Abstract
Type 1 diabetes (T1D) is a chronic autoimmune disease associated with complications that reduce the quality of life of affected individuals and their families. The therapeutic options for T1D are limited to insulin therapy and islet transplantation; these options are not focused on preserving β-cell function and endogenous insulin. Despite the promising outcomes observed in current clinical trials involving allogeneic Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) infusion for the management of T1D, the precise underlying mechanism of action remains to be elucidated. In this correspondence, we propose prospective mechanisms of action of WJ-MSCs that may be mediating their observed capability to preserve β-cell function and prevent T1D progression and provide recommendations for further investigations in clinical settings. We also highlight the efficacy of WJ-MSCs for therapeutic applications in comparison to other adult MSCs. Finally, we recommend the participation of muti-centers governed by international organizations to implement guidelines for the safe practice of cell therapy and patients’ welfare.
Keywords: Wharton’s jelly, WJ-MSC, clinical trials, mechanism of action, ProTrans, allogeneic
Graphical Abstract
Graphical Abstract.
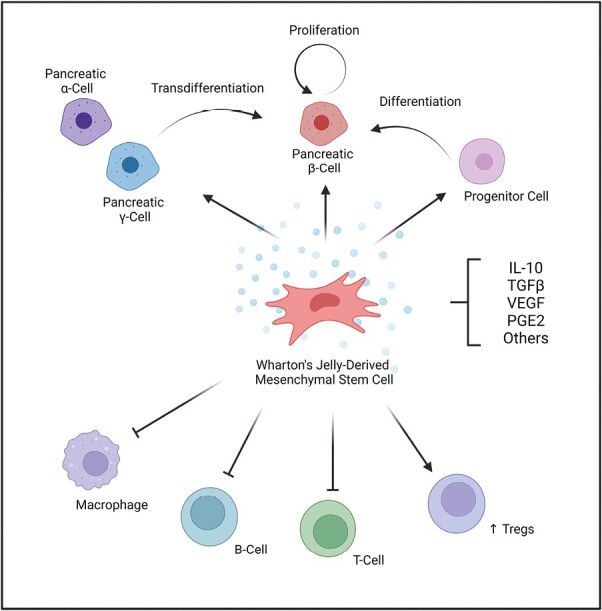
Significance Statement.
Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) have been shown to be safe and effective in the clinical trials of type 1 diabetes (T1D), with no adverse events reported. While WJ-MSCs have been shown to be effective in preserving pancreatic β-cell function, the precise mechanism of action is still unknown and requires further investigation. We hypothesized that the secretome of WJ-MSCs may play a role in enhancing β-cell proliferation, progenitor cell differentiation, and/or modulating the immune system. Future multi-center clinical studies should include cellular dynamics and kinetics studies to further elucidate the mechanism of action of WJ-MSCs in the treatment of T1D.
Introduction
Type 1 diabetes (T1D) is a chronic autoimmune disease associated with complications that reduce the quality of life of affected individuals and their families. It accounts for 5%-10% of all diabetes cases worldwide.1 The therapeutic options for T1D are limited to insulin therapy and islet transplantation2; these options are not focused on preserving β-cell function and endogenous insulin, resulting in a substantial increase in the risk of complications. To tackle this, recent approaches involve the use of cellular therapeutic interventions to prevent the progression of T1D by preserving pancreatic β-cell function. A relatively new treatment option for cellular therapy is the use of mesenchymal stem cells (MSCs), which has gained much attention in the medical field for treating several diseases including diabetes. MSCs are multipotent non-hematopoietic cells with distinctive stemness characteristics.3,4 MSCs resides primarily in the bone marrow, but they are also detected in different adult and fetal tissues.5,6 In adults, MSCs exist in adipose tissue, dental pulp, skin, hematopoietic tissues, and others.7 In fetuses, MSCs are enriched in fetal tissues including umbilical cord stroma, placenta, amniotic fluid, and endometrium.7,8 Generally, MSCs express common cell surface markers and have multipotency capacities to differentiate into the different germ layers,9,10 nevertheless, they also possess different properties depending on their tissue source.
