Abstract
Background:
Repetitive negative thinking (RNT), often referred to as rumination in the mood disorders literature, is a symptom dimension associated with poor prognosis and suicide in major depressive disorder (MDD). Given the transdiagnostic nature of RNT, this study aimed to evaluate the hypothesis that neurobiological substrates of RNT in MDD may share the brain mechanisms underlying obsessions, particularly those involving cortico-striatal-thalamic-cortical (CSTC) circuits.
Methods:
Thirty-nine individuals with MDD underwent RNT induction during fMRI. Trait-RNT was measured by the Ruminative Response Scale (RRS) and state-RNT was measured by a visual analogue scale. We employed a connectome-wide association analysis examining the association between RNT intensity with striatal and thalamic connectivity.
Results:
A greater RRS score was associated with hyperconnectivity of the right mediodorsal thalamus with prefrontal cortex, including lateral orbitofrontal cortex, along with Wernicke’s area and posterior default mode network nodes (t = 4.66 – 6.70). A greater state-RNT score was associated with hyperconnectivity of the right laterodorsal thalamus with bilateral primary sensory and motor cortices, supplementary motor area, and Broca’s area (t = 4.51 – 6.57). Unexpectedly, there were no significant findings related to the striatum.
Conclusions:
The present results suggest RNT in MDD is subserved by abnormal connectivity between right thalamic nuclei and cortical regions involved in both visceral and higher order cognitive processing. Emerging deep-brain neuromodulation methods may be useful to establish causal relationships between dysfunction of right thalamic-cortical circuits and RNT in MDD.
Keywords: thalamus, repetitive negative thinking, rumination, depression, functional connectivity, cortico-striatal-thalamic-cortical circuit
1. Introduction
Repetitive negative thinking (RNT), often equated with rumination in the context of major depressive disorder (MDD), is a pervasive symptom strongly associated with treatment-resistance and adverse prognostic features (Runia et al., 2022). RNT reflects a persistent focus on negative feelings, distress, self-perceptions and evaluations of life events (Ehring and Watkins, 2008), and manifests in various mental health dimensions, such as depression, stress, sleep issues, substance use, and trauma (Arditte Hall et al., 2019; Clohessy and Ehlers, 1999; Ehlers et al., 1998; Everaert et al., 2022; Hamonniere et al., 2022; Hummel et al., 2021; Leung et al., 2022; Michael et al., 2007).
RNT has been conceptualized as a trait-like cognitive process that involves recurrent and persistent focus on self-relevant negative thoughts because of its persistence over time and across situations (Ehring and Watkins, 2008; Treynor et al., 2003). This perspective, however, may not fully capture the multifaceted nature of RNT. Recent studies suggest that RNT intensity can fluctuate, as be influenced by depression symptom severity, mood state, and adverse environmental stimuli including relevant interpersonal interactions (Chang et al., 2023; Philippi et al., 2022). Therefore, while trait-RNT provides a measure of frequency and tendency to engage in RNT, state-RNT captures the intensity of RNT under specific conditions. This differentiation aligns with recent studies utilizing experimental induction of RNT, which demonstrates the potential independence and distinct characteristics of trait- and state-RNT (Grant et al., 2021; LeMoult et al., 2013; Robinson and Alloy, 2003; Wang et al., 2022). While trait-RNT measures an individual’s tendency to engage in RNT, the induced state-RNT enables us to probe the specific triggers and phenomenological characteristics of RNT that are not captured by trait-RNT alone. Thus, discerning the brain mechanisms that underlie both trait and state aspects of RNT could have significant implications for clinical practice.
The relevance of RNT extends beyond MDD, as it is widely considered a transdiagnostic phenomenon (McEvoy et al., 2013). For example, research has found abnormalities of cortico-striatal-thalamic-cortical (CSTC) circuits in MDD (Bora et al., 2012; Choi et al., 2020; Hong et al., 2021; Osoba et al., 2013; Sun et al., 2023), suggesting shared brain mechanisms between RNT and obsession. This observation aligns with neuromodulation treatments targeting thalamo- and striato-cortical circuits in treatment-resistant MDD (Avecillas-Chasin et al., 2021; Davidson et al., 2022; Davidson et al., 2020).
This study focuses on characterizing striatal and thalamic circuits involved in trait- and state-RNT generation in MDD. We applied multivariate distance matrix regression (MDMR) analysis, a widely used technique to describe comprehensive representations of brain connectivity in relation with behavioral variables (Anderson, 2001; Shehzad et al., 2014). Specifically, we quantified voxel-to-voxel connectivity for the regions of interest (striatum and thalamus) in relation to RNT. Since the intensity of RNT and severity of depression and anxiety can be closely related to each other, we used the severity of depression and anxiety as well as demographic variables as covariates of no interest. We predicted that greater intensity of trait- and state-RNT would be related to increased connectivity of both thalamus and dorsal striatum with prefrontal cortical areas, specifically the orbitofrontal cortex (OFC) (Braz et al., 2017; Milad and Rauch, 2012; Pittenger et al., 2011; Saxena and Rauch, 2000; Steward et al., 2022). We further predicted that the findings would show right-hemisphere lateralization, as suggested by burgeoning experimental (Costanzo et al., 2015; Murphy et al., 2020) and clinical (Baldermann et al., 2019; Lippitz et al., 1999; Riestra et al., 2011; Scangos et al., 2021) observations of a critical role of right-hemisphere structures in symptom generation in internalizing disorders characterized by prominent RNT.
2. Methods
2.1. Study Design
The study protocol was reviewed and approved by the WCG IRB (https://www.wcgirb.com) (IRB Tracking Number 20210286), and registered on ClinicalTrials.gov (NCT04941066) as a part of a real-time fMRI-neurofeedback (rtfMRI-nf) study (Tsuchiyagaito et al., 2023).
2.2. Participants
Forty-three individuals with MDD were recruited. Eligible participants were between 18 and 65 years, fluent in English, met the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DMS-5) criteria for unipolar MDD based on the Mini-International Neuropsychiatric Interview 7.0.2 (MINI) (Sheehan et al., 1998), and had current depressive symptoms with Montgomery-Åsberg Depression Rating Scale (MADRS) score > 6 (Montgomery and Asberg, 1979). Exclusion criteria were as follows: a lifetime history of bipolar disorder, schizophrenia or any psychotic disorders; DSM-5 criteria for substance abuse or dependence within six months prior to study entry; current severe suicidal ideation as indicated by the Columbia-Suicide Severity Rating Scale (C-SSRS) (Posner et al., 2011) or attempt within 12 months prior to study entry; commencement of psychotropic medication for depression and/or anxiety less than a month before the study enrollment; commencement of psychological therapy less than a month before the study enrollment; abnormal neuromorphological brain profile; pregnancy; and general contraindications for MRI. Anxiety disorders were acceptable for enrollment. All participants provided written informed consent.
2.3. Neuroimaging data acquisition
Imaging was conducted on a 3 Tesla MR750 Discovery (GE Healthcare, Milwaukee, WI) with an 8-channel receive-only head array coil. Blood-oxygen-level-dependent fMRI data were acquired using a T2*-weighted gradient echo-planar sequence with sensitivity encoding (GE-EPI SENSE) with the following parameters: TR/TE=2000/25 ms, acquisition matrix=96 × 96, FOV/slice=240/2.9 mm, flip angle=90°, voxel size 2.5×2.5×2.9 mm; 40 axial slices, SENSE acceleration R=2. To provide anatomical reference for fMRI data, T1-weighted (T1w) MRI images were acquired with a magnetization-prepared rapid gradient-echo (MPRAGE) sequence with parameters of FOV= 240×192 mm, matrix=256×256, 124 axial slices, slice thickness=1.2 mm, 0.94×0.94×1.2 mm3 voxel volume, TR/TE=5/2 ms, SENSE acceleration R=2, flip angle=8°, delay/inversion time TD/TI=1400/725 ms, sampling bandwidth=31.2 kHz, scan time = 4 min 59 s.
