Abstract
Cholangiocytes form a complex 3D network of bile ducts in the liver and contribute to liver function. The damage or destruction of cholangiocytes can lead to biliary diseases, and the shortage of cholangiocytes remains an obstacle for drug development targeting biliary diseases. Valproic acid (VPA) is a potent activator of Notch signaling pathway that is essential for cholangiocyte differentiation. Here, we report a VPA-based approach for cholangiocyte differentiation of human pluripotent stem cells. VPA activated Notch2 expression and upregulated HES-1, HEY-1, and Sox9 gene expression in hESC-derived hepatoblast. After 7 days treatment, VPA promoted successful differentiation of hepatoblast into cholangiocytes expressing cholangiocyte marker genes (AE2, AQP1, CFTR) and proteins (CK19 and CK7). In addition, the differentiated cholangiocytes formed bile duct-like structures after implantation into the spleen of NOD/SCID mice. Our results suggested that VPA can promote hESC differentiation to cholangiocyte-like cells. The induced cholangiocytes may serve as a potential cell source for both in vitro modeling and regenerative therapy of cholangiopathies. The findings can also support further development of small-molecule based differentiation protocols for cholangiocyte production.
Keywords: valproic acid, hESC, differentiation of cholangiocyte, Notch signaling pathway
Graphical Abstract
Graphical Abstract.
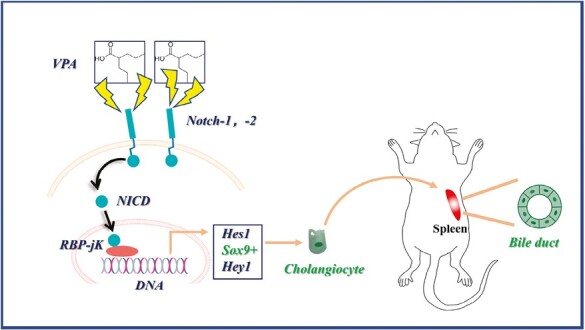
Significance Statement.
In this work, we reported the effect of valproic acid (VPA) treatment on promoting hepatoblast differentiation into cholangiocyte-like cells for the first time. Our studies contribute to the generation of cholangiocyte-like cells via a small-molecule-based approach which can facilitate the studying and development of therapies for biliary diseases.
Background
Cholangiocytes, also called biliary epithelial cells, form a complex 3D network of bile ducts in the liver.1 In humans, the total length of biliary network reaches approximately 2 km.2 It is responsible for the collection and transportation of bile acids secreted by hepatocytes.3 In addition, cholangiocytes also contribute to liver function. For example, cholangiocytes are involved in the hormone-regulated bile secretion and mucin secretion.4 Cholangiocytes also participate in liver regeneration after liver injury.5 Unfortunately, the damage or destruction of cholangiocytes can lead to biliary diseases which are difficult to treat and can lead to end-stage liver disease that are associated with high mortality.6
A variety of factors (such as chronic cholestasis, infection, and toxic injury) can increase the risk of development of biliary diseases.6,7 However, the pathogenesis of biliary diseases is still unclear due to the shortage of in vitro model.8 In addition, the shortage of in vitro biliary model also limits the progress of drug development targeting biliary diseases. Therefore, there is an urgency to generate sufficient cholangiocytes for the construction of in vitro biliary model that will facilitate the study of biliary diseases and screening of drug candidates. So far, many attempts have been investigated to culture primary cholangiocyte in vitro with little success. This is because cholangiocytes only account for approximately 3% of total liver cells, and the isolation of pure cholangiocytes from liver biopsies is challenging.9,10 Recently, using human pluripotent stem cells, including human-induced pluripotent stem cells (hiPSC) and human embryonic stem cells (hESC), to generate cholangiocyte-like cells has been investigated.
Cholangiocyte and hepatocyte share common precursors called hepatoblast. Hepatoblasts exhibit bipotent characteristics and can differentiate into hepatocytes or cholangiocytes.11,12 A 3-step differentiation protocol mimicking liver development, including definitive endoderm differentiation, hepatic specification, and hepatocyte maturation, has been widely used to generate hepatocytes via an intermediate step of hepatoblast production.13,14 In contrast, only few studies have attempted to generate cholangiocyte from hepatoblast derived from hESC and hiPSC.15,16 Previous studies have reported that activation of Notch pathway can initiate hepatoblast differentiation into cholangiocyte, as Notch signaling is required to establish cholangiocellular fate during liver development.17 Notch pathway activation induces the expression of the downstream Hes/Hey gene family, as well as the SOX9 gene, where SOX9 is a specific and early-stage marker of biliary cells in developing liver and regulates bile duct morphogenesis.18,19 In addition, Hes/Hey gene family also play important roles in regulating hepatoblast biliary differentiation. Specifically, Hes1/Hey1 act as transcriptional repressors by binding to specific DNA sequences and inhibiting the expression of genes involved in hepatocyte differentiation while promoting the differentiation of biliary cells.19-21 To promote biliary lineage commitment from hepatic progenitor cells, a number of growth factors and cytokines (such as EGF, HGF, TGFβ, and IL6) have been reported.18,22,23 However, the high cost and short half-life of these biological factors would limit the mass production of cholangiocyte. In contrast, a small-molecule based approach can be advantageous in generating cholangiocytes.
Valproic acid (VPA, 2-propylpentanoic acid) is an established small-molecule drug in epilepsy therapy with minimal cytotoxicity.24 Recently, it has also been reported that VPA can activate different signaling pathways in addition to treating epilepsy. For example, VPA can promote hair regrowth by activating the Wnt/β-catenin pathway.25,26 In addition, previous studies have reported that VPA can activate Notch signaling cascade by increasing the levels of intracellular Notch and Hes-1, and the role of VPA in promoting neural differentiation by activating the Notch signaling cascade has been extensively reported.27,28 For example, Vukićević et al reported the treatment of VPA enhances the neuronal differentiation of sympathoadrenal progenitor cells by upregulating Notch2 and Hes-1[27]. Given that Notch2 signaling pathway also plays an important role in cholangiocyte differentiation, whether VPA can promote the differentiation of hepatic progenitor cells into cholangiocyte remains to be studied. Based on the reported effect of VPA on the regulation of Notch2 signaling pathway, we hypothesized that VPA can initiate the cholangiocyte differentiation of hepatoblasts.
