Abstract
Background:
The greatest age-related weight gain occurs in the early/mid-20s. Overall dietary quality among adolescents and emerging adults (age 18-25) is poor, with ultra-processed foods (UPF) representing more than two-thirds of adolescents’ total energy intake (i.e., 68%). UPF consumption may impact cognitive and neurobiological factors that influence dietary decision-making and energy intake (EI). To date, no research has addressed this in this population.
Methods:
Participants aged 18-25 will undergo two 14-day controlled feeding periods (81% UPF, 0% UPF) using a randomly assigned crossover design, with a 4-week washout between conditions. Brain response to a UPF-rich milkshake, as well as behavioral measures of executive function, will be evaluated before and after each diet. Following each diet, measurements include ad libitum buffet meal EI, food selection, eating rate, and eating in the absence of hunger (EAH). Prior to initiating recruitment, controlled diet menus, buffet, and EAH snacks were developed and evaluated for palatability. Sensory and texture attributes of buffet and EAH snack foods were also evaluated.
Results:
Overall diet palatability was rated “like very much” (8)/”like moderately”(7) (UPF: 7.6±1.0; Non-UPF: 6.8±1.5). Subjective hardness rating (range=1-9 [1=soft, 9=hard] was similar between UPF and Non-UPF buffet and snack items (UPF:4.22± 2.19, Non-UPF: 4.70±2.03), as was the objective measure of hardness (UPF: 2874.33± 2497.06g, Non-UPF: 2243.32± 1700.51g).
Conclusions:
Findings could contribute to an emerging neurobiological understanding of the effects of UPF consumption including energy overconsumption and weight gain among individuals at a critical developmental stage.
Keywords: ultra-processed food, diet, energy intake, body weight, functional magnetic resonance imaging, cognition
1. Background
Among recommended weight individuals aged 18-30, most will transition to overweight or obesity in the next 25 years1. The greatest age-related gains occur in the early to mid-20s 1, and young adult weight gain is associated with adverse changes in many cardiovascular disease risk factors 2,3. Among adolescents In the United States (US), obesity prevalence is expected to double from levels observed in the early 2000s by 2030 4.
Dietary quality among adolescents (age 18-21) and emerging adults (age 22-25) 5 is well below the “poor” US average Healthy Eating Index-2015 scores 6. Ultra-processed foods (UPF), which are formulations of ingredients that have undergone a high degree of industrial processing and contain little or no “whole foods”, comprise more than two-thirds of adolescents’ total energy intake (i.e., 68%) 7,8. UPF consumption may adversely impact cognitive and neurobiological factors that influence dietary decision-making and energy intake (EI) 9,10. No research has addressed this issue in adolescents and young adults.
The brain’s dopamine system is a critical mediator of the neurobiological control of food intake 11. Rats exposed to a diet including bacon and frosting rapidly gained weight and showed reduced expression of the D2 dopamine receptors (D2R) in the striatum, a brain area critical for regulating reward and food intake 12. The same pattern of reduced D2R expression is seen in animals exposed to high-sugar diets 13 and humans with a high BMI show reduced D2R binding potential 12. In humans, dopamine increases in the striatum in both the tasting and post-ingestive period after consuming a high-fat, high-sugar milkshake 14. After a high-fat, high-sugar dietary intervention, brain response to milkshake cues and tasting was increased in the substantia nigra, dorsolateral prefrontal cortex, and insula compared to a high-protein intervention. However, this study did not control for the level of processing.
Only one randomized trial has tested a causal relationship between UPF and EI. Hall et al., conducted a 14-day trial in 20 adults (mean age ~31 years) that compared a high UPF diet (81% energy from UPF) to a minimally processed diet (0% energy from UPF), and reported an increase in EI with UPF exposure (508±106 kcal/d) that led to ~1 kg weight gain 10. A more rapid eating rate was noted with UPF, despite similar appetite sensations and palatability ratings across diets. The neural and physiological mechanisms underlying the greater energy intake with UPF are unclear. No study has examined brain changes and alterations in food intake in the critical period of adolescence and early adulthood. Controlled feeding studies with neural outcome measures are needed to provide a brain-based understanding of how UPF consumption drives EI and reward dysregulation.
Executive function (EF) refers to cognitive processes, such as inhibitory control, that are instrumental in our ability to learn, plan, and decide 15. Human neuroimaging research suggests that processes including prepotent response inhibition and attentional control may not mature until young adulthood 16,17. These same processes are linked with dietary behaviors 18,19. Diets high in fruits and vegetables may be associated with higher performance in these inhibitory and attentional control processes and diets higher in sweetened beverages or prepared snack foods may be associated with greater numbers of attentional issues, but the underlying neurocognitive mechanisms remains unclear 20,21. In addition to UPF consumption potentially impacting EF, issues in exercising EF such as the ability to inhibit selecting a certain food might be related to a higher UPF consumption 22. Similarly, the ability to choose a larger delayed reward over a smaller sooner reward may be impaired in people with overweight and obesity 23. This article will describe the study design and methods of a controlled feeding trial investigating the influence of UPF consumption on brain reward processing, ad libitum energy intake, food selection, EF, and delay discounting. Results of activities undertaken prior to recruitment are presented.
2. Aims and Hypotheses
The overall objective of this crossover-design controlled feeding trial is to establish proof-of-concept for altered reward processing measured by blood oxygenation level-dependent (BOLD) response to UPF, an increase in ad libitum EI, and adverse effects on EF and delay discounting in response to a UPF diet (81% total energy) compared to a diet without UPF (0% UPF; “Non-UPF”) in individuals aged 18-25. Aims are as follows:
Primary Aim:
Determine the influence of UPF consumption on brain reward response to UPF.
Secondary Aim:
Determine the influence of UPF consumption on ad libitum energy intake and food selection.
Exploratory Aim:
Explore the influence of UPF consumption on executive function, specifically inhibitory control, cognitive flexibility, and working memory, eating in the absence of hunger, and delay discounting.
First, we hypothesize that BOLD response in reward-associated brain areas (i.e., striatum and ventromedial prefrontal cortex) to UPF milkshake will be attenuated following the eucaloric UPF diet compared to the eucaloric Non-UPF diet. Second, when compared to a eucaloric Non-UPF diet, a eucaloric UPF diet will increase EI at an ad libitum buffet meal and preference for UPF will be increased. Lastly, as an exploratory aim, it is hypothesized that UPF exposure will be associated with reductions in EF performance and lower sensitivity to delayed rewards compared to no-UPF exposure and that eating in the absence of hunger will increase with UPF exposure.
3. Study Overview, Diet Design, Recruitment, and Detailed Methods
3.1. Overview
Participants aged 18-25 that are sedentary/recreationally active will undergo two controlled feeding periods, of 14 days each, in a randomly assigned order: high UPF (81% kcals from UPF) and Non-UPF (0% kcals from UPF). Diets are eucaloric and matched in nutrients, only differing in their level of food processing as described by NOVA classification 24. A four-week washout period will take place between conditions. To assess the Primary Aim of reward processing, the BOLD to a UPF-rich “milkshake” will be recorded via functional magnetic resonance imaging (fMRI) before and after each feeding period. Measurements after each diet period include ad libitum buffet meal EI, food selection, and eating rate (Secondary Aim). Eating in the absence of hunger (EAH) will be evaluated following the ad libitum meal, and additional measurements include EF and delay discounting (Exploratory Aim). See Figure 1. This trial was registered at ClinicalTrials.gov (NCT05550818).