2.4. Experimentally induced RNT and resting-state scanning
The MRI session started with a 5-min T1w MRI anatomical scan, a 6-min and 50-sec resting-state fMRI scan, and a 6-min and 50-sec experimentally induced RNT fMRI scan. Prior to the MRI session, participants identified a recent personal event that significantly triggered RNT, such as experiencing rejection by someone important to them. Participants provided a brief title for this event, which was used by research staff to prompt the participant’s recall immediately before the RNT-inducing fMRI scan. Participants were then instructed about the neurofeedback task (not analyzed in this paper but published in Tsuchiyagaito et al. (2023)), and had a rest period before the MRI session. In the scanner, the session began with a resting-state scan, where participants were instructed to clear their mind and not to think anything while viewing a fixation cross. This was followed by the RNT-inducing fMRI scan. During this scan, they were reminded of their chosen event and instructed to introspectively ruminate and ponder on it, especially focusing on their emotional reactions and why they responded the way they did, while keeping their gaze on a fixation cross. This procedure aimed to engage the participants in a state of rumination and brooding, characteristic of RNT, while inside the scanner. The MRI session ended with neurofeedback scans as described in Tsuchiyagaito et al. (2023).
2.5. Symptom measures
Trait-RNT
The 22-item Ruminative Response Scale (RRS) (Nolen-Hoeksema and Morrow, 1991) was used to measure trait-RNT. The RRS is composed of three subscales; the 5-item ‘brooding’ subscale (e.g., RRS-B item: think ‘why can’t I handle things better’), the 12-item ‘depressive rumination’ subscale (e.g., RRS-D item: think about all of your shortcomings, failings, faults and mistakes), and the 5-item ‘reflection’ subscale (e.g., RRS-R item: write down what you are thinking and analyse it). It assesses an individual’s tendency or trait to ruminate when they feel sad or faced with depressive symptoms. Participants are asked to indicate what they “generally do when feeling down, sad, or depressed” using a 4-point Likert scale ranging from 1 (never) to 4 (always), representing the trait tendency. The items in the RRS-B measure how often people engage in RNT, focusing on the causes and consequences, or a passive comparison with unachieved goals, and found to lead worse prognosis of depression (Treynor et al., 2003). The items in the RRS-D subscale are similar to the RRS-B subscale; however, this subscale measures how often people engage in RNT focusing on the depressive symptoms and having a depressed mood. We employed the RRS-B and RRS-D subscales for connectivity analyses related to trait-RNT. Of note, because the RRS-R subscale does not include pathological elements of RNT, and may even reflect protective factors against depression (Treynor et al., 2003), this subscale was not included in our main analysis (results are shown in the Supplementary materials 2.1).
State-RNT
The level of state-RNT immediately after the state-RNT fMRI scan was assessed with the visual analogue scale (VAS). Participants were requested to answer the question, “To what extent did you dwell on negative aspects of yourself?” using a button box rating from 1 (not at all) to 10 (extremely), representing the intensity of state-RNT.
Severity of depression and anxiety
Montgomery-Åsberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) and Hamilton Anxiety Scale (HAMA) (Maier et al., 1988) were assessed before the MRI session. These scores were included as covariates of no interest to control for potential confounding by overall severity of depression and anxiety, which could otherwise obscure the unique neural substrates of RNT.
2.6. Connectome-wide association analysis
Standard preprocessing was performed (Supplementary materials 1.1). We first examined the association between whole-brain patterns of functional connectivity from a seed voxel and a symptom scale using MDMR analysis (Anderson, 2001; Shehzad et al., 2014). Detailed procedures of connectome-wide association analysis are described in prior works (Misaki et al., 2018, 2020a; Misaki et al., 2020b), and in the Supplementary materials 1.2. Briefly, a connectivity map (z-transformed Pearson correlation) was made from each voxel to all other voxels in the whole-brain on the individual level. The dependent variable of MDMR is a distance matrix of the connectivity maps between participants. For the experimentally induced RNT fMRI scan, we used three variables to measure either trait- or state-RNT for the MDMR analysis: the RRS-B and RRS-D as explaining trait-RNT-specific connectivity patterns, and the VAS immediately after the RNT scan as explaining state-RNT-specific connectivity patterns. Because the resting-state fMRI scan ocurred prior to RNT induction, we focused on trait-RNT with the RRS-B and RRS-D for the resting state analyses. The MDMR model also included sex, medication status (medicated or not), age, motion (mean frame-wise displacement), depression severity (measured by MADRS) and anxiety severity (measured by HAMA) as covariates of no interest. The result of MDMR was represented with a pseudo-F value, a ratio of the variance explained by a certain regressor relative to the residual variance (Misaki et al., 2018, 2020a; Misaki et al., 2020b; Shehzad et al., 2014). These procedures were repeated for individual voxels within the striatum and thalamus masks created from the brainnetome atlas (Fan et al., 2016) (Fig. 1). The MDMR statistical map was thresholded with voxel-wise p < 0.01, and ten thousand random permutations were performed to test the significance of the statistic with cluster-size corrected p < 0.05 (Winkler et al., 2014). We selected a 4mm voxel size in this analysis to balance spatial resolution and computational demands (Al Zoubi et al., 2021; Misaki et al., 2018, 2020a; Misaki et al., 2020b). Our voxel-wise threshold of p < 0.01 for cluster formation enabled us to probe extended regions associated with RNT-related connectivity. We controlled for false positives using a cluster threshold with a permutation test (Winkler et al., 2014). The main analysis identified regions where a whole-brain multivariate connectivity pattern was significantly associated with RNT, without specifying connections. This led to a subsequent post-hoc test to identify significant voxels.
Figure 1.