In this study, we report a VPA-based approach for cholangiocyte differentiation of human pluripotent stem cells. hESC were differentiated to hepatoblast by a standard differentiation protocol followed by the treatment with VPA. VPA activated the Notch2 expression and upregulated HES-1, HEY-1, and SOX9 gene expression. After 7 days treatment, the hepatoblasts differentiated into cholangiocytes and expressed cholangiocyte marker genes (AE2, AQP1, CFTR, etc.) and proteins (CK19 and CK7). The bile duct-like structures were observed after the cholangiocytes were implanted into the spleen of NOD/SCID mice. Our results suggested that VPA-induced cholangiocyte may serve as a potential cell source for both in vitro modeling and regenerative therapy of cholangiopathies. Overall, we demonstrated that VPA could induce the differentiation of hepatoblast to cholangiocyte. The findings support further development of small-molecule based differentiation protocols for cholangiocyte production.
Methods
hESC culture and generation of hepatoblast
Culture and expansion of hESC: The hESC line cell, H9 at passages 5-10, were regularly tested mycoplasma free using mycoplasma test kit (Vazyme, China), and were maintained under feeder-free culture condition on growth factor reduced Matrigel (0.125 mg/mL) (Corning, USA) in mTeSR1 medium (STEMCELL Technologies, Canada). For hESC passaging and expansion, when hESC reached a confluence level of ~80%, hESC colonies were dissociated with Accutase (Life Technologies, USA) and resuspended as single cells in mTeSR1 medium containing 10 μM Y-27632 (Millipore, USA) and seeded in wells coated with growth factor reduced Matrigel. hESC were routinely passaged every 3-4 days.
Generation of hepatoblast from hESC: The generation of hepatoblast from hESC involves definitive endoderm differentiation and hepatic specification. For definitive endoderm differentiation, differentiation began around 18 to 20 hours after hESC seeding on Matrigel-coated surface. hESC were incubated with DE Induction Medium A (Thermofisher, USA) for the first 24 hours and then DE Incubation Medium B (Thermofisher) for another 24 hours to induce definitive endoderm differentiation. Then the cells were cultured for 5 days for hepatoblast specification in Knockout DMEM basal medium (Thermofisher) containing 20% KOSR (Thermofisher) supplemented with 1% NEAA (Thermofisher), 0.5% GlutaMAX (Thermofisher), 0.2% β-mercaptoethanol (Thermofisher), 1% Penicillin-Streptomycin (Thermofisher), and 1% Dimethylsulfoxide (Thermofisher), and the medium was replaced every 48 hours.
Cholangiocyte-Like Cell and Hepatocyte Differentiation From Hepatoblast Cell
Following endoderm differentiation and hepatoblast specification, the generated hepatoblasts were cultured in VPA free (VPA−) or VPA addition (VPA+) medium for further hepatocyte or cholangiocyte differentiation for 10 and 7 days, respectively. Hepatic differentiation medium was based on HepatoZYME medium (Thermofisher) containing 5% KOSR, 1% hydrocortisone 21-hemisuccinate (Sigma, USA), 1% GlutaMAX, 1% Pis, 1% dimethylsulfoxide, 10 ng/mL hepatocyte growth factor (Peprotech, USA), and 20 ng/mL oncostatin M (Peprotech). A 2-mM VPA concentration was used for biliary differentiation, which was based on previous reports which demonstrated effective activation of Notch signaling pathways.27 The medium was replaced every 72 hours.
RNA Extraction and Real-Time Quantitative PCR
Total RNAs were extracted with TRIzol reagent (Invitrogen, USA). cDNA was synthesized from 1000 ng of total RNA template using HiScript III RT SuperMix with gDNA Eraser (Vazyme). Real-time quantitative PCR was performed with TB Green Premix Ex Taq II (TAKARA, Japan) using the Quant Studio 7 (QS7, USA) Flex Real Time PCR System (Applied Biosystems, USA). Primers used are listed in Supplementary Table S1. Results were normalized to that of GAPDH and quantification of relative expression was determined by the 2−ΔΔCt method.
Immunofluorescence Staining
Cells were fixed with 4% paraformaldehyde (PFA, Sigma) for 15 minutes at room temperature, before permeabilized with 0.3% Triton X-100 (Sigma) for 5 minutes and blocked with 5% bovine serum albumin (BSA) for 30 minutes for immunostaining. Then the cells were incubated with primary antibodies in 1% BSA at 4 ℃ overnight, and secondary antibodies in 1% BSA for 1 hour at room temperature. Nuclear staining was performed with DAPI (Sigma) via incubation for 15 minutes. Finally, the samples were washed 3 times with PBS for 10 minutes at room temperature. All immunofluorescence images were acquired using a Leica TCS-SP8 confocal microscope. LAS X software (Leica) was used for image processing. A complete list of the primary and secondary antibodies used is provided in Supplementary Table S2.
Rhodamine 123 Uptake Assay
Cells were incubated with 100 μM rhodamine 123 (Sigma-Aldrich) in HepatoZYME medium for 30 minutes at 37 ℃ and washed 3 times with cell culture medium. Then, the cells were incubated at 37 ℃ for another 40 minutes. Images were acquired using a Nikon Inverted Microscope (ECLIPSE Ti2-A).