Figure 1.
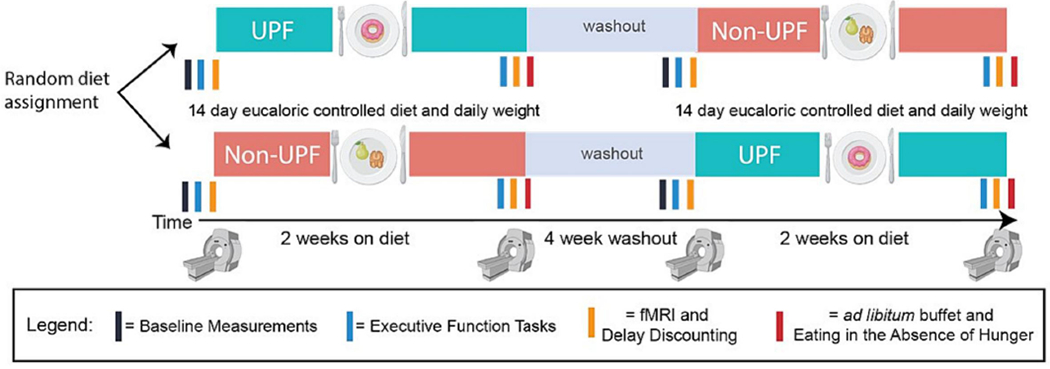
Study Design
3.2. Controlled Diet Design, Cost, and Palatability Testing
Participants will be fed a eucaloric (i.e., weight maintenance) diet that is 50% carbohydrate, 35% fat, 15% protein [10% animal/5% plant]) 25, and matched in fiber 26, added sugars, mono- and poly-unsaturated fats, saturated fat, sodium 26,27, glycemic index, and overall diet quality [HEI-2015]) 28. Prior to initiating recruitment, diets were designed to be consistent with the typical US diet 7. The 2-week high UPF diet contains 81% energy from UPF. The 2-week Non-UPF diet contains 0% UPF 29. These UPF levels are similar to those used by Hall and colleagues 10. NOVA classifications were determined using food labels and ingredient lists, the Open Food Facts app, and NDSR output files.
Diets will be prepared in the Metabolic Kitchen at Virginia Tech by ServSafe®-certified research assistants, and will consist of a 7-day cycle of menus with 3 meals, a snack, and an optional 250 kcal snack “module”. Menus were developed for 4 levels: 1500, 2000, 2500, and 3000 kcal. A sample menu for the UPF and Non-UPF 2000 kcal level is provided in Table 1. The nutrient content and targets for 2000 kcal menus are presented in Table 2.
Table 1:
Sample Daily Menu for the 2000 kcal UPF and Non-UPF Diets
| UPF (81% UPF) | Non-UPF (0% UPF) | |
|---|---|---|
| Breakfast | Scrambled eggs with Kraft American Cheese Wonder bread, toasted, with Smucker’s grape jelly |
Scrambled eggs with Kroger Natural Cheddar Cheese Homemade bread with Land O Lakes butter Whole milk |
| Lunch | Kroger Deli Black Forest Ham and Kraft American cheese sandwich with spinach and olive oil Apple with Kroger peanut butter Tropicana Orange juice |
Chicken breast and Kroger Natural Cheddar cheese sandwich with spinach Tropicana Orange juice |
| Dinner | Stouffers Chicken Fettuccini Alfredo | Chicken breast stir fry with vegetables: Kikkoman Soy sauce, green peas, onion, spinach, brown rice, olive oil |
| Snack | Chips Ahoy Cookies Minute Maid Lemonade Simple Truth Coconut Water |
Banana, fresh Quaker Rice cakes with Kroger Natural Peanut Butter Raspberries, fresh Minute Maid Lemonade |
| Optional Module | Ritz Crackers with Kraft American Cheese and Hormel Natural Choice deli Roasted Turkey Skittles Raspberries, fresh |
Good Thins Rice Crackers with Kroger Natural Cheddar Cheese and Applegate Natural Black Forest uncured Ham Cantaloupe, fresh Panda Natural Raspberry Licorice |
UPF: Ultra-processed Food
Table 2:
Planned Nutrient Targets and Actual Nutrient Content of a Sample 2000 kcal UPF and Non-UPF Menu
| 2000 kcal Diet Planned Nutrient Targets | 2000 kcal UPF Diet Menu (81%) | Absolute Difference, Target vs. Actual Values, UPF menu | 2000 kcal Non-UPF Diet Menu (0%) | Absolute Difference, Target vs. Actual Values, Non-UPF menu | |
|---|---|---|---|---|---|
| Energy (kcal) | 2000 | 1982 | 18 | 1984 | 16 |
| Total Fat (g) | 78 | 78 | 0 | 78 | 0 |
| Total Carbohydrate (g) | 250 | 254 | 4 | 251 | 1 |
| Total Protein (g) | 75 | 73 | 2 | 76 | 1 |
| Animal Protein (g) | 50 | 49 | 1 | 50 | 0 |
| Vegetable Protein (g) | 25 | 25 | 0 | 26 | 1 |
| Total Saturated Fatty Acids (SFA) (g) | 27 | 27 | 0 | 30 | 3 |
| Total Monounsaturated Fatty Acids (MUFA) (g) | 31 | 29 | 2 | 32 | 1 |
| Total Polyunsaturated Fatty Acids (PUFA) (g) | 11 | 15 | 4 | 10 | 1 |
| Total Dietary Fiber (g) | 16 | 14 | 2 | 13 | 3 |
| Soluble Dietary Fiber (g) | 4 | 5 | 1 | 4 | 0 |
| Added Sugars (g) | 70 | 72 | 2 | 70 | 0 |
| Sodium (mg) | 3400 | 3400 | 0 | 3400 | 0 |
| Potassium (mg) | 2400 | 2306 | 94 | 2237 | 163 |
| % Calories from Fat | 33 | 35 | 2 | 35 | 0 |
| % Calories from Carbohydrate | 50 | 50 | 0 | 50 | 0 |
| % Calories from Protein | 16 | 15 | 1 | 16 | 0 |
| % Calories from SFA | 12 | 12 | 0 | 13 | 1 |
| % Calories from MUFA | 13 | 13 | 0 | 14 | 1 |
| % Calories from PUFA | 8 | 7 | 1 | 4 | 4 |
| Glycemic Index (glucose reference) | 56 | 63 | 7 | 60 | 4 |
| Healthy Eating Index - 2015 | 58 | 56 | 2 | 59 | 1 |
| UPF, % energy | 81 | 0 |
UPF: Ultra-processed Food
Menus (56 daily menus and 2 modules) were developed by a research dietitian to meet the daily energy, UPF content, and nutrient values expected using NDSR 2022 software (Nutrition Coordinating Center, University of Minnesota). Modules provide extra snacks matched to the content of the total diet if needed due to changes in activity level or if the participant’s estimated energy needs are between two kcal levels (e.g., 1750 kcal). Accepted variations were ±5 g of the daily target for macronutrients and ±1 g of the daily target for soluble fiber. A list of locally available foods was developed to match the NDSR food items within menus. Non-UPF recipes were identified to provide alternatives for commercial baked goods (e.g., bread). A second dietitian reviewed the menus to verify achievement of targets. Meal and snack palatability were assessed using the adapted United States Department of Agriculture (USDA) Sensory Evaluation Form 30. Meals and snacks were rated on a 1-9 scale (1=Dislike Extremely; 9=Like Extremely). Overall menu acceptability ratings were in the Like Moderately (7)/Like Very Much (8) range, but slightly higher for the UPF menus (UPF: 7.6±1.0; Non-UPF: 6.8±1.5; P<0.05).
