Abstract
Pulmonary artery hypertension (PAH) as the group I of give pulmonary hypertension is characterized by vasoconstriction and vascular remodeling resulting in both increased pulmonary vascular resistance (PVR) and pulmonary artery pressure (PAP). The chronic and high-pressure stress experienced by endothelial cells can give rise to inflammation, oxidative stress, and infiltration by immune cells. However, there is no clearly defined mechanism for PAH and available treatment options only provide limited symptomatic relief. Due to the far-reaching effects of metal exposures, the interaction between metals and the pulmonary vasculature is of particular interest. This review will briefly introduce the pathophysiology of PAH and then focus on the potential roles of metals, including essential and non-essential metals in the pathogenic process in the pulmonary arteries and right heart, which may be linked to PAH. Based on available data from human studies of occupational or environmental metal exposure, including lead, antimony, iron, and copper, the hypothesis of metals contributing to the pathogenesis of PAH is proposed as potential risk factors and underlying mechanisms for PAH. We propose that metals may initiate or exacerbate the pathogenesis of PAH, by providing potential mechanism by which metals interact with hypoxia-inducible factor and tumor suppressor p53 to modulate their downstream cellular proliferation pathways. These need further investigation. Additionally, we present future research directions on roles of metals in PAH.
Keywords: Pulmonary hypertension, Pulmonary hypoxia, Right ventricle dysfunction, Trace elements, Mineral homeostasis, Heavy metals, Non-essential metals
1. Introduction
Pulmonary hypertension (PH) is a disease that is defined as an increase in mean pulmonary arterial pressure (mPAP) greater than 20 mmHg [3]. mPAP is measured by right ventricle (RV) catheterization at rest and further divide PH into pre-capillary and post-capillary PH based on other hemodynamic parameters, namely pulmonary arterial wedge pressure (PAWP) and pulmonary vascular resistance (PVR). Specifically, pre-capillary PH, or pulmonary arterial hypertension (PAH) a disease of the right heart, is known to have a PAWP ≤ 15 mmHg and a PVR ≥ 3 Woods Units (WU). Conversely, post-capillary PH has a PAWP > 15 mmHg and PVR < 2 WU as its defining parameters and is associated with left heart disease. Combined pre- and post-capillary PH is a combination of the two previous definitions with PAWP > 15 mmHg and PVR 3 ≥ WU [1, 2]. The prognosis for pre-capillary PH patients is very poor, with one-year mortality rates reported as high as 20% [3]. As such, currently there is no effective therapy to prevent nor treat RV failure within PAH.
Currently, PH has been classified into 5 major groups clinically: 1) PAH; 2) PH caused by left heart disease; 3) PH caused by lung diseases and/or hypoxia; 4) chronic thromboembolic PH (CTEPH), and 5) PH with unclear and/or multifactorial mechanisms [2, 3]. Therefore, the etiology of pulmonary vascular remodeling is extremely complex, involving genetic predisposition, epigenetic changes, and environmental factors potentially mediating inflammation, oxidative stress, and metabolic transformation [4]. While lifestyle choices such as smoking, diet, exercise, and environmental status [2, 3] could contribute to PH, environmental factors are considered key determinants of both PH and RV dysfunction [5-7]. Due to the relatively well-understood mechanisms of PAH and the evidence specifically reported in group 1 PH, we will use PAH as a prototype for the pathogenesis of PH as a whole.
Exposures to nonessential metals like arsenic, lead, cadmium [7, 8] and even essential metals like iron, copper and zinc, have been linked to increased incidences of cardiovascular diseases [9, 10], which have been continuously confirmed via well-designed studies [11-13]. However, the exact link between environmental metal exposure and PAH pathogenesis remains unknown. For patients diagnosed with thalassemia, the prevalence of group 1 PH, specifically, is greater than 10,000 higher than the general population. Iron overload and nitric oxide depletion are thought to be factors contributing to this increased prevalence, perhaps indicating a role of metal dyshomeostasis in the pathogenesis of PAH in this population. [14, 15]. Furthermore, an animal model of monocrotaline-induced PAH rats showed significant increases in heme oxygenase 1 (HMOX1) and iron levels in the RV alongside reduced ejection fraction of the RV, and cardiac output and diastolic function levels, measured by TAPSE (tricuspid annular plane systolic excursion). More importantly, treatment with ferrostatin-1 effectively attenuated RV hypertrophy, remodeling, myocardial fibrosis, and dysfunction in PAH rats [16]. Thus, these studies suggest the potential role of elevated iron levels in the pathogenesis of PAH.
Metal-mediated mitochondrial damage and dysfunction of endothelial cells may play crucial roles in the pathogenesis of PAH. It is known that mitochondria play vital role regarding the regulation of apoptotic cell death, calcium signaling, glucose metabolism and fatty acid oxidation. Dysfunction of any of these signaling or pathways might lead to chronic diseases, including PAH. Mitochondrial reactive oxygen or nitrogen species (ROS or RNS) and potential signaling abnormalities of calcium can synergistically activate nuclear factor κB (NF-κB), triggering inflammatory responses, leading to the death or proliferation of pulmonary artery endothelial cells (PAECs) and smooth muscle cells (PASMCs), and eventually PAH and associated RV dysfunction [2, 17, 18]. To date, there are significant knowledge gaps in the relationship between heavy metals and PAH. Therefore, the objective of this review is to abridge the evidence surrounding essential and nonessential metals, mitochondrial dysfunction, and ROS and RNS production in literature and propose the hypothesis that exposure to environmental metals is a potential contributing factor to the pathogenesis of PAH.
2. Potential contribution of ambient particulate matter to the pathogenesis of PAH
Environmental exposures can precipitate PAH [19]. Historically, silicosis (coal miner and stone worker disease) has been considered as a cause of PAH in the US and Western Europe in the early 20th century [20]. Even nowadays, PAH induced by exposure to silica is still a major problem [21, 22]. Biomass fuel exposure can not only cause obstructive and/or restrictive lung disease, but also potentially lead to systolic and diastolic RV dysfunction [23]. Additionally, exposure from smoking cigarettes is thought to be the most important trigger of PAH in chronic obstructive pulmonary disease [24]. Morphological changes in the RV (greater RV mass and end-diastolic volume) are associated with the intensity of traffic related air pollution as measured by outdoor nitric oxide (NO) concentration [25]. Furthermore, environmental exposures to silica or organic chemicals can exacerbate autoimmune diseases [25] and the development of PAH.
The relationship between air pollution, specifically particle matter (PM), and the incidence and mortality of PAH were relatively less addressed. A small study found an association between isolated RV failure and exposure to occupational dusts [26]. Furthermore, a recent study with the US-based REVEAL trial investigated the prevalence, demographics, and outcomes in everversus never-smokers with PAH. Ever-smoking status was associated with earlier hospitalization times and shorter survival after PAH diagnosis, suggesting the exacerbation of PAH pathogenesis [27]. However, it remains unclear whether ever smoking status increases the metal levels of these PAH patients. In the sections below, we will discuss the relationship between PM with the incidence and mortality of PAH.
2.1. The relationship between PM exposure and the pathogenesis of PAH within patients.
The attempt to explore the potential association between PAH and chronic exposure to ambient PM can be traced back to a 2010 case-study by Schiess et al. to assess smoking and secondhand smoke exposure in all patients with PAH [28]. In this study, 91 PAH patients were compared with 18,747 control subjects. Tobacco smoking was significantly common in male PAH patients compared to control subjects. Additionally, compared with control subjects, secondhand smoke exposure was significantly longer in nonsmokers with PAH. Therefore, this study revealed the potential risk that tobacco smoke had on men with PAH [28].
Recently Shi et al. enrolled 494,750 participants in the UK Biobank study. Exposures to PM2.5 and PM10 (PM with a diameter of less than 2.5 μm and 10 μm, respectively) were estimated by geocoded participants' residential addresses, utilizing pollution data provided by UK Department for Environment, Food and Rural Affairs (DEFRA). They found that during a median follow-up of 11.75 years, 2,517 participants developed PAH, and 696 died. Exposure to both PMs was associated with the incidence of PAH by adjusted hazard ratios (HRs) of 1.73 and 1.70, respectively. Furthermore, exposure to PM2.5 and PM10 increased the transition from PAH to death. This indicates that exposure to various ambient air pollutants might play key but differing roles in both the incidence and severity of PAH [29]. A recent study by Cui et al confirmed these findings from the UK study by analyzing the medical records of 1,755 children requiring mechanical ventilation in the ICU between December 2013 to December 2020. They found that PAH significantly increased the effects of PM2.5 on ventilator-associated pneumonia in pediatric patients [30].
