Abstract
While glycans are among the most abundant macromolecules on the cell with widespread functions, their role in immunity has historically been challenging to study. This is in part due to difficulties assimilating glycan analysis into routine approaches used to interrogate immune cell function. Despite this, recent developments have illuminated fundamental roles for glycans in host immunity. The growing field of glycoimmunology continues to leverage new tools and approaches to uncover the function of glycans and glycan-binding proteins in immunity. Here we utilize clinical vignettes to examine key roles of glycosylation in allergy, inborn errors of immunity, and autoimmunity. We will discuss the diverse functions of glycans as epitopes, modulators of antibody function, and as regulators of immune cell function. Finally, we will highlight immune modulatory therapies which harness the critical role of glycans in the immune system.
Keywords: Glycosylation, Immunomodulation, Antibody glycosylation, PGM3, Immunotherapy
Introduction
Glycosylation is among the most common post-translational modifications in the body; it is estimated that over 50% of proteins are glycosylated.1 Glycosylation refers to the addition of carbohydrate structures (glycans) to asparagine (N-glycans) or serine/threonine residues (O-glycans) of newly synthesized proteins, thereby generating glycoproteins (Figure 1). Glycolipids are formed through the addition of glycans to lipid molecules.2 Collectively these glycoconjugates (glycoproteins and glycolipids) are secreted or embedded in the cell membrane as part of the glycocalyx, a dense layer of glycoproteins, glycolipids, and proteoglycans that cover cells. Glycosylation impacts protein half-life, intrinsic aspects of their activity, and interaction with binding partners.3 In addition, glycans can represent a significant component of glycoprotein weight and, as they extend from the protein surface, can be the most accessible feature to its surroundings. Consequently, glycans influence cell and protein interaction with the environment.4–7 For example, factor H, which facilitates complement inactivation, binds to sialic acids on the cell surface thereby protecting host cells from alternative complement activation,8, 9 emphasizing the role of sialic acids in distinguishing self from non-self.10 Engaging with glycoconjugates are glycan-binding proteins (GBP), also called lectins, that are key to decoding the glycans.9, 10 As such, glycans are critical structural features of glycoconjugates that shape their overall activity.11
Figure 1: Glycans can shape immune cell activity.
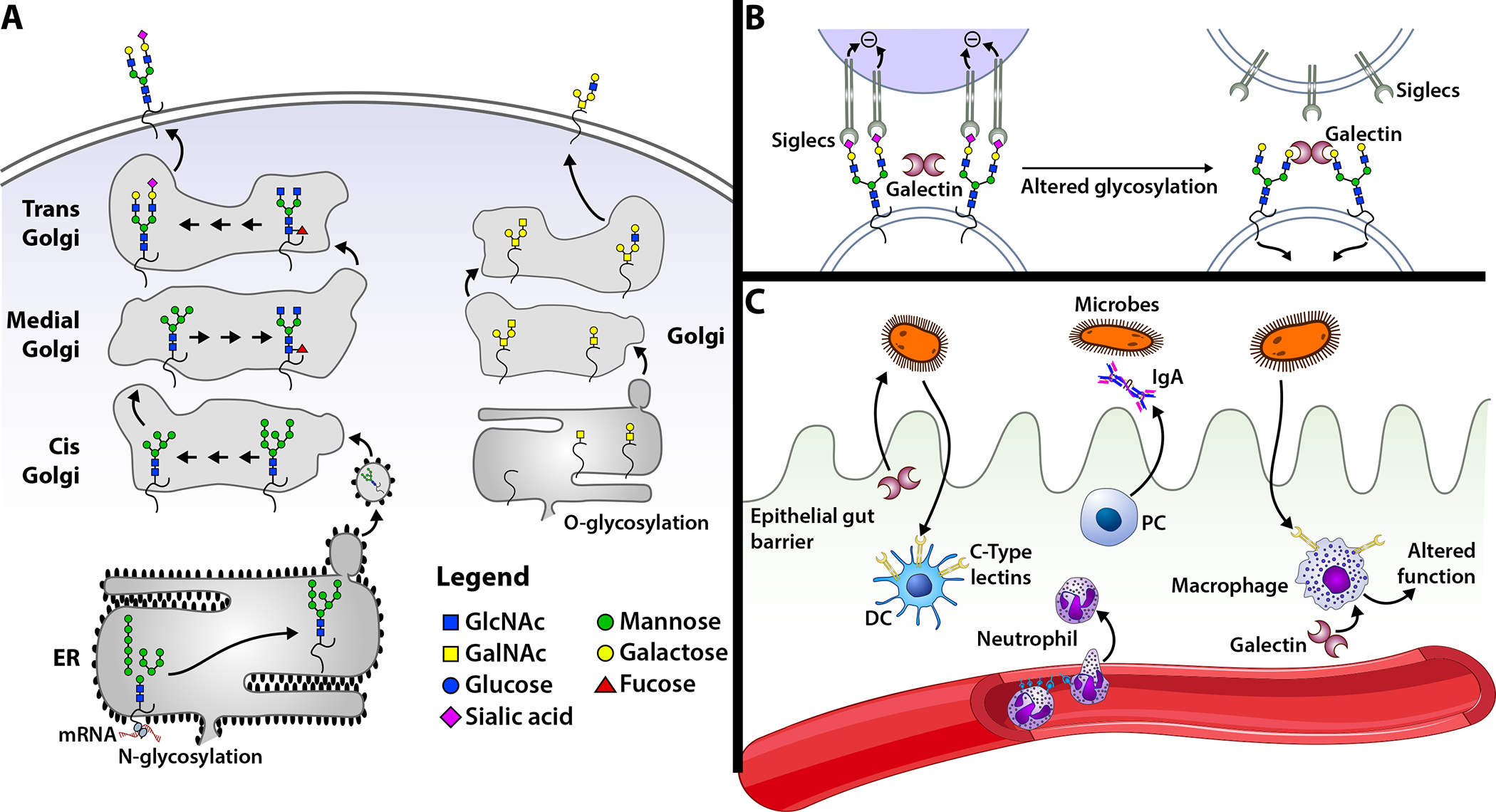
(A) Glycosylation of proteins occurs through the attachment of glycans to asparagine (N glycosylation) or serine/threonine (O glycosylation) residues. N-glycosylation occurs in the endoplasmic reticulum (ER) and Golgi, whereas O-glycosylation occurs in the Golgi. During N-glycosylation, a precursor is attached to the glycoprotein, which is then trimmed and modified by a series of glycosidases and glycosyltransferases in a sequential manner until it reaches the trans-Golgi. From the trans-Golgi, the glycoprotein is transported through secretory vesicles to its destination. In contrast to N glycosylation, O glycosylation is initiated in the Golgi by the stepwise addition of monosaccharides. (B) Changes in cell surface glycosylation can impact the sensitivity of cell interactions with a glycan-binding protein (GBP). A cell that expresses glycans terminating in α2–6 linked sialic acid can interact with the siglec, CD22. In contrast, removal of sialic acid exposes galactose residues that can serve as ligands for galectins. (C) GBP and glycan regulation of host immunity. Dendritic cells and macrophages express C-type lectins that engage microbes, which can facilitate microbe uptake and/or change the behavior of the cell. Galectins can both directly kill microbes and regulate immune cell activity. Selectins facilitate leukocyte recruitment.
Many immune regulators that have been used as CD markers are either glycoconjugates or GBPs. Although these have been used for years to identify or modify unique immune cell populations, including the use of plant-derived GBPs as T cell mitogens long before the T cell receptor was defined,12 the impact of glycans on cell surface glycoconjugates and the role of GBPs in shaping immune function have been difficult to define.7, 13–16 This does not reflect a lack of importance of glycosylation in orchestrating immune function, but is a direct outcome of the non-linear and non-templated nature of glycan synthesis and overall structure. While these very features of glycans likely contribute to their central and dynamic role in many immunological processes, from antibody effector function to chemokine receptor sensitivity ligand engagement,11, 17 these same properties have made them challenging to study.2, 3, 7, 9, 18, 19 However, recent advances in glycan sequencing and synthesis technologies, in addition to novel methods to study GBP-glycan interactions, have accelerated the field of glycosciences in general and the application of glycobiology to allergy and immunology.20–26
In this review, we seek to illustrate the critical and diverse role of glycans in the immune system. Given the diverse role glycans and GBPs play in immune function, we will not attempt to provide a comprehensive review on the subject. Instead, we will provide an overview of glycosylation, followed by vignettes highlighting key features of glycoimmunology in the context of medicine. We will focus on the role of glycans as (1) epitopes, (2) modulators of antibody functions, and (3) regulators of immune cell development, activation, and differentiation. We conclude with a discussion of potential therapeutic applications within glycoimmunology.