The UPF diet’s average daily cost (food + Labor at US$15/hour) was calculated to be $20.97 ($9.72 food cost + $11.25 labor cost). The Non-UPF diet’s average cost was calculated to be $40.23 ($10.23 food + $30 labor).
3.3. Controlled Diet Delivery
Participant’s estimated energy needs will be determined using the Mifflin St. Jeor Equation 31 and an activity factor based upon their reported usual physical activity level 32. Weight will be monitored to ensure stability. Trends of weight changes ±1.5 kg over 2-3 days will be countered by the addition/subtraction of 250 kcal modules or a change in dietary energy level.
Participants will have breakfast supervised in our laboratory daily, except for Sundays when we will provide meals in advance on Saturday. The remaining food for the day will be provided in a portable cooler. Uneaten items will be returned the following morning. The total gram weight of food provided vs total grams consumed will be the indicator of compliance. To be included in data analysis, participants must be >95% adherent to the diet. In addition, participants are asked about any deviations from the diet on a daily basis (Monday-Saturday).
3.4. Ad Libitum Buffet Meal and Eating in the Absence of Hunger (EAH): Food Item Selection and Texture Assessment
3.4.1. Buffet Meal and EAH Food Selection
Food items for the Buffet Meal and the Eating in the Absence of Hunger (EAH) snack were chosen to offer participants comparable foods that differed in UPF content. For example, the EAH snack will contain two types of potato chips - one a UPF (Pringles) and one that is not a UPF (Cape Cod kettle chip). Each UPF and Non-UPF grouping has 5 items for the buffet meal (juice, cereal, dairy, fruit, grain) and 3 items for the EAH (chips, fruit, cookies) (Figure 2). UPF and Non-UPF items were comparable in macronutrients, energy density, and hyperpalatability 33. Beverages are not included in hyperpalatability criteria. Other than fruit items, all foods met hyperpalatability criteria. Details are provided in Supplemental Tables 1 and 2.
Figure 2:
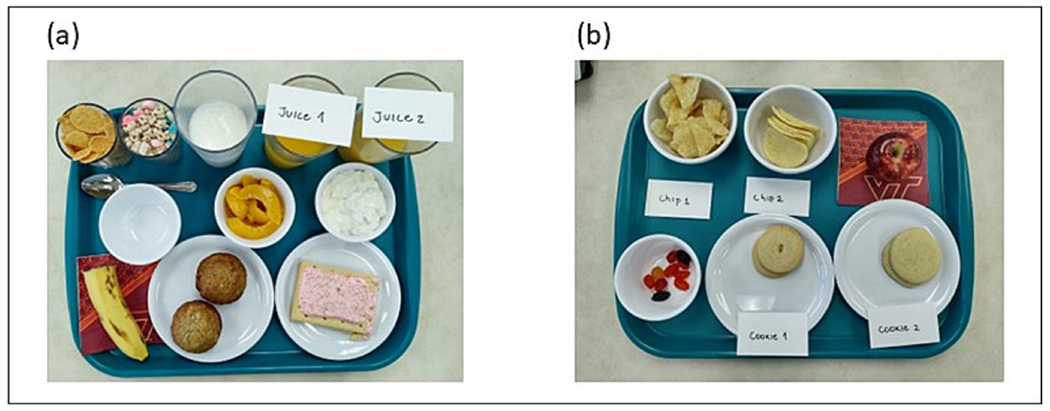
Ad Libitum Breakfast Buffet Meal and Eating in the Absence of Hunger Snack Trays.
(a) Buffet meal tray (Nature’s Path Corn Flakes and Lucky Charms, banana and Del Monte canned peaches in syrup, Homemade banana muffin and Pop Tart, 100% orange juice and Sunny Delight orange juice drink, milk and Kroger whole milk yogurt with vanilla flavoring)
(b) Eating in the absence of hunger snack tray (Cape Cod chips and Pringles, homemade sugar cookie and Keebler sugar cookie, apple and Welch’s fruit snacks)
Buffet and snack items were tested for palatability and texture by 12-14 panelists to ensure that UPF and Non-UPF items were comparable. Palatability was assessed using the USDA Sensory Evaluation form 30. Acceptability and texture ratings are presented for all items in Table 3. The acceptability scale ranged 1-9 (1=“dislike extremely”, 9=“like extremely”). For cohesiveness, defined as the amount of deformation undergone by the sample when biting through it, the scale ranged 1-15 (1=not cohesive, e.g., corn muffin; 15=cohesive e.g., chewing gum) . For hardness, defined as the force required to bite through the sample, the scale ranged 1-9 (1=low/soft, e.g., cream cheese, 9= hard, e.g., rock candy)35. To assess differences between UPF and Non-UPF in ratings, linear mixed effects models (lmerTest, R) were fit with acceptability, cohesiveness, and hardness as the outcome, type (UPF vs Non-UPF) as a fixed effect and participants as a random effect (all p>0.05; Figure 3).
Table 3.
Subjective Snack and Buffet Meal Food Palatability and Texture Ratings
| Food Group Item | Overall Acceptability | Cohesiveness | Hardness |
|---|---|---|---|
| UPF Snack food items (EAH) | 6.9 | 6.3 | 5.5 |
| Non-UPF Snack items (EAH) | 7.5 | 4.4 | 5.4 |
| UPF Buffet items | 6.9 | 4.8 | 3.3 |
| Non-UPF Buffet items | 6.8 | 4.0 | 4.0 |
UPF: Ultra-processed food; EAH: Eating in the absence of hunger.
Figure 3.
