Abstract
While osteoporosis is reliably diagnosed using dual energy X-ray absorptiometry (DXA), screening rates are alarmingly low, contributing to preventable fractures. Raman spectroscopy (RS) can detect biochemical changes that occur in bones transcutaneously and can arguably be more accessible than DXA as a fracture risk assessment. A reasonable approach to translate RS is to interrogate phalangeal bones of human hands, where the soft tissues covering the bone are less likely to hamper transcutaneous measurements. To that end, we set out to first determine whether Raman spectra obtained from phalangeal bones correlate with distal radius fracture strength, which can predict subsequent osteoporotic fractures at the spine and hip. We performed RS upon diaphyseal and epiphyseal regions of exposed proximal phalanges from 12 cadaver forearms classified as healthy (n=3), osteopenic (n=4), or osteoporotic (n=5) based on wrist T-scores measured by DXA. We observed a significant decrease in phosphate to matrix ratio and a significant increase in carbonate substitution in the osteoporotic phalanges relative to healthy and osteopenic phalanges. Multivariate regression models produced wrist T-score estimates with significant correlation to the DXA-measured values (r = 0.79). Furthermore, by accounting for phalangeal RS parameters, body mass index, and age, a multivariate regression significantly predicted distal radius strength measured in a simulated-fall biomechanical test (r = 0.81). These findings demonstrate the feasibility of interrogating the phalanges using RS for bone quality assessment of distant clinical sites of fragility fractures, such as the wrist. Future work will address transcutaneous measurement challenges as another requirement for scale-up and translation.
Keywords: fragility fracture, Raman spectroscopy, osteoporosis, distal radius fracture, DXA
Introduction
Osteoporosis is a multifactorial bone disease that causes bones to become fragile (Coughlan and Dockery, 2014; Johnell and Kanis, 2006). Even though post-menopausal women are more likely to be diagnosed than men (Force, 2018), osteoporosis affects aging individuals in both sexes (Riggs and Melton, 1995). A complication of osteoporosis is a fragility fracture at the distal forearm and wrist, vertebrae, or hip (Riggs and Melton, 1995). Clinically, osteoporosis is diagnosed by measuring bone mineral density (BMD) using dual energy X-ray absorptiometry (DXA). A T-score, used for diagnosis, is defined by the standard deviation of an individual’s BMD compared to a young, healthy, sex- and ethnicity- matched reference population (Link, 2012).
Although DXA is the clinical standard, a low percentage of the target population gets scanned. Specifically, the U.S. Preventive Services Task Force (USPSTF) recommends universal screening for women older than 65 and screening for at-risk younger postmenopausal women (Force, 2018). However, a retrospective analysis from 1,638,454 women reported the screening rates were alarmingly low: 21.1%, 26.5%, and 12.8% for the age ranges 50–60, 65–79, and older than 80 respectively (Gillespie and Morin, 2017). In efforts to help with low screening rates, Medicare has covered osteoporosis screening costs for qualified individuals since 1998 (Register, 1997), but screening rates have remained low (Gillespie and Morin, 2017; McAdam-Marx et al., 2012; Zhang et al., 2012). However, if a cost-effective and portable modality can identify at-risk osteoporotic patients, referrals and DXA screening rates may increase, allowing interventional therapy to treat patients before they experience a fracture.
A potential predictor for subsequent fragility fractures is a distal radius fracture (Cuddihy et al., 1999; Klotzbuecher et al., 2000; Mallmin and Ljunghall, 1994). Distal radius fractures (DRF) rank among the most frequent fractures in adults. Therefore, alternatives to DXA, including peripheral quantitative computed tomography (pQCT), have been investigated and shown the potential in predicting distal radius fracture risk (Arias-Moreno et al., 2019; Kazakia et al., 2011; Muller et al., 2003) as a means to identify patients at risk of subsequent osteoporotic fractures in the hip or spine (Chen et al., 2013). Since volar plating is the best practice for treating DRF to reestablish joint integrity and natural angles, most published biomechanical DRF models in the literature are designed to evaluate devices for osteosynthesis and typically involve a wedge osteotomy (Baumbach et al., 2012). Therefore, these osteotomy-based models do not replicate an osteoporosis-related fragility fracture that might occur in an intact distal radius due to a fall. The mechanical properties of the distal radius have been evaluated in 3-point bending of the intact radius, compression testing on metaphyseal bone slices machined from the distal radius, or simulated fall scenario on an intact forearm (Baumbach et al., 2012; Eckstein et al., 2004; Hudelmaier et al., 2004; Lochmüller et al., 2002). Research indicates that densitometric measurements taken at the distal radius can accurately predict its mechanical strength, but measurements taken at remote sites, even in the same bone, show significantly reduced predictive accuracy (Eckstein et al., 2004).
Alternatively, Raman spectroscopy has been investigated for decades as a potential tool for assessing bone quality but has yet to be validated as a fracture risk assessment tool. Raman spectroscopy is an optical technique that yields a biochemical signature associated with molecular vibration frequencies. It has been well established that Raman spectra collected from bones are capable of giving insights related to the bone mineralization and matrix components (Morris and Mandair, 2011; Unal et al., 2021) that are relevant to osteoporosis (McCreadie et al., 2006; Paschalis et al., 2016). Recent efforts have focused on nondestructive Raman estimation of biomechanical properties of human femurs (Ojanen et al., 2015; Unal et al., 2021; Yerramshetty and Akkus, 2008) and vertebrae (Kim et al., 2014), with subsequent destructive measurements providing reference values. Previous studies have used Raman microscopy to study cortical and trabecular bone and the differences have been well characterized (Alunni Cardinali et al., 2021; Goodyear et al., 2009; McCreadie et al., 2006; Timchenko et al., 2020). By using a non-contact Raman instrument, spectra can be measured from either epiphyseal or diaphyseal regions.
Raman can be performed transcutaneously and non-invasively by using spatially offset Raman spectroscopy (SORS), and the feasibility of using SORS to interrogate murine tibiae in vivo has been documented (Maher et al., 2013; Shu et al., 2018). In transitioning to the larger dimensions of human bodies, the bones within the hand are more suitable for in vivo measurements because of the thinner overlying soft tissue compared to tibiae (Pejović-Milić et al., 2002). Wilczec et al. demonstrated that digital X-ray radiogrammetry of the metacarpals predicted subsequent hip fractures with an accuracy of approximately 87% (Wilczek et al., 2013), indicating that metacarpals can be a clinically relevant site for fracture risk. More recently, preliminary transcutaneous data from hand cadavers suggested that measurements from phalanges yielded better bone signal to noise ratios compared to metacarpals (Chen et al., 2021).
The goal of this study was to perform Raman spectroscopy on exposed proximal phalanges to 1) characterize the spectral differences between epiphyseal and diaphyseal regions, 2) determine if either bone region is sensitive to osteoporotic related bone changes, and 3) determine if either bone region enables predictions either of the distal radial strength or of the DXA-derived T-score measured at the distal radius. The work presented here validates the phalanges as a valid anatomical region to perform future transcutaneous SORS measurements and gives insight as to what regions within the bone should be explored in future transcutaneous work.
