Abstract
Stroke is a leading cause of death in the US and around the world but with limited treatment options. Survivors often present with long-term cognitive and neurological deficits. Stem cell-based therapy has emerged as a potential treatment for stroke. While stem cell transplantation in stroke has reached clinical trials, mostly safety outcomes have been reported with efficacy readouts warranting more studies. In an effort to optimize the stem cell regimen for stroke, here we conducted vis-a-vis comparison of different routes of transplantation, namely, intracerebral, intraarterial, and intranasal delivery of expanded human CD34 + stem cells, called ProtheraCytes, in the established stroke model of transient middle cerebral artery occlusion (MCAO) using adult Sprague-Dawley rats. After adjusting for the dose and subacute timing of cell delivery, animals were randomly assigned to receive either ProtheraCytes or vehicle. Motor and neurological assays from days 7 to 28 post-stroke revealed significant functional recovery across all 3 delivery routes of ProtheraCytes compared to vehicle-treated stroke rats. Additionally, ProtheraCytes-transplanted stroke rats displayed significantly reduced infarct size and cell loss in the peri-infarct area coupled with enhanced neurogenesis and angiogenesis compared to vehicle-treated stroke rats. These results highlight the safety and efficacy of transplanting ProtheraCytes, including via the minimally invasive intranasal route, in conferring robust and stable behavioral and histological positive outcomes in experimental stroke.
Keywords: cerebral ischemia, cell transplantation, functional recovery, neurogenesis, angiogenesis, cell delivery route
Graphical Abstract
Graphical Abstract.

Stem cell-derived extracellular vesicles promote recovery of ischemic cells. The present study demonstrates a novel mechanism of action mediating stem cell-induced therapeutic effects in ischemic stroke. Whereas the long-standing view of stem cell repair of the stroke brain implicates cell replacement, our findings implicates a process of by-stander effects, whereby stem cells release extracellular vesicles (EVs), in particular CD-63-labeled EVs, which appear to target the vasculature and enhance vascular endothelial growth factor zlevels, while dampening inflammation (Iba-1 cells) and increasing neural cell proliferation (DCX). Altogether, these reparative processes rescue the neurovascular unit via a multi-pronged mechanism involving EV release, vasculogenesis, anti-inflammation, and neurogenesis.
Significance Statement.
This study provides guidance on the safe and effective route of stem cell delivery in stroke animals, with mechanistic evidence suggesting the key role of extracellular vesicles in mediating the therapeutic effects of transplanted stem cells.
Introduction
Stroke remains as one of the most detrimental diseases that cause significant neurological impairments.1-3 As the fifth leading cause of death in the US, stroke has limited treatment options, and the projected growth of vulnerable aging populations makes the search for effective stroke therapy even more urgent. Currently, endovascular thrombectomy and tissue plasminogen activator (tPA) are the only approved acute stroke treatment options. However, the short therapeutic time window and potential inducement of adverse effects limit the use of available acute stroke treatment.1,4-11 While rehabilitation offers some therapeutic effects, many chronic ischemic stroke victims are unable to fully recover cognitive and motor functions due to loss of brain cells caused by the deprivation of oxygenated blood.12,13 With 87% of all stroke cases consisting of ischemia12 and current stroke treatments offering limited use, a potent treatment that can regenerate lost brain cells is warranted.
Stem cell therapy has emerged as a potential candidate to treat stroke by restoring lost brain cells. The neuroprotective and regenerative abilities of stem cells in both acute14-17 and chronic18-20 events have marked stem cell transplantation as a novel solution to stroke-induced deficits. Neurotrophic factor secretion, restorative properties, cell replacement, and biobridge formation are some of the functional mechanisms of stem cell therapy.20-29 In middle cerebral artery occlusion (MCAO) models in rats, different types of cells, such as bone marrow and umbilical cord blood-derived mesenchymal stem cells (MSCs),30-32 demonstrate similar therapeutic effects by increasing neurotrophic growth factors, decreasing apoptosis and reducing neurological damage in infarct border zone.16,30,33-35 Bone marrow stromal cells also increase vascular endothelial growth factor (VEGF) activity and enhance angiogenesis.32 Umbilical cord blood cells have shown functional recovery in MCAO models, but there are limited clinical studies to support their use in humans.31 Similarly, MSCs have been advantageous for their accessibility, but their performance is highly dependent on their method of transplantation,30 and their safety and efficacy remain undetermined.36 Significant improvements to angiogenesis, neurogenesis, and vasculogenesis accompany MCAO mice treated with endothelial cells.14 While stem cell transplantation possesses potent mechanisms outlined in MCAO models, finding the most suitable line of stem cells proves to be a challenge.
As previously shown in acute myocardial infarction, acute cerebral ischemic attacks are followed by large and bursting mobilizations of peripheral blood derived CD34 + cells at 1-3 days and 7-10 days after the event.37 The extent of the CD34 + cell mobilization is significantly correlated to the neurological and functional recoveries observed at one and 3 months in the NIH Stroke Scale (NIHSS) and modified Rankin Scale (mRS), respectively, therefore being predictive of neurological and functional recovery.37 Indeed, a clinical study that mobilized CD34 + cells via daily subcutaneous injections of granulocyte colony stimulating factor (G-CSF) for 5 consecutive days after ischemic stroke shows functional and structural improvement in some patients.38 When 1-3 × 106 CD34 + cells were injected intraarterially by catheter angiography into the ipsilesional middle cerebral artery within 7 days of stroke onset, all patients displayed improvements in mRS and NIHSS score at 6 months.39 Additionally, when 3-8 × 106 CD34 + cells were injected intracerebrally at ≥ 6 months after stroke onset in patients with a middle cerebral artery infarct, treated patients exhibit significantly greater improvement in NIHSS, mRS, and European Stroke Scale at 12 months post-treatment compared to control patients.40 CD34 + cells have been shown to promote angiogenesis via the secretion of paracrine factors such as exosomes containing pro-angiogenic miRNAs41 and via gap junction mediated cell-cell interaction.42 Preclinical studies have shown that administration of CD34 + cells after stroke enhances neurogenesis via angiogenesis.43 CD34 + cells promote an environment conducive to neovascularization of the ischemic brain that enhances neuroplasticity and neuronal regeneration.44
While the success of stem cell therapy heavily relies on the type of stem cell used, the method of transplantation is equally, if not more, important due to different delivery routes triggering different therapeutic mechanisms and presenting unique functional benefits. Intracerebral (IC) transplantation is known for its high number of stem cells in the lesion area, significant neurological recovery, and lower peripheral side effects.45,46 However, the downside of IC delivery is its limited clinical application for stroke patients who cannot tolerate direct injection via neurosurgical operations, which may lead to surgical complications.47 Intra-arterial (IA) injection offers a less invasive method of delivery for more vulnerable stroke patients. Due to stem cells’ homing capability, indirect injection of stem cells can still penetrate the blood-brain barrier and reach the lesion site.48 IA infusions in phase I/II studies improved functional outcomes over 12 months.49 Intravenous (IV) administration also achieves similar therapeutic outcomes as IA by avoiding surgical interventions. However, both IA and IV methods have lower effectiveness, compared to IC delivery, as fewer cells arrive at the infarct area. Additionally, IA and IV injections increase the risk of pulmonary embolism and thrombosis as a large number of stem cells accumulate in the lungs and spleen.48,50,51 IA administration of some cells, in particular MSCs, has the risk of secondary cerebral infarcts/ microembolism.52 Intranasal (IN) route has emerged as a new method of stem cell transplantation. Because stem cells are administered through the olfactory system, cells can bypass the blood-brain barrier and reach the lesion site.53,54 IN administration in ischemic injury models has demonstrated improved cognitive, motor, and sensory functions through noninvasive operations,55 highlighting its safety and convenience without sacrificing its effectiveness. Further research is needed to solidify IN as a novel delivery route for stem cell therapy.