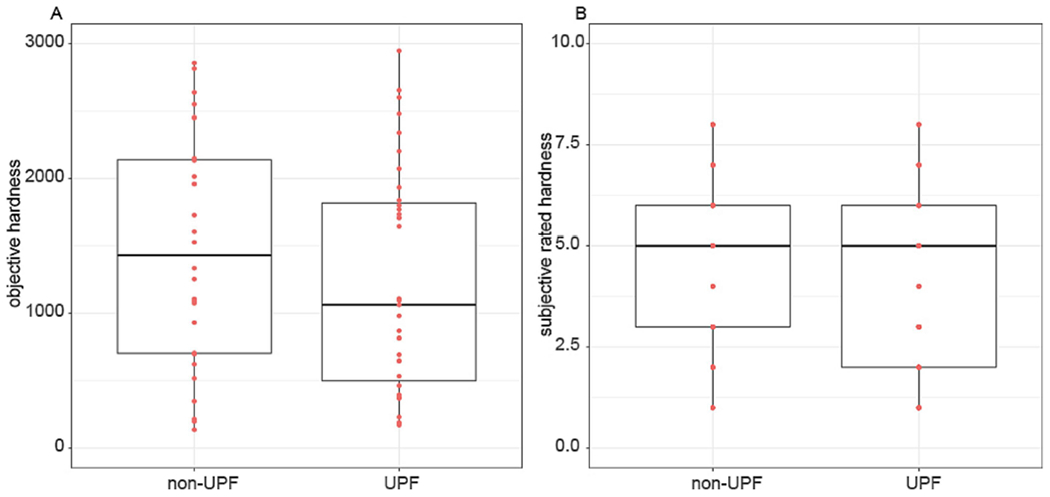
Objective (A) and Subjective (B) Hardness Ratings for UPF and Non-UPF Buffet and EAH Snack Foods
3.4.2. Texturometer Food Texture Assessment
Food texture (e.g., hardness) impacts eating rate and energy intake 36. To account for this in the non-liquid foods selected for the ad libitum meal and EAH snacks, texture was objectively measured using a TA-XT Plus Texture Analyzer, with the following settings: Pre-test speed: 2.0 mm/sec, Test speed: 5.0 mm/sec, Post-test speed: 5.0 mm/sec, Time between strokes: 5.0 sec, Target mode: stain or distance depending on product, Auto trigger force: 50.0 g, Tare mode: auto, Advanced options: on. The height of each food item was measured (mm) and was multiplied by 0.7 to determine the height of a 70% compression. This value was used as the target distance in the texturometer settings. For foods whose height could not accurately be measured (e.g., yogurt), the strain target mode was utilized and set to a 70% strain. Settings were loaded into the texture program before starting. A Texture Profile Analysis (TPA) program with a known macro was utilized. The force and height of the texturometer were calibrated before testing each new item. A flat cylindrical 2.5-inch compression probe was used for all the foods.
For the two chips (Cape Cod Kettle Chips, Pringles), Welch’s Fruit Snacks, Del Monte peaches, and Nature’s Path Cornflakes, a single item was placed into a container to prevent slipping when compressed. The Lucky Charms cereal and marshmallow pieces were tested separately, and results were averaged. The Pop Tart was cut into four equal rectangular pieces. For the yogurt, a single scoop was placed into the container. Fresh fruits were cut into even slices and placed into the container. The homemade muffin was cut in half and placed onto the metal holding plate. Both cookies (Keebler and Homemade Sugar cookies) were placed one at a time directly onto the plate. All items were placed center on either the holding plate or in the container, and in similar orientation and direction to minimize differences. Each item’s texture evaluation was performed in replicates of varying frequencies (ranged 6-15) based on the degree of differences in results.
The average, standard deviation (SD), and coefficient of variation for the texture outcomes were calculated. Outliers were defined as negative numbers or more than 3 SD from the mean and were deleted from the data set. Certain products were not applicable to all the TPA results (e.g., chips did not have measurable chewiness). Hardness could not be recorded for cookies due to exceeding the 50kg load cell (the highest available). To assess differences between UPF and Non-UPF items in objective ratings, linear mixed effects models (lmerTest, R) were fit with hardness, cohesiveness, and chewiness as the outcome variable, type (UPF vs Non_UPF) as a fixed effect and run number as a random effect (all p>0.05; Figure 3).
3.5. Participant Recruitment and Screening Procedures
Recruitment strategies will include flyers, ads in university listservs, and social media. Interested individuals will complete an online prescreening survey to evaluate eligibility. Those who may meet eligibility criteria (Table 4) will be emailed the consent form and study food list to verify the absence of food allergies. The first in-person screening visit will include informed consent, health history, menstrual cycle status, measurement of height, weight, BMI, 24-hour dietary recall, and assessment of dietary cognitive restraint 37, addictive-like eating behaviors scale 38, adverse life events checklist, and physical activity 39 using RedCap software. Two additional 24-hour recalls will be completed via phone after the initial visit. The three-day averaged recalls will represent habitual baseline intake; recalls will include two weekdays and one weekend day. Recalls will be analyzed using NDSR 2022. UPF intake will be determined manually by trained evaluators using NDSR output files and recall forms.
Table 4:
Inclusion and Exclusion criteria for the Influence of Ultra-processed Foods on Reward Processing and Energy Intake study
| Inclusion Criteria |
|---|
| • Age 18-25 years. |
| • Weight stable (±5 pounds) for the previous 6 months. |
| • No plans to gain/lose weight or change the physical activity level. |
| • Willing to pick up food daily and consume food provided for two 14-day periods. |
| • Willing to consume all study foods. |
| • Verbal and written informed consent. |
| • Unrestrained eater (Three Factor Eating Questionnaire - cognitive restraint score <11). |
| • No reported history of eating disorders. |
| • Sedentary to recreationally active. |
| Exclusion Criteria |
|
|
| • BMI >30 kg/m2. |
| • Diagnosis of endocrine disorders or other major chronic diseases (e.g., type 2 diabetes, hypothyroidism, hypertension). Individuals on a stable dose of thyroid medication (>6 months) are eligible to participate. |
| • Pregnant or plan to become pregnant. |
| • Food allergies or aversions. |
| • Contraindications to Magnetic Resonance Imaging (MRI): history of head injury with loss of consciousness for more than 10 minutes, claustrophobia, or individuals with pacemakers, aneurysm clips, neurostimulators, cochlear implants, metal in eyes, steel worker, or other implants. |
UPF: Ultra-processed Food; BMI: Body Mass Index
The second in-person screening visit will include a mock fMRI scan to verify the absence of claustrophobia in order to improve fMRI data quality and ensure participants are able to complete the fMRI measurement. The mock fMRI utilizes a simulation scanner and 64-channel head coil (Vera MRI Simulator, Psychology Software Tools, Pittsburgh, PA) that is identical to the real scanner except for the magnetic field. Participants will undergo a shortened version of the milkshake task to practice drinking liquids while supine. Participants will choose a tasteless, artificial saliva solution best suited for them to be utilized as a control against the milkshake, and this tasteless solution concentration will be confirmed at each subsequent fMRI scan.
3.6. Detailed Measurement Methods
3.6.1. Baseline measurements
Baseline measurements will include body weight, bioelectrical impedance analysis (BIA), waist/hip circumferences, computerized cognitive EF tasks, fMRI and a delay discounting task.
