Abstract
The anterior and lateral thalamus (ALT) contains head direction cells that signal the directional orientation of an animal within an environment. ALT has direct and indirect connections with the parietal cortex (PC), an area hypothesized to play a role in coordinating viewer-dependent and viewer-independent spatial reference frames. This coordination between reference frames would allow an individual to translate movements toward a desired location from memory. Thus, ALT-PC functional connectivity would be critical for moving toward remembered allocentric locations. This hypothesis was tested with a place-action task that requires associating an appropriate action (left or right turn) with a spatial location. There are four arms, each offset by 90 degrees, positioned around a central starting point. A trial begins in the central starting point. After exiting a pseudorandomly selected arm, the rat had to displace the correct object covering one of two (left versus right) feeding stations to receive a reward. For a pair of arms facing opposite directions, the reward was located on the left, and for the other pair, the reward was located on the right. Thus, each reward location had a different combination of allocentric location and egocentric action. Removal of an object was scored as correct or incorrect. Trials in which the rat did not displace any objects were scored as ‘no selection’ trials. After an object was removed, the rat returned to the center starting position and the maze was reset for the next trial. To investigate the role of the ALT-PC network, muscimol inactivation infusions targeted bilateral PC, bilateral ALT, or the ALT-PC network. Muscimol sessions were counterbalanced and compared to saline sessions within the same animal. All inactivations resulted in decreased accuracy, but only bilateral PC inactivations resulted in increased non selecting, increased errors, and longer latency responses on the remaining trials. Thus, the ALT-PC network is critical for linking an action with a spatial location for successful navigation.
Keywords: parietal cortex, thalamus, egocentric, allocentric, reference frame transformation
Introduction
It is an adaptive trait for any animal to be able to navigate through the world with purposeful goals (Gallistel, 1990; O’Keefe & Nadel, 1978). Accurate navigation is necessary to guide behavior toward sources of food or away from aversive locations, or when exploring new places and returning to a home base from these places. Goal locations are always changing depending on the needs of an animal and this requires brain circuitry that can support these navigational needs in a flexible and adaptable manner. A large body of research has shown that a cortical-limbic circuit is responsible for representing the spatial layout of the environment in a map-like (allocentric) frame of reference, thereby supporting accurate spatial navigation (McNaughton, Battaglia, Jensen, Moser, & Moser, 2006; O’Keefe & Nadel, 1978; O’Mara & Aggleton, 2019; Zhao, 2018). However, because animals interact with the environment from the perspective of their own body or their view of the environment, the brain must represent space in relation to spatial frames of reference that can accommodate different perspectives of the environment (Wang, 2012). Research has provided evidence that a variety of brain regions support the use of different spatial reference frames (Alexander, Place, Starrett, Chrastil, & Nitz, 2023; Alexander, Robinson, Stern, & Hasselmo, 2023; Alexander et al., 2022; Clark, Simmons, Berkowitz, & Wilber, 2018; Nitz, 2006, 2009, 2012; Ormond & O’Keefe, 2022; Wilber, Clark, Forster, Tatsuno, & McNaughton, 2014). Two well-studied reference frames are known as egocentric and allocentric coordinate systems. The egocentric frame of reference considers a goal location in relation to the self, while the allocentric frame of reference considers a goal location in relation to landmarks (Byrne & Crawford, 2010). Studies have employed different tasks (y-maze, Morris water maze, and the radial arm maze) to isolate and identify neural circuits and behaviors that underlie egocentric and allocentric reference frame use in navigation. However, egocentric and allocentric perspectives or strategies do not always work independently from one another, but can work in tandem, therefore, it can be difficult to isolate their respective contributions on spatial behavior (McDonald & White, 1994; Sutherland & Hamilton, 2004; Whishaw, Hines, & Wallace, 2001). Similarly, the brain circuits that underlie the use of different strategies overlap in their function (e.g., the parietal cortex and retrosplenial cortex contain cells that encode in allocentric or egocentric reference frames or both; see Alexander et al., 2022; Nitz, 2006, 2009, 2012; Wilber et al., 2014).
The rodent parietal cortex (PC) has been shown to contain both single-cells and modules (large groups of adjacent cells with consistent encoding across depth) encoding of various motion states, including running straight at a particular speed or turning at a particular angular velocity, as well as encoding the animal’s 3D body position (Mimica, Dunn, Tombaz, Bojja, & Whitlock, 2018; Nitz, 2006, 2009, 2012; Whitlock, Pfuhl, Dagslott, Moser, & Moser, 2012; Wilber et al., 2014; Wilber, Skelin, Wu, & McNaughton, 2017). However, the representation of space in the PC region is heterogeneous, meaning that it contains cells that respond to egocentric representations, allocentric representations, or both (Nitz, 2009; Wilber et al., 2014). For instance, in a task where rats were trained to run to a randomly ordered set of cue lights, recorded cells in the PC were found to be modulated by egocentric cue direction, allocentric head direction, or a conjunctive combination of this information (Wilber et al., 2014). In addition, when the PC is damaged, animals exhibit severe navigation deficits such that the path taken to goal locations is usually inefficient (reviewed in: Clark et al., 2018; Kolb, Buhrmann, McDonald, & Sutherland, 1994). Thus, the PC has a role in guiding accurate navigation toward goal locations.
The PC receives extensive input from the anterior and lateral thalamic (ALT) nuclei; both of which are thought to have a critical role in processing spatial information for navigation (Aggleton & Nelson, 2015; Clark & Harvey, 2016; Peckford et al., 2014; Perry & Mitchell, 2019). Head direction (HD) cells, which are found in these areas (particularly the anterodorsal, anteroventral, anteromedial, and laterodorsal subnuclei), fire as a function of an animal’s head direction and are anchored to a fixed position in the room or environment, but are also modulated by an animals self-motion and vestibular cues (Butler, Smith, van der Meer, & Taube, 2017; Clark & Harvey, 2016; Clark & Taube, 2012; Dudchenko, Wood, & Smith, 2019; Jankowski et al., 2015; Marchette, Vass, Ryan, & Epstein, 2014; Taube, 2007; Xu et al., 2019; Yoder & Taube, 2014). HD cell signals have also been identified in other limbic-cortical areas (e.g., medial entorhinal cortex, parietal cortex, retrosplenial cortex, parasubiculum, and postsubiculum; Taube, 2007). Research comparing the anchoring characteristics of HD cells suggest that distal cues are likely to modulate their activity more so than proximal/foreground cues (Knight & Hayman, 2014). This is likely due to the relative permanence of background cues. Further, studies have shown that damage to the ALT nuclei impairs the acquisition and retention of allocentric information, but does not impair navigation based on egocentric information or visual cues marking a goal location (Aggleton & Nelson, 2015; Clark & Harvey, 2016; Harvey, Thompson, Sanchez, Yoder, & Clark, 2017; Lopez et al., 2009; Moreau et al., 2013; O’Mara, 2013; Peckford et al., 2014; Wolff, Gibb, Cassel, & Dalrymple-Alford, 2008). These findings demonstrate a critical role for the ALT region in the navigational ability that relies on an allocentric strategy.
In many computational and theoretical models, the thalamic HD signal is critical for translating between allocentric and egocentric coordinate systems. This is because an animal’s heading position is necessary to know the relationship between itself and the surrounding world (Bicanski & Burgess, 2016, 2018; Byrne, Becker, & Burgess, 2007; Calton, Turner, Cyrenne, Lee, & Taube, 2008; Clark, Bassett, Wang, & Taube, 2010; Clark & Taube, 2012; Pouget, Deneve, & Duhamel, 2002). The ALT and PC are anatomically and functionally connected with both direct and indirect connections via the retrosplenial cortex (Clark et al., 2018; Wilber et al., 2015) and are in a prime anatomical position to serve as a translational interface between egocentric and allocentric frames of reference (Wilber et al., 2015). Although both PC and ALT contain HD cells (Taube, 1995; Wilber et al., 2014), a fundamental coding scheme in the PC is action centered (Wilber et al., 2017), positioning it as a critical structure for interfacing between allocentric representations and action. Thus, the ALT-PC circuit may be critical for interfacing between action centered and allocentric frames of reference.
