Abstract
The effect of the plasminogen activator Pla of Yersinia pestis on the adhesiveness of bacteria to the mammalian extracellular matrix was determined. Y. pestis KIM D27 harbors the 9.5-kb plasmid pPCP1, encoding Pla and pesticin; the strain efficiently adhered to the reconstituted basement membrane preparation Matrigel, to the extracellular matrix prepared from human lung NCI-H292 epithelial cells, as well as to immobilized laminin. The isogenic strain Y. pestis KIM D34 lacking pPCP1 exhibited lower adhesiveness to both matrix preparations and to laminin. Both strains showed weak adherence to type I, IV, and V collagens as well as to human plasma and cellular fibronectin. The Pla-expressing recombinant Escherichia coli LE392(pC4006) exhibited specific adhesiveness to both extracellular matrix preparations as well as to laminin. The Pla-expressing strains showed a low-affinity adherence to another basement membrane component, heparan sulfate proteoglycan, but not to chondroitin sulfate proteoglycan. The degradation of radiolabeled laminin, heparan sulfate proteoglycan, or human lung extracellular matrix by the Pla-expressing recombinant E. coli required the presence of plasminogen, and degradation was inhibited by the plasmin inhibitors aprotinin and α2-antiplasmin. Our results indicate a function of Pla in enhancing bacterial adhesion to extracellular matrices. Y. pestis also exhibits a low level of Pla-independent adhesiveness to extracellular matrices.
Yersinia pestis, the plague bacillus, is usually transmitted to humans by the bite of infected fleas; human-to-human transmission also occurs via the respiratory route. Following subcutaneous infection, the bacterium is able to invade deeper tissues and cause a highly invasive systemic infection with often fatal outcome. Death in human plague is caused by the large number of disseminated bacteria and the associated host reactions to bacterial endotoxin. Production of bacteremia also ensures infection of new fleas and subsequent transmission to other hosts. Among the many virulence determinants of Y. pestis (reviewed in reference 21), a plasmid of 9.5 kb has been associated with the invasive character of plague (34). Loss of this plasmid increases the median lethal dose 106-fold in mice infected by the subcutaneous route, whereas little effect on lethal dose is seen after intravenous infection (4). This finding indicates that the products encoded by the 9.5-kb plasmid particularly function to promote bacterial spread to cause systemic infection.
The 9.5-kb plasmid of Y. pestis (designated pPCP1) encodes three protein products: the bacteriocin pesticin, the pesticin immunity protein, and the outer membrane protease Pla (30). The pesticin activity is not correlated with virulence of Y. pestis, whereas inactivation of the pla gene results in dramatic loss of virulence in subcutaneously infected mice (33). The pla mutants cause a localized infection at the injection site and are not able to spread to the liver and spleen. Pla degrades outer membrane proteins encoded by another virulence plasmid of Y. pestis (27, 32), but its main pathogenetic function is thought to be the proteolytic activation of plasminogen into plasmin (1). Plasmin is a potent serine protease that cleaves fibrin clots (fibrinolysis) and noncollagenous proteins of the mammalian basement membrane (BM) and extracellular matrix (ECM) such as laminin and fibronectin (reviewed in reference 19). Plasmin also activates latent procollagenases, and hence it has been thought that the plasminogen activator activity of Pla might enhance the invasiveness of Y. pestis by causing damage to host tissue barriers. Pla has also been reported to cleave the C3 protein of the complement system (33), thus interfering with the complement activation and reducing chemoattractants at the infection site. Indeed, few inflammatory cells were detected at the site after a subcutaneous infection of mice with a Pla-positive strain of Y. pestis (33).
Free plasmin in plasma is rapidly inactivated by α2-antiplasmin, the main physiological inhibitor of plasmin activity (reviewed in reference 23). Binding of plasminogen to lysine-containing targets, e.g., on fibrin, is associated with dramatic changes in the conformation of the plasminogen molecule (16) which in the bound form is more readily activated by its physiological activator tissue-type plasminogen activator. On the other hand, the immobilized plasmin molecule is resistant to inactivation by α2-antiplasmin. Immobilization of plasminogen and plasmin and the associated conformational changes thus are important in the creation of transient and localized proteolytic activity. In addition to Y. pestis, several invasive human bacterial pathogens interfere with the plasminogen activation by expressing plasminogen receptors or plasminogen activators (reviewed in references 2, 8, and 12). The bacterial plasminogen receptors bind plasminogen and enhance its activation by tissue-type plasminogen activator on the bacterial surface, in essence turning a nonproteolytic bacterium into a proteolytic one with the help of a host-derived proteolytic system. Metastatic tumor cells utilize plasminogen activation to penetrate the BM (for a review, see reference 19), which has led to speculation that bacterial plasminogen receptors and activators may function in an analogous manner (2, 12).
Bacterial attachment to tissue sites is an important virulence property aiding colonization and spread of bacteria at epithelial tissues or subepithelial ECM. Many of the invasive pathogens that express plasminogen receptors and/or activators also adhere to the mammalian ECM (reviewed in reference 39), and fimbrial adhesins with affinity to ECM have been identified as a class of enterobacterial plasminogen receptors (20, 28). Our hypothesis (12) has been that bacterial adhesiveness directs the bacterium-bound plasmin activity onto the BM and ECM, leading to localized proteolysis and increased penetration of bacteria through the ECM. This has been observed in in vitro degradation and penetration assays using reconstituted BM or extracted ECM and adhesive bacteria expressing plasminogen receptors (14, 38). Plasminogen also potentiates bacterial transcytosis across an epithelial cell monolayer (41) and, under in vivo conditions, enhances spirochetemia caused by Borrelia burgdorferi in mice (5). These findings suggest that plasminogen activation and adherence to ECM act together to bring about bacterial metastasis through tissue barriers. Adhesive properties have recently been proposed for the Pla protease (11), which led us to assess the possible function of Pla in adherence of Y. pestis to the BM and ECM.