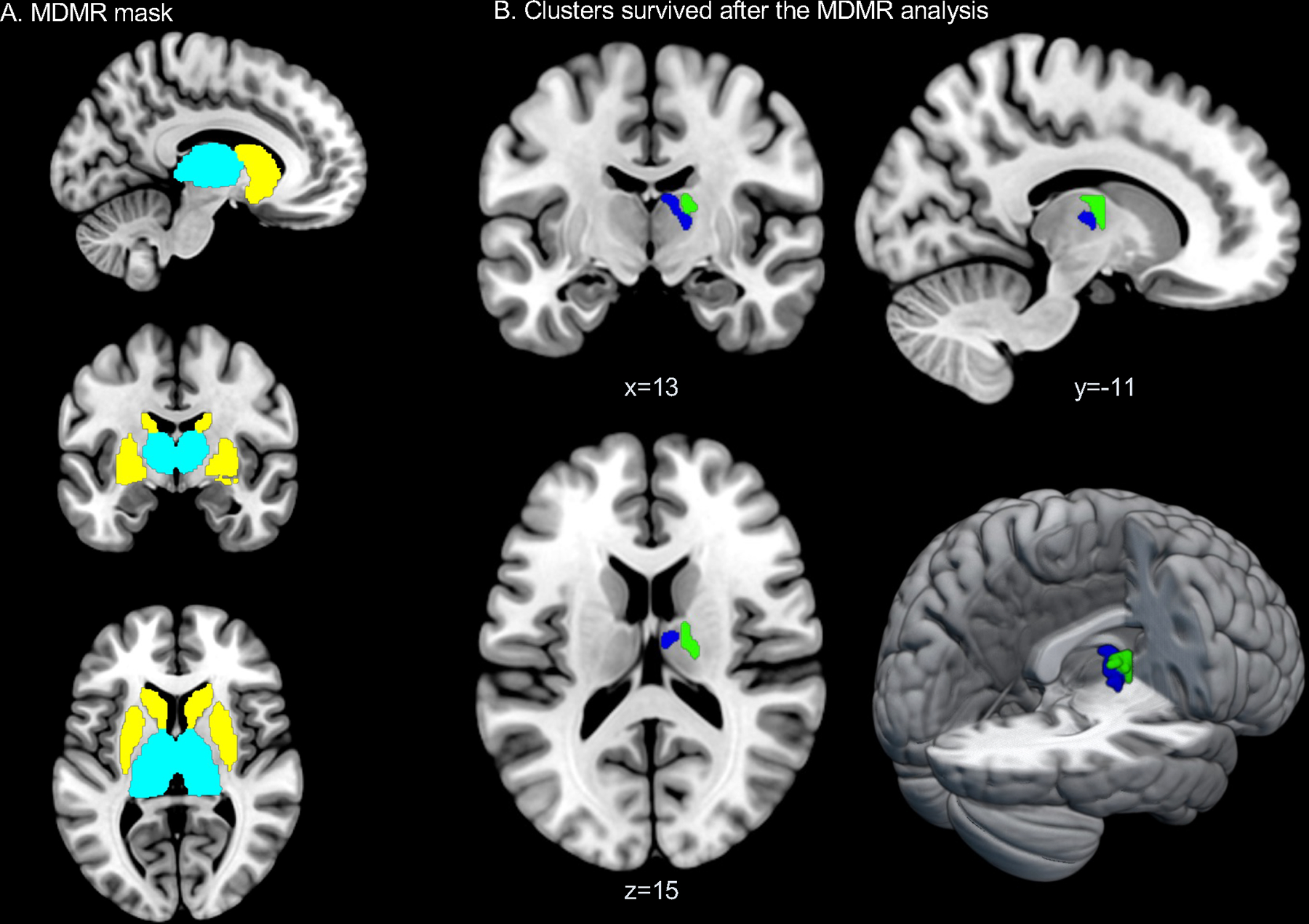
MDMR mask and results. A. Striatum (yellow) and thalamus (cyan) masks used for the MDMR analysis. The striatum and the thalamus regional masks were created from the basal-ganglia subregions and thalamus subregions in the brainnetome atlas. B. Blue: a significant cluster associated with trait-RNT (RRS-D) during the experimentally induced RNT scan. Green: a significant cluster associated with state-RNT (VAS) during the experimentally induced RNT scan.
2.7. Post-hoc analysis for the connectome-wide association results
We performed a post-hoc, seed-based connectivity analysis for significant clusters discovered in the MDMR statistical parametric map in relation to RNT intensity. The MDMR statistic, similar to the analysis of variance (ANOVA), indicates the effects of RNT intensity on the whole-brain connectivity pattern; however it does not indicate which specific connectivity is associated with RNT intensity. Therefore, the post-hoc analysis was performed to elucidate the connectivity that contributed to the significant MDMR statistics. The statistical map was thresholded with voxel-wise p < 0.001 and cluster-size correction of p < 0.05. For the post-hoc analysis, we used a 2mm voxel size to localize the effects as precisely as possible (Al Zoubi et al., 2021; Misaki et al., 2018, 2020a; Misaki et al., 2020b). We set a more stringent threshold of p < 0.001 to extract representative connectivity contributing to the significant MDMR statistic (Misaki et al., 2018, 2020a; Misaki et al., 2020b). Supplementary materials 1.3 contains the detailed description of this technique.
3. Results
3.1. Demographic and clinical measures
Four subjects did not have analyzable data during the RNT-state and resting-state fMRI scans due to excessive head motion (more than 25% fMRI data were censored with large head motions as > 0.25 mm frame-wise displacement), resulting in 39 subjects for each analysis (Table 1). The sample showed different levels of depression, anxiety and trait-RNT scores (Supplementary materials, Fig. S1). Correlations between clinical measures are in the Supplementary materials (Table S1).
Table 1.
Demographic and clinical measures (N=39).
| Demographic data | Mean/n | (SD/%) |
|---|---|---|
|
| ||
| Age | 33.7 | (11.13) |
| Female (%) | 28 | (71.79) |
| Race/Ethnicity: Non-white (%) | 9 | (23.08) |
| Black | 1 | (2.56) |
| Hispanic | 1 | (2.56) |
| Native American | 7 | (17.95) |
| White | 30 | (76.92) |
| Diagnosis (%) | ||
| Major depressive disorder (MDD) without comorbidity | 18 | (46.15) |
| MDD and anxiety disorder | 21 | (53.85) |
| Generalized anxiety disorder | 13 | (33.33) |
| Social anxiety disorder | 10 | (25.64) |
| Panic disorder | 8 | (20.51) |
| Depressive episode (%) | ||
| Single episode | 15 | (38.46) |
| Recurrent | 24 | (61.54) |
| History of trauma exposure (%) | 19 | (48.72) |
| Medicated (%) | 21 | (53.85) |
| Antidepressants | 18 | (46.15) |
| Stimulants | 2 | (5.13) |
| Benzodiazepines | 5 | (12.82) |
| Current psychotherapy (%) | 9 | (23.08) |
|
| ||
| Clinical measures | Mean | (SD) |
|
| ||
| Ruminative Response Scale Brooding subscore (RRS-B) | 12.7 | (3.10) |
| Ruminative Response Scale Depressive rumination subscore (RRS-D) | 30.6 | (6.90) |
| Ruminative Response Scale Reflection subscore (RRS-R) | 11.1 | (3.35) |
| Ruminative Response Scale Total score | 54.4 | (11.74) |
| Montgomery-Åsberg Depression Rating Scale (MADRS) | 19.9 | (6.15) |
| Hamilton Anxiety Scale (HAMA) | 16.1 | (5.50) |
3.2. Connectome-wide association analysis during the experimentally induced RNT (state) scan
MDMR analysis
Figure 1A shows the masks for the striatum and thalamus regions, where the MDMR analysis was performed. No striatum clusters were found to be associated with RNT. In the thalamus mask, a significant association between the RRS-D and functional connectivity patterns was found (pseudo-F = 3.09). The VAS score measuring state-RNT also showed a significant association with the right thalamic region (pseudo-F = 3.11) (Table 2 and Fig. 1B).
Table 2.
Peak coordinates and statistics of clusters associated with trait- and state-RNT from the MDMR analysis.
| Hemisphere/Location | Brainnetome Atlas Label | Peak MNI coordinates | Cluster size (4mm3) | Pseudo F-value | Correlated clinical measures |
|---|---|---|---|---|---|
|
| |||||
| R Mediodorsal nucleus of thalamus | cTtha_r (caudal Temporal thalamus) | 6 –10 16 | 12 | 3.09 | Trait-RNT (RRS-D) |
| R Laterodorsal nucleus of thalamus | lPFtha_r (lateral Pre-frontal thalamus) | 18 –14 16 | 11 | 3.11 | State-RNT (VAS) |
A voxel-wise threshold of p < 0.01 and a cluster size threshold of p < 0.05. L: left; R: right; MNI: Montreal Neurological Institute; RNT: Repetitive Negative Thinking; RRS-D: Ruminative Response Scale Depressive rumination subscore, VAS: Visual Analogue Scale.
Post-hoc analysis for the connectome-wide association analysis with trait-RNT
Seed-to-whole-brain connectivity analysis with the right mediodorsal thalamus cluster revealed significant positive associations of RRS-D with bilateral OFC, left superior temporal sulcus (Wernicke’s area), bilateral retrosplenial cortex, and precuneus/posterior cingulate cortex (t = 4.66 – 6.70) (Fig. 2A). The peak coordinates of the significant connectivity are shown in Table 3.
