Abstract
Objective:
Insomnia is known to exacerbate pain symptoms. The purpose of the present study was to compare the secondary effects of cognitive behavioral therapy for insomnia (CBT-I) against a novel treatment for insomnia called acceptance and behavioral changes for insomnia (ABC-I) among individuals with comorbid pain. Differences in the potential mechanisms through which these treatments impact pain were also examined.
Methods:
Data consisted of a secondary analysis from a randomized comparative effectiveness trial of CBT-I and ABC-I among women veterans with insomnia and comorbid pain. Pain outcomes, beliefs about sleep, and psychological flexibility were assessed at baseline, post-treatment, and at three-months follow-up.
Results:
At baseline, 93 women veterans reported comorbid insomnia and pain (mean age = 46.7; 33.3% Black, 24.7% Hispanic/Latina). Both CBT-I (n=48) and ABC-I (n=45) were associated with decreased pain intensity (p <.001, Cohen’s d = .41–.67) and pain interference (p <.001, Cohen’s d = .71–.77) at post-treatment and three-months follow-up, with results indicating that ABC-I was non-inferior to CBT-I for pain improvement. Both conditions were associated with greater psychological flexibility post-treatment, and CBT-I resulted in larger reductions in dysfunctional beliefs about sleep (p = .01, Cohen’s d = .59).
Conclusion:
CBT-I and ABC-I both had positive secondary effects on pain with ABC-I being non-inferior to CBT-I with respect to its impact on pain. The mechanisms of change associated with these treatments may differ with CBT-I leading to greater reduction in dysfunctional beliefs. Hybrid treatments which incorporate an acceptance and commitment approach to both insomnia and pain warrant further examination.
Keywords: insomnia, pain, CBT, ACT, Women, veterans
Insomnia and pain are heavily intertwined, with over 50% of individuals with insomnia reporting persistent pain compared to only 19% of individuals without insomnia [1]. Co-occurring insomnia and pain is not only common, but is associated with a range of adverse health consequences [2]. While there is evidence of a bi-directional relationship between sleep and pain, sleep has been identified as a stronger and more consistent predictor of pain than vice versa [3]. Given these considerations, treatments which confer beneficial effects for both conditions warrant further examination.
CBT-I and Pain
Cognitive behavioral therapy for insomnia (CBT-I) is well-established as the first-line treatment for insomnia [4,5]. This brief and structured treatment helps patients replace behaviors and cognitions that inadvertently perpetuate insomnia with strategies that improve sleep. A initial review of six randomized controlled trials (RCTs) examining CBT-I in patients with comorbid pain conditions found evidence of clinically meaningful improvements in insomnia symptoms, as well as partial support for improvements in pain functioning and intensity [6]. A more recent meta-analysis of fourteen RCTs of CBT-I found that, on average, 58% of participants with comorbid pain reported improvements in pain post-treatment [7].
A core component of CBT-I is cognitive restructuring, a skill that assists individuals in identifying and reframing dysfunctional thoughts and beliefs related to insomnia. Dysfunctional beliefs about sleep are a known predictor of poor pain outcomes [8] and are effectively reduced following CBT-I [9]. While cognitive restructuring in CBT-I is primarily focused on sleep-related thoughts, CBT-I has also been shown to decrease pain catastrophizing and reduce dysfunctional beliefs pertaining to the sleep-pain interaction [10,11]. Decreases in dysfunctional beliefs therefore appear to have important implications for the management of pain.
ACT and Pain
While CBT-I is the first-line treatment for insomnia, both CBT and acceptance and commitment therapy (ACT) are evidence-based treatments for chronic pain. ACT uses mindfulness, acceptance, and cognitive de-fusion strategies to promote psychological flexibility, which refers to the ability to be in contact with the present moment and engage in behavior which aligns with one’s personal values [12]. Specifically, ACT teaches individuals to notice and accept difficult emotions and experience while encouraging engagement in valued activities. Studies suggest that ACT may be particularly relevant for the management of pain, with treatment resulting in improved psychological flexibility and pain acceptance [13]. Additionally, acceptance has been shown to be superior to cognitive restructuring as a pain tolerance strategy [14]. A possible mechanism for this difference might be that, when pain itself cannot be reduced, it may be challenging to “think differently” about it while developing psychological flexibility around how to live with pain symptoms may lead to more improvement in pain tolerance.
While CBT-I is a highly effective insomnia treatment, it does have some limitations, especially in populations with comorbid conditions. In particular, adherence to treatment recommendations is challenging and can attenuate treatment benefits [15,16]. Acceptance and Behavioral Changes for insomnia (ABC-I) is a novel ACT-informed treatment for insomnia which attempts to address these shortcomings of CBT-I by combining evidence-based behavioral strategies to treat insomnia (e.g., stimulus control, sleep restriction, and sleep hygiene) with exercises from ACT [17]. Specifically, ACT exercises in ABC-I aim to increase adherence by promoting psychological flexibility such as increasing one’s willingness to face short-term discomfort during sleep restriction in order to pursue one’s values [17].
Empirical evidence for ABC-I is still in its infancy but appears to hold promise. For example, a small randomized controlled trial of a similar ACT-based insomnia treatment showed significant improvement in insomnia symptoms, sleep quality, and quality of life post-treatment; with gains in sleep quality maintained six months following treatment [18]. Moreover, follow-up interviews at study completion indicated that ACT-based exercises (e.g., acceptance exercises, committed action, and mindfulness) were among the most helpful strategies endorsed by participants. Another small single-arm pilot study of an ACT-based treatment for insomnia that incorporated behavioral sleep strategies displayed reductions in both insomnia and pain disability post-treatment [19]. While it is currently unknown whether the secondary effects of ABC-I on pain compare favorably to those of CBT-I, evidence supporting the effects of behavioral strategies for insomnia on pain, combined with research highlighting the benefit of ACT-based strategies for pain, suggest that ABC-I warrants further examination.
Psychological flexibility is believed to be a central mechanism of change for ACT-based treatments [20]. Existing research suggests that psychological flexibility is associated with better sleep among individuals with comorbid pain and insomnia, and may act as a buffer against pain itself [21,22]. In fact, improvements in psychological flexibility have been shown to be a stronger predictor of functional improvements in pain compared to traditional pain coping skills such as activity pacing or use of relaxation skills [23]. While ABC-I draws upon ACT-based strategies which seeks to promote psychological flexibility, it unknown whether psychological flexibility acts as a mechanism through which ABC-I might improve pain symptoms.
Present Study
Given the aforementioned considerations, the first aim of the present study was to compare the secondary effects of ABC-I and CBT-I on pain outcomes among individuals with insomnia disorder who also endorsed chronic pain. In order to examine this aim, we used data from a recently completed randomized comparative effectiveness trial which examined the relative effects of CBT-I and ABC-I among women veterans with insomnia [24]. In the current study, it was hypothesized that ABC-I would be non-inferior to CBT-I with respect to its impact on pain outcomes among the participants who also endorsed chronic pain. Given the known improvements in sleep associated with both treatment conditions [24], the second aim of the study was to assess whether the mechanisms through which CBT-I and ABC-I confer positive effects on pain varies by treatment group. Dysfunctional beliefs about sleep were hypothesized to mediate the association between treatment group and pain for participants in the CBT-I condition while psychological flexibility was hypothesized to mediate the association between treatment group and pain for participants in the ABC-I condition.
Methods
Participants
Participants included in the present study were drawn from a larger randomized controlled trial comparing CBT-I to ABC-I among women veterans with insomnia (NCT02076165). Both treatments were associated with improved sleep immediately post-treatment and at 3-month post-treatment follow-up with ABC-I demonstrating similar levels of effectiveness compared to CBT-I [24]. A detailed CONSORT flow diagram was previously published [24].
