Abstract
Nucleophilic copper-mediated radioiodination (CMRI) of organoboronic precursors with radioiodides is a promising method of radioiodination. The previously reported CMRI has demonstrated its great potential and scope of labeling for the radiosynthesis of radioiodine-labeled compounds. However, the reported protocols (using a small amount/volume of radioactivity) are practically not reproducible in large-scale CMRI, in which the radioactivity was usually provided in a bulk alkaline solution. A large amount of water and a strong base are incompatible with CMRI. To overcome these issues in large-scale CMRI, we have developed a simple protocol for large-scale CMRI. The bulk water was removed under a flow of inert gas at 110 °C, and the strong base (i.e., NaOH) was neutralized with an acid, pyridinium p-toluenesulfonate or p-toluenesulfonic acid. In the model reactions of [123I]KX-1, a PARP-1 radioligand for Auger radiotherapy, radiochemical conversions were significantly improved after neutralization of the base, and the addition of additional acids was tolerated and favorable for the reactions. Using this protocol, [123I]KX-1 was radiosynthesized from 20 mCi (0.74 GBq) of [123I]iodide in high radiochemical yields, high radiochemical purity and high molar activity. This protocol should be applicable to the radiosynthesis of other compounds with radioiodine via CMRI.
Keywords: Copper-mediated radioiodination, [123I]iodide, PARP-1, Auger radiotherapy, radiolabeling, radioligands
Graphical Abstract:
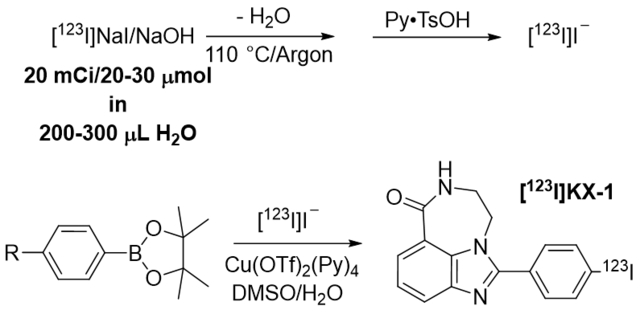
Neutralization of the strong basic solution of [123]NaI/NaOH with an acid (pyridinium p-toluenesulfonate) promotes the radiosynthesis of [123I]KX-1 on large-scale via copper-mediated radioiodination (CMRI) of the organoboronic precursor in high radiochemical yield, high radiochemical purity and high molar activity. This strategy should be applicable to the radiosynthesis of other radioiodine-labeled compounds via CMRI.
Introduction
Nucleophilic copper-mediated radioiodination (CMRI) of organoboronic precursors with radioiodides is a promising method to label an aromatic ring, compared with the conventional electrophilic radioiodination. In the past few years, CMRI with 123I, 125I, and 131I has been reported and demonstrated great potential and scope of labeling for CMRI of organoborons1–5. In these reports, only a small amount of radioactivity (up to 1 mCi (0.037 GBq)) in a small volume of water, methanol, or a methanolic 0.1 N NaOH solution was used as the source of radioactivity. It is well known that a strong base is detrimental to copper-mediated radiofluorination6,7, and so is expected. Commercial radioiodines are usually provided in an alkaline solution (e.g., 0.1 N NaOH). 123I is an Auger electron emitter and [123I]KX-18 has been used for PARP-1 targeted Auger radiotherapy. A large quantity of [123I]KX-1 is needed to evaluate its Auger therapeutic effect in small animals. The commercial [123I]iodide was delivered in up to 0.3 mL 0.1 N NaOH solution. This amount of water is not compatible with CMRI9 and the amount of NaOH (up to 30 μmol) not only requires the corresponding amount of precursor and copper catalyst but also will result in the decomposition of the copper catalyst, forming insoluble solids, which is extremely challenging for the HPLC purification. In this short note, we will report a simple strategy to overcome these issues.
Experimental
General
All chemicals were obtained from Sigma-Aldrich (Saint Louis, MO, USA) and used without further purification. Waters HLB light cartridges were obtained from Waters (Milford, MA, USA). [123I]Iodide in 0.1 N NaOH solution was purchased from BWX Technologies, Inc. (Ottawa, ON, Canada). The precursor and standard of KX-1 were prepared as previously reported2, and Cu(OTf)2(Py)4 was prepared according to the literature10. High performance liquid chromatography (HPLC) was performed with an ultraviolet detector and a well-scintillation NaI (Tl) detector. Acetonitrile and water with 0.1% TFA were used as the HPLC mobile phase. Radio-TLC (silica gel/20 % methanol in dichloromethane/Rf of KX-1: 0.9 and [123I]iodide: 0) was accomplished using a Bioscan AR-2000 imaging scanner (Bioscan, Inc., Washington, DC).