Remarkably, MSCs isolated from fetal tissues are superior for regenerative medicine applications because, unlike adult cells, they have a higher proliferation rate, longevity, and immune privilege. These unique properties are due to the absence of genetic alternations or modulations that are associated with aging and exposure to environmental toxins.11 Furthermore, fetal MSCs have a naïve embryonic nature and express pluripotency markers, such as NANOG, Oct 3/4, and Sox2, which are characteristic of human embryonic stem cells.12,13 Nevertheless, MSCs isolation from fetal tissues and placental/endometrium are less favorable for clinical applications due to ethical concern related to fetal apportionment, and perspective heterogeneity with the mother’s cells, respectively. Therefore, the use of umbilical cord tissues is appreciable since they are also considered medical waste. Worth mentioning, umbilical cord blood- and amniotic fluid-derived MSCs share similar characteristics to that of umbilical cord stroma MSCs, also known as Wharton jelly-derived MSCS (WJ-MSCs), however, they are less attractive for clinical application due to their low frequency, poor proliferation rate and culture limitations.14 Taking together, the Wharton jelly- umbilical cord stroma is an attractive source for MSCs for prospective therapeutic applications (best reviewed in15).
Clinical Evidence of the Use of WJ-MSCs
Carlsson et al. recently published the results of a clinical trial on allogeneic Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) treatment via ProTrans in adults with recent-onset T1D.16 A combined Phase I/II trial was conducted in which escalating does of allogenic WJ-MSCs were intravenously administered, followed by a randomized, double-blind, placebo-controlled study. The inclusion criteria included adult patients (age 18-40 years, BMI < 30) with no chronic complications or viral infections, who were diagnosed with type 1 diabetes (<2 years) and had a fasting plasma C-peptide concentration > 0.12 nM. Initially, patients received low dose (25 × 106) of the WJ-MSCs. Then, patients were divided into two groups, with three participants receiving 100 × 106 cells and three participants receiving 200 × 106 cells. Based on the safety results of the initial study, the randomized, double-blind, placebo-controlled trial was conducted using an interventional system.16 The ProTrans technology comprises a bag of thawed WJ-MSCs isolated from different umbilical cord donors, which is mixed with a saline solution and paired with a standard blood infusion bag and is finally introduced intravenously to the patient (nextcellpharma.com). In parallel, the control-placebo participants received 5% wt/vol human serum albumin without the cell products. The intervention design was remarkably detailed by Carlsson and Svahn.17 During 12-months follow up, the authors observed a significant decline in C-peptide levels in the placebo-treated individuals (47% to baseline values). Alternatively, patients on the ProTrans intervention were presented with only 10% decline in the C-peptide levels. In addition, the insulin requirements were notably increased in placebo-treated individuals, but not with the ProTrans-treated individuals.16
In 2013, Hu et al. conducted a randomized, double-blind study in patients with recent-onset T1D, aged < 25 years.18 Initially, all patients were treated with intensive insulin therapy to maintain glycemic baseline control. Then, the patients in the intervention group received WJ-MSCs intravenously, while the patients in the control group were treated with normal saline. The treatments were administered twice over a period of 4 weeks. All patients were followed up monthly and quarterly for a period of 24 months. No adverse conditions were detected, similar to the Carlsson et al. study.16 Clinically, patients receiving WJ-MSCs showed a significantly lower HbA1c, elevated levels of fasting C-peptide and C-peptide/glucose ratio, and significantly reduced insulin requirements during the intervention, compared to the control group.18
In 2016, Cai et al. performed a pilot randomized controlled open-label clinical study in patients aged 18-40 years with ≥ 2 and ≤ 16 years of T1D history and absence of associated complications.19 Before the intervention, all patients received intensive insulin therapy, exercise, and a healthy diet for a period of 3 months. Then, the patients were randomized into the intervention group, who received WJ-MSCs and BM-MSCs by pancreatic arterial infusion, and the control group, with no cell therapy application. Standard clinical treatment was applied to both groups.19 Patients were followed for 12 months at 3-month intervals. The authors reported a significant increase in insulin and C-peptide levels in the intervention group. Additionally, the intervention group showed a significant improvement in HbA1c, fasting glycemia, and daily insulin requirements. Notably, patients from both groups experienced severe hypoglycemia, abdominal pain, and upper respiratory infections.19
In 2021, Lu et al. conducted an open-label parallel-arm, non-randomized clinical study in patients aged 8-55 years with adult-onset (>18 years) and juvenile-onset T1D.20 All participants were treated with intensive insulin therapy. Later, the intervention group received an intravenous infusion of WJ-MSCs followed by a second dose after 3 months, while the control group was sustained on intensive insulin therapy. The patients were followed up every 3 months for a period of 12 months.20 The authors reported a 10% increase in fasting C-peptide levels from baseline in almost 50% of patients in the intervention group. Relative to the control group, the percent change of postprandial C-peptide was significantly increased in adult patients who received the WJ-MSCs treatment. However, the changes in fasting C-peptide were not significantly different between the two groups among the juvenile-onset T1D. Notably, three patients in the intervention group achieved insulin independence and maintained insulin freedom for 3 to 12 months.20
Together, despite the differences in the experimental design of the mentioned trials, all concluded the safety and efficacy of the clinical application of WJ-MSCs, along with their capability to preserve β-cell function and prevent the long-term progression of T1D. Nevertheless, the authors did not investigate the cellular dynamics and plasticity of the applied WJ-MSCs and explore how these cells mediate the observed phenotype.