MATERIALS AND METHODS
Bacterial strains, plasmids, and bacterial cultivation.
Y. pestis KIM D27 (pPCP1+ pgm pYV+) and KIM D34 (pPCP− pgm pYV+) are isogenic derivatives of Y. pestis KIM-10 (7) that have been attenuated by chromosomal deletion of the pgm locus encoding the pigmentation phenotype (37). The strains originate from R. R. Brubaker (Michigan State University, East Lansing) and were obtained from M. Skurnik (Turku Center for Biotechnology, Turku, Finland). The 102-kb chromosomal deletion to Pgm− had been selected on hemin agar (10) to yield D27, and then pPCP had been eliminated from D27 by cold curing (26) to give D34. Plasmid pC4006 harbors a 1.2-kb DNA fragment from the virulence plasmid and encodes Pla in the pUC19 vector (11); plasmid pC4007 contains a truncated pla gene in pUC19 and was used as a negative control (11). Both plasmids were transformed to and expressed in the Escherichia coli K-12 background in the nonadhesive strain LE392 (25). The Y. pestis strains were cultivated on brain heart infusion agar (BHI) plates for 36 h at 28°C and then overnight at 37°C in BHI broth under shaking. The E. coli strains were grown overnight at 37°C on Luria agar plates containing 75 μg of ampicillin per ml in the case of the recombinant strains. After cultivation, the bacteria were collected, washed twice with phosphate-buffered saline, pH 7.1 (PBS), and used for the assays.
Adherence assays.
Bacterial adherence to the reconstituted BM preparation Matrigel (Biocoat, Bedford, Mass.), to the ECM of the human lung mucoepidermoid carcinoma cell line NCI-H292 (ATCC CRL-1848), and to individual proteins of the ECM were performed as described earlier (13, 36, 38, 40). Matrigel was diluted 1:25 in PBS and reconstituted on Lab-Tek chamber slides (Nunc, Roskilde, Denmark) in a total volume of 250 μl by incubating the chambers for 1 h at 37°C and then overnight at room temperature. The NCI-H292 epithelial cells were cultivated to confluence on diagnostic slides in RPMI 1640 medium (Gibco BRL Life Technologies, Paisley, Scotland) supplemented with 10% (wt/vol) fetal calf serum (PAA Laboratories GmbH, Linz, Austria) and 2 mM l-glutamine (Gibco BRL Life Technologies). ECM was prepared by detergent treatment of the cell layer as described previously (9, 36), and the absence of epithelial cells on the glass slides was checked microscopically before the adherence assays. To analyze bacterial adherence to individual proteins of the ECM, ultrapure (entactin-free) laminin from Engelbreth-Holm-Swarm mouse tumor, human plasma fibronectin (Collaborative Biomedical Products, Bedford, Mass.), cellular fibronectin from human foreskin fibroblasts (Fibrogenex, Chicago, Ill.), and type I, IV, and V collagens isolated from human placenta (Sigma Chemical Co., St. Louis, Mo.) were coated on glass slides to obtain a 2.5 pmol per well. Data for quantitative coating were available from previous work (40). With the assays involving Matrigel, ECM, or the ECM proteins, the control surfaces were coated with bovine serum albumin (BSA) or fetuin (Sigma) from a solution of 25 μg/ml. Quenching of the target surfaces was performed by incubating the glass slides at room temperature for 2 h in 2% (wt/vol) BSA in PBS. The bacteria were tested at concentrations ranging from 107 to 5 × 109 cells/ml in PBS. Bacterial adherence to immobilized mouse heparan sulfate proteoglycan (Sigma) and chondroitin sulfate proteoglycan from bovine aorta (Biocoat) was tested as described above but on glass slides coated from a protein solution of 50 μg/ml; the same coating procedure was used for the control proteins laminin and BSA. After washing of the glass slides with 0.1% (wt/vol) BSA–PBS, the adherent bacteria were fixed with methanol for 10 min and stained with methylene blue. The bacteria were visualized in a microscope equipped with a charge-coupled device camera, and the images were digitized by using the NIH Image 1.55 program as detailed elsewhere (38). The number of bacteria in 20 randomly chosen microscopic fields of 1.6 × 104 μm2 was determined.
Degradation assays.