Intrasplenic Implantation
Non-obese diabetic severe combined immunodeficient (NOD-SCID) mice of 6-8 weeks were purchased from the Laboratory Animal Services Centre (CUHK, Hong Kong). All animal experiments were performed with the approval of the Animal Experimentation Ethics Committee of the Chinese University of Hong Kong (Ref. No. 21-249-MIS). The mice were anesthetized with ketamine (50 mg/mL, Bayer, Germany) and xylazine (20 mg/mL, Bayer, Germany). Then the skin of splenic area was transversely dissected and a small incision was created through the peritoneal layer to expose the spleen. The spleen was gently lifted before 105 cholangiocytes were implanted into spleen with tweezer. Sham operation was performed by introducing PBS. The peritoneal cavity was then closed by suturing. Regular post-operative care was performed.
Hematoxylin Eosin Staining and Immunohistochemistry
Spleen samples from different groups were fixed with 4% PFA at 4 ℃ overnight, washed with PBS and then embedded in Tissue Processor. The samples were dehydrated, embedded in paraffin, and sectioned at 5 μm thickness. Paraffin-embedded sections were dewaxed with xylene and rehydrated. Hematoxylin eosin (H&E) staining were performed in accordance with the manufacturer’s instructions. Antigen retrieval was performed in Antigen Retriever (PT Module, Thermofisher). The subsequent immunohistochemistry staining steps were performed as described above (Immunofluorescence staining).
Statistical Analysis
Data were expressed as mean ± standard deviation (SD), and each experiment was repeated 3 times. Statistical analysis was performed on the results of different groups by one-way ANOVA. All the statistical analyses were carried out using the GraphPad Prism software version 8.0.1. Statistically significant values were defined as P < 0.05.
Results
Generation and Characterization of Hepatoblast
Hepatoblasts are the common precursors of hepatocytes and cholangiocytes and can give rise to both cell types. In this study, hESC were differentiated to hepatoblasts using a published differentiation method as illustrated in Fig. 1A.13 Following hepatic specification, the morphology of hESC gradually changed from defined colonies to less dense and flatter cells containing prominent nuclei (Fig. 1B). The immunostaining results suggested that more than 80% of total cells on Day 7 expressed hepatic transcription factor HNF4α, which is crucial to regulate hepatogenesis, as well as hepatoblast markers AFP (~90% of total cells) and CK19 (~90% of total cells). On the contrary, more than 90% of cells on day 0 expressed pluripotency marker OCT4 but not HNF4α, AFP, and CK19. On day 7, OCT4 was undetectable (Fig. 1C). These results were further confirmed by qPCR analysis (Fig. 1D). The qPCR results also showed that OCT4 gene was highly expressed on day 0 and decreased during 7 days of differentiation, while the expression of DE marker FOXA2 peaked on day 2 and declined slightly on day 7. In addition, significant upregulation of HNF4A and AFP genes was observed on day 7. These results indicated successful differentiation of hESC toward the hepatoblast lineage.
Figure 1.
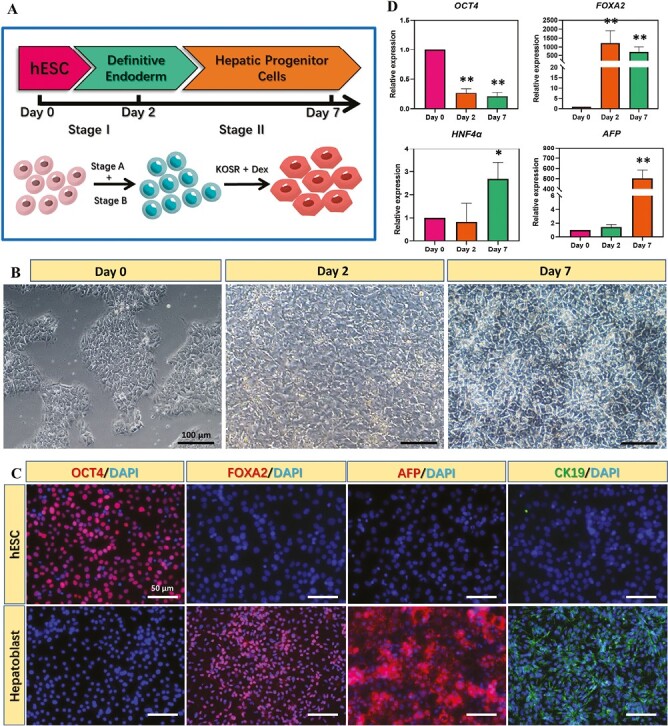
Differentiation of hESC into hepatoblasts. (A) Protocol of hepatic specification of hESC. (B) Bright field images of hESC during hepatic specification. (C) Immunostaining of marker proteins representative of different differentiation stages at various time points. (D) Expression of marker proteins representative of different differentiation stages at various time points. (n = 3, * indicates P < .05, **P < .01 vs. day 0)
VPA Treatment to Promote the Generation of Cholangiocyte-Like Cells
We then assessed whether VPA could be used to drive differentiation of hepatoblasts toward the cholangiocyte lineage. The effects of VPA treatment were comprehensively analyzed in terms of cell morphology, gene and protein expression. To begin with, dramatic morphological changes were observed between VPA- and VPA + groups. In VPA + group, differentiated cells gradually fused and organized to form cystic structure on day 3 and grew into 3D cystic and tubular structure (Fig. 2A), which resembles stem cell-derived biliary cells reported previously.29 In contrast, the cells in VPA-group appeared tightly aggregated and resembled hepatocytes.30,31 The qPCR results demonstrated significant upregulation of mature biliary markers in VPA + group on day 7, including AE2, AQP1 (a water channel), CFTR (a bile acid transporter), CK19 (a biliary cytokeratin), and SSR2 (a somatostatin receptor), compared with VPA- and day 7 (hepatoblast) groups (Fig. 2B).
Figure 2.