Glycan biosynthesis
Unlike the proteins they decorate, the composition of glycans is not directly encoded in the genome but is instead determined by a series of glycosyltransferases and glycosidases, in addition to available donor sugar nucleotides, that are present in a given cell.3, 7 This gives rise to a diverse set of glycans that can rapidly change in response to environmental cues by modulating the expression of glycan-modifying enzymes and the donor sugar nucleotides required for glycan elongation. These dynamic changes in turn affect protein function and alter a cell’s sensitivity to GBPs.3, 16, 27 N-glycan formation is initiated by the addition of a preformed oligosaccharide by the oligosaccharyltransferase (OST) complex that is then modified within the endoplasmic reticulum (ER) and Golgi apparatus (GA). O-glycans are formed stepwise in the GA, while glycolipids are likewise synthesized in a similar stepwise fashion. The final products are trafficked through secretory vesicles to the cell membrane or secreted into extracellular space (Figure 1). Monosaccharide donors are obtained through the metabolism of dietary sugars and from recycling glycans of endocytosed glycoproteins through the lysosome. Unlike secreted and membrane-bound proteins, glycosylation of intracellular proteins consists of a single monosaccharide attached to serine/threonine residues, O linked N-acetylglucosamine (GlcNAc).28–31
Glycan-binding proteins in immune regulation
The first described GBP with a physiological role in the mammalian system was the asialoglycoprotein receptor. Expressed in the liver, it binds galactose and N-acetylgalactosamine (GalNAc) residues exposed due to loss of sialic acid on glycoproteins and clears proteins and platelets from circulation. The asialoglycoprotein receptor belongs to the calcium-dependent (C) – type lectins, one family of lectins with important functions in immunity.32, 33 Together with C-type lectins, sialic acid-binding immunoglobulin-like lectins (siglecs) and galectins are examples of GBPs that modulate both innate and adaptive immunity.9
C-type lectins can act as pattern-recognition receptors that recognize pathogen- and danger-associated molecular patterns, glycans expressed on the cell surface of the pathogen or exposed following cell damage, respectively (Figure 1). Examples include macrophage-inducible C-type lectin (Mincle), Dendritic cell-associated C-type lectin (Dectin)-1, Dectin-2, and DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) which can engage extracellular bacteria and fungi. Upon recognition of their target glycan, C-type lectins facilitate phagocytosis and induction of an innate immune response. Additionally, some of these receptors, such as mannose-binding lectin, are secreted and bind extracellular pathogens and trigger complement. Pathogens can also exploit these receptors to enter the cell, for instance, DC-SIGN can be a critical vehicle for HIV infection.34 C-type lectins can also regulate key immune cell functions, such as leukocyte trafficking, where selectins (E-, P-, and L-selectin) facilitate lymphocyte homing and neutrophil extravasation at sites of inflammation.34, 35
Siglecs are membrane-embedded GBPs expressed by both innate and adaptive immune cells that interact with sialylated cellular targets. Most siglecs contain an immunoreceptor tyrosine-based inhibitory motif (ITIM) conferring suppressive activity (Figure 1). For example, CD22 (Siglec 2) recognizes sialic acid residues on host cells, inhibits B cells from responding to self-antigens by downregulating BCR signaling, and triggers apoptosis of the self-reactive B cells.36–38 Siglecs can likewise regulate T cells, macrophages, eosinophils, neutrophils, and monocytes.39, 40
Galectins are widely expressed in cells and tissues and have diverse functions. Extracellularly, galectins regulate immune cells by engaging polylactosamine (Poly-LacNAc) branches, a common modification that can be found on glycoconjugates.41–44 Intracellularly they regulate immune cells through interaction with cytosolic proteins.45 In addition to regulating host immune function, galectins can directly interact with microbes, which can protect a host through direct antimicrobial activity or can be exploited by pathogens to establish infection.19, 21, 26 Galectins negatively regulate T cell function by inhibiting activation, modulating cytokine expression, and inducing apoptosis in activated T cells.45, 46 Likewise, galectins can regulate neutrophils by inducing their turnover through apoptosis independent processes.43 In immune cell development, galectins additionally can regulate T cell, B cell, dendritic cell, and macrophage maturation.14
Glycan epitopes
Anti-A and -B blood groups
The first anti-glycan antibodies discovered in the human population were directed against A and B blood group antigens on the surface of red blood cells. ABO blood groups were the first polymorphisms described in the human population and likely evolved because of infectious disease pressures that continue to be apparent as recently as COVID-19.47–49 While other alloantibodies can certainly form against a variety of alloantigens following transfusion,50–54 testing of ABO blood group antigens prior to transfusion and transplantation is the most common example of personalized medicine.55–57 When these testing procedures fail, ABO incompatible blood transfusions can lead to rapid intravascular hemolysis, resulting in severe and often fatal transfusion reactions.53, 58 Although conflicting data exist,59, 60 the formation of anti-A and anti-B is thought to reflect exposure to glycans decorating gut microbiota.55 However, anti-carbohydrate antibodies are certainly not limited to anti-A and anti-B. Anti-glycan antibodies can form against a wide variety of glycan-based blood group antigens and related structures. Unlike many RBC-induced alloantibodies, which tend to predominantly be immunoglobulin G (IgG),50–53, 61, 62 most anti-A and anti-B antibodies are IgM but can likewise be IgG with a small proportion of IgA antibodies.50–52
Clinical allergy testing and glycan epitopes
While most anti-glycan antibodies are thought to be predominately IgM and form in the absence of CD4+ T cell help, glycan-specific antibodies have been discovered for all classes of antibodies, including IgE.63 The discovery of glycan-specific IgE antibodies is intrinsically linked to the development and widespread use of radioallergosorbent allergy testing. In 1967, Wide et al. reported a new in-vitro method, the radioallergosorbent test, to detect allergen-specific antibodies of a new immunoglobulin class,IgE.64 As this testing became widely available, multiple groups noted cross-reactivity between different types of food, grass pollen, birch, papain, and bromelain. In 1981, Aalberse et al. studied several individuals with cross-reactive serum, concluding that the IgE antibodies were likely binding to carbohydrate epitopes found in numerous foods, in particular plants, and insect venoms.65 These cross-reactive epitopes (later termed cross-reactive carbohydrate determinants or CCDs) were subsequently confirmed to be distinct carbohydrate chains (glycans) found in insect venoms, parasites, and food.66–68 Unlike the clinical relevance of anti-ABO (H) antibodies, numerous studies suggested that, despite their high prevalence, these anti-glycan IgE antibodies had little, if any, clinical relevance. Most patients with anti-plant glycan IgE antibodies do not report corresponding symptoms of food allergy.65, 66, 69 Patients with anti-plant glycan antibodies were evaluated by skin prick testing, in-vitro basophil histamine release assay, and double-blinded placebo-controlled oral challenges. In the subset of patients who underwent skin testing and/or oral challenge, there was no evidence of allergy with negative skin testing and no allergic symptoms with oral challenge. In an in vitro basophil histamine release assay, the majority of anti-glycan IgE antibodies from these patients’ serum induced histamine release but at concentrations 5–6 times the positive control used, major grass pollen allergen rPhl p 5.66, 70 Therefore, the initial focus on anti-glycan IgE antibodies was mitigating their potential for causing false positive allergy testing.71–73
Alpha-gal and Cetuximab
In contrast to these earlier studies, Chung et al. reported that IgE anti-galactose α1,3 galactose (α-Gal) antibodies could trigger anaphylaxis.74 This study stemmed from the observation of increased rate of severe hypersensitivity reactions to Cetuximab, a chimeric mouse-human IgG1 monoclonal antibody used in colorectal and squamous cell cancers, in North Carolina, Arkansas, Missouri, Virginia and Tennessee.75, 76 One study found a 22% rate of severe hypersensitivity reactions in Tennessee and North Carolina, in contrast to a rate of <1% in the Northeast.77
Chung et al. hypothesized that the increased rate of sever hypersensitivity reactions was due to preexisting IgE anti-Cetuximab antibodies. Their study confirmed this hypothesis and identified the epitope as a glycan, α-Gal. First described by Karl Landsteiner as the “B-like antigen”, α-Gal is a terminal glycan modification expressed in all mammals, except old world monkeys and humans, and has structural similarities to the blood group B antigen.78, 79 Cetuximab has an α-Gal moiety on each of its fragment antigen-binding regions (Fab region). The structure and number of α-Gal molecules on cetuximab results in increased avidity, allowing for cross-linking of IgE and subsequent severe allergic symptoms.80, 81
Given that the glycosylation of proteins is determined by the cell line expressing it, Chung et al. expressed Cetuximab in a cell line lacking α-1,3-galactosyltransferase. The patients’ anti-Cetuximab antibodies no longer bound to it, confirming that the epitope for Cetuximab is α-Gal. They also found that anti-cetuximab IgE antibodies bound to cat, dog, and beef proteins, all of which are non-primate mammalian proteins.74 Based on these findings, Commins et al. hypothesized that IgE antibodies binding to α-Gal could cause food allergy to red meat. Indeed, they found specific IgE antibodies against α-Gal in patients who developed urticaria, angioedema, and/or anaphylaxis most commonly 3–6 hours after eating red meat. This IgE-mediated reaction was not typical of anaphylaxis due to the delayed timing of the reaction and the epitope, which was a glycan rather than a protein.82–84 Commins et al. subsequently demonstrated that these patients were sensitized to α-Gal after multiple bites from the Lone Star tick, which has a high concentration of α-Gal in its saliva. Lone star ticks are most prevalent in the southeastern United States, likely explaining the increased prevalence of hypersensitivity to Cetuximab in that region.81, 85 Alpha-Gal syndrome thus highlights how the environment interplays with the body’s response to glycoconjugates (Figure 2). Anti-α-Gal antibodies not only can contribute to drug and red meat allergies, but recent studies have shown that they can also facilitate IgG anti-drug antibody development and may be responsible for the premature failure of porcine-derived heart valve replacement.86,87
Figure 2: Glycans as epitopes, glycan regulation of antibody activity, and the impact of glycosylation on immune cell function.