Degradation of ECM proteins and of ECM was analyzed by a modification of the method used in tumor cell metastasis studies (19) as recently adapted for bacteriology (14, 38). Briefly, laminin and heparan sulfate proteoglycan were labeled with 125I (Amersham International, Amersham, England) by the Iodogen method (17). The specific activities obtained were 5 × 106 cpm/μg for laminin and 107 cpm/μg for heparan sulfate proteoglycan. For both proteins, we used an amount corresponding to 6 × 105 cpm/well. Samples of the labeled proteins taken before and after the degradation assays were subjected to sodium dodecyl sulfate-gel electrophoresis in 5 to 18% polyacrylamide gels to analyze the molecular sizes of the peptides. Radiolabeled ECM was prepared by detergent extraction (9) from NCI-H292 cells metabolically labeled with l-[35S]methionine and l-[35S]cysteine (Amersham) as described elsewhere (38). Bacteria (2 × 109 cells/ml in PBS) were added onto the glass slides coated with radiolabeled ECM or ECM components either in PBS alone or in PBS supplemented with (i) human Glu-plasminogen (20 μg/ml in PBS; Bio-Pool, Umeå, Sweden); (ii) Glu-plasminogen and aprotinin (500 KIU/ml in PBS; Sigma), or (iii) Glu-plasminogen and α2-antiplasmin (tested at 7, 14, and 50 μg/ml in PBS; Bio-Pool). The slides with the bacteria were incubated under gentle shaking at 37°C, and samples were taken from the buffer at time intervals to measure the released radioactivity. The results shown are from a representative assay performed with independent duplicates.
Measurement of plasminogen activation.
An aliquot (200 μl) of the bacterial suspensions used in the degradation assays (see above) was incubated with 30 μl of the chromogenic plasmin substrate S-2251 solution (2.5 mg/ml; Kabivitrum, Stockholm, Sweden) for 1 h at 37°C. The bacteria were pelleted, and the absorbance at 405 nm of the supernatant was measured spectrophotometrically (20). The ratio of the cell-bound and the soluble plasmin activity was assessed as described elsewhere (14).
RESULTS
Adhesiveness to ECM preparations.
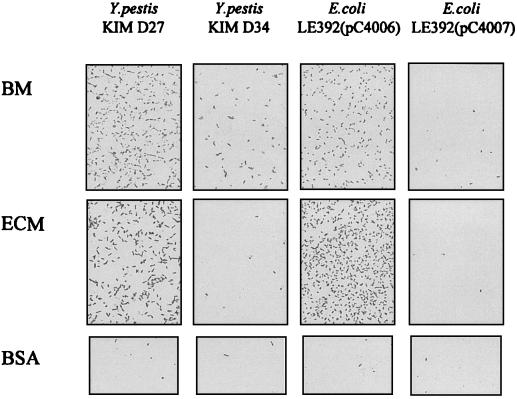
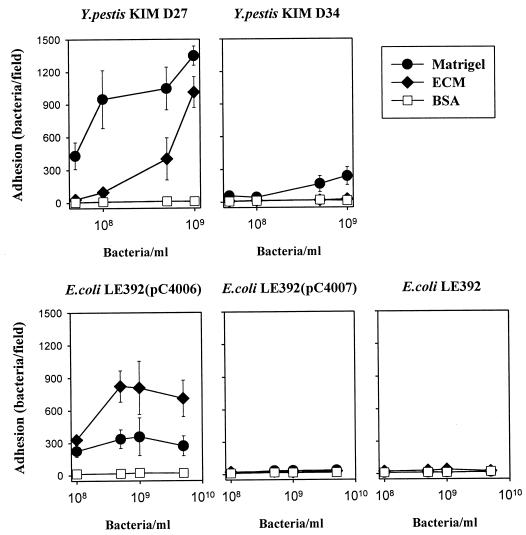
We initially assessed the ECM adhesiveness of two isogenic, attenuated Y. pestis strains, KIM D27 and KIM D34. Strain KIM D27 harbors the 9.5-kb pPCP1 plasmid encoding Pla, pesticin, and pesticin immunity, whereas strain KIM D34 has been cured of the plasmid. The strain KIM D27 exhibited a highly efficient adhesion to the reconstituted mouse BM preparation Matrigel and to the ECM prepared from the human lung cell line NCI-H292 (Fig. 1). Adhesiveness of Y. pestis KIM D34 to the BM and ECM surfaces was weaker, and neither Y. pestis strain exhibited significant adhesiveness to the control surface coated with BSA. The role of Pla in the observed bacterial adhesion was suggested by the efficient adhesion to BM and ECM by the recombinant E. coli strain LE392(pC4006) expressing Pla, whereas the Pla-negative strains E. coli LE392(pC4007) (Fig. 1) and LE392 (see below) showed only weak adhesion. E. coli LE392(pC4006) expresses pla on a 1.2-kb DNA insert in plasmid pUC19, whereas the control plasmid pC4007 encodes a truncated and inactive form of Pla. Plasminogen activator activity was detected with E. coli LE392(pC4006) but not with E. coli LE392(pC4007) or E. coli LE392 (see below).
FIG. 1.
Adherence of Pla+ Y. pestis KIM D27, Pla− Y. pestis KIM D34, Pla+ E. coli LE392(pC4006), and Pla− E. coli LE392(pC4007) to the reconstituted mouse BM preparation Matrigel and to the ECM prepared by detergent extraction from human lung NCI-H292 epithelial cells. The control surface was coated with BSA. The bacteria were tested at a concentration of 109 cells/ml.
We next quantitatively analyzed the adhesiveness of the Y. pestis and the E. coli strains to mouse BM and human ECM (Fig. 2). Y. pestis KIM D27 exhibited a significantly greater adhesion to BM and ECM than did strain Y. pestis KIM D34; the latter strain, however, consistently exhibited a low level of adhesiveness to BM, whereas it adhered only weakly to the ECM preparation. E. coli LE392(pC4006) also exhibited efficient adhesion to BM and ECM, whereas no adhesion was detected with the strains E. coli LE392(pC4007) and E. coli LE392.