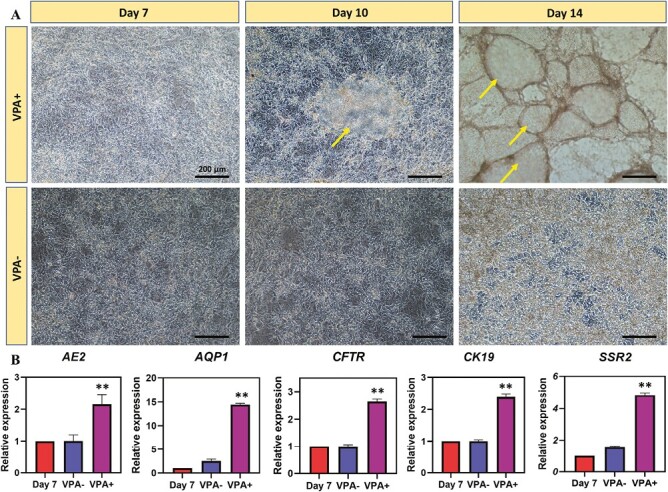
VPA promoted the differentiation of hepatoblasts into cholangiocyte-like cells. (A) Phenotypic changes of hepatoblasts with or without VPA treatment. Arrow: cholangiocyte-like cells. (B) Real-time PCR showing the expression of cholangiocyte marker genes with or without VPA treatment. (n = 3, *P < .05, **P < .01 vs. day 7)
In addition, we observed that the number of cystic and tubular structures increased during culture (Supplementary Fig. S1), where the average diameter of cystic structures reached ~531 μm on day 14 (Fig. 3A). Phalloidin immunostaining also confirmed that VPA- (hepatocyte-like) cells and VPA + (cholangiocyte like) cells exhibited different cell morphologies and further validated the formation of 3D cystic and tubular structure in VPA + group (Fig. 3C). To demonstrate the successful differentiation of hepatoblasts to biliary lineage cells under the treatment of VPA, immunostaining of hepatoblast marker AFP and biliary markers CK7 and CK19 was performed (Fig. 4A and B). Reduced AFP expression and increased CK7 and CK19 expression were observed after the treatment of VPA. In particular, the expression of CK7 and CK19 was mainly distributed around the biliary cystic structures. In addition, uptakes of acetylated α-tubulin and rhodamine123 were also observed after VPA treatment (Supplementary Figs. S2 and S3). Taken together, these results demonstrated that VPA promoted the generation of cholangiocyte-like cells from hepatoblasts and the formation of biliary cystic and tubular structures.
Figure 3.
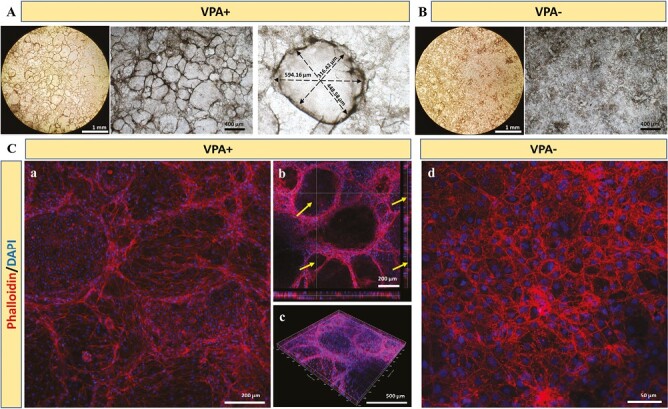
The morphological characterization of the differentiated cells with or without VPA treatment. (A) Bright field images of the morphology of cholangiocyte-like cells after the treatment of VPA for 7 days. (B) Bright field images of the morphology of cells without VPA treatment. (C) Phalloidin and DAPI staining of the differentiated cells with or without VPA treatment. Arrow: biliary cystic-like structures.
Figure 4.
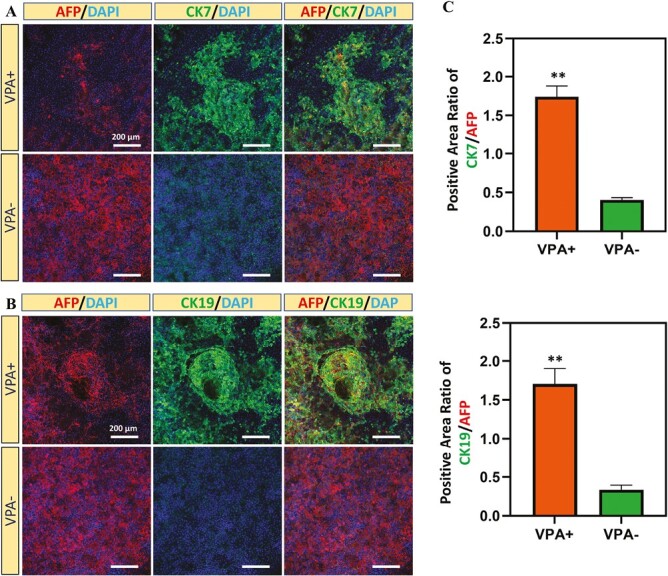
Immunostaining of cholangiocyte marker proteins in differentiated cells with or without VPA treatment. (A) Immunostaining of CK7 in differentiated cells with or without VPA treatment. (B) Immunostaining of CK19 in differentiated cells with or without VPA treatment. (C) Quantification of positive area ratio of CK7, CK19 and AFP in the differentiated cells with or without VPA treatment. (n = 3, *P < .05, **P < .01).