3.6.2. Body Mass and Composition
Participants will be weighed on a scale accurate to ±0.1 kg (Scale-Tronix Inc.; White Plains, New York). Circumferences will be measured based on World Health Organization instructions 40. Body composition will be assessed using BIA (InBody 770 scan).
3.6.3. Cognitive Tasks
Executive function will be evaluated before and after each diet period using computerized tasks (E-prime 3.0, Psychology Software Tools, Pittsburgh, PA). The Go-NoGo task evaluates inhibitory control 41,42. A modified version of the Flanker task 43 will be used to assess participants’ selective attention and interference control 44. The Corsi Block-Tapping Backward Task will be used to measure visuospatial working memory 45,46.
3.6.4. fMRI Scan
BOLD response to a UPF-rich milkshake will be acquired via fMRI before and after each diet period. To avoid habituation, participants will choose two flavors of milkshake from chocolate, strawberry, and caramel. An artificial saliva or “tasteless” solution will be used as a control condition instead of water, as water has a flavor and activates gustatory cortex 47. Milkshakes and tasteless solutions will be delivered through a custom manifold, gustometer, fitted to the head coil and connected to a pump system that allows precisely timed and measured delivery of liquids 48. Two 11-minute scans will be performed. Each milkshake or tasteless presentation will be preceded by a 2 second visual cue (blue or red; counterbalanced across participants) projected on a screen behind the bore and viewed through a mirror 49. Following a jittered interstimulus interval (ISI), 3mls of milkshakes or tasteless solution will be delivered over 4 seconds. A variable jittered ISI of 5-12 seconds will follow stimulus delivery, allowing time to swallow. A total of 18 each milkshake and tasteless cue and deliveries will occur over two runs. Following each milkshake presentation, a water rinse will occur over 4 seconds to clear remaining solution from the mouth. PsychoPy 50 will be used to control all visual cues and pump triggers. Following the session, participants will perform an additional set of ratings of all solutions using PsychoPy.
For this study, a Magnetom Prisma whole-body 3T Prisma scanner with a 64-channel head coil (Siemens AG, Medical Solutions, Erlangen, Germany) will be used. The two echo planar image (EPI) runs (TR: 1500.0 ms, TE: 34.00 ms, field of view:192 mm, flip angle: 70 deg, voxel size: 2.0×2.0×2.0 mm3, 72 axial slices, ascending interleaved in-plane acquisition, anterior to posterior phase encoding directions and a multiband acceleration factor of 4) were collected. Two images with identical parameters but opposite phase encoding (posterior to anterior) will be collected to estimate and correct the susceptibility-induced distortion. Structural images will be collected using a T1-weighted sequence (TR: 2300 ms, TE: 2.32 ms, field of view: 240 mm, flip angle: 8 deg, 0.9×0.9×0.9 mm3, and 192 sagittal slices). The parameters used here are very similar to those used in Oren et al 51. We use a slightly longer TR and a multiband acceleration factor of 4. No acceleration other than multiband is used.
3.6.5. Delay Discounting
To examine the role that UPF plays on cognition and impulsivity, delay discounting will be measured to capture discount rate. This will be done with a short task that asks participants about their preference for different amounts of money over different amounts of time 52.
3.6.6. Two-Week Controlled Feeding Period
For 12 days participants will come into the dining lab each morning in a fasting state, be weighed, eat breakfast, and be provided with a cooler containing food for the rest of the day. They will be asked about side effects and diet adherence. On day 13, participants will repeat the computerized EF tests, BIA, and circumference measures. On day 14, participants will complete an fMRI session.
3.6.7. Accelerometry
Participants will wear an accelerometer (wGT3X-BT, Actigraph, Inc.) on their wrist for 4 days (1 weekend day and 3 weekdays) 53. Physical Activity (PA) measurements will be made during each controlled feeding period to ensure consistency in PA during the two feeding periods.
3.6.8. Buffet Meal and Eating in the Absence of Hunger
Following the controlled feeding period (day 15), participants will come to the laboratory fasting an ad libitum buffet meal. Visual Analog Scales (VAS) 54 will be completed before and 30 minutes after the meal to assess sensations of fullness and hunger. Time spent eating (meal start/stop time), kcal and grams of food consumed will be recorded to calculate the meal eating rate (g/min, kcal/min). Food selection will also be recorded (UPF, Non-UPF).
Eating in the absence of hunger will be assessed immediately after the meal. Participants will take one bite of each item and rate it’s palatability and familiarity using the Labeled Hedonic Scale (LHS) 55. They will be left to consume the remaining snacks if they wish to do so or relax for 15 minutes. Snack selection and consumption will be covertly recorded. Following this test, participants will be given a take-home bag with the same snacks and a link to the LHS page in RedCap to assess EAH outside the laboratory. The amount and type of snacks consumed will be evaluated.
3.6.9. Washout Period Measures
Participants will undergo a 4-week washout where they will be asked to consume their habitual diet. In the last week of the washout, baseline measurements will be repeated.
3.7.0. Second Controlled Feeding Period Measures
Participants will complete the second feeding period, and repeat all baseline and post-tests as previously described.
4. Data analysis
4.1. Power analysis
Prior to study implementation, effect sizes were extracted from the effects of sugar-sweetened beverage (SSB) consumption intervention on caudate and ventromedial prefrontal cortex (cohen’s d= 1.036 and 0.980, respectively) response to SSB delivery 56. This intervention and task design of that study is similar to one proposed here. The average cohen’s d of 1.008 was computed and power analysis was conducted using the pwr toolbox in R. For a two-sided paired t-test with an alpha of 0.001 and power of 90% to account for whole brain corrections, a sample size of 26 is needed.
Data from Hall et al., 2019 were downloaded from the Open Science Framework. The effect size for breakfast was calculated using the effsize package in R as 0.825. It was estimated that for a two-sided paired t-test with an alpha of 0.05 and 80% power, 13 individuals are needed to detect this difference.
The enrollment goal of 32 participants will account for 20% attrition to retain the 26 needed to detect differences in outcomes with sufficient power from the primary and secondary aims.
4.2. Statistical analysis
Imaging data will be preprocessed (motion correction, distortion correction, registration, slice timing correction) using fMRIprep 57. Data will be preprocessed within 24 hours of data collection. fMRIprep output, motion parameters, and carpet plots will be inspected. Participants with movement greater than 2mm in a single direction will be asked to repeat the scan. If a scan cannot be rescheduled only runs with movement less than 2mm will be included. The resulting images will be smoothed using a 6mm full width half maximum gaussian kernel in SPM12 (Wellcome Trust Center for Neuroimaging, London, UK). All subsequent analyses will be performed in SPM12.
On the first level a box-car model for each stimulus type, milkshake cue, tasteless cue, milkshake receipt, and tasteless receipt will be created. Framewise displacement, standard motion parameters, and cerebrospinal fluid signal fluctuation will be entered as regressors of no interest in this first level model. This allows for the creation of contrasts of interest: milkshake cue > tasteless cue, milkshake receipt > tasteless receipt, and their inverses.