The present study was aimed at experimentally testing this anatomical and theoretical hypothesis using a novel place-action task (similar to: Grieves, Jenkins, Harland, Wood, & Dudchenko, 2016 except action and place are paired here and odor and place were paired in the previous study) along with disconnection of the ALT and PC through muscimol inactivation. Briefly, the place-action task requires that rats perform a specific action when at a specific orientation/place in the environment. Thus, we specifically hypothesize that disruption of functional connectivity between dorsal-medial thalamus and PC will impair performance in this task. Functional disconnection was performed by selectively inactivating the ALT and PC contralaterally using muscimol infusions targeting each region in one hemisphere (e.g. right PC and left ALT; Fresno, Parkes, Faugère, Coutureau, & Wolff, 2019; Hernandez et al., 2017; Jo & Lee, 2010). The ALT has dense ipsilateral (but not contralateral) projections to PC, which does not have many reciprocal connections to ALT (Wilber et al., 2015). Thus, we took advantage of the primarily ipsilateral anatomical connectivity between ALT and PC to disrupt this circuit with contralateral or “cross” infusions. This enables us to evaluate the role of the PC, ALT, and the ALT-PC network in egocentric and allocentric coordination, thus allowing us to test the hypothesis that the ALT-PC circuit is critical for allocentric-egocentric coordination, a hypothesis with robust theoretical and computational modeling support, but with little or no direct empirical evidence to date.
Methods
The study used 11 Long Evans rats, comprising 6 females and 5 males, with ages ranging from 2 to 11 months. The rats were individually housed and maintained on a 12-hour light and dark cycle. All animals were behaviorally naïve at the start of the experiment. Food deprivation was implemented during behavioral training and experimentation, with rats being maintained at no less than 80% of their ad libitum body weight. Water was accessible to the rats throughout all phases of experimentation. All procedures carried out were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Florida State University Animal Care and Use Committee.
Pre-training
The experiment used a maze apparatus that was secured on top of a circular arena measuring 5 feet in diameter and consisted of 4 walled pathways or “arms” leading out from a central chamber. Each arm was positioned at a 90-degree angle from the adjacent arms and was parallel to the walls in the room, resulting in four arms of equal length centered around the middle of the arena (Fig. 1A). This layout created 4 paths, or arms, of equal length that were centered around the middle of the circular arena. The width of each arm was equivalent to the width of its corresponding door, creating a square region in the center of the arena when all doors were closed. This center region served as the starting point for each trial. At the end of each arm, a weigh boat was secured to the arena’s surface and covered with a disc. For pretraining, rats were progressively trained to run through a maze arm and remove a plastic disc that rested on top of a small, square weigh boat. Pretraining typically takes approximately 10 sessions.
Figure 1. Place-action apparatus, experimental timeline and surgical targets.

A. Four arms of equal length each bisect a pair of feeding stations - one rewarded, ‘correct’ and one non-rewarded, ‘incorrect’ location. Each reward station is associated with a different combination of allocentric place and egocentric action. B. Behavioral sessions and pharmacological inactivation timeline. C. Double bilateral cannulae targets: PC (magenta) and ALT (blue). Projections between the ALT and PC are laregly ipsilateral, so the network can be tested with contralateral inactivations.
For the first phase of pre-training, the doors remained open, and 2 Froot Loops were placed in each weigh boat under the discs. The rats began each session at the center starting point and were allowed to explore the maze with little intervention. Whenever the rats removed a disc to eat a Froot Loop underneath, the experimenter re-covered the weigh boat containing the remaining Froot Loop. Once a weigh boat was emptied, it was refilled with 2 more Froot Loops. Each 1st phase pre-training session was conducted for 20 min every day until the rat removed the disc 5 times from each weigh boat. The first phase of training takes approximately 7 sessions.
In the second phase of pre-training, the doors remained open, but the weigh boats were never filled with Froot Loops. Rats were hand fed a Froot Loop after removing any disc from any weigh boat. Each 2nd phase pre-training session was conducted for 20 min and continued every day until the rats removed discs 5 times from each weigh boat. The second phase of pretraining takes approximately 3 sessions.
In the third and final phase of pre-training, the sessions were conducted as they were in the 2nd phase pre-training, except that only 1 door, selected randomly by the experimenter, was open to a single arm per trial. The rats were trained to run down an open arm, remove the disc at the end of the arm, and return to the center starting point after receiving a reward at the location of the removed disk. This was repeated for the entire 20-minute session. The 3rd phase pre-training sessions continued every day until the rat again removed discs 5 times from each weigh boat. The third phase of pretraining takes approximately 10 sessions.
Place-Action Task
To perform the place-action task, the maze apparatus was set up as previously described. The task was performed in a manner similar to the 3rd pretraining phase, but now involved 8 disc-covered weigh boats positioned to the left and right sides of each arm and secured toward the edge of the arena (Fig. 1A). Each pair of weigh boats, bisected by an arm, consisted of a rewarded ‘correct’ zone and an ‘incorrect’ zone. For two of these weigh boat pairs, the reward zone was located on the right side of the arm and for the other two pairs, the reward zone was located on the left side of the arm. Each of the rewarded zones were assigned a numerical label (1, 2, 3, 4) and were input into a random list generator (Random.org) in order to generate a randomized 40-item list containing each reward zone number exactly 10 times. The list was then rearranged manually to avoid single (4-4) and double (1-2-1-2) zone repeats. This pseudorandomized list was generated for each behavioral session and determined the arm order for each of the 40 trials.
The rats began every trial in the center of the maze. To start each trial, a door was opened leading into the pseudo-randomly selected arm. Rats were rewarded for removing the disc covering the weigh boat on the correct side. If a rat removed a disc from the incorrect side or did not remove a covering after 1 min, the trial was marked as incorrect or as no selection, respectively. After a correct response, an incorrect response, or no selection, the trial was completed, and the rat returned to the center starting point. Before beginning the next trial, the experimenter would sanitize the traversed area with ethanol and rotate the disc covering to prevent aromatic cues from being used. The session ended when all 40 trials on the list were completed. The rats received cannula implantation once they reached a criterion performance of at least 85% correct for two consecutive days. The animals were given full access to food after this criterion was met and scheduled for surgery.
Cannula Implantation Surgery
All 11 rats were surgically implanted with two sets of cannula bilaterally targeting both the PC (anterior-posterior −4.5 mm, medial-lateral ± 3 mm, dorsal-ventral −0.1 mm) and the ALT (anterior-posterior −1.74 mm, medial-lateral ± 1.25 mm, dorsal-ventral −5.23 mm). After surgery, all animals were given 7 days to recover with full access to food and water.
Infusion and Behavior Timeline
After the post-surgical recovery period, rats continued the behavioral sessions as previously described. When the animal again reached the criterion performance of at least 85% correct for two consecutive days, it received an infusion the following day. For the first infusion, saline or muscimol (ordered randomly, with pairs counterbalanced) was infused bilaterally into the PC before performing a behavioral session (Fig. 1B–C). For the days following, each rat continued the behavioral task until criterion performance was again reached. The animal was then scheduled to receive a second infusion with the counterbalanced solution (saline or muscimol) the following day, prior to a behavioral session. This procedure was continued until the bilateral PC, contralateral PC and ALT pairs (Left PC + Right ALT, Right PC + Left ALT), and bilateral ALT all received successful pairs of muscimol and saline infusions. However, if one of the infusion sites became obstructed (such as due to dura regrowth), the procedure was halted for that particular site. The behavioral performance between the saline and muscimol infusion days were then compared for each pair of sessions. Note, in the case that a cannula became obstructed and infusions could not continue for that particular cannula (e.g., bilateral PC obstruction from dura regrowth), infusions would be repeated several times for saline and muscimol pairs for the remaining cannula options (e.g., bilateral ALT).
Infusion Details
All infusions were done with two 10 ml, 22 gauge Hamilton syringes held in a Model ‘22’ Harvard Apparatus motorized syringe pump. The rats were lightly restrained, and the dummy cannula were removed before inserting the infusion cannula into the infusion guides. All PC infusions were done 45 min before the behavior session began at a rate of 0.3 ml/min for 1 min (Raposo, Kaufman, & Churchland, 2014; but note we were targeting a larger region than Raposo et al. and thus used a longer delay after the infusion). Infusion cannula were kept inside the guides for 1 min after infusion before removing and reinserting the dummy cannula. All ALT infusions were done 30 min before the behavior session began at a rate of 0.167 ml/min for 1.5 min (Harvey et al., 2017). ALT infusion cannula were kept inside the guides for 30 sec after infusion and then removed before reinserting the dummy cannula. For the network infusions, a unilateral PC infusion was completed 15 min prior to a subsequent unilateral ALT infusion, which was always contralateral to the hemisphere that received the PC infusion. Again, differences between ALT and PC infusion parameters were due to the smaller structure to optimize sufficient time for coverage of the structure, but prior to significant diffusion into adjacent structures. The effects from muscimol begin almost immediately and are stable for several hours, thus the differential timing necessary due to differential size of the structures and different infusion rates was not expected to differentially impact behavior (Allen et al., 2008; Hikosaka & Wurtz, 1985; Krupa, Ghazanfar, & Miguel, 1999).