Extracellular vesicles (EV) play a notable role in intercellular communications, such as coagulation and immune responses.56 Cells undergoing apoptosis tend to release vesicles to the extracellular environment, but recent discoveries suggest that healthy cells also release vesicles to the extracellular environment. Aging animals treated with small EVs (sEVs) derived from adipose mesenchymal stem cells (ADSCs) demonstrated improvement in age-dependent functions.57 ADSC-sEVs also promoted regenerative effects in the kidneys and muscles by lowering inflammatory activity, oxidative stress, and senescence markers.57 Furthermore, tissues of aged mice treated with ADSC-sEVs had lower predicted epigenetic age and metabolome similar to younger mice.57 MicroRNAs in sEVs may be responsible for the therapeutic effects.57
EVs can be classified based on their cellular origin: exosomes, microvesicles, and apoptotic bodies.56,58 Healthy, functional cells release both exosomes and microvesicles. Exosomes are endocytic vesicles that form through inward budding of multivesicular endosomes while microvesicles bud from the cell surface. Exosomes are favored over microvesicles in identifying healthy cell activity due to their consistent size and known protein content.59 Tetraspanins, such as CD63, are a branch of membrane proteins that congregate into the microdomains of the plasma membrane.58,60,61 They can be used as markers for EV due to their abundance in exosomes, signifying healthy cell activity.
This study was designed to investigate the different routes of transplantation, namely IC, IA, and IN delivery of expanded human CD34 + stem cells ProtheraCytes .62 Here, we assess the safety and efficacy of ProtheraCytes transplantation, including via the minimally invasive IN route, in behavioral and histological outcomes in the MCAO stroke model.
Methods
Animals
All experiments were conducted in accordance with the National Institutes of Health Guide and Use of Laboratory Animals. The experiments were approved by Institutional Animal Care and Use committee (IACUC approval number: IS00009075) of the University of South Florida, Morsani College of Medicine. All animals had free access to food and water while housed under normal conditions (20 °C, 50% relative humidity, and a 12 hours light/dark cycle). A total of 60 adult Sprague-Dawley rats (250 g male, approximately 8 weeks old) equally divided in 6 groups were used (Figure 1). Of all the animals, 3 rats died at day 7, 2 of which without undergoing any transplantation.
Figure 1.

A timeline of the experimental procedures. Stem cell transplantation was performed on day 3. Behavior testing was performed on day −1 (baseline), 0, 7, 14, and 28. Middle cerebral arterial occlusion (MCAO) was performed on day 0.
MCAO model
Adult Sprague-Dawley rats were subjected to stroke (n = 60) and anesthetized by a mixture of 1%–2% isoflurane in nitrous oxide/oxygen (69%/30%) via face mask. Body temperatures were maintained at 37 ± 0.3 °C during the surgical procedures. A midline skin incision was made in the neck with subsequent isolation of the left common carotid artery, the external carotid artery (ECA), and internal carotid artery. Thereafter, a 4-0 monofilament nylon suture (4-0 Medium B MCAO suture, Doccol Co.) was advanced from the common carotid artery bifurcation until it blocked the origin of the MCA. The skin incision was closed with surgical clips. Animals were allowed to recover from anesthesia during the 1-hour MCAO. At 1 hour after MCAO, animals were re anesthetized, and reperfusion commenced with the withdrawal of the suture. Thereafter, surgical incisions were closed, and animals were allowed to recover from anesthesia. Regarding pain management, animals underwent a single injection of Meloxicam ER (2 mg/mL) before surgery and a single injection of Buprenorphine SR (1.2 mg/kg) after surgery.
CD34 + Cell Culture for Collection of ProtheraCytes
ProtheraCytes were obtained after expansion of mobilized CD34+ cells from healthy donors (Lonza) as previously described.63 The transport of ProtheraCytes from CellProthera (Mulhouse) to the USF Health Center was conducted at +4/10 °C with temperature monitoring.
Transplantation of ProtheraCytes
At 3 days after MCAO, animals were randomly assigned to receive transplantation of ProtheraCytes at either IA (3 million cells in 1 mL vehicle; n = 10), IC (300k cells in 10 μL vehicle; n = 10), or IN (1 million cells in 36 μL vehicle; n = 10) or vehicle alone (saline intraarterially, intracerebrally, or intranasally; n = 30). IA was via the carotid artery. Since the animals received right MCAO, animals also received infusion of cells/saline into the carotid artery. Animals were anesthetized with a mixture of 1%-2% isoflurane in nitric oxide/oxygen (69%/30%) via a face mask, and body temperature was maintained at 37 ± 0.3 °C during the surgical procedures. Infusion of cells/saline was performed via a bolus injection delivered from a 25G needle of a 1 mL syringe inserted into the carotid artery. The syringe was filled with cells/saline. After dosing, the needle was removed and the carotid artery pressed for 30 seconds to prevent any bleeding, and the skin wound reclosed with surgical clips. For IC transplantation, stereotaxic surgery targeted the right striatum. All surgical procedures were conducted under aseptic conditions. Animals were anesthetized with a mixture of 1%-2% isoflurane in nitric oxide/oxygen (69%/30%) via a face mask, and body temperature was maintained at 37 ± 0.3 °C during the surgical procedures. Once deep anesthesia was achieved (by checking for pain reflexes), hair was shaved around the area of surgical incision (skull area) with enough border to prevent contaminating the operative site, followed by 2 surgical germicidal scrubs of site, and draping with sterile drapes. The animal was then fixed to a stereotaxic apparatus (Kopf Instruments). A 26-gauge Hamilton syringe was then lowered into a small, burred skull opening (transplant coordinates were adjusted to correspond with the striatal area adjacent to the infarcted site: 0.5 mm anterior and 1.0 mm lateral to bregma and 4.0 mm, 3.5 mm, and 3.0 mm below the dural surface). Within this single needle pass, 3 deposits of the test article (100 000 cells in 3 μL per deposit or a total of 300 000 cells in 9 μL of saline for 3 deposits) were made. The target area was the medial striatum which corresponds to the peri-infarcted striatal area, based on previously established target sites for similar stereotaxic implants. Each deposit consisted of 100,000 viable cells in 3 μL volume infused over a period of 3 minutes. Following an additional 2-minute absorption time, the needle was retracted, and the wound closed with stainless steel wound clips. A heating pad and a rectal thermometer allowed maintenance of body temperature at approximately 37 °C throughout surgery and following recovery from anesthesia. For IN transplantation, animas were held with a hand grip that allowed the animals to recline on their backs while immobilizing the skull, and the nose drop containing the substance/cell suspension was carefully placed on one nostril allowing it to be snorted naturally, and then the other nostril. One hundred units of hyaluronidase (Sigma-Aldrich Chemie GmbH, H3506) dissolved in 24 μL sterile PBS was administered to the rat nostrils (6 μL/nostril, repeat once after 2 minutes) 30 minutes prior to the administration of ProtheraCytes or vehicle. One million cells resuspended in 36 μL were applied to each rat using the same method as described for hyaluronidase, while control group received the same amount of vehicle only. Immunosuppressants were not required after cell transplantation for MSCs therapeutic effects, as already demonstrated.14,24
Behavioral Tests
All investigators were blinded to the treatment conditions when testing the animals. Each rat was subjected to a series of behavioral tests to assess the motor and neurological conditions of the animals before (baseline) and after MCAO (day 0) and after perfusion (days 7, 14, and 28). Behavioral tests included the elevated body swing test (EBST), forelimb akinesia test, bilateral forepaw grasp, and beam walking ability tests.
Elevated Body Swing Test (EBST)
EBST is a measure of asymmetrical motor behavior that does not require animal training or drug injection. The rat was held in the vertical axis approximately 1 inch from the base of its tail and then elevated to an inch above the surface on which it had been resting. The frequency and direction of the swing behavior were recorded over 20 tail elevations. A swing was counted when the head of the rat moved more than 10° from the vertical axis to either side. Normally, intact rats displayed a 50% swing bias, that is, the same number of swings to the left and to the right. A 75% swing bias toward one direction was used as a criterion of motor deficit. The total number of swings made to the biased side was added per animal and divided by 20, providing the average number of biased swings per individual animal.
Forelimb Akinesia
Forelimb akinesia measures the ability of the animal to hold on to a 2 mm diameter steel rod to assess the ipsilateral and contralateral forepaw strength and motility. Grades were as follows: 0 for normal grasping behavior, 1 for slow grasping without rigidity, 2 for slow grasping with rigidity, and 3 for no grasping with forelimbs.
