Abstract
Gluten challenge is an essential clinical tool that involves reintroducing or increasing the amount of gluten in the diet to facilitate diagnostic testing in celiac disease (CD). Nevertheless, there is no consensus regarding the applications of gluten timing, dosing, and duration in children. This review aims to summarize the current evidence, discuss practical considerations, and proposes a clinical algorithm to help guide testing in pediatric patients.
Childhood development, social circumstances, and long-term health concerns must be considered when identifying a candidate for gluten challenge. Based on previous studies, the authors suggest baseline serology followed by a minimum of 3–6 grams of gluten per day for over 12 weeks to optimize diagnostic accuracy for evaluation of CD. A formal provider check-in at 4–6 weeks is essential so the provider and family can adjust dosing or duration as needed. Increasing the dose of gluten further may improve diagnostic yield if tolerated, although in select cases a lower dose and shorter course (6–12 weeks) may be sufficient.
There is consensus that mild elevations in celiac serology (<10 times the upper limit of normal) or symptoms, while supportive are not diagnostic for CD. Current North American Society for Pediatric Gastroenterology, Hepatology and Nutrition guidelines recommend histologic findings of intraepithelial lymphocytosis, crypt hyperplasia, and villous atrophy as the accurate and most appropriate endpoint for gluten challenge.
Keywords: celiac, coeliac, gluten-free
Graphical Abstract:
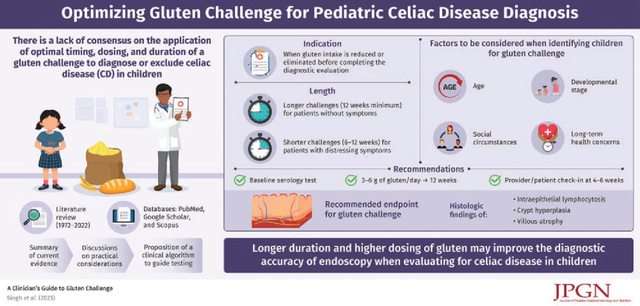
Celiac disease (CD) is a chronic gluten driven immune-mediated enteropathy (1) that can present with gastrointestinal symptoms, extraintestinal manifestations, or no symptoms at all (2). Diagnosis of CD involves assessment of symptoms, serology, and duodenal histology (3–5), which normalize on a gluten-free diet (GFD) (6,7). Therefore, accurate evaluation requires patients to be ingesting sufficient gluten when tested. Frequently, patients reduce their gluten intake to ameliorate symptoms or to treat presumed disease. Consequently, physicians may recommend a gluten challenge, which involves reintroducing or increasing the amount of gluten consumed to facilitate diagnostic testing. Although adult and pediatric studies (8–14) have attempted to identify how much gluten must be ingested to ensure serologic and endoscopic findings are reliable, consensus has not been reached (15–17). Here, we outline indications for gluten challenge, review current evidence that informs how to perform a challenge, and discuss practical considerations.
METHODS
PubMed, Google Scholar, and Scopus (1972–2022) were searched using the terms “celiac,” “coeliac,” “gluten,” and “challenge” alone and in combination. Highly cited publications and those published in 2000 or later were prioritized. Authors are members of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Celiac Disease Special Interest Group.
CLINICAL INDICATIONS
Gluten challenge is most commonly employed when gluten intake is reduced or eliminated before a definitive diagnostic evaluation of CD was completed (Table 1). Gluten restriction may precede testing or occur in response to abnormal celiac serology titers below the threshold for non-biopsy diagnosis. The European Society of Pediatric Gastroenterology, Hepatology and Nutrition published guidelines for serologic diagnosis without biopsy if the tissue transglutaminase-immunoglobulin A (tTG-IgA) is greater than 10 times above the upper limit of normal and there is a positive endomysial antibody on a second blood draw (18), however NASPGHAN has not adopted this diagnostic algorithm to date. In certain situations, gastroenterologists may discuss these European guidelines with families, however according to NASPGHAN (19–21), to complete appropriate diagnostic testing, one needs to restart a gluten-containing diet, repeat serology, and in most cases perform an endoscopy.
TABLE 1.