FIG. 2.
Adherence of Pla+ Y. pestis KIM D27 and E. coli LE392(pC4006) as well as of Pla− Y. pestis KIM D34, E. coli LE392(pC4007), and E. coli LE392 to the BM preparation Matrigel reconstituted on glass slides as well as to the ECM prepared by detergent extraction from human lung NCI-H292 cells. The bacteria were tested at four different concentrations as indicated, and the data shown are means ± SDs for 20 randomly chosen microscopic fields of 1.6 × 104 μm2. The control surface was coated with BSA.
Adhesion to ECM components.
We analyzed adhesion of the strains to isolated components of the ECM. Figure 3 shows adhesiveness to the glycosylated proteins mouse laminin and the plasma and the cellular forms of human fibronectin, as well as to the less glycosylated type I, type IV, and type V collagens. These proteins were coated to obtain a surface concentration of 2.5 pmol. As control targets, we used fetuin (a highly glycosylated protein) and the nonglycosylated protein BSA coated from a solution of 25 μg/ml. Y. pestis KIM D27 exhibited efficient adhesiveness to laminin, whereas its adhesiveness to the other target proteins was lower but above the background level seen with immobilized fetuin or BSA. The adhesiveness to laminin of the strain Y. pestis KIM D34 was lower than that shown by KIM D27, whereas the other target proteins were recognized with weak affinity by both Y. pestis strains. The recombinant strain E. coli LE392(pC4006) expressing Pla adhered efficiently to laminin and poorly to the other target proteins. No significant adhesiveness was seen with the other E. coli strains.
FIG. 3.
Adherence of Pla+ Y. pestis KIM D27 and E. coli LE392(pC4006) as well as of Pla− Y. pestis KIM D34, E. coli LE392(pC4007), and E. coli LE392 to immobilized proteins of the ECM. Bacterial concentration varied from 108 to 5 × 109 cells/ml, and the surface concentration of the target proteins was 2.5 pmol. The control surfaces were coated with the highly glycosylated fetuin and with the nonglycosylated BSA from a solution of 25 μg/ml. Data are means ± SDs for 20 randomly chosen microscopic fields of 1.6 × 104 μm2.
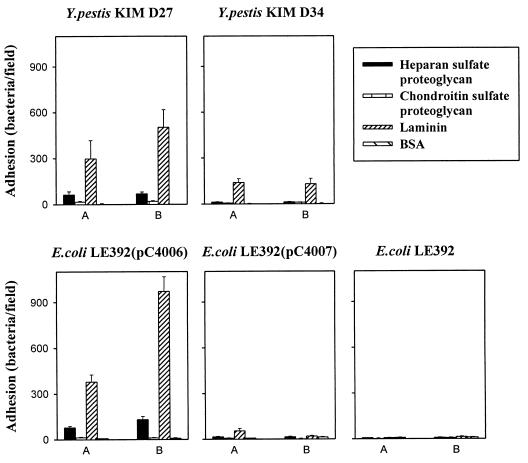
We next assessed bacterial adhesiveness to the BM proteoglycans mouse heparan sulfate and bovine chondroitin sulfate. As these two preparations consist of molecular species heterogeneous in molecular size, we used them as well as the positive (laminin) and negative (BSA) control proteins from a constant coating solution of 50 μg/ml. Y. pestis KIM D27 exhibited adhesiveness to the heparan sulfate proteoglycan that was fivefold lower than that to laminin but higher than the adhesiveness to BSA-coated glass (Fig. 4). No adherence of the Y. pestis strains to chondroitin sulfate proteoglycan was detected. Similarly, the Pla+ recombinant strain E. coli LE392(pC4006) exhibited a low level of adhesiveness to heparan sulfate proteoglycan but not to chondroitin sulfate. No adhesiveness of the other E. coli strains to the proteoglycans was detected.
FIG. 4.
Adherence of Pla+ Y. pestis KIMD27 and E. coli LE392(pC4006) as well as of Pla− Y. pestis KIM D34, E. coli LE392(pC4007), and E. coli LE392 to immobilized proteoglycans. For comparison, bacterial adhesiveness to laminin and BSA is also shown. All target proteins were coated from a solution of 50 μg/ml, and the bacteria were tested at the concentrations of 108 cells/ml (A) and 109 cells/ml (B). Data are means ± SDs for 20 randomly chosen fields of 1.6 × 104 μm2.
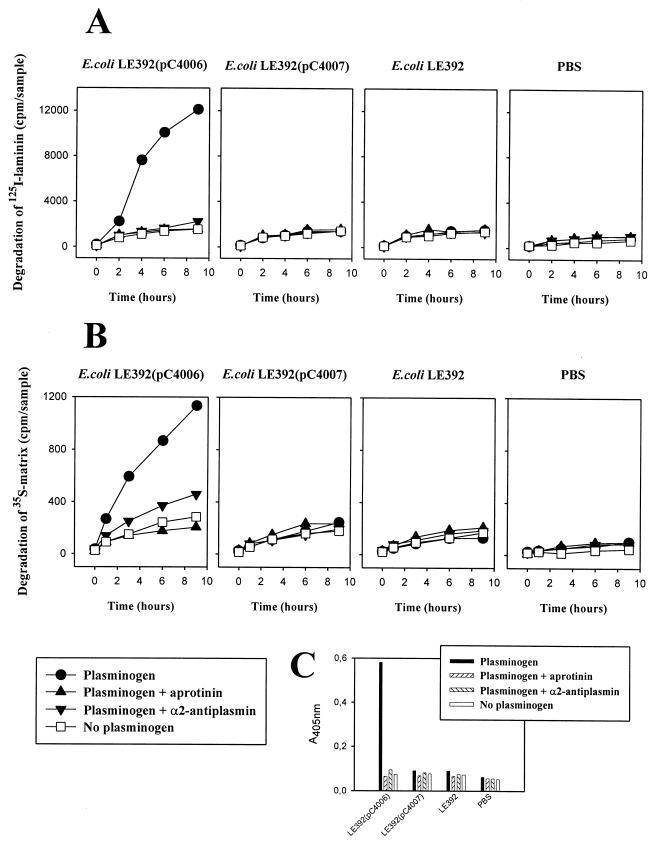
Degradation of radiolabeled ECM proteins and human ECM.