Bilateral Forepaw Grasp
Bilateral forepaw grasp measures the ability of a rat to hold onto a 2 mm diameter steel rod. Grade 0 was used for rats with normal forepaw grasping behavior, 1 for rapid grasping but with rigidity, 2 for slow grasping with rigidity, and 3 for a rat unable to grasp with the forepaw.
Beam Walking
Beam walking ability uses a beam apparatus that is 80 cm long with a flat surface of 2-4 cm width resting at least 40 cm above the table/surface top on 2 poles. The animal was placed at one end of the beam then the ability to traverse the beam and reach the other end was assessed. The grades were as follows: 0 for a rat that easily traverses the beam, 1 if the rat slowly traverses the beam, 2 for partially traversing the beam but falls off, and 3 for a rat unable to stay on the beam for 10 seconds.
The scores from all 3 tests were added to give a total neurologic deficit score (maximum possible score is 9 with mean composite neurologic score of 3). A score of 2.5 was set as a criterion to be considered a “stroke” animal.
Euthanasia and Perfusion
To perform immunofluorescent analysis, animals were euthanized under deep anesthesia. Animals were briefly perfused through the ascending aorta with cold PBS (200 mL), followed by 4% paraformaldehyde in phosphate buffer (200 mL). Rat brains were harvested and postfixed in the same fixative (72 hours), followed by complete submersion in 30% sucrose in phosphate buffer. Cryostats were used to cut multiple coronal sections at 40 μm thickness, which were stored at −20 °C.
Nissl Staining
Nissl staining was performed with 0.1% cresyl violet solution (Sigma-Aldrich) using a standard protocol to evaluate the peri-infarct injury of our MCAO model. From each perfused brain, 6 coronal sections between the anterior edge and posterior edge of the MCAO infarct area were collected and processed for Nissl staining. Every 6th coronal tissue section was chosen at random to quantify cell survival in the peri-infarct area. Brain sections were examined using a light microscope (Keyence). Neuronal survival in the peri-infarct area of the brain was quantified using a computer-assisted image analysis system (NIH Image Software) and was expressed as a percentage of the ipsilateral hemisphere compared to the contralateral hemisphere. The infarction area ratio calculated defining left hemisphere (LT), right hemisphere (RT), and infarction area (RI) in mm2. Infarction area ratio = [LT − (RT − RI)] × 100/LT (%).64 To control for edema, the following edema correction formula was performed in this study. The volume of brain damage was measured in each slice and quantified by a computer assisted image analysis system (NIH Image Software) and calculated by the following formula: 2.0 mm (thickness of the slice) × (sum of the damaged area per slice or in all brain slices) then expressed as percentage. To minimize artifacts produced by post-TBI edema in the injured area, the brain damage volume was calculated as described previously.65 Briefly, the injured area in the ipsilateral hemisphere was indirectly measured by subtracting the non-injured area in the ipsilateral hemisphere from the total intact area of the contralateral hemisphere. We counted positive live cells based on their morphological features, in particular taking into consideration their round and oval shape soma with a healthy nucleus as opposed to those cells with dense, compact, or fragmented nucleus representative of a pyknotic cell.
Immunohistochemistry
Staining for vascular endothelial growth factor receptor 1 (VEGFr1) (1:200; Abcam; AB32152), ionized calcium binding adaptor molecule-1 (IBA-1) (1:500; Wako; 019-19741), doublecortin (DCX) (1:500; Abcam; AB18723), CD63 (1:200; Novus Biologicals; NB100-77913) were conducted on every 1 of 6 sections, 40 mm in thickness of brain. From each section, approximately 4-6 images at 20× magnification were taken using a confocal microscope (Zeiss) and analyzed with ImageJ (National Institutes of Health). All photomicrographs were converted to gray scale. Background was selected from blank control images and subsequently used to subtract the background from all images. The same threshold was used for all images. Thereafter, the labeling intensity of each section was quantified as the average optical density readings of 4-6 randomly selected areas within that section. The final labeling intensity of each group was expressed as the average of each labeling intensity per section. All sections were washed in PBS for 5 minutes 3 separate times. After washing, samples were then blocked for 60 minutes at room temperature with 5% normal goat serum (Invitrogen) and 0.1% triton X-100 in 0.1 M PBS. Endothelial progenitor cell marker with transplantation cell marker mouse monoclonal human CD34 (1:200; Thermo Fisher Scientific, MA5-15331), which specifically cross-reacted only with human samples without rat cross-reactivity, were double-labeled to assess ProtheraCytes. Then, brain slices were incubated overnight with the listed primary antibodies at 4 ˚C. Afterward, sections were washed 3 times for 10 minutes in 0.1 M PBS and soaked in 5% normal goat serum and 0.1% triton X-100 in 0.1 M PBS with corresponding secondary antibodies, goat anti-mouse IgG-Alexa 488 (green) (1:500; Invitrogen), or goat anti-rabbit IgG-Alexa 594 (red) (1:500; Invitrogen) for 2 hours. Sections were again washed 3 times for 5 minutes in PBS, and cover-slipped with Vectashield hardset with DAPI (H-1500, Vector Laboratories, California). All sections were examined on the ipsilateral corpus callosum using a confocal microscope (Zeiss) and cell counting was done by a blinded team member. Control studies included exclusion of primary antibody substituted with 5% normal goat serum in 0.1 M PBS.
Exosome Isolation From ProtheraCytes
ProtheraCytes were seeded in a serum-free medium (DMEM medium) in a bioreactor for extracellular vesicle release by exerting a controlled mechanical stimulation on cells for 30 minutes to 2 hours according to Everzom’s proprietary method. The size distribution and concentration measurements of ProtheraCytes-derived exosomes were performed using the Nanosight (NS300). The exosome membrane markers were quantified by flow cytometry using the MACSPlex Exosome kit (Miltenyi).
For miRNA analysis, ProtheraCytes were cultured at the concentration of 2.5 × 105 cells/mL in StemSpan-AOF (STEMCELL Technologies) supplemented with cytokines for 40 hours. Then, cells were collected by centrifugation at 400g for 10 minutes; and exosomes were purified from the supernatant by precipitation using the ExoQuick-TC kit (System Biosciences) according to the manufacturer’s instructions. After isolation, exosomes were characterized by flow cytometry using the ExoStep kit with a bead-bound anti-CD63 capture (ImmunoStep, Spain) confirming the identity of the exosomes secreted by ProtheraCytes.
Oxygen Glucose Deprivation (OGD)
Once confluence of primary rat cortical cells was achieved, we initiated the OGD. The cells were initially exposed to Dulbecco’s phosphate-buffered saline, then placed in an anaerobic chamber (Plas-Labs, Inc) containing nitrogen (95%) and carbon dioxide (5%) for 15 minutes at 37 °C, and finally, the chamber was sealed and incubated for 90 minutes at 37 °C (hypoxic–ischemic condition). OGD was terminated by changing normal media, and cell cultures reintroduced to the regular CO2 incubator (normoxic condition) at 37 °C for 1 h, which represented a model of reperfusion. After reperfusion, wells were randomly cocultured with ProtheraCytes or standard DMEM medium, at a concentration of 40 000 cells per well overnight. This coculture set-up, the primary rat cortical cells were suspended in the treatment condition using 8-well poly-L-lysine plates, with each treatment condition done in 6 biological samples. The coculture was created using a 2-chamber system with the primary rat cortical cells in lower chamber and the ProtheraCytes in the upper chamber, allowing us to conduct accurate assessments of the primary rat cortical cells without contamination from the ProtheraCytes population. Cell viability (Trypan blue) and metabolic activity (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide or MTT) levels were examined in neurons.
Trypan Blue Assay
Trypan blue (0.2%) exclusion method was conducted and mean viable cell counts were calculated in 4 randomly selected areas (1 mm2, n = 10) to reveal the cell viability after the ischemic-reperfusion condition. Briefly, within 5 minutes after adding trypan blue, we digitally captured under microscope (×200) 10 pictures (approximately 100 cells per picture) for each condition, then randomly selected 5 pictures, and counted the number of cells for each individual treatment condition. Normalized cell viability was calculated from the following equation: viable cells (%) = [1.00 − (number of blue cells/number of total cells)] × 100.