Clinical contexts for pursuing a gluten challenge
| The new diagnosis | Gluten causing symptoms | Potential celiac or unknown diagnosis | |
|---|---|---|---|
|
| |||
| Clinical scenario | Positive celiac serology <10 times the upper limit of normal + no duodenal biopsy + already on a gluten-free diet | No celiac serology +/− no endoscopy and clear symptoms with gluten and on a gluten-free diet | Positive celiac serology and normal endoscopy with 1. Concern for insufficient gluten prior to scope 2. Opted for gluten-free diet without confirmation of celiac disease |
| Proceed to gluten challenge | Yes, if patient amenable to confirm celiac disease | Yes, challenge helps differentiate gluten-related disorder to clarify diagnosis and goals of care | Yes, can identify if patient now has active celiac disease |
Gluten challenge should also be considered when diagnostic studies yield discordant results such as in “potential CD” (eg, positive celiac autoantibodies in the absence of duodenal enteropathy (22)) or in the presence of gluten induced symptoms without positive serology (23). Prudent diagnosis of potential CD requires examination of whether gluten consumption prior to endoscopy was sufficient to exclude CD and if adequate biopsies were taken (24). Gluten challenge may be indicated so that inappropriate diagnosis of potential CD in such cases does not delay diagnosis and treatment.
ASSESSMENT
Assessments during gluten challenge include symptoms, autoantibodies, and histopathology (12,13). Baseline evaluation by the gastroenterologist includes estimating current gluten intake, screening gastrointestinal and extraintestinal manifestations, and establishing goals of gluten consumption for the challenge. Human leukocyte antigen (HLA) genotyping can be informative as HLA DQ2, DQ7, and/or DQ8 are necessary for CD susceptibility, albeit not diagnostic (25–27). Thus, HLA testing may identify who could potentially forgo a gluten challenge given the low occurrences of HLA-DQ2/7/8-negative CD (16). Test costs and clinical context should be considered.
Baseline serologies prior to challenge may be particularly helpful in patients without previous serologic testing, patients ingesting minimal amounts of gluten, or patients who have recently started a GFD. Elevated serologies may be helpful, but normal celiac serologies in patients taking little or no gluten cannot be used to confidently exclude CD (Fig. 1). tTG-IgA (4) with total IgA to exclude IgA deficiency are recommended. Deamidated gliadin antibodies, tissue transglutaminase IgG, and/or endomysial antibody may be used depending on the IgA status and clinical context (28).