It has been postulated that Pla enhances bacterial migration by causing damage of tissue barriers, such as BM, through plasmin formation. The foregoing results gave evidence that expression of Pla enhances bacterial adhesiveness to BM and to laminin, suggesting the possibility that Pla degrades these tissue barriers. We therefore assessed whether bacteria expressing Pla are able to directly degrade radiolabeled laminin, heparan sulfate proteoglycan, or ECM, or whether the bacteria need conversion of plasminogen to plasmin for such activity. We also evaluated the effects on the degradation by α2-antiplasmin, the main physiological inhibitor of circulating, nonimmobilized plasmin, as well as by aprotinin, an inhibitor of both bound and soluble forms of plasmin.
The plasmin activity formed from plasminogen in the presence of E. coli LE392(pC4006), E. coli LE392(pC4007), and E. coli LE392 and the effects of α2-antiplasmin and aprotinin are shown in Fig. 5C. Plasmin was formed effectively in the presence of the Pla+ E. coli LE392(pC4006) but only in trace amounts in the presence of the other strains. Plasmin activity was inhibited by aprotinin as well as by α2-antiplasmin close to the background level seen without added bacteria (Fig. 5C). We estimated the proportion of the cell-bound plasmin to the soluble plasmin activity formed in the presence of E. coli LE392(pC4006) cells; in different experiments, the cell-bound activity comprised 1 to 3% of the total plasmin activity (details not shown). No endogenous plasminogen activator activity was observed with the E. coli strains.
FIG. 5.
Degradation of 125I-labeled laminin (A) and 35S-labeled extracellular matrix prepared from human lung NCI-H292 cells (B) by Pla+ or Pla− recombinant E. coli in the presence of plasminogen and inhibitors of plasmin activity. (C) Plasmin activity associated with the E. coli cells or in PBS without added bacteria, measured with the chromogenic substrate. Bacteria were used at a concentration of 2 × 109 cells/ml.
In the presence of plasminogen, 125I-labeled laminin was effectively degraded with E. coli LE392(pC4006) but not with the other E. coli cells (Fig. 5A). We confirmed by autoradiography that the radioactivity released from the coated glass surface indeed represented degraded laminin (results not shown). No degradation of 125I-laminin by E. coli LE392(pC4006) was detected in the absence of plasminogen. The degradation of laminin was nearly completely inhibited by the plasmin inhibitors aprotinin and α2-antiplasmin. Essentially similar results were obtained with the degradation of 125I-heparan sulfate immobilized on glass (data not shown). In analyzing the effect of α2-antiplasmin on degradation, we used plasminogen and α2-antiplasmin in the molar ratios of 2:1, 1:1, and 1:3; in all cases, equally effective inhibition by α2-antiplasmin of the degradation was observed (details not shown).
Using the same experimental design, we analyzed the degradation of a more complex target, the 35S-labeled ECM prepared from human lung NCI-H292 cells (Fig. 5B). Again, significant degradation was observed only in the presence of plasminogen and E. coli LE392(pC4006) cells, and the process was inhibited in the presence of aprotinin or α2-antiplasmin.
DISCUSSION
Association of the pla gene with the virulence and invasiveness of Y. pestis is well established (21, 33), but the mechanism(s) by which Pla enhances bacterial migration remains uncertain (8). Various virulence roles for Pla have been proposed (8, 21). Pla confers Y. pestis a mechanism for producing host-derived proteolytic activity that can degrade ECM and thus potentiate invasion. Pla has also been suggested to cleave fibrin deposits that trap Y. pestis and to reduce chemoattractants at the infection site by degrading C3 and thus interfering with the complement system. Pla also expresses a weak coagulase activity (1). An adhesive function for Pla has been proposed (11), and our present results demonstrate that Pla efficiently enhances bacterial adherence to human ECM and mouse BM. We did not detect any degradation of the adhesion targets by Pla-expressing bacteria directly, whereas the radiolabeled ECM and ECM proteins were degraded in the presence of plasminogen.
