Abstract
Infectious syphilis, caused by the spirochete bacterium Treponema pallidum subsp. pallidum, remains a public health concern worldwide. The immune-response evasion mechanisms employed by T. pallidum are poorly understood, and prior attempts to identify immunoprotective antigens for subsequent vaccine design have been unsuccessful. Previous investigations conducted in our laboratory identified the T. pallidum glycerophosphodiester phosphodiesterase as a potential immunoprotective antigen by using a differential immunologic expression library screen. In studies reported here, heterologous expression of the T. pallidum glycerophosphodiester phosphodiesterase in Escherichia coli yielded a full-length, enzymatically active protein. Characterization of the recombinant molecule showed it to be bifunctional, in that it exhibited specific binding to human immunoglobulin A (IgA), IgD, and IgG in addition to possessing enzymatic activity. IgG fractionation studies revealed specific binding of the recombinant enzyme to the Fc fragment of human IgG, a characteristic that may play a role in enabling the syphilis spirochete to evade the host immune response. In further investigations, immunization with the recombinant enzyme significantly protected rabbits from subsequent T. pallidum challenge, altering lesion development at the sites of challenge. In all cases, animals immunized with the recombinant molecule developed atypical pale, flat, slightly indurated, and nonulcerative reactions at the challenge sites that resolved before lesions appeared in the control animals. Although protection in the immunized rabbits was incomplete, as demonstrated by the presence of T. pallidum in the rabbit infectivity test, glycerophosphodiester phosphodiesterase nevertheless represents a significantly immunoprotective T. pallidum antigen and thus may be useful for inclusion in an antigen cocktail vaccine for syphilis.
Treponema pallidum subsp. pallidum establishes a lifelong chronic infection in the absence of appropriate antibiotic treatment. In the early 1990s, the rate of infectious syphilis in the United States reached its highest level in 40 years. Present-day syphilis continues to be a medically relevant disease affecting both the southern United States and developing nations, with an estimated 3.5 million cases occurring annually worldwide (35). Apart from the serious nature of the disease itself, a number of studies suggest that syphilis infections may increase the risk of acquisition and transmission of human immunodeficiency virus (HIV) (15, 16, 43). Furthermore, syphilis may be more difficult to eradicate from HIV-infected individuals, thus increasing the direct morbidity and mortality associated with treponemal infections (7, 21, 27). The apparent failure of public health efforts to control syphilis worldwide and the increased potential for HIV acquisition have renewed interest in the development of a vaccine against this disease.
Previous attempts to design a subunit syphilis vaccine have met with limited success (25). Immunization protocols performed in the experimental rabbit model using recombinant or native T. pallidum proteins, including protein 4D (9) and T. pallidum endoflagella (13), have provided at best partial protection against intradermal T. pallidum challenge. Moreover, immunization of experimental rabbits with various electroeluted and recombinant T. pallidum proteins, such as the 47-, 37-, 34.5-, 33-, 30-, 17-, and 15-kDa molecules (as designated in Table 3 in reference 33) and T. pallidum rare outer-membrane protein 1 (Tromp1) (8), did not provide any significant protection upon intradermal challenge (24). Complete protection against challenge has been reported only following prolonged, complex immunization regimes using gamma-irradiated (30), antiformin-treated (45), or 4°C “aged” (29) whole organisms. Although these immunization protocols represent impractical methods of vaccination, they do demonstrate that successful vaccination against syphilis may be achieved upon elucidation of the appropriate immunoprotective antigens.
To enable rational vaccine design for syphilis, more information is needed about treponemal interaction with the immune system and, specifically, the immune-response evasion mechanisms employed by T. pallidum. One of the central paradoxes of syphilis is the induction of a rapid humoral and cellular immune response that is capable of eliminating millions of treponemes from primary syphilitic lesions but is incapable of eradicating the few organisms present during latency. Macrophages are believed to be responsible for this rapid clearance of T. pallidum from early lesions, presumably through antibody-mediated treponemal opsonization and subsequent phagocytosis and killing by macrophages. In support of this, antibody has been demonstrated to be required for phagocytosis of treponemes by macrophages in vitro (28) and for macrophage-mediated killing of T. pallidum (5). In addition, the systemic appearance of opsonic antibody has been shown to immediately precede bacterial clearance in the experimental rabbit model (6).
Collectively, these observations demonstrate the importance of identifying the target antigens of T. pallidum-specific opsonic antibody. To accomplish this, we made use of the fact that rabbit macrophages phagocytize T. pallidum in vitro by using antiserum from T. pallidum-infected rabbits as a source of opsonizing antibody (opsonic rabbit serum [ORS]) (28), while antiserum from rabbits immunized with heat-killed T. pallidum fails to opsonize T. pallidum (nonopsonic rabbit serum [NORS]) (23). This observed differential reactivity was exploited to identify putative target antigens of opsonic antibody by immunologically screening a T. pallidum Lambda ZAP II genomic expression library in duplicate with either ORS or NORS. In other organisms, opsonic antibodies generally recognize bacterial peptidoglycan, lipopolysaccharide, capsular polysaccharides, or proteins. Thus, since T. pallidum does not have an accessible peptidoglycan layer nor does it contain known lipopolysaccharide or capsular material, the opsonic targets were predicted to be surface-exposed outer-membrane proteins.
The identification of T. pallidum rare outer-membrane proteins (Tromps) has been technically problematic due in part to the fragile nature of the treponeme outer membrane, the low density of outer-membrane-spanning proteins (34, 48) and the presence of a cell-surface-masking slime layer of host and/or bacterial origin (49). The most compelling candidates for surface-exposed outer-membrane proteins to date are Tromps 1 and 2 (8, 12), although controversy exists as to whether Tromp1 actually resides on the outer leaflet of the treponeme outer membrane (1), and the cellular location of Tromp2 has not been independently confirmed. Differential screening of the T. pallidum genomic expression library with functional antibodies was an alternative approach for the identification of such proteins and had the added benefit of simultaneously targeting potentially immunoprotective proteins involved in opsonization and subsequent bacterial clearance.
As reported previously, this method identified glycerophosphodiester phosphodiesterase (Gpd) from four independent plaques that reacted solely with the ORS (44). Homologues of this molecule have previously been identified in Haemophilus influenzae (Hpd [19, 31]), Escherichia coli (GlpQ [46]), Bacillus subtilis (GlpQ [32]), and in the relapsing fever spirochete Borrelia hermsii (GlpQ [38], Gpd [40]). In E. coli, GlpQ is periplasmic and functions by hydrolyzing glycerophosphodiesters from phospholipid and triglyceride metabolism to glycerol 3-phosphate (22). The Gpd homologues of H. influenzae and B. hermsii are lipoproteins (18, 38, 40), and studies have indicated that at least a subset of these molecules is exposed on the surface of these bacteria (2, 41). Similarly, the T. pallidum Gpd is also predicted to be a lipoprotein (42, 44) and has been isolated from T. pallidum outer-membrane preparations (42). These observations, combined with the immunological screening method we used to isolate the T. pallidum Gpd, suggest that this molecule may be an outer-membrane protein with potential immunoprotective capabilities and, since no such proteins have been definitively identified in T. pallidum, Gpd thus warranted further investigation. Results reported here address the putative cellular location, function, and immunoprotective potential of the T. pallidum Gpd.