FIGURE 1.
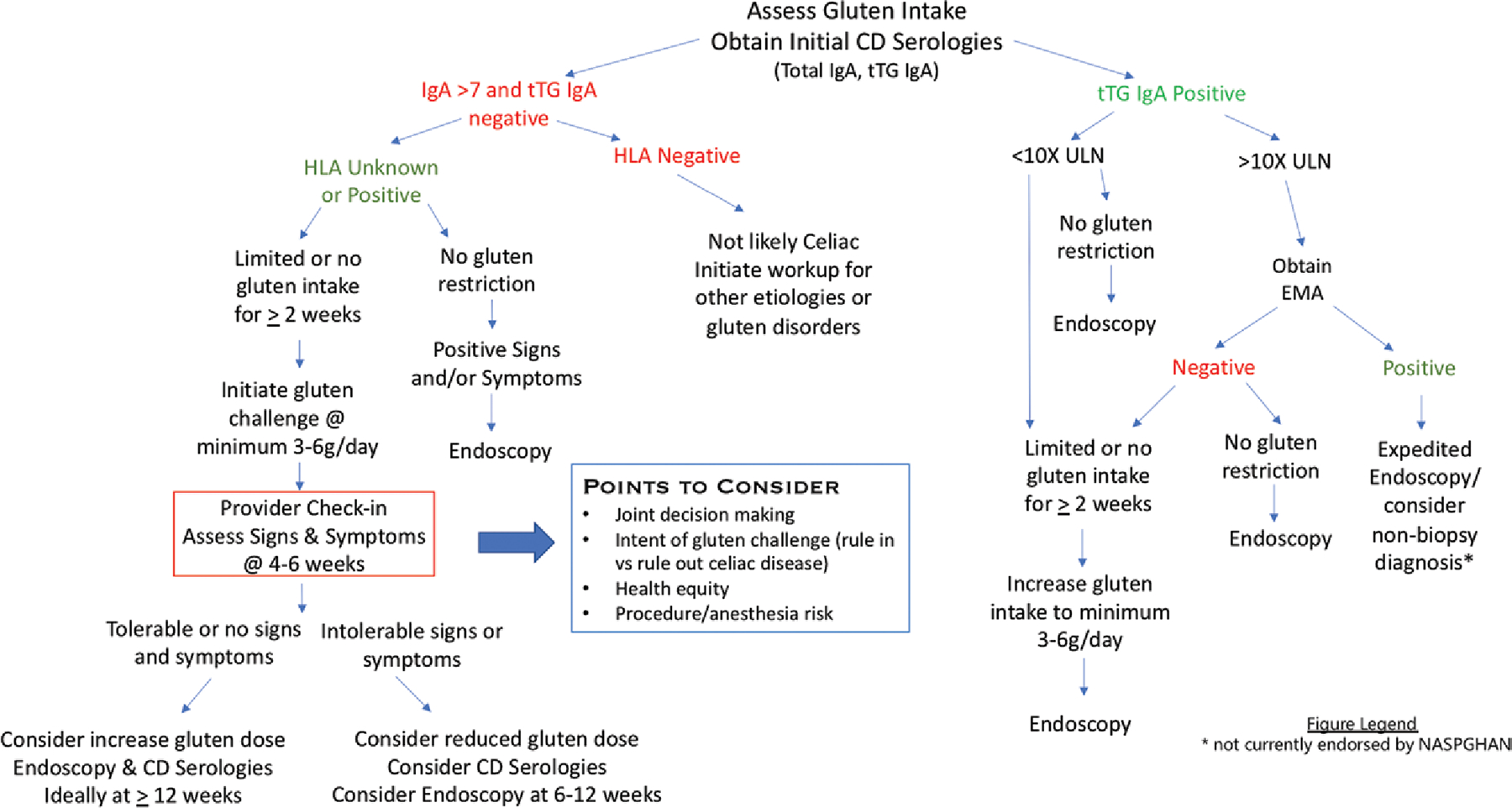
A clinical approach to gluten challenge for the diagnosis or exclusion of CD in children. Goals of testing should be clear with shared decision making to balance quality of life, patient safety, and accuracy of diagnosis. In addition to assessment of gluten intake, celiac serology titers, and HLA genotyping when applicable, the framework of the challenge requires communication and monitoring of signs and symptoms. A formal provider check-in at 4–6 weeks allows potential modification to the duration of the gluten challenge and may guide timing of repeat serology and subsequent endoscopy. CD = celiac disease; EMA = endomysial antibody; HLA = human leukocyte antigen; IgA = immunoglobulin A; tTG = tissue transglutaminase; ULN = upper limit of normal.
INITIATING A GLUTEN CHALLENGE
Gluten challenge should only be undertaken after a comprehensive clinical assessment and discussion about the benefits versus risks. Contraindications include known anaphylactic response to gluten, severe neurological manifestations, or debilitating symptoms. Given the importance of clinical context and heterogeneity of responses to gluten, it is critical to discuss the intended dosing and duration of gluten ingestion prior to the start of the challenge. These parameters may be influenced by age, pubertal development, potential for symptom severity, and duration of prior gluten restriction.
Previous gluten challenge studies (Table 1, Supplemental Digital Content, http://links.lww.com/MPG/D275) have used a range of gluten intake (1–30 grams per day) and weight based dosing (0.2–1 grams gluten/kg/day) (29). Historically, when gluten challenge was part of the diagnostic algorithm for all patients, consumption of 10 grams gluten/day for 6–8 weeks was recommended (30–32). In 2013, Leffler et al (13) suggested a change to the clinical practice paradigm based on a study in adults in which 3 grams gluten/day for 14 days was sufficient to confirm histologic relapse in healed CD patients. A subsequent double-blind study of a 14-day gluten challenge demonstrated dose responsiveness as those who received 10 grams gluten/day had greater reduction in villous height to crypt depth ratio than those who received 3 grams gluten/day. Notably, more symptoms were reported by those who received the lower dose (12).