Methods
Cadaver samples
We obtained twelve fresh-frozen cadaver arms from the Anatomy Gifts Registry (AGR) (Hanover, MD), with corresponding wrist DXA scans. The summary of the cadavers is shown in Table 1. Detailed sample demographics are shown in Supplementary Table 1.
Table 1:
Demographics of distal arm cadaver donors (mean ± std).
| WHO Classification | Sex | Ethnicity | Age (Years) | BMI | Wrist BMD (g/cm2) | Wrist T-score |
|---|---|---|---|---|---|---|
| Normal | Males: n = 2 Females: n = 1 |
Black: n = 1 White: n = 2 |
35.7 ± 8.0 | 24.7 ± 3.0 | 0.789 ± 0.104 | 0.27 ± 0.58 |
| Osteopenia | Males: n = 2 Females: n = 2 |
White: n = 4 | 50.3 ± 22.9 | 31.0 ± 12.2 | 0.690 ± 0.075 | −1.15 ± 0.13 |
| Osteoporosis | Males: n = 2 Females: n = 3 |
Black: n = 1 White: n = 4 |
83.6 ± 6.8 | 24 ± 4.80 | 0.536 ± 0.054 | −3.64 ± 0.67 |
Distal radial biomechanical testing
Mechanical testing was performed using methods adapted from Lochmuller et al. (Lochmüller et al., 2002). Cadaver arm samples were thawed at room temperature. Soft tissues 3 inches proximal to the wrist were dissected to expose the ulna and radius, which were cut approximately 6 inches proximal to the wrist. The exposed ulna and radius were embedded in a self-curing acrylic (Bosworth Fastray, Keystone Industries, Singen, Germany), with 15° radial abduction and with the forearm in pronation. Using an Instron system (Electroplus10000; Norwood, MA) a load was applied to the palm in displacement control at a rate of 3.3 mm/s to induce a wrist fracture that would result from breaking a fall with the hand. The distal radius strength, defined as maximum load (N), was measured.
Raman spectroscopy
As described previously (Maher et al., 2011; Massie et al., 2021), Raman measurements were performed ex vivo on the excised, thawed proximal phalanges using an 830 nm laser delivering 120 mW to a spot of approximately 1.0 mm in diameter. From each cadaver, the individual proximal phalanges 2–4 were measured and averaged. The mean length of phalanges was 45 mm (± 4.5 mm). For each bone, 5 adjacent measurements spaced 1 mm apart along the bone axis were collected from both the distal epiphysis and mid-diaphysis regions. Individual spectra were acquired from a sum of five 60-second exposures. All fifteen epiphyseal spectra (5 spectra/bone × 3 bones/cadaver) were averaged to produce an overall epiphyseal spectrum; the same was done for the diaphyseal spectra. The completed dataset thus consisted of 2 spectra per cadaver: 1 diaphysis spectrum and 1 epiphysis spectrum. In addition to this general protocol, one excised proximal phalanx was measured along the entire length of the bone axis in 1.5 mm step increments to document the spatial evolution of the Raman spectral lineshape.
Spectral pre-processing involved cosmic ray removal, readout and dark current subtraction, image aberration correction, fluorescence removal, and smoothing (Maher et al., 2011; Massie et al., 2021). All spectra were normalized to their mean absolute deviation (Massie et al., 2021). Commonly reported Raman peak areas were calculated for matrix and mineral components by computing the area between each peak and the line connecting its two spectral endpoints (see Figure S2). Matrix components were defined by spectral regions associated with contributions from CH2 (1410–1496 cm−1), amide III (1218–1358 cm−1), and amide 1 (1596–1720 cm−1). Phosphate mineral (924–986 cm−1) to matrix (PTMR), carbonate (1054–1098 cm−1) to matrix (CTMR) and carbonate to phosphate (CTPR) peak area ratios were calculated, all of which have been useful in predicting mechanical properties (Carretta et al., 2015; Makowski et al., 2017; Unal et al., 2019; Yerramshetty and Akkus, 2008). Additionally, matrix spectral regions were fit to subpeaks. CH2 and amide I were fit to 4 pre-defined sub-peaks and amide III was fit to 3 sub-peaks. Details on the sub-peak fitting are described in Supplementary material.
Micro-computed tomography
The phalanges were scanned by micro-computed tomography (μCT; VivaCT 40; Scanco Medical; Bassersdorf, Switzerland) at 21 μm isotropic resolution using an integration time of 200 ms, energy of 55 kV, and intensity of 145 μA. The Scanco trabecular analysis was used to determine the trabecular properties, by averaging over 2709 μm at the distal epiphysis. The Scanco midshaft analysis was used to determine the cortical thickness, by averaging over a length of 0.609 mm.
Statistical analysis
Statistical analysis was performed in MATLAB®, GraphPad Prism®, and JMP16. To compare the average effects of osteoporosis, an ordinary one-way ANOVA was conducted followed by a Tukey’s multiple comparisons test.
To create a prediction model for distal radius fracture, we used linear regressions using partial least squares regression (PLSR) with a leave one out cross-validated (LOOCV) approach. We performed a standard least squares regression in JMP16 to determine what sample demographics were correlated with the maximum load (Supplementary Table 4). Commonly reported ratios such as PTMR, CTMR, CTPR were calculated since they are relevant for predicting mechanical properties (Carretta et al., 2015; Makowski et al., 2017; Unal et al., 2019; Yerramshetty and Akkus, 2008). Three LOOCV-PLSR models were performed independently using inputs of 1) BMI, age, and radius BMD from DXA (12×3 matrix), 2) BMI, age, epiphyseal PTMR, CTMR, and CTPR (12×5 matrix), and 3) BMI, age, diaphyseal PTMR, CTMR, and CTPR (12×5 matrix). To compare the three models on equal footing, the model rank was fixed at 3 in all cases regardless of the number of input variables (Qi and Berger, 2007). To quantify the success of the model, the RMSECV and Pearson correlation coefficient (p < 0.05) were calculated.
Results
Figure 1B shows a stack of Raman spectra acquired at 1.5 mm sampling intervals, spanning the length of a representative proximal phalanx. The spectra exhibit smooth evolution along this sequence, with the two epiphyseal regions sharing features that the mid-diaphysis lacks. Of note, the solid boxes highlight a spectral region where epiphyses have two sharp peaks, 1268 cm−1 and 1302 cm−1, that are much less prominent in the diaphysis measurements. The dashed boxes similarly indicate a region where a peak at 1658 cm−1 is higher and narrower at the epiphyses.