MATERIALS AND METHODS
Bacterial strains.
T. pallidum subsp. pallidum (Nichols strain) was propagated in New Zealand White rabbits as previously described (26). E. coli XL-1 Blue and BL21 (DE3) pLysS were obtained from Stratagene (La Jolla, Calif.).
Overexpression studies.
The entire 1,071-bp open reading frame encoding the T. pallidum Gpd (44) (GenBank accession no. AF004286) was PCR amplified from T. pallidum genomic DNA by using primers designed from the 5′ (5′-TATAACAGGGGAGGAGAGAAGCATATGCG-3′; contains a NdeI site) and 3′ (5′-CGGGATCCTCAATAGCGGGCGGGTTTG-3′; contains a BamHI site) ends of the Gpd coding region. Following PCR (60°C annealing, 74°C extension, 30 cycles) the amplification product was digested with NdeI and BamHI, ligated to a similarly digested pET-3a T7 expression vector (Novagen, Madison, Wis.), and transformed first into E. coli XL-1 Blue and then into the E. coli expression strain BL21 (DE3) pLysS. The reading frame and sequence of the expression construct were verified by DNA sequencing using the T7 promoter primer (Pharmacia, Piscataway, N.J.) and internal primers designed from the Gpd DNA sequence, the Applied Biosystems dye terminator sequencing kit, and the ABI 373A DNA sequencer in accordance with the manufacturer’s instructions. Expression, differential fractionation of transformed bacteria into soluble and insoluble fractions, and subsequent purification of inclusion bodies containing the overexpressed recombinant T. pallidum Gpd were performed in accordance with the manufacturer’s instructions (Novagen). Control soluble fractions and inclusion bodies were prepared from E. coli BL21 (DE3) pLysS expressing the pET-3a vector alone.
Enzyme assays.
E. coli BL21 (DE3) pLysS expressing the Gpd-pET-3a construct and control E. coli cells transformed with the pET-3a vector alone were analyzed for Gpd-specific activity by a coupled spectrophotometric assay as described previously (22). Briefly, enzyme activity was determined at 25°C in a 0.5-ml assay mixture containing 0.45 ml of 1 M hydrazine-glycine (pH 9.0) buffer (4.86 ml of hydrazine hydrate plus 1.5 g of glycine diluted to 100 ml with water), 0.5 mM NAD, 10 mM CaCl2, 20 U of glycerol-3-phosphate dehydrogenase (Sigma, St. Louis, Mo.)/ml, 0.5 mM glycerophosphorylcholine (Sigma), and 0.1 to 10 μg of E. coli BL21 (DE3) pLysS lysate prepared from cells transformed with either the Gpd-pET-3a construct or the pET-3a vector alone. The rate of NAD reduction was monitored by recording the increase in absorbance at 340 nm. One unit of phosphodiesterase activity is defined as the amount of enzyme required to hydrolyze 1 μmol of glycerophosphodiester/min under these conditions. A molar absorbance coefficient of 6,300 M−1 cm−1 for NADH at 340 nm was used to calculate specific activity. Lysates were prepared by inducing protein expression from the T7 promoter in bacterial cells with an optical density at 600 nm of 0.5 with 0.4 mM IPTG (isopropyl-β-d-thiogalactopyranoside) followed by growth for an additional 3 h to allow maximal protein expression. Bacteria were harvested by centrifugation, washed once with 50 mM Tris-HCl (pH 8.0)–2 mM EDTA and resuspended in the hydrazine-glycine buffer for subsequent inclusion at the appropriate concentration in the enzyme assay.
Antisera.
Immune rabbit serum (IRS) was collected from rabbits that had been chronically infected with T. pallidum for >90 days. Anti-Gpd polyclonal antiserum was raised in a New Zealand White rabbit by immunizing three times with 100 μg of each of the inclusion bodies purified from E. coli BL21 (DE3) pLysS transformed with the Gpd-pET-3a construct, emulsified in the Ribi adjuvant MPL + TDM + CWS (monophosphoryl lipid A + trehalose dicorynomycolate + cell wall skeleton) (Sigma). Immunizations were administered intradermally (ID), subcutaneously (SC), intramuscularly (IM), and intraperitoneally (IP) at 3-week intervals as outlined by the Ribi adjuvant system, and antiserum for use in subsequent experiments was collected 1 week after the final immunization.
Opsonization assay.
Anti-Gpd polyclonal antiserum and the corresponding control preimmune serum and IRS were tested in three separate experiments with a total number of replicate assays of eight (preimmune serum), seven (anti-Gpd serum), and nine (IRS) for their ability to opsonize T. pallidum by using a standard phagocytosis assay as previously described (39). All antisera were used at a 1:100 dilution and incubated for 4 h with rabbit peritoneal macrophages and T. pallidum prior to determination of the percentage of macrophages phagocytosing treponemes. Statistical analysis was performed using the two-tailed Student t test.