2.2. Association of exposure to PM with the pathogenesis of PAH in animal models
Grunig et al. reported that PM2.5 exacerbated antigen-induced pulmonary arterial remodeling in mice. Exposure to PM2.5 alone at a dose of 25 μg per instillation (the equivalent of 1.25 mg/kg body weight) in mice did not cause arterial remodeling. However, low dose PM2.5 exposure (approximately 0.625 mg/kg) significantly exacerbated pulmonary arterial remodeling in antigen treated mice. They further showed that the combination of PM2.5 and antigen significantly increased RV pressures, compared to those with the antigen or PM2.5 alone, suggesting that even low-dose PM2.5 could worsen PAH if PM2.5 exposure is added to another inflammatory condition [19].
Continuing with this aspect, Chen et al. examined the influence of exposure to PM2.5 on the PAH murine model induced by left ventricular (LV) failure. They randomly divided 30 ten-week-old C57BL/6 mice to four groups: sham group, sham + PM2.5 group, transverse aortic constriction (TAC) group, and TAC + PM2.5 group). Eight weeks post TAC surgery, RV and lung remodeling and functions were measured. Although PM2.5 exposure alone did not directly induce PAH, exposure to PM2.5 augmented TAC-induced PAH as evidenced by decreased ejection fraction, increased RV systolic pressure, increased RV cardiomyocytes size, and overall RV remodeling [31].
Other preliminary experiments with animal models also demonstrated similar findings as mentioned above: exposure to ambient PM caused significant changes in the pulmonary vasculature on the morphological, functional, and molecular levels, and even demonstrated RV dysfunction [32-34]. Diesel exhaust, one of the main sources of urban air pollution, was found to stimulate PASMC proliferation and induced endothelial cell apoptosis [35]. Furthermore, diesel exhaust was also found to increased RV systolic pressure and cause RV hypertrophy and PAH within a mouse model [35].
In summary, exposure to PMs exacerbates the pathogenesis of PAH. The evidence from preclinical animal models supports the strong associations between PM exposure and PAH observed in humans from different data bases or populations. However, whether PM contains metals and whether these metals play a role in PAH pathogenesis remain largely unclear. In the following section, we will discuss the potential effects of metals on the pathogenesis of PAH.
3. Potential effects of metals on the pathogenesis of PAH
3.1. Evidence indicating the potential roles of metals in the pathogenesis of PAH
Currently, studies involving the relationship between metal exposure or essential metal dyshomeostasis and PAH and/or RV dysfunction are less investigated [5, 7, 36]. An early study determined that serum levels of cadmium, cobalt, and iron were significantly higher in chronic obstructive pulmonary disease (COPD) cases with PAH compared to COPD patients without PAH [37], suggesting the potential associations of these metals with PAH.
Our recent prospective, single center pilot study investigated metal levels between 20 PAH patients and 10 healthy controls by measuring 25 metal levels in blood, plasma, and urine samples using an X Series II quadrupole inductively coupled plasma mass spectrometry (ICP-MS). In plasma samples, significantly increased silver and copper levels in PAH patients showed a significant correlation between cardiac output and cardiac index. Plasma chromium levels determined a significant correlation between mixed venous saturation, 6-min walk distance, and B-type natriuretic peptide [6]. These two studies showed significant differences between PAH and control groups in terms of metal concentrations. Therefore, we will specifically discuss the potential association of each metal with the pathogenesis of PAH in the following sections.
3.1.1. Evidence for the potential role of lead in the pathogenesis of PAH
Lead is one of the nonessential metals, metals that we do not need physiologically for our body, but has toxic effects on many visceral organs and disrupts physiological functions. Occupational exposure to lead significantly increases blood lead levels, causes small airway obstructions, and poor pulmonary function in lead-acid battery recyclers [38]. The decreased lung function could cause Group 3 PAH, derived from lung diseases and/or hypoxia. For this study, of course, two questions need to be addressed: first is determining the main exposure source of the increased lead levels in the blood as it could not be completely attributed to inhalation of lead and subjects might have dietary intake of lead contaminated food or drinking water. Second, is determining whether other metals or compounds in the polluted environments might have caused lung function to decrease.
These questions were addressed in animal models. Rats were exposed to lead acetate by daily intramuscular injections (40 μg/kg on the 1st day and 0.5 μg/kg subsequently) for 7 days, which significantly increased blood lead levels and lead deposition along with superoxide anions production in the pulmonary arteries. These changes were accompanied with increased RV pressures and contractile/relaxing dysfunctions of pulmonary vessels [39], suggesting the potential pathogenic role of lead in the development of PAH and RV dysfunction.
3.1.2. Evidence for the potential role of antimony in the pathogenesis of PAH
We recently found levels of antimony, a nonessential metal, were significantly higher in the blood and plasma of patients with PAH when compared to controls. In this pilot study, we evaluated antimony levels in the blood, plasma, and urine of 20 PAH patients and 10 controls, which showed significantly higher blood and plasma levels of antimony in PAH patients when compared to controls. Antimony blood and plasma levels were also significantly higher in both idiopathic PAH patients and non-idiopathic PAH when compared with controls. But these differences were not significantly observed in the urine levels of antimony. Additionally, there was a significant correlation between plasma antimony levels and nearly all the PAH prognostic hemodynamic parameters, including mean right atrial pressure (mRAP), cardiac output (CO), cardiac index (CI), pulmonary vascular resistance (PVR), and mixed venous oxygenation (SvO2) [5].
This pilot study suggested potential roles of increased antimony levels in the pathogenesis of PAH. However, whether exposure to these nonessential metals directly causes toxicities to pulmonary vessels and RV or is indirectly mediated by their interactions with essential metals, leading to essential metal dyshomeostasis, is unclear.
3.1.3. Evidence for the potential role of iron in the pathogenesis of PH
Iron as an essential metal is involved in many metabolic processes such as electron transport, nucleotide synthesis, and oxygen transport. Therefore, maintaining normal iron levels is crucial for maintaining our health. In the human body, iron is stored in hemoglobin, ferritin, transferrin, intracellular proteins, and a labile iron pool. This labile iron pool, also referred to as non-transferrin bound iron, is a reactive pool of relatively free-floating iron, and can generate ROS through a Fenton reaction [40].
Iron deficiency is found in PAH patients (38.25%, with the highest prevalence in connective tissue disease associated PAH) and is associated with worse PAH clinical outcomes [41, 42]. The effect of hypoxia on pulmonary arterial pressure depends on the iron status possibly through the transcription factor hypoxia-inducible factor, HIF [41, 43]. Despite the connections between iron homeostasis and PAH, interventions targeting iron pathways in PAH patients showed varying results. Supplementation with slow-release iron in idiopathic or heritable PAH patients with iron deficiency improved their iron status but failed to improve their exercise capacity, pro-BNP, and cardiopulmonary hemodynamic testing [44].
However, administration of intravenous iron attenuated the rise in pulmonary artery pressures with hypoxia in animal models [45]. It has been noticed that intracellular iron deficiency alters pulmonary vascular function and iron-deficient mice and rats exhibited abnormal pulmonary artery pressure, pulmonary vascular remodeling, and RV hypertrophy [45, 46].
The association between iron overload and PAH may also be implicated by the fact that the thalassemia syndrome patients have a higher risk of developing PAH and that PAH was almost absent in patients with thalassemia major if they strictly adhered to standard transfusions with iron chelation therapy [47-51].
Animal models also suggested a direct role of iron overload in PAH development of monocrotaline-induced rats [52] and hypoxia-induced mice [53]. In these two studies, monocrotaline-treated rats and hypoxia-exposed mice showed pulmonary vascular remodeling, increased RV pressure, RV hypertrophy, and decreased RV ejection fraction with normal diet conditions. However, PAH pathogenesis and RV dysfunctions were attenuated with iron restriction in their diets, suggesting the possible involvement of iron in these two PAH animal models. Furthermore, the direct causative effect of iron on PAH was confirmed via a study by Bertoli et al. Injection of iron-dextran (200 mg/kg/day i.p.) for 28 days showed increased circulating iron levels, lung iron deposits, RV dysfunction, RV hypertrophy, pulmonary artery remodeling and vasoconstriction [54]. These experiments indicated that iron might be involved in the development of monocrotaline- or hypoxia-induced PAH and could directly induce the pathogenesis of PAH.
3.1.4. Evidence for the potential role of copper in the pathogenesis of PAH
Copper is an important essential metal for our body and its abnormality is linked to several chronic diseases. Since 1980s, the significant increase in serum copper levels were noticed in PAH patients compared to controls [55]. Later, patients with both systemic sclerosis and PAH were also found to have elevated ratios of copper to zinc, copper to selenium, and ceruloplasmin/selenoprotein as compared to controls [56]. Our recent pilot study showed significantly higher plasma copper levels in PAH patients than control [6]. Therefore, more studies to directly examine serum or plasma copper levels in patients with PAH are needed. Along this line of work, a few biomarkers related to copper metabolism were found to be significantly increased in PAH patients and could be used as new diagnostic biomarkers and/or targets for PAH patients [57].