MTT-cell Viability Assay
The colorimetric 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide or MTT reduction assay was conducted by following the instructions for use of Promega Corporation products (Cell Titer 96, Non-Radioactive Cell Proliferation Assay, Promega Corporation). This method assessed mitochondrial activity, thus an indirect measure of cell viability by measuring the ability of cultured cells to convert yellow MTT to purple formazan dye. The supernatant and the cells were separated from the mixed culture at the end of the 3-hours exposure time. Approximately 100 μL DMEM without phenol red was added, then 20 μL of the dye solution was added to each well, and the mixture was incubated on the plate at 37 °C for 3 hours in a humidified, 5% CO2 atmosphere. After incubation, 100 μL of the solubilization solution/stop mix was added to each well, and the plate was allowed to stand overnight in the humidified, 5% CO2 incubator at 37 °C. The absorbance was quantified spectrophotometrically at a wavelength of 570 nm and with a reference wavelength of 900 nm in the BioTek Synergy HT 96-well microplate reader (BioTek Instruments, Inc.).
Statistical Analysis
The statistical data was obtained using one-way analysis of variance (ANOVA) and subsequent post hoc Bonferroni’s test for behavior tests. Statistical significance was preset at P < .05. (GraphPad version 9.0) (means ± S.D.). The Kolmogorov-Smirnov test was used to assess normality and the resulting values were <5% of the critical values.66
Results
ProtheraCytes ameliorate stroke-induce behavioral deficits
A combination of regular motor and neurological tests vulnerable to stroke was conducted to examine the post-stroke functions and stem cell treatment. All animals displayed a strong swing bias in EBST and impaired scores in balance beam, paw grasp, and forelimb akinesia tests on day 0 after MCAO. Neurological and motor tests were also performed on days 7, 14, and 28 after transplanting treatment groups with ProtheraCytes on day 3. Post-transplantation results revealed that animals treated with ProtheraCytes displayed significantly improved functional outcomes in all motor tests examined here (Fig. 2). EBST was performed to evaluate locomotor performance after MCAO and cell transplantation. Stroke animal groups treated with ProtheraCytes had significantly lower percent swing bias (F20,270 = 19.69, ****P < .0001) compared to the vehicle-treated animal groups. No significant difference was found among IC, IA, and IN transplanted groups. The beam walking test showed that ProtheraCytes-treated stroke groups showed significantly prolonged time spent balancing on the rod (F20,270 = 3.568, ****P < .0001) compared to vehicle-treated stroke animals while no significant difference among IC, IA, and IN transplanted cell groups was noted. Paw grasp test revealed significantly lower stroke severity scores in ProtheraCytes-treated stroke animals (F20,270 = 3.480, ****P < .0001) compared to vehicle-treated stroke animals even after 28 days. There was no significant difference among the IC, IA, and IN transplanted groups in paw grasp tests. Similarly, the forelimb test revealed lower stroke severity scores in cell transplanted stroke animal groups (F20,270 = 2.996, ****P < .0001) compared to vehicle-treated groups. Again, no significant differences were found among IC, IA, and IN treatment groups. These results highlight the significant improvement in motor and neurological performances in stroke animals treated with ProtheraCytes compared to vehicle-treated stroke animals without significant differences in the delivery routes of the stem cells.
Figure 2.

Behavioral tests. (a) Motor activity revealed by EBST. MCAO + cell groups displayed significantly less asymmetry on days 7, 14, and 28 (*P < .05, **P < .01, ****P < .0001). (b) Motor activity revealed by cylinder test. MCAO + cell groups demonstrated significantly more use of impaired forelimb (*P < .05, **P < .01, ***P < .001, ****P < .0001). (c) Motor activity revealed by Grip Strength. MCAO + cell groups presented significantly less impaired paw grasp (*P < .05, **P < .01, ***P < .001). (d) Motor activity revealed by balance beam. MCAO + cell showed significantly better motor coordination during beam walks (**P < .01, ***P < .001).
ProtheraCytes Reduce Cerebral Infarcts and Peri-Infarct Cell Loss
Measurement of the infarct area and evaluation of neuronal survival in the peri-infarct area of the cortex was performed through Nissl staining with 0.1% cresyl violet to stain neuronal Nissl bodies. Compared to the control group, the stroke animals transplanted with ProtheraCytes presented with a reduced cerebral infarct area (F5,196 = 14.64, *P < .05, **P < .01, ***P < .001, ****P < .0001; Fig. 3). No significant difference in the infarct area was found among IC, IA, and IN transplanted groups. Additionally, ProtheraCytes-treated groups displayed significantly higher percentage of live cells in the peri-infarct cortex (F5,206 = 15.14, *P < .05, **P < .01, ***P < .001, ****P < .0001) compared to vehicle-treated stroke animals (Fig. 4). There was no significant difference in the peri-infarct area live cell percentage among IC, IA, and IN delivered cell transplantation. Observations made in the infarct and peri-infarct areas indicate a significant reduction in neuronal damage in ProtheraCytes-treated stroke animals compared to vehicle-treated stroke animals without difference in the method of transplantation.
Figure 3.
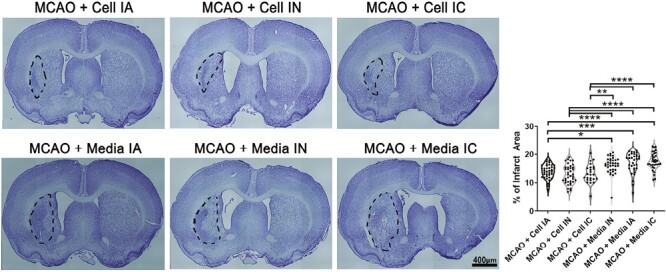
Infarct area. Nissl staining for coronal brain sections showing infarct areas (black outline) of MCAO + Cell IA, MCAO + Cell IN, MCAO + Cell IC, MCAO + Media IA, MCAO + Media IN, MCAO + Media IC. MCAO + Cell groups displayed significantly smaller infarcts (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Figure 4.

Peri-infarct area. Nissl staining for quantitative analysis of live cells (black arrow heads) in the peri-infarct area for MCAO + Cell IA, MCAO + Cell IN, MCAO + Cell IC, MCAO + Media IA, MCAO + Media IN, MCAO + Media IC. MCAO + Cell groups showed significantly more living cells in the peri-infarct (*P < .05, **P < .01, ****P < .0001). Scale bar = 50 µm.
ProtheraCytes Dampen Stroke-Induced Inflammatory Response in the Brain
Iba-1 with endothelial progenitor cell marker with transplantation cell marker (CD34) staining was used to quantify neuroinflammation and microglial cell activity in the peri-infarct area (Fig. 5). Stroke animals treated with IC transplanted ProtheraCytes displayed a significant reduction in Iba-1 positive activated microglial cells in the peri-infarct cortex (F5,114 = 7.130, ***P < .001). Reduced Iba-1 positive cells were also seen in treatment groups via IA and IN administration, no significance was found when compared to vehicle-treated stroke animals. In other words, our results suggest that the inflammatory response in the brain was reduced in ProtheraCytes-treated stroke animals, but significant difference was only observed when ProtheraCytes was delivered intracerebrally. Immunohistochemical analyses of Iba-1 and CD34 in the peri-infarct area of ischemic brain revealed higher quantities (F5,114 = 274.7, ****P < .0001) of CD34 marker in transplantation cell group of stroke animals (Fig. 5). IC transplanted treatment group possessed the highest quantity of CD34 + cells, with IA transplanted treatment group the second highest, and IN transplanted treatment group had the lowest number of CD34 + cell count in the peri-infarct area.
Figure 5.

Inflammation. Iba-1 double stained with CD34 to measure inflammatory activity. MCAO + Cell IC had significantly less Iba-1 positive cells than MCAO + media IN, MCAO + media IA, and MCAO + media IC groups (**P < 0.01). Treatment groups had significantly more CD34 expression (***P < .001) with MCAO + Cell IC having the highest cell count within the treatment group (*P < .05; ***P < .001). Scale bar = 50 µm.