Radiosynthesis of [123I]KX-1
[123I]Iodide (20 mCi (0.74 GBq)) in 0.1 N NaOH solution (0.27 mL, 27 μmol) was transferred from the shipping vial to a 5 mL Wheaton V-vial. The shipping vial was rinsed with water (100 μL), and all the radioactivity was combined in the V-vial. Water was then removed at 110 °C under a flow of argon via a charcoal trap until less than 5 μL of water was left. At room temperature, the argon atmosphere was replaced with air and water (50 μL) was added to the V-vial, which was vortexed before and after the addition of pyridinium p-toluenesulfonate (PPTS) (10 mg, 40 μmol) in DMSO (100 μL). The precursor (0.5 mg, 1.3 μmol) and Cu(OTf)2(Py)4 (3.7 mg, 5.5 μmol) in DMSO (300 μL) was added to the above solution in the V-vial, which was then capped, vortexed and vented via an alumina cartridge as a trap. The vial was then heated at 110 °C and the radiolabeling was monitored by radio-HPLC or radio-TLC analysis of the reaction mixture. Upon completion of the reaction (10–20 min), the reaction mixture was allowed to cool down and then diluted with 0.1 % TFA (4 mL) for HPLC purification (Column: Agilent SB-C18 250 × 9.4 mm 5 μm; Mobile phase: 21 % MeCN/ 79 % water/0.1 % TFA; Flow rate: 4 mL/min; UV: 270 nm). The radioactive peak of [123I]KX-1 was collected at 20–21 min, and then diluted with water (40 mL). The diluted solution was passed through a Waters HLB light cartridge under vacuum, followed by water (10 mL) to rinse the cartridge. [123I]KX-1 was eluted from the cartridge with ethanol (3 × 0.2 mL). To prepare the dose for Auger radiotherapy study in small animals, ethanol was removed at 110 °C under an argon flow. The final dose was formulated with ethanol (100 μL) and saline (900 μL) and analyzed by radio-HPLC (Column: Alltima C18 250 × 4.6 mm 10 μm; Mobile phase: 35 % MeCN/ 65 % water/0.1 % TFA; Flow rate: 2 mL/min; UV: 250 nM) for identity, molar activity and radiochemical purity of the final dose.
Results and discussion
The strategy of treating [123I]iodide/NaOH with acids (PPTS or TsOH) was explored in model reactions (Table 1). The labeling reaction was carried out in aqueous DMSO (50 μL water/400 μL DMSO) with 5 μmol NaOH added. As shown in Table 1. the neutralization with one equivalent of acids significantly improved the radiochemical conversion (RCC), from 44 % to over 70 %. It appears that much more acids favor the labeling reaction, which went to completion with the addition of four equivalent acids. Under this condition, reducing the amount of precursor to 0.125 mg (0.32 μmol) resulted in minimum RCC, indicating that a sufficient amount of precursor is needed to complete the reaction.
Table 1.
Exploration of copper-mediated radioiodination with the addition of acids to neutralize strong basic conditions.

| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Experimenta | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|
| |||||||||
| Precursor (μmol) | 0.64 | 0.64 | 0.64 | 0.64 | 0.64 | 0.64 | 0.64 | 0.32 | 0.32 |
| [Cu] (μmol) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| NaOH (μmol) | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| PPTS (μmol) | / | 5 | 10 | 20 | / | / | / | 20 | / |
| TsOH·H2O (μmol) | / | / | / | / | 5 | 10 | 20 | / | 20 |
| RCC (%) | 44 | 73 | 79 | 98 | 72 | 79 | 99 | 13 | 5 |
Note:
Reaction condition: [123I]NaI (15 μCi), [Cu]: Cu(OTf)2(Py)4, DMSO (400 μL) and water (50 μL) as the reaction solvents, 110 °C/10 min in air atmosphere. RCC was determined by radio-HPLC.
For the radiosynthesis of [123I]KX-1 on large scales, the bulk water in the [123I]iodide solution was first removed under a general argon flow at 110 °C in about 20 min. This is a typical procedure to remove water; no radioactivity was lost during this drying process. A small amount of water is tolerated in CMRI, so water (50 μL) was added to solubilize the dried residue/solid for the following neutralization and labeling reaction. Up to 10 mg of PPTS (40 μmol) was added to the radioactivity with NaOH (up to 27 μmol), and 0.5 mg of precursor (1.3 μmol) was used to ensure high reproducibility and RCC of the labeling reaction. The labeling reaction was carried out in air atmosphere according to our experience with copper-mediated radiobromination11, which did not process under an argon atmosphere, and this is consistent with what is reported for copper-mediated radiofluorination12. DMSO has not been used for CMRF of organoborons in literature, however, excellent RCC was obtained with DMSO for the copper-mediated radiobromination of the bromo-derivative of KX-111, so DMSO was used for the CMRI of [123I]KX-1. When DMSO was used as the solvent, heating is required to drive the reaction to completion. After heating, the reaction mixture remained clear in the color of the copper catalyst and was free of insoluble solids after dilution with water for HPLC injection. Besides the mechanical loss of 123I (up to 10 %), almost all radioactivity was converted to [123I]KX-1 according to the analytical radio-HPLC of the reaction mixture and the semi-preparation radio-HPLC purification (Figure 1A). The radiochemical yield, determined by counting of the final dose (~1 mL in a 5 mL polyprolyene vial) and the starting [123I]iodide (~0.2 mL in a small glass vial) by a dose calibrator, is 107.5 ± 1.2 % (n = 6). The radiochemical purity is over 99 %, and the molar activity is 23 ± 12 Ci/μmol (843 ± 453 GBq/ μmol) (n = 6) at the end of synthesis. This result is much better than the radiosynthesis of [123I]KX-1 using electrophilic aromatic destannylation (60–70 % radiochemical yields and >90 % chemical and radiochemical purity)8. This strategy should be applicable to the direct radiosynthesis of other radioiodine-labeled compounds via CMRI (e.g., 123I-MAPi, which was radiosynthesized via a two-step strategy: electrophilic aromatic destannylation and coupling reaction13).