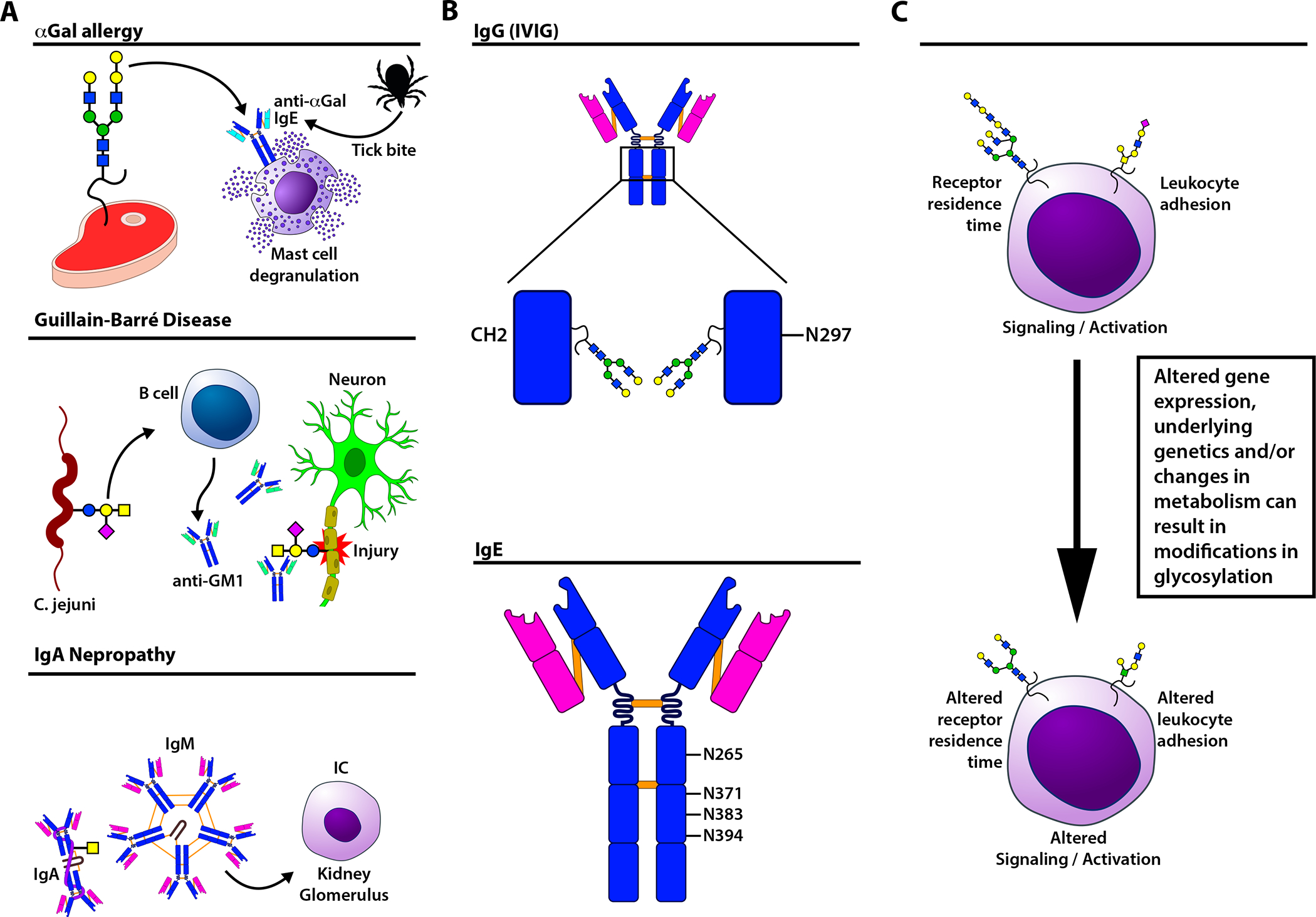
(A) Upper panel: In α-galactose (Gal) allergy, an IgE anti-α-Gal antibody response is triggered by a tick bite. Subsequently, IgE-mediated activation of mast cells triggers an allergic response to α-Gal epitopes found in dietary meat. Middle panel: In Guillain-Barre Syndrome, anti-carbohydrate antibodies form following exposure to Campylobacter jejuni, which expresses surface glycans similar to the ganglioside GM1 found on neurons. This results in cross-reactivity with neuronal GM1, causing neuronal injury. Lower panel: In IgA nephropathy, increases in total IgA result in a corresponding increase in Tn-bearing IgA. The latter are bound by naturally occurring anti-Tn IgM antibodies which form immune complexes that can deposit in glomeruli, causing kidney injury. (B) IgG antibodies possess an asparagine (N) 297 glycan in the Fc domain that can regulate Fc gamma receptor engagement and complement activation. IgE can also be glycosylated on N265, N371, N383, and N394. (C) Changes in glycosylation on the proteins and lipids expressed on the cell surface can impact a variety of fundamental cellular activities. These include receptor residence time, receptor sensitivity to ligand-induced signaling, cellular activation states, and leukocyte trafficking.
Unanswered questions regarding glycan epitopes in allergy
The sharp contrast in immunogenicity of α-Gal compared to most IgE glycan epitopes raises several questions. First, are there other glycan epitopes that lead to clinically significant allergic symptoms? There is evidence for glycans acting as clinically significant epitopes, e.g. peanut allergy88 and insect venom allergy.66 Glycan modifications can also act as adjuvants, increasing the immunogenicity of diverse antigens including dust, 89 peanut, 90 microbes,91 and therapeutic recombinant factor VIII in hemophilia patients.87 This is important in the context of using recombinant proteins for allergy testing. Depending on the cell line used to express a recombinant allergen, its glycosylation may differ from the natural allergen.92 Therefore, glycan epitopes can both be the cause of false positive and, more rarely, false negative allergy testing.66, 72, 93 Antibodies can also form against N-glycolylneuraminic acid (Neu5Gc), another glycan present in red meat. In contrast to α-Gal, although Neu5Gc is not synthesized by human cells, if introduced through oral intake it can become incorporated into human cell glycoconjugates. These anti-Neu5Gc antibodies may contribute to chronic inflammation and cancer.94 Emerging data suggests a role for Neu5GC in allergy, but future studies are certainly needed.95
Secondly, how are anti-glycan IgE antibodies formed? For most anti-glycan IgE’s specific for CCD’s the route of sensitization is thought to be inhalant allergens, or insect stings.66, 96, 97 The exact mechanism for anti-α-Gal IgE antibody formation remains a debated question. While additional studies are needed, early studies suggest that the microbiota influence anti-α-Gal IgG antibody formation, similar to their potential role in anti-ABO antibody development. 26, 63, 98, 99 It may be that the Lone Star tick bite triggers class switching of IgG to IgE anti-α-Gal antibodies although the mechanism for this remains poorly understood.80, 100 Alternatively, repeated Lone Star tick bites may lead to anti-alpha gal IgE antibodies, IgE sensitization, which subsequently can trigger allergic reactions to red meat or cetuximab.81, 85, 101–104 Third, why do some glycans induce severe allergic responses while the majority do not? There are several potential explanations for this, none of which have been fully tested. One compelling argument is that the ability to cross-link IgE determines the potential pathogenicity of a glycan epitope. This means that the number of glycan binding sites on a protein as well as their steric location determines if a glycan epitope can cause IgE cross-linking and subsequent histamine release.74, 105 In this model, if glycans bind with lower affinity to IgE or the density of the glycan epitope is suboptimal, cross-linking may not occur. However, even with lower affinity IgE interactions, optimal glycan epitope presentation may allow for sufficient avidity to drive histamine release.66 Of note, although most CCDs are thought to have low affinity for IgE, multiple studies have suggested this is not always the case.106, 107 Clinically, anti-glycan epitopes remain a diagnostic challenge.66, 68, 71–73, 108
Glycan epitopes and autoimmunity
IgA nephropathy
In addition to serving as targets in allergies, glycan modifications can also be the primary epitopes in a variety of autoimmune diseases. IgA nephropathy, first described in 1968, is a clinical diagnosis based on IgA and IgG deposits in the glomeruli.109, 110 Anti-glycan antibodies against the hypoglycosylated Tn antigen, a simple monosaccharide O-glycan modification within the hinge region of IgA, can result in immune complex formation that leads to antibody deposition in glomeruli, resulting in renal pathology.111 The level of anti-Tn antibodies correlates with disease severity and predicts relapse post kidney transplant. Pathogenesis is thought to follow a two-step process. First, elevations in total IgA increase the relative abundance of hypoglycosylated IgA1, resulting in higher exposure to cryptic Tn antigen GalNAc residues. Second, anti-Tn autoantibodies, which are mainly IgM, cause immune complex formation that can deposit in glomeruli leading to kidney damage (Figure 2). These immune complexes can trigger complement activation, which may contribute to the underlying pathophysiology.
It is unclear how hypoglycosylated IgA antibodies are formed. IgA is galactosylated by core 1β1,3-galactosyltransferases (C1GalT1) with the help of a chaperone, Cosmc.112 Early hypotheses suggested Tn formation on IgA was a product of reduced Cosmc expression. More recent data suggests that the IgA1 hinge region is less accessible to C1GalT1. Consistent with this, while total IgA is increased in patients with IgA nephropathy, the percentage of Tn+ IgA does not change.113 Environmental triggers which lead to acute increases in IgA1 production coupled with the presence of naturally occurring anti-Tn antibodies may ultimately converge to drive anti-Tn-IgA1 antibody immune complex formation and associated pathology.
Molecular mimicry and Guillain-Barre syndrome
One of the classic examples of the breakdown of identifying self vs. non-self toward carbohydrate antigens is Guillain-Barre syndrome (GBS), the most common cause of pediatric acute flaccid paralysis. Like the formation of anti-A and anti-B blood group antibodies, microbial molecular mimicry is thought to be the root of the pathogenesis of GBS. The majority of patients with GBS have an antecedent microbial infection, most commonly Campylobacter jejuni. It is thought that lipo-oligosaccharides and lipopolysaccharides found on the cell membrane of C. jejuni mimic the structure of GM1 gangliosides, a type of glycolipid found on the cell surface of neural axons. Anti-GM1 glycolipid antibodies that form in response to C. jejuni can engage neurons, leading to neuronal damage and severe life-threatening peripheral neuropathy (Figure 2)114. Intravenous immunoglobulin (IVIG), as well as plasmapheresis, are used to treat GBS.115
Glycans modify protein (immunoglobulin) function
IVIG
Glycans not only function as epitopes, but they also play a critical role in modulating the function of immunoglobulins. Although all immunoglobulins are glycosylated, this has been best studied for IgG. IgG antibodies are N-glycosylated on their Fc portion with a biantennary glycan modifying each of the two heavy chains at asparagine 297 (ASN297) (Figure 2), although Fab fragments can be variably glycosylated as noted above for Cetuximab. The immune modulatory role of immunoglobulin glycosylation was first identified for IVIG.116 First given to patients with deficiencies in humoral immunity, including X-linked agammaglobulinemia and common variable immunodeficiency, IVIG was originally purified as a therapeutic strategy to restore antibody levels.117 Two children with congenital agammaglobulinemia and severe thrombocytopenia treated with IVIG had resolution of thrombocytopenia. This led to the hypothesis that IVIG alone may enhance platelet counts in patients with immune thrombocytopenia (ITP). Treatment of 13 patients with ITP with IVIG lead to significant increases in platelet counts,118 providing some of the earliest data suggesting that IVIG could be used to treat autoimmunity. However, the features of IVIG responsible for favorably manipulating autoimmunity were unknown.
Using a murine model of ITP to investigate the anti-inflammatory mechanism of IVIG, the Fc portion of IgG was found to be sufficient for its anti-inflammatory activity. Additional studies demonstrated that Fc region sialyation, which is a terminal glycan modification, is a key mediator of its anti-inflammatory properties.116, 119 Although the anti-inflammatory mechanisms of IVIG remain an active debate, in animal models of arthritis and ITP, hyper-sialylated IVIG had enhanced anti-inflammatory activity.120 In addition, using multiple autoimmune models, Pagan et al. converted endogenous IgG to anti-inflammatory IgG through sialylation.121 It is now well-established that N-glycan modification of the Fc portion of immunoglobulins impacts the conformation, stability, binding affinity, and effector functions of IgG antibodies.122, 123 However, the impact of distinct glycoforms on individual IgG isotypes remains incompletely defined. Recent findings also demonstrate that Fc receptor glycosylation itself not only can impact IgG engagement, but that the glycosylation of recombinant forms of Fc receptors commonly used to study IgG-Fc receptor interactions may not always faithfully recapitulate native glycosylation states.124 As a result, additional studies are needed to fully define the complex regulation of IgG and Fc receptor glycosylation on the outcomes of IgG interactions.