Previous animal models have shown that copper plays into the pathogenesis of PAH as implicated by a combined copper-depleted diet and copper chelation therapy via tetrathiomolybdate which either prevented or reversed the development of severe PAH induced by SU5416 and hypoxia (SuHx) [58]. Furthermore, Zimnicka et al., showed up-regulation of the Cu-uptake transporter 1, CTR1, and the Cu-efflux pump, ATP7A, in the pulmonary arteries of hypoxia-induced PAH mice [59]. Poels et al showed copper depleted diet could prevent the development of PAH in hypoxia induced mice [60], suggesting the systemic level of copper might be related to the PAH pathogenesis. All these experimental studies strongly suggested the potential involvement of copper in the pathogenesis of PAH.
3.1.5. Evidence for the potential role of vanadium in the pathogenesis of PAH
Vanadium is a relatively abundant element in soil, water, and the atmosphere, as it is widely used by many industries and medical fields. Occupational inhalation of vanadium was found to induce acute respiratory symptoms in boiler makers, causing safety regulations to be put into place decreasing overall vanadium exposure. [61, 62]. However, the exposure to metals/metalloids from environmental sources are more relevant and complex. There was a report that exposure to vanadium oxides attached to fine PM were associated with increased risk of respiratory symptoms in children [63] and older people in US counties [64]. There is no study to address whether vanadium exposure is related to the pathogenesis of PAH in epidemiological study; however, in an animal model, rats that were treated with dietary vanadate for 2 months and proceeded to develop PAH, as evidenced by significantly higher mean and systolic RV pressures when compared to rats without vanadium treatment. RV hypertrophy was also noted in vanadium-treated rats [65] which was further confirmed by two later studies [66, 67]. Li et al. determined that vanadium induced acute pulmonary vasoconstriction was partially through the inhibition of endothelial NO production via PKC-dependent phosphorylation of Thr495 of eNOS [66].
3.1.6. Evidence for the potential role of cadmium in the pathogenesis of PAH
Cadmium is another nonessential metal worth mentioning. Although there was no evidence to link exposure to cadmium to the pathogenesis of PAH, a few features of cadmium and its potential impact on health are relevant. The first question is whether cadmium distributes into the lungs and could potentially induce PAH. In animal models, cadmium levels in blood, aortic walls, lungs, and livers were increased significantly in rats exposed to cadmium via inhalation [68]. Later studies showed the increased cadmium deposition in the lungs after inhalational exposure to cadmium [69, 70] along with increased lung oxidative stress and damages [70]. To determine how smoking affects the organ distribution and accumulation of cadmium, male C57B1 mice and Sprague-Dawley rats were exposed daily for 52-60 consecutive weeks to mainstream smoke using a nose-only exposure system. Cadmium levels were 5-6- and 2-3-fold greater in the exposed animals than the control levels in the lungs and kidneys, respectively. In contrast, the liver did not show increased cadmium levels in exposed mice or rats, suggesting the low-dose chronic inhalational exposure to cigarette smoking leads to the highest cadmium accumulation in the lung, followed by the kidney, with minimal distribution to other organs [71]. Therefore, cadmium could potentially contribute to smoking-related lung diseases such as emphysema, possibly via an altered redox balance and by macrophage dysfunctions [72]. Mice with low-dose cadmium by drinking water (10 mg/L in drinking water) for 20 weeks increased lung cadmium to a level of non-occupationally exposed adult humans, with increased airway hyper-responsiveness to methacholine challenge, suggesting the potential development of Group 1 and 3 PAH derived from chronic lung diseases in response to inhalational exposure to cadmium [73].
4. Hypothesis
PH is a complex disorder characterized by increased pulmonary artery pressure and RV dysfunction. While the exact causes of PAH remain unknown, it is generally considered to result from a combination of genetic, epigenetic, and environmental factors. Metals may contribute to the pathogenesis of PAH as one of the environmental factors. Currently, there is limited direct evidence linking metals to the pathogenesis of PH in humans. However, certain occupational conditions increase the exposure to certain metals, such as lead, antimony, copper, and iron, which were found to be associated with the development of PAH. Some studies, for instance, suggested that iron overload, as seen in conditions like hereditary hemochromatosis or thalassemia, may assist in PAH development. Iron accumulation in the lungs could promote inflammation and oxidative stress, possibly leading to vascular dysfunction and PAH.
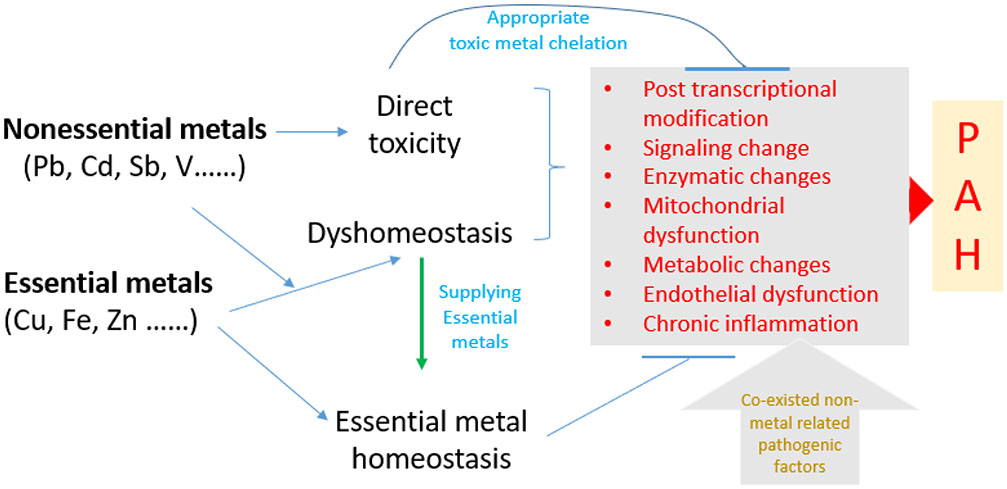
It's important to note that most PAH cases are not directly related to metal exposure or accumulation. In most cases, the disease is classified as idiopathic (of unknown cause) or correlates other conditions like connective tissue diseases, left heart and lung diseases, or genetic mutations. The primary pathological processes in these cases involve abnormal PASMC proliferation, PAEC dysfunction, and vascular remodeling, rather than metal-induced changes. Under these conditions, exposure to metal either via inhalational or systemic intake, may worsen or accelerate the existed pathogenesis of PAH. Therefore, we propose a plausible hypothesis, as illustrated in Figure 1, that exposure to non-essential metals can either directly damage PAECs and PASMCs via the Fenton reaction to generate free radicals, or interact with essential metals via competing for their transporters to cause intracellular essential metal dyshomeostasis, which in turn cause dysfunctions of essential metal-dependent enzymes, transcriptional factors, and signaling pathways as well as cellular metabolic abnormalities, resulting in the development of PAH.
Figure 1. The proposed hypothesis.
We hypothesize that exposure to metals, particularly non-essential metal, that either (1) directly damages endothelial cells of pulmonary arteries via Fenton reaction to generate free radicals, or (2) interacts with essential metals via competing for their transporters to cause intracellular essential metal dyshomeostasis that can lead essential metals-dependent enzyme, transcriptional factors, signaling pathway kinase dysfunction, and mitochondrial dysfunction or damage, all which can cause mitochondria-mediated metabolic changes, oxidative stress, chronic inflammation. Therefore, specific chelation of nonessential metal or specific supplementation of certain deficient essential metals may prevent these metal effects on PAH pathogenesis. In addition, non-essential metals or/and essential metals dyshomeostasis may accelerate PAH pathogenesis from other nonmetal PAH risk factors. PAH=Pulmonary artery hypertension.
Although there was no direct evidence from human studies to indicate the direct role of exposure to metals in the development of PAH, in the animal models as mentioned above intramuscular injections of lead acetate for 7 days increased RV pressures and contractile/relaxing dysfunctions of pulmonary vessels [39]. This suggests the potential development of PAH and RV dysfunction. Direct dietary intake of vanadate for 2 months induced the develop PAH and RV hypertrophy [65-67]. Injection of iron-dextran for 28 days induced PAH along with RV remodeling and dysfunction [54] and iron restriction in the diet could prevent monocrotaline- and hypoxia-induced PAH [52, 53]. All these suggest the direct role of metals in the pathogenesis of PAH in the experimental model. In term of the possible mechanisms, the following two hypothetic mechanisms may explain how metals may directly induce or indirectly accelerate/worsen the pathogenesis of PAH.