Figure 1:
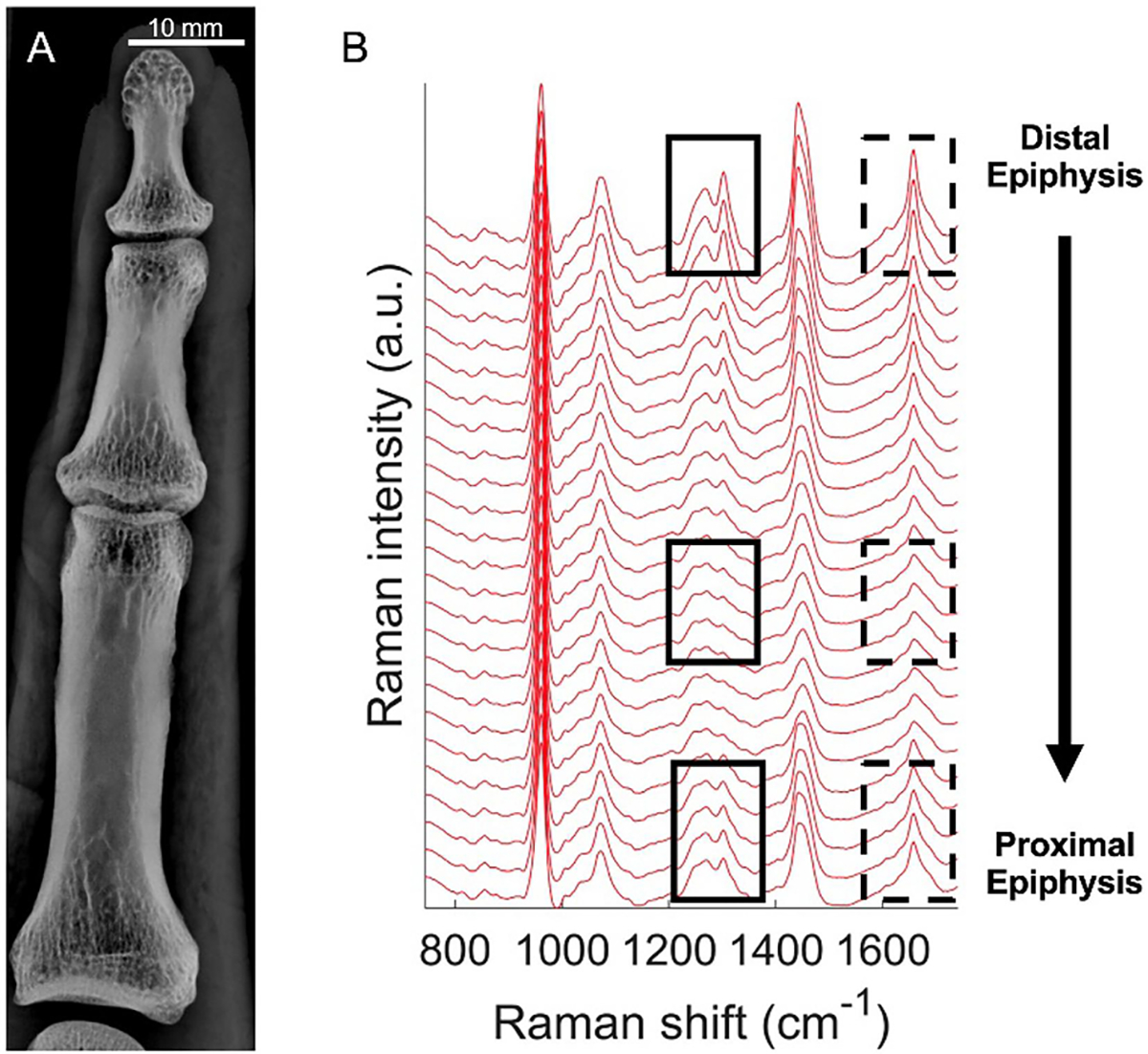
Spectral differences of a single bone measured by Raman spectroscopy. (A) X-ray image of a finger from a healthy subject, indicating distal and proximal epiphysis regions. (B) Raman spectra measured along the length of the bone (spaced 1.5 mm apart). Two spectral regions (solid, dashed boxes) are highlighted to show distinctive differences at the epiphyseal regions and in the diaphysis.
We investigated if either region could detect osteoporosis-related bone changes (Figure 2A and C). The mean healthy spectrum (green) was different from the mean osteopenic (blue) and osteoporotic (red) spectra in both the diaphyseal (Figure 2A) and epiphyseal (Figure 2C) regions. To further characterize these differences, the mean healthy spectrum was subtracted from the mean osteopenic and osteoporotic spectra in the diaphyseal and epiphyseal regions (Figures 2B and D, respectively). These residual plots revealed that osteoporotic samples had relatively lower signal in the phosphate spectral region and higher signal in the regions where matrix contributions exist. Osteopenic samples exhibited the same trend but less strongly. Additionally, we observed the matrix component regions within the residual spectra closely resembled spectral features found in adipose tissue (Figures 2B and D).
Figure 2:

Effects of osteoporosis detected from proximal phalanx Raman spectra. (A) Mean healthy (n = 3), osteopenia (n = 4), osteoporosis (n = 5) spectra from diaphysis. (B) Spectral difference plots showing osteopenia (red) and osteoporosis (blue) have relatively lower phosphate signal and higher matrix signal when compared to healthy. The matrix components in the residual plots have spectral features that resemble pure adipose tissue (black dashed) (C) Mean spectra from epiphysis. (D) Spectral differences from epiphysis show the same trends in diaphysis (relatively lower phosphate and higher matrix).
To further investigate specific bone changes to which the spectra were sensitive, we quantified various peak ratios (Figure 3). Osteoporosis resulted in a significant decrease in PTMR (using the amide I region as the matrix component) in the diaphysis and epiphysis when compared to the healthy subjects (p < 0.01). Additionally, the diaphysis measurements showed a significant decrease between osteopenia to osteoporosis (p < 0.05). While not statistically significant, the CTMR (using amide I as the matrix component) increased between healthy and osteoporosis by 6.9% in the diaphysis measurements (p = 0.092) and 2.5% in the epiphysis measurements (p = 0.74). Osteoporosis resulted in a significant increase in CTPR in the diaphysis and epiphysis measurements when compared to the healthy subjects (p < 0.05); trends in crystallinity exhibited correlation with CTPR, as expected, although with less significant difference between the T-score categories. Additionally, the diaphysis measurements showed a significant increase from osteopenia to osteoporosis (p < 0.05). Similar trends were observed using the other matrix components, CH2 or amide III, and these results are detailed in Supplementary Table 2.