IgE glycosylation
Although IgE has the most glycosylation sites of the immunoglobulins (Figure 2), less is known about its glycosylation. To investigate the clinical relevance of IgE glycosylation, Shade et al. studied the glycosylation content of IgE molecules in a cohort of individuals with and without atopy. They found that those with peanut allergy had increased sialylation of IgE, while those without atopic disease had increased bisecting GlcNAc and terminal galactose, exposed when sialic acid is absent. In addition, galactose and sialic acid content of total IgE were predictive for allergic disease. Using passive immunization in mice, removal of sialic acid from IgE prior to sensitization weakened the allergic response. Removal of the sialic acid did not impact binding to the allergen, but instead negatively modulated signaling through the FcεRI receptor. Ultimately, using an enzyme that cleaves sialic acid (sialidase), removal of sialic acid from IgE-bearing cells in-vivo attenuated anaphylaxis, suggesting a potential therapeutic strategy of glycoengineering immunoglobulins as a treatment for atopic disease.125
Congenital disorders of glycosylation (CDG)
Glycans can also regulate fundamental aspects of cellular function. However, as changes in cellular glycosylation can impact many different cellular processes, the consequences of glycan changes are highly pleiotropic and therefore much more complex than those observed for an individual class of proteins, such as immunoglobulins (Figure 2).92, 126 CDGs are a rare group of monogenic disorders caused by pathogenic variants in pathways important for glycan biosynthesis. In 2020, Pascoal et al reviewed immune involvement in CDGs. Of the 133 CDGs described at that time, 23 had an immune phenotype including immunodeficiency, autoinflammation, or autoimmunity. The immunodeficient phenotype ranges from mild, susceptibility to specific pathogens, to severe, severe combined immunodeficiency (SCID). There is a wide spectrum of autoimmune and autoinflammatory phenotypes including inflammatory bowel disease (IBD), celiac disease, recurrent fever, and aphthous ulcer.127
Leukocyte Adhesion Deficiency (LAD) type II
LAD type II is a well-described CDG with a severe and distinct immune phenotype. Two unrelated boys from consanguineous families presented with short stature, intellectual disability, recurrent bacterial infections without pus, distinct facial appearance, elevated neutrophil count, and the Bombay (hh) blood type. Their recurrent bacterial infections without pus formation and elevated neutrophil count were reminiscent of a recently described disorder termed leukocyte adhesion deficiency (LAD). In LAD, the integrin CD18 is not expressed leading to defective neutrophil adhesion. Neutrophil studies of the two boys demonstrated intact CD18. The researchers hypothesized it might be due to a defect in glycosylation because of the presence of the Bombay blood type. The Bombay blood type is characterized by a lack of the H antigen due to a defect in fucosylation critical for ABO blood group formation. Ultimately, a lack of sialyl lewis-X, a fucosylated glycan and ligand for selectins, was found on the boys’ neutrophils leading to a neutrophil trafficking defect. 128, 129
As discussed before, leukocyte extravasation is mediated by selectins. Selectins are membrane-bound proteins; L-selectin resides on leukocytes, whereas E-selectin and P-selectin are expressed on activated vascular endothelial cells. Selectins mediate rolling of leukocytes along vascular endothelial, which is followed by integrin-mediated firm adhesion and subsequent extravasation.35, 130 LAD type II illustrates that sialyl lewis-X is required for neutrophil adhesion to endothelial cells through binding to selectins. Deficiency of sialyl lewis-X therefore leads to a severe defect in neutrophil trafficking.
X-linked immunodeficiency with magnesium defect, EBV infection, and Neoplasia (XMEN)
In 2011, XMEN, a new inborn error of immunity, was described. XMEN is caused by a hemizygous loss-of-function mutation in magnesium transporter 1 (MAGT1). Patients can present with combined immunodeficiency marked by persistent EBV infection, EBV-associated B cell malignancies, and lymphoproliferation. Immune phenotyping demonstrated an inverted CD4/CD8 ratio, elevated B cell counts, and decreased surface expression of NKG2D (natural killer group 2) on NK cells and CD8 T cells.131 Initially, these findings were attributed to a defect in magnesium transport. Subsequently, a larger cohort of patients was described with a broader range of phenotypes including lymphadenopathy, cytopenias, liver disease, cavum septum pellucidum, and increased CD3+CD4−CD8−TCRαβ+ (double negative) T cells.132 Investigators hypothesized that a magnesium defect was not sufficient to fully explain this complex phenotype. As MAGT1 is also known to be a subunit of the OST complex that initiates N-glycosylation, the complex phenotype might be due to abnormal N-glycosylation. Indeed, loss of MAGT1 leads to hypoglycosylation of multiple immune proteins. Most notably, decreased glycan expression leads to reduced cell surface retention of NKG2D, which results in decreased cytotoxic effector function of NK and CD8+ T cells. 133–135
Phosphoglucomutase 3 (PGM3) deficiency
Another example of a CDG with a dominant immune phenotype is PGM3 deficiency. In 2014, Sassi et al. reported 9 individuals from 4 consanguineous families with Hyper-IgE syndrome (HIES) and biallelic hypomorphic variants in PGM3. All individuals in the study had markedly increased serum IgE levels, recurrent skin abscesses, and pneumonia with no variants identified in genes associated with HIES. Functional testing demonstrated defective catalytic activity of PGM3 with reduced production of complex branched N-glycans.136 Stray-Pedersen et al. subsequently reported three unrelated patients with biallelic variants in PGM3 deficiency with T−B−NK+ SCID, congenital neutropenia with progression to bone marrow failure, two of the three patients having skeletal dysplasia. Only one patient had elevated serum IgE levels. Two of the patients underwent curative hematopoietic stem cell transplantation (HSCT). The third most severely affected patient died as an infant from severe infection. Functional testing again demonstrated reduced enzymatic activity of PGM3, with one variant completely inactivating the enzyme.137 To date, four patients with PGM3 deficiency have received HSCT, three of which with good outcomes. The fourth had severe neurological impairment and multiple post-HSCT complications, dying from infection 8 months post-HSCT. There is some evidence that residual PGM3 activity correlates with severity of presentation but why some patients develop SCID while others present with HIES remains incompletely understood. 136–138
Diminished levels of PGM3 disrupt the hexosamine pathway of glycolysis, which is important for generating a substrate, uridine diphosphate (UDP)–GlcNAc, needed for protein glycosylation, which leads reduced N- and O-glycans on immune proteins.139 Patients with PGM3 deficiency have reduced numbers of specific N- and O-glycans that are dependent on UDP-GlcNAc. Specifically, neutrophils and B cells in these patients have reduced branched N-glycans, glycan moieties that are important in immune regulation.136 Such changes likely influence the surface resident time of key glycoprotein receptors and sensitivity to GBPs, culminating in immune dysregulation.
Polygenic/complex trait diseases and N-glycan branching
Ulcerative Colitis (UC)
Defective N-glycan branching has also been implicated in polygenic diseases such as ulcerative colitis (UC) and multiple sclerosis (MS). T cells from patients with active UC carry less branched N-glycans compared to those with well-controlled disease. Supplementing patient T cells in vitro with GlcNAc restored N-glycan branching and inhibited activation and proliferation of T cells.140, 141 In patients with severe UC, the level of branched N-glycans was predictive of response to standard therapy, defined as 5-aminosalicylate, corticosteroids, and immunosuppressants. When looking at the disease course on a 5-year timespan 80% of patients with high levels of branched N-glycans had a good response to standard therapy over the course of 5 years. In contrast, of patients with low levels of branched N-glycans, 88% of patients needed to divert from standard therapy and receive anti-TNF therapy. In a separate study, T cells isolated from colon biopsies from patients with UC had lower expression of glycosyltransferase Mgat5 and fewer N-glycans compared to healthy controls.142 Finally, single nucleotide polymorphisms (SNPs) in Mgat5 were associated with reduced expression of Mgat5 in T cells of UC patients. These SNPs correlated with disease severity and the need for biologics.143 Although these research studies are promising, the use of branched N-glycans as biomarkers has never been tested in a clinical trial.144
Multiple Sclerosis (MS)
In MS patients, SNPs in Mgat5 have also been associated with MS disease severity; SNPs that correlated with reduced branched N-glycans were enriched in patients with the most severe disease.145 PL/J mice develop spontaneous autoimmunity that is similar to chronic MS. Grigorian et al. showed that in these mice development of disease was suppressed following adoptive transfer of T cells fed in vitro with GlcNAc, which raised levels of UDP-GlcNAc and increased N-glycan branching by Mgat5.146 Oral treatment with GlcNAc increased branched N-glycans and was able to suppress EAE progression in mice.147 In the same mouse model of MS, N-glycan branching was eliminated through KO of glycosyltransferases Mgat1 or Mgat2 in B cells, eliminating or truncating branched N-glycans respectively. These mice had increased demyelination driven by Th1 and Th17 cells that were activated by autoreactive B cells.148 Deficient N-glycan branching is likely an important risk factor for these polygenic diseases.