Clinical Translation Perspectives
Remarkably, the current approach establishes a foundation for the development of clinical programs for treating T1D. In the current trial of Carlsson et al.,16 a single infusion of WJ-MSCs was sufficient to preserve endogenous insulin for a year in adult patients with recent-onset T1D. These results are unprecedented because in other existing clinical trials, several infusions have been required to achieve improvements in postprandial C-peptide and Hb1Ac levels.18-20
Despite the notable use of MSCs in successful clinical trials for the treatment of various diseases, there is a critical concern related to the fact that the majority of intravenously administered MSCs end up in the lungs21,22 and are also detected in the liver and spleen tissues.23 In fact, the biodistribution of systematically applied MSCs remains elusive and requires further intensive investigations to justify the observed healing capacity of MSCs.24 Worth mentioning, homing a small number of MSCs within the target organ could be sufficient to stimulate the recovery process due to their enriched exosomes, signaling proteins, and microRNAs contents.15 Therefore, the most predominant therapeutic effect of systematically administrated MSCs could be through their paracrine signaling, cell-cell interactions, and possible differentiation into specialized cell types to replace damaged or diseased cells in the body. In general, it is likely that the route of administration of MSCs plays a role in determining which of these mechanisms of action are most important. For example, intravenously administered MSCs are more likely to exert their effects through paracrine signaling, while MSCs that are injected directly into a tissue are more likely to differentiate into specialized cell types. It is important to note that these approaches are still in the early stages of development. More research is needed to determine the optimal route of administration and dose of MSCs for clinical applications.
Another key factor in the success of clinical applications of MSCs is their source.25 To elucidate the fundamental importance of the MSCs source for prospective clinical trials and regenerative potential, Ganguly et al. compared the transcriptome-proteome integrity of WJ-MSCs, BM-MSCs, and adipose tissue AD-MSCs.26 The multi-parametric analyses revealed that WJ-MSCs promote a robust host innate immune response by activating the anti-inflammatory markers and secreting immune-cell mediators. On the other hand, adult-MSCs were found to stimulate the host angiogenic signaling cascades due to their enrichment in factors related to extracellular matrix remodeling.26 Together, these data highlight the importance of molecular signatures to characterize MSC therapies for clinical applications.
From this perspective, WJ-MSCs are more suitable for clinical studies associated with systemic cell infusion due to their molecular signature, juvenility, and minimal exposure to environmental toxins and associated genetic modulation.27 Alternatively, adult-MSCs are more suitable for clinical trials associated with topical application such as wound healing and diabetes foot ulcers. In support to this notation, a similar study was previously reported by Carlsson and his team, who infused autologous bone marrow MSCs in patients with T1D.28 In this study, the patients’ inclusion and exclusion criteria were identical to that of the ProTrans study.16 The bone marrow MSCs were isolated from each patient, characterized for authenticity, and infused to back to the same patient, however, no ProTrans intervention was applied.28 During the first year, the authors reported significant elevation in C-peptide levels in BM-MSC-treated patients, as compared to the control group.28 Together, despite differences in cell infusion approaches, both clinical trials concluded the safety and efficacy of the clinical application of MSCs, along with their capability to preserve β-cell function and prevent the long-term progression of T1D. Notably, both the WJ-MSCs16 and BM-MSCs28 clinical trials were successfully conducted by the same research group at Karolinska University Hospital, Stockholm, Sweden. We anticipate that the rationale behind WJ-MSCs utilization in the ProTrans clinical trial is due to their superior characteristics, non-invasive isolation protocol, and facilitated purification procedures, as well as their high titer and proliferation capacities relative to that of BM-MSCs.