Statistics
A paired Hotelling’s T-Square test was performed to investigate the relationship between the PC inactivation (muscimol vs saline) and accuracy performance (measured with number of trials or percentage) on the place-action task when animals were used as the sample. Note, each animal can have more than one pair of infusions for a particular inactivation condition (e.g., 2 pairs of Saline and Muscimol sessions for Bilateral ALT). In these instances multiple paired saline muscimol sessions were averaged for a given inactivation condition. When session was used as the sample, a mixed effects model was used to account for the repeated testing within an animal. Since the summation of the three dependent variables (correct, incorrect and no selection) is a constant, this test cannot be directly used (violation of multicollinearity assumption among the dependent variables). To address this problem, the three dependent variables were first transformed to two unconstrained variables via the Isometric log-ratio (ILR) transformation (Egozcue, Pawlowsky-Glahn, Mateu-Figueras, & Barceló-Vidal, 2003), and then the paired Hotelling’s T-square test was implemented on the transformed data. Since the ILR transformation is a bijective mapping between constrained composite variables and unconstrained free variables, the test result can be directly transferred back to the original data. For mixed effects models, the ILR transformation was performed separately for saline and muscimol paired sessions and the difference between the session pairs was calculated so that the mixed effects model could assess if this differed from zero. Significant Hotelling’s T-square tests and mixed effects models were followed by planned comparisons. For Hotelling’s T-square tests planned comparisons consisted of two-group paired t-tests (or two-group mixed models tests) done within the context of the overall test (Maxwell & Delaney, 2003), comparing the saline condition to the inactivation condition for each performance category (correct, incorrect, and no selection). For data where the animal was the sample, paired t-tests within each region were also used to assess for possible effects of muscimol on trial duration, side bias, head scanning, and procedural errors. For data where the session was the sample, mixed effects models within each region were used to assess for possible effects of muscimol on trial duration, side bias, head scanning and procedural errors. For all statistical analyses, p ≤ 0.05 was considered significant. Statistical analyses were performed using MATLAB (MathWorks) or StatView (SAS Institute).
Histology
Once all sets of infusions (PC, ALT, ALT-PC and unilateral) and behavioral sessions were completed, the rats infused with fluorescent muscimol or labeled AAV into PC and ATN. Rats were then deeply anesthetized with an IP injection of a sodium pentobarbital solution. Following anesthetization, rats were perfused transcardially with a 1X phosphate-buffered saline solution (PBS), followed by a 4% paraformaldehyde (PFA) in 1X PBS solution. The whole head was removed and preserved in the 4% PFA solution for 24 h before extracting the brain and further fixing in the 4% PFA solution for another 24 h. The brain was then cryoprotected in a 30% solution of sucrose. The brain was frozen and cut coronally at 40 μm thick with a sliding microtome. Sections were mounted onto slides with a mounting media containing DAPI and then coverslipped. Cannula placements were verified using a scanning microscope (Zeiss Axio Imager M2).
Results
Histological analysis confirmed that most cannula were placed within the ALT and PC (Fig. 2). The center point and spread were determined using a YFP-tagged AAV or fluorescent muscimol. In cases where cannula became clogged before final analyses, the anterior/posterior spread was estimated using muscimol spread from the functional cannula tracts as a guide. Note, muscimol is lighter than fluorescent muscimol and thus actual spread of the inactivating agent is likely larger than the spread we observed from our histological observations. ALT placements typically included a combination of anterior (anterodorsal, anteroventral) and lateral thalamic (laterodorsal) nuclei. For most infusions, muscimol likely spread into the adjacent centrolateral and lateral mediodorsal thalamic nuclei. Because these lateral thalamic regions also have interconnectivity with PC (Wilber et al., 2015), and have been linked with spatial processing (Gibb, Wolff, & Dalrymple-Alford, 2006; Wilber et al., 2015), these data were included in our analyses.
Figure 2. Histological verification of cannulae placements.

Depiction of ALT (Middle) and PC (Right) cannulae placement overlayed on atlas images. Each color represents the verified range of fluorescent muscimol or damage for one animal. Left. Example of tissue imaged in ALT (Left Top Two Images) or PC (Middle Bottom) illustrating the range of likely fluorescent muscimol spread. Colored dots next to histology images and on the schematics denote animal identity. Anterior/Posterior from bregma values are listed for each schematic and histology image. Same animal is color coded with the same color on subsequent figures. Note, the Bottom Right ALT image is Left/Right reflected to save space.
One rat was excluded from further ALT and network analyses due to a mistakenly placed ALT cannula in the fimbria of the hippocampus. As a result, 7 rats (4 males and 3 females) and 8 paired data sets (i.e., pairs of 1 saline and 1 muscimol infusions) were available for ALT only inactivation. Note, each animal can have more than one pair of infusions for a particular inactivation condition (e.g., Bilateral ALT), and thus can contribute multiple data sets (see also Infusion and Behavioral Timeline). Four animals were excluded from further PC and network analyses because of PC guide cannula blockage prior to the first PC infusion, making it impossible for muscimol (and saline) to diffuse into the cortical tissue. Therefore, 7 rats (4 males and 3 females) and 8 paired data sets remained for PC only inactivation. Due to missed placement for ALT and additional clogging of PC cannula after bilateral PC inactivation, 4 rats (2 males and 2 females) and 7 paired data sets were available for ALT-PC network inactivation. The proportion of male and female rats across the inactivation conditions (PC, ALT or ALT-PC) did not significantly differ (χ2 = 0.05, p = 0.82). Additionally, the ages of rats were highly similar across inactivation conditions, with the mean age ranging from 7.8 to 8.0 months (Mean ± SEM: PC: 7.9 ± 0.65; ALT: 7.8 ± 0.9; ALT-PC: 8.0 ± 1.2; Range: PC: 5.8-10.5; ALT: 6.0-9.8; ALT-PC: 5.9-10.9). Overall, each animal provided 1-4 datasets per inactivation condition.
As a control for the contralateral network inactivations, unilateral inactivations were performed. This was done to confirm that the changes in behavior resulting from contralateral ALT-PC inactivation were not due to a general inactivation of two unilateral regions, but rather the disruption of the network formed between the two regions. However, because this manipulation was performed last, there was a higher likelihood that the PC cannula were obstructed, resulting in only 2 paired data sets from 1 animal being available for further unilateral infusion analyses. Although these results supported the specificity of the effects observed, the sample size was too small to draw firm conclusions and thus is not reported.
Parietal Cortex
A mixed effects model was performed to investigate the relationship between the PC inactivation (muscimol vs saline) and performance accuracy (measured by percentage) on the place-action task. The percentage (Fig. 3 Top Left) varied across performance category (correct, incorrect, or no selection) following inactivation (t(7) = −3.32, p = 0.01). The linear mixed model can be characterized using the following classical form,
where the subscript ij didcates the jth observation in the ith subject, is the overall intercept, denotes the difference of the (transformed) percentage vectors between muscimol and saline conditions, denotes the random effect for the ith subject, and is the noise term. In this study, the mixed model focuses on the observed variable and random effect . Specifically, PC inactivation significantly reduced the percentage of correct trials (t(7) = −4.26, p<0.01), and increased both incorrect (t(7) = 3.02, p < 0.05) and no selection (t(7) = 3.00, p < 0.05) percentage as compared to saline. The observed variables in these three mixed models are differences of the percentage values between muscimol and saline, respectively, and the random effect is still the intercept for each subject. In addition, the average trial duration for PC inactivation sessions was significantly longer than saline sessions (Fig. 3 Top Right; t(7) = 2.61, p < 0.05). Thus, PC inactivation impaired performance on the place-action task by increasing both errors and no selection trials.
Figure 3. Inactivation of PC, ALT, and the PC-ALT Network Impaired Performance.

When data set is used as the sample, inactivation of the PC (Top Left), ALT (Bottom Left), and the ALT-PC network (Bottom Right) all produce impairments in the task. Specifically, for each inactivation condition (PC, ALT & ALT-PC network) there was a significant reduction in percent correct and a significant increase in percent incorrect. PC inactivation, but not ALT inactivation, increase the duration of each trial (Top Right/Inset) and the number of no selection trials, consistent with impaired linking of the correct action to the allocentric location (n=8 PC, n=8 ALT, & n=7 ALT-PC network data sets). Each rat is one color and data set pairs are connected with dashed lines. Note, rat color coding is consistent across Fig. 2 and Fig. 3 so cannula placements can be compared to the data for each rat. * p < 0.05, ** p < 0.01.
To ensure that a small number of data sets were not driving the observed effects we also performed the analyses using animals as the sample. For animals with more than one dataset per inactivation condition, we averaged the datasets to obtain one dataset per animal per condition. We found that inactivation produced variation in the percentage of trials across the categories (Correct, Incorrect and No Selection; Fig. 4 Top; F(2,5) = 10.98, p ≤ 0.01). Similarly, PC inactivation significantly reduced the percentage of correct trials (t(7) = −4.37, p<0.01), and increased both incorrect (t(7) = 3.17, p < 0.05) and no selection (t(7) = 2.81, p < 0.05) percentage as compared to saline. In addition, the average trial duration for PC inactivation sessions was significantly longer than saline sessions (not shown; t(6) = 2.44, p = 0.05).