Figure 1.

(A). Semi-preparative radio-HPLC purification of [123I]KX-1. After collecting [123I]KX-1, the column was rinsed with 80 % MeCN/20 % water/0.1 % TFA. [123I]KX-1 is the major radioactive product; (B). Analytical radio-HPLC of [123I]KX-1, one hour after formulation in 10 % ethanol/saline. The first injection is the animal dose (0.74 GBq/mL), showing the high chemical purity of [123I]KX-1 according to the UV trace. The second injection at 5 min is a co-injection with the authentic KX-1, indicating its high radiochemical purity (> 99.3 %).
Conclusion
We have developed a simple strategy for large-scale copper-mediated radioiodination (CMRI) by removing the bulk water and neutralizing the base from the radioiodine solution before CMRI. Using this strategy, [123I]KX-1 was radiosynthesized in high radiochemical yields, high radiochemical purity, and high molar activity, demonstrating its great potential in preparing radioiodine-labeled compounds on a large scale.
Acknowledge
This work was partly supported by the National Institutes of Health (EB029752).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Reference
- 1.Wilson TC, McSweeney G, Preshlock S, et al. Radiosynthesis of SPECT tracers via a copper mediated 123I iodination of (hetero)aryl boron reagents. Chem Comm. 2016;52(90):13277–13280. [DOI] [PubMed] [Google Scholar]
- 2.Reilly SW, Makvandi M, Xu K, Mach RH. Rapid Cu-catalyzed [211At]astatination and [125I]iodination of boronic esters at room temperature. Org Lett. 2018;20(7):1752–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang P, Zhuang R, Guo Z, Su X, Chen X, Zhang X. A highly efficient copper-mediated radioiodination approach using aryl boronic acids. Chem Eur J. 2016;22(47):16783–16786. [DOI] [PubMed] [Google Scholar]
- 4.Kondo Y, Kimura H, Sasaki I, et al. Copper-mediated radioiodination and radiobromination via aryl boronic precursor and its application to 125I/77Br–labeled prostate-specific membrane antigen imaging probes. Bioorg Med Chem. 2022;69:116915. [DOI] [PubMed] [Google Scholar]
- 5.Kondo Y, Kimura H, Fukumoto C, et al. Copper-mediated radioiodination reaction through aryl boronic acid or ester precursor and its application to direct radiolabeling of a cyclic peptide. J Labeled Comp Radiopharm. 2021;64(8):336–345. [DOI] [PubMed] [Google Scholar]
- 6.Zlatopolskiy BD, Zischler J, Krapf P, et al. Copper-mediated aromatic radiofluorination revisited: Efficient production of PET tracers on a preparative scale. Chem Eur J. 2015;21(15):5972–5979. [DOI] [PubMed] [Google Scholar]
- 7.Mossine AV, Brooks AF, Makaravage KJ, et al. Synthesis of [18F]arenes via the copper-mediated [18F]fluorination of boronic acids. Org Lett. 2015;17(23):5780–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riad A, Gitto SB, Lee H, et al. PARP theranostic Auger emitters are cytotoxic in BRCA mutant ovarian cancer and viable tumors from ovarian cancer patients enable ex-vivo screening of tumor response. Molecules. 2020;25(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo Y, Kimura H, Sasaki M, et al. Effect of water on direct radioiodination of small molecules/peptides using copper-mediated iododeboronation in water–alcohol solvent. ACS Omega. 2023;8(27):24418–24425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haynes JS, Rettig SJ, Sams JR, Trotter J, Thompson RC. Pyrazine and pyridine complexes of copper(II) trifluoromethanesulfonate. Crystal structure of tetrakis(pyridine)bis(trifluoromethanesulfonato-O)copper(II) and magnetic exchange in (pyrazine)bis(trifluoromethanesulfonato-O)copper(II). Inorg Chem. 1988;27(7):1237–1241. [Google Scholar]
- 11.Sreekumar S, Zhou D, Mpoy C, et al. Preclinical efficacy of a PARP-1 targeted Auger-emitting radionuclide in prostate cancer. Int J Mol Sci. 2023;24(4):3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright JS, Kaur T, Preshlock S, et al. Copper-mediated late-stage radiofluorination: five years of impact on preclinical and clinical PET imaging. Clin Trans Imaging. 2020;8(3):167–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirovano G, Jannetti SA, Carter LM, et al. Targeted brain tumor radiotherapy using an Auger emitter. Clin Cancer Res. 2020;26(12):2871. [DOI] [PMC free article] [PubMed] [Google Scholar]