PAGE and immunoblot analyses.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting were performed as previously described (4), except that samples were blotted onto an Immobilon-polyvinylidene fluoride membrane (Millipore Corp., Bedford, Mass.). Heterologous expression of the recombinant T. pallidum Gpd was monitored by SDS-PAGE analysis of approximately 2 μg of total bacterial lysate or soluble and insoluble bacterial fractions and subsequent staining with Coomassie blue R-250. The level of immunoreactivity of anti-Gpd polyclonal antiserum on Gpd-pET-3a- or pET-3a-transformed E. coli was assayed by electrophoresis and blotting of 2 μg of total bacterial lysate or purified inclusion bodies and probing with a 1:1,000 dilution of anti-Gpd polyclonal rabbit serum followed by a 1:3,000 dilution of horseradish peroxidase-labeled goat F(ab′)2 anti-rabbit immunoglobulin G (IgG; Sigma). Standard techniques were used to prepare anti-Gpd polyclonal antiserum from which anti-E. coli antibodies had been removed (36). For analysis of the level of immunoreactivity of anti-Gpd antiserum on washed and unwashed treponemes, T. pallidum was extracted from infected testes as previously described (28) and either immediately resuspended in SDS-PAGE sample buffer (unwashed preparation) or washed one time or three times with 10 mM Tris-HCl (pH 7.5) by centrifugation (15,000 × g) prior to resuspension of the treponemes in sample buffer. Approximately 1.4 × 107 T. pallidum were electrophoresed for each sample (unwashed, washed one time, washed three times), blotted, and probed with a 1:1,000 dilution of anti-Gpd polyclonal rabbit serum followed by a 1:3,000 dilution of horseradish peroxidase-labeled goat F(ab′)2 anti-rabbit IgG. For analysis of the Ig binding capability of T. pallidum Gpd, inclusion bodies were purified from E. coli BL21 (DE3) pLysS expressing the Gpd-pET-3a construct or the pET-3a vector alone, and 2 μg of the inclusion preparation was separated by SDS-PAGE, blotted, and analyzed for Ig binding by sequential incubations with primary Ig and the corresponding horseradish peroxidase-conjugated secondary antibody. To investigate the binding specificity of T. pallidum Gpd for human IgG, IgG papain (Sigma) fractionation and subsequent Fc and Fab fragment purification were performed by standard techniques (17). Primary Igs were used at a concentration of 1 μg/ml and included human IgA (Sigma), IgD (The Binding Site, Birmingham, United Kingdom), intact IgG (Sigma), IgG Fc and Fab fragments, and IgM (Sigma). Corresponding horseradish peroxidase-conjugated secondary antibodies (Sigma) were used at a concentration of 0.2 μg/ml and included goat F(ab′)2 anti-human IgA (alpha chain specific), goat F(ab′)2 anti-human IgD (delta chain specific), goat F(ab′)2 anti-human IgG (gamma chain specific) and goat F(ab′)2 anti-human IgM (mu chain specific). All immunoblots were blocked with 5% gelatin in Tris-buffered saline with 0.1% Tween 20 and developed by using enhanced chemiluminescence detection (Amersham, Cleveland, Ohio). Rainbow high-range molecular mass markers (Amersham) were used as standards.
Immunization and challenge experiments.
In the initial protection experiment, one New Zealand White rabbit was immunized three times (IM, SC, IP, and ID) at 3-week intervals with the Ribi MPL + TDM + CWS adjuvant and 100 μg of purified inclusion bodies from E. coli expressing the Gpd-pET-3a construct. Three weeks after administration of the final immunization, the immunized rabbit and an unimmunized control rabbit were challenged ID at each of six sites on their shaved backs with 103 T. pallidum per site. In a second experiment, two groups of four rabbits each were immunized with 100 μg of purified inclusion bodies from E. coli expressing either the pET-3a vector alone or the Gpd-pET-3a construct. Immunizations were performed using the Ribi adjuvant and an immunization regime similar to that outlined above. These groups of rabbits, as well as an additional group of four rabbits that had received no prior immunization, were challenged with virulent T. pallidum as in the initial protection experiment 2 weeks after the final immunization.
Protection analyses.
For both protection experiments, rabbits were examined daily to monitor the development, appearance, and progression of lesions appearing at the challenge sites. Lesions were designated typical if they were red, raised, indurated, and generally progressed to ulceration, while atypical lesions were pale, flat, only slightly indurated and nonulcerative. Prior to lesion ulceration on the control animals (31 days postinfection), lesion aspirates were collected from all challenge sites and examined by dark-field microscopy for viable treponemes. The serological status of all challenged rabbits was determined by using the Venereal Disease Research Laboratory (VDRL) test at 5 weeks postchallenge. The degree of protection in the immunized animals was assayed by the rabbit infectivity test (47). For this assay, rabbits immunized with either the pET-3a vector alone or the Gpd-pET-3a construct were sacrificed at 10 weeks postchallenge, and their lymph nodes and testes were collected and minced. Tissues deemed negative for treponemes by dark-field microscopic analysis were subsequently transferred to a naive animal by intratesticular infection. Tissue recipient animals were assayed for serological evidence of infection at monthly intervals by VDRL and fluorescent treponemal antibody absorption (FTA-ABS) tests. Statistical analyses were performed using the two-tailed Student t test and analysis of variance with repeated measures.
RESULTS
Overexpression and characterization of the T. pallidum Gpd.
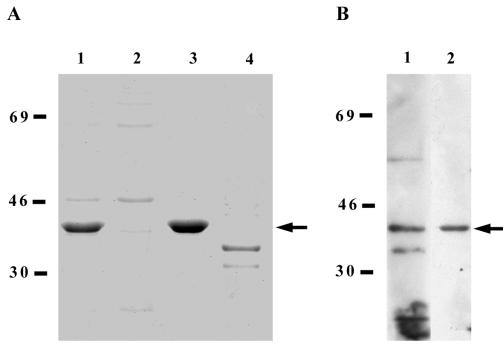
Heterologous expression of the full-length T. pallidum gpd gene in E. coli BL21 (DE3) pLysS by using the inducible pET-3a T7 expression system resulted in efficient production of a 41-kDa recombinant molecule, as assayed by SDS-PAGE and subsequent Coomassie blue staining (Fig. 1A, lane 1). Differential fractionation of the transformed bacteria into soluble and insoluble fractions showed that the majority of the recombinant 41-kDa molecule was found in the inclusion body preparation (lanes 2 and 3, respectively) and that the recombinant 41-kDa molecule represented the major protein in the inclusion body preparation (at least 90% of the total protein). No corresponding protein within this molecular mass range was observed in the insoluble fraction of control E. coli BL21 (DE3) pLysS transformed with the pET-3a vector alone (lane 4). Although the Gpd-pET-3a expression construct contained the entire Gpd open reading frame, including the putative lipoprotein leader sequence (44), the unusual signal peptidase II site (LVAGC) may not be efficiently recognized in E. coli. This would explain the predominant localization of the recombinant T. pallidum Gpd to the cytoplasmic inclusion bodies.
FIG. 1.
Overexpression of the recombinant T. pallidum Gpd and analysis of anti-Gpd immunoreactivity. (A) Coomassie blue-stained SDS-PAGE analysis of E. coli BL21 (DE3) pLysS expressing either the Gpd-pET-3a construct (lane 1, crude lysate; lane 2, soluble fraction; lane 3, insoluble fraction) or the pET-3a vector alone (lane 4, insoluble fraction). (B) Immunoblot analysis of anti-Gpd immunoreactivity on purified inclusion bodies from E. coli BL21 (DE3) pLysS expressing the Gpd-pET-3a construct. Lanes: 1, anti-Gpd polyclonal antiserum, 2, E. coli-adsorbed anti-Gpd polyclonal antiserum. Each lane contains approximately 2 μg of total bacterial lysate and soluble or insoluble bacterial fractions, and molecular mass standards in kilodaltons are indicated at the left of each panel. In each panel, the recombinant T. pallidum Gpd is indicated by an arrow.