Figure 4. Percentage of correct, incorrect, and no selection trials with animal as sample.
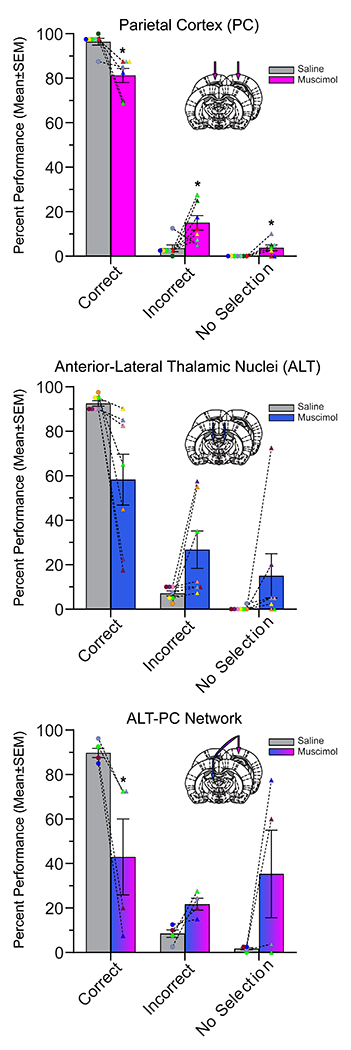
Inactivation of the PC (Top) and the ALT-PC network (Bottom), but not the ALT (Middle), produced impairments with the animals as the sample (n=7 animals each for PC, & ALT, & n=4 animals for network). Specifically, for PC and ALT-PC inactivation condition there was a significant reduction in percent correct. Thus, results were similar for PC and ALT-PC, but not ALT, when the animal is the sample. Each rat data point is color coded with the same color as prior figures. * p < 0.05, ** p < 0.01.
To rule out the possibility that reduced motivation following PC inactivation was responsible for the increased number of no selection trials, we measured the percentage of trials in which the animal left the start box without prodding. We found that for PC inactivation no selection trials, every rat left the start box without prodding 100% of the time for every session, suggesting that reduced motivation did not explain the reduced selecting following PC inactivation. Thus, PC inactivation impaired performance on the place-action task by increasing both errors and non selecting.
Anterior-Lateral Thalamus
We conducted a mixed effects model to investigate the relationship between ALT inactivation and place-action task performance. Consistent with our hypothesis, inactivation produced variation in percentage of trials across categories (Correct, Incorrect and No Selection; Fig. 3 Bottom Left; t(7) = −3.41, p = 0.01). In this mixed model, the variable is the difference of the (transformed) percentage vectors between muscimol and saline conditions, and the random effect is the intercept for each subject. Specifically, ALT inactivation significantly reduced the percentage of correct trials (t(7) = −3.18, p < 0.05) and increased incorrect percentage compared to saline (t(7) = 2.42, p < 0.05), but not no selection percentage (t(7) = 1.69, p = 0.13). The observed variables in these three mixed models are differences of the percentage values between muscimol and saline, respectively, and the random effect is still the intercept for each subject. Unlike PC, the average trial duration for ALT inactivation sessions was not significantly different from saline sessions (Fig. 3 Top Right; t(7) = 1.63, p = 0.15). Thus, ALT inactivation impaired performance on the place-action task by increasing incorrect responses, but not no selection trials.
To further verify the observed effects, we also performed the ALT analyses with animals as the sample. Surprisingly, inactivation did not produce variation in percentage of trials across the categories (Correct, Incorrect and No Selection; Fig. 4 Middle; F(2,5) = 3.98, p = 0.09). As with mixed effects model with data sets as a sample, the average trial duration for ALT inactivation sessions was not significantly different than saline sessions (not shown; t(6) = 1.42, p = 0.20). Thus, ALT inactivation impaired performance on the place-action task by reducing correct responses, but only when data set was used as the sample and repeated samples within an animal were controlled for using a mixed effects model.
Parietal-Anterior-Lateral Thalamic Network
We also performed a mixed effects model to account for repeated testing within an animal for contralateral ALT-PC inactivations and the effect on the place-action task performance when the data set was the sample. The percentage (Fig. 3 Bottom Right; t(6) = −8.24, p < 0.0001) varied across performance category (Correct, Incorrect, and No Selection). In this mixed model, the variable is the difference of the (transformed) percentage vectors between muscimol and saline conditions, and the random effect is the intercept for each subject. Specifically, ALT-PC network inactivation reduced the number of correct trials (t(6) = −3.66, p = 0.01) and increased the percentage of incorrect trials (t(6) = 3.45, p = 0.01) compared to saline, but did not increase the no selection percentage as compared to saline (t(6) = 2.02, p = 0.09). The observed variables in these three mixed models are differences of the percentage values between muscimol and saline, respectively, and the random effect is still the intercept for each subject. As with PC inactivation sessions, the average trial duration for ALT-PC inactivation sessions was greater than saline (Fig. 3 Top Right; t(6) = 2.56, p < 0.05). Thus, ALT-PC network inactivation impaired performance on the place-action task by increasing incorrect responses. Unlike with PC inactivation, non selecting was not significantly increased; however, as with PC inactivation, trial duration was significantly increased. This suggests that ALT-PC inactivation may have both produced a hesitancy to respond but that the effect was stronger with PC inactivation leading to more trials being classified as no response trials. In summary, PC, ALT, and network inactivation all produced a slightly different pattern of results (see below for a direct test of this observation).
We also performed the network analyses using animals as the sample. As before, inactivation produced variation in percentage of trials across the categories (Correct, Incorrect and No Selection; Fig. 4 Bottom; F(2,2) = 336.49, p < 0.01). Specifically, network inactivation reduced the percentage of correct trials (t(3) = −3.22, p<0.05), but did not increase incorrect percentage (t(3) = 3.01, p = 0.06) or no selection percentage when compared to saline (t(3) = 1.77, p = 0.18). Unlike PC, the average trial duration for ALT-PC inactivation sessions was not significantly different than saline sessions (not shown; t(3) = 1.97, p = 0.14). Thus, network inactivation impaired performance on the place-action task by increasing incorrect responses, but not non selection trials.
We conducted an additional analysis to ensure that the observed impairment with network inactivation versus ALT inactivation was not due to the smaller number of animals used for the ALT-PC network analysis. We selected ALT data sets for all animals that had ALT-PC inactivations (four data sets from three out of the four ALT-PC animals). As with ALT inactivations with animal as the sample, ALT inactivation for this subset of the data did not produce variations in percentage of trials across the categories (Correct, Incorrect and No Selection; t(3) = −3.05, p = 0.06). Thus, differences in the subset of animals used for the ALT-PC animals are unlikely to explain the differential effect for network versus ALT inactivation.
Bilateral PC, Bilateral ALT, and ALT-PC inactivation each produced a different pattern of impairment.
Finally, we conducted an additional mixed effects model with the animal or the session as the sample comparing the pattern of inactivation effects on performance across the three inactivation types (PC, ALT, & ALT-PC) and found that inactivation effects varied across the three types of inactivation for both session as the sample and animals as the sample (t(17) > 5.84, ps < 0.0001). The observed variables in these three mixed models are differences of the averaged percentage values (within each subject) between muscimol and saline, respectively, and the random effect is the intercept for each brain area. Specifically, mixed models planned comparisons on each pairwise combination of inactivation types revealed that each inactivation type differed from each of the other inactivation types (t(10-13) > 4.69, ps < 0.001; Same variables and random effects as the above full model except for two of three brain areas). Thus, network inactivation produced a different pattern of impairment than inactivating each region individually, and inactivating PC alone produced a different pattern of impairment than inactivating ALT alone.
Bilateral ALT and ALT-PC inactivation (but not Bilateral PC) induced Impairments may be larger in female rats.
When comparing the raw data across inactivation conditions it was noted that while there was no indication of sex differences following PC inactivation; however, following ALT and ALT-PC inactivation 3 female rats had the lowest performance (percent correct; see raw data: https://osf.io/4p34s/), suggesting a possible sex by inactivation condition interaction. Unfortunately, our study is not sufficiently powered to investigate this potential interaction with sex further. In order to ensure that these outliers were not responsible for impairments observed for these inactivation conditions, we removed these data points and re-ran the omnibus mixed models test with data set as the sample. For ALT-PC network inactivations, the percentage again varied across performance category (Correct, Incorrect, and No Selection; t(4) = −5.84, p < 0.01). However, for bilateral ALT inactivation with data set as the sample, the percentage no longer significantly varied across performance category (t(5) = −2.27, p = 0.07) similar to what was observed when animal was used as the sample. Thus, removing the outliers did not significantly change the overall pattern of the data suggesting that if effects are larger in female rats when these regions are inactivated, males are also impaired (at least for ALT-PC network inactivations).