Participants from the parent study were recruited using a multiple-step process. First, women veterans who received outpatient care from a large urban healthcare system within the past six months were invited to participate in a postal screening survey about insomnia. Veterans who endorsed symptoms of insomnia, operationalized as symptoms of poor sleep with daytime consequences at least three days per week over the past three months, were subsequently assessed for further eligibility. Details of this postal survey are published elsewhere [25]. Veterans with unstable housing, lack of transportation, severe mental health conditions, untreated severe sleep apnea were excluded from the study. Detailed descriptions of the recruitment process are available elsewhere [26,27]. To be included in the secondary data analysis, participants needed to meet the aforementioned study criteria and endorse comorbid pain operationalized as a score of one or greater on the Brief Pain Inventory at baseline. Ultimately, 93 women veterans met criteria and are included here.
Procedure
The parent study was approved by the Institutional Review Board at the VA Greater Los Angeles Healthcare System. Participants who met initial eligibility criteria completed baseline assessments of their sleep, physical health, and mental health. Stratified random assignment, based on participants’ average reported sleep duration (≥6 hours vs. <6 hours) was used to balance the severity of sleep problems in each treatment group. Participants completed comprehensive assessments of their sleep, physical, and mental health immediately following the five-week intervention (post-treatment), as well as three months after completing the intervention (three-month follow-up).
Measures
Pain.
Participants’ pain symptoms were measured using the Brief Pain Inventory (BPI) [28]. Pain intensity on the BPI was measured using the mean of four questions rated on an 11-point Likert scale from 0 (no pain) to 10 (pain as bad as you can imagine). The pain interference subscale of the BPI measures how much pain impairs seven daily activities over the past week. Each item is rated on an 11-point Likert scale from 0 (does not interfere) to 10 (completely interferes). Total pain interference is calculated using the average of these seven items.
Selected items from the Comorbidity Index were used to identify participants with pre-existing chronic pain conditions [29]. Specifically, the index inquires about the presence or absence of 30 common physical conditions and 6 common mental health conditions. The index also queries about the presence of three pain conditions including chronic back pain, arthritis, and chronic headaches. These three conditions are among the most prevalent chronic pain conditions reported by women [30,31].
Sleep.
Participants’ sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI) [32]. The PSQI consists of 19 items that captures seven sleep domains and produces total score ranging from 0 to 21 with higher scores representing poorer sleep quality. In addition to the PSQI, the Insomnia Severity Index (ISI) was used to measure symptoms of insomnia [33]. The ISI consists of seven items and produces a total score ranging from 0 to 28. Higher scores on the ISI indicate greater symptoms of insomnia.
Mental Health.
Anxiety, depression, and PTSD symptoms were assessed via the Generalized Anxiety Disorder-7 (GAD-7), Patient Health Questionnaire-9 (PHQ-9), and the PTSD Checklist for DSM-5 (PCL-5), respectively [34–36]. The GAD-7 consists of seven items rated on 4-point Likert scale ranging from 0 (not at all) to 3 (nearly every day). Total scores range from 0 to 21 with higher scores corresponding with greater anxiety. The PHQ-9 consists of 9 items rated on the same Likert scale and produces a total score ranging from 0 to 27. Finally, veterans who endorsed exposure to trauma completed the PCL-5, a 20-item questionnaire that assesses for DSM-5 symptoms of PTSD. Items are rated on a five-point Likert scale ranging from 0 (not at all) to 4 (extremely). Scores greater than 31 are indicative of probable PTSD.
Dysfunctional Beliefs about Sleep.
Negative or unrealistic beliefs about sleep were assessed using the Dysfunctional Beliefs About Sleep–10 item scale (DBAS-10) [37] Items are rated on a visual analog scale from 0 (strongly disagree) to 10 (strongly agree). The sum score across all items was used to calculate a respondent’s total score on the DBAS, with higher scores representing greater dysfunctional beliefs about sleep.
Psychological Flexibility.
Psychological flexibility was assessed using the Acceptance and Action Questionnaire-II (AAQ-II) [38]. The measure consists of 7-items related to psychological flexibility as conceptualized by ACT. Each item is rated on a 7-point Likert scale from 1 (never true) to 7 (always true). Total scores range from 7 to 49, with lower scores indicating greater psychological flexibility.
Intervention Overview
Both arms of the intervention consisted of five, 60-minute, weekly individual sessions led by a trained study interventionist. Each treatment condition included behavioral strategies for insomnia (i.e., sleep restriction, stimulus control, and sleep hygiene) and required participants to complete a daily sleep diary.
CBT-I.
Session 1 of CBT-I focused on providing psychoeducation about sleep (3P model), introducing stimulus control, and reviewing proper sleep hygiene habits. Session 2 provided a rationale for sleep restriction, reviewed participants’ sleep diaries, and developed a sleep restriction action plan. During session 3, participants’ sleep diaries were reviewed and time in bed was adjusted. The usefulness of sleep-related cognitions was discussed and participants were asked to create positive coping cards. Session 4 focused on addressing obstacles to progress and using cognitive strategies, such as cognitive restructuring, to address issues related to treatment adherence. Finally, session 5 focused on relapse prevention strategies and consolidating treatment gains.
ABC-I.
ABC-I treatment sessions covered the same general topics but infused sessions with ACT-based strategies. The first session provided psychoeducation about sleep (3P model), introduced stimulus control, reviewed sleep hygiene practices, and used ACT-based metaphors to enhance participants’ motivation for behavioral prescriptions. Session 2 provided a rationale for sleep restriction, emphasizing the benefit of short-term discomfort versus long-term benefit using ACT metaphors. Participants were asked to implement the sleep restriction schedule while continuing to complete a daily sleep diary. Session 3 focused on introducing additional ACT-based concepts to assist patients in managing insomnia symptoms (e.g., cognitive defusion, dirty vs. clean discomfort). During session 4, time in bed was adjusted according to participants’ sleep diaries and participants were encouraged to view and manage obstacles through an ACT-based lens. Finally, session 5 was dedicated to reviewing relapse prevention strategies.
Data Analyses
Participants who endorsed pain as measured by the BPI at baseline were included in the current analyses. All analyses were completed using SPSS version 27 [39]. Pain intensity and pain interference were analyzed using a two by three factorial mixed-effects model with a fixed intercept in which treatment group was a two-level between-subjects factor (CBT-I vs. ABC-I) and time was a three-level repeated-measures factor (baseline, post-treatment, and three-month follow-up). Mixed effects models conducted did not include specification of random effects. Using these mixed-effects models, average pain intensity and pain interference for each time point was calculated. In addition, differences in the average change of pain intensity and pain interference from baseline to each follow-up time point were calculated for the two treatment groups. Thus, the mixed-effects models allowed for the examination of both group differences, as well as the rate of change over time. Bonferroni-corrected post-hoc tests were conducted to examine differences in pain intensity and inference across different timepoints.
In order to determine whether changes in pain intensity and pain interference were the same between the two treatment groups, two non-inferiority tests, using the two one-sided tests (TOST) procedure [40], specifying an upper and lower equivalence bound based on the smallest effect size of interest for pain intensity and interference were estimated. Among 11-point numerical pain rating scales, such as those contained within the BPI, a 2-point difference in pain has been identified as the minimally clinically important difference [41]. Thus, for the purposes of this study, the non-inferiority margin was defined as a mean difference in pain intensity or interference ±2. Specifically, non-inferiority was established if the 95% confidence interval of the difference between mean pain of the CBT-I group and mean pain of the ABC-I group was completely contained between the lower bound of the non-inferiority margin (i.e., −2) and zero.
In order to examine mechanisms which mediated the relationship between group assignment and pain outcomes, two mediational analyses using Model 4 in PROCESS macro v3.0 were conducted in SPSS [42]. Group assignment (CBT-I vs. ABC-I) was entered as the independent variable, while dysfunctional beliefs about sleep and psychological flexibility at post-treatment only, as reported by the DBAS-10 and AAQ-II, were entered as parallel mediators. Pain intensity and pain interference at the three-month follow-up were included as the respective dependent variable for each model. Finally, two repeated-measures ANOVAs were also conducted to examine changes in dysfunctional beliefs and psychological flexibility by treatment group. Once again, treatment group was included as a two-level between-subjects factor (CBT-I vs. ABC-I) while time was entered as a three-level repeated-measures factor (baseline, post-treatment, and three-month follow-up). Bonferroni-corrected post-hoc tests were conducted to examine differences in dysfunctional beliefs and psychological flexibility across different timepoints.
