011;108(51):E1441–E1450. doi: 10.1073/pnas.1115267108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujisaki W, Shimojo S, Kashino M, Nishida S. Recalibration of audiovisual simultaneity. Nat Neurosci. 2004;7(07):773–778. doi: 10.1038/nn1268. [DOI] [PubMed] [Google Scholar]
- 62.Navarra J, Vatakis A, Zampini M, Soto-Faraco S, Humphreys W, Spence C. Exposure to asynchronous audiovisual speech extends the temporal window for audiovisual integration. Brain Res Cogn Brain Res. 2005;25(02):499–507. doi: 10.1016/j.cogbrainres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 63.Stevenson R A, Wilson M M, Powers A R, Wallace M T. The effects of visual training on multisensory temporal processing. Exp Brain Res. 2013;225(04):479–489. doi: 10.1007/s00221-012-3387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vatakis A, Navarra J, Soto-Faraco S, Spence C. Audiovisual temporal adaptation of speech: temporal order versus simultaneity judgments. Exp Brain Res. 2008;185(03):521–529. doi: 10.1007/s00221-007-1168-9. [DOI] [PubMed] [Google Scholar]
- 65.Sugano Y, Keetels M, Vroomen J. Adaptation to motor-visual and motor-auditory temporal lags transfer across modalities. Exp Brain Res. 2010;201(03):393–399. doi: 10.1007/s00221-009-2047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petrini K, Dahl S, Rocchesso Det al. Multisensory integration of drumming actions: musical expertise affects perceived audiovisual asynchrony Exp Brain Res 2009198(2-3):339–352. [DOI] [PubMed] [Google Scholar]