Glycosylation in cancer
Abnormal glycosylation is a hallmark of cancer.144 Changes in glycosylation can result in the expression of non-self-glycans, termed tumor-associated carbohydrate antigens (TACAs). TACAs can intrinsically enhance neoplastic transformation.149 These modifications can also engage immune modulatory receptors on immune cells, possibly impeding anticancer immune responses and create immune suppressive environments.150 Localized changes in the expression of GBPs, particularly galectins, can likewise serve to modulate the tumor microenvironment.151 The expression of these neoantigens is often restricted to the tumor cells, and may therefore also be specifically targeted without harming healthy cells.150, 152 In addition to triggering direct cytotoxicity, anti-TACA therapies may improve anti-cancer immune responses by alleviating immune suppression.150, 152
Therapeutic applications of glycobiology
Dinutuximab
The first FDA approved immunotherapy targeting a glycan epitope, Dinutuximab, was also the first immunotherapy to be approved for pediatric cancer. It is a chimeric monoclonal antibody against glycolipid ganglioside G2 (GD2). GD2 expression is mainly restricted to cancer cells with the exception of the central nervous system (CNS). GD2 is expressed on the surface of neuroectodermal tumors, such as neuroblastomas, gliomas, melanomas, sarcomas, as well as triple-negative breast cancer cells. Upon binding GD2 on neuroblastoma cells, Dinutuximab induces potent antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).153, 154 The relatively restricted expression of GD2 in healthy cells allows anti-GD2 monoclonals to more effectively target cancer cells, although there are significant side effects due to binding of the antibody to normal CNS cells.155 The underlying features of anti-GD2 therapy that facilitate effective treatment outcomes remains a matter of active study.155–157
Sweet CAR T cells
In 2016, Posey et al. reported a glycan-binding chimeric antigen receptor (CAR) T cell targeting a widely expressed tumor-specific epitope, GalNAxa1-O-Ser/Thr (Tn) - Mucin1 (Tn-MUC1). In vitro these CAR T cells had no reactivity against healthy human cells, but effectively induced tumor cell lysis in vitro and survival in murine models of T cell leukemia and disseminated pancreatic cancer. These CAR T cells can target multiple types of cancer which express Tn-MUC1. This method is being harnessed in phase 1 clinical trials for several solid tumors.158, 159 The unique expression of the glycan Tn antigen in cancer cells is particularly promising, given that it is highly expressed in the majority of carcinomas, and is rarely expressed in normal tissue.160, 161 It is a good potential biomarker given that it correlates with poor prognosis in a number of cancer types.161 While therapeutics targeting Tn have been hindered by a historical lack of high affinity specific antibodies against Tn, newly developed anti-Tn antibodies are promising.162, 163
Glycoengineering of antibodies: Benralizumab
Glycans, in addition to serving as targets for therapeutics, can also be modified to optimize the function of the therapeutics themselves. As previously discussed, N-glycan modifications of the Fc portion of IgGs are critical for their confirmation, binding affinity, and effector function.122 This has led to glycoengineering of monoclonal antibodies by changing the Fc N-glycan to optimize their function. The most successful example of this to date has been the removal of core fucose on a monoclonal antibody (afucosylation) that increased ADCC activity.164 In most cases, the N-glycan modification impacts conformation stability leading to differential affinity and binding to Fc receptors. In these cases, the N-glycan does not directly interact with the Fc receptor. In contrast, for both naturally occurring and glycoengineered antibodies, afucosylated antibodies bind with higher affinity to FcγRIIIa and FcγRIIIb through direct carbohydrate-carbohydrate interaction.122, 165
Reduced or afucosylated anti-CD20 monoclonal antibodies are one of the best examples of deliberate monoclonal glycoengineering improving therapeutic potential.166 These glycoengineered therapeutics show increased efficacy with improved B cell depletion and tumor growth inhibition in B cell malignancies.166 Benralizumab is an afucosylated humanized anti-IL-5a antibody that triggers ADCC-mediated lysis of eosinophils and blocks IL-5R signaling. Clinically, it reduces asthma exacerbations in patients with severe eosinophilic asthma and is currently being evaluated in clinical trials against eosinophilic chronic rhinosinusitis, chronic obstructive pulmonary disease, and chronic idiopathic urticaria. 167
Modulating cell behavior with glycan-binding proteins (Siglecs)
Changes in the glycans expressed on the cell surface can alter the sensitivity to glycan-binding proteins such as galectins and siglecs. In turn, these GBPs can modulate immune cell signaling. For example, therapeutics have been developed to recruit siglecs to immune-activating receptors and inhibit downstream cell signaling. Islam et al. used anti-IgD antibodies linked to a high-affinity siglec ligand (anti-IgD-CD22L) to inhibit B cell signaling in-vitro. In their in-vitro model, calcium flux was used as a measure of B cell activation. They showed inhibition of calcium flux when stimulating murine B cells with anti-IgD-CD22L. By repeating the experiment using B cells from a CD22 knockout mouse they demonstrated the specificity of this inhibition, with anti-IgD-CD22L now inducing robust calcium flux.168 To further leverage siglec biology to treat allergic disease, the ability of anti-human anti-IgE antibodies bound to a high-affinity siglec ligand (anti-IgE-CD33L) was tested in an in vivo anaphylaxis model. In both the passive cutaneous anaphylaxis model and a passive systemic anaphylaxis model in “humanized” mice, anti-IgE-CD33L inhibited mast cell degranulation and prevented degranulation when mice were re-challenged with anti-IgE antibodies. Numerous studies have also demonstrated therapeutic potential for galectins in a broad range of diseases, including infection, autoimmunity, and even cancer. We anticipate that these strategies can be used to modulate cell signaling in many other disorders of allergy and immunology.168, 169.
Targeting protein-glycan interactions using monoclonal antibodies has been proven effective for other diseases as well. Crizanlizumab, a P-selectin inhibitor, was one of the first FDA-approved drugs for the treatment of vaso-occlusive crisis in patients with sickle cell disease. Vaso-occlusive crises occur when sickled red cells adhere to the endothelium and block blood flow in the vessels of patients with sickle cell disease. This in turn triggers inflammation, mainly driven by neutrophils. Blocking P-selectin interaction with neutrophils using Crizanlizumab successfully reduced the rate of pain episodes in patients with sickle cell disease.170
Conclusion
The examples outlined above are just a few examples of glycans’ diverse roles in the immune system. As noted earlier, many questions remain. The overall mechanisms responsible for the formation of anti-glycan antibodies, including exposures and immune regulators that govern class switching to IgE, remain relatively unexplored. Similarly, while the impact of glycosylation on IgG function has been extensively examined, the factors that regulate alterations in immunoglobulin glycosylation, in addition to the role of glycans in regulating IgE function is only beginning to emerge. Given the ability of glycosylation to impact numerous cellular functions, including cellular engagement by entire families of GBPs, immune regulation by cell surface glycosylation remains a formidable, yet exciting frontier. With new technologies becoming readily available, the application of glycosciences to understanding fundamental features of immune function holds significant promise in providing new therapies and understanding fundamental features of allergy and immunology.
Funding
National Institutes of Health (5T32AI007512-37 to RH; R01 HL165975 and R01 HL138714 to SRS)
Abbreviations:
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- α-Gal
alpha-galactose
- C1GalT1
core 1β1,3-galactosyltransferases
- C3
Complement factor 3
- C-type lectin
Calcium-dependent type lectin
- CAR T
Chimeric Antigen Receptor T cell
- CCD
cross-reactive carbohydrate determinants
- CD
Cluster of Differentiation
- CD3+CD4−CD8−TCRαβ+
Double Negative
- CDC
Complement Dependent Cytotoxicity
- CDG
Congenital Disorders of Glycosylation
- CNS
Central Nervous System
- DC-SIGN
DC-specific intercellular adhesion molecule-3-grabbing non integrin
- Dectin
Dendritic cell associated C-type lectin
- EAE
Experimental Autoimmune Encephalomyelitis
- EBV
Epstein-Barr Virus
- ER
Endoplasmic Reticulum
- Fab region
Fragment antigen-binding region
- GA
Golgi Apparatus
- GalNAc
N-Acetylgalactosamine
- GBS
Guillain-Barre Syndrome
- GBP
Glycan-Binding Protein
- GD2
Glycolipid ganglioside 2
- GlcNAc
N-Acetylglucosamine
- GM1
Monosialotetrahexosylganglioside
- HIES
Hyper-IgE Syndrome
- HSCT
Hematopoietic Stem Cell Transplantation
- HSP
Henoch-Schönlein Purpura
- IBD
Inflammatory Bowel Disease
- Ig
Immunoglobulin
- ITIM
Immunoreceptor Tyrosine-based Inhibitory Motif
- ITP
Immune Thrombocytopenia
- IVIG
Intravenous Immunoglobulin
- LAD
Leukocyte Adhesion Deficiency
- LPS
Lipopolysaccharide
- MAC
Membrane Attack Complex
- MAGT1
Magnesium Transporter 1
- MBL
Mannose-Binding Lectin
- Mgat
Mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
- Mincle
Macrophage-inducible C-type lectin
- MS
Multiple Sclerosis
- Neu5Ac
N-glycolylneuraminic acid
- OST Complex
Oligosaccharyltransferase complex
- Poly-LacNAc
Polylactosamine
- PGM3
Phosphoglucomutase 3
- SCID
Severe Combined Immunodeficiency
- Siglec
Sialic acid-binding immunoglobulin-like lectins
- SNP
Single nucleotide polymorphisms
- TACA
Tumor-associated carbohydrate antigens
- UC
Ulcerative Colitis
- UDP
Uridine diphosphate
- XMEN
X-linked immunodeficiency with magnesium defect, EBV infection, and Neoplasia
Footnotes
Conflicts: Sean Stowell received Honoria from Grifols for lectures and is a consultant for Novartis, Cellics, Argenex and Alexion. He also receives research support from Alexion. No other authors report any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.An HJ, Froehlich JW, Lebrilla CB. Determination of glycosylation sites and site-specific heterogeneity in glycoproteins. Current Opinion in Chemical Biology 2009; 13:421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol 2019; 15:346–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varki A Biological roles of glycans. Glycobiology 2017; 27:3–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mockl L The Emerging Role of the Mammalian Glycocalyx in Functional Membrane Organization and Immune System Regulation. Front Cell Dev Biol 2020; 8:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varki A Evolutionary forces shaping the Golgi glycosylation machinery: why cell surface glycans are universal to living cells. Cold Spring Harb Perspect Biol 2011; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varki A, Kornfeld S. Historical Background and Overview. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al. , editors. Essentials of Glycobiology. 4th ed. Cold Spring Harbor (NY); 2022. p. 1–20. [Google Scholar]