These first level analyses will be brought up to the group level using a flexible factorial design with time (4 time points), intervention (UPF vs Non-UPF), and participant as factors. Age, sex, and BMI will be entered as regressors as well as treatment order. A family wise error (FWE) rate of p<0.05 will be calculated across all voxels of the brain to determine whole brain corrected effects. Next, a single anatomical mask of a priori regions of interest (ROIs; dorsal and ventral striatum and ventromedial prefrontal cortex) will be applied using a small volume correction (FWE p<0.05). These ROIs were chosen from two previous publications demonstrating blunted response to SSB after exposure and blunted response to milkshake after ice cream exposure 56,58. Finally, a whole brain analysis will be conducted with FWE of p<0.05. The hypothesis that brain response to the UPF milkshake will be related to EI by entering EI from the breakfast buffet meal (kcals) will be tested as a regressor at the group level. Results from both analyses, ROI and whole brain, will be reported, as well as the group level results from all contrasts and F-tests.
A paired t-test will be used to assess the primary outcome of EI during the buffet breakfast meal. Percent EI from each type of food (UPF or Non-UPF) will be calculated from total EI. A 2-way ANOVA with intervention and type will be used to assess the effect of the intervention on food choice.
Previous literature has used a paired sample t-test to test the outcome of change in EI, which will also be done here. As an exploratory analysis we propose to specify a mixed-effects ANOVA, a class of mixed models. Energy intake will be regressed on dummy-coded UPF and Non-UPF conditions as the reference category, and fixed effects for relevant biological variables (e.g., age, sex) and experimental variables such as diet order. The unique distribution of means each individual provides the random component for the mixed ANOVA model. To reliably estimate SD, all individuals are estimated to have the same SD for their own data; as such, within and between-person SD will be modeled as fixed effects. Maximum likelihood estimation will be used to obtain estimates of the model parameters, with likelihood ratio tests (LRT) used to test the significance of effects against a null distribution assuming no effect. If the data is not normally distributed, a generalized linear mixed model methodology will be applied with the appropriate distribution and link functions specified.
For the Exploratory Aim, a 2-way repeated measures ANOVA will evaluate the effects of UPF or Non-UPF on EF processes as part of a composite variable. Given that this aim is exploratory, we will also utilize three separate 2-way repeated measures ANOVAs to examine intervention effects on EF measures individually to inform an understanding of which cognitive processes might be associated with UPF consumption. Additional analyses may be used to examine the influence of individual difference variables on cognitive outcomes, as well as whether changes in EF throughout the intervention are associated with fMRI outcomes.
Following trial completion and the publication of study results, de-identified data will be posted to Virginia Tech’s data repository, VTechData, for sharing. Deidentified neuroimaging data will be made available on the OpenNeuro platform.
5. Discussion and Future Directions
Food attributes beyond macronutrients and energy density, such as food matrix and degree of commercial processing, impact EI and health. Using NOVA, research has associated UPF consumption with numerous adverse effects including, obesity 59–61 and increased CVD risk 59,62,63. Despite a growing body of evidence linking UPF to negative health outcomes, the Dietary Guidelines for American do not currently address UPF intake due to a lack of experimental research.
In 1947, Adolph fed rats diets that were isocaloric, but varied in volume and energy density, and found rodents accurately adjusted the volume of food consumed to maintain a constant EI 64. The same is not observed to those exposed to diets composed of high-UPF ingredients, which cause weight gain compared to controls 12,65–67. Thus, UPFs may override brain systems that govern intake regulation and drive intake above physiological needs.
Researchers speculate that these foods can drive addictive-like behaviors 68. The second possible driver of overconsumption is that UPFs contain combinations of macronutrients not seen in our evolutionary past. Fat and carbohydrate signal their nutrient value to the brain through separable peripheral pathways that converge on a single neural signal – dopamine in the striatum 69. It is possible UPFs exploit this signal convergence, leading to their over-valuation. UPFs can exploit the gut-brain axis in other ways potentially degrading its ability to properly signal nutrient availability. To increase palatability, non-nutritive sweeteners (UPFs) are often added to foods that already contain nutritive sugars and starches. Exposure to this “mismatch” of information from the oral cavity and later available calories in the gut impairs insulin sensitivity and decreases brain response to sweet tastes 70. Thus, UPF may exploit the gut-brain axis to change brain response to food. However, studies were not designed to test the consequences of UPF exposure and did not control for the level of processing 69 or only tested one UPF component in isolation 70. This research could contribute to an emerging neurobiological understanding of the adverse effects of UPF consumption including energy overconsumption, weight gain, and obesity risk among individuals at a critical developmental stage.
5.1. Potential challenges
Recruitment and retention may be challenges, but will be achievable due to our previous experience. Dietary adherence will be managed by supervising breakfast and reinforcing the importance of consuming study foods. Comfort in the fMRI environment and swallowing prone can affect head movement and data quality. We will include a “mock” scanning session to mitigate these effects 49.
6. Conclusions and Future Directions
If our hypotheses are supported, we will have preliminary data for a larger trial to investigate the influence of UPF on gut/brain signaling, dose/response effects, the influence of UPF intake on EI at various levels of energy density, and mechanistic studies to dissect brain reward-UPF relationships. At this time, it is unclear what mechanisms could link UPF to increased EI, weight gain, and other health outcomes 71. These findings may contribute to an emerging evidence base on the health effects of UPF and potentially inform future dietary guidelines.
Supplementary Material
Acknowledgment:
We acknowledge Julia J Scialla, MD, MHS, University of Virginia School of Medicine, for helpful conversations related to the study and medical safety oversight.