Prospective Mechanism of Action
Currently, the exact mechanism of action of WJ-MSCs is not well elucidated. We anticipate several possible explanations for how WJ-MSCs may be contributing towards the observed benefits (Figure 1). One such explanation is that WJ-MSCs release various factors that can modulate the immune system, such as interleukin-10 (IL-10), transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), and/or prostaglandin E2.29,30 These factors can help in reducing inflammation, promoting tolerance, and protecting cells from damage.15 The released growth factors may enhance the proliferation of existing β cells, or at least in part, may enrich FoxP3+ regulatory T cells (Tregs), a specialized subset of helper T cells that play a key role in attenuating T1D autoimmunity.31,32 Another possible explanation would be that WJ-MSCs may differentiate and/or mediate the differentiation of residual progenitors into new β-cells. In addition, WJ-MSCs’ factors could facilitate changes in the fate of other pancreatic cells, such as α- and δ cells, to be able to transdifferentiate into β cells. Altogether, these prospective mechanisms may lead to the observed increase in total β-cell mass and function in Carlsson et al. study.16
Figure 1.
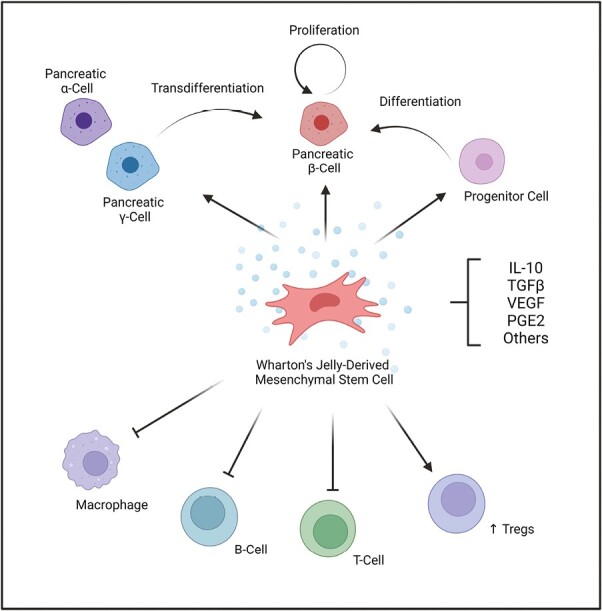
A schematic represents prospective mechanism of action of Wharton’s jelly-derived mesenchymal stromal cell infusion in preserving pancreatic beta-cell function and endogenous insulin in patients with type 1 diabetes. The secretome released by WJ-MSCs include factors such as IL-10, TGF-β, VEGF, and PGE2. These factors may (1) enhance pancreatic β-cell proliferation, progenitor cells differentiation, or pancreatic α- and/or γ-cells trans-differentiation into β-cells (2) inhibit the activity or macrophages, B-cells, and/or T-cells; (3) increase the activity of regulatory T-cells (Tregs). Abbreviations: IL-10, interleukin-10; PGE2, prostaglandin E2; TGF-β, Transforming growth factor-beta; regulatory T-cells, Tregs; VEGF, vascular endothelial growth factor; WJ-MSCs, Wharton’s jelly-derived mesenchymal stem cells.
Recommendations for future clinical trials
Lastly, the use of WJ-MSCs in clinical trials seems to be facilitating a new era in the treatment of T1D.18,33 Therefore, we encourage researchers to explore the mechanism of action of the introduced WJ-MSCs, their cytokine profile, and pancreatic β cells mass and function. In addition, clinical trials should also enroll participants with a long-term history of T1D with sustained residual functional β cells.34,35 Further, clinical studies should involve multi-centers with larger cohorts of different ethnicities. We also believe that the involvement of international societies and organizations would be highly beneficial in implementing guidelines and consensuses pertaining to the safe practice of cell therapy.
Conclusion
WJ-MSCs are promising cell therapeutic agents. Besides the therapeutic outcome, clinical trials should also focus on the cellular dynamics and kinetics to understand the mechanism of action of WJ-MSC. In addition, multi-center clinical studies should be implemented with large multi-ethnic cohorts, enrolling patients with a well-established long-term history of T1D.
Acknowledgments
The authors thank Kuwait Foundation for Advancement of Sciences (KFAS) for funding.
Contributor Information
Ashraf Al Madhoun, Genetics and Bioinformatics, Dasman Diabetes Institute, Dasman 15462, Kuwait.
Lubaina Koti, Genetics and Bioinformatics, Dasman Diabetes Institute, Dasman 15462, Kuwait.