Figure 3:
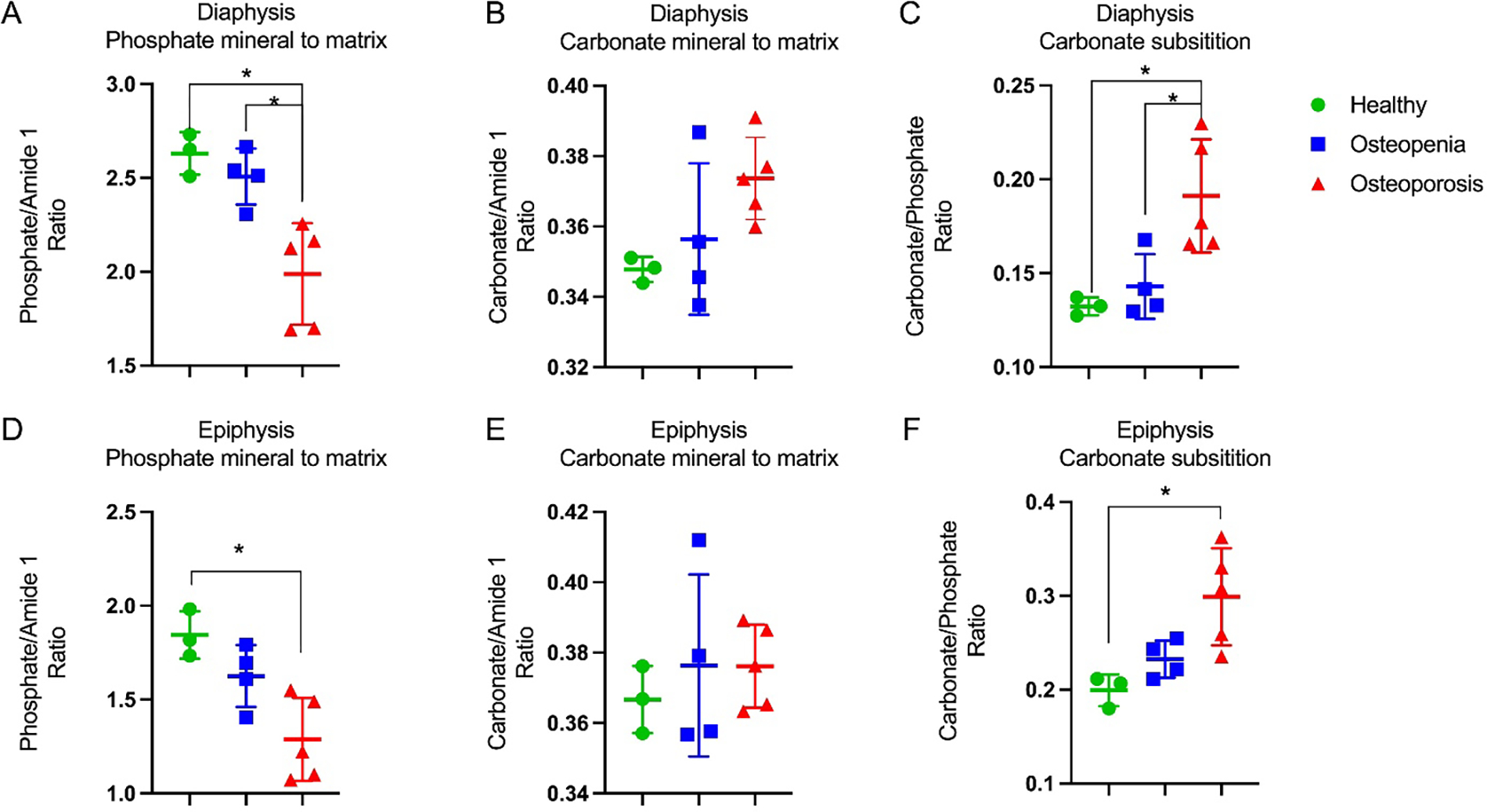
Effects of osteoporosis on proximal phalanges determined by Raman-derived bone quality metrics. (A,B,C) Diaphyseal mineral to matrix ratios and carbonate substitution, showing significant differences between healthy and osteoporosis. (D,E,F) Epiphyseal mineral to matrix ratios and carbonate substitution, showing the same trends as the diaphyseal metrics and significant differences between healthy and osteoporosis. Asterisk indicates significance determined by Tukey’s multiple comparisons test (p < 0.05).
We sought to quantify the effects that osteoporosis has on the matrix spectral signature. As shown in Supplementary Figure 1, pure adipose tissue has spectral peaks at 1264, 1302, 1438, and 1658 cm−1. The mean healthy diaphysis spectrum has peaks at 1248, 1450, 1662 cm−1 (Figure 2). By modeling the amide III, CH2, and amide I regions with pre-selected Lorentz-Gaussian sub-peaks, using the parameters summarized in Supplementary Table 2, the relative strengths of spectral features associated with adipose and mean healthy bone could be compared. For example, the CH2 region was modeled using 4 sub-peaks, two of which were located near 1438 and 1450 cm−1 (Figure 4A). Because these peaks are associated with lipid and collagen, respectively (Manoharan et al., 1992), the ratio of the areas of these sub-peaks provided a metric. The 1438 cm−1/1450 cm−1 area ratio from diaphysis measurements (Figure 4B) was significantly higher in the osteoporotic samples compared to healthy (p < 0.01) and osteopenia (p < 0.05). Although the epiphysis measurements had no significant differences, the same increasing trend was observed (Figure 4C).
Figure 4:

Raman derived matrix component differences in osteoporosis. (A) CH2 region was deconvolved into 4 subpeaks. (B, C) The ratio of 1438 cm−1 to 1450 cm−1 was significantly higher in osteoporotic samples in both diaphysis and epiphysis measurements. (D) Amide I region deconvolved into 4 subpeaks. (E) Ratio of 1660 cm−1 to 1634 cm−1 from diaphysis measurements was significantly higher in osteoporosis. (F) Ratio of 1656 to 1636 cm−1 from epiphysis measurements was significantly higher in osteoporosis. Asterisk indicates significance determined by Tukey’s multiple comparisons test (p < 0.05).
Similarly, using four Lorentz-Gaussians to model the spectral region that contains amide I vibrations, the ratio 1660 cm−1/1636 cm−1 was calculated, because the 1660 cm−1 subband matches a distinctive and sharp vibrational band of lipids (as shown in Supplementary Figure 1). Both the diaphysis and epiphysis spectra showed the osteoporotic samples had a significantly higher ratio when compared to the healthy subjects (Figure 4 E and F), suggesting either an increase in adipose signature or a change in the amide I matrix component.
To characterize the cortical bone geometry and trabecular architecture, μCT was performed on the proximal phalanges (Figure 5). The cortical thickness was significantly lower in osteoporosis when compared to healthy (p < 0.01) and osteopenia (p < 0.05). The cortical bone area fraction and vBMD significantly decreased in osteoporosis compared to healthy and osteopenia (p < 0.05). Trabecular bone analysis on the distal epiphysis showed significant differences between healthy and osteopenia and healthy and osteoporosis in (p < 0.05) in several trabecular bone properties (p < 0.05). In summary, osteopenia and osteoporosis resulted in significant reductions in bone volume fraction, trabecular number and thickness, and trabecular vBMD.