- 7.Rabinovich GA, van Kooyk Y, Cobb BA. Glycobiology of immune responses. Ann N Y Acad Sci 2012; 1253:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langford-Smith A, Day AJ, Bishop PN, Clark SJ. Complementing the Sugar Code: Role of GAGs and Sialic Acid in Complement Regulation. Front Immunol 2015; 6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnaar RL. Glycans and glycan-binding proteins in immune regulation: A concise introduction to glycobiology for the allergist. J Allergy Clin Immunol 2015; 135:609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamili NA, Arthur CM, Gerner-Smidt C, Tafesse E, Blenda A, Dias-Baruffi M, et al. Key regulators of galectin-glycan interactions. Proteomics 2016; 16:3111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhallen L, Lackman JJ, Wendt R, Gustavsson M, Yang Z, Narimatsu Y, et al. “Glyco-sulfo barcodes” regulate chemokine receptor function. Cell Mol Life Sci 2023; 80:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bevan MJ, Cohn M. Cytotoxic effects of antigen- and mitogen-induced T cells on various targets. J Immunol 1975; 114:559–65. [PubMed] [Google Scholar]
- 13.Gabius HJ, Kaltner H, Kopitz J, Andre S. The glycobiology of the CD system: a dictionary for translating marker designations into glycan/lectin structure and function. Trends Biochem Sci 2015; 40:360–76. [DOI] [PubMed] [Google Scholar]
- 14.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol 2009; 9:338–52. [DOI] [PubMed] [Google Scholar]
- 15.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol 2008; 9:593–601. [DOI] [PubMed] [Google Scholar]
- 16.Rabinovich GA, Toscano MA, Jackson SS, Vasta GR. Functions of cell surface galectin-glycoprotein lattices. Curr Opin Struct Biol 2007; 17:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol 2010; 30 Suppl 1:S9–14. [DOI] [PubMed] [Google Scholar]
- 18.Stowell SR, Arthur CM, McBride R, Berger O, Razi N, Heimburg-Molinaro J, et al. Microbial glycan microarrays define key features of host-microbial interactions. Nat Chem Biol 2014; 10:470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu SC, Jan HM, Vallecillo-Zuniga ML, Rathgeber MF, Stowell CS, Murdock KL, et al. Whole microbe arrays accurately predict interactions and overall antimicrobial activity of galectin-8 toward distinct strains of Streptococcus pneumoniae. Sci Rep 2023; 13:5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kearney CJ, Vervoort SJ, Ramsbottom KM, Todorovski I, Lelliott EJ, Zethoven M, et al. SUGAR-seq enables simultaneous detection of glycans, epitopes, and the transcriptome in single cells. Sci Adv 2021; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu SC, Kamili NA, Dias-Baruffi M, Josephson CD, Rathgeber MF, Yeung MY, et al. Innate immune Galectin-7 specifically targets microbes that decorate themselves in blood group-like antigens. iScience 2022; 25:104482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, et al. Copper-free click chemistry for dynamic in vivo imaging. Proc Natl Acad Sci U S A 2007; 104:16793–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calle B, Bineva-Todd G, Marchesi A, Flynn H, Ghirardello M, Tastan OY, et al. Benefits of Chemical Sugar Modifications Introduced by Click Chemistry for Glycoproteomic Analyses. J Am Soc Mass Spectrom 2021; 32:2366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho AD, Wu SC, Kamili NA, Blenda AV, Cummings RD, Stowell SR, et al. An Automated Approach to Assess Relative Galectin-Glycan Affinity Following Glycan Microarray Analysis. Front Mol Biosci 2022; 9:893185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arthur CM, Cummings RD, Stowell SR. Using glycan microarrays to understand immunity. Curr Opin Chem Biol 2014; 18:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stowell SR, Arthur CM, Dias-Baruffi M, Rodrigues LC, Gourdine JP, Heimburg-Molinaro J, et al. Innate immune lectins kill bacteria expressing blood group antigen. Nat Med 2010; 16:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniels MA, Hogquist KA, Jameson SC. Sweet ‘n’ sour: the impact of differential glycosylation on T cell responses. Nat Immunol 2002; 3:903–10. [DOI] [PubMed] [Google Scholar]
- 28.Magalhaes A, Duarte HO, Reis CA. The role of O-glycosylation in human disease. Mol Aspects Med 2021; 79:100964. [DOI] [PubMed] [Google Scholar]
- 29.Wopereis S, Lefeber DJ, Morava E, Wevers RA. Mechanisms in protein O-glycan biosynthesis and clinical and molecular aspects of protein O-glycan biosynthesis defects: a review. Clin Chem 2006; 52:574–600. [DOI] [PubMed] [Google Scholar]
- 30.Mannino MP, Hart GW. The Beginner’s Guide to O-GlcNAc: From Nutrient Sensitive Pathway Regulation to Its Impact on the Immune System. Front Immunol 2022; 13:828648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanley P, Moremen KW, Lewis NE, Taniguchi N, Aebi M. N-Glycans. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al. , editors. Essentials of Glycobiology. 4th ed. Cold Spring Harbor (NY); 2022. p. 103–16. [Google Scholar]
- 32.Hoffmeister KM, Falet H. Platelet clearance by the hepatic Ashwell-Morrell receptor: mechanisms and biological significance. Thromb Res 2016; 141 Suppl 2:S68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grewal PK. The Ashwell-Morell receptor. Methods Enzymol 2010; 479:223–41. [DOI] [PubMed] [Google Scholar]
- 34.Geijtenbeek TB, van Kooyk Y. DC-SIGN: a novel HIV receptor on DCs that mediates HIV-1 transmission. Curr Top Microbiol Immunol 2003; 276:31–54. [DOI] [PubMed] [Google Scholar]
- 35.Brown GD, Willment JA, Whitehead L. C-type lectins in immunity and homeostasis. Nat Rev Immunol 2018; 18:374–89. [DOI] [PubMed] [Google Scholar]
- 36.Bochner BS, Zimmermann N. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. J Allergy Clin Immunol 2015; 135:598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark EA, Giltiay NV. CD22: A Regulator of Innate and Adaptive B Cell Responses and Autoimmunity. Front Immunol 2018; 9:2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol 2014; 14:653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan S, Paulson JC. Siglecs as Immune Cell Checkpoints in Disease. Annu Rev Immunol 2020; 38:365–95. [DOI] [PubMed] [Google Scholar]
- 40.Karmakar J, Mukherjee K, Mandal C. Siglecs Modulate Activities of Immune Cells Through Positive and Negative Regulation of ROS Generation. Front Immunol 2021; 12:758588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stowell SR, Dias-Baruffi M, Penttila L, Renkonen O, Nyame AK, Cummings RD. Human galectin-1 recognition of poly-N-acetyllactosamine and chimeric polysaccharides. Glycobiology 2004; 14:157–67. [DOI] [PubMed] [Google Scholar]
- 42.Stowell SR, Karmakar S, Arthur CM, Ju T, Rodrigues LC, Riul TB, et al. Galectin-1 induces reversible phosphatidylserine exposure at the plasma membrane. Mol Biol Cell 2009; 20:1408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stowell SR, Karmakar S, Stowell CJ, Dias-Baruffi M, McEver RP, Cummings RD. Human galectin-1, −2, and −4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood 2007; 109:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stowell SR, Qian Y, Karmakar S, Koyama NS, Dias-Baruffi M, Leffler H, et al. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol 2008; 180:3091–102. [DOI] [PubMed] [Google Scholar]
- 45.Liu FT, Stowell SR. The role of galectins in immunity and infection. Nat Rev Immunol 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundblad V, Morosi LG, Geffner JR, Rabinovich GA. Galectin-1: A Jack-of-All-Trades in the Resolution of Acute and Chronic Inflammation. J Immunol 2017; 199:3721–30. [DOI] [PubMed] [Google Scholar]
- 47.Severe Covid GG, Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, et al. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N Engl J Med 2020; 383:1522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu SC, Arthur CM, Wang J, Verkerke H, Josephson CD, Kalman D, et al. The SARS-CoV-2 receptor-binding domain preferentially recognizes blood group A. Blood Adv 2021; 5:1305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu SC, Arthur CM, Jan HM, Garcia-Beltran WF, Patel KR, Rathgeber M, et al. Blood Group A Enhances SARS-CoV-2 Infection. Blood 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zerra PE, Patel SR, Jajosky RP, Arthur CM, McCoy JW, Allen JWL, et al. Marginal Zone B Cells Mediate a CD4 T Cell Dependent Extrafollicular Antibody Response Following RBC Transfusion in Mice. Blood 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jajosky RP, Patel SR, Wu SC, Patel KR, Covington ML, Vallecillo-Zuniga ML, et al. Prior Immunization to an Intracellular Antigen Enhances Subsequent Red Blood Cell Alloimmunization in Mice. Blood 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mener A, Patel SR, Arthur CM, Chonat S, Wieland A, Santhanakrishnan M, et al. Complement serves as a switch between CD4+ T cell-independent and -dependent RBC antibody responses. JCI Insight 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arthur CM, Stowell SR. The Development and Consequences of Red Blood Cell Alloimmunization. Annu Rev Pathol 2023; 18:537–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arthur CM, Patel SR, Smith NH, Bennett A, Kamili NA, Mener A, et al. Antigen Density Dictates Immune Responsiveness following Red Blood Cell Transfusion. J Immunol 2017; 198:2671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jajosky RP, Wu SC, Zheng L, Jajosky AN, Jajosky PG, Josephson CD, et al. ABO blood group antigens and differential glycan expression: Perspective on the evolution of common human enzyme deficiencies. iScience 2023; 26:105798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stowell CP, Stowell SR. Biologic roles of the ABH and Lewis histo-blood group antigens Part I: infection and immunity. Vox Sang 2019; 114:426–42. [DOI] [PubMed] [Google Scholar]
- 57.Stowell SR, Stowell CP. Biologic roles of the ABH and Lewis histo-blood group antigens part II: thrombosis, cardiovascular disease and metabolism. Vox Sang 2019. [DOI] [PubMed] [Google Scholar]
- 58.Stowell SR, Winkler AM, Maier CL, Arthur CM, Smith NH, Girard-Pierce KR, et al. Initiation and regulation of complement during hemolytic transfusion reactions. Clin Dev Immunol 2012; 2012:307093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adam I, Motyka B, Tao K, Jeyakanthan M, Alegre ML, Cowan PJ, et al. Sex, T Cells, and the Microbiome in Natural ABO Antibody Production in Mice. Transplantation 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Branch DR. Anti-A and anti-B: what are they and where do they come from? Transfusion 2015; 55 Suppl 2:S74–9. [DOI] [PubMed] [Google Scholar]