Little’s MCAR test was used to determine whether data were missing completely at random with respect to key variables including age, insomnia symptoms, pain intensity, pain interference, and mental health symptoms.
Results
Descriptive Statistics
Ninety-three women endorsed pain at baseline and were randomized to receive CBT-I (n=48) or ABC-I (n=45). These participants were primarily middle-aged (M=46.7, SD=12.7) and college-educated (M education=16.5 years, SD=2.8). Participants were predominately White (40.9%) or African American (33.3%). Over half were working at least part-time (51.2%) and a third were married (34.10%). Of the 93 individuals included in this analysis, 5 did not complete the post-treatment assessment (1 CBT-I; 4 ABC-I) and 7 did not complete the 3-month follow-up assessment (3 CBT-I; 4 ABC-I) because they could not be reached by telephone or mail (i.e., passive refusal to complete the study assessments).
Insomnia symptoms based on the ISI fell within the moderate range (M=15.2, SD=4.9). Pain scores were moderate with an average BPI intensity score of 4.9 (SD=1.8) and an average BPI interference score of 5.1 (SD=2.5). The majority of participants endorsed a history of chronic back pain (64.5%), arthritis (59.1%) or chronic headaches (51.2%). On average, participants endorsed having nearly six medical or psychological comorbidities. The average score on the PHQ-9 was consistent with moderate depressive symptoms while anxiety symptoms on the GAD-7 fell within the mild range. Approximately 43.5% of individuals endorsed PTSD symptoms that fell within the clinical range.
Of the 93 participants with pain who were randomized to receive either CBT-I or ABC-I, 88 completed the post-intervention assessment and 86 completed the three-month follow-up assessment. The number of participants within each group at each of the assessment periods is presented in Figure 1. Little’s MCAR test was non-significant, χ2=19.14, df =27, p=.87, suggesting that data were missing completely at random with respect to age, insomnia symptoms, pain intensity, pain interference, and mental health symptoms. Complete demographic and clinical data are presented in Table 1.
Figure 1.
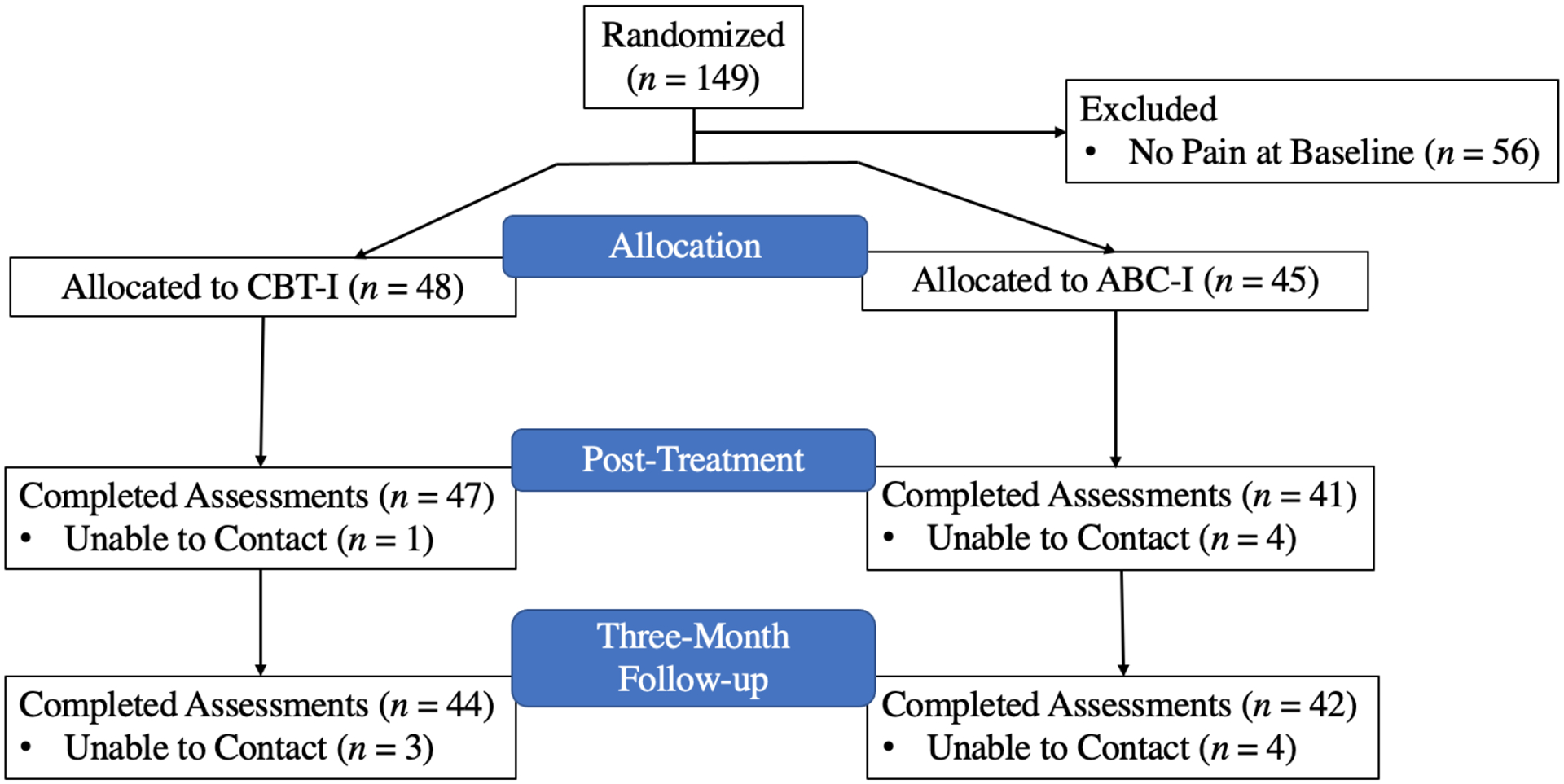
Flow diagram of the number of participants at each study assessment
Table 1.