Enzymatic analysis of crude bacterial lysates demonstrated E. coli expressing the Gpd-pET-3a construct exhibited threefold-higher Gpd-specific enzymatic activity than E. coli expressing the pET-3a vector alone (specific activities of 7.2 U mg−1 versus 2.3 U mg−1, respectively). This increase in enzymatic activity observed for Gpd-pET-3a-transformed E. coli verifies that these bacteria are overexpressing Gpd. The background level of Gpd-specific activity observed in vector-transformed bacteria results from the endogenously expressed E. coli Gpd homologue, GlpQ (22).
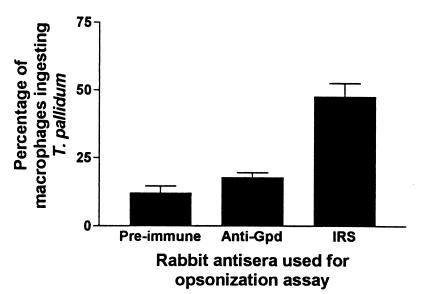
Inclusion bodies purified from E. coli expressing the Gpd-pET-3a construct were used to generate polyclonal antiserum against the recombinant T. pallidum Gpd. Immunoblot analysis showed that an immunoreactive 41-kDa protein was observed in both purified inclusion bodies (Fig. 1B, lane 1) and lysates (data not shown) of E. coli expressing the Gpd-pET-3a construct, and no corresponding immunoreactive protein in preparations of E. coli expressing the pET-3a vector alone was observed (data not shown). Although several other immunoreactive proteins were visible in the purified inclusion bodies from E. coli expressing the Gpd-pET-3a construct (lane 1), adsorption of anti-E. coli antibodies from the polyclonal antiserum removed all background reactivity, leaving only the immunoreactive 41-kDa protein (lane 2). The size of this immunoreactive protein corresponded to the predicted molecular mass of the T. pallidum Gpd (44). The ability of the anti-Gpd antiserum to opsonize T. pallidum was also investigated by using a standard phagocytosis assay. As shown in Fig. 2, no significant difference between the opsonization potential of anti-Gpd antiserum and control preimmune serum (P = 0.14) was observed, as compared to the opsonization capacity of serum collected from rabbits chronically infected with T. pallidum (immune rabbit serum).
FIG. 2.
Opsonic potential of the recombinant T. pallidum Gpd. Shown are the percentages of rabbit peritoneal macrophages phagocytosing T. pallidum after a 4-h incubation with a 1:100 dilution of either IRS, anti-T. pallidum Gpd polyclonal antiserum, or preimmune serum (normal rabbit serum) collected prior to commencement of the immunization protocol. Three separate experiments were performed to test for opsonization with a total number of replicate assays of eight (preimmune serum), seven (anti-Gpd serum) and nine (IRS). Bars show standard errors of the means.
Characterization of anti-Gpd immunoreactivity on T. pallidum lysates.
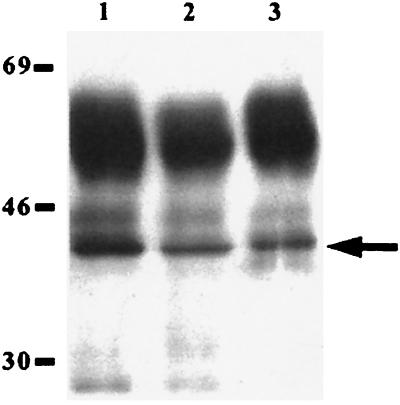
The level of reactivity of the anti-Gpd polyclonal antiserum on lysates of washed and unwashed T. pallidum preparations was investigated by immunoblot analysis. As shown in Fig. 3, a strongly immunoreactive band corresponding to the 41-kDa T. pallidum Gpd was present in lysates of unwashed treponemes extracted directly from infected rabbit testes (lane 1). Approximately equal levels of immunoreactivity were observed with the 41-kDa molecule present in lysates prepared from T. pallidum washed one time (lane 2) and three times (lane 3) following extraction from rabbit testes, although in both cases the degree of immunoreactivity was significantly less than that observed in an equal quantity of unwashed treponeme lysate. Previous investigations have demonstrated that the fragile outer membrane is partially removed during washing of T. pallidum by centrifugation (14), and thus the above results suggest concurrent loss of anti-Gpd immunoreactivity, and therefore loss of Gpd itself, with the treponeme outer membrane during washing.
FIG. 3.
Immunoblot analysis of anti-Gpd immunoreactivity on T. pallidum lysates. Antiserum raised against the recombinant T. pallidum Gpd was used to probe an unwashed T. pallidum lysate (lane 1) and lysates of T. pallidum washed one (lane 2) and three (lane 3) times. Each lane contains 1.4 × 107 treponemes, and molecular mass standards in kilodaltons are indicated at the left. The T. pallidum Gpd is indicated by an arrow. The strongly immunoreactive bands at approximately 55 and 25 kDa represent the heavy and light chains, respectively, of rabbit antibody molecules that are contaminating the T. pallidum lysate preparations and are detected by the goat F(ab′)2 anti-rabbit IgG secondary antibody. This immunoreactivity also decreases in washed treponemes.
Ig-binding capabilities of T. pallidum Gpd.
As previously reported, amino acid sequence analyses demonstrated that T. pallidum Gpd shares the highest degree of amino acid sequence similarity with the Gpd homologue from H. influenzae and not with the corresponding enzyme from the related spirochete B. hermsii, as might be expected (44). This result is interesting, especially in light of the fact that the H. influenzae Gpd homologue has been reported to have IgD-binding capability (19, 37). For these reasons, the Ig-binding capacity of the recombinant T. pallidum Gpd was also investigated. As demonstrated in Fig. 4A, the recombinant T. pallidum Gpd exhibited a broader range of Ig binding than the H. influenzae Gpd homologue, with specific binding observed for human IgA, -D, and -G but not -M (lanes 1, 3, 5, and 7, respectively). The Ig binding was specific for the T. pallidum Gpd and did not represent spurious binding by a contaminating E. coli protein, as no Ig binding was observed for similarly prepared E. coli expressing the pET-3a vector alone (lanes 2, 4, 6, and 8). IgG fractionation studies (Fig. 4B) revealed that T. pallidum Gpd specifically binds the Fc fragment of human IgG (lane 3) with an intensity similar to that observed for intact IgG (lane 1), while no binding to either the Fab fragment of human IgG (lane 5) or the secondary F(ab′)2 antibody (lane 7) was detected. Control lanes containing inclusion bodies prepared from E. coli transformed with the pET-3a vector alone once again did not exhibit binding to intact IgG (lane 2), IgG Fc and Fab fragments (lanes 4 and 6, respectively), or the secondary antibody (lane 8).
FIG. 4.