Figure 2.
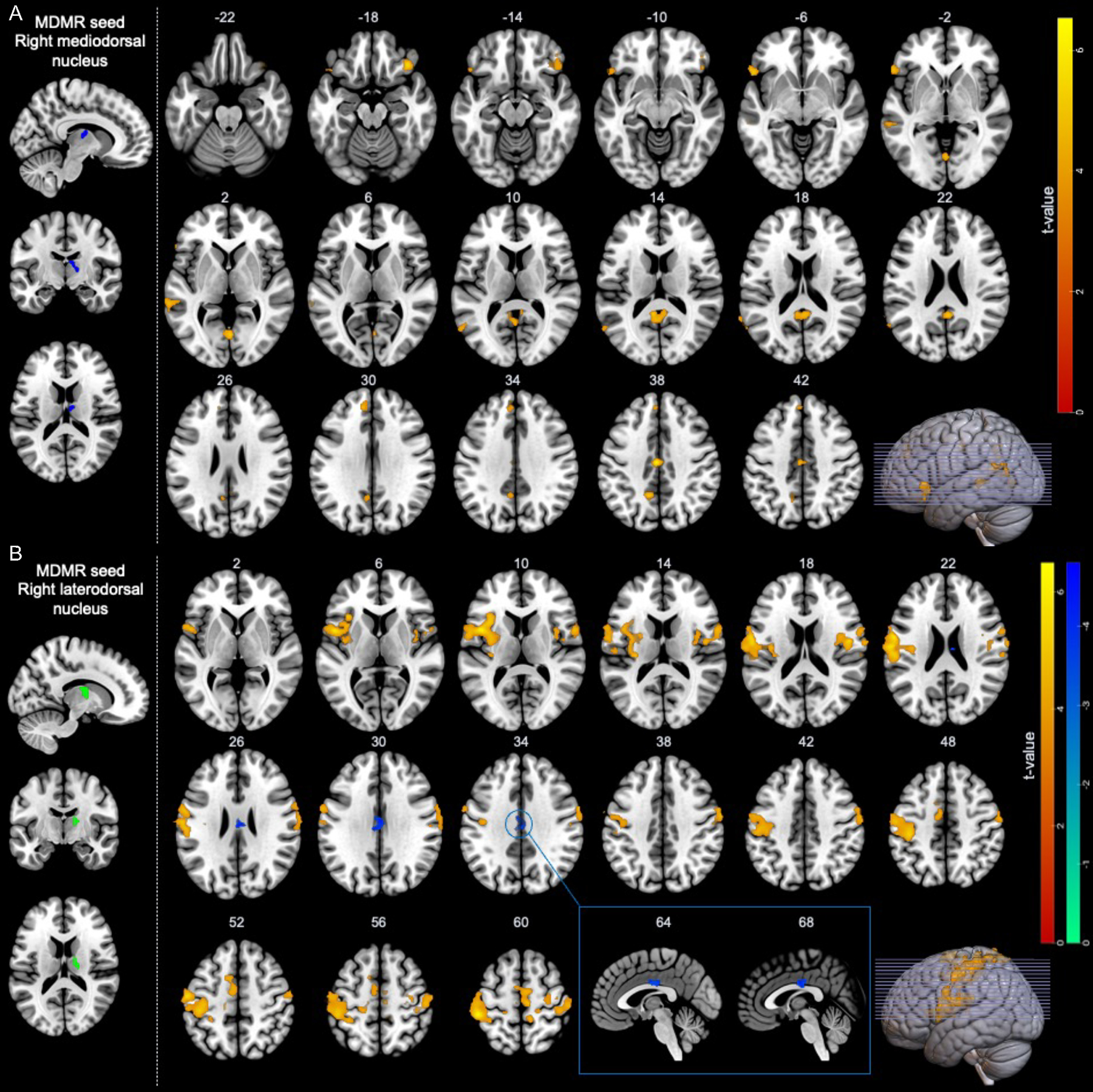
Regions showing significant functional connectivity from the right thalamus-seed associated with trait- and state-RNT during the experimentally induced RNT scan. A. Functional connectivity associated with trait-RNT (RRS-D). B. Functional connectivity associated with state-RNT (VAS).
Table 3.
Functional connectivity showing significant association with trait- and state-RNT.
| Brain regions | Brodmann Area | MNI coordinate |
Voxels (2mm3) | T-value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
|
| ||||||
| Trait-RNT (RRS-D) | ||||||
| 1. Right mediodorsal thalamus seed | ||||||
| 1.1 Bilateral retrosplenial cortex, precuneus, posterior cingulate cortex | 23, 31 | −1 | −55 | 11 | 254 | 5.11 |
| 1.2 Right lateral orbital frontal cortex | 12, 47 | 39 | 29 | −19 | 159 | 5.94 |
| 1.3 Left lateral orbital frontal cortex, inferior frontal gyrus | 44, 45, 47 | −51 | 21 | −7 | 139 | 5.19 |
| 1.4 Left superior temporal sulcus (Wernicke’s area) | 22 | −65 | −35 | 3 | 110 | 4.91 |
| 1.5 Left precuneus, posterior cingulate cortex | 23, 31 | −3 | −55 | 27 | 94 | 5.32 |
| 1.6 Bilateral lingual gyrus | 19 | 1 | −69 | 1 | 89 | 5.51 |
| 1.7 Left dorsomedial prefrontal cortex, anterior cingulate cortex | 9, 10, 32 | −5 | 41 | 33 | 87 | 4.72 |
| 1.8 Left supramarginal gyrus, superior temporal sulcus | 22, 40 | −65 | −49 | 19 | 79 | 4.66 |
| 1.9 Bilateral middle cingulate cortex | 23, 24 | −3 | −17 | 39 | 72 | 6.70 |
| State-RNT (VAS) | ||||||
| 2. Right laterodorsal thalamus seed | ||||||
| 2.1 Left primary somatosensory cortex | 1, 2, 3 | −55 | −13 | 49 | 1922 | 6.38 |
| 2.2 Left inferior frontal gyrus (Broca’s area), rolandic operculum, insula | 4, 6, 13, 43, 44 | −59 | −5 | 17 | 1705 | 6.57 |
| 2.3 Right primary somatosensory cortex, inferior frontal gyrus, rolandic operculum, insula | 1, 2, 3, 4, 6, 13, 43 | 57 | −9 | 47 | 1406 | 5.58 |
| 2.4 Bilateral primary motor area, supplementary motor area | 4, 6 | 5 | −9 | 61 | 731 | 5.25 |
| 2.5 Bilateral middle cingulate cortex | 23, 24 | −1 | −21 | 29 | 141 | −4.52 |
| 2.6 Left primary somatosensory cortex | 3 | −23 | −27 | 55 | 117 | 4.51 |
| 2.7 Right superior parietal lobule | 7 | 25 | −47 | 71 | 112 | 5.13 |
| 2.8 Right primary somatosensory cortex, superior parietal lobule | 1, 2, 3, 5 | 17 | −31 | 65 | 105 | 4.71 |
Post-hoc analysis for the connectome-wide association analysis with state-RNT
Seed-to-whole-brain connectivity analysis with the right laterodorsal thalamus cluster revealed significant associations of state-RNT (VAS) with the left inferior frontal gyrus (Broca’s area), bilateral insula, bilateral primary and supplementary motor cortex, and bilateral primary sensory cortex (t = 4.51 – 6.57), whereas significant negative association was found in the middle cingulate cortex (t = −4.52) (Fig. 2B and Table 3). Correlations between each functional connectivity and clinical measures with and without covariates can be found in the Supplementary materials (Fig. S2).