4.1. Metal effects on HIF-1α functions may be one of the possible mechanisms responsible for metal effects on the pathogenesis of PAH
4.1.1. HIF-1 and its regulation
In order to maintain homeostasis under insufficient oxygen supply, an organism requires adequate adaptive induction of angiogenesis to maintain energy homeostasis and cell survival, which is predominantly controlled by HIF-1, particularly HIF-1α [74]. Prolyl hydroxylase domain-containing protein 2 (PHD2), an enzyme encoded by the EGLN1 gene, is a α-ketoglutarate/2-oxoglutarate-dependent hydroxylase, superfamily non-heme iron-containing proteins [74]. Therefore, PHD2 enzyme chelates an Fe2, Figure 2A. PHD2 is the primary regulator of HIF-1α steady state levels in the cell by improving its degradation under normoxia and releases HIF-1α from ubiquitination under hypoxia condition (Figure 2). The von Hippel-Lindau protein (pVHL) mediates the ubiquitination and rapid degradation of HIF-α (including HIF-1α and HIF-2α). A PHD2 knockdown showed increased levels of HIF-1α even under normoxia, and an increase in HIF-1α nuclear accumulation and HIF-dependent transcription [74, 75].
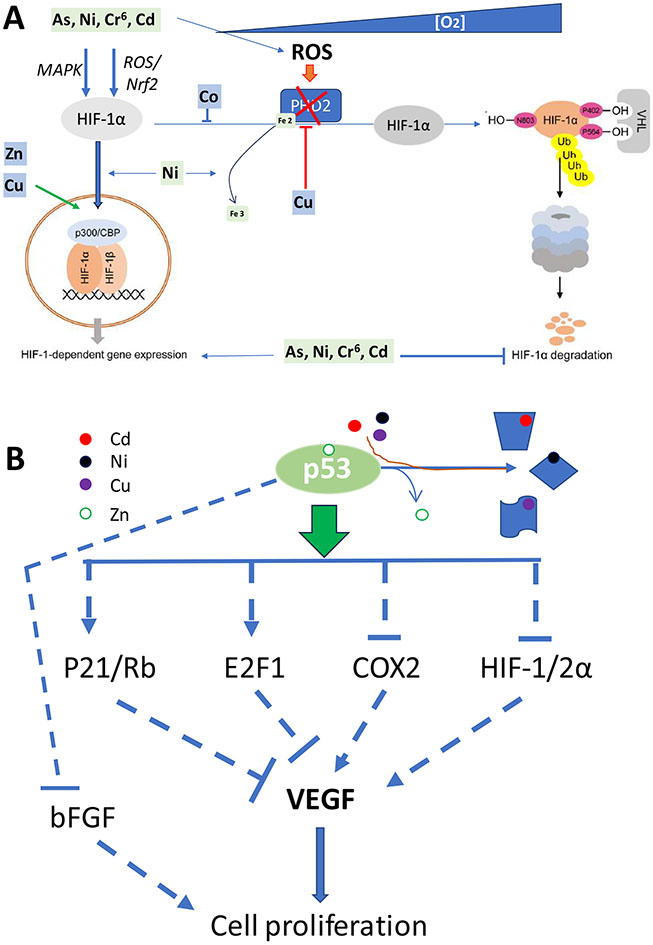
Figure 2. Metal potential effects on HIF-1α- and P53-mediated cell proliferation.
A: Illustration of oxygen-dependent regulation of HIF-1α signaling: under normoxic conditions, HIF-1α is hydroxylated by PHD2 and the hydroxylated HIF-1α binds von Hippel–Lindau (VHL) and subsequently undergoes HIF-1α poly-ubiquitylation and degradation while under hypoxic conditions, hydroxylation and acetylation are inhibited, HIF-1α accumulates and forms dimers with HIF-1β, and then translocate into nuclear to form complex with p300/CBP to transcriptionally upregulate their downstream genes, including VEGF to stimulate cell proliferation. In addition, increased ROS generation under hypoxic conditions results in disulfde bond-mediated PHD2 homo-dimer formation via thiol groups of cysteine residues in the catalytic site, thus decreasing enzyme activity. Meanwhile ROS also oxidizes PHD2 Fe2 to Fe3, leading to the reduction of PHD2 activity. HIF-1α-mediated pathways are stimulated by copper, zinc, cobalt (Co) and nonessential metals (As, Ni, Cr6 and Cd). B: Wild-type Zn-bound p53 transcriptionally upregulated P21 and E2F1 to ensue proliferate cell arrest at G1/S phase with inhibition of VEGF expression, meanwhile Zn-bound p53 can also downregulate HIF-1/2α, COX2 and bFGF to prevent their stimulating effect on VEGF or cell proliferation. Since Zn bound p53 can be replaced under certain conditions, p53 protein conformation is changed with loss of its normal function.
4.1.2. The essential role of HIF-1 in PAH
In response to hypoxia, systemic and pulmonary arterioles show different changes: the former dilate to increase perfusion with increase in O2 delivery while the latter constrict and shunt blood flow away from areas of the lung that are not ventilated. While hypoxic pulmonary vasoconstriction (HPV) is adaptive response, HPV leads to both increased hypoxemia and increased pulmonary arterial and RV pressure that, if untreated, ultimately results in RV failure. In addition to HPV as an acute response of PASMCs to hypoxia, pulmonary arterioles subjected to chronic hypoxia also undergo pathological remodeling, including PASMC proliferation and even muscularized vessels, becoming persistent PAH [76].
Studies showed that HIF-1α plays an important role in PASMCs and HIF-2α plays an important role in PAECs since mice with deletion of the Hif1a gene encoding HIF-1α or the Epas1 gene encoding HIF-2α are resistant to chronic hypoxia-induced PAH [77-79]. Pharmacological blockage of HIF-1 with the inhibitor (digoxin, [80] in mice, and HIF-2α with its inhibitor (PT-2567, [79]) in rats, blocked the effects of hypoxia on hypoxia-induced increases in pulmonary vascular muscularization and RV pressure. In parallel conditional knockout of PHD2 in endothelial cells results in the development of PAH under normoxic conditions [81, 82]. Individuals who are heterozygous for a missense mutation (Gly537Arg) in HIF-2α have pulmonary hypertension [83]. Therefore, findings from these genetic or pharmacological loss-of-function in animal models and gene mutations in humans strongly support the essential role of increased HIF-2α activity in the development of PAH.
4.1.3. Metal modification of HIFs and PHD2 pathways
Due to the important role of induced HIF-1 and/or HIF-2 in PAH pathogenesis, any molecules that affect HIF expression or function will potentially affect the pathogenesis of PAH. HIF-1α signaling under hypoxia and normoxia is strongly dependent on redox stimuli [84]. Several metals are involved in redox biology by their participating the Fenton reaction, which are discussed by several reviews [76,79,84] and not discussed here. Here we will focus on the direct role of metals on the modification of HIF expression and function. Under normal conditions a tight interaction between iron metabolism and HIF-1 pathway is required for the body, which, however, can be substituted by other biologically essential metals including copper, zinc, manganese, and cobalt [85, 86]. For instance, cobalt binds directly to the ODD domain of HIF-α to avoid the VHL-binding and thereby prevent the degradation of HIF-α [85]; therefore, Co has been used to induce a hypoxia model for the cultured cells in vitro.
As another example copper is a positive regulator of HIF-1α signaling and free copper can induce ceruloplasmin synthesis in a HIF-1–dependent way, because copper can inhibit prolyl-4-hydroxylation to functionally stabilize HIF-1α [87]. In contrast, copper deficiency prevented HIF-1α -induced VEGF protein accumulation and mRNA expression [88] by inhibiting HIF-1α binding to the critical motifs in the promotor and enhancer regions of the target genes [89]. In the review by Xiao et al. [90], the fact that copper regulates HIF-1 activity at multiple sites, including HIF-1α protein stabilization, transcriptional complex formation, and binding to the HRE sequence of target genes has been emphasized. Therefore, copper at physiologically relevant levels do not influence either the production or the stability of HIF-1α protein, but instead, it is required for HIF-1 transcriptional complex formation and binding to the HIF-HRE sequence of target genes. Copper deprivation inhibited the recruitment of cofactors, such as p300, to HIF-1 transcriptional complex, which was likely via affecting FIH activity [90]. Thus, copper is required for HIF-1 activation through regulation of HIF-1 binding to the HRE and the formation of the HIF-1 transcriptional complex. Therefore, this may explain why the elevated ratios of copper to zinc and ceruloplasmin/selenoprotein were found in the patients with both systemic sclerosis and PAH as compared to those with only sclerosis [56], and higher plasma copper levels in PAH patients than control [6]. In contrast, chelation of copper prevented or reversed the development of severe PAH induced by SU5416 and hypoxia (SuHx) [58] or by hypoxia [60].