Immunoblot analyses of the Ig-binding capability of recombinant T. pallidum Gpd. Shown are inclusion bodies purified from E. coli BL21 (DE3) pLysS transformed with either the pET-3a-Gpd construct (lanes 1, 3, 5, and 7) or with the pET-3a vector alone (lanes 2, 4, 6, and 8). Molecular mass standards in kilodaltons are indicated on the left of each panel. (A) Specificity of recombinant T. pallidum Gpd for binding various Ig classes. Lanes: 1 and 2, human IgA and goat F(ab′)2 anti-human IgA (alpha chain specific); 3 and 4, human IgD and goat F(ab′)2 anti-human IgD (delta chain specific); 5 and 6, human IgG and goat F(ab′)2 anti-human IgG (gamma chain specific); 7 and 8, human IgM and goat F(ab′)2 anti-human IgM (mu chain specific). (B) Investigation of the binding specificity of recombinant T. pallidum Gpd for human IgG. In all cases the secondary antibody used was goat F(ab′)2 anti-human IgG (gamma chain specific). Lanes: 1 and 2, intact human IgG; 3 and 4, human IgG Fc fragment; 5 and 6, human IgG Fab fragment; 7 and 8, no primary Ig.
Immunoprotective capacity of T. pallidum Gpd.
The protection afforded by immunization with Gpd was tested in the rabbit syphilis model in two separate experiments. In the first experiment, one rabbit was immunized with inclusion bodies purified from E. coli expressing the Gpd-pET-3a construct emulsified in Ribi adjuvant prior to intradermal challenge at six independent sites with 103 T. pallidum per site. A control rabbit received no prior immunization but received the same intradermal challenge. The Gpd-immunized rabbit developed atypical pale, flat, slightly indurated, and nonulcerative lesions within 7 days of challenge at two of the six challenge sites, with no lesions observed at the remaining four challenge sites. In contrast, the control rabbit developed typical red, raised, highly indurated, and ulcerative lesions at five of six challenge sites at 12 to 14 days postchallenge.
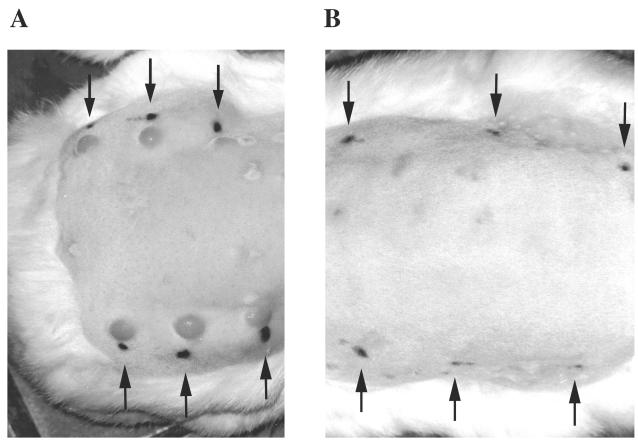
Based on these encouraging results, the above immunization and challenge protocol was repeated with four rabbits immunized with the Gpd-pET-3a inclusion body preparation prior to intradermal T. pallidum challenge. To ensure that neither a contaminating E. coli protein, the Ribi adjuvant, nor the immunization process itself influenced the outcome of T. pallidum challenge, a control group of four rabbits underwent a similar immunization protocol with inclusion bodies purified from E. coli expressing the pET-3a vector alone prior to intradermal challenge. An additional group of four rabbits received no prior immunization, once again to serve as intradermal challenge control animals. All eight control rabbits developed typical red, raised, and highly indurated lesions at each of the six challenge sites that in some cases progressed to ulceration. All four of the Gpd-immunized rabbits developed atypical pale, flat, slightly indurated, and nonulcerative reactions at each of the six challenge sites. In all cases, induration in the Gpd-immunized animals resolved before lesions appeared in the control animals and resembled delayed-type hypersensitivity responses more than typical syphilis chancres. Figure 5 shows lesion development in representative rabbits from the control pET-3a-immunized group (panel A) and the Gpd-pET-3a construct-immunized group (panel B).
FIG. 5.
Immunoprotective capacity of recombinant T. pallidum Gpd. Shown are representative rabbits from the control pET-3a immunization group (A) and the Gpd-pET-3a immunization group (B). The lesions observed on the unimmunized control rabbits were similar to those of the control pET-3a immunization group, and thus are not shown. The black ink spots indicated by arrows are adjacent to the intradermal challenge sites. The faint scars present on the back of the rabbits are sites of subcutaneous immunizations. Photographs were taken prior to lesion ulceration in the control rabbits on day 23 postchallenge.
Table 1 summarizes the postchallenge analyses performed on the rabbits to determine the degree of protection provided by immunization with the T. pallidum recombinant Gpd. As shown in the table, the maximum diameter of lesions in the Gpd-immunized animals was significantly smaller than that of lesions appearing in the control animals (P < 0.0001). In addition, dark-field microscopy examination of the challenge sites performed 31 days following infection revealed treponemes in four of four unimmunized control rabbits and three of four control pET-3a vector-immunized rabbits, while no treponemes were observed in the three Gpd-pET-3a construct-immunized animals. Serological examination of the rabbits 5 weeks postchallenge revealed a high VDRL test titer for normal, unimmunized animals compared to significantly reduced titers observed for the immunized rabbits (P < 0.02), with the lowest mean titer being recorded for the Gpd-immunized rabbits. The slight level of protection observed for the vector control-immunized animals, as demonstrated by the reduction in ulceration, dark-field-microscopy-positive lesions and VDRL titers in these animals compared to the unimmunized controls, may arise from the hyperimmune status of these animals as a result of immunization with the Ribi adjuvant MPL + TDM + CWS. Relevant to this point is the fact that immunizations with the Ribi adjuvant were performed SC and ID in close proximity to the subsequent challenge sites, thus raising the possibility that a localized influx of immune effector cells contributed to the slight level of protection observed in these animals. Although unlikely, it is also possible that an E. coli protein present in the control pET-3a inclusion body preparation used for immunization may provide a small level of protection upon subsequent challenge with T. pallidum (Fig. 1A, lane 4).
TABLE 1.
Summary of postchallenge analyses of normal control rabbits and rabbits immunized with either the T. pallidum Gpd or the pET-3a vector
| Immunization | No. of rabbits | Lesion type | Time of lesion appearance (days)
|
No. of lesions ulcerating | Maximum lesion diameter (mm) (mean ± SD)b | No. of dark-field-microscopy-positive aspirates
|
VDRL titer (5 weeks postchallenge)
|
No. of dark-field-microscopy-positive rabbits in tissue transfer assay | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean ± SD | Per rabbit | Per lesion | Range | Mean | ||||||
| None (control) | 5 | Typical | 12–16 | 14 ± 0.8 | 24/30 | 15.0 ± 1.6 | 4/4 | 21/24 | 4–32 | 16 | NDe |
| Gpd | 5 | Atypical | 7–9 | 7.1 ± 0.5a | 0/30a | 8.8 ± 2.5a | 0/3c | 0/18a | 2–8 | 4d | 3/3 |
| pET-3a vector | 4 | Typical | 14–15 | 15.4 ± 3.7 | 7/24 | 14.1 ± 2.0 | 3/4 | 11/24 | 4–8 | 5.6d | 3/3c |
P < 0.0001 compared with either normal or vector-immunized control animals (statistical analyses performed using the Student t test for the time of lesion appearance and analysis of variance with repeated measures for the number of lesions ulcerating and the lesion aspirate dark-field-microscopy analysis).