VPA Treatment to Promote Cholangiocyte Differentiation Via Activating Notch Signaling
We further investigated the effect of VPA on promoting hepatoblast differentiation into cholangiocyte-like cells. Previous reports have identified Notch signaling as an important mechanism of biliary lineage commitment.19,32 Following the activation of Notch2 receptors, the Notch intracellular domain (NICD) of Notch receptor will translocate to the nucleus, associate with the DNA-binding protein RBPJκ, and mediate changes in gene transcription,19 such as Homolog of Hairy/Enhancer-of-Split (HES1) and Hairy/enhancer-of-split related with YRPW motif 1 (HEY1), to promote biliary differentiation (Fig. 5A). After the hepatoblast were treated with VPA, increased expression of NOTCH2 was observed at both gene and protein levels (Fig. 5B, C). The gene expression level of NOTCH1 was also significantly higher in VPA + group compared with VPA− group, although the level between that of VPA + group and hepatoblast (day 7) was similar (Fig. 5C). In addition, we also observed increased expression levels of HES1 and HEY1, both of which are known to be the downstream effectors of Notch signaling pathway and regulate biliary lineage differentiation (Fig. 5C). Furthermore, the upregulation of SRY-related HMG box transcription factor 9 (SOX9) gene, which was considered a biliary marker and expressed during biliary differentiation,33 was observed in VPA + group. This indicated the successful commitment of biliary lineage after the treatment of VPA. Finally, compared with hepatoblast (Day 7), significant downregulation of NOTCH1, NOTCH2, and SOX9 genes was observed in the VPA− group. These results suggested VPA promoted biliary differentiation of hepatoblast through activation of the Notch2 signaling pathway and transcriptional activation of downstream HES/HEY family members.
Figure 5.
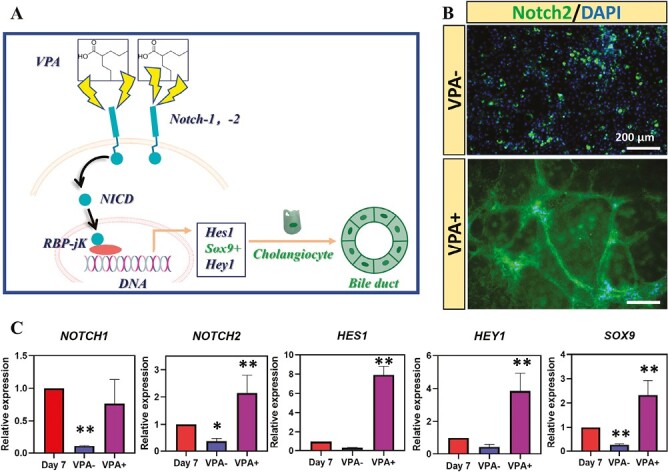
VPA promoted the differentiation of hepatoblasts into cholangiocyte-like cells via activating Notch signaling. (A) Schematic diagram of the mechanism of VPA promoting the differentiation of hepatoblasts into cholangiocyte-like cells. (B) Immunostaining of Notch2 in differentiated cells with or without VPA treatment. (C) Real-time PCR results of the expression of genes in Notch2 signaling pathway. (n = 3, *P < .05, **P < .01 vs. day 7).
The In Vivo Construction of Biliary Duct
Next, we tried to evaluate the engraftment of VPA-induced cholangiocyte-like cells in vivo by transplanting them under the splenic capsule of NOD-SCID mice (Fig. 6A). Sham operation was performed as control. At 6 weeks after transplantation, the transplanted cells were found engrafted in the spleen by expressing human specific CK19 (Fig. 6B, C). We also identified biliary duct-like structures that stained positive for hCK19, while hCK19 staining was undetectable in sham-operated spleens, confirming the specificity of the antibodies used.
Figure 6.
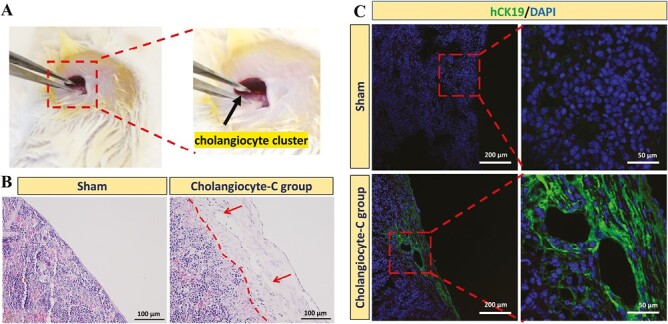
Intrasplenic implantation of hESC-derived cholangiocyte-like cells and analysis after implantation. (A) The procedure of introducing cholangiocyte-like cell cluster into the mice spleen. Black arrow: cholangiocyte-like cell cluster. (B) HE staining of the spleen section obtained from sham mice and mice receiving implantation of cholangiocyte-like cell cluster (cholangiocyte-c group) 6 weeks after implantation. (C) Immunostaining of human-specific CK19 in the spleen section 6 weeks after implantation. The shame-operated mice were used as control.
Discussion
The limited supply of functional biliary tissues or cells and the failure of animal models to accurately recapitulate human cholangiopathies have been the major obstacles to the development of effective therapies for biliary diseases.34 In this study, we explored the use of VPA to activate Notch2 signaling pathway and hence promote hepatoblast differentiation to cholangiocyte-like cells. The differentiated cells expressed biliary lineage-specific protein markers (such as CK7, CK19) and mature biliary gene markers (AQP1, CFTR, etc.). In addition, differentiated cholangiocyte-like cells were able to form biliary cystic structures and engrafted in vivo.
Previous studies suggested Notch signaling pathway regulated the differentiation of embryonic biliary precursors and promoted the formation of the second biliary layer during development.19 Several attempts have been reported to generate cholangiocyte-like cells from hepatic progenitor cells via the activation of Notch signaling pathway.23,35,36 However, most of these approaches are time-consuming and involve the use of expensive growth factors/proteins or coculture with a supporting cell type. For example, Ogawa et al reported a method to generate cholangiocyte-like cells from hiPSC by coculturing hepatoblasts generated from iPSC with OP9 stromal cells.37 This approach required a total culture time of 25 days and administration of growth factors such as TGF-β. Sampaziotis et al reported another protocol to generate cholangiocyte-like cells from iPSC-derived hepatoblasts using FGF10, EGF, and Activin.38 Compared with previous methods, our small-molecule-based approach could also activate Notch signaling pathway and generate cholangiocyte-like cells, which is more straightforward and less expensive. Besides, our approach requires a relatively short culture time. Treating hepatoblasts with VPA for 7 days resulted in the generation of cholangiocyte-like cells displaying biliary cystic and tubular structures similar to those described in previous studies (Fig. 3).29 Moreover, Sampaziotis et al reported the diameter of biliary cystic structures formed by isolated primary cholangiocytes were ~547 μm, which is similar to that formed by our hESC derived cholangiocyte-like cells (~531 μm).39 Overall, these results suggested VPA treatment is an efficient and relatively inexpensive approach to generate cholangiocyte-like cells. Future studies will focus on optimizing the small-molecule-based differentiation protocols for cholangiocyte production and benchmarking the maturity of the differentiated cells with those generated by growth factor-based approaches.