Furthermore, zinc and manganese both also play important role in modulation of HIF pathways: Several studies showed the inhibitory effect of zinc on HIF-1α nuclear translocation in astrocytes [91] and stimulating proteasomal degradation of HIF-1α [92]. In fact, zinc deficiency induced by TPEN in primary human microvascular endothelial cells is associated with HIF-1α signaling up-regulation due to increased HIF-1α nuclear translocation [93]. These suggest that zinc most likely acts as a negative regulator of HIF-1α, which may be mediated by the essential role of zinc finger domain in PHD2 that is essential for HIF-1α hydroxylation [94]. Manganese reduced HIF-1α degradation with increasing its intracellular levels by inhibiting HIF prolyl hydroxylase [95]. Concomitantly, Manganese exposure was shown to increase HIF-1 target VEGF expression both in an in vitro model of human pulmonary epithelial cells incubated with Manganese and murine lungs following Manganese inhalation [96]. These findings generally correspond to the earlier observation of increased HIF-1α expression in broilers with manganese deficiency-induced tibial dyschondroplasia [97].
In contrast to the role of essential metals in regulation of HIF-1 signaling that may mediate its role in health and disease, modulation of HIF-1α pathway by toxic metals is less studied and may be considered as the potential additional mechanism underlying its toxic effects. Based on the recent review by Aschner, up-regulation of HIF-1 signaling is mainly observed in response to exposure to chromium, arsenic, and nickel whereas cadmium and mercury may both stimulate and inhibit HIF-1 pathway. The mechanisms underlying the influence of toxic metal exposure on HIF-1 signaling involve modulation of PHD2 activity that is strongly related to prooxidant effect of metals, indirect effects [75].
4.2. Metal effects on p53 function as one of the possible mechanisms responsible for metal effects on the pathogenesis of PAH
4.2.1. P53 and its close regulation
The transcription factor p53 functions as a gatekeeper, regulating a myriad of genes to maintain normal cell functions except for its well-known function as a tumor suppressor. In the recent years, its potential roles in the cardiovascular function and pathogenesis were also gradually appreciated [98-100], and in the pathogenesis of PAH were paid attention [101], and summarized by Hsieh et al. [102]. In general, when DNA damage is detected in a cell p53 is activated by ATM/ATR and initiates a signaling cascade that leads to cell cycle arrest by activating the transcription of its downstream genes that produce proteins like p21 (also known as cyclin-dependent kinase inhibitor 1A, CDKN1A), as illustrated in figure 2, resulting in cell cycle arrest at the G1 checkpoint, or even senescence when a cell has experienced extensive damage. Meanwhile the DNA repair pathways is also active by ATM/ATR (Figure 2B). However, if the DNA damage is too severe and cannot be adequately repaired, p53 can induce apoptosis by increasing the expression of pro-apoptotic genes, such as BAX and PUMA, and repressing anti-apoptotic genes, like Bcl-2. Overall, p53 acts as a guardian of the genome by ensuring that cells with genomic instability do not continue to proliferate. Thus, dysregulation of p53 can lead to uncontrolled cell proliferation and the survival of cells with unhealthy genomics. In fact, p53 is ubiquitously expressed and has major impacts on the physiology and/or pathophysiology of virtually all organs in the body, due to the plenty of is downstream genes involved in various very fundamental aspects of cell biology. Therefore, p53 expression and function are important for the pathogenesis of CVDs and PAH [98-102].
4.2.2. Essential role of p53 and associated members in PAH
Since key pathogenesis of PAH includes abnormal proliferation of PASMCs, apoptotic resistance of PAECs and senescent cell accumulations, all these may be related to the abnormal p53 expression and function (PMID: 35740436; 35749795). As illustrated in Figure 2, HIF-1α activation is a key pathway to active VEGF and FGF2 expression that stimulate cell proliferation, as a key step of PASMC PAH pathogenesis. However, activated p53 can activate p21/Rb and E2F1 pathways to inhibit the expression of VEGF and can also inactivate HIF-1α and COX2 pathways to prevent their stimulating VEGF expression, both of which results in inhibiting VEGF function of prompting cell proliferation. In fact, decreased level of p53 protein in the lungs from both animals and subjects with PAH [103-106], even specifically in PASMCs in specimens isolated from patients with idiopathic PAH [107, 108]. More specifically, in murine and rat models of PAH, p53 expression is downregulated in PASMCs, but is upregulated in PAECs [108].
To support the above notion, a study has examined the role of p53 and HIF-1α expression on hypoxia-induced pulmonary arterial remodeling, wild-type (WT) and p53 knockout (p53KO) mice were exposed to either normoxia or hypoxia for 8 wk. Both genotypes demonstrated elevated right ventricular pressures, right ventricular hypertrophy, these changes were significantly greater in the p53KO mice than in the WT mice. The p53KO mice had lower levels of p21 expression, and higher levels of HIF-1α, VEGF, and PDGF expression than WT mice along with a higher proliferating cell nuclear antigen expression of pulmonary artery in p53KO mice. This study suggested, via interacting with p21 and HIF-1α, p53 plays a suppress role in hypoxic pulmonary arterial remodeling and pulmonary arterial smooth muscle cell proliferation under hypoxia [101]. Furthermore, another study has shown the sufficiency of p53 inactivation alone to induce development of PAH in rats, implicating the p53 pathway at the initiation stages of PAH pathogenesis [105].
Contrast to this, treatment of cultured human PASMCs with p53 activator, Nutlin-3a caused increases in phosphorylated p53 protein levels, expression of p53 target genes (p21, Bax, BTG2, and MDM2), leading to cell growth arrest with the induction of senescence but not apoptosis. Daily intraperitoneal Nutlin-3a treatment for 3 weeks dose-dependently reduced PAH, shown by pulmonary artery muscularization and RV hypertrophy in mice exposed to chronic hypoxia or SU5416/hypoxia [109]. Combined treatment of Nutlin3a with PT2385 (HIF-1α inhibitor) is more effective than monotherapy in reversing established SuHx-PH. Therefore, combination treatment confers greater therapeutic efficacy against PAH through a selective modulation of p53 and HIF-2α in PASMC and PAEC [110]. PAH prevention or reversal by Nutlin-3a required lung p53 stabilization and increased p21 expression since there was no Nutlin-3a effects in hypoxia-exposed p53-KO and p21-KO mice [109].
4.2.3. Metal effects on p53 expression and function
Metals can indeed affect the conformation and function of the p53 protein. The interaction between metals and p53 has been an active area of research and has important implications for cellular responses to metal exposure and their potential impact on human health. Zinc, for example, is known to stabilize the DNA-binding domain of p53 and is also essential for maintaining proper conformation (three-dimensional structure) and function of p53. Exposure of cultured cells to the membrane-permeable zinc chelator induced wild-type p53 to accumulate in mutant forms with low DNA-binding activity. Removal of zinc chelator from culture medium allowed p53 to refold into the immunologically wild-type form, followed by a transient increase in DNA binding, expression of the cyclin-dependent kinase inhibitor p21WAF1, and cell-cycle delay in the G1 phase. Thus, modulation of intracellular zinc induced conformational changes in p53 that activated wild-type function, suggesting that metalloregulation may play a role in controlling p53 [111-113]. The binding of magnesium significantly stimulated the binding of the protein to DNA in a sequence-independent manner, which differed from that of zinc ions in a sequence-specific manner. Therefore, magnesium likely a factor to affect or regulate the transactivation of p53 [114, 115].
Whereas other metals like cadmium [116] or nickel [117] may cause conformational changes that impact the function of p53. Disruption of the correct protein conformation can impair p53's ability to bind to DNA or interact with other proteins. Similarly, copper and iron (or heme) also impair p53's ability to bind to DNA [118-120].
5. Conclusion and perspectives
In summary, PH pathogenesis may be linked to heavy and essential metal dyshomeostasis. These metals are ubiquitous throughout the environment. In PM, specifically, they have been shown to lead to the development of PH and other lung pathologies in humans and animal studies. There are several mechanisms by which metals are thought to promote physiological conditions similar to those seen in the eventual pathophysiology of PH. This leads to our hypothesis, that inhalation and/or ingestion of heavy and essential metals via environmental exposure may be a risk factor for the eventual development of PH.
Additionally, exposure to non-essential metals or dyshomeostasis of essential metals may worsen or accelerate other PAH risk factors-induced pathologies. To comprehensively understand the involvement of metal dyshomeostasis in the pathogenesis of PAH and underlying mechanisms by which how metal dyshomeostasis exacerbates existing PAH pathophysiology is urgently needed.