Further postchallenge analyses were performed only on the four rabbits contained within the second protection experiment.
One rabbit from each of the Gpd- and vector control-immunized groups was euthanized during the course of the experiment.
P < 0.02 compared with normal control animals (statistical analysis performed using the Student t test).
ND, not determined.
At this point, the normal, unimmunized control rabbits were deemed infected with T. pallidum and were not further analyzed. Additional experiments were performed on the immunized rabbits, however; lymph node and testis tissues were collected from the Gpd- and vector control-immunized animals, shown to be dark-field-microscopy-negative for treponemes, and subsequently transferred to naive recipient animals. This assay, called the rabbit infectivity test, is designed to test for the outgrowth of low numbers of a treponeme inoculum and thus to determine the degree of protection in the immunized animals. Table 1 shows that both the Gpd- and vector control-immunized animals were infected with T. pallidum, as demonstrated by seroconversion of the tissue recipient animals.
DISCUSSION
This report further characterizes the T. pallidum Gpd, previously identified by way of differential, immunological screening of a treponeme genomic expression library (44). Heterologous expression of the open reading frame encoded by the gpd gene facilitated production of an enzymatically active recombinant Gpd for subsequent studies into the function, cellular location, and immunoprotective capacity of this molecule.
Despite the original isolation protocol used to identify the T. pallidum Gpd, namely the differential immunoreactivity of ORS, but not NORS, on plaques expressing this molecule (44), no significant enhancement of T. pallidum phagocytosis by rabbit peritoneal macrophages was conferred by the presence of anti-Gpd antiserum. Although unexpected, this lack of significant opsonic activity may be indicative of a low level of native Gpd expression on the surface of intact T. pallidum, and thus the possibility that anti-Gpd antibodies contribute to the level of opsonization observed with IRS cannot be ruled out. If several target antigens participate in the process of opsonization, then inclusion of antiserum against only a single target antigen in the phagocytosis assay might not result in the level of opsonization required for detection. Alternatively, the lack of observed opsonic activity may simply reflect the absence of appropriate conformation in the T. pallidum Gpd as a result of heterologous expression in E. coli. Experiments to further investigate the opsonic potential of the T. pallidum Gpd are currently being performed, including raising antiserum against a more conformationally correct molecule, prepared by using alternative methods of recombinant expression or by isolation of the native Gpd from T. pallidum. In addition, opsonization assays using anti-Gpd antiserum in combination with antiserum raised against other putative opsonic target antigens (11) will be conducted to test the theory that multiple target antigens are required to attain detectable levels of opsonization.
If one of the above procedures demonstrates that Gpd is a target antigen of opsonic antibody, this will provide evidence for a cell surface disposition for Gpd in T. pallidum, as the targets of opsonic antibody are thought to be outer-membrane proteins exposed on the treponeme surface. Preliminary investigations into the cellular locale of Gpd in T. pallidum by using the gel microdroplet immunofluorescence protocol of Cox et al. (14) suggest that Gpd is located on the T. pallidum surface (10), although reproducible results could not be obtained due to technical difficulties. Also, as reported here, indirect evidence for the presence of Gpd in T. pallidum outer membranes was obtained by immunoblot analysis using anti-Gpd antiserum on T. pallidum lysate preparations. A significant loss of immunoreactivity was observed in lysates prepared from treponemes whose outer membranes had been partially removed by washing prior to lysis, compared to lysates prepared from unwashed treponemes in which the fragile outer membrane and its constituent proteins remain intact prior to lysis. Additionally, in separate experiments, Shevchenko et al. isolated Gpd from T. pallidum outer-membrane preparations (42). These combined results suggest that Gpd is associated with the outer membrane, and additional studies are currently under way to investigate the potential cell surface disposition of this molecule.
The Ig-binding capacity of the T. pallidum Gpd suggests that this molecule possesses an additional functional role besides its enzymatic contribution to phosphodiester metabolism. In H. influenzae, the Gpd homologue has been linked to pathogenesis, as Gpd knockout mutants have been shown to be 100-fold less virulent in animal models (20). Similarly, Gpd may be relevant to the pathogenesis of T. pallidum. In this scenario, Gpd residing on the outer leaflet of the T. pallidum outer membrane would bind the Fc region of Igs. Early in the immune response to T. pallidum, when specific antibody is limiting and avidity is low, Gpd may preferentially bind antibody by the Fc region, thus limiting the antibodies’ cytotoxic and opsonic capacities. In addition, this interaction may sterically mask surface-exposed opsonic target antigens, which may include Gpd itself, and in this way block the binding of opsonic antibodies. Along these lines, Alderete and Baseman previously reported the presence of host IgG on the outer membrane of T. pallidum (3). They showed that removal of bound Igs could be accomplished only by trypsin treatment of washed treponeme preparations, thus indicating that host IgG was avidly bound to the T. pallidum surface. These investigators surmised that “coating of the parasite by host macromolecules may allow for in vivo survival of treponemes for extended lengths of time with subsequent manifestations of the secondary and tertiary stages of the disease. One of the virulence determinants, therefore, may be a function of the efficient masking of the treponemal surface” (3). These insightful comments mirror our proposed model for treponemal immune-response evasion and establishment of chronic infection.
Furthermore, the specificity of Ig binding may have relevance to the various stages of disease in syphilis. Treponemes present at mucosal venues in primary chancres would encounter a humoral response consisting largely of IgA and IgG, whereas IgG would be the predominant Ig encountered by secondary- and tertiary-stage treponemes. Thus, the wide range of Ig-binding capabilities exhibited by T. pallidum would contribute to treponemal immune-response evasion regardless of the stage of disease or the corresponding anatomical location of bacterial infection.
The immunoprotective capacity of the T. pallidum Gpd was tested in the rabbit syphilis model in two separate protection experiments. In each experiment, immunization with the recombinant, Gpd afforded significant protection against challenge with virulent T. pallidum, with attenuation of lesion appearance, lesion development, and treponemal proliferation being observed at the challenge sites. In particular, following challenge Gpd-immunized rabbits developed atypical small, pale, flat, slightly indurated, and nonulcerative lesions that arose early and resolved prior to the appearance of typical lesions on control unimmunized and vector-immunized rabbits. Dark-field-microscopy examination of aspirates collected from both the sites of challenge and the testes of the Gpd-immunized animals demonstrated a lack of detectable treponemes. Although complete protection in the Gpd-immunized animals was not observed, as demonstrated by both detectable VDRL serologies and infection of naive tissue recipient rabbits in the rabbit infectivity test, the degree of protection conferred by immunization with the recombinant Gpd molecule nevertheless is impressive. To our knowledge, Gpd represents the first defined antigenic vaccine to demonstrate this level of protection upon T. pallidum challenge. Further studies will be performed to determine whether alternative methods of Gpd expression, purification, or vaccine delivery, or covaccination of Gpd with other promising immunoprotective antigens as part of a vaccine cocktail, can achieve complete immunity against T. pallidum challenge.