Side bias, head scanning, and procedural errors
We also investigated whether muscimol inactivation resulted in general changes in behavioral performance by measuring the number of times the animal engaged in stereotyped behaviors relating to errors or inefficiencies in navigation and orientation. Side bias, or the ratio of preferred left or right turns toward the goal location was calculated and compared between muscimol inactivation and saline control for each performance category. The side bias was calculated by taking the absolute value of the total number of left choices minus the total number of right choices and then dividing by the total number of trials.
Paired t-tests (animals as sample) or mixed effects models (session as the sample) were performed to assess side bias ratios for each inactivation type (PC, ALT, and ALT-PC network) comparing saline versus muscimol data. There were no significant differences in side bias between muscimol and saline for any of the brain regions when the animal was used as the sample (Fig. 5 Top Left; PC: t(7) = 2.07, p = 0.18; Network: t(6) = 2.35, p = 0.06) and for PC or ALT-PC network when the session was the sample (Fig. 5 Bottom Left; PC: t(5) = 2.09, p = 0.09; ALT: t(5) = −1.70, p = 0.15; Network: t(2) = 1.13, p = 0.37). However, there was a significant side bias following muscimol inactivation for ALT (t(7) = 2.41, p < 0.05) suggesting that in the absence of an input from ALT animals switched to an egocentric strategy which was not effective. Additionally, we assessed head scanning (the number of times an animal moved its head to and from the location of the goal). Paired t-tests (animal as sample) or mixed effects models (session as the sample) were also used to assess the relationship between inactivation and head scanning. There were no significant differences in head scanning between muscimol and saline for any of the brain regions when the data set as the sample (Fig. 5 Top Middle; PC: t(7) = 1.29, p = 0.24; ALT: t(7) = −1.79, p = 0.12; Network: t(6) = 1.55, p = 0.17) and when the animal as the sample (Fig. 5 Bottom Middle; PC: t(5) = −0.68, p = 0.53; ALT: t(5) = 1.44, p = 0.21; Network: t(2) = −1.82, p = 0.21).
Figure 5. Error analysis.

Error analyses are shown for session as the sample (Top Row) and animals as the sample (Bottom Row). Left Column. Side bias scores were calculated for each session and averaged across animals. 0 is no preference for left or right turns to target location; 1 is complete preference for one turn direction to target. Side bias did not differ for any inactivation condition (ps > 0.12). Middle Column. The number of head scan movements made before a correct or incorrect decision averaged for each session did not differ for any inactivation condition (ps > 0.19). Right Column. The mean number of procedural errors per session was significantly increased following PC inactivation for both session and animals as the sample. * p < 0.05.
Lastly, we looked at procedural errors which included the number of times an animal traveled around the perimeter of the arena and past any of the three other arms. There was a significant increase in procedural errors using session as the sample for PC inactivations, but not for ALT and ALT-PC network inactivations (Fig. 5 Top Right; PC: t(7) = 3.27, p = 0.01; ALT: t(7) = 2.15, p = 0.07; Network: t(6) = 1.99, p = 0.09). Likewise, there was a significant increase in procedural errors using animal as sample for PC inactivations, but not for ALT and ALT-PC network inactivations (Fig. 5 Bottom Right; PC: t(5) = 3.40, p < 0.05; ALT: t(5) = −1.55, p = 0.18; Network: t(2) = −1.19, p = 0.36).
Discussion
The aim of this study was to test the hypothesis that functional connectivity between the ALT and PC is necessary for linking actions with allocentric spatial information. Overall, the results demonstrated that the ability to accurately perform the place-action task decreased significantly with muscimol inactivations across all inactivation types (ALT, PC, ALT-PC network). Though some effects were similar across inactivation conditions, there were several key differences and a significantly different pattern of impairment for each of the three inactivation types. First, ALT inactivation only significantly altered performance when data set was used as the sample and not when the animal was the sample. Second, only PC inactivation increased no selection trials, and procedural errors; while PC or ALT-PC network inactivation (only when the session was the sample) increased trial length. This suggests PC is essential for generating the appropriate action to the goal location. Third, when the session was used as the sample ALT inactivation led to a side bias. Thus, when ALT is intact, but PC or the ALT-PC network is inhibited, the rat has difficulty generating the appropriate action, but with ALT inhibited, the PC generates the wrong action (potentially because the PC is receiving incorrect information when the ALT is inhibited) or the animal used an egocentric strategy such as always go right. Together these findings suggest that the ALT-PC circuit is critical for transforming an allocentric location into the appropriate action.
The results of the present study are consistent with the notion that the PC has a critical role in linking egocentric action to allocentric information and potentially serves as a convergence point for these two spatial frames of reference. Supporting this view are results from previous studies showing that single-cell encoding in the PC is mixed with both egocentric and allocentric encoding, including conjunctive encoding in both reference frames. However, mesoscale encoding (multi-unit activity) in PC is organized around egocentric motion state (Kolb et al., 1994; Kolb & Walkey, 1987; Wilber et al., 2014; Wilber et al., 2017). Further, motion state encoding in PC is sometimes anticipatory, predicting the upcoming action (Whitlock et al., 2012; Wilber et al., 2014). This encoding scheme at both the single unit scale and mesoscale, combined with the present results, suggests that the PC plays a critical role in the ability to access an allocentric map and use this information to navigate towards a desired goal. Therefore, the present finding of impaired performance following PC inactivation, coupled with slow response or non-selecting, may highlight the inability to execute the proper actions toward the desired trajectory or goal location.
Although there were trends toward less accurate performance after bilateral ALT inactivation, these observations were not supported by significant impairments when the animal was used as the sample, unlike with PC or ALT-PC inactivations, which did produce significant impairments when the animal was the sample. It is worth noting that previous studies have demonstrated that the ALT has a role in allocentric spatial encoding (Aggleton & Nelson, 2015; Clark & Harvey, 2016; O’Mara, 2013; Van Der Werf, Jolles, Witter, & Uylings, 2003). Previous work, however, has shown that anterior thalamic inactivation can spare certain forms of place memory (Stackman, Lora, & Williams, 2012), which may explain the milder deficits observed with ALT inactivation in the present study.
HD cells have also been identified in several limbic brain regions, and while damage to the anterior thalamus is known to disrupt HD signaling in some of these other regions (Clark & Taube, 2012; Taube, 2007), it is possible that circuits independent of the anterior thalamus may compensate. HD cells are often linked to allocentric spatial processing (Dudchenko et al., 2019; Taube, 1995, 2007) which is supported by observations that experimental manipulation of this neural signal and damage to the ALT produces impairments very similar to hippocampal inactivation or lesions (Aggleton, Hunt, Nagle, & Neave, 1996; Butler et al., 2017).
In the present study, ALT infusions largely targeted the anterodorsal and anteroventral thalamic nuclei, but did not encroach on the anteromedial where HD cells have also been identified along with other spatial signals (Jankowski et al., 2015; Taube, 2007; Vertes, Linley, Groenewegen, & Witter, 2015). Our infusions also included the lateral thalamus in addition to the anterior thalamus, the adjacent laterodorsal thalamus contains HD information while other regions of the lateral thalamic aggregate (centrolateral, lateral mediodorsal nuclei) have also been linked to spatial navigation and memory (Clark & Harvey, 2016; Lopez et al., 2009; Mitchell & Dalrymple-Alford, 2006; Mizumori & Williams, 1993; Perry & Mitchell, 2019; Taube, 1995; Taube & Bassett, 2003).
Interestingly, network inactivation, but not ALT inactivation, impaired performance when the animal was the sample, despite less animals in the network inactivation condition. Similarly, ALT inactivation also did not impair performance when a subset of the data was used that matched the ALT-PC data set as closely as possible (i.e., came from as close to the same set of animals as possible). This suggests that the impairment from network inactivation was not due to targeting a subset of ALT that differed from the additional animals included for ALT only inactivation.
Based on our literature review, we used different delays for muscimol diffusion for ALT versus PC, designed to allow greater spread in the larger PC structure. Thus, it is possible that the differences in timing for the ALT and PC inactivation may have contributed to observed differences in their effects. However, effects of muscimol typically manifest immediately and remain stable for several hours, regardless of the size of the targeted structure or the infusion rate (Allen et al., 2008; Hikosaka & Wurtz, 1985; Krupa et al., 1999). Therefore, it is unlikely that the differential timing required for the ALT and PC inactivation procedures significantly impacted behavior in a differential manner.