Neus Carrió, Department of Periodontology, Universitat Internacional de Catalunya (UIC), C/Josep Trueta s/n, Sant Cugat del Valles, 08195 Barcelona, Spain.
Maher Atari, Biointelligent Technology Systems SL, C/Diputaccion 316, 3D, 08009 Barcelona, Spain.
Fahd Al-Mulla, Genetics and Bioinformatics, Dasman Diabetes Institute, Dasman 15462, Kuwait.
Funding
This study was supported by Kuwait Foundation for Advancement of Sciences (KFAS) (grant no. RA CB-2021-007). The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Conflict of Interest
The authors declare no potential conflicts of interest.
Author Contributions
A.A.M. wrote the manuscript, L.K., N.C., M.A., and F. Al-M. revised and edited the manuscript. All authors approved the manuscript for submission.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Abela AG, Fava S.. Why is the incidence of type 1 diabetes increasing? Curr Diabetes Rev. 2021;17(8):e030521193110. 10.2174/1573399817666210503133747 [DOI] [PubMed] [Google Scholar]
- 2. Walker S, Appari M, Forbes S.. Considerations and challenges of islet transplantation and future therapies on the horizon. Am J Physiol Endocrinol Metab. 2022;322(2):E109-E117. 10.1152/ajpendo.00310.2021 [DOI] [PubMed] [Google Scholar]
- 3. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147. 10.1126/science.284.5411.143 [DOI] [PubMed] [Google Scholar]
- 4. Ali H, Al-Yatama MK, Abu-Farha M, Behbehani K, Al Madhoun A.. Multi-lineage differentiation of human umbilical cord Wharton’s Jelly Mesenchymal Stromal Cells mediates changes in the expression profile of stemness markers. PLoS One. 2015;10(4):e0122465. 10.1371/journal.pone.0122465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedenstein AJ, Chailakhjan RK, Lalykina KS.. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393-403. 10.1111/j.1365-2184.1970.tb00347.x [DOI] [PubMed] [Google Scholar]
- 6. Uder C, Brückner S, Winkler S, Tautenhahn HM, Christ B.. Mammalian MSC from selected species: features and applications. Cytometry A. 2018;93(1):32-49. 10.1002/cyto.a.23239 [DOI] [PubMed] [Google Scholar]
- 7. da Silva Meirelles L, Chagastelles PC, Nardi NB.. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204-2213. 10.1242/jcs.02932 [DOI] [PubMed] [Google Scholar]
- 8. Jiang R, Han Z, Zhuo G, et al. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Front Med. 2011;5(1):94-100. 10.1007/s11684-011-0116-z [DOI] [PubMed] [Google Scholar]
- 9. Ghaneialvar H, Soltani L, Rahmani HR, Lotfi AS, Soleimani M.. Characterization and classification of mesenchymal stem cells in several species using surface markers for cell therapy purposes. Indian J Clin Biochem. 2018;33(1):46-52. 10.1007/s12291-017-0641-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carvalho MM, Teixeira FG, Reis RL, Sousa N, Salgado AJ.. Mesenchymal stem cells in the umbilical cord: phenotypic characterization, secretome and applications in central nervous system regenerative medicine. Curr Stem Cell Res Ther. 2011;6(3):221-228. 10.2174/157488811796575332 [DOI] [PubMed] [Google Scholar]
- 11. Barkholt L, Flory E, Jekerle V, et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy. 2013;15(7):753-759. 10.1016/j.jcyt.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 12. Nekanti U, Rao VB, Bahirvani AG, et al. Long-term expansion and pluripotent marker array analysis of Wharton’s jelly-derived mesenchymal stem cells. Stem Cells Dev. 2010;19(1):117-130. 10.1089/scd.2009.0177 [DOI] [PubMed] [Google Scholar]
- 13. Higuchi O, Okabe M, Yoshida T, et al. Stemness of human Wharton’s jelly mesenchymal cells is maintained by floating cultivation. Cell Reprogram. 2012;14(5):448-455. 10.1089/cell.2012.0020 [DOI] [PubMed] [Google Scholar]
- 14. Zeddou M, Briquet A, Relic B, et al. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol Int. 2010;34(7):693-701. 10.1042/CBI20090414 [DOI] [PubMed] [Google Scholar]
- 15. Drobiova H, Sindhu S, Ahmad R, et al. Wharton’s jelly mesenchymal stem cells: a concise review of their secretome and prospective clinical applications. Front Cell Dev Biol. 2023;11:1211217. 10.3389/fcell.2023.1211217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carlsson PO, Espes D, Sisay S, et al. Umbilical cord-derived mesenchymal stromal cells preserve endogenous insulin production in type 1 diabetes: a phase I/II randomised double-blind placebo-controlled trial. Diabetologia. 2023;66(8):1431-1441. 10.1007/s00125-023-05934-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carlsson P-O, Svahn MG.. Wharton’s jelly derived allogeneic mesenchymal stromal cells for treatment of type 1 diabetes: study protocol for a double-blinded, randomized, parallel, placebo-controlled trial. Clin Trials Degener Dis. 2018;3(2):32. 10.4103/2542-3975.235141 [DOI] [Google Scholar]