ProtheraCytes Promote the Formation of De Novo Neurons After Stroke
DCX double stained with CD34 was used to assess neurogenesis in the peri-infarct area (Fig. 6). ProtheraCytes-treated stroke animals showed significant improvement of DCX positive cell count compared to vehicle-treated stroke animals (F5,114 = 81.65, ****P < .0001). Meanwhile, there was no significant difference in DCX positive cells within treatment group of different delivery routes. Altogether, the results indicate that significantly more de novo neurons were made in ProtheraCytes-treated stroke animals compared to vehicle-treated stroke animals, regardless of the method of transplantation. Immunohistochemical analyses of DCX and CD34 in the peri-infarct area of ischemic brain revealed larger number (F5,114 = 212.6, ****P < .0001) of CD34 marker in transplantation cell group of stroke animals compared to vehicle-treated groups (Fig. 6). IA transplanted treatment group displayed the highest quantity of CD34 + cells, while IC transplanted treatment group was the second highest, and IN transplanted treatment group had the lowest number of CD34 + cell count in the peri-infarct area.
Figure 6.

Neurogenesis. DCX double stained with CD34 measured neurogenesis differences between groups. All treatment groups MCAO + Cell IA, MCAO + Cell IN, and MCAO + Cell IC had significantly higher DCX expression than control groups MCAO + Media IA, MCAO + Media IN, and MCAO + Media IC (**P < .01). Treatment groups had significantly more CD34 expression (**P < .01). MCAO + Cell IN displayed significantly lower CD34 expression compared to MCAO + Cell IC (*P < .05). Scale bar = 50 µm.
ProtheraCytes Induce Vasculogenesis in Stroke-Affected Brains
Angiogenesis in the peri-infarct area was assessed by marking VEGFr1 double stained with CD34 (Fig. 7). Stroke animals receiving ProtheraCytes through IA or IC methods showed significantly higher VEGFr1 positive cell counts compared to all vehicle-treated animal groups (F5,161 = 21.30, ****P < .0001). While stroke animals treated with IN-delivered ProtheraCytes had more VEGFr1 positive cells than vehicle-treated stroke animals, no significant difference was found. Additionally, within treatment groups, IC-delivered ProtheraCytes had significantly more VEGFr1 expression than in ProtheraCytes-treated stroke animals via IA (*P < .05) and IN (****P < .0001). Stroke animals administered with IA ProtheraCytes also had significantly higher VEGFr1 positive cell counts compared to stroke animals treated with IN ProtheraCytes. Our results display increased angiogenesis activity in ProtheraCytes-treated stroke animals compared to vehicle-treated stroke animals, where significant improvements were observed in animals transplanted with ProtheraCytes intracerebrally and intraarterially. Immunohistochemical analyses of VEGFr1 and CD34 in the peri-infarct area of ischemic brain showed positive stain in transplantation cell groups of stroke rats. The high magnification of staining showed CD34 neighboring VEGFr1 markers in positive cells.
Figure 7.

Angiogenesis. VEGFr1 double stained with CD34 quantified angiogenesis activity. MCAO + Cell IC and MCAO + Cell IA had significantly higher VEGFr1 expression than all MCAO + Media groups (**P < .01). MCAO + Cell IC had significantly more VEGFr1 positive cell counts than MCAO + Cell IA (*P < .05) and MCAO + Cell IN (**P < .01). MCAO + Cell IN had significantly less angiogenesis activity when compared to MCAO + Cell IA (*P < .05). CD34 expression was significantly higher in all treatment groups than in all media groups (**P < .01). Scale bar = 50 µm and 10 µm.
ProtheraCytes Possess Extracellular Cesicles
Extracellular vesicles were assessed by utilizing CD63 markers (Fig. 8).67 Stroke animals treated with stem cells (IC, IN, and IA) showed significantly higher CD63 positive stain count compared to vehicle-treated stroke animals (F5,174 = 197.6, ****P < 0.0001). The results indicate that significantly more EVs and exosome activity were seen in ProtheraCytes-treated stroke animals compared to vehicle-treated stroke animals, regardless of the method of transplantation. Immunohistochemical analyses of CD63 revealed significantly higher quantities of healthy cells in the peri-infarct area. In parallel, under the in vitro stroke model of OGD, cocultures of ProtheraCytes with primary neurons protected against OGD as evidenced by increased neuronal cell viability and metabolic activity using trypan blue and MTT assays, respectively (Fig. 9A, 9B). Subsequent analysis of exosome marker expression revealed that ProtheraCytes expressed the exosomal marker CD63 (Fig. 9C), further supporting our postulated EV-mediated mechanism underlying the therapeutic effects of ProtheraCytes.
Figure 8.

Extracellular vesicles. CD63 staining was used as a marker to quantify extracellular vesicles and healthy stem cell activity. All treatment groups MCAO + Cell IA, MCAO + Cell IN, and MCAO + Cell IC had significantly higher CD63 positive stain count than control groups MCAO + Media IA, MCAO + Media IN, and MCAO + Media IC (*p < 0.05). Scale bar = 50 µm.
Figure 9.

ProtheraCytes protect against in vitro stroke model of oxygen glucose deprivation. (A) Trypan blue. ANOVA revealed significant treatment effects. Post hoc Bonferonni’s tests revealed significant differences in cell survival, with primary neurons exposed to OGD and cocultured with ProtheraCytes rescuing against OGD-induced cell death significantly better than primary neurons subjected to OGD. (B) MTT. Similarly, ANOVA showed significant treatment effects, with post hoc Bonferonni’s tests detecting significant differences in metabolic activity, again with primary neurons exposed to OGD and cocultured with ProtheraCytes reducing the OGD-induced metabolic impairment significantly better than primary neurons subjected to OGD. (C) CD63. Analysis of exosome marker expression revealed that ProtheraCytes express the exosomal marker CD63, which was not detectable in the standard medium. Statistical significance is depicted as follows: *P < .05; **P < .01; ***P < .001.
Discussion
The present study investigated the potential therapeutic effects of ProtheraCytes through different transplantation paths, namely IC, IA, and IN. Transplanting ProtheraCytes significantly reduced behavioral and motor deficits in rats induced with transient MCAO when compared to the media groups, and there was no significant difference between treatment groups even after day 28. Behavioral improvements of ProtheraCytes-treated animals were reflected in Nissl staining of the ipsilateral striatum, where the infarct area was significantly reduced, and significantly higher percentage of live cells was seen in cell-treated groups compared to vehicle-treated groups. Despite similarities found in behavioral tests and Nissl staining, differences in immunohistochemistry were found between each transplantation method. Although neuroinflammatory marker Iba-1 was reduced in all MCAO + Cell groups, only IC transplantation significantly reduced Iba-1. Other reports have shown that CD34 + cells decreased sustained pro-inflammatory activity of NF-κB and its downstream effector molecules TNF-α, IL-1β, and IL-6 at the wound bed of diabetic NOD/SCID mice.68 Additionally, angiogenesis marker VEGFr1 expression were significantly higher in IC and IA cell-treated groups than vehicle-treated stroke animals but not IN. This suggests that IC and IA routes enhanced VEGF receptor phosphorylation, possibly by activating pro-angiogenesis signaling cascades in the ischemic brain injury.69,70 Angiogenesis could also be strengthened by direct cell-cell contacts leading to increased propagation of pro-angiogenetic stimuli.42 On the other hand, transplanting ProtheraCytes via IC, IA, and IN compared to all vehicle groups significantly enhanced DCX expression, a notable cytoskeleton associated protein that is transiently expressed during generation of neuronal cells. Furthermore, cell-treated groups, regardless of delivery route, had significantly higher CD63 marker quantities compared to vehicle-treated groups, indicating improved healthy cell activity. Despite varying results in the immunofluorescence analyses, IC, IA, and IN cell-treated stroke animals all displayed co-localization of CD34 with DCX and Iba-1, and CD34 was observed to be neighboring VEGFr1 markers in positive cells.