- 61.Zerra PE, Patel SR, Jajosky RP, Arthur CM, McCoy JW, Allen JWL, et al. Marginal zone B cells mediate a CD4 T-cell-dependent extrafollicular antibody response following RBC transfusion in mice. Blood 2021; 138:706–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jajosky RP, Patel KR, Allen JWL, Zerra PE, Chonat S, Ayona D, et al. Antibody-Mediated Antigen Loss Switches Augmented Immunity to Antibody-Mediated Immunosuppression. Blood 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kappler K, Hennet T. Emergence and significance of carbohydrate-specific antibodies. Genes Immun 2020; 21:224–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wide L, Bennich H, Johansson SG. Diagnosis of allergy by an in-vitro test for allergen antibodies. Lancet 1967; 2:1105–7. [DOI] [PubMed] [Google Scholar]
- 65.Aalberse RC, Koshte V, Clemens JG. Immunoglobulin E antibodies that crossreact with vegetable foods, pollen, and Hymenoptera venom. J Allergy Clin Immunol 1981; 68:356–64. [DOI] [PubMed] [Google Scholar]
- 66.Homann A, Schramm G, Jappe U. Glycans and glycan-specific IgE in clinical and molecular allergology: Sensitization, diagnostics, and clinical symptoms. J Allergy Clin Immunol 2017; 140:356–68. [DOI] [PubMed] [Google Scholar]
- 67.Petersen A, Vieths S, Aulepp H, Schlaak M, Becker WM. Ubiquitous structures responsible for IgE cross-reactivity between tomato fruit and grass pollen allergens. J Allergy Clin Immunol 1996; 98:805–15. [DOI] [PubMed] [Google Scholar]
- 68.Altmann F Coping with cross-reactive carbohydrate determinants in allergy diagnosis. Allergo J Int 2016; 25:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Veen M Poor biological activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. Immunology Letters 1997; 56:183–4. [DOI] [PubMed] [Google Scholar]
- 70.Mari A, Ooievaar-de Heer P, Scala E, Giani M, Pirrotta L, Zuidmeer L, et al. Evaluation by double-blind placebo-controlled oral challenge of the clinical relevance of IgE antibodies against plant glycans. Allergy 2008; 63:891–6. [DOI] [PubMed] [Google Scholar]
- 71.Jappe U, Raulf-Heimsoth M, Hoffmann M, Burow G, Hubsch-Muller C, Enk A. In vitro hymenoptera venom allergy diagnosis: Improved by screening for cross-reactive carbohydrate determinants and reciprocal inhibition. Allergy 2006; 61:1220–9. [DOI] [PubMed] [Google Scholar]
- 72.Sinson E, Ocampo C, Liao C, Nguyen S, Dinh L, Rodems K, et al. Cross-reactive carbohydrate determinant interference in cellulose-based IgE allergy tests utilizing recombinant allergen components. PLOS ONE 2020; 15:e0231344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ansotegui IJ, Melioli G, Canonica GW, Caraballo L, Villa E, Ebisawa M, et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organization Journal 2020; 13:100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med 2008; 358:1109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol 2005; 23:1803–10. [DOI] [PubMed] [Google Scholar]
- 76.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004; 351:337–45. [DOI] [PubMed] [Google Scholar]
- 77.O’Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol 2007; 25:3644–8. [DOI] [PubMed] [Google Scholar]
- 78.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem 1988; 263:17755–62. [PubMed] [Google Scholar]
- 79.Landsteiner K, Miller CP. Serological Studies on the Blood of the Primates : Iii. Distribution of Serological Factors Related to Human Isoagglutinogens in the Blood of Lower Monkeys. J Exp Med 1925; 42:863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roman-Carrasco P, Hemmer W, Cabezas-Cruz A, Hodzic A, de la Fuente J, Swoboda I. The alpha-Gal Syndrome and Potential Mechanisms. Front Allergy 2021; 2:783279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steinke JW, Platts-Mills TA, Commins SP. The alpha-gal story: lessons learned from connecting the dots. J Allergy Clin Immunol 2015; 135:589–96; quiz 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol 2009; 123:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Platts-Mills TAE, Li R-c, Keshavarz B, Smith AR, Wilson JM. Diagnosis and Management of Patients with the α-Gal Syndrome. The Journal of Allergy and Clinical Immunology: In Practice 2020; 8:15–23.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chakrapani N, Fischer J, Swiontek K, Codreanu-Morel F, Hannachi F, Morisset M, et al. α-Gal present on both glycolipids and glycoproteins contributes to immune response in meat-allergic patients. J Allergy Clin Immunol 2022; 150:396–405.e11. [DOI] [PubMed] [Google Scholar]
- 85.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol 2011; 127:1286–93 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Senage T, Paul A, Le Tourneau T, Fellah-Hebia I, Vadori M, Bashir S, et al. The role of antibody responses against glycans in bioprosthetic heart valve calcification and deterioration. Nat Med 2022; 28:283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arthur CM, Zerra PE, Shin S, Wang J, Song X, Doering CB, et al. Nonhuman glycans can regulate anti-factor VIII antibody formation in mice. Blood 2022; 139:1312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Md A, Maeda M, Matsui T, Takasato Y, Ito K, Kimura Y. Purification and molecular characterization of a truncated-type Ara h 1, a major peanut allergen: oligomer structure, antigenicity, and glycoform. Glycoconj J 2021; 38:67–76. [DOI] [PubMed] [Google Scholar]
- 89.Al-Ghouleh A, Johal R, Sharquie IK, Emara M, Harrington H, Shakib F, et al. The glycosylation pattern of common allergens: the recognition and uptake of Der p 1 by epithelial and dendritic cells is carbohydrate dependent. PLoS One 2012; 7:e33929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol 2006; 177:3677–85. [DOI] [PubMed] [Google Scholar]
- 91.Okano M, Satoskar AR, Nishizaki K, Abe M, Harn DA. Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J Immunol 1999; 163:6712–7. [PubMed] [Google Scholar]
- 92.Cummings RD. The repertoire of glycan determinants in the human glycome. Mol Biosyst 2009; 5:1087–104. [DOI] [PubMed] [Google Scholar]
- 93.Westphal S, Kolarich D, Foetisch K, Lauer I, Altmann F, Conti A, et al. Molecular characterization and allergenic activity of Lyc e 2 (beta-fructofuranosidase), a glycosylated allergen of tomato. Eur J Biochem 2003; 270:1327–37. [DOI] [PubMed] [Google Scholar]
- 94.Samraj AN, Pearce OM, Läubli H, Crittenden AN, Bergfeld AK, Banda K, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci U S A 2015; 112:542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frei R, Ferstl R, Roduit C, Ziegler M, Schiavi E, Barcik W, et al. Exposure to nonmicrobial N-glycolylneuraminic acid protects farmers’ children against airway inflammation and colitis. J Allergy Clin Immunol 2018; 141:382–90 e7. [DOI] [PubMed] [Google Scholar]
- 96.Hemmer W, Focke M, Kolarich D, Wilson IB, Altmann F, Wöhrl S, et al. Antibody binding to venom carbohydrates is a frequent cause for double positivity to honeybee and yellow jacket venom in patients with stinging-insect allergy. J Allergy Clin Immunol 2001; 108:1045–52. [DOI] [PubMed] [Google Scholar]
- 97.Ebo DG, Hagendorens MM, Bridts CH, De Clerck LS, Stevens WJ. Sensitization to cross-reactive carbohydrate determinants and the ubiquitous protein profilin: mimickers of allergy. Clin Exp Allergy 2004; 34:137–44. [DOI] [PubMed] [Google Scholar]
- 98.Montassier E, Al-Ghalith GA, Mathé C, Le Bastard Q, Douillard V, Garnier A, et al. Distribution of Bacterial α1,3-Galactosyltransferase Genes in the Human Gut Microbiome. Front Immunol 2019; 10:3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun 1988; 56:1730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chandrasekhar JL, Cox KM, Loo WM, Qiao H, Tung KS, Erickson LD. Cutaneous Exposure to Clinically Relevant Lone Star Ticks Promotes IgE Production and Hypersensitivity through CD4. J Immunol 2019; 203:813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cabezas-Cruz A, Hodžić A, Román-Carrasco P, Mateos-Hernández L, Duscher GG, Sinha DK, et al. Environmental and Molecular Drivers of the α-Gal Syndrome. Front Immunol 2019; 10:1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Platts-Mills TAE, Commins SP, Biedermann T, van Hage M, Levin M, Beck LA, et al. On the cause and consequences of IgE to galactose-α-1,3-galactose: A report from the National Institute of Allergy and Infectious Diseases Workshop on Understanding IgE-Mediated Mammalian Meat Allergy. J Allergy Clin Immunol 2020; 145:1061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rispens T, Derksen NI, Commins SP, Platts-Mills TA, Aalberse RC. IgE production to α-gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS One 2013; 8:e55566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choudhary SK, Karim S, Iweala OI, Choudhary S, Crispell G, Sharma SR, et al. Tick salivary gland extract induces alpha-gal syndrome in alpha-gal deficient mice. Immun Inflamm Dis 2021; 9:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wicklein D, Lindner B, Moll H, Kolarich D, Altmann F, Becker WM, et al. Carbohydrate moieties can induce mediator release: a detailed characterization of two major timothy grass pollen allergens. Biol Chem 2004; 385:397–407. [DOI] [PubMed] [Google Scholar]
- 106.Jin C, Altmann F, Strasser R, Mach L, Schähs M, Kunert R, et al. A plant-derived human monoclonal antibody induces an anti-carbohydrate immune response in rabbits. Glycobiology 2008; 18:235–41. [DOI] [PubMed] [Google Scholar]
- 107.Plum M, Tjerrild L, Raiber T, Bantleon F, Bantleon S, Miehe M, et al. Structural and functional analyses of antibodies specific for modified core N-glycans suggest a role in T. Allergy 2023; 78:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holzweber F, Svehla E, Fellner W, Dalik T, Stubler S, Hemmer W, et al. Inhibition of IgE binding to cross-reactive carbohydrate determinants enhances diagnostic selectivity. Allergy 2013; 68:1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Novak J, Barratt J, Julian BA, Renfrow MB. Aberrant Glycosylation of the IgA1 Molecule in IgA Nephropathy. Semin Nephrol 2018; 38:461–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Novak J, Julian BA, Tomana M, Mestecky J. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol 2008; 28:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiang SH, Kennard AL, Walters GD. Recurrent glomerulonephritis following renal transplantation and impact on graft survival. BMC Nephrol 2018; 19:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci U S A 2002; 99:16613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Matsumoto Y, Aryal RP, Heimburg-Molinaro J, Park SS, Wever WJ, Lehoux S, et al. Identification and characterization of circulating immune complexes in IgA nephropathy. Sci Adv 2022; 8:eabm8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yuki N, Yoshino H, Sato S, Miyatake T. Acute axonal polyneuropathy associated with anti-GM1 antibodies following Campylobacter enteritis. Neurology 1990; 40:1900–2. [DOI] [PubMed] [Google Scholar]
- 115.van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol 2014; 10:469–82. [DOI] [PubMed] [Google Scholar]