ACKNOWLEDGMENTS
Many thanks to Lynn Barrett, Anna Dukes, and Kindra Mikota for excellent technical assistance.
This work was supported by grants AI 34616, AI 18988, AI 34615, and AI 31448 from the National Institutes of Health (W.C.V.V. and S.A.L.) and postdoctoral fellowships from the Natural Sciences and Engineering Research Council of Canada and the Medical Research Council of Canada (C.E.C.).
REFERENCES
- 1.Akins D R, Robinson E, Shevchenko D, Elkins C, Cox D L, Radolf J D. Tromp1, a putative rare outer membrane protein, is anchored by an uncleaved signal sequence to the Treponema pallidum cytoplasmic membrane. J Bacteriol. 1997;179:5076–5086. doi: 10.1128/jb.179.16.5076-5086.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akkoyunlu M, Ruan M, Forsgren A. Distribution of protein D, an immunoglobulin D-binding protein, in Haemophilus strains. Infect Immun. 1991;59:1231–1238. doi: 10.1128/iai.59.4.1231-1238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alderete J F, Baseman J B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979;26:1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker-Zander S A, Hook E W, Bonin P, Handsfield H H, Lukehart S A. Antigens of Treponema pallidum recognized by IgG and IgM antibodies during syphilis infection in humans. J Infect Dis. 1985;151:264–272. doi: 10.1093/infdis/151.2.264. [DOI] [PubMed] [Google Scholar]
- 5.Baker-Zander S A, Lukehart S A. Macrophage-mediated killing of opsonized Treponema pallidum. J Infect Dis. 1992;165:69–74. doi: 10.1093/infdis/165.1.69. [DOI] [PubMed] [Google Scholar]
- 6.Baker-Zander S A, Shaffer J M, Lukehart S A. Characterization of the serum requirement for macrophage-mediated killing of Treponema pallidum ssp. pallidum: relationship to the development of opsonizing antibodies. FEMS Immunol Med Microbiol. 1993;6:273–280. doi: 10.1111/j.1574-695X.1993.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 7.Berry C D, Hooton T M, Collier A C, Lukehart S A. Neurological relapse after benzathine penicillin therapy for secondary syphilis in a patient with HIV infection. N Engl J Med. 1987;316:1587–1589. doi: 10.1056/NEJM198706183162507. [DOI] [PubMed] [Google Scholar]
- 8.Blanco D R, Champion C I, Exner M M, Erdjument-Bromage H, Hancock R E, Tempst P, Miller J N, Lovett M A. Porin activity and sequence analysis of a 31-kilodalton Treponema pallidum subsp. pallidum rare outer membrane protein (Tromp1) J Bacteriol. 1995;177:3556–3562. doi: 10.1128/jb.177.12.3556-3562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borenstein L A, Radolf J D, Fehniger T E, Blanco D R, Miller J N, Lovett M A. Immunization of rabbits with recombinant Treponema pallidum surface antigen 4D alters the course of experimental syphilis. J Immunol. 1988;140:2415–2421. [PubMed] [Google Scholar]
- 10.Cameron, C. E., C. Castro, S. A. Lukehart, and W. C. Van Voorhis. Unpublished data.
- 11.Cameron, C. E., A. Centurion-Lara, S. A. Lukehart, and W. C. Van Voorhis. Unpublished data.
- 12.Champion C I, Blanco D R, Exner M M, Erdjument-Bromage H, Hancock R E, Tempst P, Miller J N, Lovett M A. Sequence analysis and recombinant expression of a 28-kilodalton Treponema pallidum subsp. pallidum rare outer membrane protein (Tromp2) J Bacteriol. 1997;179:1230–1238. doi: 10.1128/jb.179.4.1230-1238.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Champion C I, Miller J N, Borenstein L A, Lovett M A, Blanco D R. Immunization with Treponema pallidum endoflagella alters the course of experimental rabbit syphilis. Infect Immun. 1990;58:3158–3161. doi: 10.1128/iai.58.9.3158-3161.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox D L, Akins D R, Porcella S F, Norgard M V, Radolf J D. Treponema pallidum in gel microdroplets: a novel strategy for investigation of treponemal molecular architecture. Mol Microbiol. 1995;15:1151–1164. doi: 10.1111/j.1365-2958.1995.tb02288.x. [DOI] [PubMed] [Google Scholar]
- 15.Darrow W W, Echenberg D F, Jaffe H W, O’Malley P M, Byers R H, Getchell J P, Curran J W. Risk factors for human immunodeficiency virus (HIV) infection in homosexual men. Am J Public Health. 1987;77:479–483. doi: 10.2105/ajph.77.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenblatt R M, Lukehart S A, Plummer F A, Quinn T C, Critchlow C W, Ashley R L, D’Costa L J, Ndinya-Achola J O, Corey L, Ronald A R, Holmes K K. Genital ulceration as a risk factor for human immunodeficiency virus infection. AIDS. 1988;2:47–50. doi: 10.1097/00002030-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Harlow E, Lane D, editors. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 18.Janson H, Heden L, Forsgren A. Protein D, the immunoglobulin D-binding protein of Haemophilus influenzae, is a lipoprotein. Infect Immun. 1992;60:1336–1342. doi: 10.1128/iai.60.4.1336-1342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janson H, Heden L, Grubb A, Ruan M, Forsgren A. Protein D, an immunoglobulin D-binding protein of Haemophilus influenzae: cloning, nucleotide sequencing, and expression in Escherichia coli. Infect Immun. 1991;59:119–125. doi: 10.1128/iai.59.1.119-125.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janson H, Melhus A, Hermansson A, Forsgren A. Protein D, the glycerophosphodiester phosphodiesterase from Haemophilus influenzae with affinity for human immunoglobulin D, influences virulence in a rat otitis model. Infect Immun. 1994;62:4848–4854. doi: 10.1128/iai.62.11.4848-4854.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johns D R, Tierney M, Felsenstein D. Alteration in the natural history of neurosyphilis by concurrent infection with the human immunodeficiency virus. N Engl J Med. 1987;316:1569–1572. doi: 10.1056/NEJM198706183162503. [DOI] [PubMed] [Google Scholar]
- 22.Larson T J, Ehrmann M, Boos W. Periplasmic glycerophosphodiester phosphodiesterase of Escherichia coli, a new enzyme of the glp regulon. J Biol Chem. 1983;258:5426–5432. [PubMed] [Google Scholar]
- 23.Lukehart, S. A. 1993. Unpublished data.