To these ends, the following issues need to be addressed: 1) Although epidemiologically there are several large cohorts currently investigating the potential effects of environmental exposure to metals on human health such as National Health and Nutrition Examination Survey (NHANES), which have included wide ranges of measurements and variables for many diseases, there are very few studies including the measurements and variables for PAH and RV dysfunction. Therefore, large epidemiological cohort with PAH and RV information should be developed in the future. 2) Preclinical studies with animal models for the metal effects on PAH have been started but remain limited. Therefore, animal models with different routes of exposure to, particularly inhalational exposure to various metals including single metal or cluster of metals including both nonessential and essential metals should be specifically investigated to define its roles to induce PAH pathogenesis and consequently RV dysfunction. Therefore, specific chelation of nonessential metal or specific supplementation of certain deficient essential metals may prevent these metal effects on the development of PAH pathogenesis. In summary, essential and nonessential metals might play important roles in PAH pathogenesis and could be utilized for future therapeutic targets.
Acknowledgements
This work was supported in part by the University of Louisville Executive Vice President for Research and Innovation Internal Grant (JH, LC); University of Louisville School of Medicine Basic Grant (JH, LC); National Institute of Environmental Health Sciences (P30ES030283 to JH, LC, MCC; T32ES011564 to DC; and R35ES028373, R01ES032189, P42ES023716, and R21ES031510 to MCC); Gilead Sciences COMMIT COVID-19 RFP Program grant (Gilead IN-US-983-6063 to JH); National Center for Advancing Translational Sciences grant (1U18TR003787-01 to JH); and the National Institute of General Medical Sciences (P20GM113226 to MCC). National Heart, Lung, and Blood Institute (R01HL158779 to JH), National Institute of Allergy and Infectious Diseases (R01AI172873 to JH). Dr. El-Kersh has received institutional research grants from UT and J&J Actelion, has participated in advisory boards for UT and J&J Actelion, and has acted as a consultant for Acceleron and UT. Dr. Cave discloses institutional research grants and/or has received honoraria for speaking/consulting from Intercept, Gilead, and Abbvie.
Footnotes
Availability of data and materials: Available upon request
Competing interests: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement
Data for this review is based published in PubMed, all which are available and were appropriately cited.
References
- 1.Frost A., et al. , Diagnosis of pulmonary hypertension. Eur Respir J, 2019. 53(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert M., et al. , 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J, 2023. 61(1). [DOI] [PubMed] [Google Scholar]
- 3.Anderson JJ and Lau EM, Pulmonary Hypertension Definition, Classification, and Epidemiology in Asia. JACC Asia, 2022. 2(5): p. 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q., et al. , Circular RNAs in pulmonary hypertension: Emerging biological concepts and potential mechanism. Animal Model Exp Med, 2022. 5(1): p. 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Kersh K., et al. , Plasma level of antimony correlates with pulmonary arterial hypertension severity. Curr Res Toxicol, 2022. 3: p. 100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Kersh K., et al. , Metallomics in pulmonary arterial hypertension patients. Pulm Circ, 2023. 13(1): p. e12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang J., et al. , Overview of the cardiovascular effects of environmental metals: New preclinical and clinical insights. Toxicol Appl Pharmacol, 2022. 454: p. 116247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Q., et al. , HIMF (Hypoxia-Induced Mitogenic Factor) Signaling Mediates the HMGB1 (High Mobility Group Box 1)-Dependent Endothelial and Smooth Muscle Cell Crosstalk in Pulmonary Hypertension. Arterioscler Thromb Vasc Biol, 2019. 39(12): p. 2505–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatnagar A., Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res, 2006. 99(7): p. 692–705. [DOI] [PubMed] [Google Scholar]
- 10.Navas-Acien A., et al. , Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation, 2004. 109(25): p. 3196–201. [DOI] [PubMed] [Google Scholar]
- 11.Domingo-Relloso A., et al. , The association of urine metals and metal mixtures with cardiovascular incidence in an adult population from Spain: the Hortega Follow-Up Study. Int J Epidemiol, 2019. 48(6): p. 1839–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhury R., et al. , Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ, 2018. 362: p. k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nigra AE, et al. , Environmental Metals and Cardiovascular Disease in Adults: A Systematic Review Beyond Lead and Cadmium. Curr Environ Health Rep, 2016. 3(4): p. 416–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derchi G., et al. , Prevalence and risk factors for pulmonary arterial hypertension in a large group of beta-thalassemia patients using right heart catheterization: a Webthal study. Circulation, 2014. 129(3): p. 338–45. [DOI] [PubMed] [Google Scholar]
- 15.Wood JC, Pulmonary hypertension in thalassemia: a call to action. Blood, 2022. 139(13): p. 1937–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song J., et al. , Ferrostatin-1 Blunts Right Ventricular Hypertrophy and Dysfunction in Pulmonary Arterial Hypertension by Suppressing the HMOX1/GSH Signaling. J Cardiovasc Transl Res, 2023. [DOI] [PubMed] [Google Scholar]
- 17.Michelakis ED, Spatio-temporal diversity of apoptosis within the vascular wall in pulmonary arterial hypertension: heterogeneous BMP signaling may have therapeutic implications. Circ Res, 2006. 98(2): p. 172–5. [DOI] [PubMed] [Google Scholar]
- 18.Dromparis P, Sutendra G, and Michelakis ED, The role of mitochondria in pulmonary vascular remodeling. J Mol Med (Berl), 2010. 88(10): p. 1003–10. [DOI] [PubMed] [Google Scholar]
- 19.Grunig G., et al. , Perspective: ambient air pollution: inflammatory response and effects on the lung's vasculature. Pulm Circ, 2014. 4(1): p. 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scano G., et al. , Cardiopulmonary adaptation to exercise in coal miners. Arch Environ Health, 1980. 35(6): p. 360–6. [DOI] [PubMed] [Google Scholar]
- 21.Luhadia K., et al. , Type 1 Pulmonary Hypertension and Silicosis in a Bluestone Cutter: A Case Report on Raising Awareness. Cureus, 2023. 15(2): p. e35425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourlier D., et al. , A rare case of sarcoidosis-associated pulmonary hypertension in a patient exposed to silica. Eur Respir Rev, 2016. 25(139): p. 93–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emiroglu Y., et al. , BNP levels in patients with long-term exposure to biomass fuel and its relation to right ventricular function. Pulm Pharmacol Ther, 2010. 23(5): p. 420–4. [DOI] [PubMed] [Google Scholar]
- 24.Leary PJ, et al. , Traffic-related air pollution and the right ventricle. The multi-ethnic study of atherosclerosis. Am J Respir Crit Care Med, 2014. 189(9): p. 1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaisson NF and Hassoun PM, Systemic sclerosis-associated pulmonary arterial hypertension. Chest, 2013. 144(4): p. 1346–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagat DK, et al. , Factors associated with isolated right heart failure in women: a pilot study from western Kenya. Glob Heart, 2014. 9(2): p. 249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frost AE, et al. , Smoking history and pulmonary arterial hypertension: Demographics, onset, and outcomes. J Heart Lung Transplant, 2023. 42(3): p. 377–389. [DOI] [PubMed] [Google Scholar]
- 28.Schiess R., et al. , Tobacco smoke: a risk factor for pulmonary arterial hypertension? A case-control study. Chest, 2010. 138(5): p. 1086–92. [DOI] [PubMed] [Google Scholar]
- 29.Shi H., et al. , Dynamic association of ambient air pollution with incidence and mortality of pulmonary hypertension: A multistate trajectory analysis. Ecotoxicol Environ Saf, 2023. 262: p. 115126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui Z., et al. , Short-term exposure to ambient fine particulate pollution aggravates ventilator-associated pneumonia in pediatric intensive care patients undergoing cardiovascular surgeries. Environ Health, 2023. 22(1): p. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen JJ, et al. , PM2.5 exposure aggravates left heart failure induced pulmonary hypertension. Acta Cardiol, 2019. 74(3): p. 238–244. [DOI] [PubMed] [Google Scholar]