This work was supported by the National Institutes of Health (grant number: R21HD109722).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Dutton GR, Kim Y, Jacobs DR, et al. 25-year weight gain in a racially balanced sample of U.S. adults: The CARDIA study. Obesity (Silver Spring). 2016;24(9):1962–1968. doi: 10.1002/oby.21573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Truesdale KP, Stevens J, Lewis CE, Schreiner PJ, Loria CM, Cai J. Changes in risk factors for cardiovascular disease by baseline weight status in young adults who maintain or gain weight over 15 years: the CARDIA study. Int J Obes (Lond). 2006;30(9):1397–1407. doi: 10.1038/sj.ijo.0803307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei GS, Coady SA, Reis JP, et al. Duration and Degree of Weight Gain and Incident Diabetes in Younger Versus Middle-Aged Black and White Adults: ARIC, CARDIA, and the Framingham Heart Study. Diabetes Care. 2015;38(11):2042–2049. doi: 10.2337/dc14-2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring). 2008;16(10):2323–2330. doi: 10.1038/oby.2008.351 [DOI] [PubMed] [Google Scholar]
- 5.Arnett JJ. Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist. 2000;55:469–480. doi: 10.1037/0003-066X.55.5.469 [DOI] [PubMed] [Google Scholar]
- 6.HEI Scores for Americans | Food and Nutrition Service. Accessed April 28, 2023. https://www.fns.usda.gov/hei-scores-americans
- 7.Baraldi LG, Martinez Steele E, Canella DS, Monteiro CA. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study. BMJ Open. 2018;8(3):e020574. doi: 10.1136/bmjopen-2017-020574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Martínez Steele E, Du M, et al. Trends in Consumption of Ultraprocessed Foods Among US Youths Aged 2-19 Years, 1999-2018. JAMA. 2021;326(6):519–530. doi: 10.1001/jama.2021.10238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes Gonçalves N, Vidal Ferreira N, Khandpur N, et al. Association Between Consumption of Ultraprocessed Foods and Cognitive Decline. JAMA Neurology. 2023;80(2):142–150. doi: 10.1001/jamaneurol.2022.4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall KD, Ayuketah A, Brychta R, et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2020;32(4):690. doi: 10.1016/j.cmet.2020.08.014 [DOI] [PubMed] [Google Scholar]
- 11.Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30(8):375–381. doi: 10.1016/j.tins.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 12.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–641. doi: 10.1038/nn.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colantuoni C, Schwenker J, McCarthy J, et al. Excessive sugar intake alters binding to dopamine and muopioid receptors in the brain. Neuroreport. 2001;12(16):3549–3552. doi: 10.1097/00001756-200111160-00035 [DOI] [PubMed] [Google Scholar]
- 14.Thanarajah SE, Backes H, DiFeliceantonio AG, et al. Food Intake Recruits Orosensory and Post-ingestive Dopaminergic Circuits to Affect Eating Desire in Humans. Cell Metabolism. 2019;29(3):695–706.e4. doi: 10.1016/j.cmet.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 15.Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1231–1238. doi: 10.1097/00004583-200210000-00013 [DOI] [PubMed] [Google Scholar]
- 17.Cohen Kadosh K, Heathcote LC, Lau JYF. Age-related changes in attentional control across adolescence: how does this impact emotion regulation capacities? Front Psychol. 2014;5:111. doi: 10.3389/fpsyg.2014.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen JFW, Gorski MT, Gruber SA, Kurdziel LBF, Rimm EB. The effect of healthy dietary consumption on executive cognitive functioning in children and adolescents: a systematic review. Br J Nutr. 2016;116(6):989–1000. doi: 10.1017/S0007114516002877 [DOI] [PubMed] [Google Scholar]
- 19.Fieldhouse JLP, Doorduijn AS, de Leeuw FA, et al. A Suboptimal Diet Is Associated with Poorer Cognition: The NUDAD Project. Nutrients. 2020;12(3):703. doi: 10.3390/nu12030703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riggs N, Chou CP, Spruijt-Metz D, Pentz MA. Executive cognitive function as a correlate and predictor of child food intake and physical activity. Child Neuropsychol. 2010;16(3):279–292. doi: 10.1080/09297041003601488 [DOI] [PubMed] [Google Scholar]
- 21.Farsad-Naeimi A, Asjodi F, Omidian M, et al. Sugar consumption, sugar sweetened beverages and Attention Deficit Hyperactivity Disorder: A systematic review and meta-analysis. Complement Ther Med. 2020;53:102512. doi: 10.1016/j.ctim.2020.102512 [DOI] [PubMed] [Google Scholar]
- 22.Bartholdy S, Dalton B, O’Daly OG, Campbell IC, Schmidt U. A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task. Neuroscience & Biobehavioral Reviews. 2016;64:35–62. doi: 10.1016/j.neubiorev.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 23.Tang J, Chrzanowski-Smith OJ, Hutchinson G, Kee F, Hunter RF. Relationship between monetary delay discounting and obesity: a systematic review and meta-regression. Int J Obes. 2019;43(6):1135–1146. doi: 10.1038/s41366-018-0265-0 [DOI] [PubMed] [Google Scholar]
- 24.Monteiro CA, Cannon G, Levy RB, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936–941. doi: 10.1017/S1368980018003762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shan Z, Rehm CD, Rogers G, et al. Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality Among US Adults, 1999-2016. JAMA. 2019;322(12):1178–1187. doi: 10.1001/jama.2019.13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King DE, Mainous AG, Lambourne CA. Trends in Dietary Fiber Intake in the United States, 1999-2008. Journal of the Academy of Nutrition and Dietetics. 2012;112(5):642–648. doi: 10.1016/j.jand.2012.01.019 [DOI] [PubMed] [Google Scholar]
- 27.Cheteu Wabo TM, Wu X, Sun C, et al. Association of dietary calcium, magnesium, sodium, and potassium intake and hypertension: a study on an 8-year dietary intake data from the National Health and Nutrition Examination Survey. Nutr Res Pract. 2022;16(1):74–93. doi: 10.4162/nrp.2022.16.1.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comparing Versions of the HEI | EGRP/DCCPS/NCI/NIH. Accessed May 23, 2023. https://epi.grants.cancer.gov/hei/comparing.html
- 29.Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21(1):5–17. doi: 10.1017/S1368980017000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sensory Evaluation Form Usda - Fill and Sign Printable Template Online. Accessed June 6, 2023. https://www.uslegalforms.com/form-library/99614-sensory-evaluation-form-usda [Google Scholar]
- 31.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–247. doi: 10.1093/ajcn/51.2.241 [DOI] [PubMed] [Google Scholar]
- 32.Academy of Nutrition and Dietetics. Accessed September 21, 2023. https://www.eatrightpro.org/news-center/practice-trends/adjusted-or-ideal-body-weight-for-nutrition-assessment
- 33.Fazzino TL, Rohde K, Sullivan DK. Hyper-Palatable Foods: Development of a Quantitative Definition and Application to the US Food System Database. Obesity. 2019;27(11):1761–1768. doi: 10.1002/oby.22639 [DOI] [PubMed] [Google Scholar]
- 34.Muñoz AM. Development and Application of Texture Reference Scales. Journal of Sensory Studies. 1986;1(1):55–83. doi: 10.1111/j.1745-459X.1986.tb00159.x [DOI] [Google Scholar]
- 35.Lawless HT, Heymann H. Sensory Evaluation of Food: Principles and Practices. Springer New York; 2010. doi: 10.1007/978-1-4419-6488-5 [DOI] [Google Scholar]