- 116.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 2001; 291:484–6. [DOI] [PubMed] [Google Scholar]
- 117.Bruton OC. Agammaglobulinemia. Pediatrics 1952; 9:722–8. [PubMed] [Google Scholar]
- 118.Imbach P, Barandun S, d’Apuzzo V, Baumgartner C, Hirt A, Morell A, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet 1981; 1:1228–31. [DOI] [PubMed] [Google Scholar]
- 119.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006; 313:670–3. [DOI] [PubMed] [Google Scholar]
- 120.Washburn N, Schwab I, Ortiz D, Bhatnagar N, Lansing JC, Medeiros A, et al. Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proc Natl Acad Sci U S A 2015; 112:E1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pagan JD, Kitaoka M, Anthony RM. Engineered Sialylation of Pathogenic Antibodies In Vivo Attenuates Autoimmune Disease. Cell 2018; 172:564–77.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang LX, Tong X, Li C, Giddens JP, Li T. Glycoengineering of Antibodies for Modulating Functions. Annu Rev Biochem 2019; 88:433–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anthony RM, Wermeling F, Ravetch JV. Novel roles for the IgG Fc glycan. Ann N Y Acad Sci 2012; 1253:170–80. [DOI] [PubMed] [Google Scholar]
- 124.Patel KR, Roberts JT, Subedi GP, Barb AW. Restricted processing of CD16a/Fc gamma receptor IIIa N-glycans from primary human NK cells impacts structure and function. J Biol Chem 2018; 293:3477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shade KC, Conroy ME, Washburn N, Kitaoka M, Huynh DJ, Laprise E, et al. Sialylation of immunoglobulin E is a determinant of allergic pathogenicity. Nature 2020; 582:265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vicente MM, Leite-Gomes E, Pinho SS. Glycome dynamics in T and B cell development: basic immunological mechanisms and clinical applications. Trends Immunol 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pascoal C, Francisco R, Ferro T, Dos Reis Ferreira V, Jaeken J, Videira PA. CDG and immune response: From bedside to bench and back. J Inherit Metab Dis 2020; 43:90–124. [DOI] [PubMed] [Google Scholar]
- 128.Etzioni A, Frydman M, Pollack S, Avidor I, Phillips ML, Paulson JC, et al. Brief report: recurrent severe infections caused by a novel leukocyte adhesion deficiency. N Engl J Med 1992; 327:1789–92. [DOI] [PubMed] [Google Scholar]
- 129.Gazit Y, Mory A, Etzioni A, Frydman M, Scheuerman O, Gershoni-Baruch R, et al. Leukocyte adhesion deficiency type II: long-term follow-up and review of the literature. J Clin Immunol 2010; 30:308–13. [DOI] [PubMed] [Google Scholar]
- 130.McEver RP, Cummings RD. Perspectives series: cell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest 1997; 100:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011; 475:471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li FY, Chaigne-Delalande B, Su H, Uzel G, Matthews H, Lenardo MJ. XMEN disease: a new primary immunodeficiency affecting Mg2+ regulation of immunity against Epstein-Barr virus. Blood 2014; 123:2148–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Freeze HH. XMEN: welcome to the glycosphere. J Clin Invest 2020; 130:80–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ravell JC, Matsuda-Lennikov M, Chauvin SD, Zou J, Biancalana M, Deeb SJ, et al. Defective glycosylation and multisystem abnormalities characterize the primary immunodeficiency XMEN disease. J Clin Invest 2020; 130:507–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Matsuda-Lennikov M, Biancalana M, Zou J, Ravell JC, Zheng L, Kanellopoulou C, et al. Magnesium transporter 1 (MAGT1) deficiency causes selective defects in N-linked glycosylation and expression of immune-response genes. J Biol Chem 2019; 294:13638–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sassi A, Lazaroski S, Wu G, Haslam SM, Fliegauf M, Mellouli F, et al. Hypomorphic homozygous mutations in phosphoglucomutase 3 (PGM3) impair immunity and increase serum IgE levels. Journal of Allergy and Clinical Immunology 2014; 133:1410–9.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stray-Pedersen A, Backe Paul H, Sorte Hanne S, Mørkrid L, Chokshi Niti Y, Erichsen Hans C, et al. PGM3 mutations cause a congenital disorder of glycosylation with severe immunodeficiency and skeletal dysplasia. The American Journal of Human Genetics 2014; 95:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Greig KT, Antonchuk J, Metcalf D, Morgan PO, Krebs DL, Zhang J-G, et al. Agm1/Pgm3-Mediated sugar nucleotide synthesis is essential for hematopoiesis and development. Molecular and Cellular Biology 2007; 27:5849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Akella NM, Ciraku L, Reginato MJ. Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol 2019; 17:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dias AM, Correia A, Pereira MS, Almeida CR, Alves I, Pinto V, et al. Metabolic control of T cell immune response through glycans in inflammatory bowel disease. Proc Natl Acad Sci U S A 2018; 115:E4651–E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dias AM, Dourado J, Lago P, Cabral J, Marcos-Pinto R, Salgueiro P, et al. Dysregulation of T cell receptor N-glycosylation: a molecular mechanism involved in ulcerative colitis. Hum Mol Genet 2014; 23:2416–27. [DOI] [PubMed] [Google Scholar]
- 142.Pereira MS, Maia L, Azevedo LF, Campos S, Carvalho S, Dias AM, et al. A [Glyco]biomarker that Predicts Failure to Standard Therapy in Ulcerative Colitis Patients. J Crohns Colitis 2019; 13:39–49. [DOI] [PubMed] [Google Scholar]
- 143.Pereira MS, Duraes C, Catarino TA, Costa JL, Cleynen I, Novokmet M, et al. Genetic Variants of the MGAT5 Gene Are Functionally Implicated in the Modulation of T Cells Glycosylation and Plasma IgG Glycome Composition in Ulcerative Colitis. Clin Transl Gastroenterol 2020; 11:e00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stowell SR, Ju T, Cummings RD. Protein glycosylation in cancer. Annu Rev Pathol 2015; 10:473–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li CF, Zhou RW, Mkhikian H, Newton BL, Yu Z, Demetriou M. Hypomorphic MGAT5 polymorphisms promote multiple sclerosis cooperatively with MGAT1 and interleukin-2 and 7 receptor variants. J Neuroimmunol 2013; 256:71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grigorian A, Araujo L, Naidu NN, Place DJ, Choudhury B, Demetriou M. N-acetylglucosamine inhibits T-helper 1 (Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis. J Biol Chem 2011; 286:40133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee SU, Li CF, Mortales CL, Pawling J, Dennis JW, Grigorian A, et al. Increasing cell permeability of N-acetylglucosamine via 6-acetylation enhances capacity to suppress T-helper 1 (TH1)/TH17 responses and autoimmunity. PLoS One 2019; 14:e0214253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mortales CL, Lee SU, Manousadjian A, Hayama KL, Demetriou M. N-Glycan Branching Decouples B Cell Innate and Adaptive Immunity to Control Inflammatory Demyelination. iScience 2020; 23:101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jiang Y, Wen T, Yan R, Kim SR, Stowell SR, Wang W, et al. O-glycans on death receptors in cells modulate their sensitivity to TRAIL-induced apoptosis through affecting on their stability and oligomerization. FASEB J 2020; 34:11786–801. [DOI] [PubMed] [Google Scholar]
- 150.Sun R, Kim AMJ, Lim SO. Glycosylation of Immune Receptors in Cancer. Cells 2021; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer 2005; 5:29–41. [DOI] [PubMed] [Google Scholar]
- 152.Rodrigues Mantuano N, Natoli M, Zippelius A, Laubli H. Tumor-associated carbohydrates and immunomodulatory lectins as targets for cancer immunotherapy. J Immunother Cancer 2020; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010; 363:1324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu AL, Gilman AL, Ozkaynak MF, Naranjo A, Diccianni MB, Gan J, et al. Long-Term Follow-up of a Phase III Study of ch14.18 (Dinutuximab) + Cytokine Immunotherapy in Children with High-Risk Neuroblastoma: COG Study ANBL0032. Clin Cancer Res 2021; 27:2179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shao C, Anand V, Andreeff M, Battula VL. Ganglioside GD2: a novel therapeutic target in triple-negative breast cancer. Ann N Y Acad Sci 2022; 1508:35–53. [DOI] [PubMed] [Google Scholar]
- 156.Durbas M, Horwacik I, Boratyn E, Kamycka E, Rokita H. GD2 ganglioside specific antibody treatment downregulates PI3K/Akt/mTOR signaling network in human neuroblastoma cell lines. Int J Oncol 2015; 47:1143–59. [DOI] [PubMed] [Google Scholar]
- 157.Liu X, Wills CA, Chen L, Zhang J, Zhao Y, Zhou M, et al. Small extracellular vesicles induce resistance to anti-GD2 immunotherapy unveiling tipifarnib as an adjunct to neuroblastoma immunotherapy. J Immunother Cancer 2022; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Posey AD, Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B, et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity 2016; 44:1444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Raglow Z, McKenna MK, Bonifant CL, Wang W, Pasca di Magliano M, Stadlmann J, et al. Targeting glycans for CAR therapy: The advent of sweet CARs. Mol Ther 2022; 30:2881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Matsumoto Y, Jia N, Heimburg-Molinaro J, Cummings RD. Targeting Tn-positive tumors with an afucosylated recombinant anti-Tn IgG. Sci Rep 2023; 13:5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kudelka MR, Gu W, Matsumoto Y, Ju T, Barnes Ii RH, Kardish RJ, et al. Targeting altered glycosylation in secreted tumor glycoproteins for broad cancer detection. Glycobiology 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kudelka MR, Ju T, Heimburg-Molinaro J, Cummings RD. Simple sugars to complex disease--mucin-type O-glycans in cancer. Adv Cancer Res 2015; 126:53–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ju T, Aryal RP, Kudelka MR, Wang Y, Cummings RD. The Cosmc connection to the Tn antigen in cancer. Cancer Biomark 2014; 14:63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 2003; 278:3466–73. [DOI] [PubMed] [Google Scholar]
- 165.Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A 2011; 108:12669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marshall MJE, Stopforth RJ, Cragg MS. Therapeutic Antibodies: What Have We Learnt from Targeting CD20 and Where Are We Going? Front Immunol 2017; 8:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pereira NA, Chan KF, Lin PC, Song Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs 2018; 10:693–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Islam M, Arlian BM, Pfrengle F, Duan S, Smith SA, Paulson JC. Suppressing Immune Responses Using Siglec Ligand-Decorated Anti-receptor Antibodies. J Am Chem Soc 2022; 144:9302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Marino KV, Cagnoni AJ, Croci DO, Rabinovich GA. Targeting galectin-driven regulatory circuits in cancer and fibrosis. Nat Rev Drug Discov 2023; 22:295–316. [DOI] [PubMed] [Google Scholar]
- 170.Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N Engl J Med 2017; 376:429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]