We successfully balanced the number of male and female rats across the inactivation conditions as the proportion of male and female rats did not differ for any inactivation condition (PC, ALT or ALT-PC). We also pretrained rats to criterion before each inactivation so that any sex difference in performing the task would be minimized. As a result there were no sex differences following saline infusion. Similarly, there was no indication of sex differences following PC inactivation; however, following ALT and ALT-PC inactivation, 3 female rats had the lowest performance (percent correct; see raw data: https://osf.io/4p34s/) suggesting a possible sex by inactivation condition interaction. Note, removing the data from these rats did not significantly change the pattern of the results. Though we cannot rule out the possibility that muscimol happened to diffuse better in these rats resulting in more complete inactivation we also cannot rule out the possibility that there is a sex by inactivation condition interaction. There are numerous reports of sex differences in rodents for spatial navigation tasks with most, but not all studies, finding that male rats perform better at allocentric tasks (Cimadevilla et al., 1999; Forcano, Santamaría, Mackintosh, & Chamizo, 2009; Isgor & Sengelaub, 2003; Korol, Malin, Borden, Busby, & Couper-Leo, 2004; Koss & Frick, 2017; Willing, Drzewiecki, Cuenod, Cortes, & Juraska, 2016) and that males prefer geometric cues while female rats prefer landmarks (Kanit et al., 2000; Keeley, Tyndall, Scott, & Saucier, 2013; Rodríguez, Torres, Mackintosh, & Chamizo, 2010). However, to our knowledge there are no reports of an interaction between inactivating specific brain regions (but not others) and sex. Thus, future studies should investigate this potential interaction.
The pattern of results for ALT inactivation, PC inactivation and ALT-PC network inactivation supports the hypothesis that the ALT-PC circuit is critical for transforming spatially relevant contextual demands into the appropriate actions. This transformation would be critical for generating a route to a goal location based on allocentric information and executing the proper movements toward the goal (McNaughton, Knierim, & Wilson, 1995; Sutherland & Hamilton, 2004; Wilber et al., 2014). The connections between ALT and PC are largely ipsilateral, so the effect we observed from disconnecting the circuit with contralateral infusions (right PC and left ALT) is consistent with effects observed from circuit disconnection in other regions with similar structural connectivity (Fresno et al., 2019; Hernandez et al., 2017; Jo & Lee, 2010; Wilber et al., 2015). While these previous studies with similar anatomical connectivity observed a significantly greater impact from contralateral than unilateral inactivations (often with little if any impact from unilateral infusions), the present study did not include a unilateral control condition. Thus, given the similarity between PC and network inactivations, we cannot rule out the possibility that all observed effects are actually the result of unilateral PC inactivation.
The place-action task used in this study requires a combination of both allocentric location and egocentric action in order to reach one of four fixed goal locations. In contrast, other “cross-maze” task variations (similar maze layout, but different task rules) force the animal to utilize a specific strategy, either allocentric or egocentric. In these cross-maze variations, ALT inactivations produced deficits only when an allocentric strategy was employed, but not when an egocentric strategy was employed (Aggleton et al., 1996). The present task does not distinguish between allocentric heading and allocentric location, therefore, animals may be solving the task by transforming a place into action or by transforming a heading into action. Nevertheless, since PC and ALT-PC network inactivation types both produced a deficit in the place-action task, these results suggest that the ALT-PC circuit is critical for translating, or at the very least, coordinating between allocentric goal location (or heading) and egocentric action. Similarly, since goal locations are in unique (i.e., slightly differing) spatial locations, it cannot be fully ruled out that animals are using an allocentric only strategy to learn the goal locations. However, the impairment following PC inactivation suggests that egocentric information was used by the animals, particularly since previous work has shown that animals can still learn an allocentric location following PC lesions, though, the route to the location is less direct (Kolb et al., 1994). Future research could further our understanding of allocentric-egocentric coordination by using a paradigm in which there are distinct allocentric, egocentric and transformation components in which allocentric location is dissociated from allocentric heading. Such work would rule out the possibility (though unlikely given the previous research outlined above) that memory for reward locations or altered reward sensitivity might explain the present results.
Although the present study found a drop in correct responding with PC and ALT inactivations when data sets were the sample, only PC inactivations were significant when animals were the sample. One additional notable difference is that following ALT inactivation, animals proceeded to the incorrect location (sometimes with a side bias such as always going to the right), while PC inactivation leads to increased non selecting. This could mean that allocentric information was not being translated properly in the absence of the HD signal from ALT, leading to an inability to select the correct action or that in the absence of the HD signal the animal developed an egocentric strategy (e.g., always go to the right).
The ALT-PC circuit is likely a component of a larger network for coordination of spatial information that includes the retrosplenial cortex and hippocampus (which would both be intact following ALT inactivation). It is important to note that several other regions contribute to egocentric and allocentric spatial information processing. For instance, the hippocampus and entorhinal cortex have specific cell types, place cells and grid cells respectively, that are thought to be the neural substrate of an allocentric cognitive map-like representation of the environment for navigation (Moser, Moser, & McNaughton, 2017; O’Keefe & Nadel, 1978). The hippocampus has direct connectivity to the retrosplenial cortex, allowing the transfer of hippocampal place information to a brain region that contains a mixture of allocentric and egocentric encoding cells (Alexander & Nitz, 2017; Wyss & Van Groen, 1992). Finally, the retrosplenial cortex sends and receives many projections to both ALT and PC, making it a critical component of a network for processing egocentric and allocentric spatial information. Coordination across this brain network would be essential in order to provide flexibility and efficiency when coordinating allocentric and egocentric information to travel toward a goal location as in the place-action task. Thus, it is likely that multiple regions support coordination between ego- and allo-centric representations for navigation and that here we have dissected the contribution of the ALT-PC circuit to this larger brain network.
Together the evidence here suggests that the ALT-PC circuit is critical for the coordination between allocentric location and egocentric action in order to reach a goal. Thus, the ALT-PC circuit may be critical for transformation of allocentric place into egocentric action.
Supplementary Material
Acknowledgments:
This research was supported by grants from the National Institutes of Health (R01 AA029700 to BJC, and R00 AG049090 and R01 AG070094 to AAW) and Florida Department of Health (FL DOH 20A09 to AAW).
Funding:
This research was supported by grants from the National Institutes of Health (R01 AA029700 to BJC, and R00 AG049090 and R01 AG070094 to AAW) and Florida Department of Health (FL DOH 20A09 to AAW).
Data Availability:
The data that support the findings of this study will be made openly available in OSF at https://osf.io/4p3rs/ upon acceptance for publication.