In this study, the concentration of VPA was chosen according to previous studies about applying VPA to activate Notch2 signaling pathway in neural differentiation.40,41 Vukićević et al reported that significant upregulation of NOTCH2 and its downstream effector HES1 gene was observed in chromospheres cells 24 hours after 2mM VPA treatment. This in turn enhanced the neural differentiation of sympathoadrenal progenitor cells.27 VPA was originally approved by the FDA for the treatment of seizures and is now widely applied in neural differentiation. Tao et al reported VPA activated Notch signaling pathway via Notch1 in MC3TE‐E1 cells. The downstream effectors HES1, HEY1 were also upregulated after VPA treatment.42 While Liu et al reported that Notch1 and Notch2 display equivalent function during mice development,43 our results showed that only NOTCH2 upregulation was observed after VPA treatment, while the expression level of NOTCH1 remained the same (Fig. 5). In contrast, downregulation of both NOTCH1 and NOTCH2 was observed in VPA− (hepatocyte differentiation) group. These results demonstrated VPA promoted cholangiocyte differentiation via upregulation of NOTCH2 instead of NOTCH1, while downregulation of both NOTCH2 and NOTCH1 occurred during hepatocyte differentiation. Nevertheless, it is of note that one of the limitations of employing a single concentration of VPA in our study is the inability to establish a dose-response relationship.44 A dose-response analysis would provide a comprehensive understanding of VPA’s impact on biliary differentiation of hepatoblast, enabling the determination of a minimum effective dose to activate the major signaling pathways (eg, Notch) while mitigating unintended effects on other ones. Consequently, further dosage optimization is necessary to identify the minimum effective dose.
In addition to Notch signaling pathways, expression of marker genes of BMP, Hedgehog, TGF-β, and Wnt signaling pathways was also evaluated by RT-qPCR (Supplementary Fig. S4). The results suggested VPA also promoted the expression of gene (GLI1) of the Hedgehog signaling pathway while downregulated BMP2 expression. This is consistent with previous report by Omenetti et al who showed higher expression of Hedgehog signaling pathway genes in cholangiocyte compared with hepatocyte,45 while it is shown that BMP signaling is necessary for liver specification.46 No significant difference was observed in AXIN, PTCH1, and TGFBR2 gene expression between VPA− and VPA+ groups. These suggested VPA treatment may influence multiple signaling pathways.
Generation of bipotent hepatoblast is prerequisite to the successful promotion of biliary differentiation using VPA. In this study, we have generated hepatoblasts with high expression of AFP gene and protein (Fig. 1C, D). Previous studies have reported the important roles of this state in cholangiocyte differentiation. For example, Lim et al reported using HNF1β and FOXA3 to promote mouse fibroblast trans-differentiation into cholangiocyte progenitor cells, and only progenitor cells with high AFP expression could differentiate to cholangiocyte-like cells efficiently, while those with low AFP expression were associated with low differentiation efficiency.47 Therefore, evaluation of AFP expression level of hepatoblast may be a key step before cholangiocyte differentiation induced by VPA treatment.
Conclusion
In this work, we reported the effect of VPA treatment on promoting hepatoblast differentiation into cholangiocyte-like cells for the first time. VPA efficiently activated Notch signaling pathway in hepatoblasts to induce cholangiocyte differentiation. The differentiated cholangiocyte-like cells expressed cholangiocyte marker genes (AE2, AQP1, CFTR, etc.) and proteins (CK19 and CK7), and were able to form biliary cystic structures. Our studies contribute to the generation of cholangiocyte-like cells via a small-molecule-based approach which can facilitate the development of therapies for biliary diseases.
Supplementary Material
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2019YFA0111300 and 2018YFA0903400); Research Grants Council of Hong Kong (ECS (24204819), National Natural Science Foundation of China and the Research Grants Council of Hong Kong Special Administrative Region, China (N_CUHK432/20); Sichuan Science and Technology Program (2022YFS0610); Health@InnoHK, Innovation and Technology Commission, the Government of Hong Kong SAR of the People's Republic of China; VC discretionary Fund (8601014), and Direct Grant (4054596) of The Chinese University of Hong Kong.
Contributor Information
Shuai Deng, Laboratory of Molecular Pharmacology, Department of Pharmacology, School of Pharmacy, Southwest Medical University, Luzhou, People’s Republic of China; Key Laboratory for Regenerative Medicine of the Ministry of Education of China, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, People’s Republic of China; Institute for Tissue Engineering and Regenerative Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, People’s Republic of China.
Xiaoyu Zhao, Key Laboratory for Regenerative Medicine of the Ministry of Education of China, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, People’s Republic of China; Institute for Tissue Engineering and Regenerative Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, People’s Republic of China.
Ziyan Kou, Key Laboratory for Regenerative Medicine of the Ministry of Education of China, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, People’s Republic of China; Institute for Tissue Engineering and Regenerative Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, People’s Republic of China.
Yanlun Zhu, Key Laboratory for Regenerative Medicine of the Ministry of Education of China, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, People’s Republic of China; Institute for Tissue Engineering and Regenerative Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, People’s Republic of China; Center for Neuromusculoskeletal Restorative Medicine, Hong Kong SAR, People’s Republic of China.
Xuerao Zhang, Key Laboratory for Regenerative Medicine of the Ministry of Education of China, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, People’s Republic of China; Institute for Tissue Engineering and Regenerative Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, People’s Republic of China.