Pediatric gluten challenge trials are limited. Meyer et al challenged children with biopsy-proven CD in remission with 10 grams gluten/day. A challenge–duration response relationship was observed with 68% having histologic relapse at 8 weeks, 84% at 3 months, and 97% at 2 years (33). Other pediatric studies have shown dose-dependence of serologic and histologic relapse with chronic daily ingestion of small amounts of gluten (~200 mg and 1 gram) (33,34). In another study, sustained exposure to 10 mg/kg/day of gluten led to villous atrophy in 9.2% at 6 months and 53.7% by 36 months (35).
Finally, ensuring consistent gluten ingestion and the form in which the gluten is delivered can be problematic. “Slice of bread” equivalents are often suggested, but concrete recommendations may be more practical as children typically consume a variety of gluten-containing foods. Objective data regarding the gluten content of specific foods is limited, but calculations have been employed to estimate gluten content (36–38). The University of Minnesota Nutrient Coordinating Center (NCC) Food and Nutrient Database calculates gluten content based upon estimating the fraction of vegetable protein in food presumed to be gluten (37). Mean gluten content (rounded to nearest 0.5 grams) of seven common foods included in the NCC Database are listed in Table 2. There was insufficient data to provide reliable estimates for other gluten-containing staple foods. The NCC approach is limited by variability in ingredients and preparation. Therefore, we also calculated the estimated gluten content in vital wheat gluten flour and all-purpose baking flour using the same method as previous translational studies (12,39).
TABLE 2.
Estimated gluten content in common foods
| Food category | Subcategory | Estimated gluten content | Serving size (weight) |
|---|---|---|---|
|
| |||
| Bagel | Multigrain, whole wheat, wheat | 7 | Medium; 3.4—4 inch diameter (105 grams) |
| Bread | White | 5 | |
| Multigrain | 2.5 | ||
| Whole wheat | 3 | Medium slice (range: 28–44 grams) | |
| Wheat | 2 | ||
| Tortilla | White | 1.5 | |
| Wheat | 3 | 7.5–8 inch diameter (46–49 grams) | |
| White | 2.5 | ||
| Ramen noodles | n/a | 3 | 1 cup |
| Pasta | Wheat | 6 | 1 cup (140 grams) |
| Crackers | Graham cracker | 1.6 | Four 2.5 inch squares |
| Brownie | n/a | 0.4 | Single 2 inch square vital wheat |
| Flour | Gluten flour | 6–7 | 1 tablespoon |
| Baking or all purpose flour | 0.6 | 1 tablespoon | |
MAXIMIZING ACCURACY
Clinically, the gluten challenge is dynamic, as patient intake varies from day to day. Clinical monitoring is critical as either the dose or duration of gluten challenge may need to be adjusted based on signs and symptoms. Based on previous studies, we suggest a minimum of 3–6 grams of gluten per day for 12 weeks to optimize diagnostic accuracy of the CD evaluation. If gluten is causing mild or no symptoms, then more gluten (up to 10 grams/day or more) for longer is encouraged to increase confidence that the challenge was sufficient to induce relapse and CD can be appropriately excluded (Fig. 1). If gluten is causing distressing symptoms an abbreviated challenge of 6–12 weeks is acceptable. However, a shorter gluten challenge may not be able to rule out CD. Thus, a longer challenge improves the chance to rule in CD and avoid multiple endoscopies with anesthesia. Duration may be adjusted for those who have restricted gluten for a short period (<2 weeks) as villous architecture is unlikely to be restored in this time frame and high levels of circulating T cells may prime the response.
Regardless of dosing and duration, there is consensus that mild elevations in serology or symptoms, albeit supportive, have limited diagnostic value. Current NASPGHAN guidelines recommend endoscopy and duodenal histopathology with intraepithelial lymphocytosis, crypt hyperplasia, and villous atrophy the most appropriate endpoint for gluten challenge (5).
MANAGING SYMPTOMS
Symptoms are expected during a gluten challenge. These may include gastrointestinal (eg, abdominal pain, diarrhea, bloating) and extraintestinal manifestations (eg, fatigue, headaches, brain fog). Early studies suggested that symptomatic response worsened over time and was more frequent with higher gluten intake. A pediatric study in 1972 with 2 g/day gluten found 4% of subjects reported symptoms within 4 days, and 25% by 6 months (40). When higher doses (minimum gluten 10 g/day) were used, 13% of children developed symptoms within 12 hours, increasing to 33% at 4 weeks (41). If symptoms develop, it is important for the patient/family to notify the clinician so they can guide whether to adjust the gluten dose and continue the challenge or expedite lab testing and/or endoscopy.