- 32.Batalha JR, et al. , Concentrated ambient air particles induce vasoconstriction of small pulmonary arteries in rats. Environ Health Perspect, 2002. 110(12): p. 1191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Courtois A., et al. , Impairment of NO-dependent relaxation in intralobar pulmonary arteries: comparison of urban particulate matter and manufactured nanoparticles. Environ Health Perspect, 2008. 116(10): p. 1294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomson EM, et al. , Toxicogenomic analysis of susceptibility to inhaled urban particulate matter in mice with chronic lung inflammation. Part Fibre Toxicol, 2009. 6: p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J., et al. , Diesel exhaust inhalation exposure induces pulmonary arterial hypertension in mice. Environ Pollut, 2018. 237: p. 747–755. [DOI] [PubMed] [Google Scholar]
- 36.Yang AM, et al. , Environmental heavy metals and cardiovascular diseases: Status and future direction. Chronic Dis Transl Med, 2020. 6(4): p. 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asker S., et al. , Serum levels of trace minerals and heavy metals in severe COPD patients with and without pulmonary hypertension. Int J Chron Obstruct Pulmon Dis, 2018. 13: p. 1803–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yadav SK, et al. , Occupational lead exposure is an independent modulator of hypertension and poor pulmonary function: A cross-sectional comparative study in lead-acid battery recycling workers. Toxicol Ind Health, 2022. 38(3): p. 139–150. [DOI] [PubMed] [Google Scholar]
- 39.Covre EP, et al. , Low-level lead exposure changes endothelial modulation in rat resistance pulmonary arteries. Vascul Pharmacol, 2016. 85: p. 21–28. [DOI] [PubMed] [Google Scholar]
- 40.Sonnweber T., et al. , Anaemia, iron homeostasis and pulmonary hypertension: a review. Intern Emerg Med, 2020. 15(4): p. 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson JC, et al. , The crossroads of iron with hypoxia and cellular metabolism. Implications in the pathobiology of pulmonary hypertension. Am J Respir Cell Mol Biol, 2014. 51(6): p. 721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu X., et al. , Prevalence of iron deficiency in different subtypes of pulmonary hypertension. Heart Lung, 2018. 47(4): p. 308–313. [DOI] [PubMed] [Google Scholar]
- 43.Smith TG, et al. , Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: two randomized controlled trials. JAMA, 2009. 302(13): p. 1444–50. [DOI] [PubMed] [Google Scholar]
- 44.Howard L., et al. , Supplementation with Iron in Pulmonary Arterial Hypertension. Two Randomized Crossover Trials. Ann Am Thorac Soc, 2021. 18(6): p. 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lakhal-Littleton S., et al. , Intracellular iron deficiency in pulmonary arterial smooth muscle cells induces pulmonary arterial hypertension in mice. Proc Natl Acad Sci U S A, 2019. 116(26): p. 13122–13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cotroneo E., et al. , Iron homeostasis and pulmonary hypertension: iron deficiency leads to pulmonary vascular remodeling in the rat. Circ Res, 2015. 116(10): p. 1680–90. [DOI] [PubMed] [Google Scholar]
- 47.Aessopos A and Farmakis D, Pulmonary hypertension in beta-thalassemia. Ann N Y Acad Sci, 2005. 1054: p. 342–9. [DOI] [PubMed] [Google Scholar]
- 48.Aessopos A., et al. , Thalassemia heart disease: a comparative evaluation of thalassemia major and thalassemia intermedia. Chest, 2005. 127(5): p. 1523–30. [DOI] [PubMed] [Google Scholar]
- 49.Aessopos A., et al. , Cardiac status in well-treated patients with thalassemia major. Eur J Haematol, 2004. 73(5): p. 359–66. [DOI] [PubMed] [Google Scholar]
- 50.Anthi A, Orfanos SE, and Armaganidis A, Pulmonary hypertension in beta thalassaemia. Lancet Respir Med, 2013. 1(6): p. 488–96. [DOI] [PubMed] [Google Scholar]
- 51.Hagar RW, Morris CR, and Vichinsky EP, Pulmonary hypertension in thalassaemia major patients with normal left ventricular systolic function. Br J Haematol, 2006. 133(4): p. 433–5. [DOI] [PubMed] [Google Scholar]
- 52.Naito Y., et al. , Impact of dietary iron restriction on the development of monocrotaline-induced pulmonary vascular remodeling and right ventricular failure in rats. Biochem Biophys Res Commun, 2013. 436(2): p. 145–51. [DOI] [PubMed] [Google Scholar]
- 53.Naito Y., et al. , Iron is associated with the development of hypoxia-induced pulmonary vascular remodeling in mice. Heart Vessels, 2016. 31(12): p. 2074–2079. [DOI] [PubMed] [Google Scholar]
- 54.Bertoli SR, et al. , Chronic iron overload induces vascular dysfunction in resistance pulmonary arteries associated with right ventricular remodeling in rats. Toxicol Lett, 2018. 295: p. 296–306. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed T and Sackner MA, Increased serum copper in primary pulmonary hypertension: a possible pathogenic link? Respiration, 1985. 47(4): p. 243–6. [DOI] [PubMed] [Google Scholar]
- 56.Sun Q., et al. , Selenium and Copper as Biomarkers for Pulmonary Arterial Hypertension in Systemic Sclerosis. Nutrients, 2020. 12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L., et al. , Identification of biomarkers related to copper metabolism in patients with pulmonary arterial hypertension. BMC Pulm Med, 2023. 23(1): p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bogaard HJ, et al. , Copper dependence of angioproliferation in pulmonary arterial hypertension in rats and humans. Am J Respir Cell Mol Biol, 2012. 46(5): p. 582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zimnicka AM, et al. , Upregulated copper transporters in hypoxia-induced pulmonary hypertension. PLoS One, 2014. 9(3): p. e90544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poels EM, et al. , Supplementing exposure to hypoxia with a copper depleted diet does not exacerbate right ventricular remodeling in mice. PLoS One, 2014. 9(4): p. e92983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hauser R., et al. , Airway obstruction in boilermakers exposed to fuel oil ash. A prospective investigation. Am J Respir Crit Care Med, 1995. 152(5 Pt 1): p. 1478–84. [DOI] [PubMed] [Google Scholar]
- 62.Woodin MA, et al. , Acute respiratory symptoms in workers exposed to vanadium-rich fuel-oil ash. Am J Ind Med, 2000. 37(4): p. 353–63. [DOI] [PubMed] [Google Scholar]
- 63.Patel MM, et al. , Ambient metals, elemental carbon, and wheeze and cough in New York City children through 24 months of age. Am J Respir Crit Care Med, 2009. 180(11): p. 1107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bell ML, et al. , Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med, 2009. 179(12): p. 1115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Susic D and Kentera D, Effect of chronic vanadate administration on pulmonary circulation in the rat. Respiration, 1986. 49(1): p. 68–72. [DOI] [PubMed] [Google Scholar]
- 66.Li Z., et al. , Vanadyl sulfate inhibits NO production via threonine phosphorylation of eNOS. Environ Health Perspect, 2004. 112(2): p. 201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang YC and Ghio AJ, Vascular effects of ambient pollutant particles and metals. Curr Vasc Pharmacol, 2006. 4(3): p. 199–203. [DOI] [PubMed] [Google Scholar]
- 68.Baranski B., et al. , Effect of inhalation exposure to cadmium oxide on arterial blood pressure, lipid metabolism and tissue cadmium concentration in rats. Med Pr, 1983. 34(1): p. 11–9. [PubMed] [Google Scholar]
- 69.Prasada Rao PV and Gardner DE, Effects of cadmium inhalation on mitochondrial enzymes in rat tissues. J Toxicol Environ Health, 1986. 17(2-3): p. 191–9. [DOI] [PubMed] [Google Scholar]
- 70.Grose EC, et al. , A comparative study of the effects of inhaled cadmium chloride and cadmium oxide: pulmonary response. J Toxicol Environ Health, 1987. 21(1-2): p. 219–32. [DOI] [PubMed] [Google Scholar]
- 71.Gairola CG and Wagner GJ, Cadmium accumulation in the lung, liver and kidney of mice and rats chronically exposed to cigarette smoke. J Appl Toxicol, 1991. 11(5): p. 355–8. [DOI] [PubMed] [Google Scholar]
- 72.Ganguly K., et al. , Cadmium in tobacco smokers: a neglected link to lung disease? Eur Respir Rev, 2018. 27(147). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chandler JD, et al. , Low-dose oral cadmium increases airway reactivity and lung neuronal gene expression in mice. Physiol Rep, 2016. 4(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xia Y, Choi HK, and Lee K, Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem, 2012. 49: p. 24–40. [DOI] [PubMed] [Google Scholar]
- 75.Aschner M., et al. , The role of hypoxia-inducible factor 1 alpha (HIF-1alpha) modulation in heavy metal toxicity. Arch Toxicol, 2023. 97(5): p. 1299–1318. [DOI] [PubMed] [Google Scholar]