While transplanting stem cells 24 hours after MCAO is proposed as the most optimal time to treat stroke animals compared to earlier timeframes,71 broader time windows of stem cell therapy have been shown to have the same therapeutic effects in stroke models. Previously, we have shown significant reduction in infarct and peri-infarct areas, and decreased loss of hippocampal neurons upon treatment of stroke animals 60 days after the injury,72 suggesting a flexible timeframe of stem cell therapy and addressing the limited time window in current stroke treatments. Other studies demonstrated that IA delivery of mononuclear human umbilical cord blood cells (hUCB) significantly improved neurological performances in rats treated 24 hours or 7 days after stroke.35 Similar improvements in behavioral and physiological outcomes have been observed in IV administration at 48 hours after MCAO.73 To expand our earlier findings, our study treated animals with ProtheraCytes at 3 days after MCAO. In line with previous studies, stroke animals transplanted with stem cells at this delayed period displayed significant behavioral and neurological outcomes.
Currently, numerous routes of stem cell transplantation have been examined in MCAO models, but the most optimal method is yet to be determined. IC delivery of stem cells has been suggested as the most effective route as direct injection migrates cells to the infarct area and may improve neurological functions.74 Our present findings align with this claim as IC delivered ProtheraCytes -treated stroke animals had significant improvement in behavioral tests and surviving neurons, and significant reduction in the infarct area. Immunohistochemistry also revealed anti-inflammatory effects, which was significant in the IC delivered cell group, coupled with neurogenic and angiogenic properties in all transplanted groups. However, IC administration may not be suitable in clinical settings because of the invasiveness of the procedure and the intolerance of some stroke patients, which may lead to severe surgical side effects.47,75-77 Additionally, uncontrolled volumes of neural stem cells may cause adverse effects, further injuring healthy brain tissue.78 IV delivery was introduced as a less invasive method of stem cell transplantation, making it a safer clinical option compared to the IC route. Unfortunately, IV as well as IA administration sacrifices efficacy as stem cells typically migrate to organs other than the brain, such as the spleen, liver, lungs, heart, or kidney.77,79,80 Despite displaying endogenous regeneration in vesicles and neurons in IA transplanted cell groups, the IA route poses a risk of thrombus. Although studies have suggested that optimizing the stem cell and vehicle volumes and rate of injection, as in the present study, may limit the risk of microthrombus,16,81-83 IA routes still harbor a risk that limit its candidacy as a safe and effective stroke treatment method. IN administration is a relatively new administration route that has shown promising results. IN delivered bone marrow-derived MSCs were reported to pass through the blood-brain barrier and reach the lesion site in the brain without migrating to peripheral organs.53 Our results indicate therapeutic outcomes in motor functions and histopathology in IN-transplanted stroke-induced animals, suggesting IN as a potent delivery route. By overcoming major clinical hurdles in common delivery routes, IN transplantation may be the most practical method of transplanting stem cells, warranting further research should explore the potential use of the IN pathway.
Ongoing studies have revealed that ProtheraCytes secrete vascular endothelial growth factor (VEGF) and its concentration is significantly correlated with the number of CD34 + cells obtained after expansion (unpublished data). ProtheraCytes secrete exosomes with an average size of 86.7 ± 10.17 nm that express the exosomal markers CD9, CD63, and CD81. These exosomes contain proangiogenic miRNAs (126, 130a, 378, 26a), antiapoptotic miRNAs (21 and 146a), and antifibrotic miRNAs (133a, 24, 29b, 132). ProtheraCytes also exhibit in vitro angiogenic activity, express surface markers of endothelial progenitor cells, and can differentiate in vitro into endothelial cells.
The present study demonstrated that ProtheraCytes can be used as an effective stem cell treatment to reduce motor and histopathological deficits in ischemic stroke. Even through minimally invasive routes, such as IN transplantation, ProtheraCytes promote neuroprotective activities, including anti-inflammation, angiogenesis, neurogenesis, and extracellular vesicle activity. In the clinical setting, IN stem cell transplantation may be most suitable for treating ischemic stroke injuries especially in severely debilitated patients.
Funding
This study was funded by CellProthera.
Contributor Information
Jea-Young Lee, USF Health Center of Excellence for Aging and Brain Repair, Tampa, FL, USA.
Justin Cho, USF Health Center of Excellence for Aging and Brain Repair, Tampa, FL, USA.
Francesco D’Egidio, USF Health Center of Excellence for Aging and Brain Repair, Tampa, FL, USA.
Christine Vignon, CellProthera 12 Rue du Parc, 68100 Mulhouse, France.
Hendrik Streefkerk, CellProthera 12 Rue du Parc, 68100 Mulhouse, France.
Matthieu de Kalbermatten, CellProthera 12 Rue du Parc, 68100 Mulhouse, France.
Ibon Garitaonandia, CellProthera 12 Rue du Parc, 68100 Mulhouse, France.
Cesar V Borlongan, USF Health Center of Excellence for Aging and Brain Repair, Tampa, FL, USA.
Author Contributions
J.-Y.L.: conceptualization, data curation, formal analysis, funding acquisition, investigation, resources, software, visualization, writing; J.C.: conceptualization, data curation, formal analysis, methodology, resources, software, visualization, writing; F.D’E.: data curation, formal analysis, investigation, methodology, writing; C.V.: funding acquisition, investigation, writing; H.S., M.de K.: funding acquisition, methodology, resources, writing; I.G.: funding acquisition, project administration, resources, writing; C.V.B.: conceptualization, data curation, methodology, project administration, resources, software, writing.
Conflict of Interest
C.V., I.G. declared employment, patent holder and stock ownership with CellProthera. C.B. declared research funding from NIH. The other authors declared no potential conflicts of interest.
Data Availability
All data reported in this study are available from the corresponding author on reasonable request.
References
- 1. Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292. Epub 20131218. 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meschia JF, Brott T.. Ischaemic stroke. Eur J Neurol. 2018;25(1):35-40. Epub 20170921. 10.1111/ene.13409 [DOI] [PubMed] [Google Scholar]
- 3. Ovbiagele B, Goldstein LB, Higashida RT, et al. ; American Heart Association Advocacy Coordinating Committee and Stroke Council. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44(8):2361-75. Epub 20130522. 10.1161/STR.0b013e31829734f2 [DOI] [PubMed] [Google Scholar]
- 4. Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists' Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-35. Epub 20140805. 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Primiani CT, Vicente AC, Brannick MT, et al. Direct aspiration versus stent retriever thrombectomy for acute stroke: a systematic review and meta-analysis in 9127 patients. J Stroke Cerebrovasc Dis. 2019;28(5):1329-1337. Epub 20190214. 10.1016/j.jstrokecerebrovasdis.2019.01.034 [DOI] [PubMed] [Google Scholar]
- 6. Mokin M, Dumont TM, Veznedaroglu E, et al. Solitaire flow restoration thrombectomy for acute ischemic stroke: retrospective multicenter analysis of early postmarket experience after FDA approval. Neurosurgery. 2013;73(1):19-25; discussion 25. 10.1227/01.neu.0000429859.96652.57 [DOI] [PubMed] [Google Scholar]
- 7. Campbell BCV, Donnan GA, Lees KR, et al. Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol. 2015;14(8):846-854. Epub 20150625. 10.1016/S1474-4422(15)00140-4 [DOI] [PubMed] [Google Scholar]
- 8. Furlan AJ. Endovascular therapy for stroke—it’s about time. N Engl J Med. 2015;372(24):2347-9. Epub 20150417. 10.1056/NEJMe1503217 [DOI] [PubMed] [Google Scholar]
- 9. Cohen DL, Kearney R, Griffiths M, Nadesalingam V, Bathula R.. Around 9% of patients with ischaemic stroke are suitable for thrombectomy. BMJ. 2015;351:h4607. Epub 20150828. 10.1136/bmj.h4607 [DOI] [PubMed] [Google Scholar]