Symptoms with gluten reintroduction have limited specificity and do not equate to a diagnosis of CD. Celiac serologies may also be collected, but have limited value in the abbreviated timespan and are not required to guide planning for endoscopic evaluation. Applying patterns found in the abbreviated gluten challenge study in adults (13), even a minimum of 2 weeks gluten exposure at ≥3 grams dose may be sufficient to demonstrate villous atrophy and confirm CD. However, if villous atrophy is not present a 2-week challenge may not be sufficient to conclude CD is not present. Thus, one should consider continuing the gluten challenge and monitoring the patient on a gluten-containing diet with serology.
Prior to aborting gluten challenge, patients with severe symptoms may consider adjusting the gluten dose to reduce symptoms, although previous studies have not demonstrated a symptom correlation in patients undergoing challenge with reduced gluten load (12). Nevertheless, we suggest consideration of a reduced gluten load as well as expedited endoscopy in those with gluten-related symptoms significantly affecting their quality of life. In addition, symptom-targeted medications (eg, antispasmodics, antiemetics, acid suppressants) or reduction in lactose intake may be helpful while the patient awaits endoscopy.
SPECIAL CONSIDERATIONS IN PEDIATRICS
Given the limited data to support an optimal gluten challenge amount or duration in pediatrics, childhood development, social circumstances, and long-term health concerns must be considered. Shared decision making with the family and child are essential.
Age and developmental stage are particularly important when considering reintroduction of gluten for a patient who is thriving on a GFD. Previous guidelines discourage a gluten challenge in children less than 5 years or during pubertal development (42). However, we suggest that patients who have minimal to no symptoms and normal growth may undergo a gluten challenge at any age. Patients with poor weight gain on a GFD who have not had an improvement in growth may benefit from a gluten challenge as ruling out CD may permit safe introduction of high-calorie gluten-containing foods. On the other hand, patients with poor weight gain or growth failure who demonstrated improvement in weight or height gain associated with a GFD may benefit from a deferred gluten challenge occurring after catch-up growth and pubertal growth spurts have occurred. An individualized approach is paramount given the variability in the necessary length and response to the gluten challenge. While complications of the challenge need to be considered in key aspects of growth, and development, we suggest that there is no age or developmental period after infancy during which a gluten challenge is absolutely contraindicated.
Our recommendations fall between 2 other recently published statements, one which suggested a gluten challenge of up to 12 months with serial serologies (16,17) and another which suggested a period of 6 weeks may be sufficient (15). These discrepancies highlight the importance of tailoring the challenge to the individual patient and to whether the goal is to rule in or to rule out CD. While we agree that higher dosing and prolonged durations increases diagnostic yield and that prolonged challenge is feasible for those without symptoms, based on review of the literature we believe a 12-week endpoint will be reliable while decreasing loss to follow-up and increasing patient and family acceptance.
CONCLUSIONS
It is of utmost importance that clinicians not remove gluten from the patient’s diet before diagnostic testing for CD is complete and consultation with a gastroenterologist has occurred. When a gluten challenge is necessary, we suggest a minimum of 3–6 grams of gluten per day for 12 weeks. A formal provider check-in at 4–6 weeks is a key to allow for adjustments in dose and duration. Challenges as short as 6–12 weeks may be necessary in patients with symptoms however higher gluten doses for a longer period for patients with no or minimal symptoms improves accuracy.
Supplementary Material
What Is Known
Gluten challenge is a diagnostic tool used to confirm or exclude a diagnosis of CD.
There is no consensus regarding the application of gluten timing, dosing, and duration of a gluten challenge in children.
What Is New
Longer duration (≥12 weeks) and higher dosing of gluten may improve diagnostic accuracy of CD in children.
Clinical algorithm for a gluten challenge includes symptom assessment and serology, but the key endpoint remains histologic findings of intraepithelial lymphocytosis, crypt hyperplasia, and villous atrophy.