Hon Fai Chan, Key Laboratory for Regenerative Medicine of the Ministry of Education of China, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, People’s Republic of China; Institute for Tissue Engineering and Regenerative Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, People’s Republic of China; Center for Neuromusculoskeletal Restorative Medicine, Hong Kong SAR, People’s Republic of China; Hong Kong Branch of CAS Center for Excellence in Animal Evolution and Genetics, Hong Kong SAR, People’s Republic of China.
Conflict of Interest
Shuai Deng and Hon Fai Chan are inventors on a patent application describing the use of valproic acid for biliary differentiation of stem cells. The authors declared they have no other competing interests.
Author Contributions
Conception and design: Hon Fai Chan, Shuai Deng; Financial support: Hon Fai Chan; Collection and/or assembly of data: Shuai Deng, Xiaoyu Zhao, Ziyan Kou; Data analysis and interpretation: Shuai Deng, Xiaoyu Zhao, Yanlun Zhu, Xuerao Zhang; Manuscript writing and revision: Shuai Deng; Hon Fai Chan; Final approval of manuscript: Shuai Deng, Xiaoyu Zhao, Ziyan Kou, Yanlun Zhu, Xuerao Zhang, Hon Fai Chan.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Jones H, Alpini G, Francis H.. Bile acid signaling and biliary functions. Acta Pharmaceut Sin B 2015;5(2):123-128. 10.1016/j.apsb.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banales JM, Huebert RC, Karlsen T, et al. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol 2019;16(5):269-281. 10.1038/s41575-019-0125-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strazzabosco M, Fabris L.. Functional anatomy of normal bile ducts. Anat Rec (Hoboken). 2008;291(6):653-660. 10.1002/ar.20664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoo K-S, Lim WT, Choi HS.. Biology of cholangiocytes: from bench to bedside. Gut Liver 2016;10(5):687-698. 10.5009/gnl16033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dickson I. Cholangiocytes regenerate hepatocytes during severe liver injury. Nat Rev Gastroenterol Hepatol 2017;14(9):503-503. 10.1038/nrgastro.2017.108 [DOI] [PubMed] [Google Scholar]
- 6. Santiago P, Scheinberg AR, Levy C.. Cholestatic liver diseases: new targets, new therapies. Therap Adv Gastroenterol 2018;11:1756284818787400. 10.1177/1756284818787400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang F, Gaudio E, Onori P, et al. Mechanisms of biliary damage. J Cell Death 2010;3:13-21. 10.4137/JCD.S2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasegawa S, Yoneda M, Kurita Y, et al. Cholestatic liver disease: current treatment strategies and new therapeutic agents. Drugs. 2021;81(10):1181-1192. 10.1007/s40265-021-01545-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Machhua S, Kumar Y, Prakash Kanojia R, et al. An improved and easy protocol for primary epithelial cell culture from atretic tissue in biliary atresia. Tissue Cell. 2019;56:83-89. 10.1016/j.tice.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 10. Lewis PL, Yan M, Su J, Shah RN.. Directing the growth and alignment of biliary epithelium within extracellular matrix hydrogels. Acta Biomater. 2019;85:84-93. 10.1016/j.actbio.2018.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang L, Wang W-H, Qiu W-L, et al. A single-cell transcriptomic analysis reveals precise pathways and regulatory mechanisms underlying hepatoblast differentiation. Hepatology. 2017;66(5):1387-1401. 10.1002/hep.29353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng S, Wu J, Qiu WL, et al. Large-scale generation of functional and transplantable hepatocytes and cholangiocytes from human endoderm stem cells. Cell Rep 2020;33(10):108455. 10.1016/j.celrep.2020.108455 [DOI] [PubMed] [Google Scholar]
- 13. Deng S, Zhao X, Zhu Y, et al. Efficient hepatic differentiation of hydrogel microsphere-encapsulated human pluripotent stem cells for engineering prevascularized liver tissue. Biofabrication 2022;15:015016. [DOI] [PubMed] [Google Scholar]
- 14. Zhao X, Zhu Y, Laslett AL, Chan HF.. Hepatic differentiation of stem cells in 2D and 3D biomaterial systems. Bioengineering (Basel, Switzerland) 2020;7(2):47. 10.3390/bioengineering7020047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takayama K, Mitani S, Nagamoto Y, et al. Laminin 411 and 511 promote the cholangiocyte differentiation of human induced pluripotent stem cells. Biochem Biophys Res Commun. 2016;474(1):91-96. 10.1016/j.bbrc.2016.04.075 [DOI] [PubMed] [Google Scholar]
- 16. Sampaziotis F, Cardoso de Brito M, Madrigal P, et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol. 2015;33(8):845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ogawa M, Jiang J-X, Xia S, et al. Generation of functional ciliated cholangiocytes from human pluripotent stem cells. Nat Commun. 2021;12(1):6504. 10.1038/s41467-021-26764-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dianat N, Dubois-Pot-Schneider H, Steichen C, et al. A Dubart-Kupperschmitt, generation of functional cholangiocyte-like cells from human pluripotent stem cells and HepaRG cells. Hepatology. 2014;60(2):700-714. 10.1002/hep.27165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zong Y, Panikkar A, Xu J, et al. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136(10):1727-1739. 10.1242/dev.029140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kodama Y, Hijikata M, Kageyama R, Shimotohno K, Chiba T.. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology. 2004;127(6):1775-1786. 10.1053/j.gastro.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 21. Tanimizu N, Miyajima A.. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J Cell Sci. 2004;117(Pt 15):3165-3174. 10.1242/jcs.01169 [DOI] [PubMed] [Google Scholar]