Numerous invasive bacterial pathogens adhere to ECM, and the in vivo function of ECM adherence has been demonstrated in a few cases (39). ECM adhesiveness is generally thought to enhance bacterial colonization at damaged tissue sites, such as wounds, but there is evidence suggesting that it is important for the establishment of a systemic infection as well. The YadA surface protein of Yersinia enterocolitica mediates bacterial adhesion to laminin and collagens of BM and ECM (35). Specific mutations in YadA that abolish collagen binding have been introduced into clinical isolates of Y. enterocolitica, and such strains show decreased adhesiveness to BM as well as dramatically reduced virulence in orally infected mice (22, 35). The YadA mutant strains fail to disseminate from the intestine to liver and spleen; i.e., they are greatly impaired in ability to spread from the primary infection site (22). YadA is expressed by Y. enterocolitica and Yersinia pseudotuberculosis but not by Y. pestis, which carries a frameshift mutation in the yadA homolog gene (29). In analogy to the role of YadA-mediated adherence in Y. enterocolitica infections, the adhesiveness to BM and ECM associated with Pla may contribute to its ability to confer an invasive phenotype on Y. pestis.
An important question arising from this study is whether the Pla molecule itself acts as an adhesin or whether it modifies cryptic adhesion molecules on the bacterial surface. A function of Pla in the Yersinia background is to proteolytically degrade plasmid-encoded outer membrane proteins (27, 32); such proteolysis could alter cell surface properties to give the adhesive phenotype of Pla-positive bacteria. However, in the E. coli K-12 background in strain LE392, used as a host strain also in this study, no significant Pla-induced changes in cell wall protein profiles were detected by Sodeinde et al. (32). Our electrophoretic analyses of the E. coli LE392 derivatives used in this study also failed to reveal gross differences in the outer membrane protein profiles from Pla+ and Pla− E. coli strains (data not shown). The Pla-expressing recombinant E. coli exhibited efficient adhesion to ECM and laminin, which favors the hypothesis that the Pla molecule is directly involved in adhesion. Resolution of this question, however, requires detailed analysis of the structure-function relationships in the Pla molecule.
We identified laminin and heparan sulfate proteoglycan as ECM and BM targets for Pla-associated adhesion. The adherence was much higher to laminin than to the proteoglycan. The assays involving proteoglycans were not based on a molar coating procedure, in part due to the heterogeneity of the proteoglycan preparations, which complicates comparison of the adherence affinities to laminin and on the other hand to heparan sulfate proteoglycan. However, the observed fivefold difference in the adhesiveness to laminin and to heparan sulfate suggests that laminin is the major ECM target for Pla-associated adhesion. Adherence to laminin and heparan sulfate proteoglycan may also be involved in the previously observed adhesion of Pla-expressing bacteria to cultured epithelial cell lines (11). Type IV collagen had previously been implicated as a binding target for Pla (11), but we detected only low-level adhesiveness to the collagen types that we tested. Furthermore, this adhesiveness appeared not to involve Pla, as it was also observed with Y. pestis KIM D34 lacking Pla, and the Pla+ recombinant E. coli strain did not adhere to collagens. An explanation for the different results might be that we used human collagens, whereas Kienle et al. (11) used murine collagen. The finding that strain KIM D34 adhered, although with low affinity, to BM as well as to laminin and type IV collagen, however, indicates that Y. pestis expresses a low level of adhesiveness to BM that is independent of Pla expression.
Adherence of Pla-expressing bacteria to laminin and heparan sulfate proteoglycan raised the possibility that these molecules also are targets for the proteolytic activity of Pla. This was not, however, detected, and degradation of the ECM proteins as well as of the lung ECM by Pla-expressing bacteria required adding plasminogen into the test suspension. Furthermore, two inhibitors of plasmin activity, aprotinin and α2-antiplasmin, effectively abolished the observed degradation. These results indicate that Pla cannot by itself degrade these adhesion targets, which are well-known targets for plasmin proteolysis (19). Structural analysis of the catalytic sites on serine proteases has revealed different peptide regions responsible for proteolytic cleavage and for substrate binding (3). Such structural features are used in the design of protease inhibitors and may also provide a physical basis for the binding of Pla to certain proteins without degrading them. On the other hand, it is possible that adhesion and proteolytic activity are mutually independent properties residing on different parts of the Pla molecule. The primary structure of Pla is known (31), but it is not known which regions of the molecule are surface exposed. We are currently using genetic modification of the pla gene to define whether the enzymatic and adhesive functions can be dissected; such constructs will also be useful in determining whether the adhesive function of Pla potentiates degradation of laminin and BMs.
Our results raise a question about the pathogenetic significance of the Pla-mediated plasminogen activation in the virulence of Y. pestis. We detected that the plasmin activity formed by Pla, as measured either with the chromogenic substrate or as the degradation of radiolabeled ECM, was nearly completely inhibited by α2-antiplasmin. This finding is in accordance with our finding that only a small amount of plasmin remained bound on the bacterial surface, where it is protected against inhibitors of plasmin activity (2, 14). At present, we cannot rule out the possibility that aprotinin and α2-antiplasmin inhibit Pla rather than the formed plasmin activity; the former alternative, however, seems unlikely in view of the well-characterized action of aprotinin and α2-antiplasmin on plasmin (19, 23). In human serum, the molar ratio of the plasminogen and α2-antiplasmin is approximately 2:1, which we also used in our assays. It is unclear how the Pla-activated plasmin is able to enhance bacterial spread and cause tissue damage if it is inhibited so effectively. We can propose two possibilities for how the formed plasmin could overcome the inhibition. First, it might be that Pla produces massive amounts of plasmin that locally overcome the inhibitor levels and are functional. This alternative stresses the plasminogen activator function of Pla. Second, BM act as reservoirs for plasminogen, metalloproteinases (collagenases), as well as plasminogen activators (15, 18). Matrigel has been shown to contain plasminogen that can be activated and remains active (6), and in particular, plasminogen interacts with laminin (24). It is possible that within tissues a close association of Y. pestis with BM and laminin brings the bacterium into a microenvironment where the formed plasmin can remain active inside the BM. This alternative stresses the importance of Pla-mediated adhesion to laminin and BM in enhancing bacterial migration through tissue barriers.