Acknowledgments
Dr Silvester has served on advisory boards for Alimentiv, Mozart Therapeutics, Takeda Pharmaceuticals, and Teva Pharmaceuticals. She has served as site-PI for clinical trials sponsored by Cour Pharmaceuticals and Takeda Pharmaceuticals and has research support from Milky Way Life Sciences. Dr Leonard has served on an advisory board for Mozart Therapeutics and 9Meters Biopharma and acted as a consultant for Anokion. She has served as site-PI for Takeda Pharmaceuticals. The remaining authors report no conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
REFERENCES
- 1.Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut 2013;62:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almallouhi E, King KS, Patel B, et al. Increasing incidence and altered presentation in a population-based study of pediatric celiac disease in North America. J Pediatr Gastroenterol Nutr 2017;65:432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebwohl B, Rubio-Tapia A, Assiri A, Newland C, Guandalini S. Diagnosis of celiac disease. Gastrointest Endosc Clin N Am 2012;22:661–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Husby S, Murray JA, Katzka DA. AGA clinical practice update on diagnosis and monitoring of celiac disease-changing utility of serology and histologic measures: expert review. Gastroenterology 2019;156:885–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill ID, Dirks MH, Liptak GS, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2005;40:1–19. [DOI] [PubMed] [Google Scholar]
- 6.Gidrewicz D, Trevenen CL, Lyon M, Butzner JD. Normalization time of celiac serology in children on a gluten-free diet. J Pediatr Gastroenterol Nutr 2017;64:362–7. [DOI] [PubMed] [Google Scholar]
- 7.Midhagen G, Åberg A-K, Olcén P, et al. Antibody levels in adult patients with coeliac disease during gluten-free diet: a rapid initial decrease of clinical importance. J Intern Med 2004;256:519–24. [DOI] [PubMed] [Google Scholar]
- 8.Goel G, Tye-Din JA, Qiao SW, et al. Cytokine release and gastrointestinal symptoms after gluten challenge in celiac disease. Sci Adv 2019;5:eaaw7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daveson AJM, Tye-Din JA, Goel G, et al. Masked bolus gluten challenge low in FODMAPs implicates nausea and vomiting as key symptoms associated with immune activation in treated coeliac disease. Aliment Pharmacol Ther 2020;51:244–52. [DOI] [PubMed] [Google Scholar]
- 10.Dale HF, Biesiekierski JR, Lied GA. Non-coeliac gluten sensitivity and the spectrum of gluten-related disorders: an updated overview. Nutr Res Rev 2018;32:28–37. [DOI] [PubMed] [Google Scholar]
- 11.Catassi C, Elli L, Bonaz B, et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): the Salerno experts’ criteria. Nutrients 2015;7:4966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonard MM, Silvester JA, Leffler D, et al. Evaluating responses to gluten challenge: a randomized, double-blind, 2-dose gluten challenge trial. Gastroenterology 2021;160:720–733.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leffler D, Schuppan D, Pallav K, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut 2013;62:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) institute technical review on the diagnosis and management of celiac disease. Gastroenterology 2006;131:1981–2002. [DOI] [PubMed] [Google Scholar]
- 15.Rubio-Tapia A, Hill ID, Semrad C, et al. American College of Gastroenterology guidelines update: diagnosis and management of celiac disease. Am J Gastroenterol 2023;118:59–76. [DOI] [PubMed] [Google Scholar]
- 16.Mearin ML, Agardh D, Antunes H, et al. ESPGHAN Position paper on management and follow-up of children and adolescents with celiac disease. J Pediatr Gastroenterol Nutr 2022;75:369–86. [DOI] [PubMed] [Google Scholar]
- 17.Popp A, Laurikka P, Czika D, Kurppa K. The role of gluten challenge in the diagnosis of celiac disease: a review. Expert Rev Gastroenterol Hepatol 2023;17:691–700. [DOI] [PubMed] [Google Scholar]
- 18.Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr 2020;70:141–56. [DOI] [PubMed] [Google Scholar]
- 19.Gould MJ, Brill H, Marcon MA, Munn NJ, Walsh CM. In screening for celiac disease, deamidated gliadin rarely predicts disease when tissue transglutaminase is normal. J Pediatr Gastroenterol Nutr 2019;68:20–5. [DOI] [PubMed] [Google Scholar]