Descriptive Statistics for Randomized Participants Endorsing Pain at Baseline
| Total (N = 93) |
CBT-I (N = 48) |
ABC-I (N = 45) |
Sig. Difference | |
|---|---|---|---|---|
| Age | 46.7 (12.7) | 45.0 (12.5) | 48.6 (12.7) | NS |
| Race/Ethnicity | ||||
| African American | 31 (33.3%) | 21 (67.7%) | 10 (32.3%) | p = .03 |
| American Indian /Alaska Native | 6 (6.4%) | 0 (0%) | 6 (100%) | p = .01 |
| Asian | 4 (4.3%) | 2 (50.0%) | 2 (50.0%) | NS |
| Hispanic | 23 (24.7%) | 11 (47.8%) | 12 (52.2%) | NS |
| Native Hawaiian/Pacific Islander | 2 (2.2%) | 0 (0%) | 2 (100%) | NS |
| Other | 5 (5.4%) | 1 (20.0%) | 4 (80.0%) | NS |
| White | 38 (40.9%) | 15 (39.5%) | 23 (60.5%) | NS |
| Employment Status | ||||
| Employed for wages | 43 (46.2%) | 22 (51.2%) | 21 (48.8%) | NS |
| Unable to work | 11 (11.8%) | 5 (45.4%) | 6 (54.5%) | NS |
| Unemployed | 3 (9.7%) | 1 (33.3%) | 2 (66.6%) | NS |
| Retired | 15 (16.1%) | 7 (46.7%) | 8 (53.3%) | NS |
| Student | 8 (8.6%) | 6 (75.0%) | 2 (25.0%) | NS |
| Homemaker | 4 (4.3%) | 2 (50.0%) | 2 (50.0%) | NS |
| Sleep | ||||
| PSQI Total Score | 11.2 (3.7) | 11.9 (3.7) | 10.5 (3.6) | NS |
| ISI Total Score | 15.2 (4.9) | 16.0 (5.0) | 14.4 (4.8) | NS |
| Pain | ||||
| BPI-Pain Intensity | 4.9 (1.8) | 4.8 (1.8) | 5.0 (1.8) | NS |
| BPI-Pain Interference | 5.1 (2.5) | 5.2 (2.5) | 5.0 (2.6) | NS |
| Chronic Back Pain | 60 (64.5%) | 33 (55.0%) | 27 (45.0%) | NS |
| Arthritis | 55 (59.1%) | 30 (54.5%) | 25 (45.5%) | NS |
| Chronic Headaches | 48 (51.2%) | 23 (47.9%) | 25 (52.1%) | NS |
| Mental Health | ||||
| PHQ-9 | 10.7 (5.3) | 10.81 (5.0) | 10.6 (5.7) | N S |
| GAD-7 | 10.1 (5.7) | 11.7 (5.1) | 8.5 (5.9) | p = .01 |
| PCL-5 | 27.7 (22.1) | 28.7 (21.1) | 26.7 (23.3) | NS |
| General Health | ||||
| BMI | 29.8 (11.2) | 31.7 (14.3) | 27.8 (6.1) | N S |
| Comorbidity Index Total | 5.8 (2.9) | 5.4 (2.8) | 6.2 (3.0) | NS |
| Treatment Mechanisms | ||||
| DBAS-10 | 63.2 (17.2) | 64.2 (16.2) | 62.3 (16.2) | NS |
| AAQ-II | 25.8 (11.1) | 25.0 (9.8) | 26.6 (12.5) | NS |
Note. Age measured in years. Significance difference is between the CBT-I and ABC-I groups. NS = not significant. AAQ-II = Acceptance and Action Questionnaire-II, AHI = Apnea-Hypopnea Index, BPI = Brief Pain Inventory, BMI = Body Mass Index, DBAS-10 = Dysfunctional Beliefs about Sleep-10, ESS = Epworth Sleepiness Scale, GAD-7 = Generalized Anxiety Disorder-7, ISI = Insomnia Severity Index, PCL-5 = PTSD Checklist for the DSM-5, PHQ-9= Patient Health Questionnaire-9, PSQI = Pittsburgh Sleep Quality Index.
Pain Intensity by Treatment Group.
Results revealed a statistically significant main effect for time, F(2,78)=12.99 p<.001, η2=.14, but no main effect for treatment condition, F(1,79)=.02, p=.89, η2 <.001, nor for a treatment condition*time interaction, F(2,75)=.48, p=.62, η2=.01. Bonferroni-corrected post-hoc comparisons revealed significant differences in pain intensity between time 1 (baseline) and time 2 (follow-up) (p < .001, Cohen’s d = .67), as well as between time 1 and time 3 (three-month follow-up) (p=.001, Cohen’s d = .41), but no statistically significant differences between time 2 and 3 (p=.45). Thus, while pain intensity improved in the overall sample, there was no evidence to support treatment group differences with regards to pain intensity, nor was there evidence to support differences in the rate of pain intensity change by treatment group. As depicted in Figure 2, mean differences in participants’ average level of pain intensity between treatment conditions at post-treatment and three-month follow-up are completely contained within the pre-established equivalence interval indicating that ABC-I is non-inferior to CBT-I with regards to its impact on pain intensity.
Figure 2.
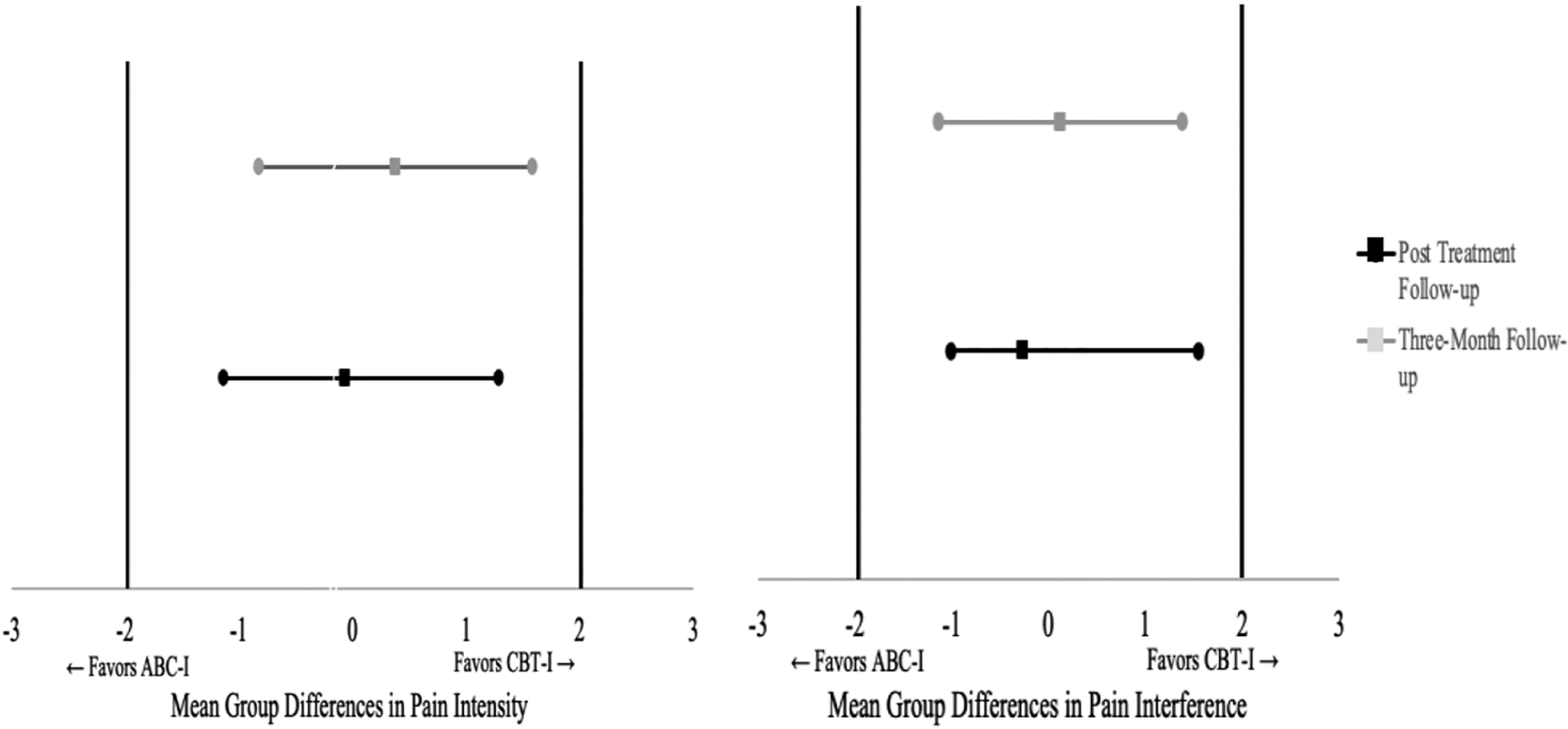
Non-inferiority tests for pain intensity and interference at post-treatment and three-month follow-up. Note. ABC-I is considered non-inferior to CBT-I with respect to pain interference if the 95% confidence interval is completely contained between zero and the lower bound of the clinical threshold (depicted as bold horizontal lines).
Pain Interference by Treatment Group.