- 10. Sheth SA, Jahan R, Gralla J, et al. ; SWIFT-STAR Trialists. Time to endovascular reperfusion and degree of disability in acute stroke. Ann Neurol. 2015;78(4):584-93. Epub 20150817. 10.1002/ana.24474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Josephson SA, Kamel H.. The acute stroke care revolution: enhancing access to therapeutic advances. JAMA. 2018;320(12):1239-1240. 10.1001/jama.2018.11122 [DOI] [PubMed] [Google Scholar]
- 12. Benjamin EJ, Muntner P, Alonso A, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 13. Sacco RL, Kasner SE, Broderick JP, et al. ; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064-89. Epub 20130507. 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishikawa H, Tajiri N, Shinozuka K, et al. Vasculogenesis in experimental stroke after human cerebral endothelial cell transplantation. Stroke. 2013;44(12):3473-81. Epub 20131015. 10.1161/STROKEAHA.113.001943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen J, Ning R, Zacharek A, et al. MiR-126 contributes to human umbilical cord blood cell-induced neurorestorative effects after stroke in type-2 diabetic mice. Stem Cells. 2016;34(1):102-113. 10.1002/stem.2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang B, Migliati E, Parsha K, et al. Intra-arterial delivery is not superior to intravenous delivery of autologous bone marrow mononuclear cells in acute ischemic stroke. Stroke. 2013;44(12):3463-72. Epub 20131010. 10.1161/STROKEAHA.111.000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tuazon JP, Castelli V, Borlongan CV.. Drug-like delivery methods of stem cells as biologics for stroke. Expert Opin Drug Deliv. 2019;16(8):823-833. Epub 20190805. 10.1080/17425247.2019.1645116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelly S, Bliss TM, Shah AK, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004;101(32):11839-44. Epub 20040727. 10.1073/pnas.0404474101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kondziolka D, Steinberg GK, Wechsler L, et al. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg. 2005;103(1):38-45. 10.3171/jns.2005.103.1.0038 [DOI] [PubMed] [Google Scholar]
- 20. Nishino H, Borlongan CV.. Restoration of function by neural transplantation in the ischemic brain. Prog Brain Res. 2000;127:461-476. 10.1016/s0079-6123(00)27022-2 [DOI] [PubMed] [Google Scholar]
- 21. Andres RH, Choi R, Pendharkar AV, et al. The CCR2/CCL2 interaction mediates the transendothelial recruitment of intravascularly delivered neural stem cells to the ischemic brain. Stroke. 2011;42(10):2923-31. Epub 20110811. 10.1161/STROKEAHA.110.606368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sullivan R, Duncan K, Dailey T, et al. A possible new focus for stroke treatment—migrating stem cells. Expert Opin Biol Ther. 2015;15(7):949-958. 10.1517/14712598.2015.1043264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xin H, Li Y, Cui Y, et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33(11):1711-5. Epub 20130821. 10.1038/jcbfm.2013.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yasuhara T, Hara K, Maki M, et al. Mannitol facilitates neurotrophic factor up-regulation and behavioural recovery in neonatal hypoxic-ischaemic rats with human umbilical cord blood grafts. J Cell Mol Med. 2010;14(4):914-921. 10.1111/j.1582-4934.2008.00671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shahaduzzaman MD, Mehta V, Golden JE, et al. Human umbilical cord blood cells induce neuroprotective change in gene expression profile in neurons after ischemia through activation of Akt pathway. Cell Transplant. 2015;24(4):721-35. Epub 20150226. 10.3727/096368914X685311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lindvall O, Kokaia Z.. Stem cells for the treatment of neurological disorders. Nature. 2006;441(7097):1094-1096. 10.1038/nature04960 [DOI] [PubMed] [Google Scholar]
- 27. Song CG, Zhang YZ, Wu HN, et al. Stem cells: a promising candidate to treat neurological disorders. Neural Regen Res. 2018;13(7):1294-1304. 10.4103/1673-5374.235085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kondziolka D, Wechsler L, Goldstein S, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55(4):565-569. 10.1212/wnl.55.4.565 [DOI] [PubMed] [Google Scholar]
- 29. Lippert T, Crowley M, Liska MG, Borlongan CV.. Stem cell-mediated biobridge: crossing the great divide between bench and clinic in translating cell therapy for stroke. Mole, Gen, Cell Adv Cerebrovas Dis. 2018:285-307. 10.1142/9789814723305_0011 [DOI]
- 30. Lim JY, Jeong CH, Jun JA, et al. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia. Stem Cell Res Ther. 2011;2(5):38. Epub 20110922. 10.1186/scrt79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vendrame M, Cassady J, Newcomb J, et al. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35(10):2390-5. Epub 20040819. 10.1161/01.STR.0000141681.06735.9b [DOI] [PubMed] [Google Scholar]
- 32. Zacharek A, Chen J, Cui X, et al. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27(10):1684-91. Epub 20070314. 10.1038/sj.jcbfm.9600475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Borlongan CV, Lind JG, Dillon-Carter O, et al. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res. 2004;1010(1-2):108-116. 10.1016/j.brainres.2004.02.072 [DOI] [PubMed] [Google Scholar]
- 34. Li Y, Chen J, Wang L, Lu M, Chopp M.. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56(12):1666-1672. 10.1212/wnl.56.12.1666 [DOI] [PubMed] [Google Scholar]
- 35. Chen J, Sanberg PR, Li Y, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32(11):2682-2688. 10.1161/hs1101.098367 [DOI] [PubMed] [Google Scholar]
- 36. Napoli E, Borlongan CV.. Recent advances in stem cell-based therapeutics for stroke. Transl Stroke Res. 2016;7(6):452-457. Epub 20160812. 10.1007/s12975-016-0490-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dunac A, Frelin C, Popolo-Blondeau M, et al. Neurological and functional recovery in human stroke are associated with peripheral blood CD34+ cell mobilization. J Neurol. 2007;254(3):327-332. 10.1007/s00415-006-0362-1 [DOI] [PubMed] [Google Scholar]
- 38. Boy S, Sauerbruch S, Kraemer M, et al. ; RAIS (Regeneration in Acute Ischemic Stroke) Study Group. RAIS (Regeneration in Acute Ischemic Stroke) Study Group Mobilisation of hematopoietic CD34+ precursor cells in patients with acute stroke is safe--results of an open-labeled non randomized phase I/II trial. PLoS One. 2011;6(8):e23099. Epub 2011 Aug 26. 10.1371/journal.pone.0023099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Banerjee S, Bentley P, Hamady M, et al. Intra-arterial immunoselected CD34+ stem cells for acute ischemic stroke. Stem Cells Transl Med. 2014;3(11):1322-30. Epub 2014 Aug 8. 10.5966/sctm.2013-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen DC, Lin SZ, Fan JR, et al. Intracerebral implantation of autologous peripheral blood stem cells in stroke patients: a randomized phase II study. Cell Transplant. 2014;23(12):1599-1612. 10.3727/096368914X678562 [DOI] [PubMed] [Google Scholar]
- 41. Sahoo S, Klychko E, Thorne T, et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109(7):724-728. 10.1161/CIRCRESAHA.111.253286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kikuchi-Taura A, Okinaka Y, Takeuchi Y, et al. Bone marrow mononuclear cells activate angiogenesis via gap junction–mediated cell-cell interaction. Stroke. 2020;51(4):1279-1289. 10.1161/STROKEAHA.119.028072 [DOI] [PubMed] [Google Scholar]
- 43. Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114(3):330-338. 10.1172/JCI20622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shyu W-C, Lin S-Z, Chiang M-F, Su C-Y, Li H.. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J Neurosci. 2006;26(13):3444-3453. 10.1523/JNEUROSCI.5165-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheng Z, Wang L, Qu M, et al. Mesenchymal stem cells attenuate blood-brain barrier leakage after cerebral ischemia in mice. J Neuroinflammation. 2018;15(1):135. Epub 20180503. 10.1186/s12974-018-1153-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ruan GP, Han YB, Wang TH, et al. Comparative study among three different methods of bone marrow mesenchymal stem cell transplantation following cerebral infarction in rats. Neurol Res. 2013;35(2):212-220. 10.1179/1743132812Y.0000000152 [DOI] [PubMed] [Google Scholar]
- 47. Vu Q, Xie K, Eckert M, Zhao W, Cramer SC.. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82(14):1277-86. Epub 20140307. 10.1212/WNL.0000000000000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jolien De M, Jolien De P, Said H-I.. Stem cell therapy for ischemic stroke: from bench to bedside. Int J Critical Care Emerg Med. 2018;4(2):058. 10.23937/2474-3674/1510058 [DOI] [Google Scholar]