ACKNOWLEDGMENTS
This study was supported by the Finnish Academy of Sciences (grants 29346 and 42103), the University of Helsinki, and the Sigrid Jusélius Foundation.
We thank Juha-Matti Aalto for technical assistance.
REFERENCES
- 1.Beesley E D, Brubaker R R, Jansen W A, Surgalla M J. Pesticins. III. Expression of coagulase and mechanisms of fibrinolysis. J Bacteriol. 1967;94:19–26. doi: 10.1128/jb.94.1.19-26.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle M D P, Lottenberg R. Plasminogen activation by invasive human pathogens. Thromb Haemost. 1997;77:1–10. [PubMed] [Google Scholar]
- 3.Branden C, Tooze J. Introduction to protein structure. New York, N.Y: Garland Publishing Inc.; 1991. [Google Scholar]
- 4.Brubaker R R, Beesely E D, Surgalla M J. Pasteurella pestis: role of pesticin I and iron in experimental plague. Science. 1965;149:422–424. doi: 10.1126/science.149.3682.422. [DOI] [PubMed] [Google Scholar]
- 5.Coleman J L, Gebbia J A, Piesman J, Degen J L, Buggs T H, Benach J L. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 6.Farina A R, Tiberio A, Tacconelli A, Cappabianca L, Gulino A, Mackay A R. Identification of plasminogen in Matrigel and its activation by reconstitution of this basement membrane extract. BioTechniques. 1996;21:904–909. doi: 10.2144/96215rr03. [DOI] [PubMed] [Google Scholar]
- 7.Finegold M J, Petery J J, Berendt R F, Adams H R. Studies of the pathogenesis of plague: blood coagulation and tissue responses of Macaca mulatta following exposure to aerosols of Pasteurella pestis. Am J Pathol. 1968;53:99–114. [PMC free article] [PubMed] [Google Scholar]
- 8.Goguen J D, Hoe N P, Subrahmanyam Y V B K. Proteases and bacterial virulence: a view from the trenches. Infect Agents Dis. 1995;4:47–54. [PubMed] [Google Scholar]
- 9.Hedman K, Johansson S, Vartio T, Kjellen L, Vaheri A, Höök M. Structure of the pericellular matrix: association of heparan and chondroitin sulfates with fibronectin-procollagen fibers. Cell. 1982;28:663–671. doi: 10.1016/0092-8674(82)90221-5. [DOI] [PubMed] [Google Scholar]
- 10.Jackson S, Burrows T W. The pigmentation of Pasteurella pestis on a defined medium containing hemin. Br J Exp Pathol. 1956;37:577–583. [PMC free article] [PubMed] [Google Scholar]
- 11.Kienle Z, Emödy L, Svanborg C, O’Toole P W. Adhesive properties conferred by the plasminogen activator of Yersinia pestis. J Gen Microbiol. 1992;138:1679–1687. doi: 10.1099/00221287-138-8-1679. [DOI] [PubMed] [Google Scholar]
- 12.Korhonen T K, Virkola R, Lähteenmäki K, Björkman Y, Kukkonen M, Raunio T, Tarkkanen A-M, Westerlund B. Penetration of fimbriate enteric bacteria through basement membranes: a hypothesis. FEMS Microbiol Lett. 1992;100:307–312. doi: 10.1111/j.1574-6968.1992.tb14057.x. [DOI] [PubMed] [Google Scholar]
- 13.Kukkonen M, Raunio T, Virkola R, Lähteenmäki K, Mäkelä P H, Klemm P, Clegg S, Korhonen T K. Basement membrane carbohydrate as a target for bacterial adhesion: binding of type 1 fimbriae of Salmonella enterica and Escherichia coli to laminin. Mol Microbiol. 1993;7:229–237. doi: 10.1111/j.1365-2958.1993.tb01114.x. [DOI] [PubMed] [Google Scholar]
- 14.Lähteenmäki K, Virkola R, Pouttu R, Kuusela P, Kukkonen M, Korhonen T K. Bacterial plasminogen receptors: in vitro evidence for a role in degradation of the mammalian extracellular matrix. Infect Immun. 1995;63:3659–3664. doi: 10.1128/iai.63.9.3659-3664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackay A R, Gomez D E, Cottam D W, Rees R C, Nason A M, Thorgeirsson U P. Identification of the 72-kDa (MMP-2) and 92-kDa (MMP-9) gelatinase/type IV collagenase in preparations of laminin and Matrigel. BioTechniques. 1993;15:1048–1051. [PubMed] [Google Scholar]
- 16.Mangel W F, Lin B, Ramakrishnan V. Characterization of an extremely large, ligand-induced conformational change in plasminogen. Science. 1990;248:69–73. doi: 10.1126/science.2108500. [DOI] [PubMed] [Google Scholar]
- 17.Markwell M A K, Fox C F. Surface-specific iodination of membrane proteins of viruses and eukaryotic cells using 1,3,4,6-tetrachloro-3α,6α-diphenylglycouril. Biochemistry. 1978;17:4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- 18.McGuire P G, Seed N W. The interaction of plasminogen activator with reconstituted basement membrane matrix and extracellular macromolecules produced by cultured epithelial cells. J Cell Biochem. 1989;40:215–227. doi: 10.1002/jcb.240400210. [DOI] [PubMed] [Google Scholar]
- 19.Mignatti P, Rifkin D B. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993;73:161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- 20.Parkkinen J, Hacker J, Korhonen T K. Enhancement of tissue type plasminogen activator-catalyzed plasminogen activation by Escherichia coli S fimbriae associated with neonatal septicaemia and meningitis. Thromb Haemost. 1991;65:483–486. [PubMed] [Google Scholar]