Results from the repeated measures ANOVA once again revealed a statistically significant main effect for time, F(2,78)=24.70, p<.001, η2=.24, but no effect for treatment condition, F(1,79)= .05, p=.82, η2=.001, nor for a treatment condition*time interaction, F(2,78)=.21, p=.81 η2=.003. Bonferroni-corrected post-hoc comparisons revealed significant reductions in pain interference between time 1 and time 2 (p<.001, Cohen’s d = .77), as well as significant reductions between time 1 and time 3 (p<.001, Cohen’s d = .71) but no differences between time 2 and 3 (p=.98). Figure 3 displays the average level of pain intensity and pain interference by treatment group over time. Differences in pain interference among the two treatment groups at post-treatment and three-month follow-up are completely contained within the pre-established equivalence interval indicating that ABC-I is non-inferior to CBT-I with respect to reductions in pain interference.
Figure 3.
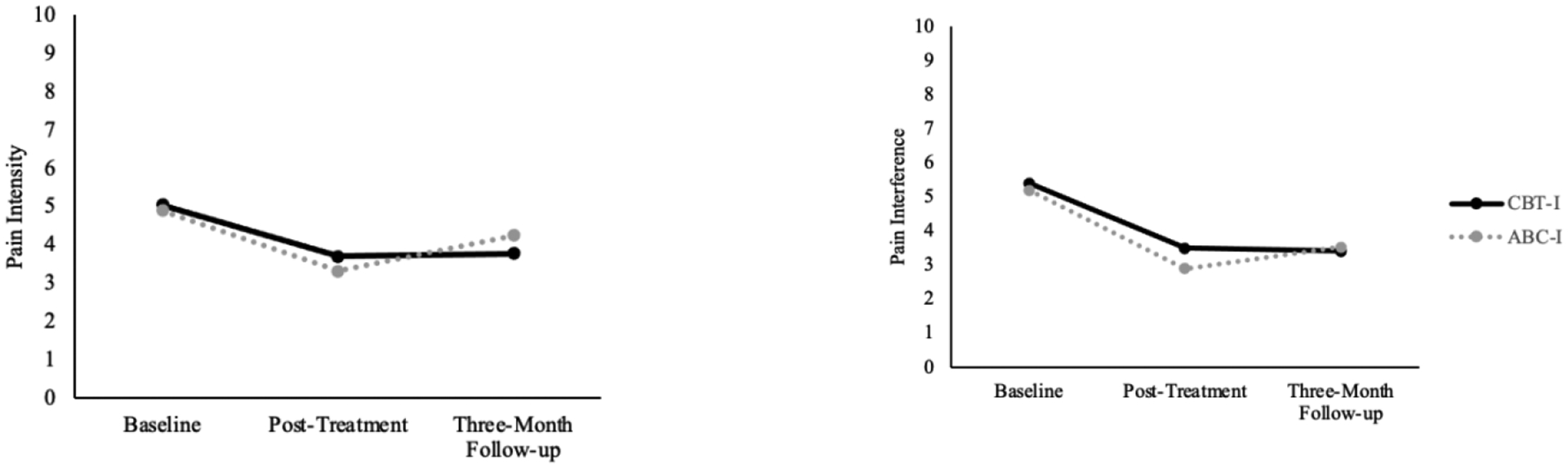
BPI Pain Intensity and Interference by Treatment Group Across Time.
Psychological mechanisms linking insomnia treatments to pain outcomes.
A mediation analysis found no direct effect for treatment group on pain severity (c’: β=.09, p=.68). However, psychological factors indirectly mediated the relationship between treatment group and pain interference [a × b: β=−.24, CI: −.51, −.02). Treatment group indirectly impacted pain severity via dysfunctional beliefs about sleep [a1 × b1: β=−.24, CI: (−.52, −.03)] but not psychological flexibility [a2 × b2: β=−.001, CI: (−.08, .06)]. That is, relative to the ABC-I condition, the CBT-I condition was negatively associated with dysfunctional beliefs about sleep post-treatment (β=−.53, p<.05) and these beliefs were positively associated with pain severity at the three-month follow-up (β=.44, p≤.001).
Treatment group also did not predict pain interference at the three-month follow-up (c’: β=.23, p=.28). In contrast to pain intensity, there was no overall indirect relationship between treatment group and pain interference via overall psychological factors (a × b: β=−.22, CI: −.48, .01). However, the relationship between treatment group and pain interference was indirectly mediated by dysfunctional beliefs about sleep (a1 × b1: β=−.21 CI: −.43, −.03) but not psychological flexibility (a2 × b2: β=−.01, CI:−.14, .08). Thus, once again, being assigned to the CBT-I condition was associated with decreased dysfunctional beliefs about sleep post-treatment (β=−.53, p<.05) and these beliefs were positively associated with pain interference at the three-month follow-up assessment (β=.38, p≤.001). Results of the aforementioned mediation analyses for pain intensity and interference are presented in Figure 4.
Figure 4.
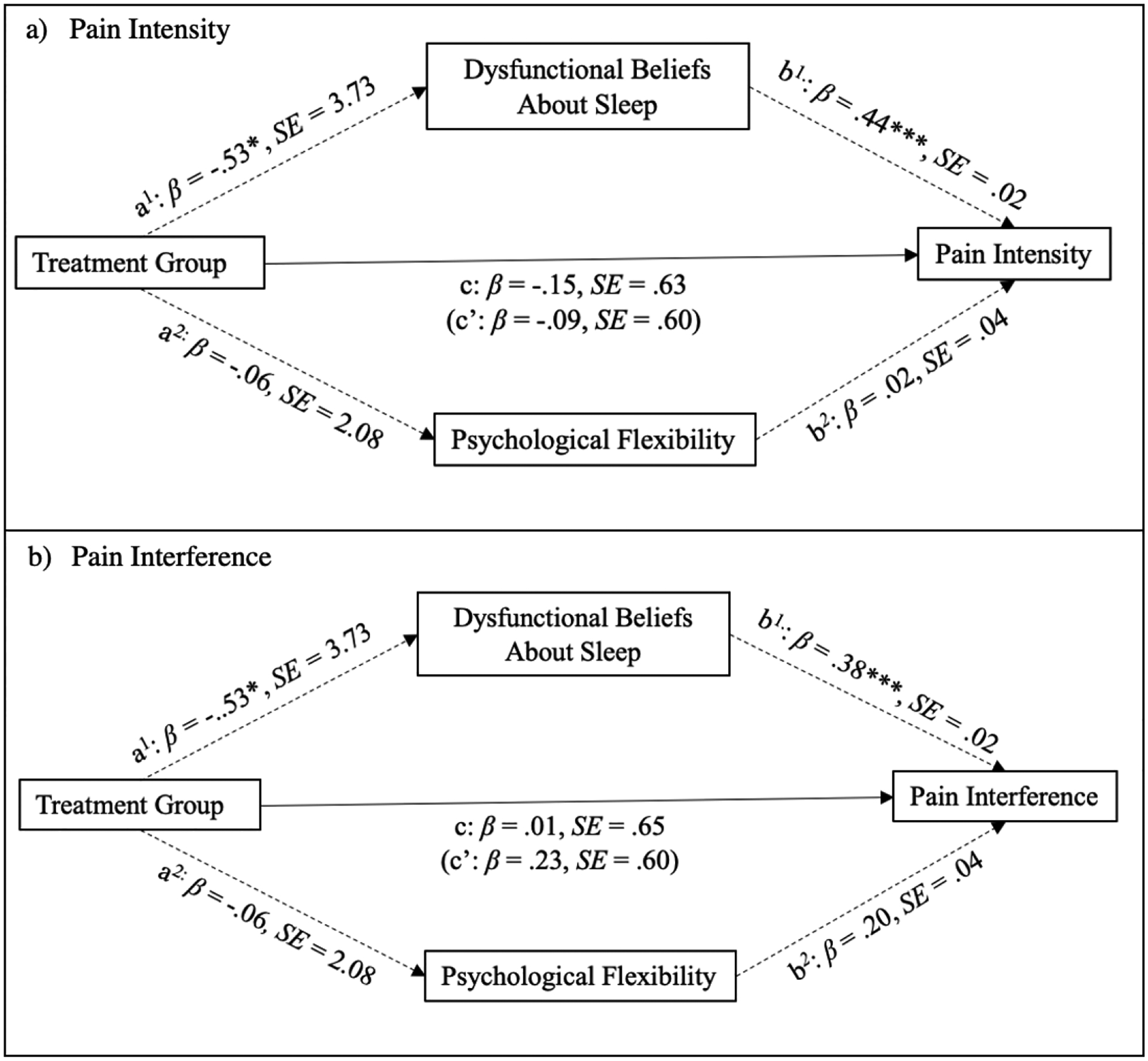
Mediational analyses of treatment mechanisms. Notes. * p < .05, **p ≤ .01, ***p <.001. Treatment group was dummy coded as follows: CBT-I = 1, ABC-I = 0. Standardized coefficients for dichotomous variables are in partially standardized form. Total and specific indirect effects are not represented in the figure.