- 49. Levy ML, Crawford JR, Dib N, et al. Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke. 2019;50(10):2835-2841. Epub 20190909. 10.1161/STROKEAHA.119.026318 [DOI] [PubMed] [Google Scholar]
- 50. Argibay B, Trekker J, Himmelreich U, et al. Intraarterial route increases the risk of cerebral lesions after mesenchymal cell administration in animal model of ischemia. Sci Rep. 2017;7:40758. Epub 20170116. 10.1038/srep40758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rascon-Ramirez FJ, Esteban-Garcia N, Barcia JA, et al. Are we ready for cell therapy to treat stroke? Front Cell Dev Biol. 2021;9:621645. Epub 20210623. 10.3389/fcell.2021.621645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cui L, Kerkelä E, Bakreen A, et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015;6(1):11. 10.1186/scrt544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Danielyan L, Schafer R, von Ameln-Mayerhofer A, et al. Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88(6):315-24. Epub 20090325. 10.1016/j.ejcb.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 54. Brooks B, Ebedes D, Usmani A, et al. Mesenchymal stromal cells in ischemic brain injury. Cells. 2022;11(6):1013. Epub 20220317. 10.3390/cells11061013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Donega V, van Velthoven CT, Nijboer CH, et al. Intranasal mesenchymal stem cell treatment for neonatal brain damage: long-term cognitive and sensorimotor improvement. PLoS One. 2013;8(1):e51253. Epub 20130103. 10.1371/journal.pone.0051253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Borges FT, Reis LA, Schor N.. Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz J Med Biol Res. 2013;46(10):824-30. Epub 20131002. 10.1590/1414-431X20132964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sanz-Ros J, Romero-Garcia N, Mas-Bargues C, et al. Small extracellular vesicles from young adipose-derived stem cells prevent frailty, improve health span, and decrease epigenetic age in old mice. Sci Adv. 2022;8(42):eabq2226. Epub 20221019. 10.1126/sciadv.abq2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yanez-Mo M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4(1):27066. Epub 20150514. 10.3402/jev.v4.27066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gould SJ, Raposo G.. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2(1):Epub 20130215. 10.3402/jev.v2i0.20389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Raposo G, Stoorvogel W.. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-383. 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zoller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009;9(1):40-55. Epub 20081211. 10.1038/nrc2543 [DOI] [PubMed] [Google Scholar]
- 62. Pasquet S, Sovalat H, Hénon P, et al. Long-term benefit of intracardiac delivery of autologous granulocyte-colony-stimulating factor-mobilized blood CD34+ cells containing cardiac progenitors on regional heart structure and function after myocardial infarct. Cytotherapy. 2009;11(8):1002-1015. 10.3109/14653240903164963 [DOI] [PubMed] [Google Scholar]
- 63. Saucourt C, Vogt S, Merlin A, et al. Design and validation of an automated process for the expansion of peripheral blood-derived CD34+ cells for clinical use after myocardial infarction. Stem Cells Transl Med. 2019;8(8):822-832. 10.1002/sctm.17-0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kawauchi S, Yasuhara T, Kin K, et al. Transplantation of modified human bone marrow-derived stromal cells affords therapeutic effects on cerebral ischemia in rats. CNS Neurosci Ther. 2022;28(12):1974-1985. 10.1111/cns.13947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xia C-F, Smith RS, Shen B, et al. Postischemic brain injury is exacerbated in mice lacking the kinin B2 receptor. Hypertension. 2006;47(4):752-761. 10.1161/01.HYP.0000214867.35632.0e [DOI] [PubMed] [Google Scholar]
- 66. Pianta S, Lee JY, Tuazon JP, et al. A short bout of exercise prior to stroke improves functional outcomes by enhancing angiogenesis. Neuromolecular Med. 2019;21(4):517-528. 10.1007/s12017-019-08533-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Development of a potency assay for CD34+ cell-based therapy for post-acute myocardial infarction 2023. Available at https://www.researchsquare.com Accessed September 19, 2023. 10.1038/s41598-023-47079-8 [DOI] [PMC free article] [PubMed]
- 68. Kanji S, Das M, Joseph M, et al. Nanofiber-expanded human CD34+ cells heal cutaneous wounds in streptozotocin-induced diabetic mice. Sci Rep. 2019;9(1):8415. 10.1038/s41598-019-44932-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cydzik M, Abdul-Wahid A, Park S, et al. Slow binding kinetics of secreted protein, acidic, rich in cysteine-VEGF interaction limit VEGF activation of VEGF receptor 2 and attenuate angiogenesis. FASEB J. 2015;29(8):3493-505. Epub 20150428. 10.1096/fj.15-271775 [DOI] [PubMed] [Google Scholar]
- 70. Larpthaveesarp A, Ferriero DM, Gonzalez FF.. Growth factors for the treatment of ischemic brain injury (growth factor treatment). Brain Sci. 2015;5(2):165-77. Epub 20150430. 10.3390/brainsci5020165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Arumugam TV, Toyoshima A, Yasuhara T, et al. Intra-arterial transplantation of allogeneic mesenchymal stem cells mounts neuroprotective effects in a transient ischemic stroke model in rats: analyses of therapeutic time window and its mechanisms. PLoS One. 2015;10(6):e0127302. 10.1371/journal.pone.0127302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Acosta SA, Tajiri N, Hoover J, Kaneko Y, Borlongan CV.. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke. 2015;46(9):2616-27. Epub 20150728. 10.1161/STROKEAHA.115.009854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Newcomb JD, Ajmo CT Jr, Sanberg CD, et al. Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell Transplant. 2006;15(3):213-223. 10.3727/000000006783982043 [DOI] [PubMed] [Google Scholar]
- 74. Guo Y, Peng Y, Zeng H, Chen G.. Progress in mesenchymal stem cell therapy for ischemic stroke. Stem Cells Int. 2021;2021:9923566. Epub 20210615. 10.1155/2021/9923566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu H, Reiter S, Zhou X, et al. Insight into the mechanisms and the challenges on stem cell-based therapies for cerebral ischemic stroke. Front Cell Neurosci. 2021;15:637210. Epub 20210225. 10.3389/fncel.2021.637210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rodriguez-Frutos B, Otero-Ortega L, Gutierrez-Fernandez M, et al. Stem cell therapy and administration routes after stroke. Transl Stroke Res. 2016;7(5):378-87. Epub 20160707. 10.1007/s12975-016-0482-6 [DOI] [PubMed] [Google Scholar]
- 77. Misra V, Ritchie MM, Stone LL, Low WC, Janardhan V.. Stem cell therapy in ischemic stroke: role of IV and intra-arterial therapy. Neurology. 2012;79(13 Suppl 1):S207-S212. 10.1212/WNL.0b013e31826959d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Darsalia V, Allison SJ, Cusulin C, et al. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J Cereb Blood Flow Metab. 2011;31(1):235-42. Epub 20100609. 10.1038/jcbfm.2010.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hauger O, Frost EE, van Heeswijk R, et al. MR evaluation of the glomerular homing of magnetically labeled mesenchymal stem cells in a rat model of nephropathy. Radiology. 2006;238(1):200-210. 10.1148/radiol.2381041668 [DOI] [PubMed] [Google Scholar]
- 80. Kraitchman DL, Tatsumi M, Gilson WD, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112(10):1451-61. Epub 20050829. 10.1161/CIRCULATIONAHA.105.537480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Eckert MA, Vu Q, Xie K, et al. Evidence for high translational potential of mesenchymal stromal cell therapy to improve recovery from ischemic stroke. J Cereb Blood Flow Metab. 2013;33(9):1322-34. Epub 20130612. 10.1038/jcbfm.2013.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gutierrez-Fernandez M, Rodriguez-Frutos B, Alvarez-Grech J, et al. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience. 2011;175(4):394-405. Epub 20101207. 10.1016/j.neuroscience.2010.11.054 [DOI] [PubMed] [Google Scholar]
- 83. Sammali E, Alia C, Vegliante G, et al. Intravenous infusion of human bone marrow mesenchymal stromal cells promotes functional recovery and neuroplasticity after ischemic stroke in mice. Sci Rep. 2017;7(1):6962. Epub 20170731. 10.1038/s41598-017-07274-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data reported in this study are available from the corresponding author on reasonable request.