Figure 5:
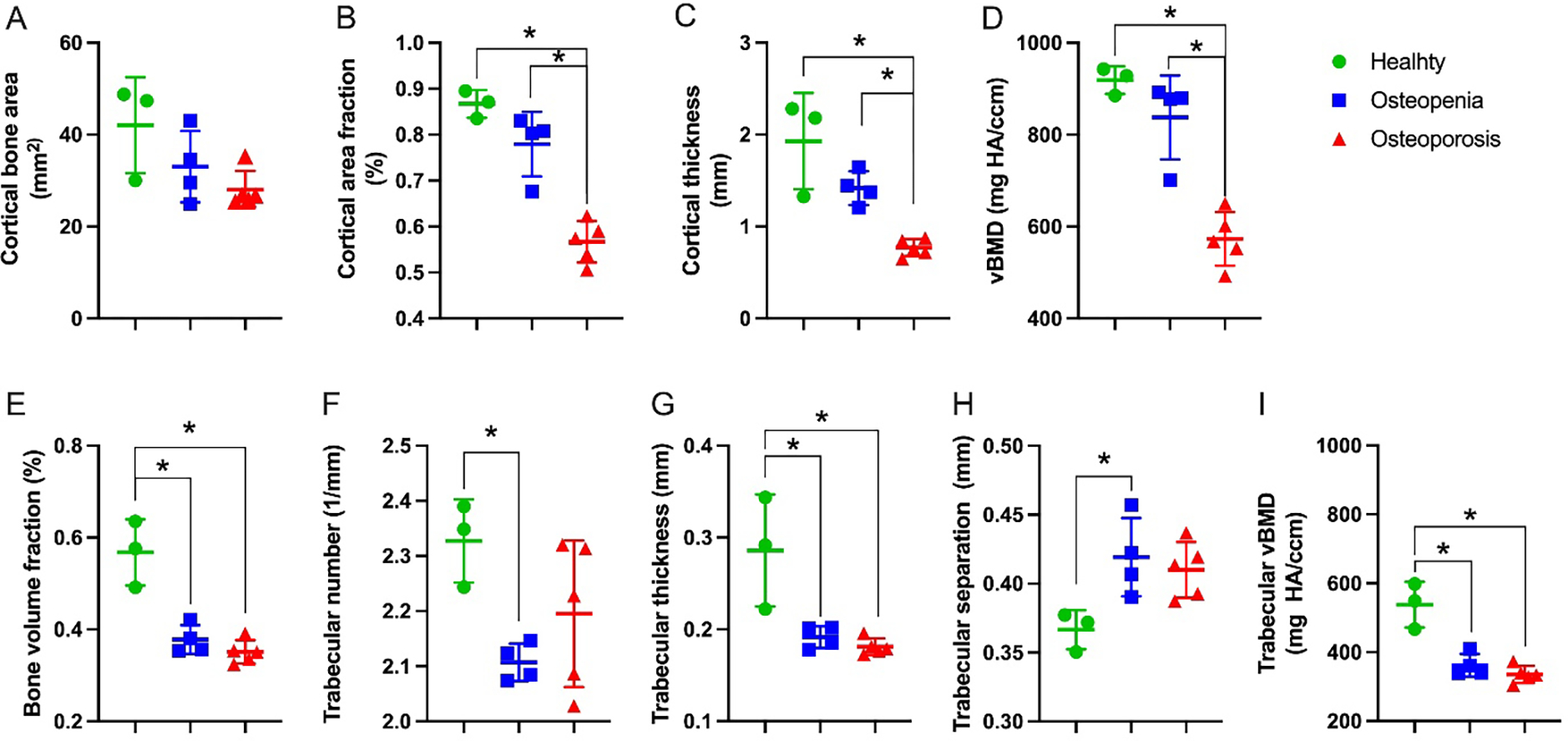
Osteoporotic related changes on proximal phalanges measured through μCT. (A-D) Cortical bone microarchitecture measured on the distal epiphyseal region. (E-I) Trabecular bone morphology measured on the mid-diaphysis. Asterisk indicates significance determined by Tukey’s multiple comparisons test (p < 0.05).
To assess whether Raman spectroscopy measurements taken from the phalanges could be used as a diagnostic or pre-screening tool for osteoporosis in the wrist, a PLSR-LOOCV regression was carried out. The analysis utilized spectra from both the proximal phalanx diaphysis (as shown in Figure 6A and B) and the epiphysis (as shown in Figure 6C and D) to predict the T-score from the wrist DXA scan. Both sets of spectra yielded predictive models with a correlation between the predicted and measured values of r = 0.60 (p < 0.05) and r = 0.79 (p < 0.05) for the diaphysis and epiphysis spectra, respectively. The mean of the predicted wrist T-scores for samples diagnosed with osteoporosis was significantly lower than those of healthy samples (Figure 6B and D) (p < 0.05).
Figure 6:

PLSR results for predicting osteoporotic diagnostic metric, T-score, derived from a wrist DXA scan using Raman spectra with a leave one out cross validation. (A) Diaphysis Raman spectra results that yielded a RMSECV of 1.46 and a significant correlation (r = 0.6, p <0.05). (Note: two data points from the healthy cohort overlap to within the plot resolution.) (B) Predicted and measured T-scores grouped by cohort. The predicted T-scores for the osteoporotic samples have a significantly lower mean T-score compared to the healthy samples. (C,D) Epiphysis Raman spectra yielded similar results with a RMSECV of 1.12 and a significant prediction model (r = 0.79, p <0.05) and significant differences between the predicted T-scores in healthy and osteoporosis. @ indicates significance compared to predicted healthy, determined by Tukey’s multiple comparisons test (p < 0.05).
In order to determine whether Raman spectroscopy measurements obtained from the phalanges could predict the fracture strength of the radius bone in the wrist, we conducted biomechanical testing to simulate a low-energy fracture resulting from a fall (as depicted in Figure 7A–C). The fracture strength was defined as the maximum force to induce a radial fracture and the fracture energy was estimated from the area under the force-displacement curves at the maximum load. The osteoporotic samples exhibited a significant 35% reduction in Fracture strength and energy compared to healthy samples (p < 0.05, Figures 7D and 7E, respectively). We then focused on developing a non-destructive model that could accurately predict fracture strength.
Figure 7:

Effects of osteoporosis on distal radius biomechanical properties. (A) Experimental set up to simulate a fall which results in a distal radius fracture. (B) X-ray confirmation showing distal radius fracture. (C) Representative Force-Displacement curves of healthy, osteopenic, and osteorporotic samples, (D) Fracture force was significantly higher in healthy samples than in osteoporosis samples (p < 0.05), determined by Tukey’s multiple comparisons test. (E) Fracture energy was significantly higher in healthy samples than in osteoporosis samples (p < 0.05), determined by Tukey’s multiple comparisons test. PLSR results for predicting fracture force using: (F) BMI, age, radius BMD that yielded a RMSECV of 245 N and a correlation coefficient of 0.86 showing a significant correlation (p < 0.05), (G) BMI, age, diaphysis proximal phalanx Raman measurements (PTMR, CTMR, CTPR) that yielded a RMSECV of 309 N and a correlation coefficient (r) of 0.79 showing a significant correlation (p < 0.05), and (H) BMI, age, epiphysis proximal phalanx Raman measurements (PTMR, CTMR, CTPR) that yielded a RMSECV of 274 N and a correlation coefficient (r) of 0.81, showing a significant correlation (p < 0.05).
Through standard least squares regression, we identified BMI and age as significant predictors of fracture strength (Supplementary Table 4). By incorporating BMI, age, and radius BMD (measured from DXA) into a PLSR-LOOCV regression approach, we generated a significant prediction model of radial fracture strength with a RMSECV of 245 (N) and a correlation coefficient of 0.86 (p < 0.05, Figure 7F). When we replaced wrist BMD with Raman ratios measured from the phalangeal diaphysis spectra (as depicted in Figure 3A–C) we achieved a comparable prediction model of radial fracture strength with a RMSECV of 309 (N) and a correlation coefficient of 0.79 (p < 0.05, Figure 7G). Similarly, by utilizing Raman ratios from the phalangeal epiphysis spectra, we generated another comparable prediction model of radial fracture strength with a RMSECV of 274 (N) and a correlation coefficient of 0.81 (p < 0.05, Figure 7H). We also explored the use of alternative matrix components, such as amide III in place of amide I, and found that Raman measurements from both the phalangeal diaphysis and epiphysis yielded significant correlations with radial fracture strength (r = 0.76, r = 0.63 respectively). However, while CH2 did not yield a significant correlation for the diaphysis (r = 0.38), it resulted in significant correlations for the epiphysis (r = 0.56).