Two additional 2 × 2 repeated-measures ANOVAs were conducted to further elucidate changes in dysfunctional beliefs and psychological flexibility by treatment group. While the repeated-measures ANOVA examining changes in dysfunctional beliefs did not reveal a significant main effect for treatment group, F(1, 81)=1.20, p=.27, η2=.02, there was a significant main effect for time, F(1,81)=145.14, p< .001, η2=.64, as well as a time*treatment group interaction effect, F(1,81)=7.53, p=.01, η2=.09. Thus, although dysfunctional beliefs about sleep decreased over time as a whole, this change was stronger for participants receiving CBT-I. At post-treatment, participants in the CBT-I group reported statistically lower levels of dysfunctional beliefs about sleep compared to the ABC-I group, F(1, 82)=7.14, p=.01, Cohen’s d = .59.
The repeated-measures ANOVA examining changes in psychological flexibility by treatment group produced a significant main effect for time, F(1,81)=54.99, p<.001, η2=.40, but no time*treatment group interaction effect, F(1,81)=.05, p=.83, η2=.001, nor any main effect for treatment group, F(1,81)=.34, p=.56, η2=.004. Pairwise comparisons revealed a significant increase in psychological flexibility from baseline to post-treatment for all participants (p<.001, Cohen’s d = .67) but there was no difference between the two groups at post-treatment (p=. 61). Changes in dysfunctional beliefs about sleep and psychological flexibility are presented as a figure in the supplemental materials.
Discussion
Consistent with our hypotheses, results from the present study suggest that both CBT-I and ABC-I were associated with small, but significant, decreases in pain over time with ABC-I being non-inferior to CBT-I with regards to its effect on pain intensity and interference among women veterans with comorbid insomnia and pain. Additionally, while CBT-I and ABC-I were both associated with greater psychological flexibility following treatment, participants assigned to the CBT-I condition reported greater decreases in dysfunctional beliefs about sleep than ABC-I.
Pain Outcomes and Treatment Non-Inferiority
The present findings contribute to existing knowledge in several important ways. Early research suggested that CBT-I was mostly associated with decreases in pain interference or improved functioning post-intervention [6]. However, coinciding with more recent studies [7], the current findings suggest that behavioral sleep interventions may also lead to small, but clinically meaningful, improvements in pain intensity post-intervention. Additionally, the current findings build on preliminary findings supporting the secondary effect of ABC-I on pain by suggesting that ABC-I may lead to similar pain outcomes as CBT-I, the gold-standard behavioral intervention for insomnia. This finding is particularly noteworthy given that examinations of other non-pharmacological interventions for comorbid insomnia and pain, such as hydro-therapy, massage myofascial therapy, or manual therapy, have been limited and produced inconsistent effects [43–45]. By leveraging the proven behavioral components of CBT-I with ACT-based strategies and techniques, ABC-I may serve as a useful non-pharmacological treatment for individuals with comorbid pain and insomnia. Finally, the current findings extend our understanding of treatments that may be beneficial for women veterans – a growing and under-studied segment of the veteran population prone to high levels of morbidity [46].
Treatment Mechanisms
Decreases in dysfunctional beliefs about sleep following treatment is consistent with previous research which found that this construct mediates the impact of CBT-I on sleep outcomes [47,48]. The present study extends these findings by indicating that decreased dysfunctional beliefs about sleep might be one mechanism through which CBT-I also improves pain. Though the cognitive techniques in CBT-I focus on challenging dysfunctional thoughts that interfere with sleep, it is possible that these techniques generalize to pain-related dysfunctional cognitions. Previous research lends support to this hypothesis, with evidence that CBT-I is associated with decreased pain catastrophizing – a common cognitive distortion associated with adverse pain outcomes [10]. Notably, cognitive techniques contained within CBT-I are also a central part of other cognitive behavioral treatment frameworks, such as CBT for chronic pain.
While it may be tempting to believe that dysfunctional beliefs about sleep decreased in the CBT-I purely as a result of the cognitive restructuring skills introduced to patients, research suggests that these beliefs decrease even when patients only receive the behavioral component of CBT-I [49]. It is possible that the implementation of the various behavioral strategies indirectly challenged previously held beliefs about sleep, some of which were linked to pain symptoms. This process may also account for the small, but significant, decreases in dysfunctional beliefs observed among participants in the ABC-I condition.
Our finding that both treatment groups reported similar improvements in psychological flexibility post-treatment coincides with research indicating that, despite having different theoretical underpinnings, CBT and ACT may influence the same shared mechanisms [50]. For example, among patients receiving either CBT or ACT for anxiety or depression, decreases in dysfunctional thinking, cognitive defusion, and patients’ willingness to engage in activities despite the presence of unpleasant emotions were equivalent among both forms of treatment [51]. Given the strong association between psychological flexibility and mental health symptoms [52], improvements in mood commonly observed following treatment may also account for why the treatment groups did not differ with respect to their impact on psychological flexibility. Unfortunately, the lack of research directly comparing the effectiveness of ACT and CBT-based approaches to insomnia obscures our understanding of the unique and shared ways in which these treatments may impact hypothesized mechanisms.
This study also adds to the available evidence regarding providing comprehensive care for women veterans to address chronic pain. Studies show that women veterans with chronic pain use more healthcare services than men veterans with chronic pain [53], and that women may benefit from different types of treatment including yoga [54]. It may be possible to implement CBT-I or ABC-I into existing programs that assist women veterans with chronic pain conditions.
Limitations & Future Directions
The present study is not without limitations. First, the parent study was designed to recruit women veterans seeking treatment for insomnia. Additional research is needed to know whether the current results would generalize to women veterans with comorbid insomnia and pain who were actively recruited based on pain criteria. Relatedly, as the parent study was focused on sleep, we have limited information about pain-related factors such as whether participants viewed pain as a primary concern. Future studies are needed to explore the relative effects of CBT-I vs. ABC-I among individuals with specific chronic pain conditions. Other factors which are known to predict pain outcomes, including pain catastrophizing and pain acceptance, should also be examined as potential treatment mechanisms. Additionally, the current findings were limited to women veterans. Although this population is prone to comorbidity and has been historically overlooked by past research, further work is needed to examine whether the current findings extend to other populations. Despite these limitations, ABC-I appears to hold important secondary effects on chronic pain and future research may benefit from exploring the effect of a hybrid ACT treatment targeting both insomnia and chronic pain.
Conclusions
Given the high prevalence of comorbidities among veterans, it is essential to prioritize interventions that provide symptom relief across different conditions. The present study provides valuable insights into treatments that may benefit individuals experiencing insomnia and comorbid pain. ABC-I was found to be non-inferior to CBT-I with respect to its impact on pain symptoms with psychological flexibility improving after both treatments while CBT-I was associated with greater reductions in dysfunctional beliefs about sleep. Future research should build on the present results by examining whether ACT interventions targeting both insomnia and pain lead to greater reduction in symptoms.
Supplementary Material
Highlights.
Women veterans are prone to both insomnia and pain.
Cognitive behavioral therapy for insomnia (CBT-I) can improve pain.
CBT-I was compared to Acceptance and Behavioral Changes for Insomnia (ABC-I).
ABC-I combines behavioral sleep strategies with Acceptance and Commitment Therapy.