Bibliography
- Aggleton JP, Hunt PR, Nagle S, & Neave N (1996). The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behavioural Brain Research, 81(1), 189–198. doi: 10.1016/S0166-4328(96)89080-2 [DOI] [PubMed] [Google Scholar]
- Aggleton JP, & Nelson AJD (2015). Why do lesions in the rodent anterior thalamic nuclei cause such severe spatial deficits? Neuroscience & Biobehavioral Reviews, 54, 131–144. doi: 10.1016/j.neubiorev.2014.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AS, & Nitz DA (2017). Spatially Periodic Activation Patterns of Retrosplenial Cortex Encode Route Sub-spaces and Distance Traveled. Current Biology, 27(11), 1551–1560.e1554. doi: 10.1016/j.cub.2017.04.036 [DOI] [PubMed] [Google Scholar]
- Alexander AS, Place R, Starrett MJ, Chrastil ER, & Nitz DA (2023). Rethinking retrosplenial cortex: Perspectives and predictions. Neuron, 111(2), 150–175. doi: 10.1016/j.neuron.2022.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AS, Robinson JC, Stern CE, & Hasselmo ME (2023). Gated transformations from egocentric to allocentric reference frames involving retrosplenial cortex, entorhinal cortex, and hippocampus. Hippocampus, 33(5), 465–487. doi: 10.1002/hipo.23513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AS, Tung JC, Chapman GW, Conner AM, Shelley LE, Hasselmo ME, & Nitz DA (2022). Adaptive integration of self-motion and goals in posterior parietal cortex. Cell Rep, 38(10). doi: 10.1016/j.celrep.2022.110504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, & Brown TH (2008). Imaging the spread of reversible brain inactivations using fluorescent muscimol. Journal of Neuroscience Methods, 171(1), 30–38. doi: 10.1016/j.jneumeth.2008.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicanski A, & Burgess N (2016). Environmental Anchoring of Head Direction in a Computational Model of Retrosplenial Cortex. The Journal of Neuroscience, 36(46), 11601–11618. doi: 10.1523/jneurosci.0516-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicanski A, & Burgess N (2018). A neural-level model of spatial memory and imagery. eLife, 7. doi: 10.7554/eLife.33752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler WN, Smith KS, van der Meer MAA, & Taube JS (2017). The Head-Direction Signal Plays a Functional Role as a Neural Compass during Navigation. Current Biology, 27(9), 1259–1267. doi: 10.1016/j.cub.2017.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne PA, Becker S, & Burgess N (2007). Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol Rev, 114(2), 340–375. doi: 10.1037/0033-295x.114.2.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne PA, & Crawford JD (2010). Cue Reliability and a Landmark Stability Heuristic Determine Relative Weighting Between Egocentric and Allocentric Visual Information in Memory-Guided Reach. Journal of Neurophysiology, 103(6), 3054–3069. doi: 10.1152/jn.01008.2009 [DOI] [PubMed] [Google Scholar]
- Calton JL, Turner CS, Cyrenne D-LM, Lee BR, & Taube JS (2008). Landmark control and updating of self-movement cues are largely maintained in head direction cells after lesions of the posterior parietal cortex. Behavioral Neuroscience, 122(4), 827–840. doi: 10.1037/0735-7044.122.4.827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadevilla JM, González-Pardo H, López L, Díaz F, Cueto EG, García-Moreno LM, & Arias JL (1999). Sex-related differences in spatial learning during the early postnatal development of the rat. Behavioural Processes, 46(2), 159–171. doi: 10.1016/S0376-6357(99)00034-0 [DOI] [PubMed] [Google Scholar]
- Clark BJ, Bassett JP, Wang SS, & Taube J (2010). Impaired head direction cell representation in the anterodorsal thalamus after lesions of the retrosplenial cortex. Journal of Neuroscience, 30(15), 5289–5302. doi: 10.1523/jneurosci.3380-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, & Harvey RE (2016). Do the anterior and lateral thalamic nuclei make distinct contributions to spatial representation and memory? Neurobiology of Learning & Memory, 133, 69–78. doi: 10.1016/j.nlm.2016.06.002 [DOI] [PubMed] [Google Scholar]
- Clark BJ, Simmons CM, Berkowitz LE, & Wilber AA (2018). The retrosplenial-parietal network and reference frame coordination for spatial navigation. Behav Neurosci, 132(5), 416–429. doi: 10.1037/bne0000260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, & Taube J (2012). Vestibular and attractor network basis of the head direction cell signal in subcortical circuits. Frontiers in Neural Circuits, 6. doi: 10.3389/fncir.2012.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER, & Smith A (2019). A new perspective on the head direction cell system and spatial behavior. Neuroscience & Biobehavioral Reviews, 105, 24–33. doi: 10.1016/j.neubiorev.2019.06.036 [DOI] [PubMed] [Google Scholar]
- Egozcue JJ, Pawlowsky-Glahn V, Mateu-Figueras G, & Barceló-Vidal C (2003). Isometric Logratio Transformations for Compositional Data Analysis. Mathematical Geology, 35(3), 279–300. doi: 10.1023/A:1023818214614 [DOI] [Google Scholar]
- Forcano L, Santamaría J, Mackintosh NJ, & Chamizo VD (2009). Single landmark learning in rats: Sex differences in a navigation task. Learning and Motivation, 40(1), 46–61. doi: 10.1016/j.lmot.2008.05.003 [DOI] [Google Scholar]
- Fresno V, Parkes SL, Faugère A, Coutureau E, & Wolff M (2019). A thalamocortical circuit for updating action-outcome associations. eLife, 8, e46187. doi: 10.7554/eLife.46187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR (1990). The Organization of Learning. Cambridge: Bradform Books/MIT Press. [Google Scholar]
- Gibb SJ, Wolff M, & Dalrymple-Alford JC (2006). Odour–place paired-associate learning and limbic thalamus: Comparison of anterior, lateral and medial thalamic lesions. Behavioural Brain Research, 172(1), 155–168. doi: 10.1016/j.bbr.2006.05.017 [DOI] [PubMed] [Google Scholar]
- Grieves RM, Jenkins BW, Harland BC, Wood ER, & Dudchenko PA (2016). Place field repetition and spatial learning in a multicompartment environment. Hippocampus, 26(1), 118–134. doi: 10.1002/hipo.22496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RE, Thompson SM, Sanchez LM, Yoder RM, & Clark BJ (2017). Post-training Inactivation of the Anterior Thalamic Nuclei Impairs Spatial Performance on the Radial Arm Maze. Frontiers in Neuroscience, 11. doi: 10.3389/fnins.2017.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AR, Reasor JE, Truckenbrod LM, Lubke KN, Johnson SA, Bizon JL, … Burke SN (2017). Medial prefrontal-perirhinal cortical communication is necessary for flexible response selection. Neurobiology of Learning & Memory, 137, 36–47. doi: 10.1016/j.nlm.2016.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, & Wurtz RH (1985). Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. Journal of Neurophysiology, 53(1), 292–308. doi: 10.1152/jn.1985.53.1.292 [DOI] [PubMed] [Google Scholar]
- Isgor C, & Sengelaub DR (2003). Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. Journal of Neurobiology, 55(2), 179–190. doi: 10.1002/neu.10200 [DOI] [PubMed] [Google Scholar]
- Jankowski MM, Passecker J, Islam MN, Vann S, Erichsen JT, Aggleton JP, & O’Mara SM (2015). Evidence for spatially-responsive neurons in the rostral thalamus. Front Behav Neurosci, 9. doi: 10.3389/fnbeh.2015.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YS, & Lee I (2010). Disconnection of the hippocampal-perirhinal cortical circuits severely disrupts object-place paired associative memory. Journal of Neuroscience, 30(29), 9850–9858. doi: 10.1523/jneurosci.1580-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanit L, Taskiran D, Yilmaz ÖA, Balkan B, Demirgören S, Furedy JJ, & Pögün S (2000). Sexually dimorphic cognitive style in rats emerges after puberty. Brain Research Bulletin, 52(4), 243–248. doi: 10.1016/S0361-9230(00)00232-X [DOI] [PubMed] [Google Scholar]
- Keeley RJ, Tyndall AV, Scott GA, & Saucier DM (2013). Sex Difference in Cue Strategy in a Modified Version of the Morris Water Task: Correlations between Brain and Behaviour. PLoS ONE, 8(7), e69727. doi: 10.1371/journal.pone.0069727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R, & Hayman R (2014). Allocentric directional processing in the rodent and human retrosplenial cortex. Frontiers in Human Neuroscience, 8. doi: 10.3389/fnhum.2014.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Buhrmann K, McDonald R, & Sutherland RJ (1994). Dissociation of the Medial Prefrontal, Posterior Parietal, and Posterior Temporal Cortex for Spatial Navigation and Recognition Memory in the Rat. Cerebral Cortex, 4(6), 664–680. doi: 10.1093/cercor/4.6.664 [DOI] [PubMed] [Google Scholar]
- Kolb B, & Walkey J (1987). Behavioural and anatomical studies of the posterior parietal cortex in the rat. Behavioural Brain Research, 23(2), 127–145. doi:Doi: 10.1016/0166-4328(87)90050-7 [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, & Couper-Leo J (2004). Shifts in preferred learning strategy across the estrous cycle in female rats. Hormones and Behavior, 45(5), 330–338. doi: 10.1016/j.yhbeh.2004.01.005 [DOI] [PubMed] [Google Scholar]
- Koss WA, & Frick KM (2017). Sex differences in hippocampal function. Journal of Neuroscience Research, 95(1-2), 539–562. doi: 10.1002/jnr.23864 [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Ghazanfar AA, & Miguel ALN (1999). Immediate Thalamic Sensory Plasticity Depends on Corticothalamic Feedback. Proceedings of the National Academy of Sciences of the United States of America, 96(14), 8200–8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J, Wolff M, Lecourtier L, Cosquer B, Bontempi B, Dalrymple-Alford J, & Cassel J-C (2009). The Intralaminar Thalamic Nuclei Contribute to Remote Spatial Memory. The Journal of Neuroscience, 29(10), 3302–3306. doi: 10.1523/jneurosci.5576-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchette SA, Vass LK, Ryan J, & Epstein RA (2014). Anchoring the neural compass: coding of local spatial reference frames in human medial parietal lobe. Nature Neuroscience, 17(11), 1598–1606. doi: 10.1038/nn.3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, & Delaney HD (2003). Designing experiments and analyzing data: A model comparison perspective. Mahwah, New Jersey: Lawrence Erlbaum Associates. [Google Scholar]