Discussion
The alarmingly low screening rates for osteoporotic fractures have led to a pressing need for a pre-screening modality that can provide an indication for the clinical gold standard DXA screening. Raman spectroscopy has been evaluated for decades as a promising technology to assess bone health due to its ability to measure the biochemical composition of bone non-invasively. Although Raman spectroscopy has shown promising results in diagnosing bone health and osteoporosis in animal models and humans, it has been challenging to translate it as a clinical diagnostic tool in the clinic. Some of the main limitations include difficulties in standardizing measurement techniques, limitations in the depth of tissue penetration, and the need for further validation studies in large human populations (Boskey and Mendelsohn, 2005; Carden and Morris, 2000; Morris and Mandair, 2011). To address these difficulties, this study tested the feasibility of detecting bone changes related to osteoporosis by measuring Raman spectra from excised proximal phalanges from human cadaver donors with various osteoporotic classifications based on wrist DXA measurements. Our findings revealed distinctive spectral features from phalanges’ epiphyseal and diaphyseal regions, with both regions exhibiting sensitivity to osteoporotic-wrist bone changes. Furthermore, we demonstrated the potential of Raman spectroscopy as a diagnostic tool of distal radius T-score (BMD) and strength based on predictive regression models that incorporate age, BMI, and Raman-derived bone health metrics measured in the phalanges.
Regardless of bone health, we observed a notable increase in adipose signal in epiphyseal regions compared to diaphyseal regions, regardless of bone health status (Figure 2). Potential explanations for this observation are that the epiphyseal region has a thinner cortical shell compared to the diaphysis (Downey and Siegel, 2006) and that the cavities of trabecular bone are filled with fatty marrow (Cordes et al., 2016; Wang et al., 2018). This suggests that the signal collected from the epiphyseal regions likely represents a combination of altered cortical and trabecular bone structure and composition as well as increased bone marrow fat mass due to a shift in differentiation of mesenchymal stem cells to adipocytes rather than to osteoblasts (Cordes et al., 2016; Wang et al., 2018). When comparing differences in disease classification, the osteoporotic samples had an increased adipose signal compared to healthy samples at both the diaphysis and the epiphyses (Figure 2 and 4). This is consistent with the known association between osteoporosis and increased bone marrow fat (Fazeli et al., 2013; Paccou et al., 2015; Rosen and Bouxsein, 2006).
According to recent epidemiologic studies, the prevalence of wrist (distal radius) fractures is on the order of 12% for Americans aged 50 and above, with comparable fracture rates between men (12.8%) and women (11.4%). The fracture rates, primarily resulting from falls from a standing height and associated with osteoporosis, increase for women aged over 60 years to 14.3% (Ye et al., 2022). We tested whether Raman measurements of a finger bone, an easily accessible site, were able to predict metrics relevant to osteoporosis and fragility fracture risk at the wrist. Specifically, we used Raman metrics to predict 1) T-score measured from a wrist DXA scan and 2) maximum load from a distal radius fracture. We were able to construct significantly predictive models for the wrist T-score using the spectra from the diaphysis and epiphysis regions of the proximal phalanges (Figure 6A+C). Grouping the predicted wrist T-scores by diagnosis, these models predicted the osteoporotic samples’ average T score to be significantly lower than the healthy samples’ average (Figure 6B and D), which agrees with the wrist DXA ground truth. These findings demonstrate the potential of using Raman spectroscopy as a pre-screening tool, which could be used to indicate the need for a DXA scan. While others have shown that Raman features can predict biomechanical properties of human bone (Makowski et al., 2017; Unal et al., 2021), this is the first report to our knowledge showing that an easily accessible bone (proximal phalanges) can predict mechanical properties of a different, clinically-relevant region of the skeleton (distal radius). These results are highly encouraging as they achieved comparable predictions of radial fracture strength to the model using the BMD of the bone that was fractured (Figure 7).
This study is not without limitations. First, while our study may have had a small sample size, our rigorous statistical analysis methods using LOOCV-PLSR predictive models mitigate this concern. However, the classification accuracy achieved in this study highlights the need for further investigation and validation. Future work will increase the sample size and potentially focus on post-menopausal osteoporosis, since women are at a higher risk of developing osteoporosis and fragility fractures. In fact, according to the International Osteoporosis Foundation, 1 in 3 women over the age of 50 will experience osteoporotic fractures, whereas the number for men is 1 in 5 (Chen et al., 2013; Curtis et al., 2016; Kanis et al., 2000; Melton et al., 1998; Melton et al., 1992). Furthermore, a larger sample size would enable separate identification of age, sex, BMI, and ethnicity related effects that contribute to the etiology of osteoporosis (Lane, 2006). Second, while more serious osteoporosis fractures occur in the spine and hip (Dimai and Fahrleitner-Pammer, 2022), in this work, we focused on wrist fractures because we could only obtain wrist T-scores from the cadaver supplier. This allowed us to correlate our Raman parameters from the digits with wrist T-scores and distal radius fracture toughness. Future work should attempt to obtain cadaver specimens from donors with hip or spine T-scores to investigate whether Raman measurements in the peripheral digits correlate with hip or spine T-scores.
Finally, we obtained Raman spectra from exposed proximal phalanges. For clinical translation, larger studies will need to perform similar measurements transcutaneously. Our published preliminary studies have already demonstrated the feasibility of transcutaneously measuring Raman spectra of human hand bones using spatially offset Raman spectroscopy (SORS) (Chen et al., 2021) and the ability to extract faithful estimates of ex vivo bone Raman spectra from multi-offset in situ measurements of intact murine hindlegs (Maher et al., 2013). Future studies will focus on optimizing the bone signal to noise ratio (BNR) measured transcutaneously to build reliable Raman models to predict gold-standard bone metrics currently provided by DXA and fracture tests. In addition, we could investigate whether proximal phalanges can predict the compositional properties of metacarpals using Raman spectroscopy, as there is evidence digital X-ray radiogrammetry of the metacarpals can predict subsequent hip fractures (Bouxsein et al., 2002).
A portable in vivo RS instrument could be transported by a regular van to primary care offices or to pop-up clinics in remote locations. Pre-screening via RS could identify subjects most in need of a gold-standard DXA scan at a central facility.
Conclusion
We demonstrated the potential of Raman spectroscopy measurements in the proximal phalanges for detecting osteoporotic bone changes and fracture strength in the distal radius. Human cadaver specimens with various bone health conditions allowed us to address intermediate steps in support of future transcutaneous in vivo measurements. We determined that Raman spectral features obtained from both diaphyseal and epiphyseal regions of the proximal phalanges correlated with osteoporosis-related changes and predicted the DXA T-score of the distal radius with significant accuracy. By calculating common Raman metrics from either diaphyseal or epiphyseal bones and combining them with donor biometrics (BMI and age), the maximum load of distal radius fractures in simulated falls could be predicted via linear regression with high accuracy. To the best of our knowledge, this is the first demonstration of using Raman spectroscopy on the proximal phalanges in detecting osteoporosis and associated fracture properties of a clinically-relevant site such as the wrist.