3.3. Connectome-wide association analysis for the resting-state scan
No significant clusters associated with trait-RNT were found in the striatum or the thalamus masks with the resting-state scan. The same MDMR analysis for the default mode network (DMN) masks (i.e., ventral medial prefrontal cortex (vmPFC) and posterior cingulate cortex from the brainnetome atlas (see details in the Supplementary materials 2.3 and 3.1) revealed no significant clusters either.
4. Discussion
This study investigated the hypothesis that greater intensity of trait- and state-RNT in MDD would be related to increased connectivity between the thalamus or dorsal striatum with prefrontal areas. We observed two main findings. First, greater trait-RNT (as assessed with RRS-D score) was associated with hyperconnectivity of the right mediodorsal thalamus to the prefrontal cortex including bilateral OFC, Wernicke’s area, and posterior DMN nodes (posterior cingulate cortex, precuneus, and retrosplenial cortex). Second, a more intense state-RNT was associated with hyperconnectivity of the right laterodorsal thalamus with bilateral primary sensory and motor cortices, supplementary motor areas, and Broca’s area. Taken together, these findings support the hypothesis that RNT in MDD is related to dysfunction in cortical regions involved in visceral control and higher-order cognitive processing including language processing (Tsuchiyagaito et al., 2022).
4.1. The role of the right thalamus in RNT
As predicted, we found significant connectivity patterns associated with RNT in the anterior and medial nucleus of the right thalamus. However, contrary to our hypothesis, neither trait- nor state-RNT was associated with striatal functional connectivity. RNT was related to regions involved in episodic memory and language, possibly reflecting the clinical phenomenology of this important symptom (i.e., repeatedly recalling the negative event and evaluating themselves), as we discussed in a previous communication (Tsuchiyagaito et al., 2022). RNT is usually related to vivid sensory perceptions, including visual, auditory, and interoceptive modalities (e.g., re-experiencing the moment when one was rejected by a significant other and rehearsing the adverse interpersonal interaction) (Brewin et al., 2010). RNT also involves dysfunction in social perceptions such as negative self-perception and a passive comparison with others (Flynn et al., 2010; Nepon et al., 2011). Current views of the thalamus conceptualize it as essential for sensory perception, initiation of complex behaviors and higher-order cognitive functions, and for regulating interoceptive information reaching cortical areas (Guillery, 1995; La Terra et al., 2022; Mitchell, 2015; Saalmann and Kastner, 2015; Sherman, 2007; Steward et al., 2022). Additionally, the ventral anterior and mediodorsal thalamic nuclei have been shown to display hyperconnectivity with prefrontal, temporal, sensorimotor and anterior cingulate cortices in MDD (Brown et al., 2017; Kong et al., 2018). In our study, the right mediodorsal and laterodorsal thalamus showed hyperconnectivity with regions in the DMN, salience, and language networks and with sensorimotor cortices, in relation with trait- and state-RNT. This is in line with studies reporting increased thalamo-cortical connectivity in depression and obsession (Ahmari and Dougherty, 2015; Brown et al., 2017; Kong et al., 2018; Pauls et al., 2014; Peters et al., 2016; Rotge et al., 2012). Thus, RNT may conceivably relate to a dysfunction in the gateway transferring internally generated information to the higher-order cognitions (e.g., spontaneous internal sensations and thoughts suddenly come to one’s mind as a form of unwanted, intrusive, and obsessive thoughts).
4.2. Striatal connectivity and RNT
The null findings in the striatum region were unexpected, considering prior studies highlighting the dual valence model, where the ventral and dorsal striatum play distinct roles in internalizing disorders (Admon et al., 2015; Chantiluke et al., 2012; Furman et al., 2011; Graybiel and Rauch, 2000; Kerestes et al., 2015; Manelis et al., 2016; Pechtel et al., 2013; Pittenger et al., 2011; Smoski et al., 2011; Treadway and Zald, 2011). Instead, we found significant connectivity patterns associated with RNT in the thalamus. This divergence may be due to individual differences in response to RNT induction, such as varying sensitivity to sensory perceptions involved in re-experiencing and contemplating the significant negative event, a characteristic of RNT we discussed earlier. Limited power and absence of structural connectivity assessments may further contribute to these null results in the striatum. A more detailed comparison with previous findings in internalizing disorders can be found in the Supplementary materials (Supplemental Discussion 3.1).
4.3. Trait-RNT and state-RNT
Another unexpected finding of the present study is hyperconnectivity between right thalamus and language processing areas in the dominant hemisphere. Thus, during RNT induction, we observed hyperconnectivity of the right thalamus with Wernicke’s area (in relation to trait-RNT), and with Broca’s area (in relation to state-RNT). Classically, Wernicke’s area is considered to play a significant role in listening and comprehension of verbal expressions, while Broca’s area is considered to play a pivotal role in speech production. Burgeoning evidence indicates that the thalamus has a significant role on lexical-semantic processes (Crosson, 2013, 2021; Fritsch et al., 2022; Wahl et al., 2008). In fact, in a previous resting-state fMRI data analysis, we found that functional connectivity of the bilateral superior temporal sulcus, a verbal comprehension-related region, distinguished high and low RNT individuals with MDD (Tsuchiyagaito et al., 2022). Conceivably, individuals evoking a more intense RNT state might have actually employed greater speech production (i.e., RNT as an inner-speech phenomenon(Tsuchiyagaito et al., 2022). Although this interpretation remains speculative, prior research showed that among subcortical structures only the thalamus was involved with lexical-semantic processes, which led to the proposition that syntactic and semantic language processing primarily occurs within thalamo-cortical networks, whereas a cohesive cortico-striatal network is not involved in these essential operations of language processing (Wahl et al., 2008). This is also partially in line with our null findings in the striatum.
Although the present findings offer insights regarding an RNT-related circuit, it is important to point out that the RRS-B subscale score did not show specific functional connectivity patterns of the striatum and thalamus. While the RRS-D has been observed to overlap with depression severity (Treynor et al., 2003), our results held after controlling the severity of depression and anxiety. Moreover, since RRS-D consists of 12 items, this subscale might have been more sensitive to detect individual differences in trait-RNT in our study.
4.4. Lack of associations in resting-state scan and RNT
In our analyses of resting-state scans, we did not identify any significant associations between RNT and clusters in the striatum, thalamus, or within the DMN masks. These null findings warrant careful interpretation. One possible interpretation of these null findings could be that there may not be a significant relationship between the resting-state functional connectivity and RNT in these regions. Specifically, our observation replicates similar findings in diverse large-scale studies on this subject (Goldstein-Piekarski et al., 2022; Tozzi et al., 2021). This does not, however, entirely rule out the potential involvement of these regions in RNT. Alternatively, these findings may reflect the limitations inherent in our study design. For example, the observation of positive findings in the RNT induction state and the absence of such findings in the resting-state does not conclusively suggest a RNT induction task effect, as we did not conduct a statistical comparison between the two states. The resting-state scan inherently lacks constraints, which means that participants may have been engaged in a wide range of cognitive activities. This potentially increases variability in the data, which may have reduced our ability to detect significant associations. Our study was also limited by its modest sample size. With a larger sample, we might have had more statistical power to detect subtle associations between RNT and functional connectivity in these regions during resting-state.