- 24.Lukehart, S. A. 1995. Unpublished data.
- 25.Lukehart S A. Prospects for development of a treponemal vaccine. Rev Infect Dis. 1985;7:S305–S313. doi: 10.1093/clinids/7-supplement_2.s305. [DOI] [PubMed] [Google Scholar]
- 26.Lukehart S A, Baker-Zander S A, Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J Immunol. 1980;124:454–460. [PubMed] [Google Scholar]
- 27.Lukehart S A, Hook III E W, Baker-Zander S A, Collier A C, Critchlow C W, Handsfield H H. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and therapy. Ann Intern Med. 1988;109:855–862. doi: 10.7326/0003-4819-109-11-855. [DOI] [PubMed] [Google Scholar]
- 28.Lukehart S A, Miller J N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978;121:2014–2024. [PubMed] [Google Scholar]
- 29.Metzger M, Michalska E, Podwinska J, Smogór W. Immunogenic properties of the protein component of Treponema pallidum. Br J Vener Dis. 1969;45:299–303. doi: 10.1136/sti.45.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller J N. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by gamma-irradiation. J Immunol. 1973;110:1206–1215. [PubMed] [Google Scholar]
- 31.Munson R S, Sasaki K. Protein D, the putative immunoglobulin D-binding protein produced by Haemophilus influenzae, is glycerophosphodiester phosphodiesterase. J Bacteriol. 1993;175:4569–4571. doi: 10.1128/jb.175.14.4569-4571.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsson R P, Beijer L, Rutberg B. The glpT and glpQ genes of the glycerol regulon in Bacillus subtilis. Microbiology. 1994;140:723–730. doi: 10.1099/00221287-140-4-723. [DOI] [PubMed] [Google Scholar]
- 33.Norris S J, Alderete J F, Axelsen N H, Bailey M J, Baker-Zander S A, Baseman J B, Bassford P J, Baughn R E, Cockayne A, Hanff P A, Hindersson P, Larsen S A, Lovett M A, Lukehart S A, Miller J N, Moskophidis M A, Müller F, Norgard M V, Penn C W, Stamm L V, van Embden J D, Wicher K. Identity of Treponema pallidum subsp. pallidum polypeptides: correlation of sodium dodecyl sulfate-polyacrylamide gel electrophoresis results from different laboratories. Electrophoresis. 1987;8:77–92. [Google Scholar]
- 34.Radolf J D, Norgard M V, Schulz W W. Outer membrane ultrastructure explains the limited antigenicity of virulent Treponema pallidum. Proc Natl Acad Sci USA. 1989;86:2051–2055. doi: 10.1073/pnas.86.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rolfs T T. Surveillance for primary and secondary syphilis—United States, 1991. Morbid Mortal Weekly Rep. 1993;42:13–19. [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Sasaki K, Munson R S. Protein D of Haemophilus influenzae is not a universal immunoglobulin D-binding protein. Infect Immun. 1993;61:3026–3031. doi: 10.1128/iai.61.7.3026-3031.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwan T G, Schrumpf M E, Hinnebusch B J, Anderson D E, Konkel M E. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J Clin Microbiol. 1996;34:2483–2492. doi: 10.1128/jcm.34.10.2483-2492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaffer J M, Baker-Zander S A, Lukehart S A. Opsonization of Treponema pallidum is mediated by immunoglobulin G antibodies induced only by pathogenic treponemes. Infect Immun. 1993;61:781–784. doi: 10.1128/iai.61.2.781-784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shang E S, Skare J T, Erdjument-Bromage H, Blanco D R, Tempst P, Miller J N, Lovett M A. Sequence analysis and characterization of a 40-kilodalton Borrelia hermsii glycerophosphodiester phosphodiesterase homolog. J Bacteriol. 1997;179:2238–2246. doi: 10.1128/jb.179.7.2238-2246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shang E S, Skare K T, Exner M M, Blanco D R, Kagan B L, Miller J N, Lovett M A. Isolation and characterization of the outer membrane of Borrelia hermsii. Infect Immun. 1998;66:1082–1091. doi: 10.1128/iai.66.3.1082-1091.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shevchenko D V, Akins D R, Robinson E J, Li M, Shevchenko O V, Radolf J D. Identification of homologs for thioredoxin, peptidyl prolyl cis-trans isomerase, and glycerophosphodiester phosphodiesterase in outer membrane fractions from Treponema pallidum, the syphilis spirochete. Infect Immun. 1997;65:4179–4189. doi: 10.1128/iai.65.10.4179-4189.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simonsen J N, Cameron D W, Gakinya M N, Ndinya-Achola J O, D’Costa L J, Karasira P, Cheang M, Ronald A R, Piot P, Plummer F A. Human immunodeficiency virus infection among men with sexually transmitted diseases. Experience from a center in Africa. N Engl J Med. 1988;319:274–278. doi: 10.1056/NEJM198808043190504. [DOI] [PubMed] [Google Scholar]
- 44.Stebeck C E, Shaffer J M, Arroll T W, Lukehart S A, Van Voorhis W C. Identification of the Treponema pallidum subsp. pallidum glycerophosphodiester phosphodiesterase homologue. FEMS Microbiol Lett. 1997;154:303–310. doi: 10.1111/j.1574-6968.1997.tb12660.x. [DOI] [PubMed] [Google Scholar]
- 45.Tani T, Inoue R, Asano O. Studies on the preventive inoculation against syphilis. Jpn Med J. 1951;4:71–86. doi: 10.7883/yoken1948.4.71. [DOI] [PubMed] [Google Scholar]
- 46.Tommassen J, Eiglmeier K, Cole S T, Overduin P, Larson T J, Boos W. Characterization of two genes, glpQ and ugpQ, encoding glycerophosphodiester phosphodiesterases of Escherichia coli. Mol Gen Genet. 1991;226:321–327. doi: 10.1007/BF00273621. [DOI] [PubMed] [Google Scholar]
- 47.Turner T B, Hardy P H, Newman B. Infectivity tests in syphilis. Br J Vener Dis. 1969;45:183–196. doi: 10.1136/sti.45.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker E M, Zampighi G A, Blanco D R, Miller J N, Lovett M A. Demonstration of rare protein in the outer membrane of Treponema pallidum subsp. pallidum by freeze-fracture analysis. J Bacteriol. 1989;171:5005–5011. doi: 10.1128/jb.171.9.5005-5011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeigler J A, Jones A M, Jones R H, Kubica K M. Demonstration of extracellular material at the surface of pathogenic T. pallidum cells. Br J Vener Dis. 1976;52:1–8. doi: 10.1136/sti.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]