- 21.Perry R D, Fetherston J D. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roggenkamp A, Neuberger H-R, Flugel A, Schmoll T, Heeseman J. Substitution of two histidine residues in YadA protein of Yersinia enterocolitica abrogates collagen binding, cell adherence and mouse virulence. Mol Microbiol. 1995;16:1207–1219. doi: 10.1111/j.1365-2958.1995.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 23.Saksela O, Rifkin D B. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- 24.Salonen E-M, Zitting A, Vaheri A. Laminin interacts with plasminogen and its tissue-type activator. FEBS Lett. 1984;172:29–32. doi: 10.1016/0014-5793(84)80866-2. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 26.Sample A K, Fowler J M, Brubaker R R. Modulation of the low-calcium response in Yersinia pestis via plasmid-plasmid interaction. Microb Pathog. 1987;2:443–453. doi: 10.1016/0882-4010(87)90051-9. [DOI] [PubMed] [Google Scholar]
- 27.Sample A K, Brubaker R R. Posttranslational regulation of Lcr plasmid-mediated peptides in pesticinogenic Yersinia pestis. Microb Pathog. 1987;3:239–248. doi: 10.1016/0882-4010(87)90057-x. [DOI] [PubMed] [Google Scholar]
- 28.Sjöbring U, Pohl G, Olsén A. Plasminogen, absorbed by Escherichia coli expressing curli or by Salmonella enteritidis expressing thin aggregative fimbriae, can be activated by simultaneously captured tissue-type plasminogen activator (t-PA) Mol Microbiol. 1994;14:443–452. doi: 10.1111/j.1365-2958.1994.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 29.Skurnik M, Wolf-Watz H. Analysis of the yopA gene encoding the Yop1 virulence determinants of Yersinia spp. Mol Microbiol. 1989;3:517–529. doi: 10.1111/j.1365-2958.1989.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 30.Sodeinde O A, Goguen J D. Genetic analysis of the 9.5-kilobase virulence plasmid of Yersinia pestis. Infect Immun. 1988;56:2743–2748. doi: 10.1128/iai.56.10.2743-2748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sodeinde O A, Goguen J D. Nucleotide sequence of the plasminogen activator gene of Yersinia pestis: relationship to ompT of Escherichia coli and gene E of Salmonella typhimurium. Infect Immun. 1989;57:1517–1523. doi: 10.1128/iai.57.5.1517-1523.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sodeinde O A, Sample A K, Brubaker R R, Goguen J D. Plasminogen activator/coagulase gene of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect Immun. 1988;56:2749–2752. doi: 10.1128/iai.56.10.2749-2752.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sodeinde O A, Subrahmanyam Y V B K, Stark K, Quan T, Bao Y, Goguen J D. A surface protease and the invasive character of plague. Science. 1992;258:1004–1007. doi: 10.1126/science.1439793. [DOI] [PubMed] [Google Scholar]
- 34.Straley S C, Brubaker R R. Localization in Yersinia pestis of peptides associated with virulence. Infect Immun. 1982;57:1517–1523. doi: 10.1128/iai.36.1.129-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamm A, Tarkkanen A-M, Korhonen T K, Kuusela P, Toivanen P, Skurnik M. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol Microbiol. 1993;10:995–1011. doi: 10.1111/j.1365-2958.1993.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 36.Toba T, Virkola R, Westerlund B, Björkman Y, Sillanpää J, Vartio T, Kalkkinen N, Korhonen T K. A collagen-binding S-layer protein in Lactobacillus crispatus. Appl Environ Microbiol. 1995;28:663–671. doi: 10.1128/aem.61.7.2467-2471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Une T, Brubaker R R. In vivo comparison of avirulent vWa− and Pgm− or Pstr phenotypes of yersiniae. Infect Immun. 1984;43:805–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Virkola R, Lähteenmäki K, Eberhard T, Kuusela P, van Alphen L, Ullberg M, Korhonen T K. Interaction of Haemophilus influenzae with the mammalian extracellular matrix. J Infect Dis. 1996;173:1137–1147. doi: 10.1093/infdis/173.5.1137. [DOI] [PubMed] [Google Scholar]
- 39.Westerlund B, Korhonen T K. Bacterial proteins binding to the mammalian extracellular matrix. Mol Microbiol. 1993;9:687–694. doi: 10.1111/j.1365-2958.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 40.Westerlund B, Kuusela P, Risteli J, Risteli L, Vartio T, Rauvala H, Virkola R, Korhonen T K. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol Microbiol. 1989;3:329–337. doi: 10.1111/j.1365-2958.1989.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 41.Zavizion B, White J H, Bramley A J. Staphylococcus aureus stimulates urokinase-type plasminogen activator expression by bovine mammary cells. J Infect Dis. 1997;176:1637–1640. doi: 10.1086/517345. [DOI] [PubMed] [Google Scholar]