- McDonald RJ, & White NM (1994). Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol, 61(3), 260–270. doi: 10.1016/s0163-1047(05)80009-3 [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, & Moser M-B (2006). Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci, 7(8), 663–678. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Knierim JJ, & Wilson MA (1995). Vector encoding and the vestibular foundations of spatial cognition: Neurophysiological and computational mechanisms. In Gazzaniga MS (Ed.), The Cognitive Neurosciences (pp. 585–595). Cambridge: The MIT Press. [Google Scholar]
- Mimica B, Dunn BA, Tombaz T, Bojja VPTNCS, & Whitlock JR (2018). Efficient cortical coding of 3D posture in freely behaving rats. Science, 362(6414), 584–589. doi:doi: 10.1126/science.aau2013 [DOI] [PubMed] [Google Scholar]
- Mitchell AS, & Dalrymple-Alford JC (2006). Lateral and anterior thalamic lesions impair independent memory systems. Learn Mem, 13(3), 388–396. doi: 10.1101/lm.122206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori S, & Williams J (1993). Directionally selective mnemonic properties of neurons in the lateral dorsal nucleus of the thalamus of rats. The Journal of Neuroscience, 13(9), 4015–4028. doi: 10.1523/jneurosci.13-09-04015.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P-H, Tsenkina Y, Lecourtier L, Lopez J, Cosquer B, Wolff M, … Cassel J-C (2013). Lesions of the anterior thalamic nuclei and intralaminar thalamic nuclei: place and visual discrimination learning in the water maze. Brain Structure and Function, 218(3), 657–667. doi: 10.1007/s00429-012-0419-0 [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser M-B, & McNaughton BL (2017). Spatial representation in the hippocampal formation: a history. Nature Neuroscience, 20(11), 1448–1464. doi: 10.1038/nn.4653 [DOI] [PubMed] [Google Scholar]
- Nitz DA (2006). Tracking Route Progression in the Posterior Parietal Cortex. Neuron, 49(5), 747–756. doi:DOI: 10.1016/j.neuron.2006.01.037 [DOI] [PubMed] [Google Scholar]
- Nitz DA (2009). Parietal cortex, navigation, and the construction of arbitrary reference frames for spatial information. Neurobiology of Learning and Memory, 91(2), 179–185. doi:DOI: 10.1016/j.nlm.2008.08.007 [DOI] [PubMed] [Google Scholar]
- Nitz DA (2012). Spaces within spaces: rat parietal cortex neurons register position across three reference frames. Nature Neuroscience, 15(10), 1365–1367. doi:http://www.nature.com/neuro/journal/vaop/ncurrent/abs/nn.3213.html#supplementary-information [DOI] [PubMed] [Google Scholar]
- O’Keefe J, & Nadel L (1978). The hippocampus as a cognitive map. Oxford: Clarendon. [Google Scholar]
- O’Mara S (2013). The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Frontiers in Systems Neuroscience, 7. doi: 10.3389/fnsys.2013.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mara SM, & Aggleton JP (2019). Space and Memory (Far) Beyond the Hippocampus: Many Subcortical Structures Also Support Cognitive Mapping and Mnemonic Processing. Frontiers in Neural Circuits, 13. doi: 10.3389/fncir.2019.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormond J, & O’Keefe J (2022). Hippocampal place cells have goal-oriented vector fields during navigation. Nature, 607(7920), 741–746. doi: 10.1038/s41586-022-04913-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckford G, Dwyer JA, Snow AC, Thorpe CM, Martin GM, & Skinner DM (2014). The effects of lesions to the postsubiculum or the anterior dorsal nucleus of the thalamus on spatial learning in rats. Behavioral Neuroscience, 128, 654–665. doi: 10.1037/bne0000019 [DOI] [PubMed] [Google Scholar]
- Perry BAL, & Mitchell AS (2019). Considering the Evidence for Anterior and Laterodorsal Thalamic Nuclei as Higher Order Relays to Cortex. Frontiers in Molecular Neuroscience, 12. doi: 10.3389/fnmol.2019.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget A, Deneve S, & Duhamel J-R (2002). A computational perspective on the neural basis of multisensory spatial representations. Nature Reviews Neuroscience, 3(9), 741–747. doi: 10.1038/nrn914 [DOI] [PubMed] [Google Scholar]
- Raposo D, Kaufman MT, & Churchland AK (2014). A category-free neural population supports evolving demands during decision-making. Nature Neuroscience, 17(12), 1784–1792. doi: 10.1038/nn.3865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez CA, Torres A, Mackintosh NJ, & Chamizo VD (2010). Sex differences in the strategies used by rats to solve a navigation task. J Exp Psychol Anim Behav Process, 36(3), 395–401. doi: 10.1037/a0017297 [DOI] [PubMed] [Google Scholar]
- Stackman RW, Lora JC, & Williams SB (2012). Directional Responding of C57BL/6J Mice in the Morris Water Maze Is Influenced by Visual and Vestibular Cues and Is Dependent on the Anterior Thalamic Nuclei. The Journal of Neuroscience, 32(30), 10211–10225. doi: 10.1523/jneurosci.4868-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, & Hamilton DA (2004). Rodent spatial navigation: At the crossroads of cognition and movement. Neurosci Biobehav Rev, 28, 687–697. doi: 10.1016/j.neubiorev.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Taube JS (1995). Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. The Journal of Neuroscience, 15(1), 70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS (2007). The Head Direction Signal: Origins and Sensory-Motor Integration. Annual Review of Neuroscience, 30(1), 181–207. doi:doi: 10.1146/annurev.neuro.29.051605.112854 [DOI] [PubMed] [Google Scholar]
- Taube JS, & Bassett JP (2003). Persistent Neural Activity in Head Direction Cells. Cerebral Cortex, 13(11), 1162–1172. doi: 10.1093/cercor/bhg102 [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Jolles J, Witter MP, & Uylings HBM (2003). Contributions of Thalamic Nuclei to Declarative Memory Functioning. Cortex, 39(4), 1047–1062. doi: 10.1016/S0010-9452(08)70877-3 [DOI] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Groenewegen HJ, & Witter MP (2015). Chapter 16 - Thalamus. In Paxinos G (Ed.), The Rat Nervous System (Fourth Edition) (pp. 335–390). San Diego: Academic Press. [Google Scholar]
- Wang RF (2012). Theories of spatial representations and reference frames: What can configuration errors tell us? Psychonomic Bulletin & Review, 19(4), 575–587. doi: 10.3758/s13423-012-0258-2 [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Hines DJ, & Wallace DG (2001). Dead reckoning (path integration) requires the hippocampal formation: evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark (idiothetic) tests. Behavioural Brain Research, 127(1), 49–69. doi: 10.1016/S0166-4328(01)00359-X [DOI] [PubMed] [Google Scholar]
- Whitlock Jonathan R., Pfuhl G, Dagslott N, Moser M-B, & Moser Edvard I. (2012). Functional Split between Parietal and Entorhinal Cortices in the Rat. Neuron, 73(4), 789–802. doi: 10.1016/j.neuron.2011.12.028 [DOI] [PubMed] [Google Scholar]
- Wilber AA, Clark BJ, Demecha A, Mesina L, Vos J, & McNaughton BL (2015). Automated cortical connectivity maps reveal anatomically distinct areas in the parietal cortex of the rat. Frontiers in Neural Circuits, 8, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber AA, Clark BJ, Forster TC, Tatsuno M, & McNaughton BL (2014). Interaction of Egocentric and world centered reference frames in the rat posterior parietal cortex. The Journal of Neuroscience, 34(16), 5431–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber AA, Skelin I, Wu W, & McNaughton BL (2017). Laminar Organization of Encoding and Memory Reactivation in the Parietal Cortex. Neuron, 95(6), 1406–1419.e1405. doi: 10.1016/j.neuron.2017.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing J, Drzewiecki CM, Cuenod BA, Cortes LR, & Juraska JM (2016). A role for puberty in water maze performance in male and female rats. Behav Neurosci, 130(4), 422–427. doi: 10.1037/bne0000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M, Gibb SJ, Cassel J-C, & Dalrymple-Alford JC (2008). Anterior but not intralaminar thalamic nuclei support allocentric spatial memory. Neurobiology of Learning and Memory, 90(1), 71–80. doi: 10.1016/j.nlm.2008.01.007 [DOI] [PubMed] [Google Scholar]
- Wyss JM, & Van Groen T (1992). Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus, 2(1), 1–11. doi: 10.1002/hipo.450020102 [DOI] [PubMed] [Google Scholar]
- Xu Z, Wu W, Winter SS, Mehlman ML, Butler WN, Simmons CM, … Clark BJ (2019). A Comparison of Neural Decoding Methods and Population Coding Across Thalamo-Cortical Head Direction Cells. Frontiers in Neural Circuits, 13(75). doi: 10.3389/fncir.2019.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, & Taube JS (2014). The vestibular contribution to the head direction signal and navigation. Frontiers in Integrative Neuroscience, 8. doi: 10.3389/fnint.2014.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M (2018). Human spatial representation: what we cannot learn from the studies of rodent navigation. Journal of Neurophysiology, 120(5), 2453–2465. doi: 10.1152/jn.00781.2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study will be made openly available in OSF at https://osf.io/4p3rs/ upon acceptance for publication.