ABC-I was non-inferior to CBT-I with respect to its effect on pain outcomes.
Funding:
This work was supported by Veterans Administration Health Services Research and Development Service (VA HSR&D) IIR 16-244 (PI: Martin); VA HSR&D Research Career Scientist Award RCS 20-191(PI: Martin); VA HSR&D Senior Research Career Scientist Award RCS 05-195 (PI: Yano); National Institutes of Health/National Heart Lung Blood Institute K23HL157754 (PI: Kelly), K24 HL143055 (PI: Martin), and National Institute on Aging (K23AG049955, PI: Dzierzewski, K23AG055668, PI: Song), VA Greater Los Angeles Healthcare System Geriatric Research, Education and Clinical Center and VA Office of Academic Affiliations (Kelly, Carlson); VA HSR&D Career Development Award (Carlson).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interest Statement: The authors have no competing interests to report.
Data Availability Statement:
Data may be available through execution of a data use agreement between the requestor’s institution and the VA Greater Los Angeles Healthcare System.
References
- [1].Jank R, Gallee A, Boeckle M, Fiegl S, Pieh C, Chronic Pain and Sleep Disorders in Primary Care, Pain Res. Treat 2017 (2017). 10.1155/2017/9081802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Husak AJ, Bair MJ, Chronic pain and sleep disturbances: A pragmatic review of their relationships, comorbidities, and treatments, Pain Med. 21 (2020) 1142–1152. [DOI] [PubMed] [Google Scholar]
- [3].Finan PH, Smith MT, The comorbidity of insomnia, chronic pain, and depression: Dopamine as a putative mechanism, Sleep Med. Rev 17 (2013) 173–183. 10.1016/j.smrv.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Edinger JD, Arnedt JT, Bertisch SM, Carney CE, Harrington JJ, Lichstein KL, Sateia MJ, Troxel WM, Zhou ES, Kazmi U, Heald JL, Martin JL, Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline, J. Clin. Sleep Med 17 (2021) 255–262. 10.5664/jcsm.8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD, C.G.C. of the A.C. of Physicians*, Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians, Ann. Intern. Med 165 (2016) 125–133. [DOI] [PubMed] [Google Scholar]
- [6].Finan PH, Buenaver LF, Coryell VT, Smith MT, Cognitive-Behavioral Therapy for Comorbid Insomnia and Chronic Pain, Sleep Med. Clin 9 (2014) 261–274. 10.1016/j.jsmc.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Selvanathan J, Pham C, Nagappa M, Peng PWH, Englesakis M, Espie CA, Morin CM, Chung F, Cognitive behavioral therapy for insomnia in patients with chronic pain – A systematic review and meta-analysis of randomized controlled trials, Sleep Med. Rev 60 (2021) 101460. 10.1016/j.smrv.2021.101460. [DOI] [PubMed] [Google Scholar]
- [8].Neilson BD, Shepherd MH, Dickerson C, Chaconas EJ, Young JL, Rhon DI, Relationship Between Attitudes and Beliefs About Sleep, Sleep Disturbance, and Pain Interference in Patients With Spinal Pain, Clin. J. Pain 38 (2022) 541. 10.1097/AJP.0000000000001051. [DOI] [PubMed] [Google Scholar]
- [9].Thakral M, Von Korff M, McCurry SM, Morin CM, Vitiello MV, Changes in Dysfunctional Beliefs about Sleep after Cognitive Behavioral Therapy for Insomnia: A Systematic Literature Review and Meta-analysis, Sleep Med. Rev 49 (2020) 101230. 10.1016/j.smrv.2019.101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lerman SF, Finan PH, Smith MT, Haythornthwaite JA, Psychological Interventions that Target Sleep Reduce Pain-Catastrophizing in Knee Osteoarthritis, Pain. 158 (2017) 2189–2195. 10.1097/j.pain.0000000000001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Afolalu EF, Moore C, Ramlee F, Goodchild CE, Tang NKY, Development of the Pain-Related Beliefs and Attitudes about Sleep (PBAS) Scale for the Assessment and Treatment of Insomnia Comorbid with Chronic Pain, J. Clin. Sleep Med 12 (2016) 1269–1277. 10.5664/jcsm.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J, Acceptance and commitment therapy: model, processes and outcomes, Behav. Res. Ther 44 (2006) 1–25. 10.1016/j.brat.2005.06.006. [DOI] [PubMed] [Google Scholar]
- [13].Hughes LS, Clark J, Colclough JA, Dale E, McMillan D, Acceptance and Commitment Therapy (ACT) for Chronic Pain, Clin. J. Pain 33 (2017) 552–568. 10.1097/AJP.0000000000000425. [DOI] [PubMed] [Google Scholar]
- [14].Kohl A, Rief W, Glombiewski JA, Acceptance, Cognitive Restructuring, and Distraction as Coping Strategies for Acute Pain, J. Pain 14 (2013) 305–315. 10.1016/j.jpain.2012.12.005. [DOI] [PubMed] [Google Scholar]
- [15].Bouchard S, Bastien C, Morin CM, Self-efficacy and adherence to cognitive-behavioral treatment of insomnia, Behav. Sleep. Med 1 (2003) 187–199. [DOI] [PubMed] [Google Scholar]
- [16].van de Laar M, Pevernagie D, van Mierlo P, Overeem S, Psychiatric comorbidity and aspects of cognitive coping negatively predict outcome in cognitive behavioral treatment of psychophysiological insomnia, Behav. Sleep. Med 13 (2015) 140–156. [DOI] [PubMed] [Google Scholar]
- [17].Fiorentino L, Martin JL, Alessi CA, The ABCs of Insomnia (ABC-I): An Acceptance Commitment Therapy (ACT)-Based Insomnia Treatment Development Study: Pilot Results and Future Directions, in: Sleep Med. Ment. Health, Springer, 2020: pp. 85–100. [Google Scholar]
- [18].Chapoutot M, Peter-Derex L, Schoendorff B, Faivre T, Bastuji H, Putois B, Telehealth-delivered CBT-I programme enhanced by acceptance and commitment therapy for insomnia and hypnotic dependence: A pilot randomized controlled trial, J. Sleep Res n/a (n.d.) e13199. 10.1111/jsr.13199. [DOI] [PubMed] [Google Scholar]
- [19].Zetterqvist V, Grudin R, Rickardsson J, Wicksell RK, Holmström L, Acceptance-based behavioural treatment for insomnia in chronic pain: A clinical pilot study, J. Context. Behav. Sci 9 (2018) 72–79. 10.1016/j.jcbs.2018.07.003. [DOI] [Google Scholar]
- [20].Ciarrochi J, Bilich L, Godsell C, Psychological flexibility as a mechanism of change in Acceptance and Commitment Therapy, (n.d.).