- 20.Unal E, Demiral M, Baysal B, et al. Frequency of celiac disease and spontaneous normalization rate of celiac serology in children and adolescent patients with type 1 diabetes. J Clin Res Pediatr Endocrinol 2021;13:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potter K, de Koning L, Butzner JD, Gidrewicz D. Survey of the initial management of celiac disease antibody tests by ordering physicians. BMC Pediatr 2019;19:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson A, Arranz E, O’Mahony S. Clinical and pathological spectrum of coeliac disease—active, silent, latent, potential. Gut 1993;34:150–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharjee A, Houlder K, Isaac DM, Lacson A, Turner J. Clinical features of children with serology negative, biopsy positive celiac disease. J Pediatr Gastroenterol Nutr 2023;77:240–3. [DOI] [PubMed] [Google Scholar]
- 24.Taavela J, Koskinen O, Huhtala H, et al. Validation of morphometric analyses of small-intestinal biopsy readouts in celiac disease. PLoS One 2013;8:e76163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karell K, Louka AS, Moodie SJ, et al. HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease. Hum Immunol 2003;64:469–77. [DOI] [PubMed] [Google Scholar]
- 26.Brown NK, Guandalini S, Semrad C, Kupfer SS. A clinician’s guide to celiac disease HLA genetics. Am J Gastroenterol 2019;114:1587–92. [DOI] [PubMed] [Google Scholar]
- 27.Tinto N, Cola A, Piscopo C, et al. High frequency of haplotype HLA-DQ7 in celiac disease patients from South Italy: retrospective evaluation of 5,535 subjects at risk of celiac disease. PLoS One 2015;10:e0138324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonard MM, Sapone A, Catassi C, Fasano A. Celiac disease and non-celiac gluten sensitivity: a review. JAMA 2017;318:647–56. [DOI] [PubMed] [Google Scholar]
- 29.Bruins MJ. The clinical response to gluten challenge: a review of the literature. Nutrients 2013;5:4614–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Packer SM, Charlton V, Keeling JW, et al. Gluten challenge in treated coeliac disease. Arch Dis Child 1978;53:449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ansaldi N, Tavassoli K, Faussone D, Forni M, Oderda G. [Clinicohistological behavior of celiac patients after gluten load following the definitive diagnosis]. Pediatr Med Chir 1988;10:3–6. [PubMed] [Google Scholar]
- 32.Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 2013;108:656–76; quiz 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer M, Greco L, Troncone R, Grimaldi M, Pansa G. Early prediction of relapse during gluten challenge in childhood celiac disease. J Pediatr Gastroenterol Nutr 1989;8:474–9. [DOI] [PubMed] [Google Scholar]
- 34.Catassi C, Rossini M, Ratsch IM, et al. Dose dependent effects of protracted ingestion of small amounts of gliadin in coeliac disease children: a clinical and jejunal morphometric study. Gut 1993;34:1515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rujner J, Socha J, Romanczuk W, et al. [Individual sensitivity of jejunal mucosa to small doses of gluten in coeliac disease]. Wiad Lek 2002;55:554–60. [PubMed] [Google Scholar]
- 36.Lexhaller B, Tompos C, Scherf KA. Comparative analysis of prolamin and glutelin fractions from wheat, rye, and barley with five sandwich ELISA test kits. Anal Bioanal Chem 2016;408:6093–104. [DOI] [PubMed] [Google Scholar]
- 37.Jasthi B, Pettit J, Harnack L. Addition of gluten values to a food and nutrient database. J Food Compos Anal 2020;85:103330. [Google Scholar]
- 38.van Overbeek FM, Uil-Dieterman IG, Mol IW, Kohler-Brands L, Heymans HS, Mulder CJ. The daily gluten intake in relatives of patients with coeliac disease compared with that of the general Dutch population. Eur J Gastroenterol Hepatol 1997;9:1097–9. [DOI] [PubMed] [Google Scholar]
- 39.Pronin D, Borner A, Weber H, Scherf KA. Wheat (Triticum aestivum L.) breeding from 1891 to 2010 contributed to increasing yield and glutenin contents but decreasing protein and gliadin contents. J Agric Food Chem 2020;68:13247–56. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton JR, McNeill LK. Childhood celiac disease: response of treated patients to a small uniform daily dose of wheat gluten. J Pediatr 1972;81:885–93. [DOI] [PubMed] [Google Scholar]
- 41.Rolles CJ, McNeish AS. Standardised approach to gluten challenge in diagnosing childhood coeliac disease. Br Med J 1976;1:1309–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J 2019;7:583–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