- 22. Ghanekar A, Kamath BM.. Cholangiocytes derived from induced pluripotent stem cells for disease modeling. Curr Opin Gastroenterol. 2016;32(3):210-215. 10.1097/MOG.0000000000000260 [DOI] [PubMed] [Google Scholar]
- 23. Matsui S, Ochiai M, Yasuda K, et al. Differentiation and isolation of iPSC-derived remodeling ductal plate-like cells by use of an AQP1-GFP reporter human iPSC line. Stem Cell Res. 2019;35:101400. 10.1016/j.scr.2019.101400 [DOI] [PubMed] [Google Scholar]
- 24. Santos J, Hubert T, Milthorpe BK.. Valproic acid promotes early neural differentiation in adult mesenchymal stem cells through protein signalling pathways. Cells 2020;9(3):619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fakhraei Lahiji S, Seo SH, Kim S, et al. Transcutaneous implantation of valproic acid-encapsulated dissolving microneedles induces hair regrowth. Biomaterials. 2018;167:69-79. 10.1016/j.biomaterials.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y, Xu Y, Kong H, et al. Microneedle system for tissue engineering and regenerative medicine. Exploration (Beijing, China) 2023;3(1):20210170. 10.1002/EXP.20210170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vukićević V, Qin N, Balyura M, et al. Valproic acid enhances neuronal differentiation of sympathoadrenal progenitor cells. Mol Psychiatry. 2015;20(8):941-950. 10.1038/mp.2015.3 [DOI] [PubMed] [Google Scholar]
- 28. Stockhausen MT, Sjölund J, Manetopoulos C, Axelson H.. Effects of the histone deacetylase inhibitor valproic acid on Notch signalling in human neuroblastoma cells. Br J Cancer. 2005;92(4):751-759. 10.1038/sj.bjc.6602309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Daoud A, Múnera JO.. Generation of human colonic organoids from human pluripotent stem cells. Methods Cell Biol. 2020;159:201-227. 10.1016/bs.mcb.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rose S, Ezan F, Cuvellier M, et al. Generation of proliferating human adult hepatocytes using optimized 3D culture conditions. Sci Rep. 2021;11(1):515. 10.1038/s41598-020-80019-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zeigerer A, Wuttke A, Marsico G, et al. Functional properties of hepatocytes in vitro are correlated with cell polarity maintenance. Exp Cell Res. 2017;350(1):242-252. 10.1016/j.yexcr.2016.11.027 [DOI] [PubMed] [Google Scholar]
- 32. Mao Y, Tang S, Yang L, Li K.. Inhibition of the Notch signaling pathway reduces the differentiation of hepatic progenitor cells into cholangiocytes in biliary atresia. Cell Physiol Biochem. 2018;49(3):1115-1123. [DOI] [PubMed] [Google Scholar]
- 33. Raynaud P, Carpentier R, Antoniou A, Lemaigre FP.. Biliary differentiation and bile duct morphogenesis in development and disease. Int J Biochem Cell Biol. 2011;43(2):245-256. 10.1016/j.biocel.2009.07.020 [DOI] [PubMed] [Google Scholar]
- 34. Pollheimer MJ, Trauner M, Fickert P.. Will we ever model PSC? - “it’s hard to be a PSC model!,”. Clin Res Hepatol Gastroenterol 2011;35(12):792-804. 10.1016/j.clinre.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 35. Rizwan M, Fokina A, Kivijärvi T, et al. Photochemically activated notch signaling hydrogel preferentially differentiates human derived hepatoblasts to cholangiocytes. Adv Funct Mater. 2021;31(5):2006116. [Google Scholar]
- 36. Wang S, Wang X, Tan Z, et al. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res. 2019;29(12):1009-1026. 10.1038/s41422-019-0242-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ogawa M, Ogawa S, Bear CE, et al. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33(8):853-861. 10.1038/nbt.3294 [DOI] [PubMed] [Google Scholar]
- 38. Sampaziotis F, de Brito MC, Geti I, et al. Directed differentiation of human induced pluripotent stem cells into functional cholangiocyte-like cells. Nat Protoc 2017;12(4):814-827. 10.1038/nprot.2017.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sampaziotis F, Justin AW, Tysoe OC, et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat Med. 2017;23(8):954-963. 10.1038/nm.4360 [DOI] [PubMed] [Google Scholar]
- 40. Jeong S-G, Ohn T, Kim SH, Cho G-W.. Valproic acid promotes neuronal differentiation by induction of neuroprogenitors in human bone-marrow mesenchymal stromal cells. Neurosci Lett. 2013;554:22-27. 10.1016/j.neulet.2013.08.059 [DOI] [PubMed] [Google Scholar]
- 41. Talwadekar M, Fernandes S, Kale V, Limaye L.. Valproic acid enhances the neural differentiation of human placenta derived-mesenchymal stem cells in vitro. J Tissue Eng Regen Med. 2017;11(11):3111-3123. 10.1002/term.2219 [DOI] [PubMed] [Google Scholar]
- 42. Tao ZS, Li TL, Xu HG, Yang M.. Hydrogel contained valproic acid accelerates bone-defect repair via activating Notch signaling pathway in ovariectomized rats. J Mater Sci Mater Med. 2021;33(1):4. 10.1007/s10856-021-06627-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu Z, Brunskill E, Varnum-Finney B, et al. The intracellular domains of Notch1 and Notch2 are functionally equivalent during development and carcinogenesis. Development. 2015;142(14):2452-2463. 10.1242/dev.125492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lutz WK, Lutz RW, Gaylor DW, Conolly RB.. Dose–response relationship and extrapolation in toxicology. mechanistic and statistical considerations, in: Reichl F.-X., Schwenk M. (Eds.), Regulatory Toxicology, Springer Berlin Heidelberg, 2014, pp. 547-5–68.. [Google Scholar]
- 45. Omenetti A, Diehl AM.. Hedgehog signaling in cholangiocytes. Curr Opin Gastroenterol. 2011;27(3):268-275. 10.1097/MOG.0b013e32834550b4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shin D, Monga SP.. Cellular and molecular basis of liver development. Compr Physiol 2013;3(2):799-815. 10.1002/cphy.c120022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lim KT, Kim J, Hwang SI.et al. Direct conversion of mouse fibroblasts into cholangiocyte progenitor cells. Stem Cell Rep. 2018;10(5):1522-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