4.5. Limitations and conclusions
This study has several limitations. First, our sample size is relatively small, which might have reduced the sensitivity to capture RNT associated circuitry in the whole-brain. Second, a majority of the participants were female (71%) and only half were medicated (54%). Whereas this recruitment reflects an effort to make the studied sample similar to demographic characteristics of the local region, this composition might make our results not immediately generalizable. Third, only individuals with MDD were recruited. To fully understand RNT across various mental health conditions, further investigation should include other clinical, normal, and subclinical groups. This would emphasize the transdiagnostic nature of RNT and allow a more comprehensive, dimensional analysis. Fourth, approximately half of our participants had been exposed to trauma in the past, which may have influenced the current intensity of RNT. We did not measure specific trauma symptoms nor control for the potential effects of trauma in our analysis. Last, our cross-sectional findings of RNT associated functional connectivity certainly do not clarify the direction of influence between thalamus and prefrontal cortical areas, nor the causal relationship of observed findings with RNT.
In summary, hyperconnectivity of the right thalamus was associated with both trait- and state-RNT. The present and prior findings highlight a key role of this circuitry in the pathophysiology of RNT in MDD, and may be useful in the explanation of mechanisms of response to deep-brain neuromodulatory treatments in MDD, as the anterior limb of the internal capsule is the major surgical psychosurgical target in this disorder, and it constitutes a major output pathway for fibers leaving the thalamus and reaching areas related to visceral and higher-order cognitive processing (Figee et al., 2022; Georgiev et al., 2021; Mayberg, 2009; Riva-Posse et al., 2018; Riva-Posse et al., 2014). Emerging neuromodulation techniques such as low-intensity focused ultrasound, which permits precise and noninvasive modulation of deep brain structures (Darmani et al., 2022; Philip and Arulpragasam, 2023) may help to establish a causal relationship between RNT and function of the circuit described herein.
Supplementary Material
Right thalamic connectivity was associated with repetitive negative thinking (RNT).
Increased connectivity in perception, cognition, and lexical-semantic regions.
Right thalamo-cortical connectivity may refine RNT-specific therapeutic strategies.
Acknowledgments
We would like to express our appreciation to CoBRE NeuroMap Investigators at LIBR, and thank all the research participants. We acknowledge the contributions of Sahib S. Khalsa, M.D., Ph.D., Tim Collins, Dara Crittenden, Amy Peterson, Megan Cole, Lisa Kinyon, Lindsey Bailey, Courtney Boone, Natosha Markham, Lisa Rillo, Angela Yakshin, and the LIBR Assessment Team for diagnostic assessments and data collection, and Julie Arterbury, Leslie Walker, Amy Ginn, Bill Alden, Julie DiCarlo, and Greg Hammond for helping with MRI scanning. The authors acknowledge Jerzy Bodurka, Ph.D. (1964–2021) for his intellectual and scientific contributions to the establishment of the EEG, structural and functional MRI, and neurofeedback processes that provided the foundation for the data collection, analysis, and interpretation of findings for the present work.
Role of the Funding Sources
This work has been supported in part by the National Institute of General Medical Sciences Center Grant Award Number, 1P20GM121312 (MPP) and the William K. Warren Foundation. Work on this manuscript was also supported in part by U01 MH123427 and I50 RX002864 (NSP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or US Department of Veterans Affairs.
Footnotes
Conflict of Interest Disclosure:
Dr. Martin P. Paulus is an advisor to Spring Care, Inc., a behavioral health startup, he has received royalties for an article about methamphetamine in UpToDate. Dr. Martin P. Paulus has a consulting agreement with and receives compensation from F. Hoffmann-La Roche Ltd. The other authors report no financial relationships with commercial interests related to the present study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Admon R, Nickerson LD, Dillon DG, Holmes AJ, Bogdan R, Kumar P, Dougherty DD, Iosifescu DV, Mischoulon D, Fava M, Pizzagalli DA, 2015. Dissociable cortico-striatal connectivity abnormalities in major depression in response to monetary gains and penalties. Psychol Med 45(1), 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmari SE, Dougherty DD, 2015. Dissecting OCD Circuits: From Animal Models to Targeted Treatments. Depress Anxiety 32(8), 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Zoubi O, Misaki M, Bodurka J, Kuplicki R, Wohlrab C, Schoenhals WA, Refai HH, Khalsa SS, Stein MB, Paulus MP, 2021. Taking the body off the mind: Decreased functional connectivity between somatomotor and default-mode networks following Floatation-REST. Human Brain Mapping 42(10), 3216–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ, 2001. A new method for non-parametric multivariate analysis of variance. Austral ecology 26(1), 32–46. [Google Scholar]
- Arditte Hall KA, Davison EH, Galovski TE, Vasterling JJ, Pineles SL, 2019. Associations between trauma-related rumination and symptoms of posttraumatic stress and depression in treatment-seeking female veterans. Journal of traumatic stress 32(2), 260–268. [DOI] [PubMed] [Google Scholar]
- Avecillas-Chasin JM, Hurwitz TA, Bogod NM, Honey CR, 2021. Tractography-Guided Anterior Capsulotomy for Major Depression and Obsessive-Compulsive Disorder: Targeting the Emotion Network. Oper Neurosurg (Hagerstown) 20(4), 406–412. [DOI] [PubMed] [Google Scholar]
- Baldermann JC, Melzer C, Zapf A, Kohl S, Timmermann L, Tittgemeyer M, Huys D, Visser-Vandewalle V, Kuhn AA, Horn A, Kuhn J, 2019. Connectivity Profile Predictive of Effective Deep Brain Stimulation in Obsessive-Compulsive Disorder. Biol Psychiatry 85(9), 735–743. [DOI] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Davey CG, Yucel M, Pantelis C, 2012. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol Med 42(4), 671–681. [DOI] [PubMed] [Google Scholar]
- Braz BY, Belforte JE, Murer MG, Galinanes GL, 2017. Properties of the corticostriatal long term depression induced by medial prefrontal cortex high frequency stimulation in vivo. Neuropharmacology 121, 278–286. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Gregory JD, Lipton M, Burgess N, 2010. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychological review 117(1), 210–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Clark DL, Hassel S, MacQueen G, Ramasubbu R, 2017. Thalamocortical connectivity in major depressive disorder. J Affect Disord 217, 125–131. [DOI] [PubMed] [Google Scholar]
- Chang F, Berenz EC, Ajilore O, Langenecker SA, Burgess HJ, Phan KL, Klumpp H, 2023. Actigraphic Wake after Sleep Onset and Symptom Severity Correspond with Rumination in Trauma-Exposed Individuals. Brain Sci 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantiluke K, Halari R, Simic M, Pariante CM, Papadopoulos A, Giampietro V, Rubia K, 2012. Fronto-striato-cerebellar dysregulation in adolescents with depression during motivated attention. Biol Psychiatry 71(1), 59–67. [DOI] [PubMed] [Google Scholar]
- Choi KW, Han KM, Kim H, Kim A, Kang W, Kang Y, Tae WS, Ham BJ, 2020. Comparison of shape alterations of the thalamus and caudate nucleus between drug-naive major depressive disorder patients and healthy controls. J Affect Disord 264, 279–285. [DOI] [PubMed] [Google Scholar]
- Clohessy S, Ehlers A, 1999. PTSD symptoms, response to intrusive memories and coping in ambulance service workers. Br J Clin Psychol 38(3), 251–265. [DOI] [PubMed] [Google Scholar]
- Costanzo EY, Villarreal M, Drucaroff LJ, Ortiz-Villafane M, Castro MN, Goldschmidt M, Wainsztein AE, Ladron-de-Guevara MS, Romero C, Brusco LI, Camprodon JA, Nemeroff C, Guinjoan SM, 2015. Hemispheric specialization in affective responses, cerebral dominance for language, and handedness: Lateralization of emotion, language, and dexterity. Behav Brain Res 288, 11–19. [DOI] [PubMed] [Google Scholar]