- 36.Teo PS, Lim AJ, Goh AT, et al. Texture-based differences in eating rate influence energy intake for minimally processed and ultra-processed meals. The American Journal of Clinical Nutrition. 2022;116(1):244–254. doi: 10.1093/ajcn/nqac068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8 [DOI] [PubMed] [Google Scholar]
- 38.Schulte EM, Gearhardt AN. Development of the Modified Yale Food Addiction Scale Version 2.0. Eur Eat Disord Rev. 2017;25(4):302–308. doi: 10.1002/erv.2515 [DOI] [PubMed] [Google Scholar]
- 39.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 40.Waist circumference and waist-hip ratio: report of a WHO expert consultation. Accessed May 22, 2023. https://www.who.int/publications-detail-redirect/9789241501491
- 41.Donders FC. On the speed of mental processes. Acta Psychol (Amst). 1969;30:412–431. doi: 10.1016/0001-6918(69)90065-1 [DOI] [PubMed] [Google Scholar]
- 42.Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56(3):1655–1665. doi: 10.1016/j.neuroimage.2011.02.070 [DOI] [PubMed] [Google Scholar]
- 43.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. doi: 10.3758/BF03203267 [DOI] [Google Scholar]
- 44.Chaddock L, Erickson KI, Prakash RS, et al. Basal Ganglia Volume Is Associated with Aerobic Fitness in Preadolescent Children. Dev Neurosci. 2010;32(3):249–256. doi: 10.1159/000316648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isaacs EB, Vargha-Khadem F. Differential course of development of spatial and verbal memory span: A normative study. British Journal of Developmental Psychology. 1989;7:377–380. doi: 10.1111/j.2044-835X.1989.tb00814.x [DOI] [Google Scholar]
- 46.Kessels RPC, van den Berg E, Ruis C, Brands AMA. The backward span of the Corsi Block-Tapping Task and its association with the WAIS-III Digit Span. Assessment. 2008;15(4):426–434. doi: 10.1177/1073191108315611 [DOI] [PubMed] [Google Scholar]
- 47.de Araujo IET, Kringelbach ML, Rolls ET, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol. 2003;90(3):1865–1876. doi: 10.1152/jn.00297.2003 [DOI] [PubMed] [Google Scholar]
- 48.Veldhuizen MG, Babbs RK, Patel B, et al. Integration of Sweet Taste and Metabolism Determines Carbohydrate Reward. Curr Biol. 2017;27(16):2476–2485.e6. doi: 10.1016/j.cub.2017.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwin Thanarajah S, DiFeliceantonio AG, Albus K, et al. Habitual daily intake of a sweet and fatty snack modulates reward processing in humans. Cell Metab. 2023;35(4):571–584.e6. doi: 10.1016/j.cmet.2023.02.015 [DOI] [PubMed] [Google Scholar]
- 50.Peirce J, Gray JR, Simpson S, et al. PsychoPy2: Experiments in behavior made easy. Behav Res Methods. 2019;51(1):195–203. doi: 10.3758/s13428-018-01193-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oren S, Tittgemeyer M, Rigoux L, Schlamann M, Schonberg T, Kuzmanovic B. Neural encoding of food and monetary reward delivery. NeuroImage. 2022;257:119335. doi: 10.1016/j.neuroimage.2022.119335 [DOI] [PubMed] [Google Scholar]
- 52.Koffarnus MN, Bickel WK. A 5-trial adjusting delay discounting task: accurate discount rates in less than one minute. Exp Clin Psychopharmacol. 2014;22(3):222–228. doi: 10.1037/a0035973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu F, Wanigatunga AA, Schrack JA. Assessment of Physical Activity in Adults Using Wrist Accelerometers. Epidemiol Rev. 2021;43(1):65–93. doi: 10.1093/epirev/mxab004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindeman A, Huang M, Dawkins E. Using the Visual Analog Scale (VAS) to Measure Perceived Hunger and Satiety at Various Mealtimes and Environments. Journal of the Academy of Nutrition and Dietetics. 2016;116(9):A99. doi: 10.1016/j.jand.2016.06.359 [DOI] [Google Scholar]
- 55.Lim J. Hedonic scaling: A review of methods and theory. Food Quality and Preference. 2011;22(8):733– 747. doi: 10.1016/j.foodqual.2011.05.008 [DOI] [Google Scholar]
- 56.Burger KS. Frontostriatal and behavioral adaptations to daily sugar-sweetened beverage intake: a randomized controlled trial. The American Journal of Clinical Nutrition. 2017;105(3):555–563. doi: 10.3945/ajcn.116.140145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esteban O, Markiewicz CJ, Blair RW, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16(1):111–116. doi: 10.1038/s41592-018-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burger KS, Stice E. Frequent ice cream consumption is associated with reduced striatal response to receipt of an ice cream–based milkshake. The American Journal of Clinical Nutrition. 2012;95(4):810–817. doi: 10.3945/ajcn.111.027003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mendonça R de D, Pimenta AM, Gea A, et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr. 2016;104(5):1433–1440. doi: 10.3945/ajcn.116.135004 [DOI] [PubMed] [Google Scholar]
- 60.Rauber F, Steele EM, Louzada ML da C, Millett C, Monteiro CA, Levy RB. Ultra-processed food consumption and indicators of obesity in the United Kingdom population (2008-2016). PLoS One. 2020;15(5):e0232676. doi: 10.1371/journal.pone.0232676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rauber F, Chang K, Vamos EP, et al. Ultra-processed food consumption and risk of obesity: a prospective cohort study of UK Biobank. Eur J Nutr. 2021;60(4):2169–2180. doi: 10.1007/s00394-020-02367-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Juul F, Vaidean G, Lin Y, Deierlein AL, Parekh N. Ultra-Processed Foods and Incident Cardiovascular Disease in the Framingham Offspring Study. J Am Coll Cardiol. 2021;77(12):1520–1531. doi: 10.1016/j.jacc.2021.01.047 [DOI] [PubMed] [Google Scholar]
- 63.Srour B, Fezeu LK, Kesse-Guyot E, et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ. 2019;365:l1451. doi: 10.1136/bmj.l1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adolph EF. Urges to eat and drink in rats. Am J Physiol. 1947;151(1):110–125. doi: 10.1152/ajplegacy.1947.151.1.110 [DOI] [PubMed] [Google Scholar]
- 65.Beilharz JE, Maniam J, Morris MJ. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain Behav Immun. 2014;37:134–141. doi: 10.1016/j.bbi.2013.11.016 [DOI] [PubMed] [Google Scholar]
- 66.Beilharz JE, Maniam J, Morris MJ. Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav Brain Res. 2016;306:1–7. doi: 10.1016/j.bbr.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 67.Robinson MJF, Burghardt PR, Patterson CM, et al. Individual Differences in Cue-Induced Motivation and Striatal Systems in Rats Susceptible to Diet-Induced Obesity. Neuropsychopharmacology. 2015;40(9):2113–2123. doi: 10.1038/npp.2015.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gearhardt AN, Hebebrand J. The concept of “food addiction” helps inform the understanding of overeating and obesity: YES. Am J Clin Nutr. 2021;113(2):263–267. doi: 10.1093/ajcn/nqaa343 [DOI] [PubMed] [Google Scholar]
- 69.DiFeliceantonio AG, Coppin G, Rigoux L, et al. Supra-Additive Effects of Combining Fat and Carbohydrate on Food Reward. Cell Metab. 2018;28(1):33–44.e3. doi: 10.1016/j.cmet.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 70.Dalenberg JR, Patel BP, Denis R, et al. Short-Term Consumption of Sucralose with, but Not without, Carbohydrate Impairs Neural and Metabolic Sensitivity to Sugar in Humans. Cell Metab. 2020;31(3):493–502.e7. doi: 10.1016/j.cmet.2020.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tobias DK, Hall KD. Eliminate or reformulate ultra-processed foods? Biological mechanisms matter. Cell Metab. 2021;33(12):2314–2315. doi: 10.1016/j.cmet.2021.10.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