- 76.Pullamsetti SS, et al. , Hypoxia-inducible factor signaling in pulmonary hypertension. J Clin Invest, 2020. 130(11): p. 5638–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu AY, et al. , Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest, 1999. 103(5): p. 691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brusselmans K., et al. , Heterozygous deficiency of hypoxia-inducible factor-2alpha protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest, 2003. 111(10): p. 1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu CJ, et al. , Suppression of HIF2 signalling attenuates the initiation of hypoxia-induced pulmonary hypertension. Eur Respir J, 2019. 54(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abud EM, et al. , Digoxin inhibits development of hypoxic pulmonary hypertension in mice. Proc Natl Acad Sci U S A, 2012. 109(4): p. 1239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang S., et al. , Loss of prolyl hydroxylase domain protein 2 in vascular endothelium increases pericyte coverage and promotes pulmonary arterial remodeling. Oncotarget, 2016. 7(37): p. 58848–58861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kapitsinou PP, et al. , The Endothelial Prolyl-4-Hydroxylase Domain 2/Hypoxia-Inducible Factor 2 Axis Regulates Pulmonary Artery Pressure in Mice. Mol Cell Biol, 2016. 36(10): p. 1584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gale DP, et al. , Autosomal dominant erythrocytosis and pulmonary arterial hypertension associated with an activating HIF2 alpha mutation. Blood, 2008. 112(3): p. 919–21. [DOI] [PubMed] [Google Scholar]
- 84.Duarte TL, Talbot NP, and Drakesmith H, NRF2 and Hypoxia-Inducible Factors: Key Players in the Redox Control of Systemic Iron Homeostasis. Antioxid Redox Signal, 2021. 35(6): p. 433–452. [DOI] [PubMed] [Google Scholar]
- 85.Yuan Y., et al. , Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J Biol Chem, 2003. 278(18): p. 15911–6. [DOI] [PubMed] [Google Scholar]
- 86.Schofield CJ and Ratcliffe PJ, Oxygen sensing by HIFhydroxylases. Nat Rev Mol Cell Biol, 2004. 5(5): p. 343–54. [DOI] [PubMed] [Google Scholar]
- 87.Martin F., et al. , Copper-dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood, 2005. 105(12): p. 4613–9. [DOI] [PubMed] [Google Scholar]
- 88.Qiu L., et al. , Copper is required for cobalt-induced transcriptional activity of hypoxia-inducible factor-1. J Pharmacol Exp Ther, 2012. 342(2): p. 561–7. [DOI] [PubMed] [Google Scholar]
- 89.Wu Z, Zhang W, and Kang YJ, Copper affects the binding of HIF-1alpha to the critical motifs of its target genes. Metallomics, 2019. 11(2): p. 429–438. [DOI] [PubMed] [Google Scholar]
- 90.Xiao Y., et al. , Copper promotion of myocardial regeneration. Exp Biol Med (Maywood), 2020. 245(10): p. 911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim I., et al. , Inhibitory effect of zinc on hypoxic HIF-1 activation in astrocytes. Neuroreport, 2008. 19(10): p. 1063–6. [DOI] [PubMed] [Google Scholar]
- 92.Nardinocchi L., et al. , Zinc downregulates HIF-1alpha and inhibits its activity in tumor cells in vitro and in vivo. PLoS One, 2010. 5(12): p. e15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morand J., et al. , Zinc deficiency promotes endothelin secretion and endothelial cell migration through nuclear hypoxia-inducible factor-1 translocation. Am J Physiol Cell Physiol, 2019. 317(2): p. C270–C276. [DOI] [PubMed] [Google Scholar]
- 94.Arsenault PR, et al. , The Zinc Finger of Prolyl Hydroxylase Domain Protein 2 Is Essential for Efficient Hydroxylation of Hypoxia-Inducible Factor alpha. Mol Cell Biol, 2016. 36(18): p. 2328–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Han J., et al. , Manganese (II) induces chemical hypoxia by inhibiting HIF-prolyl hydroxylase: implication in manganese-induced pulmonary inflammation. Toxicol Appl Pharmacol, 2009. 235(3): p. 261–7. [DOI] [PubMed] [Google Scholar]
- 96.Bredow S., et al. , Subchronic inhalation of soluble manganese induces expression of hypoxia-associated angiogenic genes in adult mouse lungs. Toxicol Appl Pharmacol, 2007. 221(2): p. 148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu L., et al. , HIF-1alpha upregulation exerts the antagonistic effect against angiogenesis inhibition in manganese deficiency-induced tibial dyschondroplasia of broiler chicks. Vet Res Commun, 2022. 46(4): p. 1023–1032. [DOI] [PubMed] [Google Scholar]
- 98.Wang H., et al. , p53 contributes to cardiovascular diseases via mitochondria dysfunction: A new paradigm. Free Radic Biol Med, 2023. 208: p. 846–858. [DOI] [PubMed] [Google Scholar]
- 99.Chan GH, et al. , The role of p53 in the alternation of vascular functions. Front Pharmacol, 2022. 13: p. 981152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Men H., et al. , The regulatory roles of p53 in cardiovascular health and disease. Cell Mol Life Sci, 2021. 78(5): p. 2001–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mizuno S., et al. , p53 Gene deficiency promotes hypoxia-induced pulmonary hypertension and vascular remodeling in mice. Am J Physiol Lung Cell Mol Physiol, 2011. 300(5): p. L753–61. [DOI] [PubMed] [Google Scholar]
- 102.Hsieh MW, et al. , The Potential Application and Promising Role of Targeted Therapy in Pulmonary Arterial Hypertension. Biomedicines, 2022. 10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wakasugi T., et al. , Role of smooth muscle cell p53 in pulmonary arterial hypertension. PLoS One, 2019. 14(2): p. e0212889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu H., et al. , Up-regulation of cullin7 promotes proliferation and migration of pulmonary artery smooth muscle cells in hypoxia-induced pulmonary hypertension. Eur J Pharmacol, 2019. 864: p. 172698. [DOI] [PubMed] [Google Scholar]
- 105.Jacquin S., et al. , Inactivation of p53 Is Sufficient to Induce Development of Pulmonary Hypertension in Rats. PLoS One, 2015. 10(6): p. e0131940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abid S., et al. , P21-dependent protective effects of a carbon monoxide-releasing molecule-3 in pulmonary hypertension. Arterioscler Thromb Vasc Biol, 2014. 34(2): p. 304–12. [DOI] [PubMed] [Google Scholar]
- 107.Perros F., et al. , Smooth Muscle Phenotype in Idiopathic Pulmonary Hypertension: Hyper-Proliferative but not Cancerous. Int J Mol Sci, 2019. 20(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Z., et al. , Divergent changes of p53 in pulmonary arterial endothelial and smooth muscle cells involved in the development of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol, 2019. 316(1): p. L216–L228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mouraret N., et al. , Activation of lung p53 by Nutlin-3a prevents and reverses experimental pulmonary hypertension. Circulation, 2013. 127(16): p. 1664–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zheng Q., et al. , Established pulmonary hypertension in rats was reversed by a combination of a HIF-2alpha antagonist and a p53 agonist. Br J Pharmacol, 2022. 179(5): p. 1065–1081. [DOI] [PubMed] [Google Scholar]
- 111.Puca R., et al. , Restoring p53 active conformation by zinc increases the response of mutant p53 tumor cells to anticancer drugs. Cell Cycle, 2011. 10(10): p. 1679–89. [DOI] [PubMed] [Google Scholar]
- 112.Hainaut P and Mann K, Zinc binding and redox control of p53 structure and function. Antioxid Redox Signal, 2001. 3(4): p. 611–23. [DOI] [PubMed] [Google Scholar]
- 113.Verhaegh GW, et al. , Modulation of p53 protein conformation and DNA-binding activity by intracellular chelation of zinc. Mol Carcinog, 1998. 21(3): p. 205–14. [DOI] [PubMed] [Google Scholar]
- 114.Xue Y, Wang S, and Feng X, Influence of magnesium ion on the binding of p53 DNA-binding domain to DNA-response elements. J Biochem, 2009. 146(1): p. 77–85. [DOI] [PubMed] [Google Scholar]
- 115.Chen Y., et al. , Investigating the Influence of Magnesium Ions on p53-DNA Binding Using Atomic Force Microscopy. Int J Mol Sci, 2017. 18(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meplan C, Mann K, and Hainaut P, Cadmium induces conformational modifications of wild-type p53 and suppresses p53 response to DNA damage in cultured cells. J Biol Chem, 1999. 274(44): p. 31663–70. [DOI] [PubMed] [Google Scholar]
- 117.Kim YJ, et al. , A molecular mechanism of nickel (II): reduction of nucleotide excision repair activity by structural and functional disruption of p53. Carcinogenesis, 2018. 39(9): p. 1157–1164. [DOI] [PubMed] [Google Scholar]
- 118.Vavra J., et al. , Characterization of the interaction between the tumour suppressor p53 and heme and its role in the protein conformational dynamics studied by various spectroscopic techniques and hydrogen/deuterium exchange coupled with mass spectrometry. J Inorg Biochem, 2023. 243: p. 112180. [DOI] [PubMed] [Google Scholar]
- 119.Shen J., et al. , Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulation of p53 localization, stability, and function. Cell Rep, 2014. 7(1): p. 180–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Verhaegh GW, Richard MJ, and Hainaut P, Regulation of p53 by metal ions and by antioxidants: dithiocarbamate down-regulates p53 DNA-binding activity by increasing the intracellular level of copper. Mol Cell Biol, 1997. 17(10): p. 5699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this review is based published in PubMed, all which are available and were appropriately cited.