Supplementary Material
Table 2:
Accuracy of predicting max load for distal radius fracture.
| Inputs | RMSECV (N) | r | p |
|---|---|---|---|
| BMI, age, radius BMD | 245 | 0.86 | p < 0.05 |
| BMI, age, diaphysis proximal phalanx Raman metrics (PTMR, CTMR, CTPR) *amide I was used as matrix component |
309 | 0.79 | p < 0.05 |
| BMI, age, epiphysis proximal phalanx Raman metrics (PTMR, CTMR, CTPR) *amide I was used as matrix component |
274 | 0.81 | p < 0.05 |
Acknowledgements
The authors would like to thank Lindsay Schnur for her technical assistance with μCT. The study was supported by grant numbers F31AR079841 (CM), R01AR070613 (AB and HA), R21AR061285 (AB and HA), and P30AR069655 (HA) from NIAMS/NIH. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Conflict of Interest Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alunni Cardinali M, Morresi A, Fioretto D, Vivarelli L, Dallari D, Govoni M, 2021. Brillouin and Raman Micro-Spectroscopy: A Tool for Micro-Mechanical and Structural Characterization of Cortical and Trabecular Bone Tissues. Materials (Basel) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Moreno AJ, Hosseini HS, Bevers M, Ito K, Zysset P, van Rietbergen B, 2019. Validation of distal radius failure load predictions by homogenized- and micro-finite element analyses based on second-generation high-resolution peripheral quantitative CT images. Osteoporosis International 30, 1433–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach SF, Dall’Ara E, Weninger P, Antoni A, Traxler H, Dörr M, Zysset PK, 2012. Assessment of a novel biomechanical fracture model for distal radius fractures. BMC Musculoskeletal Disorders 13, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey AL, Mendelsohn R, 2005. Infrared spectroscopic characterization of mineralized tissues. Vib Spectrosc 38, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouxsein ML, Palermo L, Yeung C, Black DM, 2002. Digital X-ray radiogrammetry predicts hip, wrist and vertebral fracture risk in elderly women: a prospective analysis from the study of osteoporotic fractures. Osteoporos Int 13, 358–365. [DOI] [PubMed] [Google Scholar]
- Carden A, Morris MD, 2000. Application of vibrational spectroscopy to the study of mineralized tissues (review). Journal of biomedical optics 5 3, 259–268. [DOI] [PubMed] [Google Scholar]
- Carretta R, Stüssi E, Müller R, Lorenzetti S, 2015. Prediction of local ultimate strain and toughness of trabecular bone tissue by Raman material composition analysis. Biomed Res Int 2015, 457371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CW, Huang TL, Su LT, Kuo YC, Wu SC, Li CY, Chen KB, Sung FC, 2013. Incidence of subsequent hip fractures is significantly increased within the first month after distal radius fracture in patients older than 60 years. J Trauma Acute Care Surg 74, 317–321. [DOI] [PubMed] [Google Scholar]
- Chen K, Massie C, Awad HA, Berger AJ, 2021. Determination of best Raman spectroscopy spatial offsets for transcutaneous bone quality assessments in human hands. Biomedical Optics Express 12, 7517–7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes C, Baum T, Dieckmeyer M, Ruschke S, Diefenbach MN, Hauner H, Kirschke JS, Karampinos DC, 2016. MR-Based Assessment of Bone Marrow Fat in Osteoporosis, Diabetes, and Obesity. Frontiers in Endocrinology 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan T, Dockery F, 2014. Osteoporosis and fracture risk in older people. Clin Med (Lond) 14, 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddihy MT, Gabriel SE, Crowson CS, O’Fallon WM, Melton Iii LJ, 1999. Forearm Fractures as Predictors of Subsequent Osteoporotic Fractures. Osteoporosis International 9, 469–475. [DOI] [PubMed] [Google Scholar]
- Curtis EM, van der Velde R, Moon RJ, van den Bergh JP, Geusens P, de Vries F, van Staa TP, Cooper C, Harvey NC, 2016. Epidemiology of fractures in the United Kingdom 1988–2012: Variation with age, sex, geography, ethnicity and socioeconomic status. Bone 87, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimai HP, Fahrleitner-Pammer A, 2022. Osteoporosis and Fragility Fractures: currently available pharmacological options and future directions. Best Pract Res Clin Rheumatol 36, 101780. [DOI] [PubMed] [Google Scholar]
- Downey PA, Siegel MI, 2006. Bone Biology and the Clinical Implications for Osteoporosis. Physical Therapy 86, 77–91. [DOI] [PubMed] [Google Scholar]
- Eckstein F, Kuhn V, Lochmüller EM, 2004. Strength prediction of the distal radius by bone densitometry--evaluation using biomechanical tests. Ann Biomed Eng 32, 487–503. [DOI] [PubMed] [Google Scholar]
- Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, Klibanski A, 2013. Marrow fat and bone--new perspectives. J Clin Endocrinol Metab 98, 935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force USPST, 2018. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA 319, 2521–2531. [DOI] [PubMed] [Google Scholar]
- Gillespie CW, Morin PE, 2017. Trends and Disparities in Osteoporosis Screening Among Women in the United States, 2008–2014. Am J Med 130, 306–316. [DOI] [PubMed] [Google Scholar]
- Goodyear SR, Gibson IR, Skakle JM, Wells RP, Aspden RM, 2009. A comparison of cortical and trabecular bone from C57 Black 6 mice using Raman spectroscopy. Bone 44, 899–907. [DOI] [PubMed] [Google Scholar]
- Hudelmaier M, Kuhn V, Lochmüller EM, Well H, Priemel M, Link TM, Eckstein F, 2004. Can geometry-based parameters from pQCT and material parameters from quantitative ultrasound (QUS) improve the prediction of radial bone strength over that by bone mass (DXA)? Osteoporosis International 15, 375–381. [DOI] [PubMed] [Google Scholar]
- Johnell O, Kanis JA, 2006. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17, 1726–1733. [DOI] [PubMed] [Google Scholar]
- Kanis JA, Johnell O, Oden A, Sembo I, Redlund-Johnell I, Dawson A, De Laet C, Jonsson B, 2000. Long-term risk of osteoporotic fracture in Malmö. Osteoporos Int 11, 669–674. [DOI] [PubMed] [Google Scholar]
- Kazakia GJ, Burghardt AJ, Link TM, Majumdar S, 2011. Variations in morphological and biomechanical indices at the distal radius in subjects with identical BMD. J Biomech 44, 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Cole JH, Boskey AL, Baker SP, van der Meulen MC, 2014. Reduced tissue-level stiffness and mineralization in osteoporotic cancellous bone. Calcif Tissue Int 95, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M, 2000. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15, 721–739. [DOI] [PubMed] [Google Scholar]
- Lane NE, 2006. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol 194, S3–11. [DOI] [PubMed] [Google Scholar]