- [21].Gentili C, Rickardsson J, Zetterqvist V, Simons LE, Lekander M, Wicksell RK, Psychological Flexibility as a Resilience Factor in Individuals With Chronic Pain, Front. Psychol 10 (2019). https://www.frontiersin.org/articles/10.3389/fpsyg.2019.02016 (accessed April 6, 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McCracken LM, Williams JL, Tang NKY, Psychological Flexibility May Reduce Insomnia in Persons with Chronic Pain: A Preliminary Retrospective Study, Pain Med. 12 (2011) 904–912. 10.1111/j.1526-4637.2011.01115.x. [DOI] [PubMed] [Google Scholar]
- [23].Vowles KE, McCracken LM, Comparing the role of psychological flexibility and traditional pain management coping strategies in chronic pain treatment outcomes, Behav. Res. Ther 48 (2010) 141–146. 10.1016/j.brat.2009.09.011. [DOI] [PubMed] [Google Scholar]
- [24].Martin J, Carlson G, Kelly M, Song Y, Mitchell M, Josephson K, McGownan SK, Culver N, Kay M, Erickson A, Saldana K, May K, Fiorentino L, Alessi C, Washington D, Yano E, Novel Treatment Based on Acceptance and Commitment Therapy versus Cognitive-Behavioral Therapy for Insomnia: A Randomized Comparative Effectiveness Trial in Women Veterans, Journal of Consulting and Clinical Psychology. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Martin JL, Schweizer CA, Hughes JM, Fung CH, Dzierzewski JM, Washington DL, Kramer BJ, Jouldjian S, Mitchell MN, Josephson KR, Alessi CA, Estimated Prevalence of Insomnia among Women Veterans: Results of a Postal Survey, Womens Health Issues. 27 (2017) 366–373. 10.1016/j.whi.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Carlson GC, Kelly MR, Grinberg AM, Mitchell M, McGowan SK, Culver NC, Kay M, Alessi CA, Washington DL, Yano EM, Insomnia precipitating events among Women Veterans: the impact of traumatic and nontraumatic events on sleep and mental health symptoms, Behav. Sleep. Med 19 (2021) 672–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Carlson GC, Kelly MR, Mitchell M, Josephson KR, McGowan SK, Culver NC, Kay M, Alessi CA, Fung CH, Washington DL, Benefits of cognitive behavioral therapy for insomnia for women veterans with and without probable post-traumatic stress disorder, Womens Health Issues. 32 (2022) 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cleeland CS, Ryan KM, The brief pain inventory, Pain Res. Group (1991). [Google Scholar]
- [29].Selim AJ, Fincke BG, Ren XS, Lee A, Rogers WH, Miller DR, Skinner KM, Linzer M, Kazis LE, Comorbidity Assessments Based on Patient Report: Results From the Veterans Health Study, J. Ambulatory Care Manage 27 (2004) 281–295. [DOI] [PubMed] [Google Scholar]
- [30].Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL, Sex, Gender, and Pain: A Review of Recent Clinical and Experimental Findings, J. Pain Off. J. Am. Pain Soc 10 (2009) 447–485. 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mogil JS, Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon, Nat. Rev. Neurosci 13 (2012) 859–866. 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- [32].Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research, Psychiatry Res. 28 (1989) 193–213. [DOI] [PubMed] [Google Scholar]
- [33].Bastien CH, Vallières A, Morin CM, Validation of the Insomnia Severity Index as an outcome measure for insomnia research, Sleep Med. 2 (2001) 297–307. [DOI] [PubMed] [Google Scholar]
- [34].Kroencke K, Spitzer R, Williams J, The PHQ-9: validity of a brief depression severity measure [Electronic version], J. Gen. Intern. Med 16 (2001) 606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Spitzer RL, Kroenke K, Williams JB, Löwe B, A brief measure for assessing generalized anxiety disorder: the GAD-7, Arch. Intern. Med 166 (2006) 1092–1097. [DOI] [PubMed] [Google Scholar]
- [36].Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL, The posttraumatic stress disorder checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation, J. Trauma. Stress 28 (2015) 489–498. [DOI] [PubMed] [Google Scholar]
- [37].Edinger JD, Wohlgemuth WK, Psychometric comparisons of the standard and abbreviated DBAS-10 versions of the dysfunctional beliefs and attitudes about sleep questionnaire, Sleep Med. 2 (2001) 493–500. 10.1016/s1389-9457(01)00078-8. [DOI] [PubMed] [Google Scholar]
- [38].Bond FW, Hayes SC, Baer RA, Carpenter KM, Guenole N, Orcutt HK, Waltz T, Zettle RD, Preliminary Psychometric Properties of the Acceptance and Action Questionnaire–II: A Revised Measure of Psychological Inflexibility and Experiential Avoidance, Behav. Ther 42 (2011) 676–688. 10.1016/j.beth.2011.03.007. [DOI] [PubMed] [Google Scholar]
- [39].IBM SPSS Statistics for Windows, (2020).
- [40].Schuirmann DJ, A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability, J. Pharmacokinet. Biopharm 15 (1987) 657–680. [DOI] [PubMed] [Google Scholar]
- [41].Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM, Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale, Pain. 94 (2001) 149–158. 10.1016/s0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- [42].Hayes AF, Introduction to mediation, moderation, and conditional process analysis: A regression-based approach, Guilford Publications, 2017. [Google Scholar]
- [43].Castro-Sánchez AM, Aguilar-Ferrándiz ME, Matarán-Peñarrocha GA, Sánchez-Joya MDM, Arroyo-Morales M, Fernández-de-las-Peñas C, Short-term effects of a manual therapy protocol on pain, physical function, quality of sleep, depressive symptoms, and pressure sensitivity in women and men with fibromyalgia syndrome: a randomized controlled trial, Clin. J. Pain 30 (2014) 589–597. 10.1097/AJP.0000000000000008. [DOI] [PubMed] [Google Scholar]
- [44].Castro-Sánchez AM, Matarán-Peñarrocha GA, Granero-Molina J, Aguilera-Manrique G, Quesada-Rubio JM, Moreno-Lorenzo C, Benefits of massage-myofascial release therapy on pain, anxiety, quality of sleep, depression, and quality of life in patients with fibromyalgia, Evid.-Based Complement. Altern. Med. ECAM 2011 (2011) 561753. 10.1155/2011/561753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].de M. Vitorino DF, de Carvalho LBC, do Prado GF, Hydrotherapy and conventional physiotherapy improve total sleep time and quality of life of fibromyalgia patients: randomized clinical trial, Sleep Med. 7 (2006) 293–296. 10.1016/j.sleep.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [46].Bean-Mayberry B, Yano EM, Washington DL, Goldzweig C, Batuman F, Huang C, Miake-Lye I, Shekelle PG, Systematic review of women veterans’ health: update on successes and gaps, Womens Health Issues Off. Publ. Jacobs Inst. Womens Health 21 (2011) S84–97. 10.1016/j.whi.2011.04.022. [DOI] [PubMed] [Google Scholar]
- [47].Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE, Does cognitive-behavioral insomnia therapy alter dysfunctional beliefs about sleep?, Sleep. 24 (2001) 591–599. 10.1093/sleep/24.5.591. [DOI] [PubMed] [Google Scholar]
- [48].Morin CM, Blais F, Savard J, Are changes in beliefs and attitudes about sleep related to sleep improvements in the treatment of insomnia?, Behav. Res. Ther 40 (2002) 741–752. 10.1016/s0005-7967(01)00055-9. [DOI] [PubMed] [Google Scholar]
- [49].Eidelman P, Talbot L, Ivers H, Bélanger L, Morin CM, Harvey AG, Change in Dysfunctional Beliefs About Sleep in Behavior Therapy, Cognitive Therapy, and Cognitive-Behavioral Therapy for Insomnia, Behav. Ther 47 (2016) 102–115. 10.1016/j.beth.2015.10.002. [DOI] [PubMed] [Google Scholar]
- [50].Burns JW, Mechanisms, Mechanisms, Mechanisms: It Really Does All Boil Down to Mechanisms, Pain. 157 (2016) 2393–2394. 10.1097/j.pain.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Forman EM, Chapman JE, Herbert JD, Goetter EM, Yuen EK, Moitra E, Using Session-by-Session Measurement to Compare Mechanisms of Action for Acceptance and Commitment Therapy and Cognitive Therapy, Behav. Ther 43 (2012) 341–354. 10.1016/j.beth.2011.07.004. [DOI] [PubMed] [Google Scholar]
- [52].Kashdan TB, Rottenberg J, Psychological flexibility as a fundamental aspect of health, Clin. Psychol. Rev 30 (2010) 865–878. 10.1016/j.cpr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kaur S, Stechuchak KM, Coffman CJ, Allen KD, Bastian LA, Gender Differences in Health Care Utilization Among Veterans with Chronic Pain, J. Gen. Intern. Med 22 (2007) 228–233. 10.1007/s11606-006-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Groessl EJ, Weingart KR, Johnson N, Baxi S, The benefits of yoga for women veterans with chronic low back pain, J. Altern. Complement. Med 18 (2012) 832–838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be available through execution of a data use agreement between the requestor’s institution and the VA Greater Los Angeles Healthcare System.