- Crosson B, 2013. Thalamic mechanisms in language: a reconsideration based on recent findings and concepts. Brain Lang 126(1), 73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, 2021. The Role of the Thalamus in Declarative and Procedural Linguistic Memory Processes. Front Psychol 12, 682199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani G, Bergmann TO, Butts Pauly K, Caskey CF, de Lecea L, Fomenko A, Fouragnan E, Legon W, Murphy KR, Nandi T, Phipps MA, Pinton G, Ramezanpour H, Sallet J, Yaakub SN, Yoo SS, Chen R, 2022. Non-invasive transcranial ultrasound stimulation for neuromodulation. Clin Neurophysiol 135, 51–73. [DOI] [PubMed] [Google Scholar]
- Davidson B, Eapen-John D, Mithani K, Rabin JS, Meng Y, Cao X, Pople CB, Giacobbe P, Hamani C, Lipsman N, 2022. Lesional psychiatric neurosurgery: meta-analysis of clinical outcomes using a transdiagnostic approach. J Neurol Neurosurg Psychiatry 93(2), 207–215. [DOI] [PubMed] [Google Scholar]
- Davidson B, Hamani C, Huang Y, Jones RM, Meng Y, Giacobbe P, Lipsman N, 2020. Magnetic Resonance-Guided Focused Ultrasound Capsulotomy for Treatment-Resistant Psychiatric Disorders. Oper Neurosurg (Hagerstown) 19(6), 741–749. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Mayou RA, Bryant B, 1998. Psychological predictors of chronic posttraumatic stress disorder after motor vehicle accidents. Journal of abnormal psychology 107(3), 508–519. [DOI] [PubMed] [Google Scholar]
- Ehring T, Watkins ER, 2008. Repetitive negative thinking as a transdiagnostic process. International Journal of Cognitive Therapy 1(3), 192–205. [Google Scholar]
- Everaert J, Benisty H, Gadassi Polack R, Joormann J, Mishne G, 2022. Which features of repetitive negative thinking and positive reappraisal predict depression? An in-depth investigation using artificial neural networks with feature selection. J Psychopathol Clin Sci. [DOI] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, Fox PT, Eickhoff SB, Yu C, Jiang T, 2016. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex 26(8), 3508–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figee M, Riva-Posse P, Choi KS, Bederson L, Mayberg HS, Kopell BH, 2022. Deep Brain Stimulation for Depression. Neurotherapeutics 19(4), 1229–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn M, Kecmanovic J, Alloy LB, 2010. An Examination of Integrated Cognitive-Interpersonal Vulnerability to Depression: The Role of Rumination, Perceived Social Support, and Interpersonal Stress Generation. Cognit Ther Res 34(5), 456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch M, Rangus I, Nolte CH, 2022. Thalamic Aphasia: a Review. Curr Neurol Neurosci Rep 22(12), 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman DJ, Hamilton JP, Gotlib IH, 2011. Frontostriatal functional connectivity in major depressive disorder. Biol Mood Anxiety Disord 1(1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev D, Akram H, Jahanshahi M, 2021. Deep brain stimulation for psychiatric disorders: role of imaging in identifying/confirming DBS targets, predicting, and optimizing outcome and unravelling mechanisms of action. Psychoradiology 1(3), 118–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein-Piekarski AN, Ball TM, Samara Z, Staveland BR, Keller AS, Fleming SL, Grisanzio KA, Holt-Gosselin B, Stetz P, Ma J, Williams LM, 2022. Mapping Neural Circuit Biotypes to Symptoms and Behavioral Dimensions of Depression and Anxiety. Biol Psychiatry 91(6), 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant DM, Mills AC, Judah MR, White EJ, 2021. State and trait effects of rumination on inhibitory processes in memory: Rumination and retrieval inhibition processes. Current Psychology 40, 4875–4883. [Google Scholar]
- Graybiel AM, Rauch SL, 2000. Toward a neurobiology of obsessive-compulsive disorder. Neuron 28(2), 343–347. [DOI] [PubMed] [Google Scholar]
- Guillery RW, 1995. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat 187 (Pt 3)(Pt 3), 583–592. [PMC free article] [PubMed] [Google Scholar]
- Hamonniere T, Milan L, Varescon I, 2022. Repetitive negative thinking, metacognitive beliefs, and their interaction as possible predictors for problematic cannabis use. Clin Psychol Psychother 29(2), 706–717. [DOI] [PubMed] [Google Scholar]
- Hong W, Li M, Liu Z, Li X, Huai H, Jia D, Jin W, Zhao Z, Liu L, Li J, Sun F, Xu R, Zhao Z, 2021. Heterogeneous alterations in thalamic subfields in major depression disorder. J Affect Disord 295, 1079–1086. [DOI] [PubMed] [Google Scholar]
- Hummel KV, Trautmann S, Venz J, Thomas S, Schafer J, 2021. Repetitive negative thinking: transdiagnostic correlate and risk factor for mental disorders? A proof-of-concept study in German soldiers before and after deployment to Afghanistan. BMC Psychol 9(1), 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerestes R, Harrison BJ, Dandash O, Stephanou K, Whittle S, Pujol J, Davey CG, 2015. Specific functional connectivity alterations of the dorsal striatum in young people with depression. Neuroimage Clin 7, 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong QM, Qiao H, Liu CZ, Zhang P, Li K, Wang L, Li JT, Su Y, Li KQ, Yan CG, Mitchell PB, Si TM, 2018. Aberrant intrinsic functional connectivity in thalamo-cortical networks in major depressive disorder. CNS Neurosci Ther 24(11), 1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Terra D, Bjerre AS, Rosier M, Masuda R, Ryan TJ, Palmer LM, 2022. The role of higher-order thalamus during learning and correct performance in goal-directed behavior. Elife 11, e77177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J, Arditte KA, D’Avanzato C, Joormann J, 2013. State Rumination: Associations with Emotional Stress Reactivity and Attention Biases. J Exp Psychopathol 4(5), 471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung P, Li SH, Graham BM, 2022. The relationship between repetitive negative thinking, sleep disturbance, and subjective fatigue in women with Generalized Anxiety Disorder. Br J Clin Psychol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippitz BE, Mindus P, Meyerson BA, Kihlstrom L, Lindquist C, 1999. Lesion topography and outcome after thermocapsulotomy or gamma knife capsulotomy for obsessive-compulsive disorder: relevance of the right hemisphere. Neurosurgery 44(3), 452–458; discussion 458–460. [DOI] [PubMed] [Google Scholar]
- Maier W, Buller R, Philipp M, Heuser I, 1988. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord 14(1), 61–68. [DOI] [PubMed] [Google Scholar]
- Manelis A, Almeida JR, Stiffler R, Lockovich JC, Aslam HA, Phillips ML, 2016. Anticipation-related brain connectivity in bipolar and unipolar depression: a graph theory approach. Brain 139(Pt 9), 2554–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, 2009. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest 119(4), 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy PM, Watson H, Watkins ER, Nathan P, 2013. The relationship between worry, rumination, and comorbidity: Evidence for repetitive negative thinking as a transdiagnostic construct. Journal of affective disorders 151(1), 313–320. [DOI] [PubMed] [Google Scholar]