- Link TM, 2012. Osteoporosis imaging: state of the art and advanced imaging. Radiology 263, 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochmüller E-M, Lill CA, Kuhn V, Schneider E, Eckstein F, 2002. Radius Bone Strength in Bending, Compression, and Falling and Its Correlation With Clinical Densitometry at Multiple Sites. Journal of Bone and Mineral Research 17, 1629–1638. [DOI] [PubMed] [Google Scholar]
- Maher JR, Inzana JA, Awad HA, Berger AJ, 2013. Overconstrained library-based fitting method reveals age- and disease-related differences in transcutaneous Raman spectra of murine bones. Journal of biomedical optics 18, 077001–077001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher JR, Takahata M, Awad HA, Berger AJ, 2011. Raman spectroscopy detects deterioration in biomechanical properties of bone in a glucocorticoid-treated mouse model of rheumatoid arthritis. J Biomed Opt 16, 087012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski AJ, Granke M, Ayala OD, Uppuganti S, Mahadevan-Jansen A, Nyman JS, 2017. Applying Full Spectrum Analysis to a Raman Spectroscopic Assessment of Fracture Toughness of Human Cortical Bone. Appl Spectrosc 71, 2385–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallmin H, Ljunghall S, 1994. Distal radius fracture is an early sign of general osteoporosis: bone mass measurements in a population-based study. Osteoporos Int 4, 357–361. [DOI] [PubMed] [Google Scholar]
- Manoharan R, Baraga JJ, Feld MS, Rava RP, 1992. Quantitative histochemical analysis of human artery using Raman spectroscopy. J Photochem Photobiol B 16, 211–233. [DOI] [PubMed] [Google Scholar]
- Massie C, Knapp E, Chen K, Berger AJ, Awad HA, 2021. Improved prediction of femoral fracture toughness in mice by combining standard medical imaging with Raman spectroscopy. Journal of Biomechanics 116, 110243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam-Marx C, Unni S, Ye X, Nelson S, Nickman NA, 2012. Effect of Medicare reimbursement reduction for imaging services on osteoporosis screening rates. J Am Geriatr Soc 60, 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreadie BR, Morris MD, Chen T. c., Sudhaker Rao D, Finney WF, Widjaja E, Goldstein SA, 2006. Bone tissue compositional differences in women with and without osteoporotic fracture. Bone 39, 1190–1195. [DOI] [PubMed] [Google Scholar]
- Melton LJ 3rd, Atkinson EJ, O’Connor MK, O’Fallon WM, Riggs BL, 1998. Bone density and fracture risk in men. J Bone Miner Res 13, 1915–1923. [DOI] [PubMed] [Google Scholar]
- Melton LJ 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL, 1992. Perspective. How many women have osteoporosis? J Bone Miner Res 7, 1005–1010. [DOI] [PubMed] [Google Scholar]
- Morris MD, Mandair GS, 2011. Raman assessment of bone quality. Clinical orthopaedics and related research 469, 2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller ME, Webber CE, Bouxsein ML, 2003. Predicting the failure load of the distal radius. Osteoporos Int 14, 345–352. [DOI] [PubMed] [Google Scholar]
- Ojanen X, Isaksson H, Töyräs J, Turunen MJ, Malo MKH, Halvari A, Jurvelin JS, 2015. Relationships between tissue composition and viscoelastic properties in human trabecular bone. Journal of Biomechanics 48, 269–275. [DOI] [PubMed] [Google Scholar]
- Paccou J, Hardouin P, Cotten A, Penel G, Cortet B, 2015. The Role of Bone Marrow Fat in Skeletal Health: Usefulness and Perspectives for Clinicians. J Clin Endocrinol Metab 100, 3613–3621. [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Fratzl P, Gamsjaeger S, Hassler N, Brozek W, Eriksen EF, Rauch F, Glorieux FH, Shane E, Dempster D, Cohen A, Recker R, Klaushofer K, 2016. Aging Versus Postmenopausal Osteoporosis: Bone Composition and Maturation Kinetics at Actively-Forming Trabecular Surfaces of Female Subjects Aged 1 to 84 Years. J Bone Miner Res 31, 347–357. [DOI] [PubMed] [Google Scholar]
- Pejović-Milić A, Brito J, Gyorffy J, Chettle D, 2002. Ultrasound measurements of overlying soft tissue thickness at four skeletal sites suitable for in vivo x-ray fluorescence. Medical physics 29, 2687–2691. [DOI] [PubMed] [Google Scholar]
- Qi D, Berger AJ, 2007. Chemical concentration measurement in blood serum and urine samples using liquid-core optical fiber Raman spectroscopy. Appl. Opt. 46, 1726–1734. [DOI] [PubMed] [Google Scholar]
- Register, O.o.t.F., 1997. Public Law 105 – 33 - Balanced Budget Act of 1997, in: Administration, N.A.a.R. (Ed.). [Google Scholar]
- Riggs BL, Melton LJ 3rd, 1995. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 17, 505s–511s. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Bouxsein ML, 2006. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2, 35–43. [DOI] [PubMed] [Google Scholar]
- Shu C, Chen K, Lynch M, Maher JR, Awad HA, Berger AJ, 2018. Spatially offset Raman spectroscopy for in vivo bone strength prediction. Biomedical optics express 9, 4781–4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchenko EV, Timchenko PE, Pisareva EV, Vlasov MY, Volova LT, Fedotov AA, Fedorova YV, Tyumchenkova AS, Romanova DA, Daniel MA, Subatovich AN, 2020. Optical analysis of bone tissue by Raman spectroscopy in experimental osteoporosis and its correction using allogeneic hydroxyapatite. J. Opt. Technol. 87, 161–167. [Google Scholar]
- Unal M, Ahmed R, Mahadevan-Jansen A, Nyman JS, 2021. Compositional assessment of bone by Raman spectroscopy. Analyst (London) 146, 7464–7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal M, Uppuganti S, Timur S, Mahadevan-Jansen A, Akkus O, Nyman JS, 2019. Assessing matrix quality by Raman spectroscopy helps predict fracture toughness of human cortical bone. Sci Rep 9, 7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Leng Y, Gong Y, 2018. Bone Marrow Fat and Hematopoiesis. Frontiers in Endocrinology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczek ML, Kälvesten J, Algulin J, Beiki O, Brismar TB, 2013. Digital X-ray radiogrammetry of hand or wrist radiographs can predict hip fracture risk--a study in 5,420 women and 2,837 men. European radiology 23, 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Li Q, Nie J, 2022. Prevalence, Characteristics, and Associated Risk Factors of Wrist Fractures in Americans Above 50: The Cross-Sectional NHANES Study. Front Endocrinol (Lausanne) 13, 800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerramshetty JS, Akkus O, 2008. The associations between mineral crystallinity and the mechanical properties of human cortical bone. Bone 42, 476–482. [DOI] [PubMed] [Google Scholar]
- Zhang J, Delzell E, Zhao H, Laster AJ, Saag KG, Kilgore ML, Morrisey MA, Wright NC, Yun H, Curtis JR, 2012. Central DXA utilization shifts from office-based to hospital-based settings among medicare beneficiaries in the wake of reimbursement changes. Journal of Bone and Mineral Research 27, 858–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