- 18. Hu J, Yu X, Wang Z, et al. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr J. 2013;60(3):347-357. 10.1507/endocrj.ej12-0343 [DOI] [PubMed] [Google Scholar]
- 19. Cai J, Wu Z, Xu X, et al. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care. 2016;39(1):149-157. 10.2337/dc15-0171 [DOI] [PubMed] [Google Scholar]
- 20. Lu J, Shen SM, Ling Q, et al. One repeated transplantation of allogeneic umbilical cord mesenchymal stromal cells in type 1 diabetes: an open parallel controlled clinical study. Stem Cell Res Ther. 2021;12(1):340. 10.1186/s13287-021-02417-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amadeo F, Hanson V, Liptrott NJ, et al. Fate of intravenously administered umbilical cord mesenchymal stromal cells and interactions with the host’s immune system. Biomed Pharmacother. 2023;159:114191. 10.1016/j.biopha.2022.114191 [DOI] [PubMed] [Google Scholar]
- 22. Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54-63. 10.1016/j.stem.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gholamrezanezhad A, Mirpour S, Bagheri M, et al. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol. 2011;38(7):961-967. 10.1016/j.nucmedbio.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 24. Leibacher J, Henschler R.. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7(1):7. 10.1186/s13287-015-0271-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jayasinghe M, Prathiraja O, Perera PB, et al. The role of mesenchymal stem cells in the treatment of type 1 diabetes. Cureus. 2022;14(7):e27337. 10.7759/cureus.27337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ganguly A, Swaminathan G, Garcia-Marques F, et al. Integrated transcriptome-proteome analyses of human stem cells reveal source-dependent differences in their regenerative signature. Stem Cell Rep. 2023;18(1):190-204. 10.1016/j.stemcr.2022.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fong CY, Chak LL, Biswas A, et al. Human Wharton’s Jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev Rep. 2011;7(1):1-16. 10.1007/s12015-010-9166-x [DOI] [PubMed] [Google Scholar]
- 28. Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K.. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64(2):587-592. 10.2337/db14-0656 [DOI] [PubMed] [Google Scholar]
- 29. Yang G, Fan X, Liu Y, et al. Immunomodulatory mechanisms and therapeutic potential of mesenchymal stem cells. Stem Cell Rev Rep. 2023;19(5):1214-1231. 10.1007/s12015-023-10539-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao Q, Ren H, Han Z.. Mesenchymal stem cells: immunomodulatory capability and clinical potential in immune diseases. J Cel Immunother. 2016;2(1):3-20. 10.1016/j.jocit.2014.12.001 [DOI] [Google Scholar]
- 31. Viisanen T, Gazali AM, Ihantola EL, et al. FOXP3+ regulatory T cell compartment is altered in children with newly diagnosed type 1 diabetes but not in autoantibody-positive at-risk children. Front Immunol. 2019;10:19. 10.3389/fimmu.2019.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Court AC, Le-Gatt A, Luz-Crawford P, et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020;21(2):e48052. 10.15252/embr.201948052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kassem DH, Kamal MM.. Therapeutic efficacy of umbilical cord-derived stem cells for diabetes mellitus: a meta-analysis study. Stem Cell Res Ther. 2020;11(1):484. 10.1186/s13287-020-01996-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davis AK, DuBose SN, Haller MJ, et al. Prevalence of detectable C-Peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care. 2015;38(3):476-481. 10.2337/dc14-1952 [DOI] [PubMed] [Google Scholar]
- 35. Liu EH, Digon BJ 3rd, Hirshberg B, et al. Pancreatic beta cell function persists in many patients with chronic type 1 diabetes, but is not dramatically improved by prolonged immunosuppression and euglycaemia from a beta cell allograft. Diabetologia. 2009;52(7):1369-1380. 10.1007/s00125-009-1342-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.