- Michael T, Halligan SL, Clark DM, Ehlers A, 2007. Rumination in posttraumatic stress disorder. Depression and anxiety 24(5), 307–317. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, 2012. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci 16(1), 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M, Phillips R, Zotev V, Wong CK, Wurfel BE, Krueger F, Feldner M, Bodurka J, 2018. Real-time fMRI amygdala neurofeedback positive emotional training normalized resting-state functional connectivity in combat veterans with and without PTSD: a connectome-wide investigation. Neuroimage: Clinical 20, 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M, Phillips R, Zotev V, Wong CK, Wurfel BE, Krueger F, Feldner M, Bodurka J, 2020a. Connectome-wide investigation of altered resting-state functional connectivity in war veterans with and without posttraumatic stress disorder. NeuroImage. Clinical 17, 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M, Tsuchiyagaito A, Al Zoubi O, Paulus M, Bodurka J, Tulsa 1000 I, 2020b. Connectome-wide search for functional connectivity locus associated with pathological rumination as a target for real-time fMRI neurofeedback intervention. Neuroimage Clin 26, 102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS, 2015. The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neuroscience & Biobehavioral Reviews 54, 76–88. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M, 1979. A new depression scale designed to be sensitive to change. Br J Psychiatry 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Murphy JE, Yanes JA, Kirby LAJ, Reid MA, Robinson JL, 2020. Left, right, or bilateral amygdala activation? How effects of smoothing and motion correction on ultra-high field, high-resolution functional magnetic resonance imaging (fMRI) data alter inferences. Neurosci Res 150, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepon T, Flett GL, Hewitt PL, Molnar DS, 2011. Perfectionism, negative social feedback, and interpersonal rumination in depression and social anxiety. Canadian Journal of Behavioural Science/Revue canadienne des sciences du comportement 43(4), 297. [Google Scholar]
- Nolen-Hoeksema S, Morrow J, 1991. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta Earthquake. J Pers Soc Psychol 61(1), 115–121. [DOI] [PubMed] [Google Scholar]
- Osoba A, Hanggi J, Li M, Horn DI, Metzger C, Eckert U, Kaufmann J, Zierhut K, Steiner J, Schiltz K, Heinze HJ, Bogerts B, Walter M, 2013. Disease severity is correlated to tract specific changes of fractional anisotropy in MD and CM thalamus--a DTI study in major depressive disorder. J Affect Disord 149(1–3), 116–128. [DOI] [PubMed] [Google Scholar]
- Pauls DL, Abramovitch A, Rauch SL, Geller DA, 2014. Obsessive–compulsive disorder: an integrative genetic and neurobiological perspective. Nature Reviews Neuroscience 15(6), 410–424. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA, 2013. Blunted reward responsiveness in remitted depression. Journal of psychiatric research 47(12), 1864–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SK, Dunlop K, Downar J, 2016. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front Syst Neurosci 10, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Arulpragasam AR, 2023. Reaching for the unreachable: low intensity focused ultrasound for non-invasive deep brain stimulation. Neuropsychopharmacology 48(1), 251–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Leutzinger K, Pessin S, Cassani A, Mikel O, Walsh EC, Hoks RM, Birn RM, Abercrombie HC, 2022. Neural signal variability relates to maladaptive rumination in depression. Journal of psychiatric research 156, 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Bloch MH, Williams K, 2011. Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacol Ther 132(3), 314–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ, 2011. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168(12), 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riestra AR, Aguilar J, Zambito G, Galindo y Villa G, Barrios F, Garcia C, Heilman KM, 2011. Unilateral right anterior capsulotomy for refractory major depression with comorbid obsessive-compulsive disorder. Neurocase 17(6), 491–500. [DOI] [PubMed] [Google Scholar]
- Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, McIntyre CC, Gross RE, Mayberg HS, 2018. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry 23(4), 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, Crowell AL, Garlow SJ, Rajendra JK, Mayberg HS, 2014. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry 76(12), 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS, Alloy LB, 2003. Negative Cognitive Styles and Stress-Reactive Rumination Interact to Predict Depression: A Prospective Study. Cognitive Therapy and Research 27(3), 275–291. [Google Scholar]
- Rotge JY, Aouizerate B, Amestoy V, Lambrecq V, Langbour N, Nguyen TH, Dovero S, Cardoit L, Tignol J, Bioulac B, Burbaud P, Guehl D, 2012. The associative and limbic thalamus in the pathophysiology of obsessive-compulsive disorder: an experimental study in the monkey. Transl Psychiatry 2(9), e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runia N, Yucel DE, Lok A, de Jong K, Denys D, van Wingen GA, Bergfeld IO, 2022. The neurobiology of treatment-resistant depression: A systematic review of neuroimaging studies.Neurosci Biobehav Rev 132, 433–448. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S, 2015. The cognitive thalamus. Front Syst Neurosci 9, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, Rauch SL, 2000. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. The Psychiatric clinics of North America 23(3), 563–586. [DOI] [PubMed] [Google Scholar]
- Scangos KW, Khambhati AN, Daly PM, Makhoul GS, Sugrue LP, Zamanian H, Liu TX, Rao VR, Sellers KK, Dawes HE, Starr PA, Krystal AD, Chang EF, 2021. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat Med 27(10), 1696–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(20), 22–33. [PubMed] [Google Scholar]
- Shehzad Z, Kelly C, Reiss PT, Craddock RC, Emerson JW, McMahon K, Copland DA, Castellanos FX, Milham MP, 2014. A multivariate distance-based analytic framework for connectome-wide association studies. Neuroimage 93, 74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, 2007. The thalamus is more than just a relay. Current opinion in neurobiology 17(4), 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Rittenberg A, Dichter GS, 2011. Major depressive disorder is characterized by greater reward network activation to monetary than pleasant image rewards. Psychiatry Research: Neuroimaging 194(3), 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward T, Kung PH, Davey CG, Moffat BA, Glarin RK, Jamieson AJ, Felmingham KL, Harrison BJ, 2022. A thalamo-centric neural signature for restructuring negative self-beliefs. Mol Psychiatry 27(3), 1611–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Liu M, Liu P, Zhang A, Yang C, Liu Z, Li J, Li G, Wang Y, Zhang K, 2023. Abnormal cortical-striatal-thalamic-cortical circuit centered on the thalamus in MDD patients with somatic symptoms: Evidence from the REST-meta-MDD project. J Affect Disord 323, 71–84. [DOI] [PubMed] [Google Scholar]
- Tozzi L, Zhang X, Chesnut M, Holt-Gosselin B, Ramirez CA, Williams LM, 2021. Reduced functional connectivity of default mode network subsystems in depression: Meta-analytic evidence and relationship with trait rumination. Neuroimage Clin 30, 102570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH, 2011. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 35(3), 537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S, 2003. Rumination Reconsidered: A Psychometric Analysis. Cognitive Therapy and Research 27, 247–259. [Google Scholar]
- Tsuchiyagaito A, Misaki M, Kirlic N, Yu X, Sánchez SM, Cochran G, Stewart JL, Smith R, Fitzgerald KD, Rohan ML, Paulus MP, Guinjoan SM, 2023. Real-Time fMRI Functional Connectivity Neurofeedback Reducing Repetitive Negative Thinking in Depression: A Double-Blind, Randomized, Sham-Controlled Proof-of-Concept Trial. Psychother Psychosom, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiyagaito A, Sanchez SM, Misaki M, Kuplicki R, Park H, Paulus MP, Guinjoan SM, 2022. Intensity of repetitive negative thinking in depression is associated with greater functional connectivity between semantic processing and emotion regulation areas. Psychol Med, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Marzinzik F, Friederici AD, Hahne A, Kupsch A, Schneider G-H, Saddy D, Curio G, Klostermann F, 2008. The Human Thalamus Processes Syntactic and Semantic Language Violations. Neuron 59(5), 695–707. [DOI] [PubMed] [Google Scholar]
- Wang C, Song X, Lee TMC, Zhang R, 2022. Psychometric Properties of the Chinese Version of the Brief State Rumination Inventory. Front Public Health 10, 824744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE, 2014. Permutation inference for the general linear model. Neuroimage 92